Introduction
Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) are dermatologic medical emergencies characterized by rapidly progressive diffuse cutaneous necrosis most commonly caused by acute exposure to medications. These infrequent and unexpected reactions lead to significant morbidity and mortality.1 Both conditions typically begin with a prodrome of fever and malaise followed by the progressive development of cutaneous and mucosal lesions. Ocular and urogenital lesions are also common. The affected body surface area differentiates pure SJS (<10%) from pure TEN (>30%). Complications and death typically arise from infections caused by a lack of intact skin barriers.
The prevailing understanding of the pathophysiology is that an immune reaction mediates the apoptosis of keratinocytes. As such, immunosuppressants are the hallmark of treatment beyond supportive care. However, conflicting bodies of evidence support their use, and no gold standard exists.1 Older treatment options include intravenous immunoglobulins and steroids, and one emerging treatment is cyclosporine A (CsA).1
The existing literature on the benefits of CsA in adults is conflicting given that several studies support its use and others show no benefit.2,3 There is thus a need for larger cohort studies, particularly with randomization. In children, the literature on SJS/TEN in general is sparse. Regarding CsA use specifically, there are only several case reports. We thus present our experience with a pediatric SJS/TEN patient that responded rapidly to a high dose of intravenous CsA.
Case report
An 11-year-old girl of Han-Chinese origin presented to the emergency department with a rash that started on the trunk and then progressed to involve the entire face, neck, upper chest, and proximal extremities. On examination, dusky diffuse edematous patches with erosions and blisters were noted on the affected areas (Fig 1). Nikolsky sign was positive. Additionally, mucosal erosions and crusts were noted on the lips, along with a small erosion on the left labia majora. Body surface area involvement was approximately 20%. The patient's initial vital signs were blood pressure, 94/60; heart rate, 130; respiratory rate, 24; and temperature, 103.28°F. The patient's C-reactive protein was 178.20. Complete blood count, electrolytes, blood gas, liver function tests, and immunoglobulin levels were unremarkable. The patient was known to have epilepsy and was taking levetiracetam and clobazam. Two weeks before her presentation, carbamazepine was added to her therapeutic regimen.
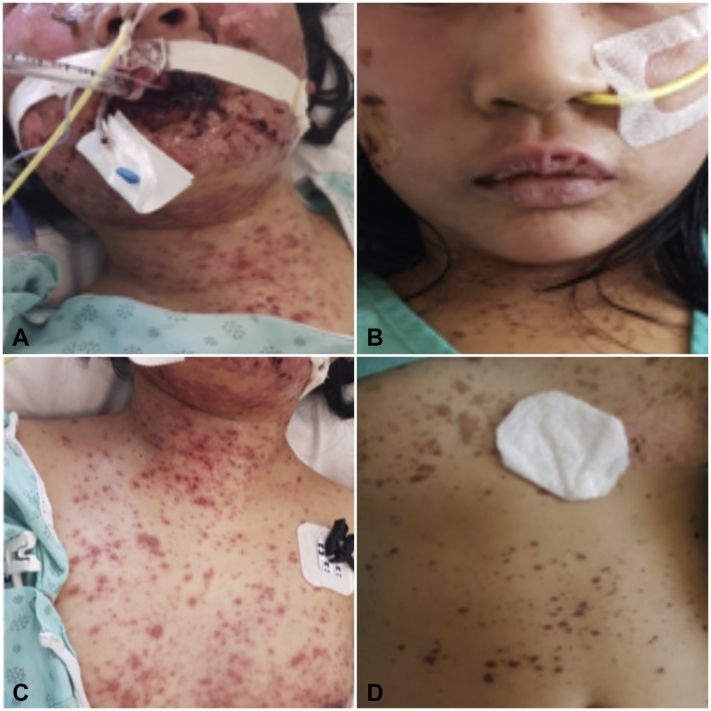
Fig 1.
Clinical progress of the SJS/TEN patient on IV CsA from day 1 to day 10. A, Facial lesions on day 1. B, facial lesions on day 10. C, Anterior torso lesions on day 1. D, Anterior torso lesions on day 10.
A 4-mm punch biopsy was conducted and the fresh-frozen section showed full-thickness epidermal necrosis out of proportion to the number of lymphocytes. This finding confirmed our clinically high suspicion of SJS/TEN overlap syndrome, most likely secondary to carbamazepine. Carbamazepine was stopped immediately, and the patient was admitted to the pediatric intensive care unit, intubated, and treated with rigorous supportive care and daily wound dressing. A multidisciplinary team was involved in her care, including the pediatric intensive care unit and the urology, ophthalmology, and gynecology departments. Blood, urine, nasopharyngeal and conjunctival cultures were ultimately negative. Chest imaging was clear. The examination by the ophthalmology department was unremarkable, and artificial tears and steroid eye drops were prescribed preventatively. The patient's severity-of-illness score for TEN was 2, corresponding with a predicted mortality of 12.1%.1 Tailoring the score to pediatric versions did not change the result.4
Following biopsy confirmation, CsA was initiated on day 1 at 3 mg/kg/d intravenously divided twice daily. Blood CsA levels were monitored to avoid toxicity. With support from the pediatric intensive care unit pharmacologist, we decided to not surpass a therapeutic level of 350 ng/mL, which is the same range used in transplant patients to avoid rejection. Our highest CsA level reached was 339 ng/mL. Most primary lesions resolved between days 5 and 7, and complete resolution was achieved by day 11. The regimen was then tapered over 10 days using oral CsA and discontinued completely on day 21. The specific tapering schedule was 3 mg/kg for 4 days, 2 mg/kg for 3 days, and finally 1 mg/kg for the last 3 days. There were no reported side effects during the therapy. Given that the patient also had erosions on her left labia majora, a mild potency topical steroid cream was applied twice daily. After 1 month of follow-up, the only abnormality noted was postinflammatory hyperpigmentation on affected regions.
Discussion
The scarcity of literature involving the pediatric population is likely related to the lower incidence of SJS/TEN in children. In the United States, the estimated incidence of SJS, SJS/TEN, and TEN among children is 5.3, 0.8, and 0.4 cases, respectively, per million children per year.5 Regarding CsA use in children, no reviews or cohort studies exist, and only 5 case studies have been published to date. Two cases demonstrate a benefit to using CsA, specifically in conjunction with intravenous immunoglobulin use. Three other cases support the use of mono CsA therapy, and our experience provides further support, uniquely for a high intravenous dose at disease onset.6
In these pediatric cases, CsA doses were between 1 and 3 mg/kg/d, and the route was either intravenous or unspecified.6, 7, 8 A proposed protocol by St John et al8 is to give pediatric patients CsA, 3 mg/kg, divided twice daily for 7 days followed by 1.5 mg/kg divided twice daily for 7 days. Although this treatment may be effective, there are not enough reports yet to create a formal guideline.
Moreover, the route of CsA administration was often unspecified and needs to be clearly described in future studies.6, 7, 8 The ideal dosage of intravenous administration needs particular delineation given that most SJS/TEN patients are unable to receive CsA orally because of severe oral erosions. Given that intravenous CsA is 3 times more bioavailable than oral CsA, it should theoretically be infused slowly over a period of 2 to 6 hours, at about one-third of the usual oral dose to avoid a nephrotoxicity effect.9 However, the fact that our patient received a full 3-mg/kg intravenous dose equivalent to 9 mg/kg orally and did not subsequently have nephrotoxicity or high blood pressure may suggest otherwise. The remarkable response achieved in our case between days 5 and 7 suggest that an initial high dose of CsA may rapidly stop the progression of the disease.
Given CsA's metabolism by the cytochrome P450 metabolic pathway, caution must be exhibited to minimize drug interactions.9 In addition to supportive therapy, our patient was concurrently receiving long-term antiepileptics (levetiracetam and clobazam) and was on empiric antibiotics (cefazolin and azithromycin). Although these are not known to interfere, we were nevertheless cautious in monitoring therapeutic CsA levels.
We draw 2 other learning points from this case. First, a well-known association exists between the human leucocyte antigen B1502 allele and carbamazepine induced SJS/TEN in the Han-Chinese.1 Unfortunately, our patient was never genetically tested. We draw attention to this lapse and concur that testing should be considered before starting carbamazepine in Han-Chinese patients. Second, vaginal stenosis and adhesions that may lead to dyspareunia or hematocolpos are known to be among the common long-term consequences in female patients with SJS/TEN, but applying topical steroid to the vulva during the acute phase has been suggested as effective in preventing this.10 Given that our patient had erosions on the labia majora, topical steroid had been applied on the affected area.
Conclusion
Our experience contributes to the growing evidence supporting CsA usage for pediatric cases of SJS/TEN. In addition, our experience highlights the possibility that a relatively high intravenous dose may be effective at rapidly achieving lesion resolution. Additional cases and randomized cohort studies are needed to better determine the appropriate dosage and route of administration.
Footnotes
Funding sources: None.
Conflicts of interest: None disclosed.
References
- 1.Schneider J.A., Cohen P.R. Stevens-Johnson syndrome and toxic epidermal necrolysis: a concise review with a comprehensive summary of therapeutic interventions emphasizing supportive measures. Adv Ther. 2017;34(6):1235–1244. doi: 10.1007/s12325-017-0530-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gonzalez-Herrada C., Rodríguez-Martín S., Cachafeiro L. Cyclosporine use in epidermal necrolysis is associated with an important mortality reduction: evidence from three different approaches. J Invest Dermatol. 2017;137(10):2092–2100. doi: 10.1016/j.jid.2017.05.022. [DOI] [PubMed] [Google Scholar]
- 3.Poizeau F., Gaudin O., Le Cleach L. Cyclosporine for epidermal necrolysis: absence of beneficial effect in a retrospective cohort of 174 patients-exposed/unexposed and propensity score-matched analyses. J Invest Dermatol. 2018;138(6):1293–1300. doi: 10.1016/j.jid.2017.12.034. [DOI] [PubMed] [Google Scholar]
- 4.Sorrell J., Anthony L., Rademaker A. Score of toxic epidermal necrosis predicts the outcomes of pediatric epidermal necrolysis. Pediatr Dermatol. 2017;34(4):433–437. doi: 10.1111/pde.13172. [DOI] [PubMed] [Google Scholar]
- 5.Hsu D.Y., Brieva J., Silverberg N.B., Paller A.S., Silverberg J.I. Pediatric Stevens-Johnson syndrome and toxic epidermal necrolysis in the United States. J Am Acad Dermatol. 2017;76(5):811–817.e4. doi: 10.1016/j.jaad.2016.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zielinska M., Matusiak Ł., Gołębiowski W. Toxic epidermal necrolysis in an 8-year-old girl successfully treated with cyclosporin A, intravenous immunoglobulin and plasma exchange. Postepy Dermatol Alergol. 2018;35(2):217–221. doi: 10.5114/ada.2018.75247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aihara Y., Ito R., Ito S., Aihara M., Yokota S. Toxic epidermal necrolysis in a child successfully treated with cyclosporin A and methylprednisolone. Pediatr Int. 2007;49(5):659–662. doi: 10.1111/j.1442-200X.2007.02439.x. [DOI] [PubMed] [Google Scholar]
- 8.St John J., Ratushny V., Liu K.J. successful use of cyclosporin A for Stevens-Johnson Syndrome and toxic epidermal necrolysis in three children. Pediatr Dermatol. 2017;34(5):540–546. doi: 10.1111/pde.13236. [DOI] [PubMed] [Google Scholar]
- 9.Kahan B.D. Cyclosporine. N Engl J Med. 1989;321(25):1725–1738. doi: 10.1056/NEJM198912213212507. [DOI] [PubMed] [Google Scholar]
- 10.Rowan D.M., Jones R.W., Oakley A., de Silva H. Vaginal stenosis after toxic epidermal necrolysis. J Low Genit Tract Dis. 2010;14(4):390–392. doi: 10.1097/LGT.0b013e3181ddf5da. [DOI] [PubMed] [Google Scholar]