Abstract
Pharmaceutical and personal care products (PPCPs) are the one of sub-class under emerging organic contaminants (EOCs). Ibuprofen is the world's third most consumable drug. This drug enters into our water system through human pharmaceutical use. It attracts the attention of environmentalist on the basis of risk associated, presence and transformation in the environment. The detection and removal are the two key area where we need to focus. The concentration of such compounds in waterbodies detected through conventional and also by the advanced methods. This review we described the available technologies including chemical, physical and biological methods, etc used the for removal of Ibuprofen. The pure culture based method, mixed culture approach and activated sludge culture approach focused and pathway of degradation of ibuprofen was deciphered by using the various methods of structure determination. The various degradation methods used for Ibuprofen are discussed. The advanced methods coupled with physical, chemical, biological, chemical methods like ozonolysis, oxidation and adsorption, nanotechnology based methods, nanocatalysis and use of nonosensors to detect the presence of small amount in waterbodies can enhance the future degradation of this drug. It is necessary to develop the new detection methods to enhance the detection of such pollutants. With the developments in new detection methods based on GC-MS//MS, HPLC, LC/MS and nanotechnology based sensors makes easier detection of these compounds which can detect even very minute amount with great sensitivity and in less time. Also, the isolation and characterization of more potent microbial strains and nano-photocatalysis will significantly increase the future degradation of such harmful compounds from the environment.
Keywords: Ibuprofen, Emerging organic pollutants, Bioremediation, Degradation pathway, Degradation intermediates, Nano based methods, Environmental science, Microbial ecology, Environmental analysis, Environmental hazard, Environmental health, Toxicology
Ibuprofen, Emerging organic pollutants, Bioremediation, Degradation pathway, Degradation intermediates, Nano based methods; Environmental science; Microbial ecology; Environmental analysis; Environmental hazard; Environmental health; Toxicology.
1. Introduction
Water is the essential substance in our ecosystem and it is required to fulfill the basic needs of all organisms from drinking, bathing, transportation, etc (Yu et al., 2006). We cannot imagine life without waterb the water quality is decreasing day by day. There are various artificial and natural causes behind the declining of the water quality but one of the major reasons is human interference. From basic need to industrial needs humans required water. But due to human interference many new pollutants are entering in our water resources which causes the declining in the rate of water quality increased many fold. The increase in world population, industrialization, increased in the area under agricultural sector and declining of green cover are the major reason behind the drastically declining water quality (Jurado et al., 2012). The entry of inorganic and organic pollutants in water bodies lead to deterioration in the water quality. Emerging organic pollutants (EOCs) are some of the artificial organic substances which are not present in the environment naturally, cause maximum damage to the ecosystem and introduced in the environment due to industrialization and manmade activity. Pharmaceutical and personal care products (PPCPs) are the one of sub-class under EOCs. The PPCPs are considered to be having more potential to degrade the environment and water quality than any other pollutants due to their bioactive nature and hazardous toxic metabolites (Chopra and Kumar, 2018, 2020). A large number of PPCPs are consumed every day, these compounds and their partial metabolic products enter into water bodies via the sewage system. However, the traditional water waste treatments plants (WWTPs) are not efficient to remove these compounds. Due to f these facts such types of pollutants are frequently observed in the WWTPs and also in surface water. Due to leaching of water, these compounds reached to the groundwater and thus may even present is drinking water (Tambosi et al., 2010).
The Ibuprofen is the third most popular, highly prescribed and most salable over the counter medicine in the world (Marchlewicz et al., 2015). It is one of the drug listed in Essential Drugs list 2010 made by World Health Organization (WHO). The toxicity and concentration of Ibuprofen in waste water treatment plants (WTPs) and water bodies are increasing day by day due to increased rate of consumption due to population pressure. This might pose a hazardous impact on the environment due to its bioactive nature. This is becoming ubiquitous to water bodies because it cannot be effectively removed by conventional water treatment methods. In recent years, its intake is increased as reported in many European Union (EU) countries (Hudec et al., 2012; Parolini et al., 2011). The therapeutic doses of Ibuprofen are ranging from 600 to 1200 mg/day. The oral dose of ibuprofen is completely (~99%) form the bond with plasma albumin rapidly and almost 15% of it is excreted by humans as unchanged molecule (conjugated with glucuronide and thiol) and also its metabolites like carboxyibuprofen, hydroxyibuprofen and carboxyhydratropic acid. The conjugates are further hydrolyzed in environment (Marchlewicz et al., 2015; Murdoch and Hay, 2015). While in WWTPs and sewage wastewater contain metabolite like 4-isobutyricbenzaldehyde, 1-(4-isobutylphenyl)-1-ethanol, 2-[4-(1-hydroxyisobutyl) phenyl] propionic acid, 4-ethylphenol, 4- ethylbenzaldehyde and 1-ethyl-4-(1-hydroxy) isobutylbenzene (Rácz et al., 2012; Zheng et al., 2011). Currently, research conducted in different parts of the world using techniques like the based on biosensors including the enzymatic biosensors, microbial biosensors and immune-sensors etc. The surface plasmon resonance (SPR) bases methods are emerging as promising technique to analyze the concentration of various emerging pollutants in various water bodies, due to their better efficiency, in real time monitoring, and economical benefits (Boruah and Biswas, 2018; Sanvicens et al., 2011). These techniques attract the attention of environmentalists for pharmaceutical and personal care products (PPCPs), a subcategory of EOCs. The PPCPs are bioactive in nature and their presence caused vast damage to water quality and adverse effect on life. The world third most consumable drug Ibuprofen is a non-steroidal anti-inflammatory drugs (NSAIDs; a subclass of PPCPs) detected in many water bodies. Presently, the concentration is varies from ppb to ppt range as per load on ecosystem (Murdoch and Hay, 2015). Ortiz de García et al. (2014) studied the ecotoxicology of 26 PPCPs drugs in aquatic systems in Spain and their environmental risk assessed. They reported that ciprofloxacin, acetaminophen, clofibrate, ibuprofen, clarithromycin, triclosan, parabens, omeprazole, and 1,4-benzoquinone has d some type of risk to the aquatic environments and/to WWTPs. This review is on the attempt for the use of current approaches for removal of Ibuprofen by various methods. The use of analytical techniques also discussed for the removal of Ibuprofen present in the environmental sources.
2. Source, occurrence and toxicity of Ibuprofen
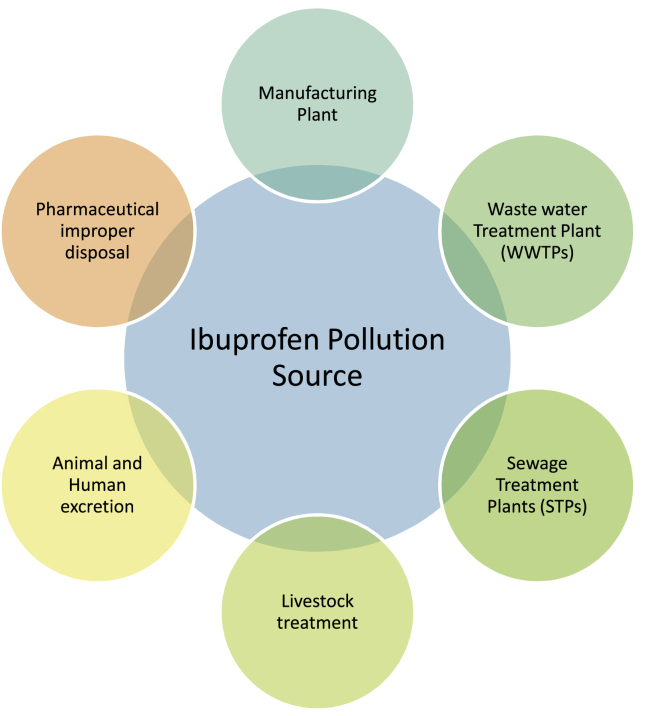
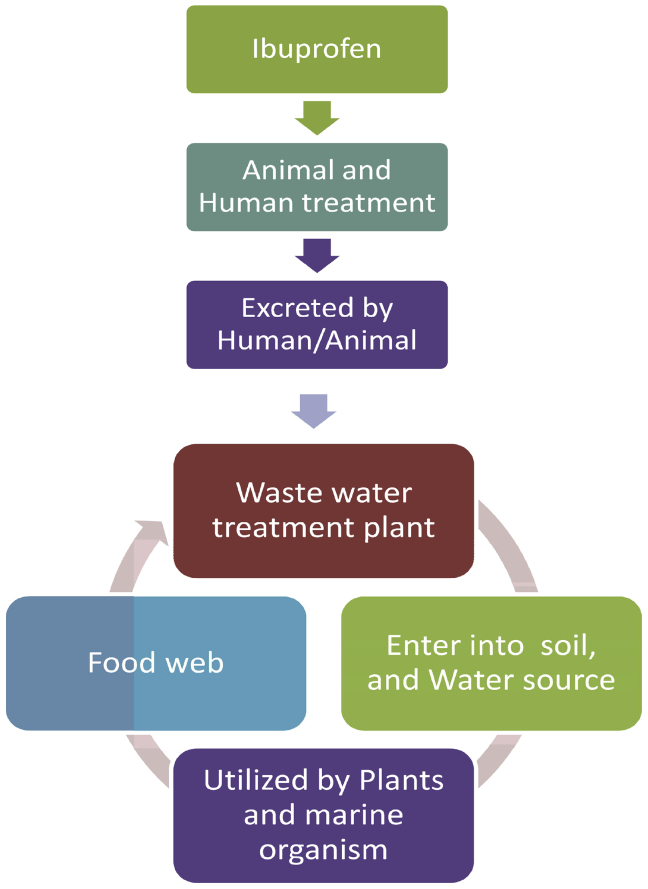
The anthropogenic activity are responsible for increasing of concentration of this drug into the environment. Although, its improper disposal, manufacturing plants, WWTPs, sewage treatments plants (STPs), livestock treatments etc., are also the reason behind the entering of Ibuprofen into environment (Figure 1). After consumption by the humans and animals, it didnot completely metabolized and gets excreted. The enzymes present in human and animals metabolize it into various metabolites. The excretion product contains both Ibuprofen and its metabolites. Its metabolites are more toxic than it parent molecule. After excretion, it enters into WWTPs, STPs, river, lake, oceans, soil, ground water etc. Further it is consumed by plans and the aquatic organisms. This gave a path it to entering into our food web (Figure 2). The main sources for monitoring it are STPs and WWTPs (Daughton and Ternes, 2001). But, it does not mean that the Ibuprofen is present only in STPs and WWTPs. Now days, it is more frequently detected in many water bodies like rivers, lake, coastal areas, ground water etc. Different technologies were used to identify the Ibuprofen in different water bodies and concentration detected different types of environmental samples (Tables 1 and 2). The concentration and its effect on aquatic organisms like adult Zebrafish (Danio rerio) studied by using UPLC-TOF/MS (Song et al., 2018).
Figure 1.
Source of occurrence of Ibuprofen.
Figure 2.
Pathway of Ibuprofen entering into food web.
Table 1.
Source of occurrence and detecting methods used to identify Ibuprofen in water bodies.
| Sr. No. | Location | Concentration | Technique Used | Reference |
|---|---|---|---|---|
| 1. | South Africa | 19.2 μg/L | HPLC equipped with photo diode array detector | (Madikizelaand Chimuka, 2017) |
| 2. | Macherey& Nagel, Düren, Germany | 3.5 μg/L | solid phase microextraction (SPME)combined with gas chromatography/mass spectrometry (GC/MSD) | (Huppert et al., 1998). |
| 3. | River Mississippi, USA | 34 ng/L | solid phase extraction using two-layer disks consisting of C18 and SDB-XC | (Zhang et al., 2007). |
| 4. | Lake Erie basin, North Ohio, USA |
1.2 μg/L | LC-MS/MS | (Wu et al., 2009) |
| 5. | Tula Valley, Mexico | 1406 ng/L | GC–MS | (Gibson et al., 2010) |
| 6. | River Mankyung, South Koria | 414 ng/L | LC-MS/MS | (Kim et al., 2009) |
| 7. | Pearl River Delta in South China | 1417 ng/L | HP 6890 GC with a Micromass Platform II massdetector | (Peng et al., 2008) |
| 8. | STP-influent stream in Taiwan | 2200 ng/L | LC/MS/MS | (Fang et al., 2012) |
| 9. | 6 WWTPs | 7800–8600 ng/L | LC/MS/MS | (Guerra et al., 2014) |
| 10. | DosacoChowk, 9–10 km Sheikhupura Road, Lahore-Pakistan | 610 μg/kg-6046 μg/kg, | LC-20A system (Shimadzu, Japan) equipped with UV detector; HPLC | (Ashfaq et al., 2017) |
| 11. | South China, the Pearl River Delta | - | Stereoisomeric profiling | (Wang et al., 2013) |
Table 2.
Ibuprofen detection in various types of environmental samples.
| S.No. | Samples | Location and concentration | Reference |
|---|---|---|---|
| 1. | Wastewater | Canada (45 μg/L) Pakistan (703–1673 μg/L) South Africa (1.38 μg/L) Belgium (5.78 μg/L) |
Guerra et al., (2014); Vergeynst et al., (2015); Ashfaq et al., (2017); Matongo et al., (2015) |
| 2. | Sludge | South Africa (0.009 μg/kg) Pakistan (2053–6064 μg/kg). |
Matongo et al., (2015); Ashfaq et al., (2017) |
| 3. | WWTPs | Greece, Sweden, Switzerland, United Kingdom, Bosnia and Herzegovina, Croatia, Serbi, China and Korea (0.004 and 603 μg/L) | Luo et al., (2014) |
| 4. | Soil and soil irrigation | Soil in Pakistan (321–610 μg/kg) Soils irrigation in eastern Spain (0.213 μg/L) |
Ashfaq et al., (2017); Vazquez-Roig et al., (2012) |
| 5. | Surface waters | Canada (0.98 μg/L) Greece (1.0–67 μg/L) Korea (<15–414 μg/L) Taiwan (5.0–280 μg/L) France (8.0 μg/L) China (1417 μg/L) |
Almeida et al., (2013); Luo et al., (2014) |
| 6. | Groundwater | Europe is 3 ng/L–395 ng/L | Luo et al. (2014) |
Their toxicity study is mainly conducted on daphnia and fish to determine the acute and chronic toxic effect. This allows identifying it's no observed effect concentration (NOEC), which used further to calculate predicted no-effect concentration (PNEC). The estimated real risk ratio of ibuprofen was ≤1, this suggested that it was an environmental risky substance (Bouissou-Schurtz et al., 2014). Its toxic effects was shown on various model organisms like Asterias rubens, Psammechinus miliaris, Arenicola marina, Allivibrio fischeri, Navicula sp., Chlorella vulgari Acutodesmus obliquus, Chlamydomonas reinhardtii, Nannochloropsis limnetica, Daphnia magna, Oryzias latipes, Oncorhynchus mykiss, Neocaridina denticulata, Scenedesmus subspicatus, Daphnia magna, Pseudokirchneriella subcapitata, Danio rerio, Rutilus rutilus, Pimephales notatus, Daphnia longispina, Menidia beryllina Oreochromis niloticus and Mytilus galloprovinciali (Mohd Zanuri et al., 2017; Di Nica et al., 2017; Ding et al., 2017; Geiger et al., 2016; Grzesiuk et al., 2016; Du et al., 2016; Jeffries et al., 2015; Sung et al., 2014; González-Naranjo and Boltes, 2014; Brozinski et al., 2013; Gonzales-Rey and Bebianno, 2012; Ragugnetti et al., 2011; Flippin et al., 2007; Fent et al., 2006). It also decreases the fish spawning and simultaneously increased the eggs number in Oryzias latipes; Japanese medaka (Flippin et al., 2007). It also caused the endocrine disruption in Mytilus galloprovincialis while present in low concentration (250 ng/L). Moreover, its presence caused the induction of antioxidative stress. It also increased the activity of catalase, superoxidase dismutase, phase II glutathione S-transferase and glutathione reductase after exposure (within 7 days). The membrane damage in digestive gland and lipid eroxidation level increased in mussels was observed (Gonzales-Rey and Bebianno, 2012). The genes down regulation caused aerobic respiration, skeletal development and immune function damage at concentration 11.5 μg/L while at higher concentrations increased expression of arachidonic acid metabolism pathway by regulatory genes and inflammatory response by immune genes (Jeffries et al., 2015). After considering these facts, it is very necessary to build up the more effective method and advance technologies for Ibuprofen removal from environment.
The acute toxicity of pharmaceutical pollutants (Ibuprofen) is calculated by short-term EC50 (10 and 100 mg/L); cyto- and genotoxic effects are analyzed with prolonged exposure to analgesics. The long exposure is mainly concerned with cell oxidative status imbalance (Parolini and Binelli, 2012). Furthermore, all additional consequences like changes in growth rate, behavior, reproduction and alterations at biochemical level are observed. Kayani et al. (2009) observed that conjugation of Ibuprofen and diacylglycerol (ibuprofen- DG) was accountable for the inhibition of cell division and non-disjunction of chromosomes to several pairs. Han et al. (2010) investigated the chronic toxicity of ibuprofen by hormonal balance under in vitro condition using the H295R cell line on Oryzias latips, Moina macrocopa and Daphenia magna. They revealed that Ibuprofen increased the 17- β estradiol production, decreased testosterone production and aromatase activity. In addition to this the 0.1 μg/L ibuprofen has resulted inr delayed hatching process. Lange et al. (2006) showed that 1–100 ng/L of ibuprofen was responsible for decrease the activity of amphipod crustacean in Gammarus pulex.
3. Removal methods of Ibuprofen
The various advance techniques such as adsorption (powdered activated carbon (PAC), granular activated carbon (GAC) etc.), biofiltration (trickling filter, sand filters, biological activated carbon (BAC) filter etc.), reverse osmosis, attached growth technology, membrane bioreactors, nanofiltration, carbon nanocomposites and magnetic nanoparticles ((AC)/CoFe2O4) are used for the removal for PPCPs (Saucier et al., 2017; Luo et al., 2014). These types of techniques are in developing phase and lot more avenues are available for research in these directions. But, due to lack of optimal treatment methods lead to release of many pollutants (PPCPs, pesticides, hydrocarbons etc.) and their metabolites into environment (Rozas et al., 2016). Theentry of these compounds into various water bodies lads to long-term adverse effects on aquatic life (primary producers, cnidarians, cladocerans, mussels, or fish) (Parolini and Binelli, 2012; Grujíć et al., 2009). In addition to this, these drugs are biological active molecules which were designed to cure disease and slowly metabolized. Oliveira et al. (2015) identified the negative impacts of the drugsin the form of biocoenosis, low biodegradability and pseudo-persistence.
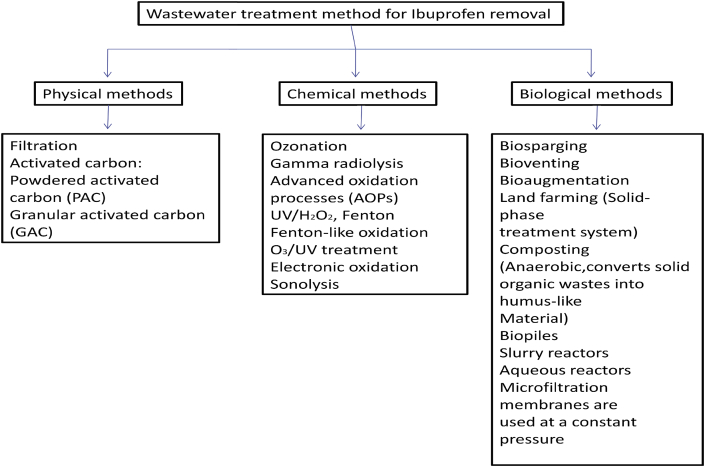
The detection of EOCs in wastes water is carried out with automatic methods which are highly sensitive, precise and selective to the many compounds (Lorenzo et al., 2018). Currently, new contaminants are entering in wastewater which led to increase or suppress the detection signals. This limits the quantification and detection of contaminants in analysis. Therefore, it is necessary to improve the technologies and pre-treatment method of samples (pure extract) to detect pollutants in waste water by easy methods (Khan et al., 2020). In the water bodies such type of pollutants makes a complex mixture along with other pollutants present in water. The techniques used to measure the water quality are based on the parameters viz. electrical conductivity, pH, turbidity, chemical oxygen demand (COD), biochemical oxygen demand (BOD), total suspended solids (TSS), total dissolved solids (TDS) and coliform count. The environmentalists major thrusts are on the nutrient load, presence of heavy metals, microbes, and other types of priority pollutants present in water (Daughton and Ternes, 2001; Daughton et al., 2009; Rodriguez-Narvaez et al., 2017). The identification of these pollutants are not possible through conventional detection methods. It is necessary to develop the new detection methods to enhance the detection of such pollutants. Therefore, new detection methods based on techniques such as GC-MSMS, HPLC, and LCMS etc deploying nanotechnology based sensors makes easier detection of these compounds. The Ibuprofen concentrations in the water bodies are low and it has been observed that their low concentration does not indicates its safe status to living system. The continuous interaction of these molecules with the living system causes the long term adverse effect. Presently, the removal method of Ibuprofen has increased attention of researcher around the world. Their r removal based methods are divided into three categories: physical, chemical and biological methods (Figure 3). There are various advanced technology are available, such as advanced oxidation processes (AOPs), coagulation-flocculation, membrane processes, membrane bioreactor (MBR), biodegradation by strain and microbial consortium for removal of Ibuprofen (Table 3). More recently, Sharma B. et al. (2019) reviewed that the organic waste including the municipal solid waste (MSW) containing diverse types of EOCs, pesticides, PPCPs, and xenobiotics. To increase the removal efficiency of Ibuprofen there are e following points which need to give attention.
-
1.
The effect of concentration of Ibuprofen on the removal efficiency of microbes. Because different microbes take different route for degradation of Ibuprofen.
-
2.
The role of microbial community in Ibuprofen degrading is accounted. It is still not clear in activated sludge system which microbial community degrades Ibuprofen.
-
3.
Biodegradation of Ibuprofen is an effective and low cost method. While removal of Ibuprofen by activated sludge biodegradation and biosorption contributed simultaneously.
-
4.
The intermediate compounds produced during degradation seems to be more toxic than the parent compound. The pathway of degradation and the intermediates produced during degradation need to be detected
Figure 3.
Different methods for Ibuprofen removal.
Table 3.
Different selective advance wastewater treatment methods for Ibuprofen removal.
| Technology used | Condition and Uses of chemicals/radiation/strains | Removal efficiency (%) | Reference |
|---|---|---|---|
| Coagulation-flocculation | Chemical: FeCl3/Al2(SO4)3; Concentration: 25, 50 mg/L |
12.0 ± 4.8% | Suárez et al. (2009), Luo et al. (2014) |
| Ozonation/AOPs | Radiation: UV254 Time: 10 min |
34% | Luo et al. (2014) |
| Ozonation/AOPs | Combine method: UV254 and H202 (50 mg/L) Time: 10 min, 30 min |
Almost 100% | Luo et al. (2014) |
| Ozone oxidation | Concentration: 1 mg/L, 160 mg/L for 20 min, | 99% | Wang and Wang (2016) |
| Membrane processes | Material: PES flat-sheet of 100 kDa Pressure = 0.5 ± 0.01 bar |
7% | Jermann et al. (2009), Luo et al. (2014) |
| Membrane processes | Material: Filmtec TW30 Pressure: 9.5–10.2 bar |
>99% | Sahar et al. (2011), Yangali-Quintanilla et al. (2011), Luo et al. (2014) |
| Membrane bioreactor | Module: Full-scale HF (Koch Puron) MAb: 235 m2 pore size: 0.1–0.2 μm |
~100% | Trinh et al. (2012), Luo et al. (2014) |
| Membrane bioreactor | Module: Lab-scale submerged HF UF module MA: 0.047 m2 Pore size: 0.04 μm SRT: 70 days HRT: 24 h MLSS: 8.6–10 g/L |
96.7 ± 0.7% | Tadkaew et al. (2011), Luo et al. (2014) |
| Membrane bioreactor | Module: Lab-scale polyvinylidene fluoride HF MA: 0.2 m2 Pore size: 0.4μm HRT: 1 or 3 days MLSS: 2.3–4.6 g/L |
Almost 100% | Bo et al. (2009), Luo et al. (2014) |
| Attached growth treatment processes | Media: bioplastic-based biofilm carriers volume: 2.5 L |
Almost 100% | Falås et al. (2012), Luo et al. (2014) |
| Activated sludge with high nitrifying activity in sequencing batch reactor (SBR) | Biodegradation time: after 24 h in water | 76% | Kruglova et al. (2016) |
| Grit channels, primary clarifies and conventional activated sludge | Initial concentration: 4500 ng/L | 99.7% | Blair et al. (2015) |
| Primary treatment + Orbal oxidation ditch + UV disinfection | Initial concentration: 130–450 ng/L | 60–90% | Sun et al. (2014) |
| Fenton oxidation | Initial concentration: 0.87 mM Temp: 30 °C pH: 3 Time: 2h Concentration: Fe2: 25% (1.2 mM), H2O2 25% (0.32 mM) |
>50% | Wang and Wang (2016) |
| Microbial biodegradation | Bacillus thuringiensis B1(2015b) | 20 mg/L in 6 days | Marchlewicz et al., 2017a, Marchlewicz et al., 2017b,Marchlewicz et al. (2016) |
| Microbial biodegradation | Patulibacter sp. I11 | 125 μg/L, 31 μg/L, 46 μg/L in 300 h, 90 h, 90 h respectively | Almeida et al. (2013) |
| Microbial biodegradation | Variovorax sp. Ibu-1 | 200 mg/L in 75 h | Murdoch and Hay (2015) |
| Microbial biodegradation | Nocardia sp. NRRL 5646 | 1000 mg/L in 120 h | Chen and Rosazza (1994) |
3.1. Removal of Ibuprofen by chemical methods
The advanced chemical processes are mainly focused on wastewater treatments. The advanced chemical processes include ozonation, gamma radiolysis, advanced oxidation processes (AOPs), UV/H2O2, Fenton and Fenton-like oxidation, O3/UV, electronic oxidation and sonolysis (Wang and Xu, 2012) and degradation of Ibuprofen from the liquid system using electro-Fenton process (Loaiza-Ambuludi et al., 2013). In ozonization the ozone is used for oxidation method for the removal of Ibuprofen. Ozone is mainly depends on the strong non-selective oxidizing activity of hydroxyl radicals (Bai et al., 2016). The mechanism behind the ozonation is the formation of hydroxyl radicals. The ultra-pure water was produced from160 mg/L to 1 mg/L and 0.1 mg/L to 12 g/L of Ibuprofen removed in 20 min at pH 9, 25 °C by ozonization (Quero-Pastor et al., 2014a, 2014b) Ozonation is an oxidation process shows efficient removal of Ibuprofen. One of the popular methods for portable water disinfection is Ultraviolet (UV) treatment. UV light disrupts the bonds of the chemical present in waterbodies so method used for removal of PPCPs (Kim et al., 2009). The gamma irradiation is one of the AOPs, rising as efficient technology for the removal of EOCs in wastewater. The removal efficiency of Ibuprofen was decreased with the increase in pH from 1.45 to 11.0 this indicating that it involved H ion radical reaction (Zheng et al., 2011). Mendez-Arriaga et al., in 2010 used Fenton technology at pH 3; 30 °C temperature for 2 h with use of Fe2+-1.2 mM, H2O2-0.32 mM for the removal of 0.87 mM Ibuprofen with >50% removal efficiency. While with Photo-Fenton at 30 °C, pH 3 for 2 h with Fe2+-1.2 mM, H2O2- 0.32 mM achieved almost 100% removal efficiency of 0.87 mM of Ibuprofen (Mendez-Arriaga et al., 2010).
Ibuprofen is more effectively removed by AOPs sub-class under physicochemical methods (Góngora et al., 2017; Iovino et al., 2016; Huang and Liu, 2015). The oxidation of Ibuprofen is started by the high reactive hydroxyl radicals during the AOPs (Góngora et al., 2017; Li et al., 2015a, Li et al., 2015b; Braz et al., 2014). Metabolites like 4-isobutylphenol, Hydratropic acid, 4-(1- carboxyethyl) benzoic acid, 4-ethylbenzaldehyde, 2-[4-(1-hydroxy-2-methylpropyl) phenyl] propanoic acid, 1-(4-isobutylphenyl-1-ethanol, 4-acetylbenzoic acid 1-isobutyl-4-vinylbenzene and 4-isobutylacetophenon are produced during this process (Ruggeri et al., 2013; Sabri et al., 2012; Caviglioli et al., 2002). However, these metabolites possess more toxicity than Ibuprofen identified in many studies (Huang and Liu, 2015; Braz et al., 2014; Quero-Pastor et al., 2014a, Quero-Pastor et al., 2014b). Du et al. (2019) used multi-walled carbon nanotubes (MWCNTs) as catalyst and ibuprofen and acetylsulfamethoxazole removal enhanced due to higher OH˙ formation.
3.2. Removal of Ibuprofen by physical method
The adsorption is most used physical process, which offers the removal of organic pollutants in wastewater. Various adsorbents are used to enhance the efficiency of removal of Ibuprofen. In tradition adsorbent the activated carbon is widely used for water treatment, it can also remove the partial concentration of Ibuprofen from waste water. The activated carbon was categorized into: powdered activated carbon (PAC) and granular activated carbon (GAC). The PAC concentration of 5 g/L and 10 mg/L was used to remove Ibuprofen concentration of 100 ng/L and 40 mg/L from surface and synthetic water respectively (Snyder et al., 2007; Mestre et al., 2007). The graphene was used by Rizzo et al., in 2015 for removal of 10 mg/L of Ibuprofen from synthetic water with the efficiency of approximate 95%. Wang et al. (2016) reported the Ibuprofen removal via multi-nanotube method from the mixture or various drugs from the river water. Hydrocar (Coal-like product) also known as hydrothermally carbonized material made by transformation of biomass through thermo-chemical process (Titirici et al., 2008). The hydrothermal carbonization (HTC) or hydrous pyrolysis is made through the decomposition of biomass in presence of subcritical water (Funke and Ziegler, 2010). The HTCs and its composites are used in Li/Na ion batteries electrodes, fuel cells and super capacitors for the adsorption, catalysis, environmental science etc (Deshmane et al., 2013; Qu et al., 2013; Titirici, 2012; Dong et al., 2011; Rillig et al., 2010). It is also potential used as contaminant remediation and soil improvement (Alatalo et al., 2016; Shi et al., 2015; Malghani et al., 2013). Previously, PPCPs removal was performed by the use of hydrocar made from sucrose or carbon products like orange peel and cork granules (Fernandez et al., 2015; Mestre et al., 2014, Mestre et al., 2015). Plósz et al. (2012) suggested a modeling framework approach using activated sludge for trace substances; which enhances the degradation of carbamazepine and diclofenac by activated sludge. The degradation of Ibuprofen can also enhance by assessing the factors which influence the degradation. Nanophoto-catalytic reactor for degradation is one of future technology for real-time degradation of pollutants (Shankar et al., 2004).
3.3. Removal of Ibuprofen by biological methods
In many toxicological studies, it is found that the intermediates formed during the advance chemical treatment pose more toxicity than parent compounds (Quero-Pastor et al., 2014a, Quero-Pastor et al., 2014b). Therefore, the biodegradation of Ibuprofen seen as a future alternative for the removal of Ibuprofen from the waterbodies. The use of biodegradation provided advantages over conventional and AOC methods like an economical, natural process and wide optimal conditions. Microbes used Ibuprofen in their metabolism as a carbon and energy source. Various microbes each other to remove the pollutants present in waterbodies. Various methods were proposed for bioremediation of various pollutants showed in Table 4. Biological treatment is considered as an important method for the micro pollutants removal (Falås et al., 2012). Although, bioremediation processes has been identified as an efficient cleaning technology. But they are characterized by some disadvantages. Such as: (1) the possible effect of bioremediation technology on the native microenvironment, (2) some xenobiotics have lower susceptibility rate during biodegradation, (3) genetics modified microbes are impossible to remove after biodegradation (4) intermediates produced during biodegradation having more toxic impact on system then their parent molecule and (6) contamination present in soil, water and gases are more feequent. The microorganisms which are able to degrade are often exposed to vibrating temperatures and pH. Although, environmental and economical conditions such as availability of additional carbon sources, high levels of xenobiotics required and price (Kumar et al., 2011; Sharma, 2012). There are many microorganisms having the capability to use Ibuprofen as carbon and energy sources. But the metabolic pathway of degradation and enzymes involved in degradation is poorly characterized. In the recent review by Zur et al. (2018), the authors described the current research and toxicity and biodegradation methods of paracetamol and ibuprofen along with the utility of bioinformatics based approaches to understand the genetic bases of microorganisms involved in degradation of such xenobiotics compounds. Bioremediation and biodegradation based methods seems to be a promising alternative to the chemical methods. But, the presence of ibuprofen in the environment, their toxic effects and mode of action of microbial degradation and as genetic mechanism need to understand in future (Zur et al., 2018).
Table 4.
Selective biological techniques for Ibuprofen degradation.
| Technique | Techniques | Applications |
|---|---|---|
| In Situ degradation | Biosparging Bioventing Bioaugmentation |
Biodegradative abilities of indigenous microorganisms in presence of Ibuprofen within environmental parameters Biodegradability of pollutants ibuprofen solubility with geological factors |
| Ex situ degradation | Land farming (Solid-phase treatment system) Composting (Anaerobic, converts solid organic wastes into humus-like material) Biopiles |
Surface application, aerobic process, application of to natural soils followed by irrigation and tilling To make plants healthier good alternative to land filling or incinerating practical and convenient. Surface application, agricultural to municipal waste |
| Bioreactors | Slurry reactors Aqueous reactors |
Bioaugmentat Toxicity of amendments Toxic concentrations of contaminants |
| Microfiltration | Microfiltration membranes are used at a constant pressure | Waste water treatment; recovery and reuse of more than 90% of original waste water |
3.3.1. Pure culture approach
There are few studies have been reported on the pure cultures isolated from soil, activated sludge, sediments, and wastewater which can use frequently detected Ibuprofen in waterbodies. Presently, there is little information about the metabolites formed during the biodegradation of Ibuprofen. So there are few pure strains are identified which have potential to degrade Ibuprofen have been described: Nocardia sp. NRRL 5646 (Chen and Rosazza, 1994; Li and Rosazza, 1997), Sphingomonas Ibu- 2 (Murdoch and Hay, 2013), Bacillus thuringiensis B1(2015b) (Marchlewicz et al., 2017a, Marchlewicz et al., 2017b), Variovorax Ibu-1 (Murdoch and Hay, 2005) and Patulibacter sp. I11 (Almeida et al., 2013). In the presence of tryptone and yeast extract the Patulibacter sp. I11 strain can degrade the Ibuprofen. This suggests that there was involvement of acyl-CoA synthetase iron, sulphur cluster and enoyl–CoA for the degradation of Ibuprofen (Almeida et al., 2013). During Ibuprofen degradation by nocardia sp. NRRL 5646the intermediate compounds like Ibuprofenol acetate and Ibuprofenol were detected (Chen and Rosazza, 1994). Murdoch and Hay (2005, 2015) proposed the pathway of degradation for Ibuprofen by Sphingomonas Ibu-2 and Variovorax Ibu-1. They suggested that meta-ring fission enzymes were involved in the degradation Variovorax Ibu-1 (Murdoch and Hay, 2015). The Bacillus thuringiensis B1(2015b) degrade the Ibuprofen in the presence of glucose. The toxicological studies showed that B. thuringiensis B1(2015b) is more resistant to Ibuprofen; The EC50 of Ibuprofen for B1 strain is 809.3 mg L−1 (Marchlewicz et al., 2017a, Marchlewicz et al., 2017b). Marco-Urrea et al., in 2010 suggested that the fungal strain like Trametes versicolor, Irpex lacteus, Ganoderma Lucidum and Phanerochaete chrysosporium also having potential to degrade Ibuprofen as a solo source of energy. The key feature for biodegradation of Ibuprofen by pure strain is enzyme induction. The biodegradation of Ibuprofen depends on potential of microbe to produced Ibuprofen degrading specific enzyme.
3.3.2. Mixed culture approach
The mixed culture approach is much easier way to achieve degradation as compared to pure culture. Because it's too difficult to isolate pure degrading strain in some; and maintenance of pure strains is also a difficult task. But there are no study is carried out on Ibuprofen degradation by mixed strain approach. But there are few studies available on the mixed culture degradation of PPCPs (Khunjar et al., 2011). The mixed culture possess move capability to remove PPCPs compare to pure culture using mixed media (Khunjar et al., 2011). Zhou et al., in 2014 reported the removal of PPCPs by mixture of culture poured in the activated sludge. Few studies demonstrated that the mixed culture biodegradation rate for removing the PPCPs is higher than the individual strain (Vasiliadou et al., 2013). The mixed strain approach is a futuristic approach for the degradation of Ibuprofen.
3.3.3. Activated sludge culture approach
The conventional WWTPs use activated sludge process for the removal of PPCPs including Ibuprofen. But, the efficiency and the removal rate of Ibuprofen is low. They remove partial concentration of Ibuprofen. And the intermediate formed during the degradation are toxic then the parent substance. However, adsorption and volatilization by using activated sludge having low contribution in the degradation of PPCPs (Li et al., 2015a, Li et al., 2015b).
4. Pathway forIibuprofen biodegradation
There is little information available how the Ibuprofen metabolized by the microbes. However, some microbes having potential to generate hydroxyl-ibuprofen and carboxylated-ibuprofen during degradation were detected (Hanlon et al., 1994; Zwiener et al., 2002; Quintana et al., 2005; Marco-Urea et al., 2009; Salgado et al., 2020). While the Murdoch and Hay tried to describe the metabolic pathway of ibuprofen with the help of Sphingomonas Ibu-2 (Murdoch and Hay, 2005, 2013, 2015). They observed a novel mechanism which involved coenzyme-A ligase. During degradation deacetylation and dioxygenation transformed into isobutylcatechol after the extradiol ring-cleavage. Chen and Rosazza (1994) reported a fungal Nocardia which having potential to transform it into carboxylic acid and further into an alcohol and acetylate.
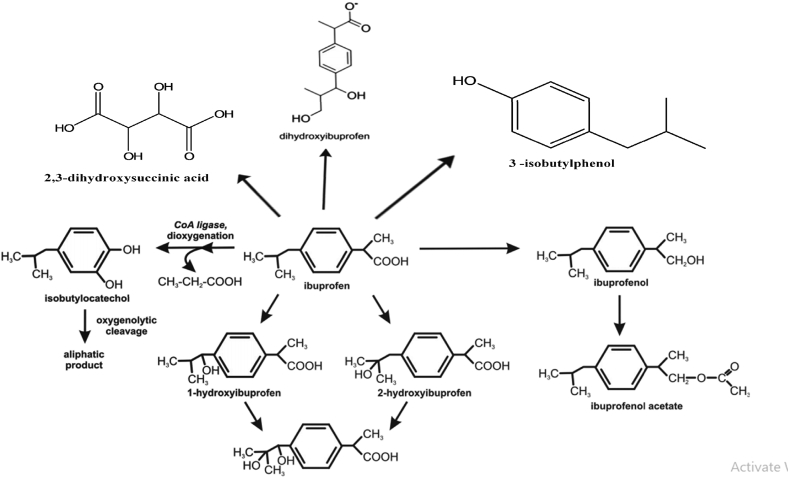
Almeida et al. (2013) also indicated involvement of this enzyme in ibuprofen decomposition by Patulibacter sp. strain I11. Salgado et al. (2020) used Patulibacter medicamentivorans strain under aerobic conditions for the degradation of Ibuprofen they suggested the two main intermediates 2-phenylpropanoic acid and isobutylbenzene were produced during the degradation of Ibuprofen. The Ibuprofen was cleaved into extradiol dioxygenase to 5-formyl-2-hydroxy-7-methylocta-2,4-dienoic acid during degradation by Bacillus thuringiensis B1(2015b) is hydroxylation of both: aromatic ring and aliphatic chain of ibuprofen (Marchlewicz et al., 2017a, Marchlewicz et al., 2017b). The main intermediate metabolites produced during Ibuprofen degradation were hydroxylated in the isopropyl chain from ibuprofen 1 and 2-hydroxyibuprofen after few hours of the experiment, and 1,2- dihydroxyibuprofen as a final metabolite. Hydroxylated and carboxylated derivatives are frequent detected during the microbial metabolism (Zwiener et al., 2002; Quintana et al., 2005). The initiation of degradation was started with 1,4-hydroquinone and the second one to 2-hydroxyquinol. 1,4-hydroquinone is a product of acyl-CoA synthase/thiolase activity and may be transform to 2-hydroxy-1,4-quinol by hydroquinone monooxygenase. Moreover, the activity of hydroxyquinol 1,2-dioxygenase after the induction by ibuprofen was observed due to presence of glucose alone the enzyme was not active at all. Hydroxyquinol 1,2-dioxygenase favorable binds 2-hydroxy-1,4-quinol and is responsible for ortho cleavage of this compound to 3-hydroxy-cis,cis-muconic acid. While during biodegradation of ibuprofen by Nocardia sp. NRRL 5646, there were two main metabolites, ibuprofenol and ibuprofenol acetate, were observed (Figure 4). Finally these products were further mineralized by bacterium (Chen and Rosazza, 1994). Murdoch and Hay (2005, 2013) characterized the most recognized Ibuprofen degradation pathways in Sphingomonas Ibu-2 bacteria, having potential to utilized Ibuprofen as sole carbon and energy source. They also provided the five-gene cluster (ipf ABDEF) involved in Ibuprofen mineralization. The genes ipfA and ipfB were coded the two subunits of dioxygenases; while the ipfD gene was coded the enzyme for the removal/addition of acyl groups acyl-CoA synthetase; the (ipfF) was coded the coenzyme A ligase gene; and finally gene ipfE function was not describe. The degradation was started with the degradation by strain Ibu-2 with coenzyme A. The enzyme removes the propionic acid chain and further dioxygenation reaction led to isobutylocatechol formation. This compound undergoes oxygenolytic cleavage (Murdoch and Hay, 2005, 2013). Whereas in various experiments, the concentration of Ibuprofen was increased with isobutyl having side-chain of hydroxyl- or carboxyl-group led to decrease in concentration of ibuprofen (Hanlon et al., 1994; Marco-Urrea et al., 2009; Quintana et al., 2005; Zwiener et al., 2002). Sharma K et al. (2019) were identified the hydroxyl, demethylation and dehydrogenation derivative during optimized the batch biodegradation using Micrococcus yunnanensis. Finally, they identified the monooxygenase enzyme which was responsible for the degradation of Ibuprofen. The degradation of ibuprofen using sludge of hospital, municipal and distillery were produced the hydroxylated ibuprofen and ibuprofen carboxylic during initial degradation period. Consequently, benzoquinone, quinone and catechol like compounds were produced during the degradation (Huang et al., 2020). Based on these studies, we have designed the pathway for degradation of ibuprofen indicating the various intermediates produced during degradation (Figure 4).
Figure 4.
Pathway of degradation of Ibuprofen purposed by different researchers ((adapted from Murdoch and Hay, 2005, 2013, 2015; Hanlon et al., 1994; Zwiener et al., 2002; Quintana et al., 2005; Marco-Urrea et al., 2009; Chen and Rosazza, 1994; Salgado et al., 2020, Sharma K et al., 2019, Huang et al., 2020).
5. Conclusion and future perspective
The conventional wastewater treatment methods are not effective for the removal of the Ibuprofen, the third most consumable drug and frequently detected water system. Moreover, the removal of Ibuprofen by activated sludge system is affected by the various physical and biological factors, such as concentration of Ibuprofen in waste water, pretreatment system, environmental conditions and microbial community present in sludge. Bioremediation based methods seems to be a promising alternative to the chemical methods. But, the toxicity and analysis of various micro-pollutants a promising field of research that needs to give attention. With the advant of nanotechnology based materials and nanosensors lead to rapid dectetion of these compounds and catalysis based methods improve the degradation. This can be possible through nanostructures, nanosphers and nanoshell which entrap the compounds in their shell and leads to further progress of the chemical based reactions. The possible reason behind the presence of Ibuprofen in environmental source due to the lack of microbial efficiency of degrading Ibuprofen. Therefore, there is need of more studies which enhance the biodegradation of Ibuprofen in water bodies to make potential strategies against the removal of Ibuprofen from waste water. Advancements in enzyme technology gives the way for the new technology of degradation and which can be preferred over conventional technology. The mixed culture approach used in reactor with nanoparticles can enhance the degradation of Ibuprofen. Therefore, more efficient technology need to be applied for more rapid and cost effective degradation of these compounds from the environment.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
S. Chopra wishes to thank UGC New Delhi India for providing research assistantship in the form of RGNF fellowship by University Grants Commission New Delhi India.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
Authors wish to thank Department of Biotechnology, DCRUST Murthal Sonipat India, for proving the necessary facilities to carry out this research.
References
- Alatalo S.-M., Mäkilä E., Repo E., Heinonen M., Salonen J., Kukk E., Sillanpää M., Titirici M.-M. Meso- and microporous soft templated hydrothermal carbons for dye removal from water. Green Chem. 2016;18:1137–1146. [Google Scholar]
- Almeida B., Kjeldal H., Lolas I., Knudsen A.D., Carvalho G., Nielsen K.L., Barreto Crespo M.T., Stensballe A., Nielsen J.L. Quantitative proteomic analysis of ibuprofen-degrading Patulibacter sp. strain I11. Biodegradation. 2013;24:615–630. doi: 10.1007/s10532-012-9610-5. [DOI] [PubMed] [Google Scholar]
- Ashfaq M., Nawaz Khan K., Saif Ur Rehman M., Mustafa G., Faizan Nazar M., Sun Q., Iqbal J., Mulla S.I., Yu C.-P. Ecological risk assessment of pharmaceuticals in the receiving environment of pharmaceutical wastewater in Pakistan. Ecotoxicol. Environ. Saf. 2017;136:31–39. doi: 10.1016/j.ecoenv.2016.10.029. [DOI] [PubMed] [Google Scholar]
- Bai Y.-Y., Lu Y., Liu J.-K. An efficient photocatalyst for degradation of various organic dyes: Ag@ Ag2MoO4–AgBr composite. J. Hazard. Mater. 2016;307:26–35. doi: 10.1016/j.jhazmat.2015.12.052. [DOI] [PubMed] [Google Scholar]
- Blair B., Nikolaus A., Hedman C., Klaper R., Grundl T. Evaluating the degradation, sorption, and negative mass balances of pharmaceuticals and personal care products during wastewater treatment. Chemosphere. 2015;134:395–401. doi: 10.1016/j.chemosphere.2015.04.078. [DOI] [PubMed] [Google Scholar]
- Bo L., Urase T., Wang X. Biodegradation of trace pharmaceutical substances in wastewater by a membrane bioreactor. Front. Environ. Sci. Eng. China. 2009;3:236–240. [Google Scholar]
- Boruah B.S., Biswas R. An optical fiber based surface plasmon resonance technique for sensing of lead ions: a toxic water pollutant. Opt. Fiber Technol. 2018;46:152–156. [Google Scholar]
- Bouissou-Schurtz C., Houeto P., Guerbet M., Bachelot M., Casellas C., Mauclaire A.-C., Panetier P., Delval C., Masset D. Ecological risk assessment of the presence of pharmaceutical residues in a French national water survey. Regul. Toxicol. Pharmacol. 2014;69:296–303. doi: 10.1016/j.yrtph.2014.04.006. [DOI] [PubMed] [Google Scholar]
- Braz F.S., Silva M.R.A., Silva F.S., Andrade S.J., Fonseca A.L., Kondo M.M. Photocatalytic degradation of ibuprofen using TiO2 and ecotoxicological assessment 2of degradation intermediates against Daphnia similis. J. Environ. Protect. 2014;5:620–626. [Google Scholar]
- Brozinski J., Lahti M., Meierjohann A., Oikari A., Kronberg L. 2013. The Anti-infl Ammatory Drugs Diclofenac, Naproxen and Ibuprofen Are Found in the Bile of Wildfish Caught Downstream of a Wastewater Treatment Plant. [DOI] [PubMed] [Google Scholar]
- Caviglioli G., Valeria P., Brunella P., Sergio C., Attilia A., Gaetano B. Identification of degradation products of Ibuprofen arising from oxidative and thermal treatments. J. Pharmaceut. Biomed. Anal. 2002;30:499–509. doi: 10.1016/s0731-7085(02)00400-4. [DOI] [PubMed] [Google Scholar]
- Chen Y., Rosazza J.P.N. Microbial transformation of ibuprofen by a nocardia species. Appl. Environ. Microbiol. 1994;60:1292–1296. doi: 10.1128/aem.60.4.1292-1296.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopra S., Kumar D. Pharmaceuticals and personal care products (PPCPs) as emerging environmental pollutants: toxicity and risk assessment. In: Gahlawat S.K., Duhan J.S., Salar R.K., Siwach P., Kumar S., Kaur P., editors. Advances in Animal Biotechnology and its Applications. Springer Singapore; Singapore: 2018. pp. 337–353. [Google Scholar]
- Chopra S., Kumar D. Characterization, optimization and kinetics study of acetaminophen degradation by Bacillus drentensis strain S1 and waste water degradation analysis. Bioresour. Bioprocess. 2020;7:9. [Google Scholar]
- Daughton C.G., Ternes T.A. 2001. Pharmaceuticals and Personal Care Products in the Nvironment 791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daughton C.G., Ternes, Thomas A. Pharmaceuticals and personal care products in the environment: agents ofsSubtle change Environ. Toxicol. 2009;28:2663–2670. doi: 10.1289/ehp.99107s6907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshmane C.A., Wright M.W., Lachgar A., Rohlfing M., Liu Z., Le J., Hanson B.E. A comparative study of solid carbon acid catalysts for the esterification of free fatty acids for biodiesel production. Evidence for the leaching of colloidal carbon. Bioresour. Technol. 2013;147:597–604. doi: 10.1016/j.biortech.2013.08.073. [DOI] [PubMed] [Google Scholar]
- Di Nica V., Villa S., Finizio A. Toxicity of individual pharmaceuticals and their mixtures to Aliivibrio fischeri: Experimental results for single compounds and considerations of their mechanisms of action and potential acute effects on aquatic organisms. Environ. Toxicol. Chem. 2017;36(3):807–814. doi: 10.1002/etc.3568. [DOI] [PubMed] [Google Scholar]
- Ding T., Yang M., Zhang J., Yang B., Lin K., Li J., Gan J. Toxicity, degradation and metabolic fate of ibuprofen on freshwater diatom Navicula sp. J. Hazard Mater. 2017;330:127–134. doi: 10.1016/j.jhazmat.2017.02.004. [DOI] [PubMed] [Google Scholar]
- Dong L., Guo S., Zhu S., Xu D., Zhang L., Huo M., Yang X. Sunlight responsive BiVO4photocatalyst: effects of pH on L-cysteine-assisted hydrothermal treatment and enhanced degradation of ofloxacin. Catal. Commun. 2011;16:250–254. [Google Scholar]
- Du J., Mei C.-F., Ying G.-G., Xu M.-Y. Toxicity thresholds for diclofenac, acetaminophen and ibuprofen in the water flea Daphnia magna. Bull. Environ. Contam. Toxicol. 2016;97:84–90. doi: 10.1007/s00128-016-1806-7. [DOI] [PubMed] [Google Scholar]
- Du M.-S., Chen K.-P., Lin Y.-P. Degradation of ibuprofen and acetylsulfamethoxazole by multi-walled carbon nanotube catalytic ozonation: surface properties{,} kinetics and modeling. Environ. Sci. Water Res. Technol. 2019;5:1758–1768. [Google Scholar]
- Falås P., Baillon-Dhumez A., Andersen H.R., Ledin A., la Cour Jansen J. Suspended biofilm carrier and activated sludge removal of acidic pharmaceuticals. Water Res. 2012;46:1167–1175. doi: 10.1016/j.watres.2011.12.003. [DOI] [PubMed] [Google Scholar]
- Fang T.-H., Nan F.-H., Chin T.-S., Feng H.-M. The occurrence and distribution of pharmaceutical compounds in the effluents of a major sewage treatment plant in Northern Taiwan and the receiving coastal waters. Mar. Pollut. Bull. 2012;64:1435–1444. doi: 10.1016/j.marpolbul.2012.04.008. [DOI] [PubMed] [Google Scholar]
- Fent K., Weston A.A., Caminada D. Ecotoxicology of human pharmaceuticals. Aquat. Toxicol. 2006;76:122–159. doi: 10.1016/j.aquatox.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Fernandez M.E., Ledesma B., Román S., Bonelli P.R., Cukierman A.L. Development and characterization of activated hydrochars from orange peels as potential adsorbents for emerging organic contaminants. Bioresour. Technol. 2015;183:221–228. doi: 10.1016/j.biortech.2015.02.035. [DOI] [PubMed] [Google Scholar]
- Flippin J.L., Huggett D., Foran C.M. Changes in the timing of reproduction following chronic exposure to ibuprofen in Japanese medaka. Oryzias latipes. Aquat. Toxicol. 2007;81:73–78. doi: 10.1016/j.aquatox.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Funke A., Ziegler F. Hydrothermal carbonization of biomass: a summary and discussion of chemical mechanisms for process engineering. Biofuels, Bioprod. Biorefining. 2010;4:160–177. [Google Scholar]
- Geiger E., Hornek-Gausterer R., Saçan M.T. Single and mixture toxicity of pharmaceuticals and chlorophenols to freshwater algae Chlorella vulgaris. Ecotoxicol. Environ. Saf. 2016;129:189–198. doi: 10.1016/j.ecoenv.2016.03.032. [DOI] [PubMed] [Google Scholar]
- Gibson R., Durán-Álvarez J.C., Estrada K.L., Chávez A., Jiménez Cisneros B. Accumulation and leaching potential of some pharmaceuticals and potential endocrine disruptors in soils irrigated with wastewater in the Tula Valley, Mexico. Chemosphere. 2010;81:1437–1445. doi: 10.1016/j.chemosphere.2010.09.006. [DOI] [PubMed] [Google Scholar]
- Góngora J.F., Elizondo P., Hernández-Ramírez A. Photocatalytic degradation of ibuprofen using TiO2 sensitized by Ru(ii) polyaza complexes. Photochem. Photobiol. Sci. 2017;16:31–37. doi: 10.1039/c6pp00222f. [DOI] [PubMed] [Google Scholar]
- González-Naranjo V., Boltes K. Toxicity of ibuprofen and perfluorooctanoic acid for risk assessment of mixtures in aquatic and terrestrial environments. Int. J. Environ. Sci. Technol. 2014;11(6):1743–1750. [Google Scholar]
- Gonzalez-Rey M., Bebianno M.J. Does non-steroidal anti-inflammatory (NSAID) ibuprofen induce antioxidant stress and endocrine disruption in mussel Mytilus galloprovincialis? Environ. Toxicol. Pharmacol. 2012;33:361–371. doi: 10.1016/j.etap.2011.12.017. [DOI] [PubMed] [Google Scholar]
- Grujić S., Vasiljević T., Laušević M. Determination of multiple pharmaceutical classes in surface and ground waters by liquid chromatography–ion trap–tandem mass spectrometry. J. Chromatogr. A. 2009;1216:4989–5000. doi: 10.1016/j.chroma.2009.04.059. [DOI] [PubMed] [Google Scholar]
- Grzesiuk M., Wacker A., Spijkerman E. Photosynthetic sensitivity of phytoplankton to commonly used pharmaceuticals and its dependence on cellular phosphorus status. Ecotoxicology. 2016;25:697–707. doi: 10.1007/s10646-016-1628-8. [DOI] [PubMed] [Google Scholar]
- Guerra P., Kim M., Shah A., Alaee M., Smyth S.A. Occurrence and fate of antibiotic, analgesic/anti-inflammatory, and antifungal compounds in five wastewater treatment processes. Sci. Total Environ. 2014;473–474:235–243. doi: 10.1016/j.scitotenv.2013.12.008. [DOI] [PubMed] [Google Scholar]
- Han S., Choi Kyungho, Kim J., Ji K., Kim S., Ahn B., Yun J., Choi Kyunghee, Khim J.S., Zhang X., Giesy J.P. Endocrine disruption and consequences of chronic exposure to ibuprofen in Japanese medaka (Oryzias latipes) and freshwater cladocerans Daphnia magna and Moina macrocopa. Aquat. Toxicol. 2010;98:256–264. doi: 10.1016/j.aquatox.2010.02.013. [DOI] [PubMed] [Google Scholar]
- Hanlon G.W., Kooloobandi A., Hutt A.J. Microbial metabolism of 2-arylpropionic acids: effect of environment on the metabolism of ibuprofen by Verticillium lecanii. J. Appl. Bacteriol. 1994;76:442–447. [Google Scholar]
- Huang C.Y., Fu L.H., Sung M.H., Huang C.F., Wu J.P., Kuo H.W. Ibuprofen biodegradation by hospital, municipal, and distillery activated sludges. Environ. Technol. 2020;41:171–180. doi: 10.1080/09593330.2018.1493146. [DOI] [PubMed] [Google Scholar]
- Huang H., Liu G. Ozone-oxidation products of ibuprofen and toxicity analysis in simulated drinking water. J. Drug Metabol. Toxicol. 2015;6:1–5. [Google Scholar]
- Hudec R., Božeková L., Tisoňová J. Consumption of three most widely used analgesics in six European countries. J. Clin. Pharm. Therapeut. 2012;37:78–80. doi: 10.1111/j.1365-2710.2011.01256.x. [DOI] [PubMed] [Google Scholar]
- Huppert N., Würtele M., Hahn H.H. Determination of the plasticizer N-butylbenzenesulfonamide and the pharmaceutical Ibuprofen in wastewater using solid phase microextraction (SPME) Fresenius’ J. Anal. Chem. 1998;362:529–536. [Google Scholar]
- Iovino Pasquale A4, Chianese Simeone A4, Canzano Silvana A4, Prisciandaro Marina A4, Musmarra, Dino P.A.-I. Degradation of ibuprofen in aqueous solution with UV light: the effect of reactor volume and pH. Water. Air. Soil Pollut. 2016;227 194-194–2016 v.227 no.6. [Google Scholar]
- Jeffries K.M., Brander S.M., Britton M.T., Fangue N.A., Connon R.E. 2015. Chronic Exposures to Low and High Concentrations of Ibuprofen Elicit Different Gene Response Patterns in a Euryhaline Fish; pp. 17397–17413. [DOI] [PubMed] [Google Scholar]
- Jermann D., Pronk W., Boller M., Schäfer A.I. The role of NOM fouling for the retention of estradiol and ibuprofen during ultrafiltration. J. Membr. Sci. 2009;329:75–84. [Google Scholar]
- Jurado A., Vàzquez-Suñé E., Carrera J., López de Alda M., Pujades E. Emerging organic contaminants in groundwater in Spain: a review of sources, recent occurrence and fate in a European context. Sci. Total Environ. 2012;440:82–94. doi: 10.1016/j.scitotenv.2012.08.029. [DOI] [PubMed] [Google Scholar]
- Kayani M.A., Parry J.M., Vickery S., Dodds P.F. In vitro genotoxic assessment of xenobiotic diacylglycerols in an in vitro micronucleus assay. Environ. Mol. Mutagen. 2009;50:277–284. doi: 10.1002/em.20445. [DOI] [PubMed] [Google Scholar]
- Khan N.A., Ullah S., Ahmed S., Haq I., Youse M., Akbar A., Changani F. 2020. Trends in Analytical Chemistry Recent Trends in Disposal and Treatment Technologies of Emerging- Pollutants- A Critical Review 122. [Google Scholar]
- Khunjar W.O., MacKintosh S.A., Skotnicka-Pitak J., Baik S., Aga D.S., Love N.G. Elucidating the relative roles of ammonia oxidizing and heterotrophic bacteria during the biotransformation of 17α-ethinylestradiol and trimethoprim. Environ. Sci. Technol. 2011;45:3605–3612. doi: 10.1021/es1037035. [DOI] [PubMed] [Google Scholar]
- Kim J.-W., Jang H.-S., Kim J.-G., Ishibashi H., Hirano M., Nasu K., Ichikawa N., Takao Y., Shinohara R., Arizono K. Occurrence of pharmaceutical and personal care products (PPCPs) in surface water from mankyung river, South Korea. J. Health Sci. 2009;55:249–258. [Google Scholar]
- Kruglova A., Kråkström M., Riska M., Mikola A., Rantanen P., Vahala R., Kronberg L. Comparative study of emerging micropollutants removal by aerobic activated sludge of large laboratory-scale membrane bioreactors and sequencing batch reactors under low-temperature conditions. Bioresour. Technol. 2016;214:81–88. doi: 10.1016/j.biortech.2016.04.037. [DOI] [PubMed] [Google Scholar]
- Kumar A., Joshi V., Dhewa T., Bisht B. Review on bioremediation of polluted environment: a management tool. Int. J. Environ. Sci. 2011;1:1079. [Google Scholar]
- Lange H.J. De, Noordoven W., Murk A.J., Lürling M., Peeters E.T.H.M. Behavioural responses of Gammarus pulex (Crustacea, Amphipoda) to low concentrations of pharmaceuticals. Aquat. Toxicol. 2006;78:209–216. doi: 10.1016/j.aquatox.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Li F.H., Yao K., Lv W.Y., Liu G.G., Chen P., Huang H.P., Kang Y.P. Photodegradation of ibuprofen under UV-VIS irradiation: mechanism and toxicity of photolysis products. Bull. Environ. Contam. Toxicol. 2015;94:479–483. doi: 10.1007/s00128-015-1494-8. [DOI] [PubMed] [Google Scholar]
- Li T., Rosazza J.P. Purification, characterization, and properties of an aryl aldehyde oxidoreductase from Nocardia sp. strain NRRL 5646. J. Bacteriol. 1997;179:3482–3487. doi: 10.1128/jb.179.11.3482-3487.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Shi Y., Gao L., Liu J., Cai Y. Occurrence, fate and risk assessment of parabens and their chlorinated derivatives in an advanced wastewater treatment plant. J. Hazard Mater. 2015;300:29–38. doi: 10.1016/j.jhazmat.2015.06.060. [DOI] [PubMed] [Google Scholar]
- Loaiza-Ambuludi S., Panizza M., Oturan N., Özcan A., Oturan M.A. Electro-Fenton degradation of anti-inflammatory drug ibuprofen in hydroorganic medium. J. Electroanal. Chem. 2013;702:31–36. [Google Scholar]
- Lorenzo M., Campo J., Picó Y. Analytical challenges to determine emerging persistent organic pollutants in aquatic ecosystems. TrAC Trends Anal. Chem. (Reference Ed.) 2018;103:137–155. [Google Scholar]
- Luo Y., Guo W., Ngo H.H., Nghiem L.D., Hai F.I., Zhang J., Liang S., Wang X.C. A review on the occurrence of micropollutants in the aquatic environment and their fate and removal during wastewater treatment. Sci. Total Environ. 2014;473–474:619–641. doi: 10.1016/j.scitotenv.2013.12.065. [DOI] [PubMed] [Google Scholar]
- Madikizela L.M., Chimuka L. Occurrence of naproxen, ibuprofen, and diclofenac residues in wastewater and river water of KwaZulu-Natal Province in South Africa. Environ. Monit. Assess. 2017;189:348. doi: 10.1007/s10661-017-6069-1. [DOI] [PubMed] [Google Scholar]
- Malghani S., Gleixner G., Trumbore S.E. Chars produced by slow pyrolysis and hydrothermal carbonization vary in carbon sequestration potential and greenhouse gases emissions. Soil Biol. Biochem. 2013;62:137–146. [Google Scholar]
- Marchlewicz A., Guzik U., Wojcieszyńska D. Over-the-counter monocyclic non-steroidal anti-inflammatory drugs in environment–sources, risks, biodegradation. Water Air Soil Pollut. 2015;226(10) doi: 10.1007/s11270-015-2622-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchlewicz A., Domaradzka D., Guzik U., Wojcieszyńska D. Bacillus thuringiensis B1(2015b) is a gram-positive bacteria able to degrade naproxen and ibuprofen. Water. Air. Soil Pollut. 2016;227:1–8. doi: 10.1007/s11270-016-2893-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchlewicz A., Guzik U., Hupert-Kocurek K., Nowak A., Wilczyńska S., Wojcieszyńska D. Toxicity and biodegradation of ibuprofen by Bacillus thuringiensis B1(2015b) Environ. Sci. Pollut. Res. 2017;24:7572–7584. doi: 10.1007/s11356-017-8372-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchlewicz A., Guzik U., Smułek W., Wojcieszyńska D. Exploring the degradation of ibuprofen by bacillus thuringiensis B1(2015b): the new pathway and factors affecting degradation. Molecules. 2017;22 doi: 10.3390/molecules22101676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marco-Urrea E., Pérez-Trujillo M., Vicent T., Caminal G. Ability of white-rot fungi to remove selected pharmaceuticals and identification of degradation products of ibuprofen by Trametes versicolor. Chemosphere. 2009;74(6):765–772. doi: 10.1016/j.chemosphere.2008.10.040. [DOI] [PubMed] [Google Scholar]
- Marco-Urrea E., Pérez-Trujillo M., Blánquez P., Vicent T., Caminal G. Biodegradation of the analgesic naproxen by Trametes versicolor and identification of intermediates using HPLC-DAD-MS and NMR. Bioresour. Technol. 2010;101:2159–2166. doi: 10.1016/j.biortech.2009.11.019. [DOI] [PubMed] [Google Scholar]
- Matongo S., Birungi G., Moodley B., Ndungu P. Pharmaceutical residues in water and sediment of msunduzi river, KwaZulu-natal, South Africa. Chemosphere. 2015;134:133–140. doi: 10.1016/j.chemosphere.2015.03.093. [DOI] [PubMed] [Google Scholar]
- Méndez-Arriaga F., Esplugas S., Giménez J. Degradation of the emerging contaminant ibuprofen in water by photo-Fenton. Water Res. 2010;44:589–595. doi: 10.1016/j.watres.2009.07.009. [DOI] [PubMed] [Google Scholar]
- Mestre A.S., Pires J., Nogueira J.M.F., Carvalho A.P. Activated carbons for the adsorption of ibuprofen. Carbon N. Y. 2007;45:1979–1988. [Google Scholar]
- Mestre A.S., Pires R.A., Aroso I., Fernandes E.M., Pinto M.L., Reis R.L., Andrade M.A., Pires J., Silva S.P., Carvalho A.P. Activated carbons prepared from industrial pre-treated cork: sustainable adsorbents for pharmaceutical compounds removal. Chem. Eng. J. 2014;253:408–417. [Google Scholar]
- Mestre A.S., Tyszko E., Andrade M.A., Galhetas M., Freire C., Carvalho A.P. Sustainable activated carbons prepared from a sucrose-derived hydrochar: remarkable adsorbents for pharmaceutical compounds. RSC Adv. 2015;5:19696–19707. [Google Scholar]
- Mohd Zanuri N.B., Bentley M.G., Caldwell G.S. Assessing the impact of diclofenac, ibuprofen and sildenafil citrate (Viagra®) on the fertilisation biology of broadcast spawning marine invertebrates. Mar. Environ. Res. 2017;127:126–136. doi: 10.1016/j.marenvres.2017.04.005. [DOI] [PubMed] [Google Scholar]
- Murdoch R.W., Hay A.G. Formation of catechols via removal of acid side chains from ibuprofen and related aromatic acids. Appl. Environ. Microbiol. 2005;71:6121–6125. doi: 10.1128/AEM.71.10.6121-6125.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdoch R.W., Hay A.G. Genetic and chemical characterization of ibuprofen degradation by Sphingomonas Ibu-2. Microbiology. 2013;159:621–632. doi: 10.1099/mic.0.062273-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdoch R.W., Hay A.G. The biotransformation of ibuprofen to trihydroxyibuprofen in activated sludge and by Variovorax Ibu-1. Biodegradation. 2015;26:105–113. doi: 10.1007/s10532-015-9719-4. [DOI] [PubMed] [Google Scholar]
- Oliveira L.L.D., Antunes S.C., Gonçalves F., Rocha O., Nunes B. Evaluation of ecotoxicological effects of drugs on Daphnia magna using different enzymatic biomarkers. Ecotoxicol. Environ. Saf. 2015;119:123–131. doi: 10.1016/j.ecoenv.2015.04.028. [DOI] [PubMed] [Google Scholar]
- Ortiz de García S.A., Pinto Pinto G., García-Encina P.A., Irusta-Mata R. Ecotoxicity and environmental risk assessment of pharmaceuticals and personal care products in aquatic environments and wastewater treatment plants. Ecotoxicology. 2014;23(8):1517–1533. doi: 10.1007/s10646-014-1293-8. [DOI] [PubMed] [Google Scholar]
- Parolini M., Binelli A. Sub-lethal effects induced by a mixture of three non-steroidal anti-inflammatory drugs (NSAIDs) on the freshwater bivalve Dreissena polymorpha. Ecotoxicology. 2012;21:379–392. doi: 10.1007/s10646-011-0799-6. [DOI] [PubMed] [Google Scholar]
- Parolini M., Binelli A., Provini A. Chronic effects induced by ibuprofen on the freshwater bivalve Dreissena polymorpha. Ecotoxicol. Environ. Saf. 2011;74:1586–1594. doi: 10.1016/j.ecoenv.2011.04.025. [DOI] [PubMed] [Google Scholar]
- Peng X., Yu Y., Tang C., Tan J., Huang Q., Wang Z. Occurrence of steroid estrogens, endocrine-disrupting phenols, and acid pharmaceutical residues in urban riverine water of the Pearl River Delta, South China. Sci. Total Environ. 2008;397:158–166. doi: 10.1016/j.scitotenv.2008.02.059. [DOI] [PubMed] [Google Scholar]
- Plósz B.G., Langford K.H., Thomas K.V. An activated sludge modeling framework for xenobiotic trace chemicals (ASM-X): assessment of diclofenac and carbamazepine. Biotechnol. Bioeng. 2012;109:2757–2769. doi: 10.1002/bit.24553. [DOI] [PubMed] [Google Scholar]
- Qu K., Wang J., Ren J., Qu X. Carbon dots prepared by hydrothermal treatment of dopamine as an effective fluorescent sensing platform for the label-free detection of iron(III) ions and dopamine. Chem. Eur J. 2013;19:7243–7249. doi: 10.1002/chem.201300042. [DOI] [PubMed] [Google Scholar]
- Quero-Pastor M., Valenzuela A., Quiroga J.M., Acevedo A. Degradation of drugs in water with advanced oxidation processes and ozone. J. Environ. Manag. 2014;137:197–203. doi: 10.1016/j.jenvman.2014.02.011. [DOI] [PubMed] [Google Scholar]
- Quero-Pastor M.J., Garrido-Perez M.C., Acevedo A., Quiroga J.M. Ozonation of ibuprofen: a degradation and toxicity study. Sci. Total Environ. 2014;466–467:957–964. doi: 10.1016/j.scitotenv.2013.07.067. [DOI] [PubMed] [Google Scholar]
- Quintana J.B., Weiss S., Reemtsma T. Pathways and metabolites of microbial degradation of selected acidic pharmaceutical and their occurrence in municipal wastewater treated by a membrane bioreactor. Water Res. 2005;39:2654–2664. doi: 10.1016/j.watres.2005.04.068. [DOI] [PubMed] [Google Scholar]
- Rácz G., Csenki Z., Kovács R., Hegyi Á., Baska F., Sujbert L., Zsákovics I., Kis R., Gustafson R., Urbányi B., Szende B. Subacute toxicity assessment of water disinfection byproducts on zebrafish. Pathol. Oncol. Res. 2012;18:579–584. doi: 10.1007/s12253-011-9479-3. [DOI] [PubMed] [Google Scholar]
- Ragugnetti M., Adams M.L., Guimarães A.T.B. 2011. Ibuprofen Genotoxicity in Aquatic Environment : an Experimental Model Using Oreochromis niloticus Ibuprofen Genotoxicity in Aquatic Environment : an Experimental Model Using Oreochromis niloticus. [Google Scholar]
- Rillig M.C., Wagner M., Salem M., Antunes P.M., George C., Ramke H.-G., Titirici M.-M., Antonietti M. Material derived from hydrothermal carbonization: effects on plant growth and arbuscular mycorrhiza. Appl. Soil Ecol. 2010;45:238–242. [Google Scholar]
- Rizzo L., Fiorentino A., Grassi M., Attanasio D., Guida M. Advanced treatment of urban wastewater by sand filtration and graphene adsorption for wastewater reuse: effect on a mixture of pharmaceuticals and toxicity. J. Environ. Chem. Eng. 2015;3:122–128. [Google Scholar]
- Rodriguez-Narvaez O.M., Peralta-Hernandez J.M., Goonetilleke A., Bandala E.R. Treatment technologies for emerging contaminants in water: a review. Chem. Eng. J. 2017;323:361–380. [Google Scholar]
- Rozas O., Vidal C., Baeza C., Jardim W.F., Rossner A., Mansilla H.D. Organic micropollutants (OMPs) in natural waters: oxidation by UV/H2O2 treatment and toxicity assessment. Water Res. 2016;98:109–118. doi: 10.1016/j.watres.2016.03.069. [DOI] [PubMed] [Google Scholar]
- Ruggeri G., Ghigo G., Maurino V., Minero C., Vione D. Photochemical transformation of ibuprofen into harmful 4-isobutylacetophenone: pathways, kinetics, and significance for surface waters. Water Res. 2013;47:6109–6121. doi: 10.1016/j.watres.2013.07.031. [DOI] [PubMed] [Google Scholar]
- Sabri N., Hanna K., Yargeau V. Chemical oxidation of ibuprofen in the presence of iron species at near neutral pH. Sci. Total Environ. 2012;427–428:382–389. doi: 10.1016/j.scitotenv.2012.04.034. [DOI] [PubMed] [Google Scholar]
- Sahar E., David I., Gelman Y., Chikurel H., Aharoni A., Messalem R., Brenner A. The use of RO to remove emerging micropollutants following CAS/UF or MBR treatment of municipal wastewater. Desalination. 2011;273:142–147. [Google Scholar]
- Salgado R., Brito D., Noronha J.P., Almeida B., Bronze M.R., Oehmen A., Carvalho G., Barreto Crespo M.T. Metabolite identification of ibuprofen biodegradation by Patulibacter medicamentivorans under aerobic conditions. Environ. Technol. 2020;41:450–465. doi: 10.1080/09593330.2018.1502362. [DOI] [PubMed] [Google Scholar]
- Sanvicens N., Mannelli I., Salvador J.-P., Valera E., Marco M.-P. Biosensors for pharmaceuticals based on novel technology. TrAC Trends Anal. Chem. (Reference Ed.) 2011;30:541–553. [Google Scholar]
- Saucier C., Karthickeyan P., Ranjithkumar V., Lima E.C., dos Reis G.S., de Brum I.A.S. Efficient removal of amoxicillin and paracetamol from aqueous solutions using magnetic activated carbon. Environ. Sci. Pollut. Res. 2017;24:5918–5932. doi: 10.1007/s11356-016-8304-7. [DOI] [PubMed] [Google Scholar]
- Shankar M.V., Anandan S., Venkatachalam N., Arabindoo B., Murugesan V. Novel thin-film reactor for photocatalytic degradation of pesticides in an aqueous solution. J. Chem. Technol. Biotechnol. 2004;79:1279–1285. [Google Scholar]
- Sharma B., Vaish B., Monika, Singh U.K., Singh P., Singh R.P. Recycling of organic wastes in agriculture: an environmental perspective. Int. J. Environ. Res. 2019;13:409–429. [Google Scholar]
- Sharma K., Kaushik G., Thotakura N., Raza K., Sharma N., Nimesh S. Fate of ibuprofen under optimized batch biodegradation experiments using Micrococcus yunnanensis isolated from pharmaceutical sludge. Int. J. Environ. Sci. Technol. 2019;16:8315–8328. [Google Scholar]
- Sharma S. Bioremediation: features, strategies and applications. Asian J. Pharm. Life Sci. ISSN. 2012;2231:4423. [Google Scholar]
- Shi Y., Zhang X., Liu G. Activated carbons derived from hydrothermally carbonized sucrose: remarkable adsorbents for adsorptive desulfurization. ACS Sustain. Chem. Eng. 2015;3:2237–2246. [Google Scholar]
- Snyder S.A., Adham S., Redding A.M., Cannon F.S., DeCarolis J., Oppenheimer J., Wert E.C., Yoon Y. Role of membranes and activated carbon in the removal of endocrine disruptors and pharmaceuticals. Desalination. 2007;202:156–181. [Google Scholar]
- Song Y., Chai T., Yin Z., Zhang X., Zhang W., Qian Y., Qiu J. Stereoselective effects of ibuprofen in adult zebrafish (Danio rerio) using UPLC-TOF/MS-based metabolomics. Environ. Pollut. 2018;241:730–739. doi: 10.1016/j.envpol.2018.06.009. [DOI] [PubMed] [Google Scholar]
- Suarez S., Lema J.M., Omil F. Pre-treatment of hospital wastewater by coagulation–flocculation and flotation. Bioresour. Technol. 2009;100:2138–2146. doi: 10.1016/j.biortech.2008.11.015. [DOI] [PubMed] [Google Scholar]
- Sun S.-P., Zeng X., Li C., Lemley A.T. Enhanced heterogeneous and homogeneous Fenton-like degradation of carbamazepine by nano-Fe3O4/H2O2 with nitrilotriacetic acid. Chem. Eng. J. 2014;244:44–49. [Google Scholar]
- Sung H.-H., Chiu Y.-W., Wang S.-Y., Chen C.-M., Huang D.-J. Acute toxicity of mixture of acetaminophen and ibuprofen to Green Neon Shrimp, Neocaridina denticulate. Environ. Toxicol. Pharmacol. 2014;38:8–13. doi: 10.1016/j.etap.2014.04.014. [DOI] [PubMed] [Google Scholar]
- Tadkaew N., Hai F.I., McDonald J.A., Khan S.J., Nghiem L.D. Removal of trace organics by MBR treatment: the role of molecular properties. Water Res. 2011;45:2439–2451. doi: 10.1016/j.watres.2011.01.023. [DOI] [PubMed] [Google Scholar]
- Tambosi J.L., Yamanaka L.Y., José H.J., Moreira R., de F.P.M., Schröder H.F. Recent research data on the removal of pharmaceuticals from sewage treatment plants (STP) Quim. Nova. 2010;33:411–420. [Google Scholar]
- Titirici M.-M. Hydrothermal carbons: synthesis, characterization, and applications. Nov. Carbon Adsorbents. 2012:351–399. [Google Scholar]
- Titirici M.-M., Antonietti M., Baccile N. Hydrothermal carbon from biomass: a comparison of the local structure from poly- to monosaccharides and pentoses/hexoses. Green Chem. 2008;10:1204–1212. [Google Scholar]
- Trinh T., van den Akker B., Stuetz R.M., Coleman H.M., Le-Clech P., Khan S.J. Removal of trace organic chemical contaminants by a membrane bioreactor. Water Sci. Technol. 2012;66:1856–1863. doi: 10.2166/wst.2012.374. [DOI] [PubMed] [Google Scholar]
- Vasiliadou I.A., Molina R., Martínez F., Melero J.A. Biological removal of pharmaceutical and personal care products by a mixed microbial culture: sorption, desorption and biodegradation. Biochem. Eng. J. 2013;81:108–119. [Google Scholar]
- Vazquez-Roig P., Andreu V., Blasco C., Picó Y. Risk assessment on the presence of pharmaceuticals in sediments, soils and waters of the Pego–Oliva Marshlands (Valencia, eastern Spain) Sci. Total Environ. 2012;440:24–32. doi: 10.1016/j.scitotenv.2012.08.036. [DOI] [PubMed] [Google Scholar]
- Vergeynst L., Haeck A., Wispelaere P. De, Langenhove H. Van, Demeestere K. Multi-residue analysis of pharmaceuticals in wastewater by liquid chromatography–magnetic sector mass spectrometry: method quality assessment and application in a Belgian case study. Chemosphere. 2015;119:S2–S8. doi: 10.1016/j.chemosphere.2014.03.069. [DOI] [PubMed] [Google Scholar]
- Wang J., Wang S. Removal of pharmaceuticals and personal care products (PPCPs) from wastewater: a review. J. Environ. Manag. 2016;182:620–640. doi: 10.1016/j.jenvman.2016.07.049. [DOI] [PubMed] [Google Scholar]
- Wang J.L., Xu L.J. Advanced oxidation processes for wastewater treatment: formation of hydroxyl radical and application. Crit. Rev. Environ. Sci. Technol. 2012;42:251–325. [Google Scholar]
- Wang Y., Ma J., Zhu J., Ye N., Zhang X., Huang H. Multi-walled carbon nanotubes with selected properties for dynamic filtration of pharmaceuticals and personal care products. Water Res. 2016;92:104–112. doi: 10.1016/j.watres.2016.01.038. [DOI] [PubMed] [Google Scholar]
- Wang Z., Huang Q., Yu Y., Wang C., Ou W., Peng X. Stereoisomeric profiling of pharmaceuticals ibuprofen and iopromide in wastewater and river water, China. Environ. Geochem. Health. 2013;35:683–691. doi: 10.1007/s10653-013-9551-x. [DOI] [PubMed] [Google Scholar]
- Wu C., Witter J.D., Spongberg A.L., Czajkowski K.P. Occurrence of selected pharmaceuticals in an agricultural landscape, western Lake Erie basin. Water Res. 2009;43:3407–3416. doi: 10.1016/j.watres.2009.05.014. [DOI] [PubMed] [Google Scholar]
- Yangali-Quintanilla V., Maeng S.K., Fujioka T., Kennedy M., Li Z., Amy G. Nanofiltration vs. reverse osmosis for the removal of emerging organic contaminants in water reuse. Desalin. Water Treat. 2011;34:50–56. [Google Scholar]
- Yu J.T., Bouwer E.J., Coelhan M. Occurrence and biodegradability studies of selected pharmaceuticals and personal care products in sewage effluent. Agric. Water Manag. 2006;86:72–80. [Google Scholar]
- Zhang S., Zhang Q., Darisaw S., Ehie O., Wang G. Simultaneous quantification of polycyclic aromatic hydrocarbons (PAHs), polychlorinated biphenyls (PCBs), and pharmaceuticals and personal care products (PPCPs) in Mississippi river water, in New Orleans, Louisiana, USA. Chemosphere. 2007;66:1057–1069. doi: 10.1016/j.chemosphere.2006.06.067. [DOI] [PubMed] [Google Scholar]
- Zheng B.G., Zheng Z., Zhang J.B., Luo X.Z., Wang J.Q., Liu Q., Wang L.H. Degradation of the emerging contaminant ibuprofen in aqueous solution by gamma irradiation. Desalination. 2011;276:379–385. [Google Scholar]
- Żur J., Piński A., Marchlewicz A., Hupert-Kocurek K., Wojcieszyńska D., Guzik U. Organic micropollutants paracetamol and ibuprofen---toxicity, biodegradation, and genetic background of their utilization by bacteria. Environ. Sci. Pollut. Res. 2018;25:21498–21524. doi: 10.1007/s11356-018-2517-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwiener C., Seeger S., Glauner T., Frimmel F. Metabolites from the biodegradation of pharmaceutical residues of ibuprofen in biofilm reactors and batch experiments. Anal. Bioanal. Chem. 2002;372:569–575. doi: 10.1007/s00216-001-1210-x. [DOI] [PubMed] [Google Scholar]