Abstract
C-tactile afferents are hypothesized to form a distinct peripheral channel that encodes the affective nature of touch. Prevailing views indicate they project, as with other unmyelinated afferents, in lamina I-spinothalamic pathways that relay homeostatically relevant information from the body toward cortical regions involved in interoceptive processing. However, in a recent study, we found that spinothalamic ablation in humans, while profoundly impairing the canonical spinothalamic modalities of pain, temperature, and itch, had no effect on benchmark psychophysical affective touch metrics. These novel findings appear to indicate that perceptual judgments about the affective nature of touch pleasantness do not depend on the integrity of the lamina I-spinothalamic tract. In this commentary, we further discuss the implications of these unexpected findings. Intuitively, they suggest that signaling of emotionally relevant C-tactile mediated touch occurs in an alternative ascending pathway. However, we also argue that the deficits seen following interruption of a putative C-tactile lamina I-spinothalamic relay might be barely perceptible—a feature that would underline the importance of the C-tactile afferent in neurodevelopment.
Keywords: C-tactile, affective touch, somatosensation, spinothalamic
Comment on: Marshall AG, Sharma ML, Marley K, Olausson H, McGlone FP. Spinal signalling of C-fiber mediated pleasant touch in humans. Elife. 2019;8: e51642. doi:10.7554/eLife.51642. PubMed PMID: 31872799; PubMed Central PMCID: PMC6964968. https://pubmed.ncbi.nlm.nih.gov/31872799/
Touch has classically been viewed as a discriminative sensation. However, propelled by the discovery of a population of slowly conducting unmyelinated low threshold mechanoreceptor (LTMR) afferents—C-tactile (CT) afferents—it has become apparent that the tactile senses also have an important affective dimension, one that is both rewarding and protective. C-tactile afferents preferentially respond to caress-like slow, stroking touch delivered at skin temperature and are poorly suited for touch discrimination (for review, see McGlone et al.1 and Ackerley et al.2). Their firing is strikingly velocity tuned, spiking most vigorously to gentle brushing at velocities of 1 to 10 cm s−1.1,2 Remarkably, this inverted U-shaped stimulus-response function positively correlates with subjective ratings of touch pleasantness.1
A dominant view of somatosensation is that information from the body that has homeostatic relevance is conveyed by unmyelinated primary afferents, terminating in laminae I and IIo of the dorsal horn. These inputs then relay in a second-order projection ascending in the lamina I-spinothalamic tract (L1-STT) pathway.3 The cutaneous sensory projections currently included in this pathway, broadly classified as interoceptive, are temperature, nociception, and pruriception, but here we posit the CT afferent would also logically project. L1-STT projection neurons in the spinal cord dorsal horn decussate to the contralateral side over 1-2 spinal segments and form an ascending relay in the anterolateral funiculus (ie, the L1-STT is a crossed projection; Figure 1A). Accordingly, targeted unilateral disruption of the anterolateral funiculus containing the L1-STT interrupts pain, temperature, and itch sensations from the opposite side of the body caudal to the lesion level. Selective activation of CTs results in activation of the dorsal posterior insular cortex, a primary cortical target of ascending L1-STT projections from other unmyelinated afferent modalities (for review, see McGlone et al.1). In contrast, no activation is seen in primary sensory cortex, which receives inputs from the Aβ-LTMR—dorsal column—medial lemniscal pathway thought to purely subserve discriminative touch. Cerebral lesions specifically involving the dorsal posterior insula disrupt touch pleasantness delivered at CT optimal velocities.4 These findings imply that Aβ- and CT-LTMR pathways are distinct with CT afferents being placed within a wider system that is more interoceptive than exteroceptive.1,3
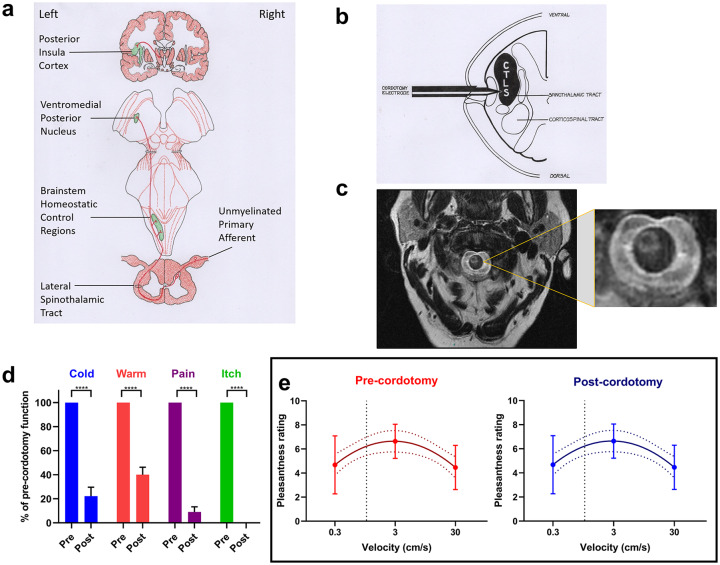
Figure 1.
Anterolateral cordotomy induces marked deficits in canonical lamina I-spinothalamic tract modalities but not in psychophysical affective touch metrics. (A) The organization of the lamina I-spinothalamic pathway. (B) Schematic showing the anterolateral cordotomy procedure. Radiofrequency lesions are given through the cordotomy electrode within the anterolateral funiculus. (C) Exemplar T2 weighted axial magnetic resonance image at the cervical-level C2 showing a lesion in the right anterolateral funiculus. (D) Bar charts showing that temperature detection, clinical pain, and cowhage-induced itch are all largely abolished by contralateral spinothalamic tract lesioning, data are presented as mean and standard error; post-cordotomy values are expressed as a percentage of pre-cordotomy function; significant differences (Related-Samples Wilcoxon Signed Rank Test) are marked with asterisks and show ****P < .0005. However, ratings of the pleasantness of gentle stroking touch show an inverted U-shaped curve that is unchanged by cordotomy (E) (Group data shown as mean and standard error. The lines of best fit with 95% confidence intervals are shown. The vertical dotted line indicates the position of a velocity of 1 cm s−1 on the logarithmic scale).
By this argument, CT projections should follow those of other unmyelinated afferents in the L1-STT, as previously stated, leading to the prediction that psychophysical measures of affective touch would be altered by lesions of this tract. This prediction was investigated in our recent study5 in patients undergoing targeted disruption of the L1-STT pathway with anterolateral cordotomy, to relieve intractable cancer-related pain on the contralateral side of the body (Figure 1B and C). Using a pre-test, post-test design psychophysical investigations demonstrated dramatic impairments in canonical L1-STT modalities of temperature, pain, and itch in all patients on the side contralateral to lesioning (Figure 1D). However, despite this unambiguous evidence of L1-STT disruption, psychophysical benchmark measures of affective touch, including the characteristic inverted U-shaped stimulus-response curve that binds such ratings to the characteristic velocity dependence of CT firing, were surprisingly unaltered (Figure 1E). These novel findings appear to indicate that perceptual judgments about the affective nature of touch pleasantness do not depend on the integrity of the L1-STT. We therefore asked the following question: “How, and in what form, might CT afferents impart their emotionally salient activity on the higher central nervous system?”
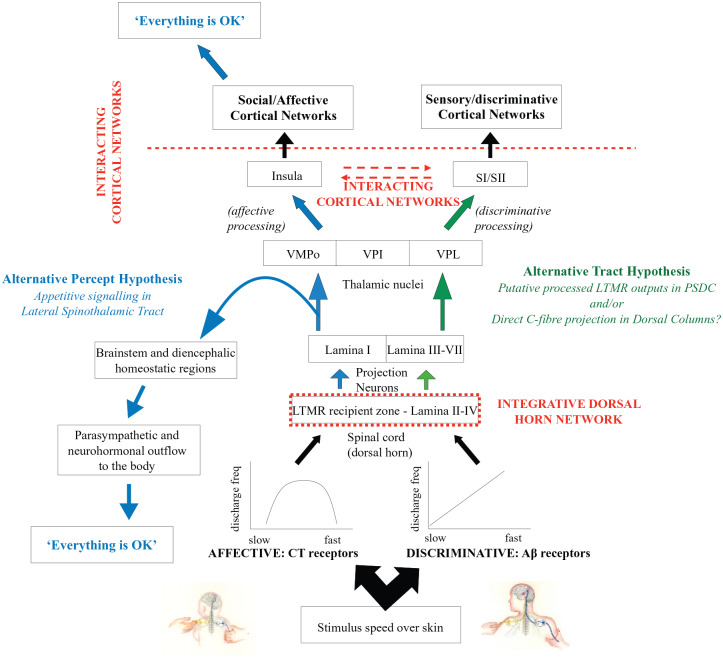
In this commentary, we will expand on the implications of these new findings and explore unanswered questions. We will also discuss how the important unresolved issues might be addressed. In the post-cordotomy state, the patients must either be making judgments about affective touch metrics based on mechanosensory information ascending in projections other than the L1-STT or reporting an associated learned response from the experience that CT stimulation is always being accompanied by Aβ-LTMR stimulation. In this respect, two broad possibilities will be discussed that we term “alternative tract” and “alternative percept” hypotheses (schematically represented in Figure 2).
Figure 2.
Schematic depicting affective and discriminative pathways in the “alternative tract” and “alternative percept” hypotheses. C-tactile afferents respond with an “inverted U”-shaped curve where the “adequate stimulus” is tuned to touch of affiliative or affective significance. Aβ afferent firing increases linearly with velocity, responding to the physical properties of the stimulus. In the “alternative tract hypothesis” (green arrows) processed CT and myelinated LTMR afferent inputs ascend in the dorsal columns. These must gain access to affective cortical regions either via forward thalamocortical or cortico-cortical connections. In the “alternative percept hypothesis” (blue arrows), CT inputs, via the L1-STT, drive an autonomic and neurohormonal outflow to the body as well as projections to social/affective cortical networks to “set the scene that all is OK.” This is anchored during early neurodevelopment. “Gestalt” touch pleasantness develops secondarily and can also, by association, be “inferred” from concomitant myelinated LTMR inputs. Dashed red lines indicate putative and established reciprocal connections that may allow mutual modulation of affective- and sensory-related processing. CT indicates C-Tactile; LTMR, low threshold mechanoreceptor; L1-STT, lamina I-spinothalamic tract; PSDC, post-synaptic dorsal column; S1/S11, primary and secondary somatosensory cortex; VMpo, ventromedial posterior nucleus; VPI, ventroposterior inferior nucleus; VPL, ventral post-lateral nucleus.
Alternative Tract Hypothesis
The most intuitive explanation for the unexpected findings is that inputs conveying peripheral information from CTs, rather than ascending the L1-STT, instead relay in an alternative tract(s), most likely including the dorsal columns. Elegant studies in rodents highlight a marked processing complexity of the panoply of primary LTMR afferent inputs, including those of C-LTMR afferents (the animal equivalent of CT afferents) in the spinal cord dorsal horn. Within this complex interconnected network, the majority of neurons are intrinsic interneurons,6 underlining the vast potential of the dorsal horn for integration of LTMR inputs into perceptually relevant ascending information. In rodents, these integrated outputs ascend the post-synaptic dorsal column (PSDC) pathway. Certainly, the firing patterns and receptive field characteristics of PSDC projection neurons are consistent with relay of processed afferent inputs.7 The PSDC is therefore a prime candidate pathway by which CT afferents could impart emotionally relevant firing patterns on ascending information. In this respect, CT afferents might not necessarily have an exclusive, unitary role in affective tactile coding, but shape ascending dorsal horn projection neuron outputs, affecting the response to a touch that was delivered at CT preferred velocity. There is also evidence that a substantial proportion, up to 25%, of directly projecting fibers within the mammalian dorsal column pathway, including in humans, are unmyelinated.8 Many of these immunostain for calcitonin gene-related peptide8 suggest that they are nociceptive. However, it is uncertain whether these direct projections represent the diversity of unmyelinated fibers, including CT afferents.
Alternative Percept Hypothesis
A further explanation for the absence of alteration in CT metrics following L1-STT lesioning is that patients have, because of lifelong integration of CT and non-CT LTMR afferent firing, learned to associate the activity pattern of myelinated LTMRs that accompanies slow gentle brushing touch with touch pleasantness. Consequently, they may be relying on ascending information derived mainly or even purely from myelinated inputs. In this case, if the perceptual consequences of a loss of ascending CT L1-STT projections are extremely subtle, the L1-STT might still be the primary ascending pathway conveying CT-related information. In this respect, it is legitimate to ask, “Are we measuring the right thing?”
In neurologically intact individuals, it is not possible to stimulate CT afferents without concomitantly activating myelinated LTMRs and, with the exception of glabrous skin, vice versa. Invaluable information about the perceptual consequences of pure CT activation comes from the investigation of two rare patients who, post-developmentally, lost all Aβ-LTMRs below cervical-level C2. In these sensory neuronopathy patients, gentle stroking of hairy skin, despite eliciting robust sympathetic skin responses, evokes no more than a barely perceptible, faintly pleasant, and poorly localized tactile sensation.1 This implies that the full expression of pleasant touch depends on concomitant Aβ-LTMR inputs. An analogy could be the perception of wetness, which depends upon integration of pressure conveyed by LTMRs and cold by Aδ fibers.9 There is no known post-developmental CT “equivalent” to Aβ denervated sensory neuronopathy patients that would enable comparison with L1-STT lesioned patients. Therefore, it is impossible to know the perceptual consequences of touch applied to hairy skin devoid of CT afferents. Gentle brushing stimulation of glabrous skin of the palm, from which CT afferent fibers have never been reported, is perceived as pleasant and also shows inverted U-shaped velocity tuning.1 However, there are differences in brain activation between glabrous and hairy skin stimulation supporting the notion that affective touch on the palm is largely attributable to secondary reinforcement.1 The putative loss of a weak CT sensation post-cordotomy, particularly in the presence of a substantial Aβ-LTMR volley that is sufficient to provide detailed information regarding force, texture, and velocity of a stimulus, might therefore be subtle/sub-perceptual. Certainly, the deficit associated with disruption of an ascending affective touch stream might not be striking enough to alter a psychophysical tuning curve for which individuals have “learned” to associate an Aβ input evoked by a 3 cm s−1 stroking stimulus with the vigorous firing of CT afferents.
In rats, a subset of lamina I projection neurons do respond to slow gentle stroking touch.10 However, these neurons are not exclusively activated by touch. Instead, they are “wide dynamic range,” also responding, indeed with considerably greater discharges, to noxious mechanical or heat stimuli. Therefore, a distinct population of lamina 1 projection neurons may exist that signal both aversive (high-intensity stimulation) and appetitive (low-intensity stimulation) states to brainstem autonomic and diencephalic structures, as well as higher central nervous system regions. The primary role of CT afferents might then not be to signal touch pleasantness per se but rather to promote a sense of “safety” or “bonding,” or to act as a stress buffer that alleviates psychological upset or pain.11 In this way, the unquestionably robust association of CT firing rates and pleasantness ratings might represent a Gestalt “affective touch” perception that is a surrogate of its true homeostatic role that, through autonomic, neurohormonal, as well as cortical signaling, lets the sentient organism know “everything is ok.”
It is in this context worth comparing the impairments after cordotomy with those associated with profound congenital loss of peripheral unmyelinated afferents, hereditary sensory and autonomic neuropathies (HSAN). Analogous to the post-L1-STT lesioned state, patients with HSAN-V12 have insensitivity to pain. However, in contrast to the post-cordotomy state, individuals with HSAN-V do not show an inverted U-shaped velocity tuning curve for ratings of pleasant touch. Instead, velocity tuning appears either flat or linearly increases with touch velocity, suggesting reliance on Aβ-LTMR inputs. These incongruous findings for affective touch likely reflect neurodevelopmental differences. The congenital small afferent deficit in HSAN must have downstream consequences for affective touch processing throughout the somatosensory neuroaxis. In HSAN patients, Aβ-LTMR activity occurs without the full homeostatically salient input from CT afferents. Therefore, unlike neurologically intact individuals, the conditioning process that occurs between Aβ-LTMR inputs and the velocity tuning of CT afferents will be underdeveloped. Intriguingly, these divergent findings suggest that the rewarding properties of touch, and the expression of the characteristic velocity tuned preference for a slow caress, might have its roots during early developmental stages where stimulation of CT afferents via nurturing touch plays a significant role in shaping the destiny of the social brain. The consequences of a lack of nurturing touch (CT stimulation) in early life, as first noted by Harry Harlow (for review, see McGlone et al.1) and more recently from rodent studies where pups from low licking grooming mothers showed increases in behavioral fearfulness and atypical hypothalamic-pituitary-adrenal (HPA) responses under conditions of stress,13 can persist throughout life and form a basis for vulnerability to stress-related diseases as found with the Romanian orphanage infants.14
Future Directions
While these two broad possibilities are not necessarily mutually exclusive, determining their relative contribution is of significant importance. If one can show that CT afferents do signal prominently, albeit in an almost subliminal manner, in the L1-STT this would not only inform our thinking about the role of CT afferents in affective touch but also provide real focus to their role in neurodevelopment. Truly delineating their projection pathways might also provide targets for neuromodulation in the treatment of pain, for example. How could these issues be addressed further? It would be of importance to assess discriminative and affective tactile sensations in patients with a range of well-defined and discrete spinal cord lesions. Isolated damage of the dorsal column pathways is rare in humans, but there might be an opportunity to study, in a pre-test, post-test manner patients undergoing midline myelotomy to treat intractable visceral pain. To determine whether the missing spinothalamic link is just “hidden,” it might be informative to use more nuanced psychophysical or psychophysiological studies investigating appetitive conditioning in these patients rather than just “crude” subjective measures of a touch pleasantness. For example, CT targeted stimulation has been shown to preferentially recruit the parasympathetic system and show distinct patterns of facial EMG activity, features in keeping with an appetitive role.15 Finally, we see a role here for neuroimaging methods (eg, functional magnetic resonance imaging [fMRI], magnetic resonance imaging [MRI] tractography, or assessment of ultra-late tactile potentials with electroencephalography [EEG]) that will bypass the uncertainty with subjective reporting of affective states.
Footnotes
Author Contributions: A.M. and F.M wrote the manuscript.
Funding:The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of Conflicting Interest:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
ORCID iD: Andrew G Marshall  https://orcid.org/0000-0001-8273-7089
https://orcid.org/0000-0001-8273-7089
References
- 1. McGlone F, Wessberg J, Olausson H. Discriminative and affective touch: sensing and feeling. Neuron. 2014;82:737-755. [DOI] [PubMed] [Google Scholar]
- 2. Ackerley R, Backlund Wasling H, Liljencrantz J, Olausson H, Johnson RD, Wessberg J. Human C-tactile afferents are tuned to the temperature of a skin-stroking caress. J Neurosci. 2014;34:2879-2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3:655-666. [DOI] [PubMed] [Google Scholar]
- 4. Kirsch LP, Besharati S, Papadaki C, et al. Damage to the right insula disrupts the perception of affective touch. Elife. 2020;9:e47895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Marshall AG, Sharma ML, Marley K, Olausson H, McGlone FP. Spinal signalling of C-fiber mediated pleasant touch in humans. Elife. 2019;8:e51642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Abraira VE, Kuehn ED, Chirila AM, et al. The cellular and synaptic architecture of the mechanosensory dorsal horn. Cell. 2017;168:295-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Noble R, Riddell JS. Cutaneous excitatory and inhibitory input to neurones of the postsynaptic dorsal column system in the cat. J Physiol. 1988;396:497-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Briner RP, Carlton SM, Coggeshall RE, Chung KS. Evidence for unmyelinated sensory fibres in the posterior columns in man. Brain. 1988;111:999-1007. [DOI] [PubMed] [Google Scholar]
- 9. Bentley I. The synthetic experiment. Am J Psychol. 1990;11:405-425. [Google Scholar]
- 10. Andrew D. Quantitative characterization of low-threshold mechanoreceptor inputs to lamina I spinoparabrachial neurons in the rat. J Physiol. 2010;588:117-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Morrison I. Keep calm and cuddle on: social touch as a stress buffer. Adapt Hum Behav Physiol. 2016;2:344-362. [Google Scholar]
- 12. Morrison I, Loken LS, Minde J, et al. Reduced C-afferent fibre density affects perceived pleasantness and empathy for touch. Brain. 2011;134:1116-1126. [DOI] [PubMed] [Google Scholar]
- 13. Meaney MJ, Szyf M, Seckl JR. Epigenetic mechanisms of perinatal programming of hypothalamic-pituitary-adrenal function and health. Trends Mol Med. 2007;13:269-277. [DOI] [PubMed] [Google Scholar]
- 14. Nelson CA, Fox NA, Zeanah CHJ. Romania’s Abandoned Children: Deprivation, Brain Development, and the Struggle for Recovery. Cambridge, MA: Harvard University Press; 2014. [Google Scholar]
- 15. Pawling R, Cannon PR, McGlone FP, Walker SC. C-tactile afferent stimulating touch carries a positive affective value. PLoS ONE. 2017;12:e0173457. [DOI] [PMC free article] [PubMed] [Google Scholar]