Abstract
Introduction: Head and neck cancer patients often suffer from physical and cognitive impairments after cancer treatment. During rehabilitation, exercise therapy can improve physical function and quality of life (QoL). Surveys demonstrated patients’ preference for home training with low- to moderate-intensity. This study was conducted in order to develope a suitable home-based training program. Therefore, the feasibility and effects of a low- to moderate-intensity exercise intervention on physical functions and QoL were evaluated. Methods: Training was conducted as supervised group training and consisted of mobilization, coordination, resistance, stretching, and relaxation exercises. The intervention lasted 12 weeks with 2 training sessions per week. Feasibility, attendance rate, physical function (eg, range of motion, 6-minute walk test [6MWT]), and QoL (eg, EORTC QLQ-30) were analyzed. Results: Ten out of 12 participants completed the intervention (83%) with an average attendance rate of 83%. Participants showed significant improvements in selected physical functions. For example, head rotation increased by 11.2° (P = .042), walking distance in the 6MWT increased by an average of 43.3 m (P = .010), and the global QoL scale improved by 8.2 points (P = .059). Additionally, there were positive changes in the physical function scale (P = .008), cognitive function scale (P = .015), and social function scale (P = .031) of the EORTC QLQ-30. Conclusion: Data indicate that the exercise program was feasible and had positive effects on physical function and QoL. Future research will analyze the effects of a home-based exercise program on physical function and QoL in a large-scale study.
Keywords: HNSCC, exercise, quality of life, physical functioning
Introduction
Worldwide, squamous cell carcinomas of the head and neck (HNSCC) account for more than 685 000 cases, with 375 000 deaths per year. HNSCC involves the oral cavity, nose/sinus, pharynx (oropharynx, nasopharynx, hypopharynx), and larynx.1 Therapeutic approaches are interdisciplinary and invasive (surgery, radiotherapy, chemotherapy, and immunotherapy). Due to the tumor’s location and the intensive local therapy procedures, patients suffer from chronic comorbidities: dysphonia, dysphagia, lymphedema, weight loss, muscle wasting, fatigue, chemotherapy-induced peripheral neuropathy (CIPN), pain, and movement restrictions. These acute and chronic side effects impair quality of life (QoL).2-5 Hence, all HNSCC patients need interdisciplinary rehabilitation including physicians, psychologists, speech and special movement/physical therapists, nutritionists, and occupational therapists.6 In general, rehabilitation aims at symptom alleviation and compensation of functional deficits.4 There is strong evidence for the benefits of exercise on body composition, muscular fitness, pain, flexibility, and QoL of HNSCC survivors.6-13 Consequently, exercise therapy is considered as a key factor for the patients’ outcome. Although standard clinical training interventions (eg, rehabilitation facilities, physiotherapy) are available, their duration is limited to a few weeks. Furthermore, some interventional studies with HNSCC patients focused on aftercare training programs without considering individual needs.11 Previous studies and standard rehabilitation often used large exercise machines, which are costly or require specialized training facilities.7,9-11 However, HNSCC survivors prefer training at home, alone, and with moderate intensity.14,15 In addition, most studies concentrated only on a single training method (eg, resistance training) and one symptom.7,9-12 Certainly, there is a need for more diverse training programs, since most patients suffer from heterogeneous impairments (movement restrictions, pain, muscle wasting, and dysfunctional sense of balance, CIPN, fatigue).2,3,5 Hence, HNSCC patients need a training program executable at home, without requiring large exercise devices and considering their physical and cognitive impairments.
In this pilot study, we evaluated the feasibility and impact of a low- to medium-intensity exercise intervention on physical function and QoL. Only exercises suitable for independent training at home and tailored to the specific needs of HNSCC patients during rehabilitation were used. In this study, the training was performed supervised by a therapist. This form was necessary, currently, studies analyzing home-based programs for HNSCC patients are rare. Due to this limitation and the lack of comparable results, 3 major issues arose that strongly indicated against a home-based setting: We did not know in the preliminary stages (1) whether the exercise program would be accepted by the patients at all, (2) whether the patients would be able to learn the selected exercises quickly, and (3) whether a 100-minute training with low- to moderate-intensity per week would have positive effects on physical functions and QoL.
Primarily, we assessed patient attendance during the 12 weeks of training. Additionally, we determined the effects of the intervention on different physical functions and overall QoL. The analysis of the compliance and effects of the training program in this pilot study are essential for designing a future large-scale study using home-based training.
Hypotheses
We predicted that our intervention will be feasible (feasibility was defined as completion of study protocol ≥70%). Furthermore, we hypothesized that a low- to moderate-intensity intervention will increase adherence due to HNSCC patients’ preferences,14,15 as well as that the comprehensive training program will have positive effects on defined physical function parameters and QoL.
Methods
Design, Recruitment, and Inclusion Criteria
This study was approved by the Ethics Committee of the University of Rostock (A 2018-0153) and conducted at the Rostock University Medical Center in Germany.
The first step was to identify training interventions in the literature that have positively influenced treatment-associated side effects in HNSCC patients. Select exercises should be performed without requiring specialists (eg, speech or physical therapists) and specific training devices (eg, large strength training devices or whole-body vibration platforms; Table 1). In the second step, a team consisting of a sports scientist and a physical therapist compiled a 12-week training program. Subsequent analysis determined the feasibility, compliance, and effects of the training program on physical function and QoL. Physical function was measured by various tests (see physical function assessments) and QoL was evaluated by patient reported outcomes (see patient reported outcomes).
Table 1.
Treatment-Related Side Effects and Suitable Exercise Interventions.
| Treatment-Associated Side Effects | Exercise Interventions |
|---|---|
| Trismus | • Mobilization exercises for the temporomandibular joints12,36 |
| Lymphedema (head, neck, arm, shoulder) | • Dynamic resistance training for the upper extremities and neck muscles37,38 |
| Restrictions of head, arm, and shoulder mobility | • Mobilization exercises of the cervical spine and shoulder11
• Progressive resistance training of the upper body and upper extremities11 • Stretching of the shoulder, neck, and chest muscles11 |
| (Scar-) Pain | • (Progressive) resistance training11
• Stretching of the relevant regions11 |
| Weight loss, muscle wasting | • Whole-body resistance training28 |
| Disturbances in the ability to balance, gait uncertainties | • Balance exercises39
• Resistance training for the lower extremities and torso39 |
| Disability in respiratory function | • Breathing exercises40
• Mobilization exercises for the thorax41 • Resistance exercises and stretching to straighten the upper body42 |
| Cognitive impairments | • Coordination exercises in combination with resistance and/or endurance training43,44 |
| Chemotherapy-induced peripheral neuropathy | • Balance exercises45 |
| Psychological impairments (depression, anxiety disorders) | • (Progressive) resistance training27
• Endurance training (outdoor)27 • Relaxation training46 |
| Fatigue | • (Progressive) resistance training27,47
• Endurance training27,47 |
| Altered body perception | • Resistance training34
• (Intensive) endurance training34 • Group training programs34 |
Due to data protection reasons in Germany, potential study participants could not be contacted directly by letter or phone. Therefore, posters and flyers were provided in the aftercare departments of the university hospital, in 3 physician practices (within a radius of 40 km), and on the home page of the Oncological Centre Rostock. Potential participants in clinical aftercare were directly asked by physicians to join the study.
Inclusion criteria were the following: (1) history of HNSCC, (2) cancer therapy completed, (3) 18 years of age or older, (4) German-speaking, and (5) able to walk without assistance. Inclusion was not linked/limited to the presence of specific deficits (eg, movement restrictions or pain). Exclusion criteria included clinically significant heart and lung diseases (eg, heart failure stage New York Heart Association III or higher, chronic obstructive pulmonary disease stage III or higher according to GOLD) or characteristics that interfere with the ability to complete the study (eg, planned interventions/rehabilitation or addiction to alcohol). Diseases that could seriously affect cognitive performance (eg, dementia, stroke, Wernicke-Korsakoff syndrome) were also excluded. Prior to participation, written informed consent was obtained from all patients.
Feasibility Outcome
Feasibility was defined as the percentage of participants able to complete the intervention and both tests before and after the intervention. The intervention was declared as feasible if ≥70% of the patients were able to complete the intervention.
Training Program
The intervention lasted 12 weeks with 2 training sessions of 50 minutes per week. The training was supervised by a therapist and performed in small groups (maximum 8 participants) in a physiotherapy room at the Rostock University Medical Center. We designed 12 training plans of which each was performed twice. The training sessions consisted of a warm-up/mobilization (eg, walking at different speeds, functional exercises for the cervical, thoracic, and lumbar spine as well as shoulder joints), coordination exercises (eg, finger coordination, balance exercises, bouncing, catching, and throwing 1 or more balls), resistance exercises (eg, pushing hands together or pulling them apart in front of the chest, pulling exercises for the back and shoulder with a rubber band, squats, exercises with a ball to strengthen chest, trunk, and leg/buttocks muscles), stretching exercises (eg, stretching of the neck, chest, and forearm muscles), and relaxation exercises (eg, mental journey through the body, fantasy travels, progressive muscle relaxation). Table 2 shows the duration of the different training exercises in minutes per training session. The training program exclusively consisted of exercises that the participants could carry out independently at home. For this reason, only small devices (eg, rubber bands, dumbbells, and gymnastic balls) were used, and exercises were selected which could be easily learned by the participants.
Table 2.
Temporal Extent (Minute) of the Different Training Contents per Training (Each Training Program Was Performed Twice per Week).
| Week |
Mean | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | ||
| Welcome, information | 3 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 5 | 2 | 2 |
| Warm-up/mobilization | 20 | 15 | 18 | 10 | 15 | 10 | 8 | 12 | 15 | 13 | 15 | 13 | 14 |
| Coordination | 5 | 10 | 5 | 8 | 20 | 10 | 10 | 8 | 8 | 15 | 5 | 5 | 9 |
| Resistance | 15 | 15 | 17 | 15 | 0 | 18 | 20 | 18 | 15 | 15 | 20 | 20 | 16 |
| Stretching | 0 | 0 | 0 | 15 | 13 | 10 | 10 | 5 | 0 | 5 | 5 | 0 | 5 |
| Relaxation | 7 | 8 | 8 | 0 | 0 | 0 | 0 | 5 | 10 | 0 | 0 | 10 | 4 |
| Total | 50 | 50 | 50 | 50 | 50 | 50 | 50 | 50 | 50 | 50 | 50 | 50 | 50 |
Each training started with a warm-up. During functional training, exercises were carried out for about 30 to 60 seconds without breaks. The participants were instructed to perform the exercises in a painless range.
The following coordination exercises were performed for 30 to 60 seconds each, with 1 (finger and arm coordination) to 3 sets (balance exercises) per exercise and 20 seconds rest in between. In total, 3 to 8 exercises with increasing difficulty were performed per training.
Then, whole-body resistance exercises were performed, focusing on neck and upper body muscles. However, the other major muscle groups, for example, the legs, were also addressed. All exercises were performed dynamically for 20 to 60 seconds or with 10 to 15 repetitions. One to 3 sets were performed per exercise with a break of 30 seconds between each set. Altogether, 5 to 8 exercises were performed per training. The resistance exercises were designed progressively (ie, increasing difficulty level of the exercises, duration of load/number of repetitions). Ratings of perceived exertion (RPE) using the 15-point Borg scale (6-20) were employed to adjust a moderately intensive level of resistance training.16 RPE should be between 11 and 15. The participants had the opportunity to adjust the intensity of the exercises according to their performance, for example, changing the range of motion (ROM), the velocity, and the resistance (rubber band).
Each training ended with stretching and/or relaxation exercises. Stretching exercises covering all large muscle groups were performed for 20 to 40 seconds with 10-second rest in between. The relaxation exercises were performed in a supine position (eg, progressive muscle relaxation) or in a freely chosen position (fantasy travels).
If single patients were unable to perform certain exercises due to movement restrictions or pain, they carried out alternative exercises. During training, the participants had the opportunity to drink and take a break if necessary. The training attendance was documented by the therapist.
Physical Function Assessments
Physical function assessments were carried out 1 week before and 1 week after the intervention. These were performed at the same time of day and in the same order. All tests were performed by the same investigator. The assessment consisted of the following measures:
State fatigue was assessed with a modified Fatigue Subscale of the Profile of Moods States (POMS-F). The POMS-F subscale consists of 7 items (eg, worn out, fatigued, exhausted, sluggish, and weary), and the participant has to rate the felt intensity on a 7-point scale (0 = not at all to 7 = extremely strong). To increase the sensitivity, a Visual Analogue Scale (0-100 mm, 0 = not at all to 100 = extremely strong) was used instead of the 7-point scale. The sum score was calculated for all items (range of 0-700). The POMS-F was used to quantify state fatigue severity on the measurement days because it has been shown that prior fatigue perception can alter the outcome of endurance and gait assessments.17,18 If a difference between pre- and posttraining would have been observed, statistical analyses would have been adjusted for that using state fatigue as a covariate.
Active ROM (°) of the shoulder joints and the cervical spine were measured in sagittal, frontal, and transversal plane using a manual goniometer. The measurements were carried out starting from the maximum ROM away from the body to the end position close to the body.
Maximum mouth opening was measured using the inter-incisor distance (cm) determined with a ruler.
Static flexibility was assessed with the stand and reach test. Subjects stood with closed/stretched legs and held 1 hand covering the other. The trunk was slowly flexed and the distance between hands and ground (which could be held for 2 s) was measured (cm).19
Fall risk was evaluated by using the short physical performance battery (SPPB). The SPPB has been shown to have predictive usefulness for the assessment of mortality risk, nursing home admission, and disability.20 The SPPB is a group of measures that combines the results of balance tests, gait speed, and repeated chair stands. The scores range from 0 (worst performance) to 12 (best performance). Test procedure: first the balance tests were carried out. The participant had to stand as stable and long as possible (maximum 10 seconds). The first position was the side by side stand. If the participant managed 10 seconds, the test was performed in a semi-tandem stand. If the test participant accomplished 10 seconds in this position, a tandem stand was performed. Afterwards, the time the participant needed to walk a distance of 4 m was recorded. Finally, the time required to perform 5 raises from a chair to an upright position as fast as possible without using the arms was measured. The seat height was 48 cm.
Aerobic performance was assessed using the 6-minute walk test (6MWT). The primary measure was the walk distance achieved within 6 minutes. The test was carried out on a 40-m long straight track (a mark was placed every 10 m) on solid ground. After the 6MWT, the participants were asked to state their RPE using the 15-point Borg scale and their exercise-induced pain in the leg muscles using a Category-Ratio (CR)–10 scale (0 = no pain at all, 10 = extremely intense pain).
Patient-Reported Outcomes
All participants completed an initial questionnaire on demographic parameters (sex, age, height, weight, family status, education, current profession status, cigarette use, alcohol use, exercise history, distance from home to training facility, and how they found out about the study) as well as disease-related information (diagnosis, year of initial diagnosis, treatment, and current impairments).
Quality of life was measured before and after the intervention using 2 established questionnaires: (1) the European Organization for Research and Treatment of Cancer (EORTC) QLQ-C30 version 3.0 questionnaire and (2) the QLQ-HN35 head and neck–specific questionnaire. The EORTC QLQ-C30 is a 30-item cancer-specific questionnaire that has a global QoL scale, 5 functional scales (physical, role, emotional, cognitive, and social), 3 symptom scales (fatigue, nausea/vomiting, and pain), and 6 single items (dyspnea, insomnia, appetite loss, constipation, diarrhea, and financial difficulties). EORTC QLQ-HN35 is a 35-item module. It contains 7 symptom scales (pain, swallowing, taste/smell, speech, social eating, social contacts, and sexuality) and 6 symptom items (teeth problems, trismus, dry mouth, sticky saliva, cough, and feeling ill). Each scale results in an average score of 0 to 100. A high value in the scale “global QoL” and in the functional scales means a high degree of subjectively perceived health and a high assessment of the QoL or a high degree of performance and function. A high value in the symptom scales correlates with a high degree of complaints and symptoms.21-23 No threshold values were set in advance to assess the clinically important difference.
Statistical Analyses
Data were analyzed using IBM SPSS 22.0 (SPSS Inc, Chicago, IL). Analyses included descriptive statistics and Wilcoxon signed rank tests. The significance criterion was P ≤ .05 (setting: exact, 1-sided).24 Cohen’s d was calculated to provide an effect size for the respective measures (0.20-0.5 = small effect, 0.5-0.8 = medium effect, ≥0.80 = large effect).
Results
Demographics
Six females and 6 males participated. The average age was 68 ± 9 years (ranging from 52 to 81 years). Eleven participants claimed to be physically active before the cancer treatment. At the time of the intervention, 4 were still physically active. They either trained independently at home or attended a rehabilitation sport group. Please see Table 3 for further demographic data and disease-related information.
Table 3.
Demographic Data and Disease-Related Information of Participants.
| Variable | n (%) or Mean (Range) |
|---|---|
| Total cohort | 12 |
| Sex | 6 females (50%) |
| Age (years) | 68 (52-81) |
| Height (cm) | 167 (152-178) |
| Weight (kg) | 70 (48-102) |
| BMI (kg/m2) | 25 (19-35) |
| Family status | |
| Single/widowed | 5 (42%) |
| Married/living with a partner | 7 (58%) |
| Education | |
| General school | 2 (17%) |
| Secondary school | 5 (42%) |
| High school | 4 (33%) |
| N/A/other | 1 (8%) |
| Current profession status | |
| Retired | 10 (83%) |
| Other | 2 (17%) |
| Tabacco consumption | |
| Nonsmokers | 6 (50%) |
| Ex-smokers | 3 (25%) |
| Smokers | 3 (25%) |
| Alcohol consumption score (frequency × amount) | |
| 0 | 12 (100%) |
| Physical training before disease | |
| Active | 11 (92%) |
| Nonactive | 1 (8%) |
| Currently physical active | |
| Active | 4 (33%) |
| Nonactive | 8 (67%) |
| Distance from home to training facility (km) | 11 (5-28) |
| Cancer site | |
| Pharynx | 5 |
| Oral cavity | 4 |
| Nose | 1 |
| Salivary gland tumor | 1 |
| SCC cervical lymph node with unknown primary | 1 |
| Year of initial diagnosis | |
| Before and in 2013 (long-term survivors) | 8 (67%) |
| After 2013 to 2018 | 4 (33%) |
| Anticancer therapy | |
| Surgery | 12 (100%) |
| Irradiation | 10 (83%) |
| Chemotherapy | 6 (50%) |
| Treatment-associated side effects | |
| Dry mouth | 9 (75%) |
| Dysphonia | 7 (58%) |
| (Scar) pain | 7 (58%) |
| Appetite loss | 6 (50%) |
| Movement restrictions | 5 (42%) |
| Lymphedema | 5 (42%) |
| Swallowing | 4 (33%) |
| Chemotherapy-induced peripheral neuropathy | 4 (33%) |
| Fatigue | 3 (25%) |
| Motor impairments | 3 (25%) |
| Altered body perception | 2 (17%) |
| Hypoglossal paresis | 2 (17%) |
| Gastric tube | 1 (8%) |
| Cognitive impairments | 1 (8%) |
| Depression | 1 (8%) |
| Disability in respiratory function | 1 (8%) |
Abbreviations: BMI, body mass index; SCC, squamous cell carcinoma.
Recruitment
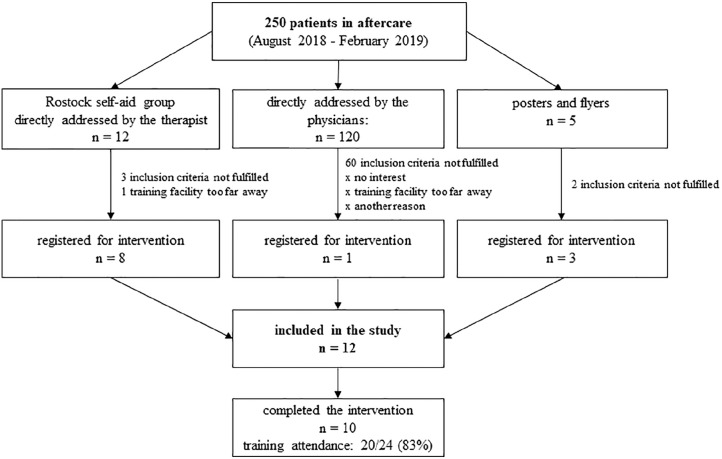
Participants were recruited between August 2018 and February 2019. A total of 12 HNSCC patients were included. Eight patients were recruited by the self-aid group “Cancer in the mouth, jaw, face, and neck area” and 3 via posters and flyers during clinical aftercare. In total, 150 HNSCC survivors were approached directly by the physicians. Only one was included in the study. Reasons for nonparticipation were the following: inclusion criteria were not met, lack of interest, distance to training facility, and others (eg, overlap with work, care/supervision of relatives/children). Figure 1 shows the flow of participants throughout the trial.
Figure 1.
Flow chart of the pilot study. x = n not indicated.
Training Attendance, Practicability, and Safety
Ten out of 12 participants completed the 12-week intervention (83%). These participants on average took part in 20 ± 3 out of 24 training sessions (83%).
All participants quickly learned and executed the mobilization, resistance, stretching, and relaxation exercises. Repeated explanations or posture corrections were not necessary. During the coordination exercises, expected problems appeared, but the content was understandable (eg, tandem stand with closed eyes). The participants rarely needed additional breaks. No adverse events occurred.
Intervention Effects on Physical Function
Table 4 presents the results for the various physical function measurements.
Table 4.
Intervention Effects on Physical Functions and Quality of Life (n = 10).
| Parameter | Pretest (mean ± SD) | Posttest (mean ± SD) | P | Effect Size Cohen’s d |
|---|---|---|---|---|
| Functional status | ||||
| POMS-F | 100.3 ± 30.5 | 110.3 ± 30.8 | .385 | 0.263 |
| ROM shoulder joint (°) | ||||
| Ante-retro version right | 163.6 ± 27.2 | 182.0 ± 17.1 | .023* | 0.646 |
| Ante-retro version left | 156.3 ± 27.3 | 186.1 ± 27.3 | .001* | 1.696 |
| Abduction-adduction right | 121.5 ± 6.5 | 129.1 ± 6.4 | .002* | 1.175 |
| Abduction-adduction left | 114.5 ± 19.3 | 116.3 ± 18.5 | .248 | 0.125 |
| Internal-external rotation right | 121.4 ± 13.9 | 126.4 ± 18.2 | .512 | 0.278 |
| Internal-external rotation left | 117.1 ± 18.0 | 120.1 ± 19.5 | .297 | 0.188 |
| ROM cervical spine (°) | ||||
| Extension-flexion | 80.4 ± 16.6 | 74.7 ± 12.9 | .100 | 0.508 |
| Lateral flexion | 39.8 ± 19.0 | 41.0 ± 13.6 | .357 | 0.065 |
| Rotation | 98.8 ± 28.3 | 110.0 ± 18.5 | .042* | 0.788 |
| Inter-incisor distance (cm) | 4.3 ± 1.0 | 3.4 ± 0.6 | .063† | 1.468 |
| Stand and reach test (cm) | −5.6 ± 2.4 | −4.3 ± 1.9 | .104 | 1.243 |
| SPPB (score) | 11.2 ± 0.3 | 12.0 ± 0.0 | .031* | — |
| 6-minute walk distance (m) | 530.2 ± 17.8 | 575.5 ± 14.8 | .010* | 3.037 |
| Rating of perceived exertion (Borg scale) | 12.1 ± 0.8 | 10.2 ± 0.6 | .047* | 1.946 |
| Exercise-induced leg muscle pain (CR-10 scale) | 2.2 ± 0.9 | 0.8 ± 0.5 | .031* | 1.831 |
| EORTC QLQ-30 | ||||
| Global QoL scale | 50.1 ± 16.4 | 58.3 ± 16.2 | .059† | 0.626 |
| Functional scales | ||||
| Physical | 80.7 ± 11.0 | 87.4 ± 11.5 | .008* | 1.077 |
| Role | 73.4 ± 31.6 | 66.6 ± 29.4 | .250 | 0.250 |
| Emotional | 66.8 ± 22.9 | 65.8 ± 21.7 | .318 | 0.041 |
| Cognitive | 28.4 ± 31.4 | 76.7 ± 27.3 | .015* | 0.829 |
| Social | 40.0 ± 38.7 | 63.4 ± 30.3 | .031* | 0.604 |
| Symptom scales | ||||
| Fatigue | 33.2 ± 21.0 | 26.6 ± 21.9 | .203 | 0.320 |
| Nausea/vomiting | 1.7 ± 5.4 | 1.7 ± 5.4 | 1.000 | — |
| Pain | 41.6 ± 33.5 | 31.6 ± 34.6 | .172 | 0.357 |
| Single items | ||||
| Dyspnea | 9.9 ± 15.9 | 3.3 ± 10.4 | .250 | 0.419 |
| Insomnia | 36.7 ± 36.8 | 46.7 ± 23.6 | .250 | 0.333 |
| Appetite loss | 37.0 ± 42.3 | 25.8 ± 32.4 | .219 | 0.273 |
| Constipation | 16.7 ± 28.4 | 10.0 ± 22.6 | .315 | 0.233 |
| Diarrhea | 6.7 ± 21.2 | 0.0 ± 0.0 | .500 | — |
| Financial difficulties | 53.4 ± 47.7 | 50.0 ± 47.8 | .500 | 0.138 |
| EOTRC QLQ-HN35 | ||||
| Symptom scales | ||||
| Pain | 26.7 ± 20.0 | 33.3 ± 32.1 | .289 | 0.409 |
| Swallowing | 37.8 ± 31.3 | 38.9 ± 29.8 | .406 | 0.064 |
| Taste/smell | 26.7 ± 35.3 | 23.3 ± 30.6 | .250 | 0.219 |
| Speech | 32.2 ± 35.0 | 33.3 ± 39.2 | .563 | 0.049 |
| Social eating | 53.4 ± 38.7 | 47.5 ± 32.8 | .156 | 0.440 |
| Social contacts | 30.0 ± 30.0 | 21.4 ± 27.2 | .068† | 0.542 |
| Sexuality | 64.5 ± 35.0 | 48.0 ± 35.1 | .031* | 0.928 |
| Symptom items | ||||
| Teeth problems | 63.3 ± 36.8 | 43.2 ± 35.4 | .063† | 0.611 |
| Trismus | 50.0 ± 47.8 | 46.7 ± 50.2 | .500 | 0.182 |
| Dry mouth | 63.4 ± 45.7 | 53.3 ± 50.2 | .250 | 0.337 |
| Sticky saliva | 29.3 ± 41.6 | 37.5 ± 51.8 | .250 | 0.805 |
| Cough | 39.9 ± 37.9 | 26.7 ± 30.8 | .328 | 0.309 |
| Feeling ill | 30.0 ± 29.3 | 16.6 ± 23.6 | .133 | 0.380 |
Abbreviations: POMS-F, Profile of Moods States–Fatigue subscale; ROM, range of motion; SPPB, short physical performance battery; CR, Category-Ratio; EORTC, European Organization for Research and Treatment of Cancer; QLQ-HN35, head and neck–specific questionnaire.
Bold values represent statistically significant values (P ≤ .05).
A statistical tendency towards a significant difference (P ≤ .10).
POMS-F
No statistically significant difference was observed. Consequently, the state fatigue was not considered as a covariate in the statistic.
ROM
Improvements in ROM were 18.4° (P = .023, d = 0.646) in ante-/retroversion and 7.6° (P = .002, d = 1.175) in abduction/adduction of the right shoulder joint. On the left side, an improvement of 29.8° (P = .001, d = 1.696) was observed in the ante-/retroversion. ROM during head rotation increased by 11.2° (P = .042, d = 0.788). There were no statistically significant changes in the ROM of the shoulder joints in internal/external rotation, of the cervical spine in the direction of flexion/extension and lateral flexion.
Inter-Incisor Distance
No statistically significant change accurred.
Stand and Reach Test
No statistically significant change was observed.
SPPB
All participants achieved the full score in the SPPB after the intervention (P = .031).
6MWT
The participants increased their walk distance by an average of 43.3 m (+8.3%, P = .010, d = 3.037). RPE (BORG scale) and exercise-induced leg muscle pain (CR-10 scale) decreased significantly (P = .047, d = 1.946/P = .031, d = 1.831, respectively).
Intervention Effects on QoL
The global QoL scale of the EORTC QLQ-30 improved by 8.2 points (P = .059, d = 0.626). The participants reported positive changes in 3 of the 5 functional scales of the EORTC QLQ-30: physical function (+6.7 points, P = .008, d = 1.077), cognitive function (+48.3 points, P = .015, d = .829), and social function (+23.4 points, P = .031, d = .604). There were no statistically significant changes in the 3 symptom scales and 6 individual elements. In the symptom scales of the EOTRC QLQ-HN35, only sexuality showed a significant improvement (−16.5 points, P = .031, d = 0.928). The social contacts scale showed a tendency toward improvement (−8.6 points, P = .068; see Table 4).
Discussion
The present pilot study evaluated a low- to moderate-intensity exercise program regarding feasibility, attendance rate, physical function, and QoL. Data indicated that the exercise program was feasible and had positive effects on physical function and QoL.
Recruitment
In this pilot study, 12 participants were recruited. Possible explanations for the low inclusion rate are discussed below.
It was not possible to write a personal invitation due to data protection reasons in Germany. Therefore, posters and flyers were provided at the centers of aftercare. This attracted 5 patients, and 3 of them were included. In addition, physicians addressed appropriate patients directly during aftercare. Approximately 250 HNSCC patients were in the aftercare during the recruitment period (Figure 1). According to the physicians, 120 of these patients were informed about the study and evaluated for eligibility. From these, approximately 50% of the patients met the inclusion criteria. Consequently, the recruitment rate for our 12 patients was about 10%. If considering only the self-aid group with 12 members, the recruitment rate was 67%. This bias confirms that patients in self-aid groups are more receptive to new treatment strategies with active participation. Additionally, the self-aid group organized a meeting with the executing therapist to present the study. There was a direct exchange between the therapist and potential participants, and questions could be answered directly.
It should also be noted that 50% males and 50% females were included. This differs from the usual sex distribution in HNSCC (90% male, 10% female).
The overall recruitment rate of the present study is also reflected in the literature. Midgley et al14 conducted a questionnaire study to establish the interest and the preferences of HNSCC survivors in participating in an exercise program. In total, 1021 patients were contacted and 437 (43%) responded. Of these, 30% declared interest, covering only 13% overall. Patients preferred exercise durations of 15 to 29 minutes. Fifty-five percent preferred to train at home, and 33% could imagine visiting a health club/gym. In our study, conditions were different: training was carried out as group training in a physiotherapy room and each training lasted 50 minutes at fixed times. The recruitment rate might be higher in a study with flexible training times and exercise durations taking the patients’ preferences into account. Therefore, a multicenter study is highly desirable to increase the sample size.
Training Attendance, Feasibility, and Safety
Our comprehensive low- to moderate-intensity exercise program is a novel exercise compilation for patients with HNSCC; and therefore, our primary objective was to determine the feasibility and compliance.
Only 2 male participants stopped the intervention for health reasons (suspected prostate cancer, cardiovascular disease) after 2 or 7 training sessions. The remaining 10 participants took part in 20/24 training sessions (83%) on average. Primary reasons for skipping sessions were: overlap with physician/physiotherapy appointments, uncomplicated infections, and holiday. In previous studies with HNSCC patients, participation was comparable (65% to 70%7,9 and >80%10,11). An advantage of intervention studies with participants after oncological treatment compared with studies with participants during treatment is the lower probability of side effects that are a barrier to exercise.25 In addition, in our study, some participants knew and motivated each other. It can be assumed that the attendance rate and the effects are lower for unsupervised training interventions.26 For this reason, the number of study participants should be significantly higher in unsupervised studies to get an adequate database for statistical analyses.
All selected exercises were quickly learned and could be executed by the participants. There were no adverse events. The participants reported subjective improvements in QoL (see section “Intervention Effects on QoL”). After completion of the 12-week intervention, the participants wished to continue the training. This feedback from the participants and the intervention effects on physical function and QoL indicates that the exercises and the exercise intensity were a good choice for HNSCC patients during rehabilitation.
Exercise Frequency, Duration, and Content
In our study, the patients were exercising twice a week for 50 minutes, respectively. This frequency and duration actually did not meet the current American College of Sports Medicine (ACSM) exercise guideline for cancer patients. ACSM recommends thrice-weekly aerobic activity for 30 minutes supplemented by 2 resistance training sessions and regular stretching of major muscle groups.25,27 Since the aim of this study was to investigate the effectiveness of the targeted training, aerobic training was deliberately omitted. Primarily, mobilization, strengthening, and stretching exercises for the neck and upper body muscles were used, as HNSCC patients have many limitations/problems in these areas.4 Akin to other cancer patients, HNSCC patients often suffer from therapy-, inactivity-, and age-associated muscle wasting.2,28 Due to these factors, we included all major muscle groups in resistance training according to the ACSM recommendations.25,27
The exercise frequency and duration of 2 times 50 minutes per week was chosen in order to match the ACSM recommendations for resistance training/stretching. However, the recommendations in the guidelines do not provide any information about the actual intensity required for resistance training and stretching. We demonstrate that low- to moderate-intensities resistance and flexibility training are sufficient to achieve positive effects in HNSCC patients. These findings are new and provide significant value for clinical care. These findings are of considerable interest as previous surveys performed in HNSCC patients showed that this patient group prefers training with moderate intensity.14,15 A further advantage is that no or only small devices are needed for the presented specific exercise program. For example, conventional dumbbells or rubber bands can be replaced by water bottles in our exercise program. Accordingly, costs for equipment can be reduced or even be totally avoided. This minimizes possible psychologic or monetary barriers when taking up an exercise program. Preferentially, the necessary equipment to support the exercise program should be provided. In contrast to the supervised training analyzed in our study, where all participants did the same exercises, home-based programs allow tailoring of the exercises to individual needs.
We believe that the advantages of this exercise program (low- to moderate-intensity, minimal cost, individual training) can result in a greater motivation of HNSCC patients to exercise.
Intervention Effects on Physical Function
ROM
A positive effect was observed in active ROM. This improvement is likely attributable to the focus on warm-up/mobilization/functional exercises, and stretching of the upper body (one third of all exercises). The exercises included mobilization of the cervical spine, which is of utmost importance for everyday life (participation in traffic, shoulder check).
As most of the participants had treatment-associated movement restrictions in the left shoulder joint, the effects were greater than on the right. The improvements in ROM are in line with the results of McNeely et al,10 who analyzed the effects of a 12-week progressive resistance training intervention in patients diagnosed with shoulder dysfunction caused by spinal accessory neurapraxia/neurectomy and evidence of trapezius dysfunction. Hence, performing progressive resistance training seems as effective as standard therapy. The weekly exercise time for ROM and stretching exercises was comparable. Based on these results, we can conclude that HNSCC patients with movement restrictions in the shoulder joints and cervical spine benefit from 30-minute functional exercises, resistance exercises, and stretching per week. The extent to which active ROM was influenced by the intake of painkillers was not assessed and should be addressed in subsequent studies.
Inter-Incisor Distance
Sixty seconds of mouth opening per training session did not induce positive changes in inter-incisor distance. The tendency to wards a decreased the mouth opening may be explained by the fact that some participants complained about increasing edema in the face and mouth, especially in the group that started training in September and finished the intervention in December.
Since not all study participants had movement restrictions in the temporomandibular joints, the training time was deliberately kept short in favor of other exercises. Individual home-based interventions could focus on temporomandibular joint mobilization.
Stand and Reach Test
No significant change could be observed for this parameter. A mean distance to the ground of 5.6 before and 4.3 cm after the intervention indicates a slight improvement. Therefore, the test seems well suited to represent changes and should be retained.
SPPB
The participants already had a very high starting score of 11 points. All participants achieved the full score after the intervention. Therefore, the SPPB was not sensitive enough to fully reflect the changes after the intervention. The SPPB is an established method for the assessment of fall risk in elderly and movement-impaired patients. However, it should be supplemented by additional tests (eg, 6MWT) for higher performing participants. Furthermore, instrumented tests (eg, gait analysis) could be used to quantify functional balance performance.
6MWT
Another result of our study was a significant improvement in the walking distance associated with a significantly lower RPE and exercise-induced leg muscle pain. The walking distance correlates with oxygen uptake, and the 6MWT is used to assess aerobic endurance performance.20 The covered 530 m (before) and 576 m (after) are above the values reported by Eades et al6 who analyzed the effect of an interdisciplinary rehabilitation program (8-week nutrition rehabilitation program) on QoL in HNSCC patients. Comparable walking distances were reported by Capozzi et al7 who analyzed the feasibility and impact of a clinic-supported 12-week progressive strength-training program for HNSCC survivors. Interestingly, study participants were significantly younger (53 ± 9 years), confirming the good performance of our participants.
The participants increased their walking distance by an average of 43.3 m (+8.3%), even though no specific endurance training was performed. This increase approaches the clinically significant change of 54 m for other clinical populations.29,30 The increase in the 6MWT performance might be attributable to improvements in balance, strength of the lower extremities, and oxygen uptake induced by the training program. Since no specific strength diagnostic (eg, determination of maximum voluntary contraction strength) and no determination of aerobic capacity (eg, maximum oxygen uptake) were carried out, it is not possible to clearly determine causality of the positive change in walking distance and degree of effort. We assume that regular balance training in combination with resistance training has increased gait stability (reduction of the fall risk). Furthermore, it cannot be ruled out that the training intervention may have increased the participants’ daily activity. This could have led to a potentiation of the intervention effects. For subsequent studies, it is recommended to monitor physical activity during the study period. Altogether it can be stated that the 6MWT is safer, easier to administer, better tolerated, and better reflects activities of daily living than other walking distance tests (eg, Shuttle Walk Test).20 For this reason, the test should be maintained.
Intervention Effects on QoL
As expected, the exercise intervention had a positive effect on overall QoL. Thus, the objective improvement of physical fitness, especially in the 6MWT, was paralleled by increased subjective outcomes. It might be that the increased physical fitness and QoL of the participants has led to an increase in daily activity.8,31 This in turn could have led to an increase in participants’ social activities, which is in line with the improvement in social function. The subjective perception of higher cognitive function probably resulted from the various coordinative exercises and the quite long training sessions (50 minutes), in which the participants were physically and cognitively challenged. In the current literature, exact threshold values for clinically important difference are discussed with some degree of controversy.32,33 Considering the small sample size of the present study, it can be assumed that changes of 7 or 8 points (physical function or global QoL) and of 23 points (social function) or 48 points (cognitive function) are clinically relevant.32 The EORTC QLQ-HN35 module measures specific symptoms/treatment-related morbidities, for example, swallowing, and dry mouth in HNSCC patients. Most of these symptoms require specific treatments and cannot be influenced by exercise programs that aim to improve physical and cognitive functions. Improvements in both areas can lead to higher self-confidence and/or a better self/body image.34 We suspect that the improvements in the symptom scale sexuality mignt be due to this.
For exercise intervention studies, the EORTC QLQ-HN35 appears to be a useful tool for symptom diagnostics. The items that can be influenced directly and indirectly by movement interventions (eg, social contacts, feeling of illness) are better represented by the EORTC QLQ-30.
Supervised Exercise Interventions Versus Home-Based Interventions
In this pilot study, the training intervention was supervised. Based on our results, the next step should be to transfer the exercise program into a home-based program. We think that the results of this study could have some clinical implications, especially if the advantages of home-based programs (eg, unlimited in time, cost-saving, adaptation on individual needs) are taken into account.
To transfer our exercise program into a home-based program, we consider the following things to be important to ensure participation and high attendance: (1) emphasize the importance of the exercise therapy in the medical consultation, (2) adapt the training contents to individual needs, (3) adapt the training frequency and duration to the current performance and preferences of the individual, independent of the recommendations. Patients with poor general condition can start, for example, with 10 to 15 minutes daily and gradually increase, (4) provide training documents (eg, exercise sheets/book, videos) including instructions for exercise execution and intensity control, (5) provide training equipment if possible, (6) offer a contact person for questions (eg, physical therapist), and (7) carry out tests regularly, for example, in the context of aftercare examinations. Improvements can increase motivation to continue.
Study Limitations
We acknowledge that our pilot study has several limitations to be considered in future research. This study is based on a small sample of HNSCC patients evaluated in a supervised group intervention setting without a control group. However, in controlled exercise interventions for HNSCC patients, no improvement in QoL of control groups have been reported.10,35 A study with an adequate sample size would allow for subgroup analyses and would strengthen our conclusions. In order to recruit enough participants, a multicenter approach has to be carried out. Studies with home-based design should record both the training frequency and duration as well as the daily physical activity. Long-term effects were not assessed in our study but should also be addressed in future designs.
Conclusion
Data of our pilot study indicate that our exercise program was feasible and effective for HNSCC patients. Carried out in a supervised group training, the exercises showed positive effects on physical function and QoL. The experiences gained in the present study suggest that a symptom-oriented exercise selection might require only short exercise times with low- to moderate-intensity to produce positive effects regarding physical function and QoL. Our results support further investigations of the feasibility and impact of independent home training in a large cohort.
In preparation for implementing a home-based intervention study, the exercises suitable for independent training at home were compiled in an exercise manual (ISBN-13: 978-1099096082, German language). For better comprehensibility and motivation, videos could also be used, which are currently available.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study was funded by the Rostock University Medical Center.
ORCID iD: Sabine Felser  https://orcid.org/0000-0002-4882-0468
https://orcid.org/0000-0002-4882-0468
References
- 1. Stewart BW, Wild CP. World Cancer Report 2014. Lyon, France: International Agency for Research on Cancer; 2014. [Google Scholar]
- 2. Couch M, Lai V, Cannon T, et al. Cancer cachexia syndrome in head and neck cancer patients: part I. Diagnosis, impact on quality of life and survival, and treatment. Head Neck. 2007;29:401-411. [DOI] [PubMed] [Google Scholar]
- 3. Epstein JB, Robertson M, Emerton S, Phillips N, Stevenson-Moore P. Quality of life and oral function in patients treated with radiation therapy for head and neck cancer. Head Neck. 2001;23:389-398. [DOI] [PubMed] [Google Scholar]
- 4. Borghardt EJ. Rehabilitation bei Patienten mit Kopf-Halstumoren. In: Schmoll HJ, ed. Indikationen, Therapiekonzepte und spezielle Therapiemodalitäten. 4th ed. Berlin, Germany: Springer Medizin Verl; 2006:1363-1372. [Google Scholar]
- 5. Langendijk JA, Doornaert P, Verdonck-de Leeuw IM, Leemans CR, Aaronson NK, Slotman BJ. Impact of late treatment-related toxicity on quality of life among patients with head and neck cancer treated with radiotherapy. J Clin Oncol. 2008;26:3770-3776. [DOI] [PubMed] [Google Scholar]
- 6. Eades M, Murphy J, Carney S, et al. Effect of an interdisciplinary rehabilitation program on quality of life in patients with head and neck cancer: review of clinical experience. Head Neck. 2013;35:343-349. [DOI] [PubMed] [Google Scholar]
- 7. Capozzi LC, Boldt KR, Lau H, Shirt L, Bultz B, Culos-Reed SN. A clinic-supported group exercise program for head and neck cancer survivors: managing cancer and treatment side effects to improve quality of life. Support Care Cancer. 2015;23:1001-1007. [DOI] [PubMed] [Google Scholar]
- 8. Capozzi LC, Nishimura KC, McNeely ML, Lau H, Culos-Reed SN. The impact of physical activity on health-related fitness and quality of life for patients with head and neck cancer: a systematic review. Br J Sports Med. 2016;50:325-338. [DOI] [PubMed] [Google Scholar]
- 9. Lønbro S, Dalgas U, Primdahl H, Overgaard J, Overgaard K. Feasibility and efficacy of progressive resistance training and dietary supplements in radiotherapy treated head and neck cancer patients—the DAHANCA 25A study. Acta Oncol. 2013;52:310-318. [DOI] [PubMed] [Google Scholar]
- 10. McNeely ML, Parliament M, Courneya KS, et al. A pilot study of a randomized controlled trial to evaluate the effects of progressive resistance exercise training on shoulder dysfunction caused by spinal accessory neurapraxia/neurectomy in head and neck cancer survivors. Head Neck. 2004;26:518-530. [DOI] [PubMed] [Google Scholar]
- 11. McNeely ML, Parliament MB, Seikaly H, et al. Effect of exercise on upper extremity pain and dysfunction in head and neck cancer survivors: a randomized controlled trial. Cancer. 2008;113:214-222. [DOI] [PubMed] [Google Scholar]
- 12. Pauli N, Fagerberg-Mohlin B, Andréll P, Finizia C. Exercise intervention for the treatment of trismus in head and neck cancer. Acta Oncol. 2014;53:502-509. [DOI] [PubMed] [Google Scholar]
- 13. Sammut L, Ward M, Patel N. Physical activity and quality of life in head and neck cancer survivors: a literature review. Int J Sports Med. 2014;35:794-799. [DOI] [PubMed] [Google Scholar]
- 14. Midgley AW, Lowe D, Levy AR, Mepani V, Rogers SN. Exercise program design considerations for head and neck cancer survivors. Eur Arch Otorhinolaryngol. 2018;275:169-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rogers LQ, Malone J, Rao K, et al. Exercise preferences among patients with head and neck cancer: prevalence and associations with quality of life, symptom severity, depression, and rural residence. Head Neck. 2009;31:994-1005. [DOI] [PubMed] [Google Scholar]
- 16. Heart Online. Rating of perceived exertion—Borg scales. http://www.heartonline.org.au/resources/documents-and-links#exercise. Accessed April 2, 2019.
- 17. Behrens M, Mau-Moeller A, Lischke A, et al. Mental fatigue increases gait variability during dual-task walking in old adults. J Gerontol A Biol Sci Med Sci. 2018;73:792-797. [DOI] [PubMed] [Google Scholar]
- 18. Van Cutsem J, Marcora S, De Pauw K, Bailey S, Meeusen R, Roelands B. The effects of mental fatigue on physical performance: a systematic review. Sports Med. 2017;47:1569-1588. [DOI] [PubMed] [Google Scholar]
- 19. Holt LE, Pelham TW, Burke DG. Modifications to the Standard Sit-and-Reach Flexibility Protocol. J Athl Train. 1999;34:43-47. [PMC free article] [PubMed] [Google Scholar]
- 20. Guralnik JM, Ferrucci L, Pieper CF, et al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci. 2000;55:M221-M231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365-376. [DOI] [PubMed] [Google Scholar]
- 22. Fayers PM. Interpreting quality of life data: population-based reference data for the EORTC QLQ-C30. Eur J Cancer. 2001;37:1331-1334. [DOI] [PubMed] [Google Scholar]
- 23. Singer S, Araújo C, Arraras JI, et al. Measuring quality of life in patients with head and neck cancer: update of the EORTC QLQ-H&N Module, Phase III. Head Neck. 2015;37:1358-1367. [DOI] [PubMed] [Google Scholar]
- 24. Cho HC, Abe S. Is two-tailed testing for directional research hypotheses tests legitimate? J Bus Res. 2013;66:1261-1266. [Google Scholar]
- 25. Schmitz KH, Campbell AM, Stuiver MM, et al. Exercise is medicine in oncology: engaging clinicians to help patients move through cancer. CA Cancer J Clin. 2019;69:468-484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sweegers MG, Altenburg TM, Brug J, et al. Effects and moderators of exercise on muscle strength, muscle function and aerobic fitness in patients with cancer: a meta-analysis of individual patient data. Br J Sports Med. 2019;53:812. [DOI] [PubMed] [Google Scholar]
- 27. Campbell KL, Winters-Stone KM, Wiskemann J, et al. Exercise guidelines for cancer survivors: consensus statement from International Multidisciplinary Roundtable. Med Sci Sports Exerc. 2019;51:2375-2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lønbro S, Dalgas U, Primdahl H, et al. Progressive resistance training rebuilds lean body mass in head and neck cancer patients after radiotherapy—results from the randomized DAHANCA 25B trial. Radiother Oncol. 2013;108:314-319. [DOI] [PubMed] [Google Scholar]
- 29. Redelmeier DA, Bayoumi AM, Goldstein RS, Guyatt GH. Interpreting small differences in functional status: the Six Minute Walk test in chronic lung disease patients. Am J Respir Crit Care Med. 1997;155:1278-1282. [DOI] [PubMed] [Google Scholar]
- 30. Wise RA, Brown CD. Minimal clinically important differences in the six-minute walk test and the incremental shuttle walking test. COPD. 2005;2:125-129. [DOI] [PubMed] [Google Scholar]
- 31. Kim JY, Lee MK, Lee DH, et al. Effects of a 12-week home-based exercise program on quality of life, psychological health, and the level of physical activity in colorectal cancer survivors: a randomized controlled trial. Support Care Cancer. 2019;27:2933-2940. [DOI] [PubMed] [Google Scholar]
- 32. Cocks K, King MT, Velikova G, Fayers PM, Brown JM. Quality, interpretation and presentation of European Organisation for Research and Treatment of Cancer quality of life questionnaire core 30 data in randomised controlled trials. Eur J Cancer. 2008;44:1793-1798. [DOI] [PubMed] [Google Scholar]
- 33. Osoba D, Rodrigues G, Myles J, Zee B, Pater J. Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol. 1998;16:139-144. [DOI] [PubMed] [Google Scholar]
- 34. Adamsen L, Andersen C, Midtgaard J, Møller T, Quist M, Rørth M. Struggling with cancer and treatment: young athletes recapture body control and identity through exercise: qualitative findings from a supervised group exercise program in cancer patients of mixed gender undergoing chemotherapy. Scand J Med Sci Sports. 2009;19:55-66. [DOI] [PubMed] [Google Scholar]
- 35. Rogers LQ, Anton PM, Fogleman A, et al. Pilot, randomized trial of resistance exercise during radiation therapy for head and neck cancer. Head Neck. 2013;35:1178-1188. [DOI] [PubMed] [Google Scholar]
- 36. Kamstra JI, Roodenburg JLN, Beurskens CHG, Reintsema H, Dijkstra PU. TheraBite exercises to treat trismus secondary to head and neck cancer. Support Care Cancer. 2013;21:951-957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Schmitz KH, Ahmed RL, Troxel AB, et al. Weight lifting for women at risk for breast cancer–related lymphedema: a randomized trial. JAMA. 2010;304:2699-2705. [DOI] [PubMed] [Google Scholar]
- 38. Schmitz KH, Ahmed RL, Troxel A, et al. Weight lifting in women with breast-cancer–related lymphedema. N Engl J Med. 2009;361:664-673. [DOI] [PubMed] [Google Scholar]
- 39. Stevens JA, Burns E. A CDC Compendium of Effective Fall Interventions: What Works for Community-Dwelling Older Adults. 3rd ed. Atlanta, GA: Centers for Disease Control and Prevention; 2015. [Google Scholar]
- 40. Liu X, Wang YQ, Xie J. Effects of breathing exercises on patients with lung cancer. Oncol Nurs Forum. 2019;46:303-317. [DOI] [PubMed] [Google Scholar]
- 41. Wada JT, Borges-Santos E, Porras DC, et al. Effects of aerobic training combined with respiratory muscle stretching on the functional exercise capacity and thoracoabdominal kinematics in patients with COPD: a randomized and controlled trial. Int J Chron Obstruct Pulmon Dis. 2016;11:2691-2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. García RS, Yáñez-Brage MI, Moolhuyzen EG, Riobo MS, Paz AL, Mate JMB. Preoperative exercise training prevents functional decline after lung resection surgery: a randomized, single-blind controlled trial. Clin Rehabil. 2017;31:1057-1067. [DOI] [PubMed] [Google Scholar]
- 43. Zimmer P, Baumann FT, Oberste M, et al. Effects of exercise interventions and physical activity behavior on cancer related cognitive impairments: a systematic review. Biomed Res Int. 2016;2016:1820954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. van Lonkhuizen PJC, Klaver KM, Wefel JS, Sitskoorn MM, Schagen SB, Gehring K. Interventions for cognitive problems in adults with brain cancer: a narrative review. Eur J Cancer Care. 2019;28:e13088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Streckmann F, Zopf EM, Lehmann HC, et al. Exercise intervention studies in patients with peripheral neuropathy: a systematic review. Sports Med. 2014;44:1289-1304. [DOI] [PubMed] [Google Scholar]
- 46. Liao J, Wu Y, Zhao Y, et al. Progressive muscle relaxation combined with Chinese medicine five-element music on depression for cancer patients: a randomized controlled trial. Chin J Integr Med. 2018;24:343-347. [DOI] [PubMed] [Google Scholar]
- 47. Mustian KM, Alfano CM, Heckler C, et al. Comparison of Pharmaceutical, Psychological, and Exercise Treatments for Cancer-Related Fatigue: A Meta-analysis. JAMA Oncology. 2017;3:961-968. [DOI] [PMC free article] [PubMed] [Google Scholar]