Abstract
SARS-CoV-2 was identified as the causative pathogen in an outbreak of viral pneumonia cases originating in Wuhan, China, with an ensuing rapid global spread that led it to be declared a pandemic by the WHO on March 11, 2020. Given the threat to public health posed by sequelae of SARS-CoV-2 infection, the literature surrounding patient presentation in severe and non-severe cases, transmission rates and routes, management strategies, and initial clinical trial results have become available at an unprecedented pace. In this review we collate current clinical and immunologic reports, comparing these to reports of previous coronaviruses to identify mechanisms driving progression to severe disease in some patients. In brief, we propose a model wherein dysregulated type I interferon signalling leads to aberrant recruitment and accumulation of innate immune lineages in the lung, impairing establishment of productive adaptive responses, and permitting a pathologic pro-inflammatory state. Finally, we extend these findings to suggest possible treatment options that may merit investigation in randomized clinical trials.
Keywords: SARS-CoV-2, COVID-19, COVID-19 pneumonia, immune response, COVID-19 mechanisms
Introduction
SARS-CoV-2 was determined to be the causative pathogen of the disease COVID-19 resulting in an outbreak of viral pneumonia cases originating in Wuhan, China in 2019. The rapid spread of the virus on a global scale led to WHO designation as a pandemic on 11 March 2020. At the time of this review, there are over 3.8 million confirmed global cases of COVID-19 and at least 270,000 attributable deaths, with over 1.2 million cases and 76,000 attributable deaths in the United States [1]. Relative to annual influenza infections, COVID-19 appears to have significantly higher rates of patient morbidity and mortality, though they remain lower than what was seen for SARS-CoV and MERS. Unfortunately, no specific therapy is available and the management of infected patients has been variable among institutions and nations. Given the rapid pace at which the global situation and our understanding of the virus is progressing, this review intends to collate currently available published case series (peer-reviewed and pre-print) and integrate these clinical findings with immunologic insights from COVID-19, SARS-CoV, and MERS studies.
Clinical characteristics among patients with COVID-19 infection
Emerging data from Wuhan, China suggests that while some patients are hospitalized with more severe COVID-19 infections (17%), most cases are not life-threatening [2]. Preliminary estimates suggest the time from infection to symptom onset to be around 5 days, and a mean duration of 11–12.5 days from the time of infection to hospitalization [3]. Despite initial controversy, viral shedding and transmission from person-to-person does occur during an asymptomatic period [4], likely contributing to the rapid global spread. Indeed, most of the viral shedding occurs before symptom onset [5]. Of note, viral shedding has been found to align with cough symptoms, in nasopharyngeal samples and sputum. Shedding can also occur through stool although infectious viral transmission has not been reported through this route [6]. A notable limitation to most studies are the relatively small sample sizes and the relative exclusion of infected patients with only mild symptoms who do not seek medical attention. The severity of patient illness has repeatedly been found to correlate with symptomology, clinical parameters, and outcome; therefore, most published reports stratify patient parameters by ‘non-severe’ versus ‘severe’ disease (studies stratifying by ‘acute respiratory distress syndrome (ARDS)’ versus ‘no ARDS’, or ‘intensive care unit (ICU)’ versus ‘no ICU’ were conformed to this strategy for ease of reporting). Published case series indicate the most common presenting symptoms to include fever in the majority and cough with fatigue, sputum production, myalgias/arthralgias, and shortness of breath less commonly present but enriched in patients with severe disease [2,7–10]. Although distinct viral strains may predominate in different geographical regions, the clinical presentations from these Chinese studies were mirrored by laboratory-confirmed COVID-19 infected patients treated at an academic health system in New York, New York, USA [11,12], though respective frequencies of fever (61%) and cough (56%) were notably lower in an Italian case series [13] (Table 1).
Table 1.
Compiled clinical data from multiple studies of severe and non-severe COVID-19 patients.
| Non-severe | Severe | Reference no. | |
|---|---|---|---|
| Symptoms (%) | |||
| Fever | 91.94 (83–97.7) | 94.29 (87–100) | [2,7–10,14,15] |
| Cough | 66.27 (33.7–82) | 71.36 (33.6–85) | [2,7–10,14,15] |
| Sputum | 31.61 (33.6–85) | 42.59 (26–64.7) | [2,7–10,14,15] |
| Fatigue | 35.54 (21–52.3) | 42.57 (22–70.6) | [2,7–10,14,15] |
| SOB | 30.18 (9.1–62) | 52.02 (17.6–87) | [7,8,10,14,15] |
| Myalgia/arthralgia | 16.23 (14.4–19.3) | 18.47 (15–23.1) | [7–9] |
| Diarrhea | 18.95 (11.4–26.5) | 16.6 (5.9–27.3) | [8,10] |
| Comorbidity (%) | |||
| Male | 59.10 (47.7–68) | 71 (58.8–85) | [2,10–12,14,15] |
| Obesity (BMI 30–40) | 32.7 (31.9–33.5) | 37.85 (32.3–43.4) | [11,12] |
| Chronic conditions | 70.70 | 73.40 | [11] |
| Cardiovascular conditions | 9.57 (0–42) | 18.23 (5.9–47.1) | [2,8,10,11,14,15] |
| Diabetes | 15.82 (4.5–22.9) | 19.41 (8–27.7) | [2,8,10–12,14,15] |
| Asthma/COPD | 5.5 (0–12.2) | 10.56 (3–17.6) | [2,10–12,14] |
| Hypertension | 22.62 (13.6–48.3) | 35.2 (15–53.8) | [2,8,10,12,14,15] |
| CKD | 2.40 | 2.10 | [8] |
| Cytokines (pg/mL) | |||
| IL-1β | 5.00 | 5.00 | [8] |
| IL-6 | 9.22 (6.3–13.41) | 18.55 (7.39–37.7) | [8,9,14–16] |
| IL-8 | 13.70 | 18.40 | [8] |
| IL-10 | 3.73 (2.4–5) | 5.6 (4.59–6.6) | [8,16] |
| TNFα | 6.24 (4.1–8.4) | 5.82 (2.9–8.7) | [8,16] |
| Labs | |||
| CRP (mg/L) | 22.6 (10.3–34.1) | 77.7 (23.5–126.6) | [10,14,15] |
| LDH (U/L) | 263.83 (253.5–281) | 439 (396–521) | [2,9,15] |
| AST (U/L) | 32 (30–34) | 41 (38–44) | [2,15] |
| ALT (U/L) | 27 (27–27) | 41.33 (35–49) | [2,9,15] |
| D-dimer (µg/mL) | 0.56 (0.52–0.6) | 3.18 (1.16–5.2) | [9,15] |
| NLR | 2.74 (2.2–3.2) | 6.53 (3.6–10.5) | [8,10,15] |
| Leukocytes (×109/L) | 4.8 (4.3–5.2) | 6 (3.7–9.8) | [7,9,10] |
| Lymphocytes (×109/L) | 1.05 (1–1.1) | 0.68 (0.4–0.9) | [2,7,9,10] |
| Platelets (×109/L) | 177.13 (149–220) | 163 (137.5–196) | [2,7,9,10] |
| Neutrophils (×109/L) | 2.89 (2.4–3.2) | 4.71 (2.8–7.04) | [8,10,15] |
| Monocytes (×109/L) | 0.37 (0.34–0.4) | 0.35 (0.29–0.4) | [8,15] |
| B cells (×106/L) | 181.05 (166–196.1) | 147.15 (125.3–169) | [8,16] |
| Total T cells (×106/L) | 648.4 (633–663.8) | 454.05 (446.5–461.6) | [8,15] |
| CD4 T cells (×106/L) | 414.27 (371–451.3) | 260.77 (234–285.1) | [8,15,16] |
| Naive CD4 T cells (× 106/L) | 35.00 | 44.50 | [8] |
| Memory CD4 T cells (×106/L) | 65.00 | 55.50 | [8] |
| CD8 T cells (×106/L) | 243.83 (201.9–288.6) | 163.73 (154.7–179) | [8,15,16] |
| Treg (×106/L) | 4.50 | 3.70 | [8] |
Data is compiled based on information provided in respective studies. Data is presented as mean (range) when applicable, only mean is presented if data is from a single study. If data conforming to the table format could not be extracted from a report then that data was excluded from the table. Abbreviations: shortness of breath (SOB), body mass index (BMI), chronic obstructive pulmonary disease (COPD), chronic kidney disease (CKD), interleukin (IL), tumour necrosis factor (TNF), C-reactive protein (CRP), lactate dehydrogenase (LDH), aspartate transaminase (AST), alanine transaminase (ALT), neutrophil-to-lymphocyte ratio (NLR), regulatory T cell (Treg).
Following hospital admission, clinical parameters tend to correlate with disease severity. The acute phase protein C-reactive protein (CRP) was significantly elevated in severe versus non-severe cases. Elevations in lactic acid dehydrogenase (LDH), aspartate aminotransferase (AST), and alanine aminotransferase (ALT) were also more frequent and more significant in severe cases, suggesting a greater extent of systemic tissue damage and corroborating a more inflammatory environment in this cohort [2,7,9,14,15].
Immunologic perturbations are increasingly recognized as a defining feature of severe infection with COVID-19. Serum cytokine derangements include a nearly invariable elevation in interleukin 6 (IL-6). IL-8 and type I interferon are also frequently elevated in the blood of patients with severe COVID-19 pneumonia [16,17]. Although increased circulating tumour necrosis factor alpha (TNFα) has been reported in more severe cases, differences in concentration appear to be inconsistent (Table 1).
Derangements in predominantly pro-inflammatory cytokines, or ‘cytokine storm’ suggest propagation of a systemic inflammatory state in hospitalized patients that develop severe disease. Specifically, elevated ferritin and IL-6 were shown to correlate with mortality in Wuhan, China [9]. The neutrophil-to-lymphocyte ratio (NLR) is a surrogate of systemic inflammation [18] and can serve as a predictor of poor prognosis in critically-ill septic [19,20] and cancer patients [21]. Extending its utility to COVID-19 patients, NLR is significantly elevated in those with severe disease [8,15,22], serving as an independent risk factor and prognosticator of patients at high risk for progression to severe pneumonia [10]. Specifically, circulating neutrophil counts are significantly elevated while nearly all adaptive populations are negatively impacted by severe disease (Table 1).
Lessons from previous coronaviruses
With our understanding still in its infancy, mechanisms contributing to severe COVID-19 pneumonia are yet to be determined; however, lessons can be gleaned from the previous coronavirus epidemics (SARS-CoV and MERS-CoV). Sequencing of the SARS-CoV-2 viral genome revealed a concordance rate of approximately 80% with SARS-CoV, its closest relative aside from bat CoVs (the likely source of COVID-19) [23,24]. Both coronaviruses also bind Angiotensin Converting Enzyme 2 (ACE2), a surface enzyme expressed predominantly on type 2 epithelial cells of the lower respiratory tract, to gain cellular entry, though SARS-CoV-2 binds with 10–20 × greater affinity [25]. Clinically, patient presentation during the SARS-CoV epidemic in 2002–2003 bore striking similarities to that being reported during the current COVID-19 pandemic. Common symptoms of SARS-CoV included fever, chills, myalgia, and dry cough [26]. LDH and CK were frequently elevated in the context of neutrophilia and lymphopenia, again reflecting a systemic inflammatory state [26–29]. Here, a dysregulated immune response frequently resulted in ARDS, a pathologic outcome similar to COVID-19 [26], and similar findings on autopsy [30,31]. Given the substantial biochemical and clinical similarities, it is logical to assume similar mechanisms dictate the immune response to these pathogens. Therefore, lessons learned in the study of SARS-CoV can be cautiously applied to our evolving understanding of COVID-19.
Putative mechanism
SARS-CoV and SARS-CoV-2 enter cells in the respiratory tract via ACE2 and release damage associated molecular patterns (DAMPs; e.g. ATP, HMGB1, nucleic acids, etc.) as well as viral particle-derived pathogen associated molecular patterns (PAMPs) into the extracellular environment. Binding of these molecules to cognate pattern recognition receptors (PRRs) stimulates an innate immune response. In this manner, lung dendritic cells recognize an infection, mature, and traffic to the draining lymph node wherein antigen is presented to T cells [32]. Stimulation of adaptive immunity then leads to viral clearance through cellular and humoral mechanisms–the likely scenario in asymptomatic patients, or with only mild disease. Progression to severe disease, however, is likely driven by dysregulation of this process.
Adaptive dysregulation
Levels of CD4 and CD8 T cells negatively correlate with disease severity in COVID-19 patients and are similarly decreased in SARS-CoV patients [27,29]. Demonstrating their central role in viral clearance, adoptive transfer of virus-specific CD4 or CD8 T cells significantly improved mortality and expedited viral clearance in a lethal challenge model of SARS-CoV. Moreover, vaccination with peptide-coated DCs one week prior to infection was able to elicit a protective CD8 T cell response [33]. In a different approach, Chen et al. depleted T cell subsets before infection and found CD4, but not CD8, T cells to be critical for efficient mouse clearance of SARS-CoV infection. In this same study, the administration of neutralising antibodies following CD4 T cell depletion promoted viral clearance, suggesting a requirement for effective B cell help and production of neutralising antibodies for viral control [34]. In line with these findings, antibodies to type A blood antigens appear to be cross-reactive and somewhat protective, as patients with type B and O blood are less frequently infected with SARS-CoV and SARS-CoV-2 [35,36]. However, declining numbers of circulating lymphocytes in severe disease seemingly suggests impairment of these responses.
In COVID-19 mediated lymphopenia, B cells, activated CD4 T cells, memory CD4 T cells, and CD8 T cells are reduced. One proposed explanation is that SARS-CoV-2 might directly infect T cells and initiate cell death by viral lysis [31]. This outcome seems unlikely, as Banerjee et al. found viral-like particles in CD4 T cells but demonstrated an absence of viral replication in healthy donor PBMCs of any lineage [37]. Furthermore, single-cell RNA-sequencing of PBMCs from hospitalized COVID-19 patients failed to find SARS-CoV-2 viral reads in any samples [17]. Although lymphopenia in the circulation could be driven by massive recruitment of these cells into the lungs, autopsy of patients having succumbed to severe COVID-19 pneumonia showed a paucity of infiltrating lymphocytes [31], rendering this an unlikely scenario as well. The systemic inflammatory state imposed by severe COVID-19 disease, much like sepsis, may then be the impetus behind observed lymphopenia and elevated NLR [38]. In sepsis, circulating lymphocytes display signs of early apoptosis, Annexin V surface expression and lymphocyte shrinkage [39], implicating loss of these populations through programmed cell death [40]. Thus, it is possible that the systemic inflammatory state during severe COVID-19 pneumonia and/or viral sepsis induces lymphocyte apoptosis and dysregulated adaptive responses.
A recent report from China has found a positive correlation between abundance of SARS-CoV-2 Nucleoprotein (NP) neutralising antibodies and disease severity, noting that earlier, stronger responders for NP specific anti-IgG and anti-IgM associate with increased diseased severity. Conversely, patients with fewer circulating neutralising antibodies were found to have a decreased viral load [6]. In agreement with this, Wu et al. reported about 30% of non-severe patients generated very low neutralising antibody titres against the spike (S) protein. It was also found that patients who were older with lower lymphocyte counts and increased CRP had increased neutralising antibody titre, however, none of these patients had severe disease [41]. Although these are small observational studies, results are consistent with reports from MERS infection, where patients having succumbed to disease had robust neutralising antibody responses during infection [42]. Similarly, in SARS-CoV infection, patients with severe illness had higher antibody titres at earlier stages during infection [43].
A macaque model of SARS-CoV proposed one explanation for an ostensible role of neutralising antibodies in disease progression. Macaques vaccinated with S protein and challenged with SARS-CoV developed diffuse alveolar damage compared to only mild pathology in controls. Disease severity was a consequence of increased inflammatory monocyte-macrophages in the lungs and was mediated in part by Fcγ receptor signalling, as the combination of blocking Fcγ receptors and giving anti-S IgG decreased macrophage production of IL-8 and MCP-1 in vitro [44]. A second explanation is that viral S protein and N protein activate complement directly and/or in complex with neutralising antibodies, contributing to the widespread complement activation that culminates in characteristic tissue damage and diffuse microthrombi [45,46]. Activated complement protein C5a is a potent anaphylatoxin that enhances FcγRIII signalling upon receptor binding (C5aR) to promote alveolar macrophage activation [47], potentially serving as a link between these two hypotheses. Indeed, this scenario accounts for observed elevations in D-dimer, reduced platelets, and diffuse tissue thrombi found on autopsy [48].
Antibody-mediated enhancement of viral disease—an increase in viral uptake, replication, and/or pathogenicity due to the binding of virions to antibodies—cannot be excluded. This mechanism has been demonstrated in various other respiratory infections, including influenza virus, respiratory syncytial virus, and is controversial in SARS-CoV and MERS [49].
It is important to consider, however, that these clinical findings are subject to a temporal sampling bias in that patients do not typically seek medical attention until symptoms are relatively progressed. Therefore, it is difficult to discern whether antibody production contributed to a dysregulated immune response and disease severity, or whether disease severity and an altered cytokine milieu drove the production of higher antibody titres. In the latter case, neutralising antibodies could still be protective if present early after infection and would help explain the potential efficacy of passive immunisation. Indeed, Hoffmann et al. found SARS-CoV neutralising antibodies directed against the viral S protein to be cross-protective, inhibiting both SARS-CoV and SARS-CoV-2 host cell entry in vitro [50].
Innate dysregulation
Frequent and significant elevations in circulating IL-6 and IL-8, which contribute to neutrophil activation and recruitment, suggest a potential pathologic role for neutrophils in patients developing severe COVID-19 pneumonia. Using a rat model of SARS-CoV, Haick et al. showed a critical contribution of neutrophils to the initial anti-viral immune response, elaborating IL-1, TNFα, CXCL1, CXCL3, CCL2, and IP10 to orchestrate the recruitment of a productive cellular response to the lungs. Expectedly, depletion of neutrophils prior to infection impaired viral clearance. However, subsequent repopulation and influx of neutrophils to the lungs in late-stage disease contributed to significant morbidity and mortality without improvement in viral clearance [51]. Bulk RNA-sequencing and pathway analysis of PBMCs from COVID-19 patients with mild versus severe symptoms revealed upregulation of neutrophil chemotaxis, neutrophil activation, and type I IFN signalling pathways, consistent with these mouse studies. Virus-host interactome analysis then indicated that non-structural proteins 9 and 10 impaired the function of NKRF (a competitive inhibitor of NFκB) to induce IL-6 and IL-8 expression in lung epithelia, which the authors postulate could incite tissue destruction and eventual ARDS [52].
A role for type I IFN signalling
What is driving this perturbed immune response that causes significantly increased rates of mortality in the elderly and those with chronic disease? Accumulating evidence suggests type I interferon signalling, or dysregulation thereof, dictates establishment of a productive immune response to then pre-empt progressive viral replication, development of severe pneumonia, and ARDS. Channappanavar et al. showed that delayed type I interferon signalling promotes lethal respiratory infection with SARS-CoV in mice, while supplementation with IFNβ prior to peak viral replication abrogated mortality with no significant difference in pulmonary viral load. Knockout of the interferon alpha receptor led to reduced expression of CCL2 (among other chemokines), which functions to recruit inflammatory monocyte-macrophages via CCR2. Antibody-mediated depletion of this population led to reduced levels of TNFα, IL-1β, IL-6, and iNOS and ameliorated disease severity, directly linking pulmonary macrophages to morbidity and mortality in SARS-CoV [53]. Extending these findings, the late administration of type I interferon led to recruitment of pathogenic neutrophils and macrophages to the lung and development of fatal pneumonia in a mouse model of MERS-CoV [54]. Single-cell RNA-sequencing of PBMCs from four COVID-19 patients at discrete stages of disease severity (pre-ICU, ICU, and post-ICU samples) also identified a relationship between dysregulated type I interferon signalling in PBMCs and clinical status. This correlated with drastically increased circulating inflammatory monocytes and fewer T and B cells while patients were in the intensive care unit [17]. In addition, autopsy of two patients having succumbed to COVID-19 infection showed significant aggregation and activation of infiltrating macrophages–homogeneously expressing IL-6, IL-10, and TNFα–amidst extensive tissue destruction, corroborating a direct role for these cells in pulmonary pathology [31].
Failure to clear viral infection early might then lead to prolonged type I signalling that can instead impair adaptive immune functions [55]. In support of this notion, flow cytometry on PBMCs from COVID-19 patients with mild versus severe disease found a significant decline in the frequency of multifunctional CD4 T cells in the severe cohort alongside the increased expression of activation and exhaustion surface proteins on CD8 T cells in the total T cell pool. The authors compare these phenotypic changes to an exhaustion-like state [56]. Indeed, prolonged type I interferon signalling is known to reinforce CD8 T cell exhaustion and impair viral control [57]. The extent to which the development of functional T cell exhaustion (that is, loss of cytokine production and cytotoxicity) contributes to disease severity on the timescale of COVID-19 infection and resolution remains to be determined.
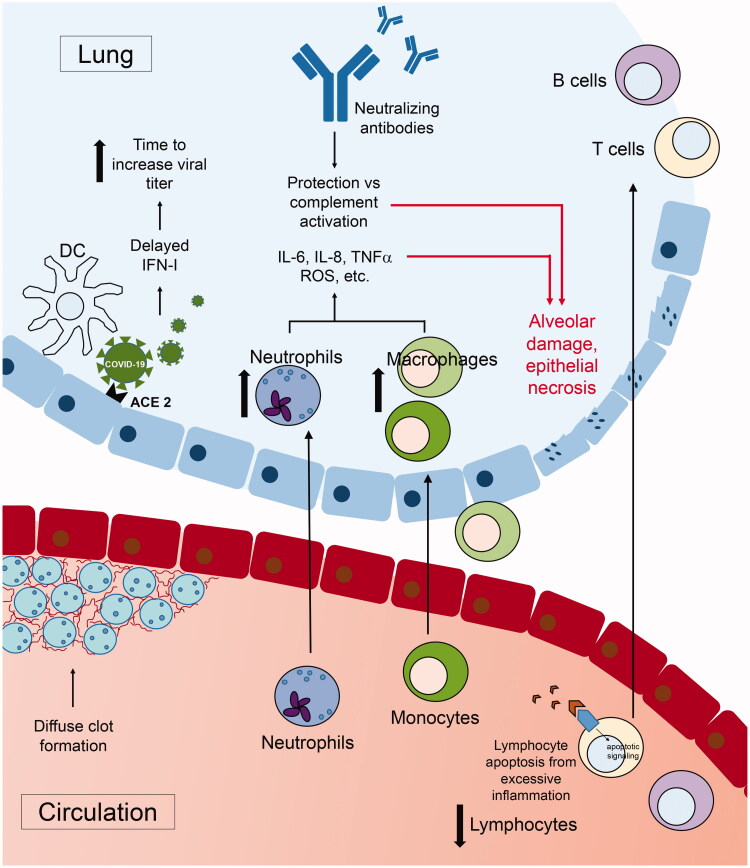
Collectively, these studies suggest a model wherein a delayed initial immune response to infection, including type I interferon signalling, permits high levels of viral replication and persistence. Neutrophil recruitment to the lungs and subsequent activation then elaborates inflammatory cytokines (IL-6 and IL-8) and chemokines (notably CCL2), driving substantial monocyte recruitment and activation. It is likely a combination of these innate populations and diffuse thrombotic complications that mediate eventual tissue destruction manifested by severe disease. The systemic inflammatory state also hinders adaptive immunity, inducing lymphocyte apoptosis, and reducing the production of canonical T cell cytokines as well as neutralising antibodies, impairing viral clearance. These factors culminate in the cytokine storm with local and systemic tissue destruction observed in patients with severe COVID-19 pneumonia. Delayed and/or blunted immune responses characteristic in the elderly [58] and an already dysregulated inflammatory state present in common comorbidities (e.g. insulin resistance, hypertension) [59,60] compromise prompt responses to infection, potentially driving the significantly increased morbidity and mortality observed in these patient populations (Figure 1).
Figure 1.
Proposed model of COVID-19 immune dysregulation leading to severe disease. Schematic of the proposed mechanism responsible for severe COVID-19 disease in some patients. Delayed type I IFN production by dendritic cells (DCs), or other IFN-producing populations, instigates a defective response that cascades into other responding immune lineages. This delay allows increased recruitment of neutrophils, secreting inflammatory cytokines and chemokines, then driving the accumulation of inflammatory monocyte-macrophages in the lungs, particularly the alveoli. Skewing of the innate response creates a potent inflammatory cytokine environment (including IL-6, IL-8, TNFα, reactive oxygen species (ROS), etc.) acting to propagate pulmonary pathology. Additionally, the systemic inflammatory state leads to lymphopenia and stymies establishment of a productive adaptive immune response.
Immunological memory to COVID-19
Whether or not protective memory responses are established following infection with COVID-19 remains uncertain. Convalescent plasma transfer from recovered to currently infected patients has shown promise in reducing clinical severity and expediting clearance of disease [61,62], implying that antibodies to at least some viral epitopes can confer protection. Because the kinetics of B cell contraction in COVID-19 are unknown, it could be that memory cells persist for some time following infection resolution, or that antibodies produced by antibody secreting cells prior to contraction are predominantly responsible for observed benefits. Importantly, if activation induced cell death is occurring in the T lymphocytes, which has been shown in CD44-positive memory or antigen experienced cells, this mechanism could also impede memory cell formation, either by depleting memory B or T cells directly or by interfering with T-B cell help [63].
It appears that memory B cell responses in SARS-CoV are relatively short lived. Patients having recovered from SARS-CoV maintained neutralising antibodies for 16 months after infection, tending to peak 4 months after infection and decreasing thereafter [64]. After 6 years, anti-SARS antibodies were undetectable in 91% of patients and no SARS specific memory B cells were found. Memory T cells were, however, detectable 6 years after infection, but their ability to confer protective immunity remains uncertain [65]. If SARS-CoV-2 proves to behave similarly, it will be important to determine the cause of this temporary persistence and approaches to address it.
Immune dysregulation and tissue pathology
Early reports from China repeatedly identified elevated d-dimer levels, alongside thrombocytopenia in severe COVID19 disease [2,9,15] consistent with formation of disseminated microthrombi and significant necrosis observed on autopsy [46]. Beyond the lungs, SARS-CoV-2 infection can cause thrombotic complications in other systems, including the heart and brain [66,67]. Large vessel clots causing ischaemic stroke in younger patients with otherwise mild symptoms can also be precipitated by infection [68]. A few potential mechanisms explaining observed hypercoagulability have been proposed. In a study of 183 patients, Tang et al. showed that 71% of COVID19 non-survivors had disseminated intravascular coagulation (DIC) [69]. This process can be initiated by pathogen-mediated activation of monocytes or epithelial cells with ensuing release of tissue factor (TF) and von Willebrand Factor (vWF) [70], Notably, proinflammatory cytokines such as TNFα, IL-6, and IL-1—frequently elevated in COVID-19—can also increase circulating vWF [71]. Observed increases in circulating vWF [72] would be expected to prolong the half-life and promote activity of factor VIII [73], potentially contributing to the diffuse microthrombi observed. Alternatively, engagement of the complement system may contribute to diffuse activation of the clotting cascade, and vice versa. N protein can directly activate complement [45] and co-localization of SARS-CoV-2 S protein with complement proteins C4d and C5b-9 (membrane attack complex) is observed in the lungs of patients having succumbed to COVID-19 [46], both suggesting a possible role for direct viral protein-mediated complement activation. The membrane attack complex components C5b-9 can then flip phosphatidylserine to the cell or platelet surface, providing support for coagulation. Moreover, C3a can induce platelet accumulation and C5a can produce pro-coagulant responses via cellular activation [74], highlighting the interplay between complement and coagulation pathways as well as potential contribution to diffuse coagulopathy. Mitigation of this induced hypercoagulable state with anticoagulants has demonstrated improved survival in patients with ‘sepsis-induced coagulopathy’ [75] and those requiring mechanical ventilation [76].
Implications for treatment strategies
As of May 8th, the NIH shows over 1,300 clinical trials registered in association with COVID-19 (search term ‘COVID19’ in clinicaltrials.gov). In addition, new studies are becoming available on preprint servers daily, giving insight into new therapy options and preliminary results thereof. Of note, the SARS-CoV-2 viral and protease structure, which was found to be highly conserved throughout CoVs, has been identified, and specific protease inhibitors with antiviral effects in vitro are currently under investigation [77,78]. The development of specific anti-viral small molecule inhibitors along with a vaccine capable of generating protective immunity is undoubtedly critical to managing the COVID-19 pandemic.
Until such targeted therapies are available, potential points of clinical intervention for patients at high risk of progression can be surmised from the available literature. Administration of type I interferon quickly after infection may be sufficient to expedite a productive immune response. Unfortunately, this is not likely to be a clinically viable approach given the brief window of opportunity between infection and intervention, well before the onset of symptoms. Interrupting early stages of the pathologic response might curtail subsequent immune derangements and facilitate viral clearance. To this end, Li et al. proposed to inhibit neutrophil production of IL-6 and IL-8 using small molecule JAK inhibitors or IL-6 neutralising antibodies [52]. Because anti-IL-6 has proven efficacious in the alleviation of chimeric antigen receptor T cell therapy cytokine release syndrome [79], it is possibly a useful adjuvant to any treatment strategy for severe COVID-19 pneumonia. Alternatively, blocking CCL2-mediated recruitment of monocytes to the lung with neutralising antibodies may be efficacious over a longer period and more closely correspond with the development of symptoms (given the role of macrophages in lung pathology). Inhibitors of activated complement components may similarly impede recruitment of these populations and limit tissue destruction [80]. Of course, a patient’s clinical picture should be evaluated when considering administration of broadly immunosuppressive agents. Giving these during the critical initial stages of response, which seem to determine whether severe disease will occur, might be detrimental to patient outcome. In these initial stages blocking monocyte recruitment, for example, may prove more advantageous.
For patients that do not come to clinical attention until pathologic inflammation has been established, or for whom clinical progression is imminent, management should focus on targeted immunosuppression and conferral of passive immunity. Early management of such patients has included broad-spectrum anti-virals, prophylactic antibiotics, and rather indiscriminate immunosuppression with corticosteroids [2]. More selective immune suppression could be achieved by specifically targeting neutrophils or macrophages. The depletion of these populations responsible for lung pathology might help to restore balance and promote an effective anti-viral immune response. Additionally, blocking type I interferons with neutralising antibodies in advanced disease may delimit lung pathology [81].
Once pulmonary pathology becomes sufficiently advanced, however, the ability to stimulate a productive immune response is compromised and passive immunisation with virus-neutralising antibodies might be of greatest benefit. This approach recently demonstrated favourable outcomes in two of three patients, while the failed case seems to have been an error in cross-matching [61]. A second study with 10 patients reported similarly auspicious outcomes [62]. According to the FDA’s Recommendations for investigational COVID-19 convalescent plasma, plasma can be used in life-threatening COVID-19 cases in the United States, but trial results are still pending [82]. While seemingly promising, plasma transfer will likely be unable to reverse lung damage from immune dysregulation; therefore, the goal must remain improvement of screening measures and early identification of patients at high risk of severe pneumonia. Furthermore, these and other potentially beneficial therapies need to be studied in randomised control studies in sufficient numbers of patients to accurately assess their utility.
Concluding remarks
Our understanding of biologic parameters governing patient presentation following infection with SARS-CoV-2 is expanding at an extraordinary pace. Here we have integrated clinical data from mild and severe COVID-19 patients with mechanistic findings from similar coronaviruses to propose a model wherein an aberrant initial innate immune response cascades into dysregulation of adaptive immunity, preventing efficient viral clearance. Exploring this possible mechanism for severe COVID-19 pathogenesis identified several treatment options meriting continued investigation.
Funding Statement
This work was supported by funds from the UAB School of Medicine Dean’s Office, University of Alabama at Birmingham.
Acknowledgments
The authors would like to thank Dr. Jeremy Walker for his clinical input reviewing this manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- 1.Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020; 3099(20):19–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel Coronavirus in Wuhan. China Lancet. 2020; 395(10223):497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li X, Geng M, Peng Y, et al. Molecular immune pathogenesis and diagnosis of COVID-19. J Pharm Anal. 2020;10(2):102–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rothe C, Schunk M, Sothmann P, et al. Transmission of 2019-NCOV infection from an asymptomatic contact in Germany. N Engl J Med. 2020; 382(10):970–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.He X, Lau EH, Wu P, et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med. 2020;26(5):672–675. https://www.medrxiv.org/content/10.1101/2020.03.15.20036707v2 [DOI] [PubMed] [Google Scholar]
- 6.Tan W, Lu Y, Zhang J, et al. Viral kinetics and antibody responses in patients with COVID-19. medRxiv [Internet]. 2020. DOI: 10.1101/2020.03.24.20042382 [DOI] [Google Scholar]
- 7.Guan W, Ni Z, Hu Y, et al. Clinical characteristics of 2019 Novel Coronavirus infection in China. N Engl J Med. 2020;382(18):1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qin C, Zhou L, Hu Z, et al. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin Infect Dis. 2020:ciaa248 DOI: 10.1093/cid/ciaa248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu J, Liu Y, Xiang P, et al. Neutrophil-to-lymphocyte ratio predicts severe illness patients with 2019 Novel Coronavirus in the early stage. medRxiv [Internet]. 2020. DOI: 10.1101/2020.02.10.20021584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Petrilli CM, Jones SA, Yang J, et al. Factors associated with hospitalization and critical illness among 4,103 patients with COVID-19 disease in New York City. medRxiv [Internet]. 2020. DOI: 10.1101/2020.04.08.20057794 [DOI] [Google Scholar]
- 12.Goyal P, Choi J, Pinheiro L, et al. Clinical characteristics of Covid-19 in New York City. N Engl J Med. 2020. DOI: 10.1056/NEJMc2010419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caruso D, Zerunian M, Polici M, et al. Chest CT Features of COVID-19 in Rome, Italy. Radiology. 2020. DOI: 10.1148/radiol.2020201237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ruan Q, Yang K, Wang W, et al. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46(5):846–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern Med. 2020:e200994 DOI: 10.1001/jamainternmed.2020.0994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wei L, Ming S, Zou B, et al. Viral invasion and type i interferon response characterize the immunophenotypes during COVID-19 infection. SSRN. 2020. [Google Scholar]
- 17.Wan S, Yi Q, Fan S, et al. Characteristics of lymphocyte subsets and cytokines in peripheral blood of 123 hospitalized patients with 2019 novel coronavirus pneumonia (NCP). medRxiv. 2020. DOI: 10.1101/2020.02.10.20021832 [DOI] [Google Scholar]
- 18.Zahorec R. Ratio of neutrophil to lymphocyte counts—rapid and simple parameter of systemic inflammation and stress in critically ill. Bratisl Lek List. 2001; 102(1):5–14. [PubMed] [Google Scholar]
- 19.Cataudella E, Giraffa CM, Di MS, et al. Neutrophil-to-lymphocyte ratio: an emerging marker predicting prognosis in elderly adults with community-acquired pneumonia. J Am Geriatr Soc. 2017; 65(8):1796–1801. [DOI] [PubMed] [Google Scholar]
- 20.Hwang SY, Shin TG, Jo IJ, et al. Neutrophil-to-lymphocyte ratio as a prognostic marker in critically-ill septic patients. Am J Emerg Med. 2017;35(2):234–239. [DOI] [PubMed] [Google Scholar]
- 21.Templeton AJ, McNamara MG, Šeruga B, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst. 2014;106(6):dju124. [DOI] [PubMed] [Google Scholar]
- 22.Zhang B, Zhou X, Zhu C, et al. Immune phenotyping based on neutrophil-to-lymphocyte ratio and IgG predicts disease severity and outcome for patients with COVID-19. medRxiv [Internet]. 2020. DOI: 10.1101/2020.03.12.20035048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou P, Yang X-L, Wang X-G, et al. Discovery of a novel coronavirus associated with the recent pneumonia outbreak in humans and its potential bat origin. Nature. 2020;579(7798):270–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boni MF, Lemey P, Jiang X, et al. Evolutionary origins of the SARS-CoV-2 sarbecovirus lineage responsible for the COVID-19 pandemic. bioRxiv [Internet]. 2020. DOI: 10.1101/2020.03.30.015008 [DOI] [PubMed] [Google Scholar]
- 25.Wrapp D, Wang N, Corbett KS, et al. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020; 367(6483):1260–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee HKK, Tso EYK, Chau TN, et al. Asymptomatic severe acute respiratory syndrome-associ-ated Coronavirus infection. Emerg Infect. 2003;9(11):1491–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.He Z, Zhao C, Dong Q, et al. Effects of severe acute respiratory syndrome (SARS) coronavirus infection on peripheral blood lymphocytes and their subsets. Int J Infect Dis. 2005; 9(6):323–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Booth CM, Matukas LM, Tomlinson GA, et al. Clinical features and short-term outcomes of 144 patients with SARS in the greater toronto area. JAMA. 2003; 289(21):2801–2809. [DOI] [PubMed] [Google Scholar]
- 29.Li T, Qiu Z, Zhang L, et al. Significant changes of peripheral T lymphocyte subsets in patients with severe acute respiratory syndrome. J Infect Dis. 2004; 189(4):648–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang C, Xie J, Zhao L, et al. Alveolar macrophage activation and cytokine storm in the pathogenesis of severe COVID-19. Res Sq. 2020. DOI: 10.21203/rs.3.rs-19346/v1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lau YL, Peiris JSM, Law H. Role of dendritic cells in SARS coronavirus infection. Hong Kong Med J. 2012;18(4):S28–S30. [PubMed] [Google Scholar]
- 33.Zhao J, Zhao J, Perlman S. T Cell responses are required for protection from clinical disease and for virus clearance in severe acute respiratory syndrome Coronavirus-infected mice. J Virol. 2010;84(18):9318–9325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen J, Lau YF, Lamirande EW, et al. Cellular immune responses to Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV) infection in senescent BALB/c Mice: CD4+ T Cells are important in control of SARS-CoV infection. J Viorl. 2010;84(3):1289–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cheng Y, Cheng G, Chui CH, et al. ABO blood group and susceptibility to severe acute respiratory syndrome. J Am Med Assoc. 2005;293(12):1450–1451. [DOI] [PubMed] [Google Scholar]
- 36.Zhao J, Yang Y, Huang H, et al. Relationship between the ABO Blood Group and the COVID-19 Susceptibility. medRxiv [Internet]. 2020. DOI: 10.1101/2020.03.11.20031096 [DOI] [Google Scholar]
- 37.Banerjee A, Nasir JA, Budylowski P, et al. Isolation, sequence, infectivity and replication kinetics of SARS-CoV-2. bioRxiv [Internet]. 2020. DOI: 10.1101/2020.04.11.037382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wesche DE, Lomas-Neira JL, Perl M, et al. Leukocyte apoptosis and its significance in sepsis and shock. J Leukoc Biol. 2005;78(2):325–337. [DOI] [PubMed] [Google Scholar]
- 39.Schroeder S, Lindemann C, Decker D, et al. Increased susceptibility to apoptosis in circulating lymphocytes of critically ill patients. Langenbeck’s Arch Surg. 2001;386(1):42–46. [DOI] [PubMed] [Google Scholar]
- 40.Roth G, Moser B, Krenn C, et al. Susceptibility to programmed cell death in T-lymphocytes from septic patients: a mechanism for lymphopenia and Th2 predominance. Biochem Biophys Res Commun. 2003; 308(4):840–846. [DOI] [PubMed] [Google Scholar]
- 41.Wu F, Wang A, Liu M, et al. Neutralizing antibody responses to SARS-CoV-2 in a COVID-19 recovered patient cohort and their implications. medRxiv [Internet]. 2020. DOI: 10.1101/2020.03.30.20047365 [DOI] [Google Scholar]
- 42.Al-Abdely HM, Midgley CM, Alkhamis AM, et al. Middle east respiratory syndrome coronavirus infection dynamics and antibody responses among clinically diverse patients, Saudi Arabia. Emerging Infect Dis. 2019;25(4):753–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ho MS, Chen WJ, Chen HY, et al. Neutralizing antibody response and SARS severity. Emerg Infect Dis. 2005; 11(11):1730–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu L, Wei Q, Lin Q, et al. Anti-spike IgG causes severe acute lung injury by skewing macrophage responses during acute SARS-CoV infection. JCI Insight. 2019;4(4):1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gao T, Hu M, Zhang X, et al. Highly pathogenic coronavirus N protein aggravates lung injury by MASP-2-mediated complement over-activation. medRxiv. 2020. DOI: 10.1101/2020.03.29.20041962 [DOI] [Google Scholar]
- 46.Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 2020. DOI: 10.1016/j.trsl.2020.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shushakova N, Skokowa J, Schulman J, et al. C5a anaphylatoxin is a major regulator of activating versus inhibitory FcgammaRs in immune complex-induced lung disease. J Clin Invest. 2002;110(12):1823–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fox SE, Akmatbekov A, Harbert JL, et al. Pulmonary and cardiac pathology in Covid-19: the first autopsy series from New Orleans medRxiv. 2020. DOI: 10.1101/2020.04.06.20050575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smatti MK, Thani AA, Al Yassine HM. Viral-induced enhanced disease illness. Front Microbiol. 2018;9:2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Haick AK, Rzepka JP, Brandon E, et al. Neutrophils are needed for an effective immune response against pulmonary rat coronavirus infection, but also contribute to pathology. J Gen Virol. 2014;95(Pt 3):578–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li J, Guo M, Tian X, et al. Virus-host interactome and proteomic survey of PMBCs from COVID-19 patients reveal potential virulence factors influencing SARS-CoV-2 pathogenesis. bioRxiv. 2020. DOI: 10.1101/2020.03.31.019216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Channappanavar R, Fehr AR, Vijay R, et al. Dysregulated Type I interferon and inflammatory monocyte-macrophage responses cause lethal pneumonia in SARS-CoV-Infected Mice. Cell Host Microbe. Cell Press. 2016; 19(2):181–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Channappanavar R, Fehr AR, Zheng J, et al. IFN-I response timing relative to Virus replication determines MERS coronavirus infection outcomes. J Clin Invest. 2019;129(9):3625–3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ivashkiv LB, Donlin LT. Regulation of type I interferon responses. Nat Rev Immunol. 2014; 14(1):36–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zheng HY, Zhang M, Yang CX, et al. Elevated exhaustion levels and reduced functional diversity of T cells in peripheral blood may predict severe progression in COVID-19 patients. Cell Mol Immunol. 2020;17(5):541–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wu T, Ji Y, Ashley Moseman E, et al. The TCF1-Bcl6 axis counteracts type I interferon to repress exhaustion and maintain T cell stemness. Sci Immunol. 2016;1(6):1–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brubaker AL, Palmer JL, Kovacs EJ. Age-related dysregulation of inflammation and innate immunity: lessons learned from rodent models. Aging Dis. 2011;2(5):346–360. [PMC free article] [PubMed] [Google Scholar]
- 59.Smith AG, Sheridan PA, Harp JB, et al. Diet-induced obese mice have increased mortality and altered immune responses when infected with Influenza Virus. J Nutr. 2007;137(5):1236–1243. [DOI] [PubMed] [Google Scholar]
- 60.Galkina E, Ley K. Immune and inflammatory mechanisms of atherosclerosis (*). Annu Rev Immunol. 2009;27(1):165–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pei S, Yuan X, Zhang Z, et al. Convalescent plasma to treat COVID-19: Chinese strategy and experiences. medRxiv. 2020. DOI: 10.1101/2020.04.07.20056440 [DOI] [Google Scholar]
- 62.Duan K, Liu B, Li C, et al. The feasibility of convalescent plasma therapy in severe COVID-19 patients: a pilot study. medRxiv. 2020. DOI: 10.1101/2020.03.16.20036145 [DOI] [Google Scholar]
- 63.Zarozinski CC, McNally JM, Lohman BL, et al. Bystander sensitization to activation-induced cell death as a mechanism of Virus-induced immune suppression. J Virol. 2000;74(8):3650–3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cao WC, Liu W, Zhang PH, et al. Disappearance of antibodies to SARS-associated coronavirus after recovery. N Engl J Med. 2007;357(11):1162–1163. http://authors.nejm.org [DOI] [PubMed] [Google Scholar]
- 65.Tang F, Quan Y, Xin Z-T, et al. Lack of peripheral memory B cell responses in recovered patients with severe acute respiratory syndrome: a six-year follow-up study. J Immunol. 2011;186(12):7264–7268. [DOI] [PubMed] [Google Scholar]
- 66.Wadman M, Couzin-Frankel J, Kaiser J, et al. How does coronavirus kill? Clinicians trace a ferocious rampage through the body, from brain to toes. Science. 2020;80. [DOI] [PubMed] [Google Scholar]
- 67.Klok FA, Kruip M, Meer NJM, van der , et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;x(xxxx):1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Oxley TJ, Mocco J, Majidi S, et al. Large vessel stroke as a presenting feature of Covid-19 in the young. N Engl J Med. 2020;382(20):e60–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tang N, Li D, Wang X, et al. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(4):844–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kitchens C. Thrombocytopenia and thrombosis in disseminated intravascular coagulation (DIC). Am Soc Hematol. 2009;2009(1):240–246. [DOI] [PubMed] [Google Scholar]
- 71.Goeijenbier M. Review: viral infections and mechanisms of thrombosis and bleeding. J Med Virol. 2012;84(10):1680–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Escher R, Breakey N, Lämmle B. Severe COVID-19 infection associated with endothelial activation. Thromb Res. 2020;190:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Terraube V, O'Donnell JS, Jenkins PV. Factor VIII and von Willebrand factor interaction: biological, clinical and therapeutic importance. Haemophilia. 2010;16(1):3–13. [DOI] [PubMed] [Google Scholar]
- 74.Kurosawa S, Stearns-Kurosawa DJ. Complement, thrombotic microangiopathy and disseminated intravascular coagulation. j Intensive Care. 2014;2(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tang N, Bai H, Chen X, et al. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18(5):1094–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Paranjpe I, Fuster V, Lala A. Association of treatment dose anticoagulation with in-hospital survival among hospitalized patients with COVID-19. J Am Coll Cardiol. 2020. DOI: 10.1016/j.jacc.2020.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bestle D, Heindl MR, Limburg, H, et al. TMPRSS2 and furin are both essential for proteolytic activation and spread of SARS-CoV-2 in human airway epithelial cells and provide promising drug targets. bioRxiv. 2020. DOI: 10.1101/2020.04.15.042085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jin Z, Du X, Xu Y. et al. Structure of Mpro from SARS-CoV-2 and discovery of its inhibitors. Nature 2020. DOI: 10.1038/s41586-020-2223-y [DOI] [PubMed] [Google Scholar]
- 79.Le RQ, Li L, Yuan W, et al. FDA approval summary: tocilizumab for treatment of chimeric antigen receptor T cell‐induced severe or life‐threatening cytokine release syndrome. The Oncol. 2018;23(8):943–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Campbell CM, Kahwash R. Will complement inhibition be the new target in treating COVID-19 related systemic thrombosis? Circulation. 2020. DOI: 10.1161/CIRCULATIONAHA.120.047419 [DOI] [PubMed] [Google Scholar]
- 81.Sallard E, Lescure F-X, Yazdanpanah Y, et al. Type 1 interferons as a potential treatment against COVID-19. Antiviral Res. 2020;178:104791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.FDA. Recommendations for Investigational COVID-19 Convalescent Plasma [Internet]. 2020; [cited 2020 May 20]. Available from: https://www.fda.gov/vaccines-blood-biologics/investigational-new-drug-ind-or-device-exemption-ide-process-cber/recommendations-investigational-covid-19-convalescent-plasma#Pathways.