Key Points
Question
Is fexofenadine use during pregnancy associated with an increased risk of adverse fetal outcomes?
Findings
This cohort study from a source cohort of 1.3 million pregnancies in Denmark from 2001 through 2016 included all pregnancies exposed to fexofenadine. No association was noted between fexofenadine use during pregnancy and risk of major birth defects, spontaneous abortion, preterm birth, small size for gestational age, or stillbirth.
Meaning
Fexofenadine use during pregnancy does not appear to be associated with a risk of adverse fetal outcomes; the results indicate that fexofenadine may have a fetal safety profile comparable to that of the currently recommended second-generation antihistamines during pregnancy and thus may be a safe choice of therapy when needed.
Abstract
Importance
Fexofenadine hydrochloride is a frequently used drug for treatment of allergic conditions during pregnancy, but the fetal safety of fexofenadine use has not been well studied.
Objective
To investigate the risk of adverse fetal outcomes associated with fexofenadine use during pregnancy.
Design, Setting, and Participants
A nationwide registry-based cohort study was conducted on pregnancies in Denmark from January 1, 2001, to December 31, 2016. Data analysis was performed from March 21, 2019, to January 29, 2020. From a cohort of 1 287 668 pregnancies, fexofenadine use was compared with cetirizine hydrochloride use during pregnancy, matched in a 1:1 ratio on propensity scores. Distinct study cohorts and exposure time periods were applied according to each outcome analysis. Sensitivity analyses included comparing pregnancies with vs without fexofenadine exposure during pregnancy but with previous use before pregnancy and with loratadine use during pregnancy as additional comparator groups.
Exposure
Filled prescription for fexofenadine.
Main Outcomes and Measures
Major birth defects and spontaneous abortion. Secondary outcomes were preterm birth, small size for gestational age (SGA), and stillbirth. Logistic regression was used to estimate prevalence odds ratios (ORs) of major birth defects, preterm birth, and SGA, and Cox proportional hazards regression was used to estimate hazard ratios (HRs) of spontaneous abortion and stillbirth.
Results
For the analyses of major birth defects and spontaneous abortion, a total of 2962 and 4901 pregnancies with fexofenadine use were included, respectively, matched in a 1:1 ratio with pregnancies with cetirizine use. Mean (SD) age of the fexofenadine cohort for analyses of major birth defects was 30.6 (4.8) years and, for analysis of spontaneous abortion, 30.4 (5.5) years. Infants born with major birth defects occurred in 118 pregnancies (4.0%) with fexofenadine use compared with 112 pregnancies (3.8%) with cetirizine use. Spontaneous abortion occurred in 413 pregnancies (8.4%) with fexofenadine use compared with 439 pregnancies (9.0%) with cetirizine use. Fexofenadine use during pregnancy was not associated with an increased risk of major birth defects (prevalence OR, 1.06; 95% CI, 0.81-1.37) or spontaneous abortion (HR, 0.93; 95% CI, 0.82-1.07) compared with cetirizine use during pregnancy. Preterm birth occurred in 370 pregnancies (7.5%) with fexofenadine use compared with 382 pregnancies (7.7%) with cetirizine use (prevalence OR, 0.97; 95% CI, 0.83-1.12), SGA occurred in 515 pregnancies (10.1%) with fexofenadine use compared with 523 pregnancies (10.2%) with cetirizine use (prevalence OR, 0.98; 95% CI, 0.87-1.12), and a total of 16 pregnancies (0.3%) with fexofenadine use ended in stillbirth compared with 24 pregnancies (0.4%) with cetirizine use (HR, 0.67; 95% CI, 0.36-1.27). Sensitivity analyses of the primary outcomes, including the comparisons of pregnancies with loratadine use and pregnancies unexposed to fexofenadine during pregnancy but with prior use of fexofenadine, showed similar results.
Conclusions and Relevance
Use of fexofenadine during pregnancy does not appear to be associated with an increased risk of adverse fetal outcomes.
This cohort study compares adverse fetal outcomes associated with fexofenadine used during pregnancy with use of cetirizine during pregnancy.
Introduction
Fexofenadine hydrochloride is a widely used, nonsedating, second-generation antihistamine for treatment of allergic disorders, including seasonal rhinitis and chronic idiopathic urticaria, estimated to affect 20% to 30% of women of childbearing age.1 Antihistamines are one of the most commonly prescribed drug classes during pregnancy, with a prevalence up to 10% to 15%.2,3 In the US, fexofenadine was the eighth most prescribed drug in the first trimester in a study period from 1999 to 2003.4 The US Food and Drug Administration approved the switch of fexofenadine from prescription-only to over-the-counter availability in 2011. Despite the broad use of fexofenadine for disorders that often also require treatment during pregnancy, data that assess the safety for the fetus are lacking. Use of cetirizine hydrochloride and loratadine during pregnancy is considered to be safe, and the drugs currently are recommended as first-line choices of second-generation antihistamines during pregnancy by European and US guidelines.5,6,7,8,9,10
In a nationwide cohort study conducted in Denmark, we investigated the association between fexofenadine use during pregnancy and adverse fetal outcomes using a propensity score–matched design, with cetirizine use during pregnancy as an active comparator group. Primary outcomes were any major birth defects and spontaneous abortions. Secondary outcomes were subgroups of birth defects, preterm birth, small size for gestational age (SGA), and stillbirth. The association between the primary outcomes and fexofenadine use during pregnancy was further examined by comparison with additional comparator groups of pregnancies with loratadine use as well as pregnancies unexposed to fexofenadine but with the women’s use of fexofenadine outside pregnancy.
Methods
Data Sources and Study Cohort
We established a registry-based cohort study from January 1, 2001, through December 31, 2016, with data linked between the different Danish nationwide registries using the unique personal identification number assigned to all inhabitants. Data analysis was conducted from March 21, 2019, to January 29, 2020. The source population consisted of all registered pregnancies in Denmark identified in the Medical Birth Registry and National Patient Registry.11,12 The Medical Birth Registry holds data on all live births and stillbirths since 1978, and the National Patient Registry contains information on all pregnancies with abortive outcomes (eg, spontaneous or induced abortion) since 1997, including estimated date of conception and date of abortion. Other data sources for this study included the National Registry of Medicinal Product Statistics for information on drug use and the Danish Civil Registration System and Statistics Denmark for information on demographic variables.13,14 The Registry of Medicinal Product Statistics13 holds information on all redeemed prescriptions from all pharmacies in Denmark, including detailed information on, for example, strength of tablet and package size. Further details on the data sources used are provided in the eMethods in the Supplement. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline for cohort studies. The study was approved by the Danish Data Protection Agency. Ethical approval and informed consent are not required for register-based research in Denmark.
We estimated pregnancy onset (ie, first day of the last menstrual period) by obtaining the gestational age from the date of birth or abortive outcome (eg, spontaneous abortion or stillbirth). This information enabled us to follow the cohort from pregnancy onset. We excluded pregnancies with multiple records on overlapping dates and pregnancy records with implausible or missing information on the gestational age. Analyses of major birth defects, preterm birth, and SGA were based on pregnancies that resulted in live births, while analyses of spontaneous abortion and stillbirth were based on all pregnancies.
Fexofenadine Exposure
Information on filled prescriptions for fexofenadine was obtained from the National Registry of Medicinal Product Statistics.13 Exposure was defined as at least 1 filled prescription for fexofenadine (Anatomical Therapeutic Chemical code R06AX26). Specific exposure time periods were defined according to the respective outcome analyses (eFigure in the Supplement): first trimester for the analyses of major birth defects, before gestational week 23 for spontaneous abortion, before end of gestational week 37 for preterm birth, and any time during pregnancy for both SGA and stillbirth. We allowed women with filled prescriptions during the 30 days before conception to be included in the study cohorts. Fexofenadine became available as an over-the-counter drug in Denmark in 2009; however, 95% of the yearly proportion of sales from the Danish pharmacies has remained personally identifiable.15 The active comparison group consisted of pregnancies with filled prescriptions for cetirizine (Anatomical Therapeutic Chemical code R06AE07).
In sensitivity analyses of the primary outcomes, we compared fexofenadine use during pregnancy with pregnancies unexposed to fexofenadine but with recent previous fexofenadine use, defined as use during the period from 6 months until 30 days before pregnancy onset. In addition, pregnancies with loratadine use during pregnancy (Anatomical Therapeutic Chemical code R06AX13) were used as a second active comparator group. Women with concurrent use of fexofenadine and cetirizine or loratadine within the same pregnancy were excluded.
Outcomes and Potential Confounders
The National Patient Registry and Medical Birth Registry were used to identify outcome cases diagnosed via inpatient or outpatient care. The primary outcomes were any major birth defects diagnosed within the first year of life and spontaneous abortion. Cases of major birth defects and subgroups for an exploratory analysis were defined according to the European Surveillance of Congenital Anomalies classification system of subgroups of major congenital anomalies, while excluding subgroups of chromosomal disorders as well as other defects of known causes (eg, Down syndrome and fetal alcohol syndrome) (eMethods in the Supplement) and minor anomalies according to the European Surveillance of Congenital Anomalies exclusion list.16 Cases of spontaneous abortions were defined as pregnancies ending in fetal death before the end of gestational week 22 with an International Statistical Classification of Diseases and Related Health Problems, Tenth Revision, code of O021 or O03, while excluding very early spontaneous abortions (ie, before gestational week 6) owing to their potential risk of misclassification. The secondary outcomes were preterm birth, defined as birth before 37 completed gestational weeks; SGA, defined as below the lowest 10th percentile of the gestational age–specific birth weight in the source cohort (pregnancies with missing information on birth weight were excluded from this analysis); and stillbirth, defined as fetal death later than the end of gestational week 22.
To account for potential confounding, we performed propensity score matching in a 1:1 ratio of pregnancies with fexofenadine use and cetirizine use to create pairwise matched cohorts for each outcome analysis according to the defined inclusion criteria and exposure time periods of the comparative drugs. The propensity scores were estimated using a logistic regression model, estimating the probability of fexofenadine use based on a range of baseline characteristics at pregnancy onset unless otherwise stated as predictors, including the week of gestation at the time of drug use initiation, demographic variables, previous pregnancy history, and prescription drug use as well as hospital care in this past year (eTable 1 in the Supplement provides covariate definitions).
Statistical Analysis
Matching was performed using the greedy nearest-neighbor matching algorithm (caliper width, 0.02 on the propensity score scale).17,18 Missing values (0%-3.2% missing) (eTable 2 in the Supplement) were imputed using the mode value. The balance of the covariate between matched groups was assessed by standardized differences. An estimate less than 10% was considered to indicate that balance of the covariate was achieved.
Logistic regression was used to estimate prevalence odds ratios (ORs) with the corresponding 95% CIs for the analyses of major birth defects, preterm birth, and SGA. We conducted exploratory analysis of subgroups of birth defects only when cells of subgroups comprised 3 or more cases owing to national regulations on data protection. A Cox proportional hazards regression model was used to estimate hazard ratios (HRs) for the analyses of spontaneous abortion and stillbirth, with gestational age (in days) as the underlying time scale. Pregnancies were censored if an abortive event (eg, induced abortion) other than the outcome of interest had occurred. A Wald test was used for assessing the interaction between time scale and exposure. All statistical tests were 2-sided; 95% CIs not overlapping 1.0 were considered to indicate statistical significance. Analyses were performed with use of SAS software, version 9.4 (SAS Institute Inc).
Prespecified sensitivity analyses of the association between pregnancies with fexofenadine use and primary outcomes included comparison with pregnancies when loratadine was used as well as pregnancies unexposed to fexofenadine but with prior fexofenadine use to test for residual confounding and confounding by indication, categorizing the fexofenadine-exposed pregnancies into 1 or 2 or more filled prescriptions during pregnancy and restricting the exposure time periods to pregnancy onset until the end of the first trimester or week 22 for analyses of major birth defects and spontaneous abortion, respectively, to increase the specificity of drug exposure. In addition, we extended the exposure times to use at any time during pregnancy to increase the sensitivity of drug exposure, restricted to singleton pregnancies for the association with major birth defects to test for any confounding related to multiple birth pregnancies, and conducted an analysis of the risk of induced abortions to test for any bias from elective abortions for the analyses of spontaneous abortion. In post hoc analyses, we ended the exposure periods before the potential risk periods of spontaneous abortion, preterm birth, SGA, and stillbirth to test for potential differential opportunity of exposure.
Results
Cohort Selection
Figure 1 presents a flowchart of the study design. During the study period, we identified 1 287 668 pregnancies eligible for study inclusion. The 5 study cohorts were subsequently constructed based on the eligibility criteria, exposure time periods, and propensity score estimation and matching for each outcome analysis. The unmatched characteristics for each cohort are reported in eTable 3 and eTable 4 in the Supplement. For pregnancies with use of fexofenadine matched in a 1:1 ratio with pregnancies with cetirizine use, the matched study cohorts included 2962 pregnancies for analysis of major birth defects (median index date, gestational day 7; interquartile range [IQR], −12 to 34) and 4901 pregnancies for analysis of spontaneous abortion (median index date, day 17; IQR, −8 to 63). The matched study cohorts for the analyses of the association between the secondary outcomes and fexofenadine use included 4933 pregnancies with fexofenadine use during pregnancy for preterm birth (median index date, day 51; IQR, 1-144), 5110 pregnancies for SGA (median index date, day 57; IQR, 2-157), and 6153 pregnancies for stillbirth (median index date, day 37; IQR, −2 to 134). In each matched cohort, baseline characteristics were well balanced between groups with standardized differences below 10% (Table 1; eTables 5, 6, and 7 in the Supplement). Mean (SD) age of the fexofenadine cohort for analyses of major birth defects was 30.6 (4.8) years and, for analysis of spontaneous abortion, 30.4 (5.5) years.
Figure 1. Flowchart of Study Design.
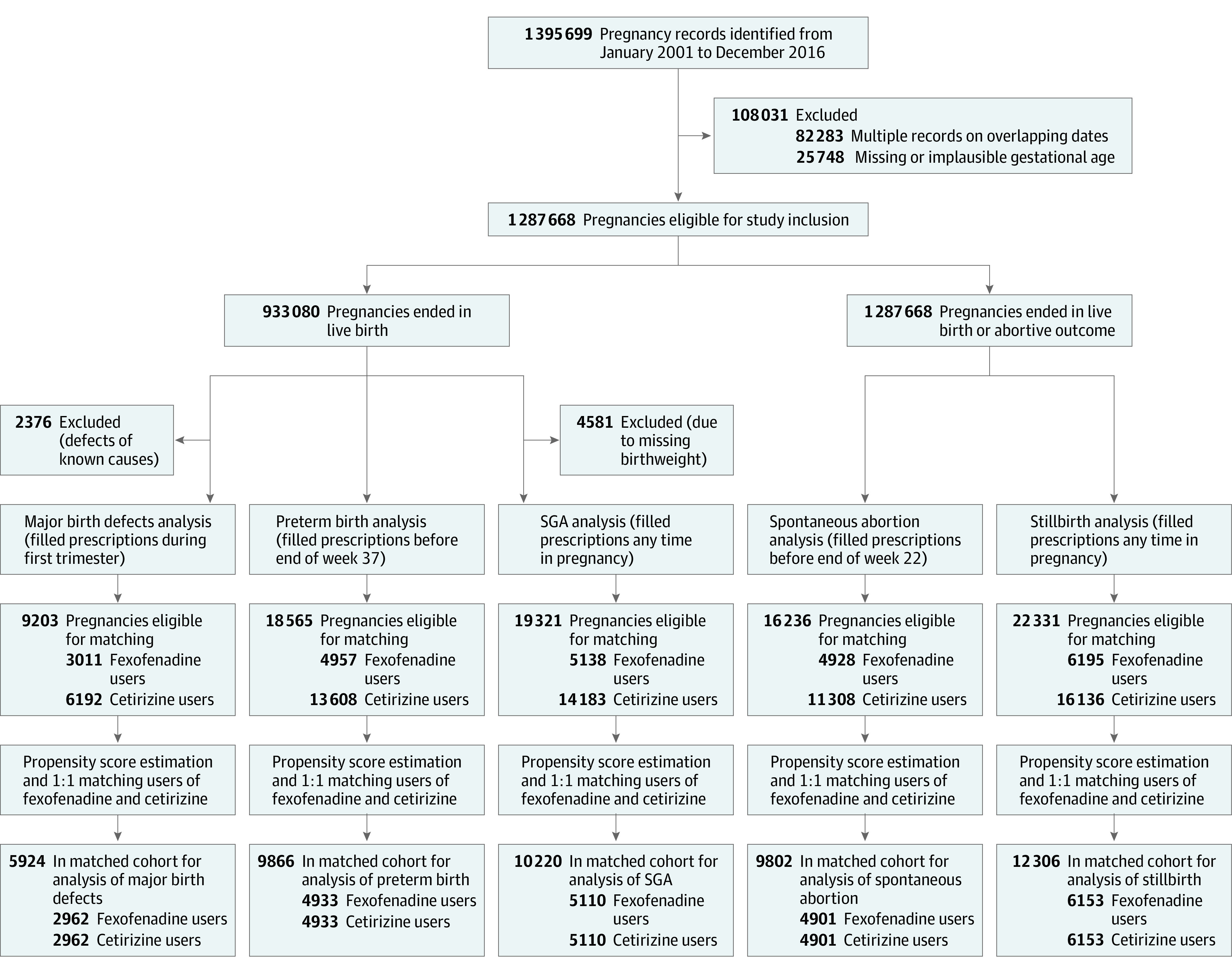
Selection process and establishment of the study cohorts included for the analyses of the primary and secondary outcomes. SGA indicates small size for gestational age.
Table 1. Baseline Characteristics of Propensity Score–Matched Pregnancy Cohorts of Fexofenadine and Cetirizine Users in 1:1 Ratioa.
| Characteristic | Matched cohort for analysis, No. (%) | |||
|---|---|---|---|---|
| Major birth defects | Spontaneous abortion | |||
| Fexofenadine users (n = 2962) | Cetirizine users (n = 2962) | Fexofenadine users (n = 4901) | Cetirizine users (n = 4901) | |
| Gestational age at first filled prescription, median (IQR) | 7 (−12 to 34) | 8 (−11 to 35) | 17 (−8 to 63) | 18 (−8 to 64) |
| Age at pregnancy onset, y | ||||
| ≤19 | 21 (0.7) | 25 (0.8) | 119 (2.4) | 131 (2.7) |
| 20-24 | 291 (9.8) | 286 (9.7) | 566 (11.6) | 558 (11.4) |
| 25-29 | 965 (32.6) | 992 (33.5) | 1483 (30.3) | 1497 (30.5) |
| 30-34 | 1070 (36.1) | 1044 (35.2) | 1598 (32.6) | 1578 (32.2) |
| ≥35 | 615 (20.8) | 615 (20.8) | 1135 (23.2) | 1137 (23.2) |
| Married or living with partner | 2574 (86.9) | 2572 (86.8) | 4002 (81.7) | 4006 (81.7) |
| Place of birth | ||||
| Denmark | 2574 (86.9) | 2593 (87.5) | 4235 (86.4) | 4236 (86.4) |
| Europe | 140 (4.7) | 130 (4.4) | 235 (4.8) | 243 (5.0) |
| Outside Europe | 248 (8.4) | 239 (8.1) | 430 (8.8) | 422 (8.6) |
| Region of residence | ||||
| Capital Region of Denmark | 905 (30.6) | 917 (31.0) | 2243 (45.8) | 2291 (46.8) |
| Region Zealand | 320 (10.8) | 329 (11.1) | 406 (8.3) | 385 (7.9) |
| Region of Southern Denmark | 601 (20.3) | 601 (20.3) | 750 (15.3) | 717 (14.6) |
| Central Denmark Region | 813 (27.4) | 794 (26.8) | 1057 (21.6) | 1057 (21.6) |
| North Denmark Region | 323 (10.9) | 321 (10.8) | 443 (9.0) | 451 (9.2) |
| Gross household income, quartile | ||||
| 1 | 732 (24.7) | 725 (24.5) | 1258 (25.7) | 1278 (26.1) |
| 2 | 757 (25.6) | 733 (24.8) | 1231 (25.1) | 1212 (24.7) |
| 3 | 726 (24.5) | 769 (26.0) | 1195 (24.4) | 1171 (23.9) |
| 4 | 747 (25.2) | 735 (24.8) | 1217 (24.8) | 1240 (25.3) |
| Education level, y | ||||
| <12 | 588 (19.9) | 595 (20.1) | 1168 (23.8) | 1208 (24.7) |
| 12-13 | 428 (14.5) | 412 (13.9) | 723 (14.8) | 692 (14.1) |
| 14-15 | 738 (24.9) | 719 (24.3) | 1134 (23.1) | 1112 (22.7) |
| >15 | 1208 (40.8) | 1236 (41.7) | 1876 (38.3) | 1889 (38.5) |
| Parity | ||||
| 1 | 1423 (48.0) | 1410 (47.6) | NA | NA |
| 2 | 1009 (34.1) | 1024 (34.6) | NA | NA |
| ≥3 | 530 (17.9) | 528 (17.8) | NA | NA |
| Multiple birth pregnancy | 109 (3.7) | 77 (2.6) | NA | NA |
| Season of conception | ||||
| Winter | 511 (17.3) | 509 (17.2) | 1132 (23.1) | 1095 (22.3) |
| Spring | 1046 (35.3) | 1013 (34.2) | 1606 (32.8) | 1620 (33.1) |
| Summer | 957 (32.3) | 994 (33.6) | 1388 (28.3) | 1410 (28.8) |
| Autumn | 448 (15.1) | 446 (15.1) | 775 (15.8) | 776 (15.8) |
| Smoking during pregnancy | 340 (11.5) | 359 (12.1) | NA | NA |
| Previous pregnancy with the same fetal outcome | 62 (2.1) | 68 (2.3) | 756 (15.4) | 755 (15.4) |
| Prescription drug use in past year prior to pregnancy onset | ||||
| Antacids, H2-blockers, and PPIs | 265 (9.0) | 273 (9.2) | 464 (9.5) | 468 (9.6) |
| Insulin | 11 (0.4) | 15 (0.5) | 22 (0.5) | 24 (0.5) |
| Oral antidiabetic drugs | 59 (2.0) | 54 (1.8) | 87 (1.8) | 73 (1.5) |
| Antihypertensives | 76 (2.6) | 67 (2.3) | 136 (2.8) | 144 (2.9) |
| Topical corticosteroids, group IIb | 302 (10.2) | 298 (10.1) | 466 (9.5) | 478 (9.8) |
| Topical corticosteroids, group IIIb | 360 (12.2) | 393 (13.3) | 567 (11.6) | 563 (11.5) |
| Topical corticosteroids, group IVb | 82 (2.8) | 95 (3.2) | 131 (2.7) | 132 (2.7) |
| Oral corticosteroids | 359 (12.1) | 347 (11.7) | 597 (12.2) | 574 (11.7) |
| Thyroid drugs | 65 (2.2) | 57 (1.9) | 101 (2.1) | 109 (2.2) |
| NSAIDs | 646 (21.8) | 641 (21.6) | 1083 (22.1) | 1094 (22.3) |
| Opiates | 165 (5.6) | 175 (5.9) | 300 (6.1) | 314 (6.4) |
| Antimigraine drugs | 108 (3.7) | 98 (3.3) | 179 (3.7) | 182 (3.7) |
| Antidepressants | 274 (9.3) | 269 (9.1) | 512 (10.5) | 535 (10.9) |
| Nasal corticosteroids | 733 (24.8) | 724 (24.3) | 1150 (23.5) | 1161 (23.7) |
| Pulmonary inhalants | 606 (20.5) | 633 (21.4) | 950 (19.4) | 975 (19.9) |
| Leukotriene receptor antagonists | 63 (2.1) | 72 (2.4) | 111 (2.3) | 114 (2.3) |
| Other second-generation antihistamines | 309 (10.4) | 325 (11.0) | 495 (10.1) | 508 (10.4) |
| Ophthalmologic antiallergics | 640 (21.6) | 637 (21.5) | 1007 (20.6) | 1004 (20.5) |
| No. of drugs used | ||||
| 1-2 | 1447 (48.9) | 1418 (47.9) | 2308 (47.1) | 2318 (47.3) |
| 3-4 | 625 (21.1) | 632 (21.3) | 1038 (21.2) | 1039 (21.2) |
| ≥5 | 163 (5.5) | 171 (5.8) | 275 (5.6) | 291 (5.9) |
| Hospital care in past year before pregnancy onset | ||||
| No. of hospitalizations | ||||
| 1 | 324 (10.9) | 333 (11.2) | 558 (11.4) | 554 (11.3) |
| 2 | 63 (2.1) | 69 (2.3) | 119 (2.4) | 118 (2.4) |
| ≥3 | 22 (0.7) | 26 (0.9) | 43 (0.9) | 42 (0.9) |
| No. of outpatient contacts | ||||
| 1 | 486 (16.4) | 496 (16.8) | 813 (16.6) | 805 (16.4) |
| 2 | 194 (6.6) | 195 (6.6) | 339 (6.9) | 356 (7.3) |
| ≥3 | 103 (3.5) | 113 (3.8) | 173 (3.5) | 180 (3.7) |
Abbreviations: IQR, interquartile range; NA, not available; NSAID, nonsteroidal anti-inflammatory drug; PPIs, proton pump inhibitors.
Percentages may not total 100 because of rounding.
Groups II to IV denotes the strength of the respective topical corticosteroid preparations.
Outcomes
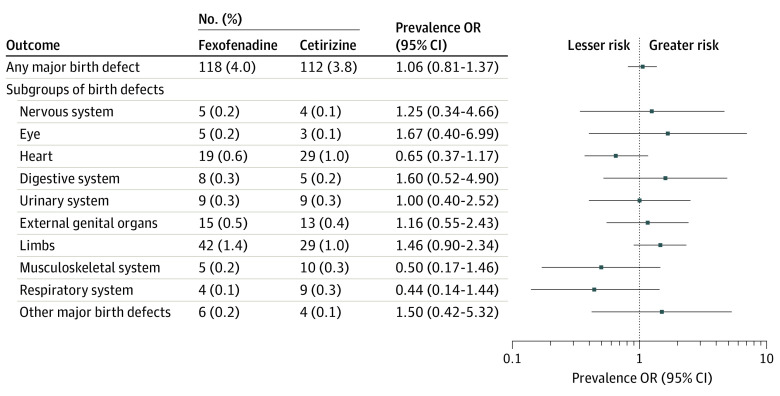
Figure 2 reports the prevalence ORs for the matched analyses of any major birth defect as well as the exploratory analysis of subgroups of major birth defects. Major birth defects in infants were diagnosed in 118 pregnancies (4.0%) with fexofenadine use compared with 112 pregnancies (3.8%) with cetirizine use (prevalence OR, 1.06; 95% CI, 0.81-1.37), corresponding to an absolute risk difference (ARD) of 0.02% (95% CI, −0.08% to 0.12%) per 1000 pregnancies. Analysis of specific subgroups of major birth defects identified no significantly increased risks associated with fexofenadine use compared with cetirizine use.
Figure 2. Association Between Fexofenadine Compared With Cetirizine Use During Pregnancy and Risk of Major Birth Defects.
Pregnancies with fexofenadine and cetirizine use in a pairwise comparison, matched on propensity scores. OR indicates odds ratio.
Table 2 reports results for the matched analyses of spontaneous abortions and secondary outcomes. The proportional hazards assumption was fulfilled for all analyses on fetal death. Spontaneous abortions occurred in 413 pregnancies (8.4%) with fexofenadine use and 439 pregnancies (9.0%) with cetirizine use (HR, 0.93; 95% CI, 0.82-1.07), corresponding to an ARD of −0.05% (95%, CI, −0.17% to 0.06%) per 1000 pregnancies.
Table 2. Association Between Fexofenadine Compared With Cetirizine Use During Pregnancy and the Primary and Secondary Outcomes.
| Outcome | Fexofenadine | Cetirizine | Measure of association (95% CI)a | ||
|---|---|---|---|---|---|
| Total No. of pregnancies | Events, No. (%) | Total No. of pregnancies | Events, No. (%) | ||
| Spontaneous abortion | 4901 | 413 (8.4) | 4901 | 439 (9.0) | 0.93 (0.82-1.07) |
| Secondary outcomes | |||||
| Preterm birth | 4933 | 370 (7.5) | 4933 | 382 (7.7) | 0.97 (0.83-1.12) |
| Small size for gestational age | 5110 | 515 (10.1) | 5110 | 523 (10.2) | 0.98 (0.87-1.12) |
| Stillbirth | 6153 | 16 (0.3) | 6153 | 24 (0.4) | 0.67 (0.36-1.27) |
Measures of association are hazard ratios for spontaneous abortion and stillbirth and prevalence odds ratios for preterm birth and small size for gestational age.
No significant associations between fexofenadine use during pregnancy and the secondary outcomes were noted compared with matched pregnancies with cetirizine use during pregnancy. These findings included risk of preterm birth (fexofenadine, 370 [7.5%] vs cetirizine, 382 [7.7%] pregnancies; prevalence OR, 0.97; 95% CI, 0.83-1.12), with an ARD of −0.02% (95% CI, −0.13% to 0.08%) per 1000 pregnancies; SGA (fexofenadine, 515 [10.1%] vs cetirizine, 523 [10.2%] pregnancies; prevalence OR, 0.98; 95% CI, 0.87-1.12), with an ARD of −0.02% (95% CI, −0.13% to 0.10%) per 1000 pregnancies; and stillbirth (fexofenadine, 16 [0.3%] vs cetirizine, 24 [0.4%] pregnancies; HR, 0.67; 95%, 0.36-1.27), with an ARD of −0.01% (95% CI, −0.03% to 0.00%) per 1000 pregnancies (Table 2).
Sensitivity Analyses
Table 3 reports results of the sensitivity analyses of the primary outcomes. For the analyses of major birth defects, the prevalence ORs were 1.12 (95% CI, 0.82-1.52) for fexofenadine use compared with loratadine use in the first trimester, 1.02 (95% CI, 0.77-1.36) for pregnancies with fexofenadine use in the first trimester compared with pregnancies unexposed to fexofenadine during pregnancy but with recent fexofenadine use before pregnancy onset, and 1.03 (95% CI, 0.84-1.26) for fexofenadine use compared with cetirizine use in pregnancy in which the exposure time period was extended throughout the entire pregnancy. For the analyses of spontaneous abortion, the HRs were 0.94 (95% CI, 0.81-1.10) for fexofenadine use compared with loratadine use in pregnancy and 0.81 (95% CI, 0.70-0.94) for pregnancies with fexofenadine use compared with pregnancies unexposed to fexofenadine during pregnancy but with recent fexofenadine use before pregnancy onset. For the analyses according to the number of filled prescriptions for fexofenadine (1 or ≥2 filled prescriptions), no differences in the estimates were identified for both major birth defects and spontaneous abortion.
Table 3. Sensitivity Analyses of the Association Between Fexofenadine Use During Pregnancy and Primary Outcomes.
| Sensitivity analysis | Major birth defects | Spontaneous abortion | ||||
|---|---|---|---|---|---|---|
| No. with outcome/total No. (%) | Prevalence OR (95% CI) | No. with outcome/total No. (%) | HR (95% CI) | |||
| Fexofenadine | Comparison group | Fexofenadine | Comparison group | |||
| Additional comparative groups | ||||||
| Compared with loratadine use during pregnancya | 89/2273 (3.9) | 80/2273 (3.5) | 1.12 (0.82-1.52) | 314/4050 (7.8) | 328/4050 (8.1) | 0.94 (0.81-1.10) |
| Compared with pregnancies unexposed to fexofenadine but with recent fexofenadine use before pregnancyb | 99/2575 (3.8) | 97/2575 (3.8) | 1.02 (0.77-1.36) | 343/4108 (8.4) | 408/4108 (9.9) | 0.81 (0.70-0.94) |
| Additional exposure definitions | ||||||
| Filled prescriptions any time during pregnancyc | 202/5122 (3.9) | 196/5122 (3.8) | 1.03 (0.84-1.26) | NA | NA | NA |
| No. of filled fexofenadine prescriptionsc | ||||||
| 1 | 96/2486 (3.9) | 112/2962 (3.8) | 1.02 (0.77-1.35) | 342/3968 (8.6) | 439/4901 (9.0) | 1.01 (0.88-1.16) |
| ≥2d | 22/476 (4.6) | 112/2962 (3.8) | 1.23 (0.77-1.97) | 71/933 (7.6) | 439/4901 (9.0) | 1.05 (0.82-1.35) |
Abbreviations: HR, hazard ratio; NA, not applicable; OR, odds ratio.
Gestational age at first filled prescription (median index date) was 15 days (interquartile range [IQR], −8 to 43) for fexofenadine use and 15 days (IQR, −9 to 44) for loratadine use during pregnancy for the analyses of major birth defects and was 29 days (IQR, −3 to 78) and 29 days (IQR, −3 to 81), respectively, for the analyses of spontaneous abortion.
For spontaneous abortion, the gestational age at first filled prescription (index date) for fexofenadine use was added as an additional matching criterion for this analysis, ie, pregnancies with no use during pregnancy were eligible for comparison as matches had they lasted until the respective index date; median, 16 days (IQR, −9 to 64).
Compared with cetirizine use during pregnancy.
For spontaneous abortion, the gestational age at the last filled prescription served as the index date for the pregnancies with use of fexofenadine for this analysis.
Results of the sensitivity analyses according to the restriction of the exposure time periods to pregnancy onset until the end of the first trimester or gestational week 22 for the analyses of major birth defects and spontaneous abortion, respectively, as well as results of the analyses of major birth defects among singleton pregnancies only and induced abortion are provided in eTable 8 in the Supplement. No substantial differences in any of these associations were identified between pregnancies with fexofenadine vs cetirizine use. Post hoc analyses in which the exposure time periods were completed before a potential outcome, did not change the results (eTable 9 in the Supplement).
Discussion
In this nationwide cohort study, we found no statistically significant differences in the risk of major birth defects, spontaneous abortion, preterm birth, SGA, and stillbirth between the use of fexofenadine and cetirizine during pregnancy. Moreover, sensitivity analyses including comparing use of fexofenadine with loratadine during pregnancy and with pregnancies unexposed to fexofenadine during pregnancy but with previous fexofenadine use provided similar results to those of the primary analyses.
Previous data are insufficient to properly assess the potential fetal risk of fexofenadine use during pregnancy. Although fexofenadine has been reported to be widely used during pregnancy,4 we are aware of only 1 retrospective case-control study that has investigated the fetal safety of fexofenadine use during pregnancy, but only in association with birth defects.2 The study included pregnancies with an outcome of birth defects with use of 13 different antihistamines during pregnancy; 54 cases of fexofenadine exposure were identified during the study period of 1997 to 2003. Although the authors emphasized that their analyses were limited by too few data to adequately estimate the risk of birth defects for fexofenadine use during pregnancy, the study estimates did not suggest a fetal risk.2 Our results may support this previous finding while enlarging the available body of epidemiologic safety data, reporting no significant differences in the risk of adverse fetal outcomes between use of fexofenadine and use of the recommended first-line, second-generation antihistamines (ie, cetirizine and loratadine) during pregnancy, as well as compared with use of fexofenadine outside of pregnancy. In addition, a cohort study included 16 pregnancies exposed to fexofenadine in a combined group of a total of 5041 pregnancies with use of antihistamines for treatment of allergic disorders, reporting no increased risk of birth defects.19 However, no separate analysis of the individual safety of fexofenadine was performed. In an exploratory analysis of subgroups of birth defects, we provide estimates for the subgroups in which cells of cases were greater than or equal to 3. Although we did not find an association between fexofenadine and any of the subgroups of birth defects compared with cetirizine, presented estimates may not be conclusive for defects that rarely occur. In addition, approximations of risk estimates for birth defects that were less prevalent and thus were not herein individually studied cannot be drawn from our results.
Fexofenadine is the active metabolite of terfenadine, and one previous cohort study investigated the fetal risk in 118 pregnancies exposed to terfenadine compared with 118 controls.20 No cases of birth defects were observed among the 65 pregnancies exposed to terfenadine in the first trimester as well as no cases of stillbirth in the control group; in addition, the findings did not suggest an association between terfenadine and preterm birth or SGA. Our study adds to these previous results by reporting what we believe to be novel data on the safety of fexofenadine use during pregnancy in association with the risk of major birth defects, spontaneous abortion, preterm birth, SGA, and stillbirth based on a large number of pregnancies with use of fexofenadine. Although the sample sizes allow precision of the results overall, cases of stillbirths were low and thus should be considered in interpretation of the findings.
Strengths and Limitations
Use of population-based registries allowed detailed characterization of included individuals, with minimal loss to follow-up and independent assessment of exposure, outcome, and covariates throughout the study period. The complete nationwide coverage of the registries permitted analyses of a large number of exposed pregnancies and included all pregnancies exposed to fexofenadine in Denmark, making the results likely generalizable to similar populations. However, use of this specific population also limits generalizability. Another limitation of the study is that ascertainment of drug use was based on filled prescriptions and not actual use. Nonadherence to dispensed drugs would bias the results toward no association with the outcomes. In sensitivity analyses according to number of filled prescriptions for fexofenadine, the results did not change for women who filled a prescription at least 2 times during pregnancy. The registrations of birth defects and spontaneous abortions in the National Patient Registry have a high validity, with positive predictive values of 88% and 97%, respectively.21,22 To reduce the possibility of factors influencing the association, we adjusted for a large set of potential confounders and baseline characteristics with use of propensity score matching, which generated similar compositions of the included variables across all of the cohorts. However, another limitation of the study is the lack of information for some variables. Data on the indication for a prescribed drug are not obtainable in Danish registries; in addition, data on smoking were not obtainable for analyses of spontaneous abortion and stillbirth, and we had no information on use of over-the-counter drugs (eg, pregnancy supplements, such as folic acid) for any of the analyses. If these factors were different between study groups or not accounted for through adjustment for correlated variables (ie, proxies) included in the propensity score matching, residual confounding may have occurred. Sensitivity analyses supported the robustness of our results of the primary analyses. In addition, to address the potential issues of residual confounding and confounding by indication, we used an active comparative design and performed sensitivity analyses with additional comparator groups. These analyses were consistent with the main findings.
Conclusions
In this nationwide cohort study of pregnant women, we found no apparent association between fexofenadine use during pregnancy and the risk of major birth defects, spontaneous abortion, preterm birth, SGA, or stillbirth. These results may suggest that fexofenadine use during pregnancy does not present an increased risk of adverse fetal outcomes compared with use of the current first-line–recommended second-generation antihistamines during pregnancy. As such, we suggest that fexofenadine can be used as equally as the other recommended antihistamines during pregnancy.
eMethods. Data Source and Excluded Birth Defects
eTable 1. Covariates Definition Included in Propensity Score and Data Sources
eTable 2. Number of Missing Values and Percentages (%) for the Unmatched Study Cohorts
eTable 3. Baseline Characteristics of Unmatched Pregnancy Cohorts of Fexofenadine and Cetirizine Users for Analyses of Primary Outcomes
eTable 4. Baseline Characteristics of Unmatched Pregnancy Cohorts of Fexofenadine and Cetirizine Users for Analyses of Secondary Outcomes
eTable 5. Baseline Characteristics of Propensity-Score Matched Pregnancy Cohorts of Fexofenadine and Cetirizine Users in 1:1 Ratio for Analyses of Secondary Outcomes
eTable 6. Standardized Differences for Comparison of Fexofenadine and Cetirizine Use During Pregnancy Before Propensity Score Matching
eTable 7. Standardized Differences for Comparison of Fexofenadine and Cetirizine Use During Pregnancy After Propensity Score Matching
eTable 8. Sensitivity Analyses of the Association Between Fexofenadine Use During Pregnancy and Primary Outcomes According to Restriction of Exposure Time Intervals, Singleton Pregnancies and Induced Abortions
eTable 9. Post hoc Analyses of the Association Between Fexofenadine Use During Pregnancy and Spontaneous Abortion, Preterm Birth, Small Size for Gestational Age, and Stillbirth According to Ending Exposure Window Prior to Start of Follow-up
eFigure. Overview of the Exposure and Outcome Time Periods for the Specific Outcome Analyses
eReferences.
References
- 1.Buhimschi CS, Weiner CP. Medications in pregnancy and lactation: part 2—drugs with minimal or unknown human teratogenic effect. Obstet Gynecol. 2009;113(2, pt 1):417-432. doi: 10.1097/AOG.0b013e31818d686c [DOI] [PubMed] [Google Scholar]
- 2.Gilboa SM, Strickland MJ, Olshan AF, Werler MM, Correa A; National Birth Defects Prevention Study . Use of antihistamine medications during early pregnancy and isolated major malformations. Birth Defects Res A Clin Mol Teratol. 2009;85(2):137-150. doi: 10.1002/bdra.20513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Werler MM, Mitchell AA, Hernandez-Diaz S, Honein MA. Use of over-the-counter medications during pregnancy. Am J Obstet Gynecol. 2005;193(3, pt 1):771-777. doi: 10.1016/j.ajog.2005.02.100 [DOI] [PubMed] [Google Scholar]
- 4.Mitchell AA, Gilboa SM, Werler MM, Kelley KE, Louik C, Hernández-Díaz S; National Birth Defects Prevention Study . Medication use during pregnancy, with particular focus on prescription drugs: 1976-2008. Am J Obstet Gynecol. 2011;205(1):51.e1-51.e8. doi: 10.1016/j.ajog.2011.02.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jáuregui Presa I, Navajas Rodríguez B, Ramos Bareño B, Gamboa Setién PM, Urrutia Etxebarria I, Antépara Ercoreca I. Chronic urticaria in special populations: children, pregnancy, lactation and elderly people. Curr Treat Options Allergy. 2016;3(4):423-438. doi: 10.1007/s40521-016-0097-x [DOI] [Google Scholar]
- 6.Powell RJ, Leech SC, Till S, Huber PAJ, Nasser SM, Clark AT; British Society for Allergy and Clinical Immunology . BSACI guideline for the management of chronic urticaria and angioedema. Clin Exp Allergy. 2015;45(3):547-565. doi: 10.1111/cea.12494 [DOI] [PubMed] [Google Scholar]
- 7.Zuberbier T, Aberer W, Asero R, et al. The EAACI/GA2LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria. Allergy. 2018;73(7):1393-1414. doi: 10.1111/all.13397 [DOI] [PubMed] [Google Scholar]
- 8.National Asthma Education and Prevention Program; Third Expert Panel on the Diagnosis and Management of Asthma Expert Panel report 3: guidelines for the diagnosis and management of asthma. National Heart, Lung, and Blood Institute. Published August 2007. Accessed April 13, 2020. https://www.ncbi.nlm.nih.gov/books/NBK7232/
- 9.Scadding GK, Durham SR, Mirakian R, et al. ; British Society for Allergy and Clinical Immunology . BSACI guidelines for the management of allergic and non-allergic rhinitis. Clin Exp Allergy. 2008;38(1):19-42. doi: 10.1111/j.1365-2222.2007.02888.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pali-Schöll I, Namazy J, Jensen-Jarolim E. Allergic diseases and asthma in pregnancy, a secondary publication. World Allergy Organ J. 2017;10(1):10. doi: 10.1186/s40413-017-0141-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Knudsen LB, Olsen J. The Danish Medical Birth Registry. Dan Med Bull. 1998;45(3):320-323. [PubMed] [Google Scholar]
- 12.Lynge E, Sandegaard JL, Rebolj M. The Danish National Patient Register. Scand J Public Health. 2011;39(7)(suppl):30-33. doi: 10.1177/1403494811401482 [DOI] [PubMed] [Google Scholar]
- 13.Kildemoes HW, Sørensen HT, Hallas J. The Danish National Prescription Registry. Scand J Public Health. 2011;39(7)(suppl):38-41. doi: 10.1177/1403494810394717 [DOI] [PubMed] [Google Scholar]
- 14.Pedersen CB. The Danish Civil Registration System. Scand J Public Health. 2011;39(7)(suppl):22-25. doi: 10.1177/1403494810387965 [DOI] [PubMed] [Google Scholar]
- 15.Danish Health Data Authority The Register of Medicinal Product Statistics. Updated June 12, 2019. Accessed February 8, 2020. http://www.medstat.dk
- 16.EUROCAT. EUROCAT guide 1.4. Instruction for the registration of congenital anomalies. EUROCAT Central Registry. Updated December 28, 2018. Accessed May 16, 2019. https://eu-rd-platform.jrc.ec.europa.eu/sites/default/files/Full_Guide_1_4_version_28_DEC2018.pdf
- 17.Austin PC. Some methods of propensity-score matching had superior performance to others: results of an empirical investigation and Monte Carlo simulations. Biom J. 2009;51(1):171-184. doi: 10.1002/bimj.200810488 [DOI] [PubMed] [Google Scholar]
- 18.Austin PC. A comparison of 12 algorithms for matching on the propensity score. Stat Med. 2014;33(6):1057-1069. doi: 10.1002/sim.6004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Källén B. Use of antihistamine drugs in early pregnancy and delivery outcome. J Matern Fetal Neonatal Med. 2002;11(3):146-152. doi: 10.1080/jmf.11.3.146.152 [DOI] [PubMed] [Google Scholar]
- 20.Loebstein R, Lalkin A, Addis A, et al. Pregnancy outcome after gestational exposure to terfenadine: a multicenter, prospective controlled study. J Allergy Clin Immunol. 1999;104(5):953-956. doi: 10.1016/S0091-6749(99)70074-6 [DOI] [PubMed] [Google Scholar]
- 21.Schmidt M, Schmidt SA, Sandegaard JL, Ehrenstein V, Pedersen L, Sørensen HT. The Danish National Patient Registry: a review of content, data quality, and research potential. Clin Epidemiol. 2015;7:449-490. doi: 10.2147/CLEP.S91125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Larsen H, Nielsen GL, Bendsen J, Flint C, Olsen J, Sørensen HT. Predictive value and completeness of the registration of congenital abnormalities in three Danish population-based registries. Scand J Public Health. 2003;31(1):12-16. doi: 10.1080/14034940210134194 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Data Source and Excluded Birth Defects
eTable 1. Covariates Definition Included in Propensity Score and Data Sources
eTable 2. Number of Missing Values and Percentages (%) for the Unmatched Study Cohorts
eTable 3. Baseline Characteristics of Unmatched Pregnancy Cohorts of Fexofenadine and Cetirizine Users for Analyses of Primary Outcomes
eTable 4. Baseline Characteristics of Unmatched Pregnancy Cohorts of Fexofenadine and Cetirizine Users for Analyses of Secondary Outcomes
eTable 5. Baseline Characteristics of Propensity-Score Matched Pregnancy Cohorts of Fexofenadine and Cetirizine Users in 1:1 Ratio for Analyses of Secondary Outcomes
eTable 6. Standardized Differences for Comparison of Fexofenadine and Cetirizine Use During Pregnancy Before Propensity Score Matching
eTable 7. Standardized Differences for Comparison of Fexofenadine and Cetirizine Use During Pregnancy After Propensity Score Matching
eTable 8. Sensitivity Analyses of the Association Between Fexofenadine Use During Pregnancy and Primary Outcomes According to Restriction of Exposure Time Intervals, Singleton Pregnancies and Induced Abortions
eTable 9. Post hoc Analyses of the Association Between Fexofenadine Use During Pregnancy and Spontaneous Abortion, Preterm Birth, Small Size for Gestational Age, and Stillbirth According to Ending Exposure Window Prior to Start of Follow-up
eFigure. Overview of the Exposure and Outcome Time Periods for the Specific Outcome Analyses
eReferences.