Abstract
Human body is colonized by a huge amount of microorganisms mostly located in the gastrointestinal tract. These dynamic communities, the environment and their metabolites constitute the microbiota. Growing data suggests a causal role of a dysbiotic microbiota in several pathologies, such as metabolic and neurological disorders, immunity dysregulations and cancer, especially the well-studied colorectal cancer development. However, many were preclinical studies and a complete knowledge of the pathogenetic mechanisms in humans is still absent. The gut microbiota can exert direct or indirect effects in different phases of colorectal cancer genesis. For example, Fusobacterium nucleatum promotes cancer through cellular proliferation and some strains of Escherichia coli and Bacteroides fragilis produce genotoxins. However, dysbiosis may also cause a pro-inflammatory state and the stimulation of a Th17 response with IL-17 and IL-22 secretion that have a pro-oncogenic activity, as demonstrated for Fusobacterium nucleatum. Microbiota has a crucial role in several stages of postoperative course; dysbiosis in fact seems related with surgical site infections and Enterococcus faecalis (and other collagenase-producers microbes) are suggested as a cause of anastomotic leak. Consequently, unbalanced presence of some species, together with altered immune response may also have a prognostic role. Microbiota has also a substantial role in effectiveness of chemotherapy, chemoresistance and in the related side effects. In other words, a complete knowledge of the fine pathological mechanisms of gut microbiota may provide a wide range of new diagnostic tools other than therapeutic targets in the light of tailored medicine.
Keywords: Intestinal microbiota, Colorectal cancer, Chemo-resistance, Therapeutic strategies
Core tip: Microbiome and immunity sciences are fields in rapid evolution gaining growing attention. The gut microbiota-immunity interplay seems to have a very important role in all the different phases of colorectal cancer process from oncogenesis to treatment and prognosis. However, many aspects have been studied only in experimental models and many theories must still be proved in humans. Providing the actual state of art of this interplay on the different steps involved in colorectal cancer, new multidisciplinary studies in humans according to this perspective may be drafted with the purpose of widening the possibilities of treatment against this frequently diagnosed pathology.
INTRODUCTION
A large and diversified group of microorganisms comprehending bacteria, viruses and fungi normally populates intestinal mucosa, such as every other epithelial surfaces. These microbes, together with their metabolic products and their local microenvironment compose the so-called microbiota[1,2]. Although most of microbiota strains are not cultivable, recent technologies of genomic sequencing, proteomics and metagenomic analysis of DNA and RNA allowed the initial identification of this population of microorganisms, along with their metabolic production and signal pathways[1-4].
Nevertheless, these commensal microbes are normally symbiotic and the immune system has established various tolerance mechanisms[2]; but, in specific conditions, the equilibrium break of microbiota-immunity axis can be responsible for several pathologies[2]. The hypothesis that a microorganism could be the cause of a surgical disease started with the discovery of the Helicobacter pylori role in the genesis of peptic ulcer. However, since the prevalence of this infection is much more higher than the incidence of peptic ulcer and since peptic ulcer may present without this infection, Helicobacter pylori was considered a “not necessary” nor “sufficient” agent to cause this pathology[1].
Similarly, the potential pathogenetic role of gut microbiota (GM) alteration in the initiation and progression of colorectal cancer (CRC) has been recently discussed[5]. For this purpose, the microorganisms may have a direct causal role or act perturbing the local immune response[2]. However, this complex relation is still far from being completely understood: The microbiota is dynamic, varying on hourly basis and the “current” microbiota of every person is the result of the individual past exposure to external agents, making the task to draft general conclusions even more challenging[3].
Several prognostic factors for CRC, for both short-term postoperative outcomes and long-term oncological outcomes, have undoubtedly been recognized[6], but, new potential prognostic factors have been proposed along the years and, in particular, the potential prognostic role of the microbiota is attracting much attention[3]. However, differences in microbiota may be at least a part of the cause of different outcomes achieved in a group of patients treated with the same protocols[3].
Although surgical resection is the cornerstone in the CRC management, whenever technically feasible, chemotherapy has a complementary role in advanced stages of disease. Relationship between chemo-resistance and intestinal microbiota has been advocated[5] but the fine mechanisms still remain unknown. Since chemo-resistance reduces the survival expectancy, the understanding of the causes of this phenomenon would be extremely important[5].
The aim of this review is a summary of the actual state of art on a developing research field: The interplay between microbiota and inflammatory/immune response applied on patients undergoing surgery for colorectal cancer, which is a pathology with high incidence and not negligible morbidity and mortality rates. Microbiota-based approach may provide a wide and quite revolutionary range of possibilities to interfere with the different phases of CRC management. Particular attention was set on postoperative outcomes in order to provide inspiration for further studies and for new potential strategies for the treatment, but also for the prevention of colorectal cancer.
GUT MICROBIOTA-IMMUNITY AXIS IN HEALTH
Advent of new technologies in metagenomic field and mass spectrometry pushed the investigators to analyze the possibility of the existence of both “health-promoting” and “disease-promoting” ecosystem of microorganisms[1]. Comprising almost 99% of the total amount of human-associated microbial mass, thousands of different species of commensal bacteria are required for a healthy gastrointestinal tract[2,7,8]. These microorganisms are members of different domains comprehending Bacteria, Archaea and Eukarya while the four most represented phyla of bacteria are Firmicutes, Bacteroidetes, Actinobacteria and Proteobacteria[2]. In particular, about 90% of gut bacteria belongs to Bacteroidetes and Firmicutes[8,9]. The gut microbiota help in several host tasks such as in digestion of complex foods (e.g., pectins), vitamins production and metabolism of glycans and fats[9]. Nevertheless, they have a role in protection against external pathogens or toxic compounds[2,7,8].
A strong relationship between intestinal microbiota and immunity system has been described[1,2,5,10]. The innate immune system associated with the mucosal surfaces accounts for approximately 80% of the active immune system and the great majority of them are located in the gastrointestinal tract[2].
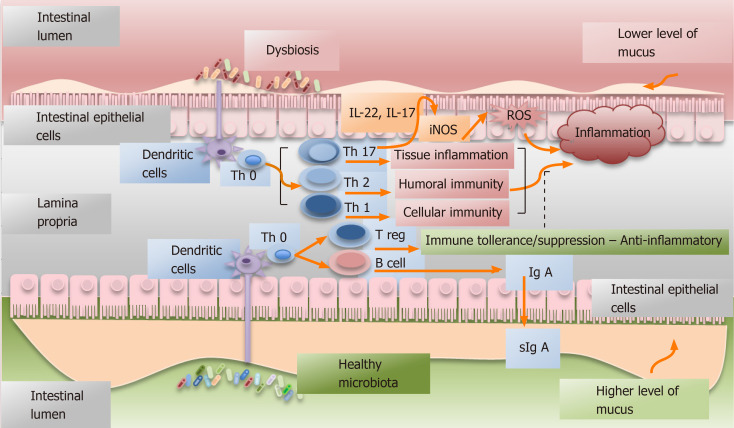
In addition, the microbiota largely contributes to the development of the lymphoid tissue[11] and it can modulate host immune system, both innate and adaptive[12]. Intestinal microbiota interacts with the immune response elements of the whole body through dendritic cells or through the stimulation of epithelial receptors, even in absence of bacterial translocation[1]. Consequently, intestinal microbes may also have either a negative or a positive effect on the immunity[11]. The Figure 1 represents a simplified summary of these interactions.
Figure 1.
Simplified graphic depicting the interactions between the healthy or dysbiotic microbiota and immune system. Dysbiosis is defined as the abnormal and predominant presence of pathogens in an environment or as alterations of the considered normal proportion of the different specimens composing the microbiota[1]. The microbiota largely contributes to the development of the lymphoid tissue[11] and it can modulate host immune system (innate and adaptive)[12]. Intestinal microbiota interacts with the immune response elements through dendritic cells. In dysbiosis, a Th17-type of immune response may be activated with consequent production of IL-17 and IL-22, both having a pro-inflammatory and pro-tumoral effect[2]. Furthermore, IL-22 can favor the expression of inducible nitric oxide synthase and the subsequently production of oxygen reactive species that are linked to cancer promotion[45].
INTESTINAL MICROBIOTA AND IMMUNITY DYSREGULATION IN COLORECTAL CANCER
The colorectal cancer is the third most frequent cancer worldwide[13] and there is a probability 4%-5% of a having a CRC in the life span[9]. Various risk factors for CRC have already been described such as life and dietary style or some comorbidities (e.g., ulcerous colitis or other conditions) leading to a persistent and prolonged colon inflammation[14,15]. Some bacteria with pro-inflammatory activities may modify the permeability of the intestinal mucosa easing the translocation of pathogens and their toxins[2,16]. Furthermore, protracted inflammation causes prolonged oxidative stress that may be responsible for DNA damages[17], as demonstrated for Escherichia coli in animal models[18].
Several papers have already highlighted a potential role of intestinal dysbiosis in the initiation and progression of human CRC[14] taking advantage of previously published studies on animal models[19,20]. Dysbiosis is defined as (1) The abnormal and predominant presence of pathogens in an environment or (2) Alterations of the considered normal proportion of the different specimens composing the microbiota[1]. This new “ecosystem” is also called pathobiome[21]. Moreover, the modifications within the microbiota related with a particular disease may take place at every taxonomic level, from the phylum to species making the discovery of these modifications and of their causal effect, an extremely challenging task[4].
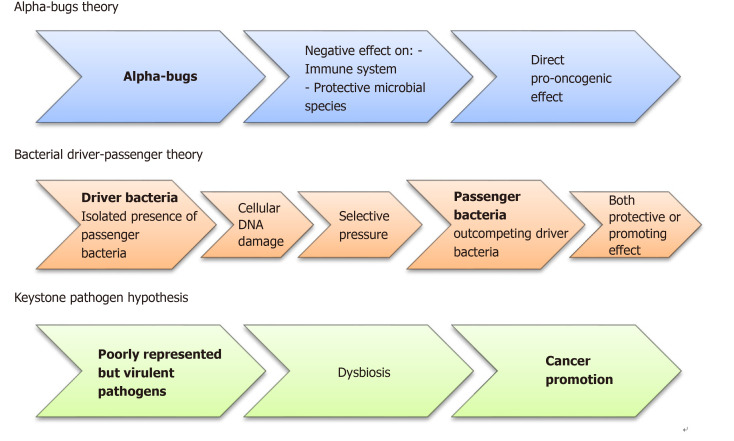
Three different pathogenetic models have been proposed. According to the “alfa-bugs” model, some species (e.g., enterotoxigenic Bacteroides fragilis-ETBF) may have direct pro-oncogenic effect acting against both immune system and protective microbial species[22]. The “bacterial driver-passenger” model suggests that some “driver bacteria” promote cancer development through DNA damage. Subsequently, as consequence of new selective pressures, “passenger bacteria” replace them having protective or cancer-promoting activities. The results of this new balance will determine tumor progression or tissue healing[23]. To note that, according to this model, microbes responsible for tumor initiation may be absent during the subsequent phases[24]. In the “keystone pathogen” model, some poorly represented pathogens have the unbalanced ability to alter the equilibrium within the normal microbiota causing a dysbiosis[25]. These theories are depicted in Figure 2.
Figure 2.
Diagram showing the three pathogenetic models involved in colorectal cancer initiation and promotion. Currently, to explain the colorectal cancer development, three different pathogenetic models have been suggested. According to the “alfa-bugs” model, some species (e.g., Bacteroides fragilis) may have direct pro-oncogenic effect acting against both immune system and protective microbial species[22]. The “bacterial driver-passenger” model suggests that some “driver bacteria” promote cancer development through DNA damage. Subsequently, the “passenger bacteria” replace them having protective or cancer-promoting activities. The results of this new balance will determine tumor progression or tissue healing[23]. Finally, in the “keystone pathogen” model, some poorly represented pathogens have the unbalanced ability to alter the equilibrium within the normal microbiota causing a dysbiosis[25].
Alterations in microbiota composition have been found in samples from normal colorectal mucosa, feces and tumor specimen in patients affected by CRC[5,26-28]. Interestingly, in a case-control study of Flemer, significative differences in microbiota composition were found between healthy volunteers and people affected by intestinal polyps[28], suggesting GM alteration in a very early stage of disease. In CRC patients, higher presence of some microorganisms (e.g., Fusobacterium, Enterococcus faecalis, Staphylococcaceae or Coriobacteridae) has been reported in previously published papers together with a lower presence of other microbes including Enterobacteria, Bifidobacterium, Lactobacillus and Treponema[29,30]. Coherently with the well-known different behavior of the right or left colon cancer, different GM modifications have been found in proximal and distal CRC[28]. Microbiota composition found in right colonic cancer was more similar to that found in control group with lower activation of Th17 response[28]. However, further clinical implications are still missing[28].
In particular, an association between Fusobacterium nucleatum (F. nucleatum) and CRC has been proposed and a suggested pathogenic mechanism involves the activation of β-catenin signal pathway causing cellular proliferation (as consequence of the bindings between FadA and E-cadherin, located on the cells of the intestinal epithelium)[31]. The F. nucleatum resulted much more represented in CRC patients when compared with healthy people[5,17]. Furthermore, its presence seems related with high-level of instability of microsatellites (MSI)[32,33] and with a CpG island methylator phenotype[34]. Nevertheless, the number of F. nucleatum and of Bacteroides fragilis (B. fragilis) (both in stool sample and tumor tissue) seems to increase along with the progression from adenoma to adenocarcinoma[35-37].
Similarly, a relation between the population number of B. fragilis and Escherichia coli (E. Coli) (adherent-invasive ones) and tumor size has been reported[38]. Possible pathogenetic mechanisms include bacterial production of toxins, known as genotoxins, able to generate damage to DNA[39]. Examples of these toxins are B. fragilis toxin, the cytolethal distending toxin or colibactin toxin produced by polyketide synthase (pks) positive E. coli or the cytotoxic necrotizing factor 1[24,39]. Higher genes’ expression of B. fragilis toxin and colibactin toxin has been found in patients affected by familial adenomatous polyposis (FAP) when compared with healthy people[40].
Furthermore, some microbial metabolites derived from alimentary intake may result genotoxic and cytotoxic[41]. Clostrudium, Bacteroides and E. coli have been reported to have this capacity[23]. In addition to direct promoting effects, intestinal microbiota may interfere in cancer proliferation through the interplay with the immune response. F. nucleatum resulted associated with lower level of CD3+ T cells[32,33], increased production of TNF-α, IL-6, IL-12 and IL-17 (all having a pro-tumoral effect), upregulation of myeloid-derived cells, and indirect suppression of CD4+ T cells activity[9,17,31]. Nonetheless, Fap2 protein produced by this microorganism is able to prevent the antitumor effect of NK cells and other T cells binding with inhibitory receptors[32,42].
In animal models, B. fragilis toxin can activate the signal transducer and transcription-3 (STAT3) pathway that is related with a specific Th17 differentiation. On the contrary, inhibition of IL-17 and IL-23 with antibodies has an antitumor action[39]. Furthermore, in presence of ETBF, regulatory T cells, which are usually related with an antitumor effect, seem to promote cancer progression through Th17 expansion[43]. Nonetheless, B. fragilis can promote inflammatory response inducing the signaling NF-κB pathway[44].
Some species of Clostridium (segmented filamentous bacteria) are other microbes able to activate Th17 producing IL-17 and IL-22 with a pro-inflammatory and pro-tumor effect[2]. Furthermore, IL-22 can favor the expression of inducible nitric oxide synthase (iNOS) and subsequently, the production of oxygen reactive species that are linked to cancer promotion[45]. High levels of IL-23 have also been found in human CRC and they seem able to activate Th17 response with further production of IL-17 e IL-22[46]. Other promoting cancer cytokines are IL-6 and 1[17]. Further specific details will not be object of this review and can be found elsewhere[17].
Despite the crucial role of inflammation in CRC development, use of non-steroidal anti-inflammatory drugs is not routinely indicated due to their potentially severe side effects[9]. The Figure 3 shows some examples of suggested mechanisms. On the contrary, some microorganisms seem to have a direct protective effect against tumor growth, for example, those producing short-chain fatty acid (e.g., butyrate or acetate)[5]. Accordingly with previously published data, Bifidobacterium seems able to inhibit tumor progression reducing the infection rate from enteropathic microorganism and decreasing the production of bile products[47,48]. Moreover, some microbes may exhibit an anticancer activity through the interaction with immune system. This positive effect is related with the phagocyte stimulation, the enhancement of NK cytotoxicity and an incremented production of immunoglobulins, including IgA (that contributes to the mucosal barrier activity)[10,11]. Evidences from experimental studies suggest that Bifidobacterium may also favors and antitumor immune response, inhibiting NF-κB signaling pathway[17,49]. Similarly, Faecalibacterium prausnitzii may have a positive effect through the induction of IL-10 secretion and the modulation of Treg response[24]. IL-10 is able to control the proliferation of Th17 cells stopping cancer progression[2,50]. Furthermore, IL-10 downregulates TNF-α production and iNOS expression[17].
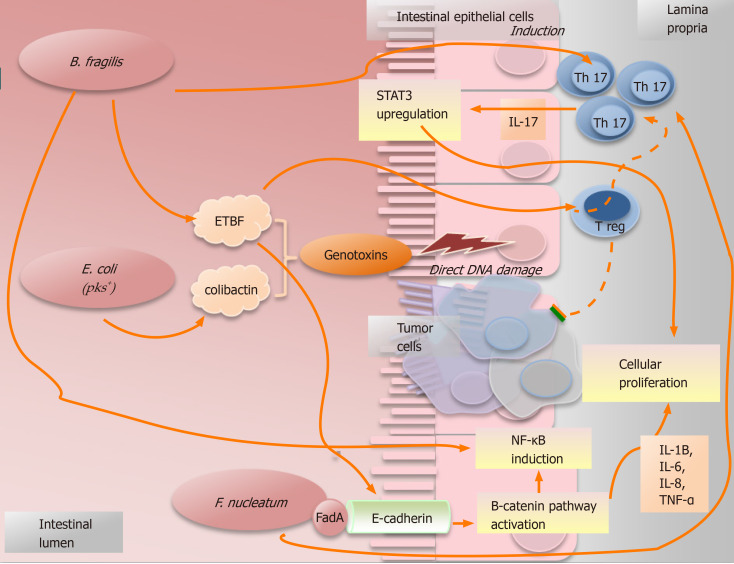
Figure 3.
Simplified picture illustrating some examples of how microorganisms promote the cancer. Bacteroides fragilis can cause the induction of Th17-type immune response with upregulation of signal transducer and transcription-3. Moreover, some subtypes of Bacteroides fragilis can secrete the toxin EBTF that can cause cancer in different ways: (1) Through direct DNA damage; (2) By Treg cells, which in presence of EBTF, seem to promote cancer progression through Th17 expansion[43]; and (3) Through the stimulation of the cleavage of E-cadherin which causes cellular proliferation and intestinal barrier breakage[23]. Nonetheless, B. fragilis can promote an inflammatory response inducing the signaling NF-κB pathway[44]. The particular group of polyketide synthase (pks) positive Escherichia coli (E. coli pks+) maintained a genomic island called “pks”. These bacteria can produce the genotoxin “colibactin”, that is able to induce direct DNA damage and, consequently, to increase the frequency of gene mutations[24,39]. Fusobacterium nucleatum can activate the β-catenin signal pathway causing cellular proliferation as consequence of the bindings between the bacterial adhesin FadA and E-cadherin which is located on the cells of the intestinal epithelium[31]. Furthermore, it is related to an increased production of some pro-cancer cytokines, such as TNF-α, IL-6, IL-12 and IL-17. STAT3: Signal transducer and transcription-3; ETBF: Enterotoxigenic Bacteroides fragilis; E. coli: Escherichia coli; F. nucleatum: Fusobacterium nucleatum.
SURGICAL TREATMENT OF CRC: GUT MICROBIOTA-IMMUNITY AXIS IN SHORT AND LONG-TERM OUTCOMES
Surgical stress determined by treatments before and after surgery (including bowel preparation, antibiotic exposure, proton-pump inhibitors’ administration and fasting) and the operation for CRC itself seem to reduce the GM biodiversity. Consequently, the balance within the intestinal microbiota and its environment results altered[51].
Deng et al[5] reported a reduction of Bacteroidetes and Firmicutes and an increase of Proteobacteria in patients surgically treated for CRC when compared to healthy volunteers. However, although this study is focused in evaluating the microbiota in fecal samples from 4 different groups (healthy controls, CRC patients, CRC patients operated and CRC patients treated with chemotherapy), the sample size is very small (5 patients operated within a total of 69 people involved). However, similar studies on patients after surgery for CRC confirmed a reduction of obligate anaerobes including several species of Clostridium, Bacteroides and Prevotella together with a reduction of Bifidobacterium. On the contrary, Enterococcus, Staphylococcus and Pseudomonas resulted enriched after surgical treatment[52].
Sze et al[53] evaluated GM changes before and after surgery in patients treated for adenomas, advanced adenomas or carcinomas. Carcinoma group had the major significant variation in microbial composition before and after surgical treatment and, interestingly, microbiota after surgery resulted quite similar to healthy people. Consequently, microbial alteration found during the follow-up may be considered as a potential biomarker for tumor recurrence and may be used to stratify the recurrence risk[53]. However, the real impact of these findings remains unknown and definitive conclusions cannot be drawn. The Table 1 summarizes the GM changes in healthy people and in patients with CRC or surgically treated.
Table 1.
Composition of gut microbiota in healthy people, in patients with colorectal cancer and after colorectal cancer surgery
| Healthy | Colorectal cancer | Post-operation |
| Bacteroidetes[5] | ↑ Staphylococcaceae[28-30] | ↑ Proteobacteria[5], ↑ Pseudomonas aeruginosa |
| Firmicutes[5], ↑ Lachnospiraceae ↑ Clostridium | ↑ Coriobacteridae[28-30], ↑ Fusobacterium nucleatum[5,32,33] | ↓ Bacteroidetes[52], ↓ Bacteroides ovatus, ↓ Prevotella |
| ↑ Bifidobacterium[52], ↓ Fusobacterium nucleatum[5,17] | ↑ Enterococcus faecalis[28-30], ↑ Bacteroides fragilis[35-38] ↑ Escherichia coli pks+[38] | ↓ Firmicutes[52], ↓ Clostridium but, ↑ Enterococcus, ↑ Staphylococcus |
| ↓ Bacteroides ovatus[37] | ↓ Bifidobacterium[52] | |
| ↓ Bifidobacterium[28-30] | ||
| ↓ Lactobacillus[28-30] | ||
| ↓ Treponema[28-30] |
↑: Higher abundance; ↓: Lower abundance; pks+: Polyketide synthase positive.
Short-term outcomes
The well-known better outcomes following bloodless interventions or postoperative course not requiring an antibiotic therapy may also be related with a better preservation of the pre-existing microbiota equilibrium[1]. Nevertheless, the good results obtained following the application of the enhanced recovery after surgery programs may also be related to a virtuous interaction with the microbiota, as well[1]. Obviously, the ability of the single individual’s microbiota to “refaunate” is of pivotal importance and many “molecular-level” aspects remain unevaluated. Consequently, further investigations according with these new perspectives are needed. Nonetheless, the analysis of the different outcomes within a group treated according to a specific protocol may become more interesting than the comparison between application or not of the protocol itselfs[1,3].
Postoperative infections
In general surgery and especially in colorectal surgery, the infection rate is still high (about 15%), being a common readmission cause with an increase in the costs, as well[3]. Obviously, intraoperative direct contamination is an unquestionable risk factor for infections. However, after elective surgery, during which the level of contamination is low, surgical site infections (SSIs) do exist and Staphylococcus aureus strains (which belong to cutaneous resident flora) represent the most frequently found species[3]. Furthermore, cultures from surgical site at the surgery-end are often poor efficient in predicting a future SSIs and, eventually, the related causal pathogens. This may have several explanations: (1) The actual instruments are not able to detect the whole composition or (2) Other still unknown mechanisms may also have a causal role[3]. According to the proposed “Trojan Horse hypothesis”, some low-abundant pathogens including methicillin-resistant Staphylococcus aureus or E. faecalis are carried on by macrophages or neutrophils from the gut to distant sites such as surgical wound[3,54].
Nonetheless, in patients considered at high risk for SSIs, these pathogens may have a particular high virulence that resulted no more balanced by the other microorganisms after surgical insult[3,54]. Prolonged postoperative fasting, not adequately compensated with enteral nutrition, may alter the GM composition causing a higher surgical site infection rate[55]. Opioid drugs seem to both directly inhibit immune system and cause microbiota changes, favoring a greater virulence of the microbes[1,56,57]. On the contrary, the use of competitive opioids antagonists seems related to a reduction of morbidity rates after colorectal surgery[58]. Consequently, reducing preoperative fasting and decreasing the use of opioids should be encouraged.
Finally, the intestinal microbiota may also have a role in wound healing[57,59]. A possible mechanism is related to the fermentation of lactic acid from specific species of the intestinal microbiota that enables the production of the neuropeptide oxytocin through a stimulation of the vagus nerve. This peptide allows the recruitment of T cells for an enhanced healing of surgical incision[59].
Anastomotic leak
Anastomotic leak (AL) is the most feared surgery-related complication. It is defined as a spillage of intestinal material outside the sutured bowel at the anastomotic site, caused by a defect of intestinal wall. Depending on its severity, it may be related to further complications (including death) and its management may range from observation to surgical treatments[60]. Anastomotic leak rates are reported to range between 1% and 19%[60]. The well-known risk factors for intestinal AL are: Tension between the intestinal edges, reduced blood supply to the viscera and technical errors.
However, surgery causes inflammation and inflammatory response may induce gut dysbiosis. Consequently, a causal role of intestinal pathogens has been reported. In 1954, Cohn conducted a study on dogs demonstrating that colon decontamination totally avoided anastomotic leak and resulted able to reverse colonic ischemia[61]. More recently, the causal role of some microorganisms including Enterococcus faecalis and Pseudomonas aeruginosa, has been suggested[1,62]. In animal models, these species can cause the leak of the anastomosis producing high level of collagenase (matrix metalloproteinase-MMP), mostly MMP-2 and MMP-9[62]. Abundance of these species has been found in humans who had surgery complicated by anastomotic leak[1,62] and copious presence of E. faecalis seems to persist into the colon despite bowel preparation[52]. Moreover, in animal models, morphine administration has been shown to increase the presence of more adhesive E. faecalis within anastomotic tissue. Consequently, there is greater collagenase production favoring higher anastomotic leak rates[63].
Anyhow, collagenase-producing bacteria seem to be necessary but insufficient in causing an anastomotic leak[3]. The other conditions needed to cause an anastomotic leak are: (1) The microbiota unbalancing (with protective microbes reduced enough to unleash pathogens); (2) Inflammatory response from anastomotic tissue is also required to make the pathogens sense and respond to a such modified environment, and (3) Pathogens must be virulent enough to overcome host defenses[3]. Low rectal resections and neoadjuvant radiotherapy are independently associated with a higher anastomotic leak rate. In patients undergoing neoadjuvant radiotherapy, interplay between radiation and GM alteration has also been advocated as a cause for higher AL rate. Radiotherapy seems to promote higher levels of virulent anaerobes in the treated site[21]. On the contrary, there are commensal GM bacteria, which have a pivotal role in defending epithelial cells of the intestine from apoptosis induced by radiations[64].
Even in this condition, microbiota seems to have both a protective and harmful role. Further studies in humans should be performed in order to assess which are health-promoting or noxious species. Again, the global result of complex modifications within this environment is more important than the single pathogen itself confirming the Koch’s postulates[1,65]. The simple analysis of the mere presence of a pathogen in a stool sample may seem quite reductive to achieve a complete understanding of a multifactorial event[3].
Although some recent and apparently countercurrent studies suggesting that oral antibiosis and complete mechanic bowel preparation allow the reduction of postoperative morbidity[66], a definitive response whether and, above all, how bowel preparation and/or antibiotic therapy resulted in a modified rate of the anastomosis leakage is still lacking and level 1 evidence are still missing[1,67]. Finally, independently from dysbiosis, a causal inflammation role in the AL at the anastomotic site has been advocated[68]. Involved innate immune response, mostly composed of neutrophils, may exacerbate hypoxia in damaged tissues, consuming oxygen during oxidative burst[69]. In emphasis, the use of non-steroidal anti-inflammatory drugs may seem intriguing; however, they are not routinely indicated, essentially for their relation with higher bleeding rate.
Other short-term outcomes
Ileus is any reduction of the normal propulsive activity of the bowel. After surgery, a transient impairment of the peristalsis always happens for few days. For postoperative ileus, undeniable causal factors are the manipulation of the bowel together with an excessive use of opioid drugs[3]. Nonetheless, experimental studies with animal models revealed a potential role of the interplay microbiota-immune system in postoperative ileus[70]. A potential pathogenetic mechanism involves a peristaltic dysfunction, caused by intestinal nervous system response to intestinal macrophages activated by the microbiota[71]. A recent study reported a reduction of postoperative ileus rate after administration of oral non-absorbable antibiotics[66]. Similarly, preoperative oral intake of probiotics (Lactobacillus and Bifidobacterium) seems to enhance the return to the normal bowel function[51]. However, a deeper understanding of this relation is required to draft further studies[1].
In prolonged postoperative ileus, high levels of IL-6 and leucocyte bowel infiltration have been found, suggesting also a direct role of inflammation. These data are coherent with the reduced duration of postoperative ileus after minimally invasive surgery[68]. Finally, in animal models, it was also reported a possible causal GM role in the formation of postoperative adhesions[70].
Long-term outcomes: Intestinal microbiota and immunity as prognostic factors
Recurrence rate after curative CRC surgery is reported to be up to 40% and the great majority of recurrence appears within 3 years from operation[6,72]. Local recurrence, mostly perianastomotic in the extraluminal space, shows a reported rate ranging between 1% and 23%[21,73]. A higher incidence of local recurrence rate has been observed after anastomotic leak. We can hypothesize different explanations: (1) A delayed start of adjuvant therapy; (2) Implant of exfoliated tumor cells that are invariably persistent in the colon after resection[21]; and (3) A process of metachronous initiation of a new tumor and new tumor promotion by a persistent inflammatory status[74]. The last two possible mechanisms, together with the consequent reduction of oral intakes, longer hospital stay (with major probabilities of nosocomial infections) and aggravated surgical stress, have a strong relation with the GM alterations[21]. As previously stated, anastomotic leak is related with the presence of microbes producing MMPs[1,62]. Nevertheless, preoperative high serum level of MMP-2 and MMP-9 has been reported to be an independent marker of a worse oncological outcome[75]. Increased proteolysis is also typical of more invasive tumors and it is not only related with anastomotic leak[75]. Moreover, MMP-9 presence in tumor sample was found in the 85% of patients with high serum level of MMP-9[75].
F. nucleatum and enterotoxigenic B. fragilis seem related with more advanced CRC stages, lower rate of disease-free survival and, consequently, they appear to be responsible for a worse prognosis[33,76,77].
Kosumi and colleagues evaluated the potential prognostic influence of Bifidobacterium in a wide cohort of 1313 patients affected by CRC[78]. The Bifidobacterium presence in tumoral tissue was found in 30% of this cohort. Although previously published data suggested a protective role of this microorganism in CRC[47-49], no significant association was found between Bifidobacterium number and survival rate. Multiple hypotheses may explain this finding: (1) Bifidobacteria produce lactic acid that, in high level, may boost tumor growth reducing immune response activation; (2) Bifidobacteria can also produce acetate that is utilized by tumor tissue; and (3) Bifidobacteria may have different roles in healthy and tumoral tissue in which these microbes may act together with other species (e.g., Fusobacterium). Nonetheless, the number of these microorganisms in CRC tissue resulted associated with the extent of signet ring cells[78].
Expression levels of different cytokines seems also related to tumor prognosis[17]. High IL-17 levels predict an adverse prognosis with a rapid development of metastasis[79]. Similarly, high level of let-7a microRNA family are related with lower presence of CD3+ and CD45RO+ which significantly correlated with higher cancer-related mortality[80]. On the contrary, high density of CD45RO+ cells within tumor tissue has been proposed as an independent positive prognostic biomarker and it is related with longer survival also independently from MSI status[81]. According to these findings, traditional prognostic systems including the TNM classification system of the American Joint Committee on Cancer and Union International Cancer Control appear to be no longer sufficient to estimate patients’ oncological outcomes[82]. From the joint effort of 14 centres with a proven expertise located in 13 countries of North America, Europe and Asia, a consensus Immunoscore was created. It resulted from the assessment by digital pathology of the density of specific T-cells (CD3+ and CD8+) in the tumor sample and in the infiltrating margins. In order to assess the prognostic role of the inflammatory infiltrate in tumoral tissue from primary resected CRC, the resulting score was tested and validated in a study including 2681 patients[82]. Inter-observer reproducibility of this score resulted high. Immunoscore revealed to be so powerful in the prognostic stratification of the patients (in terms of time to recurrence, disease-free and overall survival rates) to result more reliable than the TNM classification system suggesting the necessity to create a unique and integrated classification system[82]. Moreover, it seems also a survival predictor stronger than the MSI status[83]. Overall, the highest was the Immunoscore, the lowest resulted the recurrence rate at 3 years from surgery ranging from 5% in patients having high Immunoscore to 26% in patients having low Immunoscore. Five-years overall survival rates were 82%, 77% and 62% in high, intermediate and low Immunoscore, respectively. Similar results were confirmed even after adjustment for the principals known potential prognostic factors (e.g., demographic characteristics, TNM stage, MicroSatellite Instability status). Ultimately, this score may represent a valuable tool for tailored adjuvant chemotherapy[82].
GUT MICROBIOTA-IMMUNITY INTERPLAY IN PATIENTS UNDERGOING CHEMOTHERAPY AFTER CRC SURGERY
CRC is mostly treated with cytotoxic drugs including 5-fluorouracil, capecitabine and/or platinum-based agents[21]. However, different chemotherapy regimens exist and are actually administered according to tumor stage, patient’s general conditions and mutational status. Details that are more specific will not be object of this review.
Microbiota may interfere with chemotherapy through different ways: Modification of the GM composition, metabolisms of xenobiotics and modulation of the immune response[84,85]. Consequently, the so-called “pharmacomicrobiomics” is increasingly attracting attention[85]. Unequivocally, these treatments alter the composition of intestinal microbiota. In the previously cited paper of Deng et al[5], the demonstrated reduction of Bacteroides (mostly B. ovatus) in surgically treated patients is higher when compared with the reduction of this microorganism in the patients treated only with chemotherapy. Therefore, surgery seems more effective than chemotherapy against this potential pathogen[5]. Nevertheless, other species such as Veillonella dispar or higher abundance of Prevotella copri and Bacteroides plebeius were found in only chemotherapy-treated patients[5]. However, a relation between these quantitative differences and the potential clinical impact has to be further analyzed.
Intestinal microbiota is responsible for xenobiotic metabolism. According to experimental results, a different clinical response to the treatments with fluoropyrimidines seems related also with GM modifications other than with genetic polymorphisms[86]. Previous papers have already emphasized the possible relation between intestinal microbiota and chemoresistance in CRC patients[5]. F. nucleatum has a causal role in chemoresistance via the activation of the autophagy pathway. In particular, autophagosome formation is activated in the CRC cells with the production of their related proteins[87]. Consequently, the detection of copious presence of this species may represent a prognostic biomarker for chemoresistance, suggesting a probable modification in the administered chemotherapy[87]. High levels of circulating IL-22 have also been described as associated with chemo-resistance[88]. Nevertheless, high levels of regulatory T cells create an immunosuppressive environment reducing the efficacy of the host antitumor immune system and of the antiblastic therapy[89].
Chemotherapy treatments often cause chemotoxicity and the symptoms are mostly diarrhea, nausea, mucositis and hand-foot syndrome. Dysbiosis caused by chemotherapy administration has a causal role in colitis and diarrhea[85]. Anaerobic species are usually reduced together with an inferior production of butyrate. Butyrate is a short-chain fatty acid (SCFA) responsible for the trophysm of the intestinal mucosa. Nevertheless, this SCFA has an antitumoral action blocking cellular replication, promoting apoptosis[85], stimulating the IL-10 production and inhibiting the NF-κB activation[11]. Butyrate is also implicated in mucosal barrier efficacy through higher production of mucus[11].
Finally, the microbiota appears also to have a direct causal role in chemotoxicity. For example, irinotecan-induced mucositis is the consequence of the reactivation of its liver metabolite from intestinal bacterial β-glucuronidases[85,90]. There are several isoforms of β-glucuronidases, related to different toxicity levels[90]. On the contrary, the preservation of commensal microbiota, able to modulate tumoral micro-environment, has been reported to be pivotal in an optimal response to chemotherapy with oxaliplatin[91]. Nevertheless, a favorable gut microbiota may also have a role in the reduction of these side effects[9].
The identification of specific microbes as predictor of chemotherapy response together with a complete understanding of the mechanisms causing chemotoxicity may allow a tailored therapy with the reduction of side effects[92].
FUTURES PERSPECTIVES: INNOVATIVE STRATEGIES FOR CRC PREVENTION AND TREATMENT
Gut microbiota have a causal role in all the CRC steps, from its initiation to response to chemotherapy. Therefore, new approaches microbiota-based may add further possibilities against this tumor. Nevertheless, high expertise in “bacterial management” is required.
Role of diet in cancer prevention
Since an inappropriate diet represents a major risk factor for CRC initiation, a diet rich in fibers and vegetables, especially from cruciferous family, may aid in maintaining a healthy microbiota. Nevertheless, specific supplementary nutrients may help from the cancer prevention to the avoidance of anastomotic leak after surgery[1,21]. Probiotic and prebiotic consumption may positively modulate metabolic activity of the gut microbiota with a lower production of carcinogenic compounds[41]. Moreover, according to the results of in vitro studies, some microbes are able to bind genotoxic compounds to their cellular wall[93].
SCFAs are the results of bacterial fermentation of complex carbohydrates. Conjugated linoleic acid has a suppressive action over cellular proliferation and favors cellular apoptosis in a dose-dependent proportion[11]. It is produced by several species including Lactobacillus casei and L. acidophilus[94]. Dietary supplementation with nutrients able to increase the production of butyrate or other SCFAs may help in maintaining a healthy and balanced microbiota[1]. Lactobacillus, Bifidobacterium and Faecalibacterium prausnitzii may be used as probiotic with the same purposes together with the maintenance of an active immunosurveillance[11,95]. It has also been reported that some probiotics may change the equilibrium between Th1 and Th2 cells stimulating production of anti-inflammatory cytokines and suppressing the release of pro-inflammatory cytokines [11].
Surely, a positive regulation of the gut microbiota and its metabolic activities through the diet appears to be very interesting but certainly a challenging approach[2]. Since diet is only one of the multiple recognized risk factors for CRC, specific large-scale and life-long studies on humans are extremely difficult even to draft other than to interpret, indeed. Similarly, since some microbes have been reported to have both a protective and harmful role, an excessive introduction of supplement with probiotics may have additional negative effects and the limit in a population will probably remain unknown.
New diagnostic biomarkers
Colonoscopy is a very important examination in the CRC management, allowing to locate the tumor into the colon and to obtain a preoperative histological diagnosis of malignancies. However, patient’s discomfort more than the possibility of complications may induce several patients to postpone the exam, leading to a late diagnosis. Consequently, the research of new, less invasive diagnostic biomarkers seems very important. High serum levels of MMP-2 and MMP-9 have been proposed as biomarker for CCR and they seem to relate with particular cancer aggressiveness. In a small sample study including 32 CRC patients and 11 controls (benign disease), MMP-2 and MMP-9 resulted more reliable than traditional serum markers such as CEA[75]. Analysis of microbes from saliva samples has been suggested as a non-invasive new biomarker of CRC assuming the existence of a correlation in GM composition between mouth and gut[96]. However, our pilot study failed in finding a significative difference of microbial saliva composition, comparing healthy controls with patients affected by CRC[27].
As previously stated, microbial composition varies together with the progression healthy-adenoma-adenocarcinoma[35-37,53]. On healthy volunteers, a great majority of the phylum Firmicutes has been found[27] and the genera Clostridium, family Lachnospiraceae, may represent a biomarker in stool sample for healthy people[5]. On the contrary, the identification in stool samples of higher presence of Fusobacterium nucleatum in CRC patients when compared with healthy volunteers suggests its possible role as a novel diagnostic biomarker[5,97]. Zackular et al[37] conducted a study to demonstrate the potential as a screening tool of fecal modified microbiota. Data from 90 people (30 healthy, 30 with adenomas, 30 with adenocarcinomas) were analyzed. The authors confirmed that the fecal microbial panel was more important than a single microbe suggesting CRC as polymicrobial disease and they found a significative difference between healthy and adenoma group.
However, further large-scale, case-control studies of multidisciplinary teams are still required to confirm these findings and to obtain more information about bacterial species at strain level. Nevertheless, the eventual correlation between microbiota and other not modifiable characteristics of the patients (e.g., sex or age) should be evaluated, as well.
Perioperative management
In perioperative settings, enhanced recovery after surgery programs should be encouraged. A prolonged fasting of 6-12 h may profoundly alter GM composition and it seems no more justifiable. On the contrary, supplementary food containing microbes able to ferment acid lactic may allow an enhanced healing of the surgical wound[1]. Nonetheless, in animal models, oral supplementation with non-absorbable phosphate usually lacking after surgery, reduced bacterial related anastomotic leak[98]. Antibiotic therapy should be carefully administered and, when required, for the shortest needed period. Opioid drugs should be avoided and other methods of analgesia encouraged. Bowel preparation should be modified in order to try to eliminate only, or mostly, virulent agents maintaining a helpful biodiversity[67].
The discovery of unevenness of species (i.e., V. dispar) in chemotherapy-treated patients still misses a clinical correlation[5]. Hopefully, the recognition of a such potential biomarker may represent a supplementary target in chemotherapy modulation. Unraveling at least a part of the mechanisms of chemoresistance may provide new strategies for chemotherapy optimization[5]. Similarly, the discovery of favorable or unfavorable microbiota for chemoresistance and side effects may help in tailoring chemotherapeutic regimens[85,92]. Immune system has both protective and potential harmful role so the research of strategies to enhance positive action together with the reduction of negative effects should be encouraged. Nevertheless, a deeper understanding of immune-related cancer progression may provide new target for immunotherapy in CRC[17,80].
CONCLUSION
In conclusion, microbiota-based approaches may have a huge impact on CRC initiation, progression and treatments. However, most of the previously published studies are performed on animal models or in small groups of humans. Moreover, most of them are only “quantitative” and a correlation with the clinical impact of the findings is still lacking. Finally, and above all, CRC and its “history” is multifactorial so the evaluation of the impact of modification in gut microbiota composition on CRC management is challenging but very intriguing.
Footnotes
Conflict-of-interest statement: The authors declare no conflict of interest.
Manuscript source: Invited manuscript
Peer-review started: December 30, 2019
First decision: January 19, 2020
Article in press: May 13, 2020
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gazouli M, Zhong ZH S-Editor: Wang J L-Editor: A E-Editor: Liu MY
Contributor Information
Ilenia Bartolini, Department of Experimental and Clinical Medicine, University of Florence, Florence 50134, Italy.
Matteo Risaliti, Department of Experimental and Clinical Medicine, University of Florence, Florence 50134, Italy.
Maria Novella Ringressi, Department of Experimental and Clinical Medicine, University of Florence, Florence 50134, Italy.
Filippo Melli, Department of Experimental and Clinical Medicine, University of Florence, Florence 50134, Italy.
Giulia Nannini, Department of Experimental and Clinical Medicine, University of Florence, Florence 50134, Italy.
Amedeo Amedei, Department of Experimental and Clinical Medicine, University of Florence, Florence 50134, Italy. amedeo.amedei@unifi.it.
Paolo Muiesan, Department of Experimental and Clinical Medicine, University of Florence, Florence 50134, Italy.
Antonio Taddei, Department of Experimental and Clinical Medicine, University of Florence, Florence 50134, Italy.
References
- 1.Alverdy JC, Hyoju SK, Weigerinck M, Gilbert JA. The gut microbiome and the mechanism of surgical infection. Br J Surg. 2017;104:e14–e23. doi: 10.1002/bjs.10405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Russo E, Taddei A, Ringressi MN, Ricci F, Amedei A. The interplay between the microbiome and the adaptive immune response in cancer development. Therap Adv Gastroenterol. 2016;9:594–605. doi: 10.1177/1756283X16635082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alverdy JC. Microbiome Medicine: This Changes Everything. J Am Coll Surg. 2018;226:719–729. doi: 10.1016/j.jamcollsurg.2018.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gilbert JA, Quinn RA, Debelius J, Xu ZZ, Morton J, Garg N, Jansson JK, Dorrestein PC, Knight R. Microbiome-wide association studies link dynamic microbial consortia to disease. Nature. 2016;535:94–103. doi: 10.1038/nature18850. [DOI] [PubMed] [Google Scholar]
- 5.Deng X, Li Z, Li G, Li B, Jin X, Lyu G. Comparison of Microbiota in Patients Treated by Surgery or Chemotherapy by 16S rRNA Sequencing Reveals Potential Biomarkers for Colorectal Cancer Therapy. Front Microbiol. 2018;9:1607. doi: 10.3389/fmicb.2018.01607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bartolini I, Ringressi MN, Melli F, Risaliti M, Brugia M, Mini E, Batignani G, Bechi P, Boni L, Taddei A. Analysis of Prognostic Factors for Resected Synchronous and Metachronous Liver Metastases from Colorectal Cancer. Gastroenterol Res Pract. 2018;2018:5353727. doi: 10.1155/2018/5353727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hooper LV, Gordon JI. Commensal host-bacterial relationships in the gut. Science. 2001;292:1115–1118. doi: 10.1126/science.1058709. [DOI] [PubMed] [Google Scholar]
- 8.Rajpoot M, Sharma AK, Sharma A, Gupta GK. Understanding the microbiome: Emerging biomarkers for exploiting the microbiota for personalized medicine against cancer. Semin Cancer Biol. 2018;52:1–8. doi: 10.1016/j.semcancer.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 9.Mármol I, Sánchez-de-Diego C, Pradilla Dieste A, Cerrada E, Rodriguez Yoldi MJ. Colorectal Carcinoma: A General Overview and Future Perspectives in Colorectal Cancer. Int J Mol Sci. 2017;18:197. doi: 10.3390/ijms18010197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Honda K, Littman DR. The microbiota in adaptive immune homeostasis and disease. Nature. 2016;535:75–84. doi: 10.1038/nature18848. [DOI] [PubMed] [Google Scholar]
- 11.Dos Reis SA, da Conceição LL, Siqueira NP, Rosa DD, da Silva LL, Peluzio MD. Review of the mechanisms of probiotic actions in the prevention of colorectal cancer. Nutr Res. 2017;37:1–19. doi: 10.1016/j.nutres.2016.11.009. [DOI] [PubMed] [Google Scholar]
- 12.Lee YK, Mazmanian SK. Has the microbiota played a critical role in the evolution of the adaptive immune system? Science. 2010;330:1768–1773. doi: 10.1126/science.1195568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 14.Huxley RR, Ansary-Moghaddam A, Clifton P, Czernichow S, Parr CL, Woodward M. The impact of dietary and lifestyle risk factors on risk of colorectal cancer: a quantitative overview of the epidemiological evidence. Int J Cancer. 2009;125:171–180. doi: 10.1002/ijc.24343. [DOI] [PubMed] [Google Scholar]
- 15.Nugent FW, Haggitt RC, Gilpin PA. Cancer surveillance in ulcerative colitis. Gastroenterology. 1991;100:1241–1248. [PubMed] [Google Scholar]
- 16.Fukata M, Abreu MT. Role of Toll-like receptors in gastrointestinal malignancies. Oncogene. 2008;27:234–243. doi: 10.1038/sj.onc.1210908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen J, Pitmon E, Wang K. Microbiome, inflammation and colorectal cancer. Semin Immunol. 2017;32:43–53. doi: 10.1016/j.smim.2017.09.006. [DOI] [PubMed] [Google Scholar]
- 18.Wang X, Yang Y, Moore DR, Nimmo SL, Lightfoot SA, Huycke MM. 4-hydroxy-2-nonenal mediates genotoxicity and bystander effects caused by Enterococcus faecalis-infected macrophages. Gastroenterology. 2012;142:543–551.e7. doi: 10.1053/j.gastro.2011.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klimesova K, Kverka M, Zakostelska Z, Hudcovic T, Hrncir T, Stepankova R, Rossmann P, Ridl J, Kostovcik M, Mrazek J, Kopecny J, Kobayashi KS, Tlaskalova-Hogenova H. Altered gut microbiota promotes colitis-associated cancer in IL-1 receptor-associated kinase M-deficient mice. Inflamm Bowel Dis. 2013;19:1266–1277. doi: 10.1097/MIB.0b013e318281330a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vannucci L, Stepankova R, Kozakova H, Fiserova A, Rossmann P, Tlaskalova-Hogenova H. Colorectal carcinogenesis in germ-free and conventionally reared rats: different intestinal environments affect the systemic immunity. Int J Oncol. 2008;32:609–617. [PubMed] [Google Scholar]
- 21.Gaines S, Shao C, Hyman N, Alverdy JC. Gut microbiome influences on anastomotic leak and recurrence rates following colorectal cancer surgery. Br J Surg. 2018;105:e131–e141. doi: 10.1002/bjs.10760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sears CL, Pardoll DM. Perspective: alpha-bugs, their microbial partners, and the link to colon cancer. J Infect Dis. 2011;203:306–311. doi: 10.1093/jinfdis/jiq061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tjalsma H, Boleij A, Marchesi JR, Dutilh BE. A bacterial driver-passenger model for colorectal cancer: beyond the usual suspects. Nat Rev Microbiol. 2012;10:575–582. doi: 10.1038/nrmicro2819. [DOI] [PubMed] [Google Scholar]
- 24.Gao R, Gao Z, Huang L, Qin H. Gut microbiota and colorectal cancer. Eur J Clin Microbiol Infect Dis. 2017;36:757–769. doi: 10.1007/s10096-016-2881-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hajishengallis G, Darveau RP, Curtis MA. The keystone-pathogen hypothesis. Nat Rev Microbiol. 2012;10:717–725. doi: 10.1038/nrmicro2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Geng J, Fan H, Tang X, Zhai H, Zhang Z. Diversified pattern of the human colorectal cancer microbiome. Gut Pathog. 2013;5:2. doi: 10.1186/1757-4749-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Russo E, Bacci G, Chiellini C, Fagorzi C, Niccolai E, Taddei A, Ricci F, Ringressi MN, Borrelli R, Melli F, Miloeva M, Bechi P, Mengoni A, Fani R, Amedei A. Preliminary Comparison of Oral and Intestinal Human Microbiota in Patients with Colorectal Cancer: A Pilot Study. Front Microbiol. 2017;8:2699. doi: 10.3389/fmicb.2017.02699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Flemer B, Lynch DB, Brown JM, Jeffery IB, Ryan FJ, Claesson MJ, O'Riordain M, Shanahan F, O'Toole PW. Tumour-associated and non-tumour-associated microbiota in colorectal cancer. Gut. 2017;66:633–643. doi: 10.1136/gutjnl-2015-309595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gao Z, Guo B, Gao R, Zhu Q, Qin H. Microbiota disbiosis is associated with colorectal cancer. Front Microbiol. 2015;6:20. doi: 10.3389/fmicb.2015.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Borges-Canha M, Portela-Cidade JP, Dinis-Ribeiro M, Leite-Moreira AF, Pimentel-Nunes P. Role of colonic microbiota in colorectal carcinogenesis: a systematic review. Rev Esp Enferm Dig. 2015;107:659–671. doi: 10.17235/reed.2015.3830/2015. [DOI] [PubMed] [Google Scholar]
- 31.Kostic AD, Chun E, Robertson L, Glickman JN, Gallini CA, Michaud M, Clancy TE, Chung DC, Lochhead P, Hold GL, El-Omar EM, Brenner D, Fuchs CS, Meyerson M, Garrett WS. Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell Host Microbe. 2013;14:207–215. doi: 10.1016/j.chom.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mima K, Sukawa Y, Nishihara R, Qian ZR, Yamauchi M, Inamura K, Kim SA, Masuda A, Nowak JA, Nosho K, Kostic AD, Giannakis M, Watanabe H, Bullman S, Milner DA, Harris CC, Giovannucci E, Garraway LA, Freeman GJ, Dranoff G, Chan AT, Garrett WS, Huttenhower C, Fuchs CS, Ogino S. Fusobacterium nucleatum and T Cells in Colorectal Carcinoma. JAMA Oncol. 2015;1:653–661. doi: 10.1001/jamaoncol.2015.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mima K, Nishihara R, Qian ZR, Cao Y, Sukawa Y, Nowak JA, Yang J, Dou R, Masugi Y, Song M, Kostic AD, Giannakis M, Bullman S, Milner DA, Baba H, Giovannucci EL, Garraway LA, Freeman GJ, Dranoff G, Garrett WS, Huttenhower C, Meyerson M, Meyerhardt JA, Chan AT, Fuchs CS, Ogino S. Fusobacterium nucleatum in colorectal carcinoma tissue and patient prognosis. Gut. 2016;65:1973–1980. doi: 10.1136/gutjnl-2015-310101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ito M, Kanno S, Nosho K, Sukawa Y, Mitsuhashi K, Kurihara H, Igarashi H, Takahashi T, Tachibana M, Takahashi H, Yoshii S, Takenouchi T, Hasegawa T, Okita K, Hirata K, Maruyama R, Suzuki H, Imai K, Yamamoto H, Shinomura Y. Association of Fusobacterium nucleatum with clinical and molecular features in colorectal serrated pathway. Int J Cancer. 2015;137:1258–1268. doi: 10.1002/ijc.29488. [DOI] [PubMed] [Google Scholar]
- 35.Kostic AD, Gevers D, Pedamallu CS, Michaud M, Duke F, Earl AM, Ojesina AI, Jung J, Bass AJ, Tabernero J, Baselga J, Liu C, Shivdasani RA, Ogino S, Birren BW, Huttenhower C, Garrett WS, Meyerson M. Genomic analysis identifies association of Fusobacterium with colorectal carcinoma. Genome Res. 2012;22:292–298. doi: 10.1101/gr.126573.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Castellarin M, Warren RL, Freeman JD, Dreolini L, Krzywinski M, Strauss J, Barnes R, Watson P, Allen-Vercoe E, Moore RA, Holt RA. Fusobacterium nucleatum infection is prevalent in human colorectal carcinoma. Genome Res. 2012;22:299–306. doi: 10.1101/gr.126516.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zackular JP, Rogers MA, Ruffin MT 4th, Schloss PD. The human gut microbiome as a screening tool for colorectal cancer. Cancer Prev Res (Phila) 2014;7:1112–1121. doi: 10.1158/1940-6207.CAPR-14-0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Costello EK, Lauber CL, Hamady M, Fierer N, Gordon JI, Knight R. Bacterial community variation in human body habitats across space and time. Science. 2009;326:1694–1697. doi: 10.1126/science.1177486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu S, Rhee KJ, Albesiano E, Rabizadeh S, Wu X, Yen HR, Huso DL, Brancati FL, Wick E, McAllister F, Housseau F, Pardoll DM, Sears CL. A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat Med. 2009;15:1016–1022. doi: 10.1038/nm.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dejea CM, Fathi P, Craig JM, Boleij A, Taddese R, Geis AL, Wu X, DeStefano Shields CE, Hechenbleikner EM, Huso DL, Anders RA, Giardiello FM, Wick EC, Wang H, Wu S, Pardoll DM, Housseau F, Sears CL. Patients with familial adenomatous polyposis harbor colonic biofilms containing tumorigenic bacteria. Science. 2018;359:592–597. doi: 10.1126/science.aah3648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhu Q, Gao R, Wu W, Qin H. The role of gut microbiota in the pathogenesis of colorectal cancer. Tumour Biol. 2013;34:1285–1300. doi: 10.1007/s13277-013-0684-4. [DOI] [PubMed] [Google Scholar]
- 42.Gur C, Ibrahim Y, Isaacson B, Yamin R, Abed J, Gamliel M, Enk J, Bar-On Y, Stanietsky-Kaynan N, Coppenhagen-Glazer S, Shussman N, Almogy G, Cuapio A, Hofer E, Mevorach D, Tabib A, Ortenberg R, Markel G, Miklić K, Jonjic S, Brennan CA, Garrett WS, Bachrach G, Mandelboim O. Binding of the Fap2 protein of Fusobacterium nucleatum to human inhibitory receptor TIGIT protects tumors from immune cell attack. Immunity. 2015;42:344–355. doi: 10.1016/j.immuni.2015.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Irrazabal T, Martin A. T Regulatory Cells Gone Bad: An Oncogenic Immune Response against Enterotoxigenic B. fragilis Infection Leads to Colon Cancer. Cancer Discov. 2015;5:1021–1023. doi: 10.1158/2159-8290.CD-15-0987. [DOI] [PubMed] [Google Scholar]
- 44.Sears CL. Enterotoxigenic Bacteroides fragilis: a rogue among symbiotes. Clin Microbiol Rev. 2009;22:349–369, Table of Contents. doi: 10.1128/CMR.00053-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ziesché E, Bachmann M, Kleinert H, Pfeilschifter J, Mühl H. The interleukin-22/STAT3 pathway potentiates expression of inducible nitric-oxide synthase in human colon carcinoma cells. J Biol Chem. 2007;282:16006–16015. doi: 10.1074/jbc.M611040200. [DOI] [PubMed] [Google Scholar]
- 46.Langowski JL, Zhang X, Wu L, Mattson JD, Chen T, Smith K, Basham B, McClanahan T, Kastelein RA, Oft M. IL-23 promotes tumour incidence and growth. Nature. 2006;442:461–465. doi: 10.1038/nature04808. [DOI] [PubMed] [Google Scholar]
- 47.Fukuda S, Toh H, Hase K, Oshima K, Nakanishi Y, Yoshimura K, Tobe T, Clarke JM, Topping DL, Suzuki T, Taylor TD, Itoh K, Kikuchi J, Morita H, Hattori M, Ohno H. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature. 2011;469:543–547. doi: 10.1038/nature09646. [DOI] [PubMed] [Google Scholar]
- 48.Ubeda C, Djukovic A, Isaac S. Roles of the intestinal microbiota in pathogen protection. Clin Transl Immunology. 2017;6:e128. doi: 10.1038/cti.2017.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sivan A, Corrales L, Hubert N, Williams JB, Aquino-Michaels K, Earley ZM, Benyamin FW, Lei YM, Jabri B, Alegre ML, Chang EB, Gajewski TF. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science. 2015;350:1084–1089. doi: 10.1126/science.aac4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huber S, Gagliani N, Esplugues E, O'Connor W, Jr, Huber FJ, Chaudhry A, Kamanaka M, Kobayashi Y, Booth CJ, Rudensky AY, Roncarolo MG, Battaglia M, Flavell RA. Th17 cells express interleukin-10 receptor and are controlled by Foxp3⁻ and Foxp3+ regulatory CD4+ T cells in an interleukin-10-dependent manner. Immunity. 2011;34:554–565. doi: 10.1016/j.immuni.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guyton K, Alverdy JC. The gut microbiota and gastrointestinal surgery. Nat Rev Gastroenterol Hepatol. 2017;14:43–54. doi: 10.1038/nrgastro.2016.139. [DOI] [PubMed] [Google Scholar]
- 52.Ohigashi S, Sudo K, Kobayashi D, Takahashi T, Nomoto K, Onodera H. Significant changes in the intestinal environment after surgery in patients with colorectal cancer. J Gastrointest Surg. 2013;17:1657–1664. doi: 10.1007/s11605-013-2270-x. [DOI] [PubMed] [Google Scholar]
- 53.Sze MA, Baxter NT, Ruffin MT 4th, Rogers MAM, Schloss PD. Normalization of the microbiota in patients after treatment for colonic lesions. Microbiome. 2017;5:150. doi: 10.1186/s40168-017-0366-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Krezalek MA, Hyoju S, Zaborin A, Okafor E, Chandrasekar L, Bindokas V, Guyton K, Montgomery CP, Daum RS, Zaborina O, Boyle-Vavra S, Alverdy JC. Can Methicillin-resistant Staphylococcus aureus Silently Travel From the Gut to the Wound and Cause Postoperative Infection? Modeling the "Trojan Horse Hypothesis". Ann Surg. 2018;267:749–758. doi: 10.1097/SLA.0000000000002173. [DOI] [PubMed] [Google Scholar]
- 55.Nicholson A, Lowe MC, Parker J, Lewis SR, Alderson P, Smith AF. Systematic review and meta-analysis of enhanced recovery programmes in surgical patients. Br J Surg. 2014;101:172–188. doi: 10.1002/bjs.9394. [DOI] [PubMed] [Google Scholar]
- 56.Babrowski T, Holbrook C, Moss J, Gottlieb L, Valuckaite V, Zaborin A, Poroyko V, Liu DC, Zaborina O, Alverdy JC. Pseudomonas aeruginosa virulence expression is directly activated by morphine and is capable of causing lethal gut-derived sepsis in mice during chronic morphine administration. Ann Surg. 2012;255:386–393. doi: 10.1097/SLA.0b013e3182331870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stavrou G, Kotzampassi K. Gut microbiome, surgical complications and probiotics. Ann Gastroenterol. 2017;30:45–53. doi: 10.20524/aog.2016.0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Adam MA, Lee LM, Kim J, Shenoi M, Mallipeddi M, Aziz H, Stinnett S, Sun Z, Mantyh CR, Thacker JK. Alvimopan Provides Additional Improvement in Outcomes and Cost Savings in Enhanced Recovery Colorectal Surgery. Ann Surg. 2016;264:141–146. doi: 10.1097/SLA.0000000000001428. [DOI] [PubMed] [Google Scholar]
- 59.Poutahidis T, Kearney SM, Levkovich T, Qi P, Varian BJ, Lakritz JR, Ibrahim YM, Chatzigiagkos A, Alm EJ, Erdman SE. Microbial symbionts accelerate wound healing via the neuropeptide hormone oxytocin. PLoS One. 2013;8:e78898. doi: 10.1371/journal.pone.0078898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Frasson M, Granero-Castro P, Ramos Rodríguez JL, Flor-Lorente B, Braithwaite M, Martí Martínez E, Álvarez Pérez JA, Codina Cazador A, Espí A, Garcia-Granero E ANACO Study Group. Risk factors for anastomotic leak and postoperative morbidity and mortality after elective right colectomy for cancer: results from a prospective, multicentric study of 1102 patients. Int J Colorectal Dis. 2016;31:105–114. doi: 10.1007/s00384-015-2376-6. [DOI] [PubMed] [Google Scholar]
- 61.COHN I, Jr, RIVES JD. Antibiotic protection of colon anastomoses. Ann Surg. 1955;141:707–717. doi: 10.1097/00000658-195505000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shogan BD, Belogortseva N, Luong PM, Zaborin A, Lax S, Bethel C, Ward M, Muldoon JP, Singer M, An G, Umanskiy K, Konda V, Shakhsheer B, Luo J, Klabbers R, Hancock LE, Gilbert J, Zaborina O, Alverdy JC. Collagen degradation and MMP9 activation by Enterococcus faecalis contribute to intestinal anastomotic leak. Sci Transl Med. 2015;7:286ra68. doi: 10.1126/scitranslmed.3010658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shakhsheer BA, Versten LA, Luo JN, Defazio JR, Klabbers R, Christley S, Zaborin A, Guyton KL, Krezalek M, Smith DP, Ajami NJ, Petrosino JF, Fleming ID, Belogortseva N, Zaborina O, Alverdy JC. Morphine Promotes Colonization of Anastomotic Tissues with Collagenase - Producing Enterococcus faecalis and Causes Leak. J Gastrointest Surg. 2016;20:1744–1751. doi: 10.1007/s11605-016-3237-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Garin-Laflam MP, Steinbrecher KA, Rudolph JA, Mao J, Cohen MB. Activation of guanylate cyclase C signaling pathway protects intestinal epithelial cells from acute radiation-induced apoptosis. Am J Physiol Gastrointest Liver Physiol. 2009;296:G740–G749. doi: 10.1152/ajpgi.90268.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Falkow S. Molecular Koch's postulates applied to microbial pathogenicity. Rev Infect Dis. 1988;10 Suppl 2:S274–S276. doi: 10.1093/cid/10.supplement_2.s274. [DOI] [PubMed] [Google Scholar]
- 66.Kiran RP, Murray AC, Chiuzan C, Estrada D, Forde K. Combined preoperative mechanical bowel preparation with oral antibiotics significantly reduces surgical site infection, anastomotic leak, and ileus after colorectal surgery. Ann Surg. 2015;262:416–25; discussion 423-5. doi: 10.1097/SLA.0000000000001416. [DOI] [PubMed] [Google Scholar]
- 67.Alverdy JC, Hyman N, Gilbert J, Luo JN, Krezalek M. Preparing the Bowel for Surgery: Learning from the Past and Planning for the Future. J Am Coll Surg. 2017;225:324–332. doi: 10.1016/j.jamcollsurg.2017.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lee JA, Chico TJA, Renshaw SA. The triune of intestinal microbiome, genetics and inflammatory status and its impact on the healing of lower gastrointestinal anastomoses. FEBS J. 2018;285:1212–1225. doi: 10.1111/febs.14346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Colgan SP, Taylor CT. Hypoxia: an alarm signal during intestinal inflammation. Nat Rev Gastroenterol Hepatol. 2010;7:281–287. doi: 10.1038/nrgastro.2010.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Oncel M, Kurt N, Remzi FH, Sensu SS, Vural S, Gezen CF, Cincin TG, Olcay E. The effectiveness of systemic antibiotics in preventing postoperative, intraabdominal adhesions in an animal model. J Surg Res. 2001;101:52–55. doi: 10.1006/jsre.2001.6245. [DOI] [PubMed] [Google Scholar]
- 71.Pohl JM, Gutweiler S, Thiebes S, Volke JK, Klein-Hitpass L, Zwanziger D, Gunzer M, Jung S, Agace WW, Kurts C, Engel DR. Irf4-dependent CD103+CD11b+ dendritic cells and the intestinal microbiome regulate monocyte and macrophage activation and intestinal peristalsis in postoperative ileus. Gut. 2017;66:2110–2120. doi: 10.1136/gutjnl-2017-313856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Renouf DJ, Woods R, Speers C, Hay J, Phang PT, Fitzgerald C, Kennecke H. Improvements in 5-year outcomes of stage II/III rectal cancer relative to colon cancer. Am J Clin Oncol. 2013;36:558–564. doi: 10.1097/COC.0b013e318256f5dc. [DOI] [PubMed] [Google Scholar]
- 73.Mokhles S, Macbeth F, Farewell V, Fiorentino F, Williams NR, Younes RN, Takkenberg JJ, Treasure T. Meta-analysis of colorectal cancer follow-up after potentially curative resection. Br J Surg. 2016;103:1259–1268. doi: 10.1002/bjs.10233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang S, Liu J, Wang S, Zhao H, Ge S, Wang W. Adverse Effects of Anastomotic Leakage on Local Recurrence and Survival After Curative Anterior Resection for Rectal Cancer: A Systematic Review and Meta-analysis. World J Surg. 2017;41:277–284. doi: 10.1007/s00268-016-3761-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dragutinović VV, Radonjić NV, Petronijević ND, Tatić SB, Dimitrijević IB, Radovanović NS, Krivokapić ZV. Matrix metalloproteinase-2 (MMP-2) and -9 (MMP-9) in preoperative serum as independent prognostic markers in patients with colorectal cancer. Mol Cell Biochem. 2011;355:173–178. doi: 10.1007/s11010-011-0851-0. [DOI] [PubMed] [Google Scholar]
- 76.Wei Z, Cao S, Liu S, Yao Z, Sun T, Li Y, Li J, Zhang D, Zhou Y. Could gut microbiota serve as prognostic biomarker associated with colorectal cancer patients' survival? A pilot study on relevant mechanism. Oncotarget. 2016;7:46158–46172. doi: 10.18632/oncotarget.10064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Flanagan L, Schmid J, Ebert M, Soucek P, Kunicka T, Liska V, Bruha J, Neary P, Dezeeuw N, Tommasino M, Jenab M, Prehn JH, Hughes DJ. Fusobacterium nucleatum associates with stages of colorectal neoplasia development, colorectal cancer and disease outcome. Eur J Clin Microbiol Infect Dis. 2014;33:1381–1390. doi: 10.1007/s10096-014-2081-3. [DOI] [PubMed] [Google Scholar]
- 78.Kosumi K, Hamada T, Koh H, Borowsky J, Bullman S, Twombly TS, Nevo D, Masugi Y, Liu L, da Silva A, Chen Y, Du C, Gu M, Li C, Li W, Liu H, Shi Y, Mima K, Song M, Nosho K, Nowak JA, Nishihara R, Baba H, Zhang X, Wu K, Wang M, Huttenhower C, Garrett WS, Meyerson ML, Lennerz JK, Giannakis M, Chan AT, Meyerhardt JA, Fuchs CS, Ogino S. The Amount of Bifidobacterium Genus in Colorectal Carcinoma Tissue in Relation to Tumor Characteristics and Clinical Outcome. Am J Pathol. 2018;188:2839–2852. doi: 10.1016/j.ajpath.2018.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tosolini M, Kirilovsky A, Mlecnik B, Fredriksen T, Mauger S, Bindea G, Berger A, Bruneval P, Fridman WH, Pagès F, Galon J. Clinical impact of different classes of infiltrating T cytotoxic and helper cells (Th1, th2, treg, th17) in patients with colorectal cancer. Cancer Res. 2011;71:1263–1271. doi: 10.1158/0008-5472.CAN-10-2907. [DOI] [PubMed] [Google Scholar]
- 80.Dou R, Nishihara R, Cao Y, Hamada T, Mima K, Masuda A, Masugi Y, Shi Y, Gu M, Li W, da Silva A, Nosho K, Zhang X, Meyerhardt JA, Giovannucci EL, Chan AT, Fuchs CS, Qian ZR, Ogino S. MicroRNA let-7, T Cells, and Patient Survival in Colorectal Cancer. Cancer Immunol Res. 2016;4:927–935. doi: 10.1158/2326-6066.CIR-16-0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nosho K, Baba Y, Tanaka N, Shima K, Hayashi M, Meyerhardt JA, Giovannucci E, Dranoff G, Fuchs CS, Ogino S. Tumour-infiltrating T-cell subsets, molecular changes in colorectal cancer, and prognosis: cohort study and literature review. J Pathol. 2010;222:350–366. doi: 10.1002/path.2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pagès F, Mlecnik B, Marliot F, Bindea G, Ou FS, Bifulco C, Lugli A, Zlobec I, Rau TT, Berger MD, Nagtegaal ID, Vink-Börger E, Hartmann A, Geppert C, Kolwelter J, Merkel S, Grützmann R, Van den Eynde M, Jouret-Mourin A, Kartheuser A, Léonard D, Remue C, Wang JY, Bavi P, Roehrl MHA, Ohashi PS, Nguyen LT, Han S, MacGregor HL, Hafezi-Bakhtiari S, Wouters BG, Masucci GV, Andersson EK, Zavadova E, Vocka M, Spacek J, Petruzelka L, Konopasek B, Dundr P, Skalova H, Nemejcova K, Botti G, Tatangelo F, Delrio P, Ciliberto G, Maio M, Laghi L, Grizzi F, Fredriksen T, Buttard B, Angelova M, Vasaturo A, Maby P, Church SE, Angell HK, Lafontaine L, Bruni D, El Sissy C, Haicheur N, Kirilovsky A, Berger A, Lagorce C, Meyers JP, Paustian C, Feng Z, Ballesteros-Merino C, Dijkstra J, van de Water C, van Lent-van Vliet S, Knijn N, Mușină AM, Scripcariu DV, Popivanova B, Xu M, Fujita T, Hazama S, Suzuki N, Nagano H, Okuno K, Torigoe T, Sato N, Furuhata T, Takemasa I, Itoh K, Patel PS, Vora HH, Shah B, Patel JB, Rajvik KN, Pandya SJ, Shukla SN, Wang Y, Zhang G, Kawakami Y, Marincola FM, Ascierto PA, Sargent DJ, Fox BA, Galon J. International validation of the consensus Immunoscore for the classification of colon cancer: a prognostic and accuracy study. Lancet. 2018;391:2128–2139. doi: 10.1016/S0140-6736(18)30789-X. [DOI] [PubMed] [Google Scholar]
- 83.Mlecnik B, Bindea G, Angell HK, Maby P, Angelova M, Tougeron D, Church SE, Lafontaine L, Fischer M, Fredriksen T, Sasso M, Bilocq AM, Kirilovsky A, Obenauf AC, Hamieh M, Berger A, Bruneval P, Tuech JJ, Sabourin JC, Le Pessot F, Mauillon J, Rafii A, Laurent-Puig P, Speicher MR, Trajanoski Z, Michel P, Sesboüe R, Frebourg T, Pagès F, Valge-Archer V, Latouche JB, Galon J. Integrative Analyses of Colorectal Cancer Show Immunoscore Is a Stronger Predictor of Patient Survival Than Microsatellite Instability. Immunity. 2016;44:698–711. doi: 10.1016/j.immuni.2016.02.025. [DOI] [PubMed] [Google Scholar]
- 84.Alexander JL, Wilson ID, Teare J, Marchesi JR, Nicholson JK, Kinross JM. Gut microbiota modulation of chemotherapy efficacy and toxicity. Nat Rev Gastroenterol Hepatol. 2017;14:356–365. doi: 10.1038/nrgastro.2017.20. [DOI] [PubMed] [Google Scholar]
- 85.Pouncey AL, Scott AJ, Alexander JL, Marchesi J, Kinross J. Gut microbiota, chemotherapy and the host: the influence of the gut microbiota on cancer treatment. Ecancermedicalscience. 2018;12:868. doi: 10.3332/ecancer.2018.868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.García-González AP, Ritter AD, Shrestha S, Andersen EC, Yilmaz LS, Walhout AJM. Bacterial Metabolism Affects the C. elegans Response to Cancer Chemotherapeutics. Cell. 2017;169:431–441.e8. doi: 10.1016/j.cell.2017.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yu T, Guo F, Yu Y, Sun T, Ma D, Han J, Qian Y, Kryczek I, Sun D, Nagarsheth N, Chen Y, Chen H, Hong J, Zou W, Fang JY. Fusobacterium nucleatum Promotes Chemoresistance to Colorectal Cancer by Modulating Autophagy. Cell. 2017;170:548–563.e16. doi: 10.1016/j.cell.2017.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wu T, Cui L, Liang Z, Liu C, Liu Y, Li J. Elevated serum IL-22 levels correlate with chemoresistant condition of colorectal cancer. Clin Immunol. 2013;147:38–39. doi: 10.1016/j.clim.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 89.Curiel TJ. Regulatory T cells and treatment of cancer. Curr Opin Immunol. 2008;20:241–246. doi: 10.1016/j.coi.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Guthrie L, Gupta S, Daily J, Kelly L. Human microbiome signatures of differential colorectal cancer drug metabolism. NPJ Biofilms Microbiomes. 2017;3:27. doi: 10.1038/s41522-017-0034-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Iida N, Dzutsev A, Stewart CA, Smith L, Bouladoux N, Weingarten RA, Molina DA, Salcedo R, Back T, Cramer S, Dai RM, Kiu H, Cardone M, Naik S, Patri AK, Wang E, Marincola FM, Frank KM, Belkaid Y, Trinchieri G, Goldszmid RS. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science. 2013;342:967–970. doi: 10.1126/science.1240527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Aarnoutse R, de Vos-Geelen JMPGM, Penders J, Boerma EG, Warmerdam FARM, Goorts B, Olde Damink SWM, Soons Z, Rensen SSM, Smidt ML. Study protocol on the role of intestinal microbiota in colorectal cancer treatment: a pathway to personalized medicine 2.0. Int J Colorectal Dis. 2017;32:1077–1084. doi: 10.1007/s00384-017-2819-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Burns AJ, Rowland IR. Antigenotoxicity of probiotics and prebiotics on faecal water-induced DNA damage in human colon adenocarcinoma cells. Mutat Res. 2004;551:233–243. doi: 10.1016/j.mrfmmm.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 94.Ewaschuk JB, Walker JW, Diaz H, Madsen KL. Bioproduction of conjugated linoleic acid by probiotic bacteria occurs in vitro and in vivo in mice. J Nutr. 2006;136:1483–1487. doi: 10.1093/jn/136.6.1483. [DOI] [PubMed] [Google Scholar]
- 95.Hornef MW, Pabst O. Real friends: Faecalibacterium prausnitzii supports mucosal immune homeostasis. Gut. 2016;65:365–367. doi: 10.1136/gutjnl-2015-310027. [DOI] [PubMed] [Google Scholar]
- 96.Belstrøm D. Salivary Microbiota in Oral Health and Disease. In: Lynge Pedersen AM. Oral Infections and General Health: From molecule to chairside. Springer, Cham. 2016:115–122. [Google Scholar]
- 97.Yu J, Feng Q, Wong SH, Zhang D, Liang QY, Qin Y, Tang L, Zhao H, Stenvang J, Li Y, Wang X, Xu X, Chen N, Wu WK, Al-Aama J, Nielsen HJ, Kiilerich P, Jensen BA, Yau TO, Lan Z, Jia H, Li J, Xiao L, Lam TY, Ng SC, Cheng AS, Wong VW, Chan FK, Xu X, Yang H, Madsen L, Datz C, Tilg H, Wang J, Brünner N, Kristiansen K, Arumugam M, Sung JJ, Wang J. Metagenomic analysis of faecal microbiome as a tool towards targeted non-invasive biomarkers for colorectal cancer. Gut. 2017;66:70–78. doi: 10.1136/gutjnl-2015-309800. [DOI] [PubMed] [Google Scholar]
- 98.Hyoju SK, Klabbers RE, Aaron M, Krezalek MA, Zaborin A, Wiegerinck M, Hyman NH, Zaborina O, Van Goor H, Alverdy JC. Oral Polyphosphate Suppresses Bacterial Collagenase Production and Prevents Anastomotic Leak Due to Serratia marcescens and Pseudomonas aeruginosa. Ann Surg. 2018;267:1112–1118. doi: 10.1097/SLA.0000000000002167. [DOI] [PMC free article] [PubMed] [Google Scholar]