Abstract
The aim of this review is to offer dietary advice for individuals with spinal cord injury (SCI) and neurogenic bowel dysfunction. With this in mind, we consider health conditions that are dependent on the level of lesion including skeletal muscle atrophy, autonomic dysreflexia and neurogenic bladder. In addition, SCI is often associated with a sedentary lifestyle, which increases risk for osteoporosis and diseases associated with chronic low-grade inflammation, including cardiovascular and chronic kidney diseases. The Mediterranean diet, along with exercise and dietary supplements, has been suggested as an anti-inflammatory intervention in individuals with SCI. However, individuals with chronic SCI have a daily intake of whole fruit, vegetables and whole grains lower than the recommended dietary allowance for the general population. Some studies have reported an increase in neurogenic bowel dysfunction symptoms after high fiber intake; therefore, this finding could explain the low consumption of plant foods. Low consumption of fibre induces dysbiosis, which is associated with both endotoxemia and inflammation. Dysbiosis can be reduced by exercise and diet in individuals with SCI. Therefore, to summarize our viewpoint, we developed a Mediterranean diet-based diet and exercise pyramid to integrate nutritional recommendations and exercise guidelines. Nutritional guidelines come from previously suggested recommendations for military veterans with disabilities and individuals with SCI, chronic kidney diseases, chronic pain and irritable bowel syndrome. We also considered the recent exercise guidelines and position stands for adults with SCI to improve muscle strength, flexibility and cardiorespiratory fitness and to obtain cardiometabolic benefits. Finally, dietary advice for Paralympic athletes is suggested.
Keywords: Neurogenic bowel dysfunction, Body composition, Mediterranean diet, Food–drug interactions, Microbiota, Paralympic athletes
Core tip: Dietary advice for individuals with a spinal cord injury (SCI) and with neurogenic bowel dysfunction (NBD) must be carefully considered. This advice should include: (1) Energy and nitrogen balance; (2) Under/over-nutrition; (3) Comorbidities and polypharmacy; and (4) Micronutrient deficiency. Dysbiosis and low-grade inflammation, typical consequences of SCI, can be reduced by increasing both physical exercise and fibre intake, but particular fermentable carbohydrates and source of fiber, can sometimes increase NBD symptoms. Water intake is particularly important for NBD management and can be critical during and after exercise. Multi-disciplinary cardiovascular risk reduction programs, including tailored nutrition, strength, aerobic and flexibility training, and personalized dietary supplementation are recommended for individuals with SCI and NBD.
INTRODUCTION
Spinal cord injury (SCI) may result in motor paralysis and sensory loss below the level of the lesion[1-5] and impaired function of the autonomic nervous system (ANS) originating from the spinal cord[6]. The physio-pathological consequences of the SCI are therefore manifold, including an injury level-dependent impaired function in thoracic and abdominal organs and their related tissues[6]. The lack of motor stimulation below the level of SCI induces changes that can result in neurogenic obesity via adipose tissue accumulation[7]. The latter is associated with the release of proinflammatory adipokines that lead to chronic low-grade inflammation[7,8]. Although both under-nutrition and over-nutrition can be observed in individuals with SCI[9], compared to the guidelines for general population, individuals with chronic SCI have lower fibre intake and greater energy intake relative to energy needs[10]. Among individuals with SCI, daily intakes of whole fruits, vegetables and whole grains were lower than recommended dietary allowances[11-13]. Appropriate nutritional recommendations[14] and exercise[7] for individuals with SCI could play an important role in decreasing the risk of cardiovascular disease (CVD) and car-diometabolic syndrome (CMS).
On the other hand, symptoms of bowel dysfunction, including constipation and faecal incontinence, have a high impact on quality of life of individuals with SCI[15]. It has been reported that many characteristics of abdominal pain among patients with SCI resemble those of able-bodied individuals with chronic idiopathic constipation[16]. Dysmotility and abdominal discomfort have been attributed to the ingestion of certain carbohydrates (CHO) in individuals with gastrointestinal disorders, including irritable bowel syndrome (IBS) and functional constipation[17]. In addition to dietary advice, lifestyle recommendations, including exercise, have been suggested for patients with IBS[18], in particular in those with slow or uncoordinated transit[19]. It has been suggested that exercise may modulate intestinal permeability and motility, stool transit time and consistency[20].
In this review, considering the above-mentioned issues, we will discuss dietary management of neurogenic bowel dysfunction (NBD) among individuals with SCI and suggest a Mediterranean diet (Med-D)-based pyramid and nutritional advice for Paralympic athletes with SCI.
NEUROGENIC BOWEL DYSFUNCTION IN SPINAL CORD INJURY
NBD refers to bowel dysfunction consequent to the ANS injury and/or lack of central nervous system control (mainly from hypothalamus and brainstem)[21]. Although symptoms of both upper (esophageal[22]/gastroesophageal[23]) and lower (intestinal) gastrointestinal disorders can occur, the latter are highly prevalent in SCI and include constipation (prevalence ranging between 56% and 80%) and faecal incontinence (range 42%-75%)[21]. Colon dysfunction following SCI can be divided into two main types, depending on the level of the lesion: An upper motor neuron syndrome and a lower motor neuron syndrome[21]. Typically constipation and incontinence occur for injury above and below the thoracic (T)11/T12 region, respectively[24]. On the other hand, given that traumatic SCI usually occurs at the cervical or thoracic levels, the spinal defecation centre, located in the sacral spinal cord, is typically intact in these individuals[25].
In patients with motor complete SCI, Vallès et al[26] identified three different neuropathophysiological patterns. Pattern A is characterized by frequent constipation, moderate delay in colonic transit time (CTT) and the absence of anal relaxation during the defecation. Pattern B is characterized by defecatory difficulty, moderate delay in CTT, increased anal resistance during the defecation and preserved sacral reflexes; Pattern C is characterized by severe incontinence associated with severe delay in CTT and absence of sacral reflexes.
In a long (19-year, 1996-2015) follow up study on living with SCI, no significant changes in quality of life and faecal incontinence were found, whereas a higher prevalence (from 19% to 31%) of the population with SCI considered themselves to be constipated and increased with time the use of oral laxatives[27].
It has been suggested that constipation is a major cause of abdominal pain or discomfort after SCI, rather that neuropathic pain, with pain considered more intense and unpleasant among individuals with chronic idiopathic constipation compared to patients with SCI[16]. Furthermore, there was no association between neurological level of SCI and abdominal pain as 79% of individuals with cervical and thoracic SCI and 86% with lumbar SCI had pain[16].
Sensory stimuli below the injury level due to bowel management are among the common triggers for autonomic dysreflexia (AD), which is a complex syndrome characterized by sudden episodic high vasoconstriction-induced hypertension and resulting symptoms due to massive sympathetic ANS activity deriving from the lack of hypothalamus and brainstem control. AD generally occurs among individuals with SCI at or above the T6 level[6], above splanchnic sympathetic outflow.
Inskip et al[28] found that in a group of about 150 individuals with SCI at T7 or above, 74% experienced at least one AD symptom during bowel care including “goosebumps, spasticity, flushing and sweating” in about half population, along with general unwellness (43%) and headache (38%)”. “Heart palpitations, irregular heartbeats, or a feeling of fluttering in the chest” globally considered as symptoms of arrhythmia consequent to AD were found in 45 of 141 (32%) individuals with SCI at or above T7[28]. Longer durations of bowel care due to SCI and more severe AD were associated with lower quality of life[28].
The NBD score has been identified as a condition-specific tool to assess quality of life as "subjective well-being" among individuals with SCI[29]. NBD score includes frequency of bowel movements (0-6 points), headache, perspiration or discomfort before or during defecation (0-2 points), tablets and drops against constipation (0-2 points each), time used for each defecation (0-7 points), frequency of digital stimulation or evacuation (0-6 points), frequency of faecal incontinence (0-13 points), medication against faecal incontinence (0-4 points), flatus incontinence (0-2 points) and perianal skin problems (0-3 points)[30]. According to Krogh et al[30] a severe NBD score is ≥ 14, moderate an NBD score of 10-13, minor an NBD score of 7-9 and very minor an NBD score of 0-6. Liu et al[31] reported that differences in NBD scores were dependent on both completeness and level of injury. Severe NBD was observed in 71.1% of individuals with American Spinal Injury Association (ASIA) classification A (loss of motor and sensory function at S4-5 level), whereas lower percentages were found in patients with incomplete SCI (prevalence of 32.4%, 24.2% and 27.1% in ASIA classifications B, C and D, respectively)[31]. Furthermore, a higher percentage of individuals with cervical (48.2%) and thoracic (40.0%) SCI had severe NBD, compared to those with lumbar SCI (14.3%)[31]. Other factors, including use of laxatives and fibre intake, affect NBD score among individuals with traumatic SCI and minor to moderate bowel dysfunction (NBD Score: 8.01 ± 4.49), whereas neither the use of anticholinergics nor opioid agents were significantly associated with NBD[32]. In conclusion, living with SCI and NBD has an impact on dietary intake (amount or type of foods) and habit (time restricted or skip meal)[33], with potential effects on nutritional status.
DYSBIOSIS IN SPINAL CORD INJURY: POTENTIAL TARGET FOR FIBRE, DIETARY INTERVENTIONS AND EXERCISE TRAINING
Antibiotic treatment, due to recurrent infections, and altered CTT significantly alter the composition of gut microbiota in individuals with SCI[34]. The use of antibiotics decreases microbial diversity by 25% and increases the Bacteroidetes/Firmicutes ratio[35]. In the “Guidelines for Management of Neurogenic Bowel Dysfunction in Individuals with Central Neurological Conditions” of the Multidisciplinary Association of Spinal Cord Injured Professionals[36], it is concluded that there is some evidence that the use of probiotics may help to restore colonic flora after antibiotic treatment.
Several clinical studies have evaluated the intestinal microbiota among individuals with SCI and all have reported decreased Firmicutes compared to healthy controls[37-39]. Furthermore, the reduced microbial diversity in individuals with SCI[37,38] has been associated with unfavourable metabolic profiles[37]. At the phylum level, Bacteroidetes and Firmicutes were negatively[38] and positively correlated with high density lipoprotein cholesterol (HDL)[37], respectively, and Firmicutes were negatively correlated with serum glucose (GLU)[38]. Exercise[40,41] and Med-D[42] are known to improve metabolic (serum GLU and lipids) markers and cardiorespiratory fitness was reported to be significantly correlated to both microbial diversity[20,43,44] and Firmicutes to Bacteroidetes ratio in healthy adults[45]. Animal-based diets increased the abundance of Bacteroides[46,47] and decreased Firmicutes which metabolize dietary plant polysaccharides[47]. Prevotella[48] abundance was associated with long-term fibre intake, estimated using the Diet History Questionnaire[48]. In individuals with SCI, GLU was negatively correlated with Prevotella[38] and Megamonas[37,38] (more abundant in a healthy group than in groups with SCI)[37-39]. Although, Megamonas and Prevotella may have a positive effect on CHO metabolism, they were positively correlated with the NBD score, probably due to gas and short-chain fatty acids (SCFA), the end products of microbial fibre fermentation[38]. In IBS, discomfort, constipation and altered faecal consistency can be influenced by the presence of dietary fibre and fermentable oligosaccharides, disaccharides, monosaccharides, and polyols (FODMAP)[18,49].
Reported mean fibre intake, ranging between 15.0 g/d[11] and 16.9-17.6 g/d[10], met the Academy of Nutrition and Dietetics Evidence Analysis Library minimum target range for individuals with SCI (which starts at a minimum of 15 g/d and increases up to 30 g/d as tolerated)[50]. Stoffel et al[51] reviewed studies on neurogenic bowel management and reported that a high-fibre diet prolonged constipation and increased CTT and NBD score in persons with SCI and suggested that clinicians may recommend a low-fibre diet to reduce CCT. In a recent systematic review, Yeung et al[52] concluded that consumption of about 15 g/d of dietary fibre could be beneficial in managing neurogenic bowel in SCI, whereas an increase in fibre intake from 25 g/d to 31 g/d increased CTT and evacuation time.
Increased stool consistency (looser stool with high transit time) was inversely related to microbiota diversity and positively associated with Bacteroidetes/Firmicutes ratio and increased CCT[46]. SCFA can increase levels of serotonin, triggering contraction of the smooth muscle in the gastrointestinal tract[46] and SCFA levels have been negatively correlated with CTT[53].
On the other hand, individuals with metabolic syndrome (Met-S) had reduced SCFA[54] and SFCA were positively correlated with adherence to the Med-D pattern[55]. Triglycerides (TG) in individuals with SCI were negatively correlated with Eubacterium_rectale, a butyrate-producing bacterial genus[37]. Dialister (decreased in SCI)[37,39] was negatively correlated with low density lipoprotein cholesterol and positively correlated with HDL.
Gungor et al[39] found that, compared to healthy controls, there was a preferential decrease in Marvinbryantia in the upper motor neuron group, whereas in the lower motor neuron group, Roseburia was decreased[39]. Gungor et al[39] suggested that although the identity of the genera that are dysregulated in both groups may differ, the reduction of butyrate (a SCFA) production would be expected to be similar. High fat diets decrease butyrate levels and can induce gut dysbiosis, dysmotility and constipation[46]. On the other hand, physically fit individuals had increased abundances of key butyrate-producing taxa, including Roseburia[43].
SCFA are also involved in gut-brain signalling implicated in the regulation of metabolic and immune homeostasis[53]. Sympathetic noradrenergic nerves innervate the vasculature and tissue parenchyma of the gut-associated lymphoid tissues[56]. Norepinephrine released by sympathetic post-ganglionic nerve terminals binds to beta (β)-adrenergic receptors on innate and adaptive immune cells leading to suppression of their antimicrobial functions[56].
In pre-clinical SCI models, intestinal epithelial cell permeability is increased and is associated with enhanced bacterial translocation and endotoxemia [increased lipopolysaccharide (LPS)][56]. Data from animal models suggest that gut dysbiosis, LPS and gut-associated lymphoid tissues[57] lead to the chronic low-grade inflammation associated with Met-S after SCI[53,56]. LPS elicits strong inflammatory responses by binding to extracellular toll-like receptor 4. In mouse studies, gut dysbiosis, free fatty acids, and the activation of toll-like receptor 4 are associated with enteric neuronal death, damaged function of the enteric nervous system and delayed transit time[46]. In this context, LPS was negatively associated with adherence to the Med-D[55] and cardiorespiratory fitness was negatively correlated with LPS[43]. Therefore, both exercise and diet could reduce endotoxemia and inflammation induced by dysbiosis in individuals with SCI.
DIETARY ADVICE AND MEDITERRANEAN DIET-BASED PYRAMID FOR INDIVIDUALS WITH SPINAL CORD INJURY
Individuals with SCI experience significant skeletal muscle atrophy mainly below the level of injury[58], which may contribute to decreases in resting energy expenditure[59]. On the other hand, the immune system is an energy consumer and during chronic inflammation re-allocation of energy-rich fuels to the activated immune system, called "energy appeal reaction", can lead to inadequate energy regulation, muscle protein breakdown, low vitamin D levels, cachectic obesity, insulin resistance, dyslipidaemia, Met-S and inflammation-related anaemia and osteopenia[60,61].
The SCI-SCREEN, a nutritional screening model developed in a neurorehabilitation unit, incorporated the Lagerström’s proposed body mass index (BMI) cut-offs for SCI patients, differentiating between individuals with paraplegia (estimated muscle mass reduction of 7.5%) and those with tetraplegia (estimated muscle mass reduction of 12.5%)[62]. On the other hand, the “Guidelines for Identification and Management of Cardiometabolic Risk after Spinal Cord Injury” of the Consortium for Spinal Cord Medicine expert panel suggested definitions of obesity as: > 22% of fat mass (FM) or BMI ≥ 22 kg/m2, given that waist circumference is not a validated proxy for obesity in SCI[63].
Recommendations for energy intake in individuals with SCI in the subacute and community living phases were 22.7 kcal/kg and 27.9 kcal/kg of body mass (BM) for individuals with tetraplegia and paraplegia, respectively[50]. Recommendations for protein intakes did not differ between individuals with tetraplegia and paraplegia, amounting to 0.8-1 g/kg (of BM)[50]. Higher energy and protein recommendations were suggested for individuals with pressure ulcers[50] and for Paralympic Athletes[64]. Wheelchair athletes have been reported to have low energy availability, low protein consumption and CHO intakes near the lower limit of the recommended range (3.0-12.0 g/kg)[65]. This suggests that wheelchair athletes could be at risk of post-exercise ketosis. The latter (about 0.5-1.0 mM in response to 2 h of exercise performed in an overnight fasted state) depends on the intensity and duration of the exercise performed, as well as on the nutritional status and on the ketone body uptake by skeletal muscle, which are able to extract about 50% of circulating ketone bodies when concentrations are low (0.1-0.5 mM)[66]. In a case report, a 36-year-old man, wheelchair bound secondary to genetically confirmed spinal muscular atrophy, presented to an emergency department with epigastric pain and vomiting due to ketoacidosis, and reported decreased food intake in the preceding week in an attempt to lose weight, with a diet consisting mainly of proteins and vegetables with minimal CHO in the 48 h prior to his presentation[67]. The authors suggested that the severity of the ketosis, which seemed inconsistent with moderate starvation alone, could be due to other factors, including low muscle mass[67].
On the other hand, the sublesional muscle atrophy with SCI significantly reduces the primary storage site for CHO, promoting increased circulating GLU and risk of type 2 diabetes[14]. The reduction in fat free mass (FFM) and the increase in FM are key features in people with SCI that could increase the risk for CMS[14]. In an environment of reduced mobility, energy needs are reduced, and there is an increased risk of obesity-related chronic diseases, making energy balance a critical and fundamental issue in SCI[68]. The spinal nutrition screening tool has been validated for patients with SCI to assess the malnutrition risk[9]. The spinal nutrition screening tool assesses eight criteria: History of recent weight loss, BMI, age, level of SCI, presence of com-orbidities, skin condition, appetite and ability to eat[9]. These authors reported that patients with paraplegia were less likely to be malnourished (33.7%) than those with tetraplegia (66.1%) and readmitted patients had a lower incidence of under-nutrition (41.6%) than newly injured patients (50%)[9]. On the other hand, a 67% of individuals with SCI were at potential risk of over-nutrition (BMI > 22 kg/m2)[9]. It has been reported that the components of CMS (abdominal obesity, hypertension, insulin resistance, and dyslipidaemia) are not equally weighted; sarcopenic obesity is a highly prevalent finding after SCI and appears to be the most relevant risk factor, followed by insulin resistance[63].
Among the dietary strategies there is the “adaptability of the diabetes prevention program” (DPP) for SCI, consisting of a 24-wk energy-restricted Med-D[14]. Preliminary data in men with chronic SCI who were obese and pre-diabetic, showed that a therapeutic lifestyle intervention including diet, exercise, patient education and professional support, resulted in BM reduction that exceeded the DPP criterion (7%). The intervention also demonstrated improvements in insulin resistance and lipid profile (HDL increase and TG decrease)[14]. A systematic review of dietary interventions in adults with SCI emphasized the potential of diet in conjunction with exercise in minimizing CVD risk[69], whereas the “Consortium for Spinal Cord Medicine” noted success in weight loss using the Med-D in the DPP[63]. Med-D has been suggested for Veterans with disabilities[70], patients with chronic kidney disease (CKD)[71] and individuals with chronic pain[72]. Furthermore, Allison et al[73], recently reported that in individuals with SCI, a 3-mo anti-inflammatory diet similar to the Med-D plus supplementation decreased fat intake and proinflammatory dietary components (trans fatty acids, caffeine and sodium) and increased protein intake and some nutrients with established anti-inflammatory properties, including vitamins A, C, and E, and omega (ω)-3 polyunsaturated fatty acids (PUFA), with no change in CHO or energy intake[73]. Significant reductions in the proinflammatory cytokines interferon-γ, interleukin-1β, and interleukin-6 were observed and several proinflammatory mediators were negatively correlated with anti-inflammatory nutrients, including vitamin A, carotenoids, ω-3 PUFA, and zinc, whereas the change in total calories as well as individual macronutrients were not shown to be significantly correlated with any inflammatory mediator[73]. Obesity in SCI results in a state of chronic low-grade inflammation primarily due to proinflammatory adipokines secreted from excess adipose tissue[7]. Accordingly, individuals with chronic motor complete SCI, severe lower extremity spasticity and lower FM had lower leptin and fasting GLU than patients with SCI with no or mild spasticity[74]. On the other hand, no differences in bone mineral density (BMD), low density lipoprotein cholesterol, HDL, TG and glycosylated haemoglobin were observed between the two groups[74]. Although it has been reported that protein intake is negatively associated with BMD of lumbar vertebrae in women with SCI[75], in a cross-sectional study, no significant relationships were found between BMD and intakes of protein, calcium, vitamins D and or serum 25(OH)D in individuals with chronic SCI (94% male, lesions from C1 to T12). However, significant associations were found between BMD at the femoral neck and lumbar spine with visceral adipose tissue, insulin and leptin[76]. Leptin enhances both arterial and venous thrombosis by promoting platelet adhesion, activation and aggregation[77] and it has been recently observed that some Paralympic athletes with SCI had a higher platelet-derived CVD risk rather than CMS risk[78]. Caffeine can increase platelet aggregation, which may be associated with an elevated risk of thrombosis[79]. Conversely, among natural dietary compounds and functional foods of the Med-D with antiplatelet activities (including ω-3 PUFA, olive oil, garlic, onions and tomatoes)[80], O'Kennedy et al[81] reported that a water-soluble tomato extract, having antiplatelet anti-angiotensin-converting enzyme and anti-inflammatory activities, became the first product in Europe to obtain an approved health claim[82,83].
Vitamins A, B5, B7, B9, D, E, potassium, and calcium are deficient compared to the USDA guidelines in individuals with SCI[10]. Many of these micronutrients are linked to CHO, lipid, and/or vascular dysfunction, whereas recommendations for pressure ulcer management suggest evaluation for vitamins A and C, zinc and iron[50].
It has been reported that level and completeness of lesion, injury duration, mobility, FM%, time spent outdoors and comorbidities were not associated with plasma 25(OH)D, whereas in a multivariable model (adjusting for age, planned exercise, sex, race, wine consumption, and smoking status) supplement (but not dietary) intake was significantly associated with increased 25(OH)D[84]. Similarly, in a univariable model, stretching, range of motion, or physical therapy was not associated with higher 25(OH)D level, whereas other planned exercise was associated with higher 25(OH)D levels[84].
A systematic review emphasized the potential of diet in conjunction with exercise in minimizing CVD risk in SCI[69], whereas the Consortium for Spinal Cord Medicine did not recommend a single nutritional intervention but noted success in weight loss using the Med-D in the DPP[63]. Med-D has been suggested for Veterans with disability[70], patients with CKD[71] and individuals with chronic pain[72].
In a recent meta-analysis, 86% and 43%-74% of individuals with chronic SCI had excessive intake of CHO, and 43%-74% had excessive intake of proteins[10]. These authors[10] also reported a mean of 57 kcal/d from alcohol and pointed out that participants were also likely to underreport their true alcohol consumption given its effects on weight and health and the stigma that is often associated with alcohol consumption. In a study that included only overweight and obese individuals (BMI > 22 kg/m2, mean 30.5 ± 6.33 kg/m2 with a range from 22.44 kg/m2 to 49.91 kg/m2) with SCI (50% with paraplegia and 50% with tetraplegia), although participants’ intakes were within the recommended range of amounts for all macronutrients, they were on the low end of the recommended range for protein and on the high end for total fat[11]. Furthermore, individuals with SCI report excess intake of added sugars, sodium and saturated fat and inadequate intake of healthy fats, seafood, plant protein, fruits, vegetables and whole grains, considering the 2015-2020 Dietary Guidelines for Americans[11]. Yeung et al[52] concluded that further studies are required because it is unclear from the available studies the extent to which confounding factors, such as age, gender, physical activity, level of injury, time since SCI and comorbidities affect outcomes. The prevalence of polypharmacy is expected to increase with age and concurrent urological management must be considered in individuals with SCI.
A cross-sectional analysis[85] showed that over 1 out of 3 Veterans with SCI had CKD and in a 14-year retrospective cohort study[86], individuals with SCI and CKD had a significantly shorter survival time (10.13 mo vs 10.97 mo), higher 1-year mortality (17.65% vs 8.54%), and higher risk of mortality than those with SCI but without CKD (adjusted hazard ratio, 2.25). It has been reported that the overall prevalence of CKD was 8.0% and 22.4%, by serum creatinine-based and cystatin-C-based estimated glomerular filtration rate, respectively, and was greater in individuals with neurogenic bladder (NB)[87]. However, serum creatinine was not able to detect the early deterioration of renal function in NB patients because they present muscle wasting due to disuse and/or denervation[87]. Comorbid diabetes, small bladder volume, recurrent urinary tract infection (UTI) and proteinuria were significantly associated with CKD in the multivariable analysis[87]. Key findings from the Committee of the “Joint SIU-ICUD Consultation on Urologic Management of the Spinal Cord Injured Patient” include: (1) Renal function deterioration can occur early but can also occur at later stages following SCI, and can be related to ageing, obstruction, stone disease (due to calcium mobilization from bones, reduced mobility and UTI, with urea-splitting organisms); (2) SCI patients have higher rates of prescribed medications from multiple high-risk classes (analgesic–narcotics, anticonvulsants, antidepressants and skeletal muscle relaxants); (3) Osteoporosis and complete sensory or motor injuries are associated with higher risk of fractures; and (4) Clinicians should screen SCI patients for malnutrition and obesity[88]. Patients with SCI most frequently used products to treat pain (68%), constipation (42%), muscle spasm (42%), hypertension (42%), and depression (37%). When including natural health products, vitamins and minerals, polypharmacy was present in 74% of patients with SCI[88]. In particular, NB can be observed in individuals with SCI treated with drugs metabolized by CYP3A4 (oxybutynin, solifenacin and darifenacin)[89]. Therefore, caution has been recommended when simultaneously consuming grapefruit juice during treatment with these drugs[89]. Other citrus fruit juices, cruciferous vegetables (broccoli, cabbage, and daikon radish sprouts), soy foods (soy milk, veggies slices, tofu, and roasted soy nuts), tea and cranberry and pomegranate juices can induce adverse food-drug interactions and should be avoided in individuals in treatment for comorbidity[70,90]. Individuals with SCI are at increased risk of developing symptomatic UTI[91]. The use of cranberries (particularly juice) is widely recommended to prevent and treat UTI[92], although evidence related to the prevention of UTI are inconsistent[93]. On the other hand, the European Food Safety Authority Panel on Food Additives and Nutrient Sources Added to Food, considered the possible association between the consumption of (-)-epigallocatechin-3-gallate (EGCG, the most relevant catechin in green tea) and hepatotoxicity[94]. Catechins from green tea infusion (prepared in a traditional way and reconstituted drinks with an equivalent composition to traditional green tea infusions: EGCG from 90 mg to 300 mg), are in general considered to be safe[94]. However, doses equal or above 800 mg EGCG/d have been shown to induce a significant increase of serum transaminases[94]. Furthermore, human studies have reported that consumption of polyphenol-enriched oolong tea (750 mL for 10 d)[95] or a beverage containing black tea polyphenols (55 mg, 3 times/d for 10 d)[96] increased faecal lipid excretion. Catechins are among the antinutrient antioxidants that can have pharmacological effects[97], including the inhibition of lipase[98]. The pharmacological inhibition of lipase was associated with the excretion of the inflammatory marker faecal calprotectin in healthy individuals[99]. Spices to flavour dishes and other comfort food and beverages (sweet, cocoa, coffee, tea) should be limited and alcoholic drinks should be avoided by individuals with SCI[100], due to the potential interactions of phytochemicals and alcohol with drugs and/or the effect on bowel motility, water and energy balances.
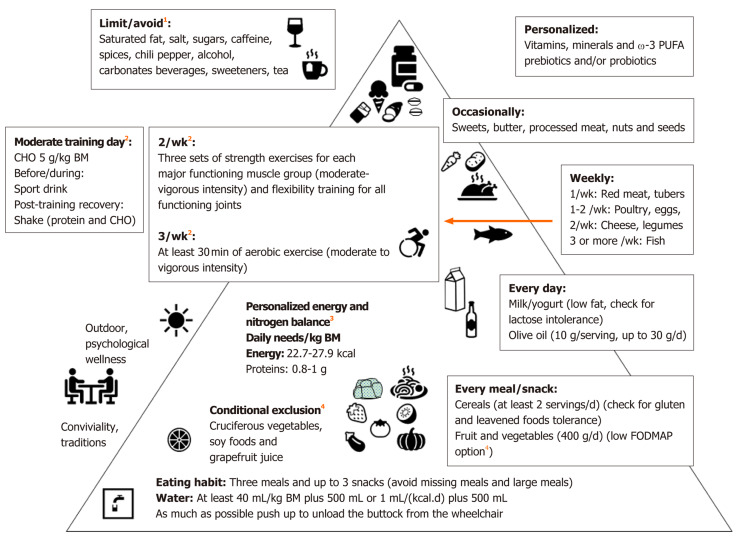
Some foods and beverages, including alcohol, caffeine, tea, coffee, cola and chocolate, prunes, figs and sorbitol containing foods, can overstimulate bowel activity or draw excessive fluid into the colon resulting in very watery stools[36]. On the other hand, individuals with SCI and NBD often have an increase in CCT, resulting in excessive fluid reabsorption and the formation of hardened stools[50]. Therefore, the recommendations for fluid intake are 1/ml fluid per kcal of estimated energy needs plus 500 mL or 40 mL per kg BM plus 500 mL[50] (Figure 1).
Figure 1.
Mediterranean diet based and exercise pyramid for individuals with spinal cord injury. Dietary advice from the previous indications for veterans[70], individuals with spinal cord injury with or without neurogenic bowel dysfunction[14,36,50,63,69,73], chronic kidney disease[71], chronic pain[72] and irritable bowel syndrome[18]. 1Limit/avoid: Potential undeliverable effects[11,36,79,94-96]; 2Exercise recommendations, derived from the integration of two recent papers[101,102] devoted to adults with spinal cord injury, are aimed to improve muscle strength, cardiorespiratory fitness, flexibility and cardiometabolic health. As a general rule, the exercise intensity should progress with time from moderate to vigorous at a rate (weeks-months) dependent from the initial level of fitness of the individual. However, personalized training is deliverable associated with sport-type-targeted nutritional recommendations (including timing and supplementation) for Paralympic athletes[64]; 3Personalized energy and nitrogen balance[50,64]: Ideal body mass 10%-15% and 5%-10% lower that for quadriplegia and paraplegia, respectively; Energy: 22.7 kcal/(kg·d) and 27.9 kcal/(kg·d) for quadriplegia and paraplegia, respectively and 30-40 kcal/(kg·d) in the presence of pressure ulcers; Proteins: Stage II pressure ulcers: 1.2-1.5 g/(kg·d), Stage III and IV pressure ulcers: 1.5-2.0 g/(kg·d); Paralympic athletes: 1.2-1.7 g/(kg·d); 4Low fermentable oligosaccharides, disaccharides, monosaccharides, and polyols: Banana, blueberry, strawberry, grape, melon, kiwi, cucumber, eggplant, green beans, lettuce, spinach, chives, pumpkin, tomato, zucchini. Cranberry juice may be associated with reduced urinary tract infection but could induce food-drug interactions, which can be observed also with other foods and beverages (conditional exclusion)[70,90]. BM: Body mass; w-3 PUFA: Omega-3 polyunsaturated fatty acids; CHO: Carbohydrates; FODMAP: Fermentable oligosaccharides, disaccharides, monosaccharides, and polyols.
For all the above-mentioned reasons, we propose a Med-D-based and exercise pyramid for individuals with SCI (Figure 1). This should be integrated with the previous indications for veterans[70], the available recommendations/suggestions for individuals with SCI with or without NBD[14,36,50,63,69,73], CKD[71], chronic pain[72] and IBS (including low FODMAP)[18], and the exercise guidelines for adults with SCI[101], along with the position statement on exercise and SCI of Exercise and sports science Australia[102]. This approach is likely to be effective in improving muscle strength, flexibility and cardiorespiratory fitness, and to obtain cardiometabolic benefits.
DIETARY ADVICE FOR PARALYMPIC ATHLETES
Applying sports nutrition plans is essential for athletes with an impairment and represents an aid when sport and exercise are practiced[103]. This is important as a component for both therapy and rehabilitation[104] in particular for individuals with SCI[101,105,106]. Paralympic athletes exhibit higher quality of life and health status associated with their opportunities to practice physical activities and to participate in sport competitions[107], and these opportunities increased considerably in recent decades[104,108]. Their levels of performance and physical fitness are increased vs. non-athletes with SCI, and many Paralympic athletes are now engaged in intense training sessions that require highly demanding nutritional needs. For example, Gerrish et al[109] reported mean energy intakes of elite Canadian and American athletes with SCI, categorized by level of injury that were above the recommendations for sedentary individuals with SCI [22.7 kcal/(kg·d) in tetraplegia and 27.9 kcal/(kg·d) in paraplegia, see Figure 1][50]. These Authors, indeed, found the following energy intakes among athletes with SCI: 26 ± 7.9 kcal/(kg·d), 28 ± 14 kcal/(kg·d), 36 ± 14 kcal/(kg·d)and 36 ± 16 kcal/(kg·d) for athletes with levels cervical, from T1 to T6, from T7 to T12 and lumbar, respectively[109].
The first step in providing nutritional advice for both health purposes and sports performance is to estimate daily energy requirements, an estimate that is often difficult to assess in Paralympic athletes. Grams et al[110], in wheelchair basketball athletes, calculated energy expenditures using values provided by Collins et al[111], Abel et al[112] and Bernardi et al[113], and found a high variability in energy intake relative to BM [ranging from 25 kcal/(kg·d) to 64 kcal/(kg·d)].
Existing equations that estimate energy expenditure at rest are based on able-bodied populations and include variables that are difficult to measure accurately in athletes with SCI, such as height or total BM[64]. The approach of Collins and collaborators is preferred in this context[111]. The daily energy requirement should be estimated by measuring the energy expenditure in basal conditions[111] and during actual sport activities[113] and training[114]. Simulations of training and competitions should be assessed on the field[114] and physiological profile of athletes determined in the laboratory[115], testing athletes repetitively to evaluate the effects of training[116]. In order to have a complete energy expenditure assessment, all other activities performed during the day which cannot be actually measured should be taken into account converting the data using at least three-days of activity. Various questionnaires for energy expenditure can be used, including Ainsworth’s tables[117], or by using accelerometers, the Physical Activity Scale for Individuals with Physical Disabilities and the Veterans Administration Physical Activity Questionnaire modified for SCI[118].
Paralympic athlete's diet must be appropriate for both the physical activity performed and the characteristics of the individual’s body composition, monitored over time. Assessment of body composition of athletes with impairments is therefore crucial to evaluate the general health status and monitor adaptation to diet and training[119,120], but in individuals with a great loss of metabolically active tissue and an asymmetric distribution of fat above and below the spinal lesion, this assessment may not be accurate[103] and can make it difficult to identify small to moderate changes in physique traits[65].
In athletes with SCI, as already pointed out in sedentary individuals with SCI, a reduction in resting energy expenditure, which depends on the level of the spinal lesion and the consequent loss of actively controllable musculature, is often observed[111]. Male and female athletes with SCI generally have lower energy requirements compared to able-bodied athletes. Despite lower energy needs, Paralympic athletes may still be consuming too few calories[65,121,122]. Aside from taking into account the previously mentioned issues and respecting an appropriate energy balance[114], in general dietary recommendations for athletes with impairments should not differ from those for able-bodied athletes[64].
Depending on the type of sport practiced, i.e., skill sports, power sports, intermittent (aerobic and anaerobic alternate metabolism) sports and endurance sports[123], because energy expenditure can vary widely[113], food intake and in particular energy requirements need to be tailored precisely to optimize performance. In particular, in endurance sports, such as Nordic sitting skiing, which requires a high energy expenditure, the competition strategy is similar, in spite of the long race duration (45-50 min) to that of an "all-out" exercise in which the highest speed is maintained from the start to the end[124]. Indeed, successful athletes need not only possess a high aerobic power but also a very high glycolytic capacity[125]. Diet and consequently GLU stores (glycogen) can become a major performance limiting factor of these competitive performances. An adequate CHO intake is essential to maintain training intensity, combat fatigue, protect immune function, sustain training adaptations and provide a key fuel for the brain and central nervous system[126-128]. Although some Paralympic athletes (such as Nordic sitting skiers) had mean FM% ranging from 14.1 (best performers) to 15.1 (others), athletes competing in power and intermittent sports, had FM% (20.9 in Alpine sitting skiers and 20.9 in Para Ice Hockey players)[125] similar to those of non-athletic able-bodied men[129]. In male individuals with a normal BMI (18.5-24.9 kg/m2) and age between 20 and 79 years, Shea et al[129] reported that both medium (FM = 15.3%-20.7%) and high (FM ≥ 20.8%) percentages of FM groups, had a significantly greater proportion of metabolically abnormal phenotypes (12.7% and 26.6% respectively), compared to individuals with low (FM ≤ 15.2%) percentage of FM, in whom this prevalence was equal to 6.2%. From that, a possible low total FFM can expose Paralympic athletes with SCI to a risk of both post-exercise ketosis (due to low ketone bodies uptake by skeletal muscles)[66,67] and type 2 diabetes (due to reduction of muscle CHO storage)[14]. Therefore, in endurance and high energy expenditure intermittent sports, CHO in Paralympic athletes with SCI is an energy source more important than in able-bodied athletes. From the above-mentioned reasons, CHO with low glycaemic index (i.e., Med-d cereals) should be preferred in the habitual diet. Adequate intake of sugars before and during exercise and sport should compensate for the low muscle glycogen stores (upper suggested range), whereas after exercise, GLU needs should be lower (lower suggested range) than able-bodied athletes. Before competition, boiled potatoes, which have a glycaemic index (78 ± 4) comparable to those of white bread (75 ± 2) and white rice boiled (73 ± 4) and higher than white spaghetti (49 ± 2)[130], could be a favourable GLU source. We therefore summarize the ACSM nutritional guidelines for able-bodied athletes[131] related to the CHO intake in table 1 that indicates the quantities (ranges) and timing of intake to provide high CHO availability for designated training or competition sessions according to athlete's BM and session characteristics. Unlike the Position statement of the International Society of Sports Nutrition regarding the timing of macronutrients in highly trained individuals, CHO re-feeding [1.2 g/(kg·h)] with a preference towards CHO sources that have a high (> 70) glycaemic index for rapid restoration of glycogen is required (< 4 h of recovery time)[132] must be evaluated on the light of possible co-morbidity in SCI[85,87,133].
Paralympic athletes’ dietary protein intake necessary to support metabolic adaptations, repair, remodelling, and for protein turnover, generally ranges from 1.2 g/(kg·d) to 1.7 g/(kg·d)[64] (Table 1). In cases of energy restriction, sudden inactivity as occurs as a result of injury, or in athletes competing in weight lifting sports (where classes depends on BM range), elevated protein intakes typically range from 2.0 g/(kg·d) to 2.3 g/(kg·d)[64,126,131] for a limited period of time (2 wk), may be advantageous in preventing FFM loss[134,135]. Mettler et al[135], after a week of habitual diet (week 1, run-in) and a week of isocaloric diet with a macronutrient energy intake composition of 50% carbohydrate, 15% protein, and 35% fat (week 2), compared two energy-restricted diets: one with 15% protein [about 1 g/kg, control group (CP)] and one with 35% protein [about 2.3 g/kg, high-protein group (HP)] for 2 wk (weeks 3 and 4). Decreases in BM (-3.0 ± 0.4 kg and -1.5 ± 0.3 kg for the CP and HP, respectively) and lean BM (-1.6 ± 0.3 kg and -0.3 ± 0.3 kg) were larger in the CP compared to those in the HP and similar FM loss were observed[135]. However, urea was higher in the HP group (8.0 ± 0.4 mmol/L and 7.9 ± 0.5 mmol/L in week 3 and 4, respectively) compared to the CP group (5.3 ± 0.4 mmol/L and 5.4 ± 0.5 mmol/L in week 3 and 4, respectively)[135]. The authors concluded that because body composition data indicate that there was no protein accretion but still a slight loss of lean BM, some of the proteins must have been metabolized, causing increased urea values[135]. Considering the risk of CKD typical among individuals with SCI[85,87], protein intakes over 2.0 g/(kg·d) should not be recommended in Paralympic athletes with SCI, even for limited period of time.
Table 1.
| Nutrients | Food sources | Quantity | Timing |
| Carbohydrate | Pasta, rice, cereals, breads, legumes, potatoes, fruit, sugar | Light: Low intensity or skill-based activities: 3-5 g/(kg·d); Moderate: Exercise program (1 h/d): 5-7 g/(kg·d); High: Endurance program (1-3 h/d moderate to high-intensity exercise): 6-10 g/(kg·d); Very high: Extreme commitment (> 4-5 h/d moderate to high-intensity exercise): 8-12 g/(kg·d) | Before or during the exercise session, during recovery from a previous session; extended (> 60 min) bouts of high intensity (> 70% VO2max): CHO at a rate of about 30-60 g of CHO/h in a 6%-8% CHO electrolyte solution (6-12 fluid ounces) every 10-15 min throughout the entire exercise bout |
| Proteins | Beef, fish, poultry, eggs, and dairy products (high biological value sources), legumes | From 1.2 g/(kg·d) to 1.7 g/(kg·d) | After exercise, co-ingestion of CHO with a high-protein recovery snack is recommended to help restore muscle glycogen effectively; Post-exercise ingestion (immediately to 2-h post) of high-quality protein sources stimulates robust increases in muscle protein synthesis; Protein-rich foods/fluids (such as milk or cheese) before bed promotes muscle protein synthesis overnight |
CHO: Carbohydrate; VO2max: Maximum oxygen uptake; >: Major.
Timing is also important for proteins (Table 1). For example, co-ingestion of CHO with protein during the recovery period resulted in improved net protein balance post-exercise. Ingesting protein (approximately 20 g to 30 g total protein, or approximately 10 g essential amino acids, especially from high biological value sources, like beef, fish, poultry, eggs, and dairy products[64] during exercise or the recovery period (post-exercise) led to increased whole body and muscle protein synthesis as well as improved nitrogen balance[136,137].
The IOS 2018[136,137] stated that protein supplements, usually low in CHO and providing 20-50 g protein per serving from high quality sources (whey, casein, milk, egg) or vegetables (e.g., soy), may contain other ingredients, some of which are not evidence-based and may increase the risk of contamination. For these reasons, protein supplements should be taken with caution.
All athletes with SCI should be encouraged to hydrate adequately (2-2.5 L/d unless other indications)[138]. Indeed, athletes with SCI are sensitive to hydration problems, in particular wheelchair-bound athletes tend to reduce their intake of liquids to avoid the complexity associated with toilet hygiene[139]. Moreover, thermoregulatory function may be impaired in Paralympic athletes and individuals with SCI[6,107,140]. In hot conditions poor thermoregulation due to impaired sweat rate can lead to overheating which can be treated by hand cooling, foot cooling, ice vests and spray bottles[102,141,142]. The fluid plan that suits most able-bodied athletes and athletic events consist of an intake of 0.4 L/h to 0.8 L/h[131], although this should be customized to the athlete’s tolerance, thermoregulatory function, and opportunities for drinking fluids. Monitoring changes in BM (before and after exercise) is an easy method to evaluate fluid needs[103,139]. Electrolyte replacement supplements (50-60 mM sodium, 10-20 mM potassium and low CHO 2-4 g/100 mL) can be used for rapid rehydration following large sodium losses during ultraendurance activities, and sport drinks (containing 5%-8% CHO, 10-35 mM sodium, 3-5 mM potassium) during exercise has been identified to be useful for post-exercise rehydration and CHO refuelling by the IOS 2018[136,137]. Although caffeine is among the few supplements (including buffering agents and nitrate) that have been shown to favourably affect performance[136,137], energy drinks containing caffeine should be avoided by athletes with SCI and NBD[36] (Figure 1).
Nitrates are popular supplements for prolonged submaximal exercise and high-intensity, intermittent, short-duration efforts. Increasing nitric oxide and enhances muscle blood flow, but potential risks for gastrointestinal upset in susceptible athletes have been suggested[136,137]. High nitrate-containing foods include leafy green and root vegetables, including spinach, rocket salad, celery, and beetroot[136,137]. It has been suggested that beetroot juice (among functional beverages) may improve performance[143,144]. However, the positive effects of beetroot juice seen in young individuals were not observed in older adults[145]. In a recent randomized (placebo-controlled) cross-over study in upper body trained able-bodied individuals and Para-cyclists with SCI (lesion level between C4 and L4), Para-cyclists showed higher nitrate concentrations after beetroot juice, whereas no differences were found in performance in either group[146]. Although a meta-analysis reported nitrate-independent blood pressure lowering effects of beetroot juice[147] and we found only a case report of interaction with methotrexate[148], supplementation in patients with in CKD requires further studies[149]. Furthermore, caution with chronic use of beetroot juice to enhance sports performances has been suggested due to increases in carcinogenic N-nitroso compounds in urine after consumption[150].
In general, an analysis of a Paralympic athlete’s sweat loss and dietary intakes is recommended to evaluate fluids, carbohydrate, protein, iron, and vitamin D status to detect nutrient insufficiencies that greatly impact athletic performance[64]. Indeed, Paralympic athletes often fail to meet the recommended dietary allowances for vitamin D, vitamin E, pantothenic acid, magnesium, potassium, iron (females), calcium (females), vitamin A (males) and folate (males)[151]. Many Paralympic athletes are at higher risk of low bone density and osteoporosis; therefore, it is important to optimize all the nutrients and factors that support bone health. Calcium-rich foods such as dairy products, fish with soft bones, and calcium-fortified foods should be encouraged, as athletes with impairments are often found to consume insufficient calcium[64]. But since Paralympic athletes are also at high risk of being deficient in vitamin D due to the nature of their health conditions or inadequate micronutrient intakes, and vitamin D can impact morphology and functionality of human skeletal muscles, testing for vitamin D status is highly recommended. In addition, promotion of safe exposure to the sunlight and vitamin D–rich food sources, such as oily fish and eggs is recommended[64]. In addition, supplementation should be provided in cases of deficiency.
Commonly consumed supplements by Paralympic athletes are vitamin D, protein powder, sport bars, and sport drinks[151,152]. In a recent study, elite athletes with SCI improved handgrip strength after a supplementation protocol based on initial 25(OH)D concentrations, whereas no change in 20-m wheelchair sprint performance was observed[153]. Vitamin D is among the nutraceuticals with moderate evidence of efficacy for immune health in athletes, along with vitamin C and probiotics[136,137]. However, according to the “IOC Consensus Statement: Dietary Supplements and the High-Performance Athlete” (IOC 2018) careful monitoring is necessary to avoid its toxicity[136,137].
The dietary recommendations, in addition to taking into account the requirements related to training and sport type, require careful consideration of the specific characteristics and physiopathology of the athlete to accommodate the unique issues of individual athletes regarding health status, nutrient needs, performance goals, physique characteristics (i.e., age, gender, stage of development, body composition), practical challenges, food preferences, and environmental conditions. Similar to able-bodied athletes there is no "one size fits all" approach. Nutrition plans need to be personalized to the individual athlete to take into account the nature of the health condition and the consequent impairment and its impact on functional capacity, the use of medications, and any coexisting medical conditions and responses to various strategies. Special nutrition recommendations are needed since athletes with impairments and in particular those with SCI are at major risk of medical complications, including low bone density and osteoporosis, epithelial wound and pressure ulcers, urolithiasis and UTI, and chronic constipation.
DISCUSSION
Bowel function, as codified by the International Classification of Functioning, Disability and Health is frequently compromised after SCI. Among International Classification of Functioning, Disability and Health ICF domains, the greatest impact of NBD is on personal and environmental factors, with 45.3% reporting need of assistance, 45.3% in emotional health and 46.9% in loss of privacy[154].
Relationships among inflammation, fatigue pain and behaviour aspects have been discussed[155]. Although a 3-mo anti-inflammatory diet with increased intake of vitamins A, C, and E, and ω-3 PUFA and reductions in trans fatty acids, caffeine and sodium, reduced chronic inflammation in individuals with SCI[73], barriers to adhering to this diet have been reported[156]. Among the reasons for low compliance are the reported increase in NBD Score after high fibre intake[51,52]. On the other hand, exercise training, reducing constipation and restoring eubiosis, can improve quality of life in individuals with SCI and NBD. Moreover, exercise improved semen quality[106] and upper limb aerobic training increased aerobic fitness[116] and reduced inflammatory cytokines[8] and oxidative stress[105]. Different sport activities have different effects on physical fitness components[113,125]. In the “Veterans Exercise Testing Study”, Myers et al[157] demonstrated that fitness is inversely related to overall health care costs among veterans.
Paralympic athletes, like individuals with SCI, had low diet quality, in terms of fruits, vegetables, legumes and cereals and there is a need for nutrition education for this population[158]. Due to a reported low nutritional knowledge[159], personalized nutrition and education related to different macro and micronutrients requirements compared to able-bodied athletes is recommended. Furthermore, mixed (outdoors and indoors) training programs[160] could improve vitamin D status[161]. Concerning sport-related muscle pain[162], we included in our Med-D-based and exercise pyramid (Figure 1) the suggestions from the Med-D for individuals with chronic pain[72].
CONCLUSION
The present review suggests dietary advice for individuals with SCI and NBD and underscores that multidisciplinary risk reduction programs, including dietary advice[114] and exercise[118], are recommended, as depicted in the Med-D-based and exercise pyramid for individuals with SCI (Figure 1). Finally, we agree with the recently suggested systematic control needed to re-adapt nutritional programs for wheelchair athletes[163].
Footnotes
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
Conflict-of-interest statement: There is no conflict of interest associated with any of the authors contributed their efforts in this manuscript.
Peer-review started: December 31, 2019
First decision: March 26, 2020
Article in press: May 13, 2020
P-Reviewer: Cerwenka H, Chiu KW, Niu ZS, Slomiany BL S-Editor: Yan JP L-Editor: A E-Editor: Zhang YL
Contributor Information
Marco Bernardi, Department of Physiology and Pharmacology "V. Erspamer", Sapienza University of Rome, Rome 00185, Italy; Italian Paralympic Committee, Rome 00191, Italy; Federazione Italiana Pallacanestro In Carrozzina (FIPIC), Rome 00188, Italy.
Anna Lucia Fedullo, Federazione Italiana Pallacanestro In Carrozzina (FIPIC), Rome 00188, Italy.
Elisabetta Bernardi, Department of Biosciences, Biotechnologies and Biopharmaceutics, University of Bari "Aldo Moro", Bari 70121, Italy.
Diego Munzi, Joint Veteran Center, Scientific Department, Army Medical Center, Rome 00184, Italy.
Ilaria Peluso, Research Centre for Food and Nutrition, Council for Agricultural Research and Economics (CREA-AN), Rome 00178, Italy. ilaria.peluso@crea.gov.it.
Jonathan Myers, VA Palo Alto Health Care System and Stanford University, Cardiology Division, Palo Alto, CA 94025, United States.
Florigio Romano Lista, Scientific Department, Army Medical Center, Rome 00184, Italy.
Tommaso Sciarra, Joint Veteran Center, Scientific Department, Army Medical Center, Rome 00184, Italy.
References
- 1.Kirshblum SC, Burns SP, Biering-Sorensen F, Donovan W, Graves DE, Jha A, Johansen M, Jones L, Krassioukov A, Mulcahey MJ, Schmidt-Read M, Waring W. International standards for neurological classification of spinal cord injury (revised 2011) J Spinal Cord Med. 2011;34:535–546. doi: 10.1179/204577211X13207446293695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burns S, Biering-Sørensen F, Donovan W, Graves DE, Jha A, Johansen M, Jones L, Krassioukov A, Kirshblum S, Mulcahey MJ, Read MS, Waring W. International standards for neurological classification of spinal cord injury, revised 2011. Top Spinal Cord Inj Rehabil. 2012;18:85–99. doi: 10.1310/sci1801-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kirshblum S, Waring W., 3rd Updates for the International Standards for Neurological Classification of Spinal Cord Injury. Phys Med Rehabil Clin N Am. 2014;25:505–517, vii. doi: 10.1016/j.pmr.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 4.ASIA and ISCoS International Standards Committee. The 2019 revision of the International Standards for Neurological Classification of Spinal Cord Injury (ISNCSCI)-What's new? Spinal Cord. 2019;57:815–817. doi: 10.1038/s41393-019-0350-9. [DOI] [PubMed] [Google Scholar]
- 5.Schuld C, Franz S, Brüggemann K, Heutehaus L, Weidner N, Kirshblum SC, Rupp R EMSCI study group. International standards for neurological classification of spinal cord injury: impact of the revised worksheet (revision 02/13) on classification performance. J Spinal Cord Med. 2016;39:504–512. doi: 10.1080/10790268.2016.1180831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hou S, Rabchevsky AG. Autonomic consequences of spinal cord injury. Compr Physiol. 2014;4:1419–1453. doi: 10.1002/cphy.c130045. [DOI] [PubMed] [Google Scholar]
- 7.Farkas GJ, Gater DR. Neurogenic obesity and systemic inflammation following spinal cord injury: A review. J Spinal Cord Med. 2018;41:378–387. doi: 10.1080/10790268.2017.1357104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosety-Rodriguez M, Camacho A, Rosety I, Fornieles G, Rosety MA, Diaz AJ, Bernardi M, Rosety M, Ordonez FJ. Low-grade systemic inflammation and leptin levels were improved by arm cranking exercise in adults with chronic spinal cord injury. Arch Phys Med Rehabil. 2014;95:297–302. doi: 10.1016/j.apmr.2013.08.246. [DOI] [PubMed] [Google Scholar]
- 9.Wong S, Derry F, Jamous A, Hirani SP, Grimble G, Forbes A. Validation of the spinal nutrition screening tool (SNST) in patients with spinal cord injuries (SCI): result from a multicentre study. Eur J Clin Nutr. 2012;66:382–387. doi: 10.1038/ejcn.2011.209. [DOI] [PubMed] [Google Scholar]
- 10.Farkas GJ, Pitot MA, Berg AS, Gater DR. Nutritional status in chronic spinal cord injury: a systematic review and meta-analysis. Spinal Cord. 2019;57:3–17. doi: 10.1038/s41393-018-0218-4. [DOI] [PubMed] [Google Scholar]
- 11.Silveira SL, Winter LL, Clark R, Ledoux T, Robinson-Whelen S. Baseline Dietary Intake of Individuals with Spinal Cord Injury Who Are Overweight or Obese. J Acad Nutr Diet. 2019;119:301–309. doi: 10.1016/j.jand.2018.08.153. [DOI] [PubMed] [Google Scholar]
- 12.Lieberman J, Goff D, Jr, Hammond F, Schreiner P, Norton HJ, Dulin M, Zhou X, Steffen L. Dietary intake and adherence to the 2010 Dietary Guidelines for Americans among individuals with chronic spinal cord injury: a pilot study. J Spinal Cord Med. 2014;37:751–757. doi: 10.1179/2045772313Y.0000000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perret C, Stoffel-Kurt N. Comparison of nutritional intake between individuals with acute and chronic spinal cord injury. J Spinal Cord Med. 2011;34:569–575. doi: 10.1179/2045772311Y.0000000026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bigford G, Nash MS. Nutritional Health Considerations for Persons with Spinal Cord Injury. Top Spinal Cord Inj Rehabil. 2017;23:188–206. doi: 10.1310/sci2303-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Emmanuel A. Neurogenic bowel dysfunction. F1000Res. 2019;8:F1000 Faculty Rev–1800. [Google Scholar]
- 16.Faaborg PM, Finnerup NB, Christensen P, Krogh K. Abdominal Pain: A Comparison between Neurogenic Bowel Dysfunction and Chronic Idiopathic Constipation. Gastroenterol Res Pract. 2013;2013:365037. doi: 10.1155/2013/365037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Okawa Y, Fukudo S, Sanada H. Specific foods can reduce symptoms of irritable bowel syndrome and functional constipation: a review. Biopsychosoc Med. 2019;13:10. doi: 10.1186/s13030-019-0152-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cozma-Petruţ A, Loghin F, Miere D, Dumitraşcu DL. Diet in irritable bowel syndrome: What to recommend, not what to forbid to patients! World J Gastroenterol. 2017;23:3771–3783. doi: 10.3748/wjg.v23.i21.3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Halmos EP. When the low FODMAP diet does not work. J Gastroenterol Hepatol. 2017;32 Suppl 1:69–72. doi: 10.1111/jgh.13701. [DOI] [PubMed] [Google Scholar]
- 20.Sohail MU, Yassine HM, Sohail A, Al Thani AA. Impact of Physical Exercise on Gut Microbiome, Inflammation, and the Pathobiology of Metabolic Disorders. Rev Diabet Stud. 2019;15:35–48. doi: 10.1900/RDS.2019.15.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qi Z, Middleton JW, Malcolm A. Bowel Dysfunction in Spinal Cord Injury. Curr Gastroenterol Rep. 2018;20:47. doi: 10.1007/s11894-018-0655-4. [DOI] [PubMed] [Google Scholar]
- 22.Radulovic M, Schilero GJ, Yen C, Bauman WA, Wecht JM, Ivan A, La Fountaine MF, Korsten MA. Greatly increased prevalence of esophageal dysmotility observed in persons with spinal cord injury. Dis Esophagus. 2015;28:699–704. doi: 10.1111/dote.12272. [DOI] [PubMed] [Google Scholar]
- 23.Silva CB, Martinez JC, Yanagita ET, Morais JF, Carvalho LB, Herani-Filho B, Moraes DG, Vianna PC, Prado GF. The repercussions of spinal cord injury on the action of the diaphragmatic crura for gastroesophageal reflux containment. Spine (Phila Pa 1976) 2008;33:2892–2897. doi: 10.1097/BRS.0b013e31818a2c59. [DOI] [PubMed] [Google Scholar]
- 24.Rodriguez GM. Bowel Function After Spinal Cord Injury. Arch Phys Med Rehabil. 2016;97:339–340. doi: 10.1016/j.apmr.2015.08.419. [DOI] [PubMed] [Google Scholar]
- 25.Callaghan B, Furness JB, Pustovit RV. Neural pathways for colorectal control, relevance to spinal cord injury and treatment: a narrative review. Spinal Cord. 2018;56:199–205. doi: 10.1038/s41393-017-0026-2. [DOI] [PubMed] [Google Scholar]
- 26.Vallès M, Vidal J, Clavé P, Mearin F. Bowel dysfunction in patients with motor complete spinal cord injury: clinical, neurological, and pathophysiological associations. Am J Gastroenterol. 2006;101:2290–2299. doi: 10.1111/j.1572-0241.2006.00729.x. [DOI] [PubMed] [Google Scholar]
- 27.Nielsen SD, Faaborg PM, Finnerup NB, Christensen P, Krogh K. Ageing with neurogenic bowel dysfunction. Spinal Cord. 2017;55:769–773. doi: 10.1038/sc.2017.22. [DOI] [PubMed] [Google Scholar]
- 28.Inskip JA, Lucci VM, McGrath MS, Willms R, Claydon VE. A Community Perspective on Bowel Management and Quality of Life after Spinal Cord Injury: The Influence of Autonomic Dysreflexia. J Neurotrauma. 2018;35:1091–1105. doi: 10.1089/neu.2017.5343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Choukou MA, Best KL, Craven BC, Hitzig SL. Identifying and Classifying Quality of Life Tools for Assessing Neurogenic Bowel Dysfunction After Spinal Cord Injury. Top Spinal Cord Inj Rehabil. 2019;25:1–22. doi: 10.1310/sci18-00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krogh K, Christensen P, Sabroe S, Laurberg S. Neurogenic bowel dysfunction score. Spinal Cord. 2006;44:625–631. doi: 10.1038/sj.sc.3101887. [DOI] [PubMed] [Google Scholar]
- 31.Liu CW, Huang CC, Chen CH, Yang YH, Chen TW, Huang MH. Prediction of severe neurogenic bowel dysfunction in persons with spinal cord injury. Spinal Cord. 2010;48:554–559. doi: 10.1038/sc.2009.181. [DOI] [PubMed] [Google Scholar]
- 32.Tate DG, Forchheimer M, Rodriguez G, Chiodo A, Cameron AP, Meade M, Krassioukov A. Risk Factors Associated With Neurogenic Bowel Complications and Dysfunction in Spinal Cord Injury. Arch Phys Med Rehabil. 2016;97:1679–1686. doi: 10.1016/j.apmr.2016.03.019. [DOI] [PubMed] [Google Scholar]
- 33.Burns AS, St-Germain D, Connolly M, Delparte JJ, Guindon A, Hitzig SL, Craven BC. Phenomenological study of neurogenic bowel from the perspective of individuals living with spinal cord injury. Arch Phys Med Rehabil. 2015;96:49–55. doi: 10.1016/j.apmr.2014.07.417. [DOI] [PubMed] [Google Scholar]
- 34.Kigerl KA, Mostacada K, Popovich PG. Gut Microbiota Are Disease-Modifying Factors After Traumatic Spinal Cord Injury. Neurotherapeutics. 2018;15:60–67. doi: 10.1007/s13311-017-0583-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Panda S, El khader I, Casellas F, López Vivancos J, García Cors M, Santiago A, Cuenca S, Guarner F, Manichanh C. Short-term effect of antibiotics on human gut microbiota. PLoS One. 2014;9:e95476. doi: 10.1371/journal.pone.0095476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Initiated by the Multidisciplinary Association of Spinal Cord Injured Professionals. Guidelines for Management of Neurogenic Bowel Dysfunction in Individuals with Central Neurological Conditions. 2012. Available from: https://www.mascip.co.uk/wp-content/uploads/2015/02/CV653N-Neurogenic-Guidelines-Sept-2012.pdf . [Google Scholar]
- 37.Zhang C, Jing Y, Zhang W, Zhang J, Yang M, Du L, Jia Y, Chen L, Gong H, Li J, Gao F, Liu H, Qin C, Liu C, Wang Y, Shi W, Zhou H, Liu Z, Yang D, Li J. Dysbiosis of gut microbiota is associated with serum lipid profiles in male patients with chronic traumatic cervical spinal cord injury. Am J Transl Res. 2019;11:4817–4834. [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang C, Zhang W, Zhang J, Jing Y, Yang M, Du L, Gao F, Gong H, Chen L, Li J, Liu H, Qin C, Jia Y, Qiao J, Wei B, Yu Y, Zhou H, Liu Z, Yang D, Li J. Gut microbiota dysbiosis in male patients with chronic traumatic complete spinal cord injury. J Transl Med. 2018;16:353. doi: 10.1186/s12967-018-1735-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gungor B, Adiguzel E, Gursel I, Yilmaz B, Gursel M. Intestinal Microbiota in Patients with Spinal Cord Injury. PLoS One. 2016;11:e0145878. doi: 10.1371/journal.pone.0145878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jelleyman C, Yates T, O'Donovan G, Gray LJ, King JA, Khunti K, Davies MJ. The effects of high-intensity interval training on glucose regulation and insulin resistance: a meta-analysis. Obes Rev. 2015;16:942–961. doi: 10.1111/obr.12317. [DOI] [PubMed] [Google Scholar]
- 41.Mann S, Beedie C, Jimenez A. Differential effects of aerobic exercise, resistance training and combined exercise modalities on cholesterol and the lipid profile: review, synthesis and recommendations. Sports Med. 2014;44:211–221. doi: 10.1007/s40279-013-0110-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tuttolomondo A, Simonetta I, Daidone M, Mogavero A, Ortello A, Pinto A. Metabolic and Vascular Effect of the Mediterranean Diet. Int J Mol Sci. 2019;20:4716. doi: 10.3390/ijms20194716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Estaki M, Pither J, Baumeister P, Little JP, Gill SK, Ghosh S, Ahmadi-Vand Z, Marsden KR, Gibson DL. Cardiorespiratory fitness as a predictor of intestinal microbial diversity and distinct metagenomic functions. Microbiome. 2016;4:42. doi: 10.1186/s40168-016-0189-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Codella R, Luzi L, Terruzzi I. Exercise has the guts: How physical activity may positively modulate gut microbiota in chronic and immune-based diseases. Dig Liver Dis. 2018;50:331–341. doi: 10.1016/j.dld.2017.11.016. [DOI] [PubMed] [Google Scholar]
- 45.Durk RP, Castillo E, Márquez-Magaña L, Grosicki GJ, Bolter ND, Lee CM, Bagley JR. Gut Microbiota Composition Is Related to Cardiorespiratory Fitness in Healthy Young Adults. Int J Sport Nutr Exerc Metab. 2019;29:249–253. doi: 10.1123/ijsnem.2018-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fayfman M, Flint K, Srinivasan S. Obesity, Motility, Diet, and Intestinal Microbiota-Connecting the Dots. Curr Gastroenterol Rep. 2019;21:15. doi: 10.1007/s11894-019-0680-y. [DOI] [PubMed] [Google Scholar]
- 47.Dudek-Wicher RK, Junka A, Bartoszewicz M. The influence of antibiotics and dietary components on gut microbiota. Prz Gastroenterol. 2018;13:85–92. doi: 10.5114/pg.2018.76005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, Ling AV, Devlin AS, Varma Y, Fischbach MA, Biddinger SB, Dutton RJ, Turnbaugh PJ. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505:559–563. doi: 10.1038/nature12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lopes SS, Miszputen SJ, Sachs A, Lima MM, Ambrogini O., Jr Evaluation of carbohydrate and fiber consumption in patients with irritable bowel syndrome in outpatient treatment. Arq Gastroenterol. 2019;56:3–9. doi: 10.1590/S0004-2803.201900000-12. [DOI] [PubMed] [Google Scholar]
- 50.Spinal Cord Injury. Evidence-Based Nutrition Practice Guideline. 2009. Available from: https://www.andeal.org/topic.cfm?menu=3485cat=3486. [Google Scholar]
- 51.Stoffel JT, Van der Aa F, Wittmann D, Yande S, Elliott S. Neurogenic bowel management for the adult spinal cord injury patient. World J Urol. 2018;36:1587–1592. doi: 10.1007/s00345-018-2388-2. [DOI] [PubMed] [Google Scholar]
- 52.Yeung HY, Iyer P, Pryor J, Nicholson M. Dietary management of neurogenic bowel in adults with spinal cord injury: an integrative review of literature. Disabil Rehabil. 2019:1–12. doi: 10.1080/09638288.2019.1652702. [DOI] [PubMed] [Google Scholar]
- 53.Noller CM, Groah SL, Nash MS. Inflammatory Stress Effects on Health and Function After Spinal Cord Injury. Top Spinal Cord Inj Rehabil. 2017;23:207–217. doi: 10.1310/sci2303-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Santos-Marcos JA, Perez-Jimenez F, Camargo A. The role of diet and intestinal microbiota in the development of metabolic syndrome. J Nutr Biochem. 2019;70:1–27. doi: 10.1016/j.jnutbio.2019.03.017. [DOI] [PubMed] [Google Scholar]
- 55.Bailey MA, Holscher HD. Microbiome-Mediated Effects of the Mediterranean Diet on Inflammation. Adv Nutr. 2018;9:193–206. doi: 10.1093/advances/nmy013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kigerl KA, Zane K, Adams K, Sullivan MB, Popovich PG. The spinal cord-gut-immune axis as a master regulator of health and neurological function after spinal cord injury. Exp Neurol. 2020;323:113085. doi: 10.1016/j.expneurol.2019.113085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wallace DJ, Sayre NL, Patterson TT, Nicholson SE, Hilton D, Grandhi R. Spinal cord injury and the human microbiome: beyond the brain-gut axis. Neurosurg Focus. 2019;46:E11. doi: 10.3171/2018.12.FOCUS18206. [DOI] [PubMed] [Google Scholar]
- 58.Gorgey AS, Dolbow DR, Dolbow JD, Khalil RK, Castillo C, Gater DR. Effects of spinal cord injury on body composition and metabolic profile - part I. J Spinal Cord Med. 2014;37:693–702. doi: 10.1179/2045772314Y.0000000245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nevin AN, Steenson J, Vivanti A, Hickman IJ. Investigation of measured and predicted resting energy needs in adults after spinal cord injury: a systematic review. Spinal Cord. 2016;54:248–253. doi: 10.1038/sc.2015.193. [DOI] [PubMed] [Google Scholar]
- 60.Straub RH. Concepts of evolutionary medicine and energy regulation contribute to the etiology of systemic chronic inflammatory diseases. Brain Behav Immun. 2011;25:1–5. doi: 10.1016/j.bbi.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 61.Straub RH, Cutolo M, Buttgereit F, Pongratz G. Energy regulation and neuroendocrine-immune control in chronic inflammatory diseases. J Intern Med. 2010;267:543–560. doi: 10.1111/j.1365-2796.2010.02218.x. [DOI] [PubMed] [Google Scholar]
- 62.Steensgaard R, Bonne S, Wojke P, Kasch H. SCI-SCREEN: A More Targeted Nutrition Screening Model to Detect Spinal Cord-Injured Patients at Risk of Malnutrition. Rehabil Nurs. 2019;44:11–19. doi: 10.1097/rnj.0000000000000108. [DOI] [PubMed] [Google Scholar]
- 63.Nash MS, Bilzon JLJ. Guideline Approaches for Cardioendocrine Disease Surveillance and Treatment Following Spinal Cord Injury. Curr Phys Med Rehabil Rep. 2018;6:264–276. doi: 10.1007/s40141-018-0203-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Scaramella J, Kirihennedige N, Broad E. Key Nutritional Strategies to Optimize Performance in Para Athletes. Phys Med Rehabil Clin N Am. 2018;29:283–298. doi: 10.1016/j.pmr.2018.01.005. [DOI] [PubMed] [Google Scholar]
- 65.Figel K, Pritchett K, Pritchett R, Broad E. Energy and Nutrient Issues in Athletes with Spinal Cord Injury: Are They at Risk for Low Energy Availability? Nutrients. 2018;10:1078. doi: 10.3390/nu10081078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pinckaers PJ, Churchward-Venne TA, Bailey D, van Loon LJ. Ketone Bodies and Exercise Performance: The Next Magic Bullet or Merely Hype? Sports Med. 2017;47:383–391. doi: 10.1007/s40279-016-0577-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lakkis B, El Chediak A, Hashash JG, Koubar SH. Severe ketoacidosis in a patient with spinal muscular atrophy. CEN Case Rep. 2018;7:292–295. doi: 10.1007/s13730-018-0345-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nightingale TE, Williams S, Thompson D, Bilzon JLJ. Energy balance components in persons with paraplegia: daily variation and appropriate measurement duration. Int J Behav Nutr Phys Act. 2017;14:132. doi: 10.1186/s12966-017-0590-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Iyer P, Beck EJ, Walton KL. A systematic review of the effect of dietary interventions on cardiovascular disease risk in adults with spinal cord injury. J Spinal Cord Med. 2019:1–20. doi: 10.1080/10790268.2019.1592926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ciccotti M, Raguzzini A, Sciarra T, Catasta G, Aiello P, Buccolieri C, Reggi R, Palmery M, Lista F, Peluso I. Nutraceutical-based Integrative Medicine: Adopting a Mediterranean Diet Pyramid for Attaining Healthy Ageing in Veterans with Disabilities. Curr Pharm Des. 2018;24:4186–4196. doi: 10.2174/1381612824666181003113444. [DOI] [PubMed] [Google Scholar]
- 71.Chauveau P, Aparicio M, Bellizzi V, Campbell K, Hong X, Johansson L, Kolko A, Molina P, Sezer S, Wanner C, Ter Wee PM, Teta D, Fouque D, Carrero JJ European Renal Nutrition (ERN) Working Group of the European Renal Association–European Dialysis Transplant Association (ERA-EDTA) Mediterranean diet as the diet of choice for patients with chronic kidney disease. Nephrol Dial Transplant. 2018;33:725–735. doi: 10.1093/ndt/gfx085. [DOI] [PubMed] [Google Scholar]
- 72.Rondanelli M, Faliva MA, Miccono A, Naso M, Nichetti M, Riva A, Guerriero F, De Gregori M, Peroni G, Perna S. Food pyramid for subjects with chronic pain: foods and dietary constituents as anti-inflammatory and antioxidant agents. Nutr Res Rev. 2018;31:131–151. doi: 10.1017/S0954422417000270. [DOI] [PubMed] [Google Scholar]
- 73.Allison DJ, Beaudry KM, Thomas AM, Josse AR, Ditor DS. Changes in nutrient intake and inflammation following an anti-inflammatory diet in spinal cord injury. J Spinal Cord Med. 2019;42:768–777. doi: 10.1080/10790268.2018.1519996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jung IY, Kim HR, Chun SM, Leigh JH, Shin HI. Severe spasticity in lower extremities is associated with reduced adiposity and lower fasting plasma glucose level in persons with spinal cord injury. Spinal Cord. 2017;55:378–382. doi: 10.1038/sc.2016.132. [DOI] [PubMed] [Google Scholar]
- 75.Sabour H, Nazari M, Latifi S, Soltani Z, Shakeri H, Larijani B, Ghodsi SM, Razavi SH. The Relationship Between Dietary Intakes of Amino Acids and Bone Mineral Density Among Individuals with Spinal Cord Injury. Oman Med J. 2016;31:22–28. doi: 10.5001/omj.2016.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Doubelt I, Totosy de Zepetnek J, MacDonald MJ, Atkinson SA. Influences of nutrition and adiposity on bone mineral density in individuals with chronic spinal cord injury: A cross-sectional, observational study. Bone Rep. 2015;2:26–31. doi: 10.1016/j.bonr.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schäfer K, Konstantinides S. Adipokines and thrombosis. Clin Exp Pharmacol Physiol. 2011;38:864–871. doi: 10.1111/j.1440-1681.2011.05589.x. [DOI] [PubMed] [Google Scholar]
- 78.Bernardi M, Fedullo AL, Di Giacinto B, Squeo MR, Aiello P, Dante D, Romano S, Magaudda L, Peluso I, Palmery M, Spataro A. Cardiovascular Risk Factors and Haematological Indexes of Inflammation in Paralympic Athletes with Different Motor Impairments. Oxid Med Cell Longev. 2019;2019:6798140. doi: 10.1155/2019/6798140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Olas B, Bryś M. Effects of coffee, energy drinks and their components on hemostasis: The hypothetical mechanisms of their action. Food Chem Toxicol. 2019;127:31–41. doi: 10.1016/j.fct.2019.02.039. [DOI] [PubMed] [Google Scholar]
- 80.Vilahur G, Badimon L. Antiplatelet properties of natural products. Vascul Pharmacol. 2013;59:67–75. doi: 10.1016/j.vph.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 81.O'Kennedy N, Raederstorff D, Duttaroy AK. Fruitflow®: the first European Food Safety Authority-approved natural cardio-protective functional ingredient. Eur J Nutr. 2017;56:461–482. doi: 10.1007/s00394-016-1265-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.EFSA Panel. Water-soluble tomato concentrate (WSTC I and II) and platelet aggregation. EFSA J. 2009 [Google Scholar]
- 83.EFSA Panel. Scientific Opinion on the modification of the authorisation of a health claim related to water-soluble tomato concentrate and helps to maintain a healthy blood flow and benefits circulation pursuant to Article 13(5) of Regulation (EC) No 1924/2006 following a request in accordance with Article 19 of the Regulation (EC) No 1924/2006. EFSA J. 2010:1689. [Google Scholar]
- 84.Koutrakis NE, Goldstein RL, Walia P, Polak MM, Lazzari AA, Tun CG, Hart JE, Garshick E. Vitamin D, diet, and lifestyle in a chronic SCI population. Spinal Cord. 2019;57:117–127. doi: 10.1038/s41393-018-0148-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fischer MJ, Krishnamoorthi VR, Smith BM, Evans CT, St Andre JR, Ganesh S, Huo Z, Stroupe KT. Prevalence of chronic kidney disease in patients with spinal cord injuries/disorders. Am J Nephrol. 2012;36:542–548. doi: 10.1159/000345460. [DOI] [PubMed] [Google Scholar]
- 86.Yu SC, Kuo JR, Shiue YL, Yu ZX, Ho CH, Wu CC, Wang JJ, Chu CC, Lim SW. One-Year Mortality of Patients with Chronic Kidney Disease After Spinal Cord Injury: A 14-Year Population-Based Study. World Neurosurg. 2017;105:462–469. doi: 10.1016/j.wneu.2017.06.009. [DOI] [PubMed] [Google Scholar]
- 87.Sung BM, Oh DJ, Choi MH, Choi HM. Chronic kidney disease in neurogenic bladder. Nephrology (Carlton) 2018;23:231–236. doi: 10.1111/nep.12990. [DOI] [PubMed] [Google Scholar]
- 88.Chan LW, Griebling TL, Arnold EP, Chu PS, New PW, Wagg A. Special considerations in the urological management of the older spinal cord injury patient. World J Urol. 2018;36:1603–1611. doi: 10.1007/s00345-018-2326-3. [DOI] [PubMed] [Google Scholar]
- 89.Paśko P, Rodacki T, Domagała-Rodacka R, Owczarek D. A short review of drug-food interactions of medicines treating overactive bladder syndrome. Int J Clin Pharm. 2016;38:1350–1356. doi: 10.1007/s11096-016-0383-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sciarra T, Ciccotti M, Aiello P, Minosi P, Munzi D, Buccolieri C, Peluso I, Palmery M, Lista F. Polypharmacy and Nutraceuticals in Veterans: Pros and Cons. Front Pharmacol. 2019;10:994. doi: 10.3389/fphar.2019.00994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Compton S, Trease L, Cunningham C, Hughes D. Australian Institute of Sport and the Australian Paralympic Committee position statement: urinary tract infection in spinal cord injured athletes. Br J Sports Med. 2015;49:1236–1240. doi: 10.1136/bjsports-2014-094527. [DOI] [PubMed] [Google Scholar]
- 92.Jepson RG, Williams G, Craig JC. Cranberries for preventing urinary tract infections. Cochrane Database Syst Rev. 2012;10:CD001321. doi: 10.1002/14651858.CD001321.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hill TC, Baverstock R, Carlson KV, Estey EP, Gray GJ, Hill DC, Ho C, McGinnis RH, Moore K, Parmar R. Best practices for the treatment and prevention of urinary tract infection in the spinal cord injured population: The Alberta context. Can Urol Assoc J. 2013;7:122–130. doi: 10.5489/cuaj.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.EFSA Panel. Scientific opinion on the safety of green tea catechins. EFSA J. 2018 doi: 10.2903/j.efsa.2018.5239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hsu TF, Kusumoto A, Abe K, Hosoda K, Kiso Y, Wang MF, Yamamoto S. Polyphenol-enriched oolong tea increases fecal lipid excretion. Eur J Clin Nutr. 2006;60:1330–1336. doi: 10.1038/sj.ejcn.1602464. [DOI] [PubMed] [Google Scholar]
- 96.Ashigai H, Taniguchi Y, Suzuki M, Ikeshima E, Kanaya T, Zembutsu K, Tomita S, Miyake M, Fukuhara I. Fecal Lipid Excretion after Consumption of a Black Tea Polyphenol Containing Beverage-Randomized, Placebo-Controlled, Double-Blind, Crossover Study. Biol Pharm Bull. 2016;39:699–704. doi: 10.1248/bpb.b15-00662. [DOI] [PubMed] [Google Scholar]
- 97.Peluso I. Dietary Antioxidants: Micronutrients and Antinutrients in Physiology and Pathology. Antioxidants (Basel) 2019;8:642. doi: 10.3390/antiox8120642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Peluso I, Serafini M. Antioxidants from black and green tea: from dietary modulation of oxidative stress to pharmacological mechanisms. Br J Pharmacol. 2017;174:1195–1208. doi: 10.1111/bph.13649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Morales P, Fujio S, Navarrete P, Ugalde JA, Magne F, Carrasco-Pozo C, Tralma K, Quezada M, Hurtado C, Covarrubias N, Brignardello J, Henriquez D, Gotteland M. Impact of Dietary Lipids on Colonic Function and Microbiota: An Experimental Approach Involving Orlistat-Induced Fat Malabsorption in Human Volunteers. Clin Transl Gastroenterol. 2016;7:e161. doi: 10.1038/ctg.2016.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Agency for Clinical Innovation. Nutrition Fact Sheet Healthy Eating for Adults with Spinal Cord Injury. 2014. Available from: https://www.aci.health.nsw.gov.au/__data/assets/pdf_file/0010/224677/ACI_Eat_for_Health_Nutrition.pdf . [Google Scholar]
- 101.Martin Ginis KA, van der Scheer JW, Latimer-Cheung AE, Barrow A, Bourne C, Carruthers P, Bernardi M, Ditor DS, Gaudet S, de Groot S, Hayes KC, Hicks AL, Leicht CA, Lexell J, Macaluso S, Manns PJ, McBride CB, Noonan VK, Pomerleau P, Rimmer JH, Shaw RB, Smith B, Smith KM, Steeves JD, Tussler D, West CR, Wolfe DL, Goosey-Tolfrey VL. Evidence-based scientific exercise guidelines for adults with spinal cord injury: an update and a new guideline. Spinal Cord. 2018;56:308–321. doi: 10.1038/s41393-017-0017-3. [DOI] [PubMed] [Google Scholar]
- 102.Tweedy SM, Beckman EM, Geraghty TJ, Theisen D, Perret C, Harvey LA, Vanlandewijck YC. Exercise and sports science Australia (ESSA) position statement on exercise and spinal cord injury. J Sci Med Sport. 2017;20:108–115. doi: 10.1016/j.jsams.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 103.Bernardi E, Bernardi M, Berni Canani R, Branca F, Garbagnati F, Scognamiglio U, Traballesi M, Bertini I, Giampietro M, Cairella G. L’alimentazione nell’atleta disabile motorio. Medicina dello Sport. 2005;58:289–301. [Google Scholar]
- 104.Egidi F, Faiola F, Guerra E, Marini C, Sardella, F, Bernardi M. Sport for disabled individuals: from rehabilitation to Paralympic Games. Med Sport. 2009;62:597–601. [Google Scholar]
- 105.Ordonez FJ, Rosety MA, Camacho A, Rosety I, Diaz AJ, Fornieles G, Bernardi M, Rosety-Rodriguez M. Arm-cranking exercise reduced oxidative damage in adults with chronic spinal cord injury. Arch Phys Med Rehabil. 2013;94:2336–2341. doi: 10.1016/j.apmr.2013.05.029. [DOI] [PubMed] [Google Scholar]
- 106.Rosety M Á, Díaz AJ, Rosety JM, Pery MT, Brenes-Martín F, Bernardi M, García N, Rosety-Rodríguez M, Ordoñez FJ, Rosety I. Exercise improved semen quality and reproductive hormone levels in sedentary obese adults. Nutr Hosp. 2017;34:603–607. doi: 10.20960/nh.549. [DOI] [PubMed] [Google Scholar]
- 107.Webborn N, Van de Vliet P. Paralympic medicine. Lancet. 2012;380:65–71. doi: 10.1016/S0140-6736(12)60831-9. [DOI] [PubMed] [Google Scholar]
- 108.Pelliccia A, Quattrini FM, Squeo MR, Caselli S, Culasso F, Link MS, Spataro A, Bernardi M. Cardiovascular diseases in Paralympic athletes. Br J Sports Med. 2016;50:1075–1080. doi: 10.1136/bjsports-2015-095867. [DOI] [PubMed] [Google Scholar]
- 109.Gerrish HR, Broad E, Lacroix M, Ogan D, Pritchett RC, Pritchett K. Nutrient Intake of Elite Canadian and American Athletes with Spinal Cord Injury. Int J Exerc Sci. 2017;10:1018–1028. doi: 10.70252/FQDQ5053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Grams L, Garrido G, Villacieros J, Ferro A. Marginal Micronutrient Intake in High-Performance Male Wheelchair Basketball Players: A Dietary Evaluation and the Effects of Nutritional Advice. PLoS One. 2016;11:e0157931. doi: 10.1371/journal.pone.0157931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Collins EG, Gater D, Kiratli J, Butler J, Hanson K, Langbein WE. Energy cost of physical activities in persons with spinal cord injury. Med Sci Sports Exerc. 2010;42:691–700. doi: 10.1249/MSS.0b013e3181bb902f. [DOI] [PubMed] [Google Scholar]
- 112.Abel T, Platen P, Rojas Vega S, Schneider S, Strüder HK. Energy expenditure in ball games for wheelchair users. Spinal Cord. 2008;46:785–790. doi: 10.1038/sc.2008.54. [DOI] [PubMed] [Google Scholar]
- 113.Bernardi M, Guerra E, Di Giacinto B, Di Cesare A, Castellano V, Bhambhani Y. Field evaluation of paralympic athletes in selected sports: implications for training. Med Sci Sports Exerc. 2010;42:1200–1208. doi: 10.1249/MSS.0b013e3181c67d82. [DOI] [PubMed] [Google Scholar]
- 114.Bernardi E, Delussu SA, Quattrini FM, Rodio A, Bernardi M. Energy balance and dietary habits of America's Cup sailors. J Sports Sci. 2007;25:1153–1160. doi: 10.1080/02640410701287180. [DOI] [PubMed] [Google Scholar]
- 115.Bernardi M, Quattrini FM, Rodio A, Fontana G, Madaffari A, Brugnoli M, Marchetti M. Physiological characteristics of America's Cup sailors. J Sports Sci. 2007;25:1141–1152. doi: 10.1080/02640410701287172. [DOI] [PubMed] [Google Scholar]
- 116.Adami PE, Delussu AS, Rodio A, Squeo MR, Corsi L, Quattrini FM, Fattorini L, Bernardi M. Upper limb aerobic training improves aerobic fitness and all-out performance of America's Cup grinders. Eur J Sport Sci. 2015;15:235–241. doi: 10.1080/17461391.2014.971878. [DOI] [PubMed] [Google Scholar]
- 117.Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, Strath SJ, O'Brien WL, Bassett DR, Jr, Schmitz KH, Emplaincourt PO, Jacobs DR, Jr, Leon AS. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32:S498–S504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 118.Myers J, Gopalan R, Shahoumian T, Kiratli J. Effects of customized risk reduction program on cardiovascular risk in males with spinal cord injury. J Rehabil Res Dev. 2012;49:1355–1364. doi: 10.1682/jrrd.2011.11.0215. [DOI] [PubMed] [Google Scholar]
- 119.Cavedon V, Zancanaro C, Milanese C. Physique and Performance of Young Wheelchair Basketball Players in Relation with Classification. PLoS One. 2015;10:e0143621. doi: 10.1371/journal.pone.0143621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Cavedon V, Zancanaro C, Milanese C. Anthropometry, Body Composition, and Performance in Sport-Specific Field Test in Female Wheelchair Basketball Players. Front Physiol. 2018;9:568. doi: 10.3389/fphys.2018.00568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Brook EM, Tenforde AS, Broad EM, Matzkin EG, Yang HY, Collins JE, Blauwet CA. Low energy availability, menstrual dysfunction, and impaired bone health: A survey of elite para athletes. Scand J Med Sci Sports. 2019;29:678–685. doi: 10.1111/sms.13385. [DOI] [PubMed] [Google Scholar]
- 122.Blauwet CA, Brook EM, Tenforde AS, Broad E, Hu CH, Abdu-Glass E, Matzkin EG. Low Energy Availability, Menstrual Dysfunction, and Low Bone Mineral Density in Individuals with a Disability: Implications for the Para Athlete Population. Sports Med. 2017;47:1697–1708. doi: 10.1007/s40279-017-0696-0. [DOI] [PubMed] [Google Scholar]
- 123.Bernardi M, Guerra E, Rodio A, Dante D, Castellano V, Peluso I, Schena F, Bhambhani Y. Assessment of Exercise Stroke Volume and Its Prediction From Oxygen Pulse in Paralympic Athletes With Locomotor Impairments: Cardiac Long-Term Adaptations Are Possible. Front Physiol. 2019;10:1451. doi: 10.3389/fphys.2019.01451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bernardi M, Janssen T, Bortolan L, Pellegrini B, Fischer G, Schena F. Kinematics of cross-country sit skiing during a Paralympic race. J Electromyogr Kinesiol. 2013;23:94–101. doi: 10.1016/j.jelekin.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 125.Bernardi M, Carucci S, Faiola F, Egidi F, Marini C, Castellano V, Faina M. Physical fitness evaluation of paralympic winter sports sitting athletes. Clin J Sport Med. 2012;22:26–30. doi: 10.1097/JSM.0b013e31824237b5. [DOI] [PubMed] [Google Scholar]
- 126.Thomas DT, Erdman KA, Burke LM. American College of Sports Medicine Joint Position Statement. Nutrition and Athletic Performance. Med Sci Sports Exerc. 2016;48:543–568. doi: 10.1249/MSS.0000000000000852. [DOI] [PubMed] [Google Scholar]
- 127.Peake JM, Neubauer O, Walsh NP, Simpson RJ. Recovery of the immune system after exercise. J Appl Physiol (1985) 2017;122:1077–1087. doi: 10.1152/japplphysiol.00622.2016. [DOI] [PubMed] [Google Scholar]
- 128.Gunzer W, Konrad M, Pail E. Exercise-induced immunodepression in endurance athletes and nutritional intervention with carbohydrate, protein and fat-what is possible, what is not? Nutrients. 2012;4:1187–1212. doi: 10.3390/nu4091187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Shea JL, King MT, Yi Y, Gulliver W, Sun G. Body fat percentage is associated with cardiometabolic dysregulation in BMI-defined normal weight subjects. Nutr Metab Cardiovasc Dis. 2012;22:741–747. doi: 10.1016/j.numecd.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 130.Atkinson FS, Foster-Powell K, Brand-Miller JC. International tables of glycemic index and glycemic load values: 2008. Diabetes Care. 2008;31:2281–2283. doi: 10.2337/dc08-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Thomas DT, Erdman KA, Burke LM. Position of the Academy of Nutrition and Dietetics, Dietitians of Canada, and the American College of Sports Medicine: Nutrition and Athletic Performance. J Acad Nutr Diet. 2016;116:501–528. doi: 10.1016/j.jand.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 132.Kerksick CM, Arent S, Schoenfeld BJ, Stout JR, Campbell B, Wilborn CD, Taylor L, Kalman D, Smith-Ryan AE, Kreider RB, Willoughby D, Arciero PJ, VanDusseldorp TA, Ormsbee MJ, Wildman R, Greenwood M, Ziegenfuss TN, Aragon AA, Antonio J. International society of sports nutrition position stand: nutrient timing. J Int Soc Sports Nutr. 2017;14:33. doi: 10.1186/s12970-017-0189-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Cao Y, Jarnecke M, Krause JS. Health-related behaviors and multiple chronic health conditions among persons with traumatic spinal cord injury. Spinal Cord. 2019;57:367–371. doi: 10.1038/s41393-018-0227-3. [DOI] [PubMed] [Google Scholar]
- 134.Phillips SM, Van Loon LJ. Dietary protein for athletes: from requirements to optimum adaptation. J Sports Sci. 2011;29 Suppl 1:S29–S38. doi: 10.1080/02640414.2011.619204. [DOI] [PubMed] [Google Scholar]
- 135.Mettler S, Mitchell N, Tipton KD. Increased protein intake reduces lean body mass loss during weight loss in athletes. Med Sci Sports Exerc. 2010;42:326–337. doi: 10.1249/MSS.0b013e3181b2ef8e. [DOI] [PubMed] [Google Scholar]
- 136.Maughan RJ, Burke LM, Dvorak J, Larson-Meyer DE, Peeling P, Phillips SM, Rawson ES, Walsh NP, Garthe I, Geyer H, Meeusen R, van Loon L, Shirreffs SM, Spriet LL, Stuart M, Vernec A, Currell K, Ali VM, Budgett RGM, Ljungqvist A, Mountjoy M, Pitsiladis Y, Soligard T, Erdener U, Engebretsen L. IOC Consensus Statement: Dietary Supplements and the High-Performance Athlete. Int J Sport Nutr Exerc Metab. 2018;28:104–125. doi: 10.1123/ijsnem.2018-0020. [DOI] [PubMed] [Google Scholar]
- 137.Maughan RJ, Burke LM, Dvorak J, Larson-Meyer DE, Peeling P, Phillips SM, Rawson ES, Walsh NP, Garthe I, Geyer H, Meeusen R, van Loon LJC, Shirreffs SM, Spriet LL, Stuart M, Vernec A, Currell K, Ali VM, Budgett RG, Ljungqvist A, Mountjoy M, Pitsiladis YP, Soligard T, Erdener U, Engebretsen L. IOC consensus statement: dietary supplements and the high-performance athlete. Br J Sports Med. 2018;52:439–455. doi: 10.1136/bjsports-2018-099027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Posthauer ME, Banks M, Dorner B, Schols JM. The role of nutrition for pressure ulcer management: national pressure ulcer advisory panel, European pressure ulcer advisory panel, and pan pacific pressure injury alliance white paper. Adv Skin Wound Care. 2015;28:175–88; quiz 189-90. doi: 10.1097/01.ASW.0000461911.31139.62. [DOI] [PubMed] [Google Scholar]
- 139.Van de Vliet P, Broad E, Strupler M. Nutrition, body composition and pharmacology. In: Vanlandewijck YC, Thompson WR, editors. In: Vanlandewijck YC, Thompson WR. The Paralympic Athlete: Handbook of Sports Medicine and Science. Wiley-Blackwell Boca Raton (FL): CRC Press. 2014: 172-197. [Google Scholar]
- 140.Price MJ, Campbell IG. Effects of spinal cord lesion level upon thermoregulation during exercise in the heat. Med Sci Sports Exerc. 2003;35:1100–1107. doi: 10.1249/01.MSS.0000074655.76321.D7. [DOI] [PubMed] [Google Scholar]
- 141.Webborn N, Price MJ, Castle PC, Goosey-Tolfrey VL. Effects of two cooling strategies on thermoregulatory responses of tetraplegic athletes during repeated intermittent exercise in the heat. J Appl Physiol (1985) 2005;98:2101–2107. doi: 10.1152/japplphysiol.00784.2004. [DOI] [PubMed] [Google Scholar]
- 142.Webborn N, Price MJ, Castle P, Goosey-Tolfrey VL. Cooling strategies improve intermittent sprint performance in the heat of athletes with tetraplegia. Br J Sports Med. 2010;44:455–460. doi: 10.1136/bjsm.2007.043687. [DOI] [PubMed] [Google Scholar]
- 143.Domínguez R, Maté-Muñoz JL, Cuenca E, García-Fernández P, Mata-Ordoñez F, Lozano-Estevan MC, Veiga-Herreros P, da Silva SF, Garnacho-Castaño MV. Effects of beetroot juice supplementation on intermittent high-intensity exercise efforts. J Int Soc Sports Nutr. 2018;15:2. doi: 10.1186/s12970-017-0204-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wylie LJ, Bailey SJ, Kelly J, Blackwell JR, Vanhatalo A, Jones AM. Influence of beetroot juice supplementation on intermittent exercise performance. Eur J Appl Physiol. 2016;116:415–425. doi: 10.1007/s00421-015-3296-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Siervo M, Oggioni C, Jakovljevic DG, Trenell M, Mathers JC, Houghton D, Celis-Morales C, Ashor AW, Ruddock A, Ranchordas M, Klonizakis M, Williams EA. Dietary nitrate does not affect physical activity or outcomes in healthy older adults in a randomized, cross-over trial. Nutr Res. 2016;36:1361–1369. doi: 10.1016/j.nutres.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 146.Flueck JL, Gallo A, Moelijker N, Bogdanov N, Bogdanova A, Perret C. Influence of Equimolar Doses of Beetroot Juice and Sodium Nitrate on Time Trial Performance in Handcycling. Nutrients. 2019;11:1642. doi: 10.3390/nu11071642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Bahadoran Z, Mirmiran P, Kabir A, Azizi F, Ghasemi A. The Nitrate-Independent Blood Pressure-Lowering Effect of Beetroot Juice: A Systematic Review and Meta-Analysis. Adv Nutr. 2017;8:830–838. doi: 10.3945/an.117.016717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Gönül M, Keseroglu H, Hacınecipoğlu F. A case of methotrexate intoxication in a patient with psoriasis who drank beetroot juice during methotrexate treatment. Clin Exp Dermatol. 2016;41:893–895. doi: 10.1111/ced.12930. [DOI] [PubMed] [Google Scholar]
- 149.Kemmner S, Lorenz G, Wobst J, Kessler T, Wen M, Günthner R, Stock K, Heemann U, Burkhardt K, Baumann M, Schmaderer C. Dietary nitrate load lowers blood pressure and renal resistive index in patients with chronic kidney disease: A pilot study. Nitric Oxide. 2017;64:7–15. doi: 10.1016/j.niox.2017.01.011. [DOI] [PubMed] [Google Scholar]
- 150.Berends JE, van den Berg LMM, Guggeis MA, Henckens NFT, Hossein IJ, de Joode MEJR, Zamani H, van Pelt KAAJ, Beelen NA, Kuhnle GG, de Kok TMCM, Van Breda SGJ. Consumption of Nitrate-Rich Beetroot Juice with or without Vitamin C Supplementation Increases the Excretion of Urinary Nitrate, Nitrite, and N-nitroso Compounds in Humans. Int J Mol Sci. 2019;20:2277. doi: 10.3390/ijms20092277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Madden RF, Shearer J, Parnell JA. Evaluation of Dietary Intakes and Supplement Use in Paralympic Athletes. Nutrients. 2017;9:1266. doi: 10.3390/nu9111266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Madden RF, Shearer J, Legg D, Parnell JA. Evaluation of Dietary Supplement Use in Wheelchair Rugby Athletes. Nutrients. 2018;10:1958. doi: 10.3390/nu10121958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Pritchett K, Pritchett RC, Stark L, Broad E, LaCroix M. Effect of Vitamin D Supplementation on 25(OH)D Status in Elite Athletes With Spinal Cord Injury. Int J Sport Nutr Exerc Metab. 2019;29:18–23. doi: 10.1123/ijsnem.2017-0233. [DOI] [PubMed] [Google Scholar]
- 154.Pires JM, Ferreira AM, Rocha F, Andrade LG, Campos I, Margalho P, Laíns J. Assessment of neurogenic bowel dysfunction impact after spinal cord injury using the International Classification of Functioning, Disability and Health. Eur J Phys Rehabil Med. 2018;54:873–879. doi: 10.23736/S1973-9087.18.04991-2. [DOI] [PubMed] [Google Scholar]
- 155.Louati K, Berenbaum F. Fatigue in chronic inflammation - a link to pain pathways. Arthritis Res Ther. 2015;17:254. doi: 10.1186/s13075-015-0784-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Bailey KA, Lenz K, Allison DJ, Ditor DS. Barriers and facilitators to adhering to an anti-inflammatory diet for individuals with spinal cord injuries. Health Psychol Open. 2018;5:2055102918798732. doi: 10.1177/2055102918798732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Myers J, Doom R, King R, Fonda H, Chan K, Kokkinos P, Rehkopf DH. Association Between Cardiorespiratory Fitness and Health Care Costs: The Veterans Exercise Testing Study. Mayo Clin Proc. 2018;93:48–55. doi: 10.1016/j.mayocp.2017.09.019. [DOI] [PubMed] [Google Scholar]
- 158.Joaquim DP, Juzwiak CR, Winckler C. Diet Quality Profile of Track-and-Field Paralympic Athletes. Int J Sport Nutr Exerc Metab. 2019:1–7. doi: 10.1123/ijsnem.2018-0361. [DOI] [PubMed] [Google Scholar]
- 159.Eskici G, Ersoy G. An evaluation of wheelchair basketball players' nutritional status and nutritional knowledge levels. J Sports Med Phys Fitness. 2016;56:259–268. [PubMed] [Google Scholar]
- 160.Fattorini L, Pittiglio G, Federico B, Pallicca A, Bernardi M, Rodio A. Workload comparison between hiking and indoor physical activity. J Strength Cond Res. 2012;26:2883–2889. doi: 10.1519/JSC.0b013e318242a61e. [DOI] [PubMed] [Google Scholar]
- 161.Pritchett K, Pritchett R, Ogan D, Bishop P, Broad E, LaCroix M. 25(OH)D Status of Elite Athletes with Spinal Cord Injury Relative to Lifestyle Factors. Nutrients. 2016;8:374. doi: 10.3390/nu8060374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Bernardi M, Castellano V, Ferrara MS, Sbriccoli P, Sera F, Marchetti M. Muscle pain in athletes with locomotor disability. Med Sci Sports Exerc. 2003;35:199–206. doi: 10.1249/01.MSS.0000048635.83126.D4. [DOI] [PubMed] [Google Scholar]
- 163.Sanz-Quinto S, Moya-Ramón M, Brizuela G, Rice I, Urbán T, López-Grueso R. Nutritional strategies in an elite wheelchair marathoner at 3900 m altitude: a case report. J Int Soc Sports Nutr. 2019;16:51. doi: 10.1186/s12970-019-0321-8. [DOI] [PMC free article] [PubMed] [Google Scholar]