Abstract
Objectives
Optimal dosage regimens of colistin for the treatment of urinary tract infections (UTI) are unknown. Colistimethate sodium (CMS), the inactive prodrug of colistin, is mainly excreted in urine and converts to colistin after filtration by glomeruli, suggesting that concentrations of colistin in urine could be much higher than in plasma. Therefore, there is a need to optimize dosage regimens of intravenous CMS for UTI. The aim of this study was to examine the relationship between AUC/MIC of formed colistin and clinical outcomes in patients with UTI caused by extremely drug resistant (XDR) Pseudomonas aeruginosa.
Methods
This prospective, observational cohort study involved patients with UTI caused by XDR P. aeruginosa. Clinical cure, bacteriological clearance and acute kidney injury (AKI) were analyzed. Steady-state colistin plasma concentrations (Css) were measured using HPLC. Based on the PK/PD of colistin in neutropenic mouse thigh infection model with P. aeruginosa, the optimal AUC/MIC should be ≥60 mg·h/L. According to the pharmacokinetics (PK) in critically-ill patients, the Css target of formed colistin in plasma was 2.5 mg/L.
Results
Thirty-three patients were included (24 lower UTI and 9 pyelonephritis). The MIC50 and MIC90 values for colistin were 0.5 and 2 mg/L respectively. Nineteen patients (57.6%) received colistin monotherapy (84.2% UTI and 15.8% pyelonephritis). Of these, clinical cure was achieved in 89.5% of cases. Among patients with clinical cure, only 5 (29.4%) attained an optimal plasma AUC/MIC and only 1 (5.9%) the therapeutic level of formed colistin (2.5 mg/L). However, 10 (58.8%) patients showed colistin plasma concentrations above the MIC of the isolated P. aeruginosa. Microbiological eradication was achieved in 76.9% of patients. AKI at the end of treatment was present in 29.4% of patients.
Conclusions
The currently recommended dosage regimens of CMS showed high efficacy for the treatment of lower complicated UTI caused by XDR P. aeruginosa in non-critically ill patients and in the case of low MIC values, but also a considerable nephrotoxicity rate. Our data suggest that the use of lower CMS doses for lower UTI should be investigated in future studies to minimize the unnecessary nephrotoxicity.
INTRODUCTION
Urinary tract infection (UTI) represents between 20–49% of all nosocomial infections and Pseudomonas aeruginosa is the responsible of 7–10% of these cases [1]. The best therapeutic approach is controversial especially for multidrug-resistant (MDR) and extensively drug-resistant (XDR) P. aeruginosa strains. Although some aminoglycosides and fosfomycin remain active against several MDR/XDR P. aeruginosa strains, the worrying increase in bacterial resistance coupled with the lack of new drugs in the pipeline has become a major clinical and public health concern globally [2]. Currently, novel agents such as ceftolozane/tazobactam and ceftazidime/avibactam have expanded the therapeutic arsenal [3]. However, both in vitro and in vivo resistance have been reported with these agents [3–5]. In this situation, until new drugs such plazomicin, meropenem-vaborbactam, imipenem-relebactam, cefiderocol, murepavadin and cefepime-zidebactam become available in daily clinical practice [6–8], polymyxins represent the only therapeutic option in many cases.
Colistin is a drug with a narrow therapeutic window and nephrotoxicity is the major dose-limiting adverse effect [9–11]. With its role as a salvage therapy for otherwise untreatable infections, it is essential to design CMS regimens that maximize its efficacy while minimizing the potential for the development of resistance and colistin-associated nephrotoxicity. The commercially available form of colistin for parenteral use is colistimethate sodium (CMS) [12], which is an inactive prodrug of colistin and ‘less’ toxic than colistin, the active compound. According to the package insert recommendations, current doses in Europe range from 1 to 3 million IU/8h in patients with normal renal function [13], regardless of the type of infection. Once administered, CMS converts into colistin in patients, and approximately 60–70% of the CMS dose is rapidly eliminated in the urine [14–16]. It is believed that conversion of CMS to colistin occurs in renal tubular cells and in the bladder [14,17,18], suggesting that concentrations of formed colistin in the urine could be much higher than those attained in plasma. Our recent clinical PK study demonstrated that colistin urinary levels were much higher than those achieved in plasma [15]. Regarding the pharmacokinetics/pharmacodynamics (PK/PD) of colistin, it is known that the index that best correlates with colistin antibacterial activity is the ratio of 24-h area under the concentration-time curve to the minimum inhibitory concentration (AUC/MIC) [19,20]. The AUC/MIC target is equal or higher than 60 mg·h/L for the treatment of infections caused by P. aeruginosa. Additionally, a recent population PK study suggested a target of 2.5 mg/L formed colistin in plasma at the steady state (Css) [21]. Considering the potentially very high concentration of formed colistin in urine, we hypothesized that for the treatment of UTI the plasma PK/PD target proposed for colistin is unnecessarily high and a lower dose of CMS may be sufficient to minimize the nephrotoxicity.
The main objective of this study was to examine the AUC/MIC value of formed colistin in plasma in a cohort of patients with symptomatic UTI (sUTI) caused by XDR P. aeruginosa and its association with clinical and microbiological outcomes. A secondary objective was to identify risk factors for colistin-associated nephrotoxicity.
MATERIAL AND METHODS
Study population
We conducted a prospective observational study in a cohort of patients with sUTI caused by XDR P. aeruginosa treated with intravenous CMS for at least 48 hours. The study was carried out at Hospital del Mar, a university tertiary care hospital in Barcelona (Spain), from January 2010 to September 2014. The local ethics committee (Comité Ètic d’Investigació Clínica del Parc de Salut Mar) approved the study. Exclusion criteria were <18 years old, pregnancy, breast feeding during the study period, to have another concomitant infection focus or to be on renal replacement therapy. A pharmacy-generated alarm system was used to identify patients being treated with CMS for UTI. The study investigators performed the assessment from the first day of the treatment and informed consent was obtained from all participants or their legal representatives.
Collected data
Patient data collected included demographic information, Charlson comorbidity index [22], the severity of disease at the time of the first CMS dose stratified according to the Acute Physiology and Chronic Health Evaluation (APACHE II) [23], clinical status at the start of treatment (defined as infection, severe sepsis or shock according to standard definitions) [24], and CMS treatment (indication, daily and total cumulative dose measured in millions of international units (IU) and duration of treatment). Other clinical data included the presence of baseline chronic kidney disease (CKD, defined as glomerular filtration rate (GFR) < 60 mL/min/1.73 m2 for ≥ 3 months), serum creatinine concentration, GFR at baseline and the end of treatment (EOT, calculated using the abbreviated Modification of Diet in Renal Disease equation (MDRD-4)) [25], and the development of acute kidney injury (AKI) at EOT according to the RIFLE criteria [26].
Combination therapy with other antibiotics that had potential activity or synergy with colistin was assessed. These combinations included colistin plus meropenem (synergistic in vitro against the most prevalent strain in our center), ceftazidime or amikacin (potential activity against P. aeruginosa). Information was collected on the concomitant use of aminoglycosides, vancomycin, angiotensin II receptor blockers, angiotensin-converting enzyme (ACE) inhibitors, loop diuretics, intravenous dye, amphotericin B and non-steroidal anti-inflammatory drugs (NSAIDs), as well as the need for vasopressor drugs or discontinuation of CMS due to nephrotoxicity. Since this study was performed in patients with XDR P. aeruginosa infections, when vancomycin was used it was as part of an empirical treatment in patients with febrile neutropenia. Vancomycin was withdrawn when the diagnosis of UTI was performed.
Definitions
XDR was defined according to Magiorakos et al. [27], as non-susceptibility to at least one agent in all but two or fewer antimicrobial categories (i.e. aminoglycosides, antipseudomonal carbapenems, antipseudomonal cephalosporins, antipseudomonal fluorquinolones, antipseudomonal penicillines + β-lactamase inhibitors, monobactams, phosphonic acids, polymyxins). UTI was defined when the patient presented with at least one of the following urinary tract symptoms: fever > 38°C in patients ≤ 65 years of age, suprapubic tenderness, costovertebral angle pain or tenderness, urinary frequency, urinary urgency or dysuria, plus positive urinary culture with no more than two species of microorganisms identified and at least one of which had a colony count of ≥ 105 CFU/mL [28]. Three UTI syndromes were considered:
Cystitis: the presence of dysuria and increased urinary frequency or urgency, with or without hematuria, in patients without fever (axillary temperature < 37.5°C).
Pyelonephritis: presence of fever (axillary temperature ≥ 38°C) and back pain or costovertebral angle tenderness.
Clinical failure was defined as when the patients showed no improvement or at least one of the initial symptoms, worsened or died. Clinical cure was defined as either the absence of symptoms or as a consistent improvement in the signs and symptoms of the infection. Bacteriological clearance and failure were defined as the eradication and persistence, respectively, of the previously isolated microorganism in a follow-up culture at the end of antibiotic treatment.
CMS administration
The dosage regimen and dose adjustments of CMS were determined by the responsible clinician. Each CMS dose (colistimethate formulation for intravenous use, GES Genéricos Españoles®) was diluted in 100 mL of physiological saline and intravenously administered over 30 min. Each vial contained 1 million international units (IU) of CMS (equivalent to 80 mg CMS).
Microbiological data
Identification and susceptibility testing of P. aeruginosa were first performed by microdilution using the Gram-negative breakpoint panel for non-fermenting gram-negative bacteria of the MicroScan® WalkAway system (Siemens Diagnostic Inc., CA). Colistin minimal inhibitory concentration (MIC) was determined by microdilution using cation-adjusted MHB; the isolate was considered susceptible if the MIC was ≤ 2 mg/L according to the Clinical and Laboratory Standards Institute (CLSI) [29].
Pharmacokinetic data
Blood samples were obtained just before the next dose on day 3–4 of the treatment. It was assumed that steady state was already achieved considering a half-life of approximately 14 h [30]. Concentrations of CMS and formed colistin in the plasma were measured using a validated HPLC method [31,32]. The limit of quantification of the HPLC methods for colistin and CMS in plasma were 0.20 and 0.50 mg/L, respectively. Considering the ‘flat’ plasma concentration-time profiles of formed colistin in critically-ill patients [10,21], measured colistin concentrations were regarded as Css,avg. Consequently, AUC24h in plasma was calculated by multiplying the obtained Css,avg value by 24 h. Since the most predictive PK/PD index for colistin is the area under unbound plasma concentration vs time curve to the minimum inhibitory concentration (fAUC/MIC) [32], we also calculated the fAUC/MIC for each patient using 0.5 as the unbound fraction for colistin in human plasma [32].
Based on recent pharmacokinetic studies in critically ill patients [21,33], we also considered Css of 2.5 mg/L as the concentration target to guide therapy. We also analyzed the percentage of patients that achieved AUC24/MIC ≥ 60 mg·h/L for the target of infections caused by P. aeruginosa[21].
The Css,avg achieved by our patients in clinical practice were compared with those that would have been obtained if the recommended CMS dosing algorithm would have been used [34].
Statistical analysis
Dichotomous data were compared using a χ2 or Fisher’s exact test. Normally distributed continuous data are expressed as means and standard deviations (SD), and compared using t-test. Otherwise, values are presented as means with interquartile range (IQR) and compared using the Mann-Whitney U-test. Baseline and clinical characteristics of patients receiving different CMS doses were compared using ANOVA or Kruskal-Wallis test. Multivariate analysis of risk factors for colistin-associated nephrotoxicity was conducted using logistic regression. Univariate analyses were performed separately for each of the risk factor variables to ascertain the odds ratio (OR) and 95% confidence interval (CI). All clinically important covariates and those with p < 0.2 in univariate analyses were included in the multivariate analysis.
RESULTS
During the study period, 33 patients with sUTI caused by XDR P. aeruginosa treated with CMS were included. Patients’ characteristics are summarized in Table 1. Sixteen (48.5%) patients had a urinary catheter at the time of infection and there were two patients (6.1%) with an indwelling urinary catheter. All catheters were removed or exchanged two days after an effective antibiotic treatment was started. Fever was present in 45.5% of the cases at the time of infectious diagnosis. However, blood cultures were only performed in 19 (57.6%) patients being only four (21.1%) positive. It’s important to point out that 14 (42.4%) of patients in this cohort received combined antibiotic treatment (33.3% of patients with lower UTI and 66.7% of patients with acute pyelonephritis).
Table 1:
Characteristics of included patients.
| Patient (n = 33) | |
|---|---|
| Age, years** | 65.1 ± 13.1 |
| Male sex, n (%) | 26 (78.8) |
| 1APACHE II** | 10.4 ± 6.83 |
| Charlson score** | 4.90 ± 2.8 |
| Urinary catheter | 16 (48.5) |
| UTI syndrome: | |
| - Cystitis | 24 (72.7) |
| - Pyelonephritis | 9 (27.3) |
| Positive blood cultures | 4 (12.1) |
| Clinical status: | |
| - Sepsis, n (%) | 10 (30.3) |
| - Shock, n (%) | 1 (3,0) |
| 2CMS daily dose (million IU)** | 5 ± 2.54 |
| CMS daily dose (in colistin base activity, (mg)/kg/day) | 2.21 ± 1.25 |
| 2CMS total cumulative dose (million IU)* | 24 (14.2–39.7) |
| 2CMS treatment duration, days** | 8.31 ± 6.43 |
| 3GFR at baseline (mL/min/1.73 m2)** | 100.2 ± 64.1 |
| Patients with 4CKD at baseline, n (%) | 8 (24.2) |
| 5Css,avg (mg/L)** | 1.19 ± 1.09 |
| 6AUC/MIC** | 60.5 ± 56.4 |
| fAUC/MIC** | 30.2 ± 28.2 |
| 6AUC/MIC ≥ 60 mg∙h/L, n (%) | 11 (33.3) |
| 5 % patients with Css,avg > 2.5 mg/L | 3 (9.1) |
| 7Expected Css, avg | 0.78 ± 0.07 |
| Css, avg above MIC | 22 (66.7) |
| Combined antibiotic therapy: | 14 (42.4) |
| - Meropenem | 6 (18.2) |
| - Amikacin | 3 (9.1) |
| - Ceftazidime | 5 (15.2) |
| 8AKI at the end of treatment, n (%) | 10 (30.3) |
| - R (Risk) | 3 (33.3) |
| - I (Injury) | 5 (55.5) |
| - F (Failure) | 2 (22.2) |
| Clinical cure, n (%) | 31 (93.9) |
| Microbiological follow up, n (%) | 23 (69.7) |
| Microbiological clearance, n (%) | 19/23 (82.6) |
Median (interquartile range).
Mean ± standard deviation.
APACHE II: Acute Physiology and Chronic Health Evaluation II.
CMS: colistin methanesulfonate
GFR: glomerular filtration rate
CKD: chronic kidney disease
Css,avg: colistin plasma concentration at steady-state
AUC: colistin area under the curve
Calculated Css,avg according the dosage algorithm of Nation et al
AKI: acute kidney injury.
Colistin MIC of the most predominant P. aeruginosa strain in our hospital was 0.5 mg/L (24 isolates with a MIC of 0.5 mg/L, 8 isolates with a MIC of 0.25 mg/L, and 1 isolate with a MIC of 2 mg/L). The MIC 50 and MIC 90 values for colistin were 0.5 and 2 mg/L respectively. This strain displayed a ceftazidime MIC of 16 mg/L, an amikacin MIC of 16 mg/L and a meropenem MIC ≥ 8 mg/L.
CMS doses were administered as follows: 8 (24.2%) patients received 1 million IU three times a day (TID); 12 (36.4%) patients received 2 million IU TID; 2 (6.1%) patients received 3 million IU TID; 2 (6.1%) patients received 4.5 million IU two times a day (BID); and in 9 (27.3%) patients different dosage adjustments were made based on patients’ renal function.
The mean plasma colistin Css was 1,19 mg/L, but individual values varied widely (0.21–5.20 mg/L) with the different selected doses. Only 3 (9.1%) patients showed colistin plasma concentrations > 2.5 mg/L and only 11 (33.3%) achieved an optimal PK/PD index (AUC/MIC ≥ 60). If we consider the cut-off point for the Css,avg of 2 mg/L (corresponding to an AUC/MIC = 48 mg·h/L) which has been recently recommended by Nation et. al [34,35], 5 (15.2%) patients achieved the therapeutic goal. However, is important to point out that 22 (66.7%) patients showed colistin plasma concentrations above the MIC of the isolated P. aeruginosa.
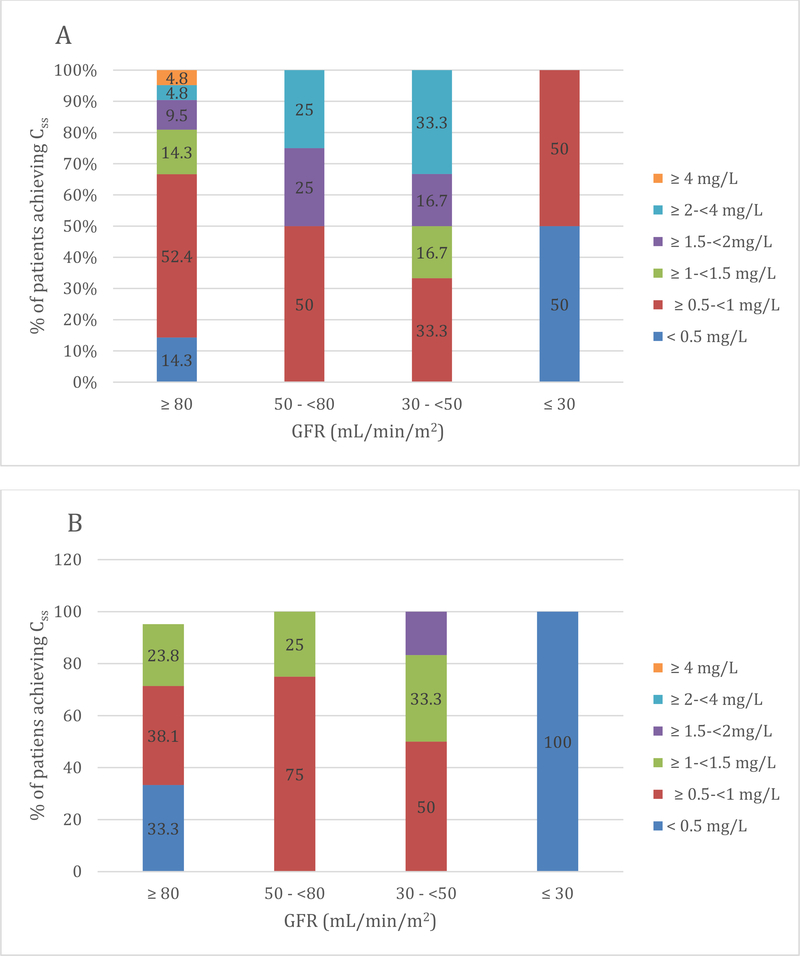
Figure 1 shows the percentage of patients with different renal function achieving the average Css of < 0.5, ≥ 0.5 to < 1, ≥ 1 to < 1.5, ≥ 1.5 to <2, ≥ 2 to < 4, ≥ 4 mg/L with the dosage regimens chosen by the responsible physician. We observed that the median values of Css achieved by our patients are quite similar to those expected by applying the dosing CMS algorithm of Nation et al [34] (0.84 [0.21–5.2] mg/L vs 0.7 [0.03–1.66] mg/L; P= 0.26).
Figure 1:
Figure A and B show the percentage of patients with different degrees of renal function achieving average steady-state plasma concentrations of colistin (Css,avg) of < 0.5, ≥0.5-< 1, ≥ 1, ≥1.5-< 2, ≥2-< 4, ≥ 4 mg/L using the real daily CMS dose selected by the responsible physician (A) and by applying the formula of Nation et al. (B) [34]
Table 2 summarizes characteristics of the study participants according to the CMS dosage regimen. CMS dosage regimens were classified in four groups as follows: < 3 million IU/day, ≥ 3 million IU/day, ≥ 6 million IU/day and ≥ 9 million IU/day. No differences were observed in any of the demographic and clinical variables with the exception of the baseline GFR that was lower in patients that received the lowest dose of CMS. No correlation was observed between Css, avg and GFR (Spearman rho, −0.07; P = 0.63) or CMS daily dose (Spearman rho, 0.30; P = 0.09).
Table 2:
Clinical and demographic characteristics of patients receiving different CMS dosage regimens.
| Variable | < 3 million IU/day (n = 6) | ≥ 3 million IU/day (n = 9) | ≥ 6 million IU/day (n = 12) | ≥ 9 million IU/ day (n = 6) | P |
|---|---|---|---|---|---|
| Age, years** | 76 (70–85) | 67 (44–82) | 61 (46–77) | 69 (52–85) | 0.06 |
| Male sex, n (%) | 6 (100) | 8 (88.9) | 8 (66.7) | 4 (66.7) | 0.08 |
| Charlson index** | 8 (4–9) | 5 (0–9) | 4 (0–9) | 6 (2–7) | 0.3 |
| Clinical status: - Severe sepsis - Shock |
2 (33.3) 1(16.7) |
3 (33.3) 0 (0) |
3 (25) 0 (0) |
2 (33.3) 0 (0) |
0.86 0.18 |
| 1CMS total dose (million IU) * | 20 (4–40) | 24 (9–112) | 24 (11–120) | 30 (27–99) | 0.20 |
| 1CMS treatment duration, days** | 10 (5–20) | 8 (3–28) | 5 (3–20) | 5 (3–11) | 0.62 |
| 2GFR at baseline (mL/min/1.73 m2)** | 33.4 (19–51) | 96.9 (39–353) | 107 (44–212) | 122 (81–138) | 0.005 |
| 3Css,avg (mg/L)** | 0.85 (0.28–3.02) | 0.57 (0.21–2.43) | 1.1 (0.5–3.64) | 1.4 (0.3–5.2) | 0.16 |
| 4Expected Css,avg (mg/L) | 0.51 (0.35–0.62) | 0.46 (0.03–1.17) | 0.83 (0.26–1.65) | 1.04 (0.88–1.66) | 0.05 |
| 5AUC/6MIC** | 40.8 (12–145 | 27.4 (9.6–116.6) | 42.7 (15.6–174.7) | 67.2 (57.6 –249.6) | 0.21 |
| AUC/MIC > 60 mg∙h/L | 2 (33.3) | 1 (11.1) | 5 (41.7) | 3 (50) | 0.37 |
| Css,avg > 2.5 mg/L Css, avg above MIC |
1 (16.7) 3 (50) |
0 (0) 6 (66.7) |
1 (8.3) 9 (75) |
1 (16.7) 4 (66.7) |
0.83 0.61 |
|
7AKI at 8EOT, n (%): - R (Risk) - I (Injury) - F (Failure) |
2 (33.3) 1 (50) 1 (50) 0 (0) |
3 (33.3) 0 (0) 2 (66.6) 1 (33.3) |
4 (33.3) 1 (25) 3 (75) 0 (0) |
1 (16.7) 0 (0) 0 (0) 1 (100) |
0.89 |
| Clinical cure, n (%) | 6 (100) | 8 (88.9) | 11 (91.7) | 6 (100) | 0.95 |
| Microbiological eradication, n (%) | 2 (50) | 7 (87.5) | 7 (87.5) | 6 (100) | 0.36 |
CMS: colistin methanesulfonate
GFR: glomerular filtration rate
Css,avg: colistin plasma concentration at steady-state
Calculated Css,avg according the algorithm of Nation et al
AUC: colistin area under the curve
MIC: minimum inhibitory concentration
AKI: acute kidney injury.
Table 3 shows the characteristics of patients with and without clinical cure in the total cohort and in the group of patients treated with colistin monotherapy. In the entire cohort, clinical cure was achieved in 31 (93.9%) patients. Within patients with clinical cure, only 10 (32.3%) achieved an AUC/MIC > 60 mg·h/L and only 2 (6.5%) a Css,avg > 2.5 mg/L. In the group treated with colistin monotherapy, clinical cure was achieved in 17 of the 19 (89.5%) patients. Of these, only 5 (29.4%) attained an optimal AUC/MIC and only 1 (5.9%) the therapeutic level of formed colistin.
Table 3:
Characteristics of patients according to the clinical outcome.
| Included patients | Patients treated with colistin alone | |||||
|---|---|---|---|---|---|---|
| Variable | Clinical cure (n = 31) | Clinical failure (n = 2) | P | Clinical cure (n=17) | Clinical failure (n=2) | P |
| Age, years | 69 (44–85) | 72.5 (72–73) | 0.5 | 68.5 (46–85) | 72.5 (72–73) | 0.35 |
| Male sex, n (%) | 24 (77.4) | 2 (100) | 1 | 14 (82.4) | 2 (100) | 1 |
| Charlson | 5 (0–9) | 6.5 (4–9) | 0.42 | 4.5 (0–9) | 6.5 (4–9) | 0.35 |
| APACHE II | 8.5 (2–28) | 11.5 (9–14) | 0.44 | 8.5 (2–28) | 11.5 (9–14) | 0.64 |
| Clinical status: | ||||||
| - Severe sepsis - Shock |
10 (32.3) 1 (3.2) |
0 (0) 0 (0) |
1 1 |
12 (70.6) 0 (0) |
2 (100) 0 (0) |
1 1 |
| Fever at start of colistin treatment, n (%) | 15 (48.4) | 0 (0) | 0.49 | 9 (52.9) | 0 (0) | 0.47 |
| UTI syndrome: | ||||||
| - Cystitis - Pyelonephritis |
22 (71) 9 (100) |
2 (100) 0 (0) |
1 | 14 (82.4) 3 (17.6) |
2 (100) 0 (0) |
1 |
| CMS1 daily dose (millions IU) | 3.5 (1–9) | 4.5 (3–6) | 0.91 | 4 (2–9) | 4.5 (3–6) | 0.95 |
| CMS1 total dose (millions IU) | 30 (12–120) | 36 (24–48) | 0.53 | 18 (6–39) | 36 (24–48) | 0.11 |
| CMS1 treatment duration, days | 5 (3–28) | 8 | 0.53 | 4 (3–10) | 8 | 0.22 |
| Colistin base activity (mg/kg/day) | 1.76 (0.52–5.1) | 2.43 (1.51–3.34) | 0.9 | 2.27 (0.8–3.64) | 2.42 (1.51–3.34) | 0.89 |
| GFR2 at baseline (ml/min/1.73 m2) | 90.3 (19–334) | 109.5 (97–122) | 0.37 | 82.02 (33–353) | 109.5 (97–122) | 0.43 |
| Patients with CKD3 at baseline, n (%) | 9 (29) | 0 (0) | 1 | 5 (29.4) | 0 (0) | 1 |
| Css,avg4 (mg/L) | 0.84 (0.21–5.2) | 1.98 (0.31–3.64) | 0.85 | 0.7 (0.21–3.02) | 1.98 (0.31–3.64) | 0.69 |
| Css,avg above MIC | 21 (67.7) | 1 (50) | 1 | 10 (58.8) | 1 (50) | 1 |
| 6AUC/MIC | 40.8 (9.6–249.6) | 94.8 (14.9–174.7) | 0.73 | 33.6 (9.6–163.2) | 94.8 (14.9–174.7) | 0.69 |
| fAUC/MIC | 21.5 (5.04–124.8) | 47.4 (7.44–87.4) | 0.85 | 16.8 (4.8–81.6) | 47.4 (7.44–87.4) | 0.69 |
| AUC/MIC ≥ 60 mg/L Css > 2,5 mg/L |
10 (32.3) 2 (6.5) |
1 (50) 1 (50) |
1 0.18 |
5 (29.4) 1 (5.9) |
1 (50) 1 (50) |
1 0.2 |
| AKI7 at end of treatment, n (%): | 9 (29) | 1 (50) | 0.52 | 5 (29.4) | 1 (50) | 1 |
Data are n (%) except indicated or median (interquartile range)
CMS: colistin methanesulfonate
GFR: glomerular filtration rate
CKD: chronic kidney disease
Css: colistin plasma concentrations at steady-state
MIC: minimum inhibitory concentration
AUC: area under the curve
AKI: acute kidney injury
At this point, we also analyzed patients with clinical failure, and we found that they were those who died of other comorbidities but not attributable to the UTI.
Despite the small sample size, we performed an analysis on the 9 patients diagnosed with pyelonephritis. All of them achieved clinical cure even though none of them reached a Css,avg above 2.5 mg/L and only 1 (11.1%) achieved an AUC/MIC > 60 mg·h/L. However, it is important to note that six (66.7%) of them were treated with combined therapy.
Microbiological follow up was available in 23 patients of whom 19 (82.6) accomplished microbiological clearance. Only 7/19 (36.8%) of these patients had reached an AUC/MIC of 60 mg·h/L and 2/19 (10.5%) a Css,avg ≥ 2.5 mg/L. No significant difference between patients who received colistin therapy and those who received combination therapy was observed for microbiological outcome (10/19 [76.9%] vs 9/14 [76.9%]; p = 0.6).
AKI at EOT was observed in 10 (30.3%) patients. The univariate analysis of risk factors for AKI showed significant differences for the Charlson score and Css,avg and a trend in the use of loop diuretics (Table 4). However, in the multivariate analysis only the Charlson score (odds ratio (OR) 1.55 (95% confidence interval (CI): 0.95–2.45, P = 0.081) and Css,avg (OR 4.36 (95% CI: 0.86–20, P = 0.074) showed a trend towards statistical significance (Table 5). There were no differences between patients treated with colistin and those treated with combined antibiotic therapy (6/19 [31.6%] vs 4/14 [28.6%]; p = 1).
Table 4:
Univariate analysis of the characteristics of patients with and without nephrotoxicity at EOT.
| Patients with AKI (n = 10) | Patients without AKI (n = 23) | P | |
|---|---|---|---|
| Male sex, n (%) | 8 (80) | 18 (78.3) | 1 |
| Age, years | 72.5 (54–82) | 65 (37–85) | 0.11 |
| Charlson index | 7.5 (4–9) | 4 (0–9) | 0.008 |
| APACHE II | 8.5 (5–14) | 9 (2–28) | 0.88 |
| Clinical status: | 0.68 | ||
| - Sepsis | 2 (20) | 8 (34.8) | |
| BMI1 (Kg/m2) | 22.6 (15.5–31.3) | 24.4 (21.1–30.12) | 1 |
| GFR2at baseline (ml/min/1.73 m2) | 96.9 (19–353) | 79.9 (33–159) | 0.6 |
| 3CMS total dose (million IU) | 22 (9–112) | 25.5 (4–120) | 0.9 |
| CMS1 treatment duration, days | 6.5 (3–28) | 6 (3–20) | 0.9 |
| Colistin base activity (mg)/kg/day | 1.81 (0.85–5.10) | 1.7 (0.52–3.4) | 0.88 |
| 4Css (mg/L) | 1.65 (0.7–5.2) | 0.57 (0.21–2.42) | 0.001 |
| 6AUC/MIC | 96.7 (28.8–249.6) | 27.4 (9.6–116.2) | 0.007 |
| fAUC/MIC | 48.4 (14.4–124.8) | 13.7 (4.8–58.1) | |
| Concomitant aminoglycoside use, n (%) | 3 (30.0) | 3 (13.0) | 0.45 |
| Concomitant vancomycin use, n (%) | 0 (0) | 3 (13.0) | 0.53 |
| Concomitant use of loop diuretic, n (%) | 5 (50.0) | 4 (17.4) | 0.09 |
Data are n (%) or median (interquartile range)
Median (interquartile range)
BMI: body mass index
GFR: glomerular filtration rate
CMS: colistin methanesulfonate
Css: colistin plasma concentrations at steady-state
MIC: minimum inhibitory concentration
AUC: area under the curve.
Table 5:
Multivariate analysis of the risk factors for nephrotoxicity at EOT.
| Variable | Odds ratio (95% CI) | P |
|---|---|---|
| Charlson score | 1.55 (0.95–2.45) | 0.081 |
| Css | 4.36 (0.86–20) | 0.074 |
| Use of loop diuretics | 0.69 (0.08–5.76) | 0.735 |
DISCUSSION
The aim of this study was to assess the plasma AUC/MIC values of formed colistin in a cohort of patients with UTI caused by XDR P. aeruginosa treated with intravenous CMS, and the association with clinical and microbiological outcomes. Although only 11 (33.3%) patients achieved the optimal plasma PK/PD target (AUC/MIC ≥ 60 mg·h/L), the clinical cure rate was 93.9% with 82.6% microbiological eradication rate. When the data were analyzed based upon colistin plasma concentration of 2.5 mg/L as a therapeutic target, no relationship was observed between the PK data and clinical outcomes, as only 6.5% of the cured patients achieved this target.
In recent clinical studies, the rate of clinical cure in UTI caused by MDR-GNB treated with CMS ranged from 50 to 92% [36–38]. Zaidi et al. performed a retrospective study of 155 patients with infections caused by MDR GNB treated with low-dose colistin (1.9 mg/kg/day of colistin base activity (CBA) [i.e. 0.063 IU/kg/day] in patients with clinical cure vs 1.7 mg CBA/kg [i.e. 0.057 IU/kg/day] in patients without clinical cure) [36]. In that study, the clinical cure rate in patients diagnosed with UTI was 83.3% [36]. Cheng et al. performed another retrospective study including patients diagnosed with MDR-GNB and treated with CMS, in whom only 5 out of 10 (50%) patients with UTI achieved clinical cure [37]. Dosage regimens of CMS in the Cheng study were based on the patient’s creatinine clearance (CLcr) as follows: CLcr ≥ 80 mL/min, 5.0 mg CBA/kg/day; CLcr 30–79 mL/min, 2.5–3.8 mg CBA/kg/day; and CLcr < 30 mL/min, 2.5 mg CBA/kg/day [37]. A previous cohort study published by our group included 13 patients with UTI caused by XDR P. aeruginosa and treated with CMS, and reported 84.6% of clinical cure [38]. In our study the average of CMS daily dose was 3 million IU per day (i.e. 1.42 mg CBA/kg/day in a patient with approximate body weight of 70 kg). Most recent clinical experience confirmed that intravenous CMS is an effective option for the treatment of UTI caused by MDR-GNB, even when it is administered at low doses. However, the limitations of most previous studies include a limited sample size, a wide range of CMS daily doses, antibiotic combination therapy and lack of pharmacokinetic data; therefore, it is difficult to draw conclusions about the optimal CMS dosage regimens for treating UTI.
In the present study, only 6 patients (18.2%) received the highest approved daily dose of CMS (i.e. 9 million IU) and 6 (18.2%) received the lowest recommended dose (i.e. < 3 million IU). These data confirms that despite recent recommendation of regulatory agencies [39] and those derived from modern population pharmacokinetic studies [34], physicians are still using outdated product or hospital CMS dosing recommendations, probably because their concern about nephrotoxicity. Nevertheless, no differences were observed in the clinical cure rate between patients receiving different CMS doses.
A key finding of this study is that the achieved Css,avg in daily clinical practice in our hospital was low. In fact, only 9% of patients achieved the therapeutic goal of Css (i.e 2.5 mg/L) and only 33.3% the optimal PK/PD index (i.e. AUC/MIC ≥ 60 mg·h/L). This finding is consistent with our previous studies [10,40]. Moreover, Css,avg achieved in this cohort match with the results of Css,avg expected by applying equation proposed by Nation et al [34], indicating that higher doses of CMS are required if higher Css,avg are needed.
The influence of renal function and hence the CMS daily dose on the Css,avg of formed colistin could not be demonstrated in this study, probably due to the small sample size. However, this study confirms the wide interpatient variability [21,34], even at a given range of GFR and after receiving the same range CMS doses, there were approximately 10-fold variations in Css,avg.
Clinical cure was achieved in 93.9% of patients. It is important to highlight that among patients achieving clinical cure, only 2 (6.5%) had achieved colistin plasma concentrations ≥ 2.5 mg/L and only 10 (32.3%) had attained an optimal AUC/MIC in plasma of 60 mg·h/L. If we focus on the group of patients treated with colistin monotherapy, clinical cure was achieved in 89.5% of patients. In the same way, only 5 (29.4%) have attained an optimal AUC/MIC and only 1 (5.9%) with the therapeutic level of formed colistin. However, it has to be considered that in almost 60% of all patients, the obtained Css,avg was above the MIC of the isolated P. aeruginosa, a fact probably related to the low MIC values of our isolates. Similar results were observed in the group of patients diagnosed with pyelonephritis since all of them were cured but none of them achieved the plasma concentration target and only 1 (11.1%), the optimal PK/PD index. Nevertheless, the fact that only 33.3% of them were treated with colistin monotherapy, prevent us from drawing consistent conclusions about the efficacy of this polymyxin in patients with pyelonephritis.
It is well known that the urinary recovery of CMS is high and CMS can be converted to colistin in vivo [18]. In a pharmacokinetic study in young volunteers treated with a single dose of 1 million IU (80 mg) CMS, Couet et al. estimated that the rate of CMS conversion into colistin was only 30% [41]. Importantly, in this study CMS was mainly excreted in urine (70% on average) and the conversion to colistin most likely occurred in the bladder [41]. A recent pharmacokinetic study performed by our group demonstrated that in 11 of 12 (91.7%) patients treated with CMS, concentrations of formed colistin in urine (up to 95.4 mg/L) were much higher than those achieved in plasma (median (IQR): 0.9 mg/L [<0.2–1.4 mg/L]) and the achieved colistin concentrations in urine were well above the MIC (0.5 mg/L) of the most predominant isolate of P. aeruginosa in our hospital [15]. Regarding the MIC of the XDR P. aeruginosa reported in the present study, it is important to point out that it is lower compared with other studies. In a study of 785 clinical isolates of XDR P. aeruginosa, Sader et. al reported an MIC50/90 of 1/2 mg/L[42]. In another study performed by the same group in 6091 bacterial isolates, MIC50/90 values were 1/1 mg/L [43]. Recently, a study performed in Spain reported MIC50/90 values of 2 mg/L [44]. In the present study, the high clinical cure rate obtained with suboptimal AUC/MIC values could be explained by the fact that a much higher colistin exposure was probably reached in the bladder and urinary tract than in plasma, but also to the low MIC of the XDR P. aeruginosa. Taken together, use of the targeted colistin AUC/MIC in plasma may be not suitable for predicting the efficacy of intravenous CMS in UTI patients and the measurement of concentrations of formed colistin in urine may be more useful. Clearly, urine samples should be collected on ice and analyzed as soon as possible to avoid any potential conversion of CMS to colistin during the sample collection procedure. In summary, our clinical experience in using intravenous CMS over the last decade supports the use of therapeutic drug monitoring (TDM) of colistin levels in plasma, as well as in urine in patients with UTI caused by XDR-GNB.
In the present study, microbiological clearance was 82.6% in the 23 patients that had microbiological follow up and no colistin-resistant isolates were detected during the next 30 days. The fact that microbiological clearance was a little lower than the clinical cure rate might be due to colonization of MDR GNB in the urinary tract of several patients. The prevalence of asymptomatic bacteriuria varies widely with age, gender and the presence of genitourinary abnormalities [45]. Patients with chronic disabilities or comorbidities characterized by impaired urinary voiding or with indwelling urinary devices are at high risk of asymptomatic bacteriuria, and in this scenario hospitalized patients are a group in which this medical condition is frequently present.
The rate of AKI at EOT in our series of patients was 30.3% and most of them were classified as “injury” by the RIFLE criteria. The reported rate of colistin-associated nephrotoxicity in recent studies using the RIFLE criteria varied from 40–50% [46,47]. In our multivariate analysis, the only factors that showed a trend towards statistically significance were the Charlson score and Css,avg. The association between colistin plasma concentrations and nephrotoxicity has previously been reported by our group that demonstrated that Css,avg > 2.42 mg/L was associated with a higher risk of AKI at EOT [46].
The high clinical response rate observed in this study and the risk of colistin-associated nephrotoxicity highlights the need for routine therapeutic drug monitoring of colistin in daily clinical practice, in order to maximize clinical efficacy while minimizing the nephrotoxicity. In addition, the measurement of colistin plasma levels in urine could be a useful tool to examine if the exposure of colistin is sufficient at the site of infection. Unfortunately, current data on TDM of colistin in clinical practice are sparse [40,48], despite that it is strongly recommended by recent consensus documents [9,13,49,50]. Currently, several new antimicrobial agents for the treatment of MDR and XDR P. aeruginosa such as ceftolozane-tazobactam and ceftazidime-avibactam are available in daily clinical practice. Overall these agents appear to be safer and more effective than colistin [51]. However, resistance to ceftolozane-tazobactam and ceftazidime-avibactam emerged, in some cases, during treatment [4,52,53] suggesting that there is still a need for the use of polymyxins and indicating that there will be a place for them in the future.
To the best of our knowledge, this is the largest study of UTI caused by XDR P. aeruginosa treated with intravenous CMS and the first clinical study assessing the relationship between colistin PK/PD target in plasma and clinical outcomes. Our study has several limitations, such as the small sample size and the fact that about 40% of the patients were treated with combined therapy, especially in patients with pyelonephritis. In addition, the lack of pharmacokinetic data in urine prevented us from calculating the AUC/MIC of formed colistin in urine. We suggest that using lower CMS doses than the currently recommended dosage regimens could be reasonable for the treatment of lower UTI caused by XDR P. aeruginosa with low colistin MIC values. This strategy could lead to lower rates of nephrotoxicity while maintaining the same effectiveness and minimizing the use of this last-line antibiotic. However, given that the microbiological clearance rate seems to be lower than the rate of clinical cure as several patients might have bacterial colonization in the urinary tract, caution is required with regard to the emergence of resistance with lower doses of CMS. Collectively, further prospective clinical studies are required and measuring colistin concentrations in urine is crucial for maximizing the efficacy of intravenous CMS against UTIs.
Acknowledgements
Funding. This work was supported by Fondo de Investigación Sanitaria (FIS) from Instituto de Salud Carlos III, Spanish Ministry of Health, FEDER, Grant numberPS09/01634 and from Spanish Ministry of Health and Social Policy, General Pharmacy Subdirection, Grant numbers EC10-165 and EC11-318. J.L. is an Australian National Health and Medical Research Council (NHMRC) Principal Research Fellow and supported by a research grant from the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (R01 AI132154).
Footnotes
Conflicts of interest. JPH has received grant from MSD, has participated in educational activities with MSD, Pfizer and Astellas and collaborated as advisor for Angelini, MSD Zambon, Shionogi and Pfizer. SG has participated in educational activities with Pfizer, Angellini, MSD and Astellas. Other authors declare that they have no competing interests. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health.
References
- [1].Lamas Ferreiro JL, Álvarez Otero J, González González L, Novoa Lamazares L, Arca Blanco A, Bermúdez Sanjurjo JR, et al. Pseudomonas aeruginosa urinary tract infections in hospitalized patients: Mortality and prognostic factors. PLoS One 2017;12:e0178178. doi: 10.1371/journal.pone.0178178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Payne DJ, Gwynn MN, Holmes DJ, Pompliano DL. Drugs for bad bugs: confronting the challenges of antibacterial discovery. Nat Rev Discov 2007;6:29–40. doi: 10.1038/nrd2201. [DOI] [PubMed] [Google Scholar]
- [3].van Duin D, Bonomo RA. Ceftazidime/Avibactam and Ceftolozane/Tazobactam: Second-generation β-Lactam/β-Lactamase Inhibitor Combinations. Clin Infect Dis 2016;63:234–41. doi: 10.1093/cid/ciw243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Haidar G, Philips NJ, Shields RK, Snyder D, Cheng S, Potoski BA, et al. Ceftolozane-tazobactam for the treatment of multidrug-resistant Pseudomonas aeruginosa infections: clinical effectiveness and evolution of resistance. Clin Infect Dis 2017;65:110–20. doi: 10.1093/cid/cix182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Shields RK, Potoski BA, Haidar G, Hao B, Doi Y, Chen L, et al. Clinical outcomes, drug toxicity, and emergence of ceftazidime-avibactam resistance among patients treated for carbapenem-resistant Enterobacteriaceae infections. Clin Infect Dis 2016;63:1615–8. doi: 10.1093/cid/ciw636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bassetti M, Vena A, Russo A, Croxatto A, Calandra T, Guery B. Rational approach in the management of Pseudomonas aeruginosa infections. Curr Opin Infect Dis 2018;31:1. doi: 10.1097/QCO.0000000000000505. [DOI] [PubMed] [Google Scholar]
- [7].Wright H, Bonomo RA, Paterson DL. New agents for the treatment of infections with Gram-negative bacteria: restoring the miracle or false dawn? Clin Microbiol Infect 2017;23:704–12. doi: 10.1016/j.cmi.2017.09.001. [DOI] [PubMed] [Google Scholar]
- [8].Jean S-S, Gould IM, Lee W-S, Hsueh P-R , International Society of Antimicrobial Chemotherapy (ISAC). New drugs for multidrug-resistant gram-negative organisms: time for stewardship. Drugs 2019. doi: 10.1007/s40265-019-01112-1. [DOI] [PubMed] [Google Scholar]
- [9].Nation RL, Li J, Cars O, Couet W, Dudley MN, Kaye KS, et al. Framework for optimisation of the clinical use of colistin and polymyxin B: the Prato polymyxin consensus. Lancet Infect Dis 2014. doi: 10.1016/S1473-3099(14)70850-3. [DOI] [PubMed] [Google Scholar]
- [10].Sorlí L, Luque S, Grau S, Berenguer N, Segura C, Montero MM, et al. Trough colistin plasma level is an independent risk factor for nephrotoxicity: a prospective observational cohort study. BMC Infect Dis 2013;13:380. doi: 10.1186/1471-2334-13-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Horcajada JP, Sorlí L, Luque S, Benito N, Segura C, Campillo N, et al. Validation of a colistin plasma concentration breakpoint as a predictor of nephrotoxicity in patients treated with colistin methanesulfonate. Int J Antimicrob Agents 2016;48:725–7. doi: 10.1016/j.ijantimicag.2016.08.020. [DOI] [PubMed] [Google Scholar]
- [12].Bergen PJ, Li J, Rayner CR, Nation RL. Colistin methanesulfonate is an inactive prodrug of colistin against Pseudomonas aeruginosa. Antimicrob Agents Chemother 2006;50:1953–8. doi: 10.1128/AAC.00035-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Pogue JM, Ortwine JK, Kaye KS. Clinical considerations for optimal use of the polymyxins: A focus on agent selection and dosing. Clin Microbiol Infect 2017;23:229–33. doi: 10.1016/j.cmi.2017.02.023. [DOI] [PubMed] [Google Scholar]
- [14].Li J, Milne RW, Nation RL, Turnidge JD, Smeaton TC, Coulthard K. Pharmacokinetics of colistin methanesulphonate and colistin in rats following an intravenous dose of colistin methanesulphonate. J Antimicrob Chemother 2004;53:837–40. [DOI] [PubMed] [Google Scholar]
- [15].Luque S, Escaño C, Sorli L, Li J, Campillo N, Horcajada JP, et al. Urinary Concentrations of colistimethate and formed colistin after intravenous administration in patients with multidrug-resistant gram-negative bacterial infections. Antimicrob Agents Chemother 2017;61:e02595–16. doi: 10.1128/AAC.02595-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Zhao M, Wu X-J, Fan Y-X, Zhang Y, Guo B-N, Yu J, et al. Pharmacokinetics of colistin methanesulfonate (CMS) in healthy Chinese subjects after single and multiple intravenous doses. Int J Antimicrob Agents 2018;51:714–20. doi: 10.1016/j.ijantimicag.2017.12.025. [DOI] [PubMed] [Google Scholar]
- [17].Couet W, Gregoire N, Marchand S, Mimoz O. Colistin pharmacokinetics: the fog is lifting. Clin Microbiol Infect 2012;18:30–9. doi: 10.1111/j.1469-0691.2011.03667.x. [DOI] [PubMed] [Google Scholar]
- [18].Li J, Nation RL, Turnidge JD, Milne RW, Coulthard K, Rayner CR, et al. Colistin: the re-emerging antibiotic for multidrug-resistant Gram-negative bacterial infections. Lancet Infect Dis 2006;6:589–601. doi: 10.1016/S1473-3099(06)70580-1. [DOI] [PubMed] [Google Scholar]
- [19].Dudhani RV, Turnidge JD, Nation RL, Li J fAUC/MIC is the most predictive pharmacokinetic/pharmacodynamic index of colistin against Acinetobacter baumannii in murine thigh and lung infection models. J Antimicrob Chemother 2010;65:1984–90. doi: 10.1093/jac/dkq226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Dudhani RV, Turnidge JD, Coulthard K, Milne RW, Rayner CR, Li J, et al. Elucidation of the pharmacokinetic/pharmacodynamic determinant of colistin activity against Pseudomonas aeruginosa in murine thigh and lung infection models. Antimicrob Agents Chemother 2010;54:1117–24. doi: 10.1128/AAC.01114-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Garonzik SM, Li J, Thamlikitkul V, Paterson DL, Shoham S, Jacob J, et al. Population pharmacokinetics of colistin methanesulfonate and formed colistin in critically ill patients from a multicenter study provide dosing suggestions for various categories of patients. Antimicrob Agents Chemother 2011;55:3284–94. doi: 10.1128/AAC.01733-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol 1994;47:1245–51. [DOI] [PubMed] [Google Scholar]
- [23].Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med 1985;13:818–29. [PubMed] [Google Scholar]
- [24].Dellinger RP, Levy MM, Carlet JM, Bion J, Parker MM, Jaeschke R, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit. Care Med, vol. 36, 2008, p. 296–327. doi: 10.1097/01.CCM.0000298158.12101.41. [DOI] [PubMed] [Google Scholar]
- [25].Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P, workgroup ADQI. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 2004;8:R204–12. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Ricci Z, Cruz D, Ronco C. The RIFLE criteria and mortality in acute kidney injury: A systematic review. Kidney Int 2008;73:538–46. doi: 10.1038/sj.ki.5002743. [DOI] [PubMed] [Google Scholar]
- [27].Magiorakos A-P, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 2012;18:268–81. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- [28].CDC, NHSN. CDC/NHSN surveillance definition of healthcare-associated infection and criteria for specific types of infections in the acute care Setting. January, 2014. Http://WwwCdcGov/Nhsn/Pdfs/Pscmanual/17pscnosinfdef_currentPdf 2014. doi: 10.1016/j.ajic.2008.03.002. [DOI] [PubMed]
- [29].Institute Clinical and Laboratory Standards. Performance Standards of Antimicrobial Susceptibility Test. Information Supplementary. M100-CLSI, Wayne, PA: 2013. [Google Scholar]
- [30].Plachouras D, Karvanen M, Friberg LE, Papadomichelakis E, Antoniadou A, Tsangaris I, et al. Population pharmacokinetic analysis of colistin methanesulfonate and colistin after intravenous administration in critically ill patients with infections caused by gram-negative bacteria. Antimicrob Agents Chemother 2009;53:3430–6. doi: 10.1128/AAC.01361-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Li J, Milne RW, Nation RL, Turnidge JD, Coulthard K, Johnson DW. A simple method for the assay of colistin in human plasma, using pre-column derivatization with 9-fluorenylmethyl chloroformate in solid-phase extraction cartridges and reversed-phase high-performance liquid chromatography. J Chromatogr Biomed Sci Appl 2001;761:167–75. [DOI] [PubMed] [Google Scholar]
- [32].Cheah S-E, Wang J, Nguyen VTT, Turnidge JD, Li J, Nation RL. New pharmacokinetic/pharmacodynamic studies of systemically administered colistin against Pseudomonas aeruginosa and Acinetobacter baumannii in mouse thigh and lung infection models: smaller response in lung infection. J Antimicrob Chemother 2015;70:3291–7. doi: 10.1093/jac/dkv267. [DOI] [PubMed] [Google Scholar]
- [33].Forrest A, Garonzik SM, Thamlikitkul V, Giamarellos-Bourboulis EJ, Paterson DL, Li J, et al. Pharmacokinetic/Toxicodynamic analysis of colistin-associated acute kidney injury in critically ill patients. Antimicrob Agents Chemother 2017;61. doi: 10.1128/AAC.01367-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Nation RL, Garonzik SM, Thamlikitkul V, Giamarellos-Bourboulis EJ, Forrest A, Paterson DL, et al. Dosing guidance for intravenous colistin in critically-ill patients. Clin Infect Dis 2016;64:ciw839. doi: 10.1093/cid/ciw839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Nation RL, Garonzik SM, Li J, Thamlikitkul V, Giamarellos-Bourboulis EJ, Paterson DL, et al. Updated US and European dose recommendations for intravenous colistin: how do they perform? Clin Infect Dis 2016;62:552–8. doi: 10.1093/cid/civ964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Zaidi STR, Al Omran S, Al Aithan ASM, Al Sultan M. Efficacy and safety of low-dose colistin in the treatment for infections caused by multidrug-resistant gram-negative bacteria. J Clin Pharm Ther 2014. doi: 10.1111/jcpt.12138. [DOI] [PubMed] [Google Scholar]
- [37].Cheng CY, Sheng WH, Wang JT, Chen YC, Chang SC. Safety and efficacy of intravenous colistin (colistin methanesulphonate) for severe multidrug-resistant Gram-negative bacterial infections. Int J Antimicrob Agents 2010;35:297–300. doi: 10.1016/j.ijantimicag.2009.11.016; 10.1016/j.ijantimicag.2009.11.016. [DOI] [PubMed] [Google Scholar]
- [38].Montero M, Horcajada JP, Sorli L, Alvarez-Lerma F, Grau S, Riu M, et al. Effectiveness and safety of colistin for the treatment of multidrug-resistant Pseudomonas aeruginosa infections. Infection 2009;37:461–5. doi: 10.1007/s15010-009-8342-x. [DOI] [PubMed] [Google Scholar]
- [39].European Medicines Agency completes review of polymyxin-based medicines | European Medicines Agency n.d. https://www.ema.europa.eu/news/european-medicines-agency-completes-review-polymyxin-based-medicines (accessed November 23, 2018).
- [40].Sorlí L, Luque S, Segura C, Campillo N, Montero M, Esteve E, et al. Impact of colistin plasma levels on the clinical outcome of patients with infections caused by extremely drug-resistant Pseudomonas aeruginosa. BMC Infect Dis 2017;17:11. doi: 10.1186/s12879-016-2117-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Couet W, Gregoire N, Gobin P, Saulnier PJ, Frasca D, Marchand S, et al. Pharmacokinetics of colistin and colistimethate sodium after a single 80-mg intravenous dose of CMS in young healthy volunteers. Clin Pharmacol Ther 2011;89:875–9. doi: 10.1038/clpt.2011.48. [DOI] [PubMed] [Google Scholar]
- [42].Sader HS, Flamm RK, Dale GE, Rhomberg PR, Castanheira M. Murepavadin activity tested against contemporary (2016–17) clinical isolates of XDR Pseudomonas aeruginosa. J Antimicrob Chemother 2018;73:2400–4. doi: 10.1093/jac/dky227. [DOI] [PubMed] [Google Scholar]
- [43].Sader HS, Farrell DJ, Flamm RK, Jones RN. Antimicrobial susceptibility of Gram-negative organisms isolated from patients hospitalized in intensive care units in United States and European hospitals (2009–2011). Diagn Microbiol Infect Dis 2014;78:443–8. doi: 10.1016/j.diagmicrobio.2013.11.025. [DOI] [PubMed] [Google Scholar]
- [44].del Barrio-Tofiño E, López-Causapé C, Cabot G, Rivera A, Benito N, Segura C, et al. Genomics and susceptibility profiles of extensively drug-resistant Pseudomonas aeruginosa isolates from Spain. Antimicrob Agents Chemother 2017;61. doi: 10.1128/AAC.01589-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Gupta K, Hooton TM, Naber KG, Wullt B, Colgan R, Miller LG, et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: A 2010 Update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis 2011;52:e103–20. doi: 10.1093/cid/ciq257. [DOI] [PubMed] [Google Scholar]
- [46].Sorlí L, Luque S, Grau S, Berenguer N, Segura C, Montero MM, et al. Trough colistin plasma level is an independent risk factor for nephrotoxicity: a prospective observational cohort study. BMC Infect Dis 2013;13:380. doi: 10.1186/1471-2334-13-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Dalfino L, Puntillo F, Mosca A, Monno R, Spada ML, Coppolecchia S, et al. High-dose, extended-interval colistin administration in critically ill patients: is this the right dosing strategy? A preliminary study. Clin Infect Dis 2012;54:1720–6. doi: 10.1093/cid/cis286; 10.1093/cid/cis286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Tafelski S, Wagner L, Angermair S, Deja M, ABx Study Group. Therapeutic drug monitoring for colistin therapy in severe multi-resistant Acinetobacter intracerebral abscess: A single case study with high-dose colistin and review of literature. SAGE Open Med Case Reports 2017;5:2050313X17711630. doi: 10.1177/2050313X17711630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Ortwine JK, Kaye KS, Li J, Pogue JM. Colistin: understanding and applying recent pharmacokinetic advances. Pharmacotherapy 2015;35:11–6. doi: 10.1002/phar.1484. [DOI] [PubMed] [Google Scholar]
- [50].Grégoire N, Aranzana-Climent V, Magréault S, Marchand S, Couet W. Clinical pharmacokinetics and pharmacodynamics of colistin. Clin Pharmacokinet 2017;56:1441–60. doi: 10.1007/s40262-017-0561-1. [DOI] [PubMed] [Google Scholar]
- [51].van Duin D, Lok JJ, Earley M, Cober E, Richter SS, Perez F, et al. Colistin versus ceftazidime-avibactam in the treatment of infections due to carbapenem-resistant Enterobacteriaceae. Clin Infect Dis 2018;66:163–71. doi: 10.1093/cid/cix783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Bassetti M, Castaldo N, Cattelan A, Mussini C, Righi E, Tascini C, et al. Ceftolozane/tazobactam for the treatment of serious P. aeruginosa infections: a multicenter nationwide clinical experience. Int J Antimicrob Agents 2018. doi: 10.1016/j.ijantimicag.2018.11.001. [DOI] [PubMed] [Google Scholar]
- [53].Humphries RM, Hemarajata P. Resistance to ceftazidime-avibactam in Klebsiella pneumoniae due to porin mutations and the increased expression of KPC-3. Antimicrob Agents Chemother 2017;61. doi: 10.1128/AAC.00537-17. [DOI] [PMC free article] [PubMed] [Google Scholar]