Abstract
Background
Congenital talipes equinovarus (CTEV), also known as clubfoot, is a common congenital orthopaedic condition characterised by an excessively turned‐in foot (equinovarus) and high medial longitudinal arch (cavus). If left untreated it can result in long‐term disability, deformity and pain. Interventions can be conservative (such as splinting or stretching) or surgical. Different treatments might be effective at different stages: at birth (initial presentation); when initial treatment does not work (resistant presentation); when the initial treatment works but the clubfoot returns (relapse/recurrent presentation); and when there has been no early treatment (neglected presentation). This is an update of a review first published in 2010 and last updated in 2014.
Objectives
To assess the effects of any intervention for any type of CTEV in people of any age.
Search methods
On 28 May 2019, we searched the Cochrane Neuromuscular Specialised Register, CENTRAL, MEDLINE, Embase, CINAHL Plus, AMED and Physiotherapy Evidence Database. We also searched for ongoing trials in the WHO International Clinical Trials Registry Platform and ClinicalTrials.gov (to May 2019). We checked the references of included studies.
Selection criteria
Randomised controlled trials (RCTs) and quasi‐RCTs evaluating interventions for CTEV, including interventions compared to other interventions, sham intervention or no intervention. Participants were people of all ages with CTEV of either one or both feet.
Data collection and analysis
Two review authors independently assessed the risks of bias in included trials and extracted the data. We contacted authors of included trials for missing information. We collected adverse event information from trials when it was available. When required we attempted to obtain individual patient data (IPD) from trial authors for re‐analysis. If unit‐of‐analysis issues were present and IPD unavailable we did not report summary data,
Main results
We identified 21 trials with 905 participants; seven trials were newly included for this update. Fourteen trials assessed initial cases of CTEV (560 participants), four trials assessed resistant cases (181 participants) and three trials assessed cases of unknown timing (153 participants). The use of different outcome measures prevented pooling of data for meta‐analysis, even when interventions and participants were comparable. All trials displayed high or unclear risks of bias in three or more domains. Twenty trials provided data. Two trials reported on the primary outcome of function using a validated scale, but the data were not suitable for inclusion because of unit‐of‐analysis issues, as raw data were not available for re‐analysis.
We were able to analyse data on foot alignment (Pirani score), a secondary outcome, from three trials in participants at initial presentation. The Pirani score is a scale ranging from zero to six, where a higher score indicates a more severe foot. At initial presentation, one trial reported that the Ponseti technique significantly improved foot alignment compared to the Kite technique. After 10 weeks of serial casting, the average total Pirani score of the Ponseti group was 1.15 points lower than that of the Kite group (mean difference (MD) −1.15, 95% confidence interval (CI) −1.32 to −0.98; 60 feet; low‐certainty evidence). A second trial found the Ponseti technique to be superior to a traditional technique, with mean total Pirani scores of the Ponseti participants 1.50 points lower than after serial casting and Achilles tenotomy (MD −1.50, 95% CI −2.28 to −0.72; 28 participants; very low‐certainty evidence). One trial found evidence that there may be no difference between casting materials in the Ponseti technique, with semi‐rigid fibreglass producing average total Pirani scores 0.46 points higher than plaster of Paris at the end of serial casting (95% CI −0.07 to 0.99; 30 participants; low‐certainty evidence).
We found no trials in relapsed or neglected cases of CTEV.
A trial in which the type of presentation was not reported showed no evidence of a difference between an accelerated Ponseti and a standard Ponseti treatment in foot alignment. At the end of serial casting, the average total Pirani score in the accelerated group was 0.31 points higher than the standard group (95% CI −0.40 to 1.02; 40 participants; low‐certainty evidence).
No trial assessed gait using a validated assessment. Health‐related quality of life was reported in some trials but data were not available for re‐analysis.
There is a lack of evidence for the addition of botulinum toxin A during the Ponseti technique, different types of major foot surgery or continuous passive motion treatment following major foot surgery. Most trials did not report on adverse events. Two trials found that further serial casting was more likely to correct relapse after Ponseti treatment than after the Kite technique, which more often required major surgery (risk differences 25% and 50%). In trials evaluating serial casting techniques, adverse events included cast slippage (needing replacement), plaster sores (pressure areas), and skin irritation. Adverse events following surgical procedures included infection and the need for skin grafting.
Authors' conclusions
From the evidence available, the Ponseti technique may produce significantly better short‐term foot alignment compared to the Kite technique. The certainty of evidence is too low for us to draw conclusions about the Ponseti technique compared to a traditional technique. An accelerated Ponseti technique may be as effective as a standard technique, but results are based on a single small comparative trial. When using the Ponseti technique semi‐rigid fibreglass casting may be as effective as plaster of Paris. Relapse following the Kite technique more often led to major surgery compared to relapse following the Ponseti technique. We could draw no conclusions from other included trials because of the limited use of validated outcome measures and the unavailability of raw data. Future RCTs should address these issues.
Plain language summary
Interventions for congenital talipes equinovarus (clubfoot)
Review question
The purpose of this review was to assess treatments for congenital talipes equinovarus (clubfoot).
Background
Clubfoot is a condition, present at birth, in which the foot is in an inturned position. There is no known cause. Different treatments might be effective at different stages: at birth (initial presentation); when initial treatment does not work (resistant presentation); when the initial treatment works but the clubfoot returns (relapse/recurrent presentation); and when there has been no early treatment (neglected presentation). Treatment aims to put the foot back into a normal position and to be pain‐free throughout life.
Treatment can be non‐surgical, surgical or both. Non‐surgical treatment (for example, casting or stretches) gently stretches the foot into a normal position. Surgery may involve the muscles, tendons, ligaments or joints. Kite and Ponseti techniques both involve prolonged joint manipulation and serial casting to correct foot alignment. The Ponseti technique involves manipulation (of the ankle joint) and usually Achilles tendon surgery, while Kite is a technique involving manipulation of the foot.
Study characteristics
From our searches we found 21 trials with 905 participants. All trials had problems of design or conduct that might have affected the results. Treatments were studied at birth (14 trials, 560 participants), during relapse (four trials, 181 participants), or at an unknown time (three trials, 153 participants). We required studies to have used 'validated' measures (i.e. shown to be reliable, consistent, and sensitive to change). Many trials did not take bilateral cases (children with two affected feet) into account during randomisation and statistical analysis. For these reasons, we were unable to include much of the data from the trials in the review.
Results and certainty of the evidence
Our main measure of the success of treatment was function (how well the foot worked in everyday life). Two trials reported on function but data were not available to re‐analyse.
Three trials that compared Ponseti with other casting techniques in children treated at birth provided data that we could analyse on foot alignment. One found that foot position may be better after Ponseti plaster casting than after Kite plaster casting. In the second trial, the evidence was uncertain whether foot position was better after Ponseti plaster casting than after a traditional technique (another type of plaster casting). One trial found that weekly Ponseti casting may be as good as Ponseti plaster casting three times a week (accelerated Ponseti). This trial did not state at which stage the treatment was done. A third trial found that the Ponseti technique may have similar results when using plaster of Paris or semi‐rigid fibreglass.
No trial assessed the quality of walking using a validated assessment. Two trials reported on the primary outcome of function using validated scales, but raw data were not available for analysis and the trials did not provide quality‐of‐life data that were suitable for reporting in the review.
We found no trials in relapsed or neglected clubfoot.
A trial in which the type of presentation was not reported found that there may be no important difference between an accelerated Ponseti or standard Ponseti treatment in foot alignment.
Relapse following the Kite technique more often required major surgery than relapse following the Ponseti technique. Data were not available to assess the results for adding botulinum toxin A, which temporarily weakens injected muscles, to the Ponseti treatment, using different types of plaster casts in the Ponseti treatment, different foot surgeries, or the treatment of relapsed or neglected clubfoot. Most trials did not report on harmful effects. When reported, harmful effects during plaster casting included casts slipping, plaster sores, and skin irritation. Harmful effects of infection and skin grafting were reported after surgery.
The searches for the review are up to date to May 2019.
Summary of findings
Background
Description of the condition
Congenital talipes equinovarus (CTEV), also known as clubfoot, is a common congenital paediatric condition, occurring in 1 to 2 per 1000 newborns (Dobbs 2006). It is characterised by an excessively turned‐in foot (equinovarus) and high medial longitudinal arch (cavus), which if left untreated leads to long‐term functional disability, deformity and pain (Ponseti 2005). CTEV is thought to begin as the limb buds form and can be diagnosed on ultrasound from 12 weeks gestation (Keret 2002). There are two types of CTEV: idiopathic (isolated) and syndromic (those associated with other syndromes or conditions). In both the cause is unknown, although emerging literature suggests a polygenic cause (Dobbs 2009; Pavone 2018), which may be influenced by external factors such as maternal smoking (Hackshaw 2011). Syndromic CTEV is often severe and more resistant to treatment (Janicki 2009).
Description of the intervention
Intervention can occur at different stages: initial presentation (where there has been no prior intervention), resistant presentation (following unsuccessful initial treatment), relapsed presentation (when there is a return of part or all components of the deformity) and neglected presentation (where no early initial intervention was undertaken).
The treatment of CTEV is usually conservative in initial cases, with surgical options reserved for correction of any resistant (remaining) deformity. Conservative treatment includes stretching, for example, the French functional method (Richards 2008); varied serial casting (e.g. plaster casts) and bracing, including Ponseti and Kite techniques (Chong 2014; Hui 2014; Pittner 2008); minor surgical intervention, for example, Achilles tenotomy (release of the heel cord), tibialis anterior tendon transfer (moving a muscle in the foot) and Achilles lengthening (lengthening of the calf muscle); the use of external fixator devices (surgical application of a metal brace) (Ponseti 2005); and botulinum toxin injections (Alvarez 2005).
The Ponseti technique is currently the most practised treatment with excellent long‐term outcomes (30 years) (Cooper 1995). This technique involves six to eight weeks of long leg plaster casts (toe to groin) with gentle manipulation around the talar head (a part of the ankle joint). Casts are changed once a week. Up to 90% of cases require an Achilles tenotomy to correct remaining equinus (heel cord tightness) deformity (Haft 2007). This is considered part of routine treatment. Patients are then required to wear boots and a bar brace for 23 hours a day for three months and then during sleep until four years of age (Ponseti 2005). The Ponseti technique has been shown to significantly reduce the need for major foot surgery (Morcuende 2004; Zionts 2010).
The Kite technique was widely practised until the emergence of the Ponseti technique. The Kite technique involves long leg plaster casts (toe to groin) with manipulation occurring around the calcaneo‐cuboid joint (a joint in the foot) (Kite 1972). Casting may continue for up to two years (Dobbs 2009), with 50% to 75% of cases requiring major surgical intervention (Lovell 1979).
Unfortunately, with all treatments relapses are common and may occur in up to 37% of children within two years (Richards 2008), and in up to 47% before four years of age (Laaveg 1980). Causes of relapse include non‐compliance with bracing regimens (such as the Ponseti method) (Morcuende 2004), relative overactivity of the tibialis anterior tendon (Ponseti 2005), and progressive neuromuscular disease (Lovell 2007; Masrouha 2012). When left untreated, the foot gradually returns to its original position. In mild cases the child may overload the lateral border of their foot during walking and in extreme cases may walk on the outside border of the foot (cuboid and fifth metatarsal) with resulting callosities and pain.
In children with relapsed CTEV, intervention is required to prevent further progressive deformity. Historically, relapses were treated with major surgical intervention including muscle, ligament and joint releases (for example, posteromedial soft tissue release) or bony operations (for example, wedge osteotomies) (Dobbs 2000). Long‐term observational studies have found poorer outcomes in those treated with major foot surgery (Dobbs 2006; Graf 2010; Ippolito 2003). Clinicians are therefore beginning to use the same conservative techniques used in initial CTEV to treat relapses (Marquez 2017; Nogueira 2009; Van Praag 2018).
How the intervention might work
Frequent stretching and active assisted movement (for example, the French functional method) have been shown to be effective in achieving good joint alignment in children less than two years old with CTEV (Richards 2007).
Serial plaster‐casting (for example, the Ponseti and Kite techniques) involving sustained stretching for an extended period, is thought to improve the extensibility of surrounding tissue and joint capsules. Magnetic resonance imaging studies of babies with CTEV show that musculature, ligament and bony changes are possible with weekly Ponseti casting (Pirani 2001). Studies have demonstrated increases in both the length and numbers of sarcomeres when a muscle is immobilised in a lengthened position for an extended period of time (Cusick 1990).
Minor joint‐sparing surgical procedures (i.e. those that do not involve the ankle or foot joints), are thought to result in good long‐term outcomes, with pain‐free feet (Dietz 2006). Examples include Achilles tenotomies (surgical release of the Achilles tendon), which have been shown in very young children to result in direct elongation of the tendon (De Gheldere 2008; Radler 2007), and tibialis anterior tendon transfer, which aims to restore the balance of musculature around the foot by making the tibialis anterior muscle pull the foot directly up rather than up and twisting in (with the big toe up) (Gray 2014b; Kuo 2001; Laaveg 1980). In severe cases, relapsed CTEV may require a combination of these procedures or major bone or joint surgery to correct the position of the foot and ankle.
Botulinum toxin, a potent neuromuscular agent, causes partial local temporary muscular weakness or paralysis, allowing for lengthening through sustained stretching (for example, serial casting). When used in the triceps surae (calf muscle), it may prevent the need for Achilles tenotomy or other major surgery (Alvarez 2005).
Why it is important to do this review
The treatment of CTEV remained varied and inconsistent until the Ponseti technique became widely practised. This technique has shown favourable long‐term outcomes (Cooper 1995), but relapses are common. A systematic review of all interventions for initial and relapsed CTEV will assist the clinician in providing the most effective treatment and allow for ongoing evaluation of these interventions in the future. This review was first published in 2012 and updated in 2014 and 2020. We undertook the update to assess recent RCTs.
Objectives
To assess the effects of any intervention for any type of congenital talipes equinovarus in people of any age.
Methods
Criteria for considering studies for this review
Types of studies
All randomised controlled trials (RCTs) or quasi‐RCTs of interventions for the treatment of CTEV. Quasi‐RCTs are those where systematic methods of allocation are used, for example, date of birth, or hospital number. Randomised cross‐over trials were eligible.
Types of participants
Any type of CTEV in people of any age.
Types of interventions
We included any intervention aimed at reducing or eliminating the deformity associated with CTEV (for example, cavus, adductus, varus and equinus). Studies could either compare an intervention with a control (sham intervention or no intervention) or with another intervention. Studies could include but were not limited to the following interventions:
stretching: for example, passive and active stretching using taping or plaster casts (serial casting);
surgery: for example, muscle lengthening, tendon transfers, osteotomies (operations on bone) and external fixators (surgically‐applied brackets which can stretch joints);
other: for example, botulinum toxin.
Types of outcome measures
The outcomes listed here are not eligibility criteria for this review, but are outcomes of interest within whichever studies are included.
Primary outcomes
Function: self‐reported or parent‐ or proxy‐reported day‐to‐day function at a minimum of one year post‐treatment, as measured by any validated assessment tool, for example, Clubfoot Disease Specific Index (DSI) (Dietz 2009); physical component of the Child Health Questionnaire (CHQ) (Landgraf 1999); and physical subscale of the Short Form 36 Health Survey (SF‐36) (Ware 1992).
Studies with different follow‐up periods were to be combined with appropriate adjustments if the assumption of steady rates of change was justified. This was not possible with the data available.
Secondary outcomes
Foot alignment: measured by any validated assessment tool, for example, radiographic, Foot Posture Index (Redmond 2006), Diméglio scale (Diméglio 1995), Pirani score (Pirani 2008).
Gait assessment: for example, pedobarography, 3D kinematics.
Parent‐ or participant‐reported health‐related quality of life: measured by any validated assessment tool, for example, Child Health Questionnaire (CHQ).
Adverse events: classified as 'any adverse event', 'adverse event leading to withdrawal of treatment', and 'life‐threatening (severe) adverse event' (requiring admission to hospital or adverse outcome leading to permanent disability or death).
We required data from valid assessments to be eligible for quantitative analysis in this review. Measures were to be assessed at a minimum of one year; however, some outcomes (for example, foot alignment) measured at the end of treatment determined whether further alternative treatment such as surgery was required. We have therefore included findings at the end of treatment, when available.
We had planned to combine studies with different follow‐up periods with appropriate adjustments if the assumption of steady rates of change was justified, but this was not possible with the data available.
Search methods for identification of studies
Electronic searches
The Cochrane Information Specialist searched the following databases on 28 May 2019.
Cochrane Neuromuscular Specialised Register via the Cochrane Register of Studies (CRS‐Web; Appendix 1).
Cochrane Central Register of Controlled Trials (CENTRAL) via CRS‐Web (Appendix 2).
MEDLINE (1946 to May 2019; Appendix 3).
Embase (1974 to May 2019; Appendix 4).
AMED (1985 to May 2019; Appendix 5).
CINAHL (1937 to May 2019; Appendix 6).
The review authors searched PEDro and the trials registries for ongoing trials.
PEDro (1929 to May 2019; Appendix 7).
World Health Organization International Clinical Trials Registry Platform (ICTRP; apps.who.int/trialsearch/; Appendix 8).
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov; Appendix 9).
There were no language or publication restrictions.
We did not search the NHS Economic Evalluation Database (NHSEED), Database of Abstracts of Reviews of Effectiveness (DARE) or Health Technology Assessment Database (HTA) for this update, as they are no longer being updated in the Cochrane Library.
Searching other resources
We reviewed the bibliographies of the trials identified and, if appropriate, contacted the trial authors as well as known experts in the field to identify additional studies. We handsearched the reference sections of retrieved articles, relevant thesis publications, and the reports or conference proceedings of relevant symposia. We contacted registered expert clinicians in the field to identify additional published or unpublished data. We specified no limitations on language or year published.
Data collection and analysis
Selection of studies
Two review authors (KG and SB, or KG and VP) independently assessed the titles and abstracts of trials identified by the search. The same two review authors checked full‐text copies of potentially relevant trials to determine eligibility based on inclusion criteria.
We did not mask study authorship and results during the study selection process, as the effect of assessor masking has not been established by empirical evidence (Higgins 2011). The review authors resolved disagreements by discussion and, if necessary, by arbitration from third or fourth review authors (JB and VP). Arbitration by the third or fourth review authors resolved all disputes so we did not contact study authors for additional information.
Data extraction and management
Two review authors (KG and SB, or KG and VP) independently extracted data using separate, standardised, prepared forms. We contacted trial authors to provide any missing information. One review author (SB or KG) entered data into the Cochrane statistical software, Review Manager 5 (RevMan 2014), and a second (KG or VP) checked the data entry. The review authors resolved disagreements by discussion and, if necessary, by arbitration from the third or fourth review authors (JB and VP). We collected data on study design and setting, participant characteristics (including disease severity and age), study eligibility criteria, details of the intervention(s) given, the outcomes assessed, the source of study funding and any conflicts of interest stated by the investigators.
Assessment of risk of bias in included studies
Two review authors (KG and SB, or KG and VP) independently rated the'Risk of bias' domains and overall risk of bias in the included studies using a standardised grading system described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2017). There were no disagreements between the review authors on assessment of risk of bias. We would have resolved any disagreements by discussion and, if necessary, by arbitration from the third or fourth review authors (JB and VP). Using the Cochrane 'Risk of bias' tool, we considered the following domains:
sequence generation;
allocation concealment;
blinding of participants and personnel
blinding of outcome assessors;
incomplete outcome data;
selective outcome reporting;
other bias.
We created a 'Risk of bias' table for each included study showing our judgement for each domain. We used judgements of 'high risk of bias', 'low risk of bias' or 'unclear risk of bias', where 'unclear risk of bias' indicates an unknown risk of bias or that an entry is not relevant to the study. We included a comment to support each of our assessments. We generated a 'Risk of bias summary figure' using RevMan to present all of the judgements in a cross‐tabulation of study by risk of bias domain.
Measures of treatment effect
Where the same outcome measures were used, we planned to calculate mean differences (MDs) and 95% confidence intervals (CIs) for continuous variables, such as foot alignment, function, gait assessment and quality of life. Where outcome measures differed but measured the same construct, we planned to calculate standardised mean differences (SMDs) and 95% CIs. For dichotomous outcomes such as adverse events we planned to determine risk ratios (RRs) and 95% CIs. We used a linear mixed model in the analysis of raw data from Harnett 2011 and Hui 2014. This assumed that outcomes were normally distributed; however, Pirani scores are not normally distributed, so we used a computed bootstrap CI as a check on the robustness of the results from this study. Exact details are provided in Appendix 10.
Unit of analysis issues
Although we planned to include cross‐over trials, there were none. If we had identified cross‐over trials we planned to use the generic inverse variance (GIV) facility in RevMan to combine the estimated difference in effects from each study with its standard error (SE).
A number of trials included data from bilateral (including both right and left feet) and unilateral cases. In bilateral cases, right and left feet from the same participant are likely to be correlated (not independent). An analysis that ignores this correlation will provide CIs and P values that are invalid, and may detect spurious significance. Where published data were unable to account for this unit‐of‐analysis issue, we requested and analysed individual patient data (IPD). One study provided such data (Harnett 2011). For this trial, we used a linear mixed model with random subject effects. As well as providing a valid analysis of this study, the linear mixed model gave an estimate of the correlation between measurements taken from left and right feet in participants who received the same intervention. Making the assumption that this correlation was consistent in other trials with the same outcome variable, we were able to adjust results and re‐analyse published data. We have provided details of each analysis in the description of each study.
If data from multiple trial arms in a single trial had been suitable for inclusion with two comparisons (e.g. intervention A versus sham and intervention B versus sham) combined in the same meta‐analysis, we would have followed guidance in Section 23.3.4 of the Cochrane Handbook for Systematic Reviews of Interventions to avoid double‐counting (Higgins 2019). Our preferred approach would be to combine intervention groups if clinically appropriate, or otherwise share a control group between comparisons.
Dealing with missing data
If the study authors had not performed an intention‐to‐treat analysis, we would have done so before entry of data into RevMan, provided sufficient data were available.
Assessment of heterogeneity
We assessed clinical heterogeneity across trials, but none of the trials were similar enough in terms of participants, interventions, and outcomes to include them in a meta‐analysis.
We planned, if meta‐analysis had been possible, to quantify inter‐trial statistical inconsistency using I2 (Deeks 2017).
We would have calculated the I2 value by: I2 = 100% [Q‐df)/Q], where Q is Cochrane's heterogeneity, Chi2 statistic, and df is the degrees of freedom. We will determine the Cochrane's Q by summing the squared deviations of each trial's estimate from the overall meta‐analytic estimate and obtain a P value by comparing the statistic with a Chi2 distribution with k‐1 degrees of freedom (where k is the number of trials). We would have used the following guide to interpret I2 values:
0% to 40% might not be important;
30% to 60% may represent moderate heterogeneity;
50% to 90% may represent substantial heterogeneity;
75% to 100% considerable heterogeneity.
We would have used a random‐effects model to incorporate heterogeneous trials in the meta‐analysis if there had been unexplained heterogeneity in meta‐analyses.
Assessment of reporting biases
We took the following measures to reduce reporting biases.
We performed comprehensive searches to identify randomised, quasi‐randomised and cross‐over trials.
We sought to include unpublished relevant studies, including those registered at inception.
If it had been possible, we planned to detect reporting biases using funnel plots to assess for small‐study effects (Sterne 2017).
Data synthesis
If there was more than one trial with a specific intervention we planned to perform a meta‐analysis using RevMan. We planned to pool data using a fixed‐effect model, where heterogeneity permitted (see Assessment of heterogeneity).
'Summary of findings' tables
We created 'Summary of findings' tables for comparisons using GRADEpro GDT software (GRADEpro GDT 2016). We have presented separate tables for each stage of treatment (i.e. initial presentations, resistant, relapsed, neglected and unknown). Owing to the paucity of data, we show comparisons for which data were available. We present the following outcomes.
Function (self‐reported or parent‐ or proxy‐reported).
Foot alignment.
Gait assessment.
Parent‐ or participant‐reported health‐related quality of life.
Adverse events.
Measures were to be assessed at a minimum of one year; however, some outcomes (for example, foot alignment) measured at the end of treatment determined whether further alternative treatment such as surgery was required. Where available, we have therefore included findings (clinically or statistically) at the end of treatment.
Two review authors (SB and KG) assessed each outcome using the GRADE working group grades of evidence (Schünemann 2017a; Schünemann 2017b). We determined that the evidence was of high certainty when further research was very unlikely to change our confidence in the estimate of effect. Moderate‐certainty evidence was when further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low‐certainty evidence was when further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. We determined evidence to be of very low certainty when we were very uncertain about the estimate. We assessed evidence using the five GRADE considerations (study limitations, imprecision, indirectness, inconsistency, and publication bias). We downgraded the certainty of evidence from high by one level if a GRADE consideration applied to a serious extent, and by two levels if very serious. We explain our reasons for downgrading in footnotes.
Subgroup analysis and investigation of heterogeneity
We would have performed the following predefined subgroup analyses if sufficient data had been available.
Age: birth to two years, two to four years, four to 10 years, 10 to 20 years, and over 20 years of age (relapses are most common in the 'birth to two years' range and decrease with age). The literature has previously reported subgroup analysis (Laaveg 1980; Richards 2008).
Unilateral versus bilateral CTEV. The literature has previously reported subgroup analysis (Gray 2014a).
Idiopathic versus syndromic (associated with other conditions) CTEV.
Initial versus relapsed CTEV.
We would have followed the same methodological principles for meta‐analysis of subgroups as for the primary analysis.
Sensitivity analysis
If clinical heterogeneity had been present, we planned to carry out a sensitivity analysis by omitting from the meta‐analysis trials at high risk of bias, or which were unpublished, or funded by industry. We would have made omissions in order, for example, we would have removed studies with unclear or inadequate allocation concealment, re‐run the analysis, then removed studies with unclear blinding and re‐run the analysis, and so on.
If data had been available, we planned to include cost‐benefit analysis of interventions for the treatment of CTEV in the Discussion, making use of the non‐randomised data where necessary.
Risk of bias in the review process
The review has a published protocol (Gray 2010). We have documented any deviations from the protocol in Differences between protocol and review.
Results
Description of studies
Results of the search
The previous version of this review included 14 studies.
In this update, the searches run by the Cochrane Neuromuscular Information Specialist retrieved 341 new citations. We removed 62 duplicated new references and CRS tracking removed a further 15. We reviewed the titles and abstracts of 264 records. We excluded 226 records and identified 38 studies as potentially relevant for inclusion. Searching reference lists of included trials did not identify any additional potentially relevant trials. We reviewed seven papers in full text and included seven new trials in this review.
The review authors conducted searches of clinical trials registries and PeDRO. The search of WHO ICTRP revealed 26 ongoing studies. ClinicalTrials.gov revealed 21 studies, and PeDRO revealed four studies. We removed one duplicated new reference, and screened 50 records from these searches, of which we excluded 46. We excluded two records reviewed in detail and selected two studies, both from ICTRP, as Ongoing studies. See Figure 1 for a flow chart illustrating the study selection process.
1.
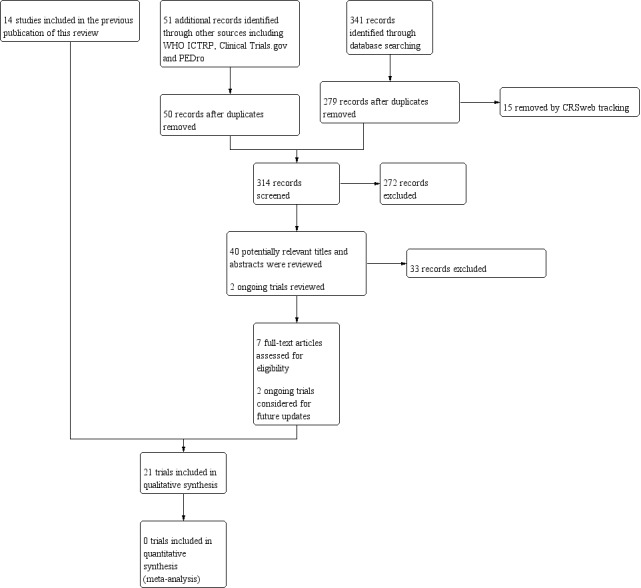
Flow diagram illustrating the study selection process.
Included studies
Twenty‐one trials met the criteria for inclusion in the review (Chen 2015; Chong 2014; Cummings 2009; El‐Deeb 2007; Elgohary 2014; Gintautiene 2016; Harnett 2011;Hui 2014; Kaewpornsawan 2007; Lahoti 2008; Manzone 1999; Maripuri 2013; Pittner 2008; Rijal 2010; Sanghvi 2009; Selmani 2012; Siddiqui 2007; Sud 2008; Svehlik 2017; Zeifang 2005; Zwick 2009). Electronic searching identified all these trials. We present details of individual trials in the tables Characteristics of included studies and 'Characteristics of included trials' (Table 6). All trials were published in the English language in peer‐reviewed journals between 1999 and 2017. We were given access to published data from two trials (Rijal 2010; Zeifang 2005), while two authors provided individual patient data (IPD) for re‐analysis (Harnett 2011; Hui 2014)
1. Characteristics of included trials.
| Study ID |
No. of participants/feet |
Randomisation of feet or participants | Combined bilateral and unilateral cases during randomisation |
CTEV case |
CTEV diagnosis |
Average start age (SD) | Treatment | Outcomes measured | Average follow‐up time (months) |
| Chen 2015 | 53/83 | Participants | Y | Initial | Idiopathic | 4.8 years | DB vs OS + DB vs OS + FAS brace | Diméglio scale 3‐dimensional foot scanning pedobarography |
78 |
| Chong 2014 | 30/45 | Participants | Y | Initial | Idiopathic | 29.6 days | Mitchell shoes vs dynamic abduction brace | Rate of recurrence | 18.7 |
| Cummings 2009 | 20/32 | Participants | Y | Initial | Not stated | 0 to 30 days | Botulinum toxin vs placebo with Ponseti technique | Time in cast for correction Need for Achilles tenotomy Relapse rate Treatment required for correction of relapse Dimeglio scale |
27 |
| El‐Deeb 2007 | 46/66 | Feet | Y | Resistant | Idiopathic | 9 months | TCIL release vs placebo during surgery for CTEV |
Radiography Modified Scoring System |
28 |
| Elgohary 2014 | 41/66 | Participants | Y | Initial | Idiopathic | 1.5 to 24.5 weeks | Traditional vs accelerated Ponseti technique | Pirani score Number of casts before tenotomy Timing of tenotomy Time from onset to complete correction |
29 |
| Gintautiene 2016 | 39/55 | Participants | Y | Initial | Idiopathic | 17 days | Ponseti vs early TATT | Pirani scale Dimiglio scale Foot range of motion Radiolography |
24 |
| Harnett 2011 | 40/51 | Participants | Y | Not stated | Idiopathic | < 90 days | Ponseti standard vs Ponseti accelerated protocol | Pirani score Number of days to correction (prior to an Achilles tenotomy) |
0 |
| Hui 2014 | 30/44 | Participants | Y | Initial | Idiopathic | 2 weeks | Semi‐rigid fibreglass plaster vs plaster of Paris | Pirani score Number of casts Total time in casts (weeks) Ease of cast removal Duration of cast removal (minutes) |
30.8 |
| Kaewpornsawan 2007 | 86/128 | Participants | Y | Resistant | Idiopathic | 5.9 months | PMR vs complete subtalar release surgery | Pirani score Turco evaluation Diméglio scale |
19.4 |
| Lahoti 2008 | 13/26 | Feet | Included bilateral cases only | Resistant | Included 2 syndromic feet (1 in each arm) | 10 months | FHL and FDL lengthening vs simple decompression in CTEV surgery | Pirani score Harrold and Walker Scale |
48 |
| Manzone 1999 | 20/30 | Feet | Y | Initial | Idiopathic | 7.7 months | PMR vs complete subtalar release surgery | Radiography Magone's Score |
27 |
| Maripuri 2013 | 26/33 | Participants | Y | Initial | Idiopathic | 11 days | Below‐knee vs above‐knee Ponseti casting | Pirani scale Time to readiness for tenotomy Time to full correction |
0 |
| Pittner 2008 | 34/42 | Participants | Y | Initial | Not stated | 10 days | Semi‐rigid cast vs plaster of Paris cast with Ponseti technique |
Diméglio scale Parent Satisfaction Questionnaire |
0 |
| Rijal 2010 | 38/60 | Feet | N | Initial | Idiopathic | 16.3 months | Ponseti vs Kite technique | Pirani score | 0 |
| Sanghvi 2009 | 42/64 | Participants | Y | Initial | Idiopathic | ≤ 9 months | Ponseti vs Kite technique | Radiography Range of movement Function using Atar et al assessment |
36 |
| Selmani 2012 | 100/150 | Participants | Y | Initial | Idiopathic | 33 days | Ponseti vs Kite technique | Pirani score Range of movement |
36 |
| Siddiqui 2007 | 60/≥ 60 | Participant | Unsure | Not stated | Not stated | 9.6 months | Window vs Turco surgery for CTEV | Diméglio scale Post‐operative assessment criteria according to Beatson 6 months after surgery Time in theatre |
0 |
| Sud 2008 | 53/81 | Participants | Y | Initial | Idiopathic | 29 days | Ponseti vs Kite technique | Diméglio scale Range of movement Functional assessment (e.g. squat) |
26 |
| Svehlik 2017 | 15/24 | Participants | Y | Initial | Idiopathic | < 2 weeks | Ponseti method vs surgical treatment |
Pirani scale FRS Ankle range of motion OFM PODCI |
117.6 |
| Zeifang 2005 | 36/36 | Participants | In bilateral cases, 1 foot was randomly selected for inclusion | Resistant | Idiopathic | 8.2 months | CPM vs immobilisation in plaster cast after surgery for CTEV | Diméglio scale | 48 |
| Zwick 2009 | 19/28 | Participants | Y | Initial | Idiopathic | < 2 weeks | Ponseti vs traditional technique (serial casting and PMR) | Pirani score PODCI Radiography Functional Rating System (FRS) Laaveg and Ponseti |
42 |
CPM: continuous passive motion CTEV: congenital talipes equinovarus DB: Denis Browne splint FAS: forefoot abduction shoe FDL: flexor digitorum longus FHL: flexor hallucis longus FRS: Functional Rating System OFM: Oxford foot model OS: orthopaedic shoe PMR: posteromedial soft tissue release PODCI: Pediatric Outcomes Data Collection Instrument SD: standard deviation TATT: tibialis anterior tendon transfer TCIL: talocalcaneal interosseus ligament vs: versus
Design
Fourteen trials were randomised controlled trials (RCTs) (Chen 2015, 53 participants; Chong 2014, 30 participants; Cummings 2009, 20 participants; Elgohary 2014, 46 participants; Gintautiene 2016, 44 participants; Harnett 2011, 40 participants; Hui 2014, 30 participants; Kaewpornsawan 2007, 86 participants; Maripuri 2013, 26 participants; Rijal 2010, 38 participants; Selmani 2012, 100 participants; Svehlik 2017, 19 participants; Zeifang 2005, 36 participants; Zwick 2009, 19 participants), three were quasi‐RCTs (El‐Deeb 2007, 46 participants; Pittner 2008, 34 participants; Sud 2008, 53 participants), and the remaining four were randomised but without a description of the method (Lahoti 2008, 13 participants; Manzone 1999, 20 participants; Sanghvi 2009, 42 participants; Siddiqui 2007, 60 participants). Four trials randomised by feet (El‐Deeb 2007; Lahoti 2008; Manzone 1999; Rijal 2010), while the remainder randomised by participants.
The duration of follow‐up ranged from end of treatment (Harnett 2011; Pittner 2008; Rijal 2010; Siddiqui 2007) to 9.8 years (in Svehlik 2017). Seven studies reported dropouts prior to data analysis (Chong 2014; Elgohary 2014; Gintautiene 2016; Pittner 2008; Sud 2008; Svehlik 2017; Zeifang 2005).
Participants
The 21 studies in this review included a total of 905 participants with CTEV.
The number of participants in each trial ranged from 13 (26 feet) to 100 (150 feet). Fourteen trials assessed treatment in participants without any prior intervention (initial presentation) (560 participants; 837 feet) (Chong 2014; Cummings 2009; Elgohary 2014; Gintautiene 2016; Hui 2014; Manzone 1999; Maripuri 2013; Pittner 2008; Rijal 2010; Sanghvi 2009; Selmani 2012; Svehlik 2017; Sud 2008; Zwick 2009), four assessed treatment in resistant cases (those who had undergone prior intervention without full correction of the deformity) (181 participants; 256 feet). (El‐Deeb 2007; Kaewpornsawan 2007; Lahoti 2008; Zeifang 2005), and three did not state the timing of the intervention or if there had been a prior intervention (153 participants; a minimum of 144 feet; Siddiqui 2007 did not state the number of included feet) (Chen 2015; Harnett 2011; Siddiqui 2007). In the 15 trials that reported adequate details, 442 participants were male and 235 were female. Hui 2014 and Lahoti 2008 did not report on sex.
Of the 14 trials assessing initial treatment, seven reported an average age of participants: 16.3 months (Rijal 2010), 4.7 years (Chen 2015), 29.6 days (Chong 2014), 17.05 days (Gintautiene 2016), two weeks (Hui 2014), 11 days (Maripuri 2013) and 7.7 months (Manzone 1999). Four trials specified age ranges: less than two weeks (Svehlik 2017; Zwick 2009), 1.5 to 24.5 weeks (Elgohary 2014) and 0 to 30 days (Cummings 2009). Sanghvi 2009 included a range of age groups from birth to more than 36 weeks. Following removal of dropout data, participants in Pittner 2008 had an average age of 10 days, Selmani 2012 an average age of 33 days, and Sud 2008, an average age of 29 days.
In the four trials that assessed treatment for resistant CTEV, participants had an average age of 8.3 months. Two trials specified idiopathic CTEV as an inclusion criterion (El‐Deeb 2007; Zeifang 2005), and one specifically excluded syndromic CTEV or similar cases (Kaewpornsawan 2007). Lahoti 2008 included two feet (one participant) with syndromic CTEV. In resistant CTEV, all cases of relapse required major foot surgery to correct.
Three trials investigated treatment of CTEV that was not stated to be initial or resistant. Harnett 2011 included participants aged less than 90 days, and participants in Siddiqui 2007 had an average age of 9.6 months. Harnett 2011 included idiopathic cases, while Siddiqui 2007 and Chen 2015 did not report on inclusion or exclusion criteria.
Inclusion of idiopathic cases was specified in 10 of the 15 trials assessing initial treatment (Chen 2015; Chong 2014; Elgohary 2014; Gintautiene 2016; Hui 2014; Manzone 1999; Maripuri 2013; Rijal 2010; Selmani 2012; Svehlik 2017). A further eight trials stated exclusion of CTEV cases associated with syndromes or with other deformities (Chong 2014; Gintautiene 2016; Hui 2014; Maripuri 2013; Svehlik 2017; Sanghvi 2009; Sud 2008; Zwick 2009). Elgohary 2014 and Chong 2014 excluded idiopathic clubfeet with previous surgical interference to the affected foot. Cummings 2009 and Pittner 2008 did not report inclusion or exclusion criteria, and Chen 2015 did not report exclusion criteria.
Interventions and comparisons
Seven trials compared other treatments versus the Ponseti technique: the Kite technique (Rijal 2010; Sanghvi 2009; Selmani 2012; Sud 2008), or traditional casting with surgery (Gintautiene 2016; Svehlik 2017; Zwick 2009). Six trials examined modification of the Ponseti technique, two by different casting materials (Hui 2014; Pittner 2008), one by the addition of botulinum toxin A (Cummings 2009), two by use of an accelerated Ponseti treatment schedule (Elgohary 2014; Harnett 2011), and one by different casting method (Maripuri 2013). Five trials compared different major surgical interventions (El‐Deeb 2007; Kaewpornsawan 2007; Lahoti 2008; Manzone 1999; Siddiqui 2007), and one compared two post‐operative regimens (Zeifang 2005). Two trials compared use of different abduction corrective methods following the Ponseti technique (Chen 2015; Chong 2014). We provide details of interventions in the Characteristics of included studies table and Table 6, 'Characteristics of included trials'.
Outcomes
Function was an outcome in seven trials (El‐Deeb 2007; Kaewpornsawan 2007; Manzone 1999; Sanghvi 2009; Sud 2008; Svehlik 2017; Zwick 2009). Two trials used a validated functional scale, the Pediatric Outcomes Data Collection Instrument (PODCI) (Svehlik 2017; Zwick 2009).
Radiography was an outcome in five trials (El‐Deeb 2007; Gintautiene 2016Manzone 1999; Sanghvi 2009; Zwick 2009).
All trials except one assessed foot alignment (Chong 2014). Sixteeen trials used validated scales specific to CTEV: the Diméglio scale (Chen 2015; Gintautiene 2016; Pittner 2008; Rijal 2010; Siddiqui 2007; Sud 2008; Zeifang 2005), and the Pirani score (Elgohary 2014; Gintautiene 2016; Harnett 2011; Hui 2014; Kaewpornsawan 2007; Lahoti 2008; Maripuri 2013; Selmani 2012; Svehlik 2017; Zwick 2009). Both scores assess several components of foot alignment. The sums of these scores form a final severity score; in both scales a higher score correlates with greater severity.
Gait assessment using pedobarography and gait analysis were investigated by Chen 2015 and Svehlik 2017 respectively.
Two trials assessed parent‐ or participant‐reported health‐related quality of life using the PODCI (Svehlik 2017; Zwick 2009).
Nineteen trials documented relapse (Chen 2015; Chong 2014; Cummings 2009; El‐Deeb 2007; Elgohary 2014; Gintautiene 2016; Harnett 2011; Hui 2014; Kaewpornsawan 2007; Lahoti 2008; Manzone 1999; Maripuri 2013; Sanghvi 2009; Selmani 2012; Siddiqui 2007; Sud 2008; Svehlik 2017; Zeifang 2005; Zwick 2009). Two trials followed up participants only to the end of initial serial casting treatment (Pittner 2008; Rijal 2010).
Eleven trials documented adverse events (Chen 2015; Elgohary 2014; Gintautiene 2016; Kaewpornsawan 2007; Manzone 1999; Maripuri 2013; Pittner 2008; Sanghvi 2009; Siddiqui 2007; Svehlik 2017; Zwick 2009).
Excluded studies
We excluded 38 studies after full‐text review as they were not RCTs. See Excluded studies for further details.
Ongoing studies
We will review two ongoing randomised controlled studies in future updates of this review. One is investigating the efficacy of a new design of foot abduction brace compared to standard foot abduction brace during Ponseti treatment of idiopathic clubfoot by measuring rates of recurrence and compliance using novel touch sensors (NCT03249805). The second study is comparing the use of two different types of splints, i.e. Dobbs splint and Denis Browne splint, in children with congenital talipes equinovarus (Madhuri 2018).
Risk of bias in included studies
We assessed risks of bias using the Cochrane 'Risk of bias' tool. We summarise our judgement about each 'Risk of bias' item for each included study in Figure 2. Two trials had an overall unclear risk of bias (Chen 2015; Cummings 2009). The remaining 19 trials had a high risk of bias (Chong 2014; El‐Deeb 2007; Elgohary 2014; Gintautiene 2016; Harnett 2011; Hui 2014; Kaewpornsawan 2007; Lahoti 2008; Manzone 1999; Maripuri 2013; Pittner 2008; Rijal 2010; Sanghvi 2009; Selmani 2012; Siddiqui 2007; Sud 2008; Svehlik 2017; Zeifang 2005; Zwick 2009).
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study. Green = low risk of bias; yellow = unclear risk of bias; red = high risk of bias.
Allocation
Eight trials were at low risk of bias, as they randomly allocated participants to treatment groups using computerised number generation (Harnett 2011; Hui 2014; Rijal 2010; Selmani 2012; Zeifang 2005), or a random‐number table (Svehlik 2017; Zwick 2009). Chong 2014 used random allocation in block sizes of four to create treatment group of similar sizes and we also assessed allocation in this trial as low risk. We assessed risk of bias as unclear in trials that used random selection of unmarked vials (Cummings 2009), or unmarked envelopes (Gintautiene 2016; Kaewpornsawan 2007). The method of randomisation was unclear in five trials that did not state the method of randomisation (Lahoti 2008; Manzone 1999; Maripuri 2013; Sanghvi 2009; Siddiqui 2007), and two trials in which the method was unclear (Elgohary 2014; Chen 2015). A high risk of bias from quasi‐randomisation occurred in two trials that used sequencing based on arrival (El‐Deeb 2007; Sud 2008), and one which used medical record numbers (Pittner 2008).
Allocation concealment was at low risk of bias in two trials (Maripuri 2013; Hui 2014). Fifteeen trials were unclear, owing to insufficient information (Chen 2015; Chong 2014; Cummings 2009; Elgohary 2014; Harnett 2011; Kaewpornsawan 2007; Lahoti 2008; Manzone 1999; Rijal 2010; Sanghvi 2009; Selmani 2012; Siddiqui 2007; Svehlik 2017; Zeifang 2005; Zwick 2009). In addition, two trials provided insufficient information on baseline characteristics which contributed to an unclear risk of bias (Gintautiene 2016; Lahoti 2008). Three trials had inadequate concealment and were at high risk of bias as a result (El‐Deeb 2007; Pittner 2008; Sud 2008).
Blinding
Blinding of participants and personnel (performance bias)
Participants were blinded to the intervention in one trial, in which participants received either botulinum toxin or a placebo, and we therefore rated it at a low risk of bias (Cummings 2009). One trial provided insufficient information on blinding of participants and was assessed as unclear (Chen 2015). A high risk of performance bias was present in the remaining 19 trials as it was not possible to blind the intervention provider (Chong 2014; El‐Deeb 2007; Elgohary 2014; Gintautiene 2016; Harnett 2011; Hui 2014; Kaewpornsawan 2007; Lahoti 2008; Manzone 1999; Maripuri 2013; Pittner 2008; Rijal 2010; Sanghvi 2009; Selmani 2012; Siddiqui 2007; Sud 2008; Svehlik 2017; Zeifang 2005; Zwick 2009).
Blinding of outcome assessment (detection bias)
Outcome assessors were blinded to the intervention in six trials and we rated them at low risk of bias (Cummings 2009; Lahoti 2008; Rijal 2010; Selmani 2012; Sud 2008; Zeifang 2005). Eleven trials provided insufficient information to permit judgement and were rated as unclear (Chen 2015; Chong 2014; El‐Deeb 2007; Gintautiene 2016; Harnett 2011; Hui 2014; Kaewpornsawan 2007; Manzone 1999; Maripuri 2013; Siddiqui 2007; Svehlik 2017). Four trials did not blind assessors and were rated as high risk of bias (Elgohary 2014; Pittner 2008; Sanghvi 2009; Zwick 2009).
Incomplete outcome data
Thirteen trials had a low risk of bias. Of these, 11 had no missing data (Chen 2015; Chong 2014; Cummings 2009; Harnett 2011; Hui 2014; Kaewpornsawan 2007; Lahoti 2008; Manzone 1999; Rijal 2010; Sanghvi 2009; Siddiqui 2007), and two had missing data addressed by an intention‐to‐treat analysis (Maripuri 2013; Svehlik 2017). Three trials were at unclear risk of bias: in Zwick 2009, several participants broke protocol by changing treatment arms; and El‐Deeb 2007 and Gintautiene 2016 provided insufficient information to determine the risk of bias from incomplete outcome data. Five trials had high risk of bias due to missing data that were not addressed (Elgohary 2014; Pittner 2008; Selmani 2012; Sud 2008; Zeifang 2005).
Selective reporting
Chen 2015, Gintautiene 2016, Maripuri 2013, and Svehlik 2017 adequately reported all outcomes and we rated them at low risk of bias. We rated 11 trials at unclear risk, as there was insufficient information to make a judgement (Cummings 2009; El‐Deeb 2007; Elgohary 2014; Harnett 2011; Kaewpornsawan 2007; Lahoti 2008; Manzone 1999; Pittner 2008; Selmani 2012; Siddiqui 2007; Zwick 2009). Six trials had identifiable selective reporting and were at high risk of bias (Chong 2014; Hui 2014; Rijal 2010; Sanghvi 2009; Sud 2008; Zeifang 2005).
Other potential sources of bias
We identified no other potential source of bias in Chong 2014, Gintautiene 2016; Lahoti 2008; Rijal 2010; Selmani 2012, and rated those studies at low risk of bias. We considered 13 trials to have an unclear risk of bias for this domain, owing to insufficient information to permit judgement (Chen 2015; Cummings 2009; El‐Deeb 2007; Elgohary 2014; Harnett 2011; Hui 2014; Kaewpornsawan 2007; Manzone 1999; Pittner 2008; Sanghvi 2009; Sud 2008; Svehlik 2017; Zeifang 2005). Three trials had identifiable other sources of bias and so were at high risk of other bias (Maripuri 2013; Siddiqui 2007; Zwick 2009). Zwick 2009 and Maripuri 2013 both stopped early. Zwick 2009 had one treatment arm with much greater rates of major surgical intervention and Maripuri 2013 had one treatment arm with higher failure rates. Siddiqui 2007 reported on a procedure that the trial authors had developed.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5
Summary of findings 1. Treatment of initial congenital talipes equinovarus (CTEV; clubfoot).
|
Patient or population: participants with CTEV at initial presentation
Settings: single centres
Intervention: various Comparison: various | |||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Certainty of the evidence (GRADE) | Comments | ||
| Assumed risk | Corresponding risk | ||||||
| Control | Intervention | ||||||
| Function | 2 trials reported on function using validated scales, but raw data were not available for analysis. 2 trials reported on function using non‐validated methods. |
||||||
| Foot alignment Pirani score. Scale from: 0 to 6. A lower score indicates better alignment | Ponseti vs Kite technique Follow‐up: 10 weeks |
Kite technique The mean foot alignment score in the Kite group was 2.12 pointsa |
Ponseti technique The mean foot alignment score in the Ponseti group was 1.15 points lower (1.32 lower to 0.98 lower) |
‐ | 38 (1 study) | ⊕⊕⊝⊝ Lowb | When treated at birth, foot alignment may be better after Ponseti plaster casting than after Kite plaster casting. |
| Ponseti technique vs traditional treatment (plaster casting and surgery) Follow‐up: 42 months |
Traditional treatment The mean foot alignment score in the traditional treatment group was 1.8 pointsc |
Ponseti technique The mean foot alignment score in the Ponseti group was 1.50 points lower (2.28 lower to 0.72 lower) |
‐ | 28 (1 study) |
⊕⊝⊝⊝ Very lowd,e | The certainty of evidence is too low to draw conclusions about foot alignment after Ponseti casting compared to traditional treatment (plaster casting and surgery) | |
| Ponseti technique, semi‐rigid fibreglass compared with plaster of Paris casting for CTEV Follow‐up: 30.8 months |
Ponseti, plaster of Paris cast The mean foot alignment score in the plaster of Paris cast group was 1.0 pointsf |
Ponseti, fibreglass cast The mean foot alignment score in the fibreglass cast group was 0.46 points higher (0.07 lower to 0.99 higher) |
‐ | 30 (1 study) | ⊕⊕⊝⊝ Lowg | When treated at birth using Ponseti casting, semi‐rigid fibreglass may be as effective as plaster of Paris. | |
| Gait assessment | Not reported ‐ no trial assessed gait using a validated measure | ||||||
| Health‐related quality of life | 2 trials assessed health‐related quality of life using a validated measure, but raw data were not available for analysis | ||||||
| Adverse events | Ponseti vs. Kite Follow‐up: 10 weeks |
In the Kite vs Ponseti comparison 1 trial reported plaster sores and skin ulceration with casting without specifying whether in the Kite or Ponseti group. The remaining trials did not report adverse events. Following relapse, the risk difference for major surgery in the Kite group was 25% and 50% higher in 2 trials. The third trial reported 11/50 relapses in the Kite group. Management was not stated. |
⊕⊕⊝⊝ Lowb | ‐ | |||
| Ponseti vs. traditional treatment (plaster casting and surgery) Follow‐up: 42 months |
Infant discomfort in orthoses was reported (1 participant, 11%). Relapse was seen in 2/9 participants in the Ponseti group within 2 months of completion of serial casting. The traditional treatment required 50% more surgical procedures on follow‐up compared to the Ponseti group. | ⊕⊝⊝⊝ Very lowd,e | ‐ | ||||
| Ponseti technique, semi‐rigid fibreglass compared with plaster of Paris casting for CTEV Follow‐up: 30.8 months |
1 trial (N = 11) reported minor skin irritation and plaster casts slippage. 1 trial (N = 30) reported a relapse rate of 1/18 in the fibreglass group and 3/12 in the Ponseti group. This trial did not report any adverse events. |
⊕⊕⊝⊝ Lowg | ‐ | ||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; CTEV: congenital talipes equinovarus | |||||||
| GRADE Working Group grades of evidence High certainty: Further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: We are very uncertain about the estimate. | |||||||
aFinal mean Ponseti score of pooled results from three strata. bDowngraded twice: once for study limitations and once for imprecision. There was insufficient information to assess allocation concealment. Blinding of providers was not possible, but observers were blinded. The study had 38 participants. cFinal mean Pirani score in control group. dDowngraded twice for study limitations: there was a high risk of performance bias, and outcome assessment was not blinded. The risk of bias was unclear in several other domains, including allocation concealment. An early stopping rule was instigated. eDowngraded for imprecision: The trial had 28 participants. fFinal mean Pirani score for control group (plaster of Paris casting). gWe downgraded the evidence twice: once for study limitations, as blinding of participants and personnel was not possible and it was unclear whether outcome assessors were blinded, and once for imprecision, as the trial included 30 participants.
Summary of findings 2. Treatment for resistant congenital talipes equinovarus (CTEV; clubfoot).
|
Patient or population: participants with resistant CTEV Settings: single centre Intervention: various Comparison: various | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| PMSTR | Subtalar release | |||||
| PMSTR | PMSTR + talocalcaneal interosseus ligament lengthening | |||||
| FHL & FDL lengthening | Simple decompression surgery | |||||
| CTEV surgery + CPM | CTEV surgery + immobilisation | |||||
| Function | Not measured | |||||
| Foot alignment | 4 trials assessed foot alignment, but data were not suitable to re‐analyse. 3 compared surgical techniques; the 4th compared CPM with immobilisation in a case post‐surgery | |||||
| Gait assessment | Not measured | |||||
| Health‐related quality of life | Not measured | |||||
| Adverse events | 1 trial reported skin infections following: PMSTR (N = 4 feet, 8.5%) and complete circumferential subtalar release (N = 2 feet, 5.1%). The remaining trials did not report adverse events. Relapses were documented in all trials but data were not available to analyse. |
⊕⊝⊝⊝ Very lowa | ‐ | |||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; CPM: continuous passive motion; PMSTR: posteromedial soft tissue release; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
aWe downgraded the evidence twice: once for study limitations, and once for blinding of intervention provider (not possible); unclear about prior treatment. The risk of bias was unclear in several other domains, including allocation concealment.
Summary of findings 3. Treatment of relapsed/recurrent congenital talipes equinovarus (CTEV; clubfoot).
|
Patient or population: participants with relapsed or recurrent CTEV Settings: ‐ Intervention: ‐ Comparison: ‐ | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Function | No trials assessed treatments for relapsed or recurrent CTEV. | |||||
| Foot alignment | ||||||
| Gait assessment | ||||||
| Health‐related quality of life | ||||||
| Adverse events | ||||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
Summary of findings 4. Treatment for neglected congenital talipes equinovarus (CTEV; clubfoot).
|
Patient or population: participants with neglected CTEV Settings: ‐ Intervention: Comparison: ‐ | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Function | No trials assessed treatments for neglected CTEV. | |||||
| Foot alignment | ||||||
| Gait assessment | ||||||
| Health‐related quality of life | ||||||
| Adverse events | ||||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
Summary of findings 5. Treatment of other congenital talipes equinovarus (CTEV; clubfoot) (timing not stated).
| Accelerated Ponseti technique versus standard Ponseti technique for CTEV | ||||||
|
Patient or population: participants with CTEV (type of case (i.e. whether initial presentation or relapsed) not specified)
Settings: single centre
Intervention: accelerated Ponseti technique Comparison: standard Ponseti technique | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Standard Ponseti technique | Accelerated Ponseti technique | |||||
| Function ‐ | Not measured | |||||
|
Foot alignment
Pirani score. A lower score indicates better alignment. Scale from: 0 to 6. Follow‐up: end of serial casting |
The median foot alignment score in the control groups was 0.5 pointsa |
The mean foot alignment score in the intervention groups was 0.31 points higher (0.40 lower to 1.02 higher) | ‐ | 40 (1 study) | ⊕⊕⊝⊝ Lowb | There is evidence that there may be no difference between an accelerated and standard Ponseti technique in foot alignment at the end of serial casting. |
| Gait assessment | Not measured | |||||
| Health‐related quality of life | Not measured | |||||
| Adverse events | 1 trial reported no complications in either group. The other trial did not report adverse events.
1 trial reported 5 x relapses and 3 x repeat tenotomies in both groups at 12‐ to 48‐month follow‐up. 1 trial reported no relapses at 6‐month follow‐up. |
⊕⊕⊝⊝ Lowb | ‐ | |||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval | ||||||
| GRADE Working Group grades of evidence High certainty: Further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: We are very uncertain about the estimate. | ||||||
aFinal mean Pirani score for the control group (standard Ponseti). bWe downgraded the certainty of the evidence twice: once for study limitations, as blinding of participants was not possible and it was unclear whether outcome assessment was blinded, and once for imprecision, as the trial included 40 participants.
The trials reported 15 comparisons. We ordered comparisons in groups: treatment for initial presentations (comparisons 1 to 9); treatment for resistant cases (comparisons 10 to 13); and other presentations (comparison 14 to 15).
Several studies were comparable, with similar treatment and participant cohorts, but they deployed different outcome measures, which precluded pooling for meta‐analysis. Thirteen authors responded to requests for additional information. Two trial authors were able to provide individual patient data for re‐analysis (Harnett 2011; Hui 2014).
Initial (treatment‐naïve) cases
Fourteen trials investigated treatment of initial presentation of CTEV (Chen 2015; Chong 2014; Cummings 2009; Gintautiene 2016; Hui 2014; Manzone 1999; Maripuri 2013; Pittner 2008; Rijal 2010; Sanghvi 2009; Selmani 2012; Sud 2008; Svehlik 2017; Zwick 2009).
Comparison 1: Ponseti versus Kite technique
Four studies (233 participants, 355 feet), compared Ponseti versus Kite techniques (Rijal 2010; Sanghvi 2009; Selmani 2012; Sud 2008). All trials only included cases of idiopathic CTEV.
Primary outcome: function
Function was an outcome in Sanghvi 2009 (functional rating system described by Atar 1992) and Sud 2008 (variety of functional measures, e.g. squatting, participation in games), but neither trial used a validated functional scale.
Secondary outcomes
Foot alignment
We could analyse foot alignment data (Pirani scores) from one trial (Rijal 2010). The Pirani score runs from zero to six, with a higher score indicating worse alignment. Results were analysed at the end of 10 weeks of serial casting and published in three categories. All categories found the Ponseti technique to be superior to the Kite technique (Analysis 1.1; Figure 3). See Table 1.
1.1. Analysis.

Comparison 1: Ponseti versus Kite technique for treatment of initial CTEV, Outcome 1: Foot alignment: Pirani score at 10 weeks
3.

Forest plot of comparison: 1 Kite versus Ponseti technique for treatment of initial CTEV. Pirani score after 10 weeks of serial casting.
To calculate a valid standard error (SE) for the difference between these means, the matching needs to be taken into account, since there may be within‐participant (intrinsic) differences (for example, ligamentous laxity). This requires knowledge of the correlation between measurements on left and right feet. Since this is unknown in this study, we used the estimate from Harnett 2011. Further detail of the following calculations can be found in Appendix 10.
Category 1 (12 participants, 24 feet) consisted of the randomised participants with bilateral CTEV, where one foot was treated with the Ponseti technique and the other with the Kite technique. At the end of serial casting, the mean (SD) of the total Pirani scores was 1.20 (0.57) in the Ponseti group versus 2.36 (0.67) in the Kite group with an estimated MD of −1.16 (95% CI −0.97 to −1.35).
Category 2 (10 participants, 20 feet) consisted of the randomised participants with bilateral CTEV, where both feet received the same treatment (four participants received the Ponseti technique and six received the Kite technique). The estimated difference in means between groups favoured the Ponseti technique with Pirani scores of −1.24 (95% CI −0.67 to −1.81).
Category 3 (16 participants, 16 feet) consisted of the randomised participants with unilateral CTEV. Ten participants were treated with the Ponseti technique and six with the Kite technique. At follow‐up, the mean (SD) favoured the Ponseti technique with total Pirani scores of 1.05 (0.49) in the Ponseti group versus 1.91 (0.73) in the Kite group. The estimated difference in the means between groups was −0.86 (95% CI −0.20 to −1.52).
Pooling the results from the three strata gave an MD of −1.15 (95% CI −0.98 to −1.32; 38 participants (60 feet); low‐certainty evidence) in favour of the Ponseti technique (Analysis 1.1; Figure 3).
Significant outcomes should be viewed with caution, as all groups contained small numbers.
Two other trials measured foot alignment using validated scales: Pirani score (Selmani 2012) and Diméglio scale (Sud 2008), but raw data (IPD) were not available for re‐analysis.
Gait assessment
None of the studies comparing Ponseti versus Kite assessed gait.
Quality of life
None of the studies comparing Ponseti versus Kite assessed quality of life.
Adverse events
Sanghvi 2009 reported plaster sores secondary to skin allergies and skin ulceration secondary to tight casts (Kite N = 3 (9%), Ponseti N = 2 (7%)). They did not state in which group each adverse event occurred. The remaining trials did not report any adverse events.
Relapses were documented in three trials. The risk difference for major surgery following relapse in the Kite group was 25% higher than in the Ponseti group in Sanghvi 2009, and 50% higher in Sud 2008. Selmani 2012 did not report what treatment was required to correct the 11 relapsed feet in the Kite group. We did not perform meta‐analysis due to small numbers. We provide details of relapse in Table 7 'Details of relapse. Ponseti versus Kite'.
2. Details of relapse: Ponseti versus Kite.
| Trial/ treatment group | No of participants with relapse (feet) | No of bilateral cases (feet) | No of unilateral cases (feet) | Treatment for bilateral relapse (feet) | Treatment for unilateral relapse (feet) | |||
|
Casting +/‐ minor surgery |
Mix | Major surgery | Casting +/‐ minor surgery | Major surgery | ||||
| Sanghvi 2009 | ||||||||
| Ponseti | 2 (3) | 1 (2) | 1 (1) | ‐ | 1 (2) | ‐ | 1 (1) | ‐ |
| Kite | 4 (5) | 1 (2) | 3 (3) | ‐ | ‐ | 1 (2) | 1 (1) | 2 (2) |
| Sud 2008 | ||||||||
| Ponseti | 5 (7) | 2 (4) | 3 (3) | 2(4) | ‐ | ‐ | 3 (3) | ‐ |
| Kite | 8 (8) | ‐ | 8 (8) | ‐ | ‐ | ‐ | 4 (4) | 4 (4) |
Comparison 2: Ponseti technique versus traditional treatment (serial casting followed by posteromedial soft tissue release)
Two trials (38 participants and 56 feet) compared Ponseti to a traditional treatment in idiopathic CTEV (Svehlik 2017; Zwick 2009).
Primary outcome: function
Function was an outcome in Svehlik 2017 and Zwick 2009, assessed using the Pediatric Outcomes Data Collection Instrument (PODCI). We were unable to estimate a treatment effect as data were not available.
Secondary outcomes
Foot alignment
We were able to analyse foot alignment data (Pirani scores). See Table 1. Further details of the following calculations can be found in Appendix 10.
Zwick 2009 randomised participants to either the Ponseti technique (9 participants, 12 feet) or a traditional technique (10 participants, 16 feet). Published mean (SD) total Pirani scores were 0.3 (0.3) in the Ponseti group (at the completion of serial casting and tenotomy) and 1.8 (1.2) in the traditional group (at the completion of a different type of serial casting), giving a difference in the means of −1.50 (95% CI −0.72 to −2.28; 28 participants; very low‐certainty evidence) in favour of the Ponseti technique (Analysis 2.1; Figure 4).
2.1. Analysis.

Comparison 2: Ponseti versus traditional treatment (plaster casting and surgery) for treatment of initial CTEV, Outcome 1: Foot alignment: Pirani score at end of serial plaster casting
4.

Forest plot of comparison: 2 Ponseti versus traditional treatment (plaster casting and surgery) for treatment of initial CTEV. Pirani score at end of initial Ponseti (serial plaster casting and tenotomy) and traditional treatment (serial plaster casting only).
Svehlik 2017 also used the Pirani scale. We were unable to estimate a treatment effect, as data were not available.
Gait assessment
Svehlik 2017 assessed gait using gait analysis. We were unable to estimate a treatment effect as data from validated outcome measures were not available.
Quality of life
Svehlik 2017 and Zwick 2009 assessed quality of life using the PODCI. We could not use the PODCI data in a meta‐analysis, as they were presented per foot with bilateral and unilateral cases combined. IPD were not available for re‐analysis.
Adverse events
Svehlik 2017 reported that the surgical group required 50% more additional surgical procedures on follow‐up compared to the Ponseti group. They also reported infant discomfort in orthoses (1 participant, 11%).
Relapse was seen in two of nine participants in the Ponseti group within two months of completion of serial casting. Both participants changed to the traditional group and underwent major surgery.
Zwick 2009 did not report any adverse events or details on relapse.
Comparison 3: Ponseti technique, semi‐rigid casting versus plaster of Paris
Two trials (64 participants, 86 feet) compared plaster of Paris to semi‐rigid casting (Hui 2014; Pittner 2008). Pittner 2008 did not state whether cases were idiopathic, while Hui 2014 included children with idiopathic clubfoot. See Table 1.
Primary outcome: function
Function was not an outcome in Hui 2014 or Pittner 2008.
Secondary outcomes
Foot alignment
IPD data were available for re‐analysis from Hui 2014. In participants who required tendo Achilles lengthening (TAL), higher mean Pirani scores of 2.2 (95% CI 1.8 to 2.6) were seen in the semi‐rigid fibreglass (SRF) group compared to plaster of Paris group 1.1 (95% CI 0.8 to 1.5) after casting. However, this model treated feet in bilateral cases as independent, and scores after casting were missing for 36% of feet. Since there is substantial correlation between feet in bilateral cases, and feet with missing scores were likely to have been those with lower scores, we performed a more rigorous analysis where we imputed missing data to a random score of 0.0, 0.5 or 1.0 with equal probability and took correlation in bilateral cases into account. Analysis of these data showed average PIrani scores as higher in the SRF group, although the 95% CI data crossed the line of no difference, suggesting that in some cases there was no difference between the two groups (MD 0.46 points higher in the SRF group than in the plaster of Paris group, 95% CI −0.07 to 0.99; 30 participants (44 feet); low‐certainty evidence) (Analysis 3.1; Figure 5).
3.1. Analysis.

Comparison 3: Ponseti technique. Comparison of semi‐rigid fibreglass and plaster of Paris., Outcome 1: Foot alignment: Pirani score at end of casting in those awaiting tenotomy
5.

Forest plot of comparison: 4 Comparison of plaster of paris and semi rigid casting at initial presentation, outcome: 4.1 Pirani score at end of casting in those awaiting tenotomy.
Pittner 2008 used the Diméglio scale to assess foot alignment, but raw data were not available.
Gait assessment
Gait was not an outcome in Hui 2014 or Pittner 2008.
Quality of life
Quality of life was not an outcome in Hui 2014 or Pittner 2008.
Adverse events
Pittner 2008 reported minor skin irritation and casts slipping (11 participants, 35%). The trial report did not identify the groups in which these events occurred.
Hui 2014 reported 8/18 and 3/12 deformity relapse cases in the semi‐rigid fibreglass and plaster of Paris groups respectively. Relapse was managed with repeat Ponseti casting, surgery or both. The type of casting and type of surgery used for each relapse was not stated. The trial authors did not report any adverse events.
Comparison 4: Ponseti technique, addition of botulinum toxin versus placebo
One study assessed the addition of botulinum toxin to the Ponseti technique (Cummings 2009; 20 participants, 32 feet). They did not state whether cases were idiopathic.
Primary outcome: function
Function was not measured.
Secondary outcomes
Foot alignment
Cummings 2009 used the Diméglio scale to assess foot alignment, but raw data were not available for analysis.
Gait assessment
Gait was not an outcome.
Quality of life
Quality of life was not an outcome.
Adverse events
The trial authors did not report any adverse events.
Relapses were reported in both groups but data were not available.
Comparison 5: Posteromedial soft tissue release versus circumferential subtalar release
One study assessed posteromedial soft tissue release versus circumferential subtalar release in idiopathic CTEV (Manzone 1999; 20 participants, 32 feet).
Primary outcome: function
Function was not measured.
Secondary outcomes
Foot alignment
Manzone 1999 measured foot alignment radiographically. We were unable to draw a conclusion for this comparison, as data from validated measures did not state when post‐operative assessment was completed, and we were unable to obtain raw data.
Gait assessment
Gait was not an outcome.
Quality of life
Quality of life was not an outcome.
Adverse events
Manzone 1999 reported superficial infections (two feet) requiring antibiotics and skin breakdown. One foot required skin grafting.
Relapse was noted in one participant (two feet) as a result of skin necrosis by the end of follow‐up (27 months).
Comparison 6: Mitchell shoes versus dynamic abduction brace
One study compared the use of Mitchell shoes (a static brace) and a dynamic abduction brace for idiopathic CTEV (Chong 2014; 30 participants, 45 feet).
Primary outcome:
Function
Function was not measured.
Secondary outcomes
Foot alignment
Foot alignment was not reported.
Gait assessment
Gait was not an outcome.
Quality of life
Quality of life was not an outcome.
Adverse events
The trial authors did not report any adverse events.
Four relapses were reported in each group, but data on the severity of the relapse or specific management to correct the relapse were not available.
Comparison 7: Ponseti technique versus early tibialis anterior tendon transfer
One study compared the Ponseti method to early tibialis anterior tendon transfer for idiopathic CTEV (Gintautiene 2016; 39 participants, 55 feet).
Primary outcome: function
Function was not measured.
Secondary outcomes
Foot alignment
Gintautiene 2016 measured foot alignment radiographically and used Pirani and Diméglio scales, but raw data were not available for analysis.
Gait assessment
Gait was not an outcome.
Quality of life
Quality of life was not an outcome.
Adverse events
The trial authors reported callus in the heel area from a foot abduction brace (FAB) in the Ponseti group, which healed without any intervention (three participants, 5.45%). Four participants (14.29%) experienced relapse in the Ponseti group but management of this was not reported. No relapses were noted in the tibialis anterior tendon group. No further adverse events were reported.
Comparison 8: Ponseti technique, above‐ versus below‐knee casting
One study compared above‐knee versus below‐knee plastering during Ponseti method (Maripuri 2013; 26 participants, 33 feet).
Primary outcome: function
Function was not measured.
Secondary outcomes
Foot alignment
Maripuri 2013 measured foot alignment using the Pirani scale, but we were unable to estimate a treatment effect, as raw data were not available for analysis.
Gait assessment
Gait was not an outcome.
Quality of life
Quality of life was not an outcome.
Adverse events
Maripuri 2013 reported a high failure rate in the below‐knee casting group (four due to plaster slippages and two due to more than eight weeks of casting), which led to early stopping of the trial. They also reported minor skin irritation and redness (five participants in the below‐knee casting group and five participants in the above‐knee casting group), which was managed by a silicone dressing and increased wool padding. Follow‐up was only to the end of casting, so relapses were not reported.
Comparison 9: Denis Browne splint versus Denis Browne with orthopaedic shoes versus forefoot abduction shoes with orthopedic shoes
One study compared the use of three different corrective methods: Denis Browne splint, Denis Browne splint with orthopaedic shoes, and a combination of forefoot abduction shoes and orthopaedic shoes for children with CTEV (Chen 2015; 53 participants, 83 feet).
Primary outcome: function
Function was not measured.
Secondary outcomes
Foot alignment
Chen 2015 assessed foot alignment and gait using the Diméglio scale, three‐dimensional foot scanning and pedobarography. We were unable to draw a conclusion for this comparison, as data from validated measures combined data from bilateral and unilateral cases, and we were unable to obtain raw data.
Gait assessment
Chen 2015 assessed gait using pedobarography, but data were not available to analyse.
Quality of life
Quality of life was not an outcome.
Adverse events
No adverse events were reported.
The trial authors reported severe equinus, adduction and varus deformities after treatment in the Denis Browne group (five participants, 33%) and the Denis Browne plus orthopaedic shoes group (one participant, 5%). However, it was not clear whether this occurred after Ponseti casting or following initiation of braces or orthoses.
Resistant cases
Four trials investigated treatment for resistant CTEV (El‐Deeb 2007; Kaewpornsawan 2007; Lahoti 2008; Zeifang 2005). See Table 2.
Comparison 10: Posteromedial soft tissue release versus subtalar release
One trial compared posteromedial soft tissue release and subtalar release in children (average age 5.9 months), who had failed to respond to prior conservative treatment (Kaewpornsawan 2007; 86 participants, 128 feet). Children were excluded if associated syndromes were present. Prior treatment was not defined.
Primary outcome: function
Function was not measured.
Secondary outcomes
Foot alignment
Kaewpornsawan 2007 measured foot alignment using Pirani and Diméglio scales, but raw data were not available for analysis.
Gait assessment
Gait was not an outcome.
Quality of life
Quality of life was not an outcome.
Adverse events
Kaewpornsawan 2007 reported skin infections in both groups: posteromedial soft tissue release (four feet, 8.5%) and complete circumferential subtalar release (two feet, 5.1%).
The trial authors noted relapse in both groups, but data were not available to analyse.
Comparison 11: Talocalcaneal interosseus ligament lengthening versus control during posteromedial soft tissue release surgery
One trial evaluated lengthening of the talocalcaneal interosseous ligament (TCIL) (El‐Deeb 2007; 46 participants, 66 feet) in idiopathic severe or very severe CTEV (grade III or IV on Diméglio scale), which had failed to respond to repeated manipulation.
Primary outcome: function
Function was not measured.
Secondary outcomes
Foot alignment
El‐Deeb 2007 measured foot alignment radiologically but we were unable to estimate a treatment effect as raw data were not available for analysis.
Gait assessment
Gait was not an outcome.
Quality of life
Quality of life was not an outcome.
Adverse events
The trial authors did not report any adverse events.
Relapse was seen in both groups, but data were not available to re‐analyse. All relapses required surgical intervention.
Comparison 12: Flexor hallucis longus and flexor digitorum longus lengthening versus simple decompression during surgery for toe flexion deformity in CTEV
One trial evaluated decompression versus lengthening of flexor hallucis longus and flexor digitorum longus in children (average age of 9.5 months) with an average Pirani score of 5.5 (Lahoti 2008; 13 participants, 26 feet). Two syndromic feet were included, one in each group. Prior treatment was not defined.
Primary outcome: function
Function was not measured
Secondary outcomes
Foot alignment
Lahoti 2008 measured foot alignment using Pirani and Harrold and Walker scales but we were unable to estimate a treatment effect, as raw data from these scales were not available.
Gait assessment
Gait was not an outcome.
Quality of life
Quality of life was not an outcome.
Adverse events:
The trial authors did not report adverse events.
Three participants presented with hindfoot relapse by the end of follow‐up (average four years), but trial authors did not state whether these participants required further treatment.
Comparison 13: Continuous passive motion (CPM) versus immobilisation in a cast post‐CTEV surgery
One trial compared CPM versus immobilisation in a cast post‐CTEV surgery in children (average age of 8.2 months) after six months of failed manipulation and casting (Zeifang 2005; 36 participants, 36 feet). All children were classified as having severe CTEV (Diméglio grade III).
Primary outcome: function
Function was not measured.
Secondary outcomes
Foot alignment
Zeifang 2005 measured foot alignment using the Diméglio scale, but we were unable to estimate a treatment effect as raw data were not available.
Gait assessment
Gait was not an outcome.
Quality of life
Quality of life was not an outcome.
Adverse events:
The trial authors did not report any adverse events.
Relapse of one participant (cast group) with bilateral deformity occurred shortly after surgery and the participant was excluded; residual deformity in each group was noted at 48 months of follow‐up. Further treatment was not documented.
Relapsed cases
No RCTs examined treatments for recurrent or relapsing CTEV.
Neglected cases
No RCTs examined treatments for neglected CTEV.
Other
Three trials investigated treatment of CTEV that was not stated to be initial or resistant (Chen 2015; Harnett 2011; Siddiqui 2007).
Comparison 14: Accelerated Ponseti technique versus standard Ponseti technique
Two trials compared an accelerated Ponseti technique (cast changes twice (Elgohary 2014; 46 participants, 74 feet), or three times a week (Harnett 2011; 40 participants, 60 feet) compared to the standard technique (weekly cast changes) in idiopathic CTEV.
Primary outcome: function
Function was not an outcome in Elgohary 2014 or Harnett 2011.
Secondary outcomes
Foot alignment
Harnett 2011 included combined unilateral and bilateral cases in published data. The trial author provided IPD on foot alignment (Pirani score), which we were able to re‐analyse. See Table 5.
Harnett 2011 randomised participants into either the accelerated Ponseti group (19 participants, 29 feet) or the standard Ponseti group (21 participants, 32 feet). Analysis using a linear mixed model with random subject effects gave an estimated difference in total mean Pirani scores at follow‐up of 0.31 (SE 0.36, 95% CI −0.40 to 1.02; 40 participants; low‐certainty evidence; Analysis 4.1, Figure 6). Using this model, the estimated correlation coefficient for left‐ and right‐foot measurements in a group which received the same treatment was 0.8704.
4.1. Analysis.

Comparison 4: Accelerated Ponseti technique versus standard Ponseti technique, Outcome 1: Foot alignment: Pirani score at the end of serial plaster casting
6.

Forest plot of comparison: 3 Accelerated Ponseti technique versus standard Ponseti technique at unknown intervention timepoint, outcome: 3.1 Foot alignment: Pirani score at the end of serial plaster casting.
As the Pirani scores were highly skewed (not normally distributed), we also constructed a bootstrap CI as a check on the robustness of these results. A non‐parametric bootstrap (stratified by group, bilateral or unilateral status and clustered by participant) constructed from 100,000 bootstrap samples gave a 95% CI extending from −0.20 to 0.86. Although this CI is narrower than that reported above, the difference is still not statistically significant (Analysis 4.1; Figure 6).
Elgohary 2014 measured foot alignment using the Pirani scale, but bilateral and unilateral cases were combined and we were unable to estimate treatment effect as raw (IPD) data were not available.
Gait assessment
Gait assessment was not an outcome in Elgohary 2014 or Harnett 2011.
Quality of life
Quality of life was not an outcome in Elgohary 2014 or Harnett 2011.
Adverse events
Harnett 2011 reported that fewer plaster casts were required in the accelerated Ponseti group. No adverse events were reported. No relapses occurred within six months.
Elgohary 2014 reported five relapses in each group. All relapses were managed with repeat traditional or accelerated Ponseti technique. Three feet in each group also required repeated tenotomy. Authors reported no complications in either treatment group.
Comparison 15: Window versus Turco surgery
One trial compared Window versus Turco surgery for children with idiopathic CTEV (mean age 9.6 months) with mild‐to‐moderate CTEV (Diméglio grade I and II) (Siddiqui 2007; 60 participants, unknown number of feet). Pre‐treatment was not defined.
Primary outcome: function
Siddiqui 2007 did not measure function.
Secondary outcomes
Foot alignment
Siddiqui 2007 measured foot alignment using the Diméglio scale, but we were unable to estimate a treatment effect, as data from validated outcome measures were not available.
Gait assessment
Gait was not an outcome.
Quality of life
Quality of life was not an outcome.
Adverse events
Siddiqui 2007 reported relapse in both groups, but raw data were not available to re‐analyse.
Siddiqui 2007 reported wound infections in both groups: Window procedure (one foot, 3%) and the Turco procedure (six feet, 20%). The Turco procedure also produced skin breakdown and wound dehiscence (opening) (two feet, 7%).
Discussion
Summary of main results
This review includes 21 trials with 905 participants. The treatment of CTEV continues to evolve. There are four distinct areas of therapeutic research: initial presentation, resistant deformity (after unsuccessful treatment), relapsed deformity (recurrence of deformity some time after initial satisfactory treatment), and neglected deformity (no early initial treatment). Within these categories, two subgroups are recognised: idiopathic CTEV and syndromic CTEV. Since different presentations of CTEV were analysed separately using a variety of validated and non‐validated outcome measures with diverse statistical approaches, we are unable to draw a single, overall conclusion about treatment for this condition.
Initial cases
Fourteen trials evaluated initial presentations of CTEV. One trial found low‐certainty evidence that the Ponseti technique may produce better foot alignment at the end of serial casting compared to the Kite technique. Adverse events were not reported (Table 1). Following relapse, the risk difference for major surgery in the Kite group was 25% and 50% higher in two trials compared to Ponseti. The certainty of the evidence is too low to draw conclusions about foot alignment after Ponseti casting compared to a traditional treatment (Table 1). This trial had small numbers, as a formal stopping rule was activated after the Kite technique was seen to lead to higher rates of major surgery than the Ponseti technique. One trial examined modification of the Ponseti technique through the use of different plaster‐casting products (semi‐rigid fibreglass casting versus plaster of Paris) and did not find any difference between the two treatment groups, based on low‐certainty evidence (Table 1). We could draw no conclusions for other interventions, i.e. surgery, and the addition of botulinum toxin A to the Ponseti technique. The reporting of adverse events was limited in all trials. In those involving serial casting (plaster casting) adverse events included pressure areas, cast slippage and skin irritations.
Resistant cases
All trials evaluating resistant cases involved major surgical procedures or post‐operative care (for example, continuous passive motion (CPM)). We could draw no conclusions from the data available (Table 2). All relapses required major surgical intervention to correct any recurrent deformity.
Other cases
One trial concluded that there may be no difference between an accelerated Ponseti technique (cast changes three times a week) and standard Ponseti technique (weekly cast changes); this evidence was of low certainty (Table 5). We could draw no conclusions about two surgical procedures (Window versus Turco procedures) due to limited available data; however, wound infections were reported to be higher in the Turco group (20% versus 3%), as were wound dehiscence (opening), skin necrosis (7%) and scarring and fibrosis (10%).
Relapsed cases
We could draw no conclusions about recurrent cases.
Neglected cases
We could draw no conclusions about neglected cases.
Overall completeness and applicability of evidence
Function and quality of life
Function was an outcome in seven trials (El‐Deeb 2007; Kaewpornsawan 2007; Manzone 1999; Sanghvi 2009; Sud 2008; Svehlik 2017; Zwick 2009), with two using a validated scale (Svehlik 2017; Zwick 2009). However, these trials combined bilateral and unilateral cases and raw data were not available to appropriately re‐analyse. Valid assessment of function is required as part of CTEV assessment because routine objective measures for CTEV (for example, x‐ray) do not reliably correlate with function (Farsetti 2006; Fridman 2007).
Foot alignment
All trials but one (Chong 2014) assessed foot alignment. Sixteen trials used validated outcome measures (Chen 2015; El‐Deeb 2007; Elgohary 2014; Gintautiene 2016; Harnett 2011; Hui 2014; Lahoti 2008; Manzone 1999; Maripuri 2013; Pittner 2008; Rijal 2010; Sanghvi 2009; Selmani 2012; Svehlik 2017; Zeifang 2005; Zwick 2009). As CTEV deformity occurs in several planes of movement (Ponseti 2005), assessment of foot alignment using valid scales is essential to report on all aspects of the deformity. Poor foot alignment correlates with the requirement for further intervention.
Gait assessment
Gait was assessed in two trials (Chen 2015; Svehlik 2017). Data were unavailable for re‐analysis. In many trials, participants were too young to undertake formalised gait assessments. Gait analysis may also be considered cost‐prohibitive and was therefore not used in many trials.
Inclusion and exclusion criteria
We did not include trials in which study design and inclusion criteria were not adequately described. Explicit inclusion and exclusion criteria about prior treatment are essential to allow the reader to judge the homogeneity of the participant population. Syndromic CTEV is known to be more resistant to treatment (Janicki 2009; Ponseti 2006), and its inclusion may influence outcomes when combined with idiopathic cases. In relapsed cases, the treatment required may be influenced by the initial treatment prescribed (Halanski 2010a).
Reporting on compliance
Compliance was not formally assessed in any of the included trials. In nine trials, different regimens of bracing and post‐operative care requiring parent or carer compliance were required (Chong 2014; Elgohary 2014; Gintautiene 2016; Sanghvi 2009; Selmani 2012; Sud 2008; Svehlik 2017; Zeifang 2005; Zwick 2009). Seven trials recorded compliance through communication with parents (Chen 2015; Chong 2014; Cummings 2009; Hui 2014; Sud 2008; Zeifang 2005; Zwick 2009). Three trials noted accurate assessment of compliance as a limitation (Chong 2014; Selmani 2012; Zeifang 2005). Cummings 2009 was unable to demonstrate an association between compliance and the rate of relapse. Compliance with bracing has been shown to influence the rate of relapse (Haft 2007; Morcuende 2004). However, compliance with bracing is very difficult to assess objectively, and no reliable and valid method has been reported.
Reporting on relapse
Many factors define relapse, making it difficult to report. In CTEV, a relapse can include multiple deformities, for example, equinus (tightness of the heel), adductus (in‐turning of the foot) or cavus (high arch). Two main types of relapse are recognised: passive and dynamic (or residual). Passive relapse refers to a loss in range of movement, whereas dynamic refers to a positional relapse where passive range is still present. Dynamic relapse, if left untreated, can lead to a passive relapse (Ponseti 2005). Treatment options depend on the type of relapse (Farsetti 2006; Haft 2007; Nogueira 2009; Ponseti 2005). Treatment to correct relapse can therefore be an indication of the severity of the deformity. Relapse may be confused with resistant deformity. There is an emerging literature attempting to discriminate relapse from mild resistant deformity (Halanski 2010b). A well‐defined outcome measure of relapsed cases will allow the reader to determine the long‐term outcome of the initial treatment.
Nineteen trials reported on relapse (Chen 2015; Chong 2014; Cummings 2009; El‐Deeb 2007; Elgohary 2014; Gintautiene 2016; Harnett 2011; Hui 2014; Kaewpornsawan 2007; Lahoti 2008; Manzone 1999; Rijal 2010; Sanghvi 2009; Selmani 2012; Siddiqui 2007; Sud 2008; Svehlik 2017; Zeifang 2005; Zwick 2009). Chong 2014 defined relapse as the need for revision surgery, repeat tenotomy or repeat casting while in the bracing phase. However, they provided no information on what determined the need for surgery, tenotomy or casting. Other trials did not define relapse in their initial protocol. We provide a summary of reported relapse in Table 8.
3. Reported details of relapses in included studies.
| Study ID | Treatment | Relapses reported | Deformity described with valid scales | Group in which relapses occurred reported | Timing of relapse reported | Treatment required to correct described? |
| Chen 2015 | DB versus OS + DB versus OS + FAS | Y | N | Y | N | N |
| Cummings 2009 | Botulinum toxin A versus placebo in Ponseti treatment | Y | N | Y | Y | Y |
| El‐Deeb 2007 | TCIL release versus control in CTEV surgery | Y | N | Y | N | Y |
| Elgohary 2014 | Traditional versus accelerated Ponseti technique | Y | N | Y | N | Y |
| Gintautiene 2016 | Ponseti versus early TATT | Y | N | Y | N | N |
| Harnett 2011 | Ponseti standard versus Ponseti accelerated protocol | Y | N | N/A | N/A | N/A |
| Hui 2014 | Plaster of paris versus semi‐rigid fibreglass | Y | N | Y | Y | Y |
| Kaewpornsawan 2007 | Modified PMR versus CCSR | Y | N | Y | N | Y |
| Lahoti 2008 | FHL and FDL lengthen versus simple decompression | Y | N | N | N | N |
| Manzone 1999 | PMR versus CCSR | Y | N | N | N | N |
| Pittner 2008 | Semi‐rigid versus POP for Ponseti treatment | No: follow‐up to end of treatment only | ||||
| Rijal 2010 | Ponseti versus Kite | No: follow‐up to end of treatment only | ||||
| Sanghvi 2009 | Ponseti versus Kite | Y | N | Y | N | Y |
| Selmani 2012 | Ponseti versus Kite | Y | N | Y | Y | Partial |
| Siddiqui 2007 | Window versus Turco surgery | Y | N | Y | N | N |
| Sud 2008 | Ponseti versus Kite | Y | Y | Y | Y | Y |
| Svehlik 2017 | Ponseti versus traditional treatment | Y | N | Y | Y | Y |
| Zeifang 2005 | CPM versus immobilisation in a cast after CTEV surgery | Y | N | Y | Y | N |
| Zwick 2009 | Ponseti versus traditional treatment | Y | N | Y | Y | Y |
CCSR: complete circumferential subtalar release CPM: continuous passive motion CTEV: congenital talipes equinovarus DB: Denis Browne splint FAS: forefoot abduction shoe FDL: flexor digitorum longus FHL: flexor hallucis longus N/A: not applicable OS: orthopaedic shoe PMR: posteromedial release POP: plaster of Paris TATT: tibialis anterior tendon transfer TCIL: talocalcaneal interosseus ligament
Cost‐benefit analysis
We could not perform a cost‐benefit analysis from data provided in the studies included in this review. Two studies external to this review have examined this.
One trial examined the cost effectiveness of Ponseti versus primary surgical management for initial treatment of idiopathic CTEV in 55 participants (86 feet) in the New Zealand socialised healthcare system (Halanski 2009). Although it was initially designed as an RCT, only nine participants agreed to randomisation, with the remainder choosing their treatment path. Cost analysis was divided into four groups: unilateral CTEV total cost, bilateral CTEV total cost, unilateral CTEV with recurrence total cost and bilateral CTEV with recurrence total cost. Secondary outcome measures of number of clinic visits, days in hospital, number of visits to operating theatres, operating room time, antibiotic doses, and pain medication doses were also examined.
During the average follow‐up period of 3½ years, the total cost of treatment per foot was significantly less in the Ponseti group for unilateral CTEV, bilateral CTEV, and bilateral CTEV with recurrence. The total cost was not significantly different between groups for unilateral CTEV with recurrence. Furthermore, the surgical group required a higher average number of days in hospital and more doses of pain medication.
This trial also calculated the equivalent cost of the above treatment in the USA healthcare system, reporting significantly higher costs in the surgical arm for unilateral CTEV, bilateral CTEV, and unilateral CTEV with recurrence.
One trial examined the cost effectiveness of CTEV management in sub‐Saharan African countries (Grimes 2016). They reported the average cost of the Ponseti treatment to be USD 167 per patient. When calculated per disability‐adjusted life year averted the cost‐effectiveness ratio was USD 22.46. The authors conclude that the Ponseti technique is a highly cost‐effective treatment compared with other health conditions, and they encourage governments to consider this management for incorporation into national health plans.
Summary
There is low‐ to very low‐certainty evidence for the Ponseti technique providing superior short‐term results to other techniques. Although these results support findings from studies which we excluded from this review, including those which were not randomised or quasi‐randomised controlled trials, it is clear that well‐powered long‐term RCTs are needed to further build this body of evidence.
Certainty of the evidence
The certainty of the existing evidence remains low to very low for several reasons. Despite identifying 21 trials for inclusion, we could only use available data for analysis from four trials. We downgraded all four studies (Harnett 2011; Hui 2014; Rijal 2010; Zwick 2009) by one level, as they were subject to a high risk of bias due to lack of blinding of participants and personnel. We also downgraded by one further level due to the imprecision of the results, e.g. single studies with a small sample size and number of events (Harnett 2011 40 participants; Hui 2014 30 participants; Rijal 2010 38 participants; Zwick 2009 19 participants). Overall, we judged the certainty of evidence to be low for all four comparisons assessed (Kite versus Ponseti technique; traditional treatment versus Ponseti techniques; semi‐rigid fibreglass compared with plaster of Paris; and accelerated Ponseti versus standard Ponseti method), which suggests that further research is very likely to have an important impact on our confidence in the estimate of effect, and is likely to change the estimate.
Despite this lack of certainty, as the body of non‐randomised evidence in CTEV supports non‐surgical management, further RCTs which compare surgical and non‐surgical management could be considered unethical and are therefore less likely to be undertaken.
Within trials, reporting of adverse events remains variable, with some trials reporting adverse events for each intervention in detail, some reporting adverse events as a whole, and others not reporting whether any adverse events occurred at all. The lack of consistency makes it difficult to ascertain the true rate of adverse events in all interventions for CTEV.
Study design
Blinding
One trial blinded participants (Cummings 2009). Hui 2014 and Pittner 2008 compared semi‐rigid (fibreglass) casting and plaster of Paris, and Rijal 2010, Selmani 2012, Sud 2008 and Sanghvi 2009 compared Kite to Ponseti techniques of casting. It is unlikely that participant or carer blinding would have affected the outcome in these trials because the care and compliance required by the participant is virtually identical. Siddiqui 2007 did not provide post‐operative care details, so we do not know whether blinding may have affected bias.
Dealing with bilateral and unilateral cases
Inclusion criteria of one or both limbs per participant is a contentious issue in many fields, including foot and ankle orthopaedics (Bryant 2006; Perera 2007). Randomisation can occur at the participant or the limb level. When randomisation occurs at the participant level but feet in bilateral and unilateral cases are pooled, a unit‐of‐analysis error occurs. In bilateral cases each limb does not respond independently of the other and therefore violates the underlying independence assumption of statistical analysis. In cases where bilateral and unilateral cases are included, disproportionate weighting is given to the bilateral cases. For example, many treatments of CTEV involve participant compliance with bracing, such that if a participant with bilateral CTEV is non‐compliant, two feet are affected by the one person. Including both feet from one participant may narrow confidence intervals and overstate findings. Results involving pooling of bilateral and unilateral cases should therefore be interpreted with caution. Seventeen trials randomised participants (Chen 2015; Chong 2014; Cummings 2009; Elgohary 2014; Gintautiene 2016; Harnett 2011; Hui 2014; Kaewpornsawan 2007; Maripuri 2013; Pittner 2008; Sanghvi 2009; Selmani 2012; Siddiqui 2007; Sud 2008; Svehlik 2017; Zeifang 2005; Zwick 2009), and four trials randomised feet (El‐Deeb 2007; Lahoti 2008; Manzone 1999; Rijal 2010). In bilateral cases all trials except one (Zeifang 2005) included all feet. Zeifang 2005 tossed a coin and only included one foot from each participant in their analysis.
Several options exist to overcome this issue. With raw data, post hoc statistical re‐analysis of one limb per person can be performed (Lesaffre 2010; Perera 2007). During study design, randomisation by exclusion of the second limb or joint, randomly selecting one limb in bilateral cases, analysing bilateral cases as a distinct subgroup or stratifying bilateral and unilateral cases can be undertaken (Bryant 2006). Alternatively, statistical techniques such as linear mixed models can be used.
Lahoti 2008 randomly allocated each foot to a different intervention. In bilateral cases where each foot is randomly assigned to a different treatment, there is less variation between individuals, allowing a more precise estimation of the treatment effect. However, the analysis of bilateral cases where each foot receives a different intervention still requires an appropriate statistical analysis to account for any potential within‐participant correlation.
To avoid unit‐of‐analysis issues we requested individual patient data for re‐analysis where required. As IPD were not available in most cases, we could not re‐analyse or include data from most trials within this review.
Potential biases in the review process
We identified all the trials in this review through electronic searching. All were published in English‐language journals. Although we made efforts to identify trials reported in languages other than English and considered some papers for possible inclusion, they did not meet our criteria. We are reasonably confident that we identified all relevant trials because RCTs in CTEV are rare and therefore typically published. We had to leave out large numbers of non‐randomised studies which were at potentially higher risk of bias through design, leaving small numbers of individual studies with higher‐quality design. We had to leave out a large amount of published data due to unit‐of analysis issues, and trials which reported data using non‐validated assessments. Where possible we sourced IPD for re‐analysis. We believe that the exclusion of these lower‐quality studies/data would not have provided evidence of sufficient certainty to outweigh the bias from design or performance in the included studies.
There are limitations to this review. We include RCTs and quasi‐RCTs, but for ethical reasons most trials investigating treatment of CTEV are not RCTs but comparisons of treatments which have been selected by the parent, carer or clinician. Inclusion of these additional trials might have allowed further analysis, but the lack of randomisation would have introduced significant bias.
Agreements and disagreements with other studies or reviews
We have identified no other systematic reviews of RCTs of interventions for CTEV.
Authors' conclusions
Implications for practice.
Evidence for the various presentations of congenital talipes equinovarus (CTEV) is accumulating; however, small sample sizes and the limited use of validated outcome measures limit clear conclusions.
In initial (treatment‐naïve) cases, the main findings from this review, based on the evidence from randomised controlled trials (RCTs), are as follows.
The Ponseti technique may produce better foot alignment in the short term compared to the Kite technique. Following relapse, the risk difference for major surgery in the Kite group was 25% and 50% higher in two trials.
The certainty of evidence was too low to draw conclusions about foot alignment in the short term following the Ponseti technique compared to a traditional technique.
When using the Ponseti technique, semi‐rigid fibreglass may be as effective as plaster of Paris.
There was a further finding in other cases, in which it was not clear whether a prior intervention had taken place.
One trial showed that there may be no evidence of a difference between an accelerated Ponseti technique and a standard Ponseti technique.
Implications for research.
To develop a strong evidence base for the treatment of various presentations of CTEV, there needs to be further evaluations in well‐designed RCTs. Long‐term high‐quality designs would be very difficult to perform. Randomisation may be considered unethical in certain circumstances and well‐designed controlled trials may provide more opportunities to analyse different treatments. The following measures would improve the quality of future trials assessing interventions for CTEV: ensuring baseline comparability by detailed inclusion and exclusion criteria; using valid and reliable outcome measures for function and quality of life; investigating robust methods to measure compliance; evaluation of treatment for relapsed cases, neglected cases, and those with non‐idiopathic CTEV. Consideration must also be given to statistical analysis, particularly when pooling unilateral and bilateral cases.
What's new
| Date | Event | Description |
|---|---|---|
| 15 May 2020 | Amended | Resolved broken links in search methods |
History
Protocol first published: Issue 7, 2010 Review first published: Issue 4, 2012
| Date | Event | Description |
|---|---|---|
| 28 May 2019 | New search has been performed | Updated search to 28 May 2019. We identified one new randomised controlled trial of the Ponseti technique, comparing semi‐rigid casting and plaster of Paris. We included five other new trials, but they did not use validated outcome tools as required by our protocol. Changes in authorship ‐ withdrawal of Professor David Little and Dr. Paul Gibbons, previous authors on this review. New authors: Shadi Bina and Elizabeth Barnes. |
| 28 May 2019 | New citation required and conclusions have changed | We have updated the conclusions to include evidence that semi‐rigid fibreglass casting is probably as effective as plaster of Paris for the Ponseti technique. |
| 21 November 2013 | New citation required but conclusions have not changed | Updated search incorporated |
| 28 September 2013 | New search has been performed | One new randomised controlled trial comparing Ponseti and Kite techniques added. Conclusions are unchanged Discussion on cost‐benefit analysis between major surgery and the Ponseti technique added, based on a new identified trial |
Acknowledgements
The review authors would like to thank the editorial team of Cochrane Neuromuscular, particularly Professor Ros Quinlivan, Professor Michael Lunn and Dr Ruth Brassington for their assistance in preparing this review; Ms Angela Gunn for assisting in developing the search strategy and conducting searches of electronic databases; and Chris Frost (an author on the first version of the review), Professor David Little and Dr. Paul Gibbons (authors on the second version of the review).
Thank you to Kristy Rose and Tamis Pin for assistance with translation of articles, and Ms Trish Bennett for assistance with the PEDro, ICTRP and clinical trials searches.
Finally, the review authors would like to thank the authors of included trials who responded to requests for additional information/data: Dr Jason Howard (Qatar), Dr Catherine Hui (Qatar), Dr Paul Harnett (UK), Dr Ernst Zwick (Austria), Dr Amish Sanghvi (India), Dr Raju Rijal (Nepal), Dr Kevin Klingele (USA), Dr Hanneke Andriesse (Sweden), Dr Khaled El‐Adwar (Egypt), Mr Om Lahoti (UK) and Mr Martin Svehlik (Austria).
We thank peer reviewers of the update: Dr Sarah Nevitt, University of Liverpool, and Dr B David Horne, Children's Hospital of Philadelphia.
This project was supported by the National Institute for Health Research via Cochrane Infrastructure funding to Cochrane Neuromuscular. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health. Cochrane Neuromuscular is also supported by the MRC Centre for Neuromuscular Disease.
Appendices
Appendix 1. Cochrane Neuromuscular Specialised Register (CRSWeb) search strategy
#1 clubfoot or clubfeet or talipes or ctev AND INREGISTER
Appendix 2. Cochrane Central Register of Controlled Trials (CENTRAL) (CRSWeb) search strategy
#1 clubfoot or clubfeet or talipes or ctev AND CENTRAL:TARGET
Appendix 3. MEDLINE OvidSP search strategy
Database: Ovid MEDLINE(R) and Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations and Daily <1946 to May 24, 2019> Search Strategy: ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 randomized controlled trial.pt. (482595) 2 controlled clinical trial.pt. (93085) 3 randomi#ed.ti,ab. (571161) 4 placebo.ab. (197897) 5 drug therapy.fs. (2109870) 6 randomly.ab. (311341) 7 trial.ab. (464408) 8 groups.ab. (1915170) 9 or/1‐8 (4469845) 10 exp animals/ not humans.sh. (4583131) 11 9 not 10 (3869280) 12 Clubfoot/ (3639) 13 (clubfoot or clubfeet or talipes or ctev).tw. (3238) 14 12 or 13 (4793) 15 11 and 14 (416) 16 remove duplicates from 15 (414)
Appendix 4. Embase OvidSP search strategy
Database: Embase Classic+Embase <1947 to 2019 May 24> Search Strategy: ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 crossover‐procedure.sh. (59573) 2 double‐blind procedure.sh. (163227) 3 single‐blind procedure.sh. (35180) 4 randomized controlled trial.sh. (552671) 5 (random$ or crossover$ or cross over$ or placebo$ or (doubl$ adj blind$) or allocat$).tw,ot. (1660896) 6 trial.ti. (278391) 7 clinical trial/ (978671) 8 or/1‐7 (2402360) 9 (animal/ or nonhuman/ or animal experiment/) and human/ (1891814) 10 animal/ or nonanimal/ or animal experiment/ (4264586) 11 10 not 9 (3560534) 12 8 not 11 (2248131) 13 limit 12 to (conference abstracts or embase) (1889627) 14 clubfoot/ (5308) 15 (clubfoot or clubfeet or talipes or ctev).mp. (7292) 16 14 or 15 (7292) 17 13 and 16 (216) 18 remove duplicates from 17 (210)
Appendix 5. AMED OvidSP search strategy
Database: AMED (Allied and Complementary Medicine) <1985 to May 2019> Search Strategy: ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 Randomized controlled trials/ (2201) 2 Random allocation/ (322) 3 Double blind method/ (717) 4 Single‐Blind Method/ (132) 5 exp Clinical Trials/ (4056) 6 (clin$ adj25 trial$).tw. (7434) 7 ((singl$ or doubl$ or treb$ or trip$) adj25 (blind$ or mask$ or dummy)).tw. (3137) 8 placebos/ (623) 9 placebo$.tw. (3341) 10 random$.tw. (19160) 11 research design/ (1996) 12 Prospective Studies/ (1264) 13 meta analysis/ (298) 14 (meta?analys$ or systematic review$).tw. (4127) 15 control$.tw. (37802) 16 (multicenter or multicentre).tw. (1105) 17 ((study or studies or design$) adj25 (factorial or prospective or intervention or crossover or cross‐over or quasi‐experiment$)).tw. (13728) 18 or/1‐17 (58626) 19 Clubfoot/ (196) 20 (clubfoot or clubfeet or talipes or ctev).tw. (235) 21 (clubfoot or clubfeet or talipes or ctev).mp. (235) 22 19 or 20 (235) 23 18 and 22 (31)
Appendix 6. CINAHL EBSCOhost search strategy
Tuesday, May 28, 2019 12:00:21 PM S25 S23 AND S24 Search modes ‐ Boolean/Phrase 18 S24 EM 20180101‐ Limiters ‐ Exclude MEDLINE records Search modes ‐ Boolean/Phrase 452,758 S23 S22 Limiters ‐ Exclude MEDLINE records Search modes ‐ Boolean/Phrase 43 S22 S18 and S21 319 S21 S19 or S20 1,040 S20 clubfoot or clubfeet or talipes or ctev 1,040 S19 (MH "Clubfoot") 844 S18 S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 or S10 or S11 or S12 or S13 or S14 or S15 or S16 or S17 1,228,343 S17 ABAB design* 119 S16 TI random* or AB random* 288,523 S15 ( TI (cross?over or placebo* or control* or factorial or sham? or dummy) ) or ( AB (cross?over or placebo* or control* or factorial or sham? or dummy) ) 576,205 S14 ( TI (clin* or intervention* or compar* or experiment* or preventive or therapeutic) or AB (clin* or intervention* or compar* or experiment* or preventive or therapeutic) ) and ( TI (trial*) or AB (trial*) ) 223,115 S13 ( TI (meta?analys* or systematic review*) ) or ( AB (meta?analys* or systematic review*) ) 81,820 S12 ( TI (single* or doubl* or tripl* or trebl*) or AB (single* or doubl* or tripl* or trebl*) ) and ( TI (blind* or mask*) or AB (blind* or mask*) ) 43,367 S11 PT ("clinical trial" or "systematic review") 165,947 S10 (MH "Factorial Design") 1,124 S9 (MH "Concurrent Prospective Studies") or (MH "Prospective Studies") 383,411 S8 (MH "Meta Analysis") 38,194 S7 (MH "Solomon Four‐Group Design") or (MH "Static Group Comparison") 97 S6 (MH "Quasi‐Experimental Studies") 10,888 S5 (MH "Placebos") 11,243 S4 (MH "Double‐Blind Studies") or (MH "Triple‐Blind Studies") 41,412 S3 (MH "Clinical Trials+") 259,816 S2 (MH "Crossover Design") 17,532 S1 (MH "Random Assignment") or (MH "Random Sample") or (MH "Simple Random Sample") or (MH "Stratified Random Sample") or (MH "Systematic Random Sample") 91,639
Appendix 7. PEDro search strategy
28 May 2019
We used a simple search strategy using the following terms separately:
clubf*
talipes
ctev
Appendix 8. WHO International Clinical Trials Registry Platform (ICTRP)
Advanced search
Condition: clubfoot OR clubfeet OR talipes OR ctev
Recruitment status: ALL
Appendix 9. Clinicaltrials.gov search strategy
28 May 2019
Advanced search
Condition: clubfoot OR clubfeet OR talipes OR ctev
Study type: interventional
Appendix 10. Calculations for results
Comparison 1
Category 1: Assuming that the correlation between means is 0.8704 (calculated from raw Harnett 2011 data), the SD of within‐subject differences is 0.330 (0.3302 = 0.572 + 0.672 ‐ 2 x 0.874 x 0.67 x 0.57), and hence the SE of the MD is 0.095 (0.330 / √12).
Category 2: Denoting the mean of the left and right scores as the combined score, analysis can compare the group‐specific means of these combined scores. Assuming that the correlation between right and left feet from the same subject is 0.8704, the mean of the combined scores ((left + right) / 2) is 0.81, and the SD was 0.430 (0.4302 = (0.482 + 0.412 + 2 x 0.8704 x 0.48 x 0.41) / 4). At the end of serial casting, the total Pirani scores in the Kite group were 2.1 (0.68) for left feet and 2.00 (0.32) for right feet. Therefore, the average combined score was 2.05 and the SD was 0.486 (0.4862 = 0.682 + 0.322 + 2 x 0.8704 x 0.68 x 0.32) / 4). Accordingly, the estimated difference of means between groups is ‐1.24 (2.05 minus 0.81) and the SE is 0.293 (0.2932 = (0.4302 / 4) + (0.4862 / 6).
Category 3: The estimated difference of the means between groups was ‐0.86 (‐1.91 to ‐1.05) and the SE was 0.336 (0.3362 = (0.492 / 10) + (0.732 / 6)), giving a 95% CI for the difference of ‐0.20 to ‐1.52.
Comparison 2
Assuming that the correlation between right and left foot scores from the same subject is r, the standard error of the mean of N observations from n subjects with SD σ, is given by σ (N + 2 r (N ‐ n))0.5/N. Therefore the estimated SE of the mean is 0.104 in the Ponseti group and 0.386 in the traditional treatment group. The estimated SE for the difference in means is 0.400 (0.4002 = 0.1042 + 0.3862).
Data and analyses
Comparison 1. Ponseti versus Kite technique for treatment of initial CTEV.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Foot alignment: Pirani score at 10 weeks | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐1.15 [‐1.32, ‐0.98] |
| 1.1.1 Bilateral cases, one foot assigned to Ponseti, one to Kite | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | ‐1.16 [‐1.35, ‐0.97] |
| 1.1.2 Bilateral cases, both feet assigned to the same treatment (Ponseti or Kite) | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐1.24 [‐1.81, ‐0.67] |
| 1.1.3 Unilateral cases, assigned to either Ponseti or Kite | 1 | 16 | Mean Difference (IV, Fixed, 95% CI) | ‐0.86 [‐1.52, ‐0.20] |
Comparison 2. Ponseti versus traditional treatment (plaster casting and surgery) for treatment of initial CTEV.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Foot alignment: Pirani score at end of serial plaster casting | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 3. Ponseti technique. Comparison of semi‐rigid fibreglass and plaster of Paris.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Foot alignment: Pirani score at end of casting in those awaiting tenotomy | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 4. Accelerated Ponseti technique versus standard Ponseti technique.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Foot alignment: Pirani score at the end of serial plaster casting | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Chen 2015.
| Study characteristics | ||
| Methods | RCT. Prospective, single‐blinded, 3‐arm parallel‐group design Randomisation of participants (not feet) |
|
| Participants | 53 children with 83 CTEV feet Inclusion criteria: children with moderate CTEV who had finished Ponseti treatment and wore DB splints for the initial period of correction Exclusion criteria: none stated PARTICIPANT CHARACTERISTICS DB group Age mean (SD): 4.7 years (0.7) Sex (male:female): 9:6 Characteristics of feet: 19 feet, 11 unilateral, 4 bilateral OS + DB group Age mean (SD): 4.9 years (1.1) Sex (male:female): 12:8 Characteristics of feet: 33 feet. 7 unilateral, 13 bilateral OS + FAS group Age mean (SD): 4.9 years (1.0) Sex (male:female): 8:10 Characteristics of feet: 31 feet. 5 unilateral, 13 bilateral |
|
| Interventions | DB versus OS + DB versus OS + FAS Participants were allocated to the groups when they began to walk, after completing a period of Ponseti treatment and wearing DB splints for initial correction. The data were collected when the children were between 4 and 5 years of age Follow‐up mean: 44 months |
|
| Outcomes | Diméglio scale 3‐dimensional foot scanning and pedobarography |
|
| Conflicts of interest | Quote: "The authors have no conflicts of interest to disclose". | |
| Funding | Supported by the National Natural Science Foundation of China | |
| Notes | Relapse reported. No information provided on management after relapse Location: China Dates conducted: The study began in 2010 and the mean follow‐up time was 44 months |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation was not stated |
| Allocation concealment (selection bias) | Unclear risk | Insufficient detail on baseline characteristics provided. No reference to allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants could not be blinded Unclear if personnel were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear whether assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts reported |
| Selective reporting (reporting bias) | Low risk | None identified |
| Other bias | Unclear risk | The trial report did not include sufficient detail to judge whether there could be other bias |
Chong 2014.
| Study characteristics | ||
| Methods | RCT. Prospective 2‐arm, parallel‐group design Randomisation of participants (not feet) |
|
| Participants | 30 participants with 45 CTEV feet who presented to a single centre Inclusion criteria: idiopathic CTEV Exclusion criteria: prior treatment (> 1 cast), prior surgical treatment, non‐idiopathic CTEV PARTICIPANT CHARACTERISTICS Age range (mean): 6 to 135 days (29.6 days) Sex (male:female): 24:6 Dynamic brace 15 participants Static brace 15 participants |
|
| Interventions | Dynamic brace versus static brace Follow‐up average (range): 18.7 months (3.0 months to 40.7 months) |
|
| Outcomes | Rate of recurrence | |
| Conflicts of interest | Quote: "There are no conflicts of interest" | |
| Funding | Kaul Pediatric Research Institute at Children's of Alabama and UAB Department of Surgery | |
| Notes | Recurrence defined as the need for revision surgery, repeat tenotomy, or repeat casting while in the bracing phase Location: USA Dates conducted: children treated between 2008 and 2012 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random allocation with block sizes of 4 to create treatment groups of similar size. Participants were randomised at the time of final Ponseti casting |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unknown if clinicians were blinded. Participants unable to be blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unknown if assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 infants were screened but dropped out prior to randomisation. One participant changed groups at 2 weeks after starting intervention. Intention‐to‐treat protocol used |
| Selective reporting (reporting bias) | High risk | Type of treatment to manage each recurrence not stated |
| Other bias | Low risk | None identified |
Cummings 2009.
| Study characteristics | ||
| Methods | RCT. 2‐arm, parallel‐group design Randomisation of participants (not feet) |
|
| Participants | 20 participants with 32 CTEV feet who presented to a single centre Inclusion criteria: full‐term infants aged 0 to 30 days, with CTEV Diméglio grade III Exclusion criteria: none stated PARTICIPANT CHARACTERISTICS Age: birth to 30 days Sex (male:female): 12:8 Botulinium toxin A group Characteristics of feet: 17 feet. 3 right, 2 left, 6 bilateral Placebo group Characteristics of feet: 15 feet. 2 right, 1 left, 6 bilateral |
|
| Interventions | Botulinum toxin A versus placebo Gastrocnemius and tibialis posterior muscles were injected under EMG (electromyography, a technique which records activity in muscles) guidance by a paediatric neurologist prior to initiation of serial casting using the Ponseti technique After the foot deformity was corrected (heel varus ≥ neutral; FFA ≥ neutral; dorsiflexion ≥ 15 °) feet were braced in reverse last shoes attached to an abduction orthosis set at 70 ° Feet that were not corrected with casting alone underwent a percutaneous Achilles tenotomy under local anaesthetic followed by further serial casting, until corrected Follow‐up average: 27 months (15 months to 4 years) |
|
| Outcomes | Time in cast for correction Need for Achilles tenotomy Relapse rate Treatment required for correction of relapse Diméglio Scale |
|
| Conflicts of interest | None declared | |
| Funding | None declared | |
| Notes | This study did not state whether children with syndromal CTEV were included or excluded Location: USA Dates conducted: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Placebo or botulinum toxin A was randomly selected by the pharmacist |
| Allocation concealment (selection bias) | Unclear risk | Both botulinum toxin A and placebo solutions manufactured and placed in identical vials by the manufacturer. Vials were coded by the manufacturer. A pharmacist at the centre randomly chose a vial and delivered it to the neurology clinic. Insufficient information about baseline characteristics provided |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Person administering treatment and participants were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessor blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | The definition of relapse was not provided |
| Other bias | Unclear risk | The trial report did not include sufficient detail to judge whether there could be other bias |
El‐Deeb 2007.
| Study characteristics | ||
| Methods | Quasi‐RCT. 2‐arm, parallel‐group design Randomisation of feet (not participants) |
|
| Participants | 46 participants with 66 feet with resistant idiopathic CTEV, referred to a single centre Inclusion criteria: idiopathic CTEV which failed conservative treatment (techniques unknown), requiring posteromedial soft tissue release Exclusion criteria: none stated PARTICIPANT CHARACTERISTICS Age mean (range) in months: 9 (3 to 24) Sex (male:female): 41:5 Characteristics of feet: 11 left, 15 right, 20 bilateral Baseline severity: 51 feet Diméglio grade IV (very severe), 15 feet Diméglio grade III (severe) Talocalcaneal interosseous ligament released Characteristics of feet: not stated Talocalcaneal interosseous ligament not released Characteristics of feet: not stated |
|
| Interventions | Talocalcaeal interosseous ligament release versus control in posteromedial soft tissue release for resistant CTEV Feet were allocated equally on an alternate basis Post‐operative care was the same in both groups. Long leg plaster in corrected position which was then changed every 3 weeks into an overcorrected position. Total time immobilised in cast was 12 weeks. Antivarus boots or splints then worn for 1 year Follow‐up average in months: 28 (24 to 36) |
|
| Outcomes | Radiological: x‐ray Radiological: MRI scans at 5 months post‐operatively in 40 participants (20 from each group) Scoring system based on combination of clinical and radiographic outcomes at an average of 28 months (range 24 to 36 months) |
|
| Conflicts of interest | None declared | |
| Funding | None declared | |
| Notes | Location: Egypt (assumed) Dates conducted: Not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Alternate sequence generation Unsure if groups were comparable at baseline |
| Allocation concealment (selection bias) | High risk | Alternate sequence generation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unable to blind intervention provider. Unclear if families were aware of which surgery was done |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Some participants had MRI scans. Unable to provide to all participants due to logistics and cost. Unsure how the limited numbers were selected |
| Selective reporting (reporting bias) | Unclear risk | The Simons system of reporting was modified. Mentioned cosmetic appearance, clinical range and strength, but did not report on these |
| Other bias | Unclear risk | The trial report did not include sufficient detail to judge whether there could be other bias |
Elgohary 2014.
| Study characteristics | ||
| Methods | RCT. Prospective 2‐arm, parallel‐group design Participants were randomised (not feet) |
|
| Participants | 46 children with 74 feet were managed by Ponseti technique. 5 participants (8 feet) were lost to follow‐up and were excluded; 41 participants with 66 feet with CTEV were included Inclusion criteria: idiopathic CTEV with Pirani score < 4 Exclusion criteria: idiopathic CTEV with previous surgical interference to the affected foot PARTICIPANT CHARACTERISTICS Traditional group Age mean (range) in weeks: 10.7 ± 6.28 (1 to 23) Sex (male:female): 14:6 Characteristics of feet: 14 bilateral, 6 unilateral (4 right and 2 left) Baseline severity: mean (SD) Pirani score 5.7 (0.62) Accelerated group Age mean (range) in weeks: 11.57 ± 6.9 (2 to 26) Sex (male:female): 12:9 Characteristics of feet: 11 bilateral, 10 unilateral (5 right and 5 left) Baseline severity: mean (SD) Pirani score 5.13 (0.61) |
|
| Interventions | Ponseti standard protocol versus Ponseti accelerated protocol for treatment of initial CTEV The Ponseti standard group underwent treatment with long leg plaster casts (toe to groin) which were changed weekly. An Achilles tenotomy was performed if dorsiflexion was < 10 °. They then wore abduction bracing for 23 hours a day for 3 months followed by night‐time wear only, until 3 years of age The Ponseti accelerated group underwent the same treatment with the exception that long leg plaster casts were changed twice a week Follow‐up: Traditional group (range): 12 ‐ 48 months (25.25 ± 8.67) Accelerated group (range): 12 ‐ 44 months (23.38 ± 9.21) |
|
| Outcomes | Pirani score Number of casts before tenotomy Timing of tenotomy Time from onset to complete correction |
|
| Conflicts of interest | Quote: "Conflict of Interest. None" | |
| Funding | Not reported | |
| Notes | Location: Mansoura University Hospital, Egypt Dates conducted: June 2010 to August 2013 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were assigned odd and even numbers in different groups. It was not stated if allocation of numbers was random |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants and personnel was not possible |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcome assessors were not blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 5 participants (8 feet) were lost to follow‐up and were excluded |
| Selective reporting (reporting bias) | Unclear risk | Unclear risk of minor adverse events |
| Other bias | Unclear risk | The trial report did not include sufficient detail to judge whether there could be other bias |
Gintautiene 2016.
| Study characteristics | ||
| Methods | RCT. Prospective 2‐arm, parallel‐group design Randomisation of participants (not feet) |
|
| Participants | 44 children with 63 feet were managed in a single centre from 2011 ‐ 2013. 5 participants (8 feet, 12.7%) dropped out and were excluded. Data from 39 children (55 feet) were collected Inclusion criteria: Idiopathic CTEV, up to 3 months of age, written consent to participate in the study, patients who underwent no other prior treatment Exclusion criteria: Patients who refused to participate in the study, severe concurrent genetic or neurological pathology that is likely to affect the child's physical development and/or the function of the foot PARTICIPANT CHARACTERISTICS Characteristics of feet: 17 right (43.59%), 6 left (15.38%), 16 bilateral (41.03%) Ponseti group Baseline severity (mean (SD)): Pirani score 5.05 (0.66), Diméglio score 11.93 (2.72) Characteristics of feet: 16 right, 12 left Age mean: 19.04 days Sex (male:female): 13:8 TATT group Baseline severity (mean (SD)): Pirani score 5.09 (0.75), Diméglio score 12.63 (2.34) Characteristics of feet: 17 right, 10 left foot Age mean: 15 days Sex: male:female: 14:4 |
|
| Interventions | Ponseti method versus early tibialis anterior tendon transfer for idiopathic CTEV Ponseti group underwent a traditional Ponseti casting. Percutaneous Achilles tenotomy was performed when equinus was persistent. Feet were immobilised for 3 weeks. Abduction brace was worn 23 hours a day up to 6 months of age, followed by 14 to 16 hours a day up to 2 years of age TATT group received the same intervention up to 6 months of age. At 6 months underwent TATT. Foot was immobilised for 5 weeks. No brace was worn after removal of plaster Follow‐up: 2 years and 5 to 12 years |
|
| Outcomes | Pirani scale, Diméglio scale Foot range of movement, e.g. dorsiflexion, plantar flexion, supination, pronation, radiological examination |
|
| Conflicts of interest | Quote: "The authors have no conflict of interest to declare" | |
| Funding | Not reported | |
| Notes | Relapse and long‐term (5‐ to 12‐year) follow‐up reported Location: Lithuania Dates conducted: 2011 to 2013 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not stated |
| Allocation concealment (selection bias) | Unclear risk | Quote: "At baseline patients were allocated randomly by the sealed envelope technique to one of two groups" Comment: Unclear how many bilateral and unilateral cases were allocated to each group |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants and personnel was not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of assessors was not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 8 feet dropped out, but it was unclear if an intention‐to‐treat analysis was used |
| Selective reporting (reporting bias) | Low risk | No selective reporting |
| Other bias | Low risk | |
Harnett 2011.
| Study characteristics | ||
| Methods | RCT. 2‐arm, parallel‐group design Participants were randomised (not feet) |
|
| Participants | 40 participants with 60 feet who presented to a single centre Inclusion criteria: idiopathic CTEV, < 90 days of age, local residency, informed consent Exclusion criteria: none stated PARTICIPANT CHARACTERISTICS Age mean (range): 31 days (7 to 55) Ponseti standard group Sex (male:female): 10:11 Characteristics of feet: 21 participants (32 feet). 11 bilateral cases Baseline severity: median Pirani score 5 (range 4 to 6) Ponseti accelerated group Sex male:female: 10:9 Characteristics of feet: 19 participants (29 feet), 9 bilateral cases Baseline severity: median Pirani score 5.5 (range 4.5 to 6) |
|
| Interventions | Ponseti standard protocol versus Ponseti accelerated protocol for treatment of initial CTEV The Ponseti standard group underwent treatment with long leg plaster casts (toe to groin) which were changed weekly. An Achilles tenotomy was performed if dorsiflexion was < 10 °. They then wore abduction bracing for 23 hours a day for 3 months followed by night‐time wear only, until 3 years of age The Ponseti accelerated group underwent the same treatment with the exception that long‐leg plaster casts were changed 3 times a week. If the deformity did not correct within 21 days the participant reverted to the standard protocol of weekly changes Follow‐up: minimum 6 months (average 251 days) |
|
| Outcomes | Pirani score Number of days to correction (prior to an Achilles tenotomy) |
|
| Conflicts of interest | Quote: "No benefits have been received or will be received from a commercial party related directly or indirectly to the subject of this article" | |
| Funding | Fellowship funding from Furlong Research Charitable Foundation. | |
| Notes | Information on relapses at follow‐up provided Location: Malawi Dates conducted: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer number generation |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unable to blind participants |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated if assessor was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 child died during treatment. Intention‐to‐treat analysis used |
| Selective reporting (reporting bias) | Unclear risk | It would have been useful to report pain as an outcome in this study |
| Other bias | Unclear risk | The trial report did not include sufficient detail to judge whether there could be other bias |
Hui 2014.
| Study characteristics | ||
| Methods | RCT. Prospective 2‐arm, parallel‐group design Participants were randomised (not feet) |
|
| Participants | 30 participants (44 feet) who presented to a regional tertiary‐level children's hospital Inclusion criteria: diagnosis of idiopathic CTEV Exclusion criteria: children with non‐idiopathic cause of CTEV ( e.g. arthrogryposis), children previously treated for CTEV, positional deformity PARTICIPANT CHARACTRISTICS Age mean (range): SRF (semi‐rigid fibreglass) group 2 weeks (1 to 11.7); plaster of Paris group 2.3 weeks (0.7 to 5.7) Plaster of Paris casting group Characteristics of feet: 12 participants (18 feet), 6 bilateral Baseline severity: mean Pirani score 4.9 (range 3 to 6) SRF casting group Characteristics of feet: 18 participants (26 feet), 8 bilateral Baseline severity: mean Pirani score 5.3 (range 2 to 6) |
|
| Interventions | Comparison of cast materials: plaster of Paris versus SRF using the Ponseti method Both plaster of Paris and SRF groups received weekly above‐knee plasters according to Ponseti method. Achilles tenotomy was performed if dorsiflexion was < 15 ° and after sufficient abduction of the foot, approximately 60 ° was achieved. Participants in both groups were fitted with FAO at the end of casting. Follow‐up: mean for SRF group, 35.8 ± 11.3 months, mean plaster of Paris group 23.7 ± 14.4 months |
|
| Outcomes | Pirani score Number of casts required for correction of clubfoot Need for percutaneous tendo‐achilles tenotomy Total time in casts (weeks) Ease of cast removal Duration of cast removal (minutes) Methods of cast removal Complications relating to the casting material Compliance with post‐correction FAO Deformity relapse Need for repeat Ponseti casting Need for ancillary surgical procedures. |
|
| Conflicts of interest | Quote: "Competing Interests: None declared" | |
| Funding | Not stated | |
| Notes | Location: Canada Dates conducted: July 2007 to December 2008 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Randomisation was performed using concealed number‐tracked envelopes according to a computer‐generated randomisation list |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unable to blind participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated if assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There was 1 dropout in each group |
| Selective reporting (reporting bias) | High risk | Insufficient reporting of relapse data |
| Other bias | Unclear risk | The trial report did not include sufficient detail to judge whether there could be other bias |
Kaewpornsawan 2007.
| Study characteristics | ||
| Methods | RCT. 2‐arm, parallel‐group design Randomisation of participants (not feet) |
|
| Participants | 86 participants with 128 CTEV feet Inclusion criteria: idiopathic CTEV which failed conservative treatment (treatment unknown), requiring surgery Exclusion criteria: children with arthrogryposis multiplex congenita, myelomeningocoele, cerebral palsy, syndromic clubfoot. Failed previous CTEV surgery PARTICIPANT CHARACTERISTICS Modified posteromedial release Age mean (range) in months: 5.8 (3 to 12) Sex (male:female): 26:21 Characteristics of feet: 25 unilateral, 22 bilateral Baseline severity: Diméglio grade 1, 1 foot; Diméglio grade 2, 26 feet; Diméglio grade 3, 35 feet; Diméglio grade 4, 7 feet Modified complete subtalar release Age mean (range) in months: 6 (3 to 12) Sex (male:female): 22:17 Characteristics of feet: 19 unilateral, 20 bilateral Baseline severity: Diméglio grade 1, 2 feet; Diméglio grade 2, 28 feet; Diméglio grade 3, 29 feet; Diméglio grade 4, 0 feet |
|
| Interventions | Modified posteromedial release versus modified complete subtalar release for clubfoot after failed conservative treatment Modified posteromedial release: standard posteromedial approach. Lengthening of tendo Achilles and tibialis posterior. Release of the origin of abductor hallucis, capsulotomy of the talonavicular, posterior tibiotalar, the talocalcaneal and medial calcaneocuboid joints. Division of plantar, calcaneofibular, superficial deltoid, spring ligament and master knot of Henry. In cases with residual toe flexion, FHL and FDL were lengthened. Kirschener wires were inserted through the talonavicular and talocalcaneal joint Modified subtalar release: a Cincinnati incision was used. The talocalcaneal and deep deltoid ligament were preserved. The talonavicular and calcaneocuboid joint were opened medially and laterally. Kirschener wires were inserted through the talonavicular and talocalcaneal joint Both groups had the same post‐operative care. Kirschner wires were removed at 6 weeks post‐operatively. Long leg casts remained in situ for 12 weeks post‐operatively After cast removal, orthopaedic shoes or Denis Browne boots were prescribed (length of time not stated) Follow‐up average: 19.4 months |
|
| Outcomes | Ponseti score Turco evaluation Diméglio scale |
|
| Conflicts of interest | None declared | |
| Funding | None declared | |
| Notes | Baseline assessment of groups P = 0.06 Location: Thailand Dates conducted: operations performed between 1996 and 2006 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Simple randomisation by envelope. Prior treatment was not outlined, so insufficient information on baseline characteristics |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The surgeon blindly opened the envelope that indicated the type of surgery." Comment: Unsure if sequentially‐numbered or opaque |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Intervention provider could not be blinded. Participant blinding unlikely to affect outcome |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Unclear risk | The trial report did not include sufficient detail to judge selective reporting |
| Other bias | Unclear risk | The trial report did not include sufficient detail to judge whether there could be other bias |
Lahoti 2008.
| Study characteristics | ||
| Methods | RCT Randomisation of feet (not participants), each participant had one foot randomised to each arm of the trial |
|
| Participants | 13 participants with bilateral CTEV requiring soft tissue release Inclusion criteria: bilateral resistant CTEV undergoing soft tissue release Exclusion criteria: none stated PARTICIPANT CHARACTERISTICS Age mean (range) in months: 10 (9 to 12) Baseline severity: 11 idiopathic cases, 2 syndromal cases. Pirani score 5.5 in 6 children and 6 in 7 children FHL and FDL lengthening Age mean: 10 months Sex (male:female): 10:3 Characteristics of feet: 13 feet. 5 right, 8 left Baseline severity: 5.5 average Pirani score FHL and FDL decompression Age mean: 10 months Sex (male:female): 10:3 Characteristics of feet: 13 feet. 8 left, 5 right Baseline severity: 5.5 average Pirani score |
|
| Interventions | Lengthening of FHL and FDL versus simple decompression of the same muscles during soft tissue release for resistant CTEV All participants had bilateral CTEV requiring surgery All feet underwent a complete soft tissue release through the Cinicinnati incision. 1 side was randomly selected to undergo FHL and FDL lengthening, the other side simple decompression Post‐operative management the same in all feet Follow‐up average: 48 months |
|
| Outcomes | Harrold and Walker scale (Harrold 1983) Pirani score |
|
| Conflicts of interest | Quote: "No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article" | |
| Funding | None declared | |
| Notes | Location: UK (assumed) Dates conducted: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not stated |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information on baseline characteristics. 2 syndromic feet were included. No reference to allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Surgeon not blinded to intervention. Blinding of participants unlikely to affect outcome |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information on outcome measures |
| Other bias | Low risk | |
Manzone 1999.
| Study characteristics | ||
| Methods | RCT. 2‐arm, parallel‐group design Randomisation of feet |
|
| Participants | 20 participants with 30 CTEV feet Inclusion criteria: resistant CTEV which had not undergone prior conservative treatment Exclusion criteria: none stated PARTICIPANT CHARACTERISTICS Age mean (range) in months: 7.7 (3.5 to 19) Sex (male:female): 15:5 Characteristics of feet: 13 right, 17 left; 20 of these were bilateral, 10 were unilateral Basline severity: unknown. Groups were matched for birthweight, age of treatment (between 3 months and walking age), weight at surgery, neurologic skills development at surgery, geographic origin, socioeconomic status and associated pathologies PMR Characteristics of feet: 15 feet in total (12 participants), 6 bilateral, 5 unilateral. The remaining 4 feet (2 participants) were bilateral cases where 1 foot was randomised to each group Baseline severity: not stated CCSR Characteristics of feet: 15 feet in total (12 participants), 6 bilateral, 5 unilateral. The remaining 4 feet (2 participants) were bilateral cases where 1 foot was randomised to each group Baseline severity: not stated |
|
| Interventions | PMR versus CCSR The PMR was completed as described by Turco with some modifications. A long‐leg cast is worn for 6 weeks post‐operatively. Kirschner wires are then removed and a short leg plaster is worn for a further 4 weeks. Following this an AFO is worn at night The CCSR was completed according to McKay and Simmons (McKay 1983), using a Cincinnati approach with modifications. The Achilles tendon underwent Z‐lengthening, all posteromedial tendons underwent z‐plasty. The posterior tibiofibular ligament and plantar fascia were only occasionally cut. The interosseous talocalcaneal ligament was never incised. A long‐leg plaster with the foot in equinus was in situ for 7 to 10 days post‐operatively. This was then changed to a long‐leg cast with the foot in dorsiflexion for a further 5 weeks. The Kirschner wires were then withdrawn and a short leg cast was worn for a further 4 weeks All participants underwent the same long‐term post‐operative care, but they did not state if AFO worn in CCSR group Follow‐up in months (range): 27 (18 to 40) |
|
| Outcomes | Radiographic Magone's score |
|
| Conflicts of interest | None declared | |
| Funding | None declared | |
| Notes | Location: Argentina (assumed) Dates conducted: 1 January 1993 to 31 December 1994 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not described |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Intervention provider could not be blinded. Participant blinding unlikely to affect outcome |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information regarding assessor blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Unclear risk | Unsure of time of post‐operative x‐ray; unsure which groups had adverse outcomes |
| Other bias | Unclear risk | The trial report did not include sufficient detail to judge whether there could be other bias |
Maripuri 2013.
| Study characteristics | ||
| Methods | RCT. Prospective 2‐arm, parallel‐group design Randomisation of participants (not feet) |
|
| Participants | 26 children with 33 CTEV feet Inclusion criteria: idiopathic clubfeet, no previous treatment, no contraindications to Ponseti treatment Exclusion criteria: clubfoot associated with an overall genetic syndrome (syndromic club foot); teratologic clubfoot associated with a neurological disorder such as meningomyelocoele; parents or guardians declined to participate; no valid consent could be obtained or an eligibility assessment was not performed by the lead clinician PARTICIPANT CHARACTERISTICS Below‐knee casting group Median age (range): 13 (1 to 40) days Sex (male:female): 10:3 Characteristics of feet: 16 feet in total (13 participants), 3 bilateral, 10 unilateral (7 right, 3 left) Baseline severity: mean Pirani score at presentation (range): 4.3 (2.5 to 5.5) Above‐knee casting group Median age (range): 10 (5 to 20) days Sex (male:female): 10:3 Characteristics of feet: 17 feet in total (13 participants), 4 bilateral, 9 unilateral (4 right, 5 left) Baseline severity: mean Pirani score at presentation (range): 4.05 (2.5 to 6) |
|
| Interventions | Above‐knee casting versus below‐knee casting All feet were evaluated to decide how many casts were required to correct the deformity and need for tendo Achilles tenotomy. Criteria for tenotomy was minimum abduction of 40 °, the heel in valgus and the anterior process of the calcaneum lateral to the tala head. 2 slips (plaster displacement far enough to retract the toes or the plaster falling off) or a plaster treatment over 8 weeks without achieving correction were considered as a treatment failure. If failure occurred in a foot treated by a below‐knee plaster, that foot was reverted to above‐knee plaster Follow‐up: Trial stopped at the point half the planned sample size had been recruited due to high failure rate in the below‐knee group |
|
| Outcomes | Pirani scale Time to readiness for tenotomy Time to full correction Risk of failure due to displacement of the cast, over‐long treatment |
|
| Conflicts of interest | Quote: "No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of the article" | |
| Funding | Financial support provided by Orthopaedic Institute at the RJAH Orthopaedic Hospital in Oswestry, UK | |
| Notes | 6 failures in the below‐knee group (4 plaster slippages within 8 weeks, 2 casting more than 8 weeks) 1 failure in the above‐knee group (casting more than 8 weeks) Location: UK Dates conducted: between 2010 and 2012 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The details of how the randomisation was undertaken was not stated |
| Allocation concealment (selection bias) | Low risk | Opaque sealed envelope was drawn by research nurse in front of parents, number given and participants allocated to a group |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unable to blind participants or personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Low risk | No selective reporting |
| Other bias | High risk | Following an interim analysis the trial was stopped due to a high failure rate in above‐knee group |
Pittner 2008.
| Study characteristics | ||
| Methods | RCT. 2‐arm, parallel‐group design Randomisation was done according to medical record number. Participants were randomised (not feet) |
|
| Participants | 34 participants with 42 CTEV feet who attended author's outpatient clinics Inclusion criteria: initial presentation of CTEV Exclusion criteria: none stated PARTICIPANT CHARACTERISTICS 3 participants excluded prior to data analysis. 1 lost to follow‐up, 1 had medical complications, and 1 switched groups during treatment Fibreglass Age mean (SD) weeks: 1.07 (0.57) Sex male:female: 9:4 Characteristics of feet: 13 participants. 16 feet (8 left, 8 right) Baseline severity: Diméglio scale score average: 13.1 Plaster Age mean (SD) weeks: 1.89 (1.88) Sex male:female: 18:0 Characteristics of feet: 18 participants. 23 feet: 9 left, 14 right Baseline severity: Diméglio scale average: 12.3 |
|
| Interventions | Semi‐rigid (fibreglass) casts versus plaster of Paris casts for Ponseti treatment of initial presentation of CTEV In the semi‐rigid (fibreglass) group, casting was done using Scotchcast Softcast (3M). In the control group, plaster of Paris was used Follow‐up: end of treatment |
|
| Outcomes | Diméglio scale Parent satisfaction questionnaire |
|
| Conflicts of interest | None declared | |
| Funding | None declared | |
| Notes | Location: USA Dates conducted: 15 month period (dates not stated) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Sequence generated by medical record number. Not stated how this was done |
| Allocation concealment (selection bias) | High risk | Sequence generated by medical record number |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unable to blind intervention providers. Participant blinding unlikely to affect outcome |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Assessors not blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Several participants were excluded after randomisation or lost to follow‐up. Their data were removed from the study |
| Selective reporting (reporting bias) | Unclear risk | Objective was to compare materials ‐ primary/secondary outcomes were not stated in Methods section. |
| Other bias | Unclear risk | The trial report did not include sufficient detail to judge whether there could be other bias |
Rijal 2010.
| Study characteristics | ||
| Methods | RCT. 2‐arm, parallel‐group design Randomisation of feet (not participants) |
|
| Participants | 38 participants with 60 CTEV feet who presented to 1 outpatient clinic Inclusion criteria: CTEV Exclusion criteria: prior intervention for CTEV, over 2 years old PARTICIPANT CHARACTERISTICS Age mean (SD, range) days: 195.7 (202.81 3 to 720 days) Sex male:female: 29:9 Basline severity: unclear (report states the groups were equal at baseline for age, sex and Pirani scores) Ponseti Characteristics of feet: 30 feet. 10 unilateral, 8 (4 participants) bilateral. The remaining 12 feet (12 participants) were bilateral cases where 1 foot was randomised to each group Kite Characteristics of feet: 30 feet. 6 feet unilateral, 12 feet (6 participants) bilateral. The remaining 12 feet (12 participants) were bilateral cases where 1 foot was randomised to each group |
|
| Interventions | Ponseti versus Kite technique in initial treatment of CTEV Casts were changed in both groups at weekly intervals for 10 weeks. Tendo Achilles tenotomy was undertaken in both groups for those with residual equinus deformity. Feet which were not corrected at the end of 10 weeks were subject to surgical correction Follow‐up: end of treatment |
|
| Outcomes | Pirani score | |
| Conflicts of interest | Quote: "Conflict of Interest: None" | |
| Funding | Quote: "Source of Support: No" | |
| Notes | Location: Nepal Dates conducted: July 2005 to May 2006 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomized....using computerized random number generation technique on Microsoft Office Excel 2007" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unable to blind intervention providers. Participant blinding unlikely to affect outcome |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Observers blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | High risk | Insufficient information on adverse events. Unsure of operative intervention required after each intervention |
| Other bias | Low risk | |
Sanghvi 2009.
| Study characteristics | ||
| Methods | RCT. 2‐arm, parallel‐group design Randomisation of participants to each group (not feet) |
|
| Participants | 42 participants with 64 CTEV feet Inclusion criteria: idiopathic CTEV, initial presentation Exclusion criteria: myelocele, meningomyelocoele, arthrogryposis multiplex congenital, other neuromuscular disorders PARTICIPANT CHARACTERISTICS Basline severity: not stated Ponseti 21 participants Age mean (SD): 13.2 (11.9) weeks Sex male:female: 13:8 Characteristics of feet: 30 feet. 18 bilateral (9 participants): 6 right, 6 left Baseline severity: not stated Kite 21 participants Age mean (SD): 12.2 (10) weeks Sex male:female: 14:7 Characteristics of feet: 34 feet. 26 bilateral (13 participants): 5 right, 3 left Baseline severity: not stated |
|
| Interventions | Ponseti versus Kite technique for treatment of initial CTEV In the Ponseti group, casts were changed every 7 to 10 days. Achilles tenotomy was performed in those with residual equinus. Bracing in abduction orthosis using Denis Browne splints was done with the affected foot at 70 ° of external rotation and the unaffected foot at 40 ° to 45 ° of external rotation. Splints were worn full‐time until walking age, and then at night only. During the day, shoes with an open toe box, straight medial border Lateral flaring of the sole and reverse Thomas heels were used until the age of 4 to 5 years In the Kite group, toe‐to‐groin casts were changed every 7 to 10 days until full correction. The final position was maintained in full‐time bracing in a neutral position with a heel lock and straight medial bar. Once the participant began walking, the brace was used at night only. During the day, shoes with an open toe box, straight medial border, lateral flaring of the sole and reverse Thomas heels were used until the age of 4 to 5 years Follow‐up average: 36 months |
|
| Outcomes | Radiographic Range of movement Scoring system according to Atar 1992 |
|
| Conflicts of interest | None stated | |
| Funding | None stated | |
| Notes | Location: India (assumed) Dates conducted: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information on baseline assessment for both groups The details of randomisation were not stated |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to determine whether allocation concealment was undertaken |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unable to blind intervention provider. Blinding of participant unlikely to affect outcome |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Assessor not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | High risk | Incomplete outcome reporting, e.g. radiographic. Cannot be entered into the meta‐analysis |
| Other bias | Unclear risk | The trial report did not include sufficient detail to judge whether there could be other bias |
Selmani 2012.
| Study characteristics | ||
| Methods | RCT. 2‐arm, parallel‐group design Randomisation of participants to each group (not feet) |
|
| Participants | 100 participants with 150 CTEV feet Inclusion criteria: idiopathic CTEV,< 3 months of age, initial presentation (no prior treatment) Exclusion criteria: myelocele, meningomyeloceles, arthrogryposis multiplex congenital, other neuromuscular causes PARTICIPANT CHARACTERISTICS Ponseti 50 participants Age mean (SD): 35.3 (25.4) days Sex male:female: 30:20 Characteristics of feet: 76 feet Baseline severity: Pirani Score mean (SD): 5.2 (0.8) Kite 50 participants Age mean (SD): 32.45 (26.3) days Sex male:female: 28:22 Characteristics of feet: 74 feet Baseline severity: Pirani Score mean (SD): 5.1 (0.7) |
|
| Interventions | Ponseti versus Kite technique for treatment of initial CTEV In the Ponseti group, casts were changed every 7 to 10 days until the foot was corrected or the participant was 1 year old. Achilles tenotomy was performed in those with residual equinus. Bracing in abduction orthosis using Denis Browne splints was done with the affected foot at 70 ° of external rotation and the unaffected foot at 40 ° to 45 ° of external rotation. Splints were worn full‐time until walking age, and then at night only. During the day, shoes with an open toe box, straight medial border. Lateral flaring of the sole and reverse Thomas heels were used until the age of 4 years Follow‐up average (SD): 36.2 (3.2) months In the Kite group, toe‐to‐groin casts were changed every 7 to 10 days until full correction or the participant was 1 year old. The final position was maintained in full‐time bracing in a neutral position with a heel lock and straight medial bar. Once the participant began walking, the brace was used at night only. During the day, shoes with an open‐toe box, straight medial border, lateral flaring of the sole and reverse Thomas heels were used until the age of 4 years |
|
| Outcomes | Pirani score, range of movement Follow‐up average (SD): 35.1 (2.5) months |
|
| Conflicts of interest | Quote: "Conflict of Interest: None" | |
| Funding | Not stated | |
| Notes | Location: University Hospital Centre, Tirana, Albania Dates conducted: January 2006 through February 2009 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised number generation |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Intervention provider could not be blinded. Participant and carer blinding not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Several participants were excluded after randomisation, or lost to follow‐up. Their data were excluded from final analysis Described functional outcome of corrected feet only |
| Selective reporting (reporting bias) | Unclear risk | Adverse events not documented. Treatment for relapsed cases in the Kite group not stated, so unknown if mild or severe relapse |
| Other bias | Low risk | |
Siddiqui 2007.
| Study characteristics | ||
| Methods | RCT. 2‐arm, parallel‐group design Participants were randomised equally into both groups |
|
| Participants | 60 infants who presented to a single centre (number of CTEV feet unknown) Inclusion criteria: CTEV with Diméglio grade I and II undergoing surgical correction Exclusion criteria: CTEV with Diméglio grade III and IV PARTICIPANT CHARACTERISTICS Age range in months: 6 to 18 Sex male:female: 37:23 Basline severity: Diméglio grade I, 20 participants; Diméglio grade II, 40 participants Window procedure Age mean: 9.5 months Characteristics of feet: 30 participants (bilateral and unilateral numbers not reported) Baseline severity: not stated Turco procedure Age mean: 9.6 months Characteristics of feet: 30 participants (bilateral and unilateral numbers not reported) Baseline severity: not stated |
|
| Interventions | Window procedure versus Turco procedure for treatment of CTEV The Window procedure was not described in detail in the article. It uses 5 or 6 small incisions rather than a long posteromedial incision Post‐operatively the foot is placed in a plaster in the corrected position. Details of the plaster were not provided In the Turco group, post‐operatively a cast was not applied for 15 days because of oedema Follow‐up: not stated |
|
| Outcomes | Diméglio scale Post‐operative assessment criteria according to Beatson (Beatson 1966) 6 months after surgery Time in theatre |
|
| Conflicts of interest | None stated | |
| Funding | None stated | |
| Notes | Location: Civil Hospital, Karachi Dates conducted: 1 June 2002 to 30 May 2005 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unable to blind intervention provider. Participant blinding unlikely to affect outcome |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Unclear risk | Follow‐up time not reported |
| Other bias | High risk | Procedure was developed, used and assessed by the same team within the same population. Bilateral and unilateral numbers not reported |
Sud 2008.
| Study characteristics | ||
| Methods | Quasi‐RCT. 2‐arm, parallel‐group design Randomisation of participants (not feet) |
|
| Participants | 53 participants with 81 CTEV feet who presented to a single centre Inclusion criteria: < 3 months of age, idiopathic CTEV Exclusion criteria: non‐idiopathic CTEV, > 3 months of age PARTICIPANT CHARACTERISTICS 8 participants lost to follow‐up and excluded prior to data analysis Ponseti Age mean (SD) days: 31.75 (27.4) Sex male:female: 14:9 Characteristics of feet: 23 participants, 36 feet. 26 bilateral (13 participants), 4 right, 6 left Baseline severity (Diméglio scale score mean (SD)): 14.39 (3.2) Kite Age mean (SD) days: 26.06 (21.4) Sex male:female: 17:5 Characteristics of feet: 22 participants, 31 feet. 18 bilateral (9 participants), 5 right, 8 left Baseline severity (Diméglio scale score mean (SD)): 16.19 (2.8) |
|
| Interventions | Ponseti versus Kite In the Ponseti group, weekly manipulation and casting was done until correction or 1 year (whichever came first). Correction was defined as 50 ° to 60 ° external rotation and 15 ° dorsiflexion with or without an Achilles tenotomy. Following correction, feet were placed in abduction bracing at 50 ° to 60 ° of external rotation, worn full time for 2 to 3 months then at night until 2 to 4 years of age In the Kite group, manipulation and casting was done until the foot was corrected. Correction was maintained in a night brace in dorsiflexion and slight valgus Follow‐up average: 26 months |
|
| Outcomes | Diméglio scale Range of movement Function ‐ squat, independent walking, pain, participation in games |
|
| Conflicts of interest | None stated | |
| Funding | None stated | |
| Notes | Location: India Dates conducted: March 2003 through February 2004 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Alternate allocation |
| Allocation concealment (selection bias) | High risk | Alternate allocation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Intervention provider unable to be blinded. Participant blinding unlikely to affect outcome |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessor blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 8 participants were excluded or lost to follow‐up and excluded from analysis |
| Selective reporting (reporting bias) | High risk | Prespecified outcomes were unclear in Methods |
| Other bias | Unclear risk | Insufficient information to permit judgment |
Svehlik 2017.
| Study characteristics | ||
| Methods | RCT. Prospective 2‐arm, parallel‐group design Randomisation of participants (not feet) |
|
| Participants | 19 participants with 28 CTEV feet Inclusion criteria: idiopathic CTEV, infants < 2 weeks of age with no other congenital deformities Exclusion criteria: perinatal problems, suspicion of neurologic or metabolic disorders PARTICIPANT CHARACTERISTICS 2 infants (4 clubfeet) in Ponseti group opted out of allocated treatment but were included in analysis 3 infants lost to follow‐up in surgical group (moved out of area) Ponseti 10 participants, 16 clubfeet Age at follow‐up, mean (SD): 9.81 (0.78) years Characteristics of feet at follow‐up: 12 feet. 3 bilateral: 6 unilateral Sex: male:female 7:5 Baseline severity: median Pirani score at birth: 3.25 Surgical 9 participants,12 clubfeet Age at follow‐ up, mean (SD): 9.85 (0.39) years Sex: male:female: 11:1 Characteristics of feet: 12 feet. 5 bilateral: 2 unilateral Baseline severity: median Pirani score at birth: 3.75 |
|
| Interventions | Ponseti versus surgical intervention In the Ponseti group, weekly manipulation and above‐knee casting as in Ponseti method followed by percutaneous Achilles tenotomy and a final cast for 3 weeks. Orthotic management once correction achieved until 2 years of age. Feet were placed in abduction bracing at 70 ° and 45 ° of external rotation for the club foot and the healthy foot in unilateral cases, respectively. Custom‐moulded shoes were provided after 2 years for daily use In the surgical group, casting according to the technique of Johann Bosch until 6 to 8 months with residual foot deformity corrected by posteromedial release (Cincinnati approach) followed by 6 weeks in a plaster cast. Night‐time rigid AFOs were provided after removal of plaster up to 36 months Follow‐up average (SD): 9.8 years (0.6) |
|
| Outcomes | Pirani scale FRS Ankle range of motion Oxford Food Model (OFM) PODCI |
|
| Conflicts of interest | Quote: "Conflict of Interest: None" | |
| Funding | One or more of the authors has received funding from Land Steiermark, Graz, Austria | |
| Notes | Recruitment was stopped after an interim report indicated a higher number of surgical procedures were required to achieve correction of the clubfoot deformity in the surgical group We could not use functional outcome data (PODCI) in a meta‐analysis as data were presented by foot, and bilateral and unilateral cases were combined. IPD were not available for re‐analysis Location: Austria Dates conducted: started 2001, completion date not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation table |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unable to blind participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear whether assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4 feet were lost to follow‐up. Intention‐to‐treat protocol was used |
| Selective reporting (reporting bias) | Low risk | No selective reporting |
| Other bias | Unclear risk | The trial report did not include sufficient detail to judge whether there could be other bias |
Zeifang 2005.
| Study characteristics | ||
| Methods | RCT. 2‐arm, parallel‐group design Randomisation of participants. However, bilateral feet received the same post‐operative management. |
|
| Participants | 36 participants with 36 CTEV feet who presented to a single centre Inclusion criteria: idiopathic CTEV. Failed conservative treatment for 6 months. Diméglio grade III. Underwent posteromedial lateral release Exclusion criteria: none stated PARTICIPANT CHARACTERISTICS In bilateral cases, 1 foot was randomly selected by tossing a coin 1 bilateral case with early relapse was excluded prior to data analysis (leaving 37 participants with 37 feet for analysis) Age mean (range) in months: 8.2 (5 to 12) Sex male:female: 27:11 CPM Characteristics of feet: 18 feet Immobilisation in a cast Characteristics of feet: 19 feet |
|
| Interventions | CPM versus immobilisation in a cast, after surgery for resistant CTEV In both groups a cast was applied post‐operatively for the first 10 days. Kirschner wires were removed from all feet 2 weeks post‐operatively In the casting group, casting for another 4½ weeks was undertaken In the CPM group, computer‐assisted 3‐dimensional therapy using a Kinetic 5090 Ankle CPM machine was used with a standardised protocol. CPM was applied for 4 hours a day. During rest periods, removable splints were applied 6 weeks after surgery, all feet were treated with a brace at night. Physiotherapy was provided to both groups for a further 6 months. When the participants began to walk, they were provided with heel cups to place in conventional shoes Follow‐up: 48 months |
|
| Outcomes | Diméglio scale | |
| Conflicts of interest | Quote: "No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article" | |
| Funding | None described | |
| Notes | Location: Germany Dates conducted: interventions subsequent to surgery between 1998 to 2001 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised number generation |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Intervention provider could not be blinded. Participant and carer blinding not possible, which could affect outcome |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 2 feet lost to follow‐up. 1 participant was excluded after randomisation and removed from data analysis |
| Selective reporting (reporting bias) | High risk | No measurement of pain post‐operatively |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Zwick 2009.
| Study characteristics | ||
| Methods | RCT. 2‐arm, parallel‐group design Randomisation of participants |
|
| Participants | 19 participants with 28 CTEV feet who presented to a single centre Inclusion criteria: idiopathic CTEV, < 2 weeks of age Exclusion criteria: none stated PARTICIPANT CHARACTERISTICS Ponseti Age mean (SD) weeks: 0.7 (0.8) Sex male:female: 3:6 Characteristics of feet: 9 participants (12 feet) 2 participants (2 feet) opted out Baseline severity: Pirani score 4.6 (1.5) Posteromedial soft tissue release Age mean (SD) weeks: 0.4 (0.4) Sex male:female: 7:3 Characteristics of feet: 10 participants (16 feet) Baseline severity: Pirani score 4.5 (1.1) |
|
| Interventions | Ponseti versus surgical intervention Treatment using the Ponseti technique involved long leg casts changed weekly. All participants required an Achilles tenotomy, done under general anaesthesia and then they were placed back into a long leg cast for a further 3 weeks. Correction was maintained in a brace with external rotation of 70 ° for affected feet and 45 ° for unaffected feet. The brace was worn full‐time until 6 months of age, then for 18 hours a day until the child started standing. Once standing, the brace was worn at night until 2 years of age. Following this, participants were placed into custom‐moulded shoes with an insole with a heel counter, and moderate flange at the medial aspect of the cuboid and medial aspect of the first metatarsal head Participants in the surgical group underwent similar weekly manipulative casting as those in the Ponseti group until 6 to 8 months of age. All residual deformities were then treated with a posteromedial release by a Cincinnati incision and fixated with Kirschner wires and long leg casts. Kirschner wires were removed at 4 weeks and casts removed at 6 weeks post‐operatively. Correction was maintained with rigid knee AFOs worn at night until 3 years of age. Moulded orthoses were prescribed once the participant was able to stand and walk Follow‐up average: 42 months |
|
| Outcomes | Pirani score PODCI FRS; Laaveg and Ponseti |
|
| Conflicts of interest | Quote: "Each author certifies that he or she has no commercial associations that might pose a conflict of interest in connected with the submitted article" | |
| Funding | None reported | |
| Notes | Stopped early secondary to ethical implications. The traditional technique was leading to greater rates of major surgical intervention compared to the Ponseti technique We could not use functional outcome data (PODCI) in a meta‐analysis as data were presented by foot, and bilateral and unilateral cases were combined. IPD were not available for re‐analysis Location: Austria Dates conducted: 2001 to 2003 (end of recruitment) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random‐number table |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of intervention provider not possible. Unable to blind participants or families |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Assessor not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "2 patients were not treated per protocol and underwent posteromedial release." Quote: "According to the intention‐to‐treat protocol, these two patients (two feet) remained assigned to the Ponseti group for further assessments and analysis." |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | High risk | Quote: "Because the rate of surgery was higher for the traditional therapy group, patient acquisition was terminated after the preliminary evaluation." |
AFO: ankle foot orthosis CCSR: complete circumferential subtalar release CPM: continuous passive motion CTEV: congenital talipes equinovarus DB: Denis Browne FAS: forefoot abduction shoe FDL: flexor digitorum longus FHL: flexor hallucis longus FRS: Functional Rating System MRI: magnetic resonance imaging OS: orthopaedic shoe OFM: Oxford Foot Model PMR: posteromedial release PODCI: Pediatric Outcomes Data Collection Instrument RCT: randomised controlled trial SD: standard deviation SRF: semi‐rigid fibreglass TATT: tibialis anterior tendon transfer
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Andriesse 2008 | Not an RCT. Allocation was consecutive |
| Atar 1993 | Not an RCT |
| Aurell 2005 | Not an RCT. Treatment was allocated according to which hospital the participant attended |
| Chang 1991 | Not an RCT |
| Chhina 2013 | Not an RCT |
| DePuy 1989 | Not an RCT. |
| DeRosa 1986 | Not an RCT |
| Derzsi 2015 | Not an RCT |
| Diméglio 1996 | Not an RCT |
| Dobbs 2017 | Not an RCT |
| Doğan 2002 | Not an RCT |
| Farsetti 2009 | Not an RCT |
| Faulks 2009 | Not an RCT. A controlled clinical trial |
| Gupta 2014 | Not an RCT |
| Halanski 2010 | Not an RCT. A controlled clinical trial |
| Hallaj‐Moghadam M 2015 | Not an RCT |
| Howren 2015 | Not an RCT |
| Ippolito 2003 | Not an RCT |
| Janicki 2011 | Not an RCT |
| Kesemenli 2003 | Not an RCT |
| Kuo 2001 | Not an RCT |
| Li 2007 | Not an RCT |
| Lohia 2014 | Not an RCT |
| Matuszewski 2012 | Not an RCT |
| Miura 2005 | No mention of randomisation |
| Napiontek 2000 | Not an RCT |
| Narang 2011 | Not an RCT |
| Nilgün 2011 | Not an RCT |
| O'Brien 2004 | Not an RCT. |
| Ponseti 2006 | Not an RCT. A case series |
| Richards 2008 | Not an RCT. A controlled clinical trial |
| Shingade 2014 | Not an RCT |
| Simons 1985 | Not an RCT |
| Steinman 2009 | Not an RCT. A controlled clinical trial |
| Thompson 1982 | No mention of randomisation |
| Tschopp 2002 | Not an RCT |
| Uglow 2000 | Not an RCT |
| Xu 2011 | Not an RCT |
RCT: randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
Madhuri 2018.
| Study name | Comparison of 2 different types of splints in children with corrected CTEV, Dobbs versus Denis Browne splint |
| Methods | RCT. Parallel‐group design |
| Participants | Target sample size: 48 Children will be entering the protocol at < 1 year of age Inclusion criteria: idiopathic unilateral or bilateral CTEV, after achieving the full correction of the deformity using Ponseti method i.e. a Diméglio score of < 5, ability and willingness to be followed up as per the protocol Exclusion criteria: neuromuscular condition, arthrogryposis and hyperlaxity, hip or knee dislocation, any contraindication to splinting, children from remote areas and outside India who may not be able to come for follow‐up, surgery other than open tenotomy |
| Interventions | Group 1 (intervention group): use of the Dobbs splint. Splint to be used for 23 hours a day for 3 months followed by 12 hours for 4 years Group 2 (control group): use of the Denis Browne splint. Splint to be used for 23 hours a day for 3 months followed by 12 hours for 4 years |
| Outcomes | Diméglio score Compliance questionnaire |
| Starting date | 1 November 2013 |
| Contact information | Dr. Vrisha Madhuri Paediatric Orthopaedics Unit, Department of Orthopaedics, Christian Medical College, Vellore Christian Medical College, Vellore, Tamilnadu, India Email: madhuriwalter@cmcvellore.ac.in Affiliation: Christian Medical College Vellore |
| Notes |
NCT03249805.
| Study name | Efficacy of a new design of foot abduction brace (FAB) compared to standard FAB during Ponseti treatment of idiopathic CTEV by measuring rate of recurrence and compliance using novel touch sensors |
| Methods | RCT. Parallel‐group design |
| Participants | 80 participants Inclusion criteria: unilateral or bilateral cases of idiopathic CTEV in children who have not yet started walking at first presentation (< 1 year of age), receiving a brace for the first time after successful correction with the Ponseti method of treatment Exclusion criteria: children who are already walking at presentation ( > 1 year of age); children with previous treatment; children who have used FAB previously; children treated with surgery other than tenotomy; children with syndromic or neuropathic cases of CTEV; children with atypical CTEV |
| Interventions | MiracleFeet Foot Abduction Brace (mFAB) group: The MiracleFeet foot abduction brace is an injected plastic moulded bar with fabric shoes that clip on and off. The shoes have laces and a strap. The brace provides 10 ° of dorsiflexion and 45 ° to 65 ° of abduction and will be equipped with novel touch sensors to measure brace compliance
Steenbeek Foot Abduction Brace (sFAB) group: The Steenbeek foot abduction brace is a fixed metal bar attached to 2 leather shoes with laces. The shoes have laces and a strap. The brace provides 10 ° of dorsiflexion and 45 ° to 65 ° of abduction, and will be equipped with novel touch sensors to measure brace compliance Minutes of brace usage and Orthotics Prosthetics User Survery (OPUS) measured every month for 6 months |
| Outcomes | Pirani score Minutes of brace usage Orthotics Prosthetics User Survey (OPUS) |
| Starting date | 7 June 2017 |
| Contact information | Dr Alaric Aroojis Department of Paediatric Orthopaedics, Acharya Dhonde Marg, Parel, Mumbai 400012 Mumbai, MAHARASHTRA India Email:aaroojis@gmail.com Affiliation: Bai Jerbai Wadia Hospital for Children |
| Notes |
CTEV: congenital talipes equinovarus FAB: foot abduction brace RCT: randomised controlled trial
Differences between protocol and review
The protocol discussed treatments for initial and relapsed CTEV; however, we identified a cohort of resistant cases (initial treatment which did not successfully correct the deformity) during this review. We therefore had to define this additional group in order to differentiate these participants from relapsed cases (i.e. those which had undergone prior treatment with successful outcomes and present with recurrence of deformity).
The protocol defined outcome measures at a minimum of one year. This was essential, as relapse is considered a common occurrence in the treatment of CTEV and defining end of treatment would not have encompassed this outcome. However, once the literature was identified it became apparent that end‐of‐treatment foot alignment often significantly affected further treatment. This is highlighted in particular with the identification of resistant cases, which often required significant surgical intervention. Where relevant (for example, at the end of initial treatment), we therefore included results prior to one year.
Treatment for relapse was not a prespecified outcome; however, we noted that it significantly affected further treatment in certain cohorts. Where data were available, we therefore described the treatment to correct relapse.
A number of trials included data from bilateral (including both right and left feet) and unilateral cases. In bilateral cases, right and left feet from the same person are unlikely to respond independently. When these are pooled with unilateral cases, results should be viewed with caution. As this problem became apparent only when we analysed the data, we used statistical methods not defined in the protocol to overcome this bias.
We added text on methods to be used if multiple comparisons within multi‐arm studies are suitable for inclusion.
We assessed performance bias and detection bias separately in this update, as is now recommended.
Dr Chris Frost, Dr Paul Gibbons and Dr David Little were additional authors in earlier published versions of the review.
We searched NHSEED, DARE and HTA databases for additional information to use in the Discussion and clinical trials registries for ongoing trials in a previous update. These databases are no longer being updated.
We included additional detail on GRADE assessment.
Contributions of authors
SB and KG wrote the draft of the update with the assistance of VP, EHB and JB.
Sources of support
Internal sources
The Children's Hospital at Westmead, NSW, Australia
The University of Sydney, NSW, Australia
Macquarie University, NSW, Australia
External sources
No sources of support supplied
Declarations of interest
SB: none known.
VP: has received consultancy fees, research grants and conference financial support for various activities unrelated to this study.
EHB: none known.
JB: Joshua Burns' research and clinical activities are funded by the Australian Department of Health (Medical Research Future Fund), US National Institutes of Health, Charcot‐Marie Tooth Association of Australia, Charcot‐Marie Tooth Association (USA), Diabetes Australia, Elizabeth Lottie May Rosenthal Bone Bequest, Perpetual Limited, Humpty Dumpty Foundation. Consultancies: Acceleron Pharma (Sept 2016).
KG: none known.
Edited (no change to conclusions)
References
References to studies included in this review
Chen 2015 {published data only}
- Chen W, Pu F, Yang Y, Yao J, Wang L, Liu H, et al. Correcting congenital talipes equinovarus in children using three different corrective methods. Medicine 2015;94(28):e1004. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chong 2014 {published data only}
- Chong DY, Finberg NS, Conklin MJ, Doyle JS, Khoury JG, Gilbert SR. Prospective evaluation of the use of Mitchell shoes and dynamic abduction brace for idiopathic clubfeet. Journal of Pediatric Orthopaedics Part B 2014;23(6):501-4. [PMID: ] [DOI] [PubMed] [Google Scholar]
Cummings 2009 {published data only}
- Cummings RJ. The effectiveness of botulinum A toxin as an adjunct to the treatment of clubfeet by the Ponseti method: a randomized, double blind, placebo controlled study. Journal of Pediatric Orthopaedics 2009;29(6):564-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
El‐Deeb 2007 {published data only (unpublished sought but not used)}
- El-Deeb KH, Ghoneim AS, El-Adwar KL, Khalil AA. Is it hazardous or mandatory to release the talocalcaneal interosseous ligament in clubfoot surgery?: a preliminary report. Journal of Pediatric Orthopaedics 2007;27(5):517-21. [PMID: ] [DOI] [PubMed] [Google Scholar]
Elgohary 2014 {published data only}
- Elgohary HS, Abulsaad M. Traditional and accelerated Ponseti technique: a comparative study. European Journal of Orthopaedic Surgery & Traumatology : Orthopedie Traumatologie 2015;25(5):949-53. [PMID: ] [DOI] [PubMed] [Google Scholar]
Gintautiene 2016 {published data only}
- Gintautiene J, Cekanauskas E, Barauskas V, Zalinkevicius R. Comparison of the Ponseti method versus early tibialis anterior tendon transfer for idiopathic clubfoot: A prospective randomized study. Medicina 2016;52(3):163-70. [PMID: ] [DOI] [PubMed] [Google Scholar]
Harnett 2011 {published and unpublished data}
- Harnett P, Freeman R, Harrison WJ, Brown LC, Backles V. An accelerated Ponseti versus the standard Ponseti method: a prospective randomised controlled trial. Journal of Bone and Joint Surgery. British Volume 2011;93-B(3):404-8. [DOI: 10.1302/0301-620X.93B3] [PMID: 21357965] [DOI] [PubMed] [Google Scholar]
Hui 2014 {published data only}
- Hui C, Joughin E, Nettel-Aguirre A, Goldstein S, Harder J, Kiefer G, et al. Comparison of cast materials for the treatment of congenital idiopathic clubfoot using the Ponseti method: a prospective randomized controlled trial. Canadian Journal of Surgery 2014;57(4):247-53. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kaewpornsawan 2007 {published data only}
- Kaewpornsawan K, Khuntisuk S, Jatunarapit R. Comparison of modified posteromedial release and complete subtalar release in resistant congenital clubfoot: a randomized controlled trial. Journal of the Medical Association of Thailand 2007;90(5):936-41. [PMID: ] [PubMed] [Google Scholar]
Lahoti 2008 {published and unpublished data}
- Lahoti O, Bajaj S. Is there a role for lengthening flexor hallucis and flexor digitorum longus tendons in surgery for club foot?: a preliminary report. Journal of Bone and Joint Surgery. British Volume 2008;90-B(6):801-2. [PMID: ] [DOI] [PubMed] [Google Scholar]
Manzone 1999 {published data only}
- Manzone P. Clubfoot surgical treatment: preliminary results of a prospective comparative study of two techniques. Journal of Pediatric Orthopaedics Part B 1999;8(4):246-50. [PMID: ] [PubMed] [Google Scholar]
Maripuri 2013 {published data only}
- Maripuri SN, Gallacher PD, Bridgens J, Kuiper JH, Kiely NT. Ponseti casting for club foot-above-or below-knee?: a prospective randomised clinical trial. Bone & Joint Journal 2013;95-B(11):1570-4. [PMID: ] [DOI] [PubMed] [Google Scholar]
Pittner 2008 {published data only (unpublished sought but not used)}
- Pittner DE, Klingele KE, Beebe AC. Treatment of clubfoot with the Ponseti method: a comparison of casting materials. Journal of Pediatric Orthopaedics 2008;28(2):250-3. [PMID: ] [DOI] [PubMed] [Google Scholar]
Rijal 2010 {published data only (unpublished sought but not used)}
- Rijal R, Shrestha BP, Singh GK, Singh M, Nepal P, Khanal GP, et al. Comparison of Ponseti and Kite's method of treatment for idiopathic clubfoot. Indian Journal of Orthopaedics 2010;44(2):202-7. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sanghvi 2009 {published data only (unpublished sought but not used)}
- Sanghvi AV, Mittal VK. Conservative management of idiopathic clubfoot: Kite versus Ponseti method. Journal of Orthopaedic Surgery (Hong Kong) 2009;17(1):67-71. [PMID: ] [DOI] [PubMed] [Google Scholar]
Selmani 2012 {published data only (unpublished sought but not used)}
- Selmani E. Is Ponseti's method superior to Kite's for clubfoot treatment? European Orthopaedics and Traumatology 2012;3(3):183-7. [EMBASE: 368243939] [Google Scholar]
Siddiqui 2007 {published data only}
- Siddiqui MA, Pirwani MA, Bhura S, Soomro YH. Window procedure versus Turco procedure for the treatment of talipes equino varus. Pakistan Journal of Surgery 2007;23(3):212-6. [Google Scholar]
Sud 2008 {published data only}
- Sud A, Tiwari A, Sharma D, Kapoor S. Ponseti's vs. Kite's method in the treatment of clubfoot - a prospective randomised study. International Orthopaedics 2008;32(3):409-13. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Svehlik 2017 {published data only}
- Svehlik M, Floh U, Steinwender G, Sperl M, Novak M, Kraus T. Ponseti method is superior to surgical treatment in club foot - long-term, randomized, prospective trial. Gait & Posture 2017;58:346-51. [DOI: 10.1016/j.gaitpost.2017.08.010] [PMID: ] [DOI] [PubMed] [Google Scholar]
Zeifang 2005 {published data only}
- Zeifang F, Carstens C, Schneider S, Thomsen M. Continuous passive motion versus immobilisation in a cast after surgical treatment of idiopathic club foot in infants: a prospective, blinded, randomised clinical study. Journal of Bone and Joint Surgery. British Volume 2005;87-B(12):1663-5. [PMID: ] [DOI] [PubMed] [Google Scholar]
Zwick 2009 {published data only (unpublished sought but not used)}
- Zwick EB, Kraus T, Maizen C, Steinwender G, Linhart WE. Comparison of Ponseti versus surgical treatment for idiopathic clubfoot: a short- term preliminary report. Clinical Orthopaedics and Related Research 2009;467(10):2668-76. [DOI: 10.1007/s11999-009-0819-5] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Andriesse 2008 {published data only}
- Andriesse H, Hägglund G. Comparison of serial casting and stretching technique in children with congenital idiopathic clubfoot. Evaluation of a new system. Acta Orthopaedica 2008;79(1):53-61. [DOI] [PubMed] [Google Scholar]
Atar 1993 {published data only}
- Atar D, Lehman WB, Grant AD. Complete soft-tissue clubfoot release with and without internal fixation. Orthopaedic Review 1993;22(9):1015-6. [PubMed] [Google Scholar]
Aurell 2005 {published data only}
- Aurell Y, Andriesse H, Johansson A, Jonsson K. Ultrasound assessment of early clubfoot treatment: a comparison of the Ponseti method and a modified Copenhagen method. Journal of Pediatric Orthopaedics, Part B 2005;14(5):347-57. [DOI] [PubMed] [Google Scholar]
Chang 1991 {published data only}
- Chang YL, Huang SC, Liu TK. Surgical management of resistant idiopathic congenital clubfoot. Journal of the Formosan Medical Association 1991;90(12):1186-93. [PubMed] [Google Scholar]
Chhina 2013 {published data only}
- Chhina H, Howren A, Simmonds A, Alvarez CM. Onabotulinumtoxin A injections: A safety review of children with clubfoot under 2 years of age at BC Children's Hospital. Journal of the European Paediatric Neurology Society 2014;18(2):171-5. [DOI] [PubMed] [Google Scholar]
DePuy 1989 {published data only}
- DePuy J, Drennan JC. Correction of idiopathic clubfoot: a comparison of results of early versus delayed posteromedial release. Journal of Pediatric Orthopedics 1989;9(1):44-8. [PubMed] [Google Scholar]
DeRosa 1986 {published data only}
- DeRosa GP, Stepro D. Results of posteromedial release for the resistant clubfoot. Journal of Pediatric Orthopedics 1986;6(5):590-5. [DOI] [PubMed] [Google Scholar]
Derzsi 2015 {published data only}
- Derzsi Z, Nagy O, Gozar H, Gurzu S, Pop TS. Kite versus Ponseti method in the treatment of 235 feet with idiopathic clubfoot. Medicine 2015;94(33):1-4. [DOI: 10.1097/MD.0000000000001379] [DOI] [PMC free article] [PubMed] [Google Scholar]
Diméglio 1996 {published data only}
- Dimégio A, Bonnet F, Mazeau P, De Rosa V. Orthopaedic treatment and passive motion machine: consequences for the surgical treatment of clubfoot. Journal of Pediatric Orthopaedics Part B 1996;5(3):173-80. [DOI] [PubMed] [Google Scholar]
Dobbs 2017 {published data only}
- Dobbs MB, Frick SL, Mosca VS, Raney E, VanBosse HJ, Lerman JA, et al. Design and descriptive data of the randomized Clubfoot Foot Abduction Brace Length of Treatment Study (FAB24). Journal of Pediatric Orthopaedics 2017;26(2):101-7. [DOI] [PubMed] [Google Scholar]
Doğan 2002 {published data only}
- Doğan A, Bagatur AE, Zorer G. The effect of deep deltoid ligament release on dorsiflexion in congenital clubfoot treated with complete subtalar release. Acta Orthopaedica et Traumatologica Turcica 2002;36(3):248-55. [PubMed] [Google Scholar]
Farsetti 2009 {published data only}
- Farsetti P, De Maio F, Russolillo L, Ippolito E. CT study on the effect of different treatment protocols for clubfoot pathology. Clinical Orthopaedics and Related Research 2009;467(5):1243-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Faulks 2009 {published data only}
- Faulks S, Richards BS. Clubfoot treatment: Ponseti and French functional methods are equally effective. Clinical Orthopaedics and Related Research 2009;467(5):1278-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gupta 2014 {published data only}
- Gupta S, Kanojia RK, Lohia L. Comparative study of posteromedial release and complete subtalar release in resistant/recurrent congenital talipes equinovarus foot: A 5-year follow-up study. Current Orthopaedic Practice 2014;25(1):43-52. [DOI: 10.1097/BCO.0000000000000040] [DOI] [Google Scholar]
Halanski 2010 {published data only}
- Halanski MA, Davison JE, Huang JC, Walker CG, Walsh SJ, Crawford HA. Ponseti method compared with surgical treatment of clubfoot: a prospective comparison. Journal of Bone and Joint Surgery. American Volume 2010;92(2):270-8. [DOI] [PubMed] [Google Scholar]
Hallaj‐Moghadam M 2015 {published data only}
- Hallaj-Moghadam M, Moradi A, Ebrahimzadeh MH. Clinical outcome of posteromedial versus posteromedial-lateral release for clubfoot. Journal of Pediatric Orthopaedics 2015;24(1):24-27. [DOI: 10.1097/BPB.0000000000000124] [DOI] [PubMed] [Google Scholar]
Howren 2015 {published data only}
- Howren AM, Jamieson DH, Alvarez CM. Early ultrasonographic evaluation of idiopathic clubfeet treated with manipulations, casts, and Botox: a double-blinded randomized control trial. Journal of Child Orthopaedics 2015;9(1):85-91. [DOI: 10.1007/s11832-015-0633-4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ippolito 2003 {published data only}
- Ippolito E, Farsetti P, Caterini R, Tudisco C. Long-term comparative results in patients with congenital clubfoot treated with two different protocols. Journal of Bone and Joint Surgery. American Volume 2003;85-A(7):1286-94. [DOI] [PubMed] [Google Scholar]
Janicki 2011 {published data only}
- Janicki JA, Wright JG, Weir S, Narayanan UG. A comparison of ankle foot orthoses with foot abduction orthoses to prevent recurrence following correction of idiopathic clubfoot by the Ponseti method. Journal of Bone and Joint Surgery. British Volume 2011;93-B(5):700-4. [DOI] [PubMed] [Google Scholar]
Kesemenli 2003 {published data only}
- Kesemenli CC, Kapukaya A, Subaşi M, Necmioglu S, Arslan H, Ozbag D, et al. Anthropometric study of patients treated for clubfoot. Journal of Pediatric Orthopedics 2003;23(4):498-502. [PubMed] [Google Scholar]
Kuo 2001 {published data only}
- Kuo KN, Hennigan SP, Hastings ME. Anterior tibial tendon transfer in residual dynamic clubfoot deformity. Journal of Pediatric Orthopedics 2001;21(1):35-41. [DOI] [PubMed] [Google Scholar]
Li 2007 {published data only}
- Li L, Zhang L, Wang E. Comparison of long-term results between muscle-strength balancing procedure and McKay procedure in treating congenital clubfoot. Chinese Journal of Reparative and Reconstructive Surgery 2007;21(10):1108-12. [PubMed] [Google Scholar]
Lohia 2014 {published data only}
- Lohia LK, Meena S, Kanjia RK. Comparative study of complete subtalar release and Joshi's external stabilization system in the management of neglected and resistant idiopathic clubfoot. Foot and Ankle Surgery 2014;21(1):16-21. [DOI: 10.1016/j.fas.2014.08.007] [DOI] [PubMed] [Google Scholar]
Matuszewski 2012 {published data only}
- Matuszewski L, Gil L, Karski J. Early results of treatment for congenital clubfoot using the Ponseti method. European Journal of Surgery Traumatology 2012;22(5):403-6. [DOI: 10.1007/s00590-011-0860-4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Miura 2005 {published data only}
- Miura Y, Kamegaya M, Saisu T, Moriya H. Effect of postoperative early ankle motion exercise using hinged ankle-foot orthoses in clubfoot. Journal of Pediatric Orthopedics 2005;25(4):529-32. [DOI] [PubMed] [Google Scholar]
Napiontek 2000 {published data only}
- Napiontek M. Muscular strength after extensive operative treatment of congenital talipes equinovarus. Journal of Pediatric Orthopaedics, Part B 2000;9(2):128-36. [DOI] [PubMed] [Google Scholar]
Narang 2011 {published data only}
- Narang AS, Singh H, Sharma V, Khare S. Comparison of short term results of JESS distractor and one stage posteriomedial release for neglected, resistant and relapsed or recurrent club foot. Journal of Orthopaedics 2011;8(4):e8. [Google Scholar]
Nilgün 2011 {published data only}
- Nilgün B, Suat E, Engin ŞÍ, Fatma U, Yakut Y. Short-term results of intensive physiotherapy in clubfoot deformity treated with the Ponseti method. Pediatrics International 2011;53(3):381-5. [DOI] [PubMed] [Google Scholar]
O'Brien 2004 {published data only}
- O'Brien SE, Karol LA, Johnston CE 2nd. Calcaneus gait following treatment for clubfoot: preliminary results of surgical correction. Journal of Pediatric Orthopaedics, Part B 2004;13(1):43-7. [DOI] [PubMed] [Google Scholar]
Ponseti 2006 {published data only}
- Ponseti IV, Zhivkov M, Davis N, Sinclair M, Dobbs MB, Morcuende JA. Treatment of the complex idiopathic clubfoot. Clinical Orthopaedics and Related Research 2006;451:171-6. [DOI] [PubMed] [Google Scholar]
Richards 2008 {published data only}
- Richards BS, Faulks S, Rathjen KE, Karol LA, Johnston CB, Jones SA. A comparison of two nonoperative methods of idiopathic clubfoot correction: the Ponseti method and the French functional (physiotherapy) method. Journal of Bone and Joint Surgery. American Volume 2008;90(11):2313-21. [DOI] [PubMed] [Google Scholar]
Shingade 2014 {published data only}
- Shingade VU, Shingade RV, Ughade SN. Single-stage correction for clubfoot associated with myelomeningocele in older children: early results. Current Orthopaedic Practice 2014;25(1):64-70. [DOI: 10.1097/BCO.0000000000000059] [DOI] [Google Scholar]
Simons 1985 {published data only}
- Simons GW. Complete subtalar release in club feet. Part II - Comparison with less extensive procedures. Journal of Bone and Joint Surgery. American Volume 1985;67(7):1056-65. [PubMed] [Google Scholar]
Steinman 2009 {published data only}
- Steinman S, Richards BS, Faulks S, Kaipus K. A comparison of two nonoperative methods of idiopathic clubfoot correction: the Ponseti method and the French functional (physiotherapy) method. Surgical technique. Journal of Bone and Joint Surgery. American Volume 2009;91(Suppl 2):299-312. [DOI] [PubMed] [Google Scholar]
Thompson 1982 {published data only}
- Thompson GH, Richardson AB, Westin GW. Surgical management of resistant congenital talipes equinovarus deformities. Journal of Bone and Joint Surgery. American Volume 1982;64(5):562-5. [PubMed] [Google Scholar]
Tschopp 2002 {published data only}
- Tschopp O, Rombouts JJ, Rossillon R. Comparison of posteromedial and subtalar release in surgical treatment of resistant clubfoot. Orthopedics 2002;25(5):527-9. [DOI] [PubMed] [Google Scholar]
Uglow 2000 {published data only}
- Uglow MG, Clarke NM. The functional outcome of staged surgery for the correction of talipes equinovarus. Journal of Pediatric Orthopedics 2000;20(4):517-23. [PubMed] [Google Scholar]
Xu 2011 {published data only}
- Xu RJ. A modified Ponseti method for the treatment of idiopathic clubfoot: a preliminary report. Journal of Pediatric Orthopedics 2011;31(3):317-9. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Madhuri 2018 {published data only}
- Comparison of 2 different types of splints in children with corrected CTEV, Dobbs versus Denis Browne splint. Ongoing study. 1 November 2013. Contact author for more information.
NCT03249805 {unpublished data only}
- NCT03249805. MiracleFeet foot abduction brace sensor trial (mFAB) [Efficacy of a new design of foot abduction brace (FAB) compared to standard FAB during Ponseti treatment of idiopathic clubfoot by measuring rate of recurrence and compliance using novel touch sensors]. https://clinicaltrials.gov/ct2/show/NCT03249805 (first received 15 August 2017).
Additional references
Alvarez 2005
- Alvarez CM, Tredwell SJ, Keenan SP, Beauchamp RD, Choit RL, Sawatzky BJ, et al. Treatment of idiopathic clubfoot utilizing botulinum A toxin: a new method and its short-term outcomes. Journal of Pediatric Orthopaedics 2005;25(2):229-35. [DOI] [PubMed] [Google Scholar]
Atar 1992
- Atar D, Lehman WB, Grant AD, Strongwater AM. Revision surgery in clubfeet. Clinical Orthopaedics and Related Research 1992;283:223-30. [PubMed] [Google Scholar]
Beatson 1966
- Beatson TR, Pearson JR. A method of assessing correction in club feet. Journal of Bone and Joint Surgery 1966;48(B):40-50. [PubMed] [Google Scholar]
Bryant 2006
- Bryant D, Havey TC, Roberts R, Guyatt G. How many patients? How many limbs? Analysis of the patients or limbs in the orthopaedic literature: a systematic review. Journal of Bone and Joint Surgery 2006;88(1):41-5. [DOI: 10.2106/JBJS.E.00272] [DOI] [PubMed] [Google Scholar]
Cooper 1995
- Cooper DM, Dietz FR. Treatment of idiopathic clubfoot. A thirty year follow-up note. Journal of Bone and Joint Surgery. American Volume 1995;77-A(10):1447-89. [DOI] [PubMed] [Google Scholar]
Cusick 1990
- Cusick B. Serial Casts: Their Use in the Management of Spasticity-Induced Foot Deformity. Tuscon, Arizona: Communication Skill Builders, 1990. [Google Scholar]
De Gheldere 2008
- De Gheldere A, Docquier PL. Analytical radiography of clubfoot after tenotomy. Journal of Pediatric Orthopaedics 2008;28(6):691-4. [DOI] [PubMed] [Google Scholar]
Deeks 2017
- Deeks JJ, Higgins JP, Altman DG, editor(s) on behalf of the Cochrane Statistical Methods Group. Chapter 9: Analysing data and undertaking meta-analyses. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), The Cochrane Collaboration, 2017. Available from www.training.cochrane.org/handbook.
Dietz 2006
- Dietz FR. Treatment of a recurrent clubfoot deformity after initial correction with the Ponseti technique. Instructional Course Lectures 2006;55:625-9. [PubMed]
Dietz 2009
- Dietz FR, Tyler MC, Leary KS, Damiano PC. Evaluation of a disease-specific instrument for idiopathic clubfoot outcome. Clinical Orthopaedics and Related Research 2009;467(5):1256-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Diméglio 1995
- Diméglio A, Bensahel H, Souchet P, Mazeau P, Bonnet F. Classification of clubfoot. Journal of Pediatric Orthopaedics. Part B 1995;4(2):129-36. [DOI] [PubMed] [Google Scholar]
Dobbs 2000
- Dobbs MB, Morcuende JA, Gurnett CA, Ponseti IV. Treatment of idiopathic clubfoot: an historical review. Iowa Orthopaedic Journal 2000;20:59-64. [PMC free article] [PubMed] [Google Scholar]
Dobbs 2006
- Dobbs MB, Nunley R, Schoenecker PL. Long-term follow-up of patients with clubfeet treated with extensive soft-tissue release. Journal of Bone and Joint Surgery. American Volume 2006;88(5):986-96. [DOI] [PubMed] [Google Scholar]
Dobbs 2009
- Dobbs M, Gurnett CA. Update on clubfoot: etiology and treatment. Clinical Orthopaedics and Related Research 2009;467(5):1146-53. [DOI: 10.1007/s11999-009-0734-9] [DOI] [PMC free article] [PubMed] [Google Scholar]
Farsetti 2006
- Farsetti P, Caterini R, Mancini F, Potenza V, Ippolito E. Anterior tibial tendon transfer in relapsing congenital clubfoot: long-term follow-up study of two series treated with a different protocol. Journal of Pediatric Orthopaedics 2006;26(1):83-90. [DOI] [PubMed] [Google Scholar]
Fridman 2007
- Fridman MW, De Almeida Fialho HS. The role of radiographic measurements in the evaluation of congenital clubfoot surgical results. Skeletal Radiology 2007;36(2):129-38. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT 2016 [Computer program]
- McMaster University (developed by Evidence Prime) GRADEpro GDT. Hamilton (ON): McMaster University (developed by Evidence Prime), accessed 6 August 2016.Available at gradepro.org.
Graf 2010
- Graf A, Hassani S, Krzak J, Long J, Caudill A, Flanagan A, et al. Long-term outcome evaluation in young adults following clubfoot surgical release. Journal of Pediatric Orthopaedics 2010;30(4):379-85. [DOI] [PubMed] [Google Scholar]
Gray 2014a
- Gray K, Barnes E, Gibbons P, Little D, Burns J. Unilateral versus bilateral clubfoot: an analysis of severity and correlation. Journal of Pediatric Orthopaedics B 2014;23(5):397-9. [DOI: 10.1097/BPB.0000000000000064] [DOI] [PubMed] [Google Scholar]
Gray 2014b
- Gray K, Burns J, Little D, Bellemore M, Gibbons P. Is tibialis anterior tendon transfer effective for recurrent clubfoot? Clinical Orthopaedics and Related Research 2014;472(2):750-8. [DOI: 10.1007/s11999-013-3287-x] [DOI] [PMC free article] [PubMed] [Google Scholar]
Grimes 2016
- Grimes CE, Holmer H, Maraka J, Ayana B, Hansen L, Lavy CB. Cost-effectiveness of club-foot treatment in low-income and middle-income countries by the Ponseti method. BMJ Global Health 2016;26(1):1-5. [DOI: 10.1136/bmjgh-2015-000023] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hackshaw 2011
- Hackshaw A, Rodeck C, Boniface S. Maternal smoking in pregnancy and birth defects: a systematic review based on 173 687 malformed cases and 11.7 million controls. Human Reproductiion Update 2011;17(5):589-604. [DOI: 10.1093/humupd/dmr022] [DOI] [PMC free article] [PubMed] [Google Scholar]
Haft 2007
- Haft GF, Walker CG, Crawford HA. Early clubfoot recurrence after use of the Ponseti method in a New Zealand population. Journal of Bone and Joint Surgery. American Volume 2007;89-A(3):487-93. [DOI] [PubMed] [Google Scholar]
Halanski 2009
- Halanski MA, Huang JC, Walsh SJ, Crawford HA. Resource utilization in clubfoot management. Clinical Orthopaedics and Related Research 2009;5:1171-9. Erratum in: Clinical Orthopedics and Related Research 2010 Apr;468(4):1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
Halanski 2010a
- Halanksi MA, Davison JE, Huang JC, Walker CG, Walsh SJ, Crawford HA. Ponseti method compared with surgical treatment of clubfoot: a prospective comparison. Journal of Bone and Joint Surgery. American Volume 2010;92(2):270-8. [DOI: 10.2106/JBJS.H.01560] [DOI] [PubMed] [Google Scholar]
Halanski 2010b
- Halanski MA, Maples DL, Davison JE, Huang JC, Crawford HA. Separating the chicken from the egg: an attempt to discern between clubfoot recurrences and incomplete corrections. Iowa Orthopaedic Journal 2010;20:29-34. [21045968] [PMC free article] [PubMed] [Google Scholar]
Harrold 1983
- Harrold AJ, Walker CJ. Treatment and prognosis in congenital clubfoot. Journal of Bone and Joint Surgery. British Volume. 1983;65-B:8-11. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Deeks JJ. Chapter 7: Selecting studies and collecting data. in: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions, version 5.1.0 (update March 2011). The Cochrane Collaoration, 2011. Available from www.training.cochrane.org/handbook.
Higgins 2017
- Higgins JP, Altman DG, Sterne JA, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), The Cochrane Collaboration, 2017. Available from www.training.cochrane.org/handbook.
Higgins 2019
- Higgins JP, Eldridge S, Li T, editor(s). Chapter 23: Including variants on randomized trials. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). The Cochrane Collaboration, 2019. Available from www.training.cochrane.org/handbook.
Janicki 2009
- Janicki JA, Narayanan UG, Harvey B, Roy A, Ramseier LE, Wright JG. Treatment of neuromuscular and syndrome-associated (nonidiopathic) clubfeet using the Ponseti method. Journal of Pediatric Orthopaedics 2009;29(4):393-7. [DOI] [PubMed] [Google Scholar]
Keret 2002
- Keret D, Ezra E, Lokiec F, Hayek S, Segev E, Wientroub S. Efficacy of prenatal ultrasonography in confirmed club foot. Journal of Bone and Joint Surgery. British Volume 2002;84(7):1015-9. [DOI] [PubMed] [Google Scholar]
Kite 1972
- Kite JH. Non operative treatment of congenital clubfoot. Clinical Orthopaedics and Related Research 1972;84:29-38. [DOI] [PubMed] [Google Scholar]
Laaveg 1980
- Laaveg SJ, Ponseti IV. Long-term results of treatment of congenital club foot. Journal of Bone and Joint Surgery. American Volume 1980;62(1):23-31. [PubMed] [Google Scholar]
Landgraf 1999
- Landgraf JM, Abetz L, Ware JE. The Child Health Questionnaire (CHQ): A User's Manual (2nd printing). Boston, MA: HealthAct, 1999. [Google Scholar]
Lesaffre 2010
- Lesaffre E, Philstrom B, Needleman I, Worthington H. The design and analysis of split-mouth studies: what statisticians and clinicians should know. Statistics in Medicine 2009;28(28):3470-82. [DOI: 10.1002/sim.3634] [DOI] [PubMed] [Google Scholar]
Lovell 1979
- Lovell WW, Farley D. Treatment of congenital clubfoot. ONA Journal 1979;6(11):453-6. [PubMed] [Google Scholar]
Lovell 2007
- Lovell ME, Morcuende JA. Neuromuscular disease as the cause of late clubfoot relapses: report of 4 cases. Iowa Orthopaedic Journal 2007;27:82-4. [PMC free article] [PubMed] [Google Scholar]
Marquez 2017
- Marquez E, Pacey V, Chivers A, Gibbons P, Gray K. The Ponseti technique and improved ankle dorsiflexion in children with relapsed clubfoot: a retrospective data analysis. Journal of Pediatric Orthopaedics B 2017;26(2):116-21. [DOI: ] [DOI] [PubMed] [Google Scholar]
Masrouha 2012
- Masrouha KZ, Morcuende JA. Relapse after tibialis anterior tendon transfer in idiopathic clubfoot treated by the Ponseti method. Journal of Pediatric Orthopedics 2012;32(1):81-4. [DOI] [PubMed] [Google Scholar]
McKay 1983
- McKay DW. New concept of and approach to clubfoot treatment. Section III: evaluation and results. Journal of Pediatric Orthopedics 1983;3:10-21. [DOI] [PubMed] [Google Scholar]
Morcuende 2004
- Morcuende JA, Dolan LA, Dietz FR, Ponseti IV. Radical reduction in the rate of extensive corrective surgery for clubfoot using the Ponseti method. Pediatrics 2004;113(2):376-80. [DOI] [PubMed] [Google Scholar]
Nogueira 2009
- Nogueira MP, Ey Batlle AM, Alves CG. Is it possible to treat recurrent clubfoot with the Ponseti technique after posteromedial release? Clinical Orthopaedics and Related Research 2009;467(5):1298-305. [DOI: 10.1007/s11999-009-0718-9] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pavone 2018
- Pavone V, Chisari E, Vescio A, Lucenti L, Sessa G, Testa G. The eitiology of idopathic congenital talipes equinovarus: a systematic review. Journal of Orthopaedic Surgery and Research 2018;13:206. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Perera 2007
- Perera R, Glasziou P. A simple method to correct for the design effect in systematic review of trials using paired dichotomous data. Journal of Clinical Epidemiology 2007;60(9):975-8. [DOI: 10.1016/j.jclinepi.2006.12.004] [DOI] [PubMed] [Google Scholar]
Pirani 2001
- Pirani S, Zeznik L, Hodges D. Magnetic resonance imaging study of the congenital clubfoot treated with the Ponseti method. Journal of Pediatric Orthopaedics 2001;21(6):719-26. [PubMed] [Google Scholar]
Pirani 2008
- Pirani S, Hodges D, Sekeramayi F. A reliable and valid method of assessing the amount of deformity in the congenital clubfoot deformity. Journal of Bone and Joint Surgery. British Volume 2008;90-B(Supp Ⅰ):53. [Google Scholar]
Ponseti 2005
- Ponseti I, Morcuende J, Mosca V, Pirani S, Dietz F, Herzenberg J, et al. Clubfoot: Ponseti Management. 2nd edition. Global-HELP Organization, 2005. [Google Scholar]
Radler 2007
- Radler C, Manner HM, Suda R, Burghardt R, Herzenberg JE, Ganger R, et al. Radiographic evaluation of idiopathic clubfeet undergoing Ponseti treatment. Journal of Bone and Joint Surgery. American Volume 2007;89(6):1177-83. [DOI] [PubMed] [Google Scholar]
Redmond 2006
- Redmond AC, Crosbie J, Ourvrier RA. Development and validation of a novel rating system for scoring standing foot posture: the Foot Posture Index. Clinical Biomechanics 2006;21(1):89-98. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration Review Manager (RevMan). Version 5.0. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Richards 2007
- Richards BS, Dempsey M. Magnetic resonance imaging of the congenital clubfoot treated with the French functional (physical therapy) method. Journal of Pediatric Orthopaedics 2007;27(2):214-9. [DOI] [PubMed] [Google Scholar]
Schünemann 2017a
- Schünemann HJ, Oxman AD, Higgins JP, Vist GE, Glasziou P, Akl E, et al, on behalf of the Cochrane GRADEing Methods Group and the Cochrane Statistical Methods Group. Chapter 11: Completing ‘Summary of findings’ tables and grading the confidence in or quality of the evidence. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017). The Cochrane Collaboration, 2017. Available from www.training.cochrane.org/handbook.
Schünemann 2017b
- Schünemann HJ, Oxman AD, Vist GE, Higgins JP, Deeks JJ, Glasziou P, et al, on behalf of the Cochrane Applicability and Recommendations Methods Group. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017). The Cochrane Collaboration, 2017. Available from www.training.cochrane.org/handbook.
Sterne 2017
- Sterne JA, Egger M, Moher D, Boutron I, editor(s). Chapter 10: Addressing reporting biases. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), The Cochrane Collabration, 2017. Available from www.training.cochrane.org/handbook.
Van Praag 2018
- Van Praag VM, Lysenko M, Harvey B, Yankanah R, Wright JG. Casting is effective for recurrence following Ponseti treatment of clubfoot. Journal of Bone and Joint Surgery. American Volume 2018;100(12):1001-8. [DOI: 10.2106/JBJS.17.01049] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ware 1992
- Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). Conceptual framework and item selection. Medical Care 1992;30(6):473-83. [PubMed] [Google Scholar]
Zionts 2010
- Zionts LE, Zhao G, Hitchcock K, Maewal J, Ebramzadeh E. Has the rate of extensive surgery to treat idiopathic clubfoot declined in the United States? Journal of Bone and Joint Surgery. American Volume 2010;92(4):882-9. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Gray 2010
- Gray K, Pacey V, Gibbons P, Little D, Burns J. Interventions for congenital talipes equinovarus. Cochrane Database of Systematic Reviews 2010, Issue 7. [DOI: 10.1002/14651858.CD008602] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gray 2012
- Gray K, Pacey V, Gibbons P, Little D, Frost C, Burns J. Interventions for congenital talipes equinovarus (clubfoot). Cochrane Database of Systematic Reviews 2012, Issue 4. [DOI: 10.1002/14651858.CD008602.pub2] [DOI] [PubMed] [Google Scholar]
Gray 2014
- Gray K, Pacey V, Gibbons P, Little D, Burns J. Interventions for congenital talipes equinovarus (clubfoot). Cochrane Database of Systematic Reviews 2014, Issue 8. [DOI: 10.1002/14651858.CD008602.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]


