An 82-year-old man with a past medical history significant for hypertension and Helicobacter pylori gastritis presented with 4 episodes of gross hematuria in late 2018. He had a previous episode of gross hematuria 5 years earlier and was found to have an enlarged prostate but an otherwise negative work-up at the time. His recent urine cytology was suspicious for malignancy, and his CT urogram showed multiple small filling defects in the bladder. An office cystoscopy demonstrated multiple papillary bladder masses, the largest being 3 cm on the left lateral wall (Figure 1).
Figure 1.
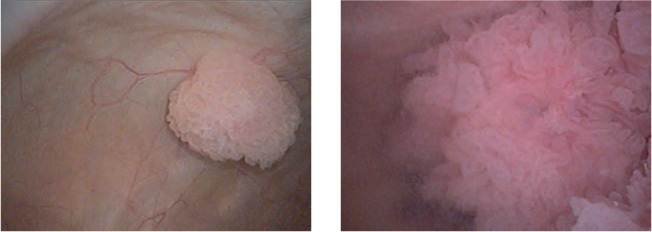
Images from office cystoscopy showing multiple papillary tumors.
Preoperative Data
Creatinine (Cr): 1.1 mg/dL
Estimated glomerular filtration rate (eGFR): >60
Urinalysis: >180 RBCs
Urine cytology: suspicious for malignancy
Management
The patient was taken to the operating room for a complete transurethral resection of his bladder tumors. His pathology report showed high-grade urothelial carcinoma involving the lamina propria with muscularis propria present but uninvolved (clinical stage T1). A repeat resection was performed 4 weeks later with no residual disease present.
The patient was started on induction full-strength intravesical bacillus Calmette-Guérin (BCG) treatment. His post-induction cystoscopy was negative. The initial plan was to continue with maintenance BCG treatment per the original SWOG protocol (weekly for 3 weeks at 3, 6, 12, 18, 24, 30, and 36 months), but there was no BCG available due to a nationwide shortage. As an alternative, the patient was started on monthly maintenance therapy with intravesical mitomycin and recently completed his ninth and final dose with no recurrence on interval quarterly cystoscopies. He will continue surveillance per American Urological Association (AUA) guidelines for high-risk non-muscle-invasive bladder cancer (NMIBC).
Comment
The initial management of all patients with NMIBC includes a transurethral resection for both diagnostic and therapeutic purposes. The AUA, as well as several other clinical societies, have created clear guidelines for NMIBC treatment, including stratification into low-, intermediate-, and high-risk groups based on pathological characteristics and history of recurrences.1–3 Adjuvant intravesical BCG is used in intermediate- and high-risk NMIBC to reduce risk of recurrence and progression.1
In recent years, the availability of BCG has fluctuated, with three major shortages occurring in the past decade.4–6 Several factors have led to the limited supply, and with each shortage, urologists are faced with difficult decisions regarding the management of their NMIBC patients. The current shortage has been ongoing for over a year with no clear indication of future ability to meet the clinical demand, despite maximized production efforts from Merck (Kenilworth, NJ), the only supplier in the United States.
In early 2019, the AUA (in conjunction with multiple collaborating organizations) released a statement to its members and the urologic community with suggested strategies for the allocation of BCG and use of alternative therapies.7 The most notable recommendations include (1) prioritizing induction BCG for high-risk, BCG-naive patients, (2) reducing doses (1/2 to 1/3 dose, with attempts to treat multiple patients in a day from a single vial), (3) limiting maintenance therapy to 1 year, and (4) using alternative agents, including mitomycin, gemcitabine, valrubicin, and docetaxel. Early cystectomy should be offered to patients with high-risk features who are surgical candidates. Intravesical chemotherapy should be the first- and second-line choice for intermediate-risk disease and as an alternative for high-risk patients when BCG is unavailable. Maintenance chemotherapy should be administered monthly for up to 1 year of total therapy.
An indirect consequence of the BCG shortages has been an overdue increase in the number of clinical trials for NMIBC. Packiam, Werntz, and Steinberg recently published an excellent review of actively accruing studies, many of which include BCG as monotherapy or in combination with other agents.8 Enrolling in a trial can be a viable option for obtaining BCG for patients if it is unavailable for non-investigational use.
Additionally, there are many promising alternatives to BCG on the horizon for NMIBC. Immune checkpoint inhibitors have shown significant activity in metastatic bladder cancer and are now being studied in several NMIBC disease states, with interim results from the KEYNOTE-057 trial demonstrating a 3-month complete response rate of 38% with pembrolizumab in BCG-unresponsive high-risk NMIBC patients with carcinoma in situ with or without papillary tumors.9 Novel intravesical delivery strategies to improve penetration and duration of exposure include thermochemotherapy, electromotive therapy, and drug-eluding implants. Targeted therapy, gene therapy, and cancer vaccines are being actively investigated as well.8
Lastly, it is worth noting that several companies are working on the production of other strains of BCG that could eventually get approved in the United States. Of note, SWOG 1602 is currently enrolling a phase 3 trial comparing the Tokyo-172 strain of BCG against TICE (the strain being produced by Merck). In the interim, urologists must continue to have informed discussions with their patients about treatment alternatives and should seek out actively enrolling clinical trials when feasible.
References
- 1.Chang SS, Boorjian SA, Chou R, et al. Diagnosis and Treatment of Non-Muscle Invasive Bladder Cancer: AUA/SUO Guideline. J Urol. 2016;196:1021–1029. doi: 10.1016/j.juro.2016.06.049. [DOI] [PubMed] [Google Scholar]
- 2.Babjuk M, Burger M, Compérat EM, et al. European Association of Urology Guidelines on Non-muscleinvasive Bladder Cancer (TaT1 and Carcinoma In Situ)-2019 Update. Eur Urol. 2019;76:639–657. doi: 10.1016/j.eururo.2019.08.016. [DOI] [PubMed] [Google Scholar]
- 3.Woldu SL, Bagrodia A, Lotan Y. Guideline of guidelines: non-muscle-invasive bladder cancer. BJU Int. 2017;119:371–380. doi: 10.1111/bju.13760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abufaraj M, Mostafid H, Shariat SF, Babjuk M. What to do during Bacillus Calmette-Guérin shortage? Valid strategies based on evidence. Curr Opin Urol. 2018;28:570–576. doi: 10.1097/MOU.0000000000000544. [DOI] [PubMed] [Google Scholar]
- 5.Messing EM. The BCG shortage. Bladder Cancer. 2017;3:227–228. doi: 10.3233/BLC-179018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Veeratterapillay R, Heer R, Johnson MI, et al. High-risk non-muscle-invasive bladder cancer-therapy options during intravesical bcg shortage. Curr Urol Rep. 2016;17:68. doi: 10.1007/s11934-016-0625-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.BCG Shortage Info, author. American Urological Association website. https://www.auanet.org/about-us/bcg-shortage-info. Accessed April 22, 2020.
- 8.Packiam VT, Werntz RP, Steinberg GD. Current clinical trials in non-muscle-invasive bladder cancer: heightened need in an era of chronic BCG shortage. Curr Urol Rep. 2019;20:84. doi: 10.1007/s11934-019-0952-y. [DOI] [PubMed] [Google Scholar]
- 9.Balar AV, Kulkarni GS, Uchio EM, et al. Keynote 057: Phase II trial of Pembrolizumab (pembro) for patients (pts) with high-risk (HR) nonmuscle invasive bladder cancer (NMIBC) unresponsive to bacillus Calmette-Guérin (BCG) J Clin Oncol. 2019;37:350. [Google Scholar]


