Abstract
Background
Cow antibodies are very unusual in having exceptionally long CDR H3 regions. The genetic basis for this length largely derives from long heavy chain diversity (DH) regions, with a single “ultralong” DH, IGHD8–2, encoding over 50 amino acids. Many bovine IGHD regions have sequence similarity but have several nucleotide repeating units that diversify their lengths. Genomically, most DH regions exist in three clusters that appear to have formed from DNA duplication events. However, the relationship between the genomic arrangement and long CDR lengths is unclear.
Results
The DH cluster containing IGHD8–2 underwent a rearrangement and deletion event in relation to the other clusters in the region corresponding to IGHD8–2, with possible fusion of two DH regions and expansion of short repeats to form the ultralong IGHD8–2 gene.
Conclusions
Length heterogeneity within DH regions is a unique evolutionary genomic mechanism to create immune diversity, including formation of ultralong CDR H3 regions.
Keywords: Bovine immunoglobulin, DH region, Ultralong CDR3, Cow antibody, Antibody diversity, Immunoglobulin repertoire, CDR3 length
Background
Adaptive immunity arose in vertebrates through the ability to somatically alter antigen receptor (antibody and T-cell receptor) genes to form diverse repertoires which are selected to bind and neutralize invading pathogens. A key component of this system is the ability to perform recombination of variable (V), diversity (D), and joining (J) gene segments through the process of V(D)J recombination [1–3]. A diversity of V, D, and J elements, along with imprecise joining at the V-D and D-J junctions enables different amino acids to be encoded in key paratopic regions which impact antigen binding.
The third complementary determining region of the heavy chain (CDR H3) is particularly important in antibody molecules as it contains the greatest diversity and also usually makes the most extensive contact with antigen. Long CDR H3 regions are often found in broadly neutralizing antibodies targeting human immunodeficiency (HIV), influenza, and polio viruses [4–8], and are also thought to be important in binding challenging antigens like G-protein coupled receptors and protease active sites [9, 10]. Thus, genetic mechanisms to form long CDR H3s may be very important in immune responses against key antigens.
In most organisms, the antibody CDR H3 forms a loop of 10–15 amino acids in length, and is encoded by the DH gene and associated recombinational junctions that form through VDJ recombination. Unusually long CDR H3s, such as those in rare broadly neutralizing anti-HIV antibodies from infected patients, are often over 20 amino acids in length [4, 11–13]. The major determinants of CDR H3 length are the length of the germline encoded DH region, as well as somatic insertion of nucleotides (e.g. N- or P- nucleotides) at the V-D and D-J junctions. In humans, the longest DH region, IGHD3–16, encodes 12 amino acids.
Bovines are remarkable in having very long CDR H3 regions [14–24], with an average length of 26 amino acids [16]but with an exceptionally long subset of the repertoire (the “ultralong” CDR H3 antibodies) that can have CDR H3 lengths of up to 70 amino acids. These CDR H3 regions form their own independently folding mini domains comprised of a β-ribbon “stalk” that protrudes far from the typical paratope surface upon which sits a disulfide-bonded “knob” [21, 23, 25, 26]. Cows are the only species thus far investigated that can produce a broadly neutralizing antibody response against HIV when immunized with an engineered spike protein immunogen as an experimental vaccine. These antibodies are characterized by ultralong CDR H3 regions that penetrate the glycan shield of the spike protein to bind a conserved broadly neutralizing epitope in the CD4 binding region [27]. Cows are therefore unusual in producing long CDR H3s, and this unique repertoire has major functional relevance in neutralizing an antigen that is extremely challenging for repertoires of other mammalian species. Therefore, understanding the natural genetic and evolutionary mechanisms behind ultralong CDR H3 generation would be important in vaccine generation as well as therapeutic antibody discovery and development.
At least two evolutionary genetic events occurred which enabled formation of ultralong CDR H3 antibodies in cows. First, a unique VH region evolved as a result of an 8-basepair duplication at the 3′ end of IGHV1–7 [16]. This particular variable region is the only VH region used in ultralong CDR H3 antibodies, and the short duplication directly encodes the ascending strand of the stalk region characteristic of these antibodies. Second, a very long DH region is found in cattle, IGHD8–2, which encodes 49 amino acids [23, 28–30]. Antibodies with ultralong CDR H3 regions invariably use IGHV1–7 and IGHD8–2 [8, 16]. Here we examine the genetic features at work in the evolution of this unusually long DH region of cattle.
Results
DH cluster 2 has a significant deletion
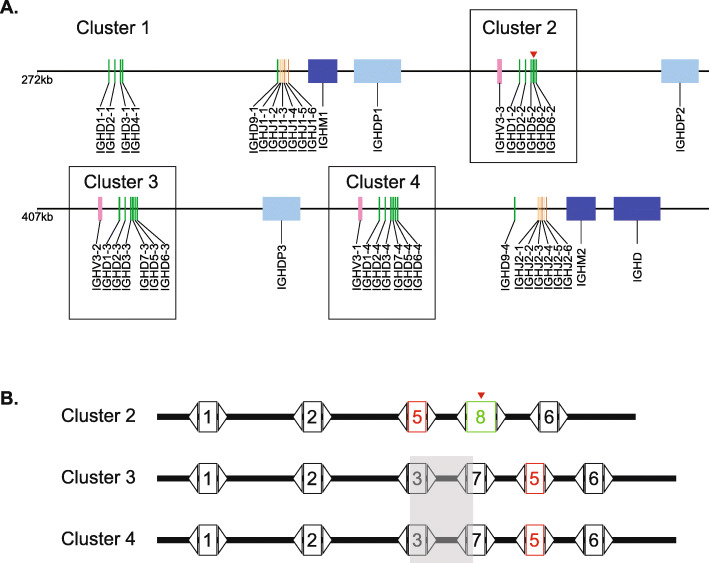
We analyzed the DH regions of the recent assembly of the Bos taurus immunoglobulin heavy chain locus [29]for features associated with the ultralong IGHD8–2 region. Of particular note, the DH regions at the heavy chain locus are divided into “clusters” that arose from duplication events through evolution. The IMGT naming nomenclature for DH regions includes numerical designations for the family and cluster of each gene; for example, IGHD3–2 is in family 3 and located in cluster 2 [16, 31–33]. There are four clusters, with clusters 2–4 being homologous with nucleotide identities of 92% (cluster 2 vs cluster 3), 99.7% (cluster 3 vs cluster 4), and 92% (cluster 2 vs cluster 4). The sequences of the DH regions located within the clusters are also homologous, with DH regions occupying analogous locations being 96 to 100% identical at the nucleotide level (Supplemental Fig. 1). A major discrepancy in the cluster sequences, however, is that cluster 2 (3480 nucleotides) is 358 and 364 nucleotides shorter than clusters 3 (3838 nt) and 4 (3844 nt), respectfully. Additionally, cluster 2 is comprised of only five DH regions, with one of them being the ultralong IGHD8–2, whereas clusters 3 and 4 are comprised of six DH regions (Fig. 1). Thus, cluster 2 appears to have a significant genomic deletion in relation to the highly homologous clusters 3 and 4. We hypothesized that this deletion might be related to formation of the ultralong IGHD8–2 region located in cluster 2. In simplistic terms, one explanation for formation of an ultralong DH region would be by fusion of two DH regions through deletion of intragenic sequence, with the fusion maintaining recombination signal sequences of each DH at both the 5′ and 3′ ends.
Fig. 1.
Schematic of D region clusters at the Bos taurus immunoglobulin heavy chain locus. a D-region cluster 2, comprising an ultralong IGHD, is shorter than highly homologous clusters. The DH regions are organized in four clusters at the immunoglobulin heavy chain locus on Bos taurus chromosome 21. Three clusters are homologous (clusters 2, 3 and 4 which are boxed). Green rectangles represent DH regions; orange, JH regions; light orange, pseudogene JH regions; blue, CH regions; light blue, pseudogene CH regions; and light pink, pseudogene VH regions. A red triangle indicates the position of the ultralong IGHD8–2. The entire locus is not shown; VH regions are upstream and remaining constant regions are downstream of the region shown. b Cluster 2 has a deletion and rearrangement in relation to clusters 3 and 4. Aligned schematic of the DH regions and their locations within the clusters. The numbers inside the boxes indicate the family members of each DH (e.g. on the first line, “1” represents IGHD1–2, and “1” on the second line represents IGHD1–3, etc.). IGHD5 is labeled in red to illustrate its unusual location in cluster 2 relative to clusters 3 and 4. The ultralong DH, IGHD8–2, is outlined in green, and indicated by a red triangle above. The transparent grey box encompassing IGHD3 and IGHD7 regions represents the approximate region of a large nucleotide deletion in cluster 2 relative to clusters 3 and 4. Open triangles represent the recombination signal sequences (RSS) containing heptamer, 12 basepair spacer, and nonamer regions
Cluster 2 has a short chromosomal rearrangement
To evaluate the location of the deletion in cluster 2 relative to clusters 3 and 4, we performed a series of sequence alignments of the clusters, the DH regions, and the intergenic regions (between DH regions). Indeed, the deletion in cluster 2 in relation to clusters 3 and 4 occurred at IGHD8–2, however the deletion was also associated with a larger chromosomal rearrangement. In this regard, IGHD5–2 in cluster 2 appears to have replaced the paralog for IGHD3–3 (cluster 3) and IGHD3–4 (cluster 4)(Fig. 1, Supplemental Figs. 2–3). The IGHD5 homologs are immediately 5′ of the IGHD6 family members in clusters 3 and 4, however IGHD5–2 is situated immediately 3′ of IGHD2–2 and immediately 5′ of the ultralong IGHD8–2 region in cluster 2 (Fig. 1). There is no IGHD3 family member in cluster 2 (Supplemental Fig. 3), with the paralog of IGHD3–3 and IGHD3–4 either deleted or fused to the adjacent DH region, which would be a paralog of IGHD7–3 (cluster 3) or IGHD7–4 (cluster 4). Global alignments of the clusters show deleted nucleotides at IGHD8–2 as well as the position occupied by family 5 genes in clusters 3 and 4 (e.g. between IGHD7 and IGHD6). Alignments of the intergenic regions show that the intergenic region corresponding to the sequence between IGHD3–3 and IGHD7–3 in cluster 3 (or IGHD3–4 and IGHD7–4 in cluster 4) is deleted in cluster 2 (Supplemental Fig. 4). While IGHD5–2 has been transposed to a location 3′ to IGHD8–2, the actual genetic material deleted clearly includes IGHD3 and its 3′ intergenic region. Thus, one possibility is that the ultralong IGHD8–2 region resulted from a deletion and associated fusion of the cluster 2 paralogs of IGHD3–3 and IGHD7–3. However, local sequence alignment reveals that the 5′ end of IGHD6–3 is 91.2% identical to IGHD8–2 (89.4% for IGHD6–2) over the first 85 nucleotides, whereas IGHD3–3 (and IGHD3–4) is only 80% identical over the first 62 residues (Supplemental Fig. 7). Of note, IGHD6 family sequences share a cysteine in the same position as the conserved cysteine in IGHD8–2, which is highly conserved in deep sequenced ultralong CDR H3 antibodies, and participates in a conserved disulfide bond at the base of the ultralong CDR H3 stalk [23, 26]. Thus, donation of an IGHD6 to the 5′ end of an IGHD7 through a recombinational or gene conversion process is a likely mechanism to produce IGHD8–2. Given the high sequence similarity of many of the DH regions and intergenic regions, we cannot definitively identify exact chromosomal breakpoints and cannot rule out that other events could have occurred in conjunction with the deletion event of the intragenic region between IGHD3 and IGHD7. For example, gene conversion could alternatively have occurred between IGHD6 and IGHD7 paralogs, or a deletion event followed by insertions of repeats into an IGHD7 paralog could have occurred. However, RSS analysis indicates that the 5′ RSS of IGHD8–2 shares identity with either IGHD3 or IGHD6 families (Table 1), thus a fusion between IGHD6 and IGHD7 or gene conversion of IGHD6 into IGHD3 followed by fusion to IGHD7 are likely mechanisms to production of the IGHD8–2 gene through a fusion event. The 3′ RSS of IGHD8–2 is identical to IGHD7 genes, and local alignments show homology between IGHD7 and IGHD8–2, suggesting that a primordial IGHD7 paralog from cluster 2 now forms the 3′ region of IGHD8–2.
Table 1.
Recombination signal sequences (RSS) of Bos Taurus IGHD regions from clusters 2–4
| 5′ RSS (heptamer spacer nonamer) |
IGHD | Cluster | 3′ RSS (heptamer spacer nonamer) |
|---|---|---|---|
| ggattttgagggtgtgcgtgtcaccctg | IGHD1–2 | 2 | cacagtgactcaggccctgacataaagt |
| ggattttgagggtgtgcgtgtcaccgtg | IGHD1–3 | 3 | cacagtgactcaggccctgacataaagt |
| ggattttgagggtgtgcgtgtcaccgtg | IGHD1–4 | 4 | cacagtgactcaggccctgacataaagt |
| gctttttgccaagggctctactgcggtg | IGHD2–2 | 2 | cacagtgagacatggggcagcaaaccct |
| gctttttgccaagggctctactgcggtg | IGHD2–3 | 3 | cacagtgagacatggggcagcaaaccct |
| gctttttgccaagggctctactgcggtg | IGHD2–4 | 4 | cacagtgagacatggggcagcaaaccct |
| ggtttctgatgccggctgtgtcacggtg | IGHD3–3 | 3 | cacagtgacactgtccaggacagaaacc |
| ggtttctgatgccggctgtgtcacggtg | IGHD3–4 | 4 | cacagtgacactgtccaggacagaaacc |
| ggtttttgatgccggctgtgtcacggtg | IGHD7–3 | 3 | cacagtgatactctctgggacaaaaacc |
| ggtttttgatgccggctgtgtcacggtg | IGHD7–4 | 4 | cacagtgatactctctgggacaaaaacc |
| ggtttctgatgccggctgtgtcacggtg | IGHD8–2 | 2 | cacagtgatactctctgggacaaaaacc |
| ggtttctgatgccggctgtgttgtggtg | IGHD5–2 | 2 | cacagtgatgctctcagtgtcagaaacc |
| ggtttctgatgccggctgtgttgtggtg | IGHD5–3 | 3 | cacagtgacgctctcagtgtcagaaacc |
| ggtttctgatgccggctgtgttgtggtg | IGHD5–4 | 4 | cacagtgacgctctcagtgtcagaaacc |
| ggtttctgatgccggctgtgtcacggtg | IGHD6–2 | 2 | cacagtgacactctctgggacaaaaacc |
| ggtttctgatgccagctgtgtcacggtg | IGHD6–3 | 3 | cacagtgacactctctgggacaaaaacc |
| ggtttctgatgccagctgtgtcacggtg | IGHD6–4 | 4 | cacagtgacactctctgggacaaaaacc |
DH genes have expanded repeats
Bovine IGHD regions are comprised of multiple repeating short sequence motifs, with the major differences between several DH regions being length differences due to variable numbers of nucleotide repeats (Fig. 2). IGHD7–4 is the second longest DH region, and only differs from IGHD7–3 (its paralog in cluster 3) by one repeat of TGGTTA, which results in a two amino acid insertion. IGHD7–3, IGHD7–4 and IGHD8–2 (the ultralong DH region) are very similar in having several repeating units, but with IGHD8–2 being dramatically longer. The 3′ ends of IGHD7–3 and IGHD7–4 are 85.6 and 77.4% identical to IGHD8–2 over the last 96 nucleotides, respectively (Supplementary Fig. 5). The longer DH regions appear to be evolutionarily active in length evolution based on expanding or contracting repeats, as polymorphisms in Bos taurus IGHD8–2 differ in repeat lengths (Fig. 2, Supplemental Fig. 6). In this regard, two IGHD8–2 polymorphisms have been reported that differ in length and cysteine position, but share similar repeating nucleotide and amino acid sequences [29, 30, 34]. Related species like Bos grunniens (domestic Yak) and Bison bison (American buffalo) also have ultralong CDR H3 regions encoded by IGHD8–2 orthologs, but differ in their lengths due to apparent differences in hexanucleotide repeat expansion within the coding regions (Fig. 2, Supplemental Fig. 6). Thus, while two DH genes may have fused to form the long IGHD8–2 gene, nucleotide repeat expansion or contraction appears to also play a role in long DH region evolution in these species.
Fig. 2.
Bovine DH regions are characterized by repetitive sequences. a Nucleotide sequences of bovine DH regions in clusters 2–4. The RSS are in lowercase letters with the heptamer and nonamers in italics and underlined. The coding region of each DH is uppercase. Repeated sequences are colored red, blue and green. b Ungulate ultralong DH regions have different repeat lengths. The nucleotide sequences of polymorphic variants IGHD8–2*01 and IGHD8–2*02 for Bos taurus are compared to domestic yak (Bos grunniens; BosGru) and American bison (Bison bison; bison) orthologs of IGHD8–2
Discussion
The antibody repertoire is a defining evolutionary feature of vertebrates. V(D)J recombination and its associated junctional diversity account for vast potential diversity in antibody receptors on naïve B-cells, with an ability to bind with low affinity to most antigens. Most species utilize many V, D, and J gene segments which produce a great combinatorial potential at the heavy and light chain loci. Some species, however, have fewer functional V, D, and J segments and may use additional mechanisms to add diversity to their repertoires. Cows, in particular, have only 10–12 functional VH regions in only one VH1 family [14, 17, 29], and 16 functional DH regions [29, 35], but have very long CDR H3 regions. The homologous DH regions are cysteine rich, and diversity can be generated through both germline and somatically generated cysteines, which can form a diverse array of potential disulfide bonded loops [23, 35, 36]. In the knob region of ultralong CDR H3s, a diversity of disulfide bond patterns has been observed in several crystal structures [23, 25, 26], and somatic mutations to and from cysteine have been confirmed through deep sequence analysis. Thus, novel cysteines encoded in the DH regions contributes to structural diversity in bovine antibodies.
The length of the DH regions in cows contributes to the overall increase in CDR H3 lengths in the antibody heavy chain repertoire. At the extreme, IGHD8–2 encodes 49 or 51 amino acids, depending on the polymorphic variant [29, 30, 34], and enables CDR H3 lengths of up to 70 amino acids. These CDR H3 regions form independently folding mini-domains comprised of a β-ribbon “stalk” and a disulfide-bonded “knob” that project far from the antigen surface. The sequence diversity of heavy chains with ultralong CDR H3 regions is enormous [16, 23], despite the fact that only one IGHD8–2 region (albeit with two polymorphic variants) is used in this entire class of antibodies. This vast diversity is explained by the fact that cattle utilize AID mediated somatic hypermutation in the pre-immune repertoire, as opposed to after antigen exposure as in other species, and this robust mutation induction substantially diversifies the repertoire through amino acid changes, cysteine mutations to alter disulfide loops, and a substantial proportion of deletional events which can also impact loop structures [16, 21]. All of these diversifying events use the germline IGHD regions as a template during repertoire formation. The IGHD templates feature multiple AID SH “hotspots” as well as nucleotide repeats that preferentially encode Ser, Gly, Tyr, and Cys, often in several repeating units like Gly-Tyr-Gly or Gly-Tyr-Ser. These amino acid repeats could be the result of nucleotide repeat expansion in the genome. Here we show that nucleotide repeating units differ between IGHD paralogs derived from different clusters, and that the unusual ultralong IGHD8–2 region likely formed from a DH-DH fusion in cluster 2 of primordial IGHD6 (or IGHD3) to IGHD7 family members. Clearly a substantial deletion event occurred in the region now encoding IGHD8–2, which can be explained by deletion of the 3′ end of primordial IGHD3, the intergenic region between IGHD3 and IGHD7, and the 5′ end of IGHD7. However, this event was also associated with a more substantial rearrangement that additionally replaced the IGHD3 paralog with IGHD5. Given that homologous variants of IGHD8–2 within Bos taurus, as well as in Bos grunniens and Bison bison differ in the length of IGHD8–2 through differences in the number of short repeats, it is likely that repeat expansion played a role in IGHD8–2 evolution either with a genetic fusion, or potentially with massive expansion in the absence of the fusion of two DH regions.
Conclusions
To summarize, our analysis indicates that the most likely origin of IGHD8–2 is through a fusion event comprising the 5′ end of IGHD6 with the 3′ end of IGHD7 based on sequence similarity analysis, as well as the preservation of the codon encoding the nearly completely conserved first cysteine of the knob domain of ultralong CDR H3 antibodies encoded by IGHD8–2. This event, however, was associated with a larger chromosomal rearrangement that replaced an IGHD3 paralog and its 3′ intergenic region in cluster 2 with IGHD5–2 (Fig. 3). The ability of the genome to diversify IGHD region lengths through genomic rearrangement and repeat expansion through evolutionary time provides a novel genetic mechanism for Darwinian diversification of the vertebrate immune system.
Fig. 3.
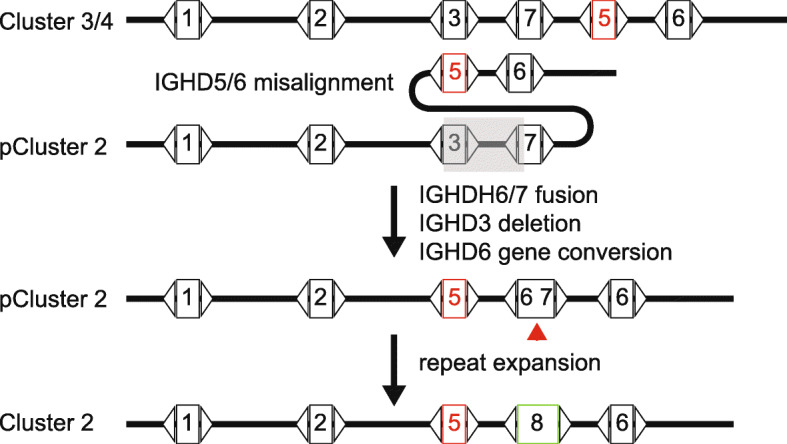
Model of deletion, fusion, and repeat expansion to form the ultralong DH region in cluster 2. Homologous sequences in clusters could misalign where IGHD5 and 6 pair with IGHD3 and 7 during replication processes. IGHD3 and its 3′ intergenic sequence is deleted (transparent grey rectangle). The short nucleotide repeats found in IGHD6 and 7 could cause mispairing and fusion, creating a fused DH-DH region of IGHD6 and IGHD7. A gene conversion or duplication event of IGHD6 would be required under this scenario. Continued repeat expansion produces IGHD8–2 homologs (red arrow). “pCluster” denotes a hypothetical precursor cluster in evolution
Methods
The DNA sequence encoding the bovine antibody heavy chain locus (accession no. KT723008) [29] was downloaded from the IMGT server (http://www.imgt.org/). Clusters 2, 3 and 4 were defined by the beginning of the Family 1 gene RSS to the end of the Family 6 gene RSS. Sequences of IGHD regions, intergenic regions, and clusters were derived using the Bioconductor program using the R statistical program language [37]. Multiple sequence alignments were done using Clustal Omega, WebPrank, or Muscle (https://www.ebi.ac.uk/services). Local sequence alignments were done using Matcher (https://www.ebi.ac.uk/Tools/psa/). The Bison bison and Bos grunniens IGHD8–2 sequences were identified by BLAST search at the ensembl genome server (www.ensembl.org) using the Bos taurus IGHD8–2 gene as query. Bison bison and Bos grunniens IGHD8–2 genes were found within the genomic sequences with accession numbers XM_010833706.1 (Bison) and CM016710.1 (Yak).
Supplementary information
Additional file 1: Figure S1. Alignment of Bos taurus DH regions.
Additional file 2: Figure S2. Alignment of DH clusters 2, 3, and 4.
Additional file 3: Figure S3. Phylogenetic of DH paralogs from clusters 2 and 3.
Additional file 4: Figure S4. Phylogenetic analysis of intergenic regions from clusters 2 and 3.
Additional file 5: Figure S5. Alignment of IGHD7 family members with IGHD8–2.
Additional file 6: Figure S6. Identification ultralong IGHD region homologs in Bos taurus, Bos grunniens and Bison bison.
Additional file 7: Figure S7. Alignment of IGHD3 and IGHD6 family members with IGHD8–2.
Additional file 8: Figure S8. Fusion analysis of IGHD members to form IGHD8–2 homologs.
Acknowledgements
We are grateful for helpful conversations regarding this work with Ali Torkamani and Michael Criscitiello.
Abbreviations
- CDR
complementarity determining region
- DH
heavy chain diversity region
- VH
heavy chain variable region
- RSS
recombination signal sequence
Authors’ contributions
Both authors performed bioinformatic analysis and made the Figures. V.V.S. wrote the manuscript with input from B.A.S. All authors have read and approved the manuscript.
Funding
This work was funded by NIH grants R01 GM105826 and R01 HD088400 to V.V.S and funded salary support and supplies.
Availability of data and materials
Not applicable.
Ethics approval
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12865-020-00359-8.
References
- 1.Fugmann SD, Lee AI, Shockett PE, Villey IJ, Schatz DG. The RAG proteins and V(D)J recombination: complexes, ends, and transposition. Annu Rev Immunol. 2000;18:495–527. doi: 10.1146/annurev.immunol.18.1.495. [DOI] [PubMed] [Google Scholar]
- 2.Smider V, Chu G. The end-joining reaction in V(D)J recombination. Semin Immunol. 1997;9(3):189–197. doi: 10.1006/smim.1997.0070. [DOI] [PubMed] [Google Scholar]
- 3.Tonegawa S. Somatic generation of antibody diversity. Nature. 1983;302:575–581. doi: 10.1038/302575a0. [DOI] [PubMed] [Google Scholar]
- 4.Burton DR, Hangartner L. Broadly neutralizing antibodies to HIV and their role in vaccine design. Annu Rev Immunol. 2016;34(1):635–659. doi: 10.1146/annurev-immunol-041015-055515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burton DR, Poignard P, Stanfield RL, Wilson IA. Broadly neutralizing antibodies present new prospects to counter highly antigenically diverse viruses. Science. 2012;337(6091):183–186. doi: 10.1126/science.1225416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kwong PD, Wilson IA. HIV-1 and influenza antibodies: seeing antigens in new ways. Nat Immunol. 2009;10(6):573–578. doi: 10.1038/ni.1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Puligedda RD, Kouiavskaia D, Al-Saleem FH, Kattala CD, Nabi U, Yaqoob H, Bhagavathula VS, Sharma R, Chumakov K, Dessain SK. Characterization of human monoclonal antibodies that neutralize multiple poliovirus serotypes. Vaccine. 2017;35(41):5455–5462. doi: 10.1016/j.vaccine.2017.03.038. [DOI] [PubMed] [Google Scholar]
- 8.Stanfield R, Wilson I. Antibody structure. Microbiolspec. 2014;2(2). 10.1128/microbiolspec.AID-0012-201310.1186/s12865-020-00359-8.
- 9.Douthwaite JA, Sridharan S, Huntington C, Hammersley J, Marwood R, Hakulinen JK, Ek M, Sjogren T, Rider D, Privezentzev C, et al. Affinity maturation of a novel antagonistic human monoclonal antibody with a long VH CDR3 targeting the class A GPCR formyl-peptide receptor 1. MAbs. 2015;7(1):152–166. doi: 10.4161/19420862.2014.985158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nam DH, Rodriguez C, Remacle AG, Strongin AY, Ge X. Active-site MMP-selective antibody inhibitors discovered from convex paratope synthetic libraries. Proc Natl Acad Sci U S A. 2016;113(52):14970–14975. doi: 10.1073/pnas.1609375114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bonsignori M, Hwang KK, Chen X, Tsao CY, Morris L, Gray E, Marshall DJ, Crump JA, Kapiga SH, Sam NE, et al. Analysis of a clonal lineage of HIV-1 envelope V2/V3 conformational epitope-specific broadly neutralizing antibodies and their inferred unmutated common ancestors. J Virol. 2011;85(19):9998–10009. doi: 10.1128/JVI.05045-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doria-Rose NA, Schramm CA, Gorman J, Moore PL, Bhiman JN, DeKosky BJ, Ernandes MJ, Georgiev IS, Kim HJ, Pancera M, et al. Developmental pathway for potent V1V2-directed HIV-neutralizing antibodies. Nature. 2014;509(7498):55–62. doi: 10.1038/nature13036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walker LM, Huber M, Doores KJ, Falkowska E, Pejchal R, Julien JP, Wang SK, Ramos A, Chan-Hui PY, Moyle M, et al. Broad neutralization coverage of HIV by multiple highly potent antibodies. Nature. 2011;477(7365):466–470. doi: 10.1038/nature10373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berens SJ, Wylie DE, Lopez OJ. Use of a single VH family and long CDR3s in the variable region of cattle Ig heavy chains. Int Immunol. 1997;9(1):189–199. doi: 10.1093/intimm/9.1.189. [DOI] [PubMed] [Google Scholar]
- 15.de los Rios M, Criscitiello MF, Smider VV. Structural and genetic diversity in antibody repertoires from diverse species. Curr Opin Struct Biol. 2015;33:27–41. doi: 10.1016/j.sbi.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deiss TC, Vadnais M, Wang F, Chen PL, Torkamani A, Mwangi W, Lefranc M-P, Criscitiello MF, Smider VV. Immunogenetic factors driving formation of ultralong VH CDR3 in Bos taurus antibodies. Cell Mol Immunol. 2017;14:1–12. doi: 10.1038/cmi.2017.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lopez O, Perez C, Wylie D. A single VH family and long CDR3s are the targets for hypermutation in bovine immunoglobulin heavy chains. Immunol Rev. 1998;162:55–66. doi: 10.1111/j.1600-065x.1998.tb01429.x. [DOI] [PubMed] [Google Scholar]
- 18.Saini SS, Allore B, Jacobs RM, Kaushik A. Exceptionally long CDR3H region with multiple cysteine residues in functional bovine IgM antibodies. Eur J Immunol. 1999;29(8):2420–2426. doi: 10.1002/(SICI)1521-4141(199908)29:08<2420::AID-IMMU2420>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 19.Saini SS, Farrugia W, Ramsland PA, Kaushik AK. Bovine IgM antibodies with exceptionally long complementarity-determining region 3 of the heavy chain share unique structural properties conferring restricted VH + Vlambda pairings. Int Immunol. 2003;15(7):845–853. doi: 10.1093/intimm/dxg083. [DOI] [PubMed] [Google Scholar]
- 20.Saini SS, Kaushik A. Extensive CDR3H length heterogeneity exists in bovine foetal VDJ rearrangements. Scand J Immunol. 2002;55(2):140–148. doi: 10.1046/j.1365-3083.2002.01028.x. [DOI] [PubMed] [Google Scholar]
- 21.Stanfield RL, Haakenson J, Deiss TC, Criscitiello MF, Wilson IA, Smider VV. The unusual genetics and biochemistry of bovine Immunoglobulins. Adv Immunol. 2018;137:135–164. doi: 10.1016/bs.ai.2017.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walther S, Czerny C-P, Diesterbeck US. Exceptionally long CDR3H are not isotype restricted in bovine immunoglobulins. PLoS One. 2013;8(5):e64234. doi: 10.1371/journal.pone.0064234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang F, Ekiert DC, Ahmad I, Yu W, Zhang Y, Bazirgan O, Torkamani A, Raudsepp T, Mwangi W, Criscitiello MF, et al. Reshaping antibody diversity. Cell. 2013;153(6):1379–1393. doi: 10.1016/j.cell.2013.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao Y, Jackson SM, Aitken R. The bovine antibody repertoire. Dev Comp Immunol. 2006;30(1–2):175–186. doi: 10.1016/j.dci.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 25.Dong J, Finn JA, Larsen PA, Smith TPL, Crowe JE., Jr Structural diversity of Ultralong CDRH3s in seven bovine antibody heavy chains. Front Immunol. 2019;10:558. doi: 10.3389/fimmu.2019.00558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stanfield RL, Wilson IA, Smider VV. Conservation and diversity in the ultralong third heavy-chain complementarity-determining region of bovine antibodies. Sci Immunol. 2016;1(1):aaf7962. doi: 10.1126/sciimmunol.aaf7962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sok D, Le KM, Vadnais M, Saye-Francisco KL, Jardine JG, Torres JL, Berndsen ZT, Kong L, Stanfield R, Ruiz J, et al. Rapid elicitation of broadly neutralizing antibodies to HIV by immunization in cows. Nature. 2017;548(7665):108–111. doi: 10.1038/nature23301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koti M, Kataeva G, Kaushik A. Organization of DH-gene locus is distinct in cattle. Dev Biol. 2008;132:307–313. doi: 10.1159/000317176. [DOI] [PubMed] [Google Scholar]
- 29.Ma L, Qin T, Chu D, Cheng X, Wang J, Wang X, Wang P, Han H, Ren L, Aitken R, et al. Internal duplications of DH, JH, and C region genes create an unusual IgH gene locus in cattle. J Immunol. 2016;196(10):4358–4366. doi: 10.4049/jimmunol.1600158. [DOI] [PubMed] [Google Scholar]
- 30.Shojaei F, Saini SS, Kaushik AK. Unusually long germline DH genes contribute to large sized CDR3H in bovine antibodies. Mol Immunol. 2003;40(1):61–67. doi: 10.1016/s0161-5890(03)00098-1. [DOI] [PubMed] [Google Scholar]
- 31.Lefranc MP. Immunoglobulin and T cell receptor genes: IMGT((R)) and the birth and rise of Immunoinformatics. Front Immunol. 2014;5:22. doi: 10.3389/fimmu.2014.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lefranc MP, Pommie C, Ruiz M, Giudicelli V, Foulquier E, Truong L, Thouvenin-Contet V, Lefranc G. IMGT unique numbering for immunoglobulin and T cell receptor variable domains and Ig superfamily V-like domains. Dev Comp Immunol. 2003;27(1):55–77. doi: 10.1016/s0145-305x(02)00039-3. [DOI] [PubMed] [Google Scholar]
- 33.Lefranc M-P, Lefranc G. The immunoglobulin FactsBook. London: Academic Press; 2001. [Google Scholar]
- 34.Liljavirta J, Niku M, Pessa-Morikawa T, Ekman A, Iivanainen A. Expansion of the preimmune antibody repertoire by junctional diversity in Bos taurus. PLoS One. 2014;9(6):e99808. doi: 10.1371/journal.pone.0099808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haakenson JK, Deiss TC, Warner GF, Mwangi W, Criscitiello MF, Smider VV. A broad role for Cysteines in bovine antibody diversity. Immunohorizons. 2019;3(10):478–487. doi: 10.4049/immunohorizons.1900058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haakenson JK, Huang R, Smider VV. Diversity in the cow Ultralong CDR H3 antibody repertoire. Front Immunol. 2018;9:1262. doi: 10.3389/fimmu.2018.01262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gentleman RC, Carey VJ, Bates DM, Bolstad B, Dettling M, Dudoit S, Ellis B, Gautier L, Ge Y, Gentry J, et al. Bioconductor: open software development for computational biology and bioinformatics. Genome Biol. 2004;5(10):R80. doi: 10.1186/gb-2004-5-10-r80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure S1. Alignment of Bos taurus DH regions.
Additional file 2: Figure S2. Alignment of DH clusters 2, 3, and 4.
Additional file 3: Figure S3. Phylogenetic of DH paralogs from clusters 2 and 3.
Additional file 4: Figure S4. Phylogenetic analysis of intergenic regions from clusters 2 and 3.
Additional file 5: Figure S5. Alignment of IGHD7 family members with IGHD8–2.
Additional file 6: Figure S6. Identification ultralong IGHD region homologs in Bos taurus, Bos grunniens and Bison bison.
Additional file 7: Figure S7. Alignment of IGHD3 and IGHD6 family members with IGHD8–2.
Additional file 8: Figure S8. Fusion analysis of IGHD members to form IGHD8–2 homologs.
Data Availability Statement
Not applicable.