Abstract
Purpose:
To review various treatment methods in thyroid eye disease (TED) related strabismus.
Methods:
We searched in PubMed and Google Scholar and Ovid MEDLINE for keywords including TED-related strabismus, strabismus in thyroid-associated ophthalmopathy, Graves' ophthalmopathy related strabismus or squint, and restrictive strabismus. Two expert strabismus specialists selected and evaluated the English articles that were related to our paper and had been published since 2000. Some articles were added based upon the references of the initial articles.
Results:
One hundred fifteen articles were found, 98 of which were mostly related to the topic of this review. Management of TED-related strabismus was reviewed and categorized in non-surgical and surgical. Botulinum toxin A (BTA) is a useful non-surgical management of strabismus in an active TED and residual deviation after strabismus surgery. Postoperative under-correction is relatively more common in TED-related esotropia. Lateral rectus resection and BTA are the options to manage the problem. Muscle rectus muscle resection should be performed after maximum recession of restricted muscles. It should be avoided on a restricted or enlarged muscle. Management of TED-related vertical deviation is challenging. In these cases, the surgical treatment selected depends on forced duction test (FDT) (pre and intraoperative), orbital imaging (which muscle is enlarged), and the amount of vertical deviation (in both down-gaze and primary position).
Conclusions:
TED-related strabismus needs careful evaluation and management to achieve optimal outcome. Different surgical and non-surgical options are available for intervention in TED-related strabismus.
Keywords: Graves' ophthalmopathy, Strabismus, Thyroid eye disease
INTRODUCTION
The thyroid eye disease (TED) is an autoimmune disorder with the presence of autoantibodies. The TED can be diagnosed when two of the following three signs are present: clinical orbital signs, laboratory tests, and typical orbital imaging finding. The typical clinical orbital signs include eyelid retraction, proptosis, compressive optic neuropathy signs, eyelid erythema, chemosis, caruncular edema, and restrictive strabismus. The laboratory tests include thyroid-stimulating hormone-receptor (TSH-R) antibody, thyroid-stimulating immunoglobulin (TSH), thyroid-binding inhibitory immunoglobulin (TBII), and anti-microsomal antibody. The typical feature in orbital imaging is enlargement of extraocular muscles with sparing of tendons at the insertion of the rectus muscles. The majority of TED patients have hyperthyroidism (90%), 6% of the patients are euthyroid, 1% have hypothyroidism, and 3% are affected by Hashimoto's thyroiditis at the time of diagnosis.1
Rundle has shown that the natural course of TED is biphasic. The initial phase (is called active or inflammatory phase) has rapid progression and reaches its peak after 6–18 months, followed by a stable phase (also called the inactive or fibrotic phase) in which a patient's symptoms regress often incompletely.2 Activity of the disease can be measured by clinical activity score (CAS).3 TED severity is assessed according to NOSPECS (no sign or symptom, only sign, soft tissue involvement, extraocular muscle involvement, corneal involvement, sight loss) score.3 Orbital imaging, particularly magnetic resonance imaging (MRI), may be useful in differentiating active thyroid-related ophthalmopathy. The increased signal intensity of muscles on T2-weighted imaging points out increased water content of the muscles that can be indicative of active inflammation.4 Short-tau inversion recovery (STIR) sequences are also useful to detect an active inflammatory process in muscles.4 The activity of disease has a high predictive value for the response to immunosuppressive treatment in TED.3 The patient with active moderate to severe TED may benefit from anti-inflammatory agents. Systemic glucocorticosteroids, rituximab, and tocilizumab have been shown to be effective in improving ocular motility and diplopia in these patients.5,6,7
Strabismus may occur in 15% of all patients with TED.8 TED most commonly involves the inferior rectus (IR) muscle, followed by the medial rectus (MR) and superior rectus (SR) muscles.9 Lateral rectus and oblique muscles are rarely involved in TED. The strabismus that can result in diplopia interferes with the patient's ability to work, read, drive, and other daily functions.10 Strabismus and diplopia management remains challenging in TED patients. In this article, the management of TED patients with strabismus is reviewed.
METHODS
We searched in PubMed and Google Scholar and Ovid MEDLINE for the following keywords: TED-related strabismus, strabismus in thyroid-associated ophthalmopathy, Grave's ophthalmopathy related strabismus or squint and restrictive strabismus. English articles published since 2000 were selected. Two expert strabismus specialists evaluated the retrieved articles. Some articles were added based upon the references of the initial articles.
RESULTS
We found 115 English articles, 98 of which were mostly related to this review. The retrieved articles had 48 surgical papers and 42 non-surgical. Eight of them had both categories. The articles included the following types of studies: 4 randomized clinical trials, 2 guidelines, 13 review articles, 19 prospective case series, 51 retrospective case series, and 9 case reports.
Management of strabismus in TED patients can be classified into two categories: non-surgical and surgical.
Non-surgical management
Diplopia, a common symptom of the strabismus, can interfere with the daily activities of the patient. Many of the patients cannot tolerate diplopia and insist on early correction of strabismus. Surgical intervention during the active phase of TED results in high reoperation (50%) and complication rates.11,12 The strabismus surgery should be postponed until inactivation of the disease and prove stability of the angle of deviation for at least 4–6 months.13,14 Orbital decompression should be done prior to strabismus surgery.15 Non-surgical management can eliminate or reduce the bothersome diplopia in some patients during active phase of the disease. These managements include prism, botulinum toxin injection, and the methods that prevent simultaneous use of two eyes (e.g., monocular occlusion).
Prism
Prism can be able to restore single binocular vision in primary or reading position in some strabismus patients. It can be used as a temporizing measure while waiting for the angle of deviation to stabilize. It can also be employed to eliminate small diplopia after surgery and for the patient who cannot undergo surgical intervention. Prism can be used for vertical and horizontal diplopia. Vertical prism may reduce the torticollis associated with restrictive vertical strabismus. Although the prism itself cannot decrease torsional diplopia, its effect on the vertical and horizontal diplopia may result in controllable torsional diplopia.16,17 TED patients usually have an incomitant deviation in primary position and reading position, so the prisms may not be able to eliminate their diplopia in both positions. However, in some TED patients with small and nearly the same angle of deviation in primary and reading position, the prism can be useful. Some TED patients may not accept the split of prism in two lenses; therefore, the prism could be put in front of the more hypotropic or restricted eye.16 Prisms can be used in two methods: ground-in prism and press-on prism (Fresnel). Ground-in prism is typically useful to correct less than 10 prisms diopter (PD) of vertical and 16 PD of horizontal diplopia.16 The press-on prism is more acceptable than ground-in prism. It can correct more deviation and is lightweight. Fresnel prism can be applied on the near or far segment of bifocal glasses. Moreover, the amount of prism needs to change because of instability in the angle of deviation in the acute phase of the disease, so the ground-in prism may be difficult and expensive in this situation. On the other hand, a large number of press-on prisms may decrease visual acuity and increase optical aberrations and light scatter.18,19
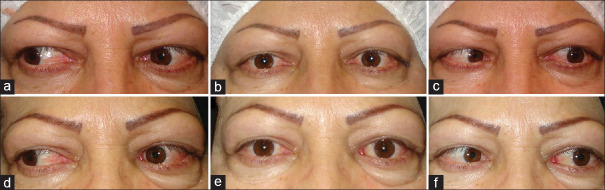
Figure 1 shows a 49-year-old woman presented with diplopia and 30 PD of esotropia one month after orbital wall decompression. Base-out Fresnel prism (12 PD) was placed in front of both eyes and her diplopia was eliminated in primary position. She underwent strabismus surgery after four months.
Figure 1.
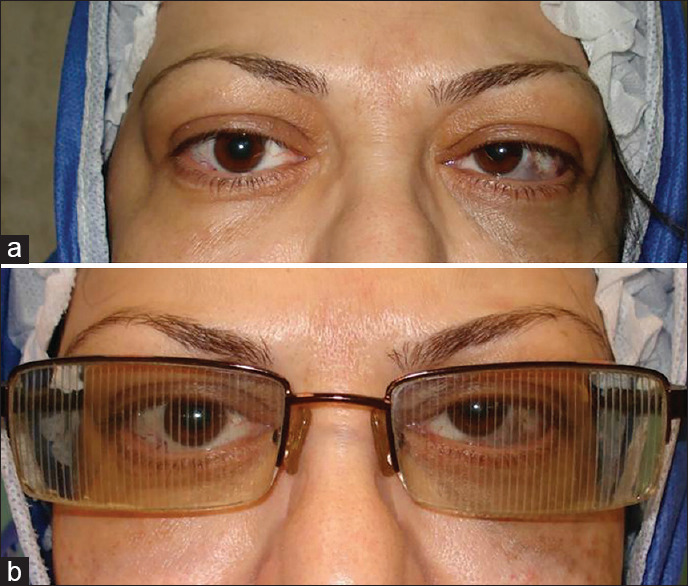
A patient with 30-prism diopter of esotropia and diplopia in the first month after orbital decompression (a). Twelve prism diopter (PD) of base-out Fresnel prism was placed in front of two eyes, and diplopia was tolerable for the patient until surgery time (b)
Botulinum toxin
Botulinum toxin A (BTA) injection in the extraocular muscles has been suggested to provide temporary relief of diplopia in selected patients during the active phase of TED.20 BTA can reduce muscle contraction and diminish strabismus in early phases of the disease. The toxin decreases limitation of ocular movements and reduces the amount of muscle recession that will be needed.20,21,22 A study reported that BTA injection had a secondary effect to decrease intraocular pressure (IOP) in patients with TED-related strabismus.23 Moreover, BTA is useful to correct small over-corrections or under-correction after strabismus surgery.24
Although some studies suggested the use of BTA within the first year of diplopia onset, several authors successfully applied the toxin for patients with chronic TED-related strabismus [Table 1].20,25,29 It is especially helpful for a patient who has systemic risk factors for strabismus surgery.26
Table 1.
Botulinum toxin A in thyroid eye disease-related strabismus
| Study | Sample size (n) | Follow-up | Preoperative deviation | Duration of TED before injection | BTA dose and mean injection per patient | Outcomes |
|---|---|---|---|---|---|---|
| Dunn et al.20 Retrospective |
8 | Average 9.8 months | Average 19.5 | Average 5.4 (1-8 months) One case 2 years |
1.25-5 unit 2.1 injection/patient |
Average deviation at last follow-up 2.25 PD (range, 0-6 PD) |
| Akbari et al.25 Prospective interventional case series |
20 | 26.8±2.8 months | - | Up to 3 years | 25 units (Dysport) 3.2±1.5 injection/patient |
Success rate 55% |
| Gair et al.26 Retrospective | 65 | 1-8 years | Hypotrpia 23 (3-60) ET 32 (4-65) |
0.5-30 years | Dysport (6.5 pg/0.1 ml) 1-11 injection/patient | 4.5% were maintained on repeated injection 78.5% poor response 4.5% declined further BTA Short duration and mild degree of strabismus have a chance of long-term benefit |
| Wu et al.27 Retrospective |
33 | 17.0±12.77 months | Horizontal 35±20.3 Vertical 33±17.27 |
- | 8.16±1.43 units 6.48±2 injection/patient |
15 cases were cured, 12 cases were partially improved, 6 cases had poor response |
| Granet et al.28 Retrospective |
22 | Up to 12 months | - | - | 5-15 units (Botox) | 59% patients benefited from BTA (success rate 37%, partial success 22%) |
PD: Prism diopter, TED: Thyroid eye disease, BTA: Botulinum toxin A, ET: Esotropia
The onset of BTA effect begins within 2–4 days after injection and lasts for 3–4 months.21,30 Several studies reported the average duration of the BTA effect was shorter than 4 months, and the mean change of deviation per injection is smaller in the patient with TED-related strabismus than others.22,27 The BTA has a longer effect in the patient with less severity and recent onset of the disease.26
Granet et al.28 reported one-third of patients with TED-related strabismus who underwent BTA injection did not need further surgical intervention. In addition, the angle of deviation reduced in 27% of patients before surgery. They suggested the BTA injection as an effective non-surgical intervention to prevent surgery in the patients with 20 PD or less deviation.
In our previous study, BTA injection was successful in 55% of patients (11 out of 25 cases).25 The toxin injection had better outcomes in the cases with esotropia, the lower initial degree of deviation, and the small magnitude of excyclotorsion.25
Figure 2 shows a 50-year-old woman who had diplopia and 20 PD of esotropia after orbital decompression. She underwent injection of BTA with a dose of 25 units in 0.1 cc (Dysport; Ipsen Biopharm Ltd., Wrexham, UK) in bilateral MR muscles. She was orthotropic and free of diplopia one year later.
Figure 2.
A patient with 20-prism diopter left esotropia at the early postoperative of orbital decompression (a-c). After botulinum toxin injection in bilateral medial rectus muscles, she was orthotropic in primary position (d-f)
The serious side effects of the BTA are uncommon. Eyelid ptosis is the most common known side effect.31 It rarely occurs in an IR injection (<1%).32 If the patient is asked to sit-up immediately after the injection, the incidence of ptosis may be decreased.28 On the other hand, the ptosis may be a temporizing treatment for corneal exposure and eyelid retraction in the TED patient.33,34
Other disadvantages of BTA injection include globe trauma, muscle damage, optic neuropathy, and the need for multiple injections.35,36 Elston et al.37 concluded that a larger dose for toxin injection is correlated with more local side effects. A study suggested the usual dose of the toxin (Dysport with a dose of 62.5 Pg or 10 units in 0.1 mL) is sufficient and results in fewer side effects in TED patients.26 In our practice, we use a larger dose of BTA (Dysport with a dose of 25 units in 0.1 cc) for TED-related-strabismus than its standard dose.
Monocular occlusion
Monocular occlusion can be another option when prism cannot correct diplopia. Several options are available for monocular occlusion including tape, foils, and contact paper.16
Surgical management
Approximately 0.6–20% of patients with TED finally require strabismus surgery.38,39,40 Although strabismus surgery can safely restore binocular single vision (BSV), achieving an optimal outcome remains challenging in these patients, particularly in cases with vertical deviation.41,42 The success rate of strabismus surgery in TED patients ranges from 43% to 100%, and reoperation rate is between 17% and 45%.43,44,45
The following points can be useful in the surgical treatment of patients with TED-related strabismus.
Before any surgery, the thyroid dysfunction should be controlled in these patients
Strabismus surgery in the active phase of TED results in significant instability in surgical outcomes.11,12 The surgeons often postpone the surgery to stabilize the strabismus for 4–6 months, though it cannot give a guarantee for the stability of the deviation after strabismus surgery. A study reported a significant change in the angle of deviation in 30% of patients who had the stable strabismus for at least 6 months46
Orbital decompression can change the initial deviation or result in new strabismus13,47,48 Strabismus surgery should be performed after orbital decompression.15 The exact role of orbital decompression is not clear in the outcomes of strabismus surgery. Some studies reported the history of previous orbital decompression associated with less favorable outcomes,49 though several studies did not demonstrate this50,51
The patient should be informed that several strabismus surgeries require restoring the BSV in the primary and reading position. The BSV may not be achieved in all gazes. Initial proptosis may increase after strabismus surgery52
The primary, secondary, and tertiary function of each muscle should be considered (e.g., the IR muscle involvement may result in hypotropia, esotropia, and excyclotropia). The surgeon should carefully assess ocular version, ocular duction, forced duction test (FDT) (preoperatively and intraoperative),53,54 and sensorimotor tests (double Maddox rod, Hess screen, and Lancaster test). Orbital imaging such as computed tomography (CT) scan and MRI can disclose which extraocular muscle is involved55
The main surgical procedure is the weakening of restricted muscles. In TED, the degree of inflammation and consequent fibrosis in involved muscles are highly variable. It has an influence on the final response to the recession procedure, so there is no general agreement for surgical dose-response in strabismus correction of the patients56
The conjunctiva of the patients is usually thin and tears easily so it should be handled carefully.
Lid surgery should be postponed to the last session because its position is often altered after orbital and strabismus surgery.
The patients may have vertical, horizontal, or mixed strabismus according to which muscle is involved.
Horizontal strabismus
The MR muscle is the second most frequently involved muscle (42–44%) in the TED.57,58 In addition to the aforementioned principles, some points should be considered in the patients with horizontal deviation.
In a small amount of esotropia, fibrotic and thickened MR muscle may resist the recession procedure, so undercorrection of the deviation is common in this setting.49,59 A study reported the rate of under-correction of 14.0% in 43 patients with TED-related esotropia who underwent MR recession.51
There is no standard surgical dose-response for MR muscle recession in TED-related esotropia. Lyu et al.51 documented the median surgical response of 2.58 PD/mm in the patients who underwent MR recession alone and 2.9 PD/mm in the patients who had additional vertical muscle recession [Table 2]. Jellema et al.60 reported the dose-response of 1.0°/mm and 1.4°/mm in unilateral and bilateral MR recession, respectively. They used the fixed suture technique in the surgery. Seventy-seven percent of the patients required one operation to correct horizontal diplopia, and 23% of the cases needed additional surgery.. In our unpublished study, dose-response was calculated by 3.62 ± 0.47 PD/mm for MR recession. In the study, we found surgical dose-response was significantly larger for patients with deviation ≥ 25 PD compared to patients with deviation <25 PD.
-
More than 6–7 mm MR recession may result in adduction deficiency. It can interfere with ability of reading and near work, even in the absence of ipsilateral antagonist muscle involvement. Although MR recession more than 7 mm in each eye would not be appropriate, this amount of recession is not enough to eliminate diplopia in some TED patients.54 A few options are available for the patients with residual esotropia after the maximum MR recession.
- The rectus muscle resection is generally avoided in TED due to aggravation of inflammation, overcorrection, and new-onset deviation. Several studies reported successful outcomes with lateral rectus resection in the patients who had residual esotropia after MR recession.61,72 They had not overcorrection and additional complications. Kim et al.62 performed bilateral lateral rectus resection using non-adjustable sutures on nine TED patients who had residual esotropia after bilateral MR recession; the patients remained under-corrected with unresolved diplopia. Greninger et al.63 reported LR muscle resection in 12 cases of TED-relatedesotropia. Diplopia was resolved in all cases with no late over-correction.
-
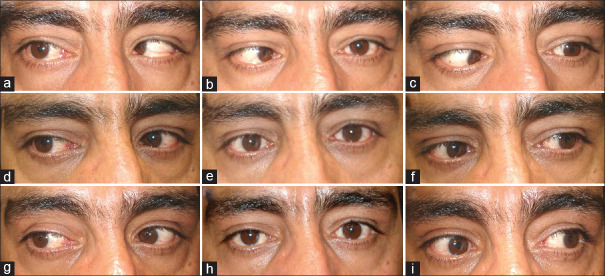
BTA injection in MR muscles is another option for residual esotropia after maximum MR muscle recession. Oeverhaus et al.73 used tendon elongation with bovine pericardium in 60 TED patients with severe esotropia after orbital decompression. They performed unilateral MR tendon elongation combined with contralateral MR recession (7 mm) in some patients and bilateral tendon elongation in others. They chose the procedures individually based on patient deviation and ocular motility. The angle of deviations within ±10 PD were reached by 59% (n = 35) at 3 months after surgery. An under-correction occurred in 22 patients (37%), significantly more frequently in cases with large deviation at baseline. Lateral rectus resection was performed in 12 (20%) of the patients. The success rate in the subgroups with esotropia ≤ 40 (after unilateral) and ≤ 50 PD (after bilateral tendon elongation) were 78% and 72%, respectively. In these subgroups for the cases with more severe esotropia, the success rate was 38% and 47%. The dose-response was 1.8 ± 0.6 PD/mm. They suggested the procedure is a safe option to correct esotropia up to 50 PD after orbital decompression. Large MR elongation may result in significant adduction limitation that may theoretically interfere with near work activity.Figure 3 shows a 34-year-old man who had diplopia and 80 PD of esotropia after bilateral orbital decompression. We performed 7 mm bilateral MR muscle recession, but he had residual esotropia (25 PD) three months after surgery. BTA was injected in bilateral MR muscles result in orthotropia at primary position. The patient had no diplopia or eye deviation in primary position in the last visit (1 year after surgery).
Restricted vertical muscles may reduce the response to MR recession in TED patiens because of adduction function of the muscles.51 On the other hand, vertical rectus muscle recession may influence on surgical dose-response of the MR recession. This topic is discussed in the section of vertical deviation.
Lateral rectus involvement is very rare in the TED patients, so in cases with exotropia, co-existing myasthenia gravis (MG) or prior orbital decompression must be ruled out. Three to 10% of patients with MG have Grave's disease,74 and up to 1% of patients with Grave's disease may develop MG.75 The presentation of ptosis and daily fluctuation of diplopia may be attributed to MG rather than TED.76 On the other hand, ptosis in TED patients could be due to compromised oculomotor nerve (compressive neuropathy caused by thyroid orbitopathy),77 idiopathic orbital inflammation,78 and other associated myopathies (including myotonic dystrophy, chronic progressive external ophthalmoplegia, and facioscapulohumeral muscular dystrophy).79 Ptosis has also been reported in a patient with levator muscle enlargement caused by TED-related inflammation and fibrosis.80
Table 2.
Strabismus surgery in thyroid eye disease (TED)
| Study | Sample size | Follow-up | Preoperative deviation | Muscle procedure | Outcomes and consideration |
|---|---|---|---|---|---|
| Dal Canto et al.50 Retrospective |
24 | Average 5.9 months | ET: 28 PD HT: 25 ET: 21, HT: 21 |
MR recession + IR recession IR recession ± IR recession SR recession IR recession + LR recession MR + IR + SR recession |
Excellent outcome (no diplopia without prism): 87.5% Reoperation rate: 8% Relaxed muscle positioning technique |
| Lyu et al.51 Retrospective |
43 | Median 14.2 months | Median 30 PD |
MR recession | Success rate: 86% Under-correction: 14% Adjustable sutures (n=35) |
| Jellema et al.60 Retrospective |
102 | 6-12 months | 8.7±4.9 degrees 18.1±7 degrees |
Unilateral MR recession BMR recession |
Success rate 77% Additional surgery 23% Surgical dose-response 1 degree/mm |
| Weldy and Kerr61 Retrospective |
11 | 2 months | 17.8 PD (8-30) | BLR resection Unilateral LR resection |
Success rate 91% Adjustable sutures Amount of resection 4-12 mm |
| Kim et al.62 Retrospective | 9 | 3 months | Distance: 23.1±10.3 (12-30) PD Near: 14.9±12.3 PD |
LR resection 5.1±1.6 (3-8) mm |
Success rate 78% Fixed sutures |
| Greninger et al.63 Retrospective |
47 | Average 19.5 months |
30-80 PD (ET before MR recession) 12-50 PD (residual ET after BMR recession) |
MR recession + LR resection LR recession |
All patients were free of diplopia |
| Peragallo et al.64 Retrospective |
Group A (n=13) Group B (n=13) Control (n=14) |
- | 17±9.2 PD 21.3±7.5 PD 11.2±1.3 PD |
IR recession (unilateral) | Over-correction A: 23% B: 14% Control: 16% Group A: Adjustable sutures in TED patients Group B: Fixed or semi-adjustable sutures in the cases with TED Control group: Adjustable sutures in patient with other forms of strabismus |
| Nicholson et al.65 Retrospective |
58 | Average 12.1 | - | MR recession MR + IR recession LR + IR recession IR recession |
Excellent outcome in 83% of the patients Relaxed muscle positioning technique Large horizontal deviation was associated with an increased chance for reoperation |
| Cruz and Davitt66 Retrospective |
8 | 18 months | - | IR recession | Seven patients were successfully aligned One case was under-corrected Adjustable sutures |
| Barker et al.67 Retrospective |
42 | 12 months | 21.1 PD | IR recession SR recession IR recession + SR recession |
71% were free of diplopia Adjustable sutures 19% over-correction |
| Kushner68 Retrospective |
57 (14 cases had TED) | 13.6±5.9 | - | IR recession MR recession |
None of them demonstrated muscle slippage Semi-adjustable sutures |
| Cestari et al.69 Retrospective |
Group 1 (n=9) Group 2 (n=9) |
4/3 months 4/8 months |
HOT: 24.2±7.2 HOT: 24.4±6.6 ET: 15.2±4.6 XT: 29.0±29.5 |
IR recession IR + SR recession IR ± SR + LR recession SR + IR + MR recession |
Group 1: Success rate 89% Group 2: Success rate 67% Postoperative vertical drift toward HT: Group 1: 1.2 PD Group 2: 6.8 PD P=0.048 |
| Kerr70 Retrospective | Group 1 n=34 (patient with TED) Group 2 n=30 (no TED) Group 3 n=13 (TED) |
2 months | Non-absorbable cases: 20±11.65 PD Absorbable cases: 20±13.8 PD |
IR recession Unilateral IR recession MR recession |
Absorbable suture and TED were associated with postoperative over-correction Non-absorbable suture was strongly correlated with the absence of over-correction |
| Tacea et al.71 Retrospective |
n=8 | 54±41 (range, 21-125 months) | Distance: 15.8±8.8 Near: 14.2±8.4 |
SR resection IR resection IR + SR resection |
Success rate 62.5% All the patients underwent prior maximal vertical rectus muscle recession. 37.5% of the patients required further surgery |
TED: Thyroid eye disease, ET: Esotropia, PD: Prism diopter, HT: Hypertropia, MR: Medial rectus, BMR: Bilateral medial rectus, HOT: Hypotropia, XT: Exotropia, IR: Inferior rectus, SR: Superior rectus, LR: Lateral rectus, BLR: Bilateral lateral rectus
Figure 3.
A patient with thyroid eye disease (TED)-related esotropia (a-c). He had residual esotropia after a 7 mm recession of bilateral medial rectus muscles (d-f). He underwent injection of botulinum toxin A (BTA) in bilateral medial rectus muscles. The patient is orthotropic in primary position 1 year after botulinum toxin injection (g-i)
Vertical deviation
The IR muscle is the most common extraocular muscle affected by TED.3 Hypotropia is the main ocular deviation in these cases. BSV in the patient may only remain in down-gaze, so some of them present with chin-up position increases with the progression of the disease. Some important points should be considered in the surgical management of TED patients with vertical strabismus.
Underestimation of contralateral IR restriction can result in postoperative over-correction. The following signs can help to discover contralateral IR involvement: enlargement of IR in orbital imaging, limitation of supra-duction in non-hypotropic eye, uncorrected chin-up position after occlusion of the hypotropic eye,81 unexpected large degrees of excyclotorsion (based on our experience), and the signs of the restricted IR in Hess screen test.
Severe restriction of IR may mask the restriction of the ipsilateral SR muscle. After IR recession, the spasm and restriction of the SR result in postoperative hypertropia (over-correction). Orbital imaging and intraoperative FDT, after dis-insertion of the IR from the sclera, can be useful to detect the involvement of the SR.
There is no standard nomogram for surgical dose-response for vertical muscle recession in TED patients.82 Jason et al.64 suggested a dose-response of 3.26 PD/mm for IR recession in these patients. In our unpublished study, dose-response was calculated 4.97 ± 1.52 PD/mm for vertical deviation in primary position. It was lower in deviation <25 PD than the deviation ≥ 25 PD. We used fixed sutures in all the patients.
Various methods have been suggested to achieve desirable surgical outcomes in these patients including relaxed muscle positioning technique,50,65 adjustable sutures,66,67 and semi-adjustable suture.68 Some studies reported over-correction rate increases with adjustable sutures.67 Barker et al.67 reported eight cases of overcorrection in 42 patients with TED-related vertical deviation who underwent strabismus surgery using adjustable sutures. They suggested eight PD under-corrections immediately after the suture adjustment decreased the chance of late over-correction. On the other hand, Cruz and Davitt66 did not find over-correction in eight patients with TED-related vertical deviation after bilateral IR recession using adjustable sutures in the hypotropic eyes. Nicholson et al.65 documented that the intraoperative relaxed muscle positioning technique (muscles are recessed to the positions where those rest freely on the globe without tension) improved ocular alignment and diplopia in most patients with TED-related strabismus. Seventy-eight percent of their cases achieved acceptable outcomes with one operation [Table 2].
-
The cases with TED-related vertical deviation may require bilateral or unilateral IR recession with or without contralateral SR recession to restore BSV. In these patients, the vertical deviation can be classified into three groups to make better decisions to treat them.
- a. In the patients with hypotropia nearly equal in down-gazeand primary position, unilateral IR recession is a good option to treat them.
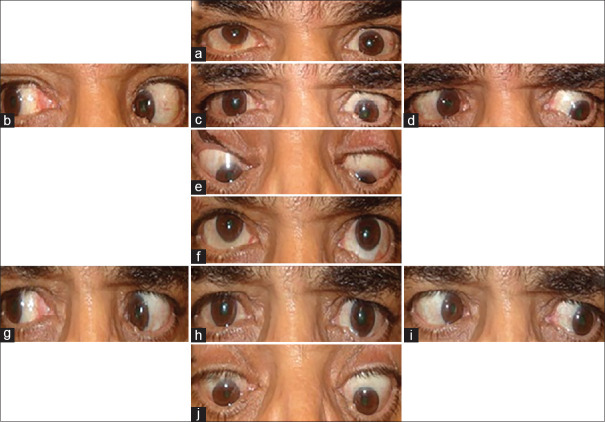
Figure 4 shows a 55-year-old man who had 20 PD left hypotropia in primary position, 35 PD in upper-gaze, and 16 PD in down-gaze. He underwent 5.5 mm left IR recession.
One year later, he was orthotropic in the primary position and down-gaze.
- b. In the cases with significant hypotropia in the primary position and up-gaze but nearly orthotropic in down-gaze, bilateral asymmetric IR recession or IR recession in the hypotropic eye in conjunction with contralateral IR posterior fixation suture are the suggested procedures.83
- c. Hypotropia present in all vertical positions but in down-gaze is the smallest and can be managed by ipsilateral IR recession to correct hypotropia in down-gaze in conjunction with contralateral SR recession to correct residual hypotropia in the primary position. In these cases, the contralateral IR restriction should be ruled out to reduce the risk of postoperative contralateral hypotropia (overcorrection)
Figure 5 shows a 55-year-old man who had 40 PD left hypotropia in primary position, 60 PD in upper-gaze, and 25 PD in down-gaze. He underwent 5 mm recession of left IR and 5 mm recession of right SR. He was orthotropic in the primary position and had no diplopia in down-gaze in the last visit (14 months after surgery).
Most surgeons prefer to weaken restricted muscles in TED patients. Some authors suggested vertical rectus recession resection in patients with a large vertical deviation.71,84 Tacea et al.71 used rectus muscle resection to overcome residual vertical deviation after the maximal recession of restricted muscle. Sixty two and half percent of eight cases achieved BSV in primary and reading position with either no prism or prism less than 5 PD. No patients were overcorrected, but 37.5% of them required further surgery for torsional diplopia, horizontal deviation, and residual vertical deviation. No patients developed inflammation or increased muscle restriction. Lee et al.84 used simultaneous vertical rectus recession and resection in six patients with large vertical deviation. Four patients (67%) had successful surgical outcome, but two patients (33%) were over-corrected such that one of them required reoperation. The resected procedure should be avoided on the muscle that has restriction and enlargement. It seems to be reasonable to postpone the resection procedure in the presence of a restricted muscle.
-
Although the restriction of the IR results in hypotropia and esotropia, TED can simultaneously involve vertical and horizontal rectus muscles. The patient can present with significant mixed hypotropia and esotropia. In these cases, vertical and horizontal muscle recession may be required to achieve BSV in primary and reading position. The dosere-sponse is more in simultaneous recession of vertical and horizontal rectus muscle than the recession of each muscle alone, so over-correction occurs more commonly in this setting.69
Cestari et al.69 reported postoperative vertical drift toward hypertropia in TED patients. There was a higher amount of vertical drift in the patients who underwent combined vertical and horizontal muscle recession compared to those who had vertical muscle recession alone (6.8 vs. 1.2 PD). In our unpublished study, each millimeter of IR recession corrected 1.64 ± 1.37 PD of esotropia. Staged surgery is helpful in these cases.
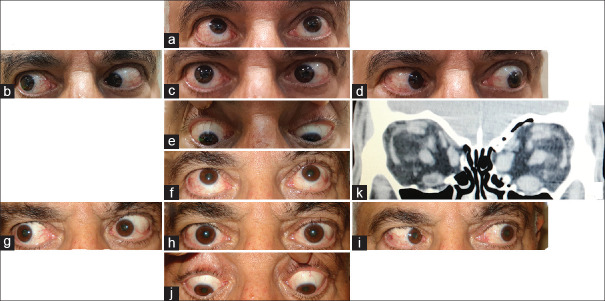
Figure 6 shows a 65-year-old man who had 30 PD left esotropia and 40 PD left hypotropia after orbital decompression. FDT was significantly positive for the left IR. The patient underwent 7 mm recession of left IR muscle. Interestingly, he was orthotropic in primary position at the last visit (7 months later).
The presence of excyclotorsion less than expected degrees or incyclotorsion (more rarely) can be a sign that indicates bilateral SR or superior oblique (SO) muscle involvement in TED patients with IR restriction. In these cases, preoperative and intraoperative (after IR dis-insertion) FDT and exaggerated FDT can be helpful to detect restriction of SR or SO.85,86 In addition, intraoperative use of skin marker and Mendez ring may be useful for the detection of torsional changes following the IR recession.87 The weakening of the restricted SO may be required to prevent development of postoperative A-pattern and incyclotorsion in this setting.85
-
Over-correction is an outcome that may occur after surgical correction of vertical deviation in TED patients. Several factors, which will be mentioned below, may increase the risk of over-correction.
- The progression of underlying thyroid myopathy after strabismus surgery (even in cases with a stable angle of deviation for 6 months) may result in postoperative instability of deviation.
Figure 7 shows a 54-year-old man who had 40 PD right hypotropia and diplopia after right medial and inferior orbital wall decompression. In his orbital CT scan, the enlargement of the right IR muscle was significant, so he underwent 7 mm recession of the right IR muscle. He became orthotropic and remained diplopia free for eight years. Eight years later, the patient underwent left orbital decompression surgery because of dysthyroid optic neuropathy. Subsequent diplopia and eye deviation occurred again. He had 12 PD of left hypotropia and 25 PD of left esotropia at six months after second orbital surgery. At that time, orbital CT scan showed left medial and IR muscles were larger than the right IR muscle. We performed 4 mm recession of the left IR muscle and 6 mm recession of the left MR muscle. The patient was orthotropic without diplopia at last examination (6 months later).
- b. Although postoperative classic muscle slippage is not common, posterior shifting of IR attachment to the sclera may occur. The predisposing factors for this situation are the gravitational force, the short arc of contact in IR muscle, and thickened tenon beneath the IR muscle in TED patients.88 Meticulous tenon dissection, semi-adjustable suture instead of adjustable suture,68,89 non-absorbable suture, and slight under-correction on immediately postoperative time have been suggested to reduce the risk of over-correction after IR recession in these cases.64,67
Kushner68 reported 100% success rate in eliminating muscle slippage by using a semi-adjustable suture technique (fixing two corners of the muscle directly to the sclera, and suspending the center of the muscle on an adjustable suture). Kerr70 reported non-absorbable sutures provided an advantage to prevent late over-correction in the patients with TED-related vertical deviation. However, non-absorbable sutures have a risk of infection and a tendency to be exposed through the conjunctiva.
- c. An overlooked contralateral restricted IR can result in over-correction (as described previously).
- d. Ipsilateral undetected SR contraction is another cause ofthe postoperative over-correction.
The over-correction after IR weakening can be treated by the recession of ipsilateral SR or contralateral restricted IR or both according to ocular ductions, FDT, and orbital imaging (CT, MRI).42,90,91
Diplopia limited to down-gaze is another postoperative problem in TED-related vertical deviation, particularly after unilateral IR recession. The options to correct the situation include Fresnel prism on the bifocal segment, ground-in prism using the slab-off technique in the bifocal segment, induced prism by glasses with an unequal bifocal height, and occlusion of bifocal near segment on the non-dominant eye. Kushner reported that the most successful non-surgical treatment for these cases was the bifocal glasses with raising of bifocal segments in the spectacles.92 For the patients who do not tolerate conservative options, posterior fixation suture (Faden operation) of the contralateral IR muscle proved to be a favorable option.83,92
Bilateral IR recession more than 6 mm can cause postoperative A-pattern and exotropia in down-gaze.93 This problem occurs due to fixation duress to SO muscles in down-gaze and SO overacting along with decrease adduction effect of IR muscle.94,95 Some options have been suggested to eliminate this problem. These include bilateral IR muscle nasalization, adjustable SO recession,85 anterior tenotomy of SO, anterior lateral transposition of inferior oblique,95 and posterior tenectomy of SO.93 The IR nasalization decreases A-pattern but increases incyclotorsion.81 Anterior SO tenotomy decreases incyclotorsion but has less effect on the A-pattern. Adjustable SO recession may prevent development of postoperative A-pattern and incyclotorsion in this setting.85
The TED itself can cause lower eyelid retraction in addition to the effect of IR muscle recession. The recession of IR more than 4 mm commonly aggravates the situation. Lockwood's ligament advancement and lower eyelid retractor lysis are the options to prevent lower eyelid retraction postoperatively.96,97 Lockwood's ligament advancement may not eliminate this problem, and its effect decreases with time.96 The correction of lower eyelid retraction in TED patients may require a spacer material.98
Figure 4.
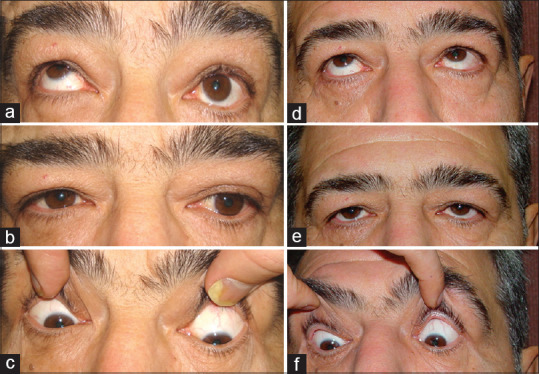
A patient with thyroid eye disease (TED) and hypotropia. He had 20-prism diopter (PD) left hypotropia in primary position, 35 PD in up-gaze, and 16 PD in down-gaze (a-c). After a 5 mm recession of left inferior rectus (IR), he was orthotropic in primary position and down-gaze (d-f)
Figure 5.
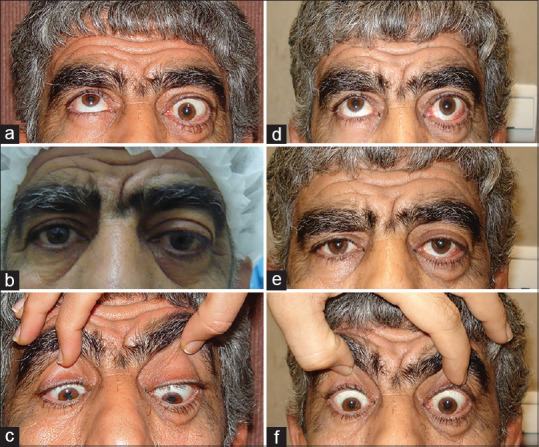
A patient with thyroid eye disease (TED)-related left hypotropia and significant limitation in elevation (a-c). He underwent 5 mm recession of left inferior rectus (IR) muscle and right superior rectus muscle; he is orthotropic in primary position without diplopia in primary position and down-gaze (d-f)
Figure 6.
A patient with 30-prism diopter (PD) left esotropia and 40 PD left hypotropia (a-e). The patient underwent 7 mm recession left inferior rectus (IR) muscle and became orthotropic postoperatively (f-j)
Figure 7.
A patient with thyroid eye disease (TED)-related strabismus. The patient had a history of right hypotropia that was corrected with recession of right inferior rectus (IR). Eight years later, he presented left hypotropia and left esotropia following left orbital decompression surgery (a-e). Orbital computed tomography (CT) scan showed left medial and IR muscle enlargement that occurred several years after right IR muscle involvement (previous orbital CT scan was not available) (k). He underwent left medial rectus and left IR recession. The patient was orthotropic in primary position and diplopia free 6 months after the surgery (f-j)
DISCUSSION
Management of strabismus is challenging in TED. The following assessments should be considered including ocular ductions, the amount of deviation, FDT (pre and intraoperative), orbital imaging, and the amount of cyclotorsion. In the patient with vertical strabismus, the angle of the deviation should be measured at the primary position, up, and down-gaze.
Strabismus surgery in TED should not be carried out until the deviation is stable for 4–6 months and after any orbital decompression. The patients should be informed about requiring several surgical and non-surgical modalities to overcome their strabismus. Muscle recession is the main surgical procedure in these form of strabismus. Staged surgical management, particularly when rectus muscle resection should be chosen, is recommended in these patients. Surgical muscle resection on the restricted muscle is not proper choice. BTA toxin and prism are the options for patients with symptomatic postoperative small strabismus. Optimal outcomes can be achieved with careful evaluations of the patients and choosing the right treatments. Successful outcome has a significant impact on improving quality of life in these patients.
Future prospective randomized studies comparing the methods of strabismus surgery in TED-related strabismus are needed to propose the optimal surgical treatment.
SUMMARY POINTS
Patients with TED-related strabismus need to be realistic about their strabismus and outcomes of the treatments.
BTA toxin may be useful in the early and chronic phases of TED-related strabismus, particularly in a small angle of deviation, as well as in the management of postoperative over-correction and under-correction.
Thyroid dysfunction should be controlled before strabismus surgery.
Strabismus surgery in TED should not be carried out until the deviation is stable for 4–6 months and after any orbital decompression.
Ocular ductions, FDT, orbital imaging, and assessment of ocular torsion are helpful in the management of TED-related strabismus.
Lateral rectus resection is an option to correct residual esotropia after the maximal recession of MR muscle.
In the patients who have hypotropia in primary position but are orthotropic in down-gaze, the recessions of unilateral IR with posterior fixation suture on the contralateral IR muscle as well as the asymmetric recession of bilateral IR muscles are good options.
Unilateral IR recession is a good option in the cases with nearly equal amounts of hypotropia in primary position and down-gaze.
Ipsilateral IR weakening with contralateral SR recession is an acceptable option for cases with hypotropia in all vertical positions but the smallest angle in down-gaze.
Surgical dose-response in combined vertical and horizontal muscle recession is more than recession of each muscle alone.
Overlooked contralateral IR restriction, ipsilateral SR contraction, and the progression of underlying myopathy are important causes of postoperative over-correction in a patient with vertical deviation.
Postoperative diplopia in down-gaze can be managed by adjustment of bifocal glasses, Fresnel prism on the bifocal, and finally, posterior fixation sutures on the contralateral IR muscle.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patients have given their consent for their images and other clinical information to be reported in the journal. The patients understands that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Authors obtained consents from the patients for publishing the photos.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Bartley GB, Fatourechi V, Kadrmas EF, Jacobsen SJ, Ilstrup DM, Garrity JA, et al. Clinical features of Graves'ophthalmopathy in an incidence cohort. Am J Ophthalmol. 1996;121:284–90. doi: 10.1016/s0002-9394(14)70276-4. [DOI] [PubMed] [Google Scholar]
- 2.Rundle FF. Management of exophthalmos and related ocular changes in Graves'disease. Metabolism. 1957;6:36–48. [PubMed] [Google Scholar]
- 3.Bartalena L, Baldeschi L, Dickinson A, Eckstein A, Kendall-Taylor P, Marcocci C, et al. Consensus statement of the European Group on Graves'orbitopathy (EUGOGO) on management of GO. Eur J Endocrinol. 2008;158:273–85. doi: 10.1530/EJE-07-0666. [DOI] [PubMed] [Google Scholar]
- 4.Lo C, Ugradar S, Rootman D. Management of graves myopathy:Orbital imaging in thyroid-related orbitopathy. J AAPOS. 2018;22:256.e1–256.pe9. doi: 10.1016/j.jaapos.2018.06.002. [DOI] [PubMed] [Google Scholar]
- 5.van Geest RJ, Sasim IV, Koppeschaar HP, Kalmann R, Stravers SN, Bijlsma WR, et al. Methylprednisolone pulse therapy for patients with moderately severe Graves'orbitopathy:A prospective, randomized, placebo-controlled study. Eur J Endocrinol. 2008;158:229–37. doi: 10.1530/EJE-07-0558. [DOI] [PubMed] [Google Scholar]
- 6.Salvi M, Vannucchi G, Currò N, Campi I, Covelli D, Dazzi D, et al. Efficacy of B-cell targeted therapy with rituximab in patients with active moderate to severe Graves'orbitopathy:A randomized controlled study. J Clin Endocrinol Metab. 2015;100:422–31. doi: 10.1210/jc.2014-3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perez-Moreiras JV, Gomez-Reino JJ, Maneiro JR, Perez-Pampin E, Romo Lopez A, Rodríguez Alvarez FM, et al. Efficacy of tocilizumab in patients with moderate-to-severe corticosteroid-resistant Graves orbitopathy:A randomized clinical trial. Am J Ophthalmol. 2018;195:181–90. doi: 10.1016/j.ajo.2018.07.038. [DOI] [PubMed] [Google Scholar]
- 8.Skov CM, Mazow ML. Managing strabismus in endocrine eye disease. Can J Ophthalmol. 1984;19:269–74. [PubMed] [Google Scholar]
- 9.Dyer JA. The oculorotary muscles in Graves'disease. Trans Am Ophthalmol Soc. 1976;74:425–56. [PMC free article] [PubMed] [Google Scholar]
- 10.Yeatts RP. Quality of life in patients with Graves ophthalmopathy. Trans Am Ophthalmol Soc. 2005;103:368–411. [PMC free article] [PubMed] [Google Scholar]
- 11.Thomas SM, Cruz OA. Comparison of two different surgical techniques for the treatment of strabismus in dysthyroid ophthalmopathy. J AAPOS. 2007;11:258–61. doi: 10.1016/j.jaapos.2006.10.021. [DOI] [PubMed] [Google Scholar]
- 12.Metz HS. Complications following surgery for thyroid ophthalmopathy. J Pediatr Ophthalmol Strabismus. 1984;21:220–2. doi: 10.3928/0191-3913-19841101-05. [DOI] [PubMed] [Google Scholar]
- 13.Nardi M. Squint surgery in TED –Hints and fints, or why Graves'patients are difficult patients. Orbit. 2009;28:245–50. doi: 10.1080/01676830903104603. [DOI] [PubMed] [Google Scholar]
- 14.Long JC. Surgical management of tropias of thyroid exophthalmos. Arch Ophthalmol. 1966;75:634–8. doi: 10.1001/archopht.1966.00970050636010. [DOI] [PubMed] [Google Scholar]
- 15.Fabian ID, Rosen N, Ben Simon GJ. Strabismus after inferior-medial wall orbital decompression in thyroid-related orbitopathy. Curr Eye Res. 2013;38:204–9. doi: 10.3109/02713683.2012.713154. [DOI] [PubMed] [Google Scholar]
- 16.Jackson JL. Nonsurgical management of diplopia after orbital decompression surgery. Am Orthopt J. 2012;62:29–33. doi: 10.3368/aoj.62.1.29. [DOI] [PubMed] [Google Scholar]
- 17.Woo SJ, Seo JM, Hwang JM. Clinical characteristics of cyclodeviation. Eye (Lond) 2005;19:873–8. doi: 10.1038/sj.eye.6701675. [DOI] [PubMed] [Google Scholar]
- 18.Véronneau-Troutman S. Fresnel prisms and their effects on visual acuity and binocularity. Trans Am Ophthalmol Soc. 1978;76:610–53. [PMC free article] [PubMed] [Google Scholar]
- 19.Gunton KB, Brown A. Prism use in adult diplopia. Curr Opin Ophthalmol. 2012;23:400–4. doi: 10.1097/ICU.0b013e3283567276. [DOI] [PubMed] [Google Scholar]
- 20.Dunn WJ, Arnold AC, O'Connor PS. Botulinum toxin for the treatment of dysthyroid ocular myopathy. Ophthalmology. 1986;93:470–5. doi: 10.1016/s0161-6420(86)33713-8. [DOI] [PubMed] [Google Scholar]
- 21.Scott AB. Botulinum toxin injection into extraocular muscles as an alternative to strabismus surgery. Ophthalmology. 1980;87:1044–9. doi: 10.1016/s0161-6420(80)35127-0. [DOI] [PubMed] [Google Scholar]
- 22.Lyons CJ, Vickers SF, Lee JP. Botulinum toxin therapy in dysthyroid strabismus. Eye (Lond) 1990;4(Pt 4):538–42. doi: 10.1038/eye.1990.74. [DOI] [PubMed] [Google Scholar]
- 23.Kikkawa DO, Cruz RC, Jr, Christian WK, Rikkers S, Weinreb RN, Levi L, et al. Botulinum A toxin injection for restrictive myopathy of thyroid-related orbitopathy:Effects on intraocular pressure. Am J Ophthalmol. 2003;135:427–31. doi: 10.1016/s0002-9394(02)02092-5. [DOI] [PubMed] [Google Scholar]
- 24.McNeer KW. An investigation of the clinical use of botulinum toxin A as a postoperative adjustment procedure in the therapy of strabismus. J Pediatr Ophthalmol Strabismus. 1990;27:3–9. doi: 10.3928/0191-3913-19900101-03. [DOI] [PubMed] [Google Scholar]
- 25.Akbari MR, Ameri A, Keshtkar Jaafari AR, Mirmohammadsadeghi A. Botulinum toxin injection for restrictive myopathy of thyroid-associated orbitopathy:Success rate and predictive factors. J AAPOS. 2016;20:126–300. doi: 10.1016/j.jaapos.2016.01.007. [DOI] [PubMed] [Google Scholar]
- 26.Gair EJ, Lee JP, Khoo BK, Maurino V. What is the role of botulinum toxin in the treatment of dysthyroid strabismus? J AAPOS. 1999;3:272–4. doi: 10.1016/s1091-8531(99)70022-4. [DOI] [PubMed] [Google Scholar]
- 27.Wu X, Lin N, Ai LK, Wang JH, Yan LJ. The application of botulinum toxin A in the treatment of restrictive strabismus in thyroid associated ophthalmopathy. Zhonghua Yan Ke Za Zhi. 2006;42:1063–7. [PubMed] [Google Scholar]
- 28.Granet DB, Hodgson N, Godfrey KJ, Ventura R, Kikkawa DO, Levi L, et al. Chemodenervation of extraocular muscles with botulinum toxin in thyroid eye disease. Graefes Arch Clin Exp Ophthalmol. 2016;254:999–1003. doi: 10.1007/s00417-016-3281-6. [DOI] [PubMed] [Google Scholar]
- 29.Fells P. Management of dysthyroid eye disease. Br J Ophthalmol. 1991;75:245–6. doi: 10.1136/bjo.75.4.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dutton JJ, Fowler AM. Botulinum toxin in ophthalmology. Surv Ophthalmol. 2007;52:13–31. doi: 10.1016/j.survophthal.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 31.Dawson EL, Sainani A, Lee JP. Does botulinum toxin have a role in the treatment of secondary strabismus? Strabismus. 2005;13:71–3. doi: 10.1080/09273970590935048. [DOI] [PubMed] [Google Scholar]
- 32.McNeer KW. Botulinum toxin injection into the superior rectus muscle of the non-dominant eye for dissociated vertical deviation. J Pediatr Ophthalmol Strabismus. 1989;26:162–4. doi: 10.3928/0191-3913-19890701-04. [DOI] [PubMed] [Google Scholar]
- 33.Morgenstern KE, Evanchan J, Foster JA, Cahill KV, Burns JA, Holck DE, et al. Botulinum toxin type a for dysthyroid upper eyelid retraction. Ophthalmic Plast Reconstr Surg. 2004;20:181–5. doi: 10.1097/00002341-200405000-00001. [DOI] [PubMed] [Google Scholar]
- 34.Uddin JM, Davies PD. Treatment of upper eyelid retraction associated with thyroid eye disease with subconjunctival botulinum toxin injection. Ophthalmology. 2002;109:1183–7. doi: 10.1016/s0161-6420(02)01041-2. [DOI] [PubMed] [Google Scholar]
- 35.Korn BS, Seo SW, Levi L, Granet DB, Kikkawa DO. Optic neuropathy associated with botulinum A toxin in thyroid-related orbitopathy. Ophthalmic Plast Reconstr Surg. 2007;23:109–14. doi: 10.1097/IOP.0b013e318032eb12. [DOI] [PubMed] [Google Scholar]
- 36.Crouch ER. Use of botulinum toxin in strabismus. Curr Opin Ophthalmol. 2006;17:435–40. doi: 10.1097/01.icu.0000243018.97627.4c. [DOI] [PubMed] [Google Scholar]
- 37.Elston JS, Lee JP, Powell CM, Hogg C, Clark P. Treatment of strabismus in adults with botulinum toxin A. Br J Ophthalmol. 1985;69:718–24. doi: 10.1136/bjo.69.10.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bartley GB, Fatourechi V, Kadrmas EF, Jacobsen SJ, Ilstrup DM, Garrity JA, et al. The treatment of Graves'ophthalmopathy in an incidence cohort. Am J Ophthalmol. 1996;121:200–6. doi: 10.1016/s0002-9394(14)70585-9. [DOI] [PubMed] [Google Scholar]
- 39.Rajendram R, Bunce C, Adams GG, Dayan CM, Rose GE. Smoking and strabismus surgery in patients with thyroid eye disease. Ophthalmology. 2011;118:2493–7. doi: 10.1016/j.ophtha.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 40.Lim NC, Sundar G, Amrith S, Lee KO. Thyroid eye disease:A Southeast Asian experience. Br J Ophthalmol. 2015;99:512–8. doi: 10.1136/bjophthalmol-2014-305649. [DOI] [PubMed] [Google Scholar]
- 41.Jellema HM, Saeed P, Mombaerts I, Dolman PJ, Garrity J, Kazim M, et al. Objective and subjective outcomes of strabismus surgery in Graves'orbitopathy:A prospective multicentre study. Acta Ophthalmol. 2017;95:386–91. doi: 10.1111/aos.13367. [DOI] [PubMed] [Google Scholar]
- 42.Flanders M, Hastings M. Diagnosis and surgical management of strabismus associated with thyroid-related orbitopathy. J Pediatr Ophthalmol Strabismus. 1997;34:333–40. doi: 10.3928/0191-3913-19971101-04. [DOI] [PubMed] [Google Scholar]
- 43.Volpe NJ, Mirza-George N, Binenbaum G. Surgical management of vertical ocular misalignment in thyroid eye disease using an adjustable suture technique. J AAPOS. 2012;16:518–22. doi: 10.1016/j.jaapos.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 44.Yan J, Zhang H. The surgical management of strabismus with large angle in patients with Graves'ophthalmopathy. Int Ophthalmol. 2008;28:75–82. doi: 10.1007/s10792-007-9114-1. [DOI] [PubMed] [Google Scholar]
- 45.Yoo SH, Pineles SL, Goldberg RA, Velez FG. Rectus muscle resection in Graves'ophthalmopathy. J AAPOS. 2013;17:9–15. doi: 10.1016/j.jaapos.2012.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee YH, Oh SY, Hwang JM. Is 6 months of stable angle of strabismus enough to perform surgery in patients with strabismus related to thyroid ophthalmopathy? Br J Ophthalmol. 2010;94:955–6. doi: 10.1136/bjo.2008.154195. [DOI] [PubMed] [Google Scholar]
- 47.Zloto O, Ben Simon G, Didi Fabian I, Sagiv O, Huna-Baron R, Ben Zion I, et al. Association of orbital decompression and the characteristics of subsequent strabismus surgery in thyroid eye disease. Can J Ophthalmol. 2017;52:264–8. doi: 10.1016/j.jcjo.2016.11.006. [DOI] [PubMed] [Google Scholar]
- 48.Nunery WR, Nunery CW, Martin RT, Truong TV, Osborn DR. The risk of diplopia following orbital floor and medial wall decompression in subtypes of ophthalmic Graves'disease. Ophthalmic Plast Reconstr Surg. 1997;13:153–60. doi: 10.1097/00002341-199709000-00001. [DOI] [PubMed] [Google Scholar]
- 49.Mocan MC, Ament C, Azar NF. The characteristics and surgical outcomes of medial rectus recessions in Graves'ophthalmopathy. J Pediatr Ophthalmol Strabismus. 2007;44:93–100. doi: 10.3928/01913913-20070301-02. [DOI] [PubMed] [Google Scholar]
- 50.Dal Canto AJ, Crowe S, Perry JD, Traboulsi EI. Intraoperative relaxed muscle positioning technique for strabismus repair in thyroid eye disease. Ophthalmology. 2006;113:2324–30. doi: 10.1016/j.ophtha.2006.04.036. [DOI] [PubMed] [Google Scholar]
- 51.Lyu IJ, Lee JY, Kong M, Park KA, Oh SY. Surgical responses of medial rectus muscle recession in thyroid eye disease-related esotropia. PLoS One. 2016;11:e0146779. doi: 10.1371/journal.pone.0146779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gomi CF, Yang SW, Granet DB, Kikkawa DO, Langham KA, Banuelos LR, et al. Change in proptosis following extraocular muscle surgery:Effects of muscle recession in thyroid-associated orbitopathy. J AAPOS. 2007;11:377–80. doi: 10.1016/j.jaapos.2007.01.115. [DOI] [PubMed] [Google Scholar]
- 53.Black BC. Treatment of incomitant hypertropia and diplopia with recession of the inferior rectus and superior rectus muscles of the same eye. J AAPOS. 2007;11:262–5. doi: 10.1016/j.jaapos.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 54.Al Qahtani ES, Rootman J, Kersey J, Godoy F, Lyons CJ. Clinical pearls and management recommendations for strabismus due to thyroid orbitopathy. Middle East Afr J Ophthalmol. 2015;22:307–11. doi: 10.4103/0974-9233.159731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nugent RA, Belkin RI, Neigel JM, Rootman J, Robertson WD, Spinelli J, et al. Graves orbitopathy:Correlation of CT and clinical findings. Radiology. 1990;177:675–82. doi: 10.1148/radiology.177.3.2243967. [DOI] [PubMed] [Google Scholar]
- 56.Ponto KA, Hommel G, Pitz S, Elflein H, Pfeiffer N, Kahaly GJ. Quality of life in a German graves orbitopathy population. Am J Ophthalmol. 2011;152:483–900. doi: 10.1016/j.ajo.2011.02.018. [DOI] [PubMed] [Google Scholar]
- 57.de Waard R, Koornneef L, Verbeeten B., Jr Motility disturbances in Graves'ophthalmopathy. Doc Ophthalmol. 1983;56:41–7. doi: 10.1007/BF00154707. [DOI] [PubMed] [Google Scholar]
- 58.Kraus DJ, Bullock JD. Treatment of thyroid ocular myopathy with adjustable and nonadjustable suture strabismus surgery. Trans Am Ophthalmol Soc. 1993;91:67–79. [PMC free article] [PubMed] [Google Scholar]
- 59.Tian S, Bolzani R, Benassi M, Lennerstrand G. Eye muscle force development in thyroid-associated ophthalmopathy in different stages of disease. Acta Ophthalmol Scand. 2007;85:431–7. doi: 10.1111/j.1600-0420.2007.00877.x. [DOI] [PubMed] [Google Scholar]
- 60.Jellema HM, Saeed P, Braaksma-Besselink Y, Schuit A, Kloos R, Mourits MP. Unilateral and bilateral medial rectus recession in Graves'orbitopathy patients. Strabismus. 2014;22:182–7. doi: 10.3109/09273972.2014.962749. [DOI] [PubMed] [Google Scholar]
- 61.Weldy E, Kerr NC. Lateral rectus muscle resection following maximal recession of the medial rectus muscle in thyroid eye disease. J AAPOS. 2017;21:291–4. doi: 10.1016/j.jaapos.2017.05.027. [DOI] [PubMed] [Google Scholar]
- 62.Kim EY, Roper-Hall G, Cruz OA. Effectiveness of bilateral lateral rectus resection for residual esotropia in dysthyroid ophthalmopathy. Am J Ophthalmol. 2016;171:84–7. doi: 10.1016/j.ajo.2016.08.029. [DOI] [PubMed] [Google Scholar]
- 63.Greninger D, Berg P, Steele E. Treatment of esotropia from thyroid eye disease by lateral rectus resection. Investig Ophthalmol Vis Sci. 2013;54:5905. [Google Scholar]
- 64.Peragallo JH, Velez FG, Demer JL, Pineles SL. Postoperative drift in patients with thyroid ophthalmopathy undergoing unilateral inferior rectus muscle recession. Strabismus. 2013;21:23–8. doi: 10.3109/09273972.2012.762533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nicholson BP, De Alba M, Perry JD, Traboulsi EI. Efficacy of the intraoperative relaxed muscle positioning technique in thyroid eye disease and analysis of cases requiring reoperation. J AAPOS. 2011;15:321–5. doi: 10.1016/j.jaapos.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 66.Cruz OA, Davitt BV. Bilateral inferior rectus muscle recession for correction of hypotropia in dysthyroid ophthalmopathy. J AAPOS. 1999;3:157–9. doi: 10.1016/s1091-8531(99)70061-3. [DOI] [PubMed] [Google Scholar]
- 67.Barker L, Mackenzie K, Adams GG, Hancox J. Long-term surgical outcomes for vertical deviations in thyroid eye disease. Strabismus. 2017;25:67–72. doi: 10.1080/09273972.2017.1318151. [DOI] [PubMed] [Google Scholar]
- 68.Kushner BJ. An evaluation of the semiadjustable suture strabismus surgical procedure. J AAPOS. 2004;8:481–7. doi: 10.1016/j.jaapos.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 69.Cestari DM, Freire MV, Chun BY. Vertical rectus muscle recession versus combined vertical and horizontal rectus muscle recession in patients with thyroid eye disease and hypotropia. J AAPOS. 2018;22:257–61. doi: 10.1016/j.jaapos.2018.04.007. [DOI] [PubMed] [Google Scholar]
- 70.Kerr NC. The role of thyroid eye disease and other factors in the overcorrection of hypotropia following unilateral adjustable suture recession of the inferior rectus (an American Ophthalmological Society thesis) Trans Am Ophthalmol Soc. 2011;109:168–200. [PMC free article] [PubMed] [Google Scholar]
- 71.Tacea F, Loane E, Grixti A, Marsh IB, Ziahosseini K. Rectus muscle resection for vertical strabismus in thyroid eye disease. Strabismus. 2018;26:71–6. doi: 10.1080/09273972.2018.1444067. [DOI] [PubMed] [Google Scholar]
- 72.Mourits MP, Koorneef L, van Mourik-Noordenbos AM, van der Meulen-Schot HM, Prummel MF, Wiersinga WM, et al. Extraocular muscle surgery for Graves'ophthalmopathy:Does prior treatment influence surgical outcome? Br J Ophthalmol. 1990;74:481–3. doi: 10.1136/bjo.74.8.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Oeverhaus M, Fischer M, Hirche H, Schlüter A, Esser J, Eckstein AK. Tendon elongation with bovine pericardium in patients with severe esotropia after decompression in Graves'orbitopathy-efficacy and long-term stability. Strabismus. 2018;26:62–70. doi: 10.1080/09273972.2018.1450430. [DOI] [PubMed] [Google Scholar]
- 74.Drachman DB. Myasthenia gravis and the thyroid gland. N Engl J Med. 1962;266:330–333. [Google Scholar]
- 75.Kiessling WR, Finke R, Kotulla P, Schleusener H. Circulating TSH-binding inhibiting immunoglobulins in myasthenia gravis. Acta Endocrinol (Copenh) 1982;101:41–6. doi: 10.1530/acta.0.1010041. [DOI] [PubMed] [Google Scholar]
- 76.Vargas ME, Warren FA, Kupersmith MJ. Exotropia as a sign of myasthenia gravis in dysthyroid ophthalmopathy. Br J Ophthalmol. 1993;77:822–3. doi: 10.1136/bjo.77.12.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Naseem M, Donker DL, Paridaens D. Blepharoptosis as a sign of severe Graves'orbitopathy. Eye (Lond) 2009;23:1743–4. doi: 10.1038/eye.2008.333. [DOI] [PubMed] [Google Scholar]
- 78.Bijlsma WR, Kalmann R. Idiopathic orbital inflammation and Graves ophthalmopathy. Arch Ophthalmol. 2010;128:131–2. doi: 10.1001/archophthalmol.2009.324. [DOI] [PubMed] [Google Scholar]
- 79.Mensink HW, van Doorn PA, Paridaens D. Concurrent myopathy in patients with Graves'orbitopathy. Orbit. 2009;28:66–70. doi: 10.1080/01676830802483157. [DOI] [PubMed] [Google Scholar]
- 80.Scruggs RT, Black EH. Thyroid eye disease with significant levator involvement and ptosis:A case report. Ophthalmic Plast Reconstr Surg. 2015;31:e153–4. doi: 10.1097/IOP.0000000000000192. [DOI] [PubMed] [Google Scholar]
- 81.Dagi LR. Management of graves myopathy:Understanding and managing vertical strabismus from thyroid eye disease. J AAPOS. 2018;22:252–5. doi: 10.1016/j.jaapos.2018.01.020. [DOI] [PubMed] [Google Scholar]
- 82.Schotthoefer EO, Wallace DK. Strabismus associated with thyroid eye disease. Curr Opin Ophthalmol. 2007;18:361–5. doi: 10.1097/ICU.0b013e32827038f2. [DOI] [PubMed] [Google Scholar]
- 83.Buckley EG, Meekins BB. Fadenoperation for the management of complicated incomitant vertical strabismus. Am J Ophthalmol. 1988;105:304–12. doi: 10.1016/0002-9394(88)90015-3. [DOI] [PubMed] [Google Scholar]
- 84.Lee JY, Park KA, Woo KI, Kim YD, Oh SY. Surgical outcomes of unilateral recession-resection for vertical strabismus in patients with thyroid eye disease. J AAPOS. 2017;21:19–22. doi: 10.1016/j.jaapos.2016.11.019. [DOI] [PubMed] [Google Scholar]
- 85.Holmes JM, Hatt SR, Bradley EA. Identifying masked superior oblique involvement in thyroid eye disease to avoid postoperative A-pattern exotropia and intorsion. J AAPOS. 2012;16:280–5. doi: 10.1016/j.jaapos.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Thacker NM, Velez FG, Demer JL, Rosenbaum AL. Superior oblique muscle involvement in thyroid ophthalmopathy. J AAPOS. 2005;9:174–8. doi: 10.1016/j.jaapos.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 87.Holmes JM, Hatt SR, Leske DA. Intraoperative monitoring of torsion to prevent vertical deviations during augmented vertical rectus transposition surgery. J AAPOS. 2012;16:136–40. doi: 10.1016/j.jaapos.2011.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chatzistefanou KI, Kushner BJ, Gentry LR. Magnetic resonance imaging of the arc of contact of extraocular muscles:Implications regarding the incidence of slipped muscles. J AAPOS. 2000;4:84–93. doi: 10.1067/mpa.2000.103434. [DOI] [PubMed] [Google Scholar]
- 89.Nihalani BR, Whitman MC, Salgado CM, Loudon SE, Hunter DG. Short tag noose technique for optional and late suture adjustment in strabismus surgery. Arch Ophthalmol. 2009;127:1584–90. doi: 10.1001/archophthalmol.2009.305. [DOI] [PubMed] [Google Scholar]
- 90.Sprunger DT, Helveston EM. Progressive overcorrection after inferior rectus recession. J Pediatr Ophthalmol Strabismus. 1993;30:145–8. doi: 10.3928/0191-3913-19930501-04. [DOI] [PubMed] [Google Scholar]
- 91.Hudson HL, Feldon SE. Late overcorrection of hypotropia in Graves ophthalmopathy. Predictive factors. Ophthalmology. 1992;99:356–60. doi: 10.1016/s0161-6420(92)31965-7. [DOI] [PubMed] [Google Scholar]
- 92.Kushner BJ. Management of diplopia limited to down gaze. Arch Ophthalmol. 1995;113:1426–30. doi: 10.1001/archopht.1995.01100110086030. [DOI] [PubMed] [Google Scholar]
- 93.Kushner BJ. Torsion and pattern strabismus:Potential conflicts in treatment. JAMA Ophthalmol. 2013;131:190–3. doi: 10.1001/2013.jamaophthalmol.199. [DOI] [PubMed] [Google Scholar]
- 94.Dagi LR, Elliott AT, Roper-Hall G, Cruz OA. Thyroid eye disease:Honing your skills to improve outcomes. J AAPOS. 2010;14:425–31. doi: 10.1016/j.jaapos.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 95.Del Monte MA. 2001 an ocular odyssey:Lessons learned from 25 years of surgical treatment for graves eye disease. Am Orthopt J. 2002;52:40–57. doi: 10.3368/aoj.52.1.40. [DOI] [PubMed] [Google Scholar]
- 96.Kushner BJ. A surgical procedure to minimize lower-eyelid retraction with inferior rectus recession. Arch Ophthalmol. 1992;110:1011–4. doi: 10.1001/archopht.1992.01080190117039. [DOI] [PubMed] [Google Scholar]
- 97.Akbari MR, Raygan F, Ameri A, Jafari A, Eshraghi B, Fard MA. Lower eyelid retractor lysis versus lock wood advancement to minimize lower eyelid retraction resulting from inferior rectus muscle recession. J AAPOS. 2013;17:445–7. doi: 10.1016/j.jaapos.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 98.Bartalena L, Baldeschi L, Boboridis K, Eckstein A, Kahaly GJ, Marcocci C, et al. The 2016 European Thyroid Association/European Group on Graves' Orbitopathy Guidelines for the Management of Graves'Orbitopathy. Eur Thyroid J. 2016;5:9–26. doi: 10.1159/000443828. [DOI] [PMC free article] [PubMed] [Google Scholar]