Abstract
Purpose:
To report a case of chronic myelogenous leukemia (CML) treatment with imatinib mesylate in the remission phase who developed unilateral macular choroidal neovascularization (CNV).
Methods:
A 45-year-old male marketer with a 5-year history of CML treated with imatinib mesylate presented with 2 months history of progressive vision loss and metamorphopsia in the right eye.
Results:
Fundus examination of the right eye revealed grey-white elevated retinal lesion with indistinct borders in the macula and retinal telangiectasia in the temporal macula. Fluorescein angiography (FA) and optical coherence tomography angiography (OCTA) confirmed the presence of CNV in the right eye. After treatment with anti-vascular endothelial growth factor (anti-VEGF), macular CNV regressed significantly.
Conclusion:
Macular CNV must be kept in mind as a rare ophthalmic manifestation of patients with CML under treatment with imatinib even in the remission phase.
Keywords: Bevacizumab, Choroidal neovascularization, Chronic myelogenous leukemia, Imatinib mesylate, Pachychoroid neovasculopathy
INTRODUCTION
Leukemic retinopathy is one of the critical causes of visual loss in patients with acute and chronic leukemia that manifests with bilateral intraretinal hemorrhages, white-centered hemorrhage, macular hemorrhage, and cotton-wool spots. In a few instances, capillary closure, retinal microaneurysms, peripheral retinal neovascularization, and massive fundus hemorrhage have been reported.1,2,3 However, single macular choroidal neovascularization (CNV) in individuals with chronic myelogenous leukemia (CML) has not been documented. Herein, we report a case with CML in the remission phase with imatinib who developed unilateral macular CNV.
CASE REPORT
A 45-year-old male marketer with a 5-year history of CML under treatment with imatinib mesylate presented with a 2 months history of progressive vision loss and metamorphopsia in the right eye. He visited in May 2018. According to the hematologic consult, CML was in remission at a daily dose of 400 mg imatinib for more than five years. Notably, the patient had no visual manifestations at routine biannual visual examinations up to 6 months before presentation. Past medical history of hypertension, diabetes mellitus, hyperlipidemia, and oronary artery disease was negative. The patient had not previously received other chemotherapeutic agents or radiotherapy, and he was not a smoker.
Ocular examination revealed visual acuity of 20/80 in the right eye and 20/20 in the left eye, and intraocular pressure was 14 and 15 mmHg respectively. Slit-lamp examinations was normal in both eyes, but fundus examination of the right eye revealed grey-white elevated retinal lesion with indistinct borders in the macula and retinal telangiectasia and scattered intraretinal hemorrhages in the right temporal macula. Fundus examination of the left eye was normal [Figure 1a].
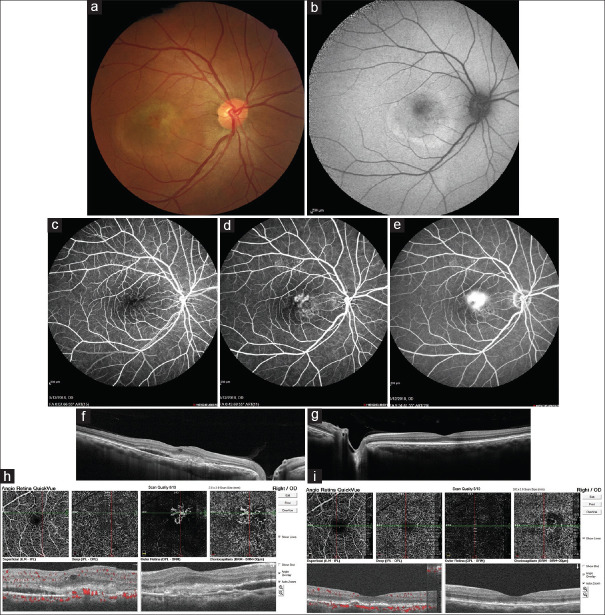
Figure 1.
(a) Fundus photograph of the right eye: Grey-white elevated retinal lesion with indistinct borders and retinal telangiectasia and scattered intraretinal hemorrhages in the temporal macula; (b) Fundus autofluorescence of the right eye shows a ring-shaped area of hyper autofluorescence with stippled central hypo autofluorescence compatible with location of choroidal neovascularization; (c-e) Fluorescein angiography of the right eye revealed a superior foci of early leakage in the macula of the right eye that through the late phases of angiography increased in size and borders faded gradually compatible with classic choroidal neovascularization and other inferior foci of stippled hyperfluorescence that was compatible with occult choroidal neovascularization; (f) Enhanced depth imaging optical coherence tomography of the right eye shows subretinal fluid and hyperreflective materials in the location of macular choroidal neovascularization with increased choroidal thickness especially in the nasal retina; (g) Enhanced depth imaging optical coherence tomography of the left eye shows relatively increased choroidal thickness with normal retinal structure; (h) Optical coherence tomography angiography confirmed the presence of choroidal neovascularization in the right eye; (i) Optical coherence tomography angiography of the right eye 1 month after 2 monthly intravitreal bevacizumab injections
Photographs of the fundus before initiation of visual symptoms were not available. However, review of regular biannual ocular examination records before initiation of visual symptoms demonstrated no abnormalities.
Multimodal imaging of patient's right fundus including fundus autofluorescence (FAF), fluorescein angiography (FA), enhanced depth optical coherence tomography (OCT), and optical coherence tomography angiography (OCTA) confirmed the presence of CNV in the right eye [Figure 1b-h]. FA of the left eye was normal.
According to his clinical examination, FA and OCTA decided to inject two monthly doses of intravitreal bevacizumab [Genentech: Avastin® (bevacizumab)]. One month after the last injection OCTA was repeated, macular CNV regressed significantly, and the patient's visual acuity rose to 20/40 [Figure 1i]. Written informed consent was obtained from the patient to report this case.
DISCUSSION
CML is a myeloproliferative neoplasm result from a reciprocal translocation of t (9; 22) (q34; q11), which is termed the Philadelphia chromosome and consists of granulocytes proliferation with fairly normal differentiation.4 First and second-generation oral tyrosine kinase inhibitors (TKIs) (e. g., imatinib, dasatinib, nilotinib, bosutinib) can accomplish long-term control of CML in the majority of patients; thus, they have become the initial treatment of choice.5,6,7
Leukemia's ophthalmic manifestations can result from primary/direct leukemic infiltration of ocular tissues (leukemic infiltrates and white-centered retinal hemorrhages) or secondary/indirect ocular involvement following systemic leukemic changes (anemia, thrombocytopenia, and hyperviscosity) and immunosuppression causing opportunistic infections.2
Peripheral retinal neovascularization has been reported in CML patients in association with peripheral capillary non-perfusion. Most cases have been associated with severe leukocytosis or thrombocytosis. Presumably, the hyperviscosity state can lead to peripheral non-perfusion and subsequent development of retinal neovascularization, as in patients with proliferative sickle retinopathy, but single macular CNV in individuals with CML has not been documented.1,8,9,10,11,12,13 Delaney and Kinsella reported a patient with CML and disc neovascularization without macular CNV who had good peripheral perfusion, although the macula did show areas of non-perfusion.14
Yang et al. recently reported a 22-year-old woman with unilateral macular CNV in recently diagnosed acute myelocytic leukemia that responded to single intravitreal ranibizumab injection.3
Leukocytosis and thrombocytosis in CML patients can lead to increased blood viscosity, and resultant slow blood flow can be associated with hypoxia and proliferative retinopathy in leukemic patients. On the other hand, anemia may likewise prompt hypoxia. However, as we mentioned, the CML patient that we presented in this case was in the remission phase under treatment with imatinib for more than five years with normal hematologic cell counts and no capillary non-perfusion areas in FA.
The increased choroidal thickness of both eyes, especially in the right eye with pachy choroidal vessels in nasal side of fovea, may suggest pachychoroid neovasculopathy (PNV) as a reason of macular CNV in this patient, but previous retinal exam of this patient six months before presentation did not show any abnormality. As a result, this scenario may suggest that structural assessment of these patients with modalities like OCT may be mandatory to find abnormalities like pachychoroid pigment epitheliopathy or pachy vessles as predisposing conditions can lead to PNV.
Recent studies have shown that leukemic cells in CML can secrete excessive vascular endothelial growth factor (VEGF), which is essential for the occurrence and development of CNV, and this increase in VEGF level may be a justification of pachychoroid in both eyes of our patient and PNV in the right eye.8 Another exciting recent study used multimodal imaging such as spectral domain optical coherence tomography (SD-OCT), OCTA, and ultra-widefield fluorescein angiography (UWF FA) in leukemic patients shows that retinal vascular pathologies either in the posterior pole or in the periphery are present frequently in these patients; however, they are asymptomatic.15
Common ocular side-effects with imatinib mesylate are periorbital edema, epiphora, extraocular muscle palsy, ptosis, and blepharoconjunctivitis, and, in rare conditions, glaucoma, papilledema, retinal hemorrhage, photosensitivity, abnormal vision, and increased intraocular pressure have been reported.16
It appears that the association of retinal neovascularization and imatinib mesylate is a very rare complication. Gulati et al. reported a case of retinal neovascularization and hemorrhage associated with the use of imatinib (Gleevec(®)) for 7 months in a 62-year-old patient being treated for gastrointestinal stromal tumor (GIST). Their patient's symptoms significantly improved with a dose reduction of imatinib from 400 mg daily to 250 mg, but they did not mention where the site of neovascularization was.17
In conclusion, although we could not directly attribute unilateral macular CNV of this patient to CML complications or imatinib side-effects, macular CNV must be kept in mind as a rare ophthalmic manifestation of patients with CML under treatment with imatinib even in the remission phase.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Macedo MS, Figueiredo AR, Ferreira NN, Barbosa IM, Furtado MJ, Correia NF, et al. Bilateral proliferative retinopathy as the initial presentation of chronic myeloid leukemia. Middle East Afr J Ophthalmol. 2013;20:353–6. doi: 10.4103/0974-9233.120016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rane PR, Barot RK, Gohel DJ, Bhagat N. Chronic Myeloid Leukaemia Presenting as Bilateral Retinal Haemorrhages with Multiple Retinal Infiltrates. J Clin Diagn Res. 2016;10:ND04–5. doi: 10.7860/JCDR/2016/18215.7822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang X, Xu J, Yang J, Zhou Y, Mei Y, Yang T, et al. Unilateral macular choroidal neovascularization-a rare manifestation in acute myelocytic leukemia:Case report. Medicine (Baltimore) 2018;97:e0344. doi: 10.1097/MD.0000000000010344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Awuah A, Asiedu K, Adanusa M, Ntodie M, Acquah E, Kyei S. A case of leukemic retinopathy mimicking common ischemic retinopathies. Clin Case Rep. 2016;4:133–7. doi: 10.1002/ccr3.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kang ZJ, Liu YF, Xu LZ, Long ZJ, Huang D, Yang Y, et al. The Philadelphia chromosome in leukemogenesis. Chin J Cancer. 2016;35:48. doi: 10.1186/s40880-016-0108-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clarkson B, Strife A, Wisniewski D, Lambek CL, Liu C. Chronic myelogenous leukemia as a paradigm of early cancer and possible curative strategies. Leukemia. 2003;17:1211–62. doi: 10.1038/sj.leu.2402912. [DOI] [PubMed] [Google Scholar]
- 7.Santos FP, Ravandi F. Advances in treatment of chronic myelogenous leukemia—new treatment options with tyrosine kinase inhibitors. Leuk Lymphoma. 2009;50(Suppl 2):16–26. doi: 10.3109/10428190903383427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Almeida DR, Chin EK, Grant LW. Chronic myelogenous leukemia presenting with bilateral optic disc neovascularization. Can J Ophthalmol. 2014;49:e68–70. doi: 10.1016/j.jcjo.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 9.Anderton L, Amoaku WM, Noble BA. Retinal and optic disc neovascularization in leukaemia. Acta Ophthalmol (Copenh) 1994;72:752–6. doi: 10.1111/j.1755-3768.1994.tb04694.x. [DOI] [PubMed] [Google Scholar]
- 10.Frank RN, Ryan SJ., Jr Peripheral retinal neovascularization with chronic myelogenous leukemia. Arch Ophthalmol. 1972;87:585–9. doi: 10.1001/archopht.1972.01000020587017. [DOI] [PubMed] [Google Scholar]
- 11.Leveille AS, Morse PH. Platelet-induced retinal neovascularization in leukemia. Am J Ophthalmol. 1981;91:640–4. doi: 10.1016/0002-9394(81)90066-0. [DOI] [PubMed] [Google Scholar]
- 12.Morse PH, McCready JL. Peripheral retinal neovascularization in chronic myelocytic leukemia. Am J Ophthalmol. 1971;72:975–8. doi: 10.1016/0002-9394(71)91701-6. [DOI] [PubMed] [Google Scholar]
- 13.Nobacht S, Vandoninck KF, Deutman AF, Klevering BJ. Peripheral retinal nonperfusion associated with chronic myeloid leukemia. Am J Ophthalmol. 2003;135:404–6. doi: 10.1016/s0002-9394(02)01956-6. [DOI] [PubMed] [Google Scholar]
- 14.Delaney WV, Jr, Kinsella G. Optic disk neovascularization in leukemia. Am J Ophthalmol. 1985;99:212–3. doi: 10.1016/0002-9394(85)90238-7. [DOI] [PubMed] [Google Scholar]
- 15.Liu TY, Smith BD, Mackey K, Linz MO, Scott AW. Retinal vascularchanges on optical coherence tomography angiography and ultra-widefield fluorescein angiography in patients with chronic leukemia. J Vitreoretin Dis. 2019;3:420–7. [Google Scholar]
- 16.Fraunfelder FW, Solomon J, Druker BJ, Esmaeli B, Kuyl J. Ocular side-effects associated with imatinib mesylate (Gleevec) J Ocul Pharmacol Ther. 2003;19:371–5. doi: 10.1089/108076803322279426. [DOI] [PubMed] [Google Scholar]
- 17.Gulati AP, Saif MW. Retinal neovascularization and hemorrhage associated with the use of imatinib (Gleevec(®)) in a patient being treated for gastrointestinal stromal tumor (GIST) Anticancer Res. 2012;32:1375–7. [PubMed] [Google Scholar]