Abstract
Purpose:
To assess the possible association between keratoconus (KC) and serum levels of 25-hydroxyvitamin D (25OHD), Selenium (Se), Zinc (Zn), and Copper (Cu) and to compare it with age-matched healthy subjects.
Methods:
One hundred patients with KC and 100 normal subjects were included. The two groups were compared for serum 25OHD and serum levels of three trace elements: Se, Zn, and Cu. These factors were also compared between groups with different KC stages.
Results:
Serum levels of vitamin D, Zn, Cu, and Se were significantly different between the KC and normal groups (P = 0.006, P = 0.015, P = 0.004, and P = 0.038, respectively). Although a lower level of 25OHD was found in severe stages of KC, it was not significantly different among different KC groups (P = 0.441). KC stage groups were not significantly different for mean serum Zn, Cu, and Se (P = 0.130, P = 0.98, P = 0.113, respectively). Although the Cu/Zn ratio was higher in cases than in controls, there was no significant difference between the two groups and between KC stages (P = 0.168, P = 0.143, respectively).
Conclusion:
Lower serum 25OHD, Cu, Zn, and Se were found in the KC group compared to the control group. The results of this study suggest that a lower antioxidative activity may be involved in the possible etiology of KC.
Keywords: Cu, Keratoconus, Se, Serum 25-hydroxyvitamin D, Zn
INTRODUCTION
Keratoconus (KC) is a common corneal ectasia which is characterized by corneal thinning, irregular astigmatism, decreased visual acuity, and scarring.1 The prevalence of KC has been reported to be higher in Asians than white people.2 In the Iranian population, KC has a prevalence that has been estimated to be about 2.5-3.3%.3,4
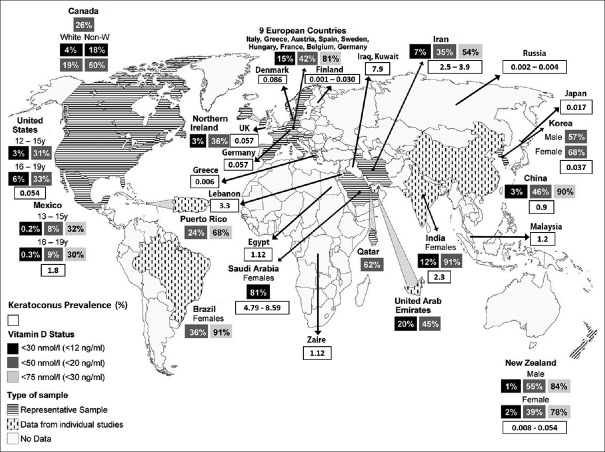
The etiopathogenesis of KC is unclear, but previous studies have shown that an interplay between genetic and environmental factors affect its occurrence.5 Some theories have proposed the critical role of oxidative stress in KC formation and progression,6,7 and molecular analysis has revealed alterations in the activity of proteases, over expression of cytokines and some matrix metalloproteinases (MMPs) in KC tear film compared with controls.8 There is alsoreportedto bea decreased activity of some proteins like catalase, glutathione, and superoxide dismutase (SOD) in KC which are involved in the process of free radical detoxification.9 Studies also have suggested the antioxidative role of 25-hydroxyvitamin D (25OHD) in amelioration of oxidative stress.10 Vitamin D receptor knockout decreases the wound healing rate of corneal epithelium in mice. It also affects the integrity of corneal tight junctions.11,12 The idea for this study came from the highly reported prevalence of KC and vitamin D deficiency in the Middle East region3,4,13,14,15,16 and the relative concordance of their prevalence in topographic maps which indicate a possible association between vitamin D deficiency and KC in people who live in this geographical area16 [Figure 1]. In 2016, a prevalence of 79% of vitamin D deficiency was reported among 846 subjects who were randomly derived from the Mashhad study.17
Figure 1.
Worldwide keratoconus (KC) prevalence has been matched to the prevalence map of Vitamin D deficiency (reproduced with permission from Palacios and Gonzales16)
Furthermore, the antioxidative role of some trace elements, especially in advanced KC, has been proposed.18 Keratoconic corneas show decreased activity of lysyl oxidase enzyme, which is important in corneal cross-linking, and Copper (Cu) is its co-factor.19,20,21 Selenium (Se) components can protect the cornea by regulating oxidative stress in the epithelium,22 and Zinc (Zn) supplementations down regulate the inflammatory cytokines and decrease oxidative stress.23 The prevalence of Zn and Cu deficiency has been reported to be 22.1% and 32.1% in Mashhad, respectively.24
Moreover, it has been shown that intra and extra cellular imbalance of Magnesium (Mg) due to Mg deficiency can be related to KC.25
To the best of the authors' knowledge, this is the first study which evaluates a possible association between 25OHD plasma level and different stages of KC. We also compared serum levels of 25OHD and three major trace elements including Cu, Zn, and Se, between the KC and normal subjects.
METHODS
This cross-sectional study adheres to the tenets of the Declaration of Helsinki and was approved by the Ethical Committee of Mashhad University of Medical Sciences. One hundred keratoconic patients who presented to the Cornea service of Khatam Al-Anbia Eye Specialty Hospital, Mashhad, Iran and had no disorder other than KC were recruited. Written informed consent was obtained from all patients before enrollment. KC was diagnosed according to topography, Orbscan, (Bausch and Lomb, Rochester, NY, USA) parameters and clinical examination based on Collaborative Longitudinal Evaluation of Keratoconus (CLEK) study criteria defined as: distortion of the keratometry mires or retinoscopic reflex which indicates an irregular cornea, showing at least 1 of the ophthalmoscopic signs of Vogt's striae, more than 2 mm arc of Fleischer's ring or corneal scarring.26 Moreover, KC patients were classified into four stages according to Krumeich criteria27 and compared to 100 age- and sex-matched healthy subjects. The control group was normal subjects in the hospital who were mostly candidates for refractive surgery and were ruled out for KC (e.g., forme fruste, subclinical, and clinical KC).
All subjects had Iranian ethnicity and were residents of an urban community. Patients with a history of penetrating keratoplasty or any ocular surgery, corneal scarring, severe dry eye disease, glaucoma, or taking any antioxidant/vitamin or anti-inflammatory treatment were excluded.
Fasting blood samples were taken in the morning from all participants. Serum was separated by centrifugation for 10 min at 3000 rate per minute and kept at -80 degrees Celsius until it was analyzed. Blood samples were examined in the same laboratory and observed with the same circumstances.
After allowing the stored samples to equilibrate to room temperature, they were diluted with deionized water (10:1) for evaluation of Zn, Cu, and Se. Serum Cu was analyzed using auto analyzer system (Premium, Biorex, Japan) with colorimetric method. Serum Zn was analyzed with the same device using a kit purchased from Grainer (Frickenhausen, Germany) with colorimetric method. For evaluation of Se level, Flame atomic absorption system (Varian, USA) was used. Serum 25OHD was measured by HPLC system (Agilent, USA) with chromatographic method.28
Statistical analysis was performed using IBM SPSS statistical software for Windows version 22 (IBM Co., Chicago, IL). Kolmogorov-Smirnov test was used to assess the normal distribution of data. Independent t-test and Mann-Whitney U tests were performed for comparison between groups. Categorical variables were analyzed using χ2 test. Mean serum trace elements were compared in four KC stages using one-way ANOVA or Kruskal Wallis-H tests. A multinomial logistic regression model was created to assess the affecting factors on severity of KC (KC stage), considering age, sex, 25OHD, Zn, Cu, Se serum level, and by convention, parameters with a significance level of P < 0.2 between 4 KC stages were entered to the model. For all results, P < 0.05 was considered statistically significant.
RESULTS
In this study, 103 (51.5%) men and 97 (48.5%) women with a mean age of 26.30 ± 7.32 years in the KC group and 27.82 ± 6.97 years in the control group (P = 0.132) participated. The two groups were not significantly different regarding gender (P = 0.572). Among 100 KC patients, 38 patients were in stage 1, 24 in stage 2, 27 in stage 3, and 11 were in stage 4 of KC.
Comparison of the serum 25-hydroxyvitamin D level between the study groups
Mean serum 25OHD was significantly lower in the case group (P = 0.006) [Table 1]. Although a lower mean serum 25OHD was also found in patients with higher KC stages, it was not significantly different between the 4 KC stage groups (P = 0.441) [Table 2]. Also, in post hoc pairwise comparison between KC stage groups, there was no significant difference for serum 25OHD (P > 0.05 for all).
Table 1.
Mean plasma 25-hydroxyvitamin D (25OHD), Zinc (Zn), Copper (Cu), Selenium (Se), Zn, and Cu in the keratoconus (KC) group compared with the control group
| Normal range | Cases (n=100) | Controls (n=100) | P | |
|---|---|---|---|---|
| 25OHD (ng/mL) | >20-25 | 11.83±7.64 | 15.51±10.95 | 0.006 |
| Se (mg/L) | 70-150 | 98.56±11.05 | 102.17±13.29 | 0.038 |
| Zn (mg/dL) | 66-110 | 58.07±18.42 | 64.28±17.17 | 0.015 |
| Cu (mg/dL) | 75-145 | 89.77±14.04 | 97.14±21.38 | 0.004 |
| Cu/Zn | 1.12-1.27 | 1.76±0.92 | 1.62±0.54 | 0.168 |
25OHD: 25-hydroxyvitamin D, Zn: Zinc, Cu: Copper, Se: Selenium
Table 2.
Mean plasma 25-hydroxyvitamin D (25OHD), Selenium (Se), Zinc (Zn), and Copper (Cu) in the keratoconus (KC) stagea groups
| Stage I (n=38) | Stage II (n=24) | Stage III (n=27) | Stage IV (n=11) | P | |
|---|---|---|---|---|---|
| Age | 25.84±6.78 | 26.75±7.37 | 25.89±7.05 | 27.81±10.05 | 0.854 |
| 25OHD (ng/mL) | 13.19±7.51 | 11.62±9.13 | 11.16±6.82 | 9.21±6.28 | 0.441 |
| Se (mg/L) | 100.70±8.58 | 93.80±13.13 | 100.50±9.76 | 100.25±14.84 | 0.113 |
| Zn (mg/dL) | 62.68±17.62 | 57.08±21.51 | 56.33±16.44 | 48.54±15.88 | 0.130 |
| Cu (mg/dL) | 89.87±13.05 | 88.96±15.80 | 89.85±16.08 | 90.95±8.43 | 0.98 |
| Cu/Zn | 1.54±0.45 | 2.00±1.53 | 1.73±0.62 | 2.10±0.88 | 0.143 |
aBased on Krumeich KC classification.27 25OHD: 25-hydroxyvitamin D, Se: Selenium, Zn: Zinc, Cu: Copper
Comparison of the serum levels of Zinc, Copper, and Selenium between the study groups
Serum level of Zn, Cu, and Se was significantly different between the case and control groups (P = 0.015, P = 0.004, and P = 0.038, respectively). Serum trace element concentrations were lower in KC patients than in the controls [Table 1].
No statistically significant difference was found between the 4 KC stages regarding mean serum Zn, Cu, and Se (P = 0.130, P = 0.98, and P = 0.113, respectively). In post hoc pairwise comparison, there was no significant difference between KC stages, for serum level of Zn, Se, and Cu (P > 0.05 for all).
According to Table 2, in comparison between KC stages, Zn and Se parameters showed P < 0.2 significance level and were entered in multinomial regression analysis. This model showed that Zn and Se serum levels were not significant affecting factors on KC stages (P = 0.08 and P = 0.072, respectively).
Cu to Zn ratio was not significantly different between the cases and controls (P = 0.168) [Table 1]. Also, the Cu/Zn ratio was not markedly different between KC stages (P = 0.143) [Table 2].
DISCUSSION
In recent years, vitamin D deficiency has been proposed as a global public health problem, and a high prevalence of hypovitaminosis D has been reported in all age groups, especially in those from the Middle East.13,14,15,16 KC is a prevalent disorder among people who live in developing countries.2,3,4,14 Vitamin D deficiency has been proven to be associated with higher risk of atopic diseases such as atopic dermatitis, some malignancies, and inflammatory and auto-immune disorders.29 Interestingly, ocular surface abnormalities like KC are more common in some of these diseases.30,31,32,33,34,35,36,37,38,39 Recent studies suggest a possible role of vitamin D in reducing oxidative stress.40 Animal studies have reported the antioxidative role of vitamin D in the treatment of diabetes mellitus in mice.10 Many parts of the eye contain vitamin D receptors, including the lens, corneal epithelium and endothelium, retinal photoreceptors, and retinal pigment epithelium.41 Vitamin D deficiency may interfere with the wound healing process, corneal epithelial and tight junction integrity, and gap junctions.11 It has been demonstrated that vitamin D deficiency can affect the ocular surface by inflammatory, apoptotic, or oxidative mechanisms.40
The results of our study showed that the mean serum level of 25OHD and Zn, in the case and control groups, were lower than normal which is in concordance with previously highly recorded vitamin D and Zn deficiency in our country. However, serum 25OHD was significantly different between the KC and normal groups. Although a lower serum 25OHD was found in patients with higher stages of KC, it was not significantly different between the KC stage groups. However, a lower vitamin D level in KC patients compared to healthy subjects, and serum level reduction of 25OHD with increasing KC stage, reveals a possible correlation between these factors. Similar to our results, Akkaya and Ulusoy,42 in a recent study, found lower serum level of 25OHD in KC patients. Although there was no significant difference between KC stages, they found higher rates of vitamin D deficiency in severe stages of KC. They stated that the low level of vitamin D can be related to the role of immunity and inflammation in the development of ectasia.
Recent studies have shown that hypovitaminosis D is highly prevalent in the Middle East13,15,16 which makes it critical to assess short and long-term outcomes of this deficiency and to investigate its probable contributions to other prevalent disorders including KC which are prevalent in this geographical area.
Antioxidant defense mechanisms are abnormal in KC corneas, and higher levels of oxidative byproducts such as nitrates are seen.43 Furthermore, it has been proposed that the accumulation of oxidative cytotoxic byproducts in KC corneas is due to the absence of defensive mechanisms such as catalase, SOD, and glutathione peroxidase which can eliminate them.44 Also, changes in the stress response of KC corneas can strongly alter the keratocytes' matrix production in stromal tissue.45 To investigate the role of oxidative stress on the development and progression of KC, several studies have investigated the serum levels of some trace elements in KC patients and compared them with healthy subjects. Cu dependent enzymes such as SODs and lysyl oxidase alter in KC corneas.44 Dudakova et al.20 proposed Cu imbalance in corneal tissue as a risk factor for KC. Kilic et al.46 found no significant difference for plasma level of Cu between KC and normal subjects, but similar to our findings, they found a lower level of Zn in KC patients. Kilic mentioned that the small sample size for SOD and Cu was a limitation for their study. However, similar to our results, they found no significant difference for Cu and Zn in different KC stages. Research has proved that Zn acts as an antioxidant and anti-inflammatory agent.23 Ortak et al.47 and his colleagues documented a significantly lower concentration of Zn and matrix metalloproteinase-2 (MMP-2) in plasma samples of KC patients. Bamdad et al.,18 reported similar findings. They found decreased serum levels of Cu, Zn, and Se in serum samples of patients with advanced KC compared to normal subjects. They also reported a marked association between serum levels of Cu and Zn in advanced KC. In our study, we included KC patients of 4 stages (according to Krumeich KC classification). In agreement with previous reports, our results showed a lower amount of Cu, Zn, and Se in the KC group compared to the normal group. Although it was not significantly different between the 4 KC stages, our findings showed an association between serum level of Zn and stage of KC. In concordance with previous reports,18 a lower serum level of Zn was seen in patients with more advanced KC, which reveals the possible role of Zn as an etiology of KC.
Previous studies have shown that, there is a relationship between Cu/Zn ratio and oxidative stress.48 It has also been reported that elevated Cu/Zn ratio is associated with increased oxidative stress and inflammation.49 Our findings showed relatively elevated Cu/Zn ratios both in controls and cases. Although it was not significant in different KC stages, higher amount of Cu/Zn ratio in KC patients can be related to oxidative stress and inflammatory nature of the KC.
Serum Se was markedly different between the normal and KC subjects. In agreement with previous reports,18 we found a lower amount of Se in serum samples of KC patients in comparison with the normal group, but it was not related to the stage of KC. The antioxidative role of Se has been proposed in the etiology of KC,18 and Higuchi et al.50 have shown that a key molecule which carries Se is selenoprotein P which plays an important role in protecting the ocular surface against oxidative stress.
In conclusion, the results of this study indicate lower serum levels of 25OHD, Cu, Zn, and Se in patients with KC, compared to the healthy control group. Considering that this is the first work to report the association between KC and vitamin D deficiency, the findings need to be verified by studies with a larger sample size with different KC stage groups. The results also strengthen the idea that supplemental use of vitamin D and aforementioned antioxidant agents may play a role in the prevention or stopping of the progression of KC.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
This work was supported by a research grant from the research deputy of Mashhad University of Medical Sciences.
REFERENCES
- 1.Romero-Jimenez M, Santodomingo-Rubido J, Wolffsohn JS. Keratoconus:A review. Contact Lens Anterior Eye. J Br Contact Lens Assoc. 2010;33:157–66. doi: 10.1016/j.clae.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 2.Georgiou T, Funnell CL, Cassels-Brown A, O'Conor R. Influence of ethnic origin on the incidence of keratoconus and associated atopic disease in Asians and white patients. Eye (Lond) 2004;18:379–83. doi: 10.1038/sj.eye.6700652. [DOI] [PubMed] [Google Scholar]
- 3.Hashemi H, Khabazkhoob M, Fotouhi A. Topographic keratoconus is not rare in an Iranian population:The Tehran eye study. Ophthalmic Epidemiol. 2013;20:385–91. doi: 10.3109/09286586.2013.848458. [DOI] [PubMed] [Google Scholar]
- 4.Hashemi H, Khabazkhoob M, Yazdani N, Ostadimoghaddam H, Norouzirad R, Amanzadeh K, et al. The prevalence of keratoconus in a young population in Mashhad, Iran. Ophthalmic Physiol Opt. 2014;34:519–27. doi: 10.1111/opo.12147. [DOI] [PubMed] [Google Scholar]
- 5.Gordon-Shaag A, Millodot M, Shneor E, Liu Y. The genetic and environmental factors for keratoconus. Biomed Res Int. 2015;2015:795738. doi: 10.1155/2015/795738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chwa M, Atilano SR, Hertzog D, Zheng H, Langberg J, Kim DW, et al. Hypersensitive response to oxidative stress in keratoconus corneal fibroblasts. Invest Ophthalmol Vis Sci. 2008;49:4361–9. doi: 10.1167/iovs.08-1969. [DOI] [PubMed] [Google Scholar]
- 7.Karamichos D, Zieske JD, Sejersen H, Sarker-Nag A, Asara JM, Hjortdal J. Tear metabolite changes in keratoconus. Exp Eye Res. 2015;132:1–8. doi: 10.1016/j.exer.2015.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Balasubramanian SA, Mohan S, Pye DC, Willcox MD. Proteases, proteolysis and inflammatory molecules in the tears of people with keratoconus. Acta Ophthalmol. 2012;90:e303–9. doi: 10.1111/j.1755-3768.2011.02369.x. [DOI] [PubMed] [Google Scholar]
- 9.Khaled ML, Helwa I, Drewry M, Seremwe M, Estes A, Liu Y. Molecular and Histopathological Changes Associated with Keratoconus. Biomed Res Int. 2017;2017:7803029. doi: 10.1155/2017/7803029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iqbal S, Khan S, Naseem I. Antioxidant Role of Vitamin D in Mice With Alloxan-Induced Diabetes. Can J Diabetes. 2018;42:412–8. doi: 10.1016/j.jcjd.2017.10.032. [DOI] [PubMed] [Google Scholar]
- 11.Elizondo RA, Yin Z, Lu X, Watsky MA. Effect of vitamin D receptor knockout on cornea epithelium wound healing and tight junctions. Invest Ophthalmol Vis Sci. 2014;55:5245–51. doi: 10.1167/iovs.13-13553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu X, Watsky MA. Effects of vitamin D receptor knockout on cornea epithelium gap junctions. Invest Ophthalmol Vis Sci. 2014;55:2975–82. doi: 10.1167/iovs.13-13788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tabrizi R, Moosazadeh M, Akbari M, Dabbaghmanesh MH, Mohamadkhani M, Asemi Z, et al. High prevalence of Vitamin D deficiency among Iranian population:A systematic review and meta-analysis. Iran J Med Sci. 2018;43:125–39. [PMC free article] [PubMed] [Google Scholar]
- 14.Assiri AA, Yousuf BI, Quantock AJ, Murphy PJ. Incidence and severity of keratoconus in Asir province, Saudi Arabia. Br J Ophthalmol. 2005;89:1403–6. doi: 10.1136/bjo.2005.074955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chakhtoura M, Rahme M, Chamoun N, El-Hajj Fuleihan G. Vitamin D in the Middle East and North Africa. Bone Rep. 2018;8:135–46. doi: 10.1016/j.bonr.2018.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Palacios C, Gonzalez L. Is vitamin D deficiency a major global public health problem? J Steroid Biochem Mol Biol. 2014;144(Pt A):138–45. doi: 10.1016/j.jsbmb.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bonakdaran S, Fakhraee F, Karimian MS, Mirhafez SR, Rokni H, Mohebati M, et al. Association between serum 25-hydroxyvitamin D concentrations and prevalence of metabolic syndrome. Adv Med Sci. 2016;61:219–23. doi: 10.1016/j.advms.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 18.Bamdad S, Owji N, Bolkheir A. Association between advanced keratoconus and serum levels of zinc, calcium, magnesium, iron, copper, and selenium. Cornea. 2018;37:1306–10. doi: 10.1097/ICO.0000000000001661. [DOI] [PubMed] [Google Scholar]
- 19.Dudakova L, Evans CJ, Liskova P. Copper in keratoconic corneas. Cornea. 2017;36:e14. doi: 10.1097/ICO.0000000000001155. [DOI] [PubMed] [Google Scholar]
- 20.Dudakova L, Liskova P, Jirsova K. Is copper imbalance an environmental factor influencing keratoconus development? Med Hypotheses. 2015;84:518–24. doi: 10.1016/j.mehy.2015.02.017. [DOI] [PubMed] [Google Scholar]
- 21.Avetisov S, Mamikonian V, Novikov I. The role of tear pH values and cucofactor of lysyl oxidase activity in the pathogenesis of keratoconus. Vestn Oftalmol. 2011;2:3–8. [PubMed] [Google Scholar]
- 22.Higuchi A, Inoue H, Kawakita T, Ogishima T, Tsubota K. Selenium compound protects corneal epithelium against oxidative stress. PLoS One. 2012;7:e45612. doi: 10.1371/journal.pone.0045612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prasad AS, Bao B, Beck FW, Kucuk O, Sarkar FH. Antioxidant effect of zinc in humans. Free Radic Biol Med. 2004;37:1182–90. doi: 10.1016/j.freeradbiomed.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 24.Parizadeh SM, Kazemi-Bajestani SM, Shapouri-Moghaddam A, Ghayour-Mobarhan M, Esmaeili H, Majdi MR, et al. Serum Zinc and Copper concentrations and socioeconomic status in a large Persian cohort. Asian Biomed. 2011;5:329–35. [Google Scholar]
- 25.Thalasselis A. The possible relationship between keratoconus and magnesium deficiency. Ophthalmic Physiol Opt. 2005;25:7–12. doi: 10.1111/j.1475-1313.2004.00232.x. [DOI] [PubMed] [Google Scholar]
- 26.Zadnik K, Barr JT, Edrington TB, Everett DF, Jameson M, McMahon TT, et al. Baseline findings in the collaborative longitudinal evaluation of keratoconus (CLEK) study. Invest Ophthalmol Vis Sci. 1998;39:2537–46. [PubMed] [Google Scholar]
- 27.Krumeich JH, Kezirian GM. Circular keratotomy to reduce astigmatism and improve vision in stage I and II keratoconus. J Refract Surg. 2009;25:357–65. doi: 10.3928/1081597X-20090401-07. [DOI] [PubMed] [Google Scholar]
- 28.Holick MF. Vitamin D status:Measurement, interpretation, and clinical application. Ann Epidemiol. 2009;19:73–8. doi: 10.1016/j.annepidem.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mesquita Kde C, Igreja AC, Costa IM. Atopic dermatitis and Vitamin D:Facts and controversies. An Bras Dermatol. 2013;88:945–53. doi: 10.1590/abd1806-4841.20132660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thyssen JP, Toft PB, Halling-Overgaard AS, Gislason GH, Skov L, Egeberg A. Incidence, prevalence, and risk of selected ocular disease in adults with atopic dermatitis. J Am Acad Dermatol. 2017;77:280–60. e1. doi: 10.1016/j.jaad.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 31.Hagan S, Martin E, Enríquez-de-Salamanca A. Tear fluid biomarkers in ocular and systemic disease:Potential use for predictive, preventive and personalised medicine. EPMA J. 2016;7:15. doi: 10.1186/s13167-016-0065-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nemet AY, Vinker S, Bahar I, Kaiserman I. The association of keratoconus with immune disorders. Cornea. 2010;29:1261–4. doi: 10.1097/ICO.0b013e3181cb410b. [DOI] [PubMed] [Google Scholar]
- 33.Arbab M, Tahir S, Niazi MK, Ishaq M, Hussain A, Siddique PM, et al. TNF-a genetic predisposition and higher expression of inflammatory pathway components in keratoconus. Invest Ophthalmol Vis Sci. 2017;58:3481–7. doi: 10.1167/iovs.16-21400. [DOI] [PubMed] [Google Scholar]
- 34.Lapp T, Maier P, Jakob T, Reinhard T. Pathophysiology of atopic blepharokeratoconjunctivitis. Ophthalmologe. 2017;114:504–13. doi: 10.1007/s00347-017-0483-1. [DOI] [PubMed] [Google Scholar]
- 35.Pahuja N, Kumar NR, Shroff R, Shetty R, Nuijts RM, Ghosh A, et al. Differential molecular expression of extracellular matrix and inflammatory genes at the corneal cone apex drives focal weakening in keratoconus. Invest Ophthalmol Vis Sci. 2016;57:5372–82. doi: 10.1167/iovs.16-19677. [DOI] [PubMed] [Google Scholar]
- 36.Galvis V, Sherwin T, Tello A, Merayo J, Barrera R, Acera A. Keratoconus:An inflammatory disorder? Eye (Lond) 2015;29:843–59. doi: 10.1038/eye.2015.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McMonnies CW. Inflammation and keratoconus. Optom Vis Sci. 2015;92:e35–41. doi: 10.1097/OPX.0000000000000455. [DOI] [PubMed] [Google Scholar]
- 38.Hagan S. Biomarkers of ocular surface disease using impression cytology. Biomark Med. 2017;11:1135–47. doi: 10.2217/bmm-2017-0124. [DOI] [PubMed] [Google Scholar]
- 39.Hashemi H, Mohebbi M, Mehravaran S, Mazloumi M, Jahanbani-Ardakani H, Abtahi SH. Hyperimmunoglobulin E syndrome:Genetics, immunopathogenesis, clinical findings, and treatment modalities. J Res Med Sci. 2017;22:53. doi: 10.4103/jrms.JRMS_1050_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cankaya C, Cumurcu T, Gunduz A. Corneal endothelial changes in patients with Vitamin D deficiency. Indian J Ophthalmol. 2018;66:1256–61. doi: 10.4103/ijo.IJO_238_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kizilgul M, Kan S, Ozcelik O, Beysel S, Apaydin M, Ucan B, et al. Vitamin D replacement improves tear osmolarity in patients with Vitamin D deficiency. Semin Ophthalmol. 2018;33:589–94. doi: 10.1080/08820538.2017.1358752. [DOI] [PubMed] [Google Scholar]
- 42.Akkaya S, Ulusoy DM. Serum Vitamin D levels in patients with keratoconus. Ocul Immunol Inflamm. 2019:1–6. doi: 10.1080/09273948.2019.1604002. doi:10.1080/09273948.2019.1604002. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 43.Toprak I, Kucukatay V, Yildirim C, Kilic-Toprak E, Kilic-Erkek O. Increased systemic oxidative stress in patients with keratoconus. Eye (Lond) 2014;28:285–9. doi: 10.1038/eye.2013.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Arnal E, Peris-Martínez C, Menezo JL, Johnsen-Soriano S, Romero FJ. Oxidative stress in keratoconus? Investig Ophthalmol Vis Sci. 2011;52:8592–7. doi: 10.1167/iovs.11-7732. [DOI] [PubMed] [Google Scholar]
- 45.Foster JW, Shinde V, Soiberman US, Sathe G, Liu S, Wan J, et al. Integrated stress response and decreased ecm in cultured stromal cells from keratoconus corneas. Invest Ophthalmol Vis Sci. 2018;59:2977–86. doi: 10.1167/iovs.18-24367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kılıç R, Bayraktar AC, Bayraktar S, Kurt A, Kavutçu M. Evaluation of serum superoxide dismutase activity, malondialdehyde, and zinc and copper levels in patients with keratoconus. Cornea. 2016;35:1512–5. doi: 10.1097/ICO.0000000000001018. [DOI] [PubMed] [Google Scholar]
- 47.Ortak H, Söğüt E, Taş U, Mesci C, Mendil D. The relation between keratoconus and plasma levels of MMP-2, zinc, and SOD. Cornea. 2012;31:1048–51. doi: 10.1097/ICO.0b013e318254c028. [DOI] [PubMed] [Google Scholar]
- 48.Wacewicz M, Socha K, Soroczyńska J, Niczyporuk M, Aleksiejczuk P, Ostrowska J, et al. Concentration of selenium, zinc, copper, Cu/Zn ratio, total antioxidant status and c-reactive protein in the serum of patients with psoriasis treated by narrow-band ultraviolet B phototherapy:A case-control study. J Trace Elem Med Biol. 2017;44:109–14. doi: 10.1016/j.jtemb.2017.06.008. [DOI] [PubMed] [Google Scholar]
- 49.Guo CH, Chen PC, Yeh MS, Hsiung DY, Wang CL. Cu/Zn ratios are associated with nutritional status, oxidative stress, inflammation, and immune abnormalities in patients on peritoneal dialysis. Clin Biochem. 2011;44:275–80. doi: 10.1016/j.clinbiochem.2010.12.017. [DOI] [PubMed] [Google Scholar]
- 50.Higuchi A, Takahashi K, Hirashima M, Kawakita T, Tsubota K. SelenoproteinPcontrols oxidative stress in cornea. PLoS One. 2010;5:e9911. doi: 10.1371/journal.pone.0009911. [DOI] [PMC free article] [PubMed] [Google Scholar]