Abstract
Acetamiprid, a selective agonist of nicotinic acetylcholine recetors, is one of the most widely used neonicotinoids. There is limited data about toxicity of acetamiprid on male reproductive system. Therefore, the study aimed to investigate the reproductive toxic potential of acetamiprid in male rats orally treated with acetamiprid with low (12.5 mg/kg) medium (25 mg/kg) or high dose (35 mg/kg) for 90 days. According to our results, sperm concentration and plasma testosterone levels decreased in dose dependent manner. Gonadotropin-releasing hormone (GnRH), follicle-stimulating hormeone (FSH), luteinizing hormone (LH) levels increased at low and medium dose groups and acetamiprid caused lipid peroxidation and glutathione (GSH) depletion in the testes. Histologic examinations revealed that acetamiprid induced apoptosis in medium and high dose groups and proliferation index dramatically decreased in high dose group. In conclusion, acetamiprid caused toxicity on male reproductive system in the high dose. The mechanism of the toxic effect may be associated with oxidative stress, hormonal disruptions and apoptosis.
Subject terms: Environmental sciences, Risk factors
Introduction
Neonicotinoids are new class of insecticides that act as selective nicotinic acetylcholine receptor (nAChR) agonist selectively in central nervous system of insects1. Acetamiprid, one of the neonicotinoid insecticides, is commonly used for agricultural and domestic purposes against a large variety of insects2,3. Acetamiprid has been reported to accumulate in plants and contaminate water and this can pose a potential risk for human health4,5.
Acetamiprid is absorbed easily after oral administration, and it is determined at the highest concentration in the liver, kidney, adrenal and thyroid glands6. Some researchers showed that acetamiprid caused toxic effects on several organ systems, including the nervous, respiratory, and immune systems in the experimental models7–9. Furthermore, it has been reported acute poisoning cases after ingestion of acetamiprid in humans10,11. Acetamiprid has also been reported to induce reproductive toxicity in different species12,13. The cross-sectional epidemiological study which was conducted in Kavar, (Iran) showed acetamiprid reduced the number of sperm in farmers who exposed to acetamiprid14.
As the use of acetamiprid is increasing, it is very important to identify the toxicity of acetamiprid. Additionally, acetamiprid can be used in combinations with other insecticide because of that, toxic effects and doses of acetamiprid are needed to elucidate well by chronic and subchronic toxicity studies. Acetamiprid has been shown toxic effects on many organs and systems. However, there is no satisfied information on the toxicity potential of acetamiprid on male reproductive system. In this study, it was aimed to examine the effects of acetamiprid on reproductive function of male rats in terms of oxidative stress, apoptosis, hormonal disruptions and histopathological changes.
Results
Effect of acetamiprid on body and testicular weights
Liver steatosis and slowness of the movements were observed at the high dose group. However, it was not found any differences in the food consumption of the groups. The body weights of control group were increased 2.14% comparing with first day of the study (p > 0.05). At the end of the 90th day, the body weights were decreased nonsignificantly 1.98, 1.60 and 3.30% for low-, medium- and high-dose groups, respectively, compared to the beginning of the experiment. Similarly, there was no significant change in the testicular weights (p > 0.05) as compared to the control group (Table 1).
Table 1.
Effect of oral acetamiprid on body weights and testicular weights of Sprague Dawley rats.
| Control group (n:10) | Low dose group (n:11) | Medium dose group (n:12) | High dose group (n:12) | |
|---|---|---|---|---|
| Body weights (g) | ||||
| Day 1 | 307.60 ± 1.46 | 329.43 ± 2.65 | 341.93 ± 3.43 | 326.60 ± 2.34 |
| Day 28 | 310.25 ± 1.65 | 326.45 ± 2.98 | 332.74 ± 2.76 | 322.15 ± 1.89 |
| Day 90 | 314.18 ± 1.60 | 322.89 ± 3.55 | 336.46 ± 3.89 | 315.82 ± 2.59 |
| Testicular weights (g) Day 90 | 1.50 ± 0.05 | 1.60 ± 0.03 | 1.55 ± 0.02 | 1.56 ± 0.05 |
Data were shown as mean ± standard error of the means (SEM). *p < 0.05, **p < 0.005, ***p < 0.0005.
Effect of acetamiprid on sperm count and morphology
Sperm count and morphology evaluations were performed according to the World Health Organization (WHO) guideline15. The number of sperm was significantly decreased in the medium- and high-dose groups (p ≤ 0.004) as compared to the control group (Table 2). However normal sperm count and abnormal sperm count did not show any significant difference among the treatment groups. In the abnormal sperm morphology evaluations, significantly increase in the flattened headed sperm was observed in the high dose treatment group (p = 0.046).
Table 2.
Evaluations of sperm morphology and counts in the Sprague Dawley rats treated orally with acetamiprid. Data were shown as mean ± standard error of the means (SEM).
| Sperm count (x106/mL) | ||||
|---|---|---|---|---|
| Control group (n:10) | Low dose group (n:11) | Medium dose group (n:12) | High dose group (n:12) | |
| Sperm count | 12.1 ± 0.4 | 11.4 ± 0.7 | 9.5 ± 0.5** | 8.6 ± 0.4*** |
| Sperm morphology (in 200 sperm) | ||||
| Control group (n:10) | Low dose group (n:11) | Medium dose group (n:12) | High dose group (n:12) | |
| Normal sperm count (mean ± SEM) | ||||
| Normal | 173.2 ± 3.8 | 174.0 ± 7.5 | 178.0 ± 3.5 | 163.6 ± 10.0 |
| Sperm count with abnormalities (mean ± SEM) | ||||
| Headless sperm | 7.3 ± 0.7 | 5.3 ± 1.7 | 5.0 ± 1.3 | 10.6 ± 2.7 |
| Detached head | 13.1 ± 0.7 | 13.6 ± 1.7 | 10.3 ± 1.3 | 15.8 ± 2.7 |
| Flattened head | 1.5 ± 0.3 | 1.5 ± 0.3 | 1.2 ± 0.2 | 3.6 ± 1.2* |
| Pinhead | 0.1 ± 0.1 | 0.3 ± 0.2 | 0.3 ± 0.1 | 0.3 ± 0.2 |
| Bent neck | 2.6 ± 0.3 | 2.7 ± 0.8 | 2.7 ± 0.6 | 3.7 ± 0.7 |
| Bent tail | 1.1 ± 0.2 | 1.2 ± 0.6 | 1.4 ± 0.5 | 1.1 ± 0.3 |
| Coiled tail | 0.7 ± 0.2 | 1.2 ± 0.4 | 0.9 ± 0.6 | 0.9 ± 0.3 |
| Multiple abnomalities | 0.3 ± 0.1 | 0.3 ± 0.1 | 0.3 ± 0.1 | 0.4 ± 0.2 |
| Total sperm count with abnormalities | 26.8 ± 3.9 | 26.0 ± 7.5 | 22.0 ± 3.5 | 36.4 ± 10.0 |
| Percentage of the sperm with abnormalities (%) | 13.4 ± 2.0 | 13.0 ± 3.8 | 11.0 ± 1.7 | 18.2 ± 5.0 |
| Percentage of the normal sperm (%) | 86.6 ± 2.0 | 87.0 ± 3.8 | 89.0 ± 1.7 | 81.8 ± 5.0 |
*p < 0.05, **p < 0.005, ***p < 0.0005.
Effect of acetamiprid on hormone levels and oxidative stress parameters
The plasma testosterone (≥ 23%) and cholesterol (≥ 12%) levels decreased in a dose dependent manner. The decrement of cholesterol level was found statistically significant in medium and high dose groups while the testosterone concentration decreased nonsignificantly. The plasma luteinizing hormone (LH), gonadotropin-releasing hormone (GnRH) and inhibin B (INHB) hormone levels increased significantly in the low and medium dose groups (≥ 2%, p < 0.05) as compared to the control group. The follicle-stimulating hormone (FSH) level also increased significantly in all the treatment groups (≥ 1.57 fold, p < 0.0005). However, it was observed that the plasma INHB levels declined 11% at the high dose of acetamiprid group.
The antioxidative parameters (Reduced glutathione (GSH) and total antioxidant status (TAS)) was found to decrease significantly in plasma and testes tissues in a dose-dependent manner (p < 0.05). Additionally, the oxidative parameters (Malondialdehyde (MDA) and Total oxidant status (TOS) of plasma and testes tissue levels were observed to increase significantly in a dose dependent manner (p < 0.05) (Table 3). The parameters were dramatically changed by 35 mg/kg acetamiprid treatment.
Table 3.
Hormones and oxidative stress parameters at the end of subchronical administration of oral acetamiprid in Sprague Dawley rats.
| Plasma levels (mean ± SEM) | ||||
|---|---|---|---|---|
| Control group (n:10) | Low dose group (n:11) | Medium dose group (n:12) | High dose group (n:12) | |
| Testosteron (ng/mL) | 2.32 ± 0.23 | 1.80 ± 0.49 | 1.65 ± 0.29 | 1.52 ± 0.17 |
| LH (mIU/mL) | 11.07 ± 0.57 | 12.65 ± 0.58* | 13.13 ± 0.17* | 11.86 ± 0.20 |
| FSH (IU/L) | 7.19 ± 0.56 | 11.87 ± 0.38*** | 14.27 ± 0.61*** | 11.30 ± 0.22*** |
| GnRH (ng/L) | 194.89 ± 11.88 | 207.72 ± 7.49 | 209.845 ± 0.95 | 200.67 ± 8.92 |
| INHB (ng/mL) | 8.25 ± 0.28 | 9.18 ± 0.16 | 10.070 ± 0.30* | 7.35 ± 0.53 |
| Cholesterol (mmol/L) | 5.46 ± 0.53 | 4.80 ± 0.34 | 4.39 ± 0.35* | 4.20 ± 0.26* |
| GSH (mmol/mg protein) | 0.47 ± 0.04 | 0.16 ± 0.01** | 0.22 ± 0.01*** | 0.21 ± 0.01*** |
| MDA (ng/mg protein) | 0.56 ± 0.01 | 0.82 ± 0.002*** | 1.03 ± 0.01*** | 1.46 ± 0.06*** |
| TAS (U/mL) | 34.67 ± 2.10 | 16.48 ± 0.78*** | 14.23 ± 0.65*** | 10.24 ± 0.82*** |
| TOS (nmol/mL) | 2.73 ± 0.26 | 2.82 ± 0.09 | 2.97 ± 0.12 | 3.68 ± 0.10*** |
| Tissue levels (mean ± SEM) | ||||
| GSH (mmol/mg protein) | 2.37 ± 0.006 | 1.99 ± 0.12*** | 1.68 ± 0.02*** | 1.52 ± 0.03*** |
| MDA (ng/mg protein) | 6.57 ± 0.46 | 6.87 ± 0.40 | 9.81 ± 1.007* | 11.30 ± 0,52*** |
| TAS (U/mL per g protein) | 46.53 ± 1.61 | 39.63 ± 1.26* | 38.03 ± 1.28*** | 30.54 ± 1.26*** |
| TOS (nmol/mL per g protein) | 12.88 ± 0.03 | 13.28 ± 0.42 | 13.73 ± 0.22 | 14.93 ± 0.20*** |
Data were shown as mean ± standard error of the means (SEM). *p < 0.05, **p < 0.005, ***p < 0.0005. LH: Luteinizing hormone, FSH: Follicle-stimulating hormone, GnRH: Gonadotrophin releasing hormone, INHB: Inhibin B, GSH: Glutathione, MDA: Malondialdehyde, TAS: Total antioxidant status, TOS: Total oxidant status.
Effects of acetamiprid on histopathology of testes
In the control group, normal testicular morphology with regular spermatogenic cells and seminiferous tubules with regular basement membrane was observed. In the low dose group (score 7.6) vacuole formation was observed in the germinal epithelium of some tubules. Regular basement membrane was observed in this group. In the medium-dose group (score 7.9), immature cells were observed in many tubule lumens. Decrease number of spermatogenic germ cells, damaged spermatogenic cells with vacuole formation and luminal immature cell rashes, were observed in a large number of seminiferous tubules in the high-dose group (score 8.1). Irregular and undulating basement membrane was observed in medium-dose and high-dose groups (Figs. 1 and 2). Seminiferous tubule score was decreased significantly all the treatment groups (p ≤ 0.01) as compared to the control group (Table 4).
Figure 1.
Representative light micrographs of experimental groups. Normal testis morphology with regular seminiferous tubules are seen in the control group (A). Normal seminiferous tubules (n) with a regular germinal epithelium and vacuolation (arrow) in the low-dose group (B). Some seminiferous tubules with immature cells (*) in their lumen as well as regular seminiferous tubules (n) in the medium-dose group (C); immature cells (*) in the lumen of some seminiferous tubules and vacuolation (arrow, inset) in the germinal epithelium in the high-dose group (D) are seen. H&E staining. Scale bars 50 µm.
Figure 2.
Representative photomicrographs of experimental groups. The regular basement membran of seminiferous tubules are seen in the control (A) and low-dose groups (B). Irregular and ondulated basement membrane (arrow) of the semniferous tubules are seen in the medium- dose (C) and high-dose groups (D). PAS reaction. Scale bars 50 µm.
Table 4.
Histopathological scoring, proliferation index and apoptotic index in the testes of Sprague Dawley rats treated with oral acetamiprid.
| Control group (n:10) | Low dose group (n:11) | Medium dose group (n:12) | High dose group (n:12) | |
|---|---|---|---|---|
| Seminiferous tubule score | 9.8 | 8.1## | 7.9## | 7.6## |
| Proliferation index | 42.0 | 37.1## | 30.5## | 24.8## |
| Apoptotic index (%) | 0 | 10.6 | 26.3# | 57.5## |
*p < 0.05, #p < 0.1, ##p < 0.01.
Proliferative cell nucleus antigen (PCNA)-positive cells were observed as dark brown in the seminiferous tubules of all groups. were observed (Fig. 3). Proliferation index was significantly decreased in all the treatment groups (p ≤ 0.01) as compared to the control group (Table 4).
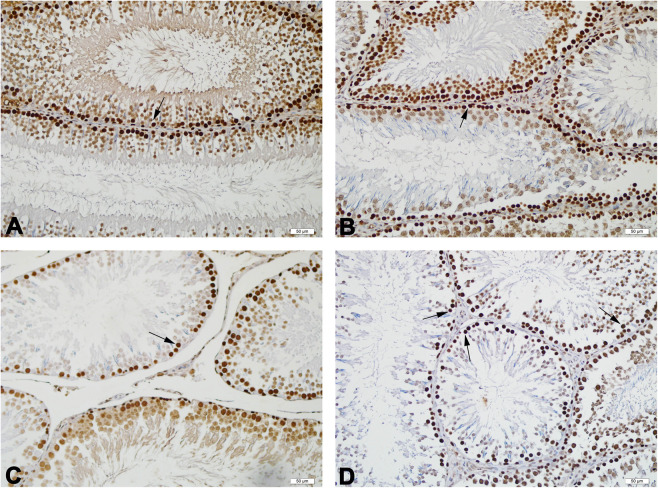
Figure 3.
Representative photomicrographs of PCNA stained testis tissues of the experimental groups. PCNA-positive cells (arrow), numerous in the control (A) and low (B)-dose groups, moderately decreased in the medium (C)-dose group, and severely decreased in the high (D)-dose group, are observed. Scale bars 50 µm.
Terminal deoxynucleotidyl transferase (TdT) dUTP nick-end labeling (TUNEL)-positive cells in the seminiferous tubules of all groups were observed as dark brown (Fig. 4). The apoptotic index was significantly increased in the medium (p < 0.1), and high-dose groups (p ≤ 0.01) compared to the control group (Table 4).
Figure 4.
Representative photomicrographs of TUNEL stained testis tissues of the experimental groups. A few number of TUNEL-positive cells (arrow), in the control (A) and low (B)-dose groups, moderately increased in the medium (C)-dose group, and severely increased in the high (D)-dose group are observed. TUNEL. Scale bars 50 µm.
Discussion
To identify the toxic effects of acetamiprid which is one of the widely used neonicotinoid insecticide on the reproductive system, sperm morphology, oxidative stress parameters, apoptosis, changes in the body and testicular weights were evaluated. In the present study, body weight decreased minimally in all acetamiprid-treated groups at the last day of experiment comparing to the beginning of the experiment except for control group and this may be associated with acetamiprid treatment and there was no significant change in testicular weights among the treatment groups. Zhang et al.7 also reported acetamiprid reduced body, and testis weights, in Kunming male mice treated with 30 mg/kg acetamiprid orally during 35 days. Similarly, Devan et al.8 observed a decrease in testicular absolute organ weight, and an increase in absolute organ weights, which of these changes are statistically non-significant in Wistar rats treated with orally acetamiprid at doses of 27.5, 55 and 110 mg/kg body weight. In the same study, NOAEL value was found to be ≤ 55 mg/kg.
It has been demonstrated that acetamiprid caused to change in serum testosterone levels and seminiferous tubule diameter and thickness in albino mice exposed to 0.16 mg/ml, and 0.22 mg/ml, by adding acetamiprid to drinking water for four weeks16. According to a study which was conducted by Rasgele17, acetamiprid did not increase the sperm abnormalities at the doses of 0.62, 1.25, and 2.50 μg/mL after 24 and 48 hours on in Mus musculus mice. A study conducted by Kong et al.18 demonstrated that acetamiprid increases LH levels and decreases testosterone levels and sperm count in Sprague Dawley rat treated with 10 mg/kg and 30 mg/kg dose. Zhang et al.7 also showed 30 mg/kg acetamiprid treatment decreased intact acrosome rate of sperms and testosterone level in Kunming male mice. In a study conducted by Mosbah et al.19, acetamiprid has been reported to increase in body weight and decline in testis weights, sperm count, plasma testosterone level, sperm motility in Wistar rats dosed by 27 mg/kg acetamiprid by gavage (5 days per week) for 45 days. In the present study, similar to Mosbah et al.19 we also found that acetamiprid induced sperm abnormalities and decreases sperm concentration and plasma testosterone levels in a dose dependent manner. Since testosterone which is main circulating androgen is synthesized from cholesterol20, the decrease of the plasma testosterone may result from decrease of plasma cholesterol level Eacker et al.21, and it was found that plasma cholesterol level declines significantly in a dose dependent manner. Besides, acetamiprid may induce oxidative stress in Leydig cells and can play a role in decreasing testosterone secretion19,22.
In addition to testosterone, GnRH, FSH, LH, and INHB hormones have important roles in spermatogenesis. The balance of these hormones is necessary for the proper functioning of the testes22. Thus, plasma levels of the hormones were also evaluated. Acetamiprid was found to disrupt the hormonal balance in the present study. It has been reported that increase in serum FSH and LH levels, and decrease in serum testosterone and INHB levels has been detected in patients who have low sperm concentration, and this can be used as biomarker for the diagnosis of the infertility in the clinic23,24. In the current study, we demonstrated that acetamiprid caused to increase FSH and LH plasma levels in all treatment groups. Although sperm count diminished in a dose dependent manner, the rate of the increase of these hormones was found to be lower in the high dose group. Interestingly, the plasma INHB level increased at the low and medium dose group whereas it decreased at the high dose group.
It has been stated that acetamiprid leads to oxidative stress in many organs such as liver, kidney, brain of different species8,25. Oxidative stress has been shown to cause damage in DNA, proteins and lipids of sperm and also induce apoptosis. As it is known, these mechanisms might play a role in decrease of sperm count and impairment of sperm function26. It is known that oxidative stress is one of the common reason of testicular dysfunction which leads to infertility27. Reactive oxygen species (ROS) production in the testis leads to oxidative damage and destroys steriodogenesis and spermatogenesis28. Some studies showed acetamiprid induced lipid peroxidation and reduced antioxidant enzymatic activity in the testes12,18. In the current study, acetamiprid led to lipid peroxidation, GSH depletion, increase in TOS, and decrease in TAS in testis homogenates in a dose dependent manner. These results were also confirmed in the plasma. However, these parameters were more affected in testis homogenate than plasma. This can be associated with high potential of ROS formation in the cells that use molecular oxygen in steroid biosynthesis in testes29. Oxidative stress also affects the regulation of proliferation, apoptosis and transcription in the testis30. Thus, acetamiprid-induced oxidative stress might result in reduced sperm count, increased sperm abnormalities and apoptosis induction in the study. Moreover, it has been stated testosterone might have antioxidant and antiapoptotic function in testes and protect sperm from DNA damage31,32 and decreased testosterone level may augment acetamiprid-induced oxidative stress in the present study. Besides, it has been stated INHB is released more in oxidative stress32. However, INHB level decreased nonsignificantly at high dose treatment group which we observed extensive oxidative stress compared to control group. This might be associated with dramatically increased apoptosis in testes at the high dose group.
As it is well known, the alterations of apoptosis have been involved in pathology of the many diseases such as cancer and neurodegenerative diseases33. It has been also showed that cell injury in testis may result in apoptosis34. In the present study, TUNEL cell count and apoptotic index were used to evaluate apoptotic cells. The number of apoptotic cell-containing seminiferous tubules was higher in the exposure groups than in the control group. The apoptotic index levels were 10.6, 26.3 and 57.5, respectively, in the low-, medium- and high-dose groups. Increased apoptosis may be a consequence of acetamiprid-induced oxidative stress. Histopathological examination revealed apoptotic cells in the tubules of all exposed groups, mostly in spermatogonia and primary spermatocytes. The number of apoptotic cells increased in a dose-dependent manner. According to the results of seminiferous tubule scoring and histopathological examination, the basement membranes of the seminiferous tubules were found to be regular in the control and low-dose exposure groups. In the medium- and high-dose exposure groups, the presence of basal membranes showing irregular and undulate structure in many seminiferous tubules was detected. In the high-dose group, a decrease in spermatogenic germ cell counts, spermatogenic cells with vacuole formation and luminal immature cell rashes were observed in most seminiferous tubules.
Spermatogenesis perturbation and weak sperm were seen in some seminiferous tubules of the Wistar rats treated with 27 mg/kg oral acetamiprid for 45 consecutive days. The presence of pelleted cell particles in the lumen, tubulous atrophy, disorganisation and degenerative direction of the seminal epithelium were observed35.
According to a study, degeneration of spermatogonia and edematous changes in seminiferous tubules and occlusion of blood vessels in the interstitial space have been reported in Swiss albino male mice treated with 2.3 mg/kg and 4.6 mg/kg acetamiprid for 30, 60 and 90 days36. In the present study, the proliferation index decreased in a dose-dependent manner, particularly in the high-dose exposure group by PCNA immunohistochemical technique.
In conclusion, acetamiprid was found to have potentially toxic to the male reproductive system on Sprague Dawley rats at the dose of 35 mg/kg. Underlying mechanisms of the toxic effects can be associated with oxidative stress and apoptosis in the testes. This data emphasizes the importance of taking precautions against environmental and occupational exposure to agricultural drugs. However, further mechanistic studies are needed to understand the molecular mechanisms of the acetamiprid induced reproductive toxicity.
Material and Method
Animals and their synchronization
A total of 48 (8–10 weeks old) male Sprague-Dawley rats with body weights of 300–350 g were obtained from Istanbul University Aziz Sancar Institute of Experimental Medicine and were divided into 4 groups, control group (n = 10), low dose group (n = 11), medium dose group (n = 12) and high dose group (n = 12). Rats were housed in polystyene standard cages with 4–5 animals in each, maintained at 21–23 °C and humidity (55 ± 5%) at Istanbul University Faculty of Pharmacy Animal Facility Unit (EDEHAB). Rats synchronized for a 12 h light/12 h dark cycle (LD 12:12). Standard pellet chow and tap water provided ad libitum throughout the experiment. Acetamiprid administration was performed during light span and commenced at 10:00 AM equal to 3 hours after light onset i.e. HALO-3 to prevent time dependent differences. The study was approved by Istanbul University Local Ethics Committee of Experimental Animals (IUHADYEK; 2016/35) and all experiments were performed in accordance with Istanbul University Animal Experiments Local Ethics Committee Guideline37,38.
Chemicals and reagents
Acetamiprid (97% of purity) was kindly gifted from Hektaş Ticaret T.A.Ş. (Istanbul, Turkey) and freshly suspended in an aqueous solution of 0.5% methylcellulose (MC) on each study day. Acetamiprid was orally administered to the rats in a fixed fluid volume (4 mL/kg body weight)37.
Sperm morphology was evaluated using phosphate-buffered saline (PBS 10×), Dulbecco’s Modified Eagle Medium/Nutrient Mixture F-12 (DMEM-F12), and fetal bovine serum (FBS) (Multicell Wisent, Quebec, Canada). For histological evaluation of testes, polylysine coated microscope slides and covers (Thermo Scientific, Munich, Germany) and microtome blades (Cologne, Germany), analytical grade chemicals (Sigma Aldrich, Munich, Germany and Merck Millipore, Munich, Germany) were used, while plastic wares from Eppendorf (Nijmegen, Netherlands) and Nest Biotechnology (Munich, Germany).
Experimental design
The threshold levels have been reported for the toxicity profiles of acetamiprid in female/male Sprague Dawley rats. No observable adverse effects level (NOAEL) and medium lethal dose (LD50) of acetamiprid were 7.1 mg/kg and 217 mg/kg, respectively39. According to the European Food Safety Authority (EFSA) 2016 data, reproductive NOAEL was 51 mg/kg40. The NOAEL of acetamiprid determined was 38.7 mg/kg mg/kg by two generation study in rats41. In our preliminary study, oral dose of 50 mg/kg of acetamiprid resulted in 25% of animal death. Accordingly, the acetamiprid dose groups were divided as highest dose group (35 mg/kg/day), medium dose group (25 mg/kg/day), and the low dose group (12.5 mg/kg/day). Acetamiprid was administered to rats during 90 days, by using oral gavage used for the rats. The control group was given only vehicle i.e., 0.5% MC.
At the end of the 90th day, the rats were exposed to inhalation anaesthesia for very short time. Then, they were sacrificed by removing large volume of blood from the orbital veins and immediately incised from abdominal to breast area. The blood samples were collected in EDTA tubes for hormone and biochemical analysis. The testes and epididymis were dissected, and sperms were collected from the epididymis. The left testis was homogenized in a sufficient amount of PBS (1×), and stored at −80 °C until biochemical analysis. The right testis was fixed in a sufficient amount of 10% neutral buffered formalin, and stored at room temperature until histological examinations.
Body weight and testis weights
Body weights of all animals were measured (Sartorius, Mettler H20, Germany) three times a week and at the end of the experiment. Mortality and clinical signs e.g. posture, locomotor activity, were controlled every day. Testicles were removed on sacrifice and weighed (Precisa XB220A, Switzerland) for the toxicological evaluation (U.S. Enviromental Protection Agency 1996). The testes were also evaluated macroscopically to reveal any enlargement, shrinkage, gaps due to tissue loss, tissue softening, tissue foreign coloring, and altered content.
The epididymis was cleaned from peripheral structures carefully, and weights were recorded. The somatic index was calculated by employing the following formula:
Epididymal sperm counting and morphology
Sperm collection and count
The caudal epididymis was minced with scissors in petri dish containing DMEM-F12 medium with 10% PBS to release sperm. The suspension was centrifuged at 2,230 rpm for 3 min. The supernatant was centrifuged again and then was added to trypan blue (1:1, v/v). The mixture was spread on the Thoma slide. The sperms were counted under a Leica microscope (Leica Microsystems Co., Wetzlar, Germany) at 20X magnification.
Sperm morphology
To assess sperm morphology, one drop of the epididymis suspension was spread on the slides and allowed to dry at room temperature. The morphological examinations of spreading samples were completed within one week. The samples were prepared in accordance with the Diff-Quick staining protocol (ADR, Istanbul, Turkey), and were carried out with 3 spreads for each sample and 200 sperm were counted for each preparation. The sperms were classified as normal, headless, detached head, flattened head, pinhead, bent neck, bent tailed, coiled tail and multiple abnormalities42,43.
Hormone and biochemical analysis in plasma and testicular tissue
The blood samples were centrifuged at 3000 rpm for 20 min −4 °C (Hettich Universal 32 R Germany). The plasma was separated and kept in −20 °C refrigerator (Arcelik 2041, Turkey). The quantitative determination of plasma cholesterol, testosterone, LH, FSH, GnRH and INHB was carried out in plasma by commercial kits according to the manufacturer’s instructions (Elabscience, Wuhan, China, and SunRed, Shanghai, China).
The testes tissues were homogenized in PBS (1:5, w/v) and kept at −80 °C (Daihan-Scientific Wisecry, South Korea). Biochemical parameters GSH, MDA, TAS and TOS indicating oxidative damage were measured by enzyme-linked immunosorbent assay (ELISA) commercial kits according to the manufacturer’s instructions (Elabscience, Wuhan, China, and SunRed, Shanghai, China). The optical density (OD) was measured at 450 nm, with a reference wavelength of 620 to 630 nm using an Epoch microplate spectrophotometer (BioTek Inc., Bad Friedrichshall, Germany).
Histological examination of testicular tissue
For the histological examination, the right testis was fixed in 10% neutral buffered formalin, dehydrated in a series of graded alcohol and embedded in paraffin44,45. Hematoxylin and eosin (H&E) staining was applied to the middle part of testis taken from paraffin blocks at a thickness of 4 μm for histopathologic analysis, and periodic acid Schiff (PAS) staining was performed to evaluate changes of seminiferous tubule basement membrane. The evaluation was performed on each of the 30 seminiferous tubules with a 20X microscopic magnification. The first evaluated seminiferous tubule was randomly selected, and the section was shifted clockwise to evaluate the others. Histopathologic scoring was assessed by the modified Johnson scoring method (Table 5)46.
Table 5.
Modified Johnson Scoring Method22.
| Score | Histological Findings |
|---|---|
| 10 | Full spermatogenesis |
| 9 | Slightly impaired spermatogenesis, many late spermatids, disorganized epithelium |
| 8 | Less than five spermatozoa per tubule, few late spermatids |
| 7 | No spermatozoa, no late spermatids, many early spermatids |
| 6 | No spermatozoa, no late spermatids, few early spermatids |
| 5 | No spermatozoa or spermatids, many spermatocytes |
| 4 | No spermatozoa or spermatids, few spermatocytes |
| 3 | Spermatogonia only |
| 2 | No germinal cells, Sertoli cells only |
| 1 | No seminiferous epithelium |
PCNA immunohistochemistry
The PCNA immunohistochemistry were done by using commercial kit from Invitrogen (Carlsbad, California, USA). For PCNA immunohistochemistry, half of the right testes were fixed for 72 h in 10% neutral buffered formalin. The paraffin sections with a thickness of 4 μm, which were collected on the positively charged slides, were done according to previous study47. Randomly selected, twenty seminiferous tubules were examined in the stained sections. The cells with brown nuclear staining were accepted PCNA positive and all of the stained and nonstained germ cells were counted. The ratio of PCNA positive cells to the total number of germ cells, “PCNA index,” was calculated for each seminiferous tubule. The average PCNA index in each case was obtained by dividing the sum of all PCNA indices by the number of seminiferous tubules in which the calculation was carried out.
Detection of apoptosis by TUNEL assay
TUNEL assay were done by using ApopTag Plus Peroxidase In Situ Apoptosis Kit (Millipore, Massachusetts-USA) according to manufacturer’s protocol with slight modifications. Twenty seminiferous tubules were examined in the stained sections. The seminiferous tubules containing 3 or more apoptotic cells were counted as TUNEL. The apoptotic index was calculated by the following formula:
Statistical evaluation
Data were expressed as means ± standard error of the means (SEM) for each studied variable. Statistical analysis was performed using the Statistical Package for Social Sciences (SPSS, v.20, Chicago, IL). The statistical significance of differences between groups was validated with one-way analysis of variance (ANOVA) followed by post hoc Tukey test for intergroup comparisons. p < 0.1, p < 0.05, p < 0.01, p < 0.005, and p < 0.0005 were considered significant.
Acknowledgements
The present work was supported by the Research Fund of Istanbul University. Project No: TDK-2016-22054.
Author contributions
Concept - Arıcan Y.E., Ozhan G.; Design - Arıcan Y.E., Ulus Karaca B., Boran T., Ozhan G.; Supervision - Ozhan G.; Resource - Ozhan G.; Materials - Ozhan G.; Data Collection and/or Processing - Arıcan Y.E., Gokceoğlu Kayalı D., Ulus Karaca B., Boran T., Ozturk N.; Analysis and/or Interpretation - Arıcan Y.E., Gokceoğlu Kayalı D., Ulus Karaca B., Boran T., Ozturk N., Okyar A., Ercan F., Ozhan G.; Literature Search - Arıcan Y.E., Gokceoğlu Kayalı D., Ulus Karaca B., Boran T., Ozturk N., Okyar A., Ercan F., Ozhan G.; Writing - Arıcan Y.E., Ulus Karaca B., Boran T., Ozhan G.; Critical Reviews - Ulus Karaca B., Boran T., Ozturk N., Okyar A., Ercan F., Ozhan G.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Han W, Tian Y, Shen X. Human Exposure to Neonicotinoid Insecticides and the Evaluation of Their Potential Toxicity: An Overview. Chemosphere. 2018;192:59–65. doi: 10.1016/j.chemosphere.2017.10.149. [DOI] [PubMed] [Google Scholar]
- 2.Brunet JL, Badiou A, Belzunces LP. In Vivo Metabolic Fate of [14C]-Acetamiprid in Six Biological Compartments of the Honeybee, Apis Mellifera L. Pest Manag Sci. 2005;61:742–748. doi: 10.1002/ps.1046. [DOI] [PubMed] [Google Scholar]
- 3.Shengyun, S., Xiaoming, L., Renfeng, W. & Yong, W. Efficacy of Several Insecticides on the Control of Myzus Persicae and Lipaphis Erysimi. Pesticide Science and Administration [accessed: 10 March 2019], http://en.cnki.com.cn/Article_en/CJFDTotal-NYKG200507004.htm (2005).
- 4.Zhou Q, Ding Y, Xiao J. Sensitive Determination of Thiamethoxam, Imidacloprid and Acetamiprid in Environmental Water Samples with Solid-Phase Extraction Packed with Multiwalled Carbon Nanotubes Prior to High-Performance Liquid Chromatography. Anal Bioanal Chem. 2006;385:1520–1525. doi: 10.1007/s00216-006-0554-7. [DOI] [PubMed] [Google Scholar]
- 5.Goulson D. An Overview of the Environmental Risks Posed by Neonicotinoid Insecticides. J Appl Ecol. 2013;50:977–987. doi: 10.1111/1365-2664.12111. [DOI] [Google Scholar]
- 6.U.S. Enviromental Protection Agency Guidelines for Reproductive Toxicity Risk Assessment Proposed Guidelines: Proposed Guidelines for Female Reproductive Risk and Proposed Guidelines for Male Reproductive Risk. Report No:EPA/630/R-96/009 [accessed: 10 March 2019] Available from, https://www.epa.gov/sites/production/files/201411/documents/guidelines_repro_toxicity.pdf. (1996).
- 7.Zhang J, et al. Oxidative Stress: Role in Acetamiprid-Induced Impairment of the Male Mice Reproductive System. Agr Sci China. 2011;10:786–796. doi: 10.1016/S1671-2927(11)60063-1. [DOI] [Google Scholar]
- 8.Devan RKS, Mishra A, Prabu PC, Mandal TK, Panchapakesan S. Sub-Chronic Oral Toxicity of Acetamiprid in Wistar Rats. Toxicol Environ Chem. 2015;97:1236–52. doi: 10.1080/02772248.2015.1092542. [DOI] [Google Scholar]
- 9.Çavaş T, Çinkılıç N, Vatan Ö, Yılmaz D. Effects of Fullerenol Nanoparticles on Acetamiprid Induced Cytoxicity and Genotoxicity in Cultured Human Lung Fibroblasts. Pestic Biochem Physiol. 2014;114:1–7. doi: 10.1016/j.pestbp.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 10.Todani M, et al. Acute Poisoning with Neonicotinoid Insecticide Acetamiprid. Chudoku Kenkyu-The Japanese Journal of Toxicology. 2008;21:387–390. [PubMed] [Google Scholar]
- 11.Imamura T, Yanagawa Y, Nishikawa K, Matsumoto N, Sakamoto T. Two Cases of Acute Poisoning with Acetamiprid in Humans. Clin Toxicol. 2010;48:851–53. doi: 10.3109/15563650.2010.517207. [DOI] [PubMed] [Google Scholar]
- 12.Kenfack A, et al. Reproductive Toxicity of Acetamiprid in Male Guinea Pig (Cavia Porcellus) JASVM. 2018;3:105–111. [Google Scholar]
- 13.Terayama. H, et al. Effect of Acetamiprid on the Immature Murine Testes. Int. J. Environ Health Res. 2018;28:683–96. doi: 10.1080/09603123.2018.1504897. [DOI] [PubMed] [Google Scholar]
- 14.Neghab M, et al. The Effects of Exposure to Pesticides on the Fecundity Status of Farm Workers Resident in a Rural Region of Fars Province, Southern Iran. Asian Pac J Trop Biomed. 2014;4:324–328. doi: 10.12980/APJTB.4.2014C586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen-Fifth Edition [accessed 2019 May 25], https://apps.who.int/iris/bitstream/handle/10665/44261/1978924157760_engpdf=1?sequence=1 (2010).
- 16.Chawseen MA. Effects Of Acetamiprid and Glyphosate Pesticides on Testis and Serum Testosterone Level in Male Mice. Journal of Duhok University. 2011;14:299–306. [Google Scholar]
- 17.Rasgele P. Abnormal Sperm Morphology in Mouse Germ Cells after Short-Term Exposures to Acetamiprid, Propineb, and Their Mixture. Arh Hig Rada Toksikol. 2014;65:47–56. doi: 10.2478/10004-1254-65-2014-2408. [DOI] [PubMed] [Google Scholar]
- 18.Kong D, et al. Acetamiprid Inhibits Testosterone Synthesis by Affecting the Mitochondrial Function and Cytoplasmic Adenosine Triphosphate Production in Rat Leydig Cells. Biol Reprod. 2016;96:254–265. doi: 10.1095/biolreprod.116.139550. [DOI] [PubMed] [Google Scholar]
- 19.Mosbah R, Djerrou Z, Mantovani A. Protective Effect of Nigella Sativa Oil against Acetamiprid Induced Reproductive Toxicity in Male Rats. Drug Chem Toxicol. 2018;41:206–212. doi: 10.1080/01480545.2017.1337127. [DOI] [PubMed] [Google Scholar]
- 20.Orta Yilmaz B, Korkut A, Erkan M. Sodium Fluoride Disrupts Testosterone Biosynthesis by Affecting the Steroidogenic Pathway in TM3 Leydig Cells. Chemosphere. 2018;212:447–455. doi: 10.1016/j.chemosphere.2018.08.112. [DOI] [PubMed] [Google Scholar]
- 21.Eacker SM, et al. Hormonal Regulation of Testicular Steroid and Cholesterol Homeostasis. Mol Endocrinol. 2008;22:623–35. doi: 10.1210/me.2006-0534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holdcraft RW, Braun RE. Hormonal Regulation of Spermatogenesis. Int J Androl. 2004;27:335–342. doi: 10.1111/j.1365-2605.2004.00502.x. [DOI] [PubMed] [Google Scholar]
- 23.Hipler UC, Hochheim B, Knöll B, Tittelbach J, Schreiber G. Serum Inhibin B as a Marker for Spermatogenesis. Arch Androl. 2001;46:217–22. doi: 10.1080/01485010151096540. [DOI] [PubMed] [Google Scholar]
- 24.Kumanov P, Nandipati K, Tomova A, Agarwal A. Inhibin B Is A Better Marker of Spermatogenesis than Other Hormones in the Evaluation of Male Factor Infertility. Fertil Steril. 2006;86:332–38. doi: 10.1016/j.fertnstert.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 25.Gasmi S, et al. Alteration of Membrane Integrity and Respiratory Function of Brain Mitochondria in the Rats Chronically Exposed to A Low Dose of Acetamiprid. ESPR. 2017;24:22258–22264. doi: 10.1007/s11356-017-9901-9. [DOI] [PubMed] [Google Scholar]
- 26.Walczak–Jedrzejowska R, Wolski JK, Slowikowska–Hilczer J. The role of oxidative stress and antioxidants in male fertility. Cent European J Urol. 2013;66:60–67. doi: 10.5173/ceju.2013.01.art19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Turner TT, Lysiak JJ. Oxidative Stress: A Common Factor in Testicular Dysfunction. J Androl. 2008;29:488–498. doi: 10.2164/jandrol.108.005132. [DOI] [PubMed] [Google Scholar]
- 28.Chainy GBN, Samantaray S, Samanta L. Testosterone-Induced Changes in Testicular Antioxidant System. Andrologia. 2009;29:343–349. doi: 10.1111/j.1439-0272.1997.tb00328.x. [DOI] [PubMed] [Google Scholar]
- 29.Zaidi SK, Shen WJ, Azhar S. Impact of Aging on Steroid Hormone Biosynthesis and Secretion. Open Longev Sci. 2012;6:1–30. doi: 10.2174/1876326X01206010001. [DOI] [Google Scholar]
- 30.Li Y, et al. Characterization of Prohibitins in Male Reproductive System and Their Expression under Oxidative Stress. J Urol. 2016;195:1160–67. doi: 10.1016/j.juro.2015.10.179. [DOI] [PubMed] [Google Scholar]
- 31.Vasconsuelo A, Pronsato L, Ronda AC, Boland R, Milanesi L. (2011). Role of 17β-estradiol and testosterone in apoptosis. Steroids. 2011;76:1223–1231. doi: 10.1016/j.steroids.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 32.Darbandi M, et al. Reactive Oxygen Species and Male Reproductive Hormones. Reprod Biol Endocrin. 2018;16:1–14. doi: 10.1186/s12958-018-0406-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Favaloro B, Allocati N, Graziano V, Di Ilio C, De Laurenzi V. Role of Apoptosis in Disease. Aging. 2012;4:330–49. doi: 10.18632/aging.100459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boekelheide K, Fleming SL, Johnson KJ, Patel SR, Schoenfeld HA. Role of Sertoli Cells in Injury-Associated Testicular Germ Cell Apoptosis. Proc Soc Exp Biol Med. 2008;225:105–115. doi: 10.1111/j.1525-1373.2000.22513.x. [DOI] [PubMed] [Google Scholar]
- 35.Rachid M, Ibrahim YM, Aziez C. Nigella Sativa Oil Protects against Reproductive Toxicity of Acetamiprid Insecticide in Male Rats. Toxicol Lett. 2013;221:S218. doi: 10.1016/j.toxlet.2013.05.652. [DOI] [Google Scholar]
- 36.Jain, S. K. Studies on Genotoxicity and Immunotoxicity of Acetamiprid - A Pyridyl Methylamine Neonicotinoid Insecticide in Mice. Thesis-PhD, Lala Lajpat Rai University of Veterinary & Animal Sciences (2015).
- 37.Karaca BU, et al. Toxic Effects of Subchronic Oral Acetamiprid Exposure in Rats. Toxicol Ind Health. 2019;35:679–687. doi: 10.1177/0748233719893203. [DOI] [PubMed] [Google Scholar]
- 38.Istanbul University Animal Experiments Local Ethic Committee Guidelines, http://cdn.istanbul.edu.tr/FileHandler2.ashx?f=istanbul-universitesi-hayvan-deneyleri-yerel-etik-kurulu-yonergesi.pdf (2019).
- 39.Yamada, T., Takahashi, H. & Hatano, R. A Novel Insecticide, Acetamiprid. In Nicotinoid Insecticides and the Nicotinic Acetylcholine Receptor, 149–76. Tokyo: Springer, 149–176 (1999).
- 40.European Food Safety Authority Peer Review of the Pesticide Risk Assessment of the Active Substance Acetamiprid. EFSA J. 2016;14:1–26. [Google Scholar]
- 41.Food and Agriculture Organization (FAO) of the United Nations and World Health Organization (WHO) Joint FAO/WHO Meeting on Pesticide Residues. Pesticide Residues in Food-2011: Part II-Toxicological Evaluations.
- 42.Industrial Reproductive Toxicology (IRDG) Computer Assisted Sperm Analysis (CASA) Group, 2000: Rat Sperm Morphological Assessment Guideline Document. Available from, http://www.irdg.co.uk/Sperm_morphology.pdf. (accessed 10 May 2019).
- 43.van Der Horst G, Maree L, Kotzé SH, O’Riain MJ. Sperm Structure and Motility in the Eusocial Naked Mole-Rat, Heterocephalus Glaber: A Case of Degenerative Orthogenesis in the Absence of Sperm Competition? BMC Evol Biol. 2011;11:351. doi: 10.1186/1471-2148-11-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Erkanlı Şentürk G, Ersoy Canillioĝlu Y, Umay C, Demiralp Eksioglu E, Ercan F. Distribution of Zonula Occludens 1 and Occludin and Alterations of Testicular Morphology after in Utero Radiation and Postnatal Hyperthermia in Rats. Int J Exp Pathol. 2012;93:438–449. doi: 10.1111/j.1365-2613.2012.00844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tamer SA, et al. Treatment with Estrogen Receptor Agonist ERβ Improves Torsion-Induced Oxidative Testis Injury in Rats. Life Sci. 2019;222:203–211. doi: 10.1016/j.lfs.2019.02.056. [DOI] [PubMed] [Google Scholar]
- 46.Gozen A, et al. Protective Activity of Ischemic Preconditioning on Rat Testicular Ischemia: Effects of Y-27632 and 5-Hydroxydecanoic Acid. J Pediatr Surg. 2013;48:1565–1572. doi: 10.1016/j.jpedsurg.2012.10.074. [DOI] [PubMed] [Google Scholar]
- 47.Köroğlu K, Çevik MÖ, Şener G, Ercan F. Apocynin Alleviates Cisplatin-induced Testicular Cytotoxicity by Regulating Oxidative Stress and Apoptosis in Rats. Andrologia. 2019;51:e13227. doi: 10.1111/and.13227. [DOI] [PubMed] [Google Scholar]