Abstract
Background
Abnormalities in mineral metabolism are common complications of organ transplantation. The role of immunosuppressive agents in alteration of mineral metabolism is not clear.
Methods
We conducted an animal study to investigate the effects of cyclosporine A (CsA), tacrolimus, and sirolimus on renal calcium, magnesium and vitamin D metabolism.
Results
CsA and tacrolimus induced a 2- to 3-fold and 1.6- to 1.8-fold increase in urinary calcium and magnesium excretion, respectively, while rapamycin had no effects on calcium, but doubled the urinary magnesium excretion. CsA and tacrolimus, but not rapamycin, elevated serum 1,25(OH)<sub>2</sub> vitamin D without affecting the parathyroid hormone level. CsA and tacrolimus reduced mRNA abundance in TRPV5 (CsA: 64 ± 3* of control; tacrolimus: 50 ± 3*) calbindin-D28k (CsA: 62 ± 4*; tacrolimus: 43 ± 3*), and vitamin D receptor (CsA: 52 ± 3*; tacrolimus: 58 ± 2*, all p < 0.05). Rapamycin did not affect gene expression in any of studied proteins. The immunofluorescence staining study demonstrated a 50* reduction of TRPV5 and calbindin-D28k by CsA and tacrolimus.
Conclusion
The suppression of VDR by calcineurin inhibitors is probably the underlying mechanism of renal calcium wasting. In spite of an increased 1,25(OH)<sub>2</sub> vitamin D level, the kidney is not able to reserve calcium, suggesting a role of vitamin D resistance that may be related to bone loss.
Key Words: Immunosuppressants, Calcium transport, Vitamin D
Introduction
Calcineurin inhibitors (CNI) such as cyclosporine A (CsA) and tacrolimus are the key immunosuppressive medications recommended by the new KDIGO guideline for initial and long-term maintenance immunosuppressive medications for kidney transplant recipients [1]. Sirolimus, a mammalian target of the rapamycin (mTOR) inhibitor, can be used as the initial immunosuppressive medication, but frequently it has been used to replace CNI for patients who develop CNI toxicity [2]. With the improvement of graft survival, side effects of long-term use of these immunosuppressive drugs become important in the clinical care of these transplant recipients. Among them, bone loss is a major concern. Both CsA and tacrolimus caused severe bone loss in experimental animals, but clinically their skeletal toxicity is less clear [3]. Bone loss has been reported in patients who were treated with high-dose CsA or tacrolimus [4]. Sirolimus does not cause bone loss in experimental animals [5]. In a recent study, sirolimus has been shown to cause less bone turnover when compared with CsA [6].
In the present study, we aim to investigate the effects of these immunosuppressive agents on calcium, magnesium and vitamin D metabolism in the kidney. We have previously reported that CsA induced hypercalciuria due to both enhanced bone resorption and decreased calcium reabsorption in the distal convoluted tubule (DCT) [7]. More recently, it has been shown that tacrolimus also induced hypercalciuria by downregulating the DCT calcium channel, transient receptor potential (TRP)V5, which is the crucial transporter regulating the final concentration of calcium in urine [8]. In the present study, we found that both CsA and tacrolimus induced hypercalciuria in mice, while rapamycin did not. CNI-induced calcium wasting is associated with downregulation of calcium-binding proteins and calcium channels in the DCT and renal vitamin D receptor (VDR), as well as upregulation of 1-alpha-hydroxylase and elevated serum 1,25-dihydroxy vitamin D (1,25(OH)2-VitD) level. These findings indicate that downregulation of VDR by CNI underlies the renal calcium wasting, and that CNI may induce a vitamin D-resistant state.
Methods
Animals and Drugs
Adult male C57BL6 mice (weight: 15–20 g) were purchased from the animal center of the National Science Council. They were maintained in a temperature-controlled and light-cycled environment. The study animals were allowed free access to water and food (calcium 1.0* and magnesium 0.5*). Animals were divided into four groups according to the study design: control group, CsA treatment (25 mg/kg/day, Sandimmun, Novartis Pharma AG, USA), tacrolimus treatment (3 mg/kg/day, Fujisawa, Killorglin Co., Kerry, Ireland), and rapamycin treatment (1 mg/kg/day, Sigma, St. Louis, Mo., USA). The administered dosage was selected based on previous studies [7,9,10,11]. All the treatment animals received daily administration via intraperitoneal injection for 1 week. Animal protocols were approved by the Institutional Animal Care and Use Committee (IACUC) of Chang-Gung Memorial Hospital, and all animal procedures were performed according to the IACUC policy.
Biochemical Data of Blood and Urine
After 1 week of treatment, urine was collected in individualized metabolic cage for each mouse. The blood samples were collected when animals were harvested. Both urine and centrifuged blood samples were then kept at -80°C in the refrigerator until assay. Biochemical data including creatinine, calcium and magnesium of serum and urine were measured as described previously [12].
Measurements of 1,25-(OH2)-VitD and Intact Parathyroid Hormone
Blood samples were collected and grouped for the determination of serum 1,25(OH)2-VitD and intact parathyroid hormone (iPTH), respectively. The serum 1,25(OH)2-VitD level was determined by the 1,25(OH)2-Vitamin D RIA kit (IBL, GmbH, Hamburg, Germany). The mouse intact PTH ELISA kit (Immutopics, Inc., San Clemente, Calif., USA) was used to determine serum iPTH level.
Gene Expression Analysis and Protein Abundance Assessment
The kidney was dissected and total RNA was isolated using TRIZOL reagent (Invitrogen, Carlsbad, Calif., USA). Reverse transcription for cDNA synthesis was performed using a reverse transcription system (Promega, Madison, Wisc., USA). Gene expression was analyzed by employing real-time polymerase chain reaction (PCR). The molecules involved in calcium transport, including TRPV5, calbindin-D28k, calbindin-D9k, paracellin-1, and calcium-sensing receptor (CaSR), and molecules involved in vitamin D metabolism, such as VDR and 1-alpha-hydroxylase were assessed in four animal groups. The mRNA abundance of β-actin was used as the internal reference for each gene evaluated. The synthesized cDNA was then subject to real-time PCR using the ABI prism 7900 HT Sequence Detection System (ABI, Foster City, Calif., USA). Primers of PCR of each gene are listed as shown in table 1. The emission signal was assessed using the fluorescent dye SYBR Green (ABI). To determine the gene expression, genes investigated in the present study were calculated as 2/(β-actin Ct – target gene Ct), where Ct represents the first cycle at which the output signal exceeds the threshold signal. PCR reaction of each gene was performed in triplicate to obtain a mean value. The alternations of gene expression are presented as percentages (*) of control group animal values.
Table 1.
Primers used for real-time PCR
| Gene | Forward primer | Reverse primer |
|---|---|---|
| Beta-actin | TTCACCACCACAGCTGAGAGG | GAACCGCTCGTTGCCAATAG |
| TRPV5 | GACGGACCTGCCAATTACAGA | TGTGGCGATGATGGCAAA |
| CBD28K | CAGAATCCCACCTGCAGTCA | TCCGGTGATAGCTCCAATCC |
| CBD9K | AGGCCAGCAAAATGTGTGCT | GTACTTGAAGCCTTCAGGAGGC |
| 1-Alpha-hydroxylase | GAGAAGGTGAAGCTGCGATGA | TCTGTCACATTCCCCACTATGG |
| VDR | GAAGAGGCCTTGAAGGACAGT | TACTTACGTCTGCACGAATTG |
| CaSR | CCTCTCGGAGCTTCGTCATC | GCACCCACTATCCCTGGCTT |
| Paracellin-1 | TTCATCACCCTGCTCCTTGG | GCGTTCGACGTAAACATCCA |
TRPV5 = Transient receptor potential V5; CBD28K = calbindin-D28k; CBD9K = calbindin-D9k; VDR = vitamin D receptor; CaSR = calcium-sensing receptor.
Immunofluorescence Study
Frozen kidney cortex tissue was used for calbindin-D28k, TRPV5 and CaSR proteins assessment. Slices of 5 µm in thickness were fixed with 4* paraformaldehyde for 15 min and incubated with primary antibody (goat anti-mouse calbindin-D28k monoclonal antibody 1:500, Sigma, rabbit anti-rat ECaC1 1:100, Alpha Diagnostic International, San Antonio, Tex., USA and mouse anti-rat CaSR monoclonal antibody 1:200, Abcam, Cambridge, UK) for 16 h, and then with FITC-conjugated secondary antibodies for calbindin-D28k (Jackson ImmunoResearch Laboratories, Inc., USA), streptavidin/FITC-conjugated secondary antibody for TRPV5 (DakoCytomation, Dako Corporation, Carpinteria, Calif., USA) and peroxidase-conjugated AffiniPure goat anti-mouse IgG for CaSR (Jackson ImmunoResearch Laboratories, West Grove, Pa., USA) for 30 min. The immunofluorescence pictures were then taken using a Zeiss fluorescence microscope connected with a digital photo camera (Evolution VF, MediaCybernetics). Semiquantitative determination of the protein expression was performed with the Image-Pro Plus 5.0 image analysis software. Amount of protein was expressed as mean of integrated optimal density. The alternation is expressed as percentage (*) of control animals.
Statistical Analyses
Data are presented as mean ± SEM. Statistical analyses of the data were performed using SPSS-PC software. Unpaired Student's t tests were used to compare differences between treatment and control groups. p < 0.05 was considered statistically significant for all tests.
Results
Biochemical Data of Blood and Urine Samples (table 2)
Table 2.
Biochemical and physiological data of the control and three drug-treatment animal groups
| Control | Cyclosporine A | Tacrolimus | Rapamycin | |
|---|---|---|---|---|
| Body weight, g | 19.5 ± 0.4 | 20.4 ± 0.5 | 19.8 ± 0.6 | 20.2 ± 0.4 |
| Serum Ca, mg/dl | 9.2 ± 1.0 | 9.4 ± 1.3 | 9.0 ± 0.8 | 9.4 ± 0.4 |
| Serum Mg, mg/dl | 2.2 ± 0.4 | 2.0 ± 0.2 | 1.9 ± 0.5 | 2.1 ± 0.4 |
| Serum creatinine, mg/dl | 0.4 ± 0.1 | 0.3 ± 0.2 | 0.5 ± 0.2 | 0.4 ± 0.1 |
| 1,25(OH)2-VitD, pg/ml | 89.0 ± 10.1 | 124.8 ± 7.5* | 131.8 ± 16.2* | 78.3 ± 12.6 |
| iPTH, pg/ml | 120 ± 12.6 | 130.4 ± 23.6 | 108.8 ± 20.4 | 122 ± 19.6 |
| Urine Ca/Cr | 0.21 ± 0.11 | 0.58 ± 0.28* | 0.46 ± 0.2* | 0.24 ± 0.18 |
| Urine Mg/Cr | 0.36 ± 0.22 | 0.59 ± 0.2* | 0.66 ± 0.14* | 0.84 ± 0.25* |
| FE(Ca), * | 0.95 ± 0.1 | 5.4 ± 1.1* | 4.78 ± 1.5* | 1.0 ± 0.42 |
| FE(Mg), * | 8.8 ± 2.1 | 18.8 ± 4.4* | 24.6 ± 5.5* | 29 ± 7.2* |
p < 0.05 vs. control group.
After 1 week treatment, there was a 2- to 3-fold increase in urinary calcium in animals treated with CsA and tacrolimus. Similarly, urinary magnesium was increased by 1- to 2-fold. The fractional excretion (FE) of calcium and magnesium was also increased. There was no significant change in urinary calcium excretion after rapamycin administration, but urinary magnesium excretion and FE of magnesium were significantly increased. The serum calcium, magnesium and creatinine levels were not affected by the administration of study drugs. The 1,25(OH)2-VitD level was increased significantly after CsA and tacrolimus treatment (176 and 119* increase, respectively), but not affected by rapamycin. The iPTH level was not changed in all three drug-treatment animal groups. The body weights of the treatment groups did not have a significant change compared with control animals.
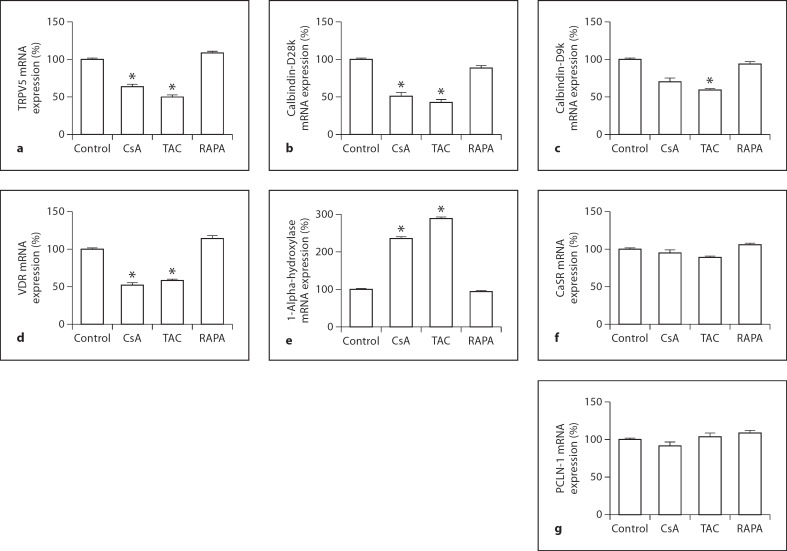
Gene Expression Analysis (fig. 1a–g)
Fig. 1.
Gene expression analysis of the effects of cyclosporine A (CsA), tacrolimus (TAC) and rapamycin (RAPA) on TRPV5 (a), calbindin-D28k (b), calbindin-D9k (c), vitamin D receptor (VDR, d), 1-alpha-hydroxylase (e), calcium-sensing receptor (CaSR, f) and paracellin-1 (PCLN-1, g). * p < 0.05.
One week of treatment with CsA diminished the mRNA abundance of TRPV5 (64 ± 3* of control, p< 0.05) and calbindin-D28k (62 ± 4*, p < 0.05). There was a trend towards decrease in calbindin-D9k mRNA, but it did not reach statistical significance. The VDR was downregulated (52 ± 3*, p < 0.05), and 1-alpha-hydroxylase was upregulated (236 ± 4*, p< 0.05). Tacrolimus inhibited the expression of TRPV5, calbindin-D28k and calbindin-D9k (50 ± 3*, 43 ± 3* and 59 ± 2*, all p < 0.05). Tacrolimus also decreased the expression of VDR (38 ± 2*) and increased 1-alpha-hydroxylase (289 ± 3*, all p < 0.05) significantly. Treatment with rapamycin did not influence the calcium-related transporters (TRPV5: 109 ± 2*; calbindin-D28k: 89 ± 3*; calbindin-D9k: 94 ± 3*, all p > 0.05). Both VDR and 1-alpha-hydroxylase were not changed by rapamycin (VDR: 114 ± 4*; 1-alpha-hydroxylase: 64 ± 3*, both p> 0.05). CaSR and paracellin-1 were not influenced by these three drugs (CsA: CaSR: 95 ± 4*; paracellin-1: 92 ± 5*; tacrolimus: 89 ± 2* and 104 ± 5*; rapamycin: 106 ± 2*; 109 ± 3*, all p > 0.05).
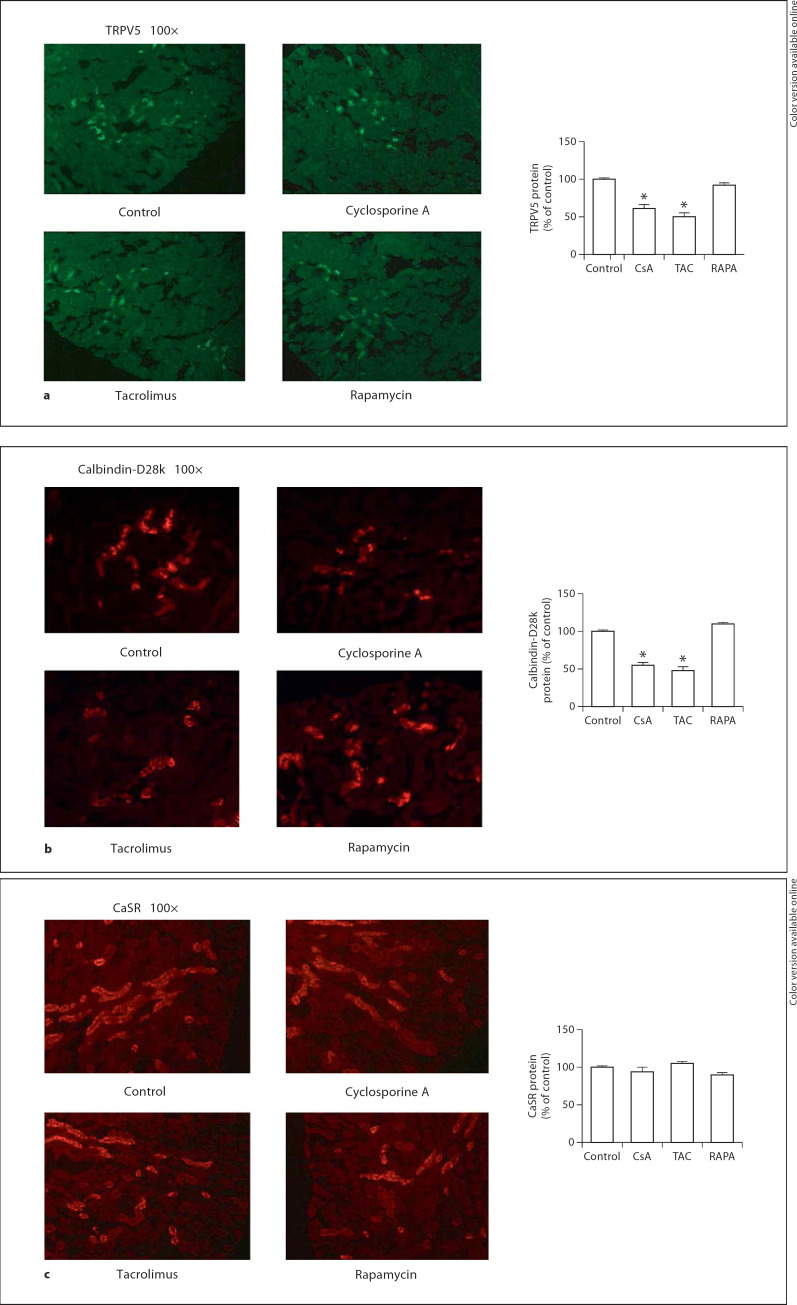
Immunofluorescence Staining (fig. 2a–c)
Fig. 2.
Immunofluorescence staining study of cyclosporine A (CsA), tacrolimus (TAC) and rapamycin (RAPA) on TRPV5 (a) and calbindin-D28k (b) in renal tissue. * p < 0.05. Immunofluorescence staining study of cyclosporine A (CsA), tacrolimus (TAC) and rapamycin (RAPA) on calcium-sensing receptor (CaSR, c) in renal tissue. * p < 0.05.
Immunofluorescence staining studies revealed that administration of CsA decreased TRPV5 (61 ± 5*) and calbindin-D28k (55 ± 4*) abundance significantly. Treatment of tacrolimus was also associated with a decreased abundance of TRPV5 (50 ± 5*) and calbindin-D28k (48 ± 5*). There was no significant alteration in TRPV5 (92 ± 3*) and calbindin-D28k (110 ± 2*) after rapamycin treatment. There was no significant change in CaSR protein abundance after treatment (CsA: 94 ± 6*; tacrolimus: 105 ± 3*; rapamycin: 90 ± 3*, all p > 0.05).
Discussion
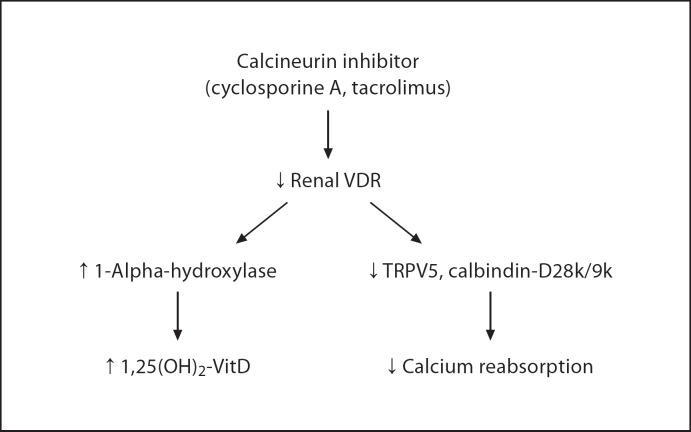
We have demonstrated that CNI treatment for 1 week induced renal calcium and magnesium wasting in spite of elevated plasma 1,25(OH)2-VitD levels. These changes are likely due to the downregulation of VDR by CNI. The loss of VDR subsequently reduces vitamin D-dependent calcium-binding proteins and calcium channels in the DCT resulting in renal calcium wasting. In addition, loss of VDR removes feedback inhibition of vitamin D on 1-alpha-hydroxylase, and results in an elevated 1,25(OH)2-VitD level. Overall, CNI creates a vitamin D-resistant state by downregulating VDR (fig. 3). In comparison, rapamycin did not have any effects on renal calcium excretion, vitamin D metabolism, or expression of DCT calcium channel and calcium-binding proteins.
Fig. 3.
Consequences of calcineurin inhibitor on renal vitamin D metabolism and distal convoluted tubule calcium transport.
The dosage of immunosuppressive agents used in this study is significantly higher than what used for kidney transplant patients. Halloran et al. [13] reported that the blood concentrations measured at a dose of 25 mg/kg CsA in the mouse are higher on average but overlap trough blood levels of 300–400 µg/l in humans. As for tacrolimus and rapamycin, 1 mg/kg/day of tacrolimus in mice gave a trough level of 25 µg/l [14] and 0.5 mg/kg/day of rapamycin gave a trough level of 20 µg/l [15]. Since we used tacrolimus 3 mg/kg/day and rapamycin 1 mg/kg/day, it is estimated that the trough levels would be 75 and 40 µg/l, respectively. These drug levels are much higher than the recommended therapeutic levels. Therefore, our findings should not be applied to patients with optimal immunosuppression, but may be applicable to those with toxic levels of CNI. In our study, the 1-week treatment with all three drugs did not raise serum creatinine levels. Since none of treatment caused significant weight changes, the serum creatinine data indicate that the dosage of CsA, tacrolimus, and rapamycin is not associated with significant renal injury in mice.
CNI causes renal wasting of both calcium and magnesium. In renal transplant patients, CNI-induced hypomagnesemia is common, while hypocalcemia is rare. It is probably due to residual hyperparathyroidism after transplantation. In our study, both CsA and tacrolimus induced a significant urinary loss of calcium and magnesium, but was not severe enough to cause a decrease in plasma levels. Although magnesium and calcium share paracellular transport in the thick ascending limb, we found that the mRNA abundance of paracellin-1 and CaSR was not affected by CNI. It is likely that magnesium wasting is due to reduced DCT reabsorption because the key transporter, TRPM6, is downregulated by CNI [8]. In addition, magnesium deficiency results in hypocalcemia, impaired PTH secretion, and low serum level of 1,25(OH)2-VitD which may cause renal wasting of calcium [16]. However, in our study, CNI-induced renal wasting of magnesium was not severe enough to lower iPTH and 1,25(OH)2-VitD levels. Therefore, it is unlikely to be a key factor in the pathogenesis of renal calcium wasting in our experimental conditions. This notion is further supported by the fact that sirolimus caused a similar degree of magnesium wasting, but did not affect renal calcium and vitamin D metabolism.
The effect of CsA on calcium homeostasis is complex. Our previous study showed that both kidney and skeleton are involved in the pathogenesis of CsA-associated renal calcium wasting. By using calbindin-D28k knockout mice, we were able to demonstrate that calciuria in these knockout mice during CsA treatment was diminished by pamidronate [7]. Our results support that CsA exerts dual effects on both renal tubule and bony tissue which contributes to negative calcium homeostasis. In the present study, we found that in addition to calbindin-D28k, CsA also inhibited TRPV5 and calbindin-D9k, although for calbindin-D9k, the difference did not reach statistical significance compared to control animals. The suppression of these calcium-binding proteins and TRPV5 is also observed in tacrolimus-treated mice, findings similar to a previous study [8].
The effects of CsA and tacrolimus on DCT calcium transport are closely related to the vitamin D signal pathway. In the kidney, calcium transport machinery in the distal tubule, such as TRPV5 and calbindins, are regulated by calcitriol. Genetic disruption on VDR is associated with a decreased expression of TRPV5, TRPV6 and calbindins [17,18]. Our findings that CsA and tacrolimus suppressed VDR expression indicate that downregulation of VDR underlies the decreased expression of calcium-related transporters of distal tubule and subsequent renal calcium wasting. The downregulation of renal VDR and calbindin-D28k by CsA has been reported previously by Grenet et al. [19].
Interestingly, we have found that CNI upregulated 1-alpha-hydroxylase in the kidney and increased plasma calcitriol levels. Since calcitriol is known to have feedback inhibition on 1-alpha-hydroxylase via VDR, when VDR is downregulated, 1,25(OH)2VitD synthesis is increased because the feedback inhibition is removed. Grenet et al. [19] previously demonstrated that CsA treatment raised both plasma and renal calcitriol levels. However, several other studies did not show consistent findings on the serum vitamin D level: the level might be unaltered or increased [10,20,21]. This variation may be due to differences in experimental designs, particularly treatment duration and the applied dosage.
Whether our findings that CNI induces vitamin D resistance have a clinical implication is not clear. Organ transplant recipients have complicated calcium and vitamin D metabolism due to concomitant medical conditions such as impaired renal function, elevated parathyroid hormone levels, simultaneous use of glucocorticoids, and other co-morbidities including cardiovascular disease and diabetes [3]. In addition, the dosage of CNI used in transplant patients is much lower than that used in animal studies. However, previous studies by Malluche's group [22,23] have demonstrated a high prevalence of low bone turnover and occurrence of osteomalacia after kidney transplantation. These mineralization defects occurred in the presence of normal serum levels of 25(OH) vitamin D and 1,25(OH)2VitD. Similar findings have been reported by another group [24]. Both groups of investigators pointed out that post-transplant mineralization defects are not related to hypophosphatemia or aluminum deposition, and suggested that these defects may be due to vitamin D resistance. Although CsA was used in the majority of patients in those studies, it is not clear whether CsA contributes to the mineralization defects. Further studies are needed to determine if CNI indeed causes a vitamin D-resistant state in transplant recipients, and explore the role of VDR in toxicities of calcineurin inhibitors.
In the present study, we also investigated whether CaSR and paracellin-1 were involved in the renal calcium wasting induced by CsA and tacrolimus. Both CaSR and paracellin-1 play important roles on calcium reabsorption in the thick ascending limb [25]. CaSR regulates sodium-potassium-chloride cotransporters and potassium channel, which control the luminal positivity. Paracellin-1 controls the paracellular transport of calcium and magnesium which is driven by the luminal positivity. Genetic abnormalities of CaSR are associated with dysregulation of calcium homeostasis and renal calcium transport [26], while patients with a mutation of paracellin-1 exhibited congenital hypomagnesemia and hypercalciuria [27]. Our study demonstrated that expression of CaSR and paracellin-1 was not affected by CsA, tacrolimus, or sirolimus.
We conclude that calcineurin inhibitors suppress VDR synthesis in the kidney with two major consequences: (1) Suppression of vitamin D-sensitive calcium-binding protein, calbindin-D28k, and epithelial calcium channel, TRPV5, in the DCT. As a result, renal calcium wasting occurs. (2) Removal of feedback inhibition on 1-alpha-hydroxylase in the kidney. As a result, the plasma 1,25(OH)2VitD level increases. Rapamycin, on the other hand, demonstrated a neutral effect on calcium homeostasis and renal calcium transport.
Acknowledgements
This work was supported by a grant from Kaohsiung Chang-Gung Memorial Hospital, Kaohsiung, Taiwan (CMRPG881191) and a grant from Dialysis Clinic Inc., a nonprofit organization, to L.-W.L.
References
- 1.Kasiske BL, Zeier MG, Chapman JR, Craig JC, Ekberg H, Garvey CA, Green MD, Jha V, Josephson MA, Kiberd BA, Kreis HA, McDonald RA, Newmann JM, Obrador GT, Vincenti FG, Cheung M, Earley A, Raman G, Abariga S, Wagner M, Balk EM. KDIGO clinical practice guideline for the care of kidney transplant recipients: a summary. Kidney Int. 2010;77:299–311. doi: 10.1038/ki.2009.377. [DOI] [PubMed] [Google Scholar]
- 2.Wong W, Venetz JP, Tolkoff-Rubin N, Pascual M. Immunosuppressive strategies in kidney transplantation: which role for the calcineurin inhibitors? Transplantation. 2005;80:289–296. doi: 10.1097/01.tp.0000168436.76784.45. [DOI] [PubMed] [Google Scholar]
- 3.Cunningham J. Posttransplantation bone disease. Transplantation. 2005;79:629–634. doi: 10.1097/01.tp.0000149698.79739.ef. [DOI] [PubMed] [Google Scholar]
- 4.Stempfle HU, Werner C, Siebert U, Assum T, Wehr U, Rambeck WA, Meiser B, Theisen K, Gärtner R. The role of tacrolimus (FK506)-based immunosuppression on bone mineral density and bone turnover after cardiac transplantation: a prospective, longitudinal, randomized, double-blind trial with calcitriol. Transplantation. 2002;73:547–552. doi: 10.1097/00007890-200202270-00010. [DOI] [PubMed] [Google Scholar]
- 5.Romero DF, Buchinsky FJ, Rucinski B, Cvetkovic M, Bryer HP, Liang XG, Ma YF, Jee WS, Epstein S. Rapamycin: a bone sparing immunosuppressant? J Bone Miner Res. 1995;10:760–768. doi: 10.1002/jbmr.5650100513. [DOI] [PubMed] [Google Scholar]
- 6.Campistol JM, Holt DW, Epstein S, Gioud-Paquet M, Rutault K, Burke JT. Bone metabolism in renal transplant patients treated with cyclosporine or sirolimus. Transplant Int. 2005;18:1028–1035. doi: 10.1111/j.1432-2277.2005.00163.x. [DOI] [PubMed] [Google Scholar]
- 7.Lee CT, Huynh VM, Lai LW, Lien YH. Cyclosporine A-induced hypercalciuria in calbindin-D28k knockout and wild-type mice. Kidney Int. 2002;62:2055–2061. doi: 10.1046/j.1523-1755.2002.00670.x. [DOI] [PubMed] [Google Scholar]
- 8.Nijenhuis T, Hoenderop JG, Bindels RJ. Downregulation of Ca2+ and Mg2+ transport proteins in the kidney explains tacrolimus (FK506)-induced hypercalciuria and hypomagnesemia. J Am Soc Nephrol. 2004;15:549–557. doi: 10.1097/01.asn.0000113318.56023.b6. [DOI] [PubMed] [Google Scholar]
- 9.Andoh TF, Burdmann EA, Fransechini N, Houghton DC, Bennett WM. Comparison of acute rapamycin nephrotoxicity with cyclosporine and FK506. Kidney Int. 1996;50:1110–1117. doi: 10.1038/ki.1996.417. [DOI] [PubMed] [Google Scholar]
- 10.Stein B, Halloran BP, Reinhardt T, Engstrom GW, Bales CW, Drezner MK, Currie KL, Takizawa M, Adams JS, Epstein S. Cyclosporin-A increases synthesis of 1,25-dihydroxyvitamin D3 in the rat and mouse. Endocrinology. 1991;128:1369–1373. doi: 10.1210/endo-128-3-1369. [DOI] [PubMed] [Google Scholar]
- 11.Wu MJ, Wen MC, Chiu YT, Chiou YY, Shu KH, Tang MJ. Rapamycin attenuates unilateral ureteral obstruction-induced renal fibrosis. Kidney Int. 2006;69:2029–2036. doi: 10.1038/sj.ki.5000161. [DOI] [PubMed] [Google Scholar]
- 12.Lee CT, Lien YH, Lai LW, Chen JB, Lin CR, Chen HC. Increased renal calcium and magnesium transporter abundance in streptozotocin-induced diabetes mellitus. Kidney Int. 2006;69:1786–1791. doi: 10.1038/sj.ki.5000344. [DOI] [PubMed] [Google Scholar]
- 13.Halloran PF, Helms LM, Kung L, Noujaim J. The temporal profile of calcineurin inhibition by cyclosporine in vivo. Transplantation. 1999;15:1356–1361. doi: 10.1097/00007890-199911150-00023. [DOI] [PubMed] [Google Scholar]
- 14.Moffatt SD, McAlister V, Calne RY, Metcalfe SM. Potential for improved therapeutic index of FK506 in liposomal formulation demonstrated in a mouse cardiac allograft model. Transplantation. 1999;15:1205–1208. doi: 10.1097/00007890-199905150-00001. [DOI] [PubMed] [Google Scholar]
- 15.Zafar I, Ravichandran K, Belibi FA, Doctor RB, Edelstein CL. Sirolimus attenuates disease progression in an orthologous mouse model of human autosomal dominant polycystic kidney disease. Kidney Int. 2010;78:754–761. doi: 10.1038/ki.2010.250. [DOI] [PubMed] [Google Scholar]
- 16.Fatemi S, Ryzen E, Flores J, Endres DB, Rude RK. Effect of experimental human magnesium depletion on parathyroid hormone secretion and 1,25-dihydroxyvitamin D metabolism. J Clin Endocrinol Metab. 1991;73:1067–1072. doi: 10.1210/jcem-73-5-1067. [DOI] [PubMed] [Google Scholar]
- 17.Li YC, Bolt MJG, Cao LP, Sitrin MD. Effects of vitamin D receptor inactivation on the expression of calbindins and calcium metabolism. Am J Physiol Endocrinol Metabol. 2001;281:E558–E564. doi: 10.1152/ajpendo.2001.281.3.E558. [DOI] [PubMed] [Google Scholar]
- 18.Zheng W, Xie Y, Li G, Kong J, Feng JQ, Li YC. Critical role of calbindin-D28K in calcium homeostasis revealed by mice lacking both vitamin D receptor and calbindin-D28K. J Biol Chem. 2004;279:52406–52413. doi: 10.1074/jbc.M405562200. [DOI] [PubMed] [Google Scholar]
- 19.Grenet O, Bobadilla M, Chibout SD, Steiner S. Evidence for the impairment of the vitamin D activation pathway by cyclosporine A. Biochem Pharmacol. 2000;59:267–272. doi: 10.1016/s0006-2952(99)00321-4. [DOI] [PubMed] [Google Scholar]
- 20.Reichel H, Grussinger A, Knehans A, Kühn K, Schmidt-Gayk H, Ritz E. Long-term therapy with cyclosporin A does not influence serum concentrations of vitamin D metabolites in patients with multiple sclerosis. Clin Invest. 1992;70:595–599. doi: 10.1007/BF00184801. [DOI] [PubMed] [Google Scholar]
- 21.Shaw AJ, Hayes ME, Davies M, Edwards BD, Ballardie FW, Chalmers RJ, Mawer EB. Cyclosporin A and vitamin D metabolism: studies in patients with psoriasis and in rats. Clin Sci. 1994;86:627–632. doi: 10.1042/cs0860627. [DOI] [PubMed] [Google Scholar]
- 22.Briner VA, Thiel G, Monier-Faugere MC, Bognar B, Landmann J, Kamber V, Malluche HH. Prevention of cancellous bone loss but persistence of renal bone disease despite normal 1,25 vitamin D levels two years after kidney transplantation. Transplantation. 1995;59:1393–1400. doi: 10.1097/00007890-199505270-00006. [DOI] [PubMed] [Google Scholar]
- 23.Monier-Faugere MC, Mawad H, Qi Q, Friedler RM, Malluche HH. High prevalence of low bone turnover and occurrence of osteomalacia after kidney transplantation. J Am Soc Nephrol. 2000;11:1093–1099. doi: 10.1681/ASN.V1161093. [DOI] [PubMed] [Google Scholar]
- 24.Montalban C, LM de Francisco A, Marinoso ML, Zubimendi JA, Unzueta MG, Amado JA, Arias M. Bone disease in long-term adult kidney transplant patients with normal renal function. Kidney Int. 2003;85:S129–S132. doi: 10.1046/j.1523-1755.63.s85.31.x. [DOI] [PubMed] [Google Scholar]
- 25.Van de Graaf SFJ, Bindels RJM, Hoenderop JGJ. Physiology of epithelial Ca2+ and Mg2+ transport. Rev Physiol Biochem Pharmacol. 2006;158:77–160. doi: 10.1007/112_2006_0607. [DOI] [PubMed] [Google Scholar]
- 26.Raue F, Haag C, Schulze E, Frank-Raue K. The role of the extracellular calcium-sensing receptor in health and disease. Exp Clin Endocrinol Diabetes. 2006;114:397–405. doi: 10.1055/s-2006-924315. [DOI] [PubMed] [Google Scholar]
- 27.Blanchard A, Jeunemaitre X, Coudol P, Dechaux M, Froissart M, May A, Demontis R, Fournier A, Paillard M, Houillier P. Paracellin-1 is critical for magnesium and calcium reabsorption in the human thick ascending limb of Henle. Kidney Int. 2007;59:2206–2215. doi: 10.1046/j.1523-1755.2001.00736.x. [DOI] [PubMed] [Google Scholar]