Abstract
Background/Aims
Epidemiologic studies suggest that cigarette smoke worsens the progression of renal injury in patients with glomerular diseases. The mechanisms involved have not been elucidated. These studies were designed to determine whether nicotine worsens markers of inflammation including glomerular cell proliferation and fibronectin deposition in an in vivomodel of glomerular injury.
Methods
Sprague-Dawley rats were injected with anti-Thy1 antibody and given either tap water or nicotine in the drinking water until sacrifice at day 14. Fibronectin expression was measured by Western blot and immunohistochemistry. COX-2 expression was also determined by Western blot in the kidney cortex of rats treated with nicotine and in cultured human mesangial cells treated with nicotine.
Results
Anti-Thy1 antibody administration resulted in a significant increase in the number of cells per glomerulus that was further increased by the administration of nicotine. In nephritic rats, the administration of nicotine significantly increased fibronectin and COX-2 expression. In cultured human mesangial cells we also demonstrated that nicotine increases COX-2 expression and activity and that COX-2 mediates mesangial cell proliferation in response to nicotine.
Conclusion
Either in vivo or in vitro treatment with nicotine leads to activation of inflammatory mediators and hallmarks of glomerular injury, which may explain the mechanisms involved in the deleterious effects of cigarette smoking on renal disease.
Key Words: Nicotine, Cigarette smoking, Kidney glomerulus, Glomerulonephritis
Introduction
Cigarette smoking is the most important cause of preventable morbidity and mortality in the US [1, 2]. Cigarette smoking is an important risk factor for atherosclerotic vascular disease [3, 4]. Endothelial dysfunction occurs early in the pathogenesis of atherosclerosis [5, 6]. We have shown that stable reactive aldehydes present in cigarette smoke promote endothelial dysfunction by increasing the production of NADPH oxidase-derived reactive oxygen species (ROS) [7, 8].
Recent epidemiologic studies have demonstrated the association between cigarette smoking and the rate of progression of renal failure, particularly among diabetics but also in patients with chronic nephritides [9,10,11]. The mechanisms by which cigarette smoking accelerates the progression of chronic kidney disease have not been established. Fibronectin is a major component of the expanded mesangial matrix, which is present in many glomerulopathies independent of their etiology [12]. We have recently shown for the first time that human mesangial cells express non-neuronal nicotinic acetylcholine receptors (nAChRs) and that nicotine increases fibronectin expression in these cells via NADPH oxidase-dependent generation of ROS [13], establishing a potential mechanism by which nicotine in cigarette smoking may participate in the progression of renal disease.
We performed in vivo studies aimed at further elucidating potential mechanisms linking cigarette smoking and progressive renal injury. We chose a well-established model of glomerular inflammation induced by antibodies that react with the glomerular mesangium in which upregulation of fibronectin synthesis has been clearly documented [14].
Methods
Rat Anti-Thy1 Nephritis Model of Mesangial Proliferative Glomerulonephritis
Sprague-Dawley rats (Harlan Sprague-Dawley, Indianapolis, Ind., USA) weighing between 200 and 225 g were kept under standard conditions and were randomly allocated to either control or experimental groups. Experimental proliferative nephritis (anti-Thy1 nephritis) was induced by a single intravenous injection of anti-Thy1 antibody (Cedarlane, Burlington, Ont., Canada, 0.5 ml/kg) [15, 16]. Rats were given either tap water or nicotine in drinking water (100 µg/ml) starting 3 days before the anti-Thy1 antibody injection and sacrificed on day 14, a time point that in this model is characterized by increased mesangial cell proliferation and extracellular matrix deposition.
Immunohistochemistry
The kidneys harvested from the rats were fixed in Tissue-Tek Express Molecular Fixative (Torrance, Calif., USA) and sectioned at 3 µm. For antigen retrieval, slides were immersed in target retrieval solution (DakoCytomation, Carpinteria, Calif., USA) for 30 min at 90°C. Fibronectin immunoreactivity was localized with specific polyclonal rabbit anti-murine antibodies (Santa Cruz Biotechnology, Santa Cruz, Calif., USA) and slides were examined in a blinded manner.
Western Blot
Fibronectin and COX-2 expression were assessed by Western blot as described before [17, 18]. Tissue or cell homogenates were washed once with PBS and resuspended in 300 µl of homogenization buffer (50 mM Tris-HCl pH 7.6, 100 mM NaCl, 2 mM EDTA, 2 mM EGTA, 1 mM DTT, 1 mM PMSF, and 1* Triton X-100) and incubated on ice for 30 min. Resulting lysates were centrifuged for 30 min at 10,000 g at 4°C, supernatants collected and protein content analyzed by Bio-Rad assay. Thirty micrograms of protein were separated by SDS-PAGE (6* acrylamide gel) and transferred to a nitrocellulose membrane. Blots were incubated overnight with antibodies for fibronectin (Aldrich, St. Louis, Mo., USA) or COX-2 (Cayman Chemicals, Ann Arbor, Mich., USA), and actin (Santa Cruz Biotechnology) was used as control for unequal loading. The blots were washed and incubated with goat anti-rabbit antibody (Santa Cruz Biotechnology) for 1 h and the signal was detected by luminol chemiluminescence.
Morphometric Analysis
Kidney sections (3 µm) were stained with periodic acid-Schiff, and glomerular nuclei counted manually in a minimum of 60 glomeruli/specimen.
Measurement of Serum Cotinine
The serum cotinine level, a metabolite of nicotine, was measured using a Cotinine Direct ELISA kit supplied by Calbiotech, Inc. (Spring Valley, Calif., USA) following the manufacturer's instructions. Serum cotinine levels are directly related to nicotine absorption and are therefore an excellent marker of the intake of nicotine by the rats.
Determination of PGE2 by Enzyme Immunoassay
The prostaglandin E2 levels in the rat urine samples were assayed using an Enzyme Immunoassay Kit from Cayman Chemical Inc. Briefly, to each well was added 50 µl of sample (or standard) and 50 µl of prostaglandin E2 AchE Tracer followed by 50 µl of prostaglandin E2 monoclonal antibody. The plates were incubated for 18 h at 4°C and washed 5 times with wash buffer. The absorbance was read at 410 nm in a Bio-Rad Benchmark Plus plate reader upon adding 200 µl Ellman's Reagent.
Mesangial Cell Culture
Human mesangial cells were purchased from Cell Systems (Kirkland, Wash., USA), grown in CSC-Complete media (Cell Systems) supplemented with 10* fetal calf serum (Cell Systems). Cells were passed by trypsinization when confluent, and used between the 3rd and 9th passages.
Mesangial Cell Proliferation
3H-Thymidine incorporation was used as an index of cell proliferation as described [19]. Briefly, cells were fasted for 72 h in Maintenance Media (Cell Systems) and stimulated for 24 h with nicotine (10−7M) with and without the COX-2 inhibitors NS-398 (10−7 to 10−6M) or nimesulide (10−7 to 10−6M). Four hours before harvesting, cells were pulsed with 3H-thymidine (1 µCi/ml). At the end of this incubation period, cells were washed 3 times with PBS; protein was precipitated with 1 ml 10* TCA for 5 min and solubilized in 1 ml 0.5 N NaOH/0.1* SDS. Duplicate aliquots (0.5 ml) were removed and counted in a liquid scintillation counter and results expressed as cpm/million cells.
Statistical Analysis
Data are expressed as means ± SE. For statistical comparisons involving 2 groups, an unpaired Student's t test was used, whereas for comparisons involving more than 2 groups, ANOVA (StatView, BrainPower, Calabasas, Calif., USA) was used. Significance was considered when p < 0.05.
Results
In vivo Studies
Nicotine Increases Glomerular Cell Number in Nephritic Rats
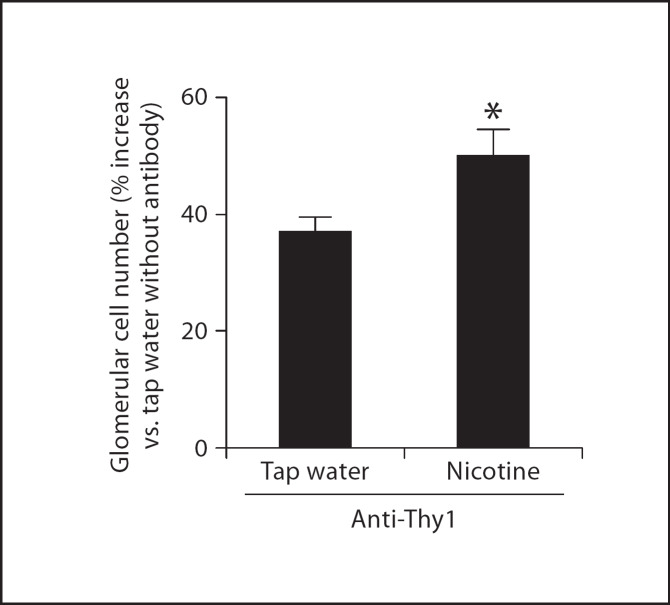
We administered nicotine in the drinking water to Sprague-Dawley rats starting 3 days prior to anti-Thy1 antibody administration and continued until sacrifice on day 14. After sacrifice, kidneys were harvested and kidney slides stained with periodic acid-Schiff. In each slide glomerular cells were counted in 60 glomeruli by an operator unaware of the different experimental conditions. As shown in figure 1, anti-Thy1 antibody administration resulted in a significant increase in glomerular cell number that was further augmented in rats that received nicotine. However, the administration of nicotine to control rats injected with vehicle did not modify glomerular cell number (not shown), suggesting that nicotine promotes but does not initiate inflammation in the glomerulus. The serum levels of cotinine (a stable nicotine metabolite) were measured by ELISA to confirm that the animals were effectively receiving nicotine. Administration of nicotine to rats resulted in an increase in serum cotinine (control 3.6 ± 1.27 ng/ml, and nicotine 56 ± 8.1 ng/ml) levels similar to those found in the plasma of active smokers.
Fig. 1.
Nicotine increases glomerular cell number in rats with anti-Thy1 nephritis (n = 6). * p < 0.05 vs. tap water.
Nicotine Increases Cortical Fibronectin Expression in Nephritic Rats
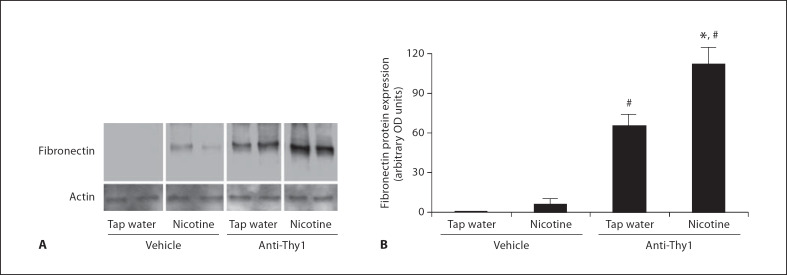
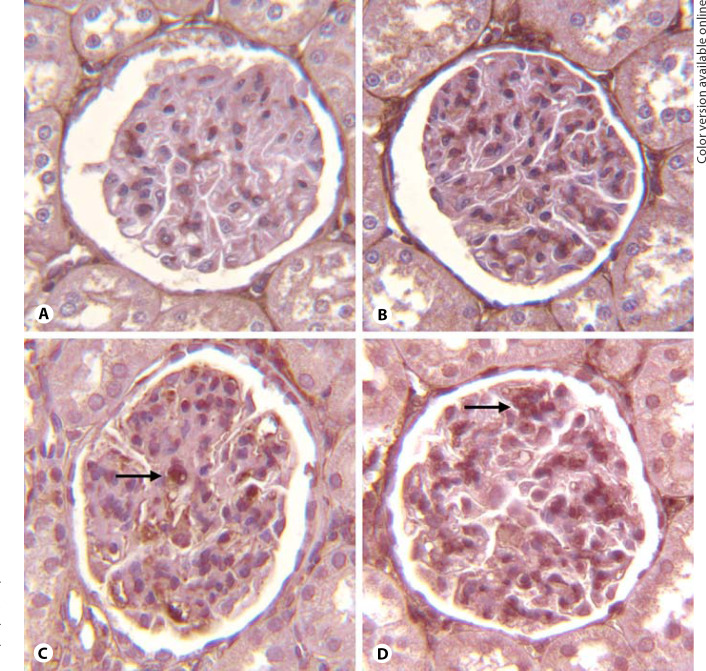
The anti-Thy1 model of nephritis is accompanied by a significant increase in the expression of cortical fibronectin [14], which is a critical matrix component. We measured fibronectin expression by Western blot and immunohistochemistry in rats with anti-Thy1 nephritis. As shown in figure 2, nicotine administration to rats injected with vehicle resulted in a small and nonsignificant increase in cortical fibronectin expression. In rats with anti-Thy1 nephritis, cortical fibronectin expression was increased with administration of vehicle (fig. 2). Nicotine administration, however, resulted in a significant further increase in fibronectin expression. Furthermore, as shown in figure 3D, we demonstrated enhanced glomerular fibronectin expression in nicotine administered anti-Thy1 nephritis as assessed by immunohistochemistry. Thus, nicotine enhanced both cell proliferation and cortical fibronectin expression in nephritic rats.
Fig. 2.
Nicotine administration increases renal cortex fibronectin expression in rats with anti-Thy1 nephritis. A Representative Western blot (in duplicates) for fibronectin and α-actin used as control for unequal loading. B Densitometry analysis after reprobing the blot with an anti-α-actin antibody (n = 6). * p <0.05 vs. tap water; # p < 0.05 vs. vehicle rats.
Fig. 3.
Nicotine increases fibronectin expression (arrows) in rats with anti-Thy1 nephritis as assessed by immunohistochemistry. A Control. B Nicotine. C Anti-Thy1 alone. D Anti-Thy1 + nicotine.
Nicotine Increases COX-2 Protein Expression in Nephritic Rats
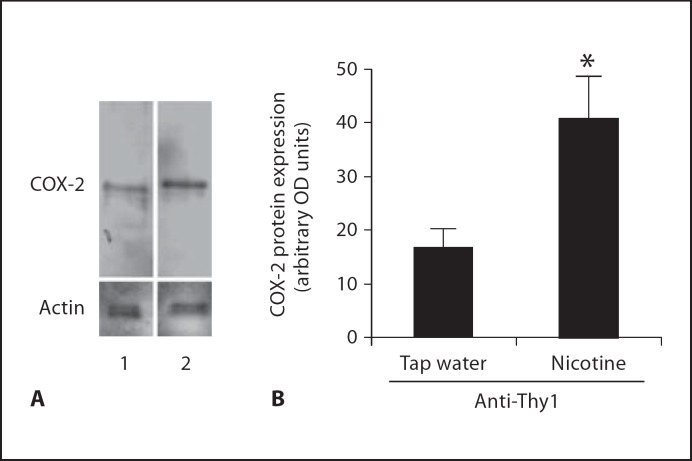
It has been observed both clinically and experimentally that the expression and activity of COX-2 was significantly upregulated in inflammatory conditions, including several forms of nephritis [20, 21]. The effects of nicotine on glomerular cell count (documented above) suggested to us that, in the setting of nephritis, nicotine may have a proinflammatory effect. We therefore investigated whether nicotine administration would increase the expression and activity of COX-2. As shown in figure 4, nicotine administration to rats with anti-Thy1 nephritis resulted in significant increases in COX-2 protein expression as determined by Western blot analysis. These changes in COX-2 expression were accompanied by a concomitant increase in urinary PGE2 excretion: baseline 2.8 ± 0.6 ng/mg creatinine; anti-Thy1 5.1 ± 0.7 ng/mg creatinine (p < 0.05 vs. control); anti-Thy1 + nicotine 7.7 ± 1.8 ng/mg creatinine (p < 0.05 vs. control and vs. anti-Thy1; n = 6).
Fig. 4.
Nicotine increases renal cortex COX-2 expression in rats with anti-Thy1 nephritis. A Representative Western blot for COX-2 and actin used as control for unequal loading. Lane 1 = Anti-Thy1; lane 2 = anti-Thy1 + nicotine. B Densitometry data analysis after reprobing the blot with an anti-α-actin antibody (n = 6). * p < 0.05 vs. tap water.
In vitro Studies
Nicotine Increases COX-2 Expression in Human Mesangial Cells
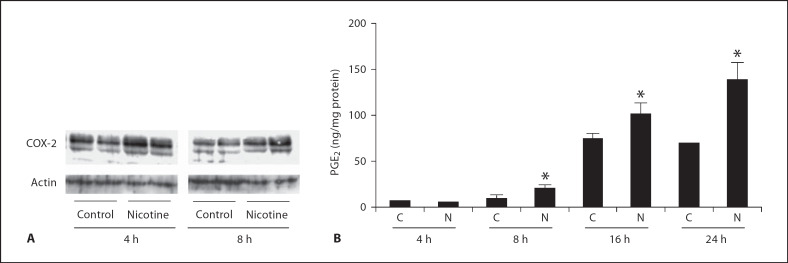
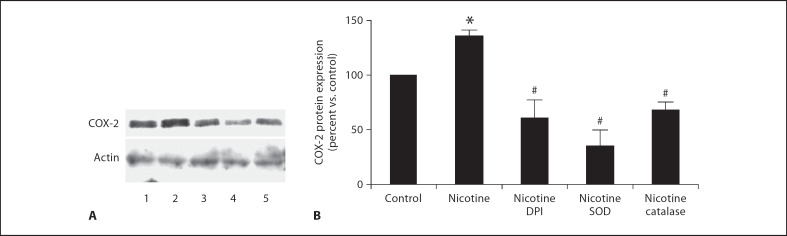
Human mesangial cells were incubated with nicotine (10−7M) for 4, 8, 16 and 24 h. As shown in figure 5A, nicotine treatment increased COX-2 expression after 4-hour exposure and this was accompanied by a concomitant increase in PGE2 production measured in culture supernatant (fig. 5B). To determine the mediation of ROS in COX-2 protein expression, human mesangial cells were exposed to nicotine (10−7M) with and without the NADPH oxidase inhibitor DPI and the ROS scavengers, SOD and catalase. As shown in figure 6, NADPH oxidase inhibition with DPI and treatment with SOD and catalase abolished nicotine-mediated COX-2 expression, suggesting that production of ROS mediates nicotine-induced COX-2 expression.
Fig. 5.
Nicotine increases COX-2 expression and activity in human mesangial cells. A Representative Western blot (in duplicates) for COX-2 and actin was used as control for unequal loading. B PGE2 production by human mesangial cells exposed to nicotine (n = 3–6). C = Control; N = nicotine. * p < 0.05 vs. respective control.
Fig. 6.
Nicotine-induced COX-2 expression in mesangial cells is prevented by the NADPH oxidase inhibitor DPI, and the ROS scavengers SOD and catalase. A Representative Western blot for fibronectin and actin used as control for unequal loading. Lane 1 = Control; lane 2 = nicotine; lane 3 = nicotine + DPI; lane 4 = nicotine + SOD; lane 5 = nicotine + catalase. B Densitometry data analysis performed after reprobing the blot with an α-actin antibody (n = 3–6). * p < 0.05 vs. control; # p < 0.05 vs. nicotine.
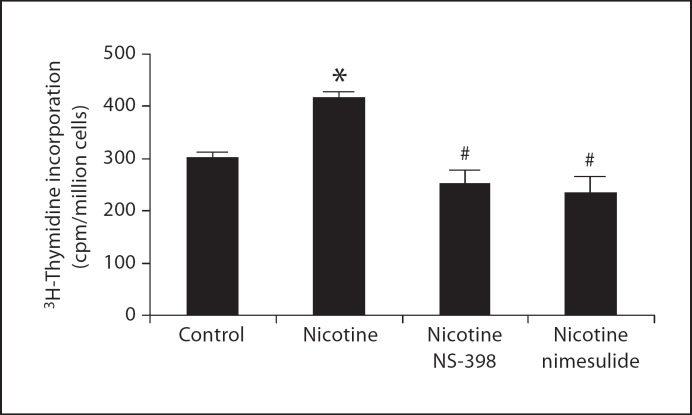
COX-2 Mediates Nicotine-Induced Cell Proliferation in Human Mesangial Cells
We have previously demonstrated that COX-2-derived prostaglandins have important growth-promoting effects in the glomerular mesangium [17]. To determine the role of COX-2 on nicotine-induced mesangial cell proliferation, human mesangial cells were stimulated with nicotine (10−7M) with and without the COX-2 inhibitors, NS-398 (10−6M) or nimesulide (10−6M) for 24 h and mesangial cell proliferation measured by 3H-thymidine incorporation. As shown in figure 7, nicotine-induced mesangial cell proliferation was prevented by COX-2 inhibition, demonstrating that COX-2 mediates in large part the effects of nicotine on mesangial cell proliferation.
Fig. 7.
Nicotine-induced mesangial cell proliferation is prevented by the COX-2 inhibitors NS-398 and nimesulide (n = 3 in duplicate). * p < 0.05 vs. control; # p < 0.05 vs. nicotine.
Discussion
We performed studies aimed at elucidating potential mechanisms by which nicotine in concentrations similar to those achieved in the plasma of smokers may initiate and/or accelerate renal injury. In the previous in vitro studies we demonstrated that nicotine, a compound present in large amounts in tobacco and responsible for the addictive effect of cigarette smoking, increases mesangial cell proliferation and extracellular matrix production, and that these effects are mediated by ROS and dependent on PKC and MAPK activation [13]. We extended these studies and here we demonstrate that nicotine increases COX-2 expression and that COX-2-derived prostaglandins mediate in large part the growth-promoting effects of nicotine in cultured human mesangial cells. In addition, our in vivo studies demonstrate that nicotine increases glomerular cell proliferation, fibronectin and COX-2 protein expression in an animal model of acute nephritis.
Epidemiologic studies have demonstrated that cigarette smoking increases the risk of progressive chronic kidney disease and accelerates the rate of progression of renal failure in diabetics [9,10,11] and in patients with various nephritides, including lupus nephritis [22] and IgA nephropathy, glomerular diseases that predominantly affect the glomerular mesangium [23, 24]. We have recently demonstrated that human mesangial cells are endowed with nAChRs and that nicotine promotes mesangial cell proliferation and extracellular matrix production [13]. The nAChRs function as agonist-regulated Ca2+ channels and are expressed not only in mesangial cells, but also in vascular endothelial cells and vascular smooth cells [25, 26]. In the vasculature, nicotine stimulates angiogenesis and accelerates atherosclerosis [3] via activation of nAChRs in a process that is partially dependent on VEGF, MAPK activation and NF-ĸB [27]. Indeed recent studies have suggested that endothelial NF-ĸB is critical in the pathogenesis of renal injury in hypertension [28] by promoting the expression of adhesion molecules and proinflammatory proteins including COX-2 and iNOS.
In the current studies we have demonstrated that nicotine increases glomerular cell proliferation and fibronectin production in the anti-Thy1 model of glomerulonephritis. This is a model characterized by initial mesangiolysis followed by transitory glomerular cellular proliferation and fibronectin production that occurs after a single injection of an anti-Thy1 antibody. These in vivo findings clearly support our previous and current studies in cultured human mesangial cells and provide mechanistic explanations for the deleterious effects of cigarette smoking in renal disease.
Clinical and experimental studies have demonstrated that COX-2 is upregulated in conditions associated with glomerular inflammation including acute glomerulonephritis and ureteral obstruction [29,30,31]. In addition, recent experimental studies have also demonstrated that COX-2 expression is upregulated in experimental models of diabetes [32, 33] and chronic kidney disease [34, 35] and that COX-2 inhibition in a model of renal ablation reduces mesangial matrix expansion, glomerular sclerosis and fibronectin and collagen IV expression, suggesting that COX-2-derived prostaglandins play an important role in the pathogenesis of renal injury [36]. To determine the role of COX-2 as a mediator of the growth-promoting effects of nicotine, we performed a series of experiments in cultured human mesangial cells. Our results show that nicotine increases COX-2 expression and activity in human mesangial cells and that COX-2 inhibition significantly reduces nicotine-induced mesangial cell proliferation. These findings suggest that COX-2-derived prostaglandins may play a major role as mediators of glomerular injury in response to nicotine. In support of our findings, COX-2 inhibition in vivo has been shown to reduce the pro-angiogenic and pro-atherosclerotic effects of nicotine. COX-2 mediates the synthesis of vasodilatory PGs and vasoconstrictive thromboxane A2 [37]. Vasodilatory PGs (PGE2 and PGI2) have been attributed with salutary effects associated with increases in blood flow and antiproliferative properties [38, 39]. However, recent evidence indicates that effects of PGE2 on growth-related responses are more complex as EP1 and EP4 receptors mediate growth-related responses [40] whereas others such as EP2 fail to do so. Within this scenario the effects of an upregulation of COX-2 may have significantly different vascular and renal effects depending on the type, distribution and quantity of the receptors present in each tissue type.
We recently demonstrated that nicotine increases the production of ROS in mesangial cells via NADPH oxidase activation [13]. We and others have shown that ROS play an important role as mediators of COX-2 induction in response to a variety of stimuli including proinflammatory cytokines and angiotensin II [17, 41]. In the current studies we now demonstrate that NADPH oxidase inhibition reduces nicotine-stimulated COX-2 expression, suggesting a role for nicotine-induced ROS as mediators of COX-2 expression.
In summary, these studies demonstrate that the systemic administration of nicotine exacerbates renal injury in a rat model of acute glomerulonephritis. In addition, we have identified COX-2-derived prostaglandins as potential mediators of these effects of nicotine. Current findings strongly support our previous studies in cultured mesangial cells and unveil novel mechanisms by which cigarette smoking accelerates the progression of renal injury.
Acknowledgements
Part of this work was presented as an abstract in the Annual Meeting of the American Society of Nephrology (2007). These studies were funded by research grants from the Flight Attendants Research Institute (FAMRI) and the James and Esther King Biomedical Research Program of the Florida Department of Health. We also thank Jessica Nigro and David Alzamora for their excellent technical assistance.
References
- 1.Boyle P. Cancer, cigarette smoking and premature death in Europe: a review including the recommendations of European cancer experts consensus meeting, Helsinki, October 1996. Lung Cancer. 1997;17:1–60. doi: 10.1016/s0169-5002(97)00648-x. [DOI] [PubMed] [Google Scholar]
- 2.McBride PE. The health consequences of smoking. Cardiovascular diseases. Med Clin North Am. 1992;76:333–353. doi: 10.1016/s0025-7125(16)30356-x. [DOI] [PubMed] [Google Scholar]
- 3.Heeschen C, Jang JJ, Weis M, Pathak A, Kaji S, Hu RS, Tsao PS, Johnson FL, Cooke JP. Nicotine stimulates angiogenesis and promotes tumor growth and atherosclerosis. Nat Med. 2001;7:833–839. doi: 10.1038/89961. [DOI] [PubMed] [Google Scholar]
- 4.Olshan AF, Li R, Pankow JS, Bray M, Tyroler HA, Chambless LE, Boerwinkle E, Pittman GS, Bell DA. Risk of atherosclerosis: interaction of smoking and glutathione s-transferase genes. Epidemiology. 2003;14:321–327. [PubMed] [Google Scholar]
- 5.Giannotti G, Landmesser U. Endothelial dysfunction as an early sign of atherosclerosis. Herz. 2007;32:568–572. doi: 10.1007/s00059-007-3073-1. [DOI] [PubMed] [Google Scholar]
- 6.Nakagami H, Kaneda Y, Ogihara T, Morishita R. Endothelial dysfunction in hyperglycemia as a trigger of atherosclerosis. Curr Diabetes Rev. 2005;1:59–63. doi: 10.2174/1573399052952550. [DOI] [PubMed] [Google Scholar]
- 7.Jaimes EA, DeMaster EG, Tian RX, Raij L. Stable compounds of cigarette smoke induce endothelial superoxide anion production via NADPH oxidase activation. Arterioscler Thromb Vasc Biol. 2004;24:1031–1036. doi: 10.1161/01.ATV.0000127083.88549.58. [DOI] [PubMed] [Google Scholar]
- 8.Raij L, DeMaster EG, Jaimes EA. Cigarette smoke-induced endothelium dysfunction: role of superoxide anion. J Hypertens. 2001;19:891–897. doi: 10.1097/00004872-200105000-00009. [DOI] [PubMed] [Google Scholar]
- 9.Orth SR. Smoking - a renal risk factor. Nephron. 2000;86:12–26. doi: 10.1159/000045708. [DOI] [PubMed] [Google Scholar]
- 10.Rossing P, Hougaard P, Parving HH. Risk factors for development of incipient and overt diabetic nephropathy in type 1 diabetic patients: a 10-year prospective observational study. Diabetes Care. 2002;25:859–864. doi: 10.2337/diacare.25.5.859. [DOI] [PubMed] [Google Scholar]
- 11.Stegmayr BG. A study of patients with diabetes mellitus (type 1) and end-stage renal failure: tobacco usage may increase risk of nephropathy and death. J Intern Med. 1990;228:121–124. doi: 10.1111/j.1365-2796.1990.tb00204.x. [DOI] [PubMed] [Google Scholar]
- 12.Border WA. Distinguishing minimal-change disease from mesangial disorders. Kidney Int. 1988;34:419–434. doi: 10.1038/ki.1988.197. [DOI] [PubMed] [Google Scholar]
- 13.Jaimes EA, Tian RX, Raij L. Nicotine: the link between cigarette smoking and the progression of renal injury? Am J Physiol Heart Circ Physiol. 2007;292:H76–H82. doi: 10.1152/ajpheart.00693.2006. [DOI] [PubMed] [Google Scholar]
- 14.Hayashi K, Osada S, Shofuda K, Horikoshi S, Shirato I, Tomino Y. Enhanced expression of membrane type-1 matrix metalloproteinase in mesangial proliferative glomerulonephritis. J Am Soc Nephrol. 1998;9:2262–2271. doi: 10.1681/ASN.V9122262. [DOI] [PubMed] [Google Scholar]
- 15.Sadlier DM, Ouyang X, McMahon B, Mu W, Ohashi R, Rodgers K, Murray D, Nakagawa T, Godson C, Doran P, Brady HR, Johnson RJ. Microarray and bioinformatic detection of novel and established genes expressed in experimental anti-thy1 nephritis. Kidney Int. 2005;68:2542–2561. doi: 10.1111/j.1523-1755.2005.00661.x. [DOI] [PubMed] [Google Scholar]
- 16.Yu L, Border WA, Anderson I, McCourt M, Huang Y, Noble NA. Combining TGF-β inhibition and angiotensin II blockade results in enhanced antifibrotic effect. Kidney Int. 2004;66:1774–1784. doi: 10.1111/j.1523-1755.2004.00901.x. [DOI] [PubMed] [Google Scholar]
- 17.Jaimes EA, Tian RX, Pearse D, Raij L. Up-regulation of glomerular COX-2 by angiotensin II: role of reactive oxygen species. Kidney Int. 2005;68:2143–2153. doi: 10.1111/j.1523-1755.2005.00670.x. [DOI] [PubMed] [Google Scholar]
- 18.Jaimes EA, Nath KA, Raij L. Hydrogen peroxide downregulates IL-1-driven mesangial iNOS activity: implications for glomerulonephritis. Am J Physiol. 1997;272:F721–F728. doi: 10.1152/ajprenal.1997.272.6.F721. [DOI] [PubMed] [Google Scholar]
- 19.Jaimes EA, Galceran JM, Raij L. Angiotensin II induces superoxide anion production by mesangial cells. Kidney Int. 1998;54:775–784. doi: 10.1046/j.1523-1755.1998.00068.x. [DOI] [PubMed] [Google Scholar]
- 20.Datta PK, Dhupar S, Lianos EA. Regulatory effects of inducible nitric oxide synthase on cyclooxygenase-2 and heme oxygenase-1 expression in experimental glomerulonephritis. Nephrol Dial Transplant. 2006;21:51–57. doi: 10.1093/ndt/gfi135. [DOI] [PubMed] [Google Scholar]
- 21.Han S, Kim K, Kim H, Kwon J, Lee YH, Lee CK, Song Y, Lee SJ, Ha N. Auranofin inhibits overproduction of pro-inflammatory cytokines, cyclooxygenase expression and PGE2 production in macrophages. Arch Pharm Res. 2008;31:67–74. doi: 10.1007/s12272-008-1122-9. [DOI] [PubMed] [Google Scholar]
- 22.Ward MM, Studenski S. Clinical prognostic factors in lupus nephritis. The importance of hypertension and smoking. Arch Intern Med. 1992;152:2082–2088. [PubMed] [Google Scholar]
- 23.Orth SR, Stockmann A, Conradt C, Ritz E, Ferro M, Kreusser W, Piccoli G, Rambausek M, Roccatello D, Schafer K, Sieberth HG, Wanner C, Watschinger B, Zucchelli P. Smoking as a risk factor for end-stage renal failure in men with primary renal disease. Kidney Int. 1998;54:926–931. doi: 10.1046/j.1523-1755.1998.00067.x. [DOI] [PubMed] [Google Scholar]
- 24.Stengel B, Couchoud C, Cenee S, Hemon D. Age, blood pressure and smoking effects on chronic renal failure in primary glomerular nephropathies. Kidney Int. 2000;57:2519–2526. doi: 10.1046/j.1523-1755.2000.00111.x. [DOI] [PubMed] [Google Scholar]
- 25.Macklin KD, Maus AD, Pereira EF, Albuquerque EX, Conti-Fine BM. Human vascular endothelial cells express functional nicotinic acetylcholine receptors. J Pharmacol Exp Ther. 1998;287:435–439. [PubMed] [Google Scholar]
- 26.Pestana IA, Vazquez-Padron RI, Aitouche A, Pham SM. Nicotinic and PDGF-receptor function are essential for nicotine-stimulated mitogenesis in human vascular smooth muscle cells. J Cell Biochem. 2005;96:986–995. doi: 10.1002/jcb.20564. [DOI] [PubMed] [Google Scholar]
- 27.Heeschen C, Weis M, Aicher A, Dimmeler S, Cooke JP. A novel angiogenic pathway mediated by non-neuronal nicotinic acetylcholine receptors. J Clin Invest. 2002;110:527–536. doi: 10.1172/JCI14676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Henke N, Schmidt-Ullrich R, Dechend R, Park JK, Qadri F, Wellner M, Obst M, Gross V, Dietz R, Luft FC, Scheidereit C, Muller DN. Vascular endothelial cell-specific NF-κB suppression attenuates hypertension-induced renal damage. Circ Res. 2007;101:268–276. doi: 10.1161/CIRCRESAHA.107.150474. [DOI] [PubMed] [Google Scholar]
- 29.Hirose S, Yamamoto T, Feng L, Yaoita E, Kawasaki K, Goto S, Fujinaka H, Wilson CB, Arakawa M, Kihara I. Expression and localization of cyclooxygenase isoforms and cytosolic phospholipase A2 in anti-Thy-1 glomerulonephritis. J Am Soc Nephrol. 1998;9:408–416. doi: 10.1681/ASN.V93408. [DOI] [PubMed] [Google Scholar]
- 30.Jerde TJ, Mellon WS, Bjorling DE, Nakada SY. Evaluation of urothelial stretch-induced cyclooxygenase-2 expression in novel human cell culture and porcine in vivo ureteral obstruction models. J Pharmacol Exp Ther. 2006;317:965–972. doi: 10.1124/jpet.105.099184. [DOI] [PubMed] [Google Scholar]
- 31.Norregaard R, Jensen BL, Topcu SO, Nielsen SS, Walter S, Djurhuus JC, Frokiaer J. Cyclooxygenase type 2 is increased in obstructed rat and human ureter and contributes to pelvic pressure increase after obstruction. Kidney Int. 2006;70:872–881. doi: 10.1038/sj.ki.5001616. [DOI] [PubMed] [Google Scholar]
- 32.Bagi Z, Erdei N, Papp Z, Edes I, Koller A. Up-regulation of vascular cyclooxygenase-2 in diabetes mellitus. Pharmacol Rep. 2006;58((suppl)):52–56. [PubMed] [Google Scholar]
- 33.Kellogg AP, Cheng HT, Pop-Busui R. Cyclooxygenase-2 pathway as a potential therapeutic target in diabetic peripheral neuropathy. Curr Drug Targets. 2008;9:68–76. doi: 10.2174/138945008783431691. [DOI] [PubMed] [Google Scholar]
- 34.Solari V, Piotrowska AP, Cascio S, Unemoto K, Chertin B, Puri P. Cyclooxygenase-2 up-regulation in reflux nephropathy. J Urol. 2003;170:1624–1627. doi: 10.1097/01.ju.0000085810.37816.65. [DOI] [PubMed] [Google Scholar]
- 35.Tokuyama H, Hayashi K, Matsuda H, Kubota E, Honda M, Okubo K, Takamatsu I, Tatematsu S, Ozawa Y, Wakino S, Saruta T. Differential regulation of elevated renal angiotensin II in chronic renal ischemia. Hypertension. 2002;40:34–40. doi: 10.1161/01.hyp.0000022060.13995.ed. [DOI] [PubMed] [Google Scholar]
- 36.Wang JL, Cheng HF, Shappell S, Harris RC. A selective cyclooxygenase-2 inhibitor decreases proteinuria and retards progressive renal injury in rats. Kidney Int. 2000;57:2334–2342. doi: 10.1046/j.1523-1755.2000.00093.x. [DOI] [PubMed] [Google Scholar]
- 37.Dubois RN, Abramson SB, Crofford L, Gupta RA, Simon LS, Van De Putte LB, Lipsky PE. Cyclooxygenase in biology and disease. FASEB J. 1998;12:1063–1073. [PubMed] [Google Scholar]
- 38.de Bittencourt PI, Jr, Miyasaka CK, Curi R, Williams JF. Effects of the antiproliferative cyclopentenone prostaglandin a1 on glutathione metabolism in human cancer cells in culture. Biochem Mol Biol Int. 1998;45:1255–1264. doi: 10.1080/15216549800203472. [DOI] [PubMed] [Google Scholar]
- 39.Haberl C, Hultner L, Flugel A, Falk M, Geuenich S, Wilmanns W, Denzlinger C. Release of prostaglandin D2 by murine mast cells: importance of metabolite formation for antiproliferative activity. Mediators Inflamm. 1998;7:79–84. doi: 10.1080/09629359891216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jabbour HN, Boddy SC. Prostaglandin E2 induces proliferation of glandular epithelial cells of the human endometrium via extracellular regulated kinase 1/2-mediated pathway. J Clin Endocrinol Metab. 2003;88:4481–4487. doi: 10.1210/jc.2003-030297. [DOI] [PubMed] [Google Scholar]
- 41.Wei Y, Sowers JR, Clark SE, Li W, Ferrario CM, Stump CS. Angiotensin II-induced skeletal muscle insulin resistance mediated by NF-κB activation via NADPH oxidase. Am J Physiol Endocrinol Metab. 2008;294:E345–E351. doi: 10.1152/ajpendo.00456.2007. [DOI] [PubMed] [Google Scholar]