Abstract
Epilepsy is a major public health concern in low and middle-income countries (LMICs) and comorbidities aggravate the burden associated with the disease. The epidemiology of these comorbidities has not been well described, although, identifying the main comorbidities of epilepsy, and their relative importance, is crucial for improving the quality of care. Comorbidities were defined as disorders coexisting with or preceding epilepsy, or else compounded or directly attributed to epilepsy or to its treatment. A meta-analysis of the proportion of main comorbidities by subcontinent as well as overall was also conducted. Out of the 2,300 papers identified, 109 from 39 countries were included in this systematic review. Four groups of comorbidities were identified: parasitic and infectious diseases (44% of comorbid conditions), somatic comorbidities (37%), psychosocial (11%), as well as psychiatric comorbidities (8%). Heterogeneity was statistically significant for most variables then random effect models were used. The most frequently studied comorbidities were: neurocysticercosis (comorbid proportion: 23%, 95% CI: 18–29), head trauma (comorbid proportion: 9%, 95% CI: 5–15) malnutrition (comorbid proportion: 16%, 95% CI: 28–40), stroke (comorbid proportion: 1.3%, 95% CI: 0.2–7.0), and discrimination for education (comorbid proportion: 34%, 95% CI: 28–40). Many comorbidities of epilepsy were identified in LMICs, most of them being infectious.
Subject terms: Medical research, Risk factors
Introduction
Epilepsy is characterized by the recurrence of seizures with neurological, cognitive, psychological and social impacts. A seizure is the transient presence of signs and/or symptoms due to a synchronous abnormal or excessive neural activity in the brain1.
In developed countries, the overall prevalence of epilepsy ranges from 5‰ to 8‰2–4 whereas in low- and middle-income countries, except in Asia, the proportion is 2–3 times higher5,6. As a consequence, among the 70 million people living with epilepsy in the world, 85% are found in countries with low income7.
Epilepsy is a major public health issue due to its medical, social, cultural and economic consequences8,9. It carries a significant burden not only due to the seizures themselves, but also to the comorbidities, the disabilities, and the stigma associated with the disease10.
In developed countries, over the last decade, researchers have established reliable and valid measures in PWE11. They explored comorbidities and grouped them into two large entities: psychiatric and somatic. In Texas, USA, an estimated 41% of PWE presented psychiatric and somatic disorders when compared to 15–20% in the general population; In England the proportion of PWE with comorbidities was 32%12. Tellez-Zenteno et al.13, using the Composit International Diagnostic Interview (CIDI) in a descriptive epidemiological survey in communities across Canada, diagnosed psychiatric disorders in 35% of PWE, compared to 20% in people without epilepsy. A cohort study in 713 adults with epilepsy in Sweden reported a comorbidity proportion of 5.9% for psychiatric disorders and 50% for somatic disorders14.
A link between epilepsy and certain comorbidities such as depression, attention deficit with hyperactivity disorder, anxiety, migraine, sleep disorders and malnutrition may exist through a common etiology or common genetic or environmental factors, as well as side effects of anti-epileptics15. Delay in starting epilepsy treatment is present in about 70% of cases where there is an occurrence of comorbidities16.
The epidemiology of the comorbidities of epilepsy in LMICs, as well as the link between these disorders and epilepsy have not been well described. There is no overall information on comorbidities affecting PWE in LMICs in the existing literature, and the data on the proportion of various comorbidities of epilepsy are fragmented. However, a good knowledge of epilepsy comorbidities is crucial for an accurate diagnosis and for delivering appropriate and comprehensive care, especially in LMICs where medical resources are scarce. Thus, we conducted a systematic review of studies on comorbidities of epilepsy in LMICs.
Methods
We referred to the recommendations of the PRISMA statement17 for drafting systematic reviews and meta-analysis in epidemiology.
Definitions
The co-occurrence of various conditions is amplified in the context of chronic diseases and the understanding of how these relate to each other is essential. Feinstein defined a comorbidity as “any separate additional clinical entity that exists (…) in the clinical course of a patient who has the index of a disease”. Comorbidity is a broad concept, including complications, causes, signs, or symptoms18. It is currently set by the experts as a disorder coexisting with epilepsy and which may precede, be compounded or be directly attributable to epilepsy or its treatment19,20. Comorbidities were split in 4 groups: psychiatric comorbidities, psychosocial comorbidities (education discrimination, marriage discrimination…), somatic comorbidities, and infectious - in particular parasitic- comorbidities.
Search strategy
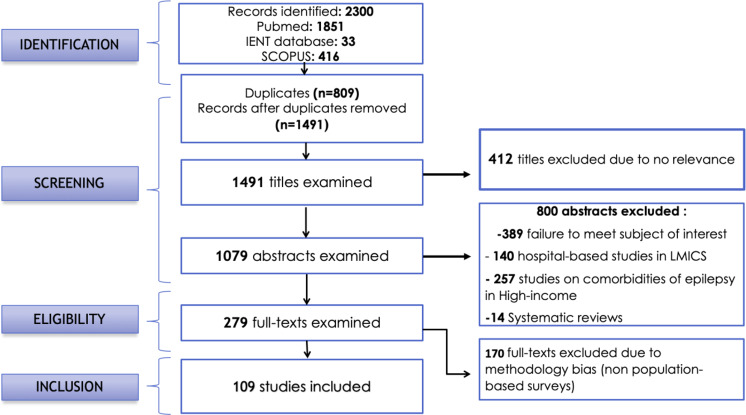
We conducted a systematic review in 3 databases: Pubmed/Medline, IENT (Institute of Epidemiology and Tropical Neurology – https://www.unilim.fr/ient) and Scopus. English keywords were used. Regarding Pubmed/Medline research the word “epilepsy” was combined with each of 62 comorbidities and was evaluated among the countries categorized as LMICs by the World Bank in 2015. At a second level the word “epilepsy” was combined with each of the 16 chapters of the international classification of Diseases (ICD-9) and each of the LMICs. We adapted this research requirements to Scopus and IENT databases. The steps followed for the selection of the studies are illustrated in the flow chart (Fig. 1). Meta-analyses and systematic reviews were used as additional sources for identifying relevant studies.
Figure 1.
Flow-chart illustrating the selection procedure.
Inclusion criteria
To be included in our study, an article had to be conducted in general population in a LMIC setting, according to a cohort, cross-sectional or case-control methodology, and to include original data on epilepsy comorbidities.
Exclusion criteria
Any study based on hospital data was excluded, as well as meta-analyses and systematic reviews.
Data extraction
In each of the papers the following data were collected: the authors, the study year, the publication year, the geographic location, the study type, the methods, the population (rural, urban, suburban), the sample size and the subgroups by sex, age classes, type of epilepsy and type of comorbidities.
Data analysis
A comorbidity included in the systematic review was considered for meta-analysis, if its link to epilepsy was physio-pathologically proven and if more than 3 studies were found on the subject. A meta-analysis of comorbidity proportions was performed using Stata software (Version 12). For each comorbidity, a ‘forest plot’ chart was produced. Then, a meta-analysis, global and stratified by sub-continents, was conducted. The pooled proportions were calculated. Data items were weighted in the computations. The weights were based on a precision estimator for each study, i.e. the opposite of the standard error of the proportion. We also calculated the I² index, which reflects the percentage of the total variation in all of the studies due to heterogeneity rather than random. Because heterogeneity was statistically significant, random effects models were used. To stabilize the variances of the proportions used in the calculations, the proportions used underwent a Logit transformation21. GPS (Global Positioning System) coordinates of the various study areas were searched http://www.gpsfrance.net/adresse-vers-coordonnees-gps enabling us to map the study sites.
Ethics
This paper does not concern directly PWE but makes use of publications concerning comorbidities of epilepsy. Informed consent is not applicable. No approval of an ethics committee is applicable is this case of the figures.
Results
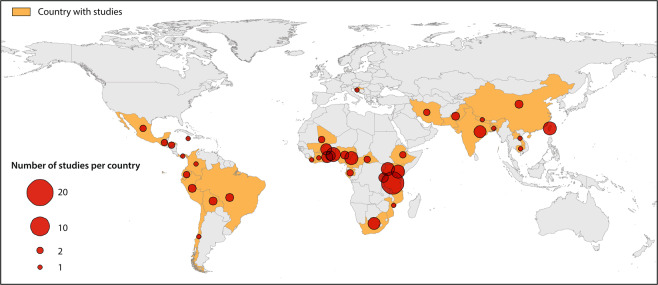
A total of 2300 publications were identified and 109 from 39 countries were included in our study. The selection process is illustrated in the flowchart (Fig. 1) and Fig. 2 shows the worldwide distribution of the included studies.
Figure 2.
Distribution of studies on comorbidities of epilepsy in low- and middle-income countries.
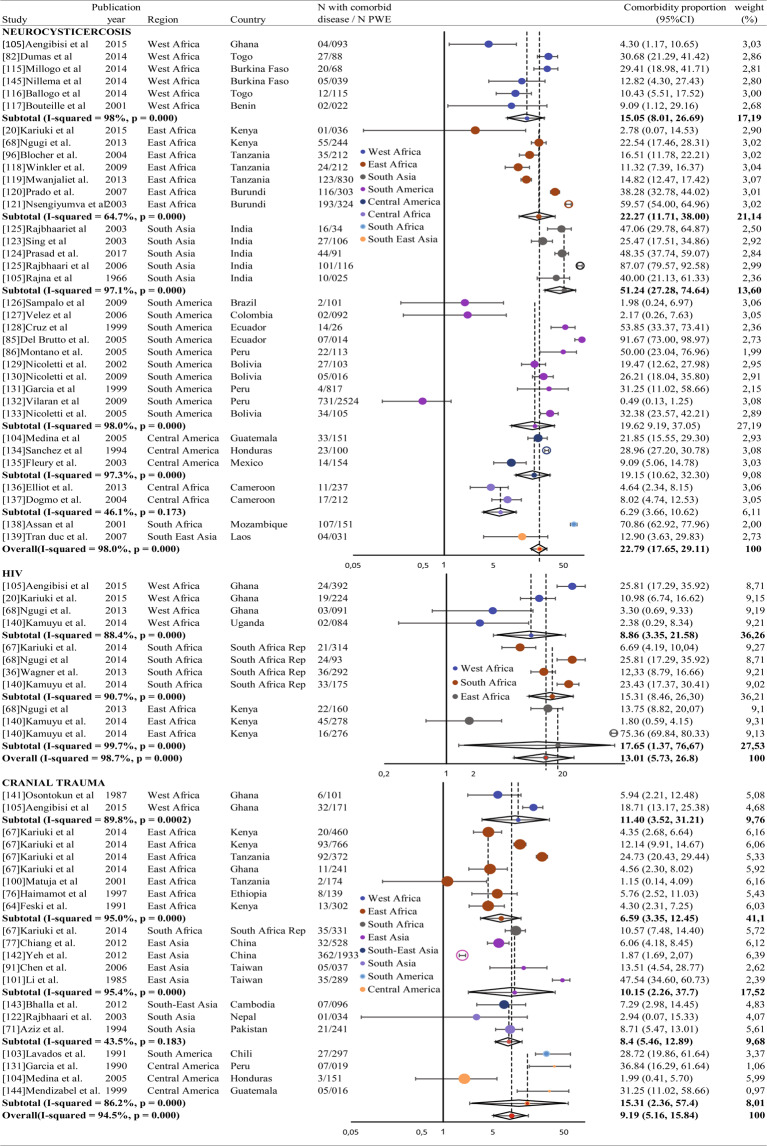
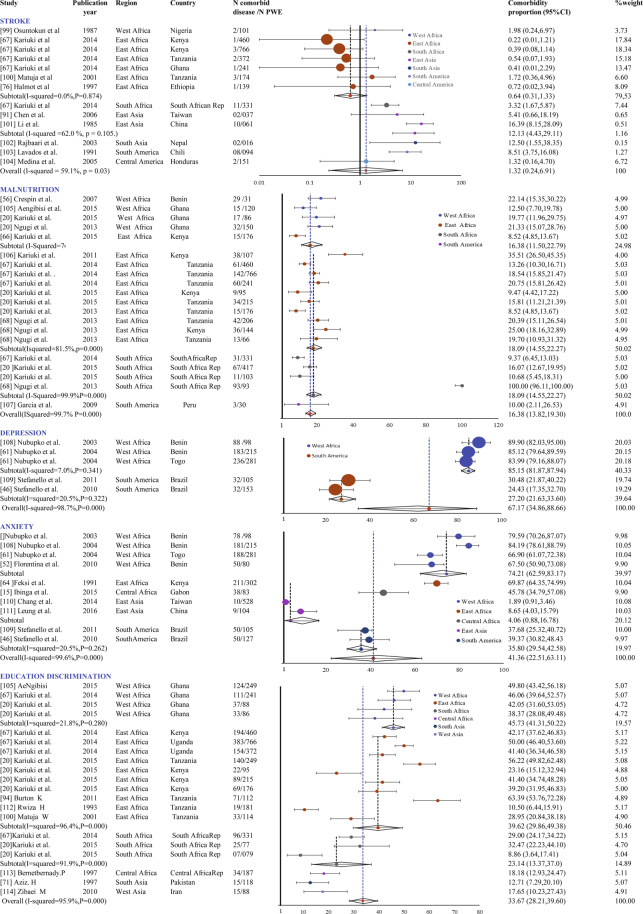
Overall, 44% of studies concerned parasitic and infectious comorbidities, 37% somatic comorbidities, 11% psychosocial comorbidities and 8% psychiatric comorbidities. Criteria for meta-analysis were met for neurocysticercosis (assessed in 35 studies), cranial trauma (18 studies), stroke (10 studies), malnutrition (9 studies), depression (4 studies) and anxiety (9 studies), HIV (5 studies) and education discrimination (9 studies). Results per comorbidity, overall and stratified by continent, are presented in Figs. 3 and 4. A significant heterogeneity between studies was found for malnutrition (I2 test: 99.7%, Q test: p < 0.0001), depression (I2 test = 98.7%, Q test: p < 0.0001) and anxiety (I2 = 99.6%, Q test p < 0.0001). The estimates were based on random effects models. It is important to note that, regarding HIV, the overall comorbidity proportion is only valid for sub-Saharan Africa. Results about comorbidities included in the review, but not in the meta-analysis, are described below:
Figure 3.
Forest plots of comorbidities in epilepsy in low- and middle-income countries (1/2).
Figure 4.
Forest plots of comorbidities in epilepsy in low- and middle-income countries (2/2).
Onchocerciasis
Thirteen studies evaluating comorbidity between epilepsy and onchocerciasis were identified. In East Africa the proportions varied between 81.8% and 98.6%22–27. In Central Africa only two studies were conducted with comorbidity proportion ranging from 22.4% to 36.9%. In West Africa the comorbidity proportions varied between 0 and 22.4%28–33.
Meningitis
Only 3 studies have been carried out to identify the link between meningitis and epilepsy. The comorbidity proportions varied between 2.2% and 3.9%.
Perinatal events
Twenty-six studies were identified (Supplementary Table 2). Perinatal trauma seems to be the most studied as 17 studies focused on the subject.
High blood pressure
Several studies revealed high blood pressure in PWE varying from 5.4% to 41.9%34–39.
Diabetes
Only two studies were identified with comorbidity proportions ranging from 5.3%40 to 26.4%41.
Employment discrimination
It was assessed in 7 studies, with comorbidity proportions between 27.4%42 and 78.0%22 (Supplementary Table 1)
Marriage discrimination
Eight studies were retrieved; the comorbidity proportions were between 46.6%43 and 88.9%44 (Supplementary Table 1).
Other diseases
Migraine,1 study, comorbidity proportion: 2.7%45; brain tumor: 11 studies, comorbidity proportion between 0.6% and 9.0%; Fractures: 1 study, comorbidity proportion: 10.4%45; Burns: 4 studies, comorbidity proportions between 0.6% and 9.0%22,46–48; mental retardation: 4 studies, comorbidity proportion between 7.9% and 11.4%45,47,49; cognitive disorders: 4 studies, comorbidity proportion between 30.6% and 69.3%22,48,50,51 and sickle cell anemia: 1 study, comorbidity proportion:1.7%8.
Discussion
Psychiatric comorbidities
It is commonly accepted that patients with epilepsy are at a greater risk of developing psychiatric disorders38 but there are very few studies conducted in general population evaluating the proportion of psychiatric comorbidities in PWE in LMICs. The wide variation of comorbid proportions between studies may be due to methodological differences between the studies, and to the variability in the sample sizes, as well as the difference between the diagnostic instruments used.
Depression
The results of this meta-analysis are in line with (and even higher than) the data reported in developed countries where the common rate of depression in PWE was high. This could be explained by the limited availability of antiepileptic drugs in LMICs. Among the risk factors for depression in PWE, the frequency of seizures and the lack of treatments are prevalent39. The link between frequency of seizures and mental illness was also found in 696 patients with epilepsy in a study by Jacoby et al.52. In Europe the overall proportion of depression in PWE is estimated to be between 20% to 30%. Thus, depression would be the most frequent psychiatric comorbidity, affecting 20% to 50% of PWE in interictal period53. Some authors suggest a two-way relationship between depression and epilepsy10,54, likely due to neuroaminergic malfunctions common to depression and epilepsy55. Furthermore, depression is more prevalent in patients with uncontrolled epilepsy.
Anxiety
There is insufficient information on the proportion of anxiety comorbid to epilepsy in LMICs. Pooled results from our study show a high proportion of anxiety compared to figures reported in developed countries. Studies in developed countries have paid more attention to depression, although anxiety could be more frequent56–58 and it is known that both disorders often co-exist42. In some circles of specialized care, the comorbid proportion of anxiety disorders may exceed 50%59–61.
Psychosocial factors
Even if it is not a disease, education discrimination can also be considered as a comorbidity because it is directly attributable to epilepsy. Education discrimination can be also explained by cognitive problems and behavioral issues which are frequent in epilepsy. They can have multiple causes, the most important being brain lesions, seizures, epileptic dysfunction, and treatment. Various social and cultural aspects in LMICS are harmful for PWE and are a source of stigma. It can be due to psychiatric disorders such as depression, anxiety or even suicide because the individual may feel useless, which would affect their self-esteem. This discrimination seems more accentuated in Africa than in Asia, and that could be explained by strong sociocultural restrictions hindering the education of PWE as they are considered as burdens to the community62. Because of the social weight, many children live hidden and cannot attend school, and in some cases, parents are forced to look for a school away from home, or even of changing schools to hide their child’s disease. Ultimately, children end up dropping out of school50. These psychosocial problems are critical obstacles in the management of epilepsy because of the public’s negative attitude towards PWE63.
Somatic comorbidities
Cranial trauma
The comorbid proportion of traumatic brain injury in LMICS could be explained by the fact that road accidents are the main causes of injury, especially in Africa. They can also be linked to work related accidents, war wounds or uncontrolled falls during seizures in PWE46 or falling while walking on slippery muddy paths. The risk of developing post-traumatic epilepsy depends on the degree and the severity of the trauma as well as the resulting complications. In a study conducted in 2000 by Farnarier in Mali, post-traumatic epilepsy represented 7% of the total patients with epilepsy2.
According to Hauser, head trauma was one of the most important risk factors for epilepsy in a general population study that he conducted in Rochester, USA. Injury was identified as the cause of epilepsy in 6% of the population64,65. Assumptions have been made concerning the mechanisms of the occurrence of epilepsy after head trauma, including the deposit of iron associated with extravasation of blood, the increase in excitotoxicity due to the accumulation of glutamate, and diffuse axonal injury edema or ischemia12, to name a few.
Stroke
Epilepsy can be an early or late complication of stroke, which is one of the most common causes of epilepsy especially in the elderly. The overall pooled comorbidity proportion from our meta-analysis seems to be underestimated when compared to comorbidity proportions found in developed countries where investigation means are more sophisticated.
Malnutrition
Although malnutrition is not considered a direct cause of epilepsy, it seems to favor the onset of epilepsy or convulsions through various nutritional deficiencies. In LMICs, the relationship between epilepsy and malnutrition has been long suspected as a potential cause66.
It has been reported by some authors that the link between epilepsy and malnutrition in developing countries is difficult to establish because few studies have explored this potential relationship, with different methods conducting to inhomogeneous conclusions66.There are several hypotheses about the possible mechanisms such as the frequency of the number of seizures which could favorize malnutrition, or the decrease of immunity, due to malnutrition66–68, that could be involved in the occurrence of epilepsy. Biochemical changes due to malnutrition, such as electrolyte abnormalities and hypoglycemia could impact on the number of occurrences69.
On the other hand, due to the attitudes towards epilepsy in sub-Saharan Africa, epilepsy can also contribute to malnutrition as people with epilepsy are often victims of food taboos that could result in malnutrition in people with epilepsy. In a study conducted by Nubukpo70, for instance, 64% of people with epilepsy in Benin and 44% in Togo were victims of food taboos.
Perinatal events
Some studies have shown that the proportion of perinatal etiologies varies from 2%to 65% of the cases of epilepsy in sub-Saharan Africa71–73. Our literature search identified perinatal trauma as one of the most common causes of epilepsy in LMICs. This is consistent with what has been described by other systematic reviews including one conducted by Ba Diop et al.4. The legacy of birth injuries, often caused by a difficult birth, could lead to epilepsy within various time lapses4. It is difficult to prematurely attach the cause of seizure to a pre-, peri- or post-natal event because this link is often only based on the examination of the subject, or possibly on the interview of relatives, and thus is subject to biases of memorization8. Tradition or even the great distance from some villages to health centers sometimes force women to undergo childbirth at home without skilled assistance74.
High blood pressure
The comorbidity proportion found in different studies appears to be higher when compared to the general population. A meta-analysis of studies on the proportion of hypertension in the general population in Nigeria reported a rate between 12.4% and 34.8% in people without epilepsy75.
The range found in our study is substantially higher than that described in a larger study in the general population in the United Kingdom whose comorbidity proportion varied between 1.45% and1.95%10. This might be explained by the lifestyle of patients with epilepsy in LMICs in terms of food hygiene and permanent stress, which can predispose the individual to hypertension leading to seizures.
The relationship between hypertension and epilepsy is an indirect causal relationship (hypertension is a major risk factor for stroke), although some authors posit that high blood pressure might have an independent effect on epilepsy75.
Diabetes
The association of diabetes, especially type 1, and epilepsy has already been mentioned. However, the pathophysiology of this association has not yet been well elucidated76. Type 1 diabetes called diabetes mellitus or MODY (Maturity-Onset Diabetes of the Young) is observed in youth population with a proportion of 0.95% in the under 20 years age group77. Several mechanisms may play a role such as autoimmunity (antibodies to Glutamic Acid Decarboxylase) and genetics76–78. In our systematic review the proportions in LMICs seem obviously higher than that described in developed countries. However, it is not possible to draw a conclusion from only two isolated studies.
Brain tumors, paradoxically, have a very low comorbidity proportion in LMICs compared to developed countries, probably due to the lack of means of imaging exploration. Because of this obvious underestimation we did not include this comorbid condition although it met the criteria for meta-analysis.
Other somatic comorbidities such as burns, mental delays, cognitive disorders18,45,79 and sickle cell disease8 were also identified as being common in PWE in LMICs. Very few studies on the comorbid relation of these diseases with epilepsy were retrieved. This has not allowed us to perform a meta-analysis.
Infectious and parasitic comorbidities
In LMICS, such as those in sub-Saharan Africa or Latin America, infections seem to explain the high proportion of epilepsy3. The Commission on tropical diseases of the International League against epilepsy has listed several diseases as being the causes of epilepsy, including malaria, tuberculosis, schistosomiasis, HIV/AIDS and neurocysticercosis. They seem to be the most frequent causes of epilepsies in a tropical environment80.
Neurocysticercosis
Cysticercosis is the most common neurological infection and is described in the literature as being a major cause of epilepsy in the tropics, particularly in Asia and Latin America81,82. It is associated with 30–50% of epilepsies in endemic areas like Peru where almost half the population lives in conditions where the transmission of Taenia solium is endemic83,84.
Onchocerciasis
Studies have confirmed an association between the proportion of filariasis such as the Loa loa, or the bancroftian infection, and the occurrence of seizures, but their causal effects or association with epilepsy are controversial85,86. The association between river blindness and epilepsy is still a matter of debate to date because there is a discrepancy between the results of different studies on the subject71.
HIV infection
Very few studies were conducted in the general population in LMICs on the comorbidity between epilepsy and HIV. Studies on this association are mostly outdated and from hospital series which are sources of substantial bias. We included HIV in the meta-analysis although the physiobiological link is not clearly proven because HIV mortality rate remains high in LMICs, We think that it would be necessary to carry out additional studies to better understand the impact of HIV on epilepsy (if there is one). The pathophysiological mechanisms involved in this association have not yet been well clarified87.
Meningitis
It is one of the most frequent causes of febrile seizures. The risk of developing epilepsy after an episode of meningitis appears to be very low, but the risk is six times higher among those who start twitching during the acute phase of the disease88. In our systematic review, very few studies were identified on this topic. The pathophysiological mechanisms involved in the epileptic process include a series of changes such as an increase in inflammatory cytokines including TNF, in response to a chronic inflammation related to activation of the immune system in response to an endotoxemia89.
Strength and limitations of this study
This is the first study to display such pooled results with worldwide data on comorbidities of epilepsy in LMICs. The application of a transformation method to stabilize logit estimates of proportions reinforces the estimates accuracy. Focusing on studies in the general population increases the external validity of our study.
Nevertheless, this study has some limitations. Firstly, using keywords only in English could be a reason for not identifying more studies conducted in Latin America. The second limitation is the high heterogeneity observed between studies. It is related to differences in methods and sample sizes, as well as the geographic variability of the studied populations. We took it into account by conducting random effects models. We have weighed studies based on a precision estimator, we could have used other methods of weights. We did not examine epilepsy phenotypes, which may underestimate comorbidities in non-convulsive epilepsies. We could have also used other databases such as African Index Medicus, which could have improved our exhaustiveness.
Conclusion
Through this study we have been able to identify the main comorbidities of epilepsy in LMICs and to determine their relative proportions. Aside from the disease itself, specific factors associated with epilepsy may have an impact on the quality of life of PWE. Most of them are preventable and treatable. The majority of these comorbid conditions share common mechanisms with epilepsy, direct or indirect, causal or through etiologies or common risk factors (genetic or environmental). It is therefore necessary to conduct further studies with valid approaches to understand their impact. Results also highlight the importance of comprehensive care for PWE, ensuring that patients and their caregivers receive correct information about the multifactorial aspect of care for epilepsy is crucial for the quality of care90–138.
Supplementary information
Acknowledgements
This research did not receive any grant from any funding agency in the public, commercial or not-for-profit sector.
Author contributions
A.M., P.M.P. and D.G. designed the study protocol. A.M. collected data. B.M. and A.M. conducted statistical analyses. C.N. and C.T. helped to configurate the forest plots. A.M. wrote the first draft. All authors reviewed the first draft. All authors contributed substantially to the content of this manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-65768-6.
References
- 1.Commission on Epidemiology and Prognosis, International League against Epilepsy (ILAE) Guidelines for epidemiologic studies on epilepsy. Epilepsia. 1993;34:92–96. doi: 10.1111/j.1528-1157.1993.tb00433.x. [DOI] [PubMed] [Google Scholar]
- 2.Farnarier G, et al. Onchocerciasis and epilepsy. Epidemiological survey in Mali. Med. Trop. 1999;60:151–155. [PubMed] [Google Scholar]
- 3.Mac TL, et al. Epidemiology, aetiology, and clinical management of epilepsy in Asia: a systematic review. Lancet Neurol. 2007;6:533–543. doi: 10.1016/S1474-4422(07)70127-8. [DOI] [PubMed] [Google Scholar]
- 4.Ba-Diop A, et al. Epidemiology, causes, and treatment of epilepsy in sub-Saharan Africa. Lancet Neurol. 2014;13:1029–1044. doi: 10.1016/S1474-4422(14)70114-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Banerjee P, Filippi D, Hauser W. The descriptive epidemiology of epilepsy—a review. Epilepsy Res. 2009;85:31–45. doi: 10.1016/j.eplepsyres.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thomas P, Arzimanoglou A. Épilepsies. Collection Abrégés de Médecine. Paris: Masson, 2e édition. 1999;40:1098–1105. [Google Scholar]
- 7.Ngugi AK, Bottomley C, Kleinschmidt I, Sander JW, Newton CR. Estimation of the burden of active and life-time epilepsy: a meta-analytic approach. Epilepsia. 2010;51:883–890. doi: 10.1111/j.1528-1167.2009.02481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ngoungou, E. B. Approche épidémiologique de la relation paludisme cérébral et épilepsie séquellaire en zone tropicale. Thèse de Doctorat d’Université, Limoges (2006). (https://www.theses.fr/2006LIMO100B)
- 9.Newton CR, Kariuki SM. Status epilepticus in sub-Saharan Africa: new findings. Epilepsia. 2013;54:50–53. doi: 10.1111/epi.12277. [DOI] [PubMed] [Google Scholar]
- 10.Gaitatzis A, Carroll K, Majeed A, Sander JW. The epidemiology of the comorbidity of epilepsy in the general population. Epilepsia. 2004;45:1613–1622. doi: 10.1111/j.0013-9580.2004.17504.x. [DOI] [PubMed] [Google Scholar]
- 11.Bijl RV, Ravelli A, Van Zessen G. Prevalence of psychiatric disorder in the general population results of The Netherlands Mental Health Survey and Incidence Study (NEMESIS) Soc. Psychiatry Psychiatr Epidemiol. 1998;33:587–595. doi: 10.1007/s001270050098. [DOI] [PubMed] [Google Scholar]
- 12.Kessler RC, Lane MC, Shahly V, Stang PE. Accounting for comorbidity in assessing the burden of epilepsy among US adults: Results from the National Comorbidity Survey Replication (NCS-R) Mol. Psychiatry. 2012;17:748–758. doi: 10.1038/mp.2011.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tellez-Zenteno JF, Patten SB, Jetté N, Williams J, Wiebe S. Psychiatric comorbidity in epilepsy: a population-based analysis. Epilepsia. 2007;48:2336–2344. doi: 10.1111/j.1528-1167.2007.01222.x. [DOI] [PubMed] [Google Scholar]
- 14.Kanner, A.M. & Schachter, S. C. Psychiatric controversies in epilepsy. San Diego, CA: Elsevier/Academic Press (2008).
- 15.Caplan R. Psychopathology and epilepsy: a two-way relationship. Epilepsy Curr. 2012;12:201–220. doi: 10.5698/1535-7511-12.5.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones JE, et al. Psychiatric comorbidity in children with new onset epilepsy. Dev Med Child Neurol. 2007;49:493–497. doi: 10.1111/j.1469-8749.2007.00493.x. [DOI] [PubMed] [Google Scholar]
- 17.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann. Intern. Med. 2009;151:264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 18.Feinstein A. Pre-therapeutic classification of comorbidity in chronic disease. J. Chronic Dis. 1970;23:455–468. doi: 10.1016/0021-9681(70)90054-8. [DOI] [PubMed] [Google Scholar]
- 19.Keezer MR, Sisodiya SM, Sander JW. Comorbidities of epilepsy: current concepts and future perspectives. Lancet Neurol. 2016;1:78–91. doi: 10.1016/S1474-4422(15)00225-2. [DOI] [PubMed] [Google Scholar]
- 20.Valderas JM, Starfield B, Sibbald B, Salisbury C, Roland M. Defining comorbidity: implications for understanding health and health services. Ann Fam Med. 2009;7:357–363. doi: 10.1370/afm.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barendregt JJ, Doi SA, Lee YY, Norman RE, Vos T. Meta-analysis of prevalence. J. Epidemiol. Community Health. 2013;67:974–978. doi: 10.1136/jech-2013-203104. [DOI] [PubMed] [Google Scholar]
- 22.Kariuki SM, et al. & SEEDS Writing Group. Prevalence and factors associated with convulsive status epilepticus in Africans with epilepsy. Neurology. 2015;84:1838–1845. doi: 10.1212/WNL.0000000000001542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaiser C, et al. The prevalence of epilepsy follows the distribution of onchocerciasis in a west Ugandan focus. Bull. World Health Organ. 1996;74:361. [PMC free article] [PubMed] [Google Scholar]
- 24.Kipp W, Kasoro S, Burnham G. Onchocerciasis and epilepsy in Uganda. Lancet Neurol. 1994;343:183–184. doi: 10.1016/s0140-6736(94)90980-6. [DOI] [PubMed] [Google Scholar]
- 25.Newell ED, Vyungimana F, Bradley JE. Epilepsy, retarded growth and onchocerciasis, in two areas of different endemicity of onchocerciasis in Burundi. Trans. R. Soc. Trop. Med. Hyg. 1997;91:525–527. doi: 10.1016/s0035-9203(97)90009-2. [DOI] [PubMed] [Google Scholar]
- 26.Kaiser C, et al. Association between onchocerciasis and epilepsy in the Itawara hyperendemic focus, West Uganda: controlling for time and intensity of exposure. Am. J. Epidemiol. 2011;85:225–228. doi: 10.4269/ajtmh.2011.10-0544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taylor MM, Meredith SE, Stufe A. The prevalence of epilepsy in an area hyperendemic for onchocerciasis in Tanzania. Am. J. Trop. Med. Hyg. 1999;61:321. [Google Scholar]
- 28.Dozie IN, Onwuliri CO, Nwoke BE. Onchocerciasis and epilepsy in parts of the Imo river basin, Nigeria: a preliminary report. Public Health. 2006;120:448–450. doi: 10.1016/j.puhe.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 29.Pion SD, Boussinesq M. Significant association between epilepsy and presence of onchocercal nodules: case-control study in Cameroon. Am. J. Trop. Med. Hyg. 2012;86:557. doi: 10.4269/ajtmh.2012.11-0603a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goudsmit J, Van der Waals FW, Gajdusek D. Epilepsy in the Gbawein and Wroughbarh clan of Grand Bassa county, Liberia: the endemic occurrence of’ See-ee’ in the native population. Neuroepidemiology. 1983;2:24–34. [Google Scholar]
- 31.Gbenou, H. D. Contribution à l'étude de l’association onchocercose-épilepsie. Résultats préliminaires d’une enquête neuroépidemiologique à Agbogbome, Commune de Paouignan, Sous-Préfecture de Dassa-Zoumé, au Bénin. Medical Thesis, National University of Benin. 126p. (1995).
- 32.Boussinesq M, Pion SD, Kamgno J. Relationship between onchocerciasis and epilepsy: a matched case-control study in the Mbam Valley, Republic of Cameroon. Trans. R. Soc. Trop. Med. Hyg. 2002;96:537–541. doi: 10.1016/s0035-9203(02)90433-5. [DOI] [PubMed] [Google Scholar]
- 33.Kabré, D. Épidémiologie de l'épilepsie dans le foyer d’onchocercose du bassin de la Bougouriba (Burkina Faso). Medical Thesis: University of Ouagadougou. 62p. (1998).
- 34.Forsgren L, Nystrom L. An incident case-referent study of epileptic seizures in adults. Epilepsy Res. 1990;6:66–81. doi: 10.1016/0920-1211(90)90010-s. [DOI] [PubMed] [Google Scholar]
- 35.Etttinger AB, et al. Symptoms of psychiatric disturbances in epilepsy. J. Epilepsy. 1998;11:10–4. [Google Scholar]
- 36.Marin-Leon L, Oliveira HB, Barros MB, Dalgalarrondo P, Botega NJ. Social inequality and common mental disorders. Braz. J. Psyquiatr. 2007;29:250–253. doi: 10.1590/s1516-44462006005000060. [DOI] [PubMed] [Google Scholar]
- 37.Wagner RG, et al. Prevalence and risk factors for active convulsive epilepsy in rural northeast South Africa. Epilepsy Res. 2014;108:782–791. doi: 10.1016/j.eplepsyres.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hermann BP, Seidenberg M, Bell B. Psychiatric comorbidity in chronic epilepsy: identification, consequences and treatment of major depression. Epilepsia. 2000;41:S31–41. doi: 10.1111/j.1528-1157.2000.tb01522.x. [DOI] [PubMed] [Google Scholar]
- 39.Adamolekun B, Meinardi H. Problems of drug therapy in epilepsy in developing countries. Trop. Geogr. Med. 1990;42:178–181. [PubMed] [Google Scholar]
- 40.Chen CC, et al. Population-based survey on prevalence of adult patients with epilepsy in Taiwan (Keelung community-based integrated screening no.12) Epilepsy Res. 2006;72:67–74. doi: 10.1016/j.eplepsyres.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 41.Tseng CH, Huang WS, Muo CH, Kao C. Increased risk of epilepsy among patients diagnosed with chronic osteomyelitis. Epilepsy Res. 2014;108:1427–1434. doi: 10.1016/j.eplepsyres.2014.06.022. [DOI] [PubMed] [Google Scholar]
- 42.Stefanello S, Marín‐Léon L, Fernandes PT, Li LM, Botega NJ. Psychiatric comorbidity and suicidal behavior in epilepsy: A community‐based case–control study. Epilepsia. 2010;51:1120–1125. doi: 10.1111/j.1528-1167.2009.02386.x. [DOI] [PubMed] [Google Scholar]
- 43.Kariuki SM, et al. & SEEDS writing group. Clinical features, proximate causes, and consequences of active convulsive epilepsy in Africa. Epilepsia. 2014;55:76–85. doi: 10.1111/epi.12392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Prischich F, et al. High prevalence of epilepsy in a village in the Littoral Province of Cameroon. Epilepsy Res. 2008;82:200–210. doi: 10.1016/j.eplepsyres.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 45.Koul R, Razdan S, Motta A. Prevalence and pattern of epilepsy (Lath/Mirgi/Laran) in rural Kashmir, India. Epilepsia. 1988;29:116–122. doi: 10.1111/j.1528-1157.1988.tb04406.x. [DOI] [PubMed] [Google Scholar]
- 46.Rwiza HT, Mteza I, Matuja WB. The clinical and social characteristics of epileptic patients in Ulanga District, Tanzania. J Epilepsy. 1993;6:162–169. [Google Scholar]
- 47.Tekle‐Haimanot R, Forsgren L, Ekstedt J. Incidence of epilepsy in rural central Ethiopia. Epilepsia. 1997;38:541–546. doi: 10.1111/j.1528-1157.1997.tb01138.x. [DOI] [PubMed] [Google Scholar]
- 48.Burton K, et al. Comorbidity of epilepsy in Tanzanian children: A community-based case–control study. Seizure. 2012;21:169–174. doi: 10.1016/j.seizure.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Blocher J, et al. A cross-sectional study of people with epilepsy and neurocysticercosis in Tanzania: clinical characteristics and diagnostic approaches. Plos Negl. Trop. Dis. 2011;5:e1185. doi: 10.1371/journal.pntd.0001185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ibinga E, et al. Impact of epilepsy on children and parents in Gabon. Epilepsy & Behav. 2015;44:110–116. doi: 10.1016/j.yebeh.2014.12.035. [DOI] [PubMed] [Google Scholar]
- 51.Powell K, et al. Cognition and behavior in a prevalent cohort of children with epilepsy in rural northern Tanzania: A three-year follow-up study. Epilepsy & Behav. 2015;51:117–123. doi: 10.1016/j.yebeh.2015.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jacoby A, Baker GA, Steen N, Potts P, Chadwick DW. The clinical course of epilepsy and its psychosocial correlates: findings from a UK community study. Epilepsia. 1996;37:148–161. doi: 10.1111/j.1528-1157.1996.tb00006.x. [DOI] [PubMed] [Google Scholar]
- 53.Nubukpo P, Houinato D, Preux P-M. Anxiety and depression among the epileptics in general population in Benin. L’Encéphale. 2003;30:214–219. doi: 10.1016/s0013-7006(04)95432-2. [DOI] [PubMed] [Google Scholar]
- 54.Vuillemier P, Jallon P. Epilepsie et troubles psychiatriques: données épidémiologiques. Rev. Neurol. 1998;154:305–317. [PubMed] [Google Scholar]
- 55.Cullère P. Épilepsie et dépression. Neuroepidemiology. 1998;17:31–54. [Google Scholar]
- 56.Kanner A, Balabanov A. Depression and epilepsy – How closely related are they? Neurology. 2002;58:527–539. doi: 10.1212/wnl.58.8_suppl_5.s27. [DOI] [PubMed] [Google Scholar]
- 57.Tegegne MT, et al. Depression and anxiety disorder among epileptic people at Amanuel Specialized Mental Hospital, Addis Ababa, Ethiopia. BMC Psychiatry. 2015;15:210. doi: 10.1186/s12888-015-0589-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Marsh L, Rao V. Psychiatric complications in patients with epilepsy: a review. Epilepsy Res. 2002;49:11–33. doi: 10.1016/s0920-1211(02)00008-6. [DOI] [PubMed] [Google Scholar]
- 59.Vazquez B, Devinsky O. Epilepsy and anxiety. Epilepsy Behav. 2003;4:S20–25. doi: 10.1016/j.yebeh.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 60.Alsaadi T, et al. Prevalence of depression and anxiety among patients with epilepsy attending the epilepsy clinic at Sheikh Khalifa Medical City, UAE: A cross-sectional study. Epilepsy Behav. 2015;52:194–199. doi: 10.1016/j.yebeh.2015.09.008. [DOI] [PubMed] [Google Scholar]
- 61.Swinkels WA, Kuyk J, de Graaf EH, van Dyck R, Spinhoven P. Prevalence of psychopathology in Dutch epilepsy inpatients: a comparative study. Epilepsy Behav. 2001;2:441–447. doi: 10.1006/ebeh.2001.0242. [DOI] [PubMed] [Google Scholar]
- 62.Munyoki G, et al. Clinical and neurophysiologic features of active convulsive epilepsy in rural Kenya: a population-based study. Epilepsia. 2010;51:2370–2376. doi: 10.1111/j.1528-1167.2010.02653.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rafael F, et al. Sociocultural and psychological features of perceived stigma reported by PWE in Benin. Epilepsia. 2010;51:1061–1068. doi: 10.1111/j.1528-1167.2009.02511.x. [DOI] [PubMed] [Google Scholar]
- 64.Hauser WA, Annegers JF, Kurland LT. Incidence of epilepsy and unprovoked seizures in Rochester, Minnesota: 1935–1984. Epilepsia. 1993;34:453–468. doi: 10.1111/j.1528-1157.1993.tb02586.x. [DOI] [PubMed] [Google Scholar]
- 65.Lowenstein DH. Epilepsy after head injury: an overview. Epilepsia. 2009;50:4–9. doi: 10.1111/j.1528-1167.2008.02004.x. [DOI] [PubMed] [Google Scholar]
- 66.Crepin S, et al. Link between epilepsy and malnutrition in a rural area of Benin. Epilepsia. 2007;48:1926–1933. doi: 10.1111/j.1528-1167.2007.01159.x. [DOI] [PubMed] [Google Scholar]
- 67.Chandra RK. Nutrition and the immune system: an introduction. Am. J. Clin. 1997;66:460–463. doi: 10.1093/ajcn/66.2.460S. [DOI] [PubMed] [Google Scholar]
- 68.Hackett R, Iype T. Malnutrition and childhood epilepsy in developing countries. Seizure. 2001;10:554–558. doi: 10.1053/seiz.2001.0532. [DOI] [PubMed] [Google Scholar]
- 69.Smith SR, Pozefsky T, Chetri MK. Nitrogen and aminoacid metabolism in adult with protein-calorie malnutrition. Metabolism. 1974;167:297–230. doi: 10.1016/s0026-0495(74)80020-x. [DOI] [PubMed] [Google Scholar]
- 70.Nubukpo P, et al. Psychosocial issues in people with epilepsy in Togo and Benin (West Africa) I. Anxiety and depression measured using Goldberg’s scale. Epilepsy & Behav. 2004;5:722–727. doi: 10.1016/j.yebeh.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 71.Preux P-M, Druet-Cabanac M. Epidemiology and aetiology of epilepsy in sub-Saharan Africa. Lancet Neurol. 2005;4:21–31. doi: 10.1016/S1474-4422(04)00963-9. [DOI] [PubMed] [Google Scholar]
- 72.Coleman R, Loppy L, Walraven G. The treatment gap and primary health care for PWE in rural Gambia. Bull World Health Organ. 2002;80:378–383. [PMC free article] [PubMed] [Google Scholar]
- 73.Feksi AT, Kaamugisha J, Gatiti S, Sander JW, Shorvon SD. A comprehensive community epilepsy programme: The Nakuru project (Kenya) Epilepsy Res. 1991;8:252–259. doi: 10.1016/0920-1211(91)90072-n. [DOI] [PubMed] [Google Scholar]
- 74.Njamnshi AK, et al. Risk factors associated with epilepsy in a rural area in Cameroun: A preliminary study. Afr. J. Neurol. Sci. 2007;2:18. [Google Scholar]
- 75.Ekwunife OI, Aguwa CN. A meta-analysis of prevalence rate of hypertension in Nigerian populations. J. Public Health Epidemiol. 2011;3:604–607. [Google Scholar]
- 76.Caietta E, et al. Association diabète de type 1 et épilepsie chez l’enfant. À propos d’une série de 10 cas. Arch. Pediatr. 2012;19:9–16. doi: 10.1016/j.arcped.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 77.Levy-Marchal, C., Fagot-Campagna, A. & Daniel, M. Surveillance épidémiologique du diabète de l’enfant. Institut national de la santé et de la recherche médicale (2007).
- 78.Baxter ER. The γ-aminobutyric acid-α-ketoglutaric acid transaminase of beef brain. J. Biol. Chem. 1958;233:1135–1139. [PubMed] [Google Scholar]
- 79.Chiang KL, Cheng C. Prevalence and neuro-psychiatric comorbidities of pediatric epilepsy in Taiwan: A national population-based study. Epilepsy Res. 2014;108:1451–1460. doi: 10.1016/j.eplepsyres.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 80.ILAE Relationship between epilepsy and tropical diseases. Epilepsia. 1994;35:89–93. [PubMed] [Google Scholar]
- 81.Dumas M, et al. Epidemiological study of neuro-cysticercosis in northern Togo (West Africa) Acta Leiden. 1989;57:191–196. [PubMed] [Google Scholar]
- 82.Bern C, et al. Magnitude of the disease burden from neurocysticercosis in a developing country. Clin. Infect. Dis. 1999;29:1203–1209. doi: 10.1086/313470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Garcia HH, et al. Cysticercosis as a major cause of epilepsy in Peru. The Cysticercosis Working Group in Peru (CWG) Lancet. 1993;41:197–200. doi: 10.1016/0140-6736(93)90064-n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Brutto OH, Santibáñez R, Idrovo L. Epilepsy and neurocysticercosis in Atahualpa: a door‐to‐door survey in rural coastal Ecuador. Epilepsia. 2005;46:583–587. doi: 10.1111/j.0013-9580.2005.36504.x. [DOI] [PubMed] [Google Scholar]
- 85.Montano SM, et al. Cysticercosis Working Group in Peru. Neurocysticercosis association between seizures, serology, and brain CT in rural Peru. Neurology. 2005;65:229–233. doi: 10.1212/01.wnl.0000168828.83461.09. [DOI] [PubMed] [Google Scholar]
- 86.Kivits M. Quatre cas d’encéphalite mortelle avec invasion du liquide céphalo-rachidien par Microfilaria Loa. Ann. Soc. Belge Méd. Trop. 1952;32:235–421. [PubMed] [Google Scholar]
- 87.Tegueu CK, Maiga Y. Epilepsy seizures linked to HIV infection in Africa. Epilepsies. 2010;22:134–142. [Google Scholar]
- 88.Annegers JF, Hauser WA, Beghi E, Nicolosi A, Kurland LT. The risk of unprovoked seizures after encephalitis and meningitis. Neurology. 1988;38:1407–1410. doi: 10.1212/wnl.38.9.1407. [DOI] [PubMed] [Google Scholar]
- 89.Hanisch UK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat.Neurosci. 2007;10:1387–1394. doi: 10.1038/nn1997. [DOI] [PubMed] [Google Scholar]
- 90.Kamuyu G, et al. Study of Epidemiology of Epilepsy in Demographic Sites (SEEDS) group. Exposure to multiple parasites is associated with the prevalence of active convulsive epilepsy in sub-Saharan Africa. Plos Negl. Trop. Dis. 2014;8:e2908. doi: 10.1371/journal.pntd.0002908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Traore H, et al. Approche socioculturelle de l’´épilepsie en Mauritanie. Med. Trop. 1998;58:365–368. [PubMed] [Google Scholar]
- 92.Edwards T, et al. Active convulsive epilepsy in a rural district of Kenya: a study of prevalence and possible risk factors. Lancet Neurol. 2008;7:50–56. doi: 10.1016/S1474-4422(07)70292-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ngugi AK, et al. & SEEDS group. Prevalence of active convulsive epilepsy in sub-Saharan Africa and associated risk factors: cross-sectional and case-control studies. Lancet. 2013;12:253–263. doi: 10.1016/S1474-4422(13)70003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Harnod T, Chen HJ, Li TC, Sung FC, Kao CH. A high risk of hyperlipidemia in epilepsy patients: a nationwide population-based cohort study. Ann. Epidemiol. 2014;24:910–914. doi: 10.1016/j.annepidem.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 95.Aziz H, Ali SM, Frances P, Khan MI, Hasan KZ. Epilepsy in Pakistan: A Population‐Based Epidemiologic Study. Epilepsia. 1994;35:950–958. doi: 10.1111/j.1528-1157.1994.tb02539.x. [DOI] [PubMed] [Google Scholar]
- 96.Osuntokun BO, et al. Prevalence of the epilepsies in Nigerian Africans: a community‐based study. Epilepsia. 1987;28:272–279. doi: 10.1111/j.1528-1157.1987.tb04218.x. [DOI] [PubMed] [Google Scholar]
- 97.Matuja WBP, et al. Risk Factors for Epilepsy in a rural area in Tanzania. Neuroepidemiology. 2001;20:242–247. doi: 10.1159/000054797. [DOI] [PubMed] [Google Scholar]
- 98.Li SC, et al. Epidemiology of epilepsy in urban areas of the People’s Republic of China. Epilepsia. 1985;26:391–394. doi: 10.1111/j.1528-1157.1985.tb05669.x. [DOI] [PubMed] [Google Scholar]
- 99.Rajbhandari KC. Epilepsy in Nepal. Can. J. Neurol. Sci. 2004;31:257–260. doi: 10.1017/s0317167100053919. [DOI] [PubMed] [Google Scholar]
- 100.Lavados J, Germain L, Morales A, Campero M, Lavados P. A descriptive study of epilepsy in the district of El Salvador, Chile, 1984–1988. Acta Neurol. Scand. 1992;85:249–256. doi: 10.1111/j.1600-0404.1992.tb04040.x. [DOI] [PubMed] [Google Scholar]
- 101.Medina MT, et al. Prevalence, incidence, and etiology of epilepsies in rural Honduras: The Salama Study. Epilepsia. 2005;46:124–131. doi: 10.1111/j.0013-9580.2005.11704.x. [DOI] [PubMed] [Google Scholar]
- 102.Ngibise, B. A. et al. Prevalence and risk factors for Active Convulsive Epilepsy in Kintampo, Ghana. Pan Afr. Med. J. 21 (2015). [DOI] [PMC free article] [PubMed]
- 103.Kariuki SM, et al. Behavioral problems in children with epilepsy in rural Kenya. Epilepsy Behav. 2012;23:41–46. doi: 10.1016/j.yebeh.2011.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Villarán MV, et al. Cysticercosis Working Group in Peru. Epilepsy and neurocysticercosis: an incidence study in a Peruvian rural population. Neuroepidemiology. 2009;33:25–31. doi: 10.1159/000210019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Stefanello S, Marin-Leon L, Fernandes PT, Li LM, Botega N. Depression and anxiety in a community sample with epilepsy in Brazil. Arq Neuropsi. 2011;69:342–348. doi: 10.1590/s0004-282x2011000300015. [DOI] [PubMed] [Google Scholar]
- 106.Chang CS, et al. Patients with epilepsy are at an increased risk of subsequent stroke: a population-based cohort study. Seizure. 2014;23:377–381. doi: 10.1016/j.seizure.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 107.Kwan P, Yu E, Leung H, Leon T, Mychaskiw MA. Association of subjective anxiety, depression, and sleep disturbance with quality‐of‐life ratings in adults with epilepsy. Epilepsia. 2009;50:1059–1066. doi: 10.1111/j.1528-1167.2008.01938.x. [DOI] [PubMed] [Google Scholar]
- 108.Bernet-Bernady P, et al. Epilepsy and its impact in northwest region of the Central African Republic] Med. Trop. 1996;57:407–411. [PubMed] [Google Scholar]
- 109.Zibaei, M., Zamani, Z., Esfahani, A. C., Anbari, K. & Nazer, M. R. Toxoplasma infection and epilepsy: A case-control study in Iran. Neurol. Asia. 16 (2011).
- 110.Millogo A, et al. Prevalence of neurocysticercosis among people with epilepsy in rural areas of Burkina Faso. Epilepsia. 2012;53:2194–2202. doi: 10.1111/j.1528-1167.2012.03687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Balogou AAK, Grunitzky KE, Beketi KA, Bouteille B, Dumas M. Cysticercose et épilepsie au nord du Togo dans le Tone. Rev. Neurol. 2000;156:270–273. [PubMed] [Google Scholar]
- 112.Bouteille, B. et al. Epidemiological study of cysticercosis/epilepsy linkage in Benin and Togo, West Africa. 19ème Réunion Scientifique de l’Association des Epidémiologistes de Langue Française (ADELF), Rennes, France, 28–30 septembre (1994).
- 113.Winkler AS, et al. Epilepsy and neurocysticercosis in rural Tanzania: an imaging study. Epilepsia. 2009;50:987–993. doi: 10.1111/j.1528-1167.2008.01867.x. [DOI] [PubMed] [Google Scholar]
- 114.Mwanjali G, et al. Prevalence and risk factors associated with human Taenia solium infections in Mbozi District, Mbeya Region, Tanzania. Plos Negl. Trop. Dis. 2013;7:2102. doi: 10.1371/journal.pntd.0002102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Prado‐Jean A, et al. Combined use of an antigen and antibody detection enzyme‐linked immunosorbent assay for cysticercosis as tools in an epidemiological study of epilepsy in Burundi. Trop. Med. Int. Health. 2007;12:895–901. doi: 10.1111/j.1365-3156.2007.01860.x. [DOI] [PubMed] [Google Scholar]
- 116.Nsengiyumva G, et al. Cysticercosis as a major risk factor for epilepsy in Burundi, East Africa. Epilepsia. 2003;44:950–955. doi: 10.1046/j.1528-1157.2003.55302.x. [DOI] [PubMed] [Google Scholar]
- 117.Singh G, et al. Association between epilepsy and cysticercosis and toxocariasis: A population-based case–control study in a slum in India. Epilepsia. 2012;53:2203–2208. doi: 10.1111/epi.12005. [DOI] [PubMed] [Google Scholar]
- 118.Prasad KN, et al. Neurocysticercosis in patients with active epilepsy from the pig farming community of Lucknow district, north India. Trans. R. Soc. Trop. Med. 2009;103:144–150. doi: 10.1016/j.trstmh.2008.07.015. [DOI] [PubMed] [Google Scholar]
- 119.Rajshekhar V, Raghava MV, Prabhakaran V, Oommen A, Muliyil J. Active epilepsy as an index of burden of neurocysticercosis in Vellore district, India. Neurology. 2006;67:2135–2139. doi: 10.1212/01.wnl.0000249113.11824.64. [DOI] [PubMed] [Google Scholar]
- 120.Sampaio LP, et al. Prevalence of epilepsy in children from a Brazilian area of high deprivation. Pediatric Neurol. 2010;42:111–117. doi: 10.1016/j.pediatrneurol.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 121.Velez A, Eslava‐Cobos J. Epilepsy in Colombia: epidemiologic profile and classification of epileptic seizures and syndromes. Epilepsia. 2006;47:193–201. doi: 10.1111/j.1528-1167.2006.00387.x. [DOI] [PubMed] [Google Scholar]
- 122.Cruz ME, et al. Epilepsy and neurocysticercosis in an Andean community. Int. J. Epidemiol. 1999;28:799–803. doi: 10.1093/ije/28.4.799. [DOI] [PubMed] [Google Scholar]
- 123.Nicoletti A, et al. Epilepsy, cysticercosis, and toxocariasis A population-based case-control study in rural Bolivia. Neurology. 2002;58:1256–1261. doi: 10.1212/wnl.58.8.1256. [DOI] [PubMed] [Google Scholar]
- 124.Nicoletti A, et al. A Natural history and mortality of chronic epilepsy in an untreated population of rural Bolivia: A follow‐up after 10 years. Epilepsia. 2009;50:2199–2206. doi: 10.1111/j.1528-1167.2009.02174.x. [DOI] [PubMed] [Google Scholar]
- 125.Garcia HH, Talley A, Gilman RH, Zorrilla L, Pretell J. Epilepsy and neurocysticercosis in a village in Huaraz, Peru. Clin. Neurol. Neurosurg. 1999;101:225–228. doi: 10.1016/s0303-8467(99)00043-8. [DOI] [PubMed] [Google Scholar]
- 126.Nicoletti A, et al. Epilepsy and Neurocysticercosis in Rural Bolivia: A Population‐based Survey. Epilepsia. 2005;46:1127–1132. doi: 10.1111/j.1528-1167.2005.67804.x. [DOI] [PubMed] [Google Scholar]
- 127.Sanchez AL, et al. A population-based, case-control study of Taenia solium taeniasis and cysticercosis. Ann. Trop. Med. Parasitol. 1999;93:247–258. [PubMed] [Google Scholar]
- 128.Fleury A, et al. High prevalence of calcified silent neurocysticercosis in a rural village of Mexico. Neuroepidemiology. 2003;22:139–145. doi: 10.1159/000068748. [DOI] [PubMed] [Google Scholar]
- 129.Elliott I, et al. Epilepsy and cysticercosis in North-West Cameroon: A serological study. Seizure. 2013;22:283–286. doi: 10.1016/j.seizure.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 130.Dongmo L, et al. Cysticercose et épilepsie: étude cas-témoins dans la Vallée du Mbam. Bull. Soc. Pathol. Exot. 2004;97:105–108. [PubMed] [Google Scholar]
- 131.Assane YA, et al. Neurocysticercosis in a rural population with extensive pig production in Angónia district, Tete Province, Mozambique. Acta Trop. 2017;165:155–160. doi: 10.1016/j.actatropica.2015.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Tran DS, et al. Risk factors for epilepsy in rural Laos: a case control study. S. East Asian J. Trop. Med. Hyg. 2007;38:537–542. [PubMed] [Google Scholar]
- 133.Kamuyu, G. et al. Study of Epidemiology of Epilepsy in Demographic Sites (SEEDS) group. Exposure to multiple parasites is associated with the prevalence of active convulsive epilepsy in sub-Saharan. Africa. Plos Negl. Trop. Dis.8, e2908 (2014). [DOI] [PMC free article] [PubMed]
- 134.Yeh CC, Chen TL, Hu CJ, Chiu WT, Liao CC. Risk of epilepsy after traumatic brain injury: a retrospective population-based cohort study. J. Neurol. Neurosurg. Psychiatry. 2013;84:441–445. doi: 10.1136/jnnp-2012-302547. [DOI] [PubMed] [Google Scholar]
- 135.Bhalla Devender, Chea Kimly, Hun Chamroeun, Vannareth Mey, Huc Pierre, Chan Samleng, Sebbag Robert, Gérard Daniel, Dumas Michel, Oum Sophal, Druet-Cabanac Michel, Preux Pierre-Marie. Population-Based Study of Epilepsy in Cambodia Associated Factors, Measures of Impact, Stigma, Quality of Life, Knowledge-Attitude-Practice, and Treatment Gap. PLoS ONE. 2012;7(10):e46296. doi: 10.1371/journal.pone.0046296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Mendizabal JE, Salguero LF. Prevalence of epilepsy in a rural community of Guatemala. Epilepsia. 1996;37:373–376. doi: 10.1111/j.1528-1157.1996.tb00574.x. [DOI] [PubMed] [Google Scholar]
- 137.Nitiéma P, et al. Prevalence case‐control study of epilepsy in three Burkina Faso villages. Acta Neurol. Scand. 2012;126:270–278. doi: 10.1111/j.1600-0404.2011.01639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Raina SK, et al. Active epilepsy as indicator of neurocysticercosis in rural northwest India. Epilepsy. Res. Treat. 2012;2012:802747. doi: 10.1155/2012/802747. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.