Abstract
Calcium-calmodulin dependent protein kinase II (CaMKII) regulates many forms of synaptic plasticity, but little is known about its functional role during plasticity induction in the cerebellum. Experiments have indicated that the β isoform of CaMKII controls the bidirectional inversion of plasticity at parallel fibre (PF)-Purkinje cell (PC) synapses in cerebellar cortex. Because the cellular events that underlie these experimental findings are still poorly understood, we developed a simple computational model to investigate how β CaMKII regulates the direction of plasticity in cerebellar PCs. We present the first model of AMPA receptor phosphorylation that simulates the induction of long-term depression (LTD) and potentiation (LTP) at the PF-PC synapse. Our simulation results suggest that the balance of CaMKII-mediated phosphorylation and protein phosphatase 2B (PP2B)-mediated dephosphorylation of AMPA receptors can determine whether LTD or LTP occurs in cerebellar PCs. The model replicates experimental observations that indicate that β CaMKII controls the direction of plasticity at PF-PC synapses, and demonstrates that the binding of filamentous actin to CaMKII can enable the β isoform of the kinase to regulate bidirectional plasticity at these synapses.
Subject terms: Cellular signalling networks, Biophysical models
Introduction
The structure and neural circuitry of the cerebellum are well understood. The precision of the cerebellar anatomy has instigated the development of many theories that attempt to unravel cerebellar function. However, although it is known that the cerebellum contributes to motor learning and cognition, there is still no general agreement about its exact functional role1–4.
Synaptic plasticity is an activity-dependent change in the strength of synaptic connections between pre and postsynaptic neurons. In cerebellar cortex, modifications in the strength of synaptic connections between parallel fibres (PFs) and Purkinje cells (PCs) such as long-term depression (LTD) and long-term potentiation (LTP) are thought to contribute to cerebellar learning5–7.
PF LTD (often called cerebellar LTD) is a process in which the strength of the PF-PC synapse is depressed by large increases in the postsynaptic calcium concentration in response to the coincident activation of PF and climbing fibre (CF) input onto the PC8–13. In addition to undergoing LTD, PF synapses can also exhibit LTP. The strengthening of excitatory synapses between PFs and PCs by PF LTP is mediated by smaller calcium concentration increases that can result from the activation of PFs without any coincident CF input to the PC. LTP is necessary to balance LTD at cerebellar PF-PC synapses in order to prevent saturation and to allow reversal of motor learning14. A large number of experimental10,15–21 and several computer simulation studies11,13,22–27 have explored the biochemical pathways involved in PF LTD. Less is known about the mechanisms underlying PF LTP, but it has been shown that PF LTP occurs after dephoshorylation of AMPA receptors by protein phosphatases leads to the insertion of additional receptors into the postsynaptic membrane, while PF LTD is triggered by the internalization and removal of AMPA receptors after their phosphorylation by protein kinases10,15–21.
Calcium-calmodulin dependent protein kinase II (CaMKII), which is one of the most abundant proteins in the brain, is a multifunctional enzyme that phosphorylates a wide range of substrates28–31. CaMKII is a critical mediator of the calcium signalling systems that underlie the induction of synaptic plasticity. Although significant progress has been made in understanding the role of CaMKII in synaptic plasticity in other brain areas, little is known about its functional role during plasticity induction in the cerebellum.
Two CaMKII isoforms, α CaMKII and β CaMKII, have been shown to mediate synaptic plasticity in the cerebellum, and therefore to be essential for cerebellar learning and memory formation29,31–33. Although β CaMKII is the predominant isoform of CaMKII in the cerebellum, the exact role of β CaMKII in cerebellar learning has yet to be established.
Experiments with Camk2b knockout mice, which have been genetically modified to prevent the expression of the gene encoding the β isoform of CaMKII, have addressed the role of β CaMKII in plasticity in cerebellar PCs. These studies demonstrated that the β CaMKII isoform regulates the direction of cerebellar plasticity at PF-PC synapses33. Stimulation protocols that induce LTD in wild-type mice result in LTP in knockout mice that lack β CaMKII, and vice versa (Fig. 1). However, the underlying mechanism that may explain these experimental findings is not clear. Van Woerden et al.33 suggested that a biochemical difference between the α CaMKII and β CaMKII isoforms could underlie the switch of the direction of synaptic plasticity. The β CaMKII, but not α CaMKII, isoforms can bind to F-actin34, which could result in sequestering of the CaMKII complex to F-actin, making it unavailable for AMPA receptor phosphorylation.
Figure 1.
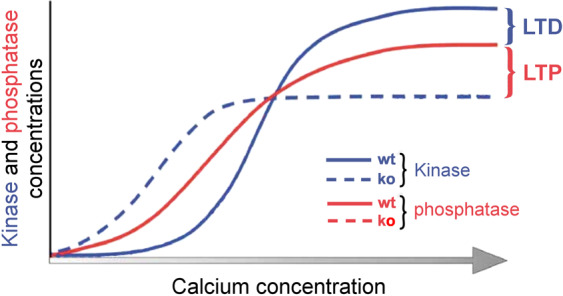
Schematic representation of bidirectional plasticity at PF-PC synapses. The experimental results obtained by Van Woerden and collaborators33 are schematically represented in this figure. The scheme illustrates how changes in the CaMKII-driven pathway could evoke different activity levels of calcium-dependent kinase (blue), resulting in the inversion of plasticity for wild-type and Camk2b knockout mice (solid and dashed lines, respectively). LTD is generated when the kinase activity (blue) surpasses the phosphatase activity (red), whereas the opposite case induces LTP.
Because experimental observations by themselves have not been able to explain how CaMKII mediates plasticity in PCs, a new dynamic model of cerebellar LTD and LTP that includes CaMKII has the potential to enhance our understanding of cerebellar plasticity. To investigate the proposed function of β CaMKII in cerebellar plasticity, we developed a mathematical model of the biochemical pathways underlying the calcium-dependent phosphorylation and dephosphorylation of AMPA receptors at PF-PC synapses. Computer simulations of our model were used to explore how β CaMKII can mediate the observed reversal of plasticity in cerebellar PCs. Our results indicate that the binding of F-actin to CaMKII can indeed enable the β CaMKII isoform to control the direction of plasticity at PF-PC synapses, as proposed by Van Woerden et al.33 Moreover, our model predicts that the sign inversion of synaptic plasticity is based on two additional mechanisms - a reduction of the overall level of CaMKII in the Camk2b knockout mice, and a resulting increase in phosphatase activity in these mice. We present the first data-driven model of intracellular signalling pathways in cerebellar PCs that includes CaMKII and that is able to replicate the induction of LTD and LTP at this important cerebellar synapse.
Methods
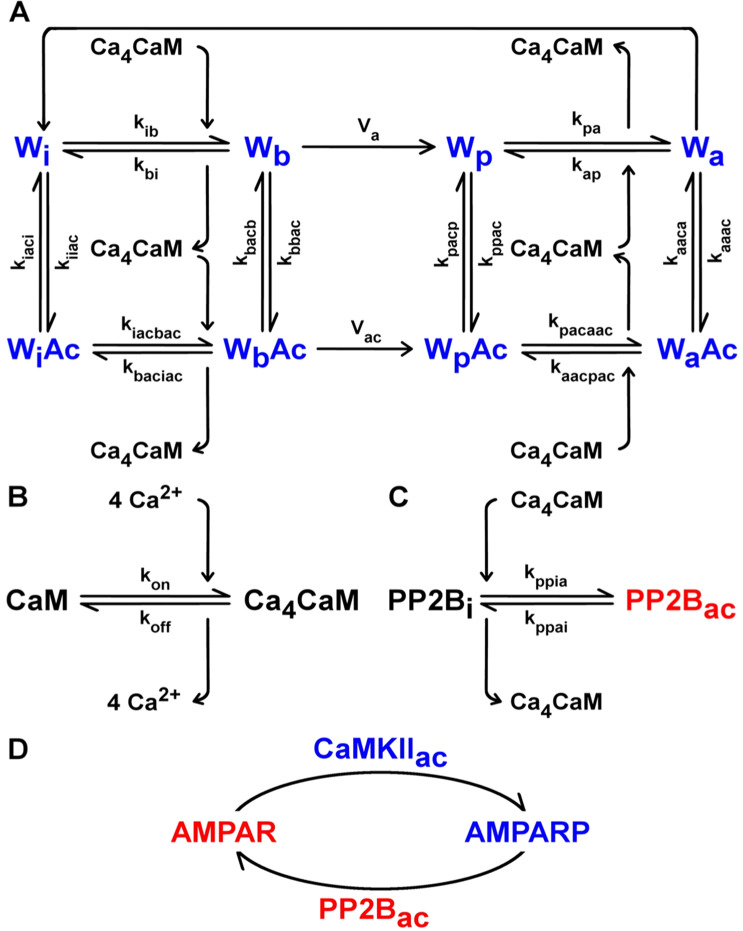
To understand the role of β CaMKII in cerebellar LTD and LTP at PF-PC synapses, we developed a mathematical model of the phosphorylation and dephosphorylation of AMPA receptors by CaMKII and protein phosphatase 2B (PP2B). The simple model of AMPA receptor phosphorylation consists of six reactions: calcium-calmodulin (Ca4CaM)-dependent activation of CaMKII, CaMKII binding to F-actin, binding of calcium to calmodulin (CaM) to form Ca4CaM, PP2B activation by Ca4CaM, and AMPA receptor phosphorylation and dephosphorylation by, respectively, CaMKII and PP2B (Fig. 2).
Figure 2.
Model of bidirectional plasticity at PF-PC synapses. (A) CaMKII activation by Ca4CaM, and its binding to F-actin (Ac). CaMKII subunits for simulations of Camk2b knockout mice are in one of four states: Wi, Wb, Wp, and Wa, where the subscripts i, b, p, and a refer to the respective subunit states: inactive, bound to Ca4CaM, phosphorylated and bound to Ca4CaM, and autonomous: phosphorylated, but dissociated from Ca4CaM. The kinase can also bind to Ac and be in the WiAc, WbAc, WpAc, and WaAc subunit states, but only in simulations of wild-type mice. Here, we allow the CaMKII subunits in the Wa form to switch to the Wi state. CaMKII is therefore gradually inactivated after calcium stimulation stops. The kinetic constants of the reversible Ca4CaM binding reactions are kib, kbi, kpa, kap, kiacbac, kbaciac, kpacaac and kaacpac, whereas kiiac, kiaci, kbbac, kbacb, kppac, kpacp, kaaac and kaaca denote the speed of the reversible Ac binding reactions. The rates of the irreversible phosphorylation of Wb and WbAc are Va and Vac, respectively. (B) Binding of four calcium ions (4Ca2+) to CaM to form Ca4CaM. kon and koff are the rate constants of the reversible 4Ca2+ binding reaction. (C) PP2B activation by Ca4CaM. PP2Bi and PP2Bac are the inactive and active forms of PP2B. The respective rate constants of PP2B activation and inactivation are kppia and kppai. (D) AMPA receptor phosphorylation and dephosphorylation by active CaMKII (CaMKIIac = Wb + Wp + Wa) and PP2Bac.
To simulate the mechanism of CaMKII autophosphorylation, we adopt a simplified version of the commonly used model of CaMKII activation by Ca4CaM developed by Dupont and collaborators35. Because Van Woerden and collaborators33 suggested that the binding of F-actin to CaMKII could underlie the switch of direction of plasticity, we incorporated the binding of F-actin to CaMKII into this model to simulate the plasticity induction in wild-type mice, whereas the F-actin binding was omitted in the knockout mice that lack β CaMKII.
In the simple model of Ca4CaM-mediated CaMKII autophosphorylation considered here, all CaMKII subunits of the simulated Camk2b knockout mice are in one of four states. Following the terminology and notational convention used by Dupont et al.35, the four states are referred to as Wi, Wb, Wp, and Wa, where the subscripts i, b, p, and a stand for inactive, bound, phosphorylated, and autonomous, respectively. Wi and Wb are unphosphorylated, and Wp and Wa are phosphorylated states, while subunits in the Wb and Wp states have Ca4CaM bound, and Wi and Wa have not. The CaMKII subunits of wild-type mice can also be in states bound to F-actin (Ac): WiAc, WbAc, WpAc, and WaAc. The CaMKII activation model developed by Dupont et al.35 was adapted here to express all kinase states in concentrations rather than fractions. Therefore, the Wi concentration may be computed from the mass conservation relation
| 1 |
where [x] denotes the concentration of substance x, e.g. [Wtot] is the total concentration of CaMKII.
Subunits in the Wb, Wp, and Wa states exhibit kinase activity, and can, therefore, phosphorylate CaMKII’s targets, including adjacent subunits in the CaMKII multimer. To be “ready” for phosphorylation, such adjacent subunits must be in the Wb state themselves. The overall phosphorylation rate associated with this process is indicated by Va, which is calculated using a phenomenological non-linear function of kinase subunits in the Wb, Wp and Wa forms as in Dupont et al.35.
| 2 |
where cb, cp and ca are weighting factors proportional to the kinase activity of each active state.
The model of Dupont et al.35 includes an empirical cubic function (Ka) to model the neighbouring autophosphorylation, allowing the mathematical model to reproduce the experimental results in De Koninck and Schulman36. The equation for Ka is
| 3 |
where the phenomenological rate for CaMKII autophosphorylation is , a, b and c are parameters that Dupont et al.35 adjusted to fit the experimental plots in De Koninck and Schulman36, and Ta is the total fraction of active subunits represented as
| 4 |
Once Ca4CaM binds to Wi, the resulting Wb form can either be phosphorylated, release Ca4CaM, or bind to Ac. Wb is an active CaMKII subunit that can also phosphorylate AMPA receptors (AMPARs). The equation for Wb is therefore
| 5 |
where [Ca4CaM] denotes the concentration of Ca4CaM and [WbAMPAR] is the concentration of Wb bound to AMPARs.
The Wp subunit can release Ca4CaM, switching to the Wa state, or bind to Ac and form WpAc. As for Wb, Wp also phosphorylates AMPARs. The amount of Wp is calculated as
| 6 |
where [WpAMPAR] is the concentration of the complex of Wp bound to AMPARs.
Wa can bind to either Ca4CaM or Ac, and phosphorylate AMPARs as well. In our model, we also allow the CaMKII subunits in the Wa form to switch to the Wi state. CaMKII is therefore gradually inactivated after calcium stimulation stops. Thus,
| 7 |
and [WaAMPAR] is the amount of Wa trapped to AMPARs.
Unlike the model by Dupont and collaborators35, the model used here does not include a separate “trapped” state in which apo-CaM (CaM without any calcium ions bound) is bound to CaMKII, mainly because dissociation of calcium and CaM cannot be distinguished experimentally (nor described thermodynamically) as two distinct processes. As a result, the values for the rate constants between the “trapped” CaM (Wt) and Wa states in Dupont et al.35 were adopted here as kap and kpa.
WiAc binds to Ca4CaM and changes to the active WbAc form, or dissociates from Ac and switches to the Wi state. Changes in WiAc concentration are
| 8 |
WbAc can be phosphorylated by neighbouring active Ac-bound subunits: WbAc itself, WpAc or WaAc. The autophosphorylation process for Ac-bound CaMKII is analogous to the mechanism of autophosphorylation of the kinase unbound to Ac (Eqs. 2, 3 and 4). The autophosphorylation rate for Ac-bound CaMKII subunits (Vac) is therefore
| 9 |
where
| 10 |
and
| 11 |
WbAc can also switch to WiAc once Ca4CaM dissociates from this kinase subunit, or dissociate from Ac and change to Wb. Thus,
| 12 |
The phosphorylated Ac- and Ca4CaM-bound form of CaMKII can release Ca4CaM and switch to the WaAc form, or dissociate from Ac and swap to the Wp state. The concentration of WpAc at each time step is expressed as
| 13 |
whereas WaAc can bind to Ca4CaM and switch to WpAc, or dissociate from Ac and turn into Wa. The equation for WaAc is
| 14 |
Ca4CaM not only activates CaMKII, but can also bind to the inactive form of PP2B (PP2Bi). The phosphatase then gets activated and switches to the PP2Bac form. PP2Bac mediates the dephosphorylation of AMPA receptors. The temporal evolution of PP2Bi concentration is
| 15 |
whereas PP2Bac concentration changes are expressed as
| 16 |
where [PP2BacAMPARP] expresses the concentration of PP2Bac bound to phosphorylated AMPA receptors (AMPARPs).
The evolution of unphosphorylated AMPA receptors at each time step is
| 17 |
where
| 18 |
| 19 |
| 20 |
and
| 21 |
Moreover, the equation that represents the evolution of AMPARPs is
| 22 |
Standard protocols of long-term depression (LTD) and long-term potentiation (LTP) induction normally involve repetitive calcium stimuli (such as 1 Hz for 300 s). To generate calcium pulses with concentrations that reflect experimental data10, our simple model that simulates the bidirectional synaptic plasticity in Purkinje cells uses a calcium dynamics model
| 23 |
where [Ca] is the calcium concentration. The term ϕ(t) − κ([Ca] − [Camin]) describes the simple model of calcium dynamics we adopted, where ϕ(t) denotes calcium concentration increases at each time step whose values originate from an input table, κ is a term that reflects the calcium removal rate through diffusion, pumps, exchanges, and [Camin] is the basal calcium concentration.
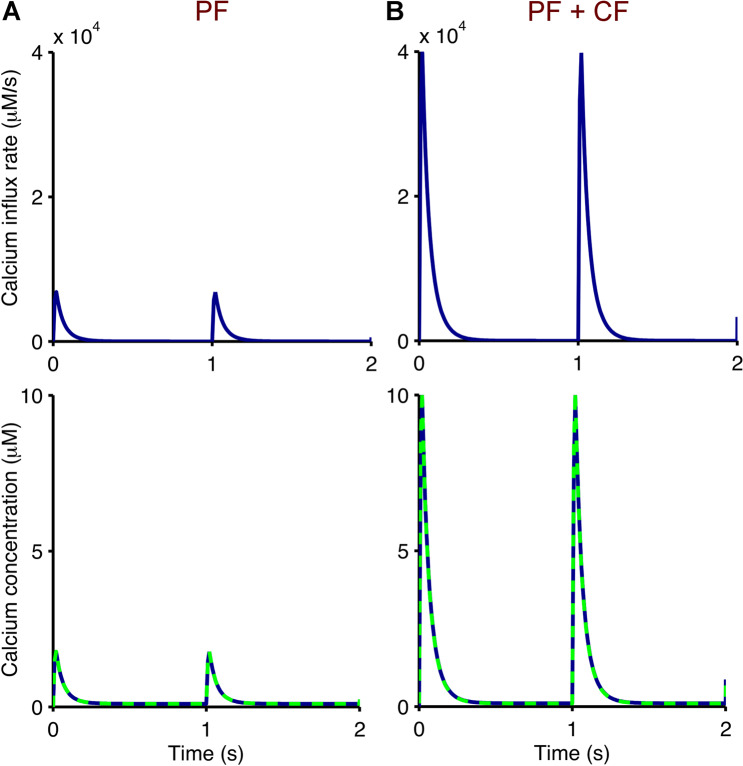
Input with high calcium influx rates (ϕ(t)) was used to stimulate the model to generate realistic amplitudes of calcium in response to parallel fibre (PF) alone and PF+ climbing fibre (CF) stimulations (Fig. 3).
Figure 3.
Pulsed calcium stimulation for cerebellar PF-PC synapses. Calcium influx rates (top, ϕ(t) in Eq. 23) are used as input to our bidirectional synaptic plasticity model to generate the desired output of calcium spikes (bottom, [Ca] in Eq. 23). These match previously published simulation data by Kuroda et al.11 (dashed), based on experimental data by Wang et al.10, which represent stimulations of PF alone (A), pulse amplitude of 1.8 μM) and paired PF and CF (B), pulse amplitude of 10 μM). Both calcium stimulations are applied at 1 Hz for 300 s.
The temporal evolution of CaM is written as
| 24 |
Ca4CaM results from the binding of four calcium ions to CaM, and activates PP2B, Wb, Wp, WbAc and WpAc. The equation that represents the evolution of Ca4CaM concentration is
| 25 |
Although the cerebellum contains four times as much β CaMKII as α CaMKII29, the actual α: β CaMKII ratio is 1:1 in PCs32,33. Therefore, the CaMKII concentration for Camk2b knockout mice is set to half of the kinase concentration for wild-type mice in our model. Experimental observations37 suggest that the affinity of CaMKII for Ca4CaM is modulated by co-assembly of α and β subunits and varies depending on the experimental preparation. In our simulations, we have assumed that α CaMKII has a greater affinity for Ca4CaM than β CaMKII. The full set of parameters adopted in the model are given in Table 1.
Table 1.
Values of kinetic parameters for modelling bidirectional plasticity at PF-PC synapses.
| Parameter description | Symbol | Value | reference |
|---|---|---|---|
| CaMKII total concentration for wild-type mice | Wtot | 26 μM | |
| CaMKII total concentration for Camk2b knockout mice | Wtot | 13 μM | 32,33 |
| F-actin total concentration | Ac | 10 μM | |
| Unphosphorylated AMPA receptor initial concentration | AMPAR | 0.5 μM | 11 |
| Phosphorylated AMPA receptor initial concentration | AMPARP | 0.5 μM | 11 |
| Basal calcium concentration | Camin | 0.045 μM | 26,42,43 |
| CaM initial concentration | CaM | 36 μM | |
| Inactive PP2B initial concentration | PP2Bi | 26 μM | |
| Phenomenological rate of CaMKII autophosphorylation | 0.29 s−1 | 35 | |
| Coefficient of CaMKII activity at Wb subunit | cb | 75% | 35 |
| Coefficient of CaMKII activity at Wp subunit | cp | 100% | 35 |
| Coefficient of CaMKII activity at Wa subunit | ca | 80% | 35 |
| Fitting parameter a | a | 0.500 | |
| Fitting parameter b | b | 1.956 | |
| Fitting parameter c | c | −1.800 | |
| Calcium removal rate | κ | 4 × 103 s−1 | |
| Rate of association of Ca4CaM to a Wi subunit | kib | 10 μM−1 s−1 | 35 |
| Rate of dissociation of Ca4CaM from a Wb subunit | kbi | 0.2 s−1 | 37 |
| Rate of association of Ca4CaM to a Wa subunit | kap | 10 μM−1 s−1 | 35 |
| Rate of dissociation of Ca4CaM from a Wp subunit | kpa | 0.004 s−1 | 44 |
| Rate of association of Ca4CaM to a WiAc subunit | kiacbac | 10 μM−1 s−1 | 35 |
| Rate of dissociation of Ca4CaM from a WbAc subunit | kbaciac | 1 s−1 | |
| Rate of association of Ca4CaM to a WaAc subunit | kaacpac | 10 μM−1 s−1 | 35 |
| Rate of dissociation of Ca4CaM from a WpAc subunit | kpacaac | 0.02 s−1 | 44 |
| Rate of CaMKII degradation | kdephos | 0.0005 s−1 | |
| Rate of association of F-actin to a Wi subunit | kiiac | 10 μM−1 s−1 | |
| Rate of dissociation of F-actin from a WiAc subunit | kiaci | 30.1 s−1 | |
| Rate of association of F-actin to a Wb subunit | kbbac | 10 μM−1 s−1 | |
| Rate of dissociation of F-actin from a WbAc subunit | kbacb | 150.5 s−1 | |
| Rate of association of F-actin to a Wp subunit | kppac | 10 μM−1 s−1 | |
| Rate of dissociation of F-actin from a WpAc subunit | kpacp | 1505 s−1 | |
| Rate of association of F-actin to a Wa subunit | kaaac | 10 μM−1 s−1 | |
| Rate of dissociation of F-actin from a WaAc subunit | kaaca | 301 s−1 | |
| Rate of association of Ca4CaM to PP2Bi | kppia | 0.15 μM−1 s−1 | |
| Rate of dissociation of Ca4CaM from PP2Bac | kppai | 0.00042 s−1 | |
| Rate of association of calcium to CaM | kon | 2 × 103 μM−4 s−1 | |
| Rate of dissociation of calcium from Ca4CaM | koff | 2.3 × 106 s−1 | |
| Rates of AMPA receptor phosphorylation by CaMKII | 0.5 μM−1 s−1 | ||
| 72.283 s−1 | 11 | ||
| 6 s−1 | 11 | ||
| Rates of AMPA receptor dephosphorylation by PP2B | 0.5 μM−1 s−1 | ||
| 72.283 s−1 | 11 | ||
| 6 s−1 | 11 |
All results presented here were obtained by numerically integrating the above coupled ODEs in XPPAUT (X-Windows Phase Plane plus Auto), a numerical tool for simulating dynamical systems, using the CVODE method with relative and absolute error tolerances of 10−10.
Results
Using simulations of our mathematical model, we first investigated the dependence of kinase and phosphatase activities on the postsynaptic intracellular calcium concentration for wild-type mice and Camk2b knockout mice. We varied the amplitude of calcium pulses, which are used as input to our model, and calculated the average concentrations of all biochemical compounds during the simulated period for each level of calcium concentration. The goal of these simulations was to compare the predictions of our computational model with the proposed explanation of the experimental findings by Van Woerden and collaborators33, which is illustrated in schematic form in Fig. 1.
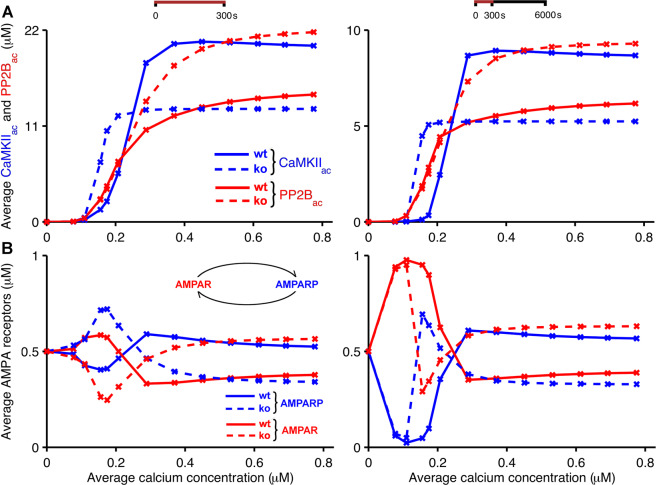
Figure 4A shows the resulting average concentrations of CaMKIIac and PP2Bac for wild-type mice and Camk2b knockout mice as we increase the average concentration of calcium during the stimulation period. To create similar conditions as in a typical cerebellar plasticity induction protocol, which was also used by Van Woerden and colleagues33, the input to the model consisted of calcium pulses applied at a rate of 1 Hz for 300 s. The figure compares the resulting kinase and phosphatase concentrations averaged over the 300 s of calcium application with concentrations averaged over a longer time period of 6000 s in order to explore the continued behaviour of the system after the offset of calcium application.
Figure 4.
Bidirectional plasticity at PF-PC synapses: kinase and phosphatase activities at different calcium concentrations. The figure shows simulation results that support the suggested explanation of the experimental observations by Van Woerden et al.33 (Fig. 1). Low calcium concentrations that induce LTP in wild-type mice (solid) lead to LTD in Camk2b knockout mice (dashed), and vice versa. In the model, the F-actin binding to CaMKII occurs in wild-type mice, whereas in knockout mice the lack of β CaMKII prevents the binding of CaMKII to F-actin. (A) Average concentrations of CaMKIIac (blue) and PP2Bac (red) as a function of average concentrations of pulsed calcium at 1 Hz for 300 s. (B) Average concentrations of unphosphorylated and phosphorylated AMPA receptors (red and blue, respectively) in response to the same stimulation protocols. In the left panels, all simulations last 300 s, that is, the panel shows average concentrations of CaMKIIac, PP2Bac and AMPA receptors in the presence of calcium signals applied at 1 Hz for 300 s. The right panels show the average concentrations of these compounds during simulations that last 6000 s, that is, continue for 5700 s after the offset of the 300 s stimulation.
In both cases, when concentrations were measured during and after the calcium stimulus, our simulation results confirm the explanation of the CaMKII-dependent switch of bidirectional plasticity that was originally put forward by Van Woerden et al.33 Low calcium concentrations, which would be expected to result from PF input alone, evoke larger average concentrations of PP2Bac than CaMKIIac in simulated wild-type mice. In contrast, for Camk2b knockout mice, in which the lack of β CaMKII prevents the binding of the CaMKII holoenzyme to F-actin, this relationship is reversed, with low calcium concentrations activating CaMKII more strongly than PP2B. Furthermore, high calcium concentrations, in the range of those expected to be caused by coincident PF and CF input, lead to higher concentrations of CaMKIIac than PP2Bac in wild-type mice, but larger phosphatase than kinase activities in Camk2b knockout mice (Fig. 4A).
However, in spite of the similarity of our simulation results with the mechanism predicted by Van Woerden and collaborators33, a comparison of Figs. 1 and 4A also reveals a subtle difference. In the schematic originally proposed by Van Woerden et al. (Fig. 1), the CaMKII-dependent synaptic switch was assumed to be entirely mediated by a change in CaMKII activity due to the genetic Camk2b knockout, with low calcium concentrations resulting in increased CaMKII activity in knockout mice, while high calcium concentrations were proposed to lead to reduced CaMKII activity compared to wild-type mice. Our simulation results (Fig. 4A) indicate that the proposed reduction in CaMKII activity for larger calcium concentrations is augmented by a coincident increase in PP2B activity. These results predict that an increase in the PP2Bac concentration acts in tandem with a decrease in the CaMKIIac concentration to convert LTD to LTP for large calcium concentrations, or coincident PF and CF input, in the Camk2b knockout mice.
The effect of CaMKII and PP2B on cerebellar synaptic plasticity is mediated by the phosphorylation and dephosphorylation of AMPA receptors, respectively, with AMPA receptor phosphorylation leading to the internalization and removal of AMPA receptors from the postsynaptic membrane and the induction of LTD. Thus, we also investigated the effect of the simulated Camk2b knockout on AMPA receptor phosphorylation for different calcium concentrations. Figure 4B shows the resulting average concentrations of phosphorylated and unphosphorylated AMPA receptors as the amount of postsynaptic intracellular calcium increases for wild-type and Camk2b knockout mice. For simplicity and in the absence of any other knowledge, we assume that the initial concentrations of phosphorylated and unphosphorylated AMPA receptors at a calcium concentration of zero are equal, that is, half of the AMPA receptors are unphosphorylated and the other half are phosphorylated. As in Fig. 4A, we compare average concentrations of AMPA receptors during the 300 s calcium application protocol (Fig. 4B, left panel) with those averaged over 6000 s (Fig. 4B, right panel), lasting for 5700 s after the offset of the calcium stimulus. When measured during the 300 s period of calcium application, the concentration of unphosphorylated AMPA receptors in wild-type mice initially increases with an increased calcium concentration, peaks at an average calcium concentration of about 0.2 μM, and drops off below its initial value for higher calcium concentrations. As expected from the simulated activation of CaMKII and PP2B (Fig. 4A), Camk2b knockout mice show the opposite behavior: the average concentration of unphosphorylated AMPA receptors during the stimulation period first decreases as the calcium concentration is raised, reaches a minimum where the unphosphorylated AMPA receptors in wild-type mice are at their maximum, and rises above its initial value for higher calcium concentrations. A similar behaviour is observed when the concentration of receptors is averaged over 6000 s, but there is a noticeable difference at very low calcium concentrations (below 0.1 μM) where the levels of unphosphorylated and phosphorylated AMPA receptors are similar for both types of mice. For these very low calcium concentrations, CaMKII is only weakly activated and the concentration of CaMKIIac rapidly drops after the calcium stimulation stops. In this case, the CaMKIIac decay is much faster than the deactivation of PP2B, favouring the dephosphorylation of AMPA receptors. In other respects, however, the results for 6000 s replicate those for 300 s, with increased (decreased) concentrations of unphosphorylated AMPA receptors for wild-type (knockout) mice at low calcium concentrations at around 0.2 μM, and decreased (increased) levels of unphosphorylated AMPA receptors for wild-type (knockout) mice for higher calcium concentrations (above 0.3 μM). Given that the level of unphosphorylated (and not internalized) AMPA receptors determines the strength of the PF-PC synapses, our simulations therefore replicate the experimental results by Van Woerden et al.33: in wild-type mice, LTP occurs at low calcium concentrations and LTD at high calcium concentrations, while knockout mice that lack β CaMKII exhibit LTD at low calcium concentrations and LTP at high calcium concentrations.
To examine in more detail how the concentrations of the different biochemical species evolve over time, we analysed the temporal evolution of kinase and phosphatase activities and AMPA receptor concentrations during and after the application of PF input alone, and compared these with their temporal evolution in response to coincident PF and CF input. The temporal evolution of unphosphorylated and phosphorylated AMPA receptors in wild-type and knockout mice in response to the two stimulation protocols is shown in Fig. 5A,B. In wild-type mice, the application of smaller calcium pulses that are triggered by PF input alone (Fig. 5A, inset) results in concentrations of unphosphorylated AMPA receptors that start exceeding their initial value after about 100 s of PF stimulation, leading to LTP induction. In Camk2b knockout mice, PF input without CF input rapidly decreases the level of unphosphorylated AMPA receptors, indicating the induction of LTD rather than LTP (Fig. 5A). In contrast, as shown in Fig. 5B, in response to the larger calcium pulses that result from the coincident activation of PF and CF input (Fig. 5B, inset), wild-type mice exhibit a decrease in unphosphorylated AMPA receptors and therefore LTD, while the Camk2b knockout leads to an increase in the concentration of unphosphorylated AMPA receptors, which corresponds to the induction of LTP.
Figure 5.
Temporal evolution of AMPA receptor phosphorylation illustrates bidirectional plasticity at PF-PC synapses in response to PF and CF input. Bidirectional plasticity at this synapse is also observed when plotting the temporal evolution of unphosphorylated and phosphorylated AMPA receptors (red and blue, respectively) for wild-type and Camk2b knockout mice (solid and dashed, respectively) in response to low calcium concentration resulting from the PF input alone (A), pulse amplitude of 1.8 μM), and high calcium concentration as a result of the paired PF and CF stimulation (B), pulse amplitude of 10 μM). The lower panels show the temporal evolution of AMPA receptor phosphorylation in simulations that lasted 6000 s, that is, continue for 5700 s after the offset of the 300 s simulation.
In some cases, the temporal evolution of the AMPA receptor concentrations in the simulations is biphasic. For example, PF input alone in wild-type mice leads to an initial decrease in the level of unphosphorylated receptors that is followed by an increase above the initial baseline (Fig. 5A, top panel). To understand the time course of the AMPA receptor phosphorylation and dephosphorylation, we analysed the temporal evolution of the activation of CaMKII and PP2B in the presence of synaptic input (Fig. 6), and their deactivation when the synaptic input ceases (Fig. 7). Figures 6A and 7A indicate that the biphasic nature of the AMPA receptor phosphorylation and dephosphorylation is based on the different rates of CaMKII and PP2B activation in the model. For both wild-type and Camk2b knockout mice, the activation of CaMKII is faster than that of PP2B, which leads an initial increase of the number of phosphorylated (and a decrease in the number of unphosphorylated) AMPA receptors in all cases (Fig. 5). However, in the presence of the F-actin binding of CaMKII in wild-type mice, the low calcium concentrations that result from PF input alone do not activate the kinase sufficiently, and the activation of PP2B surpasses the activation of CaMKII, which leads to the induction of LTP (Figs. 6A and 7A, top panel). In contrast, LTD occurs for PF input alone in knockout mice, where more CaMKII is available for activation, so that the concentration of activated CaMKII exceeds that of PP2B (Figs. 6A and 7A, bottom panel).
Figure 6.
Temporal evolution of active CaMKII and PP2B during the application of PF and CF input. Evolution of concentrations of CaMKIIac (blue) and PP2Bac (red) in wild-type mice (top, solid) and Camk2b knockout mice (bottom, dashed) in response to low calcium concentrations as a result of the PF input alone (A), pulse amplitude of 1.8 μM), and high calcium concentrations resulting from coincident PF and CF stimulation (B), pulse amplitude of 10 μM). In this figure, simulations last 300 s which is the duration of calcium stimulation.
Figure 7.
Continued temporal evolution of active CaMKII and PP2B after the application of synaptic input. Temporal evolution of CaMKIIac (blue) and PP2Bac (red) in wild-type mice (top, solid) and Camk2b knockout mice (bottom, dashed) in response to low calcium concentrations as a result of the PF input alone (A), pulse amplitude of 1.8 μM), and high calcium concentrations resulting from coincident PF and CF stimulation (B), pulse amplitude of 10 μM). Here, all simulations last 6000 s to investigate what occurs with CaMKIIac and PP2Bac concentrations after calcium input signals that last 300 s.
Figures 6B and 7B show that the opposite scenario occurs for coincident PF and CF input. The higher levels of calcium that result from the paired PF and CF stimulation result in much stronger activation of CaMKII and PP2B. In wild-type mice, the CaMKII activation exceeds the activation of PP2B, leading to LTD (Figs. 6B and 7B, top panel). However, the reduction of the overall CaMKII concentration because of the Camk2b knockout means that the level of PP2Bac in the knockout mice is higher than that of CaMKIIac and LTP occurs (Figs. 6B and 7B, bottom panel).
As previously described, the α: β CaMKII ratio in PCs is about 1:132,33. Thus, all of the previous simulations have assumed that the Camk2b knockout reduces the concentration of available CaMKII by 50%. However, it cannot be excluded that compensatory mechanisms in Camk2b knockout mice lead to an upregulation of α CaMKII. To examine whether the postulated reduction in the total CaMKII concentration in knockout mice plays a role in the reversal of synaptic plasticity, we performed a control simulation representing compensatory upregulation of α CaMKII in knockout mice, where binding of CaMKII to F-actin was disabled, but total CaMKII levels were kept the same as in wild-type mice (see Fig. 8). Figure 8 shows that this leads to a reversal of synaptic plasticity at low calcium concentrations (PF input only), but not at high calcium concentrations (PF and CF input). Thus, in order to explain the experimental results by van Woerden and collaborators33, we have to assume that the Camk2b knockout does indeed reduce the total concentration of CaMKII.
Figure 8.

Absence of bidirectional plasticity at PF-PC synapses when the CaMKII concentration is equal in wild-type and knockout mice: kinase and phosphatase activities at different calcium concentrations. The figure shows simulation results which indicate that the suggested explanation of the experimental observations by33 (Fig. 1) does not occur for higher calcium concentrations if the CaMKII concentration at knockout mice is not reduced due to a compensatory upregulation of α CaMKII. Low calcium concentrations that induce LTP in wild-type mice (solid) lead to LTD in Camk2b knockout mice (dashed), and vice versa. However, we observe that we do not see the inversion of plasticity for higher calcium concentrations if we do not reduce the CaMKII concentration for the knockout mice. Thus, we would not get the same results as in Fig. 4. The only modification was keeping the CaMKII concentration equal for both types of mice, and not reducing it for knockout mice. (A) Average concentrations of CaMKIIac (blue) and PP2Bac (red) as a function of average concentrations of pulsed calcium at 1 Hz for 300 s. (B) Average concentrations of unphosphorylated and phosphorylated AMPA receptors (red and blue, respectively) in response to the same stimulation protocols. The figures show average concentrations of CaMKIIac, PP2Bac and AMPA receptors in the presence of calcium signals applied at 1 Hz for 300 s. The panels show the average concentrations of these compounds during simulations that last 6000 s, that is, continue for 5700 s after the offset of the 300 s stimulation.
Discussion
LTD of synapses between PFs and PCs in cerebellar cortex is thought to be the basis of cerebellar learning5–7. Postsynaptic LTD at PF-PC synapses is balanced by postsynaptic LTP, but the exact conditions and cellular mechanisms that determine the direction of synaptic plasticity induction in vivo are not fully understood. PF LTD and LTP are mediated by the phosphorylation and dephosphorylation of AMPA receptors11,38 and regulated by a kinase/phosphatase switch involving enzymes such as CaMKII29,31–33 and PP2B14. The control of bidirectional plasticity by CaMKII has received particular attention, after experiments by Van Woerden and collaborators33 with Camk2b knockout mice indicated that the beta isoform of CaMKII plays a central role in determining the direction of synaptic weight change. In Camk2b knockout mice that lack β CaMKII, stimulation protocols that normally lead to the induction of LTD resulted in LTP, and vice versa33. Van Woerden et al.33 suggested that this reversal of synaptic plasticity in the Camk2b knockout mice could be based on binding of β CaMKII to F-actin in wild-type mice, which might reduce the concentration of CaMKII that is available for the phosphorylation of AMPA receptors under these normal conditions.
To test this hypothesis and investigate whether (and under which conditions) F-actin binding of CaMKII in wild-type mice, and the lack of F-actin binding in Camk2b knockout mice, can indeed lead to the observed reversal of synaptic plasticity at PF-PC synapses, we developed a simple mathematical model of AMPA receptor phosphorylation and dephosphorylation by CaMKII and PP2B. In our model, F-actin binding to CaMKII was included in wild-type mice that contain both α and β kinase isoforms. In contrast, in simulations of Camk2b knockout mice that lack β CaMKII the F-actin binding was omitted, and the total concentration of CaMKII was reduced to half of the value for wild-type mice. Simulation results of the model support the suggestion by Van Woerden and colleagues33. Moreover, they go beyond their original suggestion33 and predict that the sign reversal of synaptic plasticity is based on a combination of three mechanisms operating at different postsynaptic calcium concentrations. At low calcium concentrations such as those that result from PF input alone, and that evoke LTP in wildtype mice, the lack of F-actin binding of CaMKII in Camk2b knockout mice leads to a level of active and available CaMKII that exceeds the level of active PP2B; this results in induction of LTD in these mutant mice. For the high calcium concentrations that occur in response to a classic LTD induction protocol, that is, coincident PF and CF input in wildtype mice, two mechanisms operate together. The loss of β CaMKII in the knockout mice causes a reduction in the total concentration of CaMKII. As a consequence, less CaMKII is available for the phosphorylation of AMPA receptors, and more Ca4CaM is left available for the activation of PP2B. Thus, the model predicts that the combination of a decreased kinase activity and an increased phosphatase activity is responsible for the switch from LTD to LTP for high calcium concentrations in Camk2b knockout mice.
Although there are many computational models that investigate the biochemical processes underlying LTD at PF-PC synapses11,13,22–27, none of these models has been set up to model the induction of LTP at these synapses. Moreover, despite experimental evidence for the involvement of CaMKII in PF LTD and LTP29,31–33, the CaMKII pathway has, so far, not been included in the signalling cascades of existing PF-PC synaptic plasticity models. The present simple model is the first model of intracellular signalling at PF-PC synapses that focusses on the role of CaMKII and that models the induction of both LTD and LTP in wildtype and Camk2b knockout mice. In order to concentrate on the role of β CaMKII in bidirectional synaptic plasticity, the model has been kept intentionally simple. In future work, however, the biological realism of the model could be incrementally extended by adding components of existing models of PF LTD. A comparison of results obtained from our simple model with a more complex one could then be used to identify the potential contribution of other biochemical species to regulating the sign reversal of synaptic plasticity in cerebellar PCs.
Most modelling studies of intracellular signalling are based on the assumption that ionic and molecular concentrations can be described by mass action kinetics, and it is often assumed that reactions between chemical species occur in a single well-stirred compartment. As in the present model, this assumption leads to deterministic solutions of systems of ordinary differential equations. However, many signalling cascades involve very small numbers of molecules or ions, which are more faithfully represented by stochastic models39–41. As a complement to our deterministic model of intracellular signalling at the PF-PC synapse, a stochastic model of bidirectional plasticity at this synapse, which also accounts for spatial aspects of cellular signalling, should be developed. Results obtained from both deterministic and stochastic computational models should then be compared.
Some of the parameter values in our model could not be constrained by experimental data (see Table 1). The reason for this is that collecting experimental data about the biochemical dynamics in the cerebellum is challenging. It is difficult to measure many rates experimentally, for example the rates of association and dissociation of reactions. Thus, many of the parameters in the model were estimated in order to reproduce the mechanism we expected. Therefore, our model can be seen as a proof of existence for the suggested mechanism: there exists a reasonable set of biochemical interactions with a set of parameters such that the proposal by Van Woerden et al.33 that the binding of CaMKII to actin controls bidirectional plasticity at parallel fiber-Purkinje cell synapses can be implemented. As new and more precise experimental data become available, the parameter set of the model can be updated and the model retested. If the model predictions were falsified, this would require a re-evaluation of the model: either some of the remaining free parameters could be adjusted or new mechanisms would have to be added so that the model works.
Previous models of cerebellar LTD have measured synaptic plasticity by quantifying the concentrations or densities of phosphorylated and unphosphorylated AMPA receptors11,24. Indeed, this is the case for the simple model proposed here. However, Antunes and De Schutter have recently suggested that the extent of synaptic plasticity should be measured as a reduction in the number of synaptic AMPA receptors, rather than the concentration of phosphorylated receptors26. A next step in this research could be the inclusion of the AMPA receptor trafficking modelled by Antunes and De Schutter into the model of bidirectional plasticity at PF-PC synapses. Moreover, several concentrations of biochemical compounds and kinetic rate constants are free parameters, due to the lack of experimental data. Additional experiments will lead to an incremental improvement of the computational model, which in turn might suggest new experiments that need to be conducted.
Acknowledgements
We thank Chris De Zeeuw, Freek Hoebeek and Zhenyu Gao for helpful discussions and suggestions. TMP was supported by the São Paulo Research Foundation-FAPESP postdoctoral grant No. 2013/17063-5. ACR is part of the FAPESP research projects 2013/07699-0 and 2015/50122-0 and is partially supported by the Brazilian National Research Council-CNPq grant 306251/2014-0.
Author contributions
M.J.S. and V.S. conceived the research. T.M.P., M.J.S. and V.S. designed the experiments. T.M.P. collected data, wrote the code, performed the simulations and prepared the figures. T.M.P., M.J.S., A.C.R. and V.S. analysed and discussed the results. All authors wrote and reviewed the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Maria J. Schilstra and Volker Steuber.
References
- 1.Glickstein M. Thinking about the cerebellum. Brain. 2006;129:288–290. doi: 10.1093/brain/awh728. [DOI] [PubMed] [Google Scholar]
- 2.Glickstein M, Strata P, Voogd J. Cerebellum: history. Neurosci. 2009;162:549–559. doi: 10.1016/j.neuroscience.2009.02.054. [DOI] [PubMed] [Google Scholar]
- 3.Beaton A, Mariën P. Language, cognition and the cerebellum: Grappling with an enigma. Cortex. 2010;46:811–820. doi: 10.1016/j.cortex.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 4.D’Angelo E, Casali S. Seeking a unified framework for cerebellar function and dysfunction: from circuit operations to cognition. Front Neural Circuits. 2012;6:116. doi: 10.3389/fncir.2012.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Llinás R, Lang E, Welsh J. The cerebellum, LTD, and memory: alternative views. Learn. Mem. 1997;3:445–455. doi: 10.1101/lm.3.6.445. [DOI] [PubMed] [Google Scholar]
- 6.Hansel C, Linden DJ, D’Angelo E. Beyond parallel fiber LTD: the diversity of synaptic and non-synaptic plasticity in the cerebellum. Nat Neurosci. 2001;4:467–475. doi: 10.1038/87419. [DOI] [PubMed] [Google Scholar]
- 7.Ito M. Cerebellar circuitry as a neuronal machine. Prog Neurobiol. 2006;78:272–303. doi: 10.1016/j.pneurobio.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 8.Ito M, Kano M. Long-lasting depression of parallel fiber-Purkinje cell transmission induced by conjunctive stimulation of parallel fibers and climbing fibers in the cerebellar cortex. Neurosci Lett. 1982;33:253–258. doi: 10.1016/0304-3940(82)90380-9. [DOI] [PubMed] [Google Scholar]
- 9.Ito M. Long-term depression. Annu. Rev Neurosci. 1989;12:85–102. doi: 10.1146/annurev.ne.12.030189.000505. [DOI] [PubMed] [Google Scholar]
- 10.Wang S, Denk W, Häusser M. Coincidence detection in single dendritic spines mediated by calcium release. Nat Neurosci. 2000;12:1266–1273. doi: 10.1038/81792. [DOI] [PubMed] [Google Scholar]
- 11.Kuroda S, Schweighofer N, Kawato M. Exploration of signal transduction pathways in cerebellar long-term depression by kinetic simulation. J Neurosci. 2001;21:5693–5702. doi: 10.1523/JNEUROSCI.21-15-05693.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ito M. The molecular organization of cerebellar long-term depression. Nat Rev Neurosci. 2002;3:896–902. doi: 10.1038/nrn962. [DOI] [PubMed] [Google Scholar]
- 13.Doi T, Kuroda S, Michikawa T, Kawato M. Inositol 1,4,5-trisphosphate-dependent Ca2+ threshold dynamics detect spike timing in cerebellar Purkinje cells. J Neurosci. 2005;25:950–961. doi: 10.1523/JNEUROSCI.2727-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Belmeguenai A, Hansel C. A role for protein phosphatases 1, 2a, and 2b in cerebellar long-term potentiation. J Neurosci. 2005;25:10768–10772. doi: 10.1523/JNEUROSCI.2876-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Linden D, Connor J. Participation of postsynaptic PKC in cerebellar long-term depression in culture. Sci. 1991;254:1656–1659. doi: 10.1126/science.1721243. [DOI] [PubMed] [Google Scholar]
- 16.Lev-Ram V, Jiang T, Wood J, Lawrence D, Tsien R. Synergies and coincidence requirements between NO, cGMP, and Ca2+ in the induction of cerebellar long-term depression. Neuron. 1997;18:1025–1038. doi: 10.1016/S0896-6273(00)80340-2. [DOI] [PubMed] [Google Scholar]
- 17.Kawasaki H, et al. Requirement for mitogen-activated protein kinase in cerebellar long term depression. J Biol Chem. 1999;274:13498–13502. doi: 10.1074/jbc.274.19.13498. [DOI] [PubMed] [Google Scholar]
- 18.Ito M. Cerebellar long-term depression: characterization, signal transduction, and functional roles. Physiol Rev. 2001;81:1143–1195. doi: 10.1152/physrev.2001.81.3.1143. [DOI] [PubMed] [Google Scholar]
- 19.Chung H, Steinberg J, Huganir R, Linden D. Requirement of AMPA receptor GluR2 phosphorylation for cerebellar long-term depression. Sci. 2003;300:1751–1755. doi: 10.1126/science.1082915. [DOI] [PubMed] [Google Scholar]
- 20.Feil R, et al. Impairment of LTD and cerebellar learning by Purkinje cell-specific ablation of cGMP-dependent protein kinase i. J Cell Biol. 2003;163:295–302. doi: 10.1083/jcb.200306148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Launey T, Endo S, Sakai R, Harano J, Ito M. Protein phosphatase 2 A inhibition induces cerebellar longterm depression and declustering of synaptic ampa receptor. Proc Natl Acad Sci USA. 2004;101:676–681. doi: 10.1073/pnas.0302914101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fiala J, Grossberg S, Bullock D. Metabotropic glutamate receptor activation in cerebellar Purkinje cells as substrate for adaptive timing of the classically conditioned eye-blink response. J Neurosci. 1996;16:3760–3774. doi: 10.1523/JNEUROSCI.16-11-03760.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kotaleski J, Lester D, Blackwell K. Subcellular interactions between parallel fibre and climbing fibre signals in Purkinje cells predict sensitivity of classical conditioning to interstimulus interval. Integr Physiol Behav Sci. 2002;37:265–292. doi: 10.1007/BF02734249. [DOI] [PubMed] [Google Scholar]
- 24.Tanaka K, et al. Ca2+ requirements for cerebellar long-term synaptic depression: role for a postsynaptic leaky integrator. Neuron. 2007;54:787–800. doi: 10.1016/j.neuron.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 25.Kitagawa Y, Hirano T, Kawaguchi S. Prediction and validation of a mechanism to control the threshold for inhibitory synaptic plasticity. Mol Syst Biol. 2009;5:280. doi: 10.1038/msb.2009.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Antunes G, De Schutter E. A stochastic signaling network mediates the probabilistic induction of cerebellar long-term depression. J Neurosci. 2012;32:9288–9300. doi: 10.1523/JNEUROSCI.5976-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kawaguchi S-y, Hirano T. Gating of long-term depression by Ca2+/calmodulin-dependent protein kinase II through enhanced cGMP signalling in cerebellar Purkinje cells. J Physiol. 2013;591:1707–1730. doi: 10.1113/jphysiol.2012.245787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hanson P, Schulman H. Neuronal Ca2+/calmodulin-dependent protein kinases. Annu. Rev Biochem. 1992;61:559–601. doi: 10.1146/annurev.bi.61.070192.003015. [DOI] [PubMed] [Google Scholar]
- 29.Fink C, Meyer T. Molecular mechanisms of CaMKII activation in neuronal plasticity. Curr Opin Neurobiol. 2002;12:293–299. doi: 10.1016/S0959-4388(02)00327-6. [DOI] [PubMed] [Google Scholar]
- 30.Hudmon A, Schulman H. Structure-function of the multifunctional Ca2+/calmodulin-dependent protein kinase II. Biochem. J. 2002;364:593–611. doi: 10.1042/BJ20020228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lisman J, Schulman H, Cline H. The molecular basis of CaMKII function in synaptic and behavioural memory. Nat Rev Neurosci. 2002;3:175–190. doi: 10.1038/nrn753. [DOI] [PubMed] [Google Scholar]
- 32.Hansel C, et al. alphaCaMKII is essential for cerebellar LTD and motor learning. Neuron. 2006;51:835–843. doi: 10.1016/j.neuron.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 33.Van Woerden G, et al. [beta]CaMKII controls the direction of plasticity at parallel fiber-Purkinje cell synapses. Nat Neurosci. 2009;12:823–825. doi: 10.1038/nn.2329. [DOI] [PubMed] [Google Scholar]
- 34.Shen K, Teruel M, Subramanian K, Meyer T. Camkii[beta] functions as an f-actin targeting module that localizes CaMKII[alpha]/[beta] heterooligomers to dendritic spines. Neuron. 1998;21:593–606. doi: 10.1016/S0896-6273(00)80569-3. [DOI] [PubMed] [Google Scholar]
- 35.Dupont G, Houart G, De Koninck P. Sensitivity of CaM kinase II to the frequency of Ca2+ oscillations: a simple model. Cell Calcium. 2003;34:485–497. doi: 10.1016/S0143-4160(03)00152-0. [DOI] [PubMed] [Google Scholar]
- 36.De Koninck P, Schulman H. Sensitivity of CaM kinase II to the frequency of Ca2+ oscillations. Sci. 1998;279:227–230. doi: 10.1126/science.279.5348.227. [DOI] [PubMed] [Google Scholar]
- 37.Brocke L, Chiang L, Wagner P, Schulman H. Functional implications of the subunit composition of neuronal CaM kinase II. J Biol Chem. 1999;274:22713–22722. doi: 10.1074/jbc.274.32.22713. [DOI] [PubMed] [Google Scholar]
- 38.Lee H, Barbarosie M, Kameyama K, Bear M, Huganir R. Regulation of distinct AMPA receptor phosphorylation sites during bidirectional synaptic plasticity. Nat. 2000;405:955–959. doi: 10.1038/35016089. [DOI] [PubMed] [Google Scholar]
- 39.Bhalla U. Signaling in small subcellular volumes. I. Stochastic and diffusion effects on individual pathways. Biophys J. 2004;87:733–744. doi: 10.1529/biophysj.104.040469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bhalla U. Signaling in small subcellular volumes. II. stochastic and diffusion effects on synaptic network properties. Biophys J. 2004;87:745–753. doi: 10.1529/biophysj.104.040501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rao C, Wolf D, Arkin A. Control, exploitation and tolerance of intracellular noise. Nat. 2002;420:231–237. doi: 10.1038/nature01258. [DOI] [PubMed] [Google Scholar]
- 42.Airaksinen M, et al. Ataxia and altered dendritic calcium signaling in mice carrying a targeted null mutation of the calbindin D28k gene. Proc Natl Acad Sci USA. 1997;94:1488–1493. doi: 10.1073/pnas.94.4.1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schmidt H, Stiefel K, Racay P, Schwaller B, Eilers J. Mutational analysis of dendritic Ca2+ kinetics in rodent Purkinje cells: role of parvalbumin and calbindin D28k. J Physiol. 2003;551:13–32. doi: 10.1113/jphysiol.2002.035824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Meyer T, Hanson P, Stryer L, Schulman H. Calmodulin trapping by calcium-calmodulin-dependent protein kinase. Sci. 1992;256:1199–1201. doi: 10.1126/science.256.5060.1199. [DOI] [PubMed] [Google Scholar]