Abstract
The transcriptional coactivator YAP1 controls the balance between cell proliferation and apoptosis. YAP1 overexpression is linked to poor prognosis in many cancer types, yet its role in prostate cancer is unknown. Here, we applied YAP1 immunohistochemistry to a tissue microarray containing 17,747 clinical prostate cancer specimens. Cytoplasmic and nuclear YAP1 staining was seen in 81% and 63% of tumours. For both cytoplasmic and nuclear YAP1 staining, high levels were associated with advanced tumour stage, classical and quantitative Gleason grade, positive nodal stage, positive surgical margin, high KI67 labelling index, and early biochemical recurrence (p < 0.0001 each). The prognostic role of YAP1 staining was independent of established prognostic features in multivariate models (p < 0.001). Comparison with previously studied molecular markers identified associations between high YAP1 staining, TMPRSS2:ERG fusion (p < 0.0001), high androgen receptor (AR) expression (p < 0.0001), high Ki67 labelling index (p < 0.0001), and PTEN and 8p deletions (p < 0.0001 each). In conclusion, high YAP1 protein expression is an independent predictor of unfavourable disease course in prostate cancer. That cytoplasmic and nuclear YAP1 staining is equally linked to phenotype and prognosis fits well to a model where YAP1 activation during tumour progression includes up regulation, cytoplasmic accumulation and subsequent translocation to the nucleus.
Subject terms: Prognostic markers, Prostate
Introduction
In 2018, prostate cancer was the most common cancer in males and the third most cause of cancer related death1 with more than 1.3 million estimated newly diagnosed cases worldwide. The clinical course is variable and the currently used criteria for the distinction between high risk and low risk patients are Gleason grade, clinical stage and PSA value. To further reduce overtreatment, molecular prognostic markers would be an advance.
The transcriptional coactivator YAP1 is the critical downstream regulator of the Hippo signalling pathway that controls the balance between cell proliferation and apoptosis during embryogenesis and organ development2,3. Phosphorylation of cytoplasmic YAP1 and/or its paralogue WWTR1 by kinases of the Hippo pathway inhibits YAP1’s translocation to the nucleus where it activates target genes important for cell proliferation, cell death and cell motility4,5. Recent studies highlight a critical role of Hippo-YAP1 signalling for the biology of a wide range of cancer types. For example, in more than 90 studies published in Pubmed as to yet (March 2020), up regulation of YAP1 was reported from cancers of the cervix6, endometrium7, oesophagus8, urinary bladder9, brain10,11, skin12, head and neck13, ovary14, mesothelium15, bones16, lung17, breast18, colon19, stomach20, pancreas21 and liver22, and was linked to adverse tumour features and/or poor patient prognosis in most tumour types.
There is growing evidence that YAP1 migth also play an important role for the biology of both early and late stage prostate cancers. In vitro models suggest that YAP1 induces growth and migration in normal prostate epithelial cells5, revealed functional relationships between YAP1 activity and the prostate cancer specific TMPRSS2:ERG gene fusion23 as well as the PTEN tumour suppressor24, which is lost in about 20% of prostate cancers25, and that interaction of YAP1 with the androgen receptor may contribute to the development of castration-resistant prostate cancer26. Although these findings make YAP1 a promising candidate for a useful clinical marker in prostate cancer, five validation studies applying immunohistochemistry to 20–188 prostate cancers reported inconclusive results: There was either reduced27,28, unchanged29 or up regulated5,30 YAP1 in tumours as compared to normal or benign prostate tissues. Also, both high28,29 and low27 YAP1 protein levels have been reported to be linked with unfavourable tumour phenotype.
This study was undertaken to better understand the role of YAP1 in clinical prostate cancer samples. Here, we employed YAP1 immunohistochemistry (IHC) in a tissue microarray containing more than 14,000 prostate cancers with clinical follow-up data.
Results
Technical issues
A total of 9,571 (69%) and 9,884 (71%) tumour samples were interpretable for cytoplasmic and nuclear staining in our TMA analysis. The remaining tumors were considered non-informative because they either lacked unequivocal cancer tissue in the 0.6 mm spot or the entire tissue spot was missing on the TMA section.
YAP1 expression in normal and cancerous glands
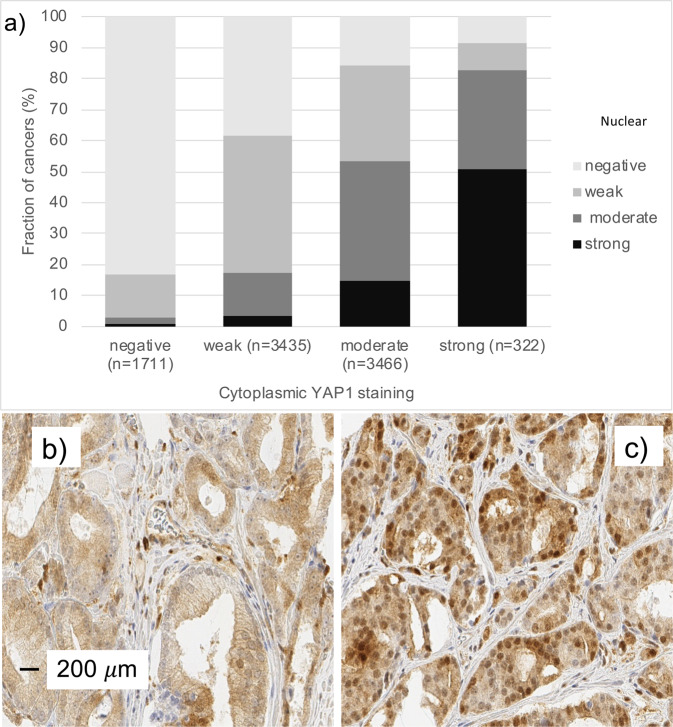
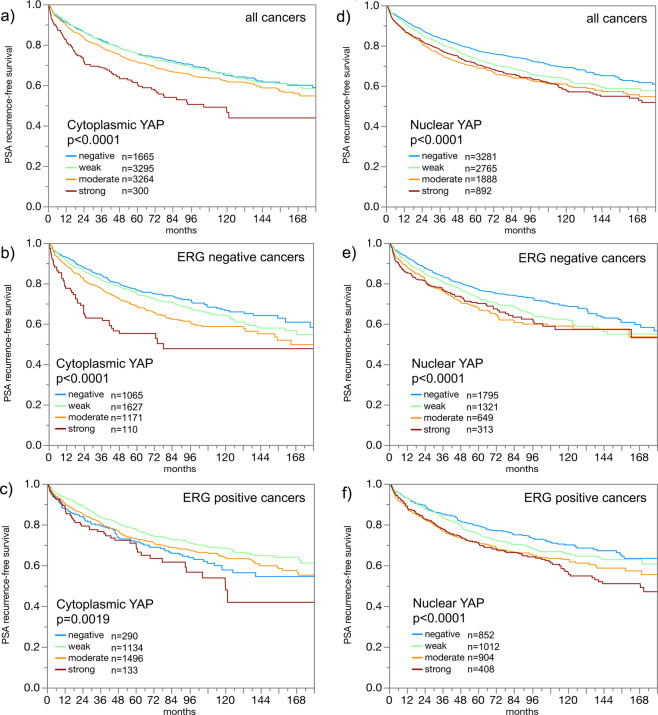
Normal prostatic glandular cells showed variable levels of cytoplasmic and nuclear staining ranging from negative to moderately positive, while basal cells always showed strong nuclear and often also cytoplasmic staining. In prostate cancers, cytoplasmic and nuclear staining was seen in 80.9% and 62.9% of tumours and was considered weak in 39%/32% (cytoplasmic/nuclear), moderate in 39%/22%, and strong in 4%/10% of cancers. Examples of cytoplasmic and nuclear YAP1 immunostainings in normal prostate and prostate cancers are shown in Figs. 1 and 2a,b. Cytoplasmic and nuclear staining was strongly linked to each other. For example, only 1% of 1,711 cancers with negative cytoplasmic staining, but 51% of 322 tumours with strong cytoplasmic staining showed strong nuclear staining (p < 0.0001, Fig. 2c). Both increased cytoplasmic and increased nuclear YAP1 staining were significantly linked to high traditional and quantitative Gleason grade (p < 0.0001), high pT category (p < 0.0001), nodal metastasis (p ≤ 0.03, Table 1, Supplementary Table S1), and early biochemical recurrence (p < 0.0001 each, Fig. 3a,b). Examples of YAP1 immunostaining in cancers with different Gleason grades are shown in Supplementary Fig. S1.
Figure 1.
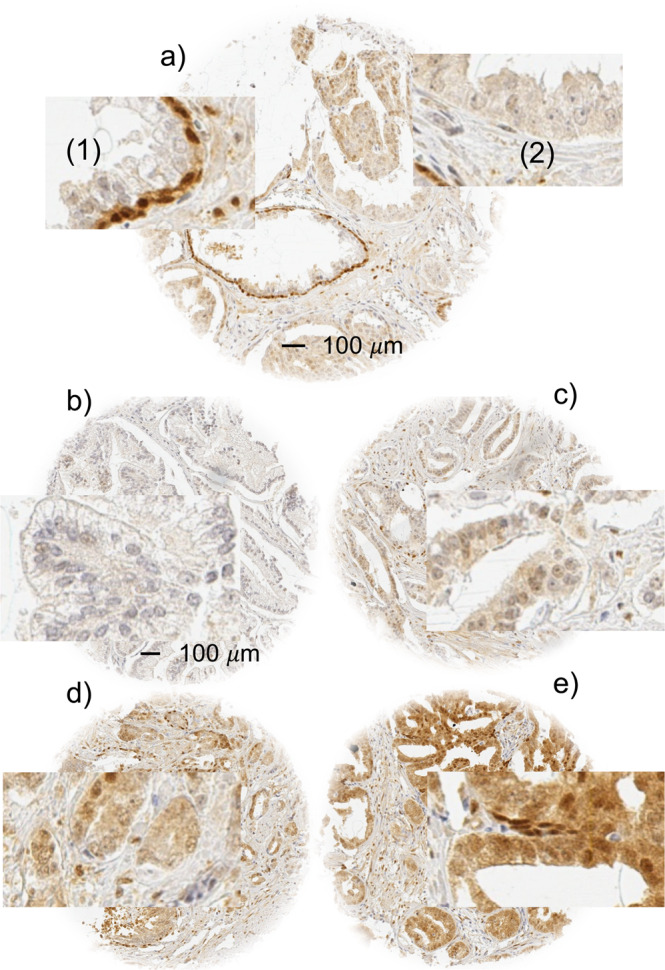
Examples of YAP1 staining in prostate tissue. (a) 0.6 mm tissue spot with normal and cancerous glands. Insets show strong YAP1 staining in (1) basal cells of the normal glands but absence of detectable staining in luminal cells (2) of tumour glands. (b–e) shows example of cancers with negative (b), weak (c), moderate (d) and strong (e) YAP1 staining.
Figure 2.
Cytoplasmic and nuclear YAP1 staining. (a) Significant correlation between cytoplasmic and nuclear YAP1 (p < 0.0001). (b) Example of a cancer with purely cytoplasmic YAP1 staining. (c) Example of a cancer with cytoplasmic and nuclear co-expression of YAP1.
Table 1.
Cytoplasmic YAP1 staining and prostate cancer phenotype.
| Cytoplasmic YAP1 staining (%) | ||||||
|---|---|---|---|---|---|---|
| N | Negative | Weak | Moderate | Strong | P | |
| All cancers | 9571 | 19.1 | 38.6 | 38.7 | 3.5 | |
| Tumour stage | <0.0001 | |||||
| pT2 | 5967 | 20.2 | 40.1 | 37.1 | 2.5 | |
| pT3a | 2261 | 17.4 | 37.0 | 40.9 | 4.8 | |
| pT3b-pT4 | 1308 | 17.0 | 34.6 | 42.7 | 5.7 | |
| Gleason grade | <0.0001 | |||||
| ≤3 + 3 | 1775 | 27.0 | 41.0 | 30.1 | 1.9 | |
| 3 + 4 | 5171 | 18.1 | 38.6 | 39.9 | 3.4 | |
| 3 + 4 Tert.5 | 440 | 17.0 | 39.8 | 42.0 | 1.1 | |
| 4 + 3 | 987 | 15.3 | 37.5 | 42.5 | 4.8 | |
| 4 + 3 Tert.5 | 679 | 12.8 | 38.7 | 42.6 | 5.9 | |
| ≥4 + 4 | 512 | 19.7 | 32.2 | 41.4 | 6.6 | |
| 3 + 4 ≤ 5% | 1292 | 20.2 | 39.2 | 38.1 | 2.5 | <0.0001 |
| 3 + 4 6–10% | 1347 | 17.1 | 39.7 | 39.9 | 3.3 | |
| 3 + 4 11–20% | 1171 | 18.6 | 36.6 | 41.4 | 3.3 | |
| 3 + 4 21–30% | 608 | 15.6 | 40.3 | 40.6 | 3.5 | |
| 3 + 4 31–49% | 520 | 17.1 | 36.2 | 41.3 | 5.4 | |
| 4 + 3 50–60% | 419 | 16.0 | 38.2 | 42.5 | 3.3 | |
| 4 + 3 61–80% | 375 | 13.6 | 38.1 | 42.1 | 6.1 | |
| 4 + 3 > 80% | 96 | 12.5 | 28.1 | 54.2 | 5.2 | |
| Lymph node metastasis | 0.0237 | |||||
| N0 | 5656 | 17.4 | 38.5 | 40.2 | 3.9 | |
| N + | 648 | 18.2 | 32.6 | 44.3 | 4.9 | |
| Preoperative PSA level (ng/ml) | <0.0001 | |||||
| <4 | 1104 | 14.8 | 36.9 | 43.7 | 4.7 | |
| 4–10 | 5714 | 18.4 | 38.8 | 39.5 | 3.3 | |
| 10–20 | 1980 | 22.3 | 40.1 | 34.3 | 3.3 | |
| >20 | 709 | 23.4 | 36.5 | 36.2 | 3.8 | |
| Surgical margin | 0.4865 | |||||
| Negative | 7522 | 19.1 | 38.8 | 38.7 | 3.4 | |
| Positive | 2011 | 19.3 | 37.8 | 38.9 | 4.0 | |
Figure 3.
Prognostic role of YAP1 in prostate cancer. (a–c) Impact of cytoplasmic staining (irrespective of nuclear staining) on PSA recurrence-free survival in (a) all cancers, (b) ERG negative cancers and (c) ERG positive cancers. (d–f): Impact of nuclear staining (irrespective of cytoplasmic staining) on PSA recurrence-free survival in (d) all cancers, (e) ERG negative cancers and (f) ERG positive cancers.
YAP1 and TMPRSS2:ERG fusion status
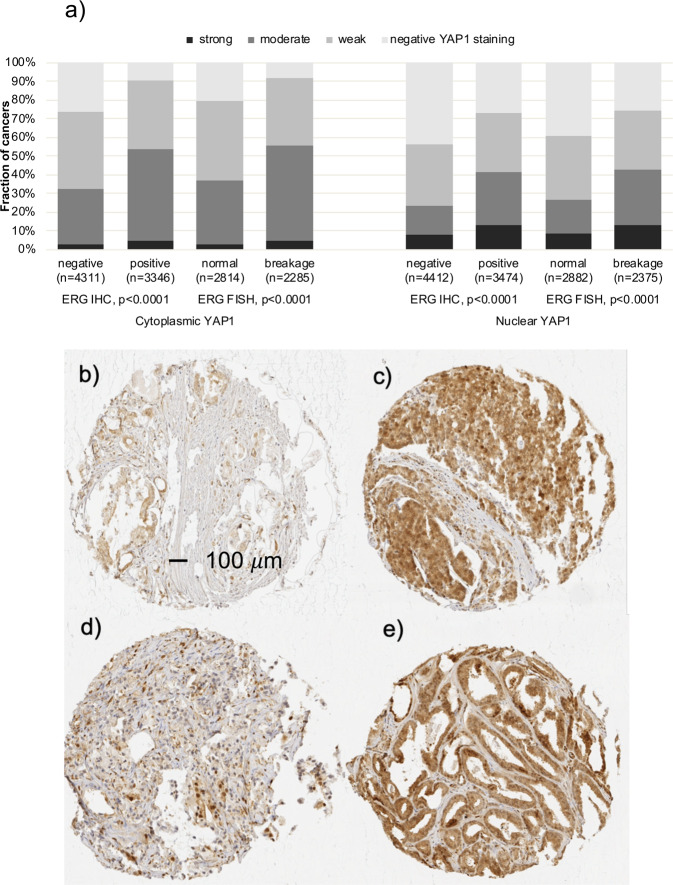
Data on both ERG break-apart fluorescence in-situ hybridization (FISH) and ERG IHC were concordant in 95.5% of these 4,617 cancers with both FISH and IHC data. High cytoplasmic and nuclear YAP1 were both significantly linked to cancers with TMPRSS2:ERG rearrangement and ERG expression (Fig. 4). Because of these differences in YAP1 staining between ERG positive and ERG negative cancers, these subsets were also evaluated separately. Associations with tumour phenotype (Supplementary Tables S2 and S3) and PSA recurrence (Fig. 3c–e; p < 0.0019 each) were largely retained in these subgroups, both for nuclear and cytoplasmic staining.
Figure 4.
YAP1 and ERG. (a) Correlation between cytoplasmic (left plot) and nuclear (right plot) YAP1 staining and ERG status assessed by immunohistochemistry (IHC) and fluorescence in situ hybridisation (FISH). (b,c) Examples of cancer spots with (b) weak and (c) strong YAP1 staining in ERG negative prostate cancers. (d,e) Examples of cancer spots with (d) weak and (e) strong YAP1 staining in ERG positive prostate cancers.
YAP1 and genomic deletion
Most deletions in prostate cancer are linked to either ERG negative cancers (i.e., deletions of 5q, 6q, 13q, 18q) or ERG positive cancers (i.e., deletions of 3p, 8p, 10q (PTEN), 12q, 16q, 17q). Because YAP1 expression was also linked to a positive ERG status, it was not surprising to find that high nuclear and cytoplasmic YAP1 staining was linked to deletions of 8p, 10q (PTEN), 16q and 17p (p < 0.0001) if all cancers were jointly analysed (Supplementary Figs. S2, S3). However, a search for associations that do not depend on ERG must be carried out in separate subsets of cancers with ERG-positive and ERG-positive cancers. For both nuclear and cytoplasmic staining, these analyses revealed that YAP1 staining is linked to deletions of 8p and PTEN (10q) in both ERG positive and ERG negative cancers (p ≤ 0.0004 each, Supplementary Figs. S2, S3).
YAP1, androgen receptor (AR) and tumour cell proliferation (Ki67 labelling index)
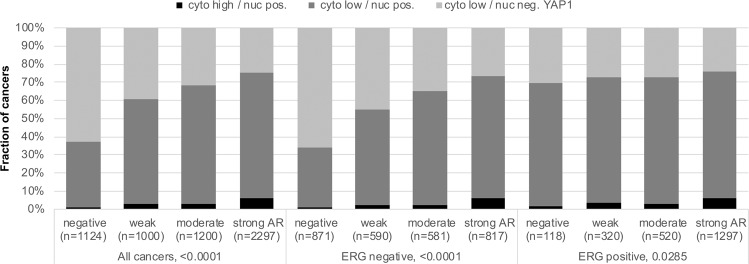
Data on YAP1 and AR expression from 7,971 cancers showed a significant association between AR expression and cytoplasmic and nuclear YAP1 staining (Fig. 5 and Supplementary Fig. 4a,b). High YAP1 staining was also significantly linked to increased cell proliferation as measured by Ki67 labelling index. These associations were statistically significant for nuclear and cytoplasmatic staining in the analysis of all cancers (p < 0.0001) and in most subsets of cancers with identical Gleason score (Table 2, Supplementary Table S4, Supplementary Fig. 4c,d).
Figure 5.
YAP1 and androgen receptor (AR). Correlation between different YAP1 staining patterns and AR expression levels in all cancers, ERG negative cancers and ERG positive cancers. “Cyto high” and “cyto low” includes cancers with moderate to strong (high) and negative to weak (low) cytoplasmic YAP staining. “Nuc pos” and “nuc neg” includes cancers with at least weak nuclear YAP1 staining (pos) and those lacking detectable nuclear YAP1 staining (neg).
Table 2.
Cytoplasmic YAP1 staining and Ki67 labelling index in all cancers, the ERG negative and positive subset.
| Gleason | Cytoplasmic | All cancers | ERG negative cancers | ERG positive cancers | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Subset | YAP1 | N | Mean ± SEM | P | n | Mean ± SEM | P | n | Mean ± SEM | P |
| Total | Negative | 1073 | 1.9 ± 0.1 | 835 | 1.7 ± 0.1 | 213 | 2.8 ± 0.2 | |||
| Weak | 2104 | 2.7 ± 0.1 | <0.0001 | 1186 | 2.7 ± 0.1 | <0.0001 | 884 | 2.7 ± 0.1 | 0.1152 | |
| Moderate | 1988 | 3.1 ± 0.1 | 829 | 3.2 ± 0.1 | 1138 | 3 ± 0.1 | ||||
| Strong | 171 | 3.4 ± 0.2 | 77 | 3.7 ± 0.3 | 90 | 3.1 ± 0.3 | ||||
| ≤3+3 | Negative | 301 | 1.6 ± 0.1 | 234 | 1.4 ± 0.1 | 53 | 2.4 ± 0.3 | |||
| Weak | 441 | 2.3 ± 0.1 | <0.0001 | 204 | 2.2 ± 0.1 | <0.0001 | 223 | 2.4 ± 0.1 | 0.9814 | |
| Moderate | 302 | 2.4 ± 0.1 | 95 | 2.5 ± 0.2 | 199 | 2.4 ± 0.1 | ||||
| Strong | 13 | 2.1 ± 0.6 | 4 | 1.8 ± 1 | 9 | 2.2 ± 0.7 | ||||
| 3+4 | Negative | 556 | 1.8 ± 0.1 | 431 | 1.6 ± 0.1 | 117 | 2.5 ± 0.2 | |||
| Weak | 1156 | 2.5 ± 0.1 | <0.0001 | 646 | 2.4 ± 0.1 | <0.0001 | 494 | 2.6 ± 0.1 | 0.0225 | |
| Moderate | 1174 | 2.9 ± 0.1 | 452 | 2.9 ± 0.1 | 712 | 3 ± 0.1 | ||||
| Strong | 101 | 2.7 ± 0.2 | 44 | 2.3 ± 0.3 | 55 | 3.1 ± 0.3 | ||||
| 3+4 Tertiary 5 | Negative | 36 | 2 ± 0.4 | 34 | 2 ± 0.4 | 2 | 2.5 ± 1.8 | |||
| Weak | 92 | 3.2 ± 0.3 | 0.0045 | 57 | 3.2 ± 0.3 | 0.0178 | 35 | 3.1 ± 0.4 | 0.3899 | |
| Moderate | 100 | 3.7 ± 0.2 | 55 | 3.7 ± 0.3 | 44 | 3.8 ± 0.4 | ||||
| Strong | 2 | 4 ± 1.7 | 2 | 4 ± 1.7 | 0 | 0 ± 0 | ||||
| 4+3 | Negative | 95 | 2.7 ± 0.3 | 70 | 2.6 ± 0.4 | 23 | 3 ± 0.6 | |||
| Weak | 216 | 3.3 ± 0.2 | 0.1992 | 143 | 3.4 ± 0.3 | 0.1452 | 71 | 3.1 ± 0.3 | 0.9742 | |
| Moderate | 213 | 3.3 ± 0.2 | 108 | 3.4 ± 0.3 | 104 | 3.2 ± 0.3 | ||||
| Strong | 26 | 4 ± 0.6 | 12 | 4.8 ± 1 | 13 | 2.9 ± 0.8 | ||||
| 4+3 Tertiary 5 | Negative | 41 | 2.9 ± 0.6 | 30 | 2.2 ± 0.7 | 10 | 4.9 ± 1.2 | |||
| Weak | 109 | 3.5 ± 0.4 | 0.0256 | 68 | 3.7 ± 0.5 | 0.0007 | 40 | 3.2 ± 0.6 | 0.46 | |
| Moderate | 115 | 4.3 ± 0.4 | 64 | 4.5 ± 0.5 | 50 | 4 ± 0.5 | ||||
| Strong | 15 | 6 ± 1 | 6 | 9.2 ± 1.6 | 8 | 2.9 ± 1.3 | ||||
| ≥4+4 | Negative | 43 | 3.2 ± 0.7 | 35 | 2.5 ± 0.7 | 8 | 6 ± 1.9 | |||
| Weak | 88 | 4.6 ± 0.5 | 0.131 | 67 | 4.3 ± 0.5 | 0.016 | 20 | 5.7 ± 1.2 | 0.3989 | |
| Moderate | 84 | 4.5 ± 0.5 | 55 | 5 ± 0.6 | 29 | 3.4 ± 1 | ||||
| Strong | 14 | 6.3 ± 1.2 | 9 | 6.6 ± 1.4 | 5 | 5.8 ± 2.4 | ||||
Multivariable analysis
YAP1 predicted biochemical recurrence independent from established prognostic parameters (Table 3). The maximal univariate hazard ratio for PSA recurrence was 1.4 for strong versus negative nuclear YAP1 expression and 1.9 for cytoplasmic YAP1 expression. In the multivariable model, YAP1 expression together with the univariably significant preoperative variables (Gleason grade, clinical stage and PSA level) showed a maximal multivariate hazard ratio of 1.3 for nuclear and 1.6 for cytoplasmic YAP1 expression. These hazard ratios were below the values for the other established parameters.
Table 3.
Multivariate hazard ratio (95% confidence interval) for biochemical relapse after prostatectomy for established risk factors and YAP1 expression in the preoperative model.
| Variable | Category | Cytoplasmic | Nuclear |
|---|---|---|---|
| n = 5,290 | n = 8,553 | ||
| Gleason grade biopsy | ≥4 + 4 vs. ≤3 + 3 | 4.1 (3.6–4.7) *** | 4.2 (3.7–4.7) *** |
| Preoperative PSA level | ≥20 vs. <4 | 3.2 (2.7–3.9) *** | 3.2 (2.7–3.9) *** |
| cT stage | T2c vs. T1c | 2.2 (1.8–2.8) *** | 2.1 (1.7–2.6) *** |
| YAP1 expression | Strong vs. negative | 1.6 (1.3–1.9) *** | 1.3 (1.1–1.5) ** |
Categories with the highest hazard ratio are shown for each variable ranked in decreasing order; *p ≤ 0.05, **p ≤ 0.001, ***p ≤ 0.0001
Discussion
The results of our study demonstrate that YAP1 up regulation is linked to prostate cancer aggressiveness independently from established prognostic markers of the disease.
Often, both nuclear and cytoplasmic YAP1 staining of cancer cells did not unequivocally differ from the staining in normal prostate glands in our study. This fits well with earlier observations by Noh et al.29, who reported strongly positive basal cells but no significant differences between the variable staining present in the normal luminal cells and in the tumour cells of 188 prostate cancers. The absence of clear-cut YAP1 expression differences between normal and cancerous prostatic glands may also explain why other studies came to contradictory conclusions. Hu et al.27 described decreased YAP1 staining in tumour cells as compared to hyperplastic or normal glands in 66 cancers. Sheng et al.30 studied YAP1 expression in 62 tissue samples obtained from tumour, tumour adjacent normal tissue and benign prostatic hyperplasia, and reported YAP1 up regulation in cancers as compared to non-neoplastic cells.
The high number of tumours in this study allowed to find a clear-cut link between higher YAP1 staining levels and adverse tumour phenotype as well as unfavourable prognosis. The functional role of YAP1 is dependent on whether it locates to the cytoplasm or to the nucleus. To activate growth-control associated genes, YAP1 must translocate from the cytoplasm to the nucleus4,31,32. The separate analysis of both nuclear and cytoplasmic staining resulted in identical associations with tumour phenotype and patient prognosis, however. This fits well to a model where YAP1 activation during tumour progression includes up regulation, cytoplasmic accumulation and subsequent translocation to the nucleus. Functional studies have shown that nuclear translocation indicates activation of YAP1, leading to induction of growth-control associated genes31,32. In line with our results, Noh et al.29 found higher levels of both cytoplasmic and nuclear YAP1 in high Gleason grade than in low grade cancers and a significant link between high YAP1 expression and early biochemical recurrence in 188 tumours. Sheng et al.30 reported links between YAP1 overexpression and higher Gleason grade as well as lymph node involvement in 32 cancers. Zhang et al.5 described high level YAP1 expression in 13 castration resistant cancers but none or only low YAP1 staining in 7 hormone naïve cancers. Only one study on 66 cancers suggested associations between decreased YAP1 expression and high Gleason score27. That YAP1 up regulation (and not down regulation) promotes cancer progression fits also well to several studies from other tumour types7,9,12,33–35.
The molecular database collected during numerous studies in the past allowed a comparison of our YAP1 data with other relevant molecular alterations. About 50% of prostate cancers contain a gene fusion involving the androgen-regulated TMPRSS2 and the transcription factor ERG36,37. Androgen dependent ERG expression results in alteration of more than 1,500 genes in affected prostate epithelial cells38. The significant up-regulation of YAP1 in cancers having a TMPRSS2:ERG fusion is consistent with data showing that ERG can activate YAP1 dependent transcription and tumour development23. The significant association of YAP1 and androgen receptor expression fits well to its known interaction. AR and YAP1 have been shown to colocalize to the nucleus, and downregulation of YAP1 leads to suppression of AR target genes, suggesting that YAP1 is important for AR signalling26. A prognostic role of YAP1 expression was observed in subsets of both ERG positive and ERG negative cancers, althougth stronger in ERG negative tumours. This makes YAP1 expression analysis a universally applicable prognostic feature that is not dependent on a particular molecular prostate cancer subtype. In earlier studies using the same prostate cancer TMA, several molecular parameters had been identified that were only prognostic in either ERG positive39,40 or ERG negative cancers41,42.
Deletions of 3p13, 8p21, 10q23 (PTEN), 12q24, 16q24, and 17p13 are linked to ERG positive cancers and 5q21, 6q15, 13q14, and 18q21 to ERG negative cancers41–47. Only the 12q13 deletion is unrelated to the ERG status40. As YAP1 was strongly associated with a positive ERG status, it is not surprising that YAP1 was either positively or inversely related to most deletions when all cancers were jointly analyzed. However, the absence of an association between most of these deletions with YAP1 expression in ERG positive and ERG negative tumour subsets argues against a direct role of YAP1 for the control of genome integrity or double strand breakage repair. The particularly strong association between YAP1 expression and PTEN deletions fits well with earlier reports describing a direct interaction between PTEN and the Hippo pathway24. Inactivation of the PTEN lipid phosphatase terminated the MOB1-LATS1/2 interaction, decreased phosphorylation of YAP1, induced YAP1 nuclear translocation, and increased the synergism between YAP1 and TEAD, thus eventually inducing cell proliferation and migration24. The strong link between YAP1 expression and 8p deletions may partly be caused by the high rate of co-deletions of 8p and PTEN. It is also possible that YAP1 has a relevant interaction with a specific 8p gene.
The present study proposes that the YAP1 protein level may be a weak, however useful, biomarker. Irrespective of whether nuclear or cytoplasmic YAP1 protein is measured, the prognostic impact of YAP1 staining was independent of conventional histo-morphological prognostic parameters. It is of note that all commonly used prognostic parameters in prostate cancer share major deficiencies. The Gleason grade, for example, suffers from very substantial interobserver variability, even between expert genitourinary pathologists48. The absence of a prognostic role of YAP1 expression in subests of cancers with identical quantitative Gleason grade highligths the power of the quantitative Gleason grading system49,50, although it is not universally applied and does not overcome all issues connected to interobserver variability in prostate cancer grading. The diagnosis of nodal metastasis is greatly dependent on the extent of surgery and the pathological work-up51. Accordingly, prognostic parameters are needed that are not necessarily statistically independent of established parameters but more reproducible and reliable than the established ones.
The polyclonal antibody against YAP1 that was used in this study strongly detected the protein in YAP1 overexpressing HeLa cells under identical experimental conditions as used for the TMA analysis. With the same protocol, non-transfected HeLa cells stained entirely negative, while HeLa cells transfected with YAP1’s paralog WWTR1 showed some faint staining with the YAP1 antibody. Given that YAP1 and WWTR1 share about 60% homology52,53, it cannot be excluded that we co-detected WWTR1 in addition to YAP1 at least in cancers with very high WWTR1 expression levels.
In summary, the data of this study show that YAP1 is a weak, however potentially useful, prognostic parameter in prostate cancer. The Hippo pathway and it’s downstream regulator YAP1 appear to play a similar important role in prostate cancer as it is known from many other solid cancer types. In the last 3 years, several Hippo pathway inhibititors that block the YAP-TEAD association have been developed and some show anti-tumour activity in-vitro54. Although still far from clinical application, such or similar substances may hold promizes for the therapy of many cancer types including early and advanced prostate cancers and prompt for diagnostic tests. For the future, we expect that panels composed of multiple antibodies, perhaps measured simultaneously by using multicolour fluorescence IHC, will be developed for prostate cancer prognosis assessment. Although analysis of the YAP1 protein alone had only a moderate – however independent – prognostic power in our study, it may represent a promising candidate for such a multiparametric prognostic approach.
Materials and Methods
Ethical statement
The study was approved by the Ethics Commission Hamburg, WF-049/09 and conducted in accordance with the Declaration of Helsinki. Informed consent has not been collected specifically for the patient samples included in this study. Usage of routinely archived formalin fixed leftover patient tissue samples for research purposes by the attending physician is approved by local laws and does not require written consent (HmbKHG, §12,1).
Patients
The study involved a total of 17,747 patients who underwent radical prostatectomy between 1992 and 2012 (Department of Urology and the Martini Clinic at the University Medical Centre Hamburg-Eppendorf). Histopathological data included pT, pN, resection margin, Gleason grade and “quantitative” Gleason grading49. Follow-up was available for a total of 14,664 patients (median 48 months; range 1 to 276 months; Supplementary Table S5). Prostate specific antigen (PSA) recurrence was defined as a postoperative PSA of 0.2 ng/ml and increasing at first of appearance. The TMA contained a single 0.6 mm core from a tumour containing tissue block for each patient55.
TMA database
The TMA was annotated with data from previous studies on Ki67 labelling Index (Ki67LI)56, androgen receptor (AR) expression36, and ERG protein expression57 that were assessed by means of immunohistochemistry (Supplementary Fig. 5). Genomic deletion of 3p13 (FOXP1)43, 5q21 (CHD1)47, 6q15 (MAP3K7)42, 8p2158, 10q23 (PTEN)25, 12p13 (CDKN1B)45, 12q2436, 13q14 (FOXO1, RB1)41, 16q2444, 17p13 (TP53)46 and 18q2159 as well as ERG rearrangement analysis57 was done by fluorescence in situ hybridization (FISH) using differentially labelled locus specific and centromere specific probes. Deletion was defined as less locus specific then centromere specific FISH probe signals in ≥60% of cancer cells.
Generation of YPA1 and WWTR1 expressing control cells
Human cervix epithelial carcinoma (HeLa) cells were cultured in DMEM (Dulbecco’s Modified Eagles Medium), 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin (P/S) at 37 °C and 5% CO2. Constructs encoding for YAP1 (cat. HG17690-UT, Sino Biological Inc., Wayne PA, USA) and WWTR1 (cat SC328639, Origene, Rockville, MD, USA) were each transfected into competent Escherichia coli cells (One ShotTM Top10, ThermoFisher Scientific, Germany). After 24 h, amplified plasmid was extracted (cat # 740579, Macherey-Nagel, Düren, Germany) and transfected into 3 × 106 HeLa cells (JetPEI DNA Transfection reagent, Polyplus-transfection S.A., Illkirch, France). Cells were grown for another 24 h, harvested, pelleted, fixed in 4% buffered formalin overnight and embedded in a paraffin block. Non-transfected HeLa cells were used as negative controls.
Immunohistochemistry
Freshly cut TMA sections were stained in a single experiment. Slides were deparaffinized and antigen was retrieved by heat (121 °C, 5 min, pH 7.8 Tris-EDTA-citrate buffer). Primary antibody specific for YAP1 (rabbit polyclonal antibody from Cell Signaling Technology, Danvers, MA, USA; cat. #4912; dilution 1:50) was applied at 37 °C for 60 min. Bound antibody was then visualized using the EnVision Kit (Dako, Glostrup, Denmark) according to the manufacturer’s directions. YAP1 showed nuclear and cytoplasmic staining. Staining of YAP1 in the positive and negative control cells is shown in Supplementary Fig. 6. A trained pathologist manually scored nuclear and cytoplasmic staining in two separate rounds of analysis according to the following criteria: The staining intensity (0, 1+, 2+, 3+) as well as the fraction of stained cells was recorded for each tissue spot. The IHC results for cytoplasmic and nuclear staining were created from these two parameters as follows: Lack of any staining (intensity 0) was considered “negative”, 1+ staining in ≤70% of tumour cells or 2+ staining in ≤30% of tumour cells was considered “weak”, 2+ staining in ≤70% of tumour cells or 2+ staining in >30% but ≤70% of tumour cells or 3+ staining in ≤30% of tumour cells was considered “moderate”, and 2+ staining in >70% of tumour cells or 3+ staining in >30% of tumour cells was considered “strong”.
Uni- and multivariable analysis
Uni- and multivariable hazard ratios for PSA recurrence were calculated for all categories. The variables significant in univariable analysis were included in the multivariable model. For comparison of the variables the categories with the maximal hazard ratio are given and ranked in decreasing order.
Statistics
Contingency tables and the chi²-test were performed to search for associations between molecular parameters and tumour phenotype. Kaplan-Meier survival curves were calculated and compared by the log-rank test. Cox proportional hazards regression analysis was performed to test for independence and significance between pathological, molecular and clinical variables. Various models combining pre- and postoperative available parameters were calculated. JPM 12 was used for calculations (SAS Institute Inc., NC, USA).
Ethical approval and informed consent
See above in Material and Methods.
Supplementary information
Acknowledgements
We are grateful to Inge Brandt, Sünje Seekamp, Melanie Witt and Maren Eisenberg for excellent technical assistance.
Author contributions
A.M., A.S., R.S., D.H., K.M., C.B., T.C., C.H.M. and A.P. contributed to conception, design, data collection, data analysis and manuscript writing. A.M.; A.S., M.C.T., F.B. and T.E. performed the immunohistochemistry analysis. M.G., H.Hu., H.He., A.H. and T.S. contributed to conception and design, collection of samples. A.S., T.S.C., S.S. and K.K. contributed to collection and data analysis. J.R.I., P.L., G.S. and A.P. supervised the study. E.B. developed the control cell lines.
Data availability
Data are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Andreas Marx and Aljoscha Schumann.
Supplementary information
is available for this paper at 10.1038/s41598-020-65772-w.
References
- 1.Bray F, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a cancer journal for clinicians. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Zheng Y, Pan D. The Hippo Signaling Pathway in Development and Disease. Dev Cell. 2019;50:264–282. doi: 10.1016/j.devcel.2019.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marti P, et al. YAP promotes proliferation, chemoresistance, and angiogenesis in human cholangiocarcinoma through TEAD transcription factors. Hepatology. 2015;62:1497–1510. doi: 10.1002/hep.27992. [DOI] [PubMed] [Google Scholar]
- 4.Hao Y, Chun A, Cheung K, Rashidi B, Yang X. Tumor suppressor LATS1 is a negative regulator of oncogene YAP. J Biol Chem. 2008;283:5496–5509. doi: 10.1074/jbc.M709037200. [DOI] [PubMed] [Google Scholar]
- 5.Zhang L, et al. The hippo pathway effector YAP regulates motility, invasion, and castration-resistant growth of prostate cancer cells. Mol Cell Biol. 2015;35:1350–1362. doi: 10.1128/MCB.00102-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu T, et al. Clinical significance of yes-associated protein overexpression in cervical carcinoma: the differential effects based on histotypes. Int J Gynecol Cancer. 2013;23:735–742. doi: 10.1097/IGC.0b013e31828c8619. [DOI] [PubMed] [Google Scholar]
- 7.Tsujiura M, et al. Yes-associated protein (YAP) modulates oncogenic features and radiation sensitivity in endometrial cancer. PLoS One. 2014;9:e100974. doi: 10.1371/journal.pone.0100974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yeo MK, et al. Correlation of expression of phosphorylated and non-phosphorylated Yes-associated protein with clinicopathological parameters in esophageal squamous cell carcinoma in a Korean population. Anticancer Res. 2012;32:3835–3840. [PubMed] [Google Scholar]
- 9.Liu JY, et al. Overexpression of YAP 1 contributes to progressive features and poor prognosis of human urothelial carcinoma of the bladder. BMC Cancer. 2013;13:349. doi: 10.1186/1471-2407-13-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ellison DW, et al. Medulloblastoma: clinicopathological correlates of SHH, WNT, and non-SHH/WNT molecular subgroups. Acta Neuropathol. 2011;121:381–396. doi: 10.1007/s00401-011-0800-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Striedinger K, et al. The neurofibromatosis 2 tumor suppressor gene product, merlin, regulates human meningioma cell growth by signaling through YAP. Neoplasia. 2008;10:1204–1212. doi: 10.1593/neo.08642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Menzel M, et al. In melanoma, Hippo signaling is affected by copy number alterations and YAP1 overexpression impairs patient survival. Pigment Cell Melanoma Res. 2014;27:671–673. doi: 10.1111/pcmr.12249. [DOI] [PubMed] [Google Scholar]
- 13.Li Z, et al. The Hippo transducer TAZ promotes epithelial to mesenchymal transition and cancer stem cell maintenance in oral cancer. Mol Oncol. 2015;9:1091–1105. doi: 10.1016/j.molonc.2015.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang X, et al. The Hippo pathway transcriptional co-activator, YAP, is an ovarian cancer oncogene. Oncogene. 2011;30:2810–2822. doi: 10.1038/onc.2011.8. [DOI] [PubMed] [Google Scholar]
- 15.Murakami H, et al. LATS2 is a tumor suppressor gene of malignant mesothelioma. Cancer Res. 2011;71:873–883. doi: 10.1158/0008-5472.CAN-10-2164. [DOI] [PubMed] [Google Scholar]
- 16.Chan LH, et al. Hedgehog signaling induces osteosarcoma development through Yap1 and H19 overexpression. Oncogene. 2014;33:4857–4866. doi: 10.1038/onc.2013.433. [DOI] [PubMed] [Google Scholar]
- 17.Xie M, et al. Prognostic significance of TAZ expression in resected non-small cell lung cancer. J Thorac Oncol. 2012;7:799–807. doi: 10.1097/JTO.0b013e318248240b. [DOI] [PubMed] [Google Scholar]
- 18.Kim SK, Jung WH, Koo JS. Yes-associated protein (YAP) is differentially expressed in tumor and stroma according to the molecular subtype of breast cancer. Int J Clin Exp Pathol. 2014;7:3224–3234. [PMC free article] [PubMed] [Google Scholar]
- 19.Lee KW, et al. Significant association of oncogene YAP1 with poor prognosis and cetuximab resistance in colorectal cancer patients. Clin Cancer Res. 2015;21:357–364. doi: 10.1158/1078-0432.CCR-14-1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu X, Xin Y, Xiao Y, Zhao J. Overexpression of YAP1 is correlated with progression, metastasis and poor prognosis in patients with gastric carcinoma. Pathol Oncol Res. 2014;20:805–811. doi: 10.1007/s12253-014-9757-y. [DOI] [PubMed] [Google Scholar]
- 21.Diep CH, et al. Down-regulation of Yes Associated Protein 1 expression reduces cell proliferation and clonogenicity of pancreatic cancer cells. PLoS One. 2012;7:e32783. doi: 10.1371/journal.pone.0032783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee K, et al. The correlation between poor prognosis and increased yes-associated protein 1 expression in keratin 19 expressing hepatocellular carcinomas and cholangiocarcinomas. BMC Cancer. 2017;17:441. doi: 10.1186/s12885-017-3431-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nguyen LT, et al. ERG Activates the YAP1 Transcriptional Program and Induces the Development of Age-Related Prostate Tumors. Cancer Cell. 2015;27:797–808. doi: 10.1016/j.ccell.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu W, et al. PTEN lipid phosphatase inactivation links the hippo and PI3K/Akt pathways to induce gastric tumorigenesis. J Exp Clin Cancer Res. 2018;37:198. doi: 10.1186/s13046-018-0795-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krohn A, et al. Genomic deletion of PTEN is associated with tumor progression and early PSA recurrence in ERG fusion-positive and fusion-negative prostate cancer. The American journal of pathology. 2012;181:401–412. doi: 10.1016/j.ajpath.2012.04.026. [DOI] [PubMed] [Google Scholar]
- 26.Kuser-Abali G, Alptekin A, Lewis M, Garraway IP, Cinar B. YAP1 and AR interactions contribute to the switch from androgen-dependent to castration-resistant growth in prostate cancer. Nat Commun. 2015;6:8126. doi: 10.1038/ncomms9126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hu X, Jia Y, Yu J, Chen J, Fu Q. Loss of YAP protein in prostate cancer is associated with Gleason score increase. Tumori. 2015;101:189–193. doi: 10.5301/tj.5000238. [DOI] [PubMed] [Google Scholar]
- 28.Collak FK, Demir U, Ozkanli S, Kurum E, Zerk PE. Increased expression of YAP1 in prostate cancer correlates with extraprostatic extension. Cancer Biol Med. 2017;14:405–413. doi: 10.20892/j.issn.2095-3941.2017.0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Noh MG, Kim SS, Hwang EC, Kwon DD, Choi C. Yes-Associated Protein Expression Is Correlated to the Differentiation of Prostate Adenocarcinoma. J Pathol Transl Med. 2017;51:365–373. doi: 10.4132/jptm.2017.05.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sheng X, et al. YAP is closely correlated with castration-resistant prostate cancer, and downregulation of YAP reduces proliferation and induces apoptosis of PC-3 cells. Mol Med Rep. 2015;12:4867–4876. doi: 10.3892/mmr.2015.4005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dong J, et al. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell. 2007;130:1120–1133. doi: 10.1016/j.cell.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao B, et al. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 2007;21:2747–2761. doi: 10.1101/gad.1602907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Song S, et al. The Hippo Coactivator YAP1 Mediates EGFR Overexpression and Confers Chemoresistance in Esophageal Cancer. Clin Cancer Res. 2015;21:2580–2590. doi: 10.1158/1078-0432.CCR-14-2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Y, Xie C, Li Q, Xu K, Wang E. Clinical and prognostic significance of Yes-associated protein in colorectal cancer. Tumour Biol. 2013;34:2169–2174. doi: 10.1007/s13277-013-0751-x. [DOI] [PubMed] [Google Scholar]
- 35.Zhang L, Song X, Li X, Wu C, Jiang J. Yes-Associated Protein 1 as a Novel Prognostic Biomarker for Gastrointestinal Cancer: A Meta-Analysis. Biomed Res Int. 2018;2018:4039173. doi: 10.1155/2018/4039173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weischenfeldt J, et al. Integrative genomic analyses reveal an androgen-driven somatic alteration landscape in early-onset prostate cancer. Cancer Cell. 2013;23:159–170. doi: 10.1016/j.ccr.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 37.Tomlins SA, et al. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science. 2005;310:644–648. doi: 10.1126/science.1117679. [DOI] [PubMed] [Google Scholar]
- 38.Brase JC, et al. TMPRSS2-ERG -specific transcriptional modulation is associated with prostate cancer biomarkers and TGF-beta signaling. BMC Cancer. 2011;11:507. doi: 10.1186/1471-2407-11-507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Krohn A, et al. Heterogeneity and chronology of PTEN deletion and ERG fusion in prostate cancer. Mod Pathol. 2014;27:1612–1620. doi: 10.1038/modpathol.2014.70. [DOI] [PubMed] [Google Scholar]
- 40.Kluth M, et al. Concurrent deletion of 16q23 and PTEN is an independent prognostic feature in prostate cancer. Int J Cancer. 2015;137:2354–2363. doi: 10.1002/ijc.29613. [DOI] [PubMed] [Google Scholar]
- 41.Kluth M, et al. 13q deletion is linked to an adverse phenotype and poor prognosis in prostate cancer. Genes, chromosomes & cancer. 2018;57:504–512. doi: 10.1002/gcc.22645. [DOI] [PubMed] [Google Scholar]
- 42.Kluth M, et al. Genomic deletion of MAP3K7 at 6q12-22 is associated with early PSA recurrence in prostate cancer and absence of TMPRSS2:ERG fusions. Mod Pathol. 2013;26:975–983. doi: 10.1038/modpathol.2012.236. [DOI] [PubMed] [Google Scholar]
- 43.Krohn A, et al. Recurrent deletion of 3p13 targets multiple tumour suppressor genes and defines a distinct subgroup of aggressive ERG fusion-positive prostate cancers. The Journal of pathology. 2013;231:130–141. doi: 10.1002/path.4223. [DOI] [PubMed] [Google Scholar]
- 44.Kluth M, et al. Deletion lengthening at chromosomes 6q and 16q targets multiple tumor suppressor genes and is associated with an increasingly poor prognosis in prostate cancer. Oncotarget. 2017;8:108923–108935. doi: 10.18632/oncotarget.22408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kluth M, et al. Genomic deletion of chromosome 12p is an independent prognostic marker in prostate cancer. Oncotarget. 2015;6:27966–27979. doi: 10.18632/oncotarget.4626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kluth M, et al. Clinical significance of different types of p53 gene alteration in surgically treated prostate cancer. Int J Cancer. 2014;135:1369–1380. doi: 10.1002/ijc.28784. [DOI] [PubMed] [Google Scholar]
- 47.Burkhardt L, et al. CHD1 is a 5q21 tumor suppressor required for ERG rearrangement in prostate cancer. Cancer Res. 2013;73:2795–2805. doi: 10.1158/0008-5472.Can-12-1342. [DOI] [PubMed] [Google Scholar]
- 48.Egevad L, et al. Standardization of Gleason grading among 337 European pathologists. Histopathology. 2013;62:247–256. doi: 10.1111/his.12008. [DOI] [PubMed] [Google Scholar]
- 49.Sauter G, et al. Clinical Utility of Quantitative Gleason Grading in Prostate Biopsies and Prostatectomy Specimens. European urology. 2016;69:592–598. doi: 10.1016/j.eururo.2015.10.029. [DOI] [PubMed] [Google Scholar]
- 50.Sauter G, et al. Integrating Tertiary Gleason 5 Patterns into Quantitative Gleason Grading in Prostate Biopsies and Prostatectomy Specimens. European urology. 2018;73:674–683. doi: 10.1016/j.eururo.2017.01.015. [DOI] [PubMed] [Google Scholar]
- 51.Wilczak W, et al. Marked Prognostic Impact of Minimal Lymphatic Tumor Spread in Prostate Cancer. European urology. 2018;74:376–386. doi: 10.1016/j.eururo.2018.05.034. [DOI] [PubMed] [Google Scholar]
- 52.Santucci M, et al. The Hippo Pathway and YAP/TAZ-TEAD Protein-Protein Interaction as Targets for Regenerative Medicine and Cancer Treatment. J Med Chem. 2015;58:4857–4873. doi: 10.1021/jm501615v. [DOI] [PubMed] [Google Scholar]
- 53.Plouffe SW, et al. The Hippo pathway effector proteins YAP and TAZ have both distinct and overlapping functions in the cell. J Biol Chem. 2018;293:11230–11240. doi: 10.1074/jbc.RA118.002715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cheng Y, Hou T, Ping J, Chen T, Yin B. LMO3 promotes hepatocellular carcinoma invasion, metastasis and anoikis inhibition by directly interacting with LATS1 and suppressing Hippo signaling. J Exp Clin Cancer Res. 2018;37:228. doi: 10.1186/s13046-018-0903-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kononen J, et al. Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat Med. 1998;4:844–847. doi: 10.1038/nm0798-844. [DOI] [PubMed] [Google Scholar]
- 56.Tennstedt P, et al. The impact of the number of cores on tissue microarray studies investigating prostate cancer biomarkers. International journal of oncology. 2012;40:261–268. doi: 10.3892/ijo.2011.1216. [DOI] [PubMed] [Google Scholar]
- 57.Minner S, et al. ERG status is unrelated to PSA recurrence in radically operated prostate cancer in the absence of antihormonal therapy. Clin Cancer Res. 2011;17:5878–5888. doi: 10.1158/1078-0432.Ccr-11-1251. [DOI] [PubMed] [Google Scholar]
- 58.Kluth M, et al. Deletion of 8p is an independent prognostic parameter in prostate cancer. Oncotarget. 2017;8:379–392. doi: 10.18632/oncotarget.13425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kluth M, et al. Deletion of 18q is a strong and independent prognostic feature in prostate cancer. Oncotarget. 2016;7:86339–86349. doi: 10.18632/oncotarget.13404. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available from the corresponding author on reasonable request.