Abstract
Growing interest lies in the assessment of the metabolic status of patients with a univentricular circulation after Fontan operation, especially in changes of amino acid metabolism. Using targeted metabolomic examinations, we investigated amino acid metabolism in a homogeneous adult Fontan-patient group with a dominant left ventricle, seeking biomarker patterns that might permit better understanding of Fontan pathophysiology and early detection of subtle ventricular or circulatory dysfunction. We compared serum amino acid levels (42 analytes; AbsoluteIDQ p180 kit, Biocrates Life Sciences, Innsbruck, Austria) in 20 adult Fontan patients with a dominant left ventricle and those in age- and sex-matched biventricular controls. Serum concentrations of asymmetric dimethylarginine, methionine sulfoxide, glutamic acid, and trans-4-hydroxyproline and the methionine sulfoxide/methionine ratio (Met-SO/Met) were significantly higher and serum concentrations of asparagine, histidine, taurine, and threonine were significantly lower in patients than in controls. Met-SO/Met values exhibited a significant negative correlation with oxygen uptake during exercise. The alterations in amino acid metabolome that we found in Fontan patients suggest links between Fontan pathophysiology, altered cell energy metabolism, oxidative stress, and endothelial dysfunction like those found in biventricular patients with congestive heart failure. Studies of extended amino acid metabolism may allow better understanding of Fontan pathophysiology that will permit early detection of subtle ventricular or circulatory dysfunction in Fontan patients.
Subject terms: Congenital heart defects, Medical research
Introduction
Ventricular dysfunction and circulatory failure with progressing end-organ impairment like renal or liver dysfunction are an important cause of morbidity and mortality in adults with complex congenital heart disease (CHD), especially in patients with single-ventricle types of CHD and Fontan circulation1,2. Besides limited cardiac output, alterations that mark Fontan hemodynamics are passive flow to the lungs, chronically elevated venous pressures, and congestion. Unfortunately, the clinical use of traditional markers such as N-terminal prohormone of brain natriuretic peptide (NT-proBNP) levels for non-invasive diagnostics and monitoring in such patients is limited3,4. Thus, for early detection of cardiac and circulatory derangement and for evaluation and tailoring of treatment options, regular functional assessment of these patients is crucial, with complete clinical examination, electrocardiogram, imaging studies, determination of values for traditional laboratory markers, or exercise capacity testing. In adult biventricular patients, novel candidate biomarkers, in addition to natriuretic peptides and troponins, for congestive heart failure and vascular perturbations have been identified via metabolomics, the study of small organic molecules, their synthesis, and their breakdown5,6. Interest has grown recently in the metabolic status of Fontan patients: besides reported abnormalities in Fontan patients’ glucose metabolism7, to date their handling of lipids has been best studied, with important changes shown, especially in the cholesterol, lipoprotein, and phospholipid pathways, hinting at chronic low-level inflammation and at altered cell signalling and energy metabolism as are found in biventricular patients with congestive heart failure8,9. In the biventricular patient with heart failure, alterations occur especially in the handling of amino acids important in both myocardium protein turnover and energy metabolism10. Additionally, the Fontan-specific characteristic of chronically elevated venous pressure favors development of a protein losing enteropathy together with end-organ, especially liver, dysfunction2. Thus, study of the metabolism of amino acids also should be a promising field in Fontan patients. With the help of targeted metabolomic examinations we hence elected to investigate amino acid metabolism in a homogeneous adult Fontan-patient group with a dominant left ventricle, seeking biomarker patterns that might permit a better understanding of Fontan pathophysiology or early detection of ventricular or circulatory dysfunction.
Results
After applying all inclusion and exclusion criteria, 20 adult Fontan patients with a systemic left ventricle were selected for the study (Supplemental Fig. 1). The results of “traditional” examinations (patient and control clinical assessment, exercise capacity testing, routine laboratory analyses) are set out in our recent work on lipid metabolism9. Table 1 lists clinical and exercise capacity testing parameters, showing as major features that in Fontan patients, minimum (at exercise) and maximum (at rest) pulse-oximeter oxygen saturation as well as O2 at the anaerobic threshold and at maximum were significantly lower than in controls.
Table 1.
Participants’ clinical characteristics9.
| Fontan patients | Controls | P-value | |
|---|---|---|---|
| Total [n] | 20 | 20 | |
| Female sex [n] | 7 | 7 | |
| Age [years] | 23.1 ± 5.1 | 24.7 ± 6.6 | 0.28 |
| After TCPC [years] | 18.8 ± 5.2 | ||
| Bodyweight [kg] | 69.8 ± 13.2 | 73.3 ± 11.7 | 0.17 |
| Height [cm] | 171.3 ± 7.4 | 174.5 ± 8.7 | 0.04 |
| Body mass index [kg/m²] | 23.8 ± 4.1 | 22.5 ± 3.3 | 0.05 |
| SpO2 at rest [%] | 93 ± 3 | 99 ± 1 | <0.00001 |
| SpO2 at exercise [%] | 90 ± 3 | 98 ± 1 | <0.00001 |
| O2 at rest [mL/kg/min] | 5.6 ± 1.7 | 5.8 ± 1.1 | 0.03 |
| O2AT [mL/kg/min] | 24.5 ± 4.9 | 30.1 ± 3.6 | <0.00001 |
| Peak O2 [mL/kg/min] | 28.8 ± 10.1 | 45.7 ± 6.4 | <0.00001 |
| Double inlet left ventricle [n] | 10 | ||
| TA + PS/PA [n] | 9 | ||
| TA + PS + VSD [n] | 1 | ||
| Extracardiac Fontan [n] | 16 | ||
| Open fenestration (at study) [n] | 3 | ||
| LPA dilation/stent [n] | 4 | ||
| Tunnel dilation/stent [n] | 6 | ||
| Closure of fenestration [n] | 1 | ||
| Closure of vv collateral [n] | 3 | ||
| Electrophysiologic examination [n] | 2 |
Values are given as mean ± standard deviation. AT, anaerobic threshold; LPA, left pulmonary artery; n, number; PA, pulmonary atresia; PS, pulmonary stenosis; SpO2, pulsoxymetric oxygen saturation; TA, tricuspid atresia; TCPC, total cavopulmonary connection; O2, oxygen uptake; VSD, ventricular septal defect; vv, veno-venous.
Routine analytes
Hematocrit, hemoglobin concentrations (“hemoglobin”), gamma glutamyl transferase and alanine aminotransferase activities, total bilirubin and creatinine concentrations (“total bilirubin” and “creatinine”), and international normalized ratio (INR) values and triglyceride and high density lipoprotein-cholesterol (HDL-C) concentrations (“triglycerides” and “HDL-C”) differed significantly between Fontan patients and controls (Table 2).
Table 2.
Values of routine analytes and of amino acids or biogenic amines and their derivatives.
| Fontan patients | Controls | P-value | |
|---|---|---|---|
| Hematocrit [%] | 47.8 ± 5.6 | 39.3 ± 4.2 | <0.00001↑ |
| Hemoglobin [g/dL] | 16.4 ± 2.1 | 12.7 ± 1.4 | <0.00001↑ |
| Total cholesterol [mg/dL] | 145.3 ± 26.5 | 149 ± 34.2 | 0.77 |
| HDL-C [mg/dL] | 42.5 ± 15.9 | 51.3 ± 12.3 | 0.03↓ |
| Non-HDL-C [mg/dL] | 85.2 ± 24.8 | 73.1 ± 20.8 | 0.2 |
| Triglycerides [mg/dL] | 128.6 ± 86.5 | 47.3 ± 22.8 | 0.003↑ |
| Total protein [g/dL] | 7.2 ± 0.5 | 7.0 ± 0.7 | 0.31 |
| Albumin [mg/dL] | 4145 ± 492 | 4215 ± 208 | 0.64 |
| Creatinine [mg/dL] | 0.8 ± 0.12 | 0.53 ± 0.18 | <0.00001↑ |
| Total bilirubin [mg/dL] | 1.22 ± 0.67 | 0.3 ± 0.29 | <0.00001↑ |
| AST [U/L] | 35.3 ± 7.7 | 31.6 ± 8.4 | 0.12 |
| ALT [U/L] | 39.4 ± 11.4 | 31.9 ± 10.1 | 0.04↑ |
| gGT [U/L] | 86.5 ± 43.6 | 35.1 ± 19.4 | 0.00002↑ |
| INR | 2.1 ± 0.76 | 1.02 ± 0.04 | <0.00001↑ |
| NT-proBNP [pg/mL] | 52.4 ± 69.2 | 39.3 ± 30.4 | 0.88 |
| CRP [mg/dL] | 0.18 ± 0.2 | 0.16 ± 0.14 | 0.47 |
Values are given as mean ± standard deviation. ALT, alanine aminotransferase; AST, aspartate aminotransferase; CRP, C-reactive protein; dL, decilitre; g, gram; gGT, gamma glutamyl transferase; HDL-C, high density lipoprotein-cholesterol; INR, international normalized ratio; NT-proBNP, N-terminal prohormone of brain natriuretic peptide; U, unit; ↑, statistically significant higher serum concentration in Fontan patients than in controls; ↓, statistically significant lower serum concentration in Fontan patients than in controls.
Metabolomic examination of serum amino acids
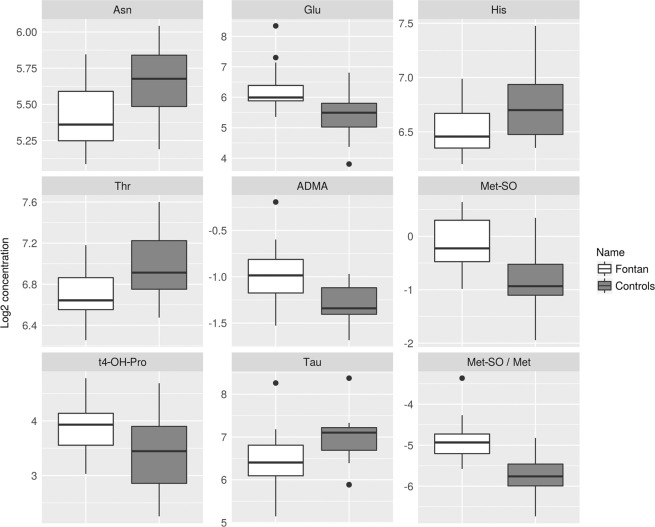
Serum concentrations of 29 amino acids or biogenic amines and their derivatives were determined, with selected sums and ratios (Table 3, Fig. 1). In Fontan patients serum concentrations of methionine (Met) sulfoxide (Met-SO), asymmetric dimethyl arginine (ADMA), glutamic acid, and trans-4-hydroxyproline and the ratio of Met-SO to Met (Met-SO/Met) were significantly higher, and serum concentrations of asparagine, histidine, taurine, and threonine were significantly lower than in controls.
Table 3.
Values of amino acids or biogenic amines and their derivatives, and of selected sums and ratios.
| Metabolite | HMBD ID | Patients | Controls | p-value | q-value | mean FC |
|---|---|---|---|---|---|---|
| Ac-Orn | HMDB0003357 | NA | NA | NA | NA | NA |
| ADMA | HMDB01539 | 0.51 ± 0.12 | 0.41 ± 0.05 | 0.0004 | 0.002↑ | 1.26 |
| Alanine | HMDB00161 HMDB01310 | 401.4 ± 84.2 | 410.5 ± 123.0 | 0.99 | 0.99 | −1.02 |
| alpha-AAA | HMDB00510 | 1.12 ± 0.61 | 0.77 ± 0.44 | 0.03 | 0.06 | 1.44 |
| Arginine | HMDB00517 HMDB03416 | 91.7 ± 26.8 | 108.2 ± 21.8 | 0.03 | 0.06 | −1.18 |
| Asparagine | HMDB00168 HMDB003378 | 43.7 ± 7.7 | 50.7 ± 8.0 | 0.007 | 0.016↓ | −1.16 |
| Aspartic acid | HMDB00191 HMDB06483 | 18.0 ± 10.4 | 17.2 ± 6.9 | 0.93 | 0.96 | 1.05 |
| c4-OH-Pro | HMDB0240251 | NA | NA | NA | NA | NA |
| Carnosine | HMDB00033 | NA | NA | NA | NA | NA |
| Citrulline | HMDB00904 | 29.4 ± 6.3 | 29.2 ± 6.6 | 0.91 | 0.95 | 1.01 |
| DOPA | HMDB00181 HMDB00609 | NA | NA | NA | NA | NA |
| Dopamine | HMDB00073 | NA | NA | NA | NA | NA |
| Glutamine | HMDB00641 HMDB03423 | 717.4 ± 134.1 | 749.0 ± 111.7 | 0.38 | 0.47 | −1.04 |
| Glutamic acid | HMDB00148 HMDB03339 | 85.9 ± 63.4 | 47.0 ± 21.8 | 0.0006 | 0.002↑ | 1.83 |
| Glycine | HMDB00123 | 321.6 ± 74.1 | 345.3 ± 91.7 | 0.41 | 0.5 | −1.07 |
| Histidine | HMDB00177 | 93.1 ± 15 | 110.4 ± 24.4 | 0.008 | 0.02↓ | −1.19 |
| Histamine | HMDB00870 | NA | NA | NA | NA | NA |
| Isoleucine | HMDB00172 HMDB0000557 | 106.6 ± 31.6 | 100.9 ± 39.5 | 0.44 | 0.53 | 1.06 |
| Kynurenine | HMDB00684 | NA | NA | NA | NA | NA |
| Leucine | HMDB00687 HMDB0013773 | 222.9 ± 78.6 | 232.8 ± 106.6 | 0.99 | 0.99 | −1.04 |
| Lysine | HMDB00182 HMDB03405 | 168.1 ± 23.5 | 165.8 ± 32.4 | 0.69 | 0.79 | 1.01 |
| Methionine | HMDB00696 | 27.9 ± 8.3 | 29.4 ± 7.8 | 0.47 | 0.55 | −1.06 |
| Met-SO | HMDB02005 | 0.94 ± 0.32 | 0.58 ± 0.23 | 0.00008 | 0.0005↑ | 1.64 |
| Nitro-Tyr | HMDB01904 | NA | NA | NA | NA | NA |
| Ornithine | HMDB00214 HMDB03374 | 139.4 ± 82.3 | 111.6 ± 40.9 | 0.22 | 0.29 | 1.25 |
| PEA | HMDB0012275 | NA | NA | NA | NA | NA |
| Phenylalanine | HMDB00159 | 77.7 ± 16.9 | 77.0 ± 12.9 | 0.99 | 0.99 | 1.01 |
| Proline | HMDB00162 HMDB03411 | 259.4 ± 63.7 | 265.7 ± 61.6 | 0.73 | 0.81 | −1.02 |
| Putrescine | HMDB01414 | NA | NA | NA | NA | NA |
| Sarcosine | HMDB00271 | 1.57 ± 0.94 | 1.62 ± 0.79 | 0.73 | 0.81 | −1.03 |
| SDMA | HMDB03334 | 0.46 ± 0.06 | 0.42 ± 0.08 | 0.1 | 0.15 | 1.08 |
| Serine | HMDB00187 HMDB03406 | 158.4 ± 33.3 | 146.5 ± 23.7 | 0.21 | 0.29 | 1.08 |
| Serotonin | HMDB00259 | 0.56 ± 0.33 | 0.7 ± 0.21 | 0.03 | 0.06 | −1.26 |
| Spermidine | HMDB01257 | NA | NA | NA | NA | NA |
| Spermine | HMDB01256 | NA | NA | NA | NA | NA |
| t4-OH-Pro | HMDB00725 HMDB0006055 | 15.8 ± 5.06 | 11.7 ± 5.2 | 0.007 | 0.02↑ | 1.35 |
| Taurine | HMDB00251 | 100.4 ± 57.1 | 132.6 ± 56.54 | 0.02 | 0.049↓ | −1.32 |
| Threonine | HMDB04041 HMDB00167 | 105.6 ± 21.0 | 127.3 ± 28.4 | 0.008 | 0.02↓ | −1.21 |
| Tryptophan | HMDB00929 HMDB0013609 | 90.5 ± 20.2 | 89.5 ± 23.3 | 0.84 | 0.89 | 1.01 |
| Tyrosine | HMDB00158 | 87.0 ± 23.0 | 83.6 ± 19.4 | 0.69 | 0.79 | 1.04 |
| Valine | HMDB00883 | 298.9 ± 72.5 | 290.7 ± 76.9 | 0.7 | 0.79 | 1.03 |
| BCAA | 628 ± 174 | 624 ± 217 | 0.8 | 0.87 | 1.01 | |
| AAA | 255 ± 53 | 250 ± 47 | 0.8 | 0.87 | 1.02 | |
| Total AA | 3544 ± 466 | 3588 ± 682 | 0.93 | 0.96 | −1.01 | |
| Essential AA | 1098 ± 223 | 1113 ± 293 | 0.97 | 0.99 | −1.01 | |
| Fischer ratio | 2.47 ± 0.52 | 2.45 ± 0.5 | 0.9 | 0.95 | 1.01 | |
| Met-SO/Met | 0.04 ± 0.016 | 0.02 ± 0.007 | <0.00001 | 0.0001↑ | 1.78 |
Metabolite concentrations for all analytes with selected sums and ratios, grouped by Fontan patients vs. controls. Values are given as mean ± standard deviation, unit of values of data [µmol/l]. FC, fold change (Fontan patients vs. controls); HMBD ID, Human Metabolome Database identification; AA, amino acids; AAA, aromatic amino acids; Ac-Orn, acetylornithine; ADMA, asymmetric dimethylarginine; alpha-AAA, alpha aminoadipic acid; BCAA, branched-chain amino acids; c4-OH-Pro, cis-4-hydroxyproline; DOPA, dihydroxyphenylalanine; Met-SO, methionine sulfoxide; Nitro-Tyr, nitrotyrosine; PEA, phenylethylamine; SDMA, symmetric dimethylarginine; t4-OH-Pro, trans-4-hydroxyproline; ↑, statistically significant higher serum concentration in Fontan patients than in controls; ↓, statistically significant lower serum concentration in Fontan patients than in controls. ‘Fischer ratio’ is the ratio of BCAA/AAA.
Figure 1.
Box-and-whisker plots of serum concentrations of amino acids or biogenic amines and their ratios that differed significantly between Fontan patients (grey boxes) and controls (white boxes). The boxes show the 1st (Q1) and 3rd quartile (Q3), the whiskers the minimum and the maximum. Black circles represent outlying values as identified by the interquartile range (IQR) rule (values smaller than (Q1-1.5*IQR) or values larger than (Q3 + 1.5*IQR)). ADMA, asymmetric dimethylarginine; Asn, asparagine; Glu, glutamic acid; His, histidine; Met, methionine; Met-SO, methionine sulfoxide; Met-SO/Met, methionine sulfoxide/methionine ratio; t4-OH-Pro, trans-4-hydroxyproline; Tau, taurine; Thr, threonine.
Correlation of routine biochemical and clinical findings with metabolomic parameters
Among routine analytes, the variables hemoglobin, albumin, and triglycerides displayed significantly positive correlations with glutamic acid. Creatinine and triglycerides displayed significantly positive correlations with ADMA, as did the variables creatinine and total bilirubin with Met-SO. Hematocrit, hemoglobin, and INR as well as minimum and maximum oxygen saturations revealed significantly positive correlations with Met-SO/Met values, and oxygen uptake at the anaerobic threshold and maximum oxygen uptake displayed significantly negative correlations with Met-SO/Met values. Single significant correlations between isolated variables included positive correlation of albumin with ornithine and serine, of triglycerides with alpha aminoadipic acid, and of the oxygen uptake at the anaerobic threshold with threonine as well as negative correlations of alanine aminotransferase activity with taurine and of INR with serotonin. No further correlations were identified, especially none with CRP or NT-proBNP (Supplemental Table 1).
Discussion
To the best of our knowledge, our study is the first clinical metabolomics study focusing on Fontan patients’ serum amino acid patterns. Its main finding is that, in comparison with controls, adult Fontan patients with a morphologically left dominant ventricle exhibit a distorted amino acid metabolome, hinting at altered (myocardial) cell energy metabolism and an elevated myocardial protein turnover as well as at oxidative stress and endothelial dysfunction, as found in biventricular patients with congestive heart failure.
Altered cell energy metabolism and elevated myocardial protein turnover
Decreased serum concentrations of taurine, asparagine, and threonine and increased concentrations of glutamic acid and hydroxyproline in our Fontan patients indicate alterations in (myocardial) cell energy metabolism and elevated myocardium protein turnover, as found in biventricular patients with congestive heart failure. Taurine is abundant in myocardial tissue, with a major function in regulation of the respiratory chain: the taurine-deficient heart suffers impaired respiratory chain function and diminished long chain fatty acid uptake by mitochondria, as found in patients with congestive heart failure10. Decreased concentrations of taurine are described in patients with dilated cardiomyopathy and in golden retrievers with heart failure10,11, and many studies report a beneficial effect of taurine supplementation on myocardial function in patients with congestive heart failure12.
Glutamic acid and asparagine also take part in central pathways of aerobic cell respiration and thereby of energy production. Via anaplerotic reactions, both amino acids are substantially involved in the tricarboxylic acid cycle13. Against the background of a switch in heart failure of preferred myocardial energy substrate from fatty acids to glucose and ketone bodies14, our findings hint at altered myocardial energy metabolism, indicating subtle ventricular dysfunction. This idea is supported by glutamic acid’s involvement in the synthesis of metabolites, e.g., by serving as a precursor for the biosynthesis of amino acids such as proline and arginine15,16. Indeed, increased serum levels of glutamic acid indicate increased protein turnover, as typical in adults with congestive heart failure or coronary heart disease17,18. The same is true for threonine, a major component of proteins, e.g., of collagen and immunoglobulins: We found decreased serum threonine levels, and decreased plasma levels are described in patients with chronic heart failure17. The increase that we observed in hydroxyproline, an analyte used to track collagen degradation, also reflects elevated protein turnover: Elevated urine and serum hydroxyproline levels are reported after muscle damage or in the bedridden and elderly19,20.
Oxidative stress and endothelial dysfunction
Protein turnover also is elevated in the presence of hyperreactive oxygen species, i.e., under oxidative stress and during endothelial dysfunction, both of which are directly linked to heart failure: Nitrous oxide (NO) synthase (NOS) isoforms are expressed not only in endothelial cells but also in cardiomyocytes, and NO regulates cardiac function through vascular-dependent and -independent effects, with, in the healthy heart, a positive inotropic effect at low NO exposure and a negative one at higher exposure21,22. In heart failure, regulation of myocardial NO production and release breaks down, with excessive release, and peripheral and vascular endothelial NOS activity is lost, resulting in endothelial dysfunction with decreased NO bioavailability attributable to increased oxidative stress23. In the course of cardiac decompensation, NO likely influences several of the core features of cardiac failure, e.g., chamber dilation, defective b-adrenergic responsiveness, and calcium cycling22.
That serum concentrations of ADMA were elevated also suggests that our Fontan patients were under oxidative stress, with altered endothelial function, consistent with subtle ventricular dysfunction. ADMA, a methyl derivate of arginine, is involved in NO-signalling and in pro- and antioxidant and -inflammatory processes. It is the major endogenous inhibitor of nitric oxide synthase (NOS). By displacing arginine, the normal NOS substrate, ADMA enhances oxidative stress by influencing NO-reactive oxygen species balance and disturbs vasodilation21,24–26. ADMA values reportedly track enhanced cardiovascular risk with endothelial dysfunction27,28, positively correlating with age, mean arterial pressure, renovascular resistance, intimal media thickness, and peripheral arterial occlusive disease5,29–33, ADMA is additionally involved in further nitric oxide (NO)-dependent signalling processes, interfering with anti-thrombotic, anti-inflammatory, and anti-apoptotic actions21.
The increases in value for Met-SO and for the Met-SO/Met ratio as indicators of systemic oxidative stress also suggest that our patients are under oxidative stress and suffer from endothelial dysfunction. Reaction with oxygen species yields Met-SO, which activates endogenous antioxidant enzymes such as Met-SO reductase A and induces synthesis of glutathione, thereby counteracting oxidative stress and inflammation34. Increased Met-SO levels have been reported in vascular disease, cardiac ischemia, and in left ventricular diastolic dysfunction35,36. The negative correlation observed between Met-SO/Met values and oxygen uptake under exercise strikingly emphasizes the role of markers of oxidative stress as indicators for subtle ventricular dysfunction in our patients. Finally, our hypothesis that oxidative stress affects our patients is supported by the decreased level found of histidine, with its anti-oxidant and anti-inflammatory properties37, and by decreases in HDL-C9,38–40. A direct interplay of lipoprotein metabolism and markers for oxidative stress exists: lipoprotein disorders are associated with increased ADMA41.
ADMA also has been used to track heart failure in patients with CHD, and with regard to heart failure is even more sensitive than NT-proBNP. However, only 13% of the patients studied had univentricular heart disease; in addition, patient exercise capacity was lower than that in our patient group33. Thus we stress that our findings of altered amino acid serum levels might imply subtle rather than frank heart failure42,43, a hypothesis supported by the fact that we found no correlation of any of the metabolites with the traditional marker for heart failure, NT-proBNP, nor with the traditional marker for inflammation C-reactive protein, and by the fact that neither by traditional clinical-laboratory means nor by metabolomic (Fischer ratio) criteria did we find any evidence for important (Fontan-associated) liver disease in our patients. Given that many adult Fontan patients will develop ventricular dysfunction, to hypothesise subtle heart failure in our patient group might well be appropriate1. Still, with respect to the definition of heart failure, which is the “inability of the heart to meet resting and exercise demands at low filling pressures”2, we cannot exclude with certainty that the metabolic alterations delineated solely reflect Fontan-specific pathophysiology with its circulatory abnormalities, with paramount abnormal systemic or pulmonary endothelial function, and with a tendency towards the formation of veno-venous or aortopulmonary collaterals44–48.
To examine the amino acid profile of Fontan patients with frank ventricular, circulatory, or hepatic impairment and to correlate increases in serum ADMA and Met-SO content with direct measurement of endothelin or with collateral vessel flow via magnetic resonance imaging studies thus would be of interest49,50.
Limitations
Because Fontan patients are few, the studied group size is small, possibly limiting the extent to which our findings can be generalized. Also limiting may be our choice of a targeted metabolomic approach and a commercial kit, as metabolites not assessed may be important. That we analyzed serum samples precludes direct comparison between our results and those of studies that used tissue samples, and we acknowledge that having assayed serum rather than vascular or myocardial tissue, we cannot rule out certain system-driven aspects of disease pathogenesis: That is, unknown confounders that affect metabolic profiles might be the true basis for the observed differences. Moreover, differences in body composition or lifestyle parameters might have influenced our results to an unknown degree. We strove to lessen the likelihood of such errors by following a strict inclusion and exclusion protocol, especially with regard to (known) comorbidities or medication.
Conclusion
The striking alterations in amino acid profile that we found may link Fontan pathophysiology with altered cell energy metabolism, oxidative stress, and endothelial dysfunction as found in biventricular patients with congestive heart failure. Markers identified through mass spectrometry-based extended amino acid metabolism might thus complement traditional diagnostic tools such as imaging, exercise capacity testing, and traditional laboratory biomarker determinations, yielding a better understanding of Fontan pathophysiology, and they are promising candidates for the early detection of ventricular or circulatory dysfunction in Fontan patients.
Methods
Like our recently published results on phospholipid and acylcarnitine metabolomic examinations, this work is a subwork of the main study protocol (Trial registration number: ClinicalTrials.gov Identifier NCT03886935)9.
Patients
Between September 2016 and March 2017, we prospectively examined adult Fontan patients with a dominant left single ventricle and age- and sex-matched healthy biventricular controls at the Center of Pediatric Cardiology and Congenital Heart Disease, Heart and Diabetes Center North Rhine-Westphalia, Ruhr-University of Bochum, Germany9. All patients had undergone two-stage palliation with partial and total cavopulmonary anastomosis. None had had aortic reconstruction or aortopulmonary shunting. Table 4 shows inclusion and exclusion criteria. See the flow chart according to STROBE (Strengthening the Reporting of Observational Studies in Epidemiology [https://strobe-statement.org/index.php?id=strobe-home]) (Supplemental Fig. 1) for details on the flow of patients through the present study.
Table 4.
Inclusion and exclusion criteria9.
| Inclusion criteria | Exclusion criteria |
|---|---|
| Written informed consent | Medication affecting metabolic state |
| Age ≥18 years | Medication affecting hemodynamic state |
| 8 hours fasting before blood sampling | Coronary artery disease |
| Dominant left ventricle (patients) | Failure of systemic ventricle |
| Biventricular healthy heart (controls) | Valvular stenosis |
| Atrioventricular regurgitation > mild | |
| Aortic regurgitation > mild | |
| Recurrent effusions | |
| Protein-losing enteropathy | |
| Metabolic disease | |
| Malignancy | |
| Other cachectic disease, malnutrition | |
| Inflammatory disease | |
| Myeloproliferative disorder | |
| Pregnancy or lactation | |
| Multiple organ failure | |
| Mental or physical handicap precluding exercise | |
| O2 AT < 20 ml/kg/min7 |
O2AT, oxygen uptake at the anaerobic threshold.
Clinical and laboratory examinations were performed as described in detail elsewhere9. Age, sex, weight, medication, and cardiac risk factor assessment, with blood sampling for routine hematological and biochemical profiling, was conducted during an outpatient visit. Fasting patients underwent phlebotomy while recumbent. Echocardiography followed, and, after a defined snack rich in carbohydrates, exercise capacity was tested. We correlated metabolic results with routine laboratory parameters and exercise capacity parameters51. All patients underwent symptom-limited treadmill exercise capacity testing with expiratory gas analysis52. A 12-lead ECG was used to determine heart rate. Oxygen uptake at rest (O2 at rest, ml/kg/min), at the anaerobic threshold (O2AT, ml/kg/min), and maximum uptake of oxygen (O2 max, ml/kg/min) were measured.
Blood studies required samples 0.5 ml greater than those for routine assessments to permit determinations of concentrations of amino acid-metabolism analytes. The blood sample was directly drawn into a tube containing a clotting activator and centrifuged within 20 min (15 °C, 10 min, 2500 × g) for separation of serum. Serum aliquots were immediately frozen and stored at -80 °C for further analyses. Frozen samples were transported on dry ice to the analysing laboratory. Analyses were performed in batches of 10 samples9.
Sample preparation
Before analysis, all serum samples were processed as described, with samples thawed on ice, then centrifuged; the supernatant was subjected to further analyses6,9. The AbsoluteIDQ p180 kit assay (Biocrates Life Sciences AG, Innsbruck, Austria) permitted targeted, fully automated quantification of 188 analytes (42 amino acids or biogenic amines and their derivatives) based on phenylisothiocyanate derivatization in the presence of internal standards followed by liquid chromatography mass spectrometry using a TSQ-Vantage (Thermo Fisher Scientific, Waltham, MA) instrument with electrospray ionization.
Statistical analysis
To exclude metabolites below the limit of detection (LOD), the raw data (µmol/L) were cleaned applying a modified 80% rule; for statistical analysis at least 80% valid values above LOD needed to be available per analyte in the samples for each group. This reduced the dataset to 143 analytes (29 amino acids or biogenic amines and their derivatives). Remaining values below LOD were imputed applying a logspline method with values between LOD and LOD/2. After log2 transformation of metabolomics data as well as of routine analytes and clinical data the dataset underwent multivariate (hierarchical cluster analysis) and univariate statistical analyses. Student’s t-testing with a Benjamini-Hochberg correction identified significant metabolite (and clinical routine-parameter) differences between patients and controls. P-values were calculated to identify significant changes between controls and Fontan patients, and were adjusted for multiple testing, or false discovery rate (FDR), according to Benjamini and Hochberg. To interpret the correlation, the significance of the correlation was calculated from r and from the degrees of freedom (a variable dependent on the sample number) (Supplemental Table 1). As a measure of linear correlation, r can have values between −1 and 1, where 1 indicates total positive linear correlation, 0 indicates no linear correlation, and −1 indicates total negative linear correlation. Correlations with FDR-adjusted p-values <0.05 and r > 0.5 (<−0.5) were considered statistically significant; in Supplemental Table 1, table cells displaying a statistically significant correlation are highlighted by a green background9.
Ethical approval and informed consent
The study protocol was approved by the local ethics committees of the Medical University of Innsbruck, Austria (AN2015-0303 357/4.3), and of the Heart and Diabetes Center North-Rhine Westphalia, Ruhr University of Bochum, Germany (AZ 52/2016), the methods were carried out in accordance with the relevant guidelines and regulations, and the subjects gave written informed consent.
Pathway analysis
Information about analytes and the pathways in which they are involved was based on https://www.metaboanalyst.ca/ and on the Kyoto Encyclopedia of Genes and Genomes (KEGG).
Supplementary information
Acknowledgements
The authors gratefully thank the laboratory team of the Erich and Hanna Klessmann Institut, Bad Oeynhausen, Germany, and the team of the “Kinderlabor” Innsbruck, Austria (Claudia Ertl, Ulrike Eichinger-Öttl, Christine Kluckner), for supporting our work by giving us the facilities to preanalyze and to store the serum samples; the team of the laboratory for exercise capacity testing in the Heart and Diabetes Center North Rhine-Westphalia, Bad Oeynhausen, Germany, for supporting us in exercise capacity testing and in blood drawing; and A.S. Knisely and E. Michel for expert comments on manuscript drafts.
Author contributions
M.M. designed the study, examined patients, prepared samples, analysed and interpreted results and wrote the manuscript; K.O.D. supervised lung function and exercise testing; K.O.D. and K.T.L. helped classify the patients; M.G.A. and U.M. performed biochemical assays and helped with statistical analysis of metabolomics-examination results; S.S.B., K.T.L., and K.O.D. helped analyze amino acids and assisted in analysis of results; M.G.A., A.E., and U.M. provided the figures; all authors proofread the manuscript and read and approved the final version of the manuscript.
Data availability
The datasets generated and analyzed during the current study are given as supplementary material (Supplemental Table 2).
Competing interests
This work is part of the project “Metabolic aspects in Fontan patients”, supported by a financial grant given to Dr. Michel from the “Tiroler Wissenschaftsförderung” (grant UNI-0404-2126). Dr. Michel has no further financial and no non-financial competing interests, or other interests that might be perceived to influence the results and/or discussion reported in this manuscript. All other authors declare no financial and no non-financial competing interests, or other interests that might be perceived to influence the results and/or discussion reported in this manuscript.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors jointly supervised this work: Kai Thorsten Laser and Sabine Scholl-Bürgi.
Supplementary information
is available for this paper at 10.1038/s41598-020-65852-x.
References
- 1.Anderson PA, et al. Contemporary outcomes after the Fontan procedure: a Pediatric Heart Network multicenter study. J Am Coll Cardiol. 2008;52(2):85–98. doi: 10.1016/j.jacc.2008.01.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rychik, J. et al. Evaluation and management of the child and adult with Fontan circulation: a scientific statement from the American Heart Association. Circulation CIR0000000000000696, 10.1161/CIR.0000000000000696 (2019). [DOI] [PubMed]
- 3.Giannakoulas G, et al. Usefulness of natriuretic peptide levels to predict mortality in adults with congenital heart disease. Am J Cardiol. 2010;105(6):869–73. doi: 10.1016/j.amjcard.2009.11.041. [DOI] [PubMed] [Google Scholar]
- 4.Larsson DA, Meurling CJ, Holmqvist F, Waktare JE, Thilén UJ. The diagnostic and prognostic value of brain natriuretic peptides in adults with a systemic morphologically right ventricle or Fontan-type circulation. Int J Cardiol. 2007;114(3):345–51. doi: 10.1016/j.ijcard.2006.01.023. [DOI] [PubMed] [Google Scholar]
- 5.Wang CH, Cheng ML, Liu MH. Amino acid-based metabolic panel provides robust prognostic value additive to B-natriuretic peptide and traditional risk factors in heart failure. Dis Markers. 2018;2018:3784589. doi: 10.1155/2018/3784589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siskos AP, et al. Interlaboratory reproducibility of a targeted metabolomics platform for analysis of human serum and plasma. Anal Chem. 2017;89(1):656–665. doi: 10.1021/acs.analchem.6b02930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ohuchi H, et al. Abnormal glucose metabolism in patients with Fontan circulation: unique characteristics and associations with Fontan pathophysiology. Am Heart J. 2019;216:125–35. doi: 10.1016/j.ahj.2019.07.013. [DOI] [PubMed] [Google Scholar]
- 8.Whiteside W, et al. Altered cholesterol metabolism and hypocholesterolemia in patients with single ventricle following Fontan palliation. J Pediatr. 2016;171:73–7. doi: 10.1016/j.jpeds.2015.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Michel M, et al. Targeted metabolomic analysis of serum phospholipid and acylcarnitine in the adult Fontan patient with a dominant left ventricle. Ther Adv Chronic Dis. 2020;11:1–25. doi: 10.1177/2040622320916031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schaffer SW, Shimada-Takaura K, Jong CJ, Ito T, Takahashi K. Impaired energy metabolism of the taurine-deficient heart. Amino Acids. 2016;48(2):549–58. doi: 10.1007/s00726-015-2110-2. [DOI] [PubMed] [Google Scholar]
- 11.Kaplan JL, et al. Taurine deficiency and dilated cardiomyopathy in golden retrievers fed commercial diets. PLoS One. 2018;13(12):e0209112. doi: 10.1371/journal.pone.0209112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ito T, Schaffer S, Azuma J. The effect of taurine on chronic heart failure: actions of taurine against catecholamine and angiotensin II. Amino Acids. 2014;46(1):111–19. doi: 10.1007/s00726-013-1507-z. [DOI] [PubMed] [Google Scholar]
- 13.Müller-Hennessen, et al. Metabolic profiles in heart failure due to non‐ischemic cardiomyopathy at rest and under exercise. ESC Heart Fail. 2017;4(2):178–89. doi: 10.1002/ehf2.12133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Neubauer S. The failing heart - an engine out of fuel. N Engl J Med. 2007;356(11):1140–51. doi: 10.1056/NEJMra063052. [DOI] [PubMed] [Google Scholar]
- 15.Murphy JM, Murch SJ, Ball RO. Proline is synthesized from glutamate during intragastric infusion but not during intravenous infusion in neonatal piglets. J Nutr. 1996;126(4):878–86. doi: 10.1093/jn/126.4.878. [DOI] [PubMed] [Google Scholar]
- 16.Wakabayashi Y, Iwashima A, Yamada E, Yamada R. Enzymological evidence for the indispensability of small intestine in the synthesis of arginine from glutamate. II. N-acetylglutamate synthase. Arch Biochem Biophys. 1991;291(1):9–14. doi: 10.1016/0003-9861(91)90098-4. [DOI] [PubMed] [Google Scholar]
- 17.Nørrelund H, et al. Abnormalities of whole body protein turnover, muscle metabolism and levels of metabolic hormones in patients with chronic heart failure. J Intern Med. 2006;260(1):11–21. doi: 10.1111/j.1365-2796.2006.01663.x. [DOI] [PubMed] [Google Scholar]
- 18.Qi L, et al. Association between a genetic variant related to glutamic acid metabolism and coronary heart disease in individuals with type 2 diabetes. JAMA. 2013;310(8):821–28. doi: 10.1001/jama.2013.276305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nogueira Ade C, Vale RG, Gomes AL, Dantas EH. The effect of muscle actions on the level of connective tissue damage. Res Sports Med. 2011;19(4):259–70. doi: 10.1080/15438627.2011.608046. [DOI] [PubMed] [Google Scholar]
- 20.Koike K, et al. Free 4-hydroxyproline content in serum of bedridden aged people is elevated due to fracture. Biol Pharm Bull. 2000;23(1):101–3. doi: 10.1248/bpb.23.101. [DOI] [PubMed] [Google Scholar]
- 21.Visser M, et al. The role of asymmetric dimethylarginine and arginine in the failing heart and its vasculature. Eur J Heart Fail. 2010;12(12):1274–81. doi: 10.1093/eurjhf/hfq158. [DOI] [PubMed] [Google Scholar]
- 22.Massion PB, Feron O, Dessy C, Balligand JL. Nitric oxide and cardiac function: ten years after, and continuing. Circ Res. 2003;93(5):388–98. doi: 10.1161/01.RES.0000088351.58510.21. [DOI] [PubMed] [Google Scholar]
- 23.Arimura K, et al. Increased inactivation of nitric oxide is involved in coronary endothelial dysfunction in heart failure. Am J Physiol Heart Circ Physiol. 2001;280(1):H68–75. doi: 10.1152/ajpheart.2001.280.1.H68. [DOI] [PubMed] [Google Scholar]
- 24.Deanfield JE, Halcox JP, Rabelink TJ. Endothelial function and dysfunction: testing and clinical relevance. Circulation. 2007;115(10):1285–95. doi: 10.1161/CIRCULATIONAHA.106.652859. [DOI] [PubMed] [Google Scholar]
- 25.Andrews KL, Triggle CR, Ellis ANO. and the vasculature: where does it come from and what does it do? Heart Fail Rev. 2002;7(4):423–45. doi: 10.1023/a:1020702215520. [DOI] [PubMed] [Google Scholar]
- 26.Cooke JP. Does ADMA cause endothelial dysfunction? Arterioscler Thromb Vasc Biol. 2000;20(9):2032–7. doi: 10.1161/01.atv.20.9.2032. [DOI] [PubMed] [Google Scholar]
- 27.Jud P, et al. Homoarginine/ADMA ratio and homoarginine/SDMA ratio as independent predictors of cardiovascular mortality and cardiovascular events in lower extremity arterial disease. Sci Rep. 2018;8(1):14197. doi: 10.1038/s41598-018-32607-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Böger RH, et al. Supplementation of hypercholesterolaemic rabbits with L-arginine reduces the vascular release of superoxide anions and restores NO production. Atherosclerosis. 1995;117(2):273–84. doi: 10.1016/0021-9150(95)05582-h. [DOI] [PubMed] [Google Scholar]
- 29.Kielstein JT, et al. Asymmetric dimethylarginine, blood pressure, and renal perfusion in elderly subjects. Circulation. 2003;107(14):1891–5. doi: 10.1161/01.CIR.0000060496.23144.A7. [DOI] [PubMed] [Google Scholar]
- 30.Miyazaki H, et al. Endogenous nitric oxide synthase inhibitor: a novel marker of atherosclerosis. Circulation. 1999;99(9):1141–6. doi: 10.1161/01.cir.99.9.1141. [DOI] [PubMed] [Google Scholar]
- 31.Böger RH, et al. Biochemical evidence for impaired nitric oxide synthesis in patients with peripheral arterial occlusive disease. Circulation. 1997;95(8):2068–74. doi: 10.1161/01.cir.95.8.2068. [DOI] [PubMed] [Google Scholar]
- 32.Surdacki A, et al. Reduced urinary excretion of nitric oxide metabolites and increased plasma levels of asymmetric dimethylarginine in men with essential hypertension. J Cardiovasc Pharmacol. 1999;33(4):652–8. doi: 10.1097/00005344-199904000-00020. [DOI] [PubMed] [Google Scholar]
- 33.Tutarel O, et al. Asymmetrical dimethylarginine–more sensitive than NT-proBNP to diagnose heart failure in adults with congenital heart disease. PLoS One. 2012;7(3):e33795. doi: 10.1371/journal.pone.0033795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martínez Y, et al. The role of methionine on metabolism, oxidative stress, and diseases. Amino Acids. 2017;49(12):2091–98. doi: 10.1007/s00726-017-2494-2. [DOI] [PubMed] [Google Scholar]
- 35.Picot CR, et al. Alterations in mitochondrial and cytosolic methionine sulfoxide reductase activity during cardiac ischemia and reperfusion. Exp Gerontol. 2006;41(7):663–67. doi: 10.1016/j.exger.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 36.Razavi AC, et al. Novel findings from a metabolomics study of left ventricular diastolic function: the Bogalusa Heart Study. J Am Heart Assoc. 2020;9(3):e015118. doi: 10.1161/JAHA.119.015118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kolho KL, Pessia A, Jaakkola T, de Vos WM, Velagapudi V. Faecal and serum metabolomics in paediatric inflammatory bowel disease. J Crohns Colitis. 2017;11(3):321–34. doi: 10.1093/ecco-jcc/jjw158. [DOI] [PubMed] [Google Scholar]
- 38.Zyblewski SC, et al. Reduction in postoperative high-density lipoprotein cholesterol levels in children undergoing the Fontan operation. Pediatr Cardiol. 2012;33(7):1154–9. doi: 10.1007/s00246-012-0274-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Whiteside W, Tan M, Yu S, Rocchini A. Low total, low-density lipoprotein, high-density lipoprotein, and non-high-density lipoprotein cholesterol levels in patients with complex congenital heart disease after Fontan palliation. J Pediatr. 2013;162(6):1199–204. doi: 10.1016/j.jpeds.2012.11.073. [DOI] [PubMed] [Google Scholar]
- 40.Whiteside W, et al. Altered cholesterol metabolism and hypocholesterolemia in patients with single ventricle following Fontan palliation. J Pediatr. 2016;171:73–7. doi: 10.1016/j.jpeds.2015.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Böger RH, et al. Asymmetric dimethylarginine (ADMA): a novel risk factor for endothelial dysfunction: its role in hypercholesterolemia. Circulation. 1998;98(18):1842–7. doi: 10.1161/01.cir.98.18.1842. [DOI] [PubMed] [Google Scholar]
- 42.von Haehling S, et al. Elevated levels of asymmetric dimethylarginine in chronic heart failure: a pathophysiologic link between oxygen radical load and impaired vasodilator capacity and the therapeutic effect of allopurinol. Clin Pharmacol Ther. 2010;88(4):506–12. doi: 10.1038/clpt.2010.116. [DOI] [PubMed] [Google Scholar]
- 43.Diller GP, et al. Exercise intolerance in adult congenital heart disease: comparative severity, correlates, and prognostic implication. Circulation. 2005;112(6):828–35. doi: 10.1161/CIRCULATIONAHA.104.529800. [DOI] [PubMed] [Google Scholar]
- 44.Oechslin E, et al. Systemic endothelial dysfunction in adults with cyanotic congenital heart disease. Circulation. 2005;112(8):1106–12. doi: 10.1161/CIRCULATIONAHA.105.534073. [DOI] [PubMed] [Google Scholar]
- 45.Celermajer DS, Cullen S, Deanfield JE. Impairment of endothelium-dependent pulmonary artery relaxation in children with congenital heart disease and abnormal pulmonary hemodynamics. Circulation. 1993;87(2):440–6. doi: 10.1161/01.cir.87.2.440. [DOI] [PubMed] [Google Scholar]
- 46.Rhodes J, et al. Effect of inhaled iloprost on the exercise function of fontan patients: A demonstration of concept. Int J Cardiol. 2013;168:2435–2440. doi: 10.1016/j.ijcard.2013.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Duncan BW, Desai S. Pulmonary arteriovenous malformations after cavopulmonary anastomosis. Ann Thorac Surg. 2003;76(5):1759–66. doi: 10.1016/s0003-4975(03)00450-8. [DOI] [PubMed] [Google Scholar]
- 48.Whitehead KK, et al. Status of systemic to pulmonary arterial collateral flow after the fontan procedure. Am J Cardiol. 2015;115(12):1739–45. doi: 10.1016/j.amjcard.2015.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Buga GM, Gold ME, Fukuto JM, Ignarro LJ. Shear stress-induced release of nitric oxide from endothelial cells grown on beads. Hypertension. 1991;17(2):187–93. doi: 10.1161/01.hyp.17.2.187. [DOI] [PubMed] [Google Scholar]
- 50.Aoki M, et al. Endothelin-1 may play an important role in the Fontan circulation. Interact Cardiovasc Thorac Surg. 2018;26(3):480–486. doi: 10.1093/icvts/ivx378. [DOI] [PubMed] [Google Scholar]
- 51.Ohuchi H, et al. Positive pediatric exercise capacity trajectory predicts better adult Fontan physiology rationale for early establishment of exercise habits. Int J Cardiol. 2018;274:80–87. doi: 10.1016/j.ijcard.2018.06.067. [DOI] [PubMed] [Google Scholar]
- 52.Dubowy KO, Baden W, Bernitzki S, Peters B. A practical and transferable new protocol for treadmill testing of children and adults. Cardiol Young. 2008;18(6):615–23. doi: 10.1017/S1047951108003181. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and analyzed during the current study are given as supplementary material (Supplemental Table 2).