Abstract
The discovery of the Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR) system has revolutionized gene editing research. Through the repurposing of programmable RNA-guided CRISPR-associated (Cas) nucleases, CRISPR-based genome editing systems allow for the precise modification of specific sites in the human genome and inspire novel approaches for the study and treatment of inherited and acquired human diseases. Here, we review how CRISPR technologies have stimulated key advances in dermatologic research. We discuss the role of CRISPR in genome editing for cutaneous disease and highlight studies on the use of CRISPR-Cas technologies for genodermatoses, cutaneous viruses and bacteria, and melanoma. Additionally, we examine key limitations of current CRISPR technologies, including the challenges these limitations pose for the widespread therapeutic application of CRISPR-based therapeutics.
Keywords: CRISPR, dermatology, gene editing, genodermatoses, viruses, cutaneous disease
Introduction
Gene editing technologies have been transformative in biological research and show immense potential for the study and treatment of inherited and acquired human diseases 1. The Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR) system uses programmable RNA-guided CRISPR-associated (Cas) nucleases to change, remove, or add genetic material to specific locations within the genome 2. In comparison with other site-specific nucleases, such as zinc finger nucleases (ZFNs), meganucleases (MNs), and transcription activator-like effector nucleases (TALENs), CRISPR-Cas nucleases are easier to design and implement through the simple manipulation of a guide RNA sequence.
Dermatologic conditions hold particular appeal as targets for CRISPR-Cas therapeutics. There are several well-described, monogenic inherited skin disorders, such as the epidermal blistering disorders, that are considered ideal candidates for genome editing therapeutics. In addition, the skin is an easily accessible organ that allows for extraction and in vitro culture of target cells as well as direct localized administration of CRISPR-Cas therapeutics through topical, grafting or injection methods 3. Lastly, for the same reason, the visibility of skin allows for simpler monitoring of the genetically edited cutaneous tissues for both efficacy and potential deleterious effects 3.
Ongoing research efforts are exploring a variety of CRISPR-Cas approaches to the development of new CRISPR-Cas therapeutics for dermatology. Though most experiments have focused on ex vivo manipulation of diseased primary cell lines, researchers are increasingly developing in vivo and ex vivo techniques with translational potential. We propose that, for a variety of reasons, dermatology is likely to continue to be at the center of the development and clinical application of CRISPR-Cas therapeutics. For example, one of the first human trials involving CRISPR-Cas9 is geared toward treating refractory melanoma, among other neoplasms 4. Therefore, in this review we will focus on the current research and potential future applications of therapeutic CRISPR-Cas nucleases in dermatology.
Mechanisms of genome engineering with CRISPR-Cas
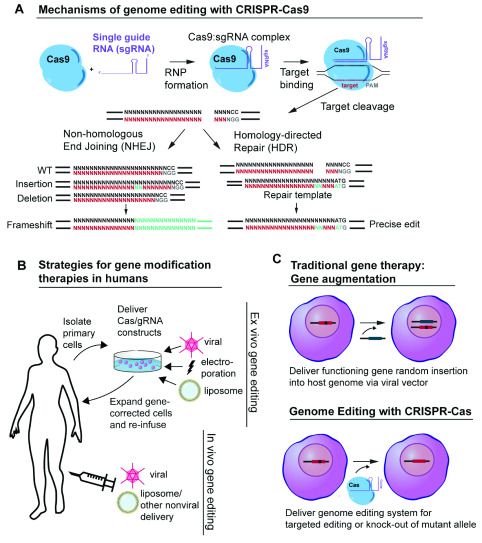
There are several types of CRISPR-Cas systems (I-III), and numerous subtypes, that have been identified in bacteria and archaea, but the type II CRISPR-Cas9 system is the best studied, particularly in terms of its application to dermatology therapeutics 5. The type II CRISPR system provides bacteria with a mechanism of immunologic memory and defense against foreign DNA 6. Using CRISPR, bacteria incorporate short sequences of exogenous DNA from invading pathogens, for example from bacteriophages or viruses that infect bacteria, into their own genome. When transcribed from the bacterial host genome, these sequences are processed into CRISPR RNAs (crRNAs) that complex with a trans-activating crRNA (tracrRNA). The crRNA/tracRNA duplex directs Cas9 to cleave target double-stranded DNA that is complementary to the 20-nucleotide guide sequence within the crRNA, creating a site-specific double strand break (DSB). In the laboratory, a single 80 to 100-nucleotide RNA transcript synthesized in the form of a single guide RNA (sgRNA) can mimic the structure and function of the crRNA/tracrRNA duplex ( Figure 1) 2. The simplicity and multiplexing capacity of CRISPR-Cas9 nuclease activity is based on the easy-to-design sgRNA or crRNA, whose RNA sequences can be modified to direct the Cas nuclease to target different sequences in the dsDNA genome.
Figure 1. CRISPR-Cas9 gene editing strategies.
( A) Mechanism of CRISPR-Cas9 genome editing. Cas9 nuclease complexes with a single guide RNA (sgRNA) to form a ribonucleoprotein (RNP). The sgRNA guides Cas9 to create a double strand break (DSB) three to four base pairs proximal to an “NGG” PAM sequence. After creating a DSB, dsDNA can be repaired by either non-homologous end joining (NHEJ) or, when a homologous dsDNA donor template is available, homology-directed repair (HDR). ( B) Strategies for gene modification therapies in humans. Ex vivo gene editing strategies involve the extraction and manipulation of patient-derived cells in vitro in cell culture. Gene-corrected cells are expanded in culture and are subsequently re-infused or grafted onto the patient. In vivo gene editing involves the direct delivery of CRISPR-Cas DNA, RNA, and/or protein via viral or nonviral means. ( C) Traditional gene therapy versus genome editing with CRISPR-Cas technology. Traditional gene therapy involves the addition of a functioning gene to replace a mutant allele. The replacement gene is usually inserted randomly into the host genome via a viral vector. In contrast, genome editing with CRISPR-Cas involves the direct, site-specific editing of the host genome.
In eukaryotic cells, following the formation of a site-specific DSB by Cas9, one of two cellular repair processes can occur: non-homologous end joining (NHEJ) or homology-directed repair (HDR) ( Figure 1) 7. NHEJ is an error-prone process that can result in mutations or nucleotide insertions and deletions (indels), interrupting the sequence of a target gene. In contrast, HDR is a high-fidelity DNA repair strategy whereby the DSB is repaired using homologous DNA as a template. HDR can be facilitated by co-administration of homologous donor DNA with the Cas nuclease. This donor sequence can be used as a synthetic template for the cell to copy when repairing the Cas-induced DSB. HDR can be used to direct the repair of a mutated gene, albeit with lower efficiency than NHEJ 8.
To date, most genome engineering strategies for dermatological disease have involved the ex vivo editing of patient-derived primary cells ( Figure 1) 9. To perform ex vivo editing, patient cells are isolated and genetically modified in vitro, potentially for subsequent autologous transplantation. This strategy allows for the clonal selection, characterization, and expansion of genetically modified cells prior to use for engraftment in the affected organ or tissue. Ex vivo approaches facilitate targeting and delivery of the CRISPR-Cas therapeutic and, by allowing for enrichment of modified cells, reduce the requirement for highly efficient and specific CRISPR-Cas editing constructs 10. However, cell expansion in culture can lead to unwanted cellular differentiation, particularly in induced pluripotent stem cells (iPSCs) 11. In addition, cell-based transplantations can be technologically challenging, especially for non-hematopoietic cells. In contrast to ex vivo gene manipulation, in vivo gene editing involves the direct modification of somatic cells in situ ( Figure 1). Using CRISPR-Cas constructs, in vivo gene editing is achieved through systemic or local administration of packaged CRISPR-Cas components (protein, DNA, and/or RNA) into the body to induce gene editing outcomes in specific organs or cells. In vivo editing requires the development of effective targeting strategies to generate cell-specific changes with minimal off-target effects and precludes comprehensive characterization of all edited cells. Safe in vivo gene editing techniques could have utility for a wide range of systemic and localized diseases, but many hurdles and concerns remain to be addressed.
Genodermatoses
Most genodermatoses are monogenic in nature and therefore serve as an attractive disease model for gene therapy 12. Because there are no widely available effective treatments for these disorders, current therapies are focused primarily on symptom management. Early success in the use of gene therapy for the treatment of the monogenic inherited epidermolysis bullosa (EB) disorders provided particular promise for the development of curative gene therapies for genodermatoses. In 2006, a patient suffering from nonlethal junctional EB (JEB) underwent successful long-term skin transplantation with epidermal sheets made from gene-corrected autologous keratinocytes 13, 14. These keratinocytes were corrected using a retroviral vector encoding the beta 3 chain of laminin-332, compensating for the mutated version of the gene in this patient. Such successes laid the groundwork for further research in not only gene replacement therapies for cutaneous disorders, but also for gene editing therapies. Unlike classically defined gene therapy, which involves the random insertion of one or more exogenous genes into cells to replace the function of a missing or mutated gene, gene editing involves the direct, site-specific manipulation of the genome using targeted nucleases such as ZFNs, TALENs, and CRISPR-Cas enzymes ( Figure 1) 15. The therapeutic potential of direct genome manipulation is immense. Researchers have leveraged CRISPR-Cas constructs to develop diverse treatment strategies for genodermatoses including the targeted addition of genes to specific genomic sites, the correction of disease-causing point mutations, and the removal of disease-causing genes or genomic sequences. Such strategies expand the scope of gene therapy beyond additive approaches, allowing for corrective gene editing and the targeted ‘knockout’ of mutant alleles in dominant negative disorders. It is also hoped that targeted gene editing strategies will reduce the risks associated with random insertion of exogenous transgenes.
EB. Of the genodermatoses, the inherited EB disorders have been the most extensively studied as potential candidates for gene editing therapy 3. The EB disorders are a diverse group of inherited blistering diseases that affect the skin and, in some subtypes, mucous membranes and other organs 16. They are caused by mutations in over 20 different genes that code for different proteins expressed at the cutaneous basement membrane zone 17.
Two EB subtypes—EB Simplex (EBS) and dominant dystrophic EB (DDEB)—are caused by dominant negative mutations that cannot be corrected with traditional additive gene therapies. Thus, these disorders are particularly well-suited for treatment with CRISPR-Cas genome editing, which allows for the direct modification of the dominant, disease-causing allele. EBS is caused by dominant negative missense mutations in either the keratin 14 ( KRT14) or keratin 5 ( KRT5) genes that code for intermediate filaments (IFs) expressed in the basal layer of the epidermis 18, 19. Kocher et al. 20 used CRISPR-Cas9-induced HDR to correct the disease-causing mutated KRT14 allele in EBS patient keratinocytes in vitro. Gene-corrected clones showed normal phenotype without characteristic mutant cytoplasmic aggregates in cell culture. DDEB is caused by dominant negative mutations in COL7A1, which codes for collagen 7 (C7) 21, 22. Shinkuma et al. 23 successfully used CRISPR-Cas9 to induce site-directed mutagenesis of the mutated COL7A1 allele in iPSCs derived from DDEB patient keratinocytes. Edited cells expressed a truncated version of C7 that was incapable of forming deleterious trimers with WT C7 and would hypothetically allow for normal anchoring fibril formation at the DEJ 23.
Unlike EBS and DDEB, JEB and RDEB are inherited in an autosomal recessive manner. Therefore, CRISPR-Cas therapeutics for these disorders must achieve gene correction to allow for production of new, functional proteins. This can be accomplished through precise correction of the disruptive mutation (i.e., via CRISPR-Cas9 induced HDR with a gene-corrected donor template) or through methods that produce a functional protein without fully correcting the disease-causing mutation. For example, in specific cases, targeted deletions can be used to either remove a premature stop codon or causative mutation directly or to disrupt splicing signals, allowing for therapeutic exon skipping. JEB is caused by autosomal recessive mutations in genes encoding subunits of the heterotrimeric laminin-5 (laminin-332) protein (e.g., LAMA3, LAMB3, and LAMC2) 24, 25, and RDEB is caused by autosomal recessive mutations in COL7A1 26, 27. For both of these conditions, CRISPR-Cas9-mediated HDR has been used to successfully correct disease-causing mutations ex vivo in patient-derived primary keratinocytes 28– 31 and iPSCs 32. When grafted onto immunodeficient mice, gene-corrected keratinocytes displayed restored WT functionality and adhesion at the DEJ 29– 31. However, the efficiency of HDR during ex vivo gene modification remains low, particularly without antibiotic section protocols or other methods of enriching for modified cells. Exon skipping approaches have been explored for the treatment of RDEB. Exon 80 contains a common disease-causing point mutation in the COL7A1 gene 33. By inducing targeted Cas9-mediated DSBs on either side of the mutation-bearing exon 80 in COL7A1, it is possible to mediate complete excision of the disease-causing mutation. Bonafant et al. delivered paired Cas9/sgRNA RNPs to patient keratinocytes ex vivo through electroporation. They were able to achieve efficient rates of deletion of exon 80, generating cells with restored C7 expression that, when grafted to immunodeficient mice, showed long-term dermal-epidermal adhesion 34.
Notably, Cas9-mediated therapies for RDEB have recently expanded to include in vivo approaches, with Wu and colleagues 35 restoring C7 function in an RDEB mouse model using the exon skipping approach. Wu et al. designed two sgRNAs targeting the 5’ and 3’ side of exon 80 and delivered them as an sgRNA/Cas9 ribonucleoprotein complex (RNP) via intradermal injection into mouse tail skin. They then directly electroporated the mouse tail to facilitate penetration of RNPs into epidermal stem cells. By causing DSBs on either side of the mutation-bearing exon 80 in COL7A1, they were able to achieve complete excision of the disease-causing mutation in epidermal stem cells. Electroporated mice displayed restored C7 function, and their dermal-epidermal adhesion area improved from 30% to 60% after one treatment 35. The success of this approach demonstrated the potential for CRISPR-Cas9-induced gene correction of epidermal stem cells in vivo without the cost and technical challenge of ex vivo cell modification. Still, however, several limitations of this method are apparent. Only 2% of epidermal cells were capable of being targeted with this novel in vivo delivery method, and no long-term follow-up to assess sustainability of the treatment could be performed. Moreover, potential off-target effects of the Cas9/sgRNA RNPs at sites other than exon 80 were not analyzed.
Epidermolytic palmoplantar keratoderma (EPPK). EPPK is an autosomal dominant keratin disease of the hereditary palmoplantar keratoderma (PPK) group characterized by an abnormal thickening of skin on the palms and soles 36. The disease is caused by dominant-negative missense mutations in the KRT9 gene, leading to the formation of a mutant keratin 9 (K9) protein that interferes with the function of the wild-type K9 37. EPPK has relatively localized disease manifestations that are well suited for targeted delivery of CRISPR therapeutics.
Luan and colleagues showed that through local delivery of a lentiviral (LV) vector carrying an sgRNA and Cas9 directed to the mutant KRT9 allele, they could disrupt the formation of the mutant K9 protein in vivo in an EPPK mouse model and induce phenotypic correction of the disease 37. Mice who received nine subcutaneous injections of the Cas9/sgRNA-containing LV particles into their right forepaw over the course of 24 days displayed restored epidermal proliferation of the forepaw and a reduction in disease-associated K9 expression. Off-target effects of the genome editing system were reportedly minimal, but analyses were limited to 10 predicted off-target sites in the mouse genome 37. In addition, long-term effects of the treatment on the mice were not recorded, and there was no analysis of potential immune response to the LV Cas9 vector—an occurrence which has been reported for other LV vector systems 38. Still, this study demonstrates the potential for robust in vivo effects of CRISPR-Cas9-mediated gene editing, particularly for diseases that have localized manifestations.
Cutaneous viruses
CRISPR-Cas systems, which evolved in bacteria to fight invading bacteriophages, have also been repurposed to serve a similar function in virally infected human cells. In human cells, CRISPR-Cas enzymes have the potential to target latent viruses that are able to escape eradication by immune surveillance and standard antiviral therapies. As such, there has been extensive research into the ability of CRISPR-Cas systems to target specific viral genomic sequences, enabling targeted disruption and even complete excision of components of the viral genome 39. In addition, researchers have leveraged the high sensitivity of certain Cas enzymes for the purposes of viral pathogen detection in human tissue samples 40. Cas12 and Cas13—two Cas enzymes that demonstrate indiscriminate trans-cleavage of ssDNA when activated by their guide-complementary target nucleotide sequence—enable ultrasensitive nucleic acid detection in viral biosensing systems such as DETECTR 41, SHERLOCK/SHERLOCKv2 42 and HOLMES/HOLMESv2 43, 44
Though most CRISPR-mediated antiviral research to date has focused on systemic viruses that lack primary cutaneous involvement, the unique accessibility of the skin suggests that CRISPR therapeutics and diagnostics might be more effectively applied to cutaneous viruses. Current research suggests CRISPR-Cas technology may be effective in detecting and modifying human papillomavirus (HPV), herpes simplex virus (HSV), and Kaposi sarcoma-associated herpesvirus (KSHV).
HPV. HPV is a dsDNA virus that infects the basal cells of stratified epithelium. Here, the virus has the potential to integrate its viral DNA into the host genome. The HPV E6 and E7 proteins of certain high-risk strains, including 16, 18, 31, 33, cause malignant transformation of epithelial cells by inactivating p53 and Rb, respectively 45, 46, and can lead to anogenital squamous cancers and other neoplasms. E7 expression from lower risk strains of HPV, particularly strains 6 and 11, is associated with the uncontrolled proliferation of epithelial cells that cause genital warts 47, 48.
Several researchers have effectively used CRISPR-Cas9 to disrupt of E6 and E7 genes in cervical cancer cells, both in vitro and in vivo in animal models 44– 46, 49– 51. One of the first antiviral CRISPR-Cas9 clinical trial in humans will involve in vivo targeting of E6/E7 in HPV-infected neoplastic cervical cells 52.
Though fewer studies have been performed with the aim of using CRISPR-Cas to cure the dermatological manifestations of HPV, researchers have begun to develop CRISPR-Cas constructs targeting the virus in HPV-associated anal cancer and genital warts. Hsu et al. 53 were able to successfully decrease tumor burden in a mouse model of HPV-16-associated anal cancer. Using an adeno-associated virus (AAV) vector, they delivered Cas9 nuclease in conjunction with two gRNAs: one gRNA specific for HPV-16 E6 and the other specific for HPV-16 E7. When delivered via three intratumoral injections over one week to mice with patient-derived xenografts of HPV-16 anal cancer cells, the CRISPR-Cas9 and dual-gRNA caused a twofold decrease in tumor volume, providing proof of concept for a novel in vivo gene editing strategy for HPV-16-associated anal cancer. For HPV-associated genital warts, CRISPR-Cas9 has been used to successfully target the E7 gene in vitro, promoting apoptosis of HPV-6 and -11-infected keratinocyte cell lines 54. Notably, however, the Cas9 and gRNAs were transfected transiently into the keratinocyte cell line and achieved only incomplete E7 deactivation. In addition, efficacy of the genome editing construct was not confirmed in vivo.
CRISPR-Cas systems hold potential for not only HPV therapeutics, but also HPV diagnostics. DETECTR is a nucleic acid biosensing system that uses the enzyme Cas12a and a ssDNA fluorescent reporting scheme to identify specific sequences in amplified dsDNA from human samples 41. Chen et al. 41 showed that by using their DETECTR platform, they could detect human papillomavirus (HPV) DNA in patient anal swab samples with attomolar sensitivity, even reliably distinguishing between different genotypes of the virus. Moreover, their assay was performed in just one hour and involved only isothermal amplification of DNA, suggesting that DETECTR could serve as a rapid, low-cost, point-of-care detection assay for HPV with similar sensitivity and specificity to conventional diagnostic PCR.
Herpesviruses. The herpesviruses are large dsDNA viruses that establish lifelong infection in human hosts 55, 56. HSV-1 and HSV-2 classically infect the oral and genital mucosal epithelium, respectively, leading to the local production of ulcers. After undergoing partial clearance by the host immune system, HSV establishes latent infection in the form of episomal DNA in sensory ganglia. KSHV, or human herpesvirus 8, infects human endothelial cells and is the causative agent of Kaposi’s sarcoma 57. Like HSV and other herpesviruses, KSHV remains latent in most infected cells. Latent herpes infections avoid immunological surveillance by limiting viral gene transcription 58 and are extremely difficult to treat.
As conventional therapies targeting viral replication are ineffective against latent virus, CRISPR-mediated targeting of viral DNA has emerged as an alternative approach for HSV, KSHV, and other herpesviruses. Roehm and colleagues 59 were able to completely abrogate HSV-1 replication in human fibroblast cells in vitro by disrupting two essential viral genes using CRISPR-Cas9. Other researchers have achieved similar success against HSV-1, inhibiting viral replication in vitro in oligodendroglioma cells and epithelial cells using Cas9/gRNA editing complexes without evident off-target effects 59, 60. For KSHV, researchers have demonstrated an ability to reduce the burden of KSHV in latently infected epithelial and endothelial cell lines by AAV-CRISPR-Cas9-mediated disruption of the KSHV latency-associated nuclear antigen (LANA) 61.
Still, challenges remain in the use of CRISPR-Cas for herpesviruses. First, despite demonstrated ability to abrogate active viral replication of HSV-1, it remains to be seen whether CRISPR-Cas can be effectively used to eradicate latent HSV-1 in neurons 62. Oh et al. were able to disrupt quiescent HSV-1 genomes, but at a much lower rate compared to HSV-1 virions in the lytic cycle 63. Because other targeted nucleases, such as MNs, have been shown to successfully target latent HSV-1 infection 64, it is suspected that current failure to eliminate latent HSV-1 using CRISPR-Cas9 may be due to epigenetic modifications of the latent HSV-1 genome that block Cas9 activity 62, 65. Moreover, further demonstration of the effectiveness of anti-HSV and anti-KSHV CRISPR-Cas systems in vivo will also be important. For HSV-1 and -2, in vivo delivery may be simpler than for other systems because latent HSV-1 and 2 have very specific tropism for the trigeminal and sacral ganglia, respectively, and possible delivery strategies could be designed to specifically home to these ganglia.
Cutaneous bacterial infections
In addition to viral infections, CRISPR-Cas technology also shows promise against resistant bacterial infections 66, 67. Bacterial infections that are resistant to common antibiotics represent a growing public health concern 68, 69. Yet, despite this risk, antibiotics continue to be overprescribed 70 while the development of novel antimicrobials for new pathogens lags behind 71. Recently, CRISPR-Cas antimicrobials have emerged as a novel treatment strategy against bacterial infections 66, 67. Specifically, CRISPR-Cas9 has been leveraged to selectively remove antimicrobial resistance genes from populations of bacteria, re-sensitizing populations of bacteria to common antimicrobials 72, 73. Though systemic delivery of CRISPR-based antimicrobials remains a challenge, the accessibility of the skin enables the delivery of CRISPR constructs via convenient topical formulations 66 and places cutaneous bacterial infections at the forefront of CRISPR antimicrobial research.
Staphylococcus aureus. Staphylococcus aureus, a common cutaneous bacterial pathogen known for its antimicrobial resistance, is responsible for 76% of all skin and soft tissue infections 74 and is associated with high morbidity and mortality 75. Antimicrobial resistance to S. aureus continues to emerge as the pathogen gains plasmids and other mobile genetic elements that confer antibiotic resistance and virulence genes 76. Moreover, outbreaks remain common as the pathogen maintains a high prevalence in the population, asymptomatically colonizing the nostrils of 20–30% of healthy adults 77.
Bikard and colleagues 72 developed a novel approach to target virulent strains of S. Aureus using CRISPR-Cas9. They developed gRNAs targeted to specific S. Aureus antimicrobial resistance genes, including the methicillin resistance gene, mecA. When delivered with Cas9 via a phage capsid to mixed populations of bacteria in vitro, these gRNA/Cas9 constructs were able to eradicate resistant S. Aureus strains and completely remove specific plasmids carrying antimicrobial resistance genes. Moreover, when delivered topically to a mouse model of S. Aureus skin colonization in vivo, these gRNA/Cas9 constructs were able to significantly decrease colonization by resistant bacteria. These results demonstrated promise for the topical application of CRISPR antimicrobials in vivo and laid the foundation for future multiplexed CRISPR antimicrobials designed to simultaneously target either several bacterial species or multiple gene sequences within the same bacterium.
Melanoma
Some of the first CRISPR-Cas clinical trials in humans have involved the use of CRISPR-Cas technology in immunotherapy for cancers, including melanoma and non-small-cell lung cancer (NSCLC) 4. Much of this research has centered on the use of gene editing to inactivate key immune checkpoint inhibitors such as programmed cell death protein-1 (PD-1) and cytotoxic T-lymphocyte-associated protein-4 (CTLA-4)—two proteins that normally inhibit the anti-tumor cytotoxic effect of endogenous and exogenous T cells 78.
Melanoma is well-known to have exceptional immunogenic potential, owing largely to the high mutational burden that drives the formation of immune-stimulating neoantigens 79. Thus, under optimal conditions, melanoma cells are particularly susceptible to destruction by the human immune system 80. Yet, clinically, the tumor microenvironment in melanoma is highly immunosuppressive 81, and advanced disease has exceptionally poor treatment response 82. Consequently, melanoma serves as an ideal target for immunotherapies that are designed to relieve tumor immunosuppression.
The first human trial designed to test the use of CRISPR-Cas for melanoma builds on the demonstrated success of previous immunotherapies, including PD-1 inhibitors 83 and T cells transduced with the NY-ESO-1 T-cell receptor (TCR) 84. Specifically, researchers aim to amplify the therapeutic effects of these existing approaches by using CRISPR-Cas9 to knock out PD-1 gene loci in autologous NY-ESO-1 TCR-transduced T cells. Autologous T cells are first taken from a patient and transduced with a LV vector that expresses the NY-ESO-1 TCR, priming them to recognize a highly immunogenic NY-ESO-1 antigen expressed on melanoma cells 85. They are then electroporated with RNA-guided CRISPR-Cas9 nucleases designed to disrupt the expression of both PD-1 and the endogenous TCR subunits, TCRα and TCRβ 4. Disrupting PD-1 prevents immune suppressive signaling, and blocking the endogenous TCR subunits inhibits aberrant immune responses that may result from TCR-mediated targeting of unknown antigens. With re-introduction of these melanoma-targeted, immune-avid T cells, researchers hope to obtain a more robust tumor-specific immune response—a response that has been difficult to achieve with previous T-cell therapies for solid tumors 86. Moreover, beyond having an enhanced anti-tumor effect, such an approach is also expected to minimize unwanted treatment side effects. Using this treatment model, PD-1 blockade will be limited to the specific TCR-transduced T-cells that are designed to home to and attack NY-ESO-1-expressing melanoma cells. This stands in contrast to traditional anti-PD1 receptor antibodies which, when administered systemically, have the potential to affect T-cells more broadly and cause widespread immunogenic side effects 87.
Perspectives and future directions
Taken together, a wide variety of studies underscore the potential for CRISPR-based therapeutics for genodermatoses, cutaneous infections, and melanoma. Future research will likely continue to expand on this success, with the aim of increasing the translatability of CRISPR therapeutics as well as developing expanded strategies to target other dermatologic diseases.
There are many other monogenic skin disorders and cutaneous infections that could be targeted with CRISPR-Cas therapeutics. Pachyonychia congenita and xeroderma pigmentosum have been targeted with RNAi-based therapies 88 and designer nucleases 89, respectively, and could likely also be targeted with CRIPSR-based genome engineering. Moreover, with the advent of hypoimmunogenic universal donor iPSCs—iPSCs that are genetically modified by CRISPR-Cas9 to avoid inciting a host immune response 90, 91—existing ex vivo gene modification strategies for genodermatoses could be applied to patients more broadly.
There are many cutaneous viruses that hold promise for targeting with gene editing technologies, including the oncoviruses, Merkel-cell polyomavirus (MCPyV), and human T-cell leukemia virus type 1 (HTLV-1). MCPyV causes up to 80% of Merkel cell carcinomas (MCC) and is randomly integrated into different sites in the MCC tumor genome 92. Excision of MCV from MCC tumor cells could represent a potential therapeutic strategy for aggressive MCCs that are often refractory to many current therapies 92– 94. Preliminary studies in Merkel cell cancer cell lines demonstrate that CRISPR-Cas9-mediated disruption of the MCPyV tumor antigens leads to diminished cell growth 95. HTLV-1, the retrovirus that causes HTLV-1-associated/tropical spastic myelopathy and adult T cell leukemia/lymphoma, has yet to be explored as a target for CRISPR-Cas therapeutics. Studies focusing on HIV 96– 99—a similarly structured retrovirus—would suggest that HTLV-1 could likely be completely excised from infected cells using CRISPR-Cas9. The relative genetic conservation of HTLV-1 relative to HIV-1 100 would make it an even more appropriate candidate for targeting by RNA-guided endonucleases.
Lastly, CRISPR-Cas technology will likely develop clinical utility in the diagnosis of dermatological disease. CRISPR-Cas diagnostic platforms using Cas9 101, Cas12 41, 43, 102, Cas13 42, and Cas14 103 have the potential to revolutionize the detection of nucleic acid sequences, allowing for the ultrasensitive, low-cost, and portable detection of cutaneous viruses and single point mutations in cutaneous tumors 103, 104.
Challenges to therapeutic application
There remain several challenges to the widespread application of CRISPR-based therapeutics. The studies reported here largely demonstrate the ability of CRISPR-Cas systems to treat human disease both in cell culture models and through ex vivo modification of primary patient cell lines. While such methods currently represent the safest approach to gene editing in humans, such a technique is technologically challenging and of limited use for routine clinical practice. In vivo treatment options would be ideal, but fewer studies have explored these possibilities. Studies on the in vivo treatment of non-dermatologic disorders, including Duchenne muscular dystrophy 105– 107, hereditary liver disease 108– 110, congenital eye disease 111, and Huntington Disease 112 have shown tremendous promise in animal models. However, of the studies in dermatology focused on in vivo delivery of CRISPR-therapeutics, all have been limited to localized effects in mouse models, and none have demonstrated high efficiency or success in long-term follow up 35, 37, 53.
For in vivo genome editing via CRISPR-Cas technology to be clinically translatable not only in dermatology but also in other fields, there are several major challenges that are yet to be effectively addressed. CRISPR-Cas guide RNAs and nucleases must 1) be optimized for specific on-target effects with minimal off-target effects, 2) be delivered efficiently to specific human cells, and 3) have minimal antigenic properties so that they are accepted by human immune systems 113. Novel CRISPR-Cas enzymes and delivery systems are being developed to tackle these challenges. To improve specificity of CRISPR-Cas9, researchers have modified Cas9 construction 114, optimized sgRNA design 115, and developed a CRISPR-Cas9 double nickase approach 116 that introduces only single-strand nicks at target sites. In addition, researchers have developed sensitive methods to scan the entire genome for unintended off-target genome editing effects, including GUIDE-Seq 117, Digenome-seq 118, SITE-seq 119, CIRCLE-Seq 120, and, most recently, VIVO 121. Such methods would allow for screening of off-target effects of a given Cas/gRNA construct prior to therapeutic use.
Novel delivery methods are also being explored that expand upon traditional AAV- and LV-associated delivery systems. Gene delivery methods with viral vectors have the potential to cause integrational mutagenesis and lifelong Cas9 expression in cells. For this reason, delivery of Cas9 nuclease by way of a transiently expressed Cas9/sgRNA RNP may be preferred. Scientists have developed lipid nanoparticles that can carry CRISPR-Cas nucleotide sequences or RNP complexes that are targeted to specific organs 122– 124. Still, it will be challenging to achieve systemic delivery of such therapies, particularly for bloodborne illnesses that would require the delivery of CRISPR-Cas constructs to every circulating B or T cell 39.
Lastly, to limit the possible immunologic response to Cas9 by preformed antibodies in human serum 125, researchers have discovered novel Cas enzymes such as the structurally distinct Cas12e and Cas12d from ground bacteria 126. CRISPR therapeutics employing nucleases from bacteria to which humans are not exposed may not be subject to pre-existing immunity, allowing for a more robust genome editing effect.
Conclusion
Though significant work remains to be done prior to its widespread therapeutic use in humans, preliminary research suggests great potential for CRISPR-Cas technology in the treatment of dermatological pathologies. As researchers continue to optimize delivery methods and off-target screening approaches, more human trials for the treatment of dermatologic diseases with CRISPR-based gene editing therapeutics will likely be initiated.
Data availability
No data are associated with this study.
Funding Statement
This work was supported by the American Skin Association (C.B.) and the Hitchcock Foundation (M.S.H.).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 1; peer review: 2 approved]
References
- 1. Finn JD, Smith AR, Patel MC, et al. : A Single Administration of CRISPR/Cas9 Lipid Nanoparticles Achieves Robust and Persistent In Vivo Genome Editing. Cell Rep. 2018;22(9):2227–2235. 10.1016/j.celrep.2018.02.014 [DOI] [PubMed] [Google Scholar]
- 2. Jinek M, Chylinski K, Fonfara I, et al. : A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science. 2012;337(6096):816–821. 10.1126/science.1225829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lwin SM, McGrath JA: Gene Therapy for Inherited Skin Disorders.ELS John Wiley & Sons.2017; 10.1002/9780470015902.a0026940 [DOI] [Google Scholar]
- 4. Stadtmauer E: Phase 1 Trial of Autologous T Cells Engineered to Express NY-ESO-1 TCR and CRISPR Gene Edited to Eliminate Endogenous TCR and PD-1 (NYCE T Cells).Clinicaltrials.Gov. NCT03399448.2018. Reference Source [Google Scholar]
- 5. Jiang F, Doudna JA: CRISPR-Cas9 Structures and Mechanisms. Annu Rev Biophys. 2017;46:505–529. 10.1146/annurev-biophys-062215-010822 [DOI] [PubMed] [Google Scholar]
- 6. Damian M, Porteus MH: A Crisper Look at Genome Editing: RNA-guided Genome Modification. Mol Ther. 2013;21(4):720–722. 10.1038/mt.2013.46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Symington LS, Gautier J: Double-Strand Break End Resection and Repair Pathway Choice. Annu Rev Genet. 2011;45:247–271. 10.1146/annurev-genet-110410-132435 [DOI] [PubMed] [Google Scholar]
- 8. Maruyama T, Dougan SK, Truttmann MC, et al. : Increasing the efficiency of precise genome editing with CRISPR-Cas9 by inhibition of nonhomologous end joining. Nat Biotechnol. 2015;33(5):538–542. 10.1038/nbt.3190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gorell E, Nguyen N, Lane A, et al. : Gene therapy for skin diseases. Cold Spring Harb Perspect Med. 2014;4(4):a015149. 10.1101/cshperspect.a015149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dai WJ, Zhu LY, Yan ZY, et al. : CRISPR-Cas9 for in vivo Gene Therapy: Promise and Hurdles. Mol Ther - Nucleic Acids. 2016;5:e349. 10.1038/mtna.2016.58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Savić N, Schwank G: Advances in therapeutic CRISPR/Cas9 genome editing. Transl Res. 2016;168:15–21. 10.1016/j.trsl.2015.09.008 [DOI] [PubMed] [Google Scholar]
- 12. Lehmann J, Seebode C, Emmert S: Research on genodermatoses using novel genome-editing tools. J Dtsch Dermatol Ges. 2017;15(8):783–789. 10.1111/ddg.13270 [DOI] [PubMed] [Google Scholar]
- 13. Mavilio F, Pellegrini G, Ferrari S, et al. : Correction of junctional epidermolysis bullosa by transplantation of genetically modified epidermal stem cells. Nat Med. 2006;12(12):1397–402. 10.1038/nm1504 [DOI] [PubMed] [Google Scholar]
- 14. De Rosa L, Carulli S, Cocchiarella F, et al. : Long-term stability and safety of transgenic cultured epidermal stem cells in gene therapy of junctional epidermolysis bullosa. Stem Cell Rep. 2014;2(1):1–8. 10.1016/j.stemcr.2013.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Maeder ML, Gersbach CA: Genome-editing Technologies for Gene and Cell Therapy. Mol Ther. 2016;24(3):430–446. 10.1038/mt.2016.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fine JD, Bruckner-Tuderman L, Eady RAJ, et al. : Inherited epidermolysis bullosa: updated recommendations on diagnosis and classification. J Am Acad Dermatol. 2014;70(6):1103–1126. 10.1016/j.jaad.2014.01.903 [DOI] [PubMed] [Google Scholar]
- 17. Uitto J, Bruckner-Tuderman L, McGrath JA, et al. : EB2017-Progress in Epidermolysis Bullosa Research toward Treatment and Cure. J Invest Dermatol. 2018;138(5):1010–1016. 10.1016/j.jid.2017.12.016 [DOI] [PubMed] [Google Scholar]
- 18. Bonifas JM, Rothman AL, Epstein EH: Epidermolysis bullosa simplex: evidence in two families for keratin gene abnormalities. Science. 1994;254(5035):1202–1205. 10.1126/science.1720261 [DOI] [PubMed] [Google Scholar]
- 19. Coulombe PA, Hutton ME, Letal A, et al. : Point mutations in human keratin 14 genes of epidermolysis bullosa simplex patients: genetic and functional analyses. Cell. 1991;66(6):1301–1311. 10.1016/0092-8674(91)90051-y [DOI] [PubMed] [Google Scholar]
- 20. Kocher T, Peking P, Klausegger A, et al. : Cut and Paste: Efficient Homology-Directed Repair of a Dominant Negative KRT14 Mutation via CRISPR/Cas9 Nickases. Mol Ther. 2017;25(11):2585–2598. 10.1016/j.ymthe.2017.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Christiano AM, Greenspan DS, Hoffman GG, et al. : A missense mutation in type VII collagen in two affected siblings with recessive dystrophic epidermolysis bullosa. Nat Genet. 1993;4(1):62–66. 10.1038/ng0593-62 [DOI] [PubMed] [Google Scholar]
- 22. Hilal L, Rochat A, Duquesnoy P, et al. : A homozygous insertion-deletion in the type VII collagen gene (COL7A1) in Hallopeau-Siemens dystrophic epidermolysis bullosa. Nat Genet. 1993;5(3):287–93. 10.1038/ng1193-287 [DOI] [PubMed] [Google Scholar]
- 23. Shinkuma S, Guo Z, Christiano AM: Site-specific genome editing for correction of induced pluripotent stem cells derived from dominant dystrophic epidermolysis bullosa. Proc Natl Acad Sci U S A. 2016;113(20):5676–81. 10.1073/pnas.1512028113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Uitto J, Pulkkinen L, Christiano AM: Molecular basis of the dystrophic and junctional forms of epidermolysis bullosa: mutations in the type VII collagen and kalinin (laminin 5) genes. J Invest Dermatol. 1994;103(5 Suppl):S39–S46. 10.1111/1523-1747.ep12398967 [DOI] [PubMed] [Google Scholar]
- 25. Nakano A, Chao SC, Pulkkinen L, et al. : Laminin 5 mutations in junctional epidermolysis bullosa: molecular basis of Herlitz vs. non-Herlitz phenotypes. Hum Genet. 2002;110(1):41–51. 10.1007/s00439-001-0630-1 [DOI] [PubMed] [Google Scholar]
- 26. Bruckner-Tuderman L, Mitsuhashi Y, Schnyder UW, et al. : Anchoring Fibrils and Type VII collagen are Absent From Skin in Severe Recessive Dystrophic Epidermolysis Bullosa. J Invest Dermatol. 1989;93(1):3–9. 10.1111/1523-1747.ep12277331 [DOI] [PubMed] [Google Scholar]
- 27. Soro L, Bartus C, Purcell S: Recessive dystrophic epidermolysis bullosa: a review of disease pathogenesis and update on future therapies. J Clin Aesthet Dermatol. 2015;8(5):41–46. [PMC free article] [PubMed] [Google Scholar]
- 28. Webber BR, Osborn MJ, McElroy AN, et al. : CRISPR/Cas9-based genetic correction for recessive dystrophic epidermolysis bullosa. NPJ Regen Med. 2016;1:16014. 10.1038/npjregenmed.2016.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Benati D, Miselli F, Cocchiarella F, et al. : CRISPR/Cas9-Mediated In Situ Correction of LAMB3 Gene in Keratinocytes Derived from a Junctional Epidermolysis Bullosa Patient. Mol Ther. 2018;26(11):2592–2603. 10.1016/j.ymthe.2018.07.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hainzl S, Peking P, Kocher T, et al. : COL7A1 Editing via CRISPR/Cas9 in Recessive Dystrophic Epidermolysis Bullosa. Mol Ther. 2017;25(11):2573–2584. 10.1016/j.ymthe.2017.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Izmiryan A, Ganier C, Bovolenta M, et al. : Ex Vivo COL7A1 Correction for Recessive Dystrophic Epidermolysis Bullosa Using CRISPR/Cas9 and Homology-Directed Repair. Mol Ther Nucleic Acids. 2018;12:554–567. 10.1016/j.omtn.2018.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jacków J, Guo Z, Hansen C, et al. : CRISPR/Cas9-based targeted genome editing for correction of recessive dystrophic epidermolysis bullosa using iPS cells. Proc Natl Acad Sci U S A. 2019;116: pii: 201907081. 10.1073/pnas.1907081116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Escámez MJ, García M, Cuadrado-Corrales N, et al. : The first COL7A1 mutation survey in a large Spanish dystrophic epidermolysis bullosa cohort: c.6527insC disclosed as an unusually recurrent mutation. Br J Dermatol. 2010;163(1):155–161. 10.1111/j.1365-2133.2010.09713.x [DOI] [PubMed] [Google Scholar]
- 34. Bonafont J, Mencía Á, García M, et al. : Clinically Relevant Correction of Recessive Dystrophic Epidermolysis Bullosa by Dual sgRNA CRISPR/Cas9-Mediated Gene Editing. Mol Ther. 2019;27(5):986–998. 10.1016/j.ymthe.2019.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wu W, Lu Z, Li F, et al. : Efficient in vivo gene editing using ribonucleoproteins in skin stem cells of recessive dystrophic epidermolysis bullosa mouse model. Proc Natl Acad Sci U S A. 2017;114(7):1660–1665. 10.1073/pnas.1614775114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Has C, Technau-Hafsi K: Palmoplantar keratodermas: clinical and genetic aspects. J Dtsch Dermatol Ges. 2016;14(2):123–140. quiz 140. 10.1111/ddg.12930 [DOI] [PubMed] [Google Scholar]
- 37. Luan XR, Chen XL, Tang YX, et al. : CRISPR/Cas9-Mediated Treatment Ameliorates the Phenotype of the Epidermolytic Palmoplantar Keratoderma-like Mouse. Mol Ther Nucleic Acids. 2018;12:220–228. 10.1016/j.omtn.2018.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nayak S, Herzog RW: Progress and prospects: immune responses to viral vectors. Gene Ther. 2010;17(3):295–304. 10.1038/gt.2009.148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. de Buhr H, Lebbink RJ: Harnessing CRISPR to combat human viral infections. Curr Opin Immunol. 2018;54:123–129. 10.1016/j.coi.2018.06.002 [DOI] [PubMed] [Google Scholar]
- 40. Li Y, Li S, Wang J, et al. : CRISPR/Cas Systems towards Next-Generation Biosensing. Trends Biotechnol. 2019;37(7):730–743. 10.1016/j.tibtech.2018.12.005 [DOI] [PubMed] [Google Scholar]
- 41. Chen JS, Ma E, Harrington LB, et al. : CRISPR-Cas12a target binding unleashes indiscriminate single-stranded DNase activity. Science. 2018;360(6387):436–439. 10.1126/science.aar6245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gootenberg JS, Abudayyeh OO, Kellner MJ, et al. : Multiplexed and portable nucleic acid detection platform with Cas13, Cas12a, and Csm6. Science. 2018;360(6387):439–444. 10.1126/science.aaq0179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Li SY, Cheng QX, Wang JM, et al. : CRISPR-Cas12a-assisted nucleic acid detection. Cell Discov. 2018;4:20. 10.1038/s41421-018-0028-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Teng F, Guo L, Cui T, et al. : CDetection: CRISPR-Cas12b-based DNA detection with sub-attomolar sensitivity and single-base specificity. Genome Biol. 2019;20(1):132. 10.1186/s13059-019-1742-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Münger K, Baldwin A, Edwards KM, et al. : Mechanisms of human papillomavirus-induced oncogenesis. J Virol. 2004;78(21):11451–60. 10.1128/JVI.78.21.11451-11460.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hoppe-Seyler K, Bossler F, Braun JA, et al. : The HPV E6/E7 Oncogenes: Key Factors for Viral Carcinogenesis and Therapeutic Targets. Trends Microbiol. 2018;26(2):158–168. 10.1016/j.tim.2017.07.007 [DOI] [PubMed] [Google Scholar]
- 47. Jacobs MV, de Roda Husman AM, van den Brule AJ, et al. : Group-specific differentiation between high- and low-risk human papillomavirus genotypes by general primer-mediated PCR and two cocktails of oligonucleotide probes. J Clin Microbiol. 1995;33(4):901–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Chelimo C, Wouldes TA, Cameron LD, et al. : Risk factors for and prevention of human papillomaviruses (HPV), genital warts and cervical cancer. J Infect. 2013;66(3):207–217. 10.1016/j.jinf.2012.10.024 [DOI] [PubMed] [Google Scholar]
- 49. Zhen S, Hua L, Takahashi Y, et al. : In vitro and in vivo growth suppression of human papillomavirus 16-positive cervical cancer cells by CRISPR/Cas9. Biochem Biophys Res Commun. 2014;450(4):1422–1426. 10.1016/j.bbrc.2014.07.014 [DOI] [PubMed] [Google Scholar]
- 50. Kennedy EM, Kornepati AV, Goldstein M, et al. : Inactivation of the human papillomavirus E6 or E7 gene in cervical carcinoma cells by using a bacterial CRISPR/Cas RNA-guided endonuclease. J Virol. 2014;88(20):11965–11972. 10.1128/JVI.01879-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zhen S, Lu JJ, Wang LJ, et al. : In Vitro and In Vivo Synergistic Therapeutic Effect of Cisplatin with Human Papillomavirus16 E6/E7 CRISPR/Cas9 on Cervical Cancer Cell Line. Transl Oncol. 2016;9(6):498–504. 10.1016/j.tranon.2016.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lau CH, Suh Y: In vivo genome editing in animals using AAV-CRISPR system: applications to translational research of human disease [version 1; peer review: 2 approved]. F1000Res. 2017;6:2153. 10.12688/f1000research.11243.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hsu DS, Kornepati AV, Glover W, et al. : Targeting HPV16 DNA using CRISPR/Cas inhibits anal cancer growth in vivo. Future Virol. 2018;13(7):475–482. 10.2217/fvl-2018-0010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Liu YC, Cai ZM, Zhang XJ: Reprogrammed CRISPR-Cas9 targeting the conserved regions of HPV6/11 E7 genes inhibits proliferation and induces apoptosis in E7-transformed keratinocytes. Asian J Androl. 2016;18(3):475–479. 10.4103/1008-682X.157399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Whitley RJ, Roizman B: Herpes simplex virus infections. Lancet. 2001;357(9267):1513–1518. 10.1016/S0140-6736(00)04638-9 [DOI] [PubMed] [Google Scholar]
- 56. Azwa A, Barton SE: Aspects of herpes simplex virus: a clinical review. J Fam Plann Reprod Health Care. 2009;35(4):237–242. 10.1783/147118909789587376 [DOI] [PubMed] [Google Scholar]
- 57. Mesri EA, Cesarman E, Boshoff C: Kaposi's sarcoma and its associated herpesvirus. Nat Rev Cancer. 2010;10(10):707–19. 10.1038/nrc2888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Grinde B: Herpesviruses: latency and reactivation - viral strategies and host response. J Oral Microbiol. 2013;5:22766. 10.3402/jom.v5i0.22766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Roehm PC, Shekarabi M, Wollebo HS, et al. : Inhibition of HSV-1 Replication by Gene Editing Strategy. Sci Rep. 2016;6:23146. 10.1038/srep23146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lin C, Li H, Hao M, et al. : Increasing the Efficiency of CRISPR/Cas9-mediated Precise Genome Editing of HSV-1 Virus in Human Cells. Sci Rep. 2016;6:34531. 10.1038/srep34531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Tso FY, West JT, Wood C: Reduction of Kaposi’s Sarcoma-Associated Herpesvirus Latency Using CRISPR-Cas9 To Edit the Latency-Associated Nuclear Antigen Gene. J Virol. 2019;93(7): pii: e02183-18. 10.1128/JVI.02183-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. van Diemen FR, Lebbink RJ: CRISPR/Cas9, a powerful tool to target human herpesviruses. Cell Microbiol. 2017;19(2):e12694. 10.1111/cmi.12694 [DOI] [PubMed] [Google Scholar]
- 63. Oh HS, Neuhausser WM, Eggan P, et al. : Herpesviral lytic gene functions render the viral genome susceptible to novel editing by CRISPR/Cas9. eLife. 2019;8: pii: e51662. 10.7554/eLife.51662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Aubert M, Madden EA, Loprieno M, et al. : In vivo disruption of latent HSV by designer endonuclease therapy. JCI Insight. 2016;1(14): pii: e88468. 10.1172/jci.insight.88468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Oh HS, Neuhausser WM, Eggan P, et al. : Herpesviral lytic gene functions render the viral genome susceptible to novel editing by CRISPR/Cas9. eLife. 2019;8: pii: e51662. 10.7554/eLife.51662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Greene AC: CRISPR-Based Antibacterials: Transforming Bacterial Defense into Offense. Trends Biotechnol. 2018;36(2):127–130. 10.1016/j.tibtech.2017.10.021 [DOI] [PubMed] [Google Scholar]
- 67. Pursey E, Sünderhauf D, Gaze WH, et al. : CRISPR-Cas antimicrobials: Challenges and future prospects. PLoS Pathog. 2018;14(6):e1006990. 10.1371/journal.ppat.1006990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Roca I, Akova M, Baquero F, et al. : The global threat of antimicrobial resistance: science for intervention. New Microbes New Infect. 2015;6:22–29. 10.1016/j.nmni.2015.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Aslam B, Wang W, Arshad MI, et al. : Antibiotic resistance: a rundown of a global crisis. Infect Drug Resist. 2018;11:1645–1658. 10.2147/IDR.S173867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Llor C, Bjerrum L: Antimicrobial resistance: risk associated with antibiotic overuse and initiatives to reduce the problem. Ther Adv Drug Saf. 2014;5(6):229–241. 10.1177/2042098614554919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Fischbach MA, Walsh CT: Antibiotics for emerging pathogens. Science. 2009;325(5944):1089–93. 10.1126/science.1176667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Bikard D, Euler CW, Jiang W, et al. : Exploiting CRISPR-Cas nucleases to produce sequence-specific antimicrobials. Nat Biotechnol. 2014;32(11):1146–1150. 10.1038/nbt.3043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Citorik RJ, Mimee M, Lu TK: Sequence-specific antimicrobials using efficiently delivered RNA-guided nucleases. Nat Biotechnol. 2014;32(11):1141–1145. 10.1038/nbt.3011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Moran GJ, Krishnadasan A, Gorwitz RJ, et al. : Methicillin-resistant S. aureus infections among patients in the emergency department. N Engl J Med. 2006;355(7):666–674. 10.1056/NEJMoa055356 [DOI] [PubMed] [Google Scholar]
- 75. Parlet CP, Brown MM, Horswill AR: Commensal Staphylococci Influence Staphylococcus aureus Skin Colonization and Disease. Trends Microbiol. 2019;27(6):497–507. 10.1016/j.tim.2019.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Weigel LM, Donlan RM, Shin DH, et al. : High-level vancomycin-resistant Staphylococcus aureus isolates associated with a polymicrobial biofilm. Antimicrob Agents Chemother. 2007;51(1):231–8. 10.1128/AAC.00576-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Graham PL, 3rd, Lin SX, Larson EL: A U.S. population-based survey of Staphylococcus aureus colonization. Ann Intern Med. 2006;144(5):318–325. 10.7326/0003-4819-144-5-200603070-00006 [DOI] [PubMed] [Google Scholar]
- 78. Buchbinder EI, Desai A: CTLA-4 and PD-1 Pathways: Similarities, Differences, and Implications of Their Inhibition. Am J Clin Oncol. 2016;39(1):98–106. 10.1097/COC.0000000000000239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Ko JS: The Immunology of Melanoma. Clin Lab Med. 2017;37(3):449–471. 10.1016/j.cll.2017.06.001 [DOI] [PubMed] [Google Scholar]
- 80. Maio M: Melanoma as a model tumour for immuno-oncology. Ann Oncol. 2012;23 Suppl 8:viii10–viii14. 10.1093/annonc/mds257 [DOI] [PubMed] [Google Scholar]
- 81. Zou W: Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer. 2005;5(4):263–274. 10.1038/nrc1586 [DOI] [PubMed] [Google Scholar]
- 82. Maverakis E, Cornelius LA, Bowen GM, et al. : Metastatic melanoma - a review of current and future treatment options. Acta Derm Venereol. 2015;95(5):516–524. 10.2340/00015555-2035 [DOI] [PubMed] [Google Scholar]
- 83. Topalian SL, Hodi FS, Brahmer JR, et al. : Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–2454. 10.1056/NEJMoa1200690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Robbins PF, Morgan RA, Feldman SA, et al. : Tumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY-ESO-1. J Clin Oncol. 2011;29(7):917–924. 10.1200/JCO.2010.32.2537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Baylis F, McLeod M: First-in-human Phase 1 CRISPR Gene Editing Cancer Trials: Are We Ready? Curr Gene Ther. 2017;17(4): 309–319. 10.2174/1566523217666171121165935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Simon B, Uslu U: CAR-T cell therapy in melanoma: A future success story? Exp Dermatol. 2018;27(12):1315–1321. 10.1111/exd.13792 [DOI] [PubMed] [Google Scholar]
- 87. Postow MA, Sidlow R, Hellmann MD: Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. N Engl J Med. 2018;378(2):158–168. 10.1056/NEJMra1703481 [DOI] [PubMed] [Google Scholar]
- 88. Bornert O, Peking P, Bremer J, et al. : RNA-based therapies for genodermatoses. Exp Dermatol. 2017;26(1):3–10. 10.1111/exd.13141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Dupuy A, Valton J, Leduc S, et al. : Targeted gene therapy of xeroderma pigmentosum cells using meganuclease and TALEN TM. PLoS One. 2013;8(11):e78678. 10.1371/journal.pone.0078678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Deuse T, Hu X, Gravina A, et al. : Hypoimmunogenic derivatives of induced pluripotent stem cells evade immune rejection in fully immunocompetent allogeneic recipients. Nat Biotechnol. 2019;37(3):252–258. 10.1038/s41587-019-0016-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Han X, Wang M, Duan S, et al. : Generation of hypoimmunogenic human pluripotent stem cells. Proc Natl Acad Sci U S A. 2019;116(21):10441–10446. 10.1073/pnas.1902566116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Feng H, Shuda M, Chang Y, et al. : Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science. 2008;319(5866):1096–1100. 10.1126/science.1152586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Martel-Jantin C, Filippone C, Cassar O, et al. : Genetic variability and integration of Merkel cell polyomavirus in Merkel cell carcinoma. Virology. 2012;426(2):134–142. 10.1016/j.virol.2012.01.018 [DOI] [PubMed] [Google Scholar]
- 94. Sastre-Garau X, Peter M, Avril MF, et al. : Merkel cell carcinoma of the skin: pathological and molecular evidence for a causative role of MCV in oncogenesis. J Pathol. 2009;218(1):48–56. 10.1002/path.2532 [DOI] [PubMed] [Google Scholar]
- 95. Temblador A, Topalis D, Andrei G, et al. : CRISPR/Cas9 Editing of the Polyomavirus Tumor Antigens Inhibits Merkel Cell Carcinoma Growth In Vitro. Cancers. 2019;11(9): pii: E1260. 10.3390/cancers11091260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Ebina H, Misawa N, Kanemura Y, et al. : Harnessing the CRISPR/Cas9 system to disrupt latent HIV-1 provirus. Sci Rep. 2013;3:2510. 10.1038/srep02510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Hu W, Kaminski R, Yang F, et al. : RNA-directed gene editing specifically eradicates latent and prevents new HIV-1 infection. Proc Natl Acad Sci U S A. 2014;111(31):11461–11466. 10.1073/pnas.1405186111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Liao HK, Gu Y, Diaz A, et al. : Use of the CRISPR/Cas9 system as an intracellular defense against HIV-1 infection in human cells. Nat Commun. 2015;6:6413. 10.1038/ncomms7413 [DOI] [PubMed] [Google Scholar]
- 99. Kaminski R, Chen Y, Fischer T, et al. : Elimination of HIV-1 Genomes from Human T-lymphoid Cells by CRISPR/Cas9 Gene Editing. Sci Rep. 2016;6:22555. 10.1038/srep22555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Gasmi M, D’Incan M, Desgranges C: Transfusion transmission of human T-lymphotropic virus type I (HTLV-I) from an asymptomatic blood donor: conservation of LTR U3, env, and tax nucleotide sequences in a recipient with HTLV-I-associated myelopathy. Transfusion. 1997;37(1):60–64. 10.1046/j.1537-2995.1997.37197176952.x [DOI] [PubMed] [Google Scholar]
- 101. Hajian R, Balderston S, Tran T, et al. : Detection of unamplified target genes via CRISPR-Cas9 immobilized on a graphene field-effect transistor. Nat Biomed Eng. 2019;3(6):427–437. 10.1038/s41551-019-0371-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Li L, Li S, Wang J: CRISPR-Cas12b-assisted nucleic acid detection platform. BioxRiv. 2018. 10.1101/362889 [DOI] [Google Scholar]
- 103. Harrington LB, Burstein D, Chen JS, et al. : Programmed DNA destruction by miniature CRISPR-Cas14 enzymes. Science. 2018;362(6416):839–842. 10.1126/science.aav4294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Teng F, Guo L, Cui T, et al. : CDetection: CRISPR-Cas12b-based DNA detection with sub-attomolar sensitivity and single-base specificity. Genome Biol. 2019;20(1):132. 10.1186/s13059-019-1742-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Nelson CE, Hakim CH, Ousterout DG, et al. : In vivo genome editing improves muscle function in a mouse model of Duchenne muscular dystrophy. Science. 2016;351(6271):403–7. 10.1126/science.aad5143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Tabebordbar M, Zhu K, Cheng JKW, et al. : In vivo gene editing in dystrophic mouse muscle and muscle stem cells. Science. 2016;351(6271):407–411. 10.1126/science.aad5177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Long C, Amoasii L, Mireault AA, et al. : Postnatal genome editing partially restores dystrophin expression in a mouse model of muscular dystrophy. Science. 2016;351(6271):400–3. 10.1126/science.aad5725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Singh K, Evens H, Nair N, et al. : Efficient In Vivo Liver-Directed Gene Editing Using CRISPR/Cas9. Mol Ther. 2018;26(5):1241–1254. 10.1016/j.ymthe.2018.02.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Yin H, Song CQ, Dorkin JR, et al. : Therapeutic genome editing by combined viral and non-viral delivery of CRISPR system components in vivo. Nat Biotechnol. 2016;34(3):328–333. 10.1038/nbt.3471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Yang Y, Wang L, Bell P, et al. : A dual AAV system enables the Cas9-mediated correction of a metabolic liver disease in newborn mice. Nat Biotechnol. 2016;34(3):334. 10.1038/nbt.3469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Ruan GX, Barry E, Yu D, et al. : CRISPR/Cas9-Mediated Genome Editing as a Therapeutic Approach for Leber Congenital Amaurosis 10. Mol Ther. 2017;25(2):331–341. 10.1016/j.ymthe.2016.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Yang S, Chang R, Yang H, et al. : CRISPR/Cas9-mediated gene editing ameliorates neurotoxicity in mouse model of Huntington’s disease. J Clin Invest. 2017;127(7):2719–2724. 10.1172/JCI92087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Cornu TI, Mussolino C, Cathomen T: Refining strategies to translate genome editing to the clinic. Nat Med. 2017;23(4):415–423. 10.1038/nm.4313 [DOI] [PubMed] [Google Scholar]
- 114. Kleinstiver BP, Pattanayak V, Prew MS, et al. : High-fidelity CRISPR-Cas9 nucleases with no detectable genome-wide off-target effects. Nature. 2016;529(7587):490–5. 10.1038/nature16526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Doench JG, Fusi N, Sullender M, et al. : Optimized sgRNA design to maximize activity and minimize off-target effects of CRISPR-Cas9. Nat Biotechnol. 2016;34(2):184–191. 10.1038/nbt.3437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Ran FA, Hsu PD, Lin CY, et al. : Double nicking by RNA-guided CRISPR Cas9 for enhanced genome editing specificity. Cell. 2013;154(6):1380–1389. 10.1016/j.cell.2013.08.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Tsai SQ, Zheng Z, Nguyen NT, et al. : GUIDE-seq enables genome-wide profiling of off-target cleavage by CRISPR-Cas nucleases. Nat Biotechnol. 2014;33(2):187–197. 10.1038/nbt.3117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Kim D, Bae S, Park J, et al. : Digenome-seq: genome-wide profiling of CRISPR-Cas9 off-target effects in human cells. Nat Methods. 2015;12(3):237–43. 10.1038/nmeth.3284 [DOI] [PubMed] [Google Scholar]
- 119. Cameron P, Settle AH, Fuller CK, et al. : SITE-Seq: A Genome-wide Method to Measure Cas9 Cleavage.2017. 10.1038/protex.2017.043 [DOI] [Google Scholar]
- 120. Tsai SQ, Nguyen NT, Malagon-Lopez J, et al. : CIRCLE-seq: a highly sensitive in vitro screen for genome-wide CRISPR-Cas9 nuclease off-targets. Nat Methods. 2017;14(6):607–614. 10.1038/nmeth.4278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Akcakaya P, Bobbin ML, Guo JA, et al. : In vivo CRISPR editing with no detectable genome-wide off-target mutations. Nature. 2018;561(7723):416–419. 10.1038/s41586-018-0500-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Zuris JA, Thompson DB, Shu Y, et al. : Cationic lipid-mediated delivery of proteins enables efficient protein-based genome editing in vitro and in vivo. Nat Biotechnol. 2015;33(1):73–80. 10.1038/nbt.3081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Jiang C, Mei M, Li B, et al. : A non-viral CRISPR/Cas9 delivery system for therapeutically targeting HBV DNA and pcsk9 in vivo. Cell Res. 2017;27(3):440–443. 10.1038/cr.2017.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Mout R, Ray M, Yesilbag Tonga G, et al. : Direct Cytosolic Delivery of CRISPR/Cas9–Ribonucleoprotein for Efficient Gene Editing. ACS Nano. 2017;11(3):2452–2458. 10.1021/acsnano.6b07600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Charlesworth CT, Deshpande PS, Dever DP, et al. : Identification of preexisting adaptive immunity to Cas9 proteins in humans. Nat Med. 2019;25(2):249–254. 10.1038/s41591-018-0326-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Burstein D, Harrington LB, Strutt SC, et al. : New CRISPR-Cas systems from uncultivated microbes. Nature. 2016;542(7640):237–241. 10.1038/nature21059 [DOI] [PMC free article] [PubMed] [Google Scholar]