Abstract
Background:
Needle-exchange programs (NEPs) reduce infections in people who inject drugs. This study assesses the impact community pharmacies have had in the Needle-Exchange Program in Portugal since 2015.
Methods:
Health gains were measured by the number of human immunodeficiency virus (HIV) and hepatitis C virus (HCV) infections averted, which were estimated, in each scenario, based on a standard model in the literature, calibrated to national data. The costs per infection were taken from national literature; costs of manufacturing, logistics and incineration of injection materials were also considered. The results were presented as net costs (i.e., incremental costs of the program with community pharmacies less the costs of additional infections avoided).
Results:
Considering a 5-year horizon, the Needle Exchange Program with community pharmacies would account for a 6.8% (n = 25) and a 6.5% reduction (n = 22) of HCV and HIV infections, respectively. The present value of net savings generated by the participation of community pharmacies in the program was estimated at €2,073,347. The average discounted net benefit per syringe exchanged is €3.01, already taking into account a payment to community pharmacies per needle exchanged.
Interpretation:
We estimate that the participation of community pharmacies in the Needle Exchange Program will lead to a reduction of HIV and HCV infections and will generate over €2 million in savings for the health system.
Conclusions:
The intervention is estimated to generate better health outcomes at lower costs, contributing to improving the efficiency of the public health system in Portugal.
Introduction
The prevalence of human immunodeficiency virus (HIV), hepatitis C virus (HCV) and hepatitis B virus (HBV) is high among people who inject drugs (PWID),1 resulting in serious public health concerns. In PWID, infectious diseases are transmitted through 2 main channels: the sharing of drug injection equipment and unprotected sexual activity. In Portugal, the last published estimates of the number of PWID ranged between 12,732 and 16,285, corresponding to a prevalence rate of 194.4 to 248.6 per 100,000 inhabitants aged 15 to 65.2 In 2016, the prevalence of HIV and HCV for PWID in treatment for addiction in Portugal varied between 7%-27% and 67%-88%, respectively, while 2.5% of screened PWID were HBV surface antigen positive.3
Knowledge Into Practice.
Needle-exchange programs are available in 90 countries around the world, and use different forms of distribution, ranging from mobile vans to home visits, with community pharmacies usually playing an important role in the program due to their accessibility in terms of opening hours and broad geographical distribution.
This study shows that, in Portugal, the participation of community pharmacies in a needle-exchange program has the potential to reduce HIV and HCV infections while saving above €2M for the health system.
Community pharmacies may increase their impact on society by joining the needle-exchange programs, which may be a cost-effective strategy even in a setting where consumption of injectable drugs is decreasing.
Mise En Pratique Des Connaissances.
Les programmes d’échange de seringues sont disponibles dans 90 pays du monde entier et utilisent différentes formes de distribution, allant des camionnettes mobiles aux visites à domicile. Les pharmacies communautaires jouent généralement un rôle important dans le programme en raison de leur accessibilité en termes d’heures d’ouverture et de leur vaste répartition géographique.
Cette étude montre qu’au Portugal, la participation des pharmacies communautaires à un programme d’échange de seringues a le potentiel de réduire les infections par le VIH et le VHC tout en permettant au système de santé d’économiser plus de 2 millions d’euros.
Les pharmacies communautaires peuvent accroître leur impact sur la société en participant aux programmes d’échange de seringues, ce qui peut constituer une stratégie rentable même dans un contexte où la consommation de drogues injectables diminue.
Needle-exchange programs (NEPs) improve access to kits with sterile drug-injecting equipment and condoms, reducing drug-related harms by decreasing transmission of infections among PWID.4-7
NEPs are available in 90 countries around the world,8 using different forms of distribution, ranging from mobile vans to home visits. Community pharmacies play an important role in these programs, mainly due to their accessibility in terms of opening hours and broad geographical distribution. In many countries, such as Australia,9 Belgium, France, Ireland, Netherlands, Slovenia, Spain and the United Kingdom,10 community pharmacies are an important distribution channel for the kits.
In Portugal, the NEP was launched in 1993 in community pharmacies. After being launched, other entities joined the program, in particular, mobile vans (1994) and both governmental and nongovernmental organizations (1999). Until 2005, community pharmacies had a dominant role in the needle distribution network.11-17
Following a request by the National Committee Against AIDS (a governmental committee), a private consulting company performed an economic evaluation of the program in 2002.18 The results showed that the NEP in community pharmacies in Portugal was cost-effective, as it generated better health outcomes while lowering costs, with cost savings due to averted HIV and HCV infections adding up to €405 million (between 1993 and 2001).
From 1993 to 2012, community pharmacies participating in the NEP did not receive any public funding and provided these services pro bono. Due to the economic crisis, the community pharmacies (which are privately owned) suspended their participation in the program in 2013, rejoining it 2 years later after signing an agreement with the government that enhanced the expansion of the role of community pharmacies, particularly in relation to the provision of health services. Currently, the following entities are actively participating in the program: primary health care centres, governmental and nongovernmental organizations, mobile vans and community pharmacies. The program allows PWID to drop off used injecting drug equipment and collect a kit containing 2 sterile needles and syringes, 2 alcohol swabs, 2 ampoules of double-distilled water, 2 citric acid sachets, 2 filters, 2 containers for drug preparation (clean cups) and 1 condom. This kit is financed by the government.
Since the first economic study in 2002,18 significant changes have occurred in areas such as the frequencies of the types of drugs consumed, consumption habits and accessibility to the NEP, as well as in the epidemiology of HIV and HCV among PWID in Portugal. Therefore, the objective of this study was to conduct a prospective cost-effectiveness analysis comparing the current (since 2015) scenario of the NEP with participation of community pharmacies (intervention scenario) versus a scenario without participation of community pharmacies (status quo scenario). The results of this analysis are relevant to all stakeholders and to inform health care policy decision-making.
Methods
The clinical benefits of the NEP were estimated as the number of HIV and HCV infections averted due to the participation of community pharmacies in the program. Therefore, health gains associated with the intervention scenario were computed as the difference between the HIV and HCV new infections in the scenarios with and without the participation of community pharmacies.
The incremental costs of including community pharmacies in the Portuguese NEP were computed as the difference between the additional costs of the NEP with community pharmacies and the savings associated with the number of additionally averted HIV and HCV infections.
A time horizon of 5 years was considered in the analysis of the number of infections. Lifetime costs associated with treatments of these infections were discounted at a 5% rate, as recommended by the National Authority of Medicines and Health Products (INFARMED) guidelines.19 The results are presented in terms of the value of the difference in costs between the scenario of the NEP with community pharmacies and the scenario without community pharmacies. There were 2 possible results: 1) the difference in costs is positive, meaning that the intervention increases costs, and 2) the difference is negative, meaning that there are (positive) “net savings” associated with the intervention.
In the base-case scenario, the participation of community pharmacists in the NEP was assumed to be complementary to the other exchange locations available in the status quo scenario. In a sensitivity analysis, a scenario where community pharmacies partially substitute for other NEP distribution sites was considered.
As this study is based on secondary and aggregate data, it was not necessary to get Institutional Ethics Review Board approval.
Health benefits
The number of new infections occurring in each scenario was separately estimated for HIV and HCV using the model presented by Jacobs et al.,20 based on the original equation by Kaplan and O’Keefe.21
The model’s structural equation is
where q is the disease prevalence in the PWID population, q is the probability of effectively cleaning the drug-injecting equipment, N is the number of circulating needles, s is the needle-sharing rate, t is the probability of infection per single injection with an infected needle and m is the number of individuals sharing the same needle.
The infection incidence depends on the susceptible population of PWID [(1 – q)], the number of unclean and shared needles in circulation [(1–θ)*N * s, and the probability of infection per sharing event [1 – (1 – q * t ) ]m, which increases nonlinearly with the number of individuals sharing the same needle (m).
The model assumes that the number of needles in circulation (N) in a given moment is stable, as in Kaplan and O’Keefe’s circulation theory.21 According to this theory, the infection incidence decreases because the intervention reduces needles’ average circulation time and therefore the exposure time, decreasing the probability of infection per sharing episode. In other words, the intervention has an impact only on the sharing rate of the needle(s) and not on the number of needles in circulation (N).
The calibration of the equations is identical for both HIV and HCV infections, except for the parameters of disease prevalence (q) and the probability of infection per shared injection (t). The equation is calibrated according to 2 principles: 1) in the status quo scenario, estimates of the number of new cases should be consistent with the historical incidence rates among PWID and their trends, and 2) calibration should use the best-available information for the equation’s epidemiological parameters.
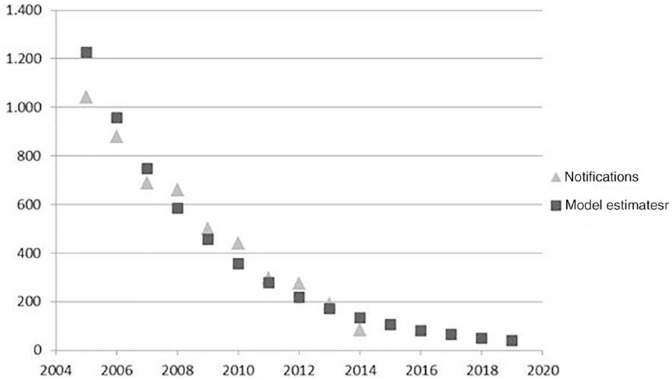
Regarding HIV incidence, national public authorities publish annually the number of notifications of HIV infections by transmission mode, including injecting drug use.22 The HIV incidence rate between 2015 and 2019 was extrapolated using a regression model where the number of new cases of HIV among PWID between 2004 and 2014 is explained by time. Figure 1 shows the model and its good fit (R2 = 92%). The registry of HIV notifications is considered the best source of information on incidence because HIV has been listed among compulsory notifiable diseases since 2005.
Figure 1.
Data and regression estimates of human immunodeficiency virus incidence in people who inject drugs
Source: INSA (2014) and authors’ estimates.20
From 2014 onwards, the number of infections was predicted to decrease. Between 2015 and 2019, the number of new cases in the status quo scenario was estimated at 81, 63, 63, 50 and 39, respectively.
The number of HIV notifications among PWID in Portugal since 2004, as well as the number predicted for the years after, is shown in Figure 1. Information regarding HCV incidence was scarcer and less systematically collected. The most recently published data by the Directorate General of Health report 45 cases of acute infections in 2008 in the general population. Other published results regarding overall HCV incidence rates in Portugal are heterogeneous, ranging between 0.37/100,000 and 8/100,000 inhabitants,22-25 corresponding to a range from a low of 37 up to a high of 810 new cases per year.
Following a personal communication with Professor Homie Razavi (from the Center for Disease Analysis [CDA]), the number of new cases of HCV among PWID in Portugal was estimated at 94 in 2014. This estimate was considered conservative given the information available and was used to calibrate the first year of the status quo scenario.
The parameters considered constant for HCV along the time horizon are the probability of infection per single injection (t), the number of individuals sharing the same needle (m) and the probability of effectively cleaning the drug-injecting equipment (q).
The model was calibrated with prevalence rates of HIV declining over time and constant rates of HCV. In line with the annual report by the General Directorate for Intervention on Addictive Behaviours and Dependencies (SICAD), published in 2014,26 and Marinho et al.,27 prevalence estimations of HIV and HCV were 24% and 92% among PWID, respectively. Additionally, the number of circulating needles in 2014 was estimated at 1,200,277, which is the moving average of the number of needles exchanged in 2013, 2014 and 2015 (an estimate was used for 2015).17
The parameters used to calibrate the model are summarized in Table 1. The sharing rate decrease associated with the intervention was assumed to be half of the percentage increase of exchanged needles in 2015 and 2016. In other words, if the number of needles exchanged increases, the circulation time drops, as does the probability of sharing a specific needle. The analysis assumes that if the number of needles exchanged increases by 10%, the sharing rate will be proportionately 5% lower. The number of needles exchanged by community pharmacies in 2015 was estimated at 87,761, based on the estimates available up to November 2015, an increase of roughly 7%. The number was estimated to reach 169,347 from 2016 onwards. The estimate of the future number of needles exchanged took into consideration the increasing trend in the number of community pharmacies participating in the program. According to data provided to the authors by the Portuguese National Pharmacy Association, in November 2015, there were 426 community pharmacies actively participating across the country and the average number of needles exchanged per month by each community pharmacy in November 2015 was 94. The resulting proportional reduction of the sharing rate in the intervention scenario was then estimated at 3.7% and 7.7% in 2015 and 2016, respectively, and assumed constant from 2016 onwards.
Table 1.
Parameters used to calibrate the model equations
| Variable | Value | Source | Constant |
|---|---|---|---|
| Probability of HIV infection per single injection (tHIV) | 0.7% | Based on Public Health Agency of Canada (2012)28 | Yes |
| Probability of HCV infection per single injection (tHCV) | 1.5% | Based on UK Ministry of Health (2009)29 | Yes |
| Number of individuals sharing the same needle (m) | 1.38 | Jacobs et al. (1999)20 | Yes |
| Probability of effectively cleaning the drug-injecting equipment (q) | 7.7% | Based on Mendes et al. (2003)30 | Yes |
| HIV prevalence among PWID (qHIV) | 14.5%-23.7% | SICAD (2014)26 and authors’ estimates | No |
| HCV prevalence among PWID (qHCV) | 92.2% | Marinho et al. (2001)27 and authors’ estimates | Yes |
| Number of circulating needles (N) | 770,555-1,200,277 | DGS (2015)17 and authors’ estimates | No |
| Sharing rate (s) in status quo scenario | 1%-9.8% | Mendes et al. (2003)30 and SICAD (2012)31 | No |
| Change in sharing rate (s) in intervention scenario | –7.7% to 3.7% | Assumption and authors’ estimates | No |
DGS, Directorate-General of Health; HCV, hepatitis C virus; HIV, human immunodeficiency virus; PWID, people who inject drugs; SICAD, General Directorate for Intervention on Addictive Behaviours and Dependencies.
Cost difference
Costs incurred by community pharmacies and other entities participating in the production—distribution, collection and incineration of the kits distributed in community pharmacies—were considered incremental costs associated with the intervention scenario. A panel of pharmacies that had previously participated in the NEP was convened and consulted to identify and estimate the costs incurred by community pharmacies, including costs related to storage, logistics and labour costs of providing the service. Costs incurred by other entities, outside the community pharmacies, were retrieved from public contracts available online32 and included production, storage, distribution and incineration of the drug-injecting equipment. Cost estimates included a predicted (but nonexistent at the time, in 2015) remuneration for community pharmacies per needle exchanged of €2.5.
Direct lifetime health care costs associated with the infections were retrieved from publications by other authors. The values retrieved are presented in Table 2. Given the estimates available,33-35 the authors considered the most conservative cost estimate for HIV (€213,251). Regarding HCV, the cost used was the most conservative cost estimate for genotype 1 (€47,422), since this is the most prevalent genotype in Portugal.23,36-39
Table 2.
Estimates of HIV and HCV treatment costs in Portugal
| Infection | Therapy | Discounted value (€) | Source |
|---|---|---|---|
| HIV | Raltegravir | 215,194 | Chaudhary et al. (2010)33 |
| Efavirenz | 213,251 | ||
| Lopinavir | 220,349 | ||
| Atazanavir | 220,934 | Carrasco et al. (2011)34 | |
| Lopinavir | 235,413 | ||
| Darunavir | 236,126 | ||
| Efavirenz | 233,425 | ||
| HCV, genotype 1 naive | Sofosbuvir/peginterferon + ribavirin | 65,519 | Félix et al. (2014)35 |
| Telaprevir/peginterferon + ribavirin | 58,059 | ||
| Boceprevir/peginterferon + ribavirin | 58,222 | ||
| Peginterferon + ribavirin | 47,422 | ||
| HCV, genotype 3 | Sofosbuvir/peginterferon + ribavirin | 63,383 | |
| Telaprevir/peginterferon + ribavirin EXP | 63,736 | ||
| Boceprevir/peginterferon + ribavirin EXP | 43,454 | ||
| Peginterferon + ribavirin | 42,582 | ||
| HCV genotype 4/5/6 | Sofosbuvir/peginterferon + ribavirin | 59,128 | |
| Peginterferon + ribavirin | 46,633 |
EXP, people with prior exposure; HCV, hepatitis C virus; HIV: human immunodeficiency virus.
The HIV and HCV cost estimates in the literature assume that the evaluation time frame starts when the treatment starts. Thus, the lifetime cost estimates were additionally discounted in order to consider a lag of 3 and 15 years between the timing of the infection and the initiation of treatment of HIV and HCV, respectively.
Univariate sensitivity analyses were performed to assess the robustness of the base case. In the sensitivity analyses, the base-case calibration was varied in terms of discount rate (3% instead of 5%), lifetime infections’ costs (–30%) and sharing rate decrease associated with intervention (–30% compared to base-case scenario). A scenario excluding the impact of the intervention on HCV incidence was analyzed. Since the substitutability between distribution channels is unknown, the possibility of some substitution between distribution sites was also explored. In particular, we considered a scenario where for each 3 needles exchanged in the community pharmacies, there is 1 less needle exchanged in the other distribution channels (1/3 substitution). This scenario translates to a reduction in the benefits associated with the intervention, since it is assumed that 1/3 of the needle exchanges in community pharmacies would have occurred anyway, with no reduction in the overall costs of running the program in the community pharmacies. Also, it was conservatively assumed that there would be no reductions in the costs associated with running the NEP in other networks.
Results
The model estimated that, in a 5-year time horizon, the participation of community pharmacies in the NEP would reduce by 25 the number of new HCV cases and by 22 the number of new HIV cases. In other words, over 5 years, community pharmacies might contribute to a reduction of 6.8% and 6.5% in HCV and HIV infections, respectively, among PWID in Portugal.
The overall costs per needle exchanged, including costs incurred outside the community pharmacies and their fees (which includes costs inside the community pharmacies), were estimated at €3.09. The number of needles exchanged was forecasted at 87,761 in the first intervention year and 169,347 in the following years. The incremental annual cost of the intervention was estimated at €271,269 in 2015 and at €523,452 from 2016 onwards.
The present value of lifetime costs associated with infections was estimated at €184,214 and €22,811 for HIV and HCV, respectively. The results in terms of net costs (i.e., the cost of the infections avoided less the incremental costs of the NEP with community pharmacy participation) are summarized in Table 3.
Table 3.
Yearly results for a 5-year time horizon (not discounted)
| Year | Needles exchanged in pharmacies | Total costs of the NEP in pharmacies | Infections avoided |
Saving associated with infections avoided (€) |
Net savings (€) | ||
|---|---|---|---|---|---|---|---|
| HCV | HIV | HCV | HIV | ||||
| 2015 | 87,761 | 271,269 | 5 | 4 | 105,359 | 700,400 | 534,489 |
| 2016 | 169,347 | 523,452 | 6 | 6 | 147,976 | 1,150,455 | 774,979 |
| 2017 | 169,347 | 523,452 | 5 | 5 | 124,300 | 894,796 | 495,644 |
| 2018 | 169,347 | 523,452 | 5 | 4 | 105,937 | 710,154 | 292,639 |
| 2019 | 169,347 | 523,452 | 4 | 3 | 89,170 | 553,918 | 119,636 |
HCV, hepatitis C virus; HIV, human immunodeficiency virus; NEP, needle exchange program.
For each period, the savings associated with the additional infections avoided were higher than the incremental costs of the intervention (i.e., the intervention scenario is a dominant strategy when compared to the status quo scenario).
The discounted savings over a 5-year horizon were estimated to be €2,073,347. Equivalently, each needle exchanged by the NEP in community pharmacies results, on average, in €3.01 of savings for taxpayers.
Sensitivity analysis proved the intervention scenario to be a robust dominant strategy. A 30% decrease in the infections’ costs had the highest impact on results, with a 61% reduction in net savings; an alternative 3% discount rate, lower than the national Portuguese guidelines’ base case but recommended by the Centers for Disease Control and Prevention for the cost-effectiveness analysis of community public health prevention interventions,40 increased the savings by 22%; reducing the impact of the intervention on the needle-sharing rate (parameter s) by 20% reduced net savings by 41%. In the scenario where HCV prevention benefits are ignored, the NEP with community pharmacies persists as a dominant intervention with discounted net savings above €1.5 million, a 25% reduction versus the base case. Finally, in the scenario where the NEP in community pharmacies is considered to substitute for other distribution networks by 1/3, the intervention is no longer dominant but the present value of the cost per averted infection was estimated at €4085.
Discussion
The results of this analysis comparing a scenario with the participation of community pharmacies in the NEP versus a status quo scenario showed that community pharmacy-based needle exchange is a dominant strategy, generating higher health benefits and simultaneously costs savings for society. This result proved to be robust through a variety of sensitivity analyses where the parameters in the model associated with the highest uncertainty were varied. In all sensitivity analyses, the participation of community pharmacies in the NEP was a cost-effective strategy (with a cost per averted infection lower than €4100 in the worst case studied and positive net savings [dominant strategy] in all other cases).
We used a model from the literature20,41,42 to estimate the number of HIV and HCV infections that were averted. We were conservative in the values used for calibrating the model. For example, HIV prevalence estimates used in the model were those leading to HIV incidence rates in line with the number of new cases notified in Portugal. This approach underestimates the potential health gains, due to the well-known notification bias. The use of a higher disease prevalence when calibrating the model would have led to higher health benefits. Furthermore, we also consider the model itself to be conservative, since it includes all costs of drug-injecting equipment but not all the benefits related to the NEP. For example, the model does not take into account the potential beneficial impact of the NEP on the transmission of other illnesses, such as HBV and tuberculosis. Moreover, the kits dispensed under the NEP also include condoms, which aim to reduce sexually transmitted diseases and unwanted pregnancies. Consideration of these potential health benefits would improve the cost-effectiveness of the intervention program.
The comparability of the results across countries is difficult to determine due to differences in costs, PWID behaviour and infections’ prevalence and time horizon considered in the analysis, among other aspects. Furthermore, most of the literature presents cost-effectiveness analyses of NEP (considered as a whole) versus no NEP,20,43-46 while the current study represents a cost-effectiveness analysis comparing the effectiveness of the Portuguese distribution network without community pharmacies with a network including community pharmacies. Nevertheless, the overall findings of the present study are in line with those reported in the literature, which also conclude that the NEP is a cost-effective strategy (regardless of the distribution site) in other countries.18,41-44
Conclusion
The estimates presented in this work show that the participation of community pharmacies in the needle-exchange program in Portugal has the potential to reduce HIV and HCV infections while saving over €2 million for the health system. Therefore, the participation of community pharmacies in this program is a dominant strategy compared to a program without them. The intervention generates better health outcomes at lower costs than those of the NEP without the community pharmacies, contributing to improving the efficiency of the public health system in Portugal. Although not easily geographically generalizable outside Portugal, our findings suggest that the inclusion of community pharmacies in the distribution network of kits in the context of a NEP is a cost-effective strategy even in a setting where the consumption of injectable drugs is decreasing.
Acknowledgments
These study results were presented in a poster and short presentation during the 12th Portuguese National Association of Pharmacies conference (April 14, 2016, Lisbon) and the ISPOR 19th Annual European Congress (November 1, 2016, Vienna).
Footnotes
Homie A. Razavi, PhD, is the manager-director of the Center for Disease Analysis (CDA) in Louisville CO (USA). In recent years, he has been leading a team of epidemiologists and modellers to quantify the epidemiology of hepatitis C virus (HCV) and forecast the future impact of HCV disease burden. The authors express their gratitude to Dr. Razavi for sharing his research group estimates of HCV incidence among people who inject drugs in Portugal, which allowed us to fine-tune the model for the HCV infection incidence.
Declaration of Conflicting Interests:This study was supported by the Portuguese National Association of Pharmacies (ANF). ANF had no control on the conduct or publication of this research. Maria Cary and José Pedro Guerreiro are researchers for the Centre for Health Research & Evaluation (CEFAR), a Contract Research Organization (CRO) of the National Association of Pharmacies (ANF), which performs health research studies. Suzete Costa was researcher and executive director of CEFAR/ANF at the time of this research. Margarida Borges, Miguel Gouveia, Francesca Fiorentino Gonçalo Jesus and António Vaz Carneiro have no competing interests to declare.
ORCID iD:Francesca Fiorentino  https://orcid.org/0000-0003-2092-3593
https://orcid.org/0000-0003-2092-3593
References
- 1. European Monitoring Centre for Drugs and Drug Addiction (EMCDDA). Drug-related infectious diseases in Europe: update from the EMCDDA expert network. Luxembourg: Publications Office of the European Union; 2015. [Google Scholar]
- 2. Ribeiro C, Carapinha L, Lavado E, Guerreiro C. Estimation of drug consumption of problematic/high risk. Portugal: 2012. Available: www.sicad.pt/BK/EstatisticaInvestigacao/EstudosConcluidos/Lists/SICAD_ESTUDOS/Attachments/146/EstimativaConsumoProblematicoAltoRiscoDrogas.pdf (accessed Nov. 15, 2019). [Google Scholar]
- 3. General Directorate for Intervention on Addictive Behaviours and Dependencies (SICAD). Annual report 2016—the situation of the country in drugs and drug addiction. Available: http://www.sicad.pt/BK/Publicacoes/Lists/SICAD_PUBLICACOES/Attachments/129/RelatorioAnual_2016_A_SituacaoDoPaisEmMateriaDeDrogas_e_Toxicodependencias.pdf (accessed Nov. 15, 2019).
- 4. Gibson DR, Flynn NM, Perales D. Effectiveness of syringe exchange programs in reducing HIV risk behavior and HIV seroconversion among injecting drug users. AIDS 2001;15(11):1329-41. [DOI] [PubMed] [Google Scholar]
- 5. Kimber J, Palmateer N, Hutchinson SJ, et al. Harm reduction among injecting drug users—evidence of effectiveness. In: Harm reduction: evidence, impacts and challenges EMCDDA. Lisbon, Portugal: European Monitoring Centre for Drugs and Drug Addiction; 2010. p. 115-63. Editors: Tim Rhodes and Dagmar Hedrich. [Google Scholar]
- 6. Palmateer N, Kimber J, Hickman M, et al. Evidence for the effectiveness of sterile injecting equipment provision in preventing hepatitis C and human immunodeficiency virus transmission among injecting drug users: a review of reviews. Addiction 2010;105(5):844-59. [DOI] [PubMed] [Google Scholar]
- 7. Van Den Berg C, Smit C, Van Brussel G, et al. Full participation in harm reduction programmes is associated to decreased risk for human immunodeficiency virus and hepatitis C virus: evidence from the Amsterdam Cohort Studies among drug users. Addiction 2007;102(9):1454-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Harm Reduction International. Global state of harm reduction 2016. Available: https://www.hri.global/contents/1739 (accessed Jan. 1, 2018).
- 9. The Pharmacy Guild of Australia. Community pharmacy roadmap program development template. Available: https://www.guild.org.au/__data/assets/pdf_file/0019/5572/needle-amp-syringe-program.pdf (accessed Feb. 2, 2017).
- 10. Harm Reduction International. Global state of harm reduction 2014. Available: https://www.hri.global/contents/1524 (accessed Jan. 1, 2018).
- 11. Directorate-General of Health (DGS). Program “Say no to a second-hand syringe.” 1993 to 2009. Available: http://www.pnvihsida.dgs.pt/programatrocaseringas/relatorios/programa-diz-nao-a-uma-seringa-em-segunda-mao-1993-20091.aspx (accessed Nov. 7, 2018).
- 12. Directorate-General of Health (DGS). Program “Say no to a second-hand syringe”—annual report 2010. Available: http://www.pnvihsida.dgs.pt/programatrocaseringas/relatorios/programa-diz-nao-a-uma-seringa-em-segunda-mao-20101.aspx (accessed Nov. 7, 2018).
- 13.Directorate-General of Health (DGS) Program “Say no to a second-hand syringe”—annual report 2011. Available: https://www.pnvihsida.dgs.pt/programatrocaseringas/relatorios/programa-diz-nao-a-uma-seringa-em-segunda-mao-20111.aspx (accessed Nov. 7, 2018).
- 14. Directorate-General of Health (DGS). Program “Say no to a second-hand syringe.” Data from January to December of 2012. Available: https://www.pnvihsida.dgs.pt/programatrocaseringas/relatorios/programa-diz-nao-a-uma-seringa-em-segunda-mao-20121.aspx (accessed Nov. 7, 2018).
- 15. Directorate-General of Health (DGS). Program “Say no to a second-hand syringe.” Annual report 2013. Available: https://www.pnvihsida.dgs.pt/programatrocaseringas/relatorios/programa-diz-nao-a-uma-seringa-em-segunda-mao-20131.aspx (accessed Nov. 7, 2018).
- 16. Directorate-General of Health (DGS). National programme for HIV/AIDS and tuberculosis. Portugal—HIV infection, AIDS and tuberculosis in numbers: 2015. Available: https://www.pnvihsida.dgs.pt/estudos-e-estatisticas111111/relatorios1/portugal-infecao-vih-sida-e-tuberculose-em-numeros-2015-pdf.aspx (accessed Nov. 25, 2018).
- 17. Directorate-General of Health (DGS). Program “Say no to a second-hand syringe.” Annual report 2015. Available: https://www.pnvihsida.dgs.pt/programatrocaseringas/relatorios/programa-diz-nao-a-uma-seringa-em-segunda-mao-20131.aspx (accessed Nov. 25, 2018).
- 18. Félix J, Inês M, Acosta C. Estimate the impact of the “Say no to a second-hand syringe” program on the risk of HIV/AIDS infection in the Portuguese injecting drug user population. Available: https://www.pnvihsida.dgs.pt/programatrocaseringas/relatorios/avaliacao-do-programa-diz-nao-a-uma-seringa-em-segunda-mao-20021.aspx (accessed Nov. 25, 2018).
- 19. Silva EA, da, Pinto CG, Sampaio C, et al. Guidelines for economic drug evaluation studies. Available: http://www.infarmed.pt/documents/281/1432055/PCAEC04_vering.pdf (accessed Dec. 10, 2018).
- 20. Jacobs P, Calder P, Taylor M, Houston S, Saunders LD, Albert T. Cost effectiveness of Streetworks’ needle exchange program of Edmonton. Can J Public Health 1999;90(3):168-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kaplan EH, O’Keefe E. Let the needles do the talking! Evaluating the new haven needle exchange. Interfaces 1993;23(1):7-26. [Google Scholar]
- 22. Unidade de Referência e Vigilância Epidemiológica, Departamento de Doenças Infeciosas. [VIH Infection/AIDS: situation in Portugal by 31st December 2014]. Available: http://hdl.handle.net/10400.18/4101 (accessed Dec. 12, 2018).
- 23. Anjo J, Café A, Carvalho A, et al. The impact of hepatitis C in Portugal. 2014;21(2):44-54. Retrieved from https://www.nice.org.uk/guidance/ph52/resources/needle-and-syringe-programmes-pdf-1996415046853 [Google Scholar]
- 24. Razavi H, Waked I, Sarrazin C, et al. The present and future disease burden of hepatitis C virus (HCV) infection with today’s treatment paradigm. J Viral Hepat 2014;21:34-59. [DOI] [PubMed] [Google Scholar]
- 25. World Health Organization (WHO). European health for all database (HFA-DB). Available: http://data.euro.who.int/hfadb/ (accessed Feb. 2, 2016).
- 26. General Directorate for Intervention on Addictive Behaviours and Dependencies (SICAD). Annual report 2013—national status regarding drugs and addictions. Available: http://www.sicad.pt/BK/Publicacoes/Lists/SICAD_PUBLICACOES/Attachments/72/RelatórioAnual_2013_A_Situação_do_País_em_matéria_de_drogas_e_toxicodependências.pdf (accessed Nov. 10, 2018).
- 27. Marinho RT, Moura MC, Giria JA, Ferrinho P. Epidemiological aspects of hepatitis C in Portugal. J Gastroenterol Hepatol 2001;16(9):1076-7. [DOI] [PubMed] [Google Scholar]
- 28. Public Health Agency of Canada. HIV transmission risk: a summary of the evidence. Available: https://www.catie.ca/sites/default/files/HIV-TRANSMISSION-RISK-EN.pdf (accessed Dec. 10, 2018).
- 29. National Institute for Health and Care Excellence. Needle and syringe programmes. 2015. Publisher and city of publication not applicable. Please add at the end of citation: Retrieved from https://www.nice.org.uk/guidance/ph52/resources/needle-and-syringe-programmes-pdf-1996415046853
- 30. Mendes Z, Costa F, Guerreiro JP. What is missing in the kit: the user perspective. Available: http://www.sicad.pt/BK/RevistaToxicodependencias/Lists/SICAD_Artigos/Attachments/187/2003_03_TXT3.pdf (accessed Oct. 8, 2018).
- 31. Carapinha L. Characterization of users included in risk reduction and harm minimization projects supported by SICAD—2011. Lisbon, Portugal: General Directorate for Intervention on Addictive Behaviours and Dependencies (SICAD); 2012. [Google Scholar]
- 32. Instituto dos Mercados Públicos, do Imobiliário e da Construção (IMPIC). Online public contracts. Available: http://www.base.gov.pt/Base/pt/Homepage (accessed Oct. 20, 2018).
- 33. Chaudhary M, Elbasha E, Pereira R, Kumar R. A continuous-time economic model to evaluate raltegravir use strategies in treatment-naive HIV-1 patients in Portugal. Value Health 2010;13(7):A436. [Google Scholar]
- 34. Carrasco J, Antunes A, Aldir, et al. Relative therapeutic value of antiretroviral drugs—analysis of clinical and economic value of antiretroviral drugs in naïve HIV patients in Portugal]. Available: http://12cnes.apes.pt/LinkClick.aspx?fileticket=aeQrPqJGu8M%3D&tabid=128&language=pt-PT (accessed Dec. 12, 2018).
- 35. Félix J, Silva MJ, Ferreira D, et al. Efectividade do tratamento com sofosbuvir na hepatite C crónica em Portugal: tradução económica [P 93]. Presented at Semana Digestiva 2014 June 5-7, 2014; Estoril, Portugal. [Google Scholar]
- 36. Areias J, Gomes H, Mocho L, Matos L, Valente C, Vale A. Epidemiological characterization of chronic hepatitis C in Portugal. J Clin Virol 2006;36:S211. [Google Scholar]
- 37. Proença L, Barroso H, Barra A, Barradas A. Characterization of a population infected with hepatitis C virus. Rev Clínica Hosp 2013;1:11-8. [Google Scholar]
- 38. Bruggmann P, Berg T, Øvrehus ALH, et al. Historical epidemiology of hepatitis C virus (HCV) in selected countries. J Viral Hepat 2014;21:5-33. [DOI] [PubMed] [Google Scholar]
- 39. Velosa J, Serejo F, Bana T, et al. Chronic hepatitis C treated with peginterferon alfa plus ribavirin in clinical practice. Hepatogastroenterology 2011;58(109):1260-6. [DOI] [PubMed] [Google Scholar]
- 40. RTI International. Guide to analyzing the cost-effectiveness of community public health prevention approaches. Available: https://aspe.hhs.gov/system/files/pdf/74686/report.pdf (accessed Oct. 12, 2018).
- 41. Andresen MA, Boyd N. A cost-benefit and cost-effectiveness analysis of Vancouver’s supervised injection facility. Int J Drug Policy 2010;21(1):70-6. [DOI] [PubMed] [Google Scholar]
- 42. Jozaghi E, Reid AA, Andresen MA, Juneau A. A cost-benefit/cost-effectiveness analysis of proposed supervised injection facilities in Ottawa, Canada. Subst Abuse Treat Prev Policy 2014;9(1):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gold M, Gafni A, Nelligan P, Millson P. Needle exchange programs: an economic evaluation of a local experience. CMAJ 1997;157(3):255-62. [PMC free article] [PubMed] [Google Scholar]
- 44. Cabasés JM, Sánchez E. Costs and effectiveness of a syringe distribution and needle exchange program for HIV prevention in a regional setting. Eur J Health Econ 2003;4(3):203-8. [DOI] [PubMed] [Google Scholar]
- 45. Kwon JA, Anderson J, Kerr CC, et al. Estimating the cost-effectiveness of needle-syringe programs in Australia. AIDS 2012;26(17):2201-10. [DOI] [PubMed] [Google Scholar]
- 46. Eythorsson ES, Asgeirsdottir TL, Gottfredsson M. Needle exchange programs are a cost-effective preventative measure against HIV in Iceland. Laeknabladid 2014;100(7-8):379-83. [DOI] [PubMed] [Google Scholar]