Abstract
Opioids are a group of analgesic agents commonly used in clinical practice. The three classical opioid receptors are MOP, DOP and KOP. The NOP (N/OFQ) receptor is considered to be a non-opioid branch of the opioid receptor family. Opioid receptors are G-protein-coupled receptors which cause cellular hyperpolarisation when bound to opioid agonists. Opioids may be classified according to their mode of synthesis into alkaloids, semi-synthetic and synthetic compounds. Opioid use disorder (OUD) is an emerging issue and important lessons can be learnt from the United States where opioid epidemic was declared as a national emergency in 2017.
Keywords: Opioid pharmacology, opioid receptors, opioid classification, analgesics, pharmacokinetics, opioid use disorder
Introduction
Morphine is commonly considered to be the archetypal opioid analgesic and the agent to which all other painkillers are compared. As long ago as 3000 BC the opium poppy, Papaver somniferum, was cultivated for its active ingredients. Morphine was isolated from opium in 1806 by Serturner. In 1847, the chemical formula for morphine was deduced and this coupled with the invention of the hypodermic needle in 1853, led to the more precise and widespread use of morphine.1,2
Classification
Though morphine is the most widely known extract of P. somniferum, four naturally occurring alkaloids (plant-derived amines) can be isolated from it: morphine, codeine, papaverine and thebaine. Simple chemical manipulations of these basic opiate alkaloids yield a range of semi-synthetic opioids which are useful in clinical medicine (agents such as diamorphine, dihydrocodeine, buprenorphine, nalbuphine, naloxone and oxycodone). During the 20th century, a number of synthetic opioids were also produced. These synthetic compounds can be divided into four chemical groupings: the morphinan derivatives (levorphanol, butorphanol), the diphenylheptane derivatives (methadone, propoxyphene), the benzomorphan derivatives (pentazocine, phenazocine) and the phenylpiperidine derivatives (pethidine, alfentanil, fentanyl, sufentanil and remifentanil) (Table 1).1,2
Table 1.
Classification of opioids by synthetic process.
| Naturally occurring compounds | Semi-synthetic compounds | Synthetic compounds |
|---|---|---|
| Morphine | Diamorphine (heroin) | Pethidine |
| Codeine | Dihydromorphone | Fentanyl |
| Thebaine | Buprenorphine | Methadone |
| Papaverine | Oxycodone | Alfentanil |
| Remifentanil | ||
| Tapentadol |
Opioids can also be classified according to their effect at opioid receptors. In this manner opioids can be considered as agonists, partial agonists, antagonists and agonist-antagonists. Agonists interact with a receptor to produce a maximal response from that receptor (analgesia following morphine administration). Conversely, antagonists bind to receptors but produce no functional response, while at the same time preventing an agonist from binding to that receptor (naloxone). Partial agonists bind to receptors but elicit only a partial functional response no matter the amount of drug administered (buprenorphine). Agonist-antagonists act as agonist at one opioid receptor but have antagonist activity at another opioid receptor (Nalbuphine).
Opioid receptors
Classically, there are considered to be three opioid receptors. These receptors are all G-protein-coupled receptors and were originally named mu (after morphine, its most commonly recognised exogenous ligand), delta (after vas deferens, the tissue within which it was first isolated) and kappa (after the first ligand to act at this receptor, ketocyclazocine). In 1996, the International Union of Basic and Clinical Pharmacology (IUPHAR) renamed the receptors OP1 (the delta receptor), OP2 (the kappa receptor) and OP3 (the mu receptor). In 2000, this nomenclature was again changed to DOP, KOP and MOP (Table 2).3 Currently, however, owing to the extensive literature previously using the Greek nomenclature for opioid receptors (δ, κ and µ), the nomenclature committee of IUPHAR (NC-IUPHAR) recommends using this classification and the DOP, KOP and MOP classification of 2000.4 Some authorities describe the existence of multiple subtypes of the three classical opioid receptors, but this is not a belief held by all researchers within the field.5 The classical opioid receptors are distributed widely within the central nervous system and, to a lesser extent, throughout the periphery, occupying sites within the vas deferens, knee joint, gastrointestinal tract, heart and immune system, among others.6
Table 2.
Changes in the classification of the classical opioid receptors over time.
| Pre cloning Before 1992 |
Post cloning 1992–1996 |
IUPHAR 1996 |
IUPHAR 2000 |
Current NC-IUPHAR |
|---|---|---|---|---|
| δ | DOR | OP1 | DOP | DOP or δ |
| κ | KOR | OP2 | KOP | KOP or κ |
| µ | MOR | OP3 | MOP | MOP or µ |
| OP4 | NOP | NOP |
In 1994, a fourth G-protein-coupled endogenous opioid like receptor was found and was subsequently named the nociceptin (NOP) receptor. Though the NOP receptor does not bind naloxone, it is a G-protein-coupled receptor system that shares a marked similarity to the known amino acid sequences of the classical opioid receptors.3,7 At a cellular level, when the NOP receptor is activated by an agonist, it produces similar actions to those described for the classical opioid receptors above. For these reasons, it has been classified as the fourth opioid receptor; however, owing to its lack of response to the classical opioid antagonist (naloxone) some pharmacologists have questioned the wisdom of this classification. NC-IUPHAR considers the NOP receptor to be a non-opioid branch of the opioid receptor family.4
Endogenous opioid ligands
Soon after the discovery of the opioid receptors, a series of endogenous ligands active at the receptors were discovered in brain extracts. Three pro-hormone precursors provide the parent compounds from which these endogenous ligands are derived. Proenkephalin is cleaved to form met-enkephalin and leu-enkephalin, which bind to the DOP receptor. Dynorphin A and B are derived from prodynorphin and are agonists at the KOP receptor. Pro-opiomelancortin (POMC) is the parent compound for β-endorphin, an agonist at the MOP receptor, though it is capable of displaying agonist activity at all three classical opioid receptors.2,3,8 Two further endogenous peptides act as agonists at the MOP receptor, endomorphin 1 and 2, but no precursor has yet been identified (Table 3). There is significant cross-talk between the endogenous agonists and the three classical receptors. The endogenous ligand of NOP receptor is nociceptin/orphanin FQ (N/OFQ), which is derived from the polypeptide precursor pre-pro-nociceptin.
Table 3.
Opioid receptors and their endogenous ligands and precursors.
| Receptor | Precursor | Peptide |
|---|---|---|
| DOP | Proenkephalin | [Met]-enkephalin |
| [Leu]-enkephalin | ||
| KOP | Pro-dynorphin | Dynorphin-A |
| Dynorphin-B | ||
| MOP | POMC | β-Endorphin |
| Unknown | Endomorphin-1 | |
| Endomorphin-2 | ||
| NOP | Pre-pro-nociceptin | N/OFQ |
In clinical practice the stimulation of the differing opioid receptors produces a range of effects, which are often dependent upon the location of the receptor. Agonists binding to MOP receptors may cause analgesia, but also sedation, respiratory depression, bradycardia, nausea and vomiting and a reduction in gastric motility. Activation of DOP receptors can cause spinal and supraspinal analgesia and reduce gastric motility, while KOP receptor stimulation may produce spinal analgesia, diuresis and dysphoria. Spinally, N/OFQ has been shown to produce analgesia and hyperalgesia, dependent upon the administered concentration, and allodynia. Supraspinally, when administered intracerebrovascularly, it is thought to produce a pro-nociceptive anti-analgesic effect, owing to an inhibition of endogenous opioid tone.3,9
Intracellular events
Though producing subtly different functional effects, all of these receptors display similar cellular responses following receptor activation. Binding of an opioid agonist to a G-protein-coupled opioid receptor on the transmembrane portion of the receptor causes the α subunit of the G-protein to exchange its bound guanosine diphosphate (GDP) molecule with intracellular guanosine triphosphate (GTP). This then allows the α-GTP complex to dissociate away from the βγ complex. Both of these complexes (α-GTP and βγ) are then free to interact with target proteins. In the case of a classical opioid agonist binding to its G-protein receptor, this results in the inhibition of adenylyl cyclase. This in turn causes a reduction in intracellular cyclic adenosine monophosphate (cAMP) levels. In addition, these complexes interact with a number of ion channels, producing activation of potassium conductance and an inhibition of calcium conductance. The net effect of these changes is a reduced intracellular cAMP, a hyperpolarisation of the cell in question and, for neuronal cells, reduced neurotransmitter release (Figure 1). In some cell types, activation of opioid receptors can also paradoxically lead to an increase in the intracellular calcium concentration.8,10
Figure 1.
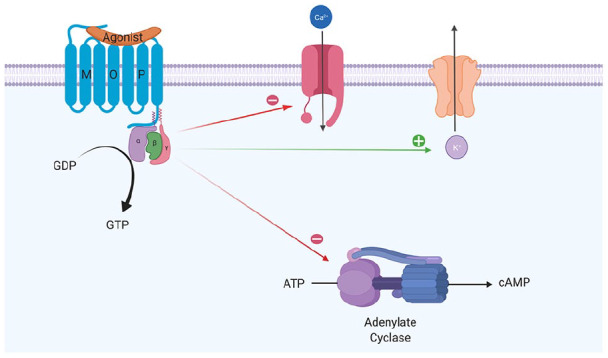
Intracellular changes occurring following the binding of an opioid agonist to a G-protein-coupled opioid receptor.
Opioid-mediated analgesia
Opioid receptors are distributed throughout the central nervous system and within peripheral tissue of neural and non-neural origin. Centrally, the periaqueductal grey (PAG), locus ceruleus and rostral ventral medulla show high concentrations of opioid receptors. Opioid receptors are also present in the substantia gelatinosa of the dorsal horn.
Within the central nervous system, activation of MOP receptors in the midbrain is thought to be a major mechanism of opioid-induced analgesia. Here, MOP agonists act by indirectly stimulating descending inhibitory pathways which act upon the periaqueductal grey (PAG) and nucleus reticularis paragigantocellularis (NRPG) with the net effect of an activation of descending inhibitory neurons. This leads to greater neuronal traffic through the nucleus raphe magnus (NRM), increasing stimulation of 5-hydroxytryptamine and enkephalin-containing neurons which connect directly with the substantia gelatinosa of the dorsal horn. This in turn results in a reduction of nociceptive transmission from the periphery to the thalamus. Exogenous and endogenous opioids can also exert a direct inhibitory effect upon the substantia gelatinosa (in the dorsal horn) and peripheral nociceptive afferent neurones, reducing nociceptive transmission from the periphery (Figure 2). This series of cellular events and mechanisms produces much of the analgesic effect commonly seen following the administration of MOP agonists.
Figure 2.
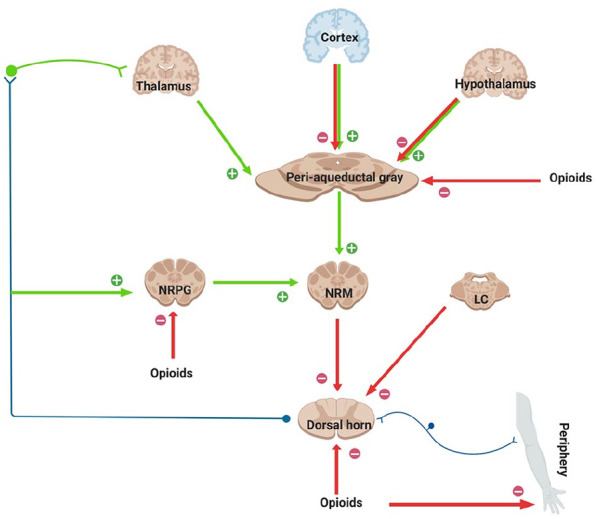
This figure shows schematically the descending inhibitory pathways. Areas shaded brown display a high expression of opioid receptors and their endogenous ligands. MOP agonists produce analgesia either by indirectly increasing neuronal traffic through the descending pathway at the NRPG and PAG, or by directly inhibiting nociceptive afferents in the periphery. MOP agonists act at the NRM to indirectly inhibit spinal pain transmission and, in addition, reduce spinal nociception.
Clinical opioids
All opioids used in clinical practice today exert their action, at least in part, at the MOP receptor, with some having additional activity at one or more further opioid receptor or receptors distinct from the opioid family. Of those drugs used in clinical practice, morphine, though generally considered to be the archetypal MOP agonist to which all other analgesics are compared, also displays some degree of activity at additional receptors, acting as an agonist at MOP receptors, but also having activity at both DOP and KOP receptors. While this MOP receptor agonism is responsible for the majority of the analgesic properties of opioids, activity at opioid receptors also accounts for many of the commonly observed side-effects seen with their use. Opioids may cause a reduction in conscious level and euphoria, making them drugs of abuse. They also exert effects on the respiratory system, reducing respiratory rate and obtunding airway reflexes, an effect which is considered advantageous during anaesthesia. Although opioids are generally considered to preserve cardiac stability, histamine release and the associated reductions in systemic vascular resistance and blood pressure are marked with morphine. Among many other side-effects, opioids can also cause constipation, nausea, vomiting, urinary retention, pruritus, muscular rigidity, miosis and dysphoria in certain individuals. This list is by no means comprehensive, but, despite their numerous drawbacks to this day, opioids remain the yardstick against which all other clinically effective analgesics are measured.
In clinical practice, morphine is frequently administered via oral or intravenous routes, although subcutaneous, transdermal, sublingual, intramuscular, epidural, intrathecal and intra-articular routes are also commonly utilised depending upon the setting. However, owing to its low lipid solubility, morphine penetrates the blood–brain barrier slowly, causing it to have a relatively slow onset of effect if administered via a route beyond this anatomical barrier. This means that even following intravenous administration, peak analgesic effect will not be achieved for some time, up to 15 minutes. Oral administration of morphine will further act to slow this onset of action and reduce morphine’s bioavailability. Typically, 40–60% of orally ingested morphine will fail to reach the systemic circulation as a result of significant first-pass metabolism in the liver and gut wall. Here, morphine is metabolised, predominantly via glucoronidation, to active metabolites excreted in urine. These metabolites, particularly in the case of morphine-6-glucuronide, can be longer lived and more potent than the parent compound, morphine. In health, therefore, morphine displays an elimination half-life of around 150 minutes, although this value may be altered by age, concomitant use of other medications and derangements of renal and hepatic function. In a clinical setting, it is often necessary to provide relatively frequent doses of morphine to allow for a consistent plasma and effect site concentration of morphine to optimise the analgesic effect.
Of the other opioids commonly encountered in an acute hospital setting, alfentanil, fentanyl and remifentanil all effectively act clinically as MOP receptor agonists, differing from morphine primarily in their pharmacokinetic properties. Alfentanil and fentanyl are both highly lipid soluble with a far more rapid onset of action than morphine. In the palliative care and chronic pain settings, such is fentanyl’s lipophilicity that it is often delivered via the sublingual or transdermal routes to avoid oral or intravenous administration. As with all highly lipid soluble drugs, prolonged administration of alfentanil or fentanyl may result in sequestration of the drug to fat stores. This in turn may result in an extended period of recovery during which the drug is cleared from the body as it is returned to the vascular compartment from the fatty tissues prior to excretion renally, on cessation of administration. Remifentanil differs in this respect as, although significantly more lipid soluble than morphine, it is rapidly metabolised extrahepatically by non-specific esterases in blood and tissue. Owing to this novel mechanism of metabolism, remifentanil is often chosen as a rapid short-acting opioid analgesic agent in theatre and intensive care, where patients may be sedated for prolonged periods of time and rapid clearance of drug is beneficial.
Opioids like codeine, oxycodone and buprenorphine are commonly used for chronic pain states. All act primarily on MOP receptors, but codeine first needs to be metabolised to morphine by the body for it to display any activity. Between 5% and 10% of the population are estimated to lack the ability to perform this conversion, so derive limited, if any, pain relief from it. Oxycodone, a potent semi-synthetic derivative of thebaine that mediates its analgesic properties through both MOP and KOP receptors, has a high oral bioavailability, which can be manufactured in a time-release preparation, allowing it to be administered twice a day.11 In contrast, buprenorphine, another agent commonly prescribed for chronic pain patients, is one of the few opioid partial agonists available for medical administration.3,12 In practice, this means that it produces analgesic effects at lower plasma concentrations via its interaction with the MOP receptor, but anti-analgesic effects at high doses through interactions with the KOP and NOP receptors. Along with the ability to deliver buprenorphine via a transdermal route, these properties make it a useful drug within the pain clinic, where its lower potential for respiratory depression and overdose than pure MOP agonists is highly advantageous.
Tramadol and methadone are two further commonly prescribed opioid receptor agonists that, in addition to MOP effects, also have activity at other non-opioid sites. Tramadol, a phenylpiperidine analogue of codeine with comparable analgesic effect, is thought to work through modulation of serotonin and norepinephrine reuptake, in addition to its action as an MOP receptor agonist. Although tramadol displays many of the side-effects associated with MOP receptor agonists, it is purported to produce less respiratory depression and fewer gastrointestinal side-effects than pure MOP agonists of comparable analgesic potency. It may, however, also interact with drugs that inhibit serotonin and noradrenaline reuptake centrally, leading to seizures. The synthetic opioid methadone, meanwhile, with its long duration of action, limited first-pass metabolism, high bioavailability and more limited potential to induce euphoria, is often used as an oral opioid substitute in individuals addicted to intravenous opioids. In addition to its role in addiction medicine, methadone is sometimes used in the treatment of chronic pain where its postulated antagonistic activity at the N-methyl-d-aspartate (NMDA) receptor may account for some of its effectiveness in neuropathic pain states. A third dual-action centrally acting analgesic agent, Tapentadol, can be used in moderate to severe acute pain and as a prolonged-release preparation for chronic pain. Tapentadol displays MOP receptor agonist and noradrenaline reuptake inhibitor properties and is purported to have comparable analgesic efficacy to controlled-release oxycodone.
In various medical settings, reversal of the effects of opioid analgesia may be required, particularly for patients with markedly depressed respiratory function or conscious level. Naloxone and naltrexone can be used to achieve this reversal through their action as antagonists at all three of the classical opioid receptors. They do not, however, bind to the NOP receptor, and therefore would not modulate the effects of any future NOP agonists or partial agonists.
Opioid use disorder (OUD)
Opioid use disorder is characterised by problematic pattern of opioid use which includes loss of control of opioid use, persistent desire, craving to use opioids despite impaired social functioning, tolerance and withdrawal.13 In 2017, a nationwide public health emergency was declared in the United States regarding opioid crisis, where increased opioid prescription has led to misuse of both prescription and non-prescription opioids, opioid overdose and death. In the United States, opioid overdoses accounted for more than 42,000 deaths in 2016 and 40% of opioid overdose deaths involved a prescription opioid. Opioid prescriptions have increased by 34% in England between 1998 and 2016. If the prescription of total oral morphine equivalency was compared between the same years, the rise was 127%.14 This opioid epidemic in the United Kingdom should be controlled and a structured approach to opioid prescription should be followed along with patient education.
Harm from long-term use of opioids for chronic non-cancer pain
The effective use of opioids for managing acute pain and in palliative care is well established. However, there is little evidence to support the use of opioids in chronic non-cancer pain,15,16 with a series of Cochrane reviews questioning the use of opioids in this setting.17–20 Despite this, a recent UK randomised controlled trial showed that 59% of chronic pain participants from 25 general practices and two community musculoskeletal services were prescribed opioids.21 There is increased risk of adverse effects like insomnia, headaches, anxiety, depression, decreased concentration and memory, inadvertent overdose14 and death with the long-term use of opioids. Tapering of opioids should be considered in such scenarios.
Conclusion
An understanding of the pharmacology of opioids would help us tailor the treatment according to clinical needs of the patient. Opioids are effective analgesic agents for acute pain and pain in palliative care. However, we must use caution when prescribing opioids for chronic non-cancer pain, given the lack of effectiveness to improve pain or quality of life and adverse effects of long-term use of opioids. Opioid use disorder is an emerging public health concern.
Acknowledgments
Figures 1 and 2 were created by the authors using Biorender.com
Footnotes
Conflict of interest: The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship and/or publication of this article.
ORCID iD: Arul James  https://orcid.org/0000-0002-2609-6456
https://orcid.org/0000-0002-2609-6456
Multiple-choice questions
Which of the following statements are correct? More than one answer may be correct for each question.
- The following intracellular changes typically happen after an opioid agonist binds to the opioid receptor:
- Adenylate cyclase is inhibited
- Decrease in cAMP
- Increase in intracellular calcium level
- Increase in intracellular potassium level
- Activation of neuronal cell
- Long-term use of opioids for chronic pain
- Is recommended by the FPM
- Increases the risk of opioid abuse, addiction and side effects
- Is always effective
- Increases the risk of harm above morphine equivalent of 120 mg/day
- Increases the risk of death
- Morphine has:
- High lipid solubility
- potent metabolites
- a high first-pass metabolism
- an analgesic effect that can be reversed with naloxone
- delayed peak effect even after intravenous administration
- Tapentadol
- can induce seizures
- is a norepinephrine re-uptake inhibitor
- acts at MOP receptor
- is a prodrug
- analgesic efficacy is comparable to that of oxycodone
- Opioid receptors:
- Are found in high concentrations in periaqueductal grey, locus ceruleus and rostral ventral medulla
- are not found in substantia gelatinosa
- the nociceptin receptor is considered a non-opioid branch of the opioid receptor family
- stimulation may produce analgesia
- all have endogenous ligands
Answers
(a) True; (b) True; (c) False; (d) False; (e) False.
(a) False; (b) True; (c) False; (d) True; (e) True.
(a) False; (b) True; (c) True; (d) True; (e) True.
(a) True; (b) True; (c) True; (d) False; (e) True.
(a) True; (b) False; (c) True; (d) True; (e) True.
References
- 1. Blakemore PR, White JD. Morphine, the Proteus of organic molecules. Chem Commun 2002; 11: 1159–1168. [DOI] [PubMed] [Google Scholar]
- 2. Charlton JE. (ed.) Opioids: core curriculum for professional education in pain. Seattle, WA: IASP Press, 2005. [Google Scholar]
- 3. McDonald J, Lambert DG. Opioid receptors. Contin Educ Anaesth Crit Care Pain 2005; 5: 22–25. [Google Scholar]
- 4. Cox BM, Christie MJ, Devi L, et al. Challenges for opioid receptor nomenclature: IUPHAR Review 9. Br J Pharmacol 2015; 172(2): 317–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dietis N, Rowbotham D, Lambert DG. Opioid receptor subtypes: fact or fiction? British J Anaesth 2011; 107: 8–18. [DOI] [PubMed] [Google Scholar]
- 6. Stein C, Schafer M, Machelska H. Attacking pain at its source: new perspectives on opioids. Nat Med 2003; 9(8): 1003–1008. [DOI] [PubMed] [Google Scholar]
- 7. Calo’ G, Guerrini R, Rizzi A, et al. Pharmacology of nociceptin and its receptor: a novel therapeutic target. Br J Pharmacol 2000; 129(7): 1261–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Corbett AD, Henderson G, McKnight AT, et al. 75 years of opioid research: the exciting but vain quest for the Holy Grail. British J Pharmacology 2006; 147: S153–S162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pan Z, Hirakawa N, Fields HL. A cellular mechanism for the bidirectional pain-modulating actions of orphanin FQ/nociceptin. Neuron May; 26: 515–522. [DOI] [PubMed] [Google Scholar]
- 10. Harrison C, Smart D, Lambert DG. Stimulatory effects of opioids. Br J Anaesth 1998; 81: 20–28. [DOI] [PubMed] [Google Scholar]
- 11. Nielsen CK, Ross FB, Lotfipour S, et al. and morphine have distinctly different pharmacological profiles: radioligand binding and behavioural studies in two rat models of neuropathic pain. Pain 2007; 132(3): 289–300. [DOI] [PubMed] [Google Scholar]
- 12. Ide S, Minami M, Satoh M, et al. Buprenorphine antinociception is abolished, but naloxone-sensitive reward is retained, in mu-opioid receptor knockout mice. Neuropsychopharmacology 2004; 29(9): 1656–1663. [DOI] [PubMed] [Google Scholar]
- 13. American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM-5). 5th ed. Washington, DC: American Psychiatric Publishing, 2013, https://www.psychiatry.org/psychiatrists/practice/dsm [Google Scholar]
- 14. Office for National Statistics (ONS). Deaths related to drug poisoning in England and Wales, 2014 registrations. 1st ed. ONS, 2014, http://www.ons.gov.uk/ons/dcp171778_414574.pdf [Google Scholar]
- 15. Ashworth J, Green DJ, Dunn KM, et al. Opioid use among low back pain patients in primary care: is opioid prescription associated with disability at 6-month follow-up? Pain 2013; 154(7): 1038–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chou R, Turner JA, Devine EB, et al. The effectiveness and risks of long-term opioid therapy for chronic pain: a systematic review for a national institutes of health pathways to prevention workshop. Ann Intern Med 2015; 162(4): 276–286, https://annals.org/aim/fullarticle/2089370/effectiveness-risks-long-term-opioid-therapy-chronic-pain-systematic-review [DOI] [PubMed] [Google Scholar]
- 17. Deshpande A, Furlan AD, Mailis-Gagnon A, et al. Opioids for chronic low-back pain. Cochrane Database Syst Rev 2007; 3: CD004959. [DOI] [PubMed] [Google Scholar]
- 18. Nüesch E, Rutjes AWS, Husni E, et al. Oral or transdermal opioids for osteoarthritis of the knee or hip. Cochrane Database Syst Rev 2009; 4: CD003115. [DOI] [PubMed] [Google Scholar]
- 19. Eisenberg E, McNicol ED, Carr DB. Opioids for neuropathic pain. Cochrane Database Syst Rev 2006; 3: CD006146. [DOI] [PubMed] [Google Scholar]
- 20. Noble M, Treadwell JR, Tregear SJ, et al. Long-term opioid management for chronic noncancer pain. Cochrane Database Syst Rev 2010; 1: CD006605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ashaye T, Hounsome N, Carnes D, et al. ; on behalf of the COPERS Study Team (ISRCTN 24426731). Opioid prescribing for chronic musculoskeletal pain in UK primary care: results from a cohort analysis of the COPERS trial. BMJ Open 2018; 8: e019491. [DOI] [PMC free article] [PubMed] [Google Scholar]
Further readings
- Opioids aware: a resource for patients and healthcare professionals to support prescribing of opioid medicines for pain, https://www.fpm.ac.uk/faculty-of-pain-medicine/opioids-aware
- Opioid receptors (version 2019.4) in the IUPHAR/BPS Guide to Pharmacology Database. IUPHAR/BPS Guide Pharmacol CITE 2019; 2019(4). 10.2218/gtopdb/F50/2019.4 [DOI] [Google Scholar]
- Busse JW, Craigie S, Juurlink DN, et al. The 2017 Canadian Guideline for Opioids for Chronic Non-Cancer Pain – Guideline for opioid therapy and chronic noncancer pain. CMAJ 2017; 189(18): E659–E666. [DOI] [PMC free article] [PubMed] [Google Scholar]


