Abstract
The gabapentinoids are often recommended as first-line treatments for the management of neuropathic pain. The differing pharmacodynamic and pharmacokinetic profiles can have implications for clinical practice. This article has summarised these key differences. In addition to their use in managing neuropathic pain, gabapentinoids are increasingly being used for off-label conditions despite the lack of evidence. Prescription rates for off-label conditions have overtaken that for on-label use. Similarly, the use of gabapentinoids in the perioperative period is now embedded in clinical practice despite conflicting evidence. This article summarises the risks associated with this increasing use. There is increasing evidence of the potential to cause harm in vulnerable populations such as the elderly and increasing prevalence of abuse. The risk of respiratory depression in combination with opioids is of particular concern in the context of the current opioid crisis. This article describes the practical considerations involved that might help guide appropriate prescribing practices.
Keywords: Gabapentin, pregabalin, pain management, adverse effects, pharmacology
Introduction
The gabapentinoid drugs gabapentin and pregabalin are antiepileptic drugs that are considered as first-line treatments for the management of neuropathic pain.1 Pregabalin is also approved for generalised anxiety disorders in the United Kingdom. The mechanisms of action are still unclear despite their widespread use. The gabapentinoids share similar mechanisms of action but differ considerably in their pharmacokinetic and pharmacodynamic characteristics. This article discusses the differences in these characteristics.
In addition to the use in neuropathic pain, off-label use in primary care is common, accounting for more than half of the prescriptions in primary care in the United Kingdom.2,3 Off-label use includes management of a wide range of conditions such as bipolar disorder, complex regional pain syndrome, attention deficit disorder, restless legs syndrome, periodic limb movement, sleep disorders, headaches, alcohol withdrawal syndrome, chronic back pain, fibromyalgia, visceral pain and acute postoperative pain.4,5 The rate of new prescriptions is increasing and tripled in England from 2007 to 2017.6 Pregabalin prescriptions in England increased from 2.7 million scripts in 2013 to 7 million scripts in 2018.6 Similarly, gabapentin prescriptions increased from 3.5 million scripts to about 7 million scripts.6 This increase in prescription rates does not correlate with the evidence for effectiveness in clinical practice. This article aims to discuss some of the evidence for efficacy and suggests strategies to promote appropriate use in clinical practice.
Development of gabapentin and pregabalin
Gabapentin was first conceptualised in the early 1970s during efforts to discover drugs for treating neurological disorders.7 Gamma-aminobutyric acid (GABA) was known to be a key inhibitory neurotransmitter, whose inhibition could cause seizures. Lipophilic groups were added to the carbon backbone to increase the bioavailability of GABA, as it does not penetrate the blood–brain barrier.8 This led to the serendipitous discovery of gabapentin as a potent anticonvulsant. The development of pregabalin was similarly fortuitous. The 3-alkyl-4-aminobutyric acids were analysed to determine their effects on glutamic acid decarboxylase (GAD) that is required for the synthesis of GABA.9 The S-enantiomer of 3-isobutyl GABA, now known as pregabalin, was found to be an effective anticonvulsant. They do not bind to the GABA receptor despite being structurally similar to GABA as can be seen in Figure 1.10 These drugs were initially marketed as off-label treatment for pain and eventually were approved by the Food and Drug Administration (FDA) for treatment of postherpetic neuralgia.
Figure 1.
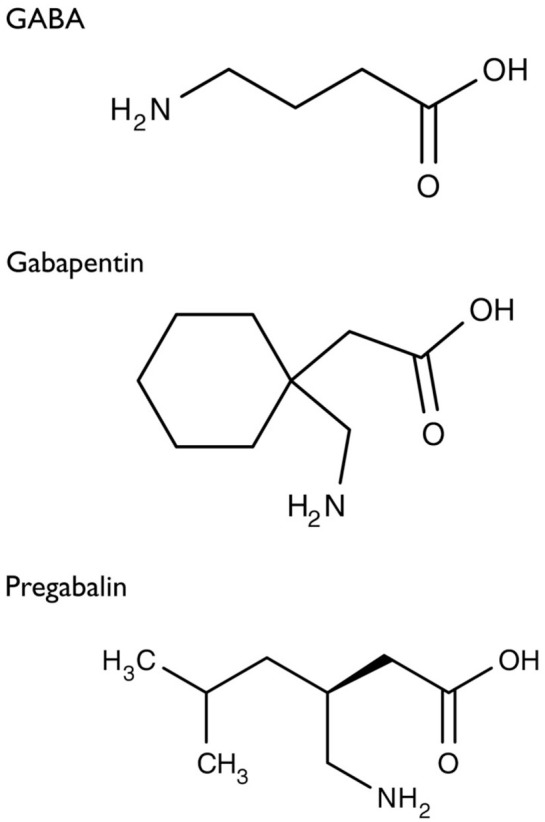
Structure of GABA: gabapentin and pregabalin.10
Pharmacokinetics
The actions of gabapentinoids are mainly at an intracellular site and require active uptake. They undergo facilitated transport across cell membranes through system l-amino acid transporters (LAT) as both drugs are structurally similar to the amino acid leucine. The effects of chronic gabapentin are blocked by an inhibitor of these transporters.11 The gabapentinoids differ in their pharmacokinetic characteristics (Table 1).
Table 1.
| Gabapentin | Pregabalin | |
|---|---|---|
| Tmax (hours) | 2–3 | 1 |
| t1/2 (hours) | 5–7 | 5.5–6.7 |
| Bioavailability | 27–60% | >90% |
| Pharmacokinetics | Nonlinear (zero-order) | Linear |
| Plasma protein binding | <3% | Assumed to be zero |
| Potency at the α2δ1 subunit | + | ++ |
| Metabolism | Nil | Very limited if any metabolism occurs. Some patients may have scant N-methylation |
| Renal excretion | 100% unchanged | 92–99% unchanged |
| Suggested dosing schedule | Three or four times daily/ | Two or three times daily |
| Usual dose | 900–3600 mg/day | 150–600 mg/day |
| Time to effective dose using recommended titrations | 14 days | 5–7 days |
| Gabapentin dosing in renal impairment (creatinine clearance, mL/min) | ||
| 50–79 | 600–1800 mg/day in three divided doses | |
| 30–49 | 300–900 mg/day in three divided doses | |
| 15–29 | 150–600 mg/day (150 mg daily dose to be given as 300 mg in three divided doses on alternate days) | |
| <15 | 150–300 mg/day in three divided doses (150 mg daily dose to be given as 300 mg in three divided doses on alternate days) | |
| Pregabalin dosing in renal impairment (eGFR, mL/min/1.73 m2) | ||
| 30–60 | Initially 75 mg daily and maximum 300 mg daily | |
| 15–30 | Initially 25–50 mg daily and maximum 150 mg daily in one to two divided doses | |
| <15 | Initially 25 mg once daily and maximum 75 mg once daily | |
Absorption and distribution
Pregabalin is rapidly and completely absorbed as compared to gabapentin. Peak plasma concentrations are seen within an hour as compared to 3 hours with gabapentin.12 Oral bioavailability for pregabalin is more than 90% as compared to 30–60% for gabapentin. These differences can be explained by the mechanism of absorption. Although both gabapentinoids are absorbed in the small intestine, pregabalin is also absorbed in the proximal colon. Absorption of gabapentin is solely dependent on LAT that are easily saturable, resulting in dose-dependent pharmacokinetics. As the dose of gabapentin increases, the area under the plasma concentration–time curve (AUC) does not increase proportionally. In contrast, pregabalin has non-saturable absorption with a linear pharmacokinetic profile and less variable bioavailability as it may be transported by carriers in addition to LAT.12 Food has only a slight effect on the rate and extent of absorption of gabapentin but can substantially delay the absorption of pregabalin without affecting the bioavailability.12
Gabapentinoids do not bind to plasma proteins. They are actively transported across the blood–brain barrier by LAT-1.13 Peak cerebrospinal fluid levels take significantly longer to achieve than peak plasma levels, with a median time of 8 hours.14 They do not influence spinal neurotransmitter concentrations of glutamate, norepinephrine, substance P and calcitonin gene–related peptide.15 Both are highly water-soluble and the volume of distribution of each is 0.8 and 0.5 L/kg for gabapentin and pregabalin, respectively.12
Metabolism and excretion
They are not metabolised by the liver and do not affect the cytochrome P450 system, major cytochrome P450 system isoenzymes; however, drug-induced hepatotoxicity has been described in case reports.16 Elimination is mostly done by the kidney and is proportional to the creatinine clearance. Accumulation can cause renal failure resulting in adverse effects.
Formulations
Gabapentin is available in two extended-release formulations in addition to the immediate release: a gastric retentive formulation (GBP-GR) and a gastro-retentive prodrug gabapentin enacarbil that are approved for the management of postherpetic neuralgia. Gabapentin enacarbil is licensed for restless leg syndrome in the United States.17 GBP-GR is administered once daily and gabapentin enacarbil is administered in two divided doses.18 GBP-GR exhibits saturable absorption similar to immediate-release gabapentin but this is enhanced by high-fat content in meals.18 Pharmacokinetic comparisons show that gabapentin enacarbil has higher bioavailability and requires a threefold lower gabapentin equivalent dose compared to other formulations.18 It also provided sustained stable levels of gabapentin exposure over 24 hours as compared to the gastroretentive and immediate-release formulations.18 Both of these formulations are not licensed in the United Kingdom.
Both gabapentinoids are also available as a liquid formulation. This can be administered in children and patients who are unable to take solid food. Capsules can be opened and their contents dissolved in water before administering via a feeding tube.
Pharmacodynamics
Mechanisms of action
Gabapentin and pregabalin do not bind to GABA receptors despite their structural similarity but have a high affinity for the α2δ-1 subunit of voltage-gated calcium channels (VGCCs).19 VGCCs are composed of multiple subunits: α1, β, γ and α2δ. The α1 subunit allows entry of calcium and the extracellular α2δ is bound to the γ subunit.20
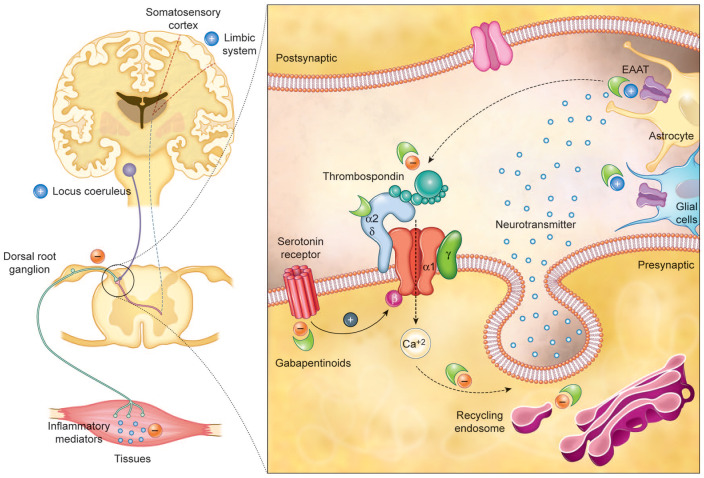
It is clear that α2δ-1 subunits are involved in nociception as levels are elevated after injury and can take several months to decline.21 Transgenic mice that express elevated levels of α2δ-1 develop neuropathic pain even in the absence of nerve damage.22 It is often assumed that the analgesic effects of gabapentinoids are due to inhibition of calcium currents by binding to the α2δ-1 subunit resulting in attenuation of postsynaptic excitability. However, this assumption is incorrect as gabapentinoids have not been shown to consistently inhibit Ca2+ currents.23 Despite this, they inhibit the release of various neurotransmitters at neuronal synapses and are effective in neuropathic pain. Several mechanisms have been postulated to explain the mechanisms of action (Figure 2):24
Figure 2.
Gabapentinoids inhibit calcium-mediated neurotransmitter release through effects on α2δ-1 subunits. They inhibit forward trafficking of α2δ-1 from the dorsal root ganglion, their recycling from endosomal compartments, thrombospondin-mediated processes and stimulate glutamate uptake by EAAT. Mechanisms not directly related to neurotransmitter release at dorsal horn include inhibition of descending serotonergic facilitation, stimulation of descending inhibition, anti-inflammatory actions and influence on the affective component of pain.24
α2δ-1 subunits are transported to the dorsal horn from their site of production in DRG (dorsal root ganglion) cell bodies. Elevated levels in the dorsal horn are associated with the development of neuropathic pain.25 Gabapentinoids inhibit the accumulation of α2δ-1 in the pre-synaptic terminals in the dorsal horn and reduce response to painful stimuli in animal models.25
α2δ-1 allows enhanced neurotransmitter release at decreased calcium influx. Gabapentinoids can influence nociception by inhibiting the α2δ-1-mediated enhanced neurotransmitter release.26
Analgesic effects are mediated by the facilitation of descending noradrenergic inhibition, inhibition of descending serotonergic facilitation and by cortical mechanisms affecting the limbic system.27–29
Gabapentinoids block the binding of thrombospondin derived from astrocytes to α2δ-1 which inhibits the formation of new excitatory synapses.30
Stimulation of the uptake of glutamate by the excitatory amino acid transporters (EAAT).31
Suppression of the inflammatory response to injury.32
Modulation of the affective component of pain.33
Relative potency
The most significant pharmacodynamic difference between the gabapentinoids relates to their potency. There are few studies comparing their relative potencies. Bockbrader and colleagues12 developed a population pharmacokinetic model and calculated EC50 values for pregabalin and gabapentin. The EC50 values of pregabalin and gabapentin were estimated to be about 9.77 and 23.9 mg/mL, respectively, based on studies in epilepsy, suggesting that pregabalin is about 2.4 times more potent than gabapentin. The EC50 of pregabalin and gabapentin was estimated to be about 4.21 and 11.7 mg/mL, respectively, in postherpetic neuralgia, suggesting pregabalin is 2.8 times more potent.12 Although potency can be a good indicator of therapeutic potential, it may not always correlate with clinical efficacy. The same study found differences in dose–response curves for analgesia. The analgesic response for gabapentin plateaued at 3600 mg/day but that for pregabalin continued to increase up to 450 mg/day.
Conversion between gabapentin and pregabalin
There is little evidence to guide conversion between gabapentin and pregabalin. The manufacturers recommend that the drugs are tapered over a minimum of 1 week. There is evidence to support direct switch between pregabalin and gabapentin but this is outside the terms of the product licence.34,35 A ratio of about 6:1 of gabapentin to pregabalin has been used based on the pharmacokinetic profiles.36 This study compared the tolerability of abrupt conversion from gabapentin to pregabalin with gradual replacement. Gabapentin was stopped with pregabalin started at the next scheduled dose in the abrupt conversion group. The other group gradually transitioned by reducing the dose of gabapentin by 50% by replacing with 50% of the desired pregabalin dose for 4 days. Both regimens were well tolerated. The dose conversion used was gabapentin 3600 mg/day to pregabalin 600 mg/day, gabapentin 1800 mg/day to pregabalin 300 mg/day and gabapentin 900 mg/day to pregabalin 150 mg/day.
Adverse effects
Adverse effects are common with gabapentinoids resulting in a discontinuation rate of at least 11%, but serious adverse events are uncommon.40,41 It is uncertain whether the incidence of adverse effects varies between the two gabapentinoids as there are no direct comparisons. The substitution of gabapentin with pregabalin in gabapentin responders resulted in improved pain relief and fewer adverse events.35 However, gabapentin non-responders who had adverse effects with gabapentin also experienced adverse effects with pregabalin.35
Central nervous system effects
Dizziness (19%), somnolescence (14%) and gait disturbances (14%) are the most common adverse effects.41 Patients must be warned about the potential to impact the ability to perform tasks that need concentration such as driving. The effects often occur during the initiation of treatment and can diminish after several weeks of treatment. Visual blurring occurs in about 7% of patients.37 Other common adverse effects affecting the central nervous system (CNS) include impaired concentration, confusion, memory loss, altered mood, movement disorders, sleep disorder, speech impairment and vertigo.38,39
Most adverse reactions involving the vestibulocerebellar/brainstem structures and higher cortical function have a clear dose–response relationship with increased risk of complications with higher doses.42 However, ocular adverse effects such as amblyopia and blurred vision appeared at lower doses of pregabalin 300 mg/day but diplopia was only seen at doses of 600 mg/day.42
Respiratory depression
Gabapentinoids do not have pharmacokinetic interactions but pharmacodynamic interactions can influence adverse effects. Respiratory depression has been described when used in combination with opioids resulting in an increased risk of accidental opioid-related mortality.43 This is of particular concern due to the increasing rates of co-prescription of these drugs. A large primary care database review showed that in 2017, 21.8% of patients with a new prescription for gabapentin and 24.1% of patients with a new prescription for pregabalin received a concomitant prescription, primarily for opioids.2 In response to increasing reports of respiratory depression, the Medicines and Healthcare Products Regulatory Agency (MHRA) released a drug safety alert regarding the rare risk of severe respiratory depression with gabapentin, with or without concomitant use of opioids.44 It recommended dose adjustments in patients with compromised respiratory function, respiratory or neurological disease, renal impairment, concomitant use of CNS depressants and elderly people as they might be at higher risk of experiencing severe respiratory depression.
Weight gain
Weight gain is common with gabapentinoids and can affect up to a quarter of all patients treated with pregabalin, resulting in non-compliance and termination of treatment.45,46 However, the extent of weight gain appears to be moderate. The majority of patients treated with pregabalin for 1 year maintain weight within ±7% baseline weight.47 Only one out of six patients gain ⩾7% weight from baseline about 2–12 months after initiation of treatment.47 Weight gain is related to dose and duration of drug exposure but not to body mass index, gender, age and development of oedema.37
Gastrointestinal effects
Gastrointestinal adverse effects such as abdominal distension, abnormal appetite, constipation, dry mouth and nausea are common and are dose-related with the exception of constipation.38,42 Peripheral oedema can affect 17% of all patients treated with pregabalin48 and has been associated with the development of heart failure in case reports.49 However, a large cohort study did not find an increased risk of heart failure with pregabalin as compared to gabapentin.50
Misuse
There is increasing awareness of the abuse potential of gabapentinoids, particularly in individuals with a history of opioid abuse.51 Both gabapentinoids have been reported to stimulate feelings of sociability, euphoria, calm and relaxation and can enhance psychoactive effects of other drugs.52 The abuse potential of pregabalin is higher as compared to gabapentin due to its pharmacokinetic properties.53 The incidence of abuse is significantly higher in secure settings. The prescription rate is double that in the general population with 2.8% of the prison population prescribed these drugs.54 Factors contributing to misuse include a high proportion of mental health disorders, substance misuse and high demand for tradable medicines.54 Two-thirds of prisoners have gabapentin or pregabalin initiated in a prison of which over half have a history of substance misuse. A total of 47% of prisoners were on concomitant opioid substitution treatments. The overdose of gabapentinoids alone is less likely to cause respiratory depression but can be lethal in combination with other CNS depressants.53 In response to concerns about medicinal misuse, diversion and addiction, pregabalin and gabapentin were reclassified as class C–controlled substances in the United Kingdom from 1 April 2019.55
Withdrawal
Withdrawal symptoms are common and appear between 12 hours and 7 days after cessation of use, with most cases occurring between 24 and 48 hours.56 More than half of the patients report agitation with confusion and disorientation reported by 45% of patients. Other symptoms that are similar to the withdrawal effects of benzodiazepines and alcohol include tachycardia, palpitations, anxiety, sweating, restlessness, hypertension, tremor, gastrointestinal symptoms, paranoia, auditory hallucinations and suicidal ideation.57 Patients with psychiatric comorbidities and the elderly may be at an increased risk of withdrawal.58 It has been hypothesised that the elderly are more vulnerable due to age-related reduction in GABA-mediated cortical inhibition and alterations in the expression of glutamate receptors.58 A slower tapering schedule such as a twice-weekly reduction of 10–25% of the dose has been suggested to minimise the risk of withdrawal effects.58
Toxicity
Gabapentin toxicity can occur in patients with chronic kidney disease.59 The risk of toxicity is higher in patients on dialysis. Patients present with symptoms such as increased sedation, confusion, unsteady gait, myoclonus, ataxia, episodic leg spasm, asterixis and tremulousness.59 Recommendations for dose reductions based on creatinine clearance are available.60 Patients on haemodialysis might require supplemental doses post-dialysis because dialysis removed approximately 35% of gabapentin and 50–60% of pregabalin. The dosing strategies are based on pharmacokinetic and toxicity studies but studies confirming their efficacy are lacking.
Efficacy in neuropathic pain
Several recommendations on the pharmacological management of neuropathic pain based on a review of randomised controlled trials are available. The Cochrane reviews of evidence for gabapentinoids in neuropathic pain have been recently updated.40,41 These reviews show moderate-quality evidence for pregabalin in postherpetic neuralgia, diabetic neuropathy and low-quality evidence for efficacy in post-stroke pain and after spinal cord injury. Pregabalin is not effective in neuropathic pain due to HIV. There is limited evidence for neuropathic back pain, neuropathic cancer pain and other forms of neuropathic pain. Gabapentin is effective to an extent in postherpetic neuralgia and diabetic neuropathy but the evidence in other forms of neuropathic pain is limited.
Clinical practice guidelines have been published by a number of international and regional professional associations, all of which recommend gabapentinoids as first-line therapy. The National Institute of Clinical Excellence (NICE) guidelines on the management of neuropathic pain recommend gabapentin, pregabalin, amitriptyline or duloxetine as the initial choice of treatment for neuropathic pain with the exception of trigeminal neuralgia.1 The guideline development group found that gabapentin was the most cost-effective followed by amitriptyline that had comparable costs per quality-adjusted life-year (QALY). Pregabalin and duloxetine were recommended as initial options due to their wider licences but did not provide the greatest net benefit at conventional QALY values. The group recommended that pregabalin and duloxetine should only be considered when gabapentin or amitriptyline is ineffective.
Despite these recommendations, the effects of most analgesics including gabapentinoids in neuropathic pain are modest with meta-analyses indicating that only a minority of patients benefit from pharmacological therapy.61,62 The combined number needed to treat (NNT) is 7.7 (6.5–9.4) for pregabalin and 7.2 (5.9–9.2) for gabapentin but this can be as high as 22 in painful diabetic neuropathy.41,62 These limited effects can be explained by the modest efficacy of drugs, high placebo response and heterogeneity in diagnostic criteria. The modest efficacy of gabapentinoids is not surprising as elevated levels of α2δ are not necessary for the development of neuropathic pain.63 Pharmacogenomic differences can also explain the inter-individual variability in responses.
Efficacy in non-neuropathic pain
The evidence for use in non-neuropathic pain and other off-label indications is poor.3 Despite the paucity of evidence, off-label prescriptions in primary care have continued to escalate and accounted for 52.0% of gabapentin and 54.8% of pregabalin prescriptions with an identified indication in 2017.2 Of these, non-neuropathic pain accounted for 80.4% of gabapentin and 58.3% of pregabalin prescriptions. It is likely that aggressive promotion by the manufacturers contributed to the rising prescription rates.64
Chronic back pain
They continue to be prescribed for the management of back pain despite evidence that they are ineffective and explicit recommendations from NICE.65 A meta-analysis of eight randomised controlled trials showed no significant improvement in pain with gabapentin over placebo in three of the studies.66 Pregabalin performed worse than other analgesics in three studies.66
Fibromyalgia
Pregabalin is often used for the management of chronic widespread pain due to conditions such as fibromyalgia. It is licensed for this indication by the FDA in the United States but not in the United Kingdom. The efficacy of pregabalin is however modest. A Cochrane review showed that it is highly effective in only a small proportion of patients with moderate to severe pain – about 10% more than placebo.67 The NNT for a reduction in pain by 30–50% in patients with moderate or severe fibromyalgia over 12–26 weeks is 10. It is likely that the efficacy is even lower as these studies are limited by their short duration of follow-up with a maximum duration of 26 weeks. The use of the last observation carried forward imputation method used in analyses of the primary outcomes could overestimate the treatment effect. There is little evidence to support the use of gabapentin in fibromyalgia.68
Perioperative pain
Gabapentinoids are often utilised perioperatively as part of multimodal analgesia to reduce opioid use that is associated with numerous adverse events such as nausea and vomiting, sedation and respiratory depression.69 They are embedded in enhanced recovery pathways, particularly for hip and knee replacement surgery. However, there is conflicting evidence to support their use.70,71 Most reviews demonstrate a reduction in perioperative opioid consumption after surgery72–76 but the quality of the trials included is moderate to very low.71 The reduction is often modest with a difference of as little as 5 mg of morphine in 24 hours.71 Although perioperative gabapentin did not affect time to postoperative pain resolution in a mixed cohort of surgical patients, it was associated with a modest increase in the rate of opioid cessation.77
This modest reduction in opioid use has to be balanced against the increased risk of adverse effects such as dizziness and increased sedation.71 Gabapentinoids increase the risk of opioid-induced respiratory depression.78 Continued use of chronic gabapentin in the postoperative period is associated with an increased rate of naloxone administration (OR: 6.30).79 Gabapentinoids are often used perioperatively to prevent chronic postsurgical pain evidence. However, the evidence is unclear with most trials limited by inadequate sample size and poor design.80 A Cochrane review concluded that gabapentinoids do not prevent chronic postsurgical pain.81
Considerations for clinical use
Pharmacological management of pain can be helpful in some patients with neuropathic pain. However, it is important to temper expectations when agreeing on a treatment plan as more than half of the patients treated for neuropathic pain with gabapentinoids do not have worthwhile pain relief.40 The rationale for why gabapentinoids are being offered should be discussed. Clinicians should emphasise that as with all medications in the management of chronic pain, gabapentinoids are used as part of a wider management plan. The goal is to achieve pain reduction so that patients can work towards functional improvement. The limited evidence for use should be discussed, particularly if they are prescribed for non-neuropathic pain. Many patients are unaware that it is impossible to predict whether medications will produce analgesia and that they may not get any pain relief, hence there is a need for a trial. Patients should be made aware of non-pharmaceutical options that might allow them to manage their pain effectively without the need for pharmacological therapies.
Adverse effects should be discussed along with the potential benefits. Patients should be warned about the risk of sedation and the impact on carrying out tasks that need them to concentrate. Significant numbers of patients discontinue therapy because of weight gain. The risk of respiratory depression should be assessed when initiating gabapentin in patients with respiratory comorbidities, neurological disease, renal impairment and in combination with other respiratory depressants such as opioids. Elderly patients and patients who misuse other drugs are at the highest risk of respiratory depression. Clinicians should assess the risk of misuse, dependence and diversion.
Patients should be made aware of the importance of dosage titration, the titration process and the requirement to take a stable regime for a few weeks before assessing for improvement in pain. Patients are often unaware that gabapentinoids cannot be taken as required and that taking an additional dose does not result in improved pain. Early clinical review after initiation of treatment will allow assessment of the effectiveness of the treatment and adverse effects.1 Each review should include an assessment of pain control, physical and psychological impact, adverse effects and need for continued treatment.1
Medications should be discontinued if there is no improvement or insufficient improvement in pain after a trial period. It has been suggested that patients should be reviewed at least annually to determine efficacy and the risk of misuse.82 A trial of reduction with possible cessation following a stable dose regime has been suggested.82
Perioperative use of gabapentinoids to reduce opioid consumption must be balanced against the risk of adverse effects such as dizziness and increased sedation, particularly in the elderly, who may be more vulnerable to the potential adverse effects.83 They should be used a part of an individualised analgesic plan taking into account comorbidities and the clinical situation. There is evidence that patients having ‘pro-nociceptive surgeries’ such as spinal surgery that may be associated with nerve damage may benefit from perioperative use.84 Opioid tolerant patients could benefit from even modest analgesic effects.85
Conclusion
Gabapentinoids can be effective in some patients with neuropathic pain but more than half of the patients fail to get worthwhile pain relief. Their efficacy in non-neuropathic pain is even less impressive. Although pregabalin has more favourable pharmacokinetics as compared to gabapentin, there is little evidence to support its preferential use. Any decision to prescribe gabapentinoids should involve consideration of the balance between the potential benefits and the risk of causing harm, particularly in vulnerable populations such as the elderly. Regular assessment of efficacy following a trial period is imperative and they should be discontinued if they fail to provide worthwhile pain relief.
Footnotes
Conflict of interest: The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Contributorship: M.C. contributed to conceptualisation, drafting, analysis and revisions of the manuscript.
Funding: The author(s) received no financial support for the research, authorship and/or publication of this article.
Guarantor: MC.
ORCID iD: Mahindra Chincholkar  https://orcid.org/0000-0002-1166-449X
https://orcid.org/0000-0002-1166-449X
References
- 1. National Institute of Clinical Excellence. Neuropathic pain in adults: pharmacological management in non-specialist settings, https://www.nice.org.uk/guidance/cg173 (2013, accessed 2 August 2019). [PubMed]
- 2. Montastruc F, Loo SY, Renoux C. Trends in first gabapentin and pregabalin prescriptions in primary care in the United Kingdom, 1993-2017. JAMA 2018; 320: 2149–2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Goodman CW, Brett AS. A clinical overview of off-label use of gabapentinoid drugs. JAMA Intern Med 2019; 179: 695–701. [DOI] [PubMed] [Google Scholar]
- 4. Mack A. Examination of the evidence for off-label use of gabapentin. J Manag Care Pharm 2003; 9: 559–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. González-Bueno J, Calvo-Cidoncha E, Desongles-Corrales T, et al. DI-024 off-label use of gabapentin and pregabalin in a tertiary hospital. Eur J Hosp Pharm 2015; 22: A84–A85. [Google Scholar]
- 6. NHS Digital. Prescription cost analysis: England, 2018 [PAS], https://digital.nhs.uk/data-and-information/publications/statistical/prescription-cost-analysis/2018 (2019, accessed 12 August 2019).
- 7. Thorpe A, Taylor C. Calcium channel α2-δ ligands: gabapentin and pregabalin. In: Taylor J, Triggle D. (eds) Comprehensive medicinal chemistry II, vol. 8 Boston, MA: Elsevier, 2007, pp. 227–246. [Google Scholar]
- 8. Satzinger G. Antiepileptics from gamma-aminobutyric acid. Arzneimittelforschung 1994; 44: 261–266. [PubMed] [Google Scholar]
- 9. Silverman RB. From basic science to blockbuster drug: the discovery of lyrica. Angew Chem Int Ed Engl 2008; 47: 3500–3504. [DOI] [PubMed] [Google Scholar]
- 10. Patel R, Dickenson AH. Mechanisms of the gabapentinoids and α2δ-1 calcium channel subunit in neuropathic pain. Pharmacol Res Perspect 2016; 4: e00205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Su TZ, Lunney E, Campbell G, et al. Transport of gabapentin, a γ-amino acid drug, by system l α-amino acid transporters: a comparative study in astrocytes, synaptosomes, and CHO Cells. J Neurochem 1995; 64: 2125–2131. [DOI] [PubMed] [Google Scholar]
- 12. Bockbrader HN, Wesche D, Miller R, et al. A comparison of the pharmacokinetics and pharmacodynamics of pregabalin and gabapentin. Clin Pharmacokinet 2010; 49: 661–669. [DOI] [PubMed] [Google Scholar]
- 13. Takahashi Y, Nishimura T, Higuchi K, et al. Transport of pregabalin via L-type amino acid transporter 1 (SLC7A5) in human brain capillary endothelial cell line. Pharm Res 2018; 35: 246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Buvanendran A, Kroin JS, Kari M, et al. Can a single dose of 300 mg of pregabalin reach acute antihyperalgesic levels in the central nervous system. Reg Anesth Pain Med 2010; 35: 535–538. [DOI] [PubMed] [Google Scholar]
- 15. Buvanendran A, Kroin JS, Della Valle CJ, et al. Cerebrospinal fluid neurotransmitter changes during the perioperative period in patients undergoing total knee replacement: a randomized trial. Anesth Analg 2012; 114: 434–441. [DOI] [PubMed] [Google Scholar]
- 16. Jackson CD, Clanahan MJ, Joglekar K, et al. Hold the gaba: a case of gabapentin-induced hepatotoxicity. Cureus 2018; 10: e2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kim ES, Deeks ED. Gabapentin enacarbil: a review in restless legs syndrome. Drugs 2016; 76: 879–887. [DOI] [PubMed] [Google Scholar]
- 18. Swearingen D, Aronoff GM, Ciric S, et al. Pharmacokinetics of immediate release, extended release, and gastric retentive gabapentin formulations in healthy adults. Int J Clin Pharmacol Ther 2018; 56: 231–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gee NS, Brown JP, Dissanayake VUK, et al. The novel anticonvulsant drug, gabapentin (neurontin), binds to the subunit of a calcium channel. J Biol Chem 1996; 271: 5768–5776. [DOI] [PubMed] [Google Scholar]
- 20. Doan L. Voltage-gated calcium channels and pain. Tech Reg Anesth Pain Manag 2010; 14: 42–47. [Google Scholar]
- 21. Luo ZD, Chaplan SR, Higuera ES, et al. Upregulation of dorsal root ganglion α2δ calcium channel subunit and its correlation with allodynia in spinal nerve-injured rats. J Neurosci 2001; 21: 1868–1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nguyen D, Deng P, Matthews EA, et al. Enhanced pre-synaptic glutamate release in deep-dorsal horn contributes to calcium channel α2δ-1 protein mediated spinal sensitization and behavioral hypersensitivity. Mol Pain 2009; 5: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Uchitel OD, Di Guilmi MN, Urbano FJ, et al. Acute modulation of calcium currents and synaptic transmission by gabapentinoids. Channels (Austin) 2010; 4: 490–496. [DOI] [PubMed] [Google Scholar]
- 24. Chincholkar M. Analgesic mechanisms of gabapentinoids and effects in experimental pain models: a narrative review. Br J Anaesth 2018; 120: 1315–1334. [DOI] [PubMed] [Google Scholar]
- 25. Bauer CS, Rahman W, Tran-Van-Minh A, et al. The anti-allodynic α2δ ligand pregabalin inhibits the trafficking of the calcium channel α2δ-1 subunit to presynaptic terminals in vivo. Biochem Soc Trans 2010; 38: 525–528. [DOI] [PubMed] [Google Scholar]
- 26. Zhou C, Luo ZD. Electrophysiological characterization of spinal neuron sensitization by elevated calcium channel alpha-2-delta-1 subunit protein. Eur J Pain 2014; 18: 649–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Suto T, Severino AL, Eisenach JC, et al. Gabapentin increases extracellular glutamatergic level in the locus coeruleus via astroglial glutamate transporter-dependent mechanisms. Neuropharmacology 2014; 81: 95–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kimura M, Eisenach JC, Hayashida K. Gabapentin loses efficacy over time after nerve injury in rats: role of glutamate transporter-1 in the locus coeruleus. Pain 2016; 157: 2024–2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lin HC, Huang YH, Chao TH, et al. Gabapentin reverses central hypersensitivity and suppresses medial prefrontal cortical glucose metabolism in rats with neuropathic pain. Mol Pain 2014; 10: 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Park J, Yu YP, Zhou C-Y, et al. Central mechanisms mediating thrombospondin-4-induced pain states. J Biol Chem 2016; 291: 13335–13348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ryu JH, Lee PB, Kim JH, et al. Effects of pregabalin on the activity of glutamate transporter type 3. Br J Anaesth 2012; 109: 234–239. [DOI] [PubMed] [Google Scholar]
- 32. Eroglu C, Allen NJ, Susman MW, et al. The gabapentin receptor α2δ-1 is a neuronal thrombospondin receptor responsible for excitatory CNS synaptogenesis. Cell 2009; 139: 380–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bannister K, Qu C, Navratilova E, et al. Multiple sites and actions of gabapentin-induced relief of ongoing experimental neuropathic pain. Pain 2017; 158: 2386–2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ifuku M, Iseki M, Hidaka I, et al. Replacement of gabapentin with pregabalin in postherpetic neuralgia therapy. Pain Med 2011; 12: 1112–1116. [DOI] [PubMed] [Google Scholar]
- 35. Toth C. Substitution of gabapentin therapy with pregabalin therapy in neuropathic pain due to peripheral neuropathy. Pain Med 2010; 11: 456–465. [DOI] [PubMed] [Google Scholar]
- 36. Bockbrader HN, Budhwani MN, Wesche DL. Gabapentin to pregabalin therapy transition: a pharmacokinetic simulation. Am J Ther 2013; 20: 32–36. [DOI] [PubMed] [Google Scholar]
- 37. Toth C. Pregabalin: latest safety evidence and clinical implications for the management of neuropathic pain. Ther Adv Drug Saf 2014; 5: 38–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. National Institute for Health and Care Excellence. British National Formulary, Pregabalin, https://bnf.nice.org.uk/drug/pregabalin.html#indicationsAndDoses (accessed 6 December 2019).
- 39. National Institute for Health and Care Excellence. British National Formulary, Gabapentin, https://bnf.nice.org.uk/drug/gabapentin.html#indicationsAndDoses (accessed 6 December 2019).
- 40. Wiffen PJ, Derry S, Bell RF, et al. Gabapentin for chronic neuropathic pain in adults. Cochrane Database Syst Rev 2017; 6: CD007938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Derry S, Bell RF, Straube S, et al. Pregabalin for neuropathic pain in adults. Cochrane Database Syst Rev 2019; 1: CD007076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zaccara G, Gangemi P, Perucca P, et al. The adverse event profile of pregabalin: a systematic review and meta-analysis of randomized controlled trials. Epilepsia 2011; 52: 826–836. [DOI] [PubMed] [Google Scholar]
- 43. Gomes T, Juurlink DN, Antoniou T, et al. Gabapentin, opioids, and the risk of opioid-related death: a population-based nested case-control study. PLoS Med 2017; 14: e1002396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Medicines and Healthcare Products Regulatory Agency. Gabapentin (Neurontin): risk of severe respiratory depression, https://www.gov.uk/drug-safety-update/gabapentin-neurontin-risk-of-severe-respiratory-depression (2017, accessed 29 September 2019).
- 45. Ryvlin P, Perucca E, Rheims S. Pregabalin for the management of partial epilepsy. Neuropsychiatr Dis Treat 2008; 4: 1211–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hoppe C, Rademacher M, Hoffmann JM, et al. Bodyweight gain under pregabalin therapy in epilepsy: mitigation by counseling patients. Seizure 2008; 17: 327–332. [DOI] [PubMed] [Google Scholar]
- 47. Cabrera J, Emir B, Dills D, et al. Characterizing and understanding body weight patterns in patients treated with pregabalin. Curr Med Res Opin 2012; 28: 1027–1037. [DOI] [PubMed] [Google Scholar]
- 48. Calkins A, Shurman J, Jaros M, et al. Peripheral edema and weight gain in adult patients with painful Diabetic Peripheral Neuropathy (DPN) receiving gabapentin enacarbil (GEn) or pregabalin enrolled in a randomized phase 2 trial (I6–1.004). Neurology 2014; 82: I6–1004. [Google Scholar]
- 49. Murphy N, Mockler M, Ryder M, et al. Decompensation of chronic heart failure associated with pregabalin in patients with neuropathic pain. J Card Fail 2007; 13: 227–229. [DOI] [PubMed] [Google Scholar]
- 50. Ho Macdonald EM, Luo J, Gomes T, et al. Pregabalin and heart failure: a population-based study. Pharmacoepidemiol Drug Saf 2017; 26: 1087–1092. [DOI] [PubMed] [Google Scholar]
- 51. Evoy KE, Morrison MD, Saklad SR. Abuse and misuse of pregabalin and gabapentin. Drugs 2017; 77: 403–426. [DOI] [PubMed] [Google Scholar]
- 52. Smith BH, Higgins C, Baldacchino A, et al. Substance misuse of gabapentin. Br J Gen Pract 2012; 62: 406–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bonnet U, Scherbaum N. How addictive are gabapentin and pregabalin? A systematic review. Eur Neuropsychopharmacol 2017; 27: 1185–1215. [DOI] [PubMed] [Google Scholar]
- 54. Specialist Pharmacy Services. Gabapentin and pregabalin offender health audit report and audit tool, https://www.sps.nhs.uk/articles/gabapentin-and-pregabalin-offender-health-audit-report-and-audit-tool/ (2013, accessed 30 September 2019).
- 55. GOV.UK. Pregabalin and gabapentin to be controlled as class C drugs, https://www.gov.uk/government/news/pregabalin-and-gabapentin-to-be-controlled-as-class-c-drugs (accessed 29 September 2019).
- 56. Mersfelder TL, Nichols WH. Gabapentin: abuse, dependence and withdrawal. Ann Pharmacother 2016; 50: 229–233. [DOI] [PubMed] [Google Scholar]
- 57. Barrett J, Kittler L, Singarajah C. Acute pregabalin withdrawal: a case report and review of the literature. Southwest J Pulm Crit Care 2015; 10: 306–310. [Google Scholar]
- 58. Mah L, Hart M. Gabapentin withdrawal: case report in an older adult and review of the literature. J Am Geriatr Soc 2013; 61: 1635–1637. [DOI] [PubMed] [Google Scholar]
- 59. Zand L, McKian KP, Qian Q. Gabapentin toxicity in patients with chronic kidney disease: a preventable cause of morbidity. Am J Med 2010; 123: 367–373. [DOI] [PubMed] [Google Scholar]
- 60. Raouf M, Atkinson TJ, Crumb MW, et al. Rational dosing of gabapentin and pregabalin in chronic kidney disease. J Pain Res 2017; 10: 275–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Attal N, Bouhassira D. Pharmacotherapy of neuropathic pain. Pain 2015; 156: S104–S114. [DOI] [PubMed] [Google Scholar]
- 62. Finnerup NB, Attal N, Haroutounian S, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol 2015; 14: 162–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Patel R, Bauer CS, Nieto-Rostro M, et al. α2δ-1 gene deletion affects somatosensory neuron function and delays mechanical hypersensitivity in response to peripheral nerve damage. J Neurosci 2013; 33: 16412–16426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Rhee TG, Ross JS. Association between industry payments to physicians and gabapentinoid prescribing. JAMA Intern Med. Epub ahead of print 8 July 2019. DOI: 10.1001/jamainternmed.2019.1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. National Institute for Health and Care Excellence. Low back pain and sciatica in over 16s: assessment and management, https://www.nice.org.uk/guidance/NG59/chapter/Recommendations#non-invasive-treatments-for-low-back-pain-and-sciatica (accessed 12 August 2019). [PubMed]
- 66. Shanthanna H, Gilron I, Rajarathinam M, et al. Benefits and safety of gabapentinoids in chronic low back pain: a systematic review and meta-analysis of randomized controlled trials. PLoS Med 2017; 14: e1002369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Derry S, Cording M, Wiffen PJ, et al. Pregabalin for pain in fibromyalgia in adults. Cochrane Database Syst Rev 2016; 9: CD011790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Cooper TE, Derry S, Wiffen PJ, et al. Gabapentin for fibromyalgia pain in adults. Cochrane Database Syst Rev 2017; 1: CD012188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Schmidt PC, Ruchelli G, Mackey SC, et al. Perioperative gabapentinoids choice of agent, dose, timing, and effects on chronic postsurgical pain. Anesthesiology 2013; 119: 1215–1221. [DOI] [PubMed] [Google Scholar]
- 70. Chou R, Gordon DB, de Leon-Casasola OA, et al. Management of postoperative pain: a clinical practice guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J Pain 2016; 17: 131–157. [DOI] [PubMed] [Google Scholar]
- 71. Fabritius ML, Strom C, Koyuncu S, et al. Benefit and harm of pregabalin in acute pain treatment: a systematic review with meta-analyses and trial sequential analyses. Br J Anaesth 2017; 119: 775–791. [DOI] [PubMed] [Google Scholar]
- 72. Zhang J, Ho KY, Wang Y. Efficacy of pregabalin in acute postoperative pain: a meta-analysis. Br J Anaesth 2011; 106: 454–462. [DOI] [PubMed] [Google Scholar]
- 73. Hurley RW, Cohen SP, Williams KA, et al. The analgesic effects of perioperative gabapentin on postoperative pain: a meta-analysis. Reg Anesth Pain Med 2006; 31: 237–247. [DOI] [PubMed] [Google Scholar]
- 74. Peng PW, Wijeysundera DN, Li CC. Use of gabapentin for perioperative pain control – a meta-analysis. Pain Res Manag 2007; 12: 85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Mishriky BM, Waldron NH, Habib AS. Impact of pregabalin on acute and persistent postoperative pain: a systematic review and meta-analysis. Br J Anaesth 2014; 114: 10–31. [DOI] [PubMed] [Google Scholar]
- 76. Doleman B, Heinink TP, Read DJ, et al. A systematic review and meta-regression analysis of prophylactic gabapentin for postoperative pain. Anaesthesia 2015; 70: 1186–1204. [DOI] [PubMed] [Google Scholar]
- 77. Hah J, Mackey SC, Schmidt P, et al. Effect of perioperative gabapentin on postoperative pain resolution and opioid cessation in a mixed surgical cohort. JAMA Surg 2018; 153: 303–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Myhre M, Diep LM, Stubhaug A. Pregabalin has analgesic, ventilatory, and cognitive effects in combination with remifentanil. Anesthesiology 2016; 124: 141–149. [DOI] [PubMed] [Google Scholar]
- 79. Deljou A, Hedrick SJ, Portner ER, et al. Pattern of perioperative gabapentinoid use and risk for postoperative naloxone administration. Br J Anaesth 2018; 120: 798–806. [DOI] [PubMed] [Google Scholar]
- 80. Reddi D. Preventing chronic postoperative pain. Anaesthesia 2016; 71: 64–71. [DOI] [PubMed] [Google Scholar]
- 81. Chaparro LE, Smith SA, Moore RA, et al. Pharmacotherapy for the prevention of chronic pain after surgery in adults. Cochrane Database Syst Rev 2013; 7: CD008307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. NHS Scotland Effective Prescribing and Therapeutics Branch. Chronic pain prescribing strategy: gabapentinoid prescribing, https://www.therapeutics.scot.nhs.uk/pain/ (accessed 6 December 2019).
- 83. Perucca E, Berlowitz D, Birnbaum A, et al. Pharmacological and clinical aspects of antiepileptic drug use in the elderly. Epilepsy Res 2006; 68: S49–S63. [DOI] [PubMed] [Google Scholar]
- 84. Eipe N, Penning J, Yazdi F, et al. Perioperative use of pregabalin for acute pain – a systematic review and meta-analysis. Pain 2015; 156: 1284–1300. [DOI] [PubMed] [Google Scholar]
- 85. Simpson G, Jackson M. Perioperative management of opioid-tolerant patients. BJA Educ 2017; 17: 124–128. [Google Scholar]