Abstract
The use of Cannabis-based preparations for medicinal use has waxed and waned in the multi-millennial history of human co-existence with the plant and its cultivation. Recorded use of preparations from Cannabis is effectively as old as recorded history with examples from China, India and Ancient Egypt. Prohibition and restriction of availability allowed a number of alternatives to take the place of Cannabis preparations. However, there has been a worldwide resurgence in medicinal Cannabis advocacy from the public. Media interest has been piqued by particular evocative cases. Altogether, therefore, there is pressure on healthcare professionals to prescribe and dispense Cannabis-based preparations. This review enunciates some of the barriers which are slowing the wider adoption of medicinal Cannabis.
Keywords: Cannabis, cannabidiol, tetrahydrocannabinol, cannabinoid
Introduction
In the 1830s–1840s, an engineer called William O’Shaughnessy, who had also trained in medicine in Edinburgh, was working in British-occupied India. Unusual for the time, he appears to have embraced the opportunity to investigate the ‘bountiful providence’ native to the subcontinent.1 His extensive writings prompted a change in the use of the Cannabis plant. In England (and elsewhere), the hemp plant had been used for centuries as a major source of fibre for rope and cloth (superseded by the availability of the finer fibres from cotton). O’Shaughnessy2 described the effects of preparations of ‘Indian hemp’ in ‘The Bengal Dispensatory and Companion to the Pharmacopoeia’, which was reviewed in the Provincial Medical and Surgical Journal of 1842, as providing ‘in a condensed and convenient form [794 pages], full information on the materia medica of the East’. O’Shaughnessy3 described investigating the effects of administering hemp extracts to three patients with rheumatism and the resultant symptoms of ‘catalepsy’ and ‘laughter . . . incontrollable’. He reported that the three were ‘relieved of their rheumatism’ and ‘discharged quite cured in 3 days thereafter’. When O’Shaughnessy returned from India, he brought with him a supply of herbal Cannabis, which was passed around to physicians as ‘Squire’s Extract’,4 prompting wider use and interest in the British Isles. Since that time, prohibition and restriction of usage in the 1920s–1930s, as well as the availability of alternative mass-produced medicines (including aspirin and heroin), limited the medicinal exploitation of Cannabis preparations. Since the turn of the 21st century, however, there has been a wider resurgence in investigating the therapeutic potential of the plant, driven in part by the public and media interest.
Current uses of Cannabis-based products for medicinal use in the United Kingdom
In the United Kingdom to date, two preparations as botanical-derived substances from the plant have been approved. Nabiximols is a combination of Δ9-tetrahydrocannabinol (THC) and cannabidiol (CBD) in a 40:40 mix, with the remainder made up of other compounds from the plant in minor (and variable) quantities. This is applied as a spray for buccal absorption and is approved as an adjunct for the treatment of the spasticity associated with multiple sclerosis. The second approved product is CBD itself. This is administered as an oral solution in sesame oil for patients suffering from seizures associated with Dravet and Lennox-Gastaut syndromes, as an adjunct treatment with clobazam.
In other parts of the world, Cannabis preparations are more widely used, including a proposed use in harm reduction associated with the US opioid crisis.5 In Canada, which has had a medicinal Cannabis programme since 2001, an online survey of 2032 patients published in 2018 identified that 42% of the patients were using it for pain syndromes, including chronic pain 29.4%; arthritis 9.3% and headache 3.7%.6 In the United Kingdom, the United Patients Alliance conducted a survey of the medicinal use of unregulated (illegal) Cannabis preparations in 2018 (https://www.upalliance.org/survey-2018). In total, 1750 respondents identified pain relief (10.7%) as a major reason, as well as a number of related symptoms, such as arthritis 3.8%, fibromyalgia 3.5%, migraine 3.1%, headache 2.7%, sciatica 2.5% and neuropathy. Other primary reasons were also cited which might have indirect influences on the subjective perception of pain, such as depression, anxiety, insomnia, muscle spasms and gut disorders. Clearly, therefore, there are people willing to break the law to make use of Cannabis-based preparations for medicinal purposes.
Advocates for medicinal Cannabis ask why should they not have more direct access to Cannabis-based preparations for medicinal use? Opponents would ask why should Cannabis be treated any differently to any other medicinal preparation in the United Kingdom (and in the wider world)?
Barrier 1: the complexity of Cannabis
There remains discussion about the taxonomy of Cannabis, which has been described as three species: Cannabis sativa, C. indica and C. ruderalis. While there are botanical descriptions that differentiate the three, there is less clarity about whether a genetic distinction exists.7 This may derive from the long history of association with humans – the plant has been exploited for medicinal purposes as well as oil, fibre and food for millennia.8 This proximity with humans has undoubtedly led to mutual changes in behaviour.
Although the names change over time and with geography, preparations from different parts of the Cannabis plant can be differentiated. The plant is an annual, which can readily grow over 3 m in height (when allowed to). The serrated edges of the leaves are a readily recognisable characteristic. It is mostly dioecious and Cannabis flowers are sticky because of the production of resinous secretions from associated trichomes.4 O’Shaughnessy described the dried flowering plant incorporating the resin as ‘Gunjah’, the larger leaves and capsules, without the stalks as ‘Bang, Subjee or Sidhee’.3 The resinous juices O’Shaughnessy referred to as ‘Churrus’ (Charas persists as a description in North Africa). We would nowadays more likely recognise these as ‘ganja’, ‘bhang’ and ‘hashish’, respectively. Alternative names abound.
The Cannabis plant generates a number of secondary metabolites (compounds not needed for common functions, such as storage or reproduction), some of which are found in a number of other plants. Terpenes are volatile hydrocarbons, which may be divided into monoterpenes derived from geranyl pyrophosphate, such as limonene, α-pinene and β-thujone, or sesquiterpenes derived from farnesyl pyrophosphate, such as bisabolol, γ-eudesmol and β-caryophyllene.9 Terpenes provide the characteristic aroma of Cannabis (and many other species of plant). The nearest neighbour genetically appears to be the hop plant (Humulus lupulus). Both Cannabis and hops accumulate terpenes and other chemicals in their flowers.
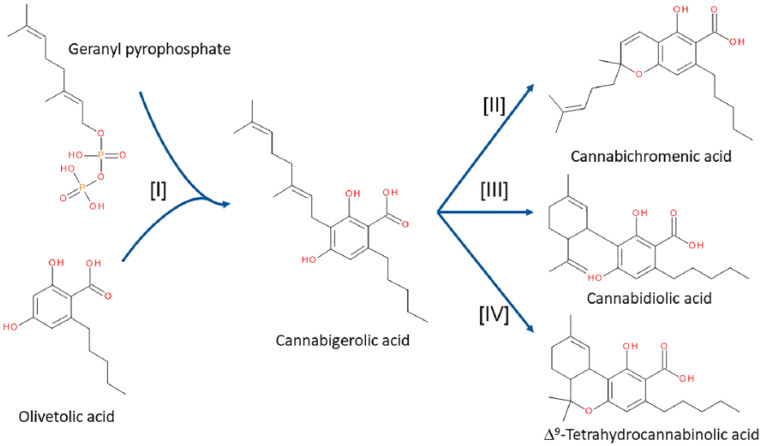
What makes Cannabis different (and probably unique) is the distinct group of enzymes which the plant expresses. The geranyl pyrophosphate used as a precursor for the monoterpenes can be conjugated to olivetolic acid to generate cannabigerolic acid (CBGA), catalysed by an enzyme termed geranyl pyrophosphate:olivetolate geranyltransferase (GOT)10 (Figure 1). Through the action of cannabichromenic acid (CBCA) synthase, cannabidiolic acid (CBDA) synthase and Δ9-tetrahydrocannabinolic acid (THCA) synthase, three distinct metabolites are formed: CBCA, CBDA and THCA, respectively (Figure 1). GOT can also make use of a shorter sidechain analogue of olivetolic acid, called divaric acid, to produce cannabigerovarinic acid (CBGVA), which acts as a precursor for cannabichromevarinic acid (CBCVA), cannabidivarinic acid (CBDVA) and Δ9-tetrahydrocannabivarinic acid (THCVA) through the same enzyme pathways. These metabolites are only part of the complexity found in the plant. Over 550 compounds have been identified in Cannabis preparations, with more than 110 being apparently unique.11 Although the vast majority of these cannabinoids are typically present to modest levels, THCA, CBDA and CBCA are often considered as the three major cannabinoids in the fresh plant.12
Figure 1.
Biosynthetic pathways of the principal cannabinoids in the Cannabis plant. CBGA is formed by conjugation of geranyl pyrophosphate to olivetolic acid under the influence of enzyme [I] GOT.10 CBGA is then the precursor for CBCA, CBDA and THCA formed by enzymes [II] CBCA synthase,13 [III] CBDA synthase14 and [IV] THCA synthase.15
Storage or heating (as in smoking, for example) of preparations from the Cannabis plant prompts decarboxylation of these acids, with the consequent accumulation of cannabichromene (CBC), CBD and THC. Over a protracted period of storage, THC itself can be oxidised further to cannabinol (CBN).16
Idiomatic usage defines two major ‘types’ of Cannabis plant based on the associated purpose of cultivation. ‘Hemp’ typically has a lower content of THCA/THC and is used for feed, fibre and/or oil isolation. ‘Marijuana’ (a term avoided by Cannabis scientists due to its racist connotations) typically has a higher content of THCA/THC and is used for spiritual and ‘recreational’ usage. Contraband samples of herbal Cannabis preparations seized in Switzerland17 and in the United States18 exhibited a highly variable range of metabolite levels, with ranges in excess of 10-fold in many cases (Table 1).
Table 1.
Cannabinoid content of confiscated Cannabis preparations as a range (% w/w).
| Metabolite | Switzerland17 | United States18 |
|---|---|---|
| CBD | 0.23–2.44 | nd–0.39 |
| CBDA | 6.66–13.77 | nd–2.11 |
| THC | 0.06–2.54 | 0.03–0.65 |
| THCA | 0.46–10.2 | 0.02–7.5 |
| CBN | 0.04–0.19 | Nd |
CBD: cannabidiol; CBDA: cannabidiolic acid; THC: Δ9-tetrahydrocannabinol; THCA: Δ9-tetrahydrocannabinolic acid; CBN: cannabinol; Nd: not detectable.
What these data illustrate is the intrinsic variability of the Cannabis plant and preparations from it. Although not expounded further here, there is a suggestion, termed the ‘entourage hypothesis’ that claims the necessity of ‘full spectrum’ sources, with minimal manipulation of the plant extracts, to achieve optimal effects. Given that the terpenes are also bioactive, there are claims that the beneficial effects are a result of the interactions and ‘synergies’ between the many hundreds of chemicals present in the plant. Testing this ‘entourage hypothesis’, with all of the multiple permutations and combinations possible, has not been conducted in a rational and systematic manner. Where more ‘natural’ preparations have been compared with isolated cannabinoids in vitro, any differences tend to be minor.19–21
So clearly one of the major obstacles to overcome is to have some precision of description. How can we compare (and expect reproducibility of) the human effects of Cannabis-based products for medicinal use (CBPM) across studies if we do not have details about the composition of active chemicals being administered?
Barrier 2: solidifying the evidence base
A number of clinical trials have investigated the potential analgesic benefits of Cannabis and related preparations. One systematic review identified that Cannabis/cannabinoids have modest/borderline benefit in a variety of chronic non-cancer pain conditions.22 Cannabinoids studies included ‘smoked Cannabis, oromucosal extracts of Cannabis-based medicine, nabilone, dronabinol and a novel THC analogue’. This is an example of the major obstacle defined above, in that like is very often not being compared to like when we consider ‘Cannabis’, since it is clearly not a single substance and the composition of Cannabis preparations can be hugely variable. In spite of that major reservation, the majority of the clinical trials meeting the inclusion criteria in the systematic review identified a significant analgesic effect with some improvement in sleep.22 A caveat of the review was that the majority of the clinical trials involved relatively few subjects and were continued for a relatively short period.
As a counterpoint to this issue of intrinsic variability, it should be noted that the mass-produced CBPM, currently available in Europe and North America, identify the content of THC and CBD in reproducible amounts from batch to batch. There is, therefore, the capacity to choose CBPM of high THC, for example, high CBD versions or alternatives with high CBD and minimal/absent THC.
Randomised controlled clinical trials (RCCT) using these defined preparations are scarce, and clearly there is a need to initiate and expand these to establish a reliable evidence base. A problem that will likely be encountered with any clinical trial is the well-recognised variability between individuals (see below). Cannabis practitioners (in parts of the world where medicinal Cannabis has been available for some time) typically attempt the administration of multiple versions/preparations before the patient identifies what appears to them to be the most effective. While this is an exemplar of the precision medicine we are striving for in many branches of healthcare, the capacity to compare like with like then becomes much more complicated when investigating whether CBPM effects are ‘real’.
A further barrier to the instigation of RCCT is the cost element. Getting support from major pharmaceutical companies (very much the experts in running large RCCT) for CBPM will be limited, since there is no clear intellectual property in the plant-derived cannabinoids, and hence no financial return on a very expensive venture.
Barrier 3: identifying the molecular mechanism/s of action
Of the multiple cannabinoids, THC is the most famous because it appears solely responsible for the well-recognised psychotropic effects of consumption of Cannabis preparations. THC was isolated/identified from hashish in 1964.23 The best-characterised molecular targets for THC are two G protein-coupled receptors called CB1 and CB2 cannabinoid receptors, which were identified almost 30 years later. The relevance of these two receptors for pain sensation is that the CB1 receptor is primarily associated with neurones, predominantly expressed presynaptically and linked to inhibition of transmitter release.24 CB1 receptors are found densely almost throughout the central nervous. Of particular relevance for the current consideration, they are present at key junctions in pain pathways, in the periphery, at primary afferent nerve terminals in the spinal cord and also in supraspinal pain nuclei.25 The CB2 receptor is associated with the immune system and so would potentially have an indirect positive effect on pain relief because of an anti-inflammatory profile.24
CB1 activation is, however, a double-edged sword. THC, through CB1 activation, evokes predictable objective symptoms that can be readily observed: a transient tachycardia and a reddening of the conjunctiva, but without changes to respiration rate, deep tendon reflexes, pupil size or responsivity.26 The tachycardia often resolves with repeated administration. More variable, however, are the subjective symptoms associated with the consumption of THC/Cannabis mediated by CB1 receptors. These include a brief period of euphoria (the ‘high’) followed by an extended period of drowsiness (the ‘dope’). The duration and extent of these are variable, both between individuals (see below for further consideration of inter-individual variability) and for the same individual with repeated dosing. Accompanying these, there is an impairment of short-term memory and dissociation from the environment.26 Occasionally, there are mild hallucinations and an increase in appetite, typically for more palatable foods (the “munchies”). Clearly, therefore, there are unwanted effects of THC, which would prompt a reluctance to prescribe and consume THC/Cannabis unless the benefits clearly outweigh the adverse effects. The use of rimonabant, a CB1-selective antagonist, which was available clinically in Europe from 2007–2008 for treating obesity/metabolic disorder, allowed confirmation of the role of CB1 receptors in peripheral27–29 and central28,30 effects of Cannabis consumption. In vitro, however, THC has been described to act through multiple other pathways, some of which are implicated in pain pathways.24 The relevance of these alternative molecular targets in pain sensation in humans has yet to be defined.
The second major cannabinoid, the levels of which are identified in CBPM, is CBD, the structure of which was identified 80 years ago.31 As identified above, CBD has recently gained approval for the treatment of specific forms of epilepsy. There are also more than 100 ongoing clinical trials investigating a variety of potential beneficial effects of CBD (see https://clinicaltrials.gov/ct2/results?cond =&term = cannabidiol&cntry =&state =&city =&dist =), some of which focus on pain relief as an outcome. Despite this extensive period of research on CBD, there is still no clear-cut molecular mechanism of action for its effects in vivo. There are, however, numerous low potency (typically 1–100 µM) in vitro effects of CBD: G protein-coupled receptor agonist32–34 or antagonist,35–37 nuclear hormone receptor agonist,38 enzyme inhibitor,39,40 transporter inhibitor,41 or ion channel agonist42–45 or antagonist/inhibitor.45–48 Both CBD and THC are non-specific antioxidants,49 which may also contribute to their effects in humans. Interestingly, CBD has been reported to ameliorate the effects of THC in humans,50,51 which has been used as an argument for explaining the adverse effects of high THC Cannabis (‘skunk’), in which the potential mitigating effects of CBD are not present.52 Of the multiple potential molecular targets described, none have yet been shown to be responsible for any effects of CBD seen in humans (admittedly a difficult task), although animal studies have implicated 5HT1A,53 CB1,54,55 CB2,54–56 TRPV155 and PPARγ57 as mediators of CBD effects in vivo. It may well be that the ‘rich pharmacology’ of CBD in having a number of low potency actions through multiple independent pathways is the actual molecular mechanism of action and also underlies the relatively minor list of adverse effects.
In animal models, CBG, CBGA, CBDA, CBC, CBCA and THCV produced complex effects,58–61 with little evidence of CB1/CB2 receptor involvement. However, there are limited reports of actions in humans. Part of the lack of an evidence base is the difficulty in isolation of individual cannabinoids in sufficient quantities for use in human/clinical investigations. CBN failed to elicit the changes in heart rate evoked by THC or modify the tachycardia when combined with THC.62 There were minor effects observed on the psychological effects of THC, without a clear effect of CBN itself.62 THCV reduced fasting plasma glucose and improved general glycaemic profile.63 When combined with THC, THCV produced a complex pattern of effects, reversing some symptoms and amplifying others.64 CBC does not appear to have been investigated as an isolated entity in humans, although varying the dose of CBC was reported not to alter the psychotropic effects of co-administered THC in a complex mixture.65
Clearly, therefore, some identification of the molecular mechanisms by which cannabinoids have their actions would greatly increase confidence in the use of CBPM.
Barrier 4: consistency of delivery and pharmacokinetics
Evidence for bioavailability of the major cannabinoids other than THC and CBD appears to be absent. The major route of delivery associated with Cannabis is smoking, although it has been suggested that only about a quarter of the THC content in a joint is delivered through the mainstream,66 making it an inefficient delivery mechanism.
Pharmacokinetic parameters for the cannabinoids are highly variable and influenced by a variety of identifiable (and less identifiable) factors. Accumulation of THC depended on the duration of smoke inhalation, draught volume inhaled and the time for holding the breath in the lungs, as well as the dose of THC/THCA present in the cigarette.67 Across nine subjects who were defined as light users of Cannabis, oral bioavailability was calculated to range from 2% to 22% and for heavy users was higher at 6–56%, with coefficients of variation (CV) of 70% for each group.68 In a group of six subjects administered small doses of THC intravenously, terminal half-lives ranged from 14.2 to 24.8 hours (and for metabolites was 43.3–54.3 hours), with a steady-state volume of distribution of 275–1005 L/70 kg.69 For these parameters, the CVs were 21%, 7% and 47%, respectively.
In a separate study, urinary THC excretion of 16.7 ± 2.6 (mean ± SD, CV = 16%) and 15.2 ± 4.2% (CV = 28%) in female and male subjects was observed in the first 72 hours following IV administration of a single dose of THC.70 Similar values were observed for oral administration (15.9 ± 3.6% and 13.4 ± 2% for women and men). Faecal excretion over 72 hours was much more variable at 25 ± 19% and 30 ± 16% for IV and 48 ± 6% and 53 ± 19% for oral administration for female and male subjects.
The pharmacokinetics of CBD has recently been reviewed, and the authors identified a paucity of information on its bioavailability.71 One study, however, identified that smoking CBD generated a bioavailability of 31 ± 13% (a coefficient of variation (CV) of 42%).72
What these numbers identify is the high levels of variability associated with THC and CBD administration. A further complication in assessing the pharmacokinetics of THC is its silo nature, in that the very hydrophobic nature of THC (and probably the majority of cannabinoids) means that it accumulates in adipose tissue.73
The metabolism of THC and CBD is initiated through cytochrome P450 activities in the liver. THC is oxidised to an 11-hydroxy metabolite, with the major isoform involved being CYP2C9.74 11–OH–THC was further metabolised to a glucuronide by UGT2B7 and UGT1A9.74 CBD is also oxidised by cytochrome P450s, 6β-hydroxy and 4ʺ-hydroxy metabolites, mainly by CYP3A4 and CYP2C19.75 A clinical trial investigated the effect of co-administration of CBD and clobazam in paediatric patients with treatment-resistant epilepsy identified that CBD administered orally led to an increase in plasma levels of clobazam.76 In a mouse model, CBD inhibited clobazam metabolism through cytochrome P450 activity and enhanced its anti-seizure capacity.77
What these data identify is that there is a high likelihood of drug–drug interactions with the principal cannabinoids (and also underline our lack of knowledge about the metabolism of the other cannabinoids). Clearly, greater information needs to be obtained on the potential interactions when co-administering CBPM with other medicinal agents, as well as other dietary supplements.
A further consideration associated with the measurement of cannabinoid pharmacokinetics is that of what levels following CBPM consumption would be considered ‘safe’ or acceptable in the context of driving or using machinery? A number of studies have attempted to assess levels of THC and its metabolites in the context of driving simulators. Despite the variation in study design, there is some correlation between blood THC levels following recent consumption of Cannabis preparations and driving impairment.78 Because blood and urinary levels of THC and/or its metabolites can result from consumption at least a week before testing, this test could not be considered as a reliable index of impairment.79
A meta-analysis of studies using the orally administered CBD: THC mixture in nabiximols identified that there appeared to be an improvement in driving ability, probably due to reduced spasticity.80
Guidance for police services and employers concerning the consumption of Cannabis and the effects of THC, in particular, tends to err towards a pragmatic approach of zero tolerance, although the practicalities of testing for relevant metabolites in biological samples are still being resolved.
Barrier 5: cost and identification of the payer
Unregulated Cannabis is a relatively cheap street drug source, as identified by the large number of consumers in the United Kingdom (see below) and elsewhere. As more reproducible sources and means of production of CBPM are involved in medicinal grade product, clearly those costs increase. A conundrum in the United Kingdom, at the moment, is who pays for these CBPM and what price should be charged? Nabiximols is typically made available for multiple sclerosis as a private prescription in England and Wales, while CBD for particular epilepsies is currently available as a ‘simple discount patient access scheme’ (https://www.nice.org.uk/guidance/TA615). It is noticeable that the introduction of wider access to non-medicinal Cannabis in Canada has not led to the eradication of the unregulated black market.81 Existing Cannabis users in the United Kingdom accessing the illegal market, and finding benefit from it, are highly likely to continue this practice unless they feel that the cost and access to regulated CBPM fit their needs.
Barrier 6: legal restrictions on fundamental research
Currently in the United Kingdom in 2020, Cannabis is listed as a Schedule 1 Controlled Drug, while CBPM were moved to Schedule 2 in November 2018 (http://www.legislation.gov.uk/uksi/2018/1055/made). While this re-scheduling has clearly facilitated the increases in clinical trials making use of CBPM, there remains a significant legal barrier in investigating preparations of Cannabis which are not designed specifically for medicinal purposes. In Germany, for example, laws limiting access to controlled drugs include an exemption for research institutions. In the United Kingdom, however, a Home Office licence, which needs to be renewed annually (costing ~£3k), is required for Universities and other academic institutions to purchase and to conduct research on compounds listed in Schedule 1. Further checks on personnel (through the Disclosure and Barring Service) also add to the costs of research. Removing these costs and reducing the associated bureaucracy, while still maintaining the good practices of record-keeping, secure storage and appropriate cheques and balances on access and usage, would be a positive step to facilitating the fundamental research needed to underpin sound clinical investigations.
The variation in legal status of Cannabis and CBPM in different countries raises two further points. One is that where individuals are moving across jurisdictions for business or tourism, there is a need to track the viability of carrying such items between countries (or between states). The second impact is on the possibility of multi-centre clinical trials, where the distribution of the potential medicinal entity will be greatly complicated having to adjust for the variation in legal status and the inevitable increase in associated paperwork.
The illegal nature of Cannabis in the United Kingdom and elsewhere has implications for the interactions between patients and healthcare professionals. Patients presenting for treatment to their physician or emergency department, or collecting medicines from their pharmacy, will be less likely to disclose their consumption of Cannabis and potentially put themselves at risk through a misdiagnosis or drug–drug interactions (see the discussion under Barrier 4).
Barrier 7: communicating the knowledgebase
There is a need to increase the level of understanding by pre-clinical and clinical scientists of which version/s of Cannabis/cannabinoids might be beneficial, how they act, as well as which dose/s and which route/s of administration are most effective. These factors are likely to be nuanced on the basis of the disease/disorder to be treated, the concurrent therapeutic agents being administered and genetic factors yet to be clarified. A final barrier to wider adoption is to get any message across to the appropriate healthcare professionals. A recent survey of healthcare professionals in hospices in the United States identified that 40% of staff and volunteers have received no education about medical Cannabis.82 Currently, there is very little, if any, teaching about Cannabis and cannabinoids on the curriculum for students of medicine, nursing and pharmacy in the United Kingdom. Part of the reason for that apparent deficiency is that curricula on those courses are already full with very high levels of contact time and a requirement for a detailed knowledge base. There is an underlying, appropriate focus on diagnostic training in medical curricula, which has squeezed the amount of pharmacology taught in medical schools. Traditionally, mechanisms of drug action were instead emphasised on pharmacy courses as important threads for future-proofing, allowing pharmacists to understand the mechanisms of drug–drug interactions and thereby improve patient safety. While that aspect of teaching continues, there is a greater emphasis on public-facing aspects of a pharmacist’s role, which is again an appropriate evolution.
A further education issue focusses on the general public and patients. Statistics from the crime survey of England and Wales (https://www.gov.uk/government/statistics/drug-misuse-findings-from-the-2018-to-2019-csew) indicate a very high proportion of respondents (~30%) had used Cannabis within their lifetime. This value has remained reasonably constant over the last 20 years (Figure 2). For those respondents who identified the use of Cannabis within the previous year, there were minor fluctuations between 6–8% over the last 10 years. In the 2018–2019 survey, 4.0% of respondents described using Cannabis in the previous month. By contrast, the numbers of respondents who identified the use of opiates in their lifetime was much lower (Figure 2), with values varying from 0.7% to 1.1% over the last two decades.
Figure 2.
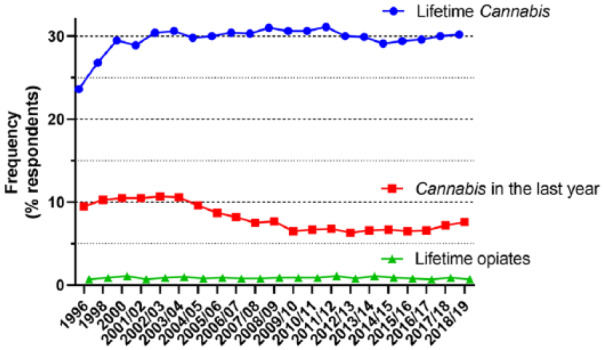
Data taken from the Crime Survey for England and Wales over the time period of 1996 to the most recent survey. Identified are the proportion of respondents who identified the use of Cannabis (blue circles) or opiates (green triangles) ever in their lifetime. Also indicated are the number of respondents who recorded Cannabis consumption in the previous year (red squares).
There are a number of potential inferences from these data. First, there is already wide experience of Cannabis consumption in the general public and, by extension, among patient populations. A second inference that ties in with a point made from the United Patients Alliance described earlier is that a large proportion of patients seeking pain relief will currently, or in recent history, be consumers of Cannabis.
Among a certain proportion of the public and patients, there will be hesitation about making use of CBPM. The logic of their position could essentially be expressed as ‘banned substances are banned for a reason’. It is possible to put a perspective to this using the UK Government’s statistics from the Home Office (https://www.ons.gov.uk/releases/deathsrelatedtodrugpoisoninginenglandandwales2018registrations). For England and Wales, on an annual basis, approximately half a million deaths are recorded. The Office for National Statistics publishes information on the numbers of deaths from drug-related poisoning and drug misuse (Figure 3). It should be stressed that naming the substance does not indicate that it was necessarily the cause of death. Many drugs are used as combinations, for instance, to either exaggerate the wanted effects or minimise unwanted effects. In the 2010s, there has been an increase in the number of non-alcohol deaths where selected substances were recorded from 2652 in 2011 to 4359 in 2018. As might be expected, the drug class with the highest recorded numbers is the opiates (increasing from 1439 in 2011 to 2208 in 2018). This is in the context of ~1% of respondents to the Crime Survey who have taken opiates ever in their lifetime and a projected estimate of 18,000 individuals who had reported opiate consumption in the last month (Figure 2). By contrast, the estimate for the number of Cannabis users in the previous month is 1.34 million. The corresponding number of deaths for 2018 where Cannabis is mentioned on the death certificate is 22. This is widely interpreted as a very low toxicity associated with acute administration of Cannabis.
Figure 3.
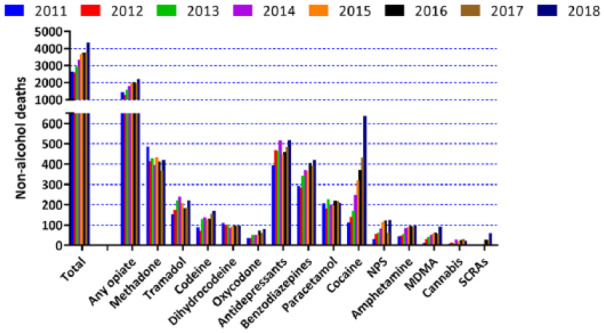
Data taken from the Office of National Statistics for the period 2011–2018 (the most recent statistics). Data shown are deaths linked to selected substances for England and Wales. Out of a total of around half a million annual deaths, about 1000–2000 are associated with opiates at the time of death, while the corresponding numbers for Cannabis are ~20.
That is not to say that Cannabis and CBPM are entirely ‘safe’, since this can only be a comparative process for any drug substance. There are risks associated with smoking Cannabis, although it remains unclear whether the risk of lung cancer is increased compared to tobacco.83 The adverse psychological impact of Cannabis is, however, a cause for concern, with a particular focus on cognitive and psychiatric disorders, such as schizophrenia.84 This issue is likely to be exacerbated in the developing brain (as with any exposure to psychoactive agents chronically) and we are likely to see a minimum age for (particularly) THC-containing CBPM. (It could be argued that the adoption of the minimum age of 21 years for access to non-medicinal Cannabis in Canada might still be too young.) It has been suggested that the risk for prompting or amplifying psychiatric issues is dose-related85 and that inter-individual variations in genes associated with dopaminergic signalling might modulate this phenomenon.86 Clearly, these risks would be minimised by pre-screening of patients to avoid exacerbation of any underlying psychiatric issues and continuous monitoring of patients during and following on from consumption of CBPM.
Conclusion
In this short review, some of the major obstacles that stand in the way of wider adoption of CBPM have been identified. These obstacles may well be surmountable, and it is encouraging to see the large numbers of clinical trials, both ongoing and planned, making use of CBPM. Given the wider availability of legal, non-medicinal Cannabis in many locations globally, there is likely to be an acceleration in the rate of investigation and, hopefully, adoption of CBPM in a variety of indications, many of which would likely include pain relief.
Footnotes
Conflict of interest: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Contributorship: SPHA
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Guarantor: SPHA is the guarantor.
ORCID iD: Stephen PH Alexander  https://orcid.org/0000-0003-4417-497X
https://orcid.org/0000-0003-4417-497X
References
- 1. MacGillivray N. Sir William Brooke O’Shaughnessy (1808–1889), MD, FRS, LRCS Ed: chemical pathologist, pharmacologist and pioneer in electric telegraphy. J Med Biogr 2017; 25(3): 186–196. [DOI] [PubMed] [Google Scholar]
- 2. O’Shaughnessy WB. The Bengal dispensatory, and companion to the pharmacopoeia. Prov Med Surgical J 1842; s1–5: 174. [Google Scholar]
- 3. O’Shaughnessy WB. The Bengal Dispensatory, and Companion to the Pharmacopoeia. Calcutta, India: Bishop’s College Press, 1841. [Google Scholar]
- 4. Russo EB. History of cannabis and its preparations in saga, science, and sobriquet. Chem Biodivers 2007; 4(8): 1614–1648. [DOI] [PubMed] [Google Scholar]
- 5. Lucas P. Rationale for cannabis-based interventions in the opioid overdose crisis. Harm Reduct J 2017; 14(1): 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Baron EP, Lucas P, Eades J, et al. Patterns of medicinal cannabis use, strain analysis, and substitution effect among patients with migraine, headache, arthritis, and chronic pain in a medicinal cannabis cohort. J Headache Pain 2018; 19(1): 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McPartland JM. Cannabis systematics at the levels of family, genus, and species. Cannabis Cannabinoid Res 2018; 33(1): 203–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pisanti S, Bifulco M. Medical cannabis: a plurimillennial history of an evergreen. J Cell Physiol 2019; 234(6): 8342–8351. [DOI] [PubMed] [Google Scholar]
- 9. Booth JK, Bohlmann J. Terpenes in Cannabis sativa – from plant genome to humans. Plant Sci 2019; 284: 67–72. [DOI] [PubMed] [Google Scholar]
- 10. Fellermeier M, Zenk MH. Prenylation of olivetolate by a hemp transferase yields cannabigerolic acid, the precursor of tetrahydrocannabinol. FEBS Lett 1998; 427(2): 283–285. [DOI] [PubMed] [Google Scholar]
- 11. Aizpurua-Olaizola O, Soydaner U, Ozturk E, et al. Evolution of the cannabinoid and terpene content during the growth of Cannabis sativa plants from different chemotypes. J Nat Prod 2016; 79(2): 324–331. [DOI] [PubMed] [Google Scholar]
- 12. Zirpel B, Kayser O, Stehle F. Elucidation of structure–function relationship of THCA and CBDA synthase from Cannabis sativa L. J Biotechnol 2018; 284: 17–26. [DOI] [PubMed] [Google Scholar]
- 13. Morimoto S, Komatsu K, Taura F, et al. Purification and characterization of cannabichromenic acid synthase from Cannabis sativa. Phytochemistry 1998; 49(6): 1525–1529. [DOI] [PubMed] [Google Scholar]
- 14. Taura F, Sirikantaramas S, Shoyama Y, et al. Cannabidiolic-acid synthase, the chemotype-determining enzyme in the fiber-type Cannabis sativa. FEBS Lett 2007; 581(16): 2929–2934. [DOI] [PubMed] [Google Scholar]
- 15. Sirikantaramas S, Morimoto S, Shoyama Y, et al. The gene controlling marijuana psychoactivity: molecular cloning and heterologous expression of Δ1-tetrahydrocannabinolic acid synthase from Cannabis sativa L. J Biol Chem 2004; 279: 39767–39774. [DOI] [PubMed] [Google Scholar]
- 16. Ross SA, Elsohly MA. CBN and D9-THC concentration ratio as an indicator of the age of stored marijuana samples. Bull Narc 1997; 49: 139–147. [Google Scholar]
- 17. Hadener M, Konig S, Weinmann W. Quantitative determination of CBD and THC and their acid precursors in confiscated cannabis samples by HPLC-DAD. Forensic Sci Int 2019; 299: 142–150. [DOI] [PubMed] [Google Scholar]
- 18. Ibrahim EA, Gul W, Gul SW, et al. Determination of acid and neutral cannabinoids in extracts of different strains of Cannabis sativa using GC-FID. Planta Med 2018; 84(4): 250–259. [DOI] [PubMed] [Google Scholar]
- 19. Blasco-Benito S, Seijo-Vila M, Caro-Villalobos M, et al. Appraising the ‘entourage effect’: antitumor action of a pure cannabinoid versus a botanical drug preparation in preclinical models of breast cancer. Biochem Pharmacol 2018; 157: 285–293. [DOI] [PubMed] [Google Scholar]
- 20. Romano B, Borrelli F, Pagano E, et al. Inhibition of colon carcinogenesis by a standardized Cannabis sativa extract with high content of cannabidiol. Phytomedicine 2014; 21(5): 631–639. [DOI] [PubMed] [Google Scholar]
- 21. Santiago M, Sachdev S, Arnold JC, et al. Absence of entourage: terpenoids commonly found in Cannabis sativa do not modulate the functional activity of Δ9-THC at human CB1 and CB2 receptors. Cannabis Cannabinoid Res 2019; 4: 165–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lynch ME, Campbell F. Cannabinoids for treatment of chronic non-cancer pain; a systematic review of randomized trials. Br J Clin Pharmacol 2011; 72: 735–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gaoni Y, Mechoulam R. Isolation, structure and partial synthesis of active constituent of hashish. J Am Chem Soc 1964; 86: 1646–1647. [Google Scholar]
- 24. Pertwee RG, Howlett AC, Abood ME, et al. International union of basic and clinical pharmacology. LXXIX. Cannabinoid receptors and their ligands: beyond CB1 and CB2. Pharmacol Rev 2010; 62: 588–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Woodhams SG, Chapman V, Finn DP, et al. The cannabinoid system and pain. Neuropharmacology 2017; 124: 105–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hollister LE. Health aspects of cannabis. Pharmacol Rev 1986; 38: 1–20. [PubMed] [Google Scholar]
- 27. Gorelick DA, Heishman SJ, Preston KL, et al. The cannabinoid CB1 receptor antagonist rimonabant attenuates the hypotensive effect of smoked marijuana in male smokers. Am Heart J 2006; 151(3): 754.e1–754.e5. [DOI] [PubMed] [Google Scholar]
- 28. Huestis MA, Boyd SJ, Heishman SJ, et al. Single and multiple doses of rimonabant antagonize acute effects of smoked cannabis in male cannabis users. Psychopharmacology (Berl) 2007; 194(4): 505–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Goodwin RS, Baumann MH, Gorelick DA, et al. CB1 – cannabinoid receptor antagonist effects on cortisol in cannabis-dependent men. Am J Drug Alcohol Abuse 2012; 38(1): 114–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gorelick DA, Goodwin RS, Schwilke E, et al. Antagonist-elicited cannabis withdrawal in humans. J Clin Psychopharmacol 2011; 31(5): 603–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Adams R, Hunt M, Clark JH. Structure of cannabidiol, a product isolated from the marihuana extract of Minnesota wild hemp. I J Am Chem Soc 1940; 62: 196–200. [Google Scholar]
- 32. Russo EB, Burnett A, Hall B, et al. Agonistic properties of cannabidiol at 5-HT1a receptors. Neurochem Res 2005; 30(8): 1037–1043. [DOI] [PubMed] [Google Scholar]
- 33. Kathmann M, Flau K, Redmer A, et al. Cannabidiol is an allosteric modulator at mu- and delta-opioid receptors. Naunyn Schmiedebergs Arch Pharmacol 2006; 372(5): 354–361. [DOI] [PubMed] [Google Scholar]
- 34. Stanley CP, Hind WH, Tufarelli C, et al. Cannabidiol causes endothelium-dependent vasorelaxation of human mesenteric arteries via CB1 activation. Cardiovasc Res 2015; 107(4): 568–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Laprairie RB, Bagher AM, Kelly ME, et al. Cannabidiol is a negative allosteric modulator of the cannabinoid CB1 receptor. Br J Pharmacol 2015; 172(20): 4790–4805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Thomas A, Baillie GL, Phillips AM, et al. Cannabidiol displays unexpectedly high potency as an antagonist of CB1 and CB2 receptor agonists in vitro. Br J Pharmacol 2007; 150(5): 613–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Laun AS, Song ZH. GPR3 and GPR6, novel molecular targets for cannabidiol. Biochem Biophys Res Commun 2017; 490: 17–21. [DOI] [PubMed] [Google Scholar]
- 38. O’Sullivan SE, Sun Y, Bennett AJ, et al. Time-dependent vascular actions of cannabidiol in the rat aorta. Eur J Pharmacol 2009; 612(1–3): 61–68. [DOI] [PubMed] [Google Scholar]
- 39. Bisogno T, Hanus L, De Petrocellis L, et al. Molecular targets for cannabidiol and its synthetic analogues: effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide. Br J Pharmacol 2001; 134(4): 845–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gilbert JC, Pertwee RG, Wyllie MG. Effects of Δ9-tetrahydrocannabinol and cannabidiol on a Mg2+-ATPase of synaptic vesicles prepared from rat cerebral cortex. Br J Pharmacol 1977; 59: 599–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Carrier EJ, Auchampach JA, Hillard CJ. Inhibition of an equilibrative nucleoside transporter by cannabidiol: a mechanism of cannabinoid immunosuppression. Proc Natl Acad Sci U S A 2006; 103(20): 7895–7900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hassan S, Eldeeb K, Millns PJ, et al. Cannabidiol enhances microglial phagocytosis via transient receptor potential (TRP) channel activation. Br J Pharmacol 2014; 171(9): 2426–2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Qin N, Neeper MP, Liu Y, et al. TRPV2 is activated by cannabidiol and mediates CGRP release in cultured rat dorsal root ganglion neurons. J Neurosci 2008; 28(24): 6231–6238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Nabissi M, Morelli MB, Santoni M, et al. Triggering of the TRPV2 channel by cannabidiol sensitizes glioblastoma cells to cytotoxic chemotherapeutic agents. Carcinogenesis 2013; 34(1): 48–57. [DOI] [PubMed] [Google Scholar]
- 45. De Petrocellis L, Ligresti A, Moriello AS, et al. Effects of cannabinoids and cannabinoid-enriched Cannabis extracts on TRP channels and endocannabinoid metabolic enzymes. Br J Pharmacol 2011; 163(7): 1479–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mahgoub M, Keun-Hang SY, Sydorenko V, et al. Effects of cannabidiol on the function of α7-nicotinic acetylcholine receptors. Eur J Pharmacol 2013; 720: 310–319. [DOI] [PubMed] [Google Scholar]
- 47. Yang KH, Galadari S, Isaev D, et al. The nonpsychoactive cannabinoid cannabidiol inhibits 5-hydroxytryptamine3A receptor-mediated currents in Xenopus laevis oocytes. J Pharmacol Exp Ther 2010; 333(2): 547–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ross HR, Napier I, Connor M. Inhibition of recombinant human T-type calcium channels by Δ9-tetrahydrocannabinol and cannabidiol. J Biol Chem 2008; 283: 16124–16134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hacke ACM, Lima D, de Costa F, et al. Probing the antioxidant activity of Δ9-tetrahydrocannabinol and cannabidiol in Cannabis sativa extracts. Analyst 2019; 144: 4952–4961. [DOI] [PubMed] [Google Scholar]
- 50. Boggs DL, Nguyen JD, Morgenson D, et al. Clinical and preclinical evidence for functional interactions of cannabidiol and delta(9)-tetrahydrocannabinol. Neuropsychopharmacology 2018; 43: 142–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Karniol IG, Shirakawa I, Kasinski N, et al. Cannabidiol interferes with the effects of Δ9-tetrahydrocannabinol in man. Eur J Pharmacol 1974; 28: 172–177. [DOI] [PubMed] [Google Scholar]
- 52. Henquet C, Kuepper R. Does cannabidiol protect against the negative effects of THC. Br J Psychiatry 2010; 197(4): 259–260. [DOI] [PubMed] [Google Scholar]
- 53. Jesus CHA, Redivo DDB, Gasparin AT, et al. Cannabidiol attenuates mechanical allodynia in streptozotocin-induced diabetic rats via serotonergic system activation through 5-HT1A receptors. Brain Res 2019; 1715: 156–164. [DOI] [PubMed] [Google Scholar]
- 54. Stern CAJ, da Silva TR, Raymundi AM, et al. Cannabidiol disrupts the consolidation of specific and generalized fear memories via dorsal hippocampus CB1 and CB2 receptors. Neuropharmacology 2017; 125: 220–230. [DOI] [PubMed] [Google Scholar]
- 55. Vilela LR, Lima IV, Kunsch EB, et al. Anticonvulsant effect of cannabidiol in the pentylenetetrazole model: Pharmacological mechanisms, electroencephalographic profile, and brain cytokine levels. Epilepsy Behav 2017; 75: 29–35. [DOI] [PubMed] [Google Scholar]
- 56. Bi GH, Galaj E, He Y, et al. Cannabidiol inhibits sucrose self-administration by CB1 and CB2 receptor mechanisms in rodents. Addict Biol 2019: e12783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Sonego AB, Prado DS, Vale GT, et al. Cannabidiol prevents haloperidol-induced vacuos chewing movements and inflammatory changes in mice via PPARγ receptors. Brain Behav Immun 2018; 74: 241–251. [DOI] [PubMed] [Google Scholar]
- 58. Anderson LL, Low IK, Banister SD, et al. Pharmacokinetics of phytocannabinoid acids and anticonvulsant effect of cannabidiolic acid in a mouse model of Dravet Syndrome. J Nat Prod 2019; 82(11): 3047–3055. [DOI] [PubMed] [Google Scholar]
- 59. Brierley DI, Harman JR, Giallourou N, et al. Chemotherapy-induced cachexia dysregulates hypothalamic and systemic lipoamines and is attenuated by cannabigerol. J Cachexia Sarcopenia Muscle 2019; 10(4): 844–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Maione S, Piscitelli F, Gatta L, et al. Non-psychoactive cannabinoids modulate the descending pathway of antinociception in anaesthetized rats through several mechanisms of action. Br J Pharmacol 2011; 162(3): 584–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Izzo AA, Capasso R, Aviello G, et al. Inhibitory effect of cannabichromene, a major non-psychotropic cannabinoid extracted from Cannabis sativa, on inflammation-induced hypermotility in mice. Br J Pharmacol 2012; 166(4): 1444–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Karniol IG, Shirakawa I, Takahashi RN, et al. Effects of Δ9-tetrahydrocannabinol and cannabinol in man. Pharmacology 1975; 13: 502–512. [DOI] [PubMed] [Google Scholar]
- 63. Jadoon KA, Ratcliffe SH, Barrett DA, et al. Efficacy and safety of cannabidiol and tetrahydrocannabivarin on glycemic and lipid parameters in patients with type 2 diabetes: a randomized, double-blind, placebo-controlled, parallel group pilot study. Diabetes Care 2016; 39(10): 1777–1786. [DOI] [PubMed] [Google Scholar]
- 64. Englund A, Atakan Z, Kralj A, et al. The effect of five day dosing with THCV on THC-induced cognitive, psychological and physiological effects in healthy male human volunteers: a placebo-controlled, double-blind, crossover pilot trial. J Psychopharmacol 2016; 30(2): 140–151. [DOI] [PubMed] [Google Scholar]
- 65. Ilan AB, Gevins A, Coleman M, et al. Neurophysiological and subjective profile of marijuana with varying concentrations of cannabinoids. Behav Pharmacol 2005; 16(5-6): 487–496. [DOI] [PubMed] [Google Scholar]
- 66. Truitt EB., Jr Biological disposition of tetrahydrocannabinols. Pharmacol Rev 1971; 23(4): 273–278. [PubMed] [Google Scholar]
- 67. Agurell S, Halldin M, Lindgren JE, et al. Pharmacokinetics and metabolism of Δ1-tetrahydrocannabinol and other cannabinoids with emphasis on man. Pharmacol Rev 1986; 38: 21–43. [PubMed] [Google Scholar]
- 68. Lindgren JE, Ohlsson A, Agurell S, et al. Clinical effects and plasma levels of Δ9-tetrahydrocannabinol (Δ9-THC) in heavy and light users of cannabis. Psychopharmacology (Berl) 1981; 74: 208–212. [DOI] [PubMed] [Google Scholar]
- 69. Hunt CA, Jones RT. Tolerance and disposition of tetrahydrocannabinol in man. J Pharmacol Exp Ther 1980; 215(1): 35–44. [PubMed] [Google Scholar]
- 70. Wall ME, Sadler BM, Brine D, et al. Metabolism, disposition, and kinetics of delta-9-tetrahydrocannabinol in men and women. Clin Pharmacol Ther 1983; 34(3): 352–363. [DOI] [PubMed] [Google Scholar]
- 71. Millar SA, Stone NL, Yates AS, et al. A systematic review on the pharmacokinetics of cannabidiol in humans. Front Pharmacol 2018; 9: 1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Ohlsson A, Lindgren JE, Andersson S, et al. Single-dose kinetics of deuterium-labelled cannabidiol in man after smoking and intravenous administration. Biomed Environ Mass Spectrom 1986; 13(2): 77–83. [DOI] [PubMed] [Google Scholar]
- 73. Rawitch AB, Rohrer R, Vardaris RM. delta-9-Tetrahydrocannabinol uptake by adipose tissue: preferential accumulation in gonadal fat organs. Gen Pharmacol 1979; 10(6): 525–529. [DOI] [PubMed] [Google Scholar]
- 74. Patilea-Vrana GI, Anoshchenko O, Unadkat JD. Hepatic enzymes relevant to the disposition of (-)-(9)-tetrahydrocannabinol (THC) and Its psychoactive metabolite, 11-OH-THC. Drug Metab Dispos 2019; 47(3): 249–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Jiang R, Yamaori S, Takeda S, et al. Identification of cytochrome P450 enzymes responsible for metabolism of cannabidiol by human liver microsomes. Life Sci 2011; 89(5–6): 165–170. [DOI] [PubMed] [Google Scholar]
- 76. Wheless JW, Dlugos D, Miller I, et al. Pharmacokinetics and tolerability of multiple doses of pharmaceutical-grade synthetic cannabidiol in pediatric patients with treatment-resistant epilepsy. CNS Drugs 2019; 33(6): 593–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Anderson LL, Absalom NL, Abelev SV, et al. Coadministered cannabidiol and clobazam: Preclinical evidence for both pharmacodynamic and pharmacokinetic interactions. Epilepsia 2019; 60(11): 2224–2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Asbridge M, Hayden JA, Cartwright JL. Acute cannabis consumption and motor vehicle collision risk: systematic review of observational studies and meta-analysis. BMJ 2012; 344: e536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Karschner EL, Schwilke EW, Lowe RH, et al. Do Δ9-tetrahydrocannabinol concentrations indicate recent use in chronic cannabis users? Addiction 2009; 104: 2041–2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Celius EG, Vila C. The influence of THC:CBD oromucosal spray on driving ability in patients with multiple sclerosis-related spasticity. Brain Behav 2018; 8(5): e00962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Mahamad S, Hammond D. Retail price and availability of illicit cannabis in Canada. Addict Behav 2019; 90: 402–408. [DOI] [PubMed] [Google Scholar]
- 82. Costantino RC, Felten N, Todd M, et al. A survey of hospice professionals regarding medical Cannabis practices. J Palliat Med 2019; 22(10): 1208–1212. [DOI] [PubMed] [Google Scholar]
- 83. Jett J, Stone E, Warren G, et al. Cannabis use, lung cancer, and related issues. J Thorac Oncol 2018; 13(4): 480–487. [DOI] [PubMed] [Google Scholar]
- 84. Krebs MO, Kebir O, Jay TM. Exposure to cannabinoids can lead to persistent cognitive and psychiatric disorders. Eur J Pain 2019; 23(7): 1225–1233. [DOI] [PubMed] [Google Scholar]
- 85. Colizzi M, Murray R. Cannabis and psychosis: what do we know and what should we do? Br J Psychiatry 2018; 212: 195–196. [DOI] [PubMed] [Google Scholar]
- 86. Colizzi M, Iyegbe C, Powell J, et al. Interaction between functional genetic variation of DRD2 and Cannabis use on risk of psychosis. Schizophr Bull 2015; 41(5): 1171–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]