Abstract
Background:
Cortical thickness (CT) and gyrification are complementary indices that assess different aspects of gray matter structural integrity. Both neurodevelopment insults and acute tissue response to antipsychotic medication could underlie the known heterogeneity of treatment response and are well-suited for interrogation into the relationship between gray matter morphometry and clinical outcomes in schizophrenia (SZ).
Methods:
Using a prospective design, we enrolled 34 unmedicated patients with SZ and 23 healthy controls. Patients were scanned at baseline and after a 6-week trial with risperidone. CT and local gyrification index (LGI) values were quantified from structural MRI scans using FreeSurfer 5.3.
Results:
We found reduced CT and LGI in patients compared to controls. Vertex-wise analyses demonstrated that hypogyrification was most prominent in the inferior frontal cortex, temporal cortex, insula, pre/postcentral gyri, temporoparietal junction, and the supramarginal gyrus. Baseline CT was predictive of subsequent response to antipsychotic treatment, and increase in CT after 6 weeks was correlated with greater symptom reductions.
Conclusions:
In summary, we report evidence of reduced CT and LGI in unmedicated patients compared to controls, suggesting involvement of different aspects of gray matter morphometry in the pathophysiology of SZ. Importantly, we found that lower CT at baseline and greater increase of CT following 6 weeks of treatment with risperidone were associated with better clinical response. Our results suggest that cortical thinning may normalize as a result of a good response to antipsychotic medication, possibly by alleviating potential neurotoxic processes underlying gray matter deterioration.
Keywords: antipsychotic, treatment response, gray matter, unmedicated, psychosis
Abstract
Contexte :
L’épaisseur corticale (EC) et la gyrification sont des indices complémentaires qui évaluent différents aspects de l’intégrité structurelle de la matière grise. Les traumatismes neurodéveloppementaux ainsi que la réponse tissulaire aiguë à la médication antipsychotique peuvent sous-tendre l’hétérogénéité connue de la réponse au traitement, et conviennent bien pour l’interrogation sur la relation entre la morphométrie de la matière grise et les résultats cliniques de la schizophrénie.
Méthodes :
À l’aide d’une méthode prospective, nous avons inscrit 34 patients non médicamentés souffrant de schizophrénie et 23 témoins en santé. Les patients ont subi un examen d’imagerie au départ et après un essai de rispéridone de 6 semaines. Les valeurs de l’épaisseur corticale (EC) et de l’indice de gyrification locale (IGL) ont été quantifiées d’après les examens par IRM structurels avec FreeSurfer.
Résultats :
Nous avons constaté des EC et des IGL réduits chez les patients comparativement aux témoins. Des analyses au sommet ont démontré que l’hypo-gyrification était surtout visible dans le cortex frontal inférieur, le cortex temporal, l’insula, les gyri pré/post centraux, l’articulation temporo-pariétale et le gyrus supramarginal. L’EC de départ prédisait la réponse subséquente au traitement antipsychotique, et l’augmentation de l’EC après six semaines était corrélée à une plus grande réduction des symptômes.
Conclusions :
En résumé, nous faisons état des preuves de la réduction de l’EC et de l’IGL chez les patients non médicamentés comparés aux témoins, ce qui suggère la participation de différents aspects de la morphométrie de la matière grise à la pathophysiologie de la schizophrénie. Surtout, nous avons constaté qu’une EC plus mince au départ, et qu’une plus grande augmentation de l’EC après six semaines de traitement à la rispéridone étaient associées à une meilleure réponse clinique. Nos résultats suggèrent que l’amincissement cortical peut être normalisé à titre de résultat d’une bonne réponse aux antipsychotiques, possiblement en atténuant les processus neurotoxiques potentiels qui sous-tendent la détérioration de la matière grise.
Introduction
Schizophrenia (SZ) is a neurodevelopmental disorder1,2,3 characterized by widespread structural brain abnormalities.4 The literature supports the theory that antipsychotic drugs (APD) affect gray matter morphometry in patients. Longitudinal and meta-analytic studies found associations between APD exposure and both increased5,6,7 and decreased8–13 gray matter volumes, and a possible link between (cumulative) APD dose and gray matter reductions.8,9 APD effects may be apparent soon after treatment is started, as suggested by a longitudinal study reporting increased gray matter volume in APD-naive patients after 8 weeks of treatment.5
While volume measures have been most widely used to assess gray matter morphometry, cortical thickness (CT) and gyrification are complementary indices that characterize different aspects of gray matter structural integrity. For example, one study found only a partial spatial overlap when comparing regions of altered gray matter volume versus altered CT in chronic SZ compared to controls.14 The authors suggested that different abnormalities in the measures could relate to different pathological mechanisms of SZ. A recent study comparing 145 APD-naive first-episode psychosis (FEP) patients and 147 controls found that CT decrease as an effect of age was more pronounced in patients,15 while another showed CT reductions were greater in treatment resistant patients with SZ than nonresistant SZ.16 As a measure, gyrification index is of particular interest due to its potential as a marker of abnormal neurodevelopment.17,18 Altered gyrification patterns have been reported across different illness stages19–22 and among first-degree relatives.23 The clinical relevance of this marker is further underscored by reports that the local gyrification index (LGI)24 showed high discriminatory ability in correctly identifying patients with a high illness burden25 and that patients with poor response to APD treatment had lower LGI compared to those who respond favorably.26
Studies measuring several gray matter morphometric features in the same SZ sample have been sparse. Perhaps the largest project to date, a single-subject meta-analysis conducted by the ENIGMA SZ work group, reported widespread cortical thinning and reduced cortical surface area in patients compared to controls. The effect size for cortical thinning was roughly twice the effect size of the surface area results,27 suggesting CT abnormalities are more prominent than surface area alterations. Palaniyappan and Liddle28 found that gray matter volume differences between SZ and healthy controls (HC) were partially mediated by LGI, CT, and surface area. Within the illness, regions of abnormal gyrification and CT only partially overlap,29 implying a regionally inhomogeneous relationship between cortical folding and gray matter thickness across the brain. Combining these complementary gray matter morphometric measures may yield additional clinically relevant insights.
Because both neurodevelopmental insults and acute tissue response to APD exposure could impact the known heterogeneity of treatment response, LGI and CT are measures well-suited in evaluating the relationship between gray matter morphometry and clinical outcomes in SZ. Presently, there are no studies that have evaluated LGI and CT in medication-naive subjects, and little is known about the longitudinal effects of APD treatment on these measures. In this study, we used a prospective design where unmedicated SZ and medication-naive FEP patients were scanned prior to APD treatment and after 6 weeks of treatment with risperidone. To control for the effect of time, we scanned a matched group of HC twice, approximately 6 weeks apart. The aims of this study were 3-fold: (1) to evaluate CT and LGI in SZ free from the confound of current APD treatment and in those with the prior exposure to any antipsychotic medication, (2) to investigate the effects of antipsychotic treatment on CT, and (3) to evaluate structural predictors of treatment response as well as structural changes over time associated with treatment response. We hypothesized that unmedicated SZ would show reduced baseline CT and LGI compared to HC and that CT would be affected by APD treatment. We also hypothesized that baseline CT and LGI would predict response to antipsychotic treatment and that greater change in CT would be correlated with greater change in clinical symptom severity.
Methods
Subjects
Thirty-seven unmedicated SZ (22 medication naive, 12 had prior APD exposure) were recruited from the emergency room, inpatient units, and outpatient clinics at the University of Alabama at Birmingham (UAB). Twenty-three HC matched on age, gender, smoking, and parental socioeconomic status (SES) were recruited by advertisements. Approval for this study was given by the UAB Institutional Review Board, and written informed consent was obtained prior to enrolment and after subjects were deemed to have capacity to provide consent.30
Diagnoses were established by review of medical records, the Diagnostic Interview for Genetic Studies,31 and consensus of 2 board-certified psychiatrists (ACL and NVK). The Brief Psychiatric Rating Scale (BPRS) was used to assess symptom severity.32
Exclusion criteria for enrollment included major neurological or medical conditions, a history of head trauma with loss of consciousness, substance use disorders (confirmed via urine drug screening) within 6 months of imaging, pregnancy or breastfeeding, or MRI contraindications. HC with a family history of a psychiatric illness in a first-degree relative were also excluded.
SZ were enrolled in a 6-week risperidone trial using a flexible dosing regimen managed by ACL and NVK. Risperidone started at 1 to 3 milligrams and titrated in 1 to 2 milligram increments; pill counts were done to monitor compliance. Use of concomitant psychotropic medications was permitted as clinically indicated. Structural scans were obtained prior to treatment (off medication) and after 6 weeks of treatment. HC were also scanned twice, with an average interval of ∼8 weeks.
MRI Acquisition
A head-only 3T MRI with a circularly polarized transmit/receive head coil was used for all imaging (Magnetom Allegra, Siemens Medical Solutions). Three-dimensional T1-weighted magnetization prepared rapid acquisition gradient echo sequence was used for structural acquisition (TR/TE = 2,300/3.93 ms, flip angle = 12°, 256 × 256 matrix, 1 mm isotropic voxels).
Structural Pre-processing
The cortical surface of each structural image was reconstructed using FreeSurfer 5.3.33 This pipeline also produces brain-wide CT values by measuring the distance between each cortical surface vertex and its closest corresponding gray/white boundary vertex. We used FreeSurfer’s QA Tools (https://surfer.nmr.mgh.harvard.edu00/fswiki/QATools) to inspect the quality of each reconstructed data set as explained in.34 Any subject’s data flagged as unsatisfactory during the quality assurance process were either manually corrected and reprocessed or excluded from final analysis due to irreparable artifacts. Three SZ were flagged during quality assurance and removed from analysis due to irreparable imaging artifacts resulting in 34 SZ (12 unmedicated and 22 medication naive) and 23 HC retained for final analysis. The remaining subject images were reprocessed using the FreeSurfer longitudinal stream.35 CT and LGI (24) values were then computed across the reconstructed cortical surface of each longitudinally processed structural image.
Demographic, Regression, and Correlational Analysis
Group differences for age, gender, smoking status, parental SES, signal to noise ratio (SNR), and estimated total intracranial volume (eTIV)36 were assessed in SPSS via independent samples t tests or chi-square tests where appropriate. Asymmetrical lateralization of functional, structural, and connectivity measures is well-documented in SZ.37,38,39 Therefore, to investigate global changes in CT and LGI, we calculated average CT and LGI per hemisphere per subject. These values were used as dependent variables in four (1 per hemisphere per measure) 2 (baseline vs. Week 6) × 2 (HC vs. SZ) mixed analysis of covariance (ANCOVA) models. Age and eTIV were included as covariates of no interest.
To investigate any direct relationship between CT and LGI, we ran partial correlation tests including averages for each measure across each hemisphere at baseline and at Week 6. We then used a Fisher r-to-z transformation to determine whether there were significant differences between baseline and Week 6 correlations.
Vertex-wise Analyses
Separate surface-based linear regression models were performed with “Query, Design, Estimate, Contrast” (QDEC) in FreeSurfer to investigate the effect of group on baseline CT and LGI at each surface vertex. Additional longitudinal two-stage linear regression models were used to calculate percent change in CT and LGI from baseline to Week 6. Similar models were used to investigate the correlation of treatment response (based on % change in BPRS positive score from (A) baseline to week 6 (B): with CT and LGI, first at baseline and then with % change over time. All QDEC models included covariates of no interest for age and eTIV. Monte Carlo null-z simulations (P < 0.05) were used to correct for multiple comparisons.
Results
No significant group differences were observed for sex, age, parental SES, packs per day, eTIV, or SNR (all P > 0.05). BPRS scores significantly decreased after 6 weeks of treatment with risperidone (Table 1). Results from a repeated demographic analysis with medication-naive SZ (n = 22), and HC did not differ from the original analysis and can be found in the Supplemental Section (Table S1).
Table 1.
Demographics, Clinical Measures, and Covariates.a
| SZ (n = 34) | HC (n = 23) | t/χ2 | P Value | |
|---|---|---|---|---|
| Gender (%male) | 73.5 | 82.6 | 0.642 | 0.423 |
| Age | 28.32 (9.42) | 27.48 (9.63) | −0.330 | 0.743 |
| Socioeconomic statusb | 5.88 (5.06)c | 4.65 (3.97) | 12.849 | 0.303 |
| Smoking (packs per day) | 0.37 (0.49) | 0.18 (0.41) | −1.564 | 0.124 |
| Illness duration (years)d | 15.00 (8.45; n = 12) | |||
| Illness onset (years)d | 22.08 (3.12) | |||
| APD naive (yes/no) | 22/12 | |||
| eTIVe | 1,593.70 (182.36) | 1,658.79 (230.71) | 1.187 | 0.240 |
| SNR | 19.93 (1.98) | 20.50 (2.07) | 1.069 | 0.290 |
| BPRSf | Baseline | Week 6 | ||
| Total | 50.91 (9.65) | 32.50 (10.45) | 7.545 | <0.001 |
| Positive | 10.44 (3.47) | 5.18 (2.41) | 7.277 | <0.001 |
| Negative | 7.44 (3.16) | 5.85 (2.74) | 2.215 | 0.030 |
Note. APD = antipsychotic drugs; BPRS = Brief Psychiatric Rating Scale; eTIV = estimated total intracranial volume; HC = healthy controls; SNR = signal to noise ratio; SZ = patients with schizophrenia.
a Mean (standard deviation) unless indicated otherwise.
b Parental socioeconomic ranks determined from Diagnostic Interview for Genetic Studies (1 to 18 scale); higher rank (lower numerical value) corresponds to higher socioeconomic status.
c Data not available for 2 SZ subjects; n = 32.
d Includes only patients who are not antipsychotic naive (n = 12), illness duration since first diagnosis.
e Estimated total intracranial volume (eTIV) in cm2.
f BPRS (1 to 7 scale); positive (conceptual disorganization, hallucinatory behavior, and unusual thought content); negative (emotional withdrawal, motor retardation, and blunted affect).
Group Differences in Global CT and LGI
Results from mixed ANCOVAs (Table 2) showed that left hemispherical CT was significant for the main effect of group (F 1, 51 = 4.918, P = 0.031), while the right hemisphere was at trend level (F 1, 51 = 3.879 P = 0.054). HC consistently showed greater CT than SZ. For LGI, the main effect of group was significant for each hemisphere (left: F 1, 51 = 15.542 P < 0.001; right: F 1, 51 = 15.619 P < 0.001; Table 2). LGI was consistently greater for HC than SZ. Results from repeat analysis with medication-naive SZ and HC showed no difference from the original results and can be found in the Supplemental Section (Table S2).
Table 2.
Mixed MANCOVA Results for CT and LGI Averages per Hemisphere.a
| SZ (n = 34) | HC (n = 23) | Left | Right | |||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Week 6 | Baseline | Week 6 | |||||
| M (SD) | M (SD) | M (SD) | M (SD) | F | P | F | P | |
| CT | ||||||||
| Left hemisphere | 2.435 (0.120) | 2.469 (0.113) | 2.514 (0.119) | 2.519 (0.108) | — | — | — | — |
| Right hemisphere | 2.432 (0.120) | 2.466 (0.120) | 2.507 (0.119) | 2.507 (0.112) | — | — | — | — |
| Group | — | — | — | — | 4.918 | 0.031 | 3.879 | 0.054 |
| Time | — | — | — | — | 0.079 | 0.780 | 0.020 | 0.887 |
| Time × Group | — | — | — | — | 1.067 | 0.306 | 1.741 | 0.193 |
| LGI | ||||||||
| Left hemisphere | 2.946 (0.105) | 2.940 (0.101) | 3.072 (0.156) | 3.065 (0.148) | — | — | — | — |
| Right hemisphere | 2.958 (0.111) | 2.956 (0.112) | 3.077 (0.146) | 3.073 (0.148) | — | — | — | — |
| Group | — | — | — | — | 15.542 | <0.001 | 15.619 | <0.001 |
| Time | — | — | — | — | 1.205 | 0.277 | 1.798 | 0.186 |
| Time × Group | — | — | — | — | 0.079 | 0.780 | 0.075 | 0.786 |
Note. CT = cortical thickness; HC = healthy controls; LGI = local gyrification index; M = mean; SD = standard deviation; SZ = patients with schizophrenia.
a Covariates accounted for include: age and estimated total intracranial volume. Repeated measures interactions and between subjects effects not included.
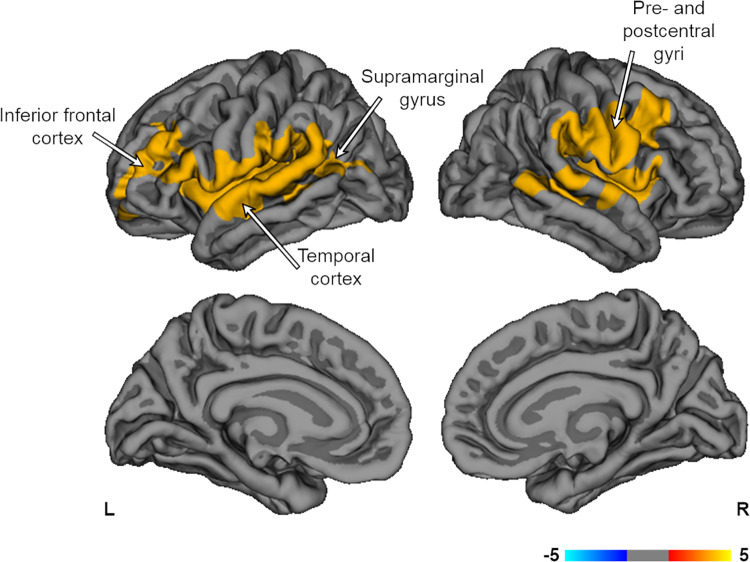
A whole brain vertex-wise group contrast of baseline CT showed no significant clusters. HC showed greater baseline LGI compared to SZ bilaterally throughout the frontotemporal areas, including the insula, and pre/postcentral gyri, as well as the left temporoparietal junction and right supramarginal gyrus (P < 0.05; Figure 1). Repeat analysis with medication-naive SZ showed HC had greater baseline LGI in the right pre/postcentral gyri (P < 0.05). These results along with a complete list of all atlas based vertex-wise results can be found in the Supplemental Section (Figure S1; Table S3).
Figure 1.
Group contrasts of baseline LGI. HC showed greater baseline LGI compared to SZ throughout frontotemporal areas, bilateral pre/post central gyri, left tempoparietal junction, and right supramarginal gyrus (P < 0.05). Models were corrected for age and eTIV. Multiple comparisons corrected with Monte Carlo null-z simulations (P < 0.05). eTIV = estimated total intracranial volume; HC = healthy controls; LGI = local gyrification index; SZ = patients with schizophrenia; L = left; R = right.
Global Correlations between CT and LGI
Neither HC nor SZ showed significant correlations between CT and LGI at either baseline or Week 6. Neither group showed a significant difference between correlation values across time points either.
Effects of Treatment Response on Global CT and LGI
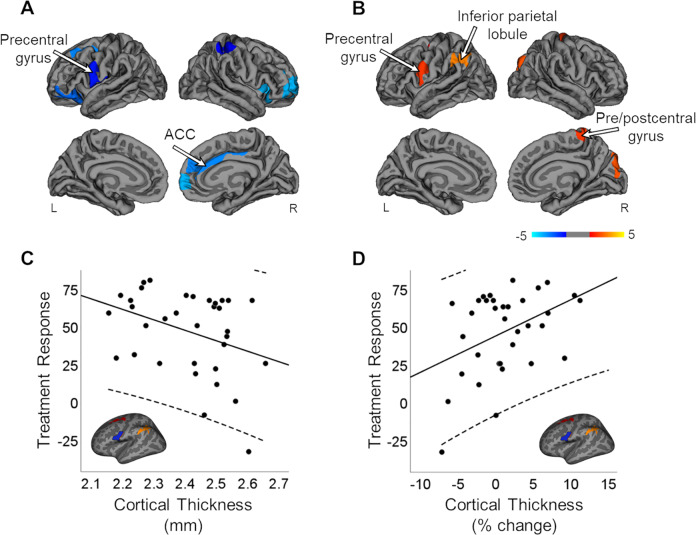
Whole brain vertex-wise correlation of treatment response with baseline CT showed significantly negative correlations bilaterally in the prefrontal cortex and postcentral gyrus, left precentral gyrus, and right cingulate and insula (P < 0.05; Figure 2A), suggesting lower CT in these regions predicted better treatment response. Treatment response was positively correlated with % change in CT over time in the precentral gyrus bilaterally, left superior frontal sulcus and inferior parietal lobule, and right postcentral gyrus and cuneus (P < 0.05; Figure 2B), suggesting that increased CT from baseline to Week 6 in those regions were associated with treatment response. To better visualize these findings, we extracted individual averages of baseline CT and % change in CT from a hand-traced precentral region of interest (ROI) defined during our analysis of treatment response and % change in CT (Figure 2C and 2D). Plots clearly show that both lower levels of baseline CT and increases in CT after treatment response are predictive of better treatment response at 6 weeks. Analyses of the relationship between treatment response and baseline CT, as well as treatment response and % change in CT, were repeated with medication-naive SZ. Results were similar to the original analyses but were more widespread (P < 0.05). These results can be found in the Supplemental Section (Figure S2).
Figure 2.
Correlation of treatment response with baseline CT and % change in CT. (A) TR was significantly correlated, negatively, with baseline CT in the prefrontal cortex, bilateral postcentral gyrus, left precentral gyrus, and right cingulate and insula (P < 0.05). (B) TR was significantly correlated, positively, with % change in CT in the precentral gyrus bilaterally, left superior frontal sulcus and inferior parietal lobule, and right postcentral gyrus and cuneus (P < 0.05). Models were corrected for age and eTIV. Multiple comparisons were corrected for with Monte Carlo null-z simulations (P < 0.05). (C) Individual averages for baseline CT and (D) % change in CT were extracted from a hand-traced precentral ROI from 2B and plotted with TR. CT = cortical thickness; eTIV = estimated total intracranial; ACC = anterior cingulate cortex; SZ = patients with schizophrenia; TR = treatment response; ROI = region of interest; L = left; R = right.
There were no significant correlations between baseline LGI and treatment response. Treatment response was negatively correlated with % change in LGI in the inferior parietal cortex bilaterally, the left middle frontal cortex, and precentral gyrus, and the right inferior frontal cortex, and cuneus (P < 0.05; Figure 3A). This indicates that, while baseline levels of LGI were not related to treatment response, reduction in LGI from baseline to posttreatment was related to better treatment response at 6 weeks. We also extracted individual averages of % change in LGI from a hand-traced ROI overlapping with the middle frontal/precentral cluster defined during initial analysis plotting this against treatment response (the plot and ROI can be seen in Figure 3B). This plot shows that change in LGI had an inverse relationship with treatment response compared to change in CT. Again, we repeated this analysis with only medication-naive SZ. As before, results were similar to the original analyses but more widespread (P < 0.05). These results along with a complete list of all atlas based vertex-wise results can be found in the Supplemental Section (Figure S3; Table S3).
Figure 3.
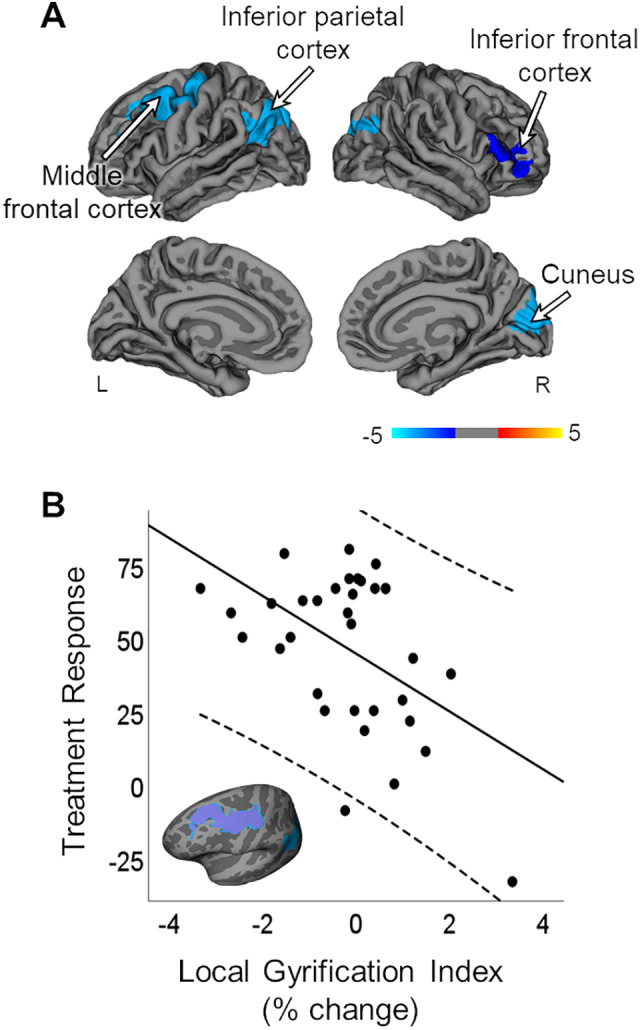
Correlation of treatment response with % change in LGI. (A) TR was significantly correlated, negatively, with % change in LGI in the inferior parietal cortex bilaterally, the left middle frontal cortex, and precentral gyrus, and the right inferior frontal cortex, and cuneus (P < .05). Models were corrected for age and eTIV. Multiple comparisons corrected for with Monte Carlo null-z simulations (P < 0.05). Individual averages for (B) % change in LGI were extracted from a hand-traced middle frontal ROI and plotted with TR. eTIV = estimated total intracranial; LGI = local gyrification index; SZ = patients with schizophrenia; TR = treatment response; ROI = region of interest; L = left; R = right.
Discussion
In this prospective, longitudinal study, we used 2 complementary gray matter morphometry indices to assess structural integrity in SZ and neurobiological signatures of APD treatment. We report evidence of reduced left hemisphere CT and global LGI in unmedicated patients compared to controls. Vertex-wise analyses suggest that LGI alterations are most prominent in frontotemporal areas, which is consistent with spatial patterns commonly detected in gray matter morphometry studies in the illness. Importantly, we found that lower CT at baseline and greater increase of CT following 6 weeks of APD treatment were associated with better clinical response. This could be interpreted as evidence of a deprived neuronal state in unmedicated SZ that may be alleviated through APD and, in turn, result in favorable clinical outcomes. Contrary to our hypothesis, we did not confirm that greater baseline LGI was predictive of better treatment response.
It is now understood that as the illness progresses, gray matter deficits become more pronounced and extensive.8,40,41 Poor clinical outcomes,42,43 duration of clinical relapses,44 cannabis use,45 and genetic liability46 are all factors associated with gray matter reductions. Because it is difficult to disentangle the effects of illness chronicity from medication effects, the extent to which APD contribute to gray matter deficits is still greatly debated.47 Here, we report global left hemisphere CT and bilateral LGI reductions in unmedicated SZ, which was true even when APD-naive SZ were taken into consideration. This indicates that antipsychotics are not the sole contributor to gray matter deficits in SZ and fits well with a number of studies reporting that reductions in gray matter volume are already observed during early stages of the illness including subjects at high risk of psychosis,48 during SZ onset,49 and even among unaffected relatives of SZ.50
While vertex-wise analyses did not detect alterations of baseline CT, we found lower LGI in the inferior frontal cortex, temporal cortex, insula and pre/postcentral gyri, the temporoparietal junction, and the supramarginal gyrus in unmedicated SZ compared to HC. Spatial patterns closely resemble those of Palaniyappan and Liddle51 who showed decreased gyrification in chronic SZ. However, these authors also found increased frontomarginal LGI which we did not. Even though imaging studies have reliably detected evidence of neurodevelopmental abnormalities in the form of altered LGI, both in FEP and in chronic SZ, findings have been inconsistent as to the directionality and spatial distribution of LGI alterations (for a recent review see the study of Matsuda and Ohi22). Heterogeneity in data analytics, cumulative APD dose, and illness chronicity may be important reasons for conflicting findings. However, there are studies with results discrepant to ours that are not explained by cumulative APD dose or illness chronicity. One such study found widespread frontal, parietal, and occipital hypergyrification among medicated FEP,52 while another showed hypergyrification across all lobes in at-risk mental state subjects; subjects who transitioned to psychosis also showed increased left occipital LGI compared to nontransitioned subjects.53 Our report extends the literature by demonstrating, for the first time, reduced LGI in medication-naive SZ, supporting this as an intrinsic illness characteristic rather than a confound of APD exposure or disease chronicity. Further underscoring the relevance of abnormal gyrification as part of the core pathology in SZ is a recent study linking genetic risk for SZ, quantified via polygenic risk score, to gyrification in 2 independent samples of healthy volunteers.54 Interestingly, a higher polygenic risk score was linked to a lower cortical LGI in bilateral inferior parietal lobes, including the supramarginal gyrus, which was one of the areas we also report LGI reductions in unmedicated SZ.
Our results showed that lower baseline CT in prefrontal, sensorimotor, cingulate, and insular cortices predicted better treatment response and that the increase in CT after 6 weeks of treatment was associated with greater alleviation of positive symptoms. Like us, a recent study in medication-naive FEP patients report a relationship between change in CT and symptom improvement after 6 weeks of treatment with amisulpride.55 Another group reported an increase in CT in the middle frontal cortex after 8 weeks of treatment with risperidone or quetiapine in antipsychotic-naive FEP.56 Our results suggest that cortical thinning may normalize as a result of a good response to medication, which is in agreement with a longitudinal study demonstrating that trajectories of cortical thinning after 5 years are less pronounced in patients with good compared to poor outcomes, as measured with the Global Assessment of Functioning Scale.41 Other findings may not support this conclusion. Lesh et al.57 found that medicated FEP patients had widespread cortical thinning compared to HC, while unmedicated FEP did not. It is difficult to discern if the difference in CT between the medicated and unmedicated FEP was from APD or initial morphological differences, since they did not account for baseline CT prior to APD. Ho et al.58 found that greater dosage and length of APD treatment were predictive of reduced gray matter volume in patients followed for an average interval of 7 years.
The literature supports the idea that APD treatment can help lessen cortical thinning in SZ, possibly by alleviating potential neurotoxic processes underlying gray matter deterioration59 and mitigating the accelerated frontotemporal cortical thinning experienced over the course of the illness.40 Second generation APD are shown to increase synaptic proteins levels and promote dendritic growth.60 Brain-derived neurotrophic factor (BDNF), one of these proteins, is an important regulator of synaptic transmission. BDNF is essential to synaptic plasticity and contributes to apoptotic protection.61,62 Evidence also shows that BDNF is related to increased spine density.63 Fernandes et al.64 conducted a meta-analysis with over 7,000 subjects showing that SZ was associated with lower peripheral BDNF levels. Furthermore, APD treatment was associated with increased BDNF plasma levels in SZ.64 Second generation APD in particular show potential for mitigating dendritic decline in the outer cortical layers.65 It could be argued that treatment with second generation APD (such as risperidone) could result in increased CT. Although this suggests that APD has a neurotrophic effect on cortical structure, APD may also affect glial cells. Animal studies have shown increased astrocyte density of the rat anterior cingulate cortex66 but also reduced astrocyte number within parietal gray matter in nonhuman primates67 after APD treatment. It should be noted that both of these studies had small sample sizes.
Somewhat surprisingly, we did not find an association between baseline LGI and subsequent response to antipsychotic treatment. Based on a study showing prominent hypogyria in the insular, frontal and temporal regions in medicated FEP patients who were categorized as poor responders to antipsychotic treatment compared to good responders (response to treatment here was assessed 12 weeks after imaging was completed), we hypothesized that LGI would be a useful predictor of antipsychotic treatment response.68 It is possible that a difference in the operational criteria for treatment response between the studies may in part explain discrepancies in findings. While the former used an absolute threshold for symptom severity to dichotomize patients into 2 categories (responders and nonresponders), we calculated individual symptom change scores to interrogate associations between baseline LGI and subsequent response to treatment. We chose this approach because we believe that treatment response to antipsychotic medications is best defined along a gradient, where one end is characterized by a very good response, the other by a very poor response, and the middle by a suboptimal response. As gyrification patterns are thought to be tightly linked to neurodevelopment, and thus likely relevant in terms of clinical outcomes, it is possible that LGI may not be predictive of the magnitude of clinical improvement across the entire range of treatment response. Instead, a certain threshold of hypogyria, indicative of a clinically relevant neurodevelopmental insult, may have to be met to identify those who will have a subsequent poor response to treatment. In other words, significant abnormalities in LGI may predict poor treatment response, but nonpathological variations in LGI may not be associated with clinical outcomes. Of course, this will need to be tested empirically in future large-scale studies.
There are several strengths and limitations that need to be considered when interpreting our data. To minimize data variance, we only enrolled unmedicated SZ, matched groups on several key factors, and used a rigorous longitudinal design with a single antipsychotic medication. On the other hand, our small sample did not allow us to fully investigate potential differences between medication-naive and chronic unmedicated patients or dichotomize SZ that responded well to antipsychotic treatment and those who did not.
In summary, we report evidence of reduced CT and LGI in unmedicated patients compared to controls, suggesting involvement of different aspects of gray matter morphometry in the pathophysiology of SZ. Importantly, we found that lower CT at baseline and greater increase of CT following 6 weeks of treatment with risperidone were associated with better clinical response. Our results suggest that cortical thinning may normalize as a result of a good response to antipsychotic medication, possibly by alleviating potential neurotoxic processes underlying gray matter deterioration.59 Despite a theoretical foundation for our a priori hypothesis, we did not find that LGI was predictive of treatment response. However, significant abnormalities in LGI may be indicative of poor treatment response, while nonpathological variations in LGI may not be associated with clinical outcomes. Of course, the significance of LGI strictly as a marker for poor antipsychotic response will have to be empirically established.
Supplemental Material
Supplemental Material, CJP_Supplimental_Final_1.29.20. for A Prospective Longitudinal Investigation of Cortical Thickness and Gyrification in Schizophrenia by Eric A. Nelson, Nina V. Kraguljac, David M. White, Ripu D. Jindal, Ah L. Shin and Adrienne C. Lahti in The Canadian Journal of Psychiatry
Footnotes
Authors’ Note: Medication for this study was donated (in part) to A.C.L. by Janssen Pharmaceuticals Inc.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Institute of Mental Health grants R01 MH 081014 and 102951 (A.C.L.).
ORCID iD: Adrienne C. Lahti  https://orcid.org/0000-0002-5565-7662
https://orcid.org/0000-0002-5565-7662
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Rapoport JL, Giedd JN, Gogtay N. Neurodevelopmental model of schizophrenia: update 2012. Mol Psychiatry. 2012;17(12):1228–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lewis DA, Levitt P. Schizophrenia as a disorder of neurodevelopment. Annu Rev Neurosci. 2002;25:409–432. [DOI] [PubMed] [Google Scholar]
- 3. Woods BT. Is schizophrenia a progressive neurodevelopmental disorder? toward a unitary pathogenetic mechanism. Am J Psychiatry. 1998;155(12):1661–1670. [DOI] [PubMed] [Google Scholar]
- 4. Birur B, Kraguljac NV, Shelton RC, Lahti AC. Brain structure, function, and neurochemistry in schizophrenia and bipolar disorder: a systematic review of the magnetic resonance neuroimaging literature. NPJ Schizophr. 2017;3:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yue Y, Kong L, Wang J, et al. Regional abnormality of grey matter in schizophrenia: effect from the illness or treatment? PLoS One. 2016;11(1):e0147204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Boonstra G, van Haren NEM, Schnack HG, et al. Brain volume changes after withdrawal of atypical antipsychotics in patients with first-episode schizophrenia. J Clin Psychopharmacol. 2011;31(2):146–153. [DOI] [PubMed] [Google Scholar]
- 7. Deng MY, McAlonan GM, Cheung C, et al. A naturalistic study of grey matter volume increase after early treatment in anti-psychotic naïve, newly diagnosed schizophrenia. Psychopharmacology (Berl). 2009;206(3):437–446. [DOI] [PubMed] [Google Scholar]
- 8. Haijma SV, Van Haren N, Cahn W, Koolschijn PCMP, Hulshoff Pol HE, Kahn RS. Brain volumes in schizophrenia: a meta-analysis in over 18 000 subjects. Schizophr Bull. 2013;39(5):1129–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fusar-Poli P, Smieskova R, Kempton MJ, Ho BC, Andreasen NC, Borgwardt S. Progressive brain changes in schizophrenia related to antipsychotic treatment? a meta-analysis of longitudinal MRI studies. Neurosci Biobehav Rev. 2013;37(8):1680–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vita A, De Peri L, Deste G, Sacchetti E. Progressive loss of cortical gray matter in schizophrenia: a meta-analysis and meta-regression of longitudinal MRI studies. Transl Psychiatry. 2012. ;2:e190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lieberman JA, Tollefson GD, Charles C, et al. Antipsychotic drug effects on brain morphology in first-episode psychosis. Arch Gen Psychiatry. 2005;62(4):361–370. [DOI] [PubMed] [Google Scholar]
- 12. Thompson PM, Bartzokis G, Hayashi KM, et al. Time-lapse mapping of cortical changes in schizophrenia with different treatments. Cereb Cortex. 2009;19(4):1107–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Leung M, Cheung C, Yu K, et al. Gray matter in first-episode schizophrenia before and after antipsychotic drug treatment. anatomical likelihood estimation meta-analyses with sample size weighting. Schizophr Bull. 2011;37:199–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kong L, Herold CJ, Zollner F, et al. Comparison of grey matter volume and thickness for analysing cortical changes in chronic schizophrenia: a matter of surface area, grey/white matter intensity contrast, and curvature. Psychiatry Res. 2015;231(2):176–183. [DOI] [PubMed] [Google Scholar]
- 15. Lin Y, Li M, Zhou Y, et al. Age-related reduction in cortical thickness in first-episode treatment-naive patients with schizophrenia. Neurosci Bull. 2019;35(4):688–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Barry EF, Vanes LD, Andrews DS, et al. Mapping cortical surface features in treatment resistant schizophrenia with in vivo structural MRI. Psychiatry Res. 2019;274:335–344. [DOI] [PubMed] [Google Scholar]
- 17. Stiles J, Jernigan TL. The basics of brain development. Neuropsychol Rev. 2010;20(4):327–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shimony JS, Smyser CD, Wideman G, et al. Comparison of cortical folding measures for evaluation of developing human brain. Neuroimage. 2016;125:780–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Harris JM, Moorhead TWJ, Miller P, et al. Increased prefrontal gyrification in a large high-risk cohort characterizes those who develop schizophrenia and reflects abnormal prefrontal development. Biol Psychiatry. 2007;62(5):722–729. [DOI] [PubMed] [Google Scholar]
- 20. Zuliani R, Delvecchio G, Bonivento C, et al. Increased gyrification in schizophrenia and non affective first episode of psychosis. Schizophr Res. 2018;193(5):269–275. [DOI] [PubMed] [Google Scholar]
- 21. White T, Hilgetag CC. Gyrification and neural connectivity in schizophrenia. Dev Psychopathol. 2011;23(1):339–352. [DOI] [PubMed] [Google Scholar]
- 22. Matsuda Y, Ohi K. Cortical gyrification in schizophrenia: current perspectives. Neuropsychiatr Dis Treat. 2018;14:1861–1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Vogeley K, Tepest R, Pfeiffer U, Schneider-Axmann T, Maier W, Falkai P. Right frontal hypergyria differentiation in affected and unaffected siblings from families multiply affected with schizophrenia: a morphometric MRI study. Am J Psychiatry. 2001;158(3):494–496. [DOI] [PubMed] [Google Scholar]
- 24. Schaer M, Bach Cuadra M, Tamarit L, Lazeyras F, Eliez S, Thiran JP. A Surface-based approach to quantify local cortical gyrification. IEEE Trans Med Imaging. 2008;27(2):161–170. [DOI] [PubMed] [Google Scholar]
- 25. Guo S, Iwabuchi S, Balain V, Feng J, Liddle P, Palaniyappan L. Cortical folding and the potential for prognostic neuroimaging in schizophrenia. Brit J Psychiatry. 2015;207:458–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Palaniyappan L, Marques TR, Taylor H, et al. Cortical folding defects as markers of poor treatment response in first-episode psychosis. JAMA Psychiatry. 2013;70(2):1031–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. van Erp TGM, Walton E, Hibar DP, et al. Cortical brain abnormalities in 4474 individuals with schizophrenia and 5098 control subjects via the enhancing neuro imaging genetics through meta analysis (ENIGMA) consortium. Biol Psychiatry. 2018;84(9):644–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Palaniyappan L, Liddle PF. Differential effects of surface area, gyrification and cortical thickness on voxel based morphometric deficits in schizophrenia. Neuroimage. 2012;60(1):693–699. [DOI] [PubMed] [Google Scholar]
- 29. Spalthoff R, Gaser C, Nenadic I. Altered gyrification in schizophrenia and its relation to other morphometric markers. Schizophr Res. 2018;202:195–202. [DOI] [PubMed] [Google Scholar]
- 30. Carpenter WT, Jr, Gold JM, Lahti AC, et al. Decisional capacity for informed consent in schizophrenia research. Arch Gen Psychiatry. 2000;57(6):533–538. [DOI] [PubMed] [Google Scholar]
- 31. Nurnberger JIJ, Blehar MC, Kaufmann CA, et al. Diagnostic interview for genetic studies. rationale, unique features, and training. NIMH genetics initiative . Arch Gen Psychiatry. 1994;51(6):849–859. [DOI] [PubMed] [Google Scholar]
- 32. Overall JE, Gorham DR. The brief psychiatric rating scale. Psychol Rep. 1962;10:799–812. [Google Scholar]
- 33. Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. Neuroimage. 1999;9(2):179–194. [DOI] [PubMed] [Google Scholar]
- 34. Nelson EA, White DM, Kraguljac NV, Lahti AC. Gyrification connectomes in unmedicated patients with schizophrenia and following a short course of antipsychotic drug treatment. Front Psychiatry. 2018;9:699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Reuter M, Schmansky NJ, Rosas HD, Fischl B. Neuro image within-subject template estimation for unbiased longitudinal image analysis. Neuroimage. 2012;61(2):1402–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Buckner RL, Head D, Parker J, et al. A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas-based head size normalization: reliability and validation against manual measurement of total intracranial volume. Neuroimage. 2004;23(2):724–738. [DOI] [PubMed] [Google Scholar]
- 37. Okada N, Fukunaga M, Yamashita F, et al. Abnormal asymmetries in subcortical brain volume in schizophrenia. Mole Psych. 2016;21(10):1460–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ribolsi M, Daskalakis ZJ, Siracusano A, Koch G. Abnormal asymmetry of brain connectivity in schizophrenia. Front Hum Neurosci. 2014;8(2):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Royer C, Delcroix N, Leroux E, et al. Functional and structural brain asymmetries in patients with schizophrenia and bipolar disorders. Schizophr Res. 2015;161(3):210–214. [DOI] [PubMed] [Google Scholar]
- 40. Cropley VL, Klauser P, Lenroot RK, et al. Accelerated gray and white matter deterioration with age in schizophrenia. Am J Psychiatry. 2017;174(4):2862–2895. [DOI] [PubMed] [Google Scholar]
- 41. van Haren NEM, Schnack HG, Cahn W, et al. Changes in cortical thickness during the course of illness in schizophrenia. Arch Gene Psychiatry. 2011;68(1):8718–8780. [DOI] [PubMed] [Google Scholar]
- 42. Van Haren NE, Cahn W, Hulshoff Pol HE, Kahn RS. Confounders of excessive brain volume loss in schizophrenia. Neurosci Biobehav Rev. 2013;37(10 Pt 1):2418–2423. [DOI] [PubMed] [Google Scholar]
- 43. Molina V, Hernandez JA, Sanz J, et al. Subcortical and cortical gray matter differences between Kraepelinian and non-Kraepelinian schizophrenia patients identified using voxel-based morphometry. Psychiatry Res. 2010;184(1):16–22. [DOI] [PubMed] [Google Scholar]
- 44. Andreasen NC, Liu D, Ziebell S, Vora A, Ho BC. Relapse duration, treatment intensity, and brain tissue loss in schizophrenia: a prospective longitudinal MRI study. Am J Psychiatry. 2013;170(6):609–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rais M, van Haren NE, Cahn W, et al. Cannabis use and progressive cortical thickness loss in areas rich in CB1 receptors during the first five years of schizophrenia. Eur Neuropsychopharmcol. 2010;20(12):855–865. [DOI] [PubMed] [Google Scholar]
- 46. Brans RGH, van Haren NEM, Van Baal GCM, Schnack HG, Kahn RS, Pol HEH. Heritability of changes in brain volume over time in twin pairs discordant for schizophrenia. Arch Gen Psychiatry. 2008;65(11):1259–1268. [DOI] [PubMed] [Google Scholar]
- 47. Goff DC, Falkai P, Fleischhacker WW, et al. The long-term effects of antipsychotic medication on clinical course in schizophrenia. Am J Psychiatry. 2017;174(9):840–849. [DOI] [PubMed] [Google Scholar]
- 48. Fusar-Poli P, Borgwardt S, Crescini A, et al. Neuroanatomy of vulnerability to psychosis: a voxel-based meta-analysis. Neurosci Biobehav Rev. 2011;35(5):1175–1185. [DOI] [PubMed] [Google Scholar]
- 49. Fusar-Poli P, Radua J, McGuire P, Borgwardt S. Neuroanatomical maps of psychosis onset: voxel-wise meta-analysis of antipsychotic-naive VBM studies. Schizophr Bull. 2012;38(1):1297–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bhojraj TS, Francis AN, Montrose DM, Keshavan MS. Grey matter and cognitive deficits in young relatives of schizophrenia patients. Neuroimage. 2011;54(Suppl 1):S287–S292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Palaniyappan L, Liddle PF. Aberrant cortical gyrification in schizophrenia: a surface-based morphometry study. J Psychiatry Neurosci. 2012;37(6):399–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sasabayashi D, Takayanagi Y, Nishiyama S, et al. Increased frontal gyrification negatively correlates with executive function in patients with first-episode schizophrenia. Cereb Cortex. 2017;27:2686–2694. [DOI] [PubMed] [Google Scholar]
- 53. Sasabayashi D, Takayanagi Y, Takahashi T, et al. Increased occipital gyrification and development of psychotic disorders in individuals with an at-risk mental state: a multicenter study. Biol Psychiatry. 2017;82(10):737–745. [DOI] [PubMed] [Google Scholar]
- 54. Liu B, Zhang X, Cui Y, et al. Polygenic risk for schizophrenia influences cortical gyrification in 2 independent general populations. Schizophr Bull. 2017;43(3):673–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Jessen K, Rostrup E, Mandl RCW, et al. Cortical structures and their clinical correlates in antipsychotic-naive schizophrenia patients before and after 6 weeks of dopamine D2/3 receptor antagonist treatment. Psychol Med. 2019;49(5):754–763. [DOI] [PubMed] [Google Scholar]
- 56. Goghari VM, Smith GN, Honer WG, et al. Effects of eight weeks of atypical antipsychotic treatment on middle frontal thickness in drug-naive first-episode psychosis patients. Schizophr Res. 2013;149(1-3):149–155. [DOI] [PubMed] [Google Scholar]
- 57. Lesh TA, Tanase C, Geib BR, et al. A multimodal analysis of antipsychotic effects on brain structure and function in first-episode schizophrenia. JAMA Psychiatry. 2015;72(3):226–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ho BC, Andreasen NC, Ziebell S, Pierson R, Magnotta V. Long-term antipsychotic treatment and brain volumes: a longitudinal study of first-episode schizophrenia. Arch Gen Psychiatry. 2011;68(2):128–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lahti AC, Reid MA. Is there evidence for neurotoxicity in the prodromal and early stages of schizophrenia? Neuropsychopharmacology. 2011;36(5):1779–1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Park SW, Lee CH, Cho HY, et al. Effects of antipsychotic drugs on the expression of synaptic proteins and dendritic outgrowth in hippocampal neuronal cultures. Synapse. 2013;67(1):224–234. [DOI] [PubMed] [Google Scholar]
- 61. Kuipers SD, Bramham CR. Brain-derived neurotrophic factor mechanisms and function in adult synaptic plasticity: new insights and implications for therapy. Curr Opin Drug Discov Devel. 2006;9(2):580–586. [PubMed] [Google Scholar]
- 62. Pandya CD, Kutiyanawalla A, Pillai A. BDNF-TrkB signaling and neuroprotection in schizophrenia. Asian J Psychiatry. 2013;6(1):22–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Tyler WJ, Pozzo-Miller L. Miniature synaptic transmission and BDNF modulate dendritic spine growth and form in rat CA1 neurones. J Physiol. 2003;553(PPt 2):497–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Fernandes BS, Steiner J, Berk M, et al. Peripheral brain-derived neurotrophic factor in schizophrenia and the role of antipsychotics: meta-analysis and implications. Mole Psychiatry. 2015;20(5):1108–1119. [DOI] [PubMed] [Google Scholar]
- 65. Lieberman JA, Bymaster FP, Meltzer HY, et al. Antipsychotic drugs: comparison in animal models of efficacy, neurotransmitter regulation, and neuroprotection. Pharm Rev. 2008;60:358–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Vernon AC, Crum WR, Lerch JP, et al. Reduced cortical volume and elevated astrocyte density in rats chronically treated with antipsychotic drugs-linking magnetic resonance imaging findings to cellular pathology. Biol Psychiatry. 2014;75(12):982–990. [DOI] [PubMed] [Google Scholar]
- 67. Konopaske GT, Dorph-Petersen KA, Sweet RA, et al. Effect of chronic antipsychotic exposure on astrocyte and oligodendrocyte numbers in macaque monkeys. Biol Psychiatry. 2008;63(8):759–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Palaniyappan L, Marques TR, Taylor H, et al. Cortical folding defects as markers of poor treatment response in first-episode psychosis. JAMA Psychiatry. 2013;70(10):1031–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, CJP_Supplimental_Final_1.29.20. for A Prospective Longitudinal Investigation of Cortical Thickness and Gyrification in Schizophrenia by Eric A. Nelson, Nina V. Kraguljac, David M. White, Ripu D. Jindal, Ah L. Shin and Adrienne C. Lahti in The Canadian Journal of Psychiatry