Abstract
Objective:
Alcohol and cannabis misuse are common in patients with early phase psychosis (EPP); however, research has tended to focus primarily on cannabis misuse and EPP outcomes, with a relative lack of data on alcohol misuse. This retrospective cross-sectional EPP study investigated the relationship between cannabis, alcohol, and cannabis combined with alcohol misuse, on age, gender, psychotic, depressive and anxiety symptom severity, and social/occupational functioning, at entry to service.
Methods:
Two-hundred and sixty-four EPP patients were divided into 4 groups based on substance use measured by the Alcohol, Smoking and Substance Involvement Screening Test: (1) no to low-level cannabis and alcohol misuse (LU), (2) moderate to high alcohol misuse only (AU), (3) moderate to high cannabis misuse only (CU), and (4) moderate to high alcohol and cannabis misuse (AU + CU).
Results:
We found significant between group differences in age (with the AU group being the oldest and AU + CU group the youngest) as well as gender (with the CU group having the highest percentage of men). There were also group differences in positive psychotic symptoms (lowest in AU group), trait anxiety (highest in AU + CU group), and social/occupational functioning (highest in AU group). Further regression analyses revealed a particularly strong relationship between AU + CU group and trait anxiety (3-fold increased odds of clinical trait anxiety for combined misuse of alcohol and cannabis compared to non/low users).
Conclusions:
This study demonstrates the unique demographic and clinical characteristics found in the EPP population at entry to care associated with alcohol and cannabis misuse both separately and in combination. This work highlights the importance of including the assessment of alcohol misuse in addition to cannabis misuse in future treatment guidelines and research.
Keywords: early psychosis, alcohol, cannabis, substance use disorder, clinical measures, social and occupational functioning
Abstract
Objectif :
L’abus d’alcool et de cannabis est commun chez les patients en phase précoce de psychose (PPP). Toutefois, la recherche a tendance à mettre l’accent surtout sur l’abus de cannabis et les résultats des PPP, et il s’ensuit un manque relatif de données sur l’abus d’alcool. Cette étude rétrospective transversale des PPP a recherché la relation entre le cannabis, l’alcool et le cannabis combiné à l’abus d’alcool, et l’âge, le sexe, la gravité des symptômes psychotiques, dépressifs et anxieux, et le fonctionnement social/professionnel à l’entrée dans le service.
Méthodes :
Deux cent soixante-quatre patients de PPP ont été répartis en quatre groupes selon l’utilisation de substance mesurée par le test de dépistage de la consommation d’alcool, de tabac et de substances (ASSIST) : 1) abus de cannabis et d’alcool de faible niveau (LU), 2) abus d’alcool modéré à élevé seulement (AU), 3) abus de cannabis modéré à élevé seulement (CU), et 4) abus d’alcool et de cannabis modéré à élevé (AU+CU).
Résultats :
Nous avons constaté des différences significatives entre les groupes en ce qui a trait à l’âge (le groupe AU étant le plus vieux et le groupe AU+CU, le plus jeune) et au sexe (le groupe CU ayant le pourcentage d’hommes le plus élevé). Il y avait aussi des différences entre les groupes au chapitre des symptômes psychotiques positifs (les plus faibles dans le groupe AU), de l’anxiété trait (la plus élevée dans le groupe AU+CU), et du fonctionnement social/professionnel (le plus élevé dans le groupe AU). D’autres analyses de régression ont révélé une relation particulièrement forte entre le groupe AU+CU et l’anxiété trait (probabilités 3 fois plus fortes d’anxiété trait clinique pour l’abus combiné d’alcool et de cannabis comparativement aux non-utilisateurs ou aux faibles utilisateurs.
Conclusions :
Cette étude démontre les caractéristiques démographiques et cliniques uniques constatées dans la population de PPP lors de l’entrée aux soins associés à l’abus d’alcool et de cannabis tant séparément qu’en combinaison. Ce travail souligne l’importance d’inclure l’évaluation de l’abus d’alcool en plus de l’abus de cannabis dans les futures lignes directrices du traitement et la recherche.
Introduction
Alcohol and cannabis are 2 of the most commonly misused substances, with approximately 18% of Canadians over the age of 15 years meeting criteria for alcohol use disorder (AUD) in their lifetime and approximately 7% with cannabis use disorder (CUD).1 People diagnosed with psychotic disorders tend to have even greater likelihood of lifetime AUD and CUD, approximately 21% for AUD and 27% for CUD.2,3 These rates may be even higher in early phase psychosis (EPP, within the first 5 years of onset), with AUD and CUD rates estimated to be approximately 20% to 33% and 21% to 45%, respectively.4,5,6
Substance misuse in the EPP population is associated with more severe psychotic and depressive symptoms, increased hospitalizations, and poorer functioning.6–10 Substance misuse at younger ages (<15 years of age) is also associated with an earlier onset of psychosis (primarily based on cannabis use data).11,12,13 Heavier substance use is associated with poorer functional and symptomatic outcomes compared to milder substance use,14 suggesting a dose–response relationship between the severity of substance misuse and clinical outcomes in psychosis. EPP treatment standards recommend accepting and treating patients with comorbid EPP and substance use disorders as an essential component of treatment.15,16,17
The research on substance misuse in EPP has focused primarily on cannabis use. There is a significant body of research connecting cannabis use with earlier onset and more severe symptoms of psychosis.13,18–21 This is in contrast to the research on alcohol use in this population. Despite the high use of alcohol in this age group, 82.8%,22 there remain relatively few studies examining the role that alcohol might play in the development and course of psychosis or how alcohol might interact with cannabis in this population. The limited evidence that is available suggests that, similar to cannabis, alcohol misuse may be associated with risk of developing schizophrenia 23 and younger age of onset7 although the evidence is somewhat mixed.24 However, this evidence almost exclusively comes from studies focusing on cannabis use or substance use in general rather than specifically on alcohol. Also of note, it has been suggested that concurrent use of alcohol in addition to cannabis may actually be a significant confounding factor in studies focusing solely on the cannabis–psychosis connection.25 That is to say that co-occurring alcohol use may be impacting the observed effects previously attributed to cannabis use alone.
Given the limited data in this area, and how common alcohol and cannabis misuse are in the EPP population as well as the potential detrimental impacts each of these substances can have on course of illness, the inclusion of alcohol along with cannabis as a focus of study is necessary. The present investigation was a retrospective cross-sectional study that aimed to characterize the demographic features of, and clinical characteristics associated with, alcohol and cannabis misuse (both individually and in combination) among patients at time of entry into an early intervention psychosis treatment program. The primary measurements included examination of the association of alcohol and cannabis misuse with age, gender, psychiatric symptomatology, and social and occupational functioning. It was hypothesized that the misuse of both alcohol and cannabis (individually and in combination) would be associated with younger age at entry to clinic, being male, and having more severe symptoms and worse social/occupational functioning.
Methods
Study Design and Sample
This was a retrospective cross-sectional study of patients from the Nova Scotia Early Psychosis Program (NSEPP), which services people with primary psychotic disorders between the ages of 15 and 35 years old, in Nova Scotia, Canada. Diagnoses were based on Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM IV TR) 26 or DSM-5 27 criteria. Cases with the following psychotic disorder diagnoses were included in this study: schizophrenia, schizophreniform disorder, schizoaffective disorder, and other/unspecified psychotic disorder (psychosis not otherwise specified). Patients with affective psychosis were excluded as the NSEPP does not follow patients with primary mood disorders. The study was approved by the Research Ethics Board of the Nova Scotia Health Authority (File #: 1021953).
Participant records were retrieved from a clinic database that records clinical and demographic information on patients over their 5-year course of treatment with the NSEPP. Admissions from January 2010 to June 2017 were included for a total of 384 new admissions for examining baseline data.
The World Health Organization’s Alcohol, Smoking and Substance Involvement Screening Test (WHO-ASSIST) was used to gather data on alcohol and substance use through a culturally sensitive lens.28 The ASSIST has good discriminative properties, with the ability to differentiate use from abuse and dependence.29 For all drugs except alcohol, a total specific substance score cutoff of ≥4 is used to determine the need for intervention; for alcohol, the cutoff score is ≥11. The present study used these conservative cutoffs for excluding other drug use, but for alcohol and cannabis, we elected to use a lower cutoff of ≥4 for alcohol and ≥2 for cannabis, in light of data suggesting a much higher likelihood of substance use disorder in the EPP population with these lower cutoffs.30 For the purposes of this study, the term “misuse” refers to substance use that is deemed to be clinically significant or reflects a likely substance use disorder, as determined by the ASSIST scores. The alcohol and cannabis ASSIST scores were used to divide patients into 4 groups for data analysis using the above cutoff scores: (1) no to low-level cannabis and alcohol misuse (LU, score <4 for alcohol and <2 for cannabis), (2) moderate to high alcohol misuse only (AU, score ≥4 for alcohol), (3) moderate to high cannabis misuse only (CU, score ≥2 for cannabis), and (4) moderate to high alcohol and cannabis misuse (AU + CU, score ≥4 for alcohol and ≥2 for cannabis). Participants were excluded if they scored ≥4 on the ASSIST for any other substances other than alcohol and cannabis. The 4 groups were modeled as a 4-level categorical variable. Alcohol and cannabis ASSIST scores were also treated as independent continuous variables.
Outcome Measures and Patient Characteristics
The primary outcomes include a list of psychotic, depressive, and anxiety symptom severity scores, and social/occupational functioning, at entry to service. The Positive and Negative Syndrome Scale (PANSS) score to assess severity of psychotic symptoms,31 Social and Occupational Functioning Assessment Scale (SOFAS) to assess overall functioning independent of symptom severity,32 the State-Trait Anxiety Inventory (STAI) measuring trait anxiety and state anxiety33—using a cutoff score of ≥40 to signify clinically significant anxiety,34 and the Calgary Depression Scale for Schizophrenia (CDSS) measuring depressive symptoms specifically in the context of psychosis35—using a cutoff score of ≥7 for clinically significant depression.36 Patient baseline demographics collected included age at admission, self-identified gender, and clinical diagnosis. All clinical scales were administered via face-to-face interviews with the participants at time of entry into the NSEPP by a masters’-level clinical affiliate and/or the patient’s primary treating clinician.
Statistical Analysis
Descriptive statistics were reported as count and percentages for categorical variables, means ± standard deviation for normally distributed continuous variables, and medians and interquartile ranges for nonnormally distributed continuous variables. Differences in baseline demographics and outcome assessment measures were examined using chi-square test, Kruskal–Wallis test or analysis of variance (ANOVA), as appropriate (Table 1).
Table 1.
Baseline Characteristics of Demographic and Clinical Information.
| Characteristic | LU (n = 44) | AU (n = 33) | CU (n = 55) | AU + CU (n = 132) | P Value |
|---|---|---|---|---|---|
| Age, mean (SD) | 23.55 (4.76) | 25.45 (4.96) | 22.74 (4.85) | 21.98 (3.55) | <0.001** |
| Gender: men, % (n) | 56.8% (25) | 63.6% (21) | 90.9% (50) | 81.8% (108) | <0.001** |
| PANSS, mean (SD) | |||||
| Positive | 17.46 (6.14) | 15.65 (5.11) | 19.85 (7.16) | 17.66 (5.48) | 0.041* |
| Negative | 16.27 (7.18) | 14.19 (5.84) | 16.55 (6.77) | 15.95 (6.06) | 0.490 |
| Total | 68.68 (19.08) | 63.88 (17.02) | 74.25 (19.28) | 68.82 (17.82) | 0.150 |
| CDSS, % clinically depressed | 9% (3/35) | 27% (6/22) | 21% (8/38) | 21% (20/97) | 0.300 |
| STAI state, % clinically anxious | 49% (20/41) | 61% (20/33) | 62% (34/55) | 57% (74/129) | 0.610 |
| STAI trait, % clinically anxious | 55% (22/40) | 73% (24/33) | 65% (36/55) | 79% (102/129) | 0.019* |
| SOFAS, mean (SD) | 45.27 (12.69) | 50.88 (12.57) | 41.83 (13.03) | 46.23 (12.55) | 0.041* |
Note. ASSIST = Alcohol, Smoking and Substance Involvement Screening Test; AU = alcohol misuse; AU + CU = alcohol and cannabis misuse; CDSS = The Calgary Depression Scale for Schizophrenia; CU = cannabis misuse; LU = no to low-level use; PANSS = Positive and Negative Syndrome Scale; SD = standard deviation; STAI state/trait = The State-Trait Anxiety Inventory state/trait subscales; SOFAS = Social and Occupational Functioning Assessment Scale.
*P < 0.05. **P < .01.
Multivariable linear regression analysis was used to examine the relationship of the independent continuous variables alcohol and cannabis to the clinical outcome measures: SOFAS, STAI state, STAI trait, PANSS positive, PANSS negative, and PANSS total. A negative binomial model was used to fit the skewed distribution of the CDSS data against continuous variables alcohol and cannabis. Logistic regression models were fit separately to dichotomized CDSS and dichotomized STAI state and trait outcomes with substance use disorder (SUD) category, age, and gender as independent predictors. The Tukey–Kramer multiple comparison adjustment for P values and confidence limits was used for comparisons across SUD groups. Correlation analysis using Spearman correlations on the rank ordered outcomes was performed to explore the relationship between the outcome measures. Results were compared to previous results in existing literature. SAS/STAT 14.3 software version 9.4 (SAS Institute, Cary, NC) was used for all statistical analysis.
Results
Demographics and Patterns of use
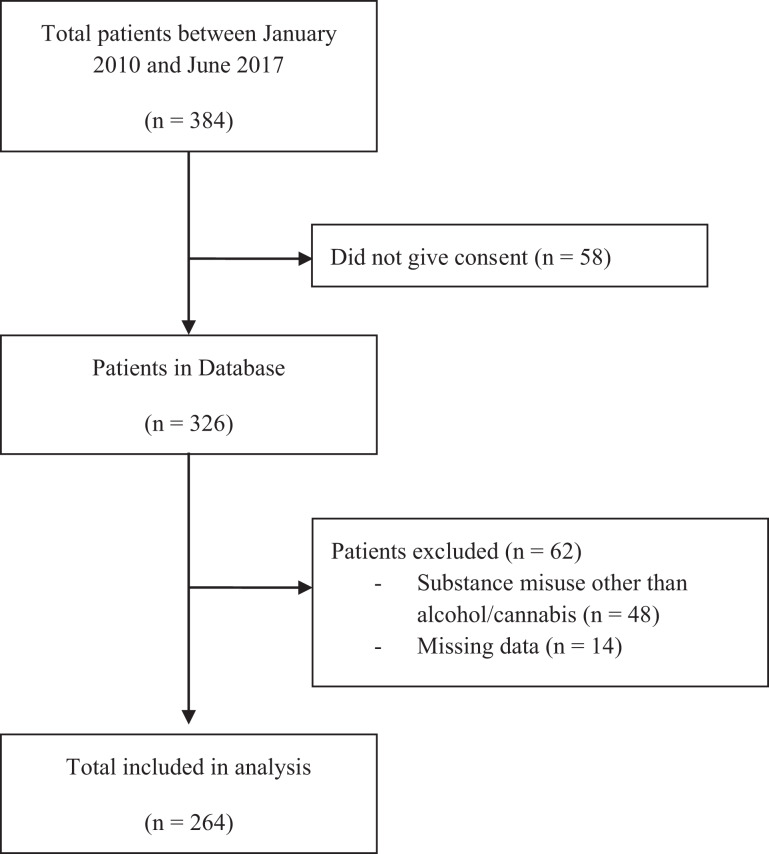
There were 264 patients included in the analysis who met the inclusion criteria (Figure 1) and with complete demographic and clinical data at baseline, with an overall mean age of 22.83 years (range = 15 to 35 years; SD = 4.37; see Table 1). In this sample, 44/264 (16.7%) were classified as LU, 33/264 (12.5%) AU, 55/264 (20.8%) CU, and 132/264 (50%) CU + AU.
Figure 1.
Flowchart of participants included in study.
There was a statistically significant between-group age difference (P = 0.002), with the AU group having the oldest average age (25.5 years [SD 5] at entry to treatment, followed by the LU (23.6 years [SD 4.8]), CU (22.7 years [SD 4.9]), and CU + AU (22.0 years [SD 3.6]) groups. Post hoc testing revealed the mean age for those in the AU group was significantly higher compared to the AU + CU group (a difference of −3.47; 95% CI, −5.60 to −1.33; P < 0.001) and compared to the CU group (a difference of −2.71; 95% CI, 0.29 to 5.13; P = .020). There was also a statistically significant gender difference (P < 0.001), with the highest men-to-women gender ratio being the CU group (10:1) and the lowest being the LU group (1.3:1).
Between-group Comparisons
There was a statistically significant between-group difference (P = 0.041) in positive symptoms with AU patients having the lowest scores. Post hoc testing revealed the mean PANSS positive symptoms score for those in the AU group was 15.65 (95% CI, 13.35 to 17.94) compared to 19.85 (95% CI, 18.01 to 21.69) in the CU group, for a difference of −4.19 (95% CI, −8.05 to −0.34; P = 0.027). Negative and total PANSS scores were not different between the 4 groups, with P values of 0.490 and 0.150, respectively. There were no between-group differences in CDSS (P = 0.300) or STAI state scores (P = 0.610). Dichotomized STAI trait scores were significantly different across SUD groups (P = 0.019), with the AU + CU group having the highest percentage of clinically significant trait anxiety. Between-group ANOVAs demonstrated a significant difference in social functioning (P = 0.041), with the AU group having the highest mean SOFAS score and the CU group having the lowest. The mean SOFAS score for those in the AU group was 50.88 (95% CI, 45.99 to 55.78) compared to the CU group, 41.83 (95% CI, 37.93 to 45.73), for a difference of 9.06 (95% CI, 0.83 to 17.28; P = 0.020).
Predictor Variables: Regression Models
Regression modeling was used to examine associations between alcohol and cannabis use on the clinical outcome measures using ASSIST scores for alcohol/cannabis as independent variables (Table 2). There was a statistically significant (P = 0.007) association between alcohol and STAI state scores, with STAI state increasing by 0.348 for each increase in alcohol ASSIST score. STAI trait (P < 0.001) increased by 0.253 per each increase in alcohol ASSIST score. There was no significant effect of alcohol or cannabis use on PANSS positive, negative, or total PANSS scores (Table 2). There was also no statistically significant association of cannabis on SOFAS score (P = 0.373) although alcohol use was associated (P = 0.020) with a 0.255 increase in SOFAS score per each increase in alcohol ASSIST score. There was no significant association for alcohol or cannabis (Wald χ2 = 1.04, P = 0.307) on CDSS scores.
Table 2.
Regression Modeling Data.
| Dependent Variable | Parameter Estimate (SE) | P Value | ||
|---|---|---|---|---|
| Alcohol | Cannabis | Alcohol | Cannabis | |
| STAI state, n = 258 | 0.348 (0.096) | −0.0378 (0.075) | 0.007 | 0.895 |
| STAI trait, n = 257 | 0.253 (0.092) | 0.0096 (0.073) | <0.001 | 0.615 |
| PANSS positive, n = 210 | −0.0329 (0.049) | 0.0122 (0.039) | 0.505 | 0.759 |
| PANSS negative, n = 210 | −0.0575 (0.053) | 0.00773 (0.042) | 0.274 | 0.854 |
| PANSS total, n = 210 | −0.159 (0.151) | 0.044 (0.121) | 0.291 | 0.716 |
| SOFAS, n = 210 | 0.255 (0.109) | −0.075 (.085) | 0.020 | 0.373 |
| CDSSa, n = 192 | 0.0069 (0.009) | 0.0075 (.007) | 0.419 | 0.307 |
Note. CDSS = The Calgary Depression Scale for Schizophrenia; PANSS = Positive and Negative Syndrome Scale; STAI state/trait = The State-Trait Anxiety Inventory state/trait subscales; SE = standard error; SOFAS = Social and Occupational Functioning Assessment Scale.
a Negative binomial regression model.
Further exploration of the STAI trait and state anxiety measures was performed to look at dichotomized outcomes for the definition of anxiety. Independent variables included 4 group categorical variables for SUD definition (reference group = LU), age, and gender. The logistic regression with clinical STAI trait scores as the outcome showed that SUD group was a statistically significant predictor (P = 0.020) while age (P = 0.186) and gender (P = 0.626) were not. The odds of clinical trait anxiety were 3.173 (95% CI, 1.452 to 6.935) times higher for the AU + CU group compared to the odds of anxiety trait in the LU group (P = 0.004). There was no statistically significant association between AU (P = 0.181) or CU (P = 0.351) when compared to LU. Estimate statements were used to generate linear functions of the parameters to look at differences in the odds of clinical trait anxiety in the AU + CU versus the other substance use groups. The odds of clinical trait anxiety was 2.092 (95% CI, 1.031 to 4.244) greater for the AU + CU group compared to the odds for the CU group (P = 0.041). However, comparison of AU + CU to AU was not statistically significant (P = 0.310). There was no statistically significant association between dichotomized STAI state anxiety and the independent variables: SUD (P = 0.611), gender (P = 0.925), and age (P = 0.650). There were no statistically significant interactions between age or gender with SUD groups, and the interaction terms were therefore removed.
Correlational Analysis: Clinical Measures
Correlational analysis using Spearman correlations were completed between clinical/functional outcome measures (Table 3). Correlations of at least moderate effect size or greater (r > .6) and with significance of P < 0.01 included PANSS positive and total scores that were both negatively associated with social and occupational functioning.
Table 3.
Correlations between Clinical Measures.
| Clinical Measures | PANSS Positive | PANSS Negative | PANSS Total | CDSS | STAI State | STAI Trait | SOFAS | |
|---|---|---|---|---|---|---|---|---|
| PANSS positive | Spearman correlation | 1 | 0.371** | 0.783** | 0.098 | 0.132 | 0.006 | −0.622** |
| Sig. (2-tailed) | <0.001 | <0.001 | 0.182 | 0.260 | 0.936 | <0.001 | ||
| N | 210 | 210 | 188 | 206 | 206 | 208 | ||
| PANSS negative | Spearman correlation | 1.000 | 0.761** | 0.243** | 0.112 | −0.020 | −0.418** | |
| Sig. (2-tailed) | <0.001 | 0.001 | 0.110 | 0.773 | <0.001 | |||
| N | 210 | 188 | 206 | 206 | 208 | |||
| PANSS total | Spearman correlation | 1.000 | 0.268** | 0.171* | 0.003 | −0.686** | ||
| Sig. (2-tailed) | <0.001 | 0.014 | 0.965 | <0.001 | ||||
| N | 188 | 206 | 206 | 208 | ||||
| CDSS | Spearman correlation | 1.000 | 0.440** | 0.538** | −0.133 | |||
| Sig. (2-tailed) | <0.001 | <0.001 | 0.070 | |||||
| N | 188 | 188 | 187 | |||||
| STAI state | Spearman correlation | 1 | 0.725** | −0.079 | ||||
| Sig. (2-tailed) | <0.001 | 0.261 | ||||||
| N | 257 | 206 | ||||||
| STAI trait | Spearman correlation | 1 | 0.060 | |||||
| Sig. (2-tailed) | 0.394 | |||||||
| N | 206 | |||||||
| SOFAS | Spearman correlation | 1 | ||||||
| Sig. (2-tailed) | ||||||||
| N | 210 | |||||||
Note. CDSS = The Calgary Depression Scale for Schizophrenia; PANSS positive/negative/total = Positive and Negative Syndrome Scale positive/negative/total subscales; SOFAS = Social and Occupational Functioning Assessment Scale; STAI state/trait = The State-Trait Anxiety Inventory state/trait subscales.
* Correlation is significant at the 0.05 level. **Correlation is significant at the 0.01 level.
Sensitivity analysis using the original alcohol and cannabis ASSIST cutoff scores was completed (Table 4). This revealed that using the original ASSIST cutoff scores, there was still a significant between-group age difference (P = 0.011) as well as gender difference (P = 0.003); as well as significant between group difference in STAI trait scores (P = 0.016). However, there were no longer between-group differences for PANSS positive or SOFAS scores.
Table 4.
Sensitivity Analysis: Group Comparisons Using Original ASSIST Cutoff Scoresa for Cannabis and Alcohol.
| Characteristic | LU (n = 93) | AU (n = 15) | CU (n = 100) | AU + CU (n = 56) | P Value |
|---|---|---|---|---|---|
| Age, mean (SD) | 23.8 (4.8) | 24.4 (4.4) | 22.2 (4.4) | 21.9 (3.0) | 0.011* |
| Gender: men, % (n) | 66% (61) | 80% (12) | 88% (88) | 77% (43) | 0.003** |
| PANSS, mean (SD) | |||||
| Positive | 16.6 (6.1) | 19.4 (5.6) | 18.7 (6.5) | 17.8 (4.7) | 0.120 |
| Negative | 16.1 (6.8) | 15.4 (6.1) | 15.7 (6.6) | 16.0 (5.6) | 0.980 |
| Total | 67.0 (19.1) | 74.2 (20.3) | 70.8 (19.2) | 68.8 (15.0) | 0.450 |
| CDSS, % clinically depressed | 14% (10/69) | 25% (3/12) | 21% (14/67) | 23% (10/44) | 0.630 |
| STAI state, % clinically anxious | 56% (50/90) | 60% (9/15) | 55% (54/99) | 65% (35/54) | 0.630 |
| STAI trait, % clinically anxious | 63% (56/89) | 80% (12/15) | 70% (69/99) | 87% (47/54) | 0.016* |
| SOFAS, mean (SD) | 46.8 (13.2) | 44.8 (12.2) | 43.3 (12.9) | 48.3 (12.1) | 0.160 |
ASSIST = Alcohol, Smoking and Substance Involvement Screening Test; AU = alcohol misuse; AU + CU = alcohol and cannabis misuse; CDSS = The Calgary Depression Scale for Schizophrenia; CU = cannabis misuse; LU = no to low-level use; PANSS = Positive and Negative Syndrome Scale; SD = standard deviation; STAI state/trait = The State-Trait Anxiety Inventory state/trait subscales; SOFAS = Social and Occupational Functioning Assessment Scale.
a Groups according to original ASSIST cutoffs: (1) no to low-level cannabis and alcohol misuse (LU, score <11 for alcohol and <4 for cannabis), (2) moderate to high alcohol misuse only (AU, score ≥11 for alcohol), (3) moderate to high cannabis misuse only (CU, score ≥4 for cannabis), and (4) moderate to high alcohol and cannabis misuse (AU + CU, score ≥11 for alcohol and ≥4 for cannabis).
*P < 0.05. *P < 0.01.
Discussion
Our results show that studying alcohol use in addition to cannabis use in EPP is important as alcohol may have significant clinical impacts both on its own and in combination with cannabis in this population. To our knowledge, this is the first time this relationship has been studied in this detail in an early psychosis population.
The first observation to note is that the vast majority of our study sample (83%) misused at least 1 substance at time of treatment entry, 60% of whom misused both alcohol and cannabis. Also, of note, is that well over half the patients (62.5%) misused alcohol. One possible reason for this estimate being higher than previous reported literature4–6 may be due to our use of the ASSIST cutoffs as a proxy for alcohol misuse rather than clinical diagnoses or DSM criteria. Nevertheless, there is evidence to suggest that the cutoff we employed for alcohol has clinical significance in that individuals who score ≥4 have a 5 to 6 times greater chance of having an AUD.30
The primary results found that AU patients were the oldest and the 2 cannabis using groups were the youngest which is consistent with previous literature11,12,37,38 although there are some mixed findings.7 Perhaps those who present at a later age have a preference for alcohol use; or, alcohol may be masking psychosis symptoms leading to delayed detection. Alternatively, there is the possibility that alcohol use could delay the onset of psychosis. This potential protective effect has been suggested previously although likely reflects the possibility that alcohol users have better social adjustment, or more positive social status, prior to the onset of illness.38,39
Regarding gender, the CU group had the highest ratio of men to women, whereas the AU group had a significantly lower men to women ratio. This gender difference suggests that EPP patients who are women tend not to prefer cannabis, while men have a significant preference for cannabis use (with or without alcohol use); a pattern also found in the general population. Overall, this data fit with previous findings in EPP where men are more likely to misuse all substances, as well as cannabis in particular.40,41
Interestingly, there was a between-group difference in severity of positive psychotic symptoms with the AU group having the lowest severity and the CU group having the highest. There were no group differences in negative or total symptoms. These findings should be interpreted with caution as we did not find a further significant effect using regression modeling. Nevertheless, the connection between cannabis use and positive psychotic symptoms has been previously found42,43 although this relationship is unclear.44,45 To our knowledge, the finding that EPP patients who use alcohol only may express fewer positive symptoms at entry to care is novel. Previous literature seems to suggest no significant association between alcohol and positive psychotic symptoms5,9,46 although 1 prior study has found that alcohol may be associated with fewer negative symptoms.44 This variation in the literature may be due to differing ways of measuring alcohol consumption and may also explain why our findings differ from previous studies. Alternatively, in our study population, it may be that those with fewer positive symptoms were more adept at acquiring and using alcohol; a similar interpretation has been made by Stone et al.44 to explain their finding that alcohol was associated with fewer negative symptoms of psychosis. However, our smaller sample size for the AU group (n = 33) also suggests caution in our interpretation and that these findings should be replicated.
Our findings also revealed that the 2 alcohol using groups (AU + CU and AU only) had the highest percentage of clinically significant STAI trait scores, with odds that were 3-fold greater in the AU + CU group compared to LU. The regression models confirmed and added confidence to this finding and suggested that alcohol use in particular was significantly associated with anxiety as opposed to the cannabis use. This association has previously been reported and seems to be even more pronounced the more severe the level of anxiety.47 This may indicate that patients with higher trait anxiety tend to use alcohol, which would be in line with the tension reduction hypothesis.48 Another possibility could be that alcohol itself exacerbates dispositional anxiety in this population. In fact, these 2 explanations are not necessarily mutually exclusive as both processes might be operative in a cyclical nature, the so called vicious cycle.49
Social and occupational functioning has generally been found to be poorer in EPP patients who misuse alcohol or cannabis.5,50 Our findings were not in line with this, and in fact, we found the opposite, with the highest functioning group being the pure alcohol misusers. In fact, we also found trend-level evidence that alcohol may be associated with higher social functioning via regression modeling which would support the between-group findings. This finding, if true, would be unique; however, again, could be explained by the findings discussed above regarding better social adjustment among alcohol users in comparison to nondrug users.38,39
Another notable negative finding was the lack of association between alcohol misuse and depressive symptoms. Previous research seems to indicate that alcohol misuse in EPP populations tends to lead to more depression.51,52 This is mirrored in the general alcohol misuse literature where there is clear evidence of a bidirectional connection between alcohol misuse and depression.53 One possibility is that our sample size may not have been large enough to detect differences in depression scores between groups. Another possibility may be a confounding factor that was not included in our analyses. For example our sample excluded affective psychoses, whereas some previous studies included this group in their population,51,52 with the possibility that a significant mood component may effect or modulate these results.
Sensitivity analysis using the original ASSIST cutoffs demonstrated that the group differences in age, gender, and trait anxiety were likely robust, as they remained significant regardless of which set of cutoffs were used. However, given that the group differences for positive psychotic symptoms and social functioning were no longer significant when the original ASSIST cutoffs were used, these findings would not be considered as robust and should be interpreted more cautiously. On the other hand, these findings may also be due to loss of statistical power to detect differences which may still exist (higher cutoffs resulting in smaller substance using groups). It is also noteworthy that using the original ASSIST cutoffs significantly shifts the number in each of the 4 groups, such that only a very small number (n = 15) remain in the AU group along with the disappearance of statistical significance in the aforementioned symptom/functional domains. Had this study used the original ASSIST cutoffs, these effects would likely have been missed which in turn raises questions about how the higher cutoffs for alcohol specifically are derived as well as the general social acceptability of alcohol in comparison with other substances. This is particularly timely in light of recent evidence that challenges the conventional wisdom that there are “safe” amounts of alcohol to consume.54
The correlation analyses confirmed a number of associations that have previously been described in the literature; however, only 2 correlations reached a moderate effect size. Namely the worse the psychotic symptomatology (positive symptoms and total symptoms), the worse the ability to function. The association between psychosis and social/occupational functioning is well established, and there is evidence that functional deficits can be detected even before the onset of illness.55 Although this correlational analysis was limited, the data that we gathered are in line with other EPP study populations in the literature.
The primary limitation of the present study is that it is retrospective and cross-sectional in nature, thereby limiting any causal interpretation or temporal prediction. A prospective study design would be preferred, ideally starting prior to the onset of illness and following participants up over the course of the illness and its treatment. This would allow for better interpretation of what seems to be a complex relationship between alcohol and cannabis use, alone and in combination, with the onset of and course of psychotic illness. Also, of note, we did not examine other commonly used substances in this populations (e.g., tobacco) as well as less commonly used but clinically concerning substances (e.g., stimulants), which would also be important to address in future work. In addition, there may be confounding factors that may affect some of our findings such as premorbid adjustment. Although this was outside of the scope of this study, it may limit the interpretation of these results and should be considered in future research. Another potential limitation is that we employed ASSIST scoring as a proxy for harmful substance use as opposed to diagnostic criteria. This, along with the fact that our data were not collected with another structured clinical interview tool, makes it difficult to directly compare our findings to some of the previous literature. However, with a general movement away from strict categorical designations in psychiatry,56,57 this may prove to be a strength moving forward. It also allows for the analysis of the data along a spectrum of substance use severity, as employed by our regression analyses, which can demonstrate dose–response relationships, as well as to help isolate the differential effect that various substances may be having on a given outcome variable.
Future studies examining the impacts of substance use in EPP would benefit from longitudinal designs as previously mentioned. Another consideration would be to include neurobiological measures. Numerous neuroimaging studies have demonstrated measurable changes that occur in the context of cannabis use58 in this population; however, evidence is limited when it comes to brain changes associated with alcohol use (or combined alcohol/cannabis use) in EPP. Collection of this data and connecting it with clinical findings would allow for a richer understanding of the relationship between substance use and psychosis and ultimately may lead to novel treatment targets that may promote recovery in this patient population. In addition, given that cannabis use was recently legalized in Canada (following the collection of this data), it would be interesting to reexamine the patterns of use and clinical outcomes in this population to see whether there are measurable changes postlegalization.
Conclusion
In conclusion, we showed that at time of entry into an early psychosis program, alcohol misuse was associated with having an older age at entry, having more dispositional anxiety symptoms, and having better social functioning. Patients using cannabis on the other hand tended to be men, tended to be younger, and had more positive symptoms of psychosis. The group with combined alcohol and cannabis use was generally intermediate in the outcomes measured other than trait anxiety where our analyses suggested the possibility that there may be additive effects between the 2 substances. These findings help better characterize the complex clinical presentations of patients with comorbid substance use and EPP. This data also underscore the possibility that alcohol may be an important confounding factor in previous studies that tend to focus primarily on the impact of cannabis in psychotic disorders and hopefully will highlight the importance of including alcohol use (and ideally all substances) in addition to cannabis use as significant clinical variables to consider in future treatment and research.
Acknowledgments
We thank Madison Holmans for her diligent work in maintaining and providing the data from the NSEPP database.
Authors’ Note: Request for access to the data can be addressed to the first author at jacob.cookey@dal.ca.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: P.G.T. has received honoraria for speaking and is on the advisory boards for Otsuka, Janssen, Inc., and Sunovion, however, not in the subject area of this article. The remaining authors have no competing financial interests.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by funding through Dalhousie University’s Department of Psychiatry Summer Studentship Program (J.M., Grant Number 990149). Also, S.H.S. is supported through a Tier 1 Canada Research Chair in Addictions and Mental Health.
ORCID iD: Jacob Cookey, MD  https://orcid.org/0000-0001-6904-7569
https://orcid.org/0000-0001-6904-7569
References
- 1. Pearson C, Janz T, Ali J. Health at a glance: mental and substance use disorders in Canada. 2013. [accessed 2018 May 31] http://www.statcan.gc.ca/pub/82-624-x/2013001/article/11855-eng.pdf.
- 2. Koskinen J, Löhönen J, Koponen H, Isohanni M, Miettunen J. Prevalence of alcohol use disorders in schizophrenia - a systematic review and meta-analysis. Acta Psychiatr Scand. 2009;120(2):85–96. [DOI] [PubMed] [Google Scholar]
- 3. Koskinen J, Löhönen J, Koponen H, Isohanni M, Miettunen J. Rate of cannabis use disorders in clinical samples of patients with schizophrenia: a meta-analysis. Schizophr Bull. 2010;36(6):1115–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wisdom JP, Manuel JI, Drake RE. Substance use disorder among people with first-episode psychosis: a systematic review of course and treatment. Psychiatr Serv. 2011;62(9):1007–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ouellet-Plamondon C, Abdel-Baki A, Salvat É, Potvin S. Specific impact of stimulant, alcohol and cannabis use disorders on first-episode psychosis: 2-year functional and symptomatic outcomes. Psychol Med. 2017;47(14):2461–2471. [DOI] [PubMed] [Google Scholar]
- 6. Turkington A, Mulholland CC, Rushe TM, et al. Impact of persistent substance misuse on 1-year outcome in first-episode psychosis. Br J Psychiatry. 2009;195(3):242–248. [DOI] [PubMed] [Google Scholar]
- 7. Addington J, Addington D. Patterns, predictors and impact of substance use in early psychosis: a longitudinal study. Acta Psychiatr Scand. 2007;115(4):304–309. [DOI] [PubMed] [Google Scholar]
- 8. Pencer A, Addington J. Substance use and cognition in early psychosis. J Psychiatry Neurosci. 2003;28(1):48–54. [PMC free article] [PubMed] [Google Scholar]
- 9. Wade D, Harrigan S, Edwards J, Burgess PM, Whelan G, McGorry PD. Substance misuse in first-episode psychosis: 15-month prospective follow-up study. Br J Psychiatry. 2006;189(3):229–234. [DOI] [PubMed] [Google Scholar]
- 10. Seddon JL, Birchwood M, Copello A, et al. Cannabis use is associated with increased psychotic symptoms and poorer psychosocial functioning in first-episode psychosis: a report from the UK National EDEN study. Schizophr Bull. 2016;42(3):619–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mauri MC, Volonteri LS, De Gaspari IF, Colasanti A, Brambilla MA, Cerruti L. Substance abuse in first-episode schizophrenic patients: a retrospective study. Clin Pract Epidemiol Ment Health. 2006;2(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Large M, Sharma S, Compton MT, Slade T, Nielssen O. Cannabis use and earlier onset of psychosis: a systematic meta-analysis. Arch Gen Psychiatry. 2011;68(6):555–561. [DOI] [PubMed] [Google Scholar]
- 13. Di Forti M, Sallis H, Allegri F, et al. Daily use, especially of high-potency cannabis, drives the earlier onset of psychosis in cannabis users. Schizophr Bull. 2014;40(6):1509–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wade D, Harrigan S, McGorry PD, Burgess PM, Whelan G. Impact of severity of substance use disorder on symptomatic and functional outcome in young individuals with first-episode psychosis. J Clin Psychiatry. 2007;68(5):767–774. [DOI] [PubMed] [Google Scholar]
- 15. Addington DE, McKenzie E, Norman R, Wang J, Bond GR. Essential evidence-based components of first-episode psychosis services. Psychiatr Serv. 2013;64(5):452–457. [DOI] [PubMed] [Google Scholar]
- 16. Ontario Ministry of Health. Early Psychosis Intervention Program Standards. Toronto: Ontario ministry of health; March 31, 2011. [accessed 2018 Jun 25] http://www.health.gov.on.ca/english/providers/pub/mental/epi_program_standards.pdf. [Google Scholar]
- 17. Crockford D, Addington D. Canadian schizophrenia guidelines: schizophrenia and other psychotic disorders with coexisting substance use disorders. Can J Psychiatry. 2017;62(9):624–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Andréasson S, Allebeck P, Engström A, Rydberg U. Cannabis and schizophrenia. a longitudinal study of Swedish conscripts. Lancet. 1987;2(8574):1483–1486. [DOI] [PubMed] [Google Scholar]
- 19. Arseneault L, Cannon M, Poulton R, Murray R, Caspi A, Moffitt TE. Cannabis use in adolescence and risk for adult psychosis: longitudinal prospective study. BMJ. 2002;325:1212–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Veen ND, Selten JP, van der Tweel I, Feller WG, Hoek HW, Kahn RS. Cannabis use and age at onset of schizophrenia. Am J Psychiatry. 2004;161(3):501–506. [DOI] [PubMed] [Google Scholar]
- 21. Casadio P, Fernandes C, Murray RM, Di Forti M. Cannabis use in young people: the risk for schizophrenia. Neurosci Biobehav Rev. 2011;35(8):1779–1787. [DOI] [PubMed] [Google Scholar]
- 22. Canadian Centre on Substance Use and Addiction. Canadian Drug Summary: Alcohol. Fall; 2017. [accessed 2018 feb 12] http://www.ccsa.ca/ResourceLibrary/CCSA-Canadian-Drug-Summary-Alcohol-2017-en.pdf.
- 23. Welch KA, McIntosh AM, Job DE, et al. The impact of substance use on brain structure in people at high risk of developing schizophrenia. Schizophr Bull. 2011;37(5):1066–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Barnes TRE, Mutsatsa SH, Hutton SB, Watt HC, Joyce EM. Comorbid substance use and age at onset of schizophrenia. Br J Psychiatry. 2006;188(3):237–242. [DOI] [PubMed] [Google Scholar]
- 25. Auther AM, Cadenhead KS, Carrión RE, et al. Alcohol confounds relationship between cannabis misuse and psychosis conversion in a high-risk sample. Acta Psychiatr Scand. 2015;132(1):60–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. American Psychiatric Association (APA). Diagnostic and Statistical Manual of Mental Disorders. 4 th ed, text rev. Washington (DC): APA; 2000. [Google Scholar]
- 27. American Psychiatric Association (APA). Diagnostic and Statistical Manual of Mental Disorders. 5 th ed Arlington (VA): APA; 2013. [Google Scholar]
- 28. WHO ASSIST Working Group. The alcohol, smoking and substance involvement screening test (ASSIST): development, reliability and feasibility. Addiction. 2002;97(9):1183–1194. [DOI] [PubMed] [Google Scholar]
- 29. Humeniuk R, Ali R, Babor TF, et al. Validation of the alcohol, smoking and substance involvement screening test (ASSIST). Addiction. 2008;103(6):1039–1047. [DOI] [PubMed] [Google Scholar]
- 30. Hides L, Cotton SM, Berger G, et al. The reliability and validity of the alcohol, smoking and substance involvement screening test (ASSIST) in first-episode psychosis. Addict Behav. 2009;34(10):821–825. [DOI] [PubMed] [Google Scholar]
- 31. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261–276. [DOI] [PubMed] [Google Scholar]
- 32. Morosini PL, Magliano L, Brambilla L, Ugolini S, Pioli R. Development, reliability and acceptability of a new version of the DSM-IV social and occupational functioning assessment scale (SOFAS) to assess routine social functioning. Acta Psychiatr Scand. 2000;101(4):323–329. [PubMed] [Google Scholar]
- 33. Spielberger CD, Gorsuch RL, Lushene R, et al. Manual for the State-Trait Anxiety Inventory. Palo Alto (CA: ): Consulting Psychologists Press; 1983. [Google Scholar]
- 34. Seedat S, Fritelli V, Oosthuizen P, Emsley RA, Stein DJ. Measuring anxiety in patients with schizophrenia. J Nerv Ment Dis. 2007;195(4):320–324. [DOI] [PubMed] [Google Scholar]
- 35. Addington D, Addington J, Schissel B. A depression rating scale for schizophrenics. Schizophr Res. 1990;3(4):247–251. [DOI] [PubMed] [Google Scholar]
- 36. Addington D, Addington J, Maticka-Tyndale E. Assessing depression in schizophrenia: the Calgary depression scale. Br J Psychiatry. 1993;163(Suppl 22):39–44. [PubMed] [Google Scholar]
- 37. Compton MT, Kelley ME, Ramsay CE, et al. Association of pre-onset cannabis, alcohol, and tobacco use with age at onset of prodrome and age at onset of psychosis in first-episode patients. Am J Psychiatry. 2009;166(11):1251–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kelley ME, Wan CR, Broussard B, et al. Marijuana use in the immediate 5-year premorbid period is associated with increased risk of onset of schizophrenia and related psychotic disorders. Schizophr Res. 2016;171(1–3):62–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Choukas-Bradley S, Giletta M, Neblett EW, Prinstein MJ. Ethnic differences in associations among popularity, likability, and trajectories of adolescents’ alcohol use and frequency. Child Dev. 2015;86(2):519–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Archie S, Rush BR, Akhtar-Danesh N, et al. Substance use and abuse in first-episode psychosis: prevalence before and after early intervention. Schizophr Bull. 2007;33(6):1354–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lange EH, Nesvåg R, Ringen PA, et al. One year follow-up of alcohol and illicit substance use in first-episode psychosis: does gender matter? Comp Psychiatry. 2014;55(2):274–282. [DOI] [PubMed] [Google Scholar]
- 42. D’Souza DC, Perry E, MacDougall L, et al. The psychotomimetic effects of intravenous delta-9-tetrahydrocannabinol in healthy individuals: implications for psychosis. Neuropsychopharmacology. 2004;29(8):1558–1572. [DOI] [PubMed] [Google Scholar]
- 43. Oluwoye O, Monroe-DeVita M, Burduli E, et al. Impact of tobacco, alcohol and cannabis use on treatment outcomes among patients experiencing first episode psychosis: data from the national RAISE-ETP study. Early Interv Psychiatry. 2018;13(1):142–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Stone JM, Fisher HL, Major B, et al. Cannabis use and first-episode psychosis: relationship with manic and psychotic symptoms, and with age at presentation. Psychol Med. 2014;44(3):499–506. [DOI] [PubMed] [Google Scholar]
- 45. Zammit S, Moore TH, Lingford-Hughes A, et al. Effects of cannabis use on outcomes of psychotic disorders: systematic review. Br J Psychiatry. 2008;193(5):357–363. [DOI] [PubMed] [Google Scholar]
- 46. Sorbara F, Liraud F, Assens F, Abalan F, Verdoux H. Substance use and the course of early psychosis: a 2-year follow-up of first-admitted subjects. Eur Psychiatry. 2003;18(3):133–136. [DOI] [PubMed] [Google Scholar]
- 47. Subramaniam M, Mahesh MV, Peh CX, et al. Hazardous alcohol use among patients with schizophrenia and depression. Alcohol. 2017. ;(65):63–69. [DOI] [PubMed] [Google Scholar]
- 48. Young RMD, Oei TPS, Knight RG. The tension reduction hypothesis revisited: an alcohol expectancy perspective. Br J Addict. 1990;85(1):31–40. [DOI] [PubMed] [Google Scholar]
- 49. Kushner MG, Abrams K, Borchardt C. The relationship between anxiety disorders and alcohol use disorders. Clin Psychol Rev. 2000;20(2):149–171. [DOI] [PubMed] [Google Scholar]
- 50. Abdel-Baki A, Ouellet-Plamondon C, Salvat É, Grar K, Potvin S. Symptomatic and functional outcomes of substance use disorder persistence 2 years after admission to a first-episode psychosis program. Psychiatry Res. 2017;(247): 113–119. [DOI] [PubMed] [Google Scholar]
- 51. Roche E, Clarke M, Browne S, et al. Prevalence and clinical correlates of depression in the acute phase of first episode schizophrenia. Ir J Psychol Med. 2010;27(1):15–18. [DOI] [PubMed] [Google Scholar]
- 52. Sönmez N, Røssberg JI, Evensen J, et al. Depressive symptoms in first-episode psychosis: a 10-year follow-up study. Early Interv Psychiatry. 2016;10(3):227–233. [DOI] [PubMed] [Google Scholar]
- 53. Boden JM, Fergusson DM. Alcohol and depression. Addiction. 2011;106(5):906–914. [DOI] [PubMed] [Google Scholar]
- 54. Griswold MG, Fullman N, Hawley C, et al. Alcohol use and burden for 195 countries and territories, 1990-2016: a systematic analysis for the global burden of disease study 2016. Lancet. 2018;392:1015–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Velthorst E, Fett AKJ, Reichenberg A, et al. The 20-year longitudinal trajectories of social functioning in individuals with psychotic disorders. Am J Psychiatry. 2017;174(11):1075–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Krueger RF, Eaton NR. Transdiagnostic factors of mental disorders. World Psychiatry. 2015;14(1):27–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Insel T, Cuthbert B, Garvey M, et al. Research domain criteria (RDOC): toward a new classification framework for research on mental disorders. Am J Psychiatry. 2010;167(7):748–751. [DOI] [PubMed] [Google Scholar]
- 58. Crocker CE, Cookey J, Tibbo PG. Neuroimaging findings in adolescent cannabis use and early phase psychosis In: Preedy VR, editor. Handbook of Cannabis and Related Pathologies: Biology, Pharmacology, Diagnosis and Treatment. Cambridge (MA): Elsevier Academic Press; 2017. [Google Scholar]