Abstract
Background:
In 2016, the global number of individuals living with dementia was 43.8 million, representing a 117% increase from 1990—mainly due to increases in aging and population growth. Up to 90% of individuals with dementia experience neuropsychiatric symptoms (NPS). However, the limitations of current treatments for NPS have drivent he search for safer pharmacotherapies—including cannabinoids.
Aim:
To assess the efficacy and acceptability of cannabinoids for the treatment of NPS in individuals with dementia.
Design:
Systematic review and meta-analysis of clinical trials.
Setting and participants:
Of 6,902 papers, 9 were eligible (n = 205, 44% female, 78 ± 7 years, 85% Alzheimer disease). Trials were in North America and Europe and explored tetrahydrocannabinol (n = 3), dronabinol (n = 5), or nabilone (n = 1).
Measurement:
Titles/abstracts were independently screened by one reviewer and reviewed by a second. Full-text screening was by two reviewers with discrepancies resolved via a third reviewer. We extracted data on the standardized mean difference (SMD) for several NPS instruments, trial completion, and adverse events. Data were pooled using random-effects models.
Findings:
Cannabinoids led to significant improvements across NPS instruments, including the Cohen Mansfield Agitation Inventory (SMD = −0.80; 95% confidence interval [CI], −1.45 to −0.16), the Neuropsychiatric Inventory (SMD = −0.61; CI, −1.07 to −0.15), and nocturnal actigraphy (SMD = −1.05; CI, −1.56 to −0.54h). Cannabinoids were well-tolerated, with an overall trial completion rate of 93% (193/205) and no serious treatment-related adverse events. Treatment efficacy was associated with baseline dementia severity and dose, but not dementia subtype, age, or sex. The overall study quality was rated as low.
Conclusions:
There is preliminary evidence for the efficacy and tolerability of cannabinoids as treatments for NPS. Population-based studies are needed to characterize their real-world effectiveness and acceptability.
Keywords: Alzheimer disease, cannabinoids, meta-analysis, systematic reviews, geriatric psychiatry, pharmacotherapy
Abstract
Contexte :
En 2016, il y avait 43,8 millions de personnes vivant avec la démence, dont 90% éprouvaient des symptômes neuropsychiatriques [SNP]. Les embûches des traitements actuels des SNP ont entraîné la recherche de pharmacothérapies plus sûres—notamment les cannabinoïdes.
Objectif :
Nous avons mené une revue systématique et une méta-analyse d’essais cliniques afin de produire des estimations globales de l’efficacité et de l’acceptabilité des cannabinoïdes pour le traitement des SNP.
Méthode :
Sur 6 902 articles, 9 étaient admissibles (n = 205, 44% de femmes, 787 ans, 85% ayant la maladie d’Alzheimer). Les essais ont été menés en Amérique du Nord et en Europe, et exploraient le tétrahydrocannabinol (n = 3), le dronabinol (n = 5), ou le nabilone (n = 1).
Mesure :
Les titres ou résumés ont été vus indépendamment par un réviseur puis par un autre. La lecture du texte intégral s’est faite par deux réviseurs dont les divergences étaient résolues par un troisième réviseur. Nous avons extrait les données de la différence moyenne normalisée [DMN] pour plusieurs instruments des SNP, la fin de l’essai, et les effets indésirables. Les données ont été regroupées à l’aide de modèles à effets aléatoires.
Résultats :
Les cannabinoïdes ont provoqué des améliorations significatives des instruments des SNP, notamment l’inventaire d’agitation de Cohen Mansfield (SNP = −0,80, IC à 95% −1,45 à −0,16), l’inventaire neuropsychiatrique (−0,61 [−1,07 à −0,15), et l’actigraphie nocturne (−1,05 [−1,56 à −0,54]). Les cannabinoïdes étaient bien tolérés, avec un taux global d’achèvement d’essai de 93% (193/205), et sans aucun essai indésirable sérieux lié au traitement. L’efficacité du traitement était associée à la gravité de la démence au départ et à la dose, mais pas au sous-type de la démence, ni à l’âge ni au sexe. La qualité générale de l’étude a été cotée faible.
Conclusions :
Il existe des données probantes préliminaires de l’efficacité et de la tolérabilité des cannabinoïdes comme traitements des SNP. Il faut des études dans la population pour en caractériser l’efficacité et l’acceptabilité réelles.
Background
Globally, the number of individuals living with dementia is increasing, which negatively affects families, communities, and health-care systems ubiquitously.1 In 2016, the global number of individuals who lived with dementia was 43.8 million (95% uncertainty interval, 37.8 to 51.0), representing a 117% increase from 1990—mainly due to increases in population ageing and growth.1
Until there are breakthroughs in preventive or curative treatment, dementia will constitute an increasing challenge to health-care systems worldwide.1 As there is presently no known cure for dementia, there is an ongoing search for effective treatments for the neuropsychiatric symptoms (NPS) of dementia, which can include agitation and aggression.2–4 These behavioral symptoms affect up to 90% of all persons with dementia and are highly predictive of caregiver stress and nursing home placement, along with acceleration of cognitive and functional decline.5
While atypical antipsychotics are the most commonly used medications for NPS, their efficacy is modest, and their use is associated with an increased risk of multiple serious adverse events, extrapyramidal symptoms, and all-cause mortality.6–9 Therefore, the limited efficacy and high-risk profiles of current pharmacotherapies for the treatment of dementia symptoms have driven the search for safer pharmacologic alternatives.10
Recently, cannabinoids have become a popular treatment for a variety of medical conditions, including chronic pain, and psychiatric disorders,11–13 including dementia.14,15 Available evidence suggests there are multiple neuroprotective properties of cannabinoids.16–20 Ligands at the CB1 cannabis receptors reduce presynaptic neurotransmitter release, including glutamate.21–23 As excessive glutamate in the synapse can lead to oxidative stress and damage to neurons promoting neurodegeneration, this is considered a therapeutic mechanism for cannabinoids in dementia.24 As well, in vitro experiments show that cannabinoids at the CB1 receptor may protect neurons from both excitotoxic25,26 and hypoxic damage.27 A role for CB2 receptors, while less apparent in neuroprotection, may also exist via reductions in neuroinflammation.28 As neurodegeneration is a feature common to the various types of dementia, the neuroprotective effects of cannabinoids may therefore be beneficial in slowing the progression of these diseases.
Evidence continues to suggest that cannabinoids, like cannabis, may be therapeutically useful in dementia as they target several underlying pathophysiological processes linked to dementia. Cannabis—notably the psychoactive substrate tetrahydrocannabinol (THC)—reduces neuroinflammation and oxidative stress, confers neuroprotective effects, induces neurogenesis, removes amyloid β, and clears neurofibrillary tangles.29–31 The hippocampus and microglia—key players in dementia pathophysiology—are concentrated in CB1 and CB2 receptors, respectively, and are modulated by exposure to cannabis.32,33 Therefore, cannabinoids have the potential to interrupt the disease process as well as treat symptoms in Alzheimer dementia. Preclinical studies in rats have demonstrated THC’s competitive inhibition of the enzyme acetylcholinesterase, paralleling anti-dementia drugs like Donepezil, diminishing acetylcholinesterase-induced amyloid β–peptide aggregation.34 Additionally, intracerebroventricular administration of a synthetic cannabinoid (WIN 55,212-2) prevented cognitive deficits and decreased neurotoxicity among rats.35 This evidence indicates therapeutic promise for cannabinoids in treating disease processes that underlie dementia.
In addition to preclinical studies, several small clinical trials have quantified the efficacy of cannabinoids for NPS among patients with dementia.36–42 While some subjective improvements were noted by patients and their caregivers, extant trials have been underpowered to detect clinically significant differences from placebo, limiting informed, evidence-based conclusions.15,43–46 Further, any therapeutic benefit of using cannabinoids must be tempered against the body of literature showing clear associations between cannabinoid use and the risk of psychosis, anxiety, and other psychiatric conditions.47,48 In addition, cannabinoid use by the elderly can lead to impairment that may lead to unintended injuries like falls.49
While several reviews4,13,15,29,30,45,50–56 have been published on the topic of cannabinoids for dementia, most have provided only systematic reviews, and not quantitative synthesis. A prior Cochrane systematic review from 200957 identified only a single trial37—precluding any formal analysis. A more recently published meta-analysis of six clinical trials declared that there was no effect of cannabinoids on agitation symptoms or NPS overall.58 However, several potential limitations were noted by the authors—such as the small number of studies, the small sample size, the lack of adequate controls, the short trial duration, and the low quality of extant trials—may have diminished their study’s statistical power to detect a significant signal to noise ratio. The authors concluded that their findings were inconclusive due to substantial heterogeneity—which justified the need for additional, rigorous studies.
To address these limitations and provide a unique contribution to the field, we conducted an updated systematic review and meta-analysis. We identified additional studies, which boosted our meta-analysis’ statistical power, which enabled the detection of significant differences in the efficacy of cannabinoids for the treatment of NPS of dementia. We also quantified the acceptability of cannabinoids for the NPS by measuring the occurrence of adverse events and trial completion rates. We identified several potential causes of heterogeneity through extensive meta-regression, subgroup, sensitivity, and publication bias analyses to help put individual trial results into perspective. We also considered a broader range of outcomes—including actigraphy, global assessments of function, and cognitive measures—to complement existing estimates obtained for agitation and aggression that have been reported by previous studies. We also offer suggestions for future studies to address limitations in the available research. For these reasons, this updated meta-analysis aims to offer unique contribution to the field of dementia research.
Methods
We undertook this review using methods consistent with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA)59 guidelines (checklist provided in Online Appendix 1). We registered our protocol with the Open Science Framework (https://osf.io/et7cm/; https://osf.io/dnsqm).
Search Strategy
We search seven electronic databases (EMBASE, MEDLINE, PsycINFO, CINAHL, PubMed, Cochrane CENTRAL, and ClinicalTrials.gov) for articles published from database inception to January 2019; the search was updated in June 2019. Sets of search strings incorporating both keywords and Medical Subject Headings (MeSH terms) reflecting cannabinoids and dementia were used and are provided in full in Online Appendix 2. Searches were limited to human literature, with no restrictions placed on language or publication type. Citations for papers in languages other than English were read via Google Translate.
Citations were imported into the web-based screening tool, Covidence,60 where duplicate citations were removed. Titles and abstracts were screened by one reviewer (AB), and all papers marked as excluded were reviewed by a second person (ACM) to ensure accuracy in first-pass screening. Full-text articles were screened by two independent reviewers (AB and ACM) with discrepancies resolved via consultation with a third reviewer (EH) when needed. Reference lists for relevant systematic reviews identified in the peer-review literature search were hand searched for additional papers not already identified, and we additionally searched table of contents of relevant journals.
Inclusion and Exclusion Criteria
The population of interest was people experiencing NPS of dementia who received cannabinoids—including whole cannabis (from plants), THC, or prescribed synthetic cannabinoids (like nabilone, cannabidiol, or dronabinol). The exact definition of NPS varied across studies, and we summarize the way in which the studies were defined and NPS operationalized in Online Appendix 3. Inclusion criteria for the study population comprised clinical trials of cannabinoid-based interventions to treat NPS (randomized or quasi-randomized controlled trials [RCTs]) where treatment efficacy and acceptability may be reported at trial completion. The primary justification for including only RCTs was to minimize the risk of bias.61 Inclusion criteria for the study outcomes comprised reporting treatment efficacy and acceptability, or studies where such data could be obtained via contacting study authors. Exclusion criteria comprised observational studies (cohort, case-control, cross-sectional, case reports, case series); works that did not present original data (e.g., letters to the editor, editorials, commentaries); works that did not present treatment efficacy or acceptability for NPS and where such data could not be obtained from study authors; systematic reviews (we hand searched reviews for relevant studies that were assessed independently against the inclusion/exclusion criteria); studies where use of cannabinoids and outcomes were not reported for the same sample of people; clinical trials of interventions with people using cannabinoids for other indications (e.g., clinical trial for epilepsy, multiple sclerosis, or palliative treatments); and studies not using validated instruments to measure NPS (e.g., Neuropsychiatric Inventory [NPI]62) or to diagnose dementia (e.g., Diagnostic and Statistical Manual of Mental Disorders [DSM]).
Data Extraction
The following data were abstracted: study information (i.e., author, journal, and year), study characteristics (i.e., mean age of participants, recruitment setting, country of study, duration of follow-up), participant characteristics (i.e., comorbidities, dementia subtype, baseline severity of NPS, ethnicity), and intervention characteristics (i.e., dementia severity, dementia subtype).
The data extraction form was developed in Microsoft Excel 2016 based on previously conducted reviews,63–65 and recommendations were outlined in the PRISMA statement.59 Data were independently extracted by one member of the research team (AB) and checked by a second (ACM). Bibliographic information was extracted in addition to study specific information. Data entry was standardized by use of a manual, which contained data entry rules. In instances where data reporting in the publications was incomplete, supplementary information and documents were sourced to locate missing data. If supplementary information could not be located or did not provide the necessary data needed, study authors were contacted by email for additional information.
A quality index based on the Cochrane Risk of Bias Tool for Randomized Controlled Trials was adopted for consistency.66 This scale uses a 7-item system to evaluate randomized studies regarding seven domains of quality: randomization, allocation, blinding of participants, blinding of outcome assessors, selection bias, selective reporting, and additional sources of bias. Each item was rated as having a high, low, or unclear risk of bias. Individual assessments for each criterion were pooled to provide an overall risk of bias assessment, where the greater the number of items with a low risk of bias, the higher the methodological quality of the study. Study information necessary for quality assessment was extracted to the Excel template by one reviewer (AB) and double-checked by a second (ACM). Discrepancies were resolved via consultation with a third reviewer (EH) where needed.
Calculation of Treatment Efficacy and Acceptability
Data from measures of NPS, including agitation (Cohen Mansfield Agitation Inventory [CMAI] and the NPI Agitation subscale), overall NPS (NPI total), and cognition (Mini-Mental Status Examination [MMSE]) were extracted from the included studies. Data on changes in weight, systolic blood pressure, and occurrence of adverse events were also extracted. For continuous data, standardized mean differences (SMDs) and 95% confidence intervals (CIs) were calculated using a random-effects model.
Data Analysis
R studio (Version 1.1.463) metafor package was used to conduct DerSimonian and Laird Mantel–Haenszel random-effects meta-analysis to pool efficacy and acceptability estimates.67 All analyses were subsequently transferred to Cochrane’s Review Manager 5.3 to generate high-quality plots and graphs.68 The random-effects model was used in all meta-analyses as we anticipated high levels of heterogeneity between cohorts. Heterogeneity was quantified using the I 2 statistic and described as low (≤25%), moderate (>25% and ≤50%), high (>50% and ≤75%), or substantial (≥75%).65,69,70 We explored the impact of study characteristics on treatment efficacy and acceptability via subgroup, sensitivity, and meta-regression analyses. Variables included percentage male, baseline age, dementia subtype, severity of dementia (MMSE), study year, and geographic region. Formal comparisons between subgroups were performed using meta-regression, via the metafor package.67 Sensitivity analyses included comparisons with leave-one-out, cumulative, subgroup, and fixed-effects meta-analyses. Bubble plots were generated where significant differences were identified between subgroups. The adjusted R 2 index was employed to quantify goodness of fit for each model. Statistical significance for all analyses was set at P < 0.05. Publication bias was assessed qualitatively, using funnel plot symmetry, as well as quantitatively, using the Egger and trim-and-fill methods.71–73
Results
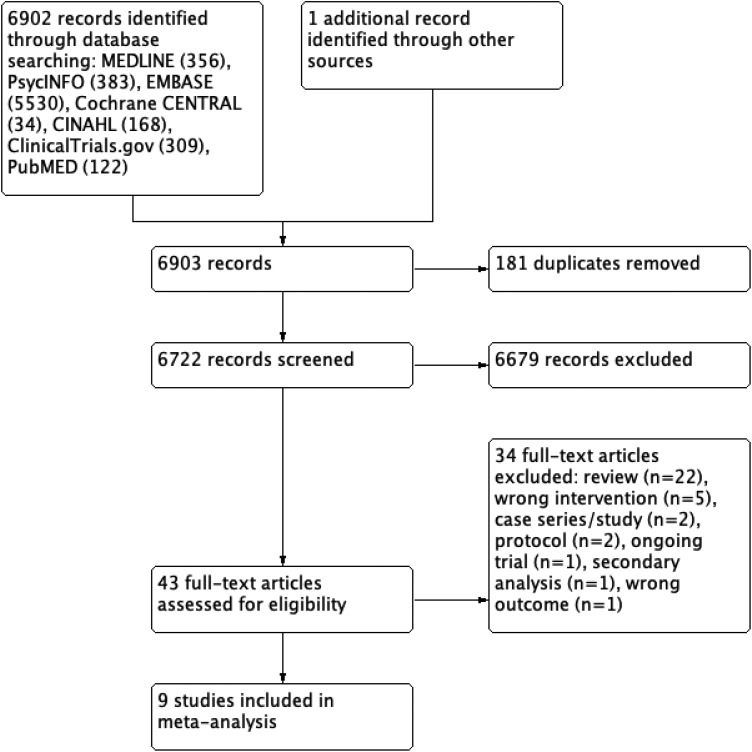
The search produced 6,903 unique papers, of which 43 were screened in full, and 936–42,74,75 were included in the review (Figure 1). Online Appendix 4 provides the list of excluded studies.
Figure 1.
PRISMA study flow diagram.
Trials were primarily undertaken in Europe36,39–42,75 or North America37,38,74 (Table 1). Six were RCTs; the remaining three were quasi-randomized studies. Trials varied widely in size (n = 2 to 50) and reporting of cohort demographics. For example, fewer than half reported the extent of previous treatments for NPS. Medical and psychiatric comorbidity among study participants was inconsistently reported, as were details regarding the duration of dementia, previous treatments, and current medications.
Table 1.
Characteristics of Included Studies.
| Study | Study Design | Study Setting | Intervention | Participants | Male (%); age (SD) | Baseline MMSE (SD) | NPS History of Participants |
|---|---|---|---|---|---|---|---|
| Volicer et al.37 | Randomized, DB, PC, crossover trial | Hospital | Dronabinol 2.5 mg po BID for 6 weeks then crossover | 15 (3 dropouts) | 91.7; 72.7 (4.9) | 4.0 (7.4) | Food refusal |
| Walther et al.36 | Retrospective systematic chart review (pre–post intervention) | Hospital | Dronabinol 2.5 mg po qHs for 14 days | 6 | 66.6; 81.5 (6.1) | 10.33 (6.28) | Nighttime agitation, daytime rhythm disturbances/sundowning |
| Mahlberg et al.75 | Randomized, SB, PC, parallel trial | Hospital | Dronabinol 2.5 mg po qHs for 14 days | 24 | 42; 78.6 (9.7) | Not reported | Agitation, aggression or resistance to care |
| Walther et al.42 | Randomized, DB, PC, crossover trial | Hospital | Dronabinol 2.5 mg po qHs for 14 days | 2 | 100; 78.0 (4.2) | 19.5 (3.5) | Circadian rhythm disturbances, verbal aggression, delusions, and apathy |
| Woodward et al.38 | Retrospective systematic chart review (pre–post intervention) | Hospital | Dronabinol 7.0 mg po daily for 4 to 50 days (average = 16.9 days) | 40 | 30; not reported | 7.0 (not reported) | Agitation, aggression or resistance to care |
| Van den Elsen et al.39 | Randomized DB PC multicentre Phase II trial | Community and nursing home | THC 1.5 mg po TID for 3 weeks | 50 | 50; 78.4 (7.4) | 15.0 (6.8) | Agitation, aggression, and aberrant motor behavior; NPI ≥ 10 |
| Van den Elsen et al.40 | Randomized DB PC repeated crossover trial | Hospital and community | THC 0.75 mg po BID for 3 days (Weeks 1 to 3) and 1.5 mg po BID for 3 days (Weeks 4 to 6) | 22 (2 dropouts) | 68; 76.4 (5.3) | 16.9 (7.8) | Agitation and aggression; NPI ≥ 10 |
| Shelef et al.41 | Open-label prospective cohort study | Hospital | THC 2.5 to 5 mg po BID for 28 days | 11 (1 dropout) | 46; 73.2 (8.6) | 10.3 (9.4) | Severe agitation and aggressive behavior resulting in hospitalization |
| Herrmann et al.74 | Randomized DB PC crossover trial | Community and nursing home | Nabilone 1 to 2 mg po qHs for 6 weeks then crossover | 38 (9 dropouts) | 77; 87 (10) | 6.5 (6.8) | NPI-A ≥3 and clinically stable |
Note. SD = standard deviation. MMSE = Mini-Mental Status Examination. NPS = Neuropsychiatric Symptom (of Dementia). DB = double-blind. PC = placebo-controlled. BID = twice daily dosing. po = by mouth. qHs = nightly dosing. SB = single blind. TID = thrice daily dosing. THC = tetrahydrocannabinol. SB = single-blind. NPI = Neuropsychiatric Inventory.
The total sample size was 208 (44% female, mean age 78 [SD = 7] years). Alzheimer disease was the most frequent dementia subtype (85%), followed by mixed (8%), vascular (6%), and frontotemporal dementia (1%). The mean baseline MMSE score of participants was 11 (SD = 7). The main indication for treatment was severe agitation or aggressive behavior (n = 8) or food refusal37 (n = 1). The mean proportion taking an antipsychotic prior to study initiation was 49% (SD = 32%).
Three trials used THC preparations,39–41 five used dronabinol,36–38,42,75 and one74 used nabilone. Outcome measures reported by studies included the NPI,36,38–42,58,75 the CMAI,36–42,74 nocturnal motor activity (as measured by actigraphy),36,38,41,42,75 the MMSE,39,41,74 and the Clinical Global Impression (CGI).38,40,41
Efficacy of Cannabinoids for NPS
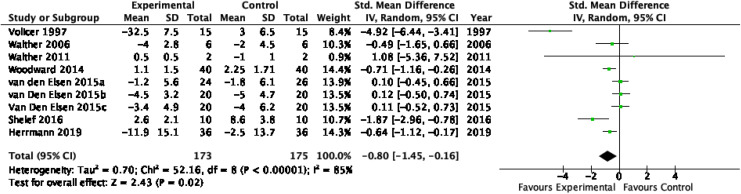
All trials (n = 208 participants) provided data to inform the pooled efficacy of cannabinoids for NPS using one or more outcome measures. For the CMAI, the SMD was −0.80 (95% CI, −1.45 to −0.16; nine studies; Figure 2), with substantial heterogeneity (I 2 = 85%). For the NPI total, the SMD was −0.61 (95% CI, −1.07 to −0.15; seven studies; Online Appendix 5), with high heterogeneity (I 2 = 67%). For the NPI-A subscore, the SMD was also −0.61 (95% CI, −0.97 to −0.25; six studies; Online Appendix 6), with moderate heterogeneity (I 2 = 50%). For nocturnal motor activity, the SMD was −1.05 (95% CI, −1.56 to −0.54; five studies; Online Appendix 7), with moderate heterogeneity (I 2 = 40%). For the MMSE, the SMD was 0.42 (95% CI, 0.07 to 0.78; three studies; Online Appendix 8), with low heterogeneity (I 2 = 3%). For the CGI, the SMD was −0.94 (95% CI, −1.24 to −0.64; three studies; Online Appendix 9), with high heterogeneity (I 2 = 70%).
Figure 2.
Forest plot from random-effects meta-analysis of the standardized mean difference in the Cohen Mansfield Agitation Inventory.
Factors Associated with Efficacy of Cannabinoids for NPS
We ran a series of meta-regressions and subgroup analyses to explore potential variables that may have accounted for the heterogeneity observed for the effect size of treatment with the CMAI, NPI, NPI-A, and nocturnal motor activity (actigraphy; Table 2). Type of cannabinoid (synthetic vs. THC), percentage male, baseline age, percentage with Alzheimer disease, and geographic region were not associated with any of the outcome measures.
Table 2.
Statistical Significance of Univariate Meta-Regression Analysis for Factors Associated with Efficacy of Cannabinoids for Neuropsychiatric Symptoms of Dementia.
| Factor | Cohen Mansfield Agitation Inventory | Neuropsychiatric Inventory | Neuropsychiatric Inventory, Agitation | Nocturnal Motor Activity |
|---|---|---|---|---|
| Agent (synthetic vs. THC) | 0.371 | 0.996 | 0.939 | 0.585 |
| Dose (mg) | 0.203 | <0.001 | 0.061 | 0.209 |
| Baseline MMSE | 0.001 | 0.636 | 0.210 | 0.830 |
| Male (%) | 0.636 | 0.261 | 0.146 | 0.675 |
| Baseline age | 0.176 | 0.208 | 0.481 | 0.061 |
| Alzheimer disease (%) | 0.393 | 0.635 | 0.833 | 0.412 |
| Region (North America vs. Europe) | 0.065 | 0.930 | 0.939 | 0.285 |
| Design (randomized vs. quasi-randomized) | 0.729 | 0.001 | 0.047 | 0.607 |
| Year of study | 0.003 | 0.966 | 0.907 | 0.407 |
Note. THC = tetrahydrocannabinol; MMSE = Mini-Mental Status Examination; bold values indicate meta-regression analyses with P values below 0.05.
For the CMAI, there was an association between effect size and baseline MMSE (with greater effect size noted in those with greater baseline MMSE) and between effect size and year of study (with greater effect sizes noted in earlier studies).
For the NPI—Total and Agitation subscale—there was a positive dose–response relationship (with higher effect sizes noted with higher total daily doses of cannabinoids) and an association with study design (with higher effect sizes noted in quasi-randomized studies relative to randomized trials). Like the CMAI, for nocturnal motor activity, there was an association between effect size and year of study (with greater effect sizes noted in earlier studies).
Sensitivity and Quality Analyses
Overall study quality was rated as low due to inconsistent methods of allocation concealment, incomplete or nonrandomization, a lack of double-blinding, and a high risk of carryover due to concurrent medication use. For summary results of the judged risk of bias across the included studies for each domain, see Online Appendices 10 and 11.
We conducted four sensitivity analyses to assess the robustness of our estimates for the CMAI, NPI, NPI-A, and nocturnal motor activity; these are described in Online Appendix 10.
First, we explored the influence of each study on pooled estimates for efficacy effect size via sensitivity analysis with leave-out-one meta-analysis, allowing the removal of each individual study from the analysis (Online Appendix 12a). This demonstrated that the effect size estimate for the CMAI was influenced by the extreme result of the Volicer 199737 study, which was an outlier from the remaining studies; when this study was excluded, the CMAI effect size was no longer statistically significant (SMD = −0.359, 95% CI [−0.782 to 0.065]). The estimates for the remaining outcome measures (NPI, NPI-A, and nocturnal motor activity) were robust to leave-out-one sensitivity analysis and remained significant with the removal of any individual study.
Second, we compared the random-effects meta-analysis estimates to a fixed-effects model, which ignores heterogeneity (Online Appendix 12b). For all outcomes, the fixed-effects SMD estimate remained statistically significant but were smaller than the random-effects estimates.
Third, we conducted cumulative meta-analyses to combine studies chronologically to identify when a characteristic or statistically significant change first occurred (Online Appendix 12c). We observed significant time trends in the CMAI, NPI-A, and nocturnal motor activity, but not the NPI total score. This was consistent with the meta-regression measuring the impact of study year on effect size.
Fourth, we conducted subgroup analyses to determine whether the type of cannabinoid preparation influenced the estimates of efficacy (Online Appendix 12d). There was no significant difference in efficacy by synthetic or THC cannabinoid preparations.
Finally, we examined evidence for potential publication bias through funnel plots (Online Appendix 13) and by applying rank correlation tests, Eggers, and the trim-and-fill method. The results did not suggest any evidence to support that a significant bias existed within this review.
Acceptability of Cannabinoids for NPS
The overall rate of completion (defined as participants reaching the primary study endpoint) was 93% (193/208). A total of 15 dropouts occurred across five studies,37,39–41,74 occurring from death (n = 2), dysphagia (n = 2), pneumonia (n = 1), malignancy (n = 2), urinary tract infection (n = 3), seizure (n = 1), elevated INR (n = 1), pneumothorax (n = 1), lethargy (n = 2), and excess as needed (PRN) usage (n = 1). Only lethargy was described by study authors as being potentially related to administration of cannabinoids.
There was no significant association between cannabinoids and weight (P = 0.76), systolic blood pressure (P = 0.20), diastolic blood pressure (P = 0.21), or the occurrence of serious adverse drug events (P = 0.51)—as demonstrated in Online Appendix 14.
Discussion
This systematic review and meta-analysis synthesized the findings of nine trials to appraise available evidence for the use of cannabinoids in the treatment of the NPS associated with dementia. Most participants completed the entirety of treatment, and few serious adverse events were reported. We found consistent evidence for the efficacy of cannabinoids in reducing NPS, which outperformed placebo on all efficacy outcome measures considered (CMAI, NPI, NPI-A, and nocturnal motor activity). Our findings are consistent with the conclusions of several previously published reviews,15,29,30,43–46,50,51,57,76–80 but these contrast the conclusions of a recent meta-analysis that declared a lack of efficacy for agitation and aggression.58
Comparison with Previous Reviews
Although the present review draws on a relatively small amount of literature that has already been subjected to review earlier this year,58 the present review aimed to yield novel information and present it in an appropriate and straightforward manner. Both reviews found significant heterogeneity across studies and neither review found differences in the occurrence of adverse events or dropouts due to an adverse event between treatment groups. However, the key differences were that the present review—based on nine studies36–42,74,75—found a significant effect of cannabinoids on NPS, while the review of Ruthirakuhan and colleagues58—which was based on six37,39,40,42,74,75 articles—did not. Of note, all six studies identified by Ruthirakuhan and colleagues were included in the present review; hence, there were differences in our conclusions despite overlaps in the studies considered. This reflects potential differences in eligibility criteria across the two reviews—with the present including quasi-randomized trials. Consequently, the three additional studies36,38,41 considered by this review that were not included by Ruthirakuhan and colleagues were quasi-randomized in design, which may explain the differences in our conclusions. Since the addition or removal of a few studies led to substantially different conclusions across reviews, the conclusions we can draw ought to be done so cautiously. In light of the growing interest in cannabinoids and the unmet need in NPS treatment, we hope this review makes a meaningful contribution to the literature, which may stimulate further research to clarify why there were discrepancies across these two reviews.
For several reasons, the finding of therapeutic efficacy here should not indiscriminately rule in the use of cannabinoids in treating NPS in dementia. Firstly, our meta-analysis was largely restricted to studies using dronabinol and THC—with only one study using nabilone. Dronabinol is a partial agonist at both CB1 and CB2 and, via second messenger pathways, inhibits adenylate cyclase and reduces concentrations of Cyclic adenosine monophosphate (cAMP).38 Dronabinol is considered the “purified” version of THC. THC, and THC-like substances alone, may be less efficacious than when paired with other cannabinoids like CBD. The conclusions we can draw are that some cannabinoids appear clinically efficacious to treat dementia-related symptoms relative to THC. Taken together, this reviews’ findings suggest that not all cannabinoids are created equally—particularly when used in the treatment of NPS of dementia. Thus, there may be potential differences between whole cannabis, THC, and synthetic cannabinoids, which may indicate that there may be broader considerations when using cannabinoids therapeutically.
Secondly, there were substantial variations in the dosing of cannabinoids used across studies. For example, dronabinol doses used across studies were moderate but higher than THC doses; however, dronabinol itself has a low bioavailability (roughly 4% to 20%) and, if combined with low dosing, might have indicated inefficacious dosing regimens.81 Nabilone, another synthetic cannabinoid analogue of THC, is both more potent than dronabinol and has greater bioavailability (approximately 60%) according to the literature—but this did not achieve statistical significance in the present review.81–83 As proposed by Russo,84 a more complete spectrum of cannabinoids—consistent with the composition of herbal cannabis—might accentuate clinical efficacy in many conditions, creating the so-called entourage effects.84–86
Finally, not all dementias have the same neurobiology. Approximately 85% of the patients across studies were diagnosed with Alzheimer; however, our meta-regression did not indicate any association between dementia subtype and treatment efficacy. However, given the small total sample size of our study (n = 208), our meta-analysis was potentially underpowered to detect significant differences across different types of dementia. Therefore, larger studies using appropriate doses of varied cannabinoids would help address the study limitations identified here and formally substantiate cannabinoid’s utility in treating NPS common to dementia.
Limitations
The findings of this meta-analysis cannot be considered without mentioning a few of its limitations. Overall, only a few small studies were identified, and fewer still could be fully meta-analyzed due to inconsistent reporting styles across studies. Most trials did not require a washout period; thus, if participants were already taking other psychotropic medication, the cannabinoid medication they received was not actually in monotherapy, therefore contaminating the effect of the intervention. In terms of aggression and agitation, the outcomes considered across studies largely looked at agitation (the Cohen Mansfield Agitation Inventory, for example). However, across trials, these terms were often used interchangeably despite their contrasting definitions.
As NPS is a broad term, considering it as a single outcome measure is somewhat problematic; however, this was tempered by the fact that the meta-analysis only pooled together trials that were using the same NPS outcome measure (e.g., the NPI), which were all validated measures for us in individuals with dementia. Further, the individuals represented across these trials may not be representative of a large, population-based sample of individuals with dementia, primarily due to a higher proportion of individuals in trials with moderate-to-severe dementia and who were institutionalized. An additional limitation was that one of the component articles37 was about the NPS of food refusal, while the others all included agitation. This is especially important because the CMAI finding of statistical significance disappeared when the paper was excluded, indicating the findings were not robust.
Further, the quality of evidence from the individual studies included in this review was low (using the Cochrane Risk of Bias Tool). In the majority of studies, both the treating clinician and participant were aware of treatment assignment because of the subjective effects of cannabinoids—even if the study was double-blinded. As many participants in the RCTs were crossed over to an open trial of cannabinoids, this precluded follow-up assessments for the control condition. Still, the lack of high-quality data and the unclear risk of bias in many key domains of the included studies prevents definitive conclusions from being made about the usefulness of dronabinol for the treatment of dementia. Further, most of the discussion section is speculative in nature as the precise mechanisms behind the observed findings cannot be confirmed with meta-analysis. As a result, the conclusions of this review should be considered tenuous.
Future Directions
This meta-analysis highlights the need for larger, high-quality, randomized, double-blind, placebo-controlled trials to determine whether additional cannabinoid preparations are clinically efficacious in the treatment of dementia. However, there is also an opportunity for future studies to address potential alternatives to RCTs for evaluating cannabinoid medicines—such as larger, Phase IV effectiveness trials using health administrative data. These studies may also provide a measure of real-world effectiveness compared to traditional efficacy measures from RCTs.
Conclusions
This systematic review and meta-analysis found consistent evidence that cannabinoids are efficacious for the treatment of NPS associated with dementia and are well-tolerated for use in individuals with dementia. However, our findings were not robust and were particularly vulnerable to the small sample sizes as demonstrated in our sensitivity analyses. Thus, while there is growing neurobiological evidence that cannabinoids may be useful in modulating disease processes in dementia, more evidence is needed before they can be recommended for routine use in clinical practice. At present, the use of cannabinoids in individuals with dementia should still be considered an experimental treatment until more clinical data are available. Population-based studies are clearly needed to characterize the real-world effectiveness and acceptability of cannabinoids for the treatment of NPS.
Supplemental Material
Supplemental Material, Data_Collection_Sheet for Cannabinoids for the Neuropsychiatric Symptoms of Dementia: A Systematic Review and Meta-Analysis by Anees Bahji, Arthi Chinna Meyyappan and Emily R. Hawken in The Canadian Journal of Psychiatry
Acknowledgments
The authors would like to thank Ms. Sandra Halliday, research librarian at Bracken Health Sciences Library at Queen’s University, for her support in developing the systematic review search strategy.
Authors’ Note: All authors are responsible for the integrity of the data and accuracy of data analyses. AB and EH conceived and designed the study. All authors acquired, analyzed, and interpreted all data. AB drafted the manuscript. All authors critically revised the manuscript. EH supervised the study.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Anees Bahji  https://orcid.org/0000-0002-3490-314X
https://orcid.org/0000-0002-3490-314X
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Nichols E, Szoeke CEI, Vollset SE, et al. Global, regional, and national burden of Alzheimer’s disease and other dementias, 1990–2016: a systematic analysis for the global burden of disease study 2016. Lancet Neurol. 2019;18(1):88–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lyketsos CG, Lopez O, Jones B, et al. Prevalence of neuropsychiatric symptoms in dementia and mild cognitive impairment: results from the cardiovascular health study. JAMA. 2002;288(12):1475–1483. [DOI] [PubMed] [Google Scholar]
- 3. Seitz DP, Brisbin S, Herrmann N, et al. Efficacy and feasibility of nonpharmacological interventions for neuropsychiatric symptoms of dementia in long term care: a systematic review. J Am Med Dir Assoc. 2012;13(6):503–506. e2. [DOI] [PubMed] [Google Scholar]
- 4. Sherman C, Ruthirakuhan M, Vieira D, et al. Cannabinoids for the treatment of neuropsychiatric symptoms, pain and weight loss in dementia. Curr Opin Psychiatry. 2018;31(2):140–146. [DOI] [PubMed] [Google Scholar]
- 5. Livingston G, Sommerlad A, Orgeta V, et al. Dementia prevention, intervention, and care. Lancet. 2017;390(10113):2673–2734. [DOI] [PubMed] [Google Scholar]
- 6. Schneider LS, Dagerman KS, Insel P. Risk of death with atypical antipsychotic drug treatment for dementia: meta-analysis of randomized placebo-controlled trials. JAMA. 2005;294(15):1934–1943. [DOI] [PubMed] [Google Scholar]
- 7. Herrmann N, Lanctôt KL. Do atypical antipsychotics cause stroke? CNS Drugs. 2005;19(2):91–103. [DOI] [PubMed] [Google Scholar]
- 8. Rochon PA, Normand SL, Gomes T, et al. Antipsychotic therapy and short-term serious events in older adults with dementia. Arch Intern Med. 2008;168(10):1090–1096. [DOI] [PubMed] [Google Scholar]
- 9. Schneider LS, Dagerman K, Insel PS. Efficacy and adverse effects of atypical antipsychotics for dementia: meta-analysis of randomized, placebo-controlled trials. Am J Geriatr Psychiatry. 2006;14(3):191–210. [DOI] [PubMed] [Google Scholar]
- 10. Garay RP, Citrome L, Grossberg GT, et al. Investigational drugs for treating agitation in persons with dementia. Expert Opin Investig Drugs. 2016;25(8):973–983. [DOI] [PubMed] [Google Scholar]
- 11. Häuser W, Finn DP, Kalso E, et al. European Pain Federation (EFIC) position paper on appropriate use of cannabis-based medicines and medical cannabis for chronic pain management. Eur J Pain. 2018;22(9):1547–1564. [DOI] [PubMed] [Google Scholar]
- 12. Yarnell S. The use of medicinal marijuana for posttraumatic stress disorder: a review of the current literature. Prim Care Companion CNS Disord. 2015;17(3). 2015. May 7 [Epub ahead of print] doi:10.4088/PCC.15r01786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nielsen S, Germanos R, Weier M, et al. The use of cannabis and cannabinoids in treating symptoms of multiple sclerosis: a systematic review of reviews. Curr Neurol Neurosci Rep. 2018;18(2):8. [DOI] [PubMed] [Google Scholar]
- 14. Aso E, Ferrer I. Cannabinoids for treatment of Alzheimer’s disease: moving toward the clinic. Front Pharmacol. 2014;5:37 2014. March 5 [Epub ahead of print] doi:10.3389/fphar.2014.00037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Walther S, Halpern M. Cannabinoids and dementia: a review of clinical and preclinical data. Pharmaceuticals. 2010;3(8):2689–2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Alvarez FJ, Lafuente H, Rey-Santano MC, et al. Neuroprotective effects of the nonpsychoactive cannabinoid cannabidiol in hypoxic-ischemic newborn piglets. Pediatr Res. 2008;64(6):653–658. [DOI] [PubMed] [Google Scholar]
- 17. Hampson AJ, Grimaldi M, Lolic M, Wink D, Rosenthal R, Axelrod J. Neuroprotective antioxidants from marijuana. Ann N Y Acad Sci. 2000;899:274–282. [PubMed] [Google Scholar]
- 18. De Lago E, Fernández-Ruiz J. Cannabinoids and neuroprotection in motor-related disorders. CNS Neurol Disord Drug Targets. 2007;6(6):377–387. [DOI] [PubMed] [Google Scholar]
- 19. Garcia-Orjuela MG, Alarcon-Franco L, Sanchez-Fernandez JC, et al. Dependence to legally prescribed opioid analgesics in a university hospital in Medellin-Colombia: an observational study. BMC Pharmacol Toxicol. 2016;17(1):42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Holgado MA, Martín-Banderas L, Álvarez-Fuentes J, et al. Neuroprotective effect of cannabinoids nanoplatforms in neurodegenerative diseases. J Drug Deliv Sci Technol. 2017;42:4284–4293. [Google Scholar]
- 21. Gerdeman G, Lovinger DM. CB1 cannabinoid receptor inhibits synaptic release of glutamate in rat dorsolateral striatum. J Neurophysiol. 2001;85(1):468–471. [DOI] [PubMed] [Google Scholar]
- 22. Rodríguez-Muñoz M, Sánchez-Blázquez P, Merlos M, et al. Endocannabinoid control of glutamate NMDA receptors: the therapeutic potential and consequences of dysfunction. Oncotarget. 2016;7(34):55840–55862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hoffman AF, Laaris N, Kawamura M, et al. Control of cannabinoid CB1 receptor function on glutamate axon terminals by endogenous adenosine acting at A1 receptors. J Neurosci. 2010;30(2):545–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Grundy RI. The therapeutic potential of the cannabinoids in neuroprotection. Expert Opin Investig Drugs. 2002;11(10):1365–1374. [DOI] [PubMed] [Google Scholar]
- 25. Hampson AJ, Grimaldi M, Axelrod J, et al. Cannabidiol and (-)Delta9-tetrahydrocannabinol are neuroprotective antioxidants. Proc Natl Acad Sci U S A. 1998;95(14):8268–8273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shen M, Thayer SA. Cannabinoid receptor agonists protect cultured rat hippocampal neurons from excitotoxicity. Mol Pharmacol. 1998;54(3):459–462. [DOI] [PubMed] [Google Scholar]
- 27. Nagayama T, Sinor AD, Simon RP, et al. Cannabinoids and neuroprotection in global and focal cerebral ischemia and in neuronal cultures. J Neurosci. 1999;19(8):2987–2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ehrhart J, Obregon D, Mori T, et al. Stimulation of cannabinoid receptor 2 (CB2) suppresses microglial activation. J Neuroinflammation. 2005;2:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Liu CS, Chau SA, Ruthirakuhan M, et al. Cannabinoids for the treatment of agitation and aggression in Alzheimer’s Disease. CNS Drugs. 2015;29(8):615–623. [DOI] [PubMed] [Google Scholar]
- 30. Ahmed AIA, van der Marck MA, van den Elsen G, et al. Cannabinoids in late-onset Alzheimer’s disease. Clin Pharmacol Ther. 2015;97(6):597–606. [DOI] [PubMed] [Google Scholar]
- 31. Panza F, Solfrizzi V, Seripa D, et al. Progresses in treating agitation: a major clinical challenge in Alzheimer’s disease. Expert Opin Pharmacother. 2015;16(17):2581–2588. [DOI] [PubMed] [Google Scholar]
- 32. Weinstein A, Livny A, Weizman A. Brain imaging studies on the cognitive, pharmacological and neurobiological effects of Cannabis in humans: evidence from studies of adult users. Curr Pharm Des. 2016;22(42):6366–6379. [DOI] [PubMed] [Google Scholar]
- 33. Nader DA, Sanchez ZM. Effects of regular cannabis use on neurocognition, brain structure, and function: a systematic review of findings in adults. Am J Drug Alcohol Abuse. 2018;44(1):4–18. [DOI] [PubMed] [Google Scholar]
- 34. Eubanks LM, Rogers CJ, Beuscher AE, et al. A molecular link between the active component of marijuana and Alzheimer’s disease pathology. Mol Pharm. 2006;3(6):773–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ramírez BG, Blázquez C, Gómez del Pulgar T, et al. Prevention of Alzheimer’s disease pathology by cannabinoids: neuroprotection mediated by blockade of microglial activation. J Neurosci. 2005;25(8):1904–1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Walther S, Mahlberg R, Eichmann U, et al. Delta-9-tetrahydrocannabinol for nighttime agitation in severe dementia. Psychopharmacology (Berl). 2006;185(4):524–528. [DOI] [PubMed] [Google Scholar]
- 37. Volicer L, Stelly M, Morris J, et al. Effects of dronabinol on anorexia and disturbed behavior in patients with Alzheimer’s disease. Int J Geriatr Psychiatry. 1997;12(9):913–919. [PubMed] [Google Scholar]
- 38. Woodward MR, Harper DG, Stolyar A, et al. Dronabinol for the treatment of agitation and aggressive behavior in acutely hospitalized severely demented patients with noncognitive behavioral symptoms. Am J Geriatr Psychiatry. 2014;22(4):415–419. [DOI] [PubMed] [Google Scholar]
- 39. van den Elsen GAH, Ahmed AIA, Verkes R-J, et al. Tetrahydrocannabinol in behavioral disturbances in dementia: a crossover randomized controlled trial. Am J Geriatr Psychiatry. 2015;23(12):1214–1224. [DOI] [PubMed] [Google Scholar]
- 40. van den Elsen GAH, Ahmed AIA, Verkes R-J, et al. Tetrahydrocannabinol for neuropsychiatric symptoms in dementia: a randomized controlled trial. Neurology. 2015;84(23):2338–2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Shelef A, Barak Y, Berger U, et al. Safety and efficacy of medical cannabis oil for behavioral and psychological symptoms of dementia: an-open label, add-on, pilot study. J Alzheimers Dis. 2016;51(1):15–19. [DOI] [PubMed] [Google Scholar]
- 42. Walther S, Schüpbach B, Seifritz E, et al. Randomized, controlled crossover trial of dronabinol, 2.5 mg, for agitation in 2 patients with dementia . J Clin Psychopharmacol. 2011;31(2):256–258. [DOI] [PubMed] [Google Scholar]
- 43. Hill KP. Medical marijuana for treatment of chronic pain and other medical and psychiatric problems: a clinical review. JAMA. 2015;313(24):2474–2483. [DOI] [PubMed] [Google Scholar]
- 44. Preuss U, Wong J, Koller G. Treatment of behavioral and psychological symptoms of dementia: a systematic review. Psychiatr Pol. 2016;50(4):679–715. [DOI] [PubMed] [Google Scholar]
- 45. van den Elsen GAH, Ahmed AIA, Lammers M, et al. Efficacy and safety of medical cannabinoids in older subjects: a systematic review. Ageing Res Rev. 2014;14:56–64. [DOI] [PubMed] [Google Scholar]
- 46. Wilkinson ST, Radhakrishnan R, D’Souza DC. A systematic review of the evidence for medical marijuana in psychiatric indications. J Clin Psychiatry. 2016;77(08):1050–1064. [DOI] [PubMed] [Google Scholar]
- 47. Gage SH, Hickman M, Zammit S. Association between cannabis and psychosis: epidemiologic evidence. Biol Psychiatry. 2016;79(7):549–556. [DOI] [PubMed] [Google Scholar]
- 48. Crippa JA, Zuardi AW, Martín-Santos R, et al. Cannabis and anxiety: a critical review of the evidence. Hum Psychopharmacol. 2009;24(7):515–523. [DOI] [PubMed] [Google Scholar]
- 49. Volkow ND, Baler RD, Compton WM, et al. Adverse health effects of marijuana use. N Engl J Med. 2014;370(23):2219–2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Santibanez RA, Sepehry AA, Hsiung G-YR. Cannabis and Alzheimer’s disease: a systematic review of the evidence. Alzheimers Dement. 2017;13(7):P614. [Google Scholar]
- 51. Tampi RR, Young JJ, Tampi DJ. Cannabinoids for the treatment of behavioral and psychological symptoms of dementia. Neurodegener Dis Manag. 2018;8(4):211–213. [DOI] [PubMed] [Google Scholar]
- 52. Amanullah S. Synthetic cannabinoids in dementia with agitation: case studies and literature review. 2013; MedicalMarijuana.eu [accessed 2018 Dec 27] http://www.medicalmarijuana.eu/research/synthetic-cannabinoids-dementia-agitation-case-studies-literature-review/.
- 53. Hillen JB, Soulsby N, Alderman C, et al. Safety and effectiveness of cannabinoids for the treatment of neuropsychiatric symptoms in dementia: a systematic review. Ther Adv Drug Saf. 2019;10 doi:10.1177/2042098619846993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Mücke M, Carter C, Cuhls H, et al. Cannabinoide in der palliativen Versorgung: Systematische Übersicht und Metaanalyse der Wirksamkeit, Verträglichkeit und Sicherheit Schmerz. 2016;30(1):25–36. [DOI] [PubMed] [Google Scholar]
- 55. Ijaopo EO. Dementia-related agitation: a review of non-pharmacological interventions and analysis of risks and benefits of pharmacotherapy. Transl Psychiatry. 2017;7(10):e1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Antonsdottir IM, Smith J, Keltz M, et al. Advancements in the treatment of agitation in Alzheimer’s disease. Expert Opin Pharmacother. 2015;16(11):1649–1656. [DOI] [PubMed] [Google Scholar]
- 57. Krishnan S, Cairns R, Howard R. Cannabinoids for the treatment of dementia. Cochrane Database Syst Rev. 2009. ;(2):CD007204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ruthirakuhan M, Lanctôt KL, Vieira D, et al. Natural and synthetic cannabinoids for agitation and aggression in Alzheimer’s Disease: a meta-analysis. J Clin Psychiatry. 2019;80(2). 2019. January 29 [Epub ahead of print]. doi: 10.4088/JCP.18r12617. [DOI] [PubMed] [Google Scholar]
- 59. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006–1012. [DOI] [PubMed] [Google Scholar]
- 60. Veritas Health Innovation. Covidence Systematic Review Software. Melbourne, Australia: Veritas Health Innovation; 2019. [Google Scholar]
- 61. Faber T, Ravaud P, Riveros C, et al. Meta-analyses including non-randomized studies of therapeutic interventions: a methodological review. BMC Med Res Methodol. 2016;16 2016. March 22 [Epub ahead of print]. doi:10.1186/s12874-016-0136-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Cummings JL. The Neuropsychiatric Inventory: assessing psychopathology in dementia patients. Neurology. 1997;48(5 Suppl 6):S10–S16. [DOI] [PubMed] [Google Scholar]
- 63. Bahji A, Mazhar MN. Treatment of Cannabis dependence with synthetic Cannabinoids: a systematic review. Can J Addict. 2016;7(4):8. [Google Scholar]
- 64. Bahji A, Mazhar MN, Hudson CC, et al. Prevalence of substance use disorder comorbidity among individuals with eating disorders: a systematic review and meta-analysis. Psychiatry Res. 2019;273:58–66. [DOI] [PubMed] [Google Scholar]
- 65. Bahji A, Hawken ER, Sepehry AA, et al. ECT beyond unipolar major depression: systematic review and meta-analysis of electroconvulsive therapy in bipolar depression. Acta Psychiatr Scand. 2019;139(3):214–226. [DOI] [PubMed] [Google Scholar]
- 66. Higgins JPT, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;34:3d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36(1):1–48. [Google Scholar]
- 68. Review Manager (RevMan) [Computer program]. Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014. [Google Scholar]
- 69. Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. [DOI] [PubMed] [Google Scholar]
- 70. Sordo L, Barrio G, Bravo MJ, et al. Mortality risk during and after opioid substitution treatment: systematic review and meta-analysis of cohort studies. BMJ. 2017;357:j1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56(2):455–463. [DOI] [PubMed] [Google Scholar]
- 73. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–1101. [PubMed] [Google Scholar]
- 74. Herrmann N, Ruthirakuhan M, Gallagher D, et al. Randomized placebo-controlled trial of nabilone for agitation in Alzheimer’s disease. Am J Geriatr Psychiatry. 2019;27(11):1161–1173. 2019. May 8 [Epub ahead of print]. doi:10.1016/j.jagp.2019.05.002 [DOI] [PubMed] [Google Scholar]
- 75. Mahlberg R, Walther S. Actigraphy in agitated patients with dementia. Monitoring treatment outcomes. Z Gerontol Geriatr. 2007;40(3):178–184. [DOI] [PubMed] [Google Scholar]
- 76. Anand A, Khurana P, Chawla J, et al. Emerging treatments for the behavioral and psychological symptoms of dementia. CNS Spectr. 2018;23(06):361–369. [DOI] [PubMed] [Google Scholar]
- 77. Lim K, See YM, Lee J. A systematic review of the effectiveness of medical cannabis for psychiatric, movement and neurodegenerative disorders. Clin Psychopharmacol Neurosci. 2017;15(4):301–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Staples H, Adcock L. Cannabinoids for behavioural symptoms in adults with dementia: a review of clinical effectiveness and guidelines. Can Agency Drugs Technol Health. 2017:11–23. https://www.cadth.ca/cannabinoids-behavioural-symptoms-adults-dementia-review-clinical-effectiveness-and-guidelines [PubMed] [Google Scholar]
- 79. Weier M, Hall W. The use of cannabinoids in treating dementia. Curr Neurol Neurosci Rep. 2017;17(8):56. [DOI] [PubMed] [Google Scholar]
- 80. Whiting PF, Wolff RF, Deshpande S, et al. Cannabinoids for medical use: a systematic review and meta-analysis. JAMA. 2015;313(24):2456–2473. [DOI] [PubMed] [Google Scholar]
- 81. Ben Amar M. Cannabinoids in medicine: a review of their therapeutic potential. J Ethnopharmacol. 2006;105(1–2):1–25. [DOI] [PubMed] [Google Scholar]
- 82. Lemberger L, Rubin A, Wolen R, et al. Pharmacokinetics, metabolism and drug-abuse potential of nabilone. Cancer Treat Rev. 1982;9(Suppl):B17–B23. [DOI] [PubMed] [Google Scholar]
- 83. McGilveray IJ. Pharmacokinetics of Cannabinoids. Pain Res Manag. 2005;10(Suppl A):15A–22A. 2005. [Epub ahead of print]. doi:10.1155/2005/242516 [DOI] [PubMed] [Google Scholar]
- 84. Russo EB. Taming THC: potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. Br J Pharmacol. 2011;163(7):1344–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Russo EB. The case for the entourage effect and conventional breeding of clinical cannabis: no “strain,” no gain. Front Plant Sci. 2018. ; 9 2018. [Epub ahead of print]. doi:10.3389/fpls.2018.01969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Massimino L. In silico discovery of terpenoid metabolism in Cannabis sativa . F1000Research. 2017;6:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, Data_Collection_Sheet for Cannabinoids for the Neuropsychiatric Symptoms of Dementia: A Systematic Review and Meta-Analysis by Anees Bahji, Arthi Chinna Meyyappan and Emily R. Hawken in The Canadian Journal of Psychiatry