Abstract
Objective
Bipolar disorder (BD) is challenging to treat, and fewer treatments are available for depressive episodes compared to mania. Light therapy is an evidence-based nonpharmacological treatment for seasonal and nonseasonal major depression, but fewer studies have examined its efficacy for patients with BD. Hence, we reviewed the evidence for adjunctive light therapy as a treatment for bipolar depression.
Methods
We conducted a systematic review of databases from inception to June 30, 2019, for randomized, double-blind, placebo-controlled trials of light therapy in patients with BD (CRD42019128996). The primary outcome was change in clinician-rated depressive symptom score; secondary outcomes included clinical response, remission, acceptability, and treatment-emergent mood switches. We quantitatively pooled outcomes using meta-analysis with random-effects models.
Results
We identified seven trials representing 259 patients with BD. Light therapy was associated with a significant improvement in Hamilton Depression Rating Scale score (standardized mean difference = 0.43, 95% confidence interval [CI], 0.04 to 0.82, P = 0.03). There was also a significant difference in favor of light therapy for clinical response (odds ratio [OR] = 2.32; 95% CI, 1.12 to 4.81; P = 0.024) but not for remission. There was no difference in affective switches between active light and control conditions (OR = 1.30; 95% CI, 0.38 to 4.44; P = 0.67). Study limitations included different light treatment parameters, small sample sizes, short treatment durations, and variable quality across trials.
Conclusion
There is positive but nonconclusive evidence that adjunctive light therapy reduces symptoms of bipolar depression and increases clinical response. Light therapy is well tolerated with no increased risk of affective switch.
Keywords: light therapy, randomized clinical trials, depression, bipolar disorder, systematic review, meta-analysis
Abstract
Objectif
Le trouble bipolaire (TB) est difficile à traiter et il y a moins de traitements offerts pour les épisodes dépressifs comparativement à la manie. La photothérapie est un traitement non pharmacologique fondé sur des données probantes pour la dépression majeure saisonnière et non saisonnière, mais moins d’études en ont examiné l’efficacité pour les patients souffrant de TB. Nous avons donc examiné les données probantes liées à la photothérapie d’appoint comme traitement de la dépression bipolaire.
Méthodes
Nous avons mené une revue systématique des bases de données, du début au 30 juin 2019, à la recherche d’essais randomisés, à double insu, contrôlés par placebo de photothérapie chez des patients souffrant de TB (CRD42019128996). Le résultat principal était le changement des scores de symptômes dépressifs évalués par un clinicien; les résultats secondaires étaient notamment la réponse clinique, la rémission, l’acceptabilité et les changements d’humeur attribuables au traitement. Nous avons regroupé les résultats quantitativement à l’aide d’une méta-analyse avec modèles à effets aléatoires.
Résultats
Nous avons repéré 7 essais représentant 259 patients souffrant de TB. La photothérapie était associée à une amélioration significative du score à l’échelle de dépression de Hamilton (différence moyenne normalisée = 0,43; intervalle de confiance à 95 % [IC] 0,04 à 0,82; p = 0,03). Il y avait aussi une différence significative en faveur de la photothérapie pour la réponse clinique (rapport de cotes [RC] = 2,32 (IC à 95 % 1,12 à 4,81; p = 0,024) mais pas pour la rémission. Il n’y avait pas de différence des changements affectifs entre la lumière active et les conditions des témoins (RC = 1,30; IC à 95 % 0,38 à 4,44; p = 0,67). Les limitations de l’étude étaient entre autres différents paramètres de la photothérapie, de petites tailles d’échantillons, des traitements de courte durée, et la qualité des variables parmi toutes les études.
Conclusion
Il y a des données positives mais non probantes indiquant que la photothérapie d’appoint réduit les symptômes de la dépression bipolaire et accroît la réponse clinique. La photothérapie est bien tolérée et ne comporte pas de risque accru de changement affectif.
Introduction
Bipolar disorder (BD), a common psychiatric condition with a prevalence of 1–2%,1 is associated with significant impairment in psychosocial functioning and is currently one of the leading causes of disability worldwide.2 Much of this disease burden is associated with depressive episodes,3,4 which dominate the episode course of BD and are more frequent and longer in duration than manic or hypomanic episodes.5,6 Bipolar depression is challenging to treat, and there are fewer available treatments for bipolar depression compared to mania.7 Hence, additional evidence-based treatment options for bipolar depression is a recognized unmet need.8
Because many patients with BD are taking mood-stabilizing and other medications, adjunctive nonpharmacological treatments for bipolar depression are particularly needed. Alternative and nonpharmacological treatments are also identified by patients as a research priority.9 Light therapy, consisting of daily exposure to bright artificial light, is an evidence-based nonpharmacological treatment for seasonal and nonseasonal major depressive disorder (MDD) that has a low side effect burden and can be used by patients alongside other treatments. Light therapy is usually administered with bright, fluorescent light delivered via a light box or other device. The parameters of light treatment include wavelength, intensity, total daily exposure time, and timing of exposure during the day. Intensity is usually measured by lux, a unit of illumination, with most studies showing therapeutic effects with white light above 2,000 lux and few effects below 500 lux.10 There is a general inverse relationship between intensity and daily exposure time, such that higher intensities require less daily exposure time. A common protocol for light therapy for MDD is 10,000 lux white fluorescent light for 30 minutes a day in the early morning. Light therapy is generally well tolerated by patients, often with milder side effects than medications.11 The mechanism of action of light therapy is not yet clearly elucidated, but major hypotheses involve chronobiological effects of light12 and effects on neurotransmitters such as serotonin.13 The positive benefit to harm ratio makes light therapy a good candidate as an adjunctive treatment for bipolar depression.
Light therapy is a recommended first- or second-line treatment for MDD in clinical guidelines14 based on many clinical studies and meta-analyses.15,16 However, there are fewer studies of light therapy for BD. A previous systematic review of light therapy studies in bipolar depression (with literature search up to October, 2015) did not identify any randomized controlled trials (RCTs), and the quantitative meta-analysis only examined pre–post effect sizes from open-label studies.17 Given that several RCTs have since been published, we sought to critically examine the efficacy and acceptability of light therapy in BD. We conducted an updated systematic review of RCTs of light therapy versus a control condition for adults with bipolar depression and quantitatively summarized results using meta-analysis to determine the efficacy of light therapy for improvement in clinician-rated depressive symptoms.
Methods
Literature Search and Study Selection
The systematic review was registered at Prospero, www.crd.york.ac.uk/prospero/, CRD42019128996. The extant literature through to June 30, 2019, was searched through the following databases: Web of Science, EMBASE (OVID), Medline (OVID), PsycInfo, and Clinicaltrials.gov. Multiple databases were also searched simultaneously using Web of Science. Search terms included MeSH terms of bipolar disorder and light therapy/phototherapy; followed by combinations of light therap*, phototherap*, light treatment*, and bipolar* (see Supplemental Information for search terms used for each database). We conducted backward reference chaining by searching through bibliographies of relevant articles and forward reference chaining by searching Google Scholar for additional articles.
Studies were selected for the review if they included (1) participants meeting validated diagnostic criteria (e.g., DSM-IV, DSM-5, ICD-10) for bipolar disorder, type I (BD-I) or type II (BD-II), currently in a depressive episode; (2) a clinician-rated measure of depressive symptomatology (e.g., Hamilton Depression Rating Scale [HAM-D],18 Montgomery Asberg Depression Rating Scale);19 (3) a specific light intervention (e.g., bright light therapy), and (4) a randomized trial design with a control condition. We excluded studies if (1) active intervention included a combination of light therapy with another treatment (e.g., light therapy and sleep deprivation) and the control condition did not include the other treatment, (2) the participants were a mix of bipolar and unipolar participants and the bipolar participant data were not separately analyzed, and (3) the study sample had other comorbidities as a primary diagnosis of interest (e.g., poststroke depression). We contacted study authors if relevant inclusion/exclusion information were not reported or were unclear.
Data Extraction
Two independent reviewers (MYT, YEJ) conducted study selection, assessed study quality, and extracted data using a data extraction form designed for the study. Any disagreement between the two independent reviewers was resolved by discussion and consensus with a third reviewer (RWL).
Risk of Bias and Quality Assessment
The criteria for quality assessment were based on recommendations in the Cochrane Handbook for Systematic Reviews of Interventions.20 We used the Cochrane Collaboration’s Risk of Bias tool21 to summarize results for the categories of random sequence generalization, allocation concealment, blinding of participants and study personnel, blinding of outcome assessment, selective reporting, and other bias.
Statistical Analysis
The primary outcome was improvement in depressive symptoms on a clinician-rated depression rating scale. We selected the HAM-D as the primary scale because all studies included HAM-D data. To account for different versions and scoring for the HAM-D, we prioritized the versions in this order: 17-item (the original version), 21-item (the 17-item HAM-D with 4 additional symptom items), 25-item (the 17-item HAM-D with 8 additional items for atypical symptoms), 23-item (the 17-item HAM-D with 6 additional items for atypical symptoms). We chose the 17-item HAM-D as the primary measure because it is the version most commonly used and for comparison to other depression treatment studies.
The primary outcome measure was difference in endpoint scores on the HAM-D between active and control conditions with intent-to-treat samples, analyzed using standardized mean differences (SMD). We used a random-effects model because of expected heterogeneity in study methodologies. To mitigate against unbalanced baselines for crossover studies, we used only the data from the first arm of the crossover.20
Secondary outcomes included clinical response (defined as 50% or greater improvement from baseline to endpoint scores), clinical remission (defined by the investigator based on endpoint scores, e.g., ≤ 7 on the 17-item HAM-D), participant self-rated symptom scales, and quality of life/functioning scales. We also examined tolerability based on proxy measures of acceptability (all-cause discontinuation rates), discontinuation rates owing to adverse events, and rates of affective switch into mania or hypomania.
Secondary outcomes and subgroup analyses with continuous data were analyzed similarly. Categorical data (e.g., response and remission, discontinuation rates) were analyzed using odds ratios (ORs). For arms with zero events, we imputed a 0.5 event score. Heterogeneity was assessed using Q statistics and I2. Presence of publication bias was assessed with funnel plots,22 Rosenthal’s fail-safe N,23 and Egger’s regression intercept.24 The meta-analytic analyses were conducted using Comprehensive Meta-Analysis (Version 2.0) software (Biostat, USA).
We anticipated heterogeneity of study methodologies with variability in diagnosis (BD-I or BD-II), light treatment parameters, study duration, and so on and hence planned several exploratory sensitivity and subgroup analyses, including low risk versus high risk of bias, low-light versus nonlight control conditions, BD-I versus BD-II participants, seasonal versus nonseasonal participants, participants taking medications (e.g., mood-stabilizers, antidepressants) versus no medications, high intensity (e.g., >1,500 lux for 2 hr or equivalent) versus low intensity (e.g., <1,000 lux) light, morning versus midday versus evening timing of light exposure, and shorter follow-up (2 weeks or less) versus longer follow-up (3 weeks or more) studies.
Results
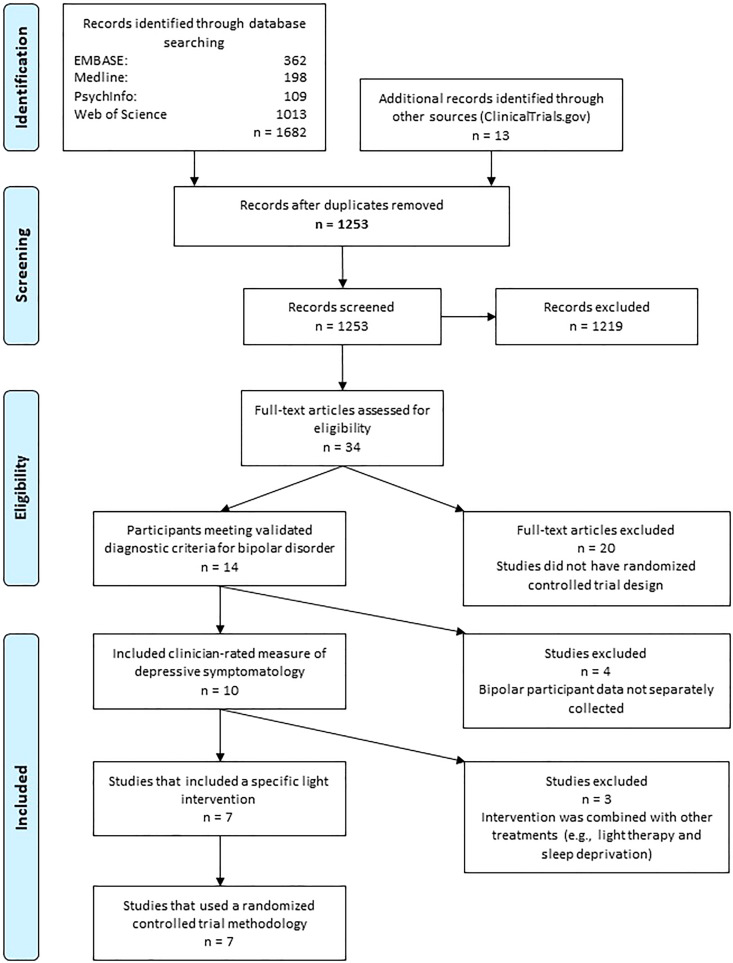
Figure 1 shows the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram for search and selection of studies. After screening and removal of duplicates, we assessed 34 full-text articles for eligibility. Twenty articles were excluded because of lack of randomized design, four articles because data for participants with BD were not separately collected, and three studies because the intervention combined light therapy with other treatments. Seven studies were eligible for inclusion; one study25 used a quasi-randomized design in which patients were randomized according to the day of week that they were admitted to an inpatient unit. We elected to retain this study in the main analysis but planned a sensitivity analysis to examine results when it was excluded.
Figure 1.
PRISMA flow diagram for study selection (www.prisma-statement.org). PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Table 1 shows the main characteristics of the included studies. Most studies had small sample sizes (range: 9–74 participants). One study26 involved participants with bipolar seasonal affective disorder (SAD), while the others excluded bipolar SAD. The ratio of BD-I to BD-II participants was variable in three studies and not specified in four; none of the studies provided separate data for the bipolar subgroups. The parameters for active light treatment (light intensity, timing during the day, duration of exposure, and duration of follow-up) also varied across studies. The active light intensities ranged from 400 lux to 10,000 lux and duration of exposure from 15 to 360 min/day. Five studies used morning timing for light exposure, one study used mid-day timing,27 and one used both morning and evening timing.26 The duration of follow-up ranged from 14 days for four studies to 28, 42, and 56 days for each of three studies. The control conditions also varied: two studies used an inactive negative ion generator,28,29 one study used a low-density negative ion generator,30 and four studies used low intensity light (100 lux or less in three studies, and 500 lux or less in one study). Note that one study29 examined 400 lux narrow-spectrum (green) light as an active treatment compared to a nonlight control condition, although 400 lux would generally be considered no brighter than illumination in a typical office setting.
Table 1.
Characteristics of Included Studies.
| Study | Bipolar I to Bipolar II Ratio | Active Light Parameters | Control Condition | Duration of Follow-up | Daily Duration of Treatment | Time of day of Treatment | Number of Participants Receiving Active Light | Number of Participants Receiving Control Condition | Outcome Measure Used in Meta-Analysis |
|---|---|---|---|---|---|---|---|---|---|
| Benedetti et al.29 | Ratio not specified | 400 lux fluorescent green light (485–515 nm, peak at 500–505 nm) | Inactive negative ion generator | 28 days | 30 min/day | Morning (according to predictive algorithm; average 6:00 a.m. for active light; 7:45 a.m. for control) | 6 | 3 | 17-item HAM-D |
| Chojnacka et al.28 | Mixed, ratio not specified (from author correspondence, mostly Bipolar II) | 10,000 lux white fluorescent light, UV filter | Inactive negative ion generator | 14 days | 30 min/day | Morning (30 min after rising, between 8:00 a.m. to 9:00 a.m.) | 29 | 21 | 21-item HAM-D |
| Dauphinais et al.30 | Mixed, ratio not specified | 7,000 lux fluorescent white light (4,000 Kelvin color temperature, UV filter) | Low-density negative ions | 56 days (8 weeks) | Began at 7.5 min/day, gradually increased to maximum of 45 min/day | Morning (shortly after rising; specific times not provided) | 18 | 20 | 25-item HAM-D |
| Rosenthal et al.26 | 2:9 | 2,500 lux full-spectrum fluorescent white light | 100 lux fluorescent yellow light | 14 days | 360 min/day (3 hr twice a day) | Morning and evening (before dawn and after dusk) | 6 | 5 | 23-item HAM-D |
| Sit et al.27 | 31:15 | 7,000 lux broad-spectrum fluorescent white light (4,000 Kelvin; UV filter) | 50 lux fluorescent red light | 42 days | 15–60 min/day flexible dose; average 45 min/day | Midday (between 12:00 p.m. to 2:30 p.m.) | 22 | 23 | 21-item HAM-D |
| Yorguner Kupeli et al.25 | 17:15 | 10,000 lux fluorescent white light | <500 lux fluorescent white light | 14 days | 30 min/day | Morning (between 8:00 a.m. to 10:00 a.m.) | 16 | 16 | 17-item HAM-D |
| Zhou et al.31 | Ratio not specified | 5,000 lux LED white light (10,000-Kelvin; UV filter) | <100 lux LED red light | 14 days | 60 min/day | Morning (between 6:30 a.m. to 9:00 a.m.) | 37 | 37 | 17-item HAM-D |
Note. nm = nanometers; UV = ultraviolet; LED = light-emitting diode; min = minutes; HAM-D = Hamilton Depression Rating Scale.
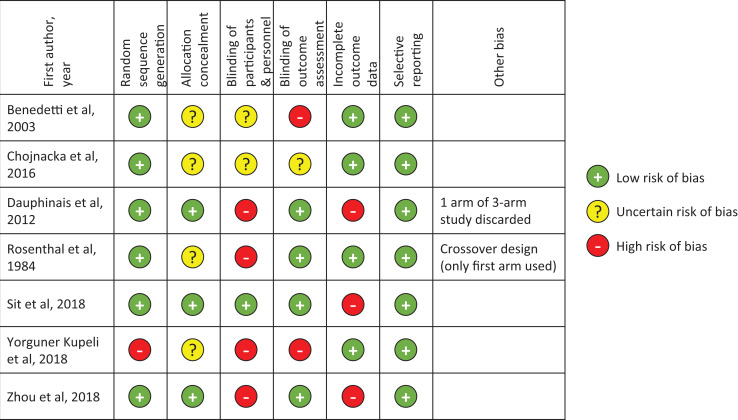
Risk of Bias Assessment
Figure 2 shows the summary of the risk of bias assessment from the Cochrane Risk of Bias tool. The studies showed high or unclear risk of bias in at least one major methodological category of risk. Four of the seven trials did not report on allocation concealment. While all of the studies included blinding of participants to treatment condition, only four trials used and reported blinded outcome raters, and six did not have or did not report on blinding of the clinical team involved in participant care. Three of the seven studies had incomplete information on dropouts. Other sources of bias were noted in the Dauphinais et al. study30 that excluded analysis of one arm of a three-arm study (an active condition receiving high-density negative ions) because of the small sample size (n = 2), and the Rosenthal et al. study26 that used a crossover design (although only first arm of the crossover was used in our analysis, there may be different expectation effects compared to a parallel design).
Figure 2.
Risk of bias assessment for included studies.
Meta-Analyses: Primary Outcomes
Depressive symptom scores at endpoint were available for all seven trials (259 participants), and all used a version of the HAM-D. Two of the studies28,29 included both unipolar and bipolar participants, but the investigators provided separate data for the BD participants. The Rosenthal et al. study26 was the only one with a crossover study design; only data from the first arm of the crossover were used in the analysis.
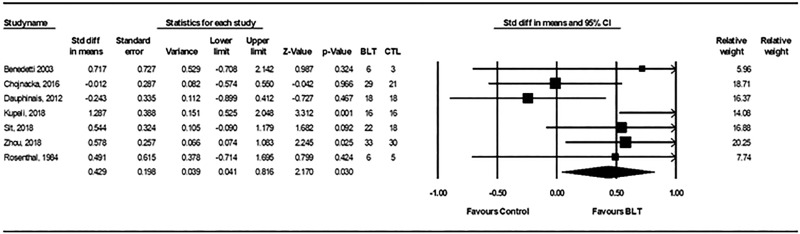
Compared with control conditions, active light therapy was associated with a significant improvement in clinician-rated depressive symptoms (SMD = 0.43; 95% confidence interval [CI], 0.04 to 0.82, P = 0.03), representing a small-to-moderate effect size (Figure 3). There was no significant heterogeneity between these trials, with Q statistics of 11.86 and I2 of 49.4% (degree of freedom = 6; P = 0.065). The fail-safe N was 11, Egger’s intercept was 1.00, t(5) = 0.53, two-tailed P = 0.62, and a funnel plot of standard errors by effect size estimates was broadly symmetrical (Supplemental Information Figure S1). These findings suggest relatively low potential for publication bias.
Figure 3.
Forest plots from meta-analysis for standardized mean difference in Hamilton Depression Rating Scale scores for active light treatment versus control condition in bipolar depression. CI: Std diff = standardized difference; CI = confidence interval; BLT = bright light treatment; CTL = control condition.
Sensitivity Analyses
Although no significant heterogeneity was found, a sensitivity analysis was conducted because of two studies that had potentially significant differences in methodology from the others. The Rosenthal et al. study26 included only bipolar SAD patients, which were excluded from the other studies, and the Yorguner Kupeli et al. study25 had a quasi-randomized study design. We conducted a sequential sensitivity analysis first excluding Rosenthal et al., that is, including only studies with nonseasonal BD participants and then excluding Yorguner Kupeli et al., that is, including only studies with full randomization.
In these sensitivity analyses (Figures S2 and S3), the effect sizes in favor of light therapy remained small to moderate but narrowly missed significance level when excluding the Rosenthal et al. study (SMD = 0.43; 95% CI, −0.002 to 0.86; P = 0.051) and was not significant when the Yorguner Kupeli et al. study was also excluded (SMD = 0.27; 95% CI, −0.09 to 0.62; P = 0.14).
Secondary Outcomes
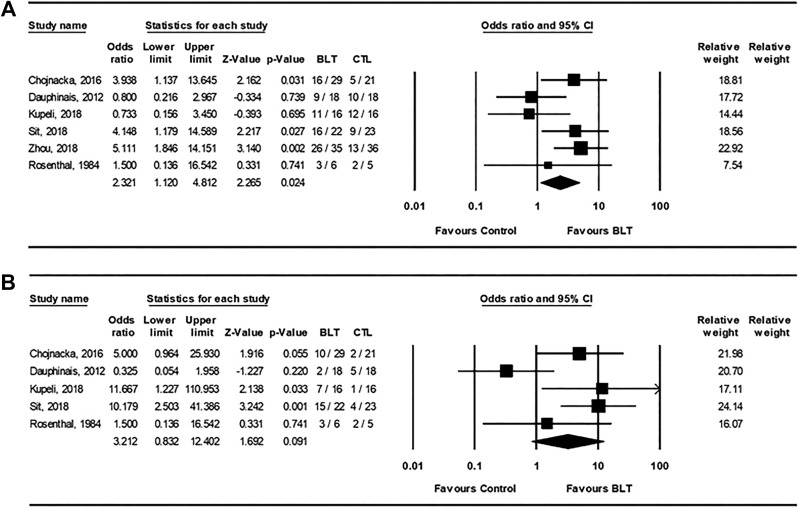
Data for clinical response and remission were available for six trials and five trials, respectively (Figure 4). The pooled OR was 2.32 (95% CI, 1.12 to 4.81; P = 0.024) for clinical response indicating a significant difference in outcome favoring light therapy. Although the pooled OR for clinical remission was higher at 3.21 (95% CI, 0.83 to 12.4; P = 0.09), the analysis showed no significant difference between conditions. The overall rates for clinical response for active light and control conditions were 64.3% and 42.9%, respectively, and for clinical remission were 40.7% and 16.9%, respectively. There were insufficient numbers of studies that reported self-rated depression measures or quality of life measures to conduct an analysis.
Figure 4.
Forest plots from meta-analyses for (A) clinical response and (B) clinical remission for active light treatment versus control condition in bipolar depression. CI = confidence interval; BLT = bright light treatment; CTL = control condition.
Study Discontinuation
Data on discontinuation rates were available for six trials. The pooled OR was 0.54 (95% CI, 0.25 to 1.14; P = 0.11) for all-cause discontinuation (Figure S4) and 0.70 (95% CI, 0.16 to 3.00; P = 0.63) for adverse event discontinuation (Figure S5), indicating no differences between active light treatment and control conditions. The rates of treatment-emergent mania or hypomania were available for all seven trials (Figure S6). Note that six studies25–29,31 did not observe any switches in either active light or control conditions, so the 0.5 event imputation rule was applied to both conditions. Active light treatment was not associated with an increased risk of affective switches compared to control conditions (OR = 1.30; 95% CI, 0.38 to 4.44; P = 0.67). The overall rates for affective switches for active light and control conditions were 3.0% and 1.6%, respectively.
Subgroup Analyses
Although we planned to conduct several subgroup analyses, sufficient data for analysis were only available for two subgroups of interest. First, we examined studies with dim light (four studies) versus nonlight (three studies) control conditions (Figures S7–S9). Active light conditions were superior to dim light control conditions in clinician-rated depressive symptoms (SMD = 0.70; 95% CI, 0.36 to 1.04; P < 0.001), clinical response (OR = 2.68; 95% CI, 1.09 to 6.63; P = 0.03), and clinical remission (OR = 7.14; 95% CI, 2.41 to 21.63; P < 0.001). However, there were no differences between active light treatment and nonlight control conditions in clinician-rated depressive symptoms (SMD = −0.04; 95% CI, −0.45 to 0.37; P = 0.84), clinical response (OR = 1.80; 95% CI, 0.38 to 8.58; P = 0.46), or clinical remission (OR = 1.31; 95% CI, 0.09 to 19.02; P = 0.85).
Second, we examined studies with follow-up duration ≤2 weeks (four studies) versus ≥3 weeks (three studies; Figures S10–S12). The studies of ≤2 weeks showed active light superior to control conditions for improvement in depressive symptoms (SMD = 0.56; 95% CI, 0.01 to 1.13; P = 0.046), response (OR = 2.66; 95% CI, 1.09 to 6.49; P = 0.032), and remission (OR = 4.72; 95% CI, 1.48 to 15.11; P = 0.009). In contrast, the studies of ≥3 weeks showed no differences between active light and control conditions in improvement in depressive symptoms (SMD = 0.24; 95% CI, −0.37 to 0.85; P = 0.44), response (OR = 1.84; 95% CI, 0.37 to 9.24; P = 0.46), or remission (OR = 1.91; 95% CI, 0.07 to 55.68; P = 0.71).
Atypical depressive symptoms have been noted to potentially predict response to light therapy in studies of SAD32,33 and MDD.34 Unfortunately, only three studies used a version of the HAM-D that included atypical symptom items, and only two studies reported responses to atypical symptom scores; hence, there were insufficient data to conduct a subgroup analysis of atypical symptoms.
Discussion
To our knowledge, this is the first systematic review and meta-analysis of RCTs of light therapy versus control conditions in patients with bipolar depression. The primary quantitative analysis of the seven identified RCTs (259 participants) found a small-to-moderate and significant effect of active light treatment in reducing depressive symptoms as assessed with the clinician-rated HAM-D. For the secondary outcomes, the comparison of active light and control conditions showed large effects in favor of light therapy for both clinical response and remission, but only the clinical response analyses were significant. The nonsignificant finding for clinical remission may be a Type II error (i.e., nonrejection of a false null hypothesis) because fewer studies included remission outcomes.
Interpretation of the positive findings are also constrained by limitations of the analysis, including variable quality and heterogeneity of the included studies, variable parameters of light treatment, small sample sizes, short duration of follow-up, and some control conditions (e.g., dim light, low-density negative ions) having potentially active treatment effects. For example, the primary studies examined mixed samples of patients with BD-I and BD-II without separate subgroup analyses. As in many other studies of add-on treatments in BD, most patients were on various concomitant medications including mood stabilizers, antipsychotics, and antidepressants, and few of the studies attempted to control for the different medications. Only one study prospectively controlled for medication use by stratifying randomization based on whether antidepressants were used or not.27 Three studies examined medication use retrospectively and found no differences between conditions.25,28,31
In the sensitivity analysis of potential sources of variability in study methodology, excluding the study with 11 participants with bipolar SAD changed the results from positive to narrowly missing statistical significance. In addition, the sensitivity analysis that excluded the quasi-randomized trial found no difference between active light and control conditions, although that analysis included only five fully randomized trials (198 participants). Thus, although the primary outcome analysis found significant effects of light therapy, these limitations indicate that the evidence for efficacy of light therapy in BD remains nonconclusive.
Light treatment was generally well tolerated in the identified studies with no differences in all-cause discontinuation and adverse event discontinuation rates compared to control conditions. Light therapy also appears to have low risk for treatment-emergent affective switch, as indicated by low switch rates with no significant differences between active light and control conditions. The low switch rates are consistent with previous reviews in nonrandomized studies of light therapy for BD.35 Our finding, however, is limited by the quantitative analysis used, in which imputation for zero event conditions may give unreliable rates for rare events with small-sample studies36 and by the short follow-up durations. In addition, most of the patients, especially those with BD-I, were on mood-stabilizing or anti-manic medications during study treatment. Of note, however, is that mild activating symptoms (agitation, anxiety, arousal) during bright light treatment were observed in several studies, although these generally resolved with reducing the duration of daily light exposure. Hence, patients with BD should be actively monitored for emergence of hypomanic symptoms that may require adjustment of light dosage.
There was considerable variability in the light treatment parameters for the active treatments, although broadly the parameters were similar to those used for light therapy of MDD, that is, 10,000 lux for 30 min/day. Most of the studies used morning timing of light exposure, as usually recommended in MDD studies, with only one study using midday timing and one older study using morning and evening timing. The durations of follow-up in these studies were also short, with only two studies using a more typical acute treatment follow up of 6–8 weeks.27,30 Given the cyclical nature of BD, future studies will need to provide longer follow-up periods for acute light treatment.
The subgroup analyses may provide useful information to guide these future studies. We found that the effects of active light therapy were significant when compared to dim light control conditions, but not when compared to non-light controls. There is no consensus for an optimal sham control condition for light treatment because the parameters for therapeutic effects of light effects are still not well understood. Dim light is often used as a plausible sham control, but some studies have shown that dim light (e.g., 400 lux) may have therapeutic effects.29 Non-light control conditions do not conflate dosing of light but they introduce a different type of treatment device that may have different expectations from participants. Our results suggest that more research is needed to compare the two types of sham conditions. We also found that shorter follow-up (2 weeks or less) studies showed superiority of active light therapy over control conditions, but longer follow-up (3weeks or more) studies showed no differences. Given that depressive episodes typically last more than 2 weeks, longer follow-up studies should be conducted to provide more information about sustained effects of light therapy for acute depressive episodes. No controlled studies have yet examined light therapy as a maintenance treatment for BD.
In summary, this meta-analysis of RCTs found positive but nonconclusive evidence that light therapy is efficacious and well tolerated as adjunctive treatment for depressive episodes in patients with BD. The parameters for light therapy in BD are similar to those for MDD. Although the risk of affective switch with light therapy is low, patients should be monitored for hypomanic or activation symptoms that may require adjusting the dose or timing of light exposure. Given the importance of finding new nonpharmacological adjunctive treatments for BD, priority should be given to further research to improve the evidence base for light therapy. Future studies would benefit from designs that include larger sample sizes, longer follow-up periods, standardization of light therapy parameters, and measures of expectation of response for sham conditions. In addition, future studies should examine subgroup analysis of response by features such as diagnosis (e.g., BD-I vs. BD-II), depressive symptoms (e.g., typical vs. atypical symptoms), and concomitant medications (e.g., mood stabilizers vs. antidepressants).
Supplemental Material
Supplemental Material, S1 for Light Therapy for Patients With Bipolar Depression: Systematic Review and Meta-Analysis of Randomized Controlled Trials by Raymond W. Lam, Minnie Y. Teng, Young-Eun Jung, Vanessa C. Evans, John F. Gottlieb, Trisha Chakrabarty, Erin E. Michalak, Jill K. Murphy, Lakshmi N. Yatham and Dorothy K. Sit in The Canadian Journal of Psychiatry
Supplemental Material, S2 for Light Therapy for Patients With Bipolar Depression: Systematic Review and Meta-Analysis of Randomized Controlled Trials by Raymond W. Lam, Minnie Y. Teng, Young-Eun Jung, Vanessa C. Evans, John F. Gottlieb, Trisha Chakrabarty, Erin E. Michalak, Jill K. Murphy, Lakshmi N. Yatham and Dorothy K. Sit in The Canadian Journal of Psychiatry
Supplemental Material, S3 for Light Therapy for Patients With Bipolar Depression: Systematic Review and Meta-Analysis of Randomized Controlled Trials by Raymond W. Lam, Minnie Y. Teng, Young-Eun Jung, Vanessa C. Evans, John F. Gottlieb, Trisha Chakrabarty, Erin E. Michalak, Jill K. Murphy, Lakshmi N. Yatham and Dorothy K. Sit in The Canadian Journal of Psychiatry
Supplemental Material, S4 for Light Therapy for Patients With Bipolar Depression: Systematic Review and Meta-Analysis of Randomized Controlled Trials by Raymond W. Lam, Minnie Y. Teng, Young-Eun Jung, Vanessa C. Evans, John F. Gottlieb, Trisha Chakrabarty, Erin E. Michalak, Jill K. Murphy, Lakshmi N. Yatham and Dorothy K. Sit in The Canadian Journal of Psychiatry
Supplemental Material, S5 for Light Therapy for Patients With Bipolar Depression: Systematic Review and Meta-Analysis of Randomized Controlled Trials by Raymond W. Lam, Minnie Y. Teng, Young-Eun Jung, Vanessa C. Evans, John F. Gottlieb, Trisha Chakrabarty, Erin E. Michalak, Jill K. Murphy, Lakshmi N. Yatham and Dorothy K. Sit in The Canadian Journal of Psychiatry
Supplemental Material, S6 for Light Therapy for Patients With Bipolar Depression: Systematic Review and Meta-Analysis of Randomized Controlled Trials by Raymond W. Lam, Minnie Y. Teng, Young-Eun Jung, Vanessa C. Evans, John F. Gottlieb, Trisha Chakrabarty, Erin E. Michalak, Jill K. Murphy, Lakshmi N. Yatham and Dorothy K. Sit in The Canadian Journal of Psychiatry
Supplemental Material, S7 for Light Therapy for Patients With Bipolar Depression: Systematic Review and Meta-Analysis of Randomized Controlled Trials by Raymond W. Lam, Minnie Y. Teng, Young-Eun Jung, Vanessa C. Evans, John F. Gottlieb, Trisha Chakrabarty, Erin E. Michalak, Jill K. Murphy, Lakshmi N. Yatham and Dorothy K. Sit in The Canadian Journal of Psychiatry
Supplemental Material, S8 for Light Therapy for Patients With Bipolar Depression: Systematic Review and Meta-Analysis of Randomized Controlled Trials by Raymond W. Lam, Minnie Y. Teng, Young-Eun Jung, Vanessa C. Evans, John F. Gottlieb, Trisha Chakrabarty, Erin E. Michalak, Jill K. Murphy, Lakshmi N. Yatham and Dorothy K. Sit in The Canadian Journal of Psychiatry
Supplemental Material, S9 for Light Therapy for Patients With Bipolar Depression: Systematic Review and Meta-Analysis of Randomized Controlled Trials by Raymond W. Lam, Minnie Y. Teng, Young-Eun Jung, Vanessa C. Evans, John F. Gottlieb, Trisha Chakrabarty, Erin E. Michalak, Jill K. Murphy, Lakshmi N. Yatham and Dorothy K. Sit in The Canadian Journal of Psychiatry
Supplemental Material, S10 for Light Therapy for Patients With Bipolar Depression: Systematic Review and Meta-Analysis of Randomized Controlled Trials by Raymond W. Lam, Minnie Y. Teng, Young-Eun Jung, Vanessa C. Evans, John F. Gottlieb, Trisha Chakrabarty, Erin E. Michalak, Jill K. Murphy, Lakshmi N. Yatham and Dorothy K. Sit in The Canadian Journal of Psychiatry
Supplemental Material, S11 for Light Therapy for Patients With Bipolar Depression: Systematic Review and Meta-Analysis of Randomized Controlled Trials by Raymond W. Lam, Minnie Y. Teng, Young-Eun Jung, Vanessa C. Evans, John F. Gottlieb, Trisha Chakrabarty, Erin E. Michalak, Jill K. Murphy, Lakshmi N. Yatham and Dorothy K. Sit in The Canadian Journal of Psychiatry
Supplemental Material, S12 for Light Therapy for Patients With Bipolar Depression: Systematic Review and Meta-Analysis of Randomized Controlled Trials by Raymond W. Lam, Minnie Y. Teng, Young-Eun Jung, Vanessa C. Evans, John F. Gottlieb, Trisha Chakrabarty, Erin E. Michalak, Jill K. Murphy, Lakshmi N. Yatham and Dorothy K. Sit in The Canadian Journal of Psychiatry
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: RWL has received ad hoc speaking/consulting fees or research grantsfrom: Akili, Allergan, Asia-Pacific Economic Cooperation, BC Leading Edge Foundation, Canadian Institutes of Health Research (CIHR), Canadian Network for Mood and Anxiety Treatments (CANMAT), Canadian Psychiatric Association, CME Institute, Hansoh, Healthy Minds Canada, Janssen, Lundbeck, Lundbeck Institute, Medscape, Mind.Me, MITACS, Ontario Brain Institute, Otsuka, Pfizer, St. Jude Medical, University Health Network Foundation, and VGH-UBCH Foundation. LNY has received speaking/consulting fees or research grants from Alkermes, Allergan, CANMAT, CIHR, Lundbeck, Otsuka, Sumitomo Dainippon Pharma, Sunovion, and Valeant.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Raymond W. Lam, MD  https://orcid.org/0000-0001-7142-4669
https://orcid.org/0000-0001-7142-4669
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Merikangas KR, Jin R, He JP, et al. Prevalence and correlates of bipolar spectrum disorder in the world mental health survey initiative. Arch Gen Psychiatry. 2011;68(3):241–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Whiteford HA, Degenhardt L, Rehm J, et al. Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet. 2013;382(9904):1575–1586. [DOI] [PubMed] [Google Scholar]
- 3. Kessler RC, Akiskal HS, Ames M, et al. Prevalence and effects of mood disorders on work performance in a nationally representative sample of U.S. workers. Am J Psychiatry. 2006;163(9):1561–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ferrari AJ, Stockings E, Khoo JP, et al. The prevalence and burden of bipolar disorder: findings from the Global Burden of Disease Study 2013. Bipolar Disord. 2016;18(5):440–450. [DOI] [PubMed] [Google Scholar]
- 5. Judd LL, Akiskal HS, Schettler PJ, et al. The long-term natural history of the weekly symptomatic status of bipolar I disorder. Arch Gen Psychiatry. 2002;59(6):530–537. [DOI] [PubMed] [Google Scholar]
- 6. Judd LL, Akiskal HS, Schettler PJ, et al. A prospective investigation of the natural history of the long-term weekly symptomatic status of bipolar II disorder. Arch Gen Psychiatry. 2003;60(3):261–269. [DOI] [PubMed] [Google Scholar]
- 7. Yatham LN, Kennedy SH, Parikh SV, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) 2018 guidelines for the management of patients with bipolar disorder. Bipolar Disord. 2018;20(2):97–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Frye MA, Prieto ML, Bobo WV, et al. Current landscape, unmet needs, and future directions for treatment of bipolar depression. J Affect Disord. 2014;169(Suppl 1):S17–S23. [DOI] [PubMed] [Google Scholar]
- 9. Nestsiarovich A, Hurwitz NG, Nelson SJ, et al. Systemic challenges in bipolar disorder management: a patient-centered approach. Bipolar Disord. 2017;19(8):676–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lam RW, Tam EM: A clinician’s guide to using light therapy. Cambridge, UK: Cambridge University Press; 2009. [Google Scholar]
- 11. Terman M, Terman JS. Light therapy for seasonal and nonseasonal depression: efficacy, protocol, safety, and side effects. CNS Spectr. 2005;10(8):647–663; quiz 672. [DOI] [PubMed] [Google Scholar]
- 12. Sit D, Haigh S. Use of “lights” for bipolar depression. Curr Psychiatry Rep. 2019;21(6):45. [DOI] [PubMed] [Google Scholar]
- 13. Tyrer AE, Levitan RD, Houle S, et al. Serotonin transporter binding is reduced in seasonal affective disorder following light therapy. Acta Psychiatr Scand. 2016;134(5):410–419. [DOI] [PubMed] [Google Scholar]
- 14. Ravindran AV, Balneaves LG, Faulkner G, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 Clinical Guidelines for the Management of Adults with Major Depressive Disorder: Section 5. Complementary and alternative medicine treatments. Can J Psychiatry. 2016;61(9):576–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Perera S, Eisen R, Bhatt M, et al. Light therapy for non-seasonal depression: systematic review and meta-analysis. B J Psych Open. 2016;2(2):116–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Penders TM, Stanciu CN, Schoemann AM, Ninan PT, Bloch R, Saeed SA. Bright light therapy as augmentation of pharmacotherapy for treatment of depression: a systematic review and meta-analysis. Prim Care Companion CNS Disord. 2016;18(5);doi:10.4088/PCC.15r01906. [DOI] [PubMed] [Google Scholar]
- 17. Tseng PT, Chen YW, Tu KY, et al. Light therapy in the treatment of patients with bipolar depression: a meta-analytic study. Eur Neuropsychopharmacol. 2016;26(6):1037–1047. [DOI] [PubMed] [Google Scholar]
- 18. Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23(4):56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. [DOI] [PubMed] [Google Scholar]
- 20. Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. London, England: The Cochrane Collaboration; 2011. [Google Scholar]
- 21. Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sterne JA, Sutton AJ, Ioannidis JP, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343:d4002. [DOI] [PubMed] [Google Scholar]
- 23. Rosenthal R. The file drawer problem and tolerance for null results. Psychol Bull. 1979;86(3):638–641. [Google Scholar]
- 24. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(109):629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yorguner Kupeli N, Bulut NS, Carkaxhiu Bulut G, Kurt E, Kora K. Efficacy of bright light therapy in bipolar depression. Psychiatry Res. 2018;260:432–438. [DOI] [PubMed] [Google Scholar]
- 26. Rosenthal NE, Sack DA, Gillin JC, et al. Seasonal affective disorder. a description of the syndrome and preliminary findings with light therapy. Arch Gen Psychiatry. 1984;41(1):72–80. [DOI] [PubMed] [Google Scholar]
- 27. Sit DK, McGowan J, Wiltrout C, et al. Adjunctive bright light therapy for bipolar depression: a randomized double-blind placebo-controlled trial. Am J Psychiatry. 2018;175(2):131–139. [DOI] [PubMed] [Google Scholar]
- 28. Chojnacka M, Antosik-Wójcińska AZ, Dominiak M, et al. A sham-controlled randomized trial of adjunctive light therapy for non-seasonal depression. J Affect Disord. 2016;203:1–8. [DOI] [PubMed] [Google Scholar]
- 29. Benedetti F, Colombo C, Pontiggia A, Bernasconi A, Florita M, Smeraldi E. Morning light treatment hastens the antidepressant effect of citalopram: a placebo-controlled trial. J Clin Psychiatry. 2003;64(6):648–653. [DOI] [PubMed] [Google Scholar]
- 30. Dauphinais DR, Rosenthal JZ, Terman M, DiFebo HM, Tuggle C, Rosenthal NE. Controlled trial of safety and efficacy of bright light therapy vs. negative air ions in patients with bipolar depression. Psychiatry Res. 2012;196(1):57–61. [DOI] [PubMed] [Google Scholar]
- 31. Zhou TH, Dang WM, Ma YT, et al. Clinical efficacy, onset time and safety of bright light therapy in acute bipolar depression as an adjunctive therapy: a randomized controlled trial. J Affect Disord. 2018;227:90–96. [DOI] [PubMed] [Google Scholar]
- 32. Terman M, Amira L, Terman JS, Ross DC. Predictors of response and nonresponse to light treatment for winter depression. Am J Psychiatry. 1996;153(11):1423–1429. [DOI] [PubMed] [Google Scholar]
- 33. Dimitrova TD, Reeves GM, Snitker S, et al. Prediction of outcome of bright light treatment in patients with seasonal affective disorder: discarding the early response, confirming a higher atypical balance, and uncovering a higher body mass index at baseline as predictors of endpoint outcome. J Affect Disord. 2017;222:126–132. [DOI] [PubMed] [Google Scholar]
- 34. Levitan RD, Levitt AJ, Michalak EE, et al. Appetitive symptoms differentially predict treatment response to fluoxetine, light, and placebo in nonseasonal major depression. J Clin Psychiatry. 2018;79(4):pii: 17m11856. [DOI] [PubMed] [Google Scholar]
- 35. Benedetti F. Rate of switch from bipolar depression into mania after morning light therapy: a historical review. Psychiatry Res. 2018;261:351–356. [DOI] [PubMed] [Google Scholar]
- 36. Efthimiou O. Practical guide to the meta-analysis of rare events. Evid Based Ment Health. 2018;21(2):72–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, S1 for Light Therapy for Patients With Bipolar Depression: Systematic Review and Meta-Analysis of Randomized Controlled Trials by Raymond W. Lam, Minnie Y. Teng, Young-Eun Jung, Vanessa C. Evans, John F. Gottlieb, Trisha Chakrabarty, Erin E. Michalak, Jill K. Murphy, Lakshmi N. Yatham and Dorothy K. Sit in The Canadian Journal of Psychiatry
Supplemental Material, S2 for Light Therapy for Patients With Bipolar Depression: Systematic Review and Meta-Analysis of Randomized Controlled Trials by Raymond W. Lam, Minnie Y. Teng, Young-Eun Jung, Vanessa C. Evans, John F. Gottlieb, Trisha Chakrabarty, Erin E. Michalak, Jill K. Murphy, Lakshmi N. Yatham and Dorothy K. Sit in The Canadian Journal of Psychiatry
Supplemental Material, S3 for Light Therapy for Patients With Bipolar Depression: Systematic Review and Meta-Analysis of Randomized Controlled Trials by Raymond W. Lam, Minnie Y. Teng, Young-Eun Jung, Vanessa C. Evans, John F. Gottlieb, Trisha Chakrabarty, Erin E. Michalak, Jill K. Murphy, Lakshmi N. Yatham and Dorothy K. Sit in The Canadian Journal of Psychiatry
Supplemental Material, S4 for Light Therapy for Patients With Bipolar Depression: Systematic Review and Meta-Analysis of Randomized Controlled Trials by Raymond W. Lam, Minnie Y. Teng, Young-Eun Jung, Vanessa C. Evans, John F. Gottlieb, Trisha Chakrabarty, Erin E. Michalak, Jill K. Murphy, Lakshmi N. Yatham and Dorothy K. Sit in The Canadian Journal of Psychiatry
Supplemental Material, S5 for Light Therapy for Patients With Bipolar Depression: Systematic Review and Meta-Analysis of Randomized Controlled Trials by Raymond W. Lam, Minnie Y. Teng, Young-Eun Jung, Vanessa C. Evans, John F. Gottlieb, Trisha Chakrabarty, Erin E. Michalak, Jill K. Murphy, Lakshmi N. Yatham and Dorothy K. Sit in The Canadian Journal of Psychiatry
Supplemental Material, S6 for Light Therapy for Patients With Bipolar Depression: Systematic Review and Meta-Analysis of Randomized Controlled Trials by Raymond W. Lam, Minnie Y. Teng, Young-Eun Jung, Vanessa C. Evans, John F. Gottlieb, Trisha Chakrabarty, Erin E. Michalak, Jill K. Murphy, Lakshmi N. Yatham and Dorothy K. Sit in The Canadian Journal of Psychiatry
Supplemental Material, S7 for Light Therapy for Patients With Bipolar Depression: Systematic Review and Meta-Analysis of Randomized Controlled Trials by Raymond W. Lam, Minnie Y. Teng, Young-Eun Jung, Vanessa C. Evans, John F. Gottlieb, Trisha Chakrabarty, Erin E. Michalak, Jill K. Murphy, Lakshmi N. Yatham and Dorothy K. Sit in The Canadian Journal of Psychiatry
Supplemental Material, S8 for Light Therapy for Patients With Bipolar Depression: Systematic Review and Meta-Analysis of Randomized Controlled Trials by Raymond W. Lam, Minnie Y. Teng, Young-Eun Jung, Vanessa C. Evans, John F. Gottlieb, Trisha Chakrabarty, Erin E. Michalak, Jill K. Murphy, Lakshmi N. Yatham and Dorothy K. Sit in The Canadian Journal of Psychiatry
Supplemental Material, S9 for Light Therapy for Patients With Bipolar Depression: Systematic Review and Meta-Analysis of Randomized Controlled Trials by Raymond W. Lam, Minnie Y. Teng, Young-Eun Jung, Vanessa C. Evans, John F. Gottlieb, Trisha Chakrabarty, Erin E. Michalak, Jill K. Murphy, Lakshmi N. Yatham and Dorothy K. Sit in The Canadian Journal of Psychiatry
Supplemental Material, S10 for Light Therapy for Patients With Bipolar Depression: Systematic Review and Meta-Analysis of Randomized Controlled Trials by Raymond W. Lam, Minnie Y. Teng, Young-Eun Jung, Vanessa C. Evans, John F. Gottlieb, Trisha Chakrabarty, Erin E. Michalak, Jill K. Murphy, Lakshmi N. Yatham and Dorothy K. Sit in The Canadian Journal of Psychiatry
Supplemental Material, S11 for Light Therapy for Patients With Bipolar Depression: Systematic Review and Meta-Analysis of Randomized Controlled Trials by Raymond W. Lam, Minnie Y. Teng, Young-Eun Jung, Vanessa C. Evans, John F. Gottlieb, Trisha Chakrabarty, Erin E. Michalak, Jill K. Murphy, Lakshmi N. Yatham and Dorothy K. Sit in The Canadian Journal of Psychiatry
Supplemental Material, S12 for Light Therapy for Patients With Bipolar Depression: Systematic Review and Meta-Analysis of Randomized Controlled Trials by Raymond W. Lam, Minnie Y. Teng, Young-Eun Jung, Vanessa C. Evans, John F. Gottlieb, Trisha Chakrabarty, Erin E. Michalak, Jill K. Murphy, Lakshmi N. Yatham and Dorothy K. Sit in The Canadian Journal of Psychiatry