Abstract
Background
Glutamatergic system has been known to play a role in the pathogenesis of major depression disorder by inducing N-methyl-d-aspartate receptor-dependent long-term depression (LTD) or metabotropic glutamate receptors (mGluR)-dependent LTD. Here, we characterized the LTD in a chronic social defeat stress (CSDS)-induced depressive mouse model.
Methods
CSDS was used to induce the depressive-like behaviors in C57BL/6 male mice, which were assessed using sucrose preference test and social interaction test. The synaptic strength including LTD and long-term potentiation (LTP) induced by paired-pulse low frequency stimulation (PP-LFS) was measured using whole-cell recording technique.
Results
CSDS induced depressive-like behaviors and facilitated PP-LFS-induced LTD in hippocampal CA3-CA1 pathway in the susceptible mice. Interestingly, mGluR5 but not N-methyl-d-aspartate receptor mediated the PP-LFS-induced LTD. In addition, mGluR5 agonist dihydroxyphenylglycine promoted PP-LFS-induced LTD specifically in susceptible mice, which was diminished by activating the BDNF/TrkB signaling pathway.
Conclusions
Our results suggest that mGluR5-dependent LTD might be responsible for the development of depressive-like behaviors in CSDS-induced depression mice model.
Keywords: MDD, mGluR5, NMDAR, CSDS, synaptic strength
Abstract
Objectif
Le glutamate est soupçonné jouer un rôle dans l’étiologie de la dépression grâce à son rôle dans la potentialisation à long terme (PLT) en conjonction avec les récepteurs NMDA, et la dépression à long terme (DLT) en conjonction avec les récepteurs mGluR. Nous caractérisons les changements PLT chez la souris avec le modèle de défaite sociale chronique.
Méthodes
Des souris C57BL/6 mâles ont subi le stress de la défaite sociale chronique (SDSC), et l’anhédonie a été quantifiée avec la préférence pour le sucrose et les comportements sociaux, avec le test d’interaction sociale. La plasticité synaptique après une stimulation pulse-paire à basse fréquence est quantifiée utilisant des enregistrements de cellule entière.
Résultats
Chez les animaux susceptibles, le SDSC diminue la préférence pour le sucrose et l’interaction sociale, mais augmente la DLT dans les afférents CA3-CA1 de l’hippocampe. Cet effet dépend des récepteurs mGluR5 et non des récepteurs NMDA, et l’agoniste dihydroxyphenylglycine augmente la DLT chez les animaux susceptibles. Nous démontrons que cet effet diminue lorsque la voie de signalisation BDNF/TrkB est activée.
Conclusions
Les Récepteurs mGluR5 Jouent un Rôle Dans Les Changements Comportementaux et la Plasticité Synaptique Chez la Souris Après le SDSC.
Introduction
Major depression disorder (MDD) is the most common mental disorder with an increasing global prevalence and a lifetime prevalence as high as 20% to 30% in women and 10% to 15% in men.1 Patients with depression suffer from anhedonia, dysphoria, suicide ideation or attempt, many other somatic symptoms, and vegetative symptoms including sleep disorder and appetite change.2,3 Furthermore, MDD can cause cognitive impairment and dementia. The disability caused by MDD exerts a huge economic burden in the world.4 According to the Diagnostic and Statistical Manual of Mental Disorders, 5th Edition, the criteria to diagnose MDD is five of these symptoms for 2 weeks.5
The investigations into the neurobiology of MDD have focused on different neurotransmitter systems. Since reserpine, a known antihypertension medication that depletes monoamines in the nerve system,6 can cause depressive symptoms, a monoamine hypothesis proposed that depression patients probably have low levels of monoamine neurotransmitters, epinephrine and serotonin.7,8 The tryptophan depletion studies further modified the monoamine hypothesis which suggests low level of serotonin might be the major culprit.9,10 In recent years, increasing evidences have suggested that glutamatergic system be implicated in the pathogenesis of depression. The earliest evidence is from the clinical observation that ketamine alleviates depressive symptoms in a remarkably short period of time by blocking one of the glutamate receptors, N-methyl-d-aspartate receptor (NMDAR),11,12 which is ion tropic. In addition, association studies have identified the metabolic glutamatergic receptors as the risk factors.13 Since long-term depression (LTD) in the hippocampus neurons has been the consistent finding in animal models with depressive-like behaviors14 and glutamate is the most common neurotransmitter that induces LTD in most synapses, numerous studies have shown that both NMDAR-dependent and mGlutR-dependent LTD are implicated in the pathogenesis of MDD.15 Furthermore, in recent years, an increasing body of clinical evidence has demonstrated that glutamate levels are significantly changed in the plasma and cerebrospinal fluid of patients with depression and suicide victims.16,17 Therefore, both the ligand and the receptors in the glutamatergic system probably play key roles in the pathogenesis of MDD. Here, we used chronic social defeat stress (CSDS) to generate a mice model of depression and investigated the molecular mechanisms underlying the LTD in the hippocampus Cornu Ammonis (CA) region in this model.
Methods and Materials
Mice
Six- to eight-week-old C57BL/6 male mice were obtained from Nanjing Model Animal Institute (Nanjing, China) and housed under standard condition with a 12-h light/dark cycle and free access to food and water. The housing temperature is 22 °C to 25 °C with humidity of 50 ± 2%. All the animals could acclimate to the housing environment for at least 1 week before experiment. All animal handling procedures were reviewed and approved by the Animal Care and Use Committee of Qingdao Mental Health Center. The behavioral testing was performed by separated experimenters who were blinded to the treatment conditions.
Depressive Model and SI Test
CSDS was conducted based on previously published protocols.18 In short, C57BL/6 mice were subjected to physical defeats that were generated by a CD-1 aggressor for 5 min every day for 10 days. The control mice received no physical treatments. All control mice were new naive mice every day. The control mice or experimental mice could have sensory contact with CD-1 mice for 24 h. During the interaction, the C57BL/6 mice and CD-1 mice were kept separately in a box by a transparent, perforated partition. All defeated and control animals received SI test on Day 11. To distinguish the susceptible animals from the resistant animals, we used the so-called microdefeat as subthreshold, as previously described.19,20 According to the microdefeat protocol, C57BL/6 mice were kept together with the CD-1 aggressor for 5 min every 15 min for three sections. Twenty-four hours later, all C57BL/6 mice were evaluated with the SI test. To assess increased susceptibility to stress, we used a subthreshold “microdefeat” model. Briefly, C57BL/6 mice were exposed to a novel CD-1 aggressor for three consecutive 5-min defeat sessions, each separated by 15 min. Twenty-four hours later, mice were subjected to the SI test. SI test was composed of two 150-s phases. In Phase 1, testing mice were allowed to explore freely in the open field arena without the presence of CD-1 mice. In Phase 2, a novel CD1 mouse was placed in the cage in the interaction zone, and the testing mice were allowed to interact with it. The interaction ratio was calculated as (time spent in the interaction zone with an aggressor)/(time spent in the interaction zone without an aggressor). And an interaction ratio of 1 was set as a cutoff: The mice with scores <1 were considered susceptible and those with scores ≥1 were considered resilient. This information has now been added into the method section.
Electrophysiology
Slices were immersed in the saline solution that contained picrotoxin (20 M) to isolate excitatory neurotransmission. The typical morphology was used to identify CA1 pyramidal neurons visually. The featured firing pattern responding to long depolarization was used to confirm the neuronal identity. Infrared differential contrast video microscopy was used (IMAGO-VGA, Till Photonics, München, Germany; Axioskop 2 FS, Zeiss, Göttingen, Germany). Glass tubing (3–6 MΩ) was used to generate patch pipettes. The whole-cell recordings were conducted according to previously published procedure.21 Briefly, recordings were generated using an EPC-9 amplifier (HEKA, Lambrecht, Germany) at 20 °C to 22 °C. The series resistance of 10 to 60 MΩ was used to compensate the bridge balance. A range of −72 to 75 mV was used as holding potentials after the resting potentials were determined. Stimulator 2100 (A-M Systems, Carlsborg, WA) was used to generate orthodromic stimulation that stimulate the Schaffer collateral pathway. Pulse and PulseFit software (version 2x90.2; HEKA) were used to acquire and analyze data. In particular, prior studies suggest that LTD induction via prolonged LFS is age limited in hippocampal area CA,22 with less response in adult animals. Therefore, a paired-pulse low frequency stimulation (PP-LFS) procedure as previously described23 was used to induce the LTD in juvenile animals.
Sucrose Preference Test (SPT)
The level of hedonia in mice was evaluated using SPT as described previously.24 Each mouse received the tests twice, before and after CSDS. Each mouse was kept in separate cages with two bottles of 1.5% sucrose solutions for 24-h adaptation. During the next 24 h, one bottle of water replaced one of the sucrose solutions. Then, the mice were starved for 18 h without water. One bottle of sucrose and water were then supplied for 1 h. All the bottles were weighted before and after exposure to the mice. The ratio of sucrose solution consumption to the total fluid intake was used to present the sucrose preference.
Chemicals
Fluvoxamine maleate (BP600), dl-2-amino-5-phosphonopentanoic acid (APV; A5282), and 6-methyl-2-(phenylethynyl) pyridine hydrochloride (MPEP; M5435) were purchased from Sigma-Aldrich (St. Louis, MO).
Statistical Analysis
All data are presented as the means ± SEM as indicated in graphs. Student’s paired or unpaired two-tailed t test was used for comparison between two groups. One-way analysis of variance followed by a Tukey’s post hoc test was used for comparison between multiple groups. All statistical tests were evaluated at the 5% significance level.
Results
CSDS Induced Depressive-Like Behaviors in Susceptible Mice
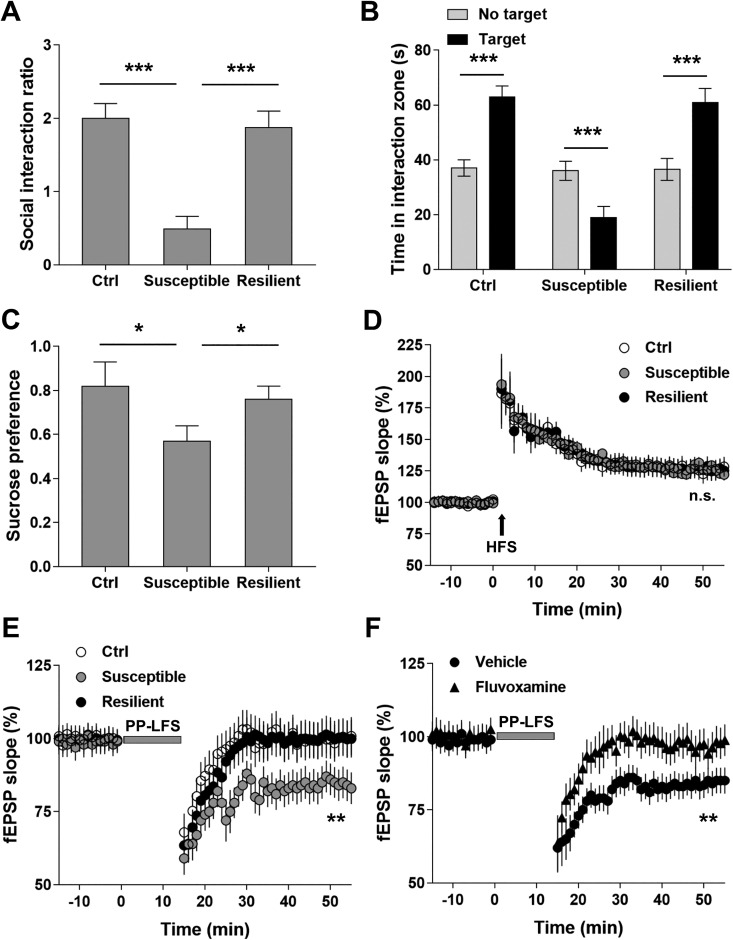
It is well known that chronic stressors are the risk factors for depression. Here, we used SI test and SPT to assess the depressive-like behaviors in mice subjected to CSDS. When exposed to CSDS, mice showed variable responses to the stressor. The microdefeat assessment distinguished the mice into two categories: susceptible and resistant. Compared with the resistant mice and controls, the susceptible mice showed significantly decreased frequency of SI (P < 0.001; Figure 1A). In addition, the interaction between the susceptible mice and CD-1 mice was much shorter than that between CD-1 mice and the resistant or control mice (P < 0.001; Figure 1B). Furthermore, susceptible mice consumed less sucrose than the resistant and control mice (P < 0.05; Figure 1C). Our results demonstrate that CSDS led to depressive-like behaviors in susceptible mice, providing a useful model to investigate the underlying molecular mechanisms.
Figure 1.
Chronic social defeat stress (CSDS) facilitated paired-pulse low frequency stimulation (PP-LFS)-induced long-term depression (LTD) in hippocampal CA3-CA1 pathway in the susceptible mice. (A) CSDS decreased social interaction ratio in the susceptible but not resilient mice. (B) CSDS significantly decreased time spent in interaction zone in the susceptible but not resilient group. (C) CSDS decreased sucrose preference of the susceptible mice. n = 12 mice per group, *P < 0.05, ***P< 0.001. (D) Long-term potentiation (LTP) induced by one train of high-frequency stimulation (1 s at 100 Hz). (E) LTD induced by PP-LFS (paired-pulse stimuli with 50-ms interpulse interval delivered at 1 Hz for 15 min) in the Ctrl, susceptible, and resilience groups. (F) CSDS-facilitated LTD in the susceptible group was alleviated by fluvoxamine treatment. Statistical difference was assessed by comparing the mean values of the magnitude of the last 10 min of field excitatory postsynaptic potential (fEPSP) recordings. **P < 0.01. n = 14 to 16 slices from 10 mice per group. One-way analysis of variance for (A) and (C), paired Student’s t test for (B), and Student’s t test for (D–F).
CSDS Facilitated PP-LFS-Induced LTD in Hippocampal CA3-CA1 Pathway in the Susceptible Mice
Previous studies have demonstrated that depressive animal model is characterized by LTD in hippocampal CA pathways. Here, we first compared the synaptic strength in hippocampal CA3-CA1 neurons among susceptible, resistant, and control mice by measuring LTP with high-frequency stimulus. No significant difference was observed (P > 0.05; Figure 1D). We then measured LTD induced by PP-LFS in hippocampal CA preps. Our results showed that PP-LFS induced LTD in all three groups, and the induced LTD was significantly stronger in susceptible mice than that in resistant and control mice (P < 0.05; Figure 1E). Interestingly, the antidepressant fluvoxamine effectively alleviated the PP-LFS-induced LTD in susceptible mice (P < 0.05; Figure 1F). Taken together, our results suggest that CSDS induced the synaptic modulations pertinent to the pathogenesis of depression.
mGluR5 but Not NMDAR Mediated the PP-LFS-Induced LTD in Susceptible Mice
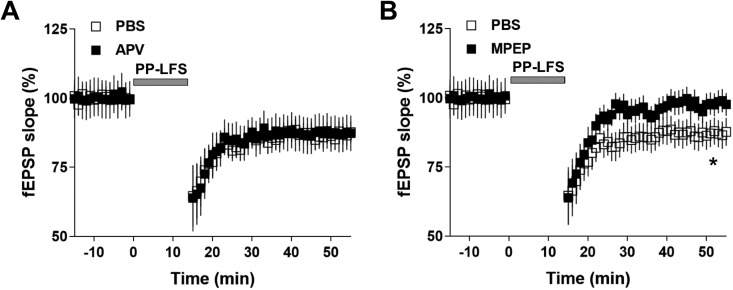
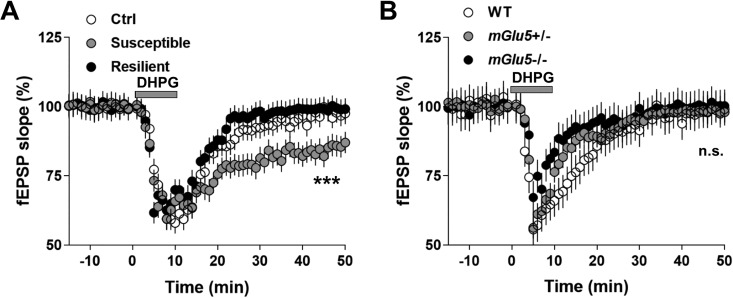
Previous studies have demonstrated that both NMDAR-LTD and mGluR-LTD play important roles in the pathogenesis of depression. Since NMDAR and mGluR mediate distinct signal transduction, the underlying molecular mechanisms regarding the pathogenesis of depression can be various across both human patients and animal models. In CSDS-induced depression model, it remains inconclusive in terms of the type of LTD. To test whether NMDAR-LTD or mGluR-LTD is present in CSDS-induced depression mice model, we treated the brain slides with either NMDAR or mGluR5 antagonist. Interestingly, mGluR5 antagonist MPEP but not NMDAR antagonist AVP successfully blocked the PP-LFS-induced LTD in susceptible mice (Figure 2A and B). Furthermore, the mGluR5 agonist dihydroxyphenylglycine (DHPG) facilitated the PP-LFS-induced LTD specifically in susceptible mice, suggesting that the level of mGluR5 protein in the cell membrane might be increased in susceptible mice (Figure 3A). Consistent with these results, DHPG failed to facilitate the PP-LFS-induced LTD in mGluR5 mutant mice (Figure 3B). Therefore, our results suggest that in CSDS-induced depression model, mGluR-LTD probably play a key role in the pathogenesis of depressive-like behaviors.
Figure 2.
Selective mGluR5 antagonist 6-methyl-2-(phenylethynyl) pyridine hydrochloride (MPEP) blocked long-term depression (LTD) expression induced by paired-pulse low frequency stimulation (PP-LFS) in susceptible mice. (A) Perfusion of N-methyl-d-aspartate receptor antagonist dl-2-amino-5-phosphonopentanoic acid (50 µM) throughout the recordings did not prevent the expression of LTD induced by PP-LFS in susceptible mice. (B) Perfusion of mGluR5 antagonist MPEP (10 µM) throughout the recordings significantly prevented the expression of LTD induced by PP-LFS in susceptible mice. Statistical difference was assessed by comparing the mean values of the magnitude of the last 10 min of fEPSP recordings. *P < 0.05. n = 14 to 16 slices from 10 mice per group. Student’s t test.
Figure 3.
Facilitated long-term depression (LTD) expression in the susceptible mice after chronic social defeat stress (CSDS) treatment is mediated by mGluR5 activation. (A) Direct application of mGluR5 agonist dihydroxyphenylglycine (DHPG; 50 µM, 10 min) significantly facilitated the expression of LTD selectively in the susceptible mice. (B) DHPG did not induce LTD expression in mGlu5+/− and mGlu5−/− mice, after CSDS treatment. Statistical difference was assessed by comparing the mean values of the magnitude of the last 10 min of fEPSP recordings. ***P < 0.001. n = 14 to 16 slices from 10 mice per group. Student’s t test.
DHPG-LTD in Susceptible Mice Was Alleviated by Activities in Brain-derived neurotrophic factor/Tropomyosin receptor kinase B (BDNF/TrkB) Signaling Pathway
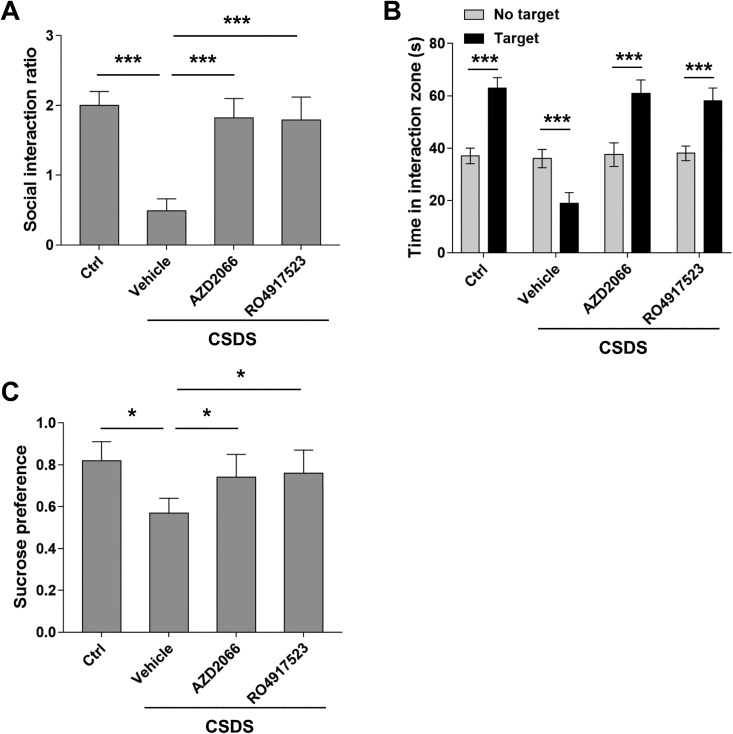
It is well known that BDNF secreted by glia cells protects the nerve system from the toxicity of excitatory neurotransmitters such as glutamate. Previous studies have shown that BDNF attenuates the signaling transduction mediated by NMDAR through activating TrkB. However, it remains unclear whether BDNF can also counteract the activities of mGluRs. It is reported that DHPG induced LTD through mGluR5. To verify, we first applied mGluR5 antagonists AZD2066 and RO4917523 to brain slide, which blocked the DHPG-LTD in susceptible mice, indicating DHPG functions through mGluR5 (Figure 4A and B). In addition, treatment with AZD2066 and RO4917523 alleviated the depressive-like behaviors in susceptible mice (Figure 5A–C). These results suggest that signaling transduction mediated by mGluR5 is the underlying molecular mechanism in CSDS-induced depression model. Particularly, the effect of AZD2066 and RO4917523 on DHPG-LTD was reversed by TrkB inhibitor ANA-12. Therefore, our results suggest that BDNF can protect the nerve system against the excitatory toxicity of glutamate by counteracting the activities of mGluR5.
Figure 4.
AZD2066 and RO4917523 alleviated dihydroxyphenylglycine (DHPG)-facilitated long-term depression (LTD) expression in chronic social defeat stress (CSDS)-treated animals via BDNF/trkB signaling pathway. (A) Perfusion of AZD2066 (10 µM) alleviated DHPG-induced mGluR-LTD in the susceptible mice. This effect was blocked by co-application of ANA-12 (50 μM). (B) Perfusion of RO4917523 (10 µM) alleviated DHPG-induced mGluR-LTD in the susceptible mice. This effect was blocked by co-application of ANA-12 (50 μM). Statistical difference was assessed by comparing the mean values of the magnitude of the last 10 min of fEPSP recordings. ** P < 0.01. n = 14 to 16 slices from 10 mice per group. Student’s t test.
Figure 5.
AZD2066 and RO4917523 alleviated chronic social defeat stress (CSDS)-induced depressive behaviors in mice. (A) AZD2066 and RO4917523 treatment (5 mg/kg i.p., 2 × 12 h) after CSDS increased social interaction ratio in mice. (B) AZD2066 and RO4917523 treatment after CSDS reversed time spent in interaction zone and restored. (C) AZD2066 and RO4917523 treatment after CSDS increased sucrose preference scores. n = 20 mice per group, unpaired two-tailed Student’s t test, *P < 0.05, ***P < 0.001. One-way ANOVA for (A) and (C) and Student’s t test for (B).
Discussions
MDD is a debilitating disease and remains to be the leading cause of disability. Animal models of depression have provided significant insights into the neurobiology of MDD. Historically, scientists have applied multiple forms of stressors to mice to illicit behavioral adaptations pertinent to depression.19 These stressors include CSDS,20 chronic unpredictable mild stress, restraint stress, or foot-shock stress followed by behavioral assessments of anhedonia such as sucrose preference or social avoidance.25 Affective-like behaviors are known to respond to social stressors. For example, repeated social stressors cause a robust depressive-like behaviors featured by social avoidance and anhedonia.26 These models make it possible to investigate the neurobiology of MDD across molecular, cellular, neural circuitry, and behavioral end points.27 In the present study, we successfully developed a mice model of depression using CSDS. Our results are consistent with previous studies regarding the behavioral phenotypes including anhedonia and social avoidance (Figure 1A–C).
Changes in synaptic plasticity have been implicated in the neurological mechanisms underlying depression. There are two types of synaptic plasticity: LTD and long-term potentiation (LTP) featured by long-lasting decreased or increased synaptic strength.15 Previous evidence has shown that in the depression rodent models, LTD is the major change in synaptic plasticity without obvious change in LTP.28 Consistent with previous studies, our results indicated that in CSDS-induced depressive mice, there was no significant change in LTP induced by prolonged LFS (Figure 1D). However, PP-LFS facilitated LTD specifically in our CSDS-induced depressive mice (Figure 1E and F). These results suggest that our model possesses the pathophysiological characteristics of depression.
Glutamate is the most common neurotransmitter that induces LTD in most synapses. It is known that there are two types of LTD mediated by two distinct groups of glutamate receptors: NMDAR-LTD and mGluR-LTD. Recently, the role of NMDAR-LTD in pathogenesis of depression is under tremendous investigation due to the rapid effectiveness of NMDAR antagonist ketamine on depressive symptoms.29 However, increasing evidence has shown that mGluR-LTD, the other major form of LTD, is also implicated in the pathogenesis of depression.30 In the present study, we showed that in our CSDS-induced depression mice model, the mGluR5 but not NMDAR facilitated the PP-LFS-induced LTD in the hippocampus CA region (Figures 2 and 3). In addition, mGluR5 agonist DHPG facilitates PP-LFS-induced LTD specifically in the brain slice of depressive mice, suggesting the expression level or sensitivity of mGluR5 increase in this group of mice (Figure 3). Further studies focusing on variations in the biochemistry features of mGluR5 would provide more insight into the molecular mechanisms underlying the specific response to DHPG in depressive mice.
Pharmacotherapy is so far one of the major treatment options in the management of depression disorder. Antidepressant medications targeting the monoamine systems have achieved moderate success. The development of serotonin–noradrenaline reuptake inhibitors (SNRIs) and selective serotonin reuptake inhibitors (SSRIs) further improve the pharmacological management of depression disorder as compared to tricyclic antidepressants (TRA) because they have more favorable side effect profile. However, similar with TRA, both SSRI and SRNI have only moderate efficacy. Furthermore, all the monoamine-based antidepressants have limited response rate around 50% and several week delayed response.31,32 More importantly, SSRI and SRNI have limited patient compliance due to some of their relatively intolerable adverse effects such as sexual dysfunction, sleep disturbances, and weight gain.33,34 In the past decade, researchers have been committing to developing new antidepressant medications that target glutamate systems. Most efforts have been focused on developing ketamine derivatives or metabolites that are not addictive.35 Interestingly, one of the ketamine metabolites (2S,6S;2R,6R)-hydroxynorketamine has antidepressant effect that is NMDAR independent. Besides NMDAR, mGluRs are another group of promising targets. In preclinical trials, multiple mGluR antagonists have shown antidepressant-like effects.36,37 In this study, we have shown that the mGluR5 antagonists AZD2066 and RO4917523 alleviated the depressive symptoms (Figure 5), which is consistent with our findings that mGluR-LTD is responsible for the development of depression in CSDS-induced mice model.
It is worth noting that there are several limitations in the current study. First, it would be nice to include other animal depression models to verify the involvement of mGluR5-dependent LTD in the depression. Second, detailed molecular mechanisms need to be thoroughly elucidated in the future studies. Last, mGluR knockout mice could be employed to further validate its role in this model.
Conclusions
Our results demonstrate that mGluR5-dependent LTD is responsible for the development of depressive symptoms in CSDS-induced depression mice model. Furthermore, our results suggest that mGluR5 is a promising target for the development of antidepressant medications.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Dongming Wang  https://orcid.org/0000-0002-6798-0889
https://orcid.org/0000-0002-6798-0889
References
- 1. Rotenstein LS, Ramos MA, Torre M, et al. Prevalence of depression, depressive symptoms, and suicidal ideation among medical students: a systematic review and meta-analysis. JAMA. 2016;316(21):2214–2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schulz PE, Arora G. Depression. Continuum (Minneap Minn). 2015;21(Behavioral Neurology and Neuropsychiatry 3):756–771. [DOI] [PubMed] [Google Scholar]
- 3. Melhem NM, Porta G, Oquendo MA, et al. Severity and variability of depression symptoms predicting suicide attempt in high-risk individuals. JAMA Psychiatry. 2019;76(6):603–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Grochtdreis T, Brettschneider C, Bjerregaard F, et al. Cost-effectiveness analysis of collaborative treatment of late-life depression in primary care (GermanIMPACT). Eur Psychiatry. 2019;57:10–18. [DOI] [PubMed] [Google Scholar]
- 5. Mitchell AJ, Smith AB, Al-salihy Z, Rahim TA, Mahmud MQ, Muhyaldin AS. Redefining diagnostic symptoms of depression using Rasch analysis: testing an item bank suitable for DSM-V and computer adaptive testing. Aust N Z J Psychiatry. 2011;45(10):846–852. [DOI] [PubMed] [Google Scholar]
- 6. Ikram H, Haleem DJ. Repeated treatment with reserpine as a progressive animal model of depression. Pak J Pharm Sci. 2017;30(3):897–902. [PubMed] [Google Scholar]
- 7. Jesulola E, Micalos P, Baguley IJ. Understanding the pathophysiology of depression: from monoamines to the neurogenesis hypothesis model—are we there yet? Behav Brain Res. 2018;341:79–90. [DOI] [PubMed] [Google Scholar]
- 8. aan het Rot M, Mathew SJ, Charney DS. Neurobiological mechanisms in major depressive disorder. CMAJ. 2009;180(3):305–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hogenelst K, Sarampalis A, Leander NP, Muller BC, Schoevers RA, aan het Rot M. The effects of acute tryptophan depletion on speech and behavioural mimicry in individuals at familial risk for depression. J Psychopharmacol. 2016;30(3):303–311. [DOI] [PubMed] [Google Scholar]
- 10. Moreno FA, Erickson RP, Garriock HA, et al. Association study of genotype by depressive response during tryptophan depletion in subjects recovered from major depression. Mol Neuropsychiatry. 2015;1(3):165–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lener MS, Kadriu B, Zarate CA., Jr Ketamine and beyond: investigations into the potential of glutamatergic agents to treat depression. Drugs. 2017;77(4):381–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Andrade C. Ketamine for depression, 5: potential pharmacokinetic and pharmacodynamic drug interactions. J Clin Psychiatry. 2017;78(7):e858–e861. [DOI] [PubMed] [Google Scholar]
- 13. Tsunoka T, Kishi T, Ikeda M, et al. Association analysis of group II metabotropic glutamate receptor genes (GRM2 and GRM3) with mood disorders and fluvoxamine response in a Japanese population. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33(5):875–879. [DOI] [PubMed] [Google Scholar]
- 14. Richter-Levin G, Xu L. How could stress lead to major depressive disorder? IBRO Rep. 2018;4:38–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Collingridge GL, Peineau S, Howland JG, Wang YT. Long-term depression in the CNS. Nat Rev Neurosci. 2010;11(7):459–473. [DOI] [PubMed] [Google Scholar]
- 16. Levine J, Panchalingam K, Rapoport A, Gershon S, McClure RJ, Pettegrew JW. Increased cerebrospinal fluid glutamine levels in depressed patients. Biol Psychiatry. 2000;47(7):586–593. [DOI] [PubMed] [Google Scholar]
- 17. Frye MA, Tsai GE, Huggins T, Coyle JT, Post RM. Low cerebrospinal fluid glutamate and glycine in refractory affective disorder. Biol Psychiatry. 2007;61(2):162–166. [DOI] [PubMed] [Google Scholar]
- 18. Liu Y, Li M, Fan M, et al. Chromodomain Y-like protein-mediated histone crotonylation regulates stress-induced depressive behaviors. Biol Psychiatry. 2019;85(8):635–649. [DOI] [PubMed] [Google Scholar]
- 19. Golden SA, Covington HE, 3rd, Berton O, Russo SJ. A standardized protocol for repeated social defeat stress in mice. Nat Protoc. 2011;6(8):1183–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schoner J, Heinz A, Endres M, Gertz K, Kronenberg G. Post-traumatic stress disorder and beyond: an overview of rodent stress models. J Cell Mol Med. 2017;21(10):2248–2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Holderbach R, Clark K, Moreau JL, Bischofberger J, Normann C. Enhanced long-term synaptic depression in an animal model of depression. Biol Psychiatry. 2007;62(1):92–100. [DOI] [PubMed] [Google Scholar]
- 22. Doyere V, Srebro B, Laroche S. Heterosynaptic LTD and depotentiation in the medial perforant path of the dentate gyrus in the freely moving rat. J Neurophysiol. 1997;77(2):571–578. [DOI] [PubMed] [Google Scholar]
- 23. Gonzalez J, Morales IS, Villarreal DM, Derrick BE. Low-frequency stimulation induces long-term depression and slow onset long-term potentiation at perforant path-dentate gyrus synapses in vivo. J Neurophysiol. 2014;111(6):1259–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhang Y, Wang Y, Lei H, et al. Optimized animal model to mimic the reality of stress-induced depression in the clinic. BMC Psychiatry. 2017;17(1):171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yan K, Chen YB, Wu JR, Li KD, Cui YL. Current rapid-onset antidepressants and related animal models. Curr Pharm Des. 2018;24(22):2564–2572. [DOI] [PubMed] [Google Scholar]
- 26. Rygula R, Abumaria N, Domenici E, Hiemke C, Fuchs E. Effects of fluoxetine on behavioral deficits evoked by chronic social stress in rats. Behav Brain Res. 2006;174(1):188–192. [DOI] [PubMed] [Google Scholar]
- 27. Covington HE, 3rd, Miczek KA. Intense cocaine self-administration after episodic social defeat stress, but not after aggressive behavior: dissociation from corticosterone activation. Psychopharmacology (Berl). 2005;183(3):331–340. [DOI] [PubMed] [Google Scholar]
- 28. Ruiz NAL, Del Angel DS, Olguin HJ, Silva ML. Neuroprogression: the hidden mechanism of depression. Neuropsychiatr Dis Treat. 2018;14:2837–2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ates-Alagoz Z, Adejare A. NMDA receptor antagonists for treatment of depression. Pharmaceuticals (Basel). 2013;6(4):480–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bernard PB, Castano AM, Bayer KU, Benke TA. Necessary, but not sufficient: insights into the mechanisms of mGluR mediated long-term depression from a rat model of early life seizures. Neuropharmacology. 2014;84:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Slowinski A, Coetzer R, Byrne C. Pharmacotherapy effectiveness in treating depression after traumatic brain injury: a meta-analysis. J Neuropsychiatry Clin Neurosci. 2019;31(3):220–227. doi: 10.1176/appineuropsych18070158. [DOI] [PubMed] [Google Scholar]
- 32. Furukawa TA, Kato T, Shinagawa Y, et al. Prediction of remission in pharmacotherapy of untreated major depression: development and validation of multivariable prediction models. Psychol Med. 2018. 10.1017/S0033291718003331. [DOI] [PMC free article] [PubMed]
- 33. Bala A, Nguyen HMT, Hellstrom WJG. Post-SSRI sexual dysfunction: a literature review. Sex Med Rev. 2018;6(1):29–34. [DOI] [PubMed] [Google Scholar]
- 34. Kirkham J, Seitz D. Evidence of ocular side effects of SSRIs and new warnings. Evid Based Ment Health. 2017;20(1):27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zanos P, Moaddel R, Morris PJ, et al. NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature. 2016;533(7604):481–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Domin H, Szewczyk B, Wozniak M, Wawrzak-Wlecial A, Smialowska M. Antidepressant-like effect of the mGluR5 antagonist MTEP in an astroglial degeneration model of depression. Behav Brain Res. 2014;273:23–33. [DOI] [PubMed] [Google Scholar]
- 37. Belozertseva IV, Kos T, Popik P, Danysz W, Bespalov AY. Antidepressant-like effects of mGluR1 and mGluR5 antagonists in the rat forced swim and the mouse tail suspension tests. Eur Neuropsychopharmacol. 2007;17(3):172–179. [DOI] [PubMed] [Google Scholar]