Abstract
A major feature of life in groups is that individuals experience social stressors of varying intensity and type. Social stress can have profound effects on health, social behavior, and ongoing relationships. Relationships can also buffer the experience of exogenous stressors. Social stress has most commonly been investigated in dyadic contexts in mice and rats that produce intense stress. Here we review findings from studies of diverse rodents and non-traditional group housing paradigms, focusing on laboratory studies of mice and rats housed in visible burrow systems, prairie and meadow voles, and mole-rats. We argue that the use of methods informed by the natural ecology of rodent species provides novel insights into the relationship between social stress, behavior and physiology. In particular, we describe how this ethologically inspired approach reveals how individuals vary in their experience of and response to social stress, and how ecological and social contexts impact the effects of stress. Social stress induces adaptive changes, as well as long-term disruptive effects on behavior and physiology.
Keywords: Stress, social behavior, group housing, visible burrow system, social hierarchy, social buffering, sociality, mice, rats, prairie voles, meadow voles, mole-rats
1. Introduction
Interactions between organisms and their environments have the capacity to shape health, physiology, and behavior. For species that live in groups, the social environment is a prominent feature of daily life. Social interactions may be beneficial, accelerating recovery from exogenous stressors, act as stressors in their own right, and everything in between. The relationship between stress and social behavior in rodents has been explored in depth elsewhere (Beery and Kaufer, 2015; Sandi and Haller, 2015). The majority of studies focus on dyadic encounters, most commonly in mice and rats. In this review, we examine the role of stress and hypothalamic-pituitary-adrenal (HPA) axis regulation in social relationships in select group-living rodents and contexts. We use examples from our own expertise to illustrate the importance of housing paradigms and consideration of variation in natural social behaviors. Specifically we explore: studies of mice and rats housed in group settings in which social hierarchies form; research on vole species that form selective social relationships; and comparative studies of African mole-rats and naked mole-rats in particular.
We begin by discussing lessons from studying mice and rats in group-housed contexts (See Figure 1). Mice (Mus musculus) and rats (Rattus norvegicus) are the dominant subjects in animal research—representing over 90% of mammalian subject use (Beery and Zucker, 2011). Unsurprisingly, the majority of research on social stress has occurred in these two species, providing the greatest depth of information upon which to build. This work has predominantly utilized social defeat and resident-intruder paradigms where one animal is physically attacked by a more dominant conspecific, and has revealed profound effects of social stress on behavioral and physiological functioning (Ambrée et al., 2018; Finnell et al., 2017; Krishnan et al., 2007; Russo and Nestler, 2013). The ready availability of genetic tools validated in these species serves as an additional advantage to working with mice, and to a lesser extent rats. Studies of mice and rats in group housing—essentially variants of a visible burrow system (VBS; described in detail in section 2.2)—provide an opportunity to study the effects of complex social interactions on stress in a dynamic and ethologically relevant context.
Figure 1. Studying social stress in rodent species.
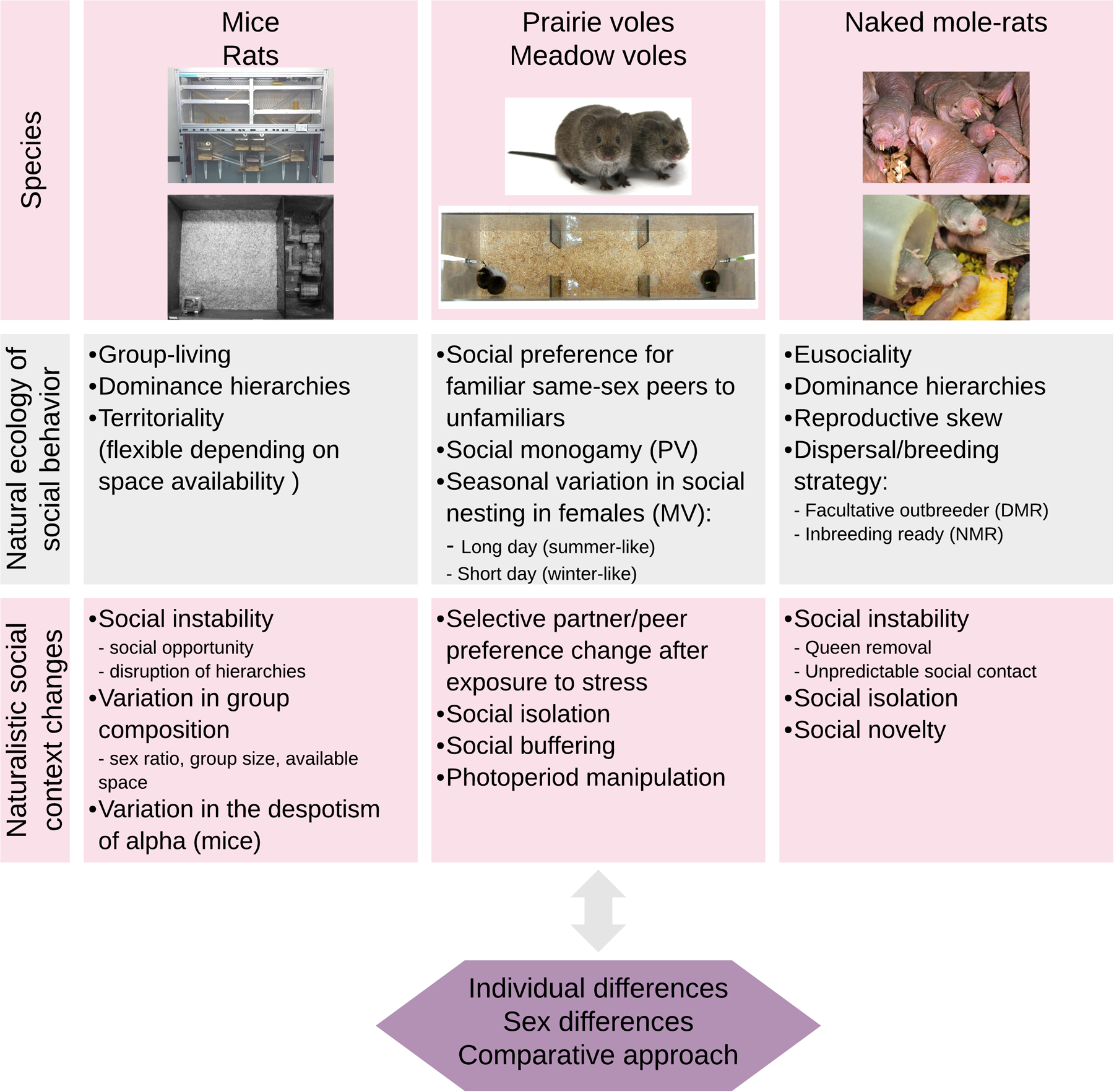
Row 1: Representative photos of each species (or housing system) discussed in sections 2, 3 & 4. Row 2: A summary of each species’ social behavior in their natural ecology. Row 3: Example social context changes related to the natural ecology that induce social stress in the lab. Photo of the rat VBS courtesy of Caroline Blanchard.
We next discuss research exploring links between stress, anxiety, and social relationships in voles. The social behavior of both prairie voles (Microtus ochrogaster) and meadow voles (Microtus pennsylvanicus) has been the focus of intense study in the field as well as the laboratory, adding important ecological context to laboratory findings (Taborsky et al., 2015). Decades of effort in multiple labs have gone into dissecting the molecular and neurobiological pathways underlying the formation of social bonds between mates in prairie voles (reviewed in Aragona and Wang, 2009; Carter and Keverne, 2009; Smith and Wang, 2018; Walum and Young, 2018). The effects of stress on social behavior have been documented in multiple domains, as well as the effects of manipulations of the social environment on measures of stress. Because prairie voles are unusual in forming selective attachments to mates, they provide an excellent model for exploring the role of these and other relationships in responding to stressors (Lieberwirth and Wang, 2016; Smith and Wang, 2018).
Around the same time monogamy was documented in prairie voles, seasonal changes in group living were identified in meadow voles (Madison, 1980; Madison et al., 1984). Meadow voles maintain exclusive territories in summer, but nest in social groups in the winter. Seasonal changes in social behavior are recapitulated with manipulations of photoperiod that mimic summer and winter day-lengths in the lab. This species can thus be used to explore the factors underlying the transition from solitary to social, and how anxiety and stress relate to social relationship formation and maintenance (Beery, 2019).
In 1981, Jennifer Jarvis published the first description of mammalian eusociality, describing the unique colony structure and reproductive skew seen in naked mole-rats (Heterocephalus glaber). Since that time, naked mole-rats have been the source of increasing curiosity - both scientifically and societally. Yet arguably the most interesting thing about naked mole-rats is that they belong to a large family of mole-rat species (family Bathyergidae). These species of African mole-rats range across the entire spectrum of sociality from completely solitary to eusocial, providing a powerful opportunity to capitalize on an evolutionary experiment and study the relationship(s) between and mechanism(s) underlying psychosocial stress and sociality in rodents.
By focusing on these diverse examples within Rodentia, we hope to highlight the complexity of social behavior and its relationship with stress and HPA axis regulation. Stress is often described in simplified and dogmatic ways (e.g. stress is bad; social subordination is always stressful), while here we show that context and species-specific ecological factors shape those relationships. Conversely, just as each of the examples presented highlights the importance of context in understanding behavior, there are common themes that appear to be shared across taxa. We highlight these findings and their implications throughout the text and in the concluding section. Finally, we emphasize the value of studying social organisms in housing paradigms that mimic natural environments, and of interpreting behavior in light of a species’ ecology.
2. Stress and social behavior in mice and rats
Mice and rats have been used in laboratory behavioral research since the first decade of the 1900s (Beck et al., 2000; Suckow et al., 2019). Rats initially gained popularity due to their docility, ability to breed well in the laboratory, and robust cognitive, emotional, social and reproductive behaviors. Rats are also relatively large, meaning that physiological measurements and manipulations (e.g. blood collection, drug infusion) as well as neuroanatomical investigations were historically more straightforward than in mice. The use of mice for behavioral and stress studies increased tremendously in the late 1990s as this species is similarly easy to breed and keep in the laboratory but is also particularly amenable to molecular and genetic manipulations such as transgenic and gene knockout studies (Rosenthal and Brown, 2007). In the last decade, with the development of the newest generation of molecular and genetic methods that allow for ever more precise physiological and neurobiological manipulations (Ellenbroek and Youn, 2016; Gerlai, 2016) there is an ever increasing interest in using both these species in behavioral and stress research.
Over most of this 120 years of laboratory research, the housing of laboratory mice and rats has become increasingly standardized with an emphasis on experimentally controlled conditions and minimal consideration for naturalistic behavior. For instance, most individuals are housed from weaning in small single-sex groups of 1–4 in small standard sized cages, in stark contrast to how wild animals live (Berdoy and Drickamer, 2007). However, although laboratory mice and rats have been undergoing a domestication process for approximately 1000 generations, there is strong evidence that key features of the social behavior of laboratory mice and rats are conserved (Adams and Boice, 1981; Berdoy and Drickamer, 2007; Boice, 1981). In the following sections we describe how housing mice and rats in enriched housing systems that mimic central elements of their natural habitat can provide unique insights into how social stress impacts behavior and physiology.
2.1. Natural Ecology of Mice and Rats
The wild ancestors of laboratory mice and rats are highly social animals that live in a diverse array of habitats across the entire world (Berdoy and Drickamer, 2007). A key feature of the social systems of both species is their flexibility. When resources are sparsely distributed, rats live at low population densities with single males each occupying their own territory. As resources become more abundant, rats live in higher densities, males have reduced home ranges and there is greater male-male competition (Calhoun, 1962). Within social groups, rats sleep together in burrows in mixed-sex groups of usually up to 15 animals (Berdoy and Drickamer, 2007; Calhoun, 1963; Lore and Flannelly, 1977), but may live in large groups of up to 100 comprised of several conjoined burrow systems (Lore and Flannelly, 1977). Although aggression within rat social groups tends to be low, when more than one adult male is present, dominance hierarchies are formed and access to females is determined based on the outcome of aggressive encounters between males (Boice and Adams, 1983). These fights are most intense when groups are forming (Calhoun, 1962). Over time, levels of aggression decrease and hierarchies appear to be relatively stable across time even if individuals change in physical capabilities such as body weight or size (Berdoy et al., 1995; Boice, 1981; Calhoun, 1962; Smith et al., 1994). Aggression between females is less well understood, but there is evidence that dominance ranks also emerge in group-housed females (Calhoun, 1963).
Similarly, wild house mice show remarkable variation in social and mating systems depending upon the distribution of resources. Most commonly, mice show male territoriality and a polygynous mating system; however, when resources are more distributed, promiscuity is reduced (Berdoy and Drickamer, 2007). Studies in the wild and in semi-natural environments reliably demonstrate that mice are extremely territorial and most male mice attempt to monopolize their own territories (Anderson, 1961; Butler, 1980; Crowcroft, 1966). Aggression can be suppressed when animals occur in high densities and individuals are forced to live in the territory of a more dominant individual (Crowcroft, 1966). However, even these animals will rapidly begin to exert territorial behavior when given more space or when space becomes compartmentalized (Crowcroft, 1966; Gray et al., 2000; Lloyd, 1975; Mackintosh, 1970). As with rats, male-male dominance dictates priority of access to resources (e.g. food, mates) as well as influencing male dispersal (Anderson and Hill, 1965; Crowcroft, 1955). However, mice are much more territorial than rats, showing much higher ongoing levels of inter-male aggression (Berdoy and Drickamer, 2007).
Aggression between female mice can also occur depending on the social context. Most commonly, females are aggressive towards unfamiliar individuals during pregnancy and lactation (Crowcroft and Rowe, 1963), but such aggression can also occur outside of this period. Female mice have also been shown to establish their own territories in wild populations (Butler, 1980; Chambers et al., 2000) and at higher population densities, aggression between females tends to increase (Chovnick et al., 1987; Yasukawa et al., 1985). Female-female aggression is reduced if individuals have prior social experience with each other (Palanza et al., 2005; Rusu and Krackow, 2004) and at low population densities (Weidt et al., 2018).
2.2. Group-housing paradigms to study social stress in mice and rats
Various paradigms have been developed to study social stress in mice and rats. The most intensively used are those that induce social defeat or subordination in subjects after being exposed either acutely or repeatedly to a more aggressive conspecific, usually in the conspecific animal’s home-cage territory but sometimes in a neutral territory (Golden et al., 2011; Malatynska and Knapp, 2005). In these paradigms, the defeated animal experiences a loss of control over their social environment, ultimately leading to dramatic changes in behavioral (e.g. social withdrawal, anhedonia), physiological (e.g. impaired cardiovascular and immune functioning) and neurobiological (e.g. down-regulation of monoaminergic and neurotrophin activity ) outcomes (Ambrée et al., 2018; Finnell et al., 2017; Krishnan et al., 2007; Russo and Nestler, 2013). The type and severity of these changes is dependent upon the frequency and intensity of the aggression received. Variations to these paradigms include those where animals are exposed to more aggressive individuals but not directly attacked (thus experiencing the psychosocial threat of attack), as well as situations where animals witness other animals undergoing social defeat. These methods also lead to several of the same phenotypic changes observed when animals directly experience physical attacks (Finnell et al., 2017; Sial et al., 2016). Although this work has primarily used male subjects, these paradigms have been adapted in mice and rats to enable female subjects to also undergo social defeat (Haller et al., 1999; Haney and Miczek, 1993; Iñiguez et al., 2018; Scholtens et al., 1990; Takahashi et al., 2017).
Though social defeat and resident-intruder paradigms are effective strategies for inducing physiological, neurobiological and behavioral changes in subjects, these paradigms are constrained by their limited ethological relevance. Though animals do experience social subordination in the wild, the aggressors experienced during lab-based social defeat are typically far more aggressive than would be expected under natural conditions. Further, socially defeated animals have no possibility of escape and are unable to interact with other conspecifics – another dissimilarity to the natural context. It has been suggested that these dyadic defeat paradigms therefore are not representative of the more variable stress that is experienced by rats and mice in the wild (Tamashiro et al., 2005). These paradigms also do not consider the stress experienced by animals in other social situations such as when dominant individuals are trying to maintain their status and territory, as well as social stress related to forming and maintaining new social relationships.
In contrast to these dyadic social stress paradigms, the short and long-term behavioral and physiological consequences of living in groups has been studied in both mice and rats. In each of these paradigms, researchers have sought to create parallel features of group-living while enabling experimental manipulation. Typically, animals are housed in single or mixed-sex groups of 4 to up to 12 animals in complex enriched housing (Blanchard et al., 1995; Ely and Henry, 1978; Williamson et al., 2016b). Animals have far more space per unit area than animals housed in standard cages and are able to avoid continuous direct contact with cage mates. As a result of this increased space and number of social relationships, another common feature of these types of housing is that these relationships become more stratified, with not just dominant and subordinate animals emerging, but also sub-dominant. Animals can often also be further rank ordered into precise social ranks. We briefly describe some examples of these paradigms below.
The behavior of group-housed rats has been studied since the pioneering work of Hebb (1947) comparing the behavior of rats housed in groups of 10–12 in so-called “free environments” which consisted of a large cage (75×75×40cm) filled with enrichment objects (e.g. wooden blocks, metal ladders and chains) to rats housed in standard or impoverished housing. Perhaps the most well-known group-housing paradigm is the visible burrow system (VBS), developed to study sociosexual behavior by Martha McClintock and Norman Adler (1978) and further utilized to study social stress by Robert and Caroline Blanchard and Randall Sakai (Blanchard and Blanchard, 1989; McEwen et al., 2015). The standard VBS, comprised of a large open area connected to side chambers by tunnels, mimics the burrow system of wild rats. Typically, four male and two female rats are housed in this system. Within days, one male rat rapidly emerges as the dominant and remains so for as long as animals are housed (usually two weeks) (Blanchard and Blanchard, 1990). The dominant animal spends most of its time in the large open area of the VBS and by the tunnel openings, and will show aggressive behavior (biting, chasing, pinning) towards the other subordinate males who spend most of their time in the smaller chambers. Critically, it appears that the emergence of male social hierarchies within the VBS is dependent upon competition for access to females, as male only groups do not readily form social hierarchies (Blanchard et al., 1995).
Group housing environments like the VBS also allow for observation of mouse social dominance hierarchies in wild, outbred, and inbred mice, similar to those formed in the wild. When housed in pens with sufficient space, individual male mice establish territories that they patrol and defend from intruders (Crowcroft, 1966; Mackintosh, 1970). As in nature, when space becomes more limited, those mice that are unable to establish their own territory become subordinate, yielding to the more dominant mouse (Brown, 1953). Over time, the intensity of fighting reduces but dominant animals continue to patrol and scent-mark their territory and enforce their dominance through non-aggressive as well as aggressive social interactions (Drickamer, 2001; Mackintosh, 1970). In established groups, subordinates tend to avoid these alpha males by fleeing from or avoiding interacting with the dominant animal. Although one male is clearly the most territorial alpha individual in each VBS, mice other than the alpha male will also engage in aggressive behavior towards more subordinate individuals (Curley, 2016). This was first described by Ulrich who recognized that laboratory mice do form social hierarchies when given sufficient space, and in many cases mice could be rank-ordered based on their relative aggression given and received (Ulrich, 1938). More recent studies of mice living in single-sex groups in larger VBS systems have shown that male mice will indeed form highly linear social hierarchies with each animal occupying its own social rank (Shemesh et al., 2013; Weissbrod et al., 2013; Williamson et al., 2016b, 2016a). In each group, the most alpha male directs aggression (biting, chasing, mounting) to all other animals and is never attacked. The second ranked animal directs aggression to all animals except the alpha to whom they are submissive, and so on. Linear hierarchies have also been found in female mice living in VBS (Williamson et al., 2019b), though much less work has been conducted thus far on group-housed female mice. It is notable however that in contrast to rats, both male and female mice are able to form dominance hierarchies in single sex housing suggesting that mice are competing over other resources than access to mates.
2.3. Stress Responsivity in VBS Housed Mice and Rats
Changes to the HPA axis regulation of stress responses have been extensively investigated in VBS male rats. Much less is currently known about the effects of group living on female physiology. Perhaps the strongest evidence for higher stress experienced by subordinate males is the increased mortality among subordinate rats even in the absence of wounding (Blanchard et al., 1985). Further, relative to dominants, subordinate males generally show elevated baseline corticosterone and adrenal hypertrophy coupled with reduced levels of corticotropin binding globulin (CBG) indicating higher levels of free corticosterone (Spencer et al., 1996), thymic atrophy and reduced body weights (McEwen et al., 2015; Tamashiro et al., 2005). These physiological outcomes are consistent with other chronic stress exposures such as chronic variable stress (CVS) (Herman and Tamashiro, 2017; McEwen et al., 2015). However, it should be noted that these differences are not found in all VBS studies, suggesting that social contexts between VBS colonies may vary and influence physiological outcomes (Buwalda et al., 2017; Dijkstra et al., 1992; Kozorovitskiy and Gould, 2004; McEwen et al., 2015). Further, differences in the central regulation of stress responses between dominants and subordinates are relatively small. Subordinates, but not dominants, show reduced levels of both glucocorticoid receptor (GR) and mineralocorticoid receptor (MR) in the CA1 of the hippocampus compared to control non-VBS housed rats, but no differences in GR or MR binding are observed (Chao et al., 1993). Subordinates also have reduced glutamic acid decarboxylase 67 (GAD67) mRNA in the paraventricular nucleus (PVN) and the bed nucleus of the stria terminalis (BNST) but increased expression in the medial prefrontal cortex (mPFC) and hippocampus, suggesting that these animals are less able to inhibit the stress response (Erlander et al., 1991; Makinson et al., 2015). Dominant and subordinate animals do not differ in CRH and AVP mRNA expression in the PVN (Albeck et al., 1997). These findings suggest that the subordinate animals housed in VBS do experience chronic stress, but it is specific to the social context and is less severe than other forms of stress such as CVS. This phenomenon may reflect the differential impact of social stress compared to non-social forms of stress.
Fewer studies have explored the stress response and HPA regulation of VBS housed mice. Subordinate male mice living in mixed-sex VBS generally do have higher baseline corticosterone than dominant males and show a greater corticosterone increase in response to ACTH administration and immobilization stress (Ely and Henry, 1978). Subordinates also have reduced glucocorticoid receptor mRNA expression in the hippocampus (So et al., 2015) and higher adrenal weights than dominants in the VBS (Ely et al., 1975), but this is not consistently observed (Ely and Henry, 1978). Notably, in male mice housed in single sex triads in standard housing, subordinates do have adrenal hypertrophy and thymic atrophy compared to dominants, congruent with findings from VBS housing (Bartoš and Brain, 1993; Turney and Harmsen, 1984). Subordinate males living in large single-sex groups have also been shown to have higher baseline corticosterone than dominant males but only in groups where alpha males are highly despotic (Williamson et al., 2017a). Further, there is little consistency in differences in plasma corticosterone between dominant and subordinate males when housed in smaller groups (Williamson et al., 2017a), indicating that social context is likely to be an important mediator between social stress and HPA axis regulation. The social stress of subordination may be higher in larger mouse groups where subordinate individuals receive more aggression from many more individuals of higher rank, and where there may be more instability in social structure. It is important to note that nearly all mouse VBS work has been carried out only in male subjects, and information regarding female social stress is limited. One study did find however that subordinate females living in large single-sex VBS groups do have significantly higher baseline corticosterone than dominant females, an effect that was even greater than observed in males (Williamson et al., 2019b). This suggests that the VBS may be an effective social stressor for female mice.
Another important source of variation in the outcomes of social stress in VBS is the background strain of rats or mice. Both rats and mice have been selectively bred in the laboratory since the early 1900s. This has led to hundreds of strains of rats and thousands of strains of mice. These inbred and outbred strains consequently differ significantly in underlying neural expression and distribution of genes, neurotransmitters and receptors that are relevant for social behavior and responses to social stress (Parmigiani et al., 1999). For instance, some mouse strains such as CD-1, Swiss and CBA/J show levels of male aggressive and territorial behavior that are similar to wild mice, whereas other strains such as C57bl/6 mice show reduced inter-male aggression and increased social tolerance (Ely and Henry, 1978; Lidster et al., 2019; Poole and Morgan, 1976; Williamson et al., 2016b). Consequently, C57bl/6 mice housed in either single-sex or mixed-sex VBS show relatively little aggression and have unclear dominant-subordinate relationships in direct contrast to group-housed CBA/J or CD1 mice (Arakawa et al., 2007; Bove et al., 2018; Ely and Henry, 1978; Pobbe et al., 2010; Williamson et al., 2016b). These differences in social organization between strains in group-housing may also explain why there are large reported differences in stress reactivity following group-housing in mice (Williamson et al., 2017a). Indeed, large strain differences in the metabolic, cardiovascular, immunological and behavioral response of male mice to social stressors such as social defeat are commonly reported (Kinsey et al., 2007; Lockwood and Turney, 1981; Razzoli et al., 2011). Much more work is needed to fully delineate the long-term effects of social stressors in the VBS on physiological and behavioral outcomes across a wider range of strains. Similarly, almost nothing is currently known about how social stress in the VBS differentially affects different strains of female mice, although this is to be expected given that strains of female mice do differ in both their aggression levels and responsiveness to standard stressors (Marchette et al., 2018; Miczek et al., 2001). Likewise, rat strains also differ in their baseline aggression levels as well as their responsiveness to social stressors (Berton et al., 1997; Pardon et al., 2002). To date, studies of rat VBS have largely focused on using the Long-Evans strain (McEwen et al., 2015) but notably strain differences in the effects of VBS housing on social stress have been observed. Rats of the Wildtype Groningen (WTG) strain also form hierarchies in the VBS, and subordinates show the characteristic loss of body weight and increased bite wounds during group housing (Buwalda et al., 2017). Despite this, dominant and subordinate WTG rats do not show any differences in HPA reactivity indicating the importance of considering strain differences. There is also evidence that the effects of social stress may be greater in wild rats than in laboratory rats. For instance, just one social defeat can lead to rapid death in rats even without signs of physical injury (Barnett, 1958a; Koolhaas et al., 1997). In sum, strain is an important consideration when interpreting the short and long-term effects of social stress in the VBS.
The timing of measures of HPA function during group housing is an important consideration when evaluating effects on stress responsivity. Both dominant and subordinate male mice living in VBS have higher plasma corticosterone levels than pair-housed animals, with levels rising up to two weeks after group housing (Ely and Henry, 1978; Williamson et al., 2017a), as well as heavier adrenals than individually housed mice (Benton et al., 1978). Similarly, dominant and subordinate VBS housed rats also show higher plasma corticosterone, adrenal hypertrophy, reduced CBG and thymic atrophy compared to standard housed males (Albeck et al., 1997; Blanchard et al., 1995; McKittrick et al., 1995; Spencer et al., 1996; Tamashiro et al., 2005). In both species, plasma corticosterone levels begin to decline after a few weeks corresponding with hierarchy stabilization and a reduction in the frequency of patrolling behavior by dominants, suggesting that differences in HPA axis activity are likely to be maximal when there are high periods of inter-male competition (Ely and Henry, 1978; McEwen et al., 2015; Williamson et al., 2017a). These data also suggest that ongoing long-term social interaction with multiple social partners may be stressful for all individuals not just subordinates, with the intensity of this stress decreasing over time.
Differential social stress experienced by dominant and subordinate animals in VBS also impacts other physiological systems. One of the most profound effects of living in the VBS for rats is that they lose 10–15% of their body weight (Blanchard et al., 1995; Melhorn et al., 2010; Nguyen et al., 2007a; Tamashiro et al., 2007a, 2004). Both dominants and subordinates lose adipose tissue in the VBS and subordinates also lose lean body mass (Melhorn et al., 2010; Nguyen et al., 2007b; Tamashiro et al., 2007a, 2007b, 2004). Both dominants and subordinates show reduced food intake during initial VBS housing, with dominants returning to basal levels of intake rapidly while subordinates continue to have fewer and smaller bouts of food intake (Melhorn et al., 2010). Moreover, subordinates have lower levels of leptin and insulin than dominants in the VBS, becoming hyperinsulinemic and hyperleptinemic when taken out of the VBS and given regular food (Tamashiro et al., 2007b). This is suggestive of the social stress experienced by subordinates leading to them expressing a phenotype akin to metabolic syndrome.
There is strong evidence from social defeat, resident-intruder and other dyadic interactions paradigms that social stress can negatively impact immune function (Takahashi et al., 2018). In these paradigms, subordinates tend to have hyperactive and dysregulated immune responses and show slower wound healing than dominants. Less is known about the immune functioning of animals housed in VBS, although subordinate VBS rats do have higher spleen weights (Blanchard et al., 1995; Tamashiro et al., 2004) and fewer available splenic glucocorticoid receptors (Spencer et al., 1996) than dominants VBS rats suggesting that subordinates may have compromised immune systems. Colony-housed subordinate male rats also have reduced CD4 and CD8 T cell as well as B cell numbers in their plasma than pair-housed rats (Stefanski et al., 2001). Mice housed in groups of three to eight in large group housing also show higher spleen weights compared to standard housed animals, with the effect being most prominent in subordinates (Turney and Harmsen, 1984; Van Loo et al., 2001). These findings are suggestive that differential social stress experienced by dominant and subordinate animals living in VBS may lead to individual differences in immune functioning, but more work is needed to determine this possibility.
2.4. Individual Differences in Stress Responsivity in Groups
Unlike several stress paradigms that emphasize controlled exposures to known stressors (e.g. restraint stress, chronic variable stress, social defeat stress) (Herman and Tamashiro, 2017), clearly the social stress exposure from living in VBS is not equivalent for all individuals. Depending upon the social context (e.g. single sex versus mixed-sex housing, number of individuals, hierarchy formation, stable versus unstable, despotic versus egalitarian individuals), different individuals are subjected to different social pressures.VBS provides an opportunity to understand how these contextual factors affect individual differences in response to stress. We have already described how the most dominant and most subordinate individuals show long-term changes in various aspects of their stress physiology; however, it is also possible to delineate further categories of animals by their social status in the VBS and examine their phenotypic plasticity.
In the rat VBS, subordinate males can be further subdivided into different subgroups. In each VBS, there is usually one subordinate that receives a disproportionately high number of attacks and wounds without being aggressive and is often referred to as the omega (Melhorn et al., 2017). These animals may even show complete social avoidance and become social outcasts (Koolhaas and Bohus, 1989). Omega males are also those who have the most dramatic body weight and lean mass reduction in the group (Melhorn et al., 2017). Dramatic changes to stress responsivity are also observed in the most subordinate males. Although they show no differences to other animals in stress response prior to hierarchy formation, following two weeks of VBS housing they display completely blunted stress responses including not showing any increase in corticosterone secretion in response to acute restraint stress (Albeck et al., 1997; Blanchard et al., 1993; McKittrick et al., 1995). Compared to other subordinates, these non-responding subordinates also have lower plasma CBG and insulin (McEwen et al., 2015; Spencer et al., 1996; Tamashiro et al., 2005) and greatly reduced CRF expression in the PVN (Albeck et al., 1997). This phenotype suggests that these subordinate rats experience chronically high levels of social stress, akin to mice who are repeatedly socially defeated and also exhibit hypocorticism (Reber et al., 2007).
Whereas the most subordinate animals undergo a complete loss of control, sub-dominant animals are tasked with having to maintain their social position above subordinates but are unable to exert control over dominant alpha individuals. Although all alpha males dominate colony life, the degree to which alphas do this varies with some being heavily despotic and others relatively more tolerant (Fokkema et al., 1995; Williamson et al., 2016b). These differences may underlie social rank effects on blood pressure. In VBS mice, the highest basal blood pressure levels is found in sub-dominants, followed by dominants and then by subordinates (Ely, 1981; Henry et al., 1986). Concomitantly, sub-dominants have activated sympathetic nervous systems compared to subordinates (Ely, 1981; Henry et al., 1986). In VBS rats, those individuals with the most elevated blood pressure are those subordinates engaged in the most aggressive behavior attempting to rise up the social hierarchy (Fokkema et al., 1995; Koolhaas and Bohus, 1989). Taken together, these data suggest that individuals of different social ranks do experience different forms of social stress at different times during colony housing, with differential consequences for their behavior and stress physiology. Further, although these findings from rats and mice indicate important differences in social stress exposures between ranks, these effects are likely to be species-specific and related to the types of hierarchy that form in each species (e.g. see section 4.2 for mole-rats). For instance, in primates low social rank appears to be associated with increased stress responsivity only in species where subordinates experience high rates of aggression and low social support (Abbott et al., 2003).
2.5. Adaptive Responses to Social Living in VBS
As hierarchies form, dominant, sub-dominant and subordinate animals all experience increases in social stress and undergo dramatic phenotypic changes. It is important to consider whether the behavioral and physiological changes observed in the VBS are adaptive or maladaptive. Not all changes observed are likely to be the result of chronic social stress, but rather are plastic changes induced by mild stress that shape an individual’s phenotype to more appropriately adapt to its current social context. For example, in the mouse VBS, dominant alpha males not only increase their aggressive and patrolling behavior (Williamson et al., 2016b), but also increase their eating and drinking frequency (Lee et al., 2018) to keep up with the energetic demands of producing and urinating major urinary proteins that are used to scent mark their territory (Lee et al., 2017). Conversely, subordinate mice inhibit their urination as has been previously shown for subordinate mice undergoing social defeat or other dyadic social stressors (Hou et al., 2016; Wood et al., 2009). VBS alpha male mice also show dysregulated circadian rhythmicity, with increased feeding and reduced quiescence during the light phase of the light cycle (Lee et al., 2018). Dominants exhibit increased sleep fragmentation and reduced REM sleep during the light phase but increased REM sleep during the dark phase (Karamihalev et al., 2019). Similarly, rats in the VBS rats also alter their feeding rhythmicity in stable hierarchies, with subordinates eating fewer meals compared to dominants as well as shifting the timing and location of their meals (Melhorn et al., 2010).
Changes in neural plasticity in VBS rats may also represent stress-induced adaptive responses to group living. Both dominant and subordinate rats show changes in neuronal morphology in hippocampal CA3 pyramidal neurons when housed in the VBS compared to controls (McKittrick et al., 2000).VBS animals show decreases in dendritic branching and dendritic length with these changes being most pronounced in dominants. Further, dominant male rats living in single-sex VBS have increased levels of new neurons in the dentate gyrus compared to subordinate males, although the levels of social stress experienced in single-sex rat VBS are likely much lower than those experienced in mixed-sex VBS (Kozorovitskiy and Gould, 2004). Dominant male mice living in VBS have reduced hippocampal expression of DNA (cytosine-5)-methyltransferases (Williamson et al., 2016a). These enzymes promote DNA methylation, suggesting that more dominant animals have generally increased hippocampal gene expression while more subordinate animals have a social suppression of gene expression. There are also several other differences in central gene expression of hormones and neurotransmitters and their receptors between dominant and subordinate rats and mice of both sexes which may be related to plastic changes in other social, emotional or cognitive behaviors (Albeck et al., 1997; McEwen et al., 2015; So et al., 2015; Williamson et al., 2017b).
The fundamental feature of social hierarchies is that more dominant animals have priority access to resources such as territory, food and mates. Although there is not a complete reproductive suppression of subordinates in either mice or rats as is seen in the social hierarchies of other species (Creel, 2001; but also see naked mole-rats described in section 4.1), dominant male mice and rats do have higher mating success (Berdoy and Drickamer, 2007). There is some evidence that these differences may be due in part to differential regulation of the HPG axis. Subordinate VBS males typically have lower plasma testosterone, smaller testes weights and reduced preputial glands (an androgen-dependent pheromone secreting organ required for scent-marking) than dominant males (Dijkstra et al., 1992; Hardy et al., 2002; Nguyen et al., 2007b; Tamashiro et al., 2004). Subordinate rats also have reduced 11βHSD protein levels and activity meaning that their Leydig cells are less protected from glucocorticoids and potentially less responsive to luteinizing hormone (Hardy et al., 2002; Monder et al., 1994). Testosterone levels are also reduced in more subordinate VBS males, with these differences being maximal when there is hierarchy disruption as these rank differences are not consistently observed in established stable hierarchies (Ely, 1981; Machida et al., 1981; Van Loo et al., 2001; Williamson et al., 2017b, 2017a). In mice housed in small groups there is also evidence that dominant animals have increased testes weights (Bronson and Eleftheriou, 1964; McKinney and Desjardins, 1973), sperm motility (Koyama and Kamimura, 2003, 1998), and preputial glands sizes (Brain et al., 1983; Bronson, 1973; Bronson and Marsden, 1973) indicating up-regulated HPG activity in dominant animals. Taken together, it is clear that dominant and subordinate animals do shift their reproductive physiology in response to their differential social experiences, but the extent to which these changes are dependent or independent upon ongoing stress exposure in the VBS remains to be more fully determined.
2.6. Social context and social stress in the VBS
Mice and rats living in the wild likely experience fluctuation in social stress depending upon various social and ecological factors. For example, although hierarchies tend to be stable in the wild, they can become destabilized through aging, loss of individuals due to death or predation, or by group composition changes (Barnett, 1958b; Crowcroft, 1966). An advantage of employing the VBS approach in the laboratory is that ethologically relevant social stressors that mirror these situations can be experimentally induced and controlled.
It is already clear that social stress is not consistent across time in VBS with it being highest during initial group formation and declining as hierarchies stabilize, but even formed hierarchies vary in the degree of social stress. Some hierarchies contain exceptionally despotic alpha individuals, as described above, that control the behavior of all other individuals in the group (Blanchard and Blanchard, 1990; Curley, 2016), whereas other hierarchies have ongoing fluctuations in social ranks (Williamson et al., 2016b). It remains to be determined how this natural variation is related to stress physiology and other phenotypic outcomes. However, it is possible to experimentally induce social instability in an ethologically relevant manner into social hierarchies in the VBS.
One way this can be achieved is by varying the initial group composition of animals. Individuals and strains vary in their baseline levels of aggression and social behaviors, which can have long-term consequences for group level behavior (Buwalda et al., 2017; Davis et al., 2009; Ely and Henry, 1978; McEwen et al., 2015). In rat VBS, manipulating the number or ratio of males to females leads to different phenotypic outcomes. Subordinate males in 2 males:4 females groups receive higher levels of aggression even from females and show significantly exaggerated stress phenotypes than subordinate males in 4 males:2 females groups (Tamashiro et al., 2004).
In mice, removing the most dominant alpha male from a group leads to increased aggression in all group members within minutes, with the beta male displaying the highest levels and eventually taking over as the alpha male (Williamson et al., 2017b). This mirrors observations in nature that sub-dominant males are primed to form their own territory and capitalize on such social opportunities (Berdoy and Drickamer, 2007; Crowcroft, 1966). This form of social instability is associated with increases in central gene expression and neuronal activity (Williamson et al., 2019a, 2017b), suggesting it could be a useful ethologically valid paradigm for studying the effects of acute social stress.
Switching animals between VBS hierarchies mimics the behavioral disruption that occurs in nature as animals move between social groups and is potentially a more dramatic social stressor. Rat VBS colonies that had males transferred between them only once a month showed increased aggression and an increase in hypertension compared to stable colonies (Henry J P et al., 1993). Further, males of all ranks living in single-sex rat VBS that have dominant males switched between groups show reduced numbers of new neurons in the hippocampus and social inhibition compared to males from stable hierarchies (Opendak et al., 2016). There is also evidence that social instability may be an even more profound stressor than status in females compared to male (Haller et al., 1999). Little is known regarding the effects of social instability induced by disrupting the hierarchy in mice VBS. However, disrupting groups of four male or four female mice in standard cages twice a week for seven weeks is chronically stressful, as evidenced by increased corticosterone levels and adrenal weights and reduced thymus weight and hippocampal corticosteroid receptors in these animals compared to non-disrupted controls (Schmidt et al., 2010, 2007; Sterlemann et al., 2008). These findings suggest that social instability induction in rat and mice VBS are robust and valid paradigms for studying social stress.
2.7. Conclusions and Future Directions
Housing mice and rats in VBS provides an ethologically relevant housing system for studying the short and long-term effects of social stress on behavior and physiology. Although the social stress experienced by animals in the VBS is not as easily controlled nor as intense as stress in other paradigms, there are clear advantages to the VBS paradigm. The VBS is an excellent paradigm for investigating individual differences in stress response related to naturalistic stressors that are in the species typical range given each species’ natural ecology. All individuals experience high social stress as groups form, but as distinct social ranks emerge, dominant, sub-dominant, and subordinate animals all have different behavioral priorities. Dominants aim to monopolize resources and defend their territories from intruders, sub-dominants aim to maintain their social status whilst being ready to take over as alpha male should the opportunity arise, and subordinates aim to avoid being continuously attacked by more dominant individuals. If hierarchies remain stable, so do the social statuses and behavior of individuals, but if the composition of hierarchies changes, then individuals of different statuses must respond rapidly and appropriately. Changes in stress responsivity, metabolism, cardiovascular and immunological function are commonly related to individual differences in rank and behavior in both mice and rats.
It still remains to be precisely determined which of these changes are maladaptive dysfunctions induced by chronic stress exposure, or plastic changes that are perhaps induced by acute stress exposure and benefit individuals to adapt to their current social status in the short-term (Kozorovitskiy and Gould, 2004; McEwen et al., 2015). Further, the vast majority of research in the VBS has focused on stress responsivity and metabolic phenotypes, and much more research is needed to more fully understand the nature of those immunological, cardiovascular, and neural outcomes that are impacted by VBS social stress. However, with the advent of video capture methods capable of tracking multiple animals in large arenas, as well as minimally invasive wireless methods for the long-term capture of physiological data, it is likely that there will be increased interest in these questions (Peleh et al., 2019; Plank et al., 2016; Redfern et al., 2017). Another area of research that is relatively little understood is how social stress impacts the formation of social hierarchies in the VBS, although studies in dyads suggest that stress may actually facilitate dominance-subordinate status in rat dyads (Cordero and Sandi, 2007; Timmer et al., 2011; see also section 3.3 in voles). Finally, both males and females living in large social groups form social hierarchies (Calhoun, 1963; Williamson et al., 2019b), yet nearly all research in VBS mice and rats has focused on male subjects. There needs to be a greatly increased focus on the social stressors experienced by females during group living and how this impacts their behavioral and physiological development, as well as how both male and female mice are affected by social stressors in mixed-sex versus single-sex VBS housing.
3. Stress and social behavior in voles
In North America, voles of the genus Microtus have been studied for fluctuations in population density for nearly a century. As researchers examined the factors that might influence population size, they discovered interesting and varied social behaviors between and among species, from seasonal variation in social nesting in meadow voles and taiga voles to the occurrence of social monogamy in prairie voles (Getz et al., 1981; Madison, 1980; Wolff and Lidicker Jr., 1980). Field studies of social behavior are ongoing (e.g., Edwards et al., 2019; Ophir et al., 2008b; Sabol et al., 2018; Solomon et al., 2009). Prairie voles have primarily become popular subjects of social neuroscience research in the laboratory, because they are one of only a small fraction of rodent species to exhibit reproductive pair-bonds. Studies of additional monogamous vole species (e.g. pine voles and Taiwan voles), as well as contrasts to promiscuous species, have contributed to our understanding of how monogamy is supported within a taxon (Fink et al., 2006; Gersten, 2008; Young, 1999).
Meadow voles have become important for understanding the mechanisms underlying group living (a.k.a. sociality), and how these compare to mechanisms involved in mate partnerships. While meadow and prairie voles have been contrasted with each other on the basis of mating system, they both share an unusual trait in exhibiting social preferences for familiar vs. unfamiliar “peers” (shorthand for same-sex age-matched conspecifics) (DeVries et al., 1997; Lee et al., 2019; Parker et al., 2001; Parker and Lee, 2003) —a behavior not typically shared by mice, rats, degus, or other social rodents tested to date (Beery et al., 2018; Schweinfurth et al., 2017). The presence of selective preferences for known peers and mates in voles provides an opportunity to better understand the role of familiarity and relationship type in the context of stress. For example, how does isolation from a bonded mate differ from isolation from a familiar peer? Following stress, does consolation behavior by a cage-mate differ by familiarity and sex? How does the experience of a stressor alter the formation of new social preferences for mates or peers? To contextualize such questions, it is helpful to first consider the ecology and behavior of these vole species in the wild in greater detail.
3.1. Prairie vole behavior: from field to lab
Prairie voles are native to grassland prairies across the Midwestern United States and into Canada (Stalling, 1990). This species has been of interest to behavioral neuroscientists since the discovery that in the wild, prairie voles often form long-term associations with mates (Getz et al., 1981)(Getz et al., 1981). In these “socially monogamous” relationships, females and males form long-term stable pair-bonds, and both sires and dams exhibit parental care, although extra-pair copulations do occur (hence not genetic monogamy) (Getz et al., 1981; Ophir et al., 2008a). Across and within populations, males exhibit a range of mating tactics, with a majority of socially monogamous “residents” as well as a smaller but significant proportion of non-pair-bonded “wanderers” (Getz et al., 1993; McGuire and Getz, 2010; Ophir et al., 2008b). Prairie voles exhibit greater polygyny in some habitats, such as more xeric regions of Kansas (Fitch, 1957), and these voles exhibit reduced paternal care when brought into the laboratory (Roberts et al., 1998). Variation of behavior with local ecology has also been demonstrated in other studies (Mabry et al., 2011).
The propensity to display social monogamy is recapitulated in the laboratory, as prairie voles form selective, enduring preferences for their opposite sex cage-mates over strangers in extended tests of social choice known as partner preference tests (Williams et al., 1992; Fig 1). In this test, a focal vole is paired with a potential mate for a period of time that is either sufficient (e.g. 24 hours) or insufficient (e.g. 6 hours) for bond formation under normal conditions. Following cohabitation, voles are tested for social preferences in a three-chambered apparatus in which both their partner and a sex-matched stranger vole are tethered at opposite ends; the test is recorded for three hours to allow for stable differences in resting social behavior (huddling) to appear (Williams et al., 1992; Winslow et al., 1993). This test has been used to explore the effects of myriad brain-region-specific manipulations of neuropeptides and neurotransmitters on the formation of social bonds.
3.2. Meadow vole behavior: from field to lab
Meadow voles are a closely related species that is sometimes sympatric with prairie voles, but their range extends into more Eastern grasslands, throughout Canada and into Alaska (Reich, 1981). Meadow voles have often been used as a contrast to prairie voles in comparative studies because they exhibit a promiscuous mating system (Boonstra et al., 1993; Getz, 1972). However, meadow voles are not asocial as sometimes described; they exhibit pronounced and well-described seasonal variation in sociality (reviewed in Beery, 2019). In summer months, female meadow voles are highly territorial and maintain home-ranges that do not overlap with other females, while male territories encompass those of multiple females (Edwards et al., 2019; Madison and Mcshea, 1987). In winter months, these territories collapse and voles begin to live and sleep in mixed-sex groups of 3–10 individuals (Madison and Mcshea, 1987). Field tests of social interactions reveal that meadow voles of both sexes are tolerant of both nest-mates and strangers in winter months, but by spring, gonadal development coincides with an increase in aggression towards strangers (McShea, 1990). Spring social groups may remain together through the first matings of the season, but they are closed to the immigration of new members (Madison and Mcshea, 1987; McShea, 1990). Because life in social groups is typically based on relationships between peers, winter sociality in meadow voles provides a useful opportunity to examine the role of stress and anxiety in seasonal transitions in group structure.
The transition from social to solitary is principally mediated by photoperiod, and manipulations of photoperiod mimic the effects of season in the field, from olfactory preferences to huddling behavior and tolerance of unfamiliar individuals (Beery et al., 2008; Ferkin and Kile, 1996; Ferkin and Zucker, 1991; Lee et al., 2019; Ondrasek et al., 2015). Voles housed in short, winter-like day lengths (short days) show strong preferences for huddling with conspecifics (Beery et al., 2009; Beery and Zucker, 2011; Parker and Lee, 2003), and can be used to probe the neurobiological pathways underlying social tolerance and life in groups (Anacker and Beery, 2013; Beery, 2019). These changes in social and aggressive behavior are most pronounced in females, both in the field and in the laboratory (Beery et al., 2009; Boonstra et al., 1993).
3.3. Effects of stress on relationship formation
The existence of familiarity preferences in voles allows for studies of how stress impacts the formation of specific relationships. In prairie voles, stress and HPA axis manipulations alter the propensity of forming a social preference for a potential mate. In females, stressful experience (a brief swim) prevented the formation of partner preference for the familiar male under conditions normally sufficient to produce a preference, but not in adrenalectomized voles (DeVries et al., 1996). Exogenous corticosterone was also capable of impairing pair-bond formation (DeVries et al., 1995), suggesting that corticosterone may mediate the effect of stress on social bonds. Interestingly, circulating corticosterone levels are naturally low immediately following cohousing with a new male, and partner preference formation occurs before they return to baseline (DeVries et al., 1995).
Similar effects of stress have been found in same-sex peer relationship formation in female meadow voles (housed in the short day lengths that promote group-living). A stressful experience (brief swim) significantly elevated corticosterone concentrations for 3 hours, and this stressor impaired the formation of a partner preference for a peer introduced immediately following the stressor. This effect was specific to the formation of relationships, as stress exposure did not impair expression of partner preferences by females in long-term cohoused pairs (Anacker et al., 2016). Thus, same-sex preferences in female meadow voles were altered in a similar manner to opposite-sex preferences in female prairie voles. Prairie voles also form same-sex preferences for peers (Beery et al., 2018; DeVries et al., 1997; Lee et al., 2019), but the role of stress in familiar peer preferences in this species has not yet been determined.
While it is tempting to conclude that stress impairs affiliation across vole species and relationship types, opposite effects of stress on social bonding were found in male prairie voles. Males typically take longer than 6h to form a partner preference for a female, but males who underwent a brief swim prior to pairing formed significant preferences after only 6h (DeVries et al., 1996). The formation of partner preferences in males was facilitated by corticosterone administration, and was impaired by adrenalectomy (Blondel and Phelps, 2016; DeVries et al., 1996). Some doses of central CRF administration also facilitated partner preference formation in males (DeVries et al., 2002).
Prairie voles thus provide a model for both stress facilitation of social bond formation, and impairment of affiliation, depending on sex. These differences in stress effects on social behavior provide an example of latent sex differences – when behavior (e.g. partner preferences) are outwardly similar, but are mediated by different mechanisms or are differently regulated by experience (Becker and Chartoff, 2019). Such differences underscore the importance of studying males and females (Beery, 2018; Beltz et al., 2019; Zucker and Beery, 2010).
3.4. Social isolation as a stressor
Social isolation is a well-described stressor in numerous rodent species (reviewed in Fone and Porkess, 2008; Mumtaz et al., 2018). Prairie voles show many isolation-related dysfunctions that highlight the importance of social relationships for this species as well. Extended social isolation from weaning in prairie voles has been associated with higher circulating corticosterone, and greater CRF immunoreactivity in the paraventricular nucleus (PVN) of the hypothalamus (Ruscio et al., 2007). In instances where separation occurs in previously paired animals, prairie voles can be used to study the effects of peer loss, mate loss, and their differences (although to date most studies have studied these separately).
In prairie voles reared with a same-sex sibling, extended (2 week to 2 month) separation from their cage-mate in adulthood has been associated with changes in neuroendocrine signaling, including increased plasma levels of corticosterone, ACTH, and oxytocin, altered expression of the immediate early gene c-Fos, and heightened activity of hypothalamic oxytocin neurons after a resident-intruder test (Grippo et al., 2007; Stowe et al., 2005). These changes co-occur with behavioral changes including altered exploratory behavior in the elevated plus maze, decreased sucrose preference (a measure of anhedonia), and increased immobility in the forced swim test (used as a measure of depressive behavior) (Grippo et al., 2008, 2007; Stowe et al., 2005). Separation from a sibling cage-mate has also been associated with higher heart rate, as in mice (Grippo et al., 2007; Späni et al., 2003), as well as changes in cardiac function associated with cardiovascular disease (Grippo et al., 2011; Peuler et al., 2012). This is also similar to findings in humans, for whom social support reduces heart rate and alters blood pressure following stressful tasks (Lepore et al., 1993; Thorsteinsson et al., 1998). Some of the physiological and behavioral sequelae of separation in prairie voles were prevented or reduced by oxytocin administration and environmental enrichment (Grippo et al., 2014, 2009), similar to findings in rats (Hellemans et al., 2004).
In prairie voles that have been paired with an opposite-sex mate, mate loss also leads to a host of endocrine, autonomic, and behavioral consequences. In both sexes, <1 week separation from a mate led to increased corticosterone and ACTH secretion, as well as increased depressive behavior (Bosch et al., 2009; McNeal et al., 2014). Examination of the effects of separation on heart rate in males revealed that isolation from a mate led to increases in heart rate, including during the forced swim test (McNeal et al., 2014).
The effects of mate- and peer-separation in prairie voles thus seem overtly similar, although changes in heart rate occurred more rapidly in males experiencing mate loss than peer loss (after 5 days vs. 2–4 weeks of separation), suggesting there may also be some differences (Grippo et al., 2007; McNeal et al., 2014). In the one study that directly compared peer and mate loss, brief separation from a mate but not a same-sex sibling resulted in increased circulating corticosterone, increased adrenal weight, and depressive-like behavior (Bosch et al., 2009). Differences in either the time-line or degree of dysfunction following separation from mates versus peers are therefore likely.
The effects of social isolation are less well understood in meadow voles. One study in long-day-housed male meadow voles found no effect of 24h or 2 week social isolation on corticosterone or behavior in the elevated plus maze, but meadow voles separated for 24h from a sibling cage-mate had increased Fos protein in several brain regions relative to controls (Stowe et al., 2005). More information is needed on the effects of social isolation in meadow voles, particularly comparing short- and long-day housing paradigms.
3.5. Social buffering of the stress response
Whenever isolation acts as a stressor, the converse is that social cohabitation can lead to improved outcomes in the face of stress, or social buffering of the stress response. Studies across a wide variety of mammals show profound effects of cohabitation on the recovery from stressors (Beery and Kaufer, 2015). As might be expected, these effects are pronounced in species that naturally cohabit with adult conspecifics in the wild. For example, social facilitation of wound healing by pair-housing occurs in socially monogamous but not in solitary Peromyscus species (Glasper and DeVries, 2005). Prairie voles have provided insight into multiple aspects of social buffering, again allowing for distinctions between familiar and unfamiliar conspecifics, as well as reproductive partners vs. familiar social peers.
The presence of a mated partner may provide social buffering from a stressor. In the first study to demonstrate this, female prairie voles were subjected to immobilization stress for 1 hour, and recovered alone or with their bonded male partner. Females recovering alone exhibited high levels of corticosterone and increased anxiety behavior, while females recovering with their male partner showed no such elevations (Smith and Wang, 2014). Microdialysis of oxytocin in the PVN of the hypothalamus revealed that oxytocin was elevated during stress, and remained elevated longer while recovering with a partner. Furthermore, administration of an oxytocin antagonist was sufficient to prevent the social buffering effect of cohousing on anxiety behavior in stressed, paired voles, and oxytocin reduced the anxiety behaviors of animals recovering from stress alone (Smith and Wang, 2014). These findings suggest that oxytocin is both necessary for social buffering, and sufficient to mimic its effects.
In another major study of social buffering in prairie voles, researchers assessed the effect of a stressor (foot-shocks paired with tones) on the behavior of stressed voles, as well as their pair-bonded mates who were not present for the stressor (Burkett et al., 2016). “Observer” voles of both sexes altered their behavior when reunited with their stressed partner “demonstrator” (but not a briefly separated unstressed partner) in the form of more rapid-onset and more extended allogrooming relative to baseline. No such increase in grooming occurred upon pairing with an unfamiliar opposite-sex conspecific. Observer voles also matched the state of stressed demonstrator voles, illustrated by correlations in circulating corticosterone levels between observers and demonstrators, and coordinated freezing to a tone in both animals (including the observer who did not receive conditioning). Observers also had elevated Fos production in the anterior cingulate cortex: a region linked to empathy in humans (Lamm et al., 2011). Injections of oxytocin receptor antagonist to this region prior to the task prevented increases in allogrooming (Burkett et al., 2016). Together, these two studies highlight that aspects of empathy-like behavior such as consolation and social contagion, as well as the role of specific brain regions and neurochemicals in these processes, can be found in wide-ranging social species.
Social buffering has not been explored in meadow voles housed in the short day lengths that coincide with group-living. However, male meadow voles housed in long day lengths do not alter their allogrooming with the stress state of a female mate/demonstrator—unlike prairie voles, but consistent with their solitary habits in summer (Burkett et al., 2016).
3.6. Seasonal changes in anxiety, corticosterone, and group living in meadow voles
As described in section 3.2, day length is the primary cue driving seasonal changes in behavior in meadow voles. Photoperiod can therefore be used to manipulate social behavior and examine the neural pathways underlying the seasonal change in sociality. Females housed in winter-like short days (versus in summer-like long days) exhibit higher huddling times, prefer huddling in larger groups, and are more socially interactive with and less aggressive toward strangers (Beery et al., 2008; Lee et al., 2019; Ondrasek et al., 2015). These changes in behavior coincide with variations in neural and endocrine signaling, some of which can mediate changes in social behavior (reviewed in Beery, 2019). Unlike prairie vole mate relationships, meadow vole familiarity preferences appear to be less about social reward (Goodwin et al., 2019), and more about social tolerance, which increases in short day lengths (Beery et al., 2008; Lee et al., 2019). Mounting evidence suggests that this tolerance is shaped by seasonal changes in anxiety and HPA axis regulation.
Anxiety-like behaviors are lower in meadow voles housed in short day lengths versus long day lengths. Short day-housed meadow voles spent more time in the light portion of a light-dark box, and this effect was especially notable in females (who undergo more dramatic seasonal change in social behaviors) (Ossenkopp et al., 2005). Short day meadow vole females were also more active and spent more time in the center of an open field (Reitz, 2014); open field exploration also differed by season in field-caught males (Turner et al., 1983). Investigation of novel conspecifics in the social interaction test was first described as a measure of anxiety (File and Seth, 2003), and female meadow voles housed in short day lengths are more interactive in this test (Lee et al., 2019). Collectively, these results reveal a pattern of increased exploratory behavior and reduced avoidance behavior in short day lengths.
Meadow voles also exhibit several seasonal and photoperiodic changes in HPA axis regulation. Corticosterone varies seasonally and with reproductive status in the field (Boonstra and Boag, 1992; Galea and McEwen, 1999), and with day length in the laboratory (Anacker et al., 2016; Pyter et al., 2005). In addition, day length-dependent variation in corticosterone binding globulin may mediate photoperiodic changes in circulating free versus total corticosterone (Beery lab; unpublished data), contributing to increased free corticosterone in short day-housed females (Anacker et al., 2016). Further up the HPA axis, CRF/urocortin pathways vary with photoperiod and may form links between anxiety and social behavior. CRF receptors were measured in female meadow voles across day lengths; in multiple brain regions, CRF2 receptors were upregulated in short day lengths, while CRF1 receptors were downregulated (Beery et al., 2014). These opposing changes, sometimes even within the same brain region, are consistent with opposing roles of these receptors, and also with decreases in behavioral anxiety (Bale and Vale, 2004). Individual differences in receptor densities in the lateral septum (increased CRF1 and decreased CRF2 receptor binding) were correlated with the amount of time spent huddling during partner preference tests, further underscoring the connection between HPA axis regulation and social behavior (Beery et al., 2014). CRF production is linked to estradiol exposure (Beery et al., 2014; Haas and George, 1989; Vamvakopoulos and Chrousos, 1993), and estradiol varies seasonally in meadow voles (Galea and McEwen, 1999), potentially mediating the connection between photoperiod and CRF distribution in the brain.
In summary, female meadow voles undergo photoperiod-induced variations in anxiety, HPA-axis regulation, and social behaviors underlying group living. Multiple aspects of HPA-axis regulation predict individual variation in social behavior, and presentation of an exogenous stressor impairs the formation of peer partner preferences (Anacker et al., 2016). The presence of strong preferences for huddling with familiar conspecifics (e.g., Beery et al., 2009; Lee et al., 2019) coupled with evidence that meadow voles experience low social reward (Beery et al., 2019; Goodwin et al., 2019) suggest that seasonal changes in anxiety and the ability to tolerate other individuals without becoming territorial or stressed may be a necessary permissive factor to promote winter sociality.
3.7. Concluding remarks and future directions
Voles have become increasingly popular subjects in studies linking stress and social behavior. Studies of prairie voles have yielded insights about broadly conserved effects of isolation in social species, as well as specific effects of social buffering and consolation behavior from known partners. Meadow voles provide an opportunity to understand how stress and anxiety relate to intraspecific changes in group living. Research in voles has benefitted from comparative approaches both within and between species, similar to those discussed for mole-rats in section 4.0. The unusual tendency for both prairie voles and short-day housed meadow voles to form selective preferences for familiar individuals sets them apart from other social rodents investigated to date. The formation of these relationships is affected by stress and HPA axis signaling. Loss of these relationships has significant physiological and behavioral consequences in prairie voles, and mate loss may be a particularly potent stressor. Future studies may determine whether loss of peer-partners in meadow voles has similar effects.
Looking ahead, more direct comparisons between different relationship types will better inform our understanding of the nature of these relationships. For example: how does long-term loss of a mate compare to loss of a peer? Would re-pairing with a new partner compensate for loss? Are peer relationships in meadow voles similarly important to measures of health and wellbeing?
Additional benefits will come from studies of stress and social behavior in voles in more naturalistic, group-housed contexts. Unlike in sections 2 and 4 of this review, the studies described in this section were almost exclusively conducted in pair-housed (or separated) voles. We know from studies of meadow voles that short-day housed meadow voles form equivalently strong preferences for multiple familiar partners, and prefer to huddle in larger groups (Beery et al., 2009; Ondrasek et al., 2015). Field and semi-natural enclosure studies of both meadow and prairie voles are ongoing, and may shed light on these topics. Advances in automated tracking and monitoring technologies (as described in section 2.7) should also enable studies of voles housed in larger groups in the laboratory.
4. Stress and sociality in mole-rats
African mole-rats (family Bathyergidae) provide a powerful comparative opportunity to study the relationship between psychosocial stress and sociality in rodents. The family is comprised of at least 30 species in 6 genera that range across the entire spectrum of sociality (Bennett and Faulkes, 2000; Faulkes and Bennett, 2013). For example, both Cape mole-rats (Georychus capensis) and Highveld mole-rats (Cryptomys hottentotus pretoriae) are seasonal breeders, but Cape mole-rats live in solitary burrows whereas Highveld mole-rats live in small, transient familial groups (Bennett and Faulkes, 2000; Bennett and Jarvis, 1988a; Jarvis and Bennett, 1991; Moolman et al., 1998). Two species within the family are extremely social and meet the criteria for eusociality: naked mole-rats (Heterocephalus glaber) and Damaraland mole-rats (Fukomys damarensis, formerly Cryptomys damarensis) (Bennett and Jarvis, 1988b; Jarvis, 1981). Despite this rich comparative potential, surprisingly little is known about how activation of the HPA axis relates to sociality in this rodent family.
4.1. Natural ecology and sociality in African mole-rats
A unifying feature of the speciose Bathyergidae family is that all species spend their lives underground, inhabiting subterranean burrow systems. They range across most of the southern half of the African continent and can be found in diverse microclimates. They excavate their own burrows in a variety of soil types (e.g., sand vs. clay), all of which contain geophytes, which are the underground storage organs of plants. These serve as the major food source for all Bathyergid species, and all foraging takes place underground. Perhaps not surprisingly, there is a relationship between soil type and species sociality where the solitary species (genera Bathyergus, Georychus, and Heliophobius) are found in mesic (moderate moisture) regions where soil excavation is easier, and social species (genera Cryptomys, Fukomys, and Heterocephalus) are found in both mesic and xeric (minimal moisture) regions (reviewed in Bennett and Faulkes, 2000; Jarvis and Bennett, 1991). Across species, burrows are organized in a similar way with networks of tunnels at varying depths: foraging paths are more superficial and permanent transit tunnels are found deeper. Species also establish set nesting and toilet chambers, which are shared among colony members in the social species. The size and complexity of the burrow systems is variable and relates to the social organization of the species and the number of animals within the colony unit (reviewed in Bennett and Faulkes, 2000).
Eusociality has evolved independently at least twice in the social mole-rats (Allard and Honeycutt, 1992; Faulkes and Bennett, 2013; Jarvis and Bennett, 1993) with naked mole-rats and Damaraland mole-rats both exhibiting cooperative brood care, overlapping generations of adults, and division of reproductive labor. In both species, reproduction is restricted to a single reproductive female – often called the queen – and a very small number of reproductive male consorts (reviewed in Holmes et al., 2009). All other adults typically remain reproductively suppressed within the colony setting. While reproductive skew is a defining feature of eusociality, the degree of skew differs between eusocial mole-rat species. In the field, naked mole-rat colonies average 60–80 individuals with a maximum of approximately 300 whereas Damaraland mole-rat colonies average 16 individuals with a maximum of approximately 40. Perhaps related to species differences in degree, the reproductive skew in naked mole-rats and Damaraland mole-rats is thought to result from distinct mechanisms. The dominant control model posits that reproductive suppression is under the control of a dominant breeding individual via direct suppression of non-breeding animals (e.g., aggression). On the other hand, the self-restraint model does not involve aggression between breeding and non-breeding animals and reproductive suppression is a consequence of inbreeding avoidance. This has important implications for understanding stress processing in social mole-rats as the dominant control model is assumed to work via stress-induced suppression of reproductive physiology while the self-restraint model is not. Comparisons between naked mole-rats and Damaraland mole-rats will be particularly useful for understanding co-evolution between stress and sociality given that naked mole-rats are proposed to fit the dominant control model (Faulkes and Abbott, 1997) whereas Damaraland mole-rats fit the self-restraint model (Clarke et al., 2001).
4.2. Stress and reproductive suppression
In contrast to the classic hypothesis that stress inhibits reproduction, chronic psychosocial stress does not appear to underlie reproductive suppression in eusocial naked mole-rats, at least as indicated by cortisol. Substantial evidence across laboratories indicates that naked mole-rats reproductively activate during periods with simultaneous increases in circulating glucocorticoids. For example, female naked mole-rats competing for dominant breeding status following removal of their queen show increases in both cortisol and progesterone (Clarke and Faulkes, 1997). In a recent case study of queen succession in naked mole-rats (Medger et al., 2019), the new queen showed mating behavior indicating reproductive activation, yet had cortisol levels that were very high (~90 ng/ml) when still in olfactory contact with the original queen. Cortisol levels then dropped to ~40 ng/ml after the original queen was completely removed. Finally, increases in circulating gonadal steroids and gene expression of their receptors in brain are coincident with increased cortisol in animals removed from colony and paired with an opposite sex animal (Edwards et al., 2020; Swift-Gallant et al., 2015).
The absence of a relationship between cortisol and reproductive suppression is also true for Damaraland mole-rats. No differences in circulating cortisol are seen between breeding and non-breeding female Damaraland mole-rats (Molteno, 1999, as cited by Bennett, 2011; Clarke et al., 2001; Medger et al., 2018). When removed from colony, females show reduced cortisol when housed alone, increased cortisol when maintained in social contact with their previous colony mates, but no change in cortisol when kept in olfactory contact with the natal colony via soiled bedding (Clarke et al., 2001). Importantly, none of these groups showed elevated progesterone until they were introduced to an unfamiliar male (Clarke et al., 2001). Consistent with this, Young et al. (2010) found that urinary cortisol is higher in female Damaraland mole-rats during the wet season compared to the dry season, meaning that increased cortisol is seen concurrent with greatest sensitivity to a GnRH challenge. Collectively, these data indicate that removal from colony does not in and of itself trigger reproductive activation in female Damaraland mole-rats and confirm there is not a causal relationship between cortisol and reproductive suppression in this species. Ansell’s mole-rats (Fukomys anselli) may also be eusocial (Patzenhauerová et al., 2013; Sichilima et al., 2011) and appear to align with patterns of cortisol seen in Damaraland mole-rats. Specifically, no effect of breeding status on urinary cortisol was detected (Novikov et al., 2015) though, as in Damaraland mole-rats (Medger et al., 2018), cortisol levels were positively correlated with age.
While the evidence suggests cortisol is not the proximate mechanism directly controlling reproductive suppression in eusocial mole-rats, it is likely that some component of HPA axis signaling is involved, at least for naked mole-rats. For this species, sex and status differences are seen in various components of the HPA axis. For example, CRF1 receptor binding density is higher in subordinates across brain regions, and particularly in the piriform cortex and cortical amygdala (Beery et al., 2016). Sex differences were found for CRF2 receptors, where expression is higher in females both globally and in the cortical amygdala and lateral amygdalar nucleus (Beery et al., 2016). Furthermore, quantification of expression of HPA relevant genes in distinct brain regions reveals that the HPA axis is changing throughout the reproductive transition in a sex-specific way (Faykoo-Martinez et al., 2018). Specifically, Crhr2 mRNA in the nucleus accumbens was highest in male subordinates removed from colony and paired with an opposite sex animal for one month, but was higher in the arcuate nucleus (favoring males) and medial amygdala (favoring females) of in-colony subordinates. Glucocorticoid receptor (Nr3c1) mRNA expression was higher in hippocampus of subordinates vs paired animals and higher in male breeders vs male subordinates in the paraventricular nucleus. Here, it is important to acknowledge that group comparisons for asking questions about stress and reproductive suppression are potentially conflated by both social and non-social factors. For example, breeders and subordinates differ in terms of reproductive activation but also reproductive experience and social status. Similarly, the opposite sex paired animals are indeed reproductively activating, and thus serve as a transitional group between subordinates and breeders, but they are in a novel environment and also comparatively socially impoverished. Thus, experimental groups differ on more than one variable, discussed more below, which needs to be considered when interpreting how HPA signaling is or is not related to reproductive suppression.
4.3. Social status and colony stability
The extent to which HPA function is associated with social status or reproductive status (or both) can be difficult to determine because these are intertwined in eusocial species. Naked and Damaraland mole-rats offer an opportunity to at least partially tease these apart because of their extreme reproductive skew and stable linear dominance hierarchies (Clarke and Faulkes, 1997; Jacobs et al., 1991). Their reproductive skew means that very few members of the social group actually achieve reproductive status; removing these animals from analyses and focusing on the hierarchy within non-reproductive members of the colony allows examination of social status independent of reproduction.
In naked mole-rats, most evidence to date suggests that no significant relationship exists between psychosocial stress and social status. Measuring urinary cortisol in established captive colonies, Clarke and Faulkes found no relationship between cortisol and dominance rank in three colonies (Clarke and Faulkes, 1998, 1997). Similarly, no relationship between cortisol and rank was detected using fecal cortisol metabolites in six established captive colonies in the Holmes laboratory (Edwards et al., 2020). Although the queens are the most aggressive animals in a naked mole-rat colony, subordinate cortisol levels are not clearly associated with rate of queen aggressive behavior (Clarke and Faulkes, 2001; Edwards et al., 2020). Clarke and Faulkes, however, detected a modest but significant negative correlation between cortisol and dominance rank (i.e., higher cortisol in more dominant animals) of both sexes in one captive colony (Clarke and Faulkes, 1997). Subordinates in this particular colony had increased cortisol following queen removal and this was also the only colony in which more than one female tried to take over breeding status. Medger et al. (2019) reported that plasma cortisol was approximately 70 ng/ml in subordinates after queen removal and during a period of fighting, with levels decreasing to approximately 40–45 ng/ml after the new queen was established. This pattern of results might reflect colony variation in social stability where no relationship between cortisol and status is seen in stable colonies but cortisol increases in animals anticipating or participating in competition for dominant breeding status.
There is no direct examination of cortisol and social status independent of reproductive status in Damaraland mole-rats. In three captive colonies of Damaraland mole-rats, no significant relationship between urinary cortisol and reproductive status was seen in females, although the queen had either the lowest or next to lowest level (Clarke et al., 2001). As with naked mole-rats, there is some evidence for social stability as a mediator of the relationship between status and stress. When subordinate females were removed from colony but kept in social contact with other non-breeding members of their natal colony, they had increased cortisol and increased aggression, which was not seen in animals exposed to soiled bedding from the colony, suggesting that the unpredictable social contact with familiar animals was serving as a psychosocial stressor. Similarly, (Medger et al., 2018) measured cortisol metabolites in feces of wild caught Damaraland mole-rats and did not see a significant effect of reproductive status in females, which they propose was due to stable colonies that were measured in the dry season.
Increased cortisol in mole-rats living in unstable colonies, particularly in individuals competing for dominance, might reflect a requirement of cortisol and associated behavioral arousal for ascending to breeding status. However, because cortisol is increased in animals experimentally removed from colony (i.e., reproductively activating in the absence of competition), it may also be the case that cortisol serves a physiological role associated with reproductive maturation and/or living outside of the colony. For example, naked mole-rats are poikilothermic and regulate their body temperature by huddling with colony mates (Buffenstein and Yahav, 1991). Animals living in isolation or with a single partner may face challenges with thermoregulation and energy balance that result in increased cortisol. Additional complexity for understanding the relationship between social status and stress in eusocial mole-rats comes from the fact that both naked mole-rats and Damaraland mole-rats show neuroanatomical differences associated with social and/or reproductive status. Notably, male and female breeders in both species have significantly larger regional volume of the paraventricular nucleus (Anyan et al., 2011; Holmes et al., 2007) and, at least in naked mole-rats, the increase in volume is triggered by removal from colony and not breeding per se (Holmes et al., 2011). Also, both species show status differences in adult hippocampal neurogenesis with breeders having lower levels of neurogenesis compared to subordinates (Oosthuizen and Amrein, 2016; Peragine et al., 2014). In contrast, subordinate male rats housed in VBS show reduced hippocampal neurogenesis relative to dominants (Kozorovitskiy and Gould, 2004), which has been attributed to the stress associated with social subordination. The relative importance of psychosocial vs physiological stress to the development and maintenance of mole-rat social groups, and how this relates to structure and function of the brain, remains to be determined.
4.4. Social isolation and social novelty
Increasing evidence indicates that, as in other species (e.g., mice, rats, and voles, discussed in section 3.4), social isolation and/or social novelty are potent stressors, depending on the mole-rat species. For social novelty, the most comprehensive direct species comparison to date examined females in solitary Cape mole-rat, transiently social Highveld mole-rats, social Mashona mole-rats (Cryptomys darlingi; Bennett et al., 1994), and eusocial Damaraland mole-rats. Testing the hypothesis that degree of sociality is related to social tolerance of unfamiliar conspecifics, Ganem and Bennett (2004) quantified occurrence of non-aggressive physical contact and aggressive/avoidance behavior during female dyadic encounters and measured variation in plasma cortisol prior to and at the end of each encounter. Mashona and Damaraland mole-rats had more variability in cortisol at baseline compared to Cape and Highveld mole-rats, but when lactating females were removed from the sample, species differences in cortisol disappeared. Only Cape mole-rat females showed increased cortisol when introduced to a novel female, suggesting social novelty is stressful or at least arousing for this solitary species; however, no significant correlations between cortisol and aggressive or affiliative behavior were detected in any species (Ganem and Bennett, 2004). More recently, Clive Coen and colleagues built on this work by directly comparing the distribution of CRF receptors in the brains of Cape mole-rats and naked mole-rats (Coen et al., 2015). As discussed in section 3.6, distribution and density of CRF1 and CRF2 receptors can relate to species specific social behavior. The most notable species differences identified in mole-rats were that CRF1 receptor binding in nucleus accumbens is lower in naked mole-rats versus Cape mole-rats, which may contribute to low tolerance of conspecifics in Cape mole-rats and high social tolerance in naked mole-rats, and that CRF2 receptor density in the lateral septum is also lower in naked mole-rats, which might reduce the anxiogenic potential of social interactions in this eusocial species. Neither mole-rat species has CRF1 receptors at measurable levels in the lateral septum or CRF2 receptors in the nucleus accumbens.
Social isolation appears to be stressful in eusocial naked mole-rats. Naked mole-rats have increased cortisol in urine and feces for up to 4 weeks after removal from colony (Edwards et al., 2020; Peragine et al., 2016). While these studies were not designed to examine the effects of social buffering and no direct statistical comparisons were made, it is interesting that in both cases, single-housed animals had higher levels of cortisol compared to pair-housed animals, suggesting some degree of social buffering. Faulkes and Abbott (1997) did not see an increase in cortisol in isolated or paired animals, though this was based on only 2 animals so results could easily be skewed by individual variability in stress responsiveness. Among naked mole-rat subordinates, animals show increased cortisol in blood two hours after exposure to an unfamiliar conspecific in a modified resident-intruder paradigm, regardless of whether they themselves are aggressive. In this case, experimental animals remained in their home caging unit and the intruder was a pre-screened non-aggressive individual. Thus, the cortisol response was independent of agonistic behaviors on the part of either the stimulus or experimental animals (Hathaway et al., 2016).
In contrast to naked mole-rats, female Damaraland mole-rats show decreased cortisol when removed from colony (Clarke et al., 2001) as long as they are not kept in social contact with former colony mates, and do not have altered cortisol when introduced to novel females (Ganem and Bennett, 2004). It is interesting to speculate that these species differences in cortisol response to removal from colony and novel conspecifics might relate to mechanism of reproductive suppression (see section 4.1) and/or dispersal strategy. Damaraland mole-rats are facultative outbreeders; both males and females will disperse after the death of a breeder, are more likely to disperse after rainfall and also permit immigration into established colonies (Jarvis and Bennett, 1993; Torrents‐Ticó et al., 2018). On the other hand, while at least some naked mole-rats show motivation to disperse both in lab and field (Braude, 2000; O’Riain et al., 1996; Toor et al., submitted), they will readily inbreed if an established breeder dies and are generally more xenophobic (Lacey and Sherman, 1991).
4.5. Conclusions and future directions
Despite offering tremendous comparative potential for understanding the relationship(s) between stress and sociality, the African mole-rats are relatively understudied. This is, of course, due in part to accessibility of study organisms but also likely influenced by long-standing biases towards studying psychosocial stress in traditional laboratory models. Broadly speaking, the relationship between stress and sociality can be classified into either 1) how an animal responds to social stimuli (e.g., is an interaction stressful or not) or 2) how social context influences how an animal responds to stress (e.g., social buffering). As discussed throughout this review, these are, in turn, critically influenced by species, sex, age, status, and experience of an individual. The evidence to date, albeit limited, indicates that mole-rats have unique neural and physiological adaptations that are associated with both species differences in social organization and individual differences in social/reproductive status.
To fully capitalize on the unique opportunity provided by this rodent family, we first and foremost need to complete basic characterization of the stress response in different species of mole-rats. How do animals of different social rank mount a physiological stress response? Does it matter if it is a social vs non-social stressor? Does it matter if it is a social vs non-social study species? A programmatic and directly comparative approach (e.g., Ganem and Bennett, 2004) will efficiently test hypotheses concerning psychosocial stress and sociality. An additional key area to pursue will be determining if and how individual differences in stress responsiveness relate to social role within the colony. Naked mole-rats show some evidence of task specialization independent of age and/or size (Mooney et al., 2015). Furthermore, when queens are removed from colony, only some animals compete for dominance (e.g., Clarke and Faulkes, 1997), and newly established opposite sex pairs show significant variability in time to first litter (Holmes, unpublished data). Essentially nothing is known about the proximate mechanisms underlying these individual differences and whether or not there is a role for HPA signaling. Collectively, this proposed work will help address the remaining unanswered questions about the relationship between stress and reproductive suppression, shedding light on the evolution of eusociality in mammals.
5. General Conclusions
In this paper we have summarized research from three distinct areas that highlight the importance and benefits of embracing ethologically relevant housing paradigms and diverse taxa in investigating the role and effects of social stress on behavior and physiology (see Figure 1). Although these literatures are diverse in their emphasis and outlook, we believe that there are several commonalities across all three that illustrate the significance of ethologically inspired behavioral neuroscience research.
Even closely related species differ in their responses to social stressors, and variation in natural ecology may underlie this variation. For example, the most potent stressor for the non-dispersing naked mole-rats appears to be socially isolating them from their colony, which can only be partially ameliorated by pair housing. Conversely, dispersing Damaraland mole-rats do not show changes in HPA activity following social isolation or when paired with conspecifics outside of the colony. Prairie and meadow voles both form selective preferences for familiar peers, but differ in mating system. Male prairie voles increased allogrooming towards their stressed female partners, while male meadow voles did not show similar consolation behavior towards their mates. Even variability in the natural ecology of rats and mice leads to differences between these species in their response to social stressors. Mice naturally engage in intra-sex conflict far more commonly than rats, so it is notable that, when forming social hierarchies, rats in the VBS show a much more profound body weight loss than mice in response to this novel social stressor. Within a species, some of the variation between individuals in the effects of social stress can also be accounted for when considering the ecological context of each species. For instance, meadow voles that are territorial in the summer but live in groups during the winter undergo changes in their stress reactivity and social behavior during short-day length photoperiods that permit social tolerance of conspecifics. To fully understand how social stress impacts behavior and physiology, there is clearly a need to study a wide range of species across ecological contexts.
Social context, which is influenced by changes in natural ecology, is also a critical factor in modulating the effects of stress across all species. For example, stress affected recently established but not long-term partner preferences in meadow voles. Social stress is not uniform across all individuals in a social group across all time periods. In both rats and mice, social rank significantly influences the timing and intensity of social stress experienced by individuals. Subordinate animals typically show elevated stress responsivity and long-term negative effects of stress on their behavior and immune, cardiovascular, metabolic and reproductive functioning. The magnitude and severity of social stress can vary dependent upon the degree of social competition within the group which is influenced by factors such as the size of groups, the group composition, space available and the despotism of the dominant individual. All of these factors can be experimentally manipulated but are also representative of natural ecological variations. Further, periods of social instability are associated with an increase in social stress experienced by all individuals compared to period of social stability. In naked mole-rats, cortisol is highest when animals are attempting to socially ascend to overtake an old queen. Likewise, in mice and in rats, social group composition changes are associated with elevated stress responses.
This review also highlights many unanswered questions. We still have much to learn about individual and species differences in the effects of social stress on many physiological systems in voles and mole-rats. Even in mouse and rat species used routinely in lab-based research, the number of studies investigating the effects of social stress in groups are relatively small compared to those that use more standard dyadic paradigms. There are even fewer studies of group housed prairie or meadow voles in the laboratory. In rats and mice, there is also a tremendous imbalance in the number of studies that have investigated male compared to female subjects. Females of both species also form social hierarchies in the VBS and appear to experience differential levels of social stress, yet almost nothing is known about the short or long-term consequences of this stress. The importance of studying both sexes is highlighted by the contrasting effects of stress on partner preference formation between male and female prairie voles. Lastly, one important domain that has been relatively under-studied is how social stress can induce adaptive physiological changes in individuals that promote short-term fitness benefits. Thus far, the majority of research has focused on disruptive long-term consequences of glucocorticoid exposure, but work across varied taxa could provide novel insights as to how social stress induces phenotypic plasticity that enables individuals to function competently in social groups.
With the advancement of tracking methods that facilitate easier study of large groups of animals and the development of wireless technologies for the logging of physiological data, we anticipate a growing interest in research on how social stress impacts social behavior and physiology. Our objective in this paper is to encourage research in this field to further promote the study of diverse taxa, and use of research designs that integrate housing and outcome measures that better mirror the natural ecology of the species under investigation.
Acknowledgements
We are grateful to Nikki Lee for feedback on this manuscript.
Funding
The authors’ current research is funded by the following sources: NIMH award R15MH113085 (AB), NSERC Discovery Grant RGPIN 2018-04780 and NSERC Discovery Accelerator Supplement RGPAS 2018-522465 (MMH), and the Samsung Scholarship Foundation (WL).
Footnotes
Conflicts of Interest
The authors report no conflicts of interest
References
- Abbott DH, Keverne EB, Bercovitch FB, Shively CA, Mendoza SP, Saltzman W, Snowdon CT, Ziegler TE, Banjevic M, Garland T, Sapolsky RM, 2003. Are subordinates always stressed? A comparative analysis of rank differences in cortisol levels among primates. Horm Behav 43, 67–82. 10.1016/s0018-506x(02)00037-5 [DOI] [PubMed] [Google Scholar]
- Adams N, Boice R, 1981. Mouse (Mus) burrows: effects of age, strain, and domestication. Animal Learning & Behavior 9, 140–144. [Google Scholar]
- Albeck DS, McKittrick CR, Blanchard DC, Blanchard RJ, Nikulina J, McEwen BS, Sakai RR, 1997. Chronic Social Stress Alters Levels of Corticotropin-Releasing Factor and Arginine Vasopressin mRNA in Rat Brain. J. Neurosci 17, 4895–4903. 10.1523/JNEUROSCI.17-12-04895.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allard MW, Honeycutt RL, 1992. Nucleotide sequence variation in the mitochondrial 12S rRNA gene and the phylogeny of African mole-rats (Rodentia: Bathyergidae). Mol Biol Evol 9, 27–40. 10.1093/oxfordjournals.molbev.a040706 [DOI] [PubMed] [Google Scholar]
- Ambrée O, Ruland C, Scheu S, Arolt V, Alferink J, 2018. Alterations of the Innate Immune System in Susceptibility and Resilience After Social Defeat Stress. Front Behav Neurosci 12. 10.3389/fnbeh.2018.00141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anacker A, Beery A, 2013. Life in groups: the roles of oxytocin in mammalian sociality. Front. Behav. Neurosci 7 10.3389/fnbeh.2013.00185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anacker AMJ, Reitz KM, Goodwin NL, Beery AK, 2016. Stress impairs new but not established relationships in seasonally social voles. Hormones and Behavior 79, 52–57. 10.1016/j.yhbeh.2016.01.004 [DOI] [PubMed] [Google Scholar]
- Anderson PK, 1961. Density, social structure, and nonsocial environment in house-mouse populations and the implications for regulation of numbers. Trans N Y Acad Sci 23, 447–451. 10.1111/j.2164-0947.1961.tb01373.x [DOI] [PubMed] [Google Scholar]
- Anderson PK, Hill JL, 1965. Mus musculus: experimental induction of territory formation. Science 148, 1753–1755. [DOI] [PubMed] [Google Scholar]
- Anyan JJ, Seney ML, Holley A, Bengston L, Goldman BD, Forger NG, Holmes MM, 2011. Social status and sex effects on neural morphology in Damaraland mole-rats, Fukomys damarensis. Brain Behav. Evol 77, 291–298. 10.1159/000328640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragona BJ, Wang Z, 2009. Dopamine regulation of social choice in a monogamous rodent species. Front. Behav. Neurosci 3 10.3389/neuro.08.015.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arakawa H, Blanchard DC, Blanchard RJ, 2007. Colony formation of C57BL/6J mice in visible burrow system: identification of eusocial behaviors in a background strain for genetic animal models of autism. Behav. Brain Res 176, 27–39. 10.1016/j.bbr.2006.07.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale TL, Vale WW, 2004. CRF and CRF Receptors: Role in Stress Responsivity and Other Behaviors. Annu. Rev. Pharmacol. Toxicol 44, 525–557. 10.1146/annurev.pharmtox.44.101802.121410 [DOI] [PubMed] [Google Scholar]
- Barnett SA, 1958a. Physiological effects of “social stress” in wild rats—I: The adrenal cortex. Journal of Psychosomatic Research 3, 1–11. 10.1016/0022-3999(58)90012-6 [DOI] [PubMed] [Google Scholar]
- Barnett SA, 1958b. An analysis of social behaviour in wild rats. Proceedings of the Zoological Society of London 130, 107–152. 10.1111/j.1096-3642.1958.tb00565.x [DOI] [Google Scholar]
- Bartoš L, Brain PF, 1993. Physiological responses to social status and housing conditions in male mice subject to food competition tests. Italian Journal of Zoology 60, 293–296. [Google Scholar]
- Beck JA, Lloyd S, Hafezparast M, Lennon-Pierce M, Eppig JT, Festing MFW, Fisher EMC, 2000. Genealogies of mouse inbred strains. Nature Genetics 24, 23–25. 10.1038/71641 [DOI] [PubMed] [Google Scholar]
- Becker JB, Chartoff E, 2019. Sex differences in neural mechanisms mediating reward and addiction. Neuropsychopharmacology 44, 166–183. 10.1038/s41386-018-0125-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beery AK, 2019. Frank Beach award winner: Neuroendocrinology of group living. Hormones and Behavior 107, 67–75. 10.1016/j.yhbeh.2018.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beery AK, 2018. Inclusion of females does not increase variability in rodent research studies. Current Opinion in Behavioral Sciences, Sex and Gender 23, 143–149. 10.1016/j.cobeha.2018.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beery AK, Bicks L, Mooney SJ, Goodwin NL, Holmes MM, 2016. Sex, social status, and CRF receptor densities in naked mole-rats. Journal of Comparative Neurology 524, 228–243. 10.1002/cne.23834 [DOI] [PubMed] [Google Scholar]
- Beery AK, Chen J, Lopez S, Lee NS, 2019. Social Selectivity and social reward in prairie voles. [Google Scholar]
- Beery AK, Christensen JD, Lee NS, Blandino KL, 2018. Specificity in Sociality: Mice and Prairie Voles Exhibit Different Patterns of Peer Affiliation. Front. Behav. Neurosci 12 10.3389/fnbeh.2018.00050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beery AK, Kaufer D, 2015. Stress, social behavior, and resilience: Insights from rodents. Neurobiology of Stress, Stress Resilience 1, 116–127. 10.1016/j.ynstr.2014.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beery AK, Loo TJ, Zucker I, 2008. Day length and estradiol affect same-sex affiliative behavior in the female meadow vole. Hormones and Behavior 54, 153–159. 10.1016/j.yhbeh.2008.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beery AK, Routman DM, Zucker I, 2009. Same-sex social behavior in meadow voles: Multiple and rapid formation of attachments. Physiology & Behavior 97, 52–57. 10.1016/j.physbeh.2009.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beery AK, Vahaba DM, Grunberg DM, 2014. Corticotropin-releasing factor receptor densities vary with photoperiod and sociality. Hormones and Behavior 66, 779–786. 10.1016/j.yhbeh.2014.08.014 [DOI] [PubMed] [Google Scholar]
- Beery AK, Zucker I, 2011. Sex bias in neuroscience and biomedical research. Neuroscience & Biobehavioral Reviews 35, 565–572. 10.1016/j.neubiorev.2010.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltz AM, Beery AK, Becker JB, 2019. Analysis of sex differences in pre-clinical and clinical data sets. Neuropsychopharmacology 44, 2155–2158. 10.1038/s41386-019-0524-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett NC, 2011. Teasing apart socially-induced infertility in non-reproductive female Damaraland mole-rats, Fukomys damarensis (Rodentia: Bathyergidae). Integrative Zoology 6, 311–320. 10.1111/j.1749-4877.2011.00263.x [DOI] [PubMed] [Google Scholar]
- Bennett NC, Faulkes CG, 2000. African Mole-Rats: Ecology and Eusociality. Cambridge University Press. [Google Scholar]
- Bennett NC, Jarvis JUM, 1988a. The reproductive biology of the Cape mole-rat, Georychus capensis (Rodentia, Bathyergidae). Journal of Zoology 214, 95–106. 10.1111/j.1469-7998.1988.tb04989.x [DOI] [Google Scholar]
- Bennett NC, Jarvis JUM, 1988b. The Social Structure and Reproductive Biology of Colonies of the Mole-Rat, Cryptomys damarensis (Rodentia, Bathyergidae). J Mammal 69, 293–302. 10.2307/1381379 [DOI] [Google Scholar]
- Bennett NC, Jarvis JUM, Cotterill FPD, 1994. The colony structure and reproductive biology of the afrotropical Mashona mole-rat, Cryptomys darlingi. Journal of Zoology 234, 477–487. 10.1111/j.1469-7998.1994.tb04861.x [DOI] [Google Scholar]
- Benton D, Goldsmith JF, Gamal-el-Din L, Brain PF, Hucklebridge FH, 1978. Adrenal activity in isolated mice and mice of different social status. Physiology & behavior 20, 459–464. [DOI] [PubMed] [Google Scholar]
- Berdoy M, Drickamer LC, 2007. Comparative social organization and life history of Rattus and Mus, in: Rodent Societies: An Ecological and Evolutionary Perspective. The University of Chicago Press, Chicago, pp. 380–392. [Google Scholar]
- Berdoy M, Smith P, Macdonald DW, 1995. Stability of social status in wild rats: Age and the role of settled dominance. Behaviour 132, 193–212. 10.1163/156853995X00694 [DOI] [Google Scholar]
- Berton O, Ramos A, Chaouloff F, Mormède P, 1997. Behavioral Reactivity to Social and Nonsocial Stimulations: A Multivariate Analysis of Six Inbred Rat Strains. Behav Genet 27, 155–166. 10.1023/A:1025641509809 [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Blanchard RJ, 1990. Behavioral correlates of chronic dominance-subordination relationships of male rats in a seminatural situation. Neuroscience & Biobehavioral Reviews 14, 455–462. 10.1016/S0149-7634(05)80068-5 [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Sakai RR, McEwen B, Weiss SM, Blanchard RJ, 1993. Subordination stress: Behavioral, brain, and neuroendocrine correlates. Behavioural Brain Research 58, 113–121. 10.1016/0166-4328(93)90096-9 [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Spencer RL, Weiss SM, Blanchard RJ, McEwen B, Sakai RR, 1995. Visible burrow system as a model of chronic social stress: Behavioral and neuroendocrine correlates. Psychoneuroendocrinology 20, 117–134. 10.1016/0306-4530(94)E0045-B [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Blanchard DC, 1989. Antipredator defensive behaviors in a visible burrow system. Journal of Comparative Psychology 103, 70–82. 10.1037/0735-7036.103.1.70 [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Blanchard DC, Flannelly KJ, 1985. Social stress, mortality and aggression in colonies and burrowing habitats. Behavioural Processes 11, 209–213. 10.1016/0376-6357(85)90062-2 [DOI] [PubMed] [Google Scholar]
- Blondel DV, Phelps SM, 2016. Effects of acute corticosterone treatment on male prairie voles (Microtus ochrogaster): Territorial aggression does not accompany induced social preference. Journal of Comparative Psychology 130, 400–406. 10.1037/com0000048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boice R, 1981. Behavioral comparability of wild and domesticated rats. Behavior genetics 11, 545–553. [DOI] [PubMed] [Google Scholar]
- Boice R, Adams N, 1983. Degrees of captivity and aggressive behavior in domestic Norway rats. Bull. Psychon. Soc 21, 149–152. 10.3758/BF03329980 [DOI] [Google Scholar]
- Boonstra R, Boag PT, 1992. Spring Declines in Microtus pennsylvanicus and the Role of Steroid Hormones. Journal of Animal Ecology 61, 339–352. 10.2307/5326 [DOI] [Google Scholar]
- Boonstra R, Xia X, Pavone L, 1993. Mating system of the meadow vole, Microtus pennsylvanicus. Behav Ecol 4, 83–89. 10.1093/beheco/4.1.83 [DOI] [Google Scholar]
- Bosch OJ, Nair HP, Ahern TH, Neumann ID, Young LJ, 2009. The CRF System Mediates Increased Passive Stress-Coping Behavior Following the Loss of a Bonded Partner in a Monogamous Rodent. Neuropsychopharmacology 34, 1406–1415. 10.1038/npp.2008.154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bove M, Ike K, Eldering A, Buwalda B, de Boer SF, Morgese MG, Schiavone S, Cuomo V, Trabace L, Kas MJH, 2018. The Visible Burrow System: A behavioral paradigm to assess sociability and social withdrawal in BTBR and C57BL/6J mice strains. Behavioural Brain Research 344, 9–19. 10.1016/j.bbr.2018.02.003 [DOI] [PubMed] [Google Scholar]
- Brain PF, Homady MH, Mainardi M, 1983. Preputial glands, dominance and aggressiveness, in mice. Italian Journal of Zoology 50, 173–187. [Google Scholar]
- Braude S, 2000. Dispersal and new colony formation in wild naked mole-rats: evidence against inbreeding as the system of mating. Behav Ecol 11, 7–12. 10.1093/beheco/11.1.7 [DOI] [Google Scholar]
- Bronson FH, 1973. Establishment of social rank among grouped male mice: Relative effects on circulating FSH, LH, and corticosterone. Physiology & Behavior 10, 947–951. 10.1016/0031-9384(73)90065-6 [DOI] [PubMed] [Google Scholar]
- Bronson FH, Eleftheriou BE, 1964. Chronic physiological effects of fighting in mice. Gen. Comp. Endocrinol 4, 9–14. 10.1016/0016-6480(64)90033-4 [DOI] [PubMed] [Google Scholar]
- Bronson FH, Marsden HM, 1973. The preputial gland as an indicator of social dominance in male mice. Behavioral biology 9, 625–628. [DOI] [PubMed] [Google Scholar]
- Brown RZ, 1953. Social behavior, reproduction, and population changes in the house mouse (Mus musculus L.). Ecological Monographs 23, 217–240. [Google Scholar]
- Buffenstein R, Yahav S, 1991. Is the naked mole-rat Hererocephalus glaber an endothermic yet poikilothermic mammal? J.THERM.BIOL 16, 227–232. 10.1016/0306-4565(91)90030-6 [DOI] [Google Scholar]
- Burkett JP, Andari E, Johnson ZV, Curry DC, Waal F.B.M. de, Young LJ, 2016. Oxytocin-dependent consolation behavior in rodents. Science 351, 375–378. 10.1126/science.aac4785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler RG, 1980. Population size, social behaviour, and dispersal in house mice: A quantitative investigation. Animal Behaviour 28, 78–85. 10.1016/S0003-3472(80)80010-8 [DOI] [Google Scholar]
- Buwalda B, Koolhaas JM, de Boer SF, 2017. Trait aggressiveness does not predict social dominance of rats in the Visible Burrow System. Physiology & Behavior, Celebrating the Science of Dr. Randall Sakai: From Salt to Stress to Obesity and Back Again 178, 134–143. 10.1016/j.physbeh.2017.01.008 [DOI] [PubMed] [Google Scholar]
- Calhoun JB, 1963. The ecology and sociology of the Norway rat. US Department of Health, Education, and Welfare, Public Health Service. [Google Scholar]
- Calhoun JB, 1962. Population Density and Social Pathology. Scientific American 206, 139–149. [DOI] [PubMed] [Google Scholar]
- Carter CS, Keverne EB, 2009. The Neurobiology of Social Affiliation and Pair Bonding, in: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT (Eds.), Hormones, Brain and Behavior (Second Edition). Academic Press, San Diego, pp. 137–166. 10.1016/B978-008088783-8.00004-8 [DOI] [Google Scholar]
- Chambers LK, Singleton GR, Krebs CJ, 2000. Movements and Social Organization of Wild House Mice (Mus Domesticus) in the Wheatlands of Northwestern Victoria, Australia. J Mammal 81, 59–69. 10.1644/1545-1542(2000)081<0059:MASOOW>2.0.CO;2 [DOI] [Google Scholar]
- Chao HM, Blanchard DC, Blanchard RJ, McEwen BS, Sakai RR, 1993. The effect of social stress on hippocampal gene expression. Molecular and Cellular Neuroscience 4, 543– 10.1006/mcne.1993.1067 [DOI] [PubMed] [Google Scholar]
- Chovnick A, Yasukawa NJ, Monder H, Christian JJ, 1987. Female behavior in populations of mice in the presence and absence of male hierarchy. Aggressive Behavior 13, 367–375. [Google Scholar]
- Clarke FM, Faulkes CG, 2001. Intracolony aggression in the eusocial naked mole-rat, Heterocephalus glaber. Animal Behaviour 61, 311–324. 10.1006/anbe.2000.1573 [DOI] [Google Scholar]
- Clarke FM, Faulkes CG, 1998. Hormonal and behavioural correlates of male dominance and reproductive status in captive colonies of the naked mole–rat, Heterocephalus glaber. Proceedings of the Royal Society of London. Series B: Biological Sciences 265, 1391–1399. 10.1098/rspb.1998.0447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke FM, Faulkes CG, 1997. Dominance and queen succession in captive colonies of the eusocial naked mole–rat, Heterocephalus glaber. Proceedings of the Royal Society of London. Series B: Biological Sciences 264, 993–1000. 10.1098/rspb.1997.0137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke FM, Miethe GH, Bennett NC, 2001. Reproductive suppression in female Damaraland mole–rats Cryptomys damarensis: dominant control or self–restraint? Proceedings of the Royal Society of London. Series B: Biological Sciences 268, 899–909. 10.1098/rspb.2000.1426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coen CW, Kalamatianos T, Oosthuizen MK, Poorun R, Faulkes CG, Bennett NC, 2015. Sociality and the telencephalic distribution of corticotrophin-releasing factor, urocortin 3, and binding sites for CRF type 1 and type 2 receptors: A comparative study of eusocial naked mole-rats and solitary Cape mole-rats. Journal of Comparative Neurology 523, 2344–2371. 10.1002/cne.23796 [DOI] [PubMed] [Google Scholar]
- Cordero MI, Sandi C, 2007. Stress amplifies memory for social hierarchy. Front Neurosci 1, 175–184. 10.3389/neuro.01.1.1.013.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creel S, 2001. Social dominance and stress hormones. Trends in ecology & evolution 16, 491–497. [Google Scholar]
- Crowcroft P, 1966. Mice All Over. Foulis. [Google Scholar]
- Crowcroft P, 1955. Territoriality in wild house mice, Mus musculus L. J Mammal 36, 299–301. 10.2307/1375908 [DOI] [Google Scholar]
- Crowcroft P, Rowe FP, 1963. Social organization and territorial behaviour in the, in: Proceedings of the Zoological Society of London. Wiley Online Library, pp. 517–531. [Google Scholar]
- Curley JP, 2016. Temporal pairwise-correlation analysis provides empirical support for attention hierarchies in mice. Biology Letters 12, 20160192 10.1098/rsbl.2016.0192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JF, Krause EG, Melhorn SJ, Sakai RR, Benoit SC, 2009. Dominant rats are natural risk takers and display increased motivation for food reward. Neuroscience 162, 23–30. 10.1016/j.neuroscience.2009.04.039 [DOI] [PubMed] [Google Scholar]
- DeVries AC, DeVries MB, Taymans S, Carter CS, 1995. Modulation of pair bonding in female prairie voles (Microtus ochrogaster) by corticosterone. PNAS 92, 7744–7748. 10.1073/pnas.92.17.7744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVries AC, DeVries MB, Taymans SE, Carter CS, 1996. The effects of stress on social preferences are sexually dimorphic in prairie voles. PNAS 93, 11980–11984. 10.1073/pnas.93.21.11980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVries AC, Guptaa T, Cardillo S, Cho M, Carter CS, 2002. Corticotropin-releasing factor induces social preferences in male prairie voles. Psychoneuroendocrinology 27, 705–714. 10.1016/S0306-4530(01)00073-7 [DOI] [PubMed] [Google Scholar]
- DeVries AC, Johnson CL, Carter CS, 1997. Familiarity and gender influence social preferences in prairie voles (Microtus ochrogaster). Can. J. Zool 75, 295–301. 10.1139/z97-037 [DOI] [Google Scholar]
- Dijkstra H, Tilders FJH, Hiehle MA, Smelik PG, 1992. Hormonal reactions to fighting in rat colonies: Prolactin rises during defense, not during offence. Physiology & Behavior 51, 961–968. 10.1016/0031-9384(92)90078-G [DOI] [PubMed] [Google Scholar]
- Drickamer LC, 2001. Urine marking and social dominance in male house mice (Mus musculus domesticus). Behavioural processes 53, 113–120. [DOI] [PubMed] [Google Scholar]
- Edwards PD, Dean EK, Palme R, Boonstra R, 2019. Assessing space use in meadow voles: the relationship to reproduction and the stress axis. J Mammal 100, 4–12. 10.1093/jmammal/gyy161 [DOI] [Google Scholar]
- Edwards PD, Mooney SJ, Bosson CO, Toor I, Palme R, Holmes MM, Boonstra R, 2020. The stress of being alone: Removal from the colony, but not social subordination, increases fecal cortisol metabolite levels in eusocial naked mole-rats. Hormones and Behavior 121, 104720 10.1016/j.yhbeh.2020.104720 [DOI] [PubMed] [Google Scholar]
- Ellenbroek B, Youn J, 2016. Rodent models in neuroscience research: is it a rat race? Disease Models & Mechanisms 9, 1079–1087. 10.1242/dmm.026120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ely DL, 1981. Hypertension, social rank, and aortic arteriosclerosis in CBA/J mice. Physiology & Behavior 26, 655–661. 10.1016/0031-9384(81)90140-2 [DOI] [PubMed] [Google Scholar]
- Ely DL, Henry JP, 1978. Neuroendocrine response patterns in dominant and subordinate mice. Hormones and Behavior 10, 156–169. 10.1016/0018-506X(78)90005-3 [DOI] [PubMed] [Google Scholar]
- Ely DL, Henry JP, Jarosz CJ, 1975. Effects of marihuana (Δ9-THC) on behavior patterns and social roles in colonies of CBA Mice. Behavioral Biology 13, 263–276. 10.1016/S0091-6773(75)91290-0 [DOI] [PubMed] [Google Scholar]
- Erlander MG, Tillakaratne NJK, Feldblum S, Patel N, Tobin AJ, 1991. Two genes encode distinct glutamate decarboxylases. Neuron 7, 91–100. 10.1016/0896-6273(91)90077-D [DOI] [PubMed] [Google Scholar]
- Faulkes CG, Abbott DH, 1997. The Physiology of a Reproductive Dictatorship: Regulation of Male and Female Reproduction by a Single Breeding Female in Colonies of Naked Mole-Rats, in: Cooperative Breeding in Mammals. Cambridge University Press, UK, pp. 302–334. 10.1017/CBO9780511574634.012 [DOI] [Google Scholar]
- Faulkes CG, Bennett NC, 2013. Plasticity and constraints on social evolution in African mole-rats: ultimate and proximate factors. Philosophical Transactions of the Royal Society B: Biological Sciences 368, 20120347 10.1098/rstb.2012.0347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faykoo-Martinez M, Monks DA, Zovkic IB, Holmes MM, 2018. Sex- and brain region-specific patterns of gene expression associated with socially-mediated puberty in a eusocial mammal. PLOS ONE 13, e0193417 10.1371/journal.pone.0193417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferkin MH, Kile JR, 1996. Melatonin Treatment Affects the Attractiveness of the Anogenital Area Scent in Meadow Voles (Microtus pennsylvanicus). Hormones and Behavior 30, 227–235. 10.1006/hbeh.1996.0027 [DOI] [PubMed] [Google Scholar]
- Ferkin MH, Zucker I, 1991. Seasonal control of odour preferences of meadow voles (Microtus pennsylvanicus) by photoperiod and ovarian hormones. Reproduction 92, 433–441. 10.1530/jrf.0.0920433 [DOI] [PubMed] [Google Scholar]
- File SE, Seth P, 2003. A review of 25 years of the social interaction test. European Journal of Pharmacology, Animal Models of Anxiety Disorders 463, 35–53. 10.1016/S0014-2999(03)01273-1 [DOI] [PubMed] [Google Scholar]
- Fink S, Excoffier L, Heckel G, 2006. Mammalian monogamy is not controlled by a single gene. PNAS 103, 10956–10960. 10.1073/pnas.0602380103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnell JE, Lombard CM, Padi AR, Moffitt CM, Wilson LB, Wood CS, Wood SK, 2017. Physical versus psychological social stress in male rats reveals distinct cardiovascular, inflammatory and behavioral consequences. PLOS ONE 12, e0172868 10.1371/journal.pone.0172868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitch HS, 1957. Aspects of Reproduction and Development in the Prairie Vole (Microtus ochrogaster). University of Kansas, Place of publication not identified. [Google Scholar]
- Fokkema DS, Koolhaas JM, van der Gugten J, 1995. Individual characteristics of behavior, blood pressure, and adrenal hormones in colony rats. Physiology & Behavior 57, 857–862. 10.1016/0031-9384(94)00333-Z [DOI] [PubMed] [Google Scholar]
- Fone KCF, Porkess MV, 2008. Behavioural and neurochemical effects of post-weaning social isolation in rodents—Relevance to developmental neuropsychiatric disorders. Neuroscience & Biobehavioral Reviews, The long-term consequences of stress on brain function: from adaptation to mental diseases 32, 1087–1102. 10.1016/j.neubiorev.2008.03.003 [DOI] [PubMed] [Google Scholar]
- Galea LAM, McEwen BS, 1999. Sex and seasonal changes in the rate of cell proliferation in the dentate gyrus of adult wild meadow voles. Neuroscience 89, 955–964. 10.1016/S0306-4522(98)00345-5 [DOI] [PubMed] [Google Scholar]
- Ganem G, Bennett NC, 2004. Tolerance to unfamiliar conspecifics varies with social organization in female African mole-rats. Physiology & Behavior 82, 555–562. 10.1016/j.physbeh.2004.05.002 [DOI] [PubMed] [Google Scholar]
- Gerlai R, 2016. Gene Targeting Using Homologous Recombination in Embryonic Stem Cells: The Future for Behavior Genetics? Front. Genet 7 10.3389/fgene.2016.00043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gersten O, 2008. Neuroendocrine biomarkers, social relations, and the cumulative costs of stress in Taiwan. Social Science & Medicine 66, 507–519. 10.1016/j.socscimed.2007.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getz LL, 1972. Social Structure and Aggressive Behavior in a Population of Microtus pennsylvanicus. J Mammal 53, 310–317. 10.2307/1379167 [DOI] [Google Scholar]
- Getz LL, Carter CS, Gavish L, 1981. The Mating System of the Prairie Vole, Microtus ochrogaster: Field and Laboratory Evidence for Pair-Bonding. Behavioral Ecology and Sociobiology 8, 189–194. [Google Scholar]
- Getz LL, McGuire B, Pizzuto T, Hofmann JE, Frase B, 1993. Social Organization of the Prairie Vole (Microtus ochrogaster). J Mammal 74, 44–58. 10.2307/1381904 [DOI] [Google Scholar]
- Glasper ER, DeVries AC, 2005. Social structure influences effects of pair-housing on wound healing. Brain, Behavior, and Immunity 19, 61–68. 10.1016/j.bbi.2004.03.002 [DOI] [PubMed] [Google Scholar]
- Golden SA, Covington HE, Berton O, Russo SJ, 2011. A standardized protocol for repeated social defeat stress in mice. Nature Protocols 6, 1183–1191. 10.1038/nprot.2011.361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin NL, Lopez SA, Lee NS, Beery AK, 2019. Comparative role of reward in long-term peer and mate relationships in voles. Hormones and Behavior, SBN/ICN Meeting 2018 111, 70–77. 10.1016/j.yhbeh.2018.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray SJ, Jensen SP, Hurst JL, 2000. Structural complexity of territories: preference, use of space and defence in commensal house mice, Mus domesticus. Animal Behaviour 60, 765–772. 10.1006/anbe.2000.1527 [DOI] [PubMed] [Google Scholar]
- Grippo A, Ihm E, Wardwell J, McNeal N, Scotti M-A, Moenk D, Chandler D, LaRocca M, Preihs K, 2014. The Effects of Environmental Enrichment on Depressive and Anxiety-Relevant Behaviors in Socially Isolated Prairie Voles. Psychosomatic Medicine 76, 277–284. 10.1097/PSY.0000000000000052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo AJ, Carter CS, McNeal N, Chandler DL, LaRocca MA, Bates SL, Porges SW, 2011. 24-hour autonomic dysfunction and depressive behaviors in an animal model of social isolation: Implications for the study of depression and cardiovascular disease. Psychosomatic Medicine 73, 59–66. 10.1097/PSY.0b013e31820019e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo AJ, Lamb DG, Carter CS, Porges SW, 2007. Social Isolation Disrupts Autonomic Regulation of the Heart and Influences Negative Affective Behaviors. Biological Psychiatry, Stress and Anxiety: Developmental and Therapeutic Perspectives 62, 1162–1170. 10.1016/j.biopsych.2007.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo AJ, Trahanas DM, Zimmerman RR, Porges SW, Carter CS, 2009. Oxytocin protects against negative behavioral and autonomic consequences of long-term social isolation. Psychoneuroendocrinology 34, 1542–1553. 10.1016/j.psyneuen.2009.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo AJ, Wu KD, Hassan I, Carter CS, 2008. Social isolation in prairie voles induces behaviors relevant to negative affect: toward the development of a rodent model focused on co-occurring depression and anxiety. Depression and Anxiety 25, E17–E26. 10.1002/da.20375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas DA, George SR, 1989. Estradiol or ovariectomy decreases crf synthesis in hypothalamus. Brain Research Bulletin 23, 215–218. 10.1016/0361-9230(89)90150-0 [DOI] [PubMed] [Google Scholar]
- Haller J, Fuchs E, Halász J, Makara GB, 1999. Defeat is a major stressor in males while social instability is stressful mainly in females: towards the development of a social stress model in female rats. Brain Research Bulletin 50, 33–39. 10.1016/S0361-9230(99)00087-8 [DOI] [PubMed] [Google Scholar]
- Haney M, Miczek KA, 1993. Ultrasounds during agonistic interactions between female rats (Rattus norvegicus). Journal of Comparative Psychology 107, 373. [DOI] [PubMed] [Google Scholar]
- Hardy MP, Sottas CM, Ge R, McKittrick CR, Tamashiro KL, McEwen BS, Haider SG, Markham CM, Blanchard RJ, Blanchard DC, Sakai RR, 2002. Trends of reproductive hormones in male rats during psychosocial stress: Role of glucocorticoid metabolism in behavioral dominance. Biol Reprod 67, 1750–1755. 10.1095/biolreprod.102.006312 [DOI] [PubMed] [Google Scholar]
- Hathaway GA, Faykoo-Martinez M, Peragine DE, Mooney SJ, Holmes MM, 2016. Subcaste differences in neural activation suggest a prosocial role for oxytocin in eusocial naked mole-rats. Hormones and Behavior 79, 1–7. 10.1016/j.yhbeh.2015.12.001 [DOI] [PubMed] [Google Scholar]
- Hebb DO, 1947. The effects of early experience on problem-solving at maturity. American Psychologist 2, 306–307. [Google Scholar]
- Hellemans KGC, Benge LC, Olmstead MC, 2004. Adolescent enrichment partially reverses the social isolation syndrome. Developmental Brain Research 150, 103–115. 10.1016/j.devbrainres.2004.03.003 [DOI] [PubMed] [Google Scholar]
- Henry JP, Liu YY, Nadra WE, Qian CG, Mormede P, Lemaire V, Ely DL, Hendley ED, 1993. Psychosocial stress can induce chronic hypertension in normotensive strains of rats. Hypertension 21, 714–723. 10.1161/01.HYP.21.5.714 [DOI] [PubMed] [Google Scholar]
- Henry JP, Stephens PM, Ely DL, 1986. Psychosocial hypertension and the defence and defeat reactions. Journal of Hypertension 4, 687–688. [DOI] [PubMed] [Google Scholar]
- Herman JP, Tamashiro KL, 2017. The visible burrow system: A view from across the hall. Physiology & Behavior, Celebrating the Science of Dr. Randall Sakai: From Salt to Stress to Obesity and Back Again 178, 103–109. 10.1016/j.physbeh.2017.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes MM, Goldman BD, Goldman SL, Seney ML, Forger NG, 2009. Neuroendocrinology and sexual differentiation in eusocial mammals. Frontiers in Neuroendocrinology, Hormones & Social Behavior 30, 519–533. 10.1016/j.yfrne.2009.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes MM, Rosen GJ, Jordan CL, de Vries GJ, Goldman BD, Forger NG, 2007. Social control of brain morphology in a eusocial mammal. Proc. Natl. Acad. Sci. U.S.A 104, 10548–10552. 10.1073/pnas.0610344104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes MM, Seney ML, Goldman BD, Forger NG, 2011. Social and hormonal triggers of neural plasticity in naked mole-rats. Behav. Brain Res 218, 234–239. 10.1016/j.bbr.2010.11.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou XH, Hyun M, Taranda J, Huang KW, Todd E, Feng D, Atwater E, Croney D, Zeidel ML, Osten P, Sabatini BL, 2016. Central Control Circuit for Context-Dependent Micturition. Cell 167, 73–86.e12. 10.1016/j.cell.2016.08.073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iñiguez SD, Flores-Ramirez FJ, Riggs LM, Alipio JB, Garcia-Carachure I, Hernandez MA, Sanchez DO, Lobo MK, Serrano PA, Braren SH, Castillo SA, 2018. Vicarious Social Defeat Stress Induces Depression-Related Outcomes in Female Mice. Biological Psychiatry, Novel Mechanisms of Antidepressant Action 83, 9–17. 10.1016/j.biopsych.2017.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs DS, Bennett NC, Jarvis JUM, Crowe TM, 1991. The colony structure and dominance hierarchy of the Damaraland mole-rat, Cryptomys damarensis (Rodentia: Bathyergidae), from Namibia. Journal of Zoology 224, 553–576. 10.1111/j.1469-7998.1991.tb03785.x [DOI] [Google Scholar]
- Jarvis JUM, 1981. Eusociality in a mammal: cooperative breeding in naked mole-rat colonies. Science 212, 571–573. 10.1126/science.7209555 [DOI] [PubMed] [Google Scholar]
- Jarvis JUM, Bennett NC, 1993. Eusociality has evolved independently in two genera of bathyergid mole-rats — but occurs in no other subterranean mammal. Behav Ecol Sociobiol 33, 253–260. 10.1007/BF02027122 [DOI] [Google Scholar]
- Jarvis JUM, Bennett NC, 1991. Ecology and behavior of the family bathyergidae, in: The Biology of the Naked Mole-Rat. Princeton University Press, USA, pp. 66–96. [Google Scholar]
- Karamihalev S, Flachskamm C, Eren N, Kimura M, Chen A, 2019. Social context and dominance status contribute to sleep patterns and quality in groups of freely-moving mice. Scientific Reports 9, 1–8. 10.1038/s41598-019-51375-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsey SG, Bailey MT, Sheridan JF, Padgett DA, Avitsur R, 2007. Repeated Social Defeat Causes Increased Anxiety-Like Behavior and Alters Splenocyte Function in C57BL/6 and CD-1 Mice. Brain Behav Immun 21, 458–466. 10.1016/j.bbi.2006.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koolhaas JM, Bohus B, 1989. Social control in relation to neuroendocrine and immunological responses, in: Stress, Personal Control and Health. John Wiley & Sons, Oxford, England, pp. 295–305. [Google Scholar]
- Koolhaas JM, De Boer SF, De Rutter AJ, Meerlo P, Sgoifo A, 1997. Social stress in rats and mice. Acta Physiol Scand Suppl 640, 69–72. [PubMed] [Google Scholar]
- Koyama S, Kamimura S, 2003. Study on the development of sperm motility and social dominance of male mice. Physiology & behavior 80, 267–272. [DOI] [PubMed] [Google Scholar]
- Koyama S, Kamimura S, 1998. Lowered sperm motility in subordinate social status of mice. Physiology & behavior 65, 665–669. [DOI] [PubMed] [Google Scholar]
- Kozorovitskiy Y, Gould E, 2004. Dominance Hierarchy Influences Adult Neurogenesis in the Dentate Gyrus. J. Neurosci 24, 6755–6759. 10.1523/JNEUROSCI.0345-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan V, Han M-H, Graham DL, Berton O, Renthal W, Russo SJ, Laplant Q, Graham A, Lutter M, Lagace DC, Ghose S, Reister R, Tannous P, Green TA, Neve RL, Chakravarty S, Kumar A, Eisch AJ, Self DW, Lee FS, Tamminga CA, Cooper DC, Gershenfeld HK, Nestler EJ, 2007. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell 131, 391–404. 10.1016/j.cell.2007.09.018 [DOI] [PubMed] [Google Scholar]
- Lacey EA, Sherman PW, 1991. Social Organization of Naked Mole-Rat Colonies: Evidence for Divisions of Labor, in: The Biology of the Naked Mole-Rat. Princeton University Press, USA, pp. 275–336. [Google Scholar]
- Lamm C, Decety J, Singer T, 2011. Meta-analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. NeuroImage 54, 2492–2502. 10.1016/j.neuroimage.2010.10.014 [DOI] [PubMed] [Google Scholar]
- Lee NS, Goodwin NL, Freitas KE, Beery AK, 2019. Affiliation, Aggression, and Selectivity of Peer Relationships in Meadow and Prairie Voles. Front. Behav. Neurosci 13 10.3389/fnbeh.2019.00052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W, Khan A, Curley JP, 2017. Major urinary protein levels are associated with social status and context in mouse social hierarchies. Proceedings of the Royal Society B: Biological Sciences 284, 20171570 10.1098/rspb.2017.1570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W, Yang E, Curley JP, 2018. Foraging dynamics are associated with social status and context in mouse social hierarchies. PeerJ 6, e5617 10.7717/peerj.5617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepore SJ, Allen KA, Evans GW, 1993. Social support lowers cardiovascular reactivity to an acute stressor. Psychosom Med 55, 518–524. 10.1097/00006842-199311000-00007 [DOI] [PubMed] [Google Scholar]
- Lidster K, Owen K, Browne WJ, Prescott MJ, 2019. Cage aggression in group-housed laboratory male mice: an international data crowdsourcing project. Scientific Reports 9, 1–12. 10.1038/s41598-019-51674-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberwirth C, Wang Z, 2016. The neurobiology of pair bond formation, bond disruption, and social buffering. Current Opinion in Neurobiology, Systems neuroscience 40, 8–13. 10.1016/j.conb.2016.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd JA, 1975. Social structure and reproduction in two freelygrowing populations of house mice (Mus musculus L.). Animal Behaviour 23, 413–424. [Google Scholar]
- Lockwood JA, Turney TH, 1981. Social dominance and stress-induced hypertension: Strain differences in inbred mice. Physiology & Behavior 26, 547–549. 10.1016/0031-9384(81)90187-6 [DOI] [PubMed] [Google Scholar]
- Lore R, Flannelly K, 1977. Rat societies. Scientific American 236, 106–118. [DOI] [PubMed] [Google Scholar]
- Mabry KE, Streatfeild CA, Keane B, Solomon NG, 2011. avpr1a length polymorphism is not associated with either social or genetic monogamy in free-living prairie voles. Animal Behaviour 81, 11–18. 10.1016/j.anbehav.2010.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machida T, Yonezawa Y, Noumura T, 1981. Age-associated changes in plasma testosterone levels in male mice and their relation to social dominance or subordinance. Hormones and Behavior 15, 238–245. 10.1016/0018-506X(81)90013-1 [DOI] [PubMed] [Google Scholar]
- Mackintosh JH, 1970. Territory formation by laboratory mice. Animal Behaviour 18, 177–183. 10.1016/0003-3472(70)90088-6 [DOI] [Google Scholar]
- Madison DM, 1980. Space use and social structure in meadow voles, Microtus pennsylvanicus. Behav Ecol Sociobiol 7, 65–71. 10.1007/BF00302520 [DOI] [Google Scholar]
- Madison DM, FitzGerald RW, McShea WJ, 1984. Dynamics of social nesting in overwintering meadow voles (Microtus pennsylvanicus): possible consequences for population cycling. Behav Ecol Sociobiol 15, 9–17. 10.1007/BF00310209 [DOI] [Google Scholar]
- Madison DM, Mcshea WJ, 1987. Seasonal Changes in Reproductive Tolerance, Spacing, and Social Organization in Meadow Voles: A Microtine Model. Integr Comp Biol 27, 899–908. 10.1093/icb/27.3.899 [DOI] [Google Scholar]
- Makinson R, Lundgren KH, Seroogy KB, Herman JP, 2015. Chronic social subordination stress modulates glutamic acid decarboxylase (GAD) 67 mRNA expression in central stress circuits. Physiology & Behavior, A Special Issue in honour of Robert Blanchard 146, 7–15. 10.1016/j.physbeh.2015.04.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malatynska E, Knapp RJ, 2005. Dominant–submissive behavior as models of mania and depression. Neuroscience & Biobehavioral Reviews, Animal Models of Depression and Antidepressant Activity 29, 715–737. 10.1016/j.neubiorev.2005.03.014 [DOI] [PubMed] [Google Scholar]
- Marchette RCN, Bicca MA, Santos E.C. da S., de Lima, T.C.M., 2018. Distinctive stress sensitivity and anxiety-like behavior in female mice: Strain differences matter. Neurobiol Stress 9, 55–63. 10.1016/j.ynstr.2018.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClintock MK, Adler NT, 1978. The role of the female during copulation in wild and domestic Norway rats (Rattus norvegicus). Behaviour 67, 67–96. 10.1163/156853978X00260 [DOI] [Google Scholar]
- McEwen BS, McKittrick CR, Tamashiro KLK, Sakai RR, 2015. The brain on stress: Insight from studies using the Visible Burrow System. Physiology & Behavior, A Special Issue in honour of Robert Blanchard 146, 47–56. 10.1016/j.physbeh.2015.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire B, Getz LL, 2010. Alternative male reproductive tactics in a natural population of prairie voles Microtus ochrogaster. Acta Theriol 55, 261–270. 10.4098/j.at.0001-7051.077.2009 [DOI] [Google Scholar]
- McKinney TD, Desjardins C, 1973. Postnatal Development of the Testis, Fighting Behavior, and Fertility in House Mice. Biol Reprod 9, 279–294. 10.1093/biolreprod/9.3.279 [DOI] [PubMed] [Google Scholar]
- McKittrick CR, Caroline Blanchard D, Blanchard RJ, McEwen BS, Sakai RR, 1995. Serotonin receptor binding in a colony model of chronic social stress. Biological Psychiatry 37, 383–393. 10.1016/0006-3223(94)00152-S [DOI] [PubMed] [Google Scholar]
- McKittrick CR, Magariños AM, Blanchard DC, Blanchard RJ, McEwen BS, Sakai RR, 2000. Chronic social stress reduces dendritic arbors in CA3 of hippocampus and decreases binding to serotonin transporter sites. Synapse 36, 85–94. 10.1002/(SICI)1098-2396(200005)36:2<85::AID-SYN1>3.0.CO;2-Y [DOI] [PubMed] [Google Scholar]
- McNeal N, Scotti M-AL, Wardwell J, Chandler DL, Bates SL, LaRocca M, Trahanas DM, Grippo AJ, 2014. Disruption of social bonds induces behavioral and physiological dysregulation in male and female prairie voles. Autonomic Neuroscience 180, 9–16. 10.1016/j.autneu.2013.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McShea WJ, 1990. Social tolerance and proximate mechanisms of dispersal among winter groups of meadow voles, Microtus pennsylvanicus. Animal Behaviour 39, 346–351. 10.1016/S0003-3472(05)80880-2 [DOI] [Google Scholar]
- Medger K, Bennett NC, Ganswindt SB, Ganswindt A, Hart DW, 2019. Changes in prolactin, cortisol and testosterone concentrations during queen succession in a colony of naked mole-rats (Heterocephalus glaber): a case study. Sci Nat 106, 26 10.1007/s00114-019-1621-1 [DOI] [PubMed] [Google Scholar]
- Medger K, Bennett NC, Lutermann H, Ganswindt A, 2018. Non-invasive assessment of glucocorticoid and androgen metabolite levels in cooperatively breeding Damaraland mole-rats (Fukomys damarensis). General and Comparative Endocrinology 266, 202–210. 10.1016/j.ygcen.2018.05.018 [DOI] [PubMed] [Google Scholar]
- Melhorn SJ, Elfers CT, Scott KA, Sakai RR, 2017. A closer look at the subordinate population within the visible burrow system. Physiology & Behavior, Celebrating the Science of Dr. Randall Sakai: From Salt to Stress to Obesity and Back Again 178, 110–116. 10.1016/j.physbeh.2017.01.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melhorn SJ, Krause EG, Scott KA, Mooney MR, Johnson JD, Woods SC, Sakai RR, 2010. Meal patterns and hypothalamic NPY expression during chronic social stress and recovery. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology 299, R813–R822. 10.1152/ajpregu.00820.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miczek KA, Maxson SC, Fish EW, Faccidomo S, 2001. Aggressive behavioral phenotypes in mice. Behavioural Brain Research 125, 167–181. 10.1016/S0166-4328(01)00298-4 [DOI] [PubMed] [Google Scholar]
- Monder C, Sakai RR, Miroff Y, Blanchard DC, Blanchard RJ, 1994. Reciprocal changes in plasma corticosterone and testosterone in stressed male rats maintained in a visible burrow system: evidence for a mediating role of testicular 11 beta-hydroxysteroid dehydrogenase. Endocrinology 134, 1193–1198. 10.1210/endo.134.3.8119159 [DOI] [PubMed] [Google Scholar]
- Moolman M, Bennett NC, Schoeman AS, 1998. The social structure and dominance hierarchy of the highveld mole-rat Cryptomys hottentotus pretoriae (Rodentia: Bathyergidae). Journal of Zoology 246, 193–201. 10.1111/j.1469-7998.1998.tb00148.x [DOI] [Google Scholar]
- Mooney SJ, Filice DCS, Douglas NR, Holmes MM, 2015. Task specialization and task switching in eusocial mammals. Animal Behaviour 109, 227–233. 10.1016/j.anbehav.2015.08.019 [DOI] [Google Scholar]
- Mumtaz F, Khan MI, Zubair M, Dehpour AR, 2018. Neurobiology and consequences of social isolation stress in animal model—A comprehensive review. Biomedicine & Pharmacotherapy 105, 1205–1222. 10.1016/j.biopha.2018.05.086 [DOI] [PubMed] [Google Scholar]
- Nguyen MMN, Tamashiro KLK, Melhorn SJ, Ma LY, Gardner SR, Sakai RR, 2007a. Androgenic Influences on Behavior, Body Weight, and Body Composition in a Model of Chronic Social Stress. Endocrinology 148, 6145–6156. 10.1210/en.2007-0471 [DOI] [PubMed] [Google Scholar]
- Nguyen MMN, Tamashiro KLK, Melhorn SJ, Ma LY, Gardner SR, Sakai RR, 2007b. Androgenic Influences on Behavior, Body Weight, and Body Composition in a Model of Chronic Social Stress. Endocrinology 148, 6145–6156. 10.1210/en.2007-0471 [DOI] [PubMed] [Google Scholar]
- Novikov EA, Kondratyuk EY, Burda H, 2015. Age-related increase of urine cortisol in non-breeding individuals of Fukomys anselli (Rodentia, Bathyergidae) from a laboratory colony. Zoologichesky Zhurnal 94, 119–124. 10.7868/S0044513415010092 [DOI] [Google Scholar]
- Ondrasek NR, Wade A, Burkhard T, Hsu K, Nguyen T, Post J, Zucker I, 2015. Environmental modulation of same-sex affiliative behavior in female meadow voles (Microtus pennsylvanicus). Physiology & Behavior 140, 118–126. 10.1016/j.physbeh.2014.12.021 [DOI] [PubMed] [Google Scholar]
- Oosthuizen MK, Amrein I, 2016. Trading new neurons for status: Adult hippocampal neurogenesis in eusocial Damaraland mole-rats. Neuroscience 324, 227–237. 10.1016/j.neuroscience.2016.03.020 [DOI] [PubMed] [Google Scholar]
- Opendak M, Offit L, Monari P, Schoenfeld TJ, Sonti AN, Cameron HA, Gould E, 2016. Lasting Adaptations in Social Behavior Produced by Social Disruption and Inhibition of Adult Neurogenesis. J. Neurosci 36, 7027–7038. 10.1523/JNEUROSCI.4435-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ophir AG, Phelps SM, Sorin AB, Wolff JO, 2008a. Social but not genetic monogamy is associated with greater breeding success in prairie voles. Animal Behaviour 75, 1143–1154. 10.1016/j.anbehav.2007.09.022 [DOI] [Google Scholar]
- Ophir AG, Wolff JO, Phelps SM, 2008b. Variation in neural V1aR predicts sexual fidelity and space use among male prairie voles in semi-natural settings. PNAS 105, 1249–1254. 10.1073/pnas.0709116105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Riain MJ, Jarvis JUM, Faulkes CG, 1996. A dispersive morph in the naked mole-rat. Nature 380, 619–621. 10.1038/380619a0 [DOI] [PubMed] [Google Scholar]
- Ossenkopp K-P, van Anders SM, Engeland CG, Kavaliers M, 2005. Influence of photoperiod and sex on locomotor behavior of meadow voles (Microtus pennsylvanicus) in an automated light–dark ‘anxiety’ test. Psychoneuroendocrinology 30, 869–879. 10.1016/j.psyneuen.2005.05.001 [DOI] [PubMed] [Google Scholar]
- Palanza P, Della Seta D, Ferrari PF, Parmigiani S, 2005. Female competition in wild house mice depends upon timing of female/male settlement and kinship between females. Animal Behaviour 69, 1259–1271. [Google Scholar]
- Pardon M-C, Gould GG, Garcia A, Phillips L, Cook MC, Miller SA, Mason PA, Morilak DA, 2002. Stress reactivity of the brain noradrenergic system in three rat strains differing in their neuroendocrine and behavioral responses to stress: implications for susceptibility to stress-related neuropsychiatric disorders. Neuroscience 115, 229–242. 10.1016/S0306-4522(02)00364-0 [DOI] [PubMed] [Google Scholar]
- Parker KJ, Lee TM, 2003. Female meadow voles (Microtus pennsylvanicus) demonstrate same-sex partner preferences. Journal of Comparative Psychology 117, 283–289. 10.1037/0735-7036.117.3.283 [DOI] [PubMed] [Google Scholar]
- Parker KJ, Phillips KM, Kinney LF, Lee TM, 2001. Day length and sociosexual cohabitation alter central oxytocin receptor binding in female meadow voles (Microtus pennsylvanicus). Behavioral Neuroscience 115, 1349–1356. 10.1037/0735-7044.115.6.1349 [DOI] [PubMed] [Google Scholar]
- Parmigiani S, Palanza P, Rodgers J, Ferrari PF, 1999. Selection, evolution of behavior and animal models in behavioral neuroscience. Neuroscience & Biobehavioral Reviews 23, 957–970. 10.1016/S0149-7634(99)00029-9 [DOI] [PubMed] [Google Scholar]
- Patzenhauerová H, Šklíba J, Bryja J, Šumbera R, 2013. Parentage analysis of Ansell’s mole-rat family groups indicates a high reproductive skew despite relatively relaxed ecological constraints on dispersal. Molecular Ecology 22, 4988–5000. 10.1111/mec.12434 [DOI] [PubMed] [Google Scholar]
- Peleh T, Ike KGO, Wams EJ, Lebois EP, Hengerer B, 2019. The reverse translation of a quantitative neuropsychiatric framework into preclinical studies: Focus on social interaction and behavior. Neuroscience & Biobehavioral Reviews, Quantitative neurosymptomatics; linking quantitative biology to Neuropsychiatry 97, 96–111. 10.1016/j.neubiorev.2018.07.018 [DOI] [PubMed] [Google Scholar]
- Peragine DE, Simpson JA, Mooney SJ, Lovern MB, Holmes MM, 2014. Social regulation of adult neurogenesis in a eusocial mammal. Neuroscience 268, 10–20. 10.1016/j.neuroscience.2014.02.044 [DOI] [PubMed] [Google Scholar]
- Peragine DE, Yousuf Y, Fu Y, Swift-Gallant A, Ginzberg K, Holmes MM, 2016. Contrasting effects of opposite- versus same-sex housing on hormones, behavior and neurogenesis in a eusocial mammal. Hormones and Behavior 81, 28–37. 10.1016/j.yhbeh.2016.03.005 [DOI] [PubMed] [Google Scholar]
- Peuler JD, Scotti M-AL, Phelps LE, McNeal N, Grippo AJ, 2012. Chronic social isolation in the prairie vole induces endothelial dysfunction: implications for depression and cardiovascular disease. Physiology & Behavior 106, 476–484. 10.1016/j.physbeh.2012.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plank A-C, von Hörsten S, Canneva F, 2016. Combining classical comprehensive with ethological based, high-throughput automated behavioral phenotyping for rodent models of stroke, in: Rodent Models of Stroke. Springer, pp. 243–261. [Google Scholar]
- Pobbe RLH, Pearson BL, Defensor EB, Bolivar VJ, Blanchard DC, Blanchard RJ, 2010. Expression of social behaviors of C57BL/6J versus BTBR inbred mouse strains in the visible burrow system. Behavioural Brain Research 214, 443–449. 10.1016/j.bbr.2010.06.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole TB, Morgan HDR, 1976. Social and territorial behaviour of laboratory mice (Mus musculus L.) in small complex areas. Animal Behaviour 24, 476–480. 10.1016/S0003-3472(76)80056-5 [DOI] [Google Scholar]
- Pyter LM, Weil ZM, Nelson RJ, 2005. Latitude affects photoperiod-induced changes in immune response in meadow voles (Microtus pennsylvanicus). Can. J. Zool 83, 1271–1278. 10.1139/z05-121 [DOI] [Google Scholar]
- Razzoli M, Carboni L, Andreoli M, Ballottari A, Arban R, 2011 Different susceptibility to social defeat stress of BalbC and C57BL6/J mice. Behavioural Brain Research 216, 100–108. 10.1016/j.bbr.2010.07.014 [DOI] [PubMed] [Google Scholar]
- Reber SO, Birkeneder L, Veenema AH, Obermeier F, Falk W, Straub RH, Neumann ID, 2007. Adrenal Insufficiency and Colonic Inflammation after a Novel Chronic Psycho-Social Stress Paradigm in Mice: Implications and Mechanisms. Endocrinology 148, 670–682. 10.1210/en.2006-0983 [DOI] [PubMed] [Google Scholar]
- Redfern WS, Tse K, Grant C, Keerie A, Simpson DJ, Pedersen JC, Rimmer V, Leslie L, Klein SK, Karp NA, Sillito R, Chartsias A, Lukins T, Heward J, Vickers C, Chapman K, Armstrong JD, 2017. Automated recording of home cage activity and temperature of individual rats housed in social groups: The Rodent Big Brother project. PLOS ONE 12, e0181068 10.1371/journal.pone.0181068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich LM, 1981. Microtus pennsylvanicus. Mamm Species 1–8. 10.2307/3503976 [DOI] [Google Scholar]
- Reitz K, 2014. Neuroendocrinology of stress and social behavior in female meadow voles. Theses, Dissertations, and Projects. [Google Scholar]
- Roberts RL, Cushing BS, Carter CS, 1998. Intraspecific variation in the induction of female sexual receptivity in prairie voles. Physiology & Behavior 64, 209–212. 10.1016/S0031-9384(98)00042-0 [DOI] [PubMed] [Google Scholar]
- Rosenthal N, Brown S, 2007. The mouse ascending: perspectives for human-disease models. Nature Cell Biology 9, 993–999. 10.1038/ncb437 [DOI] [PubMed] [Google Scholar]
- Ruscio MG, Sweeny T, Hazelton J, Suppatkul P, Sue Carter C, 2007. Social environment regulates corticotropin releasing factor, corticosterone and vasopressin in juvenile prairie voles. Hormones and Behavior 51, 54–61. 10.1016/j.yhbeh.2006.08.004 [DOI] [PubMed] [Google Scholar]
- Russo SJ, Nestler EJ, 2013. The brain reward circuitry in mood disorders. Nat. Rev. Neurosci 14, 609–625. 10.1038/nrn3381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusu AS, Krackow S, 2004. Kin-preferential cooperation, dominance-dependent reproductive skew, and competition for mates in communally nesting female house mice. Behavioral Ecology and Sociobiology 56, 298–305. [Google Scholar]
- Sabol AC, Solomon NG, Dantzer B, 2018. How to study socially monogamous behavior in secretive animals? Using social network analyses and automated tracking systems to study the social behavior of prairie voles. Front. Ecol. Evol 6 10.3389/fevo.2018.00178 [DOI] [Google Scholar]
- Sandi C, Haller J, 2015. Stress and the social brain: behavioural effects and neurobiological mechanisms. Nature Reviews Neuroscience 16, 290–304. 10.1038/nrn3918 [DOI] [PubMed] [Google Scholar]
- Schmidt MV, Scharf SH, Liebl C, Harbich D, Mayer B, Holsboer F, Müller MB, 2010. A novel chronic social stress paradigm in female mice. Hormones and Behavior 57, 415–420. 10.1016/j.yhbeh.2010.01.010 [DOI] [PubMed] [Google Scholar]
- Schmidt MV, Sterlemann V, Ganea K, Liebl C, Alam S, Harbich D, Greetfeld M, Uhr M, Holsboer F, Müller MB, 2007. Persistent neuroendocrine and behavioral effects of a novel, etiologically relevant mouse paradigm for chronic social stress during adolescence. Psychoneuroendocrinology 32, 417–429. 10.1016/j.psyneuen.2007.02.011 [DOI] [PubMed] [Google Scholar]
- Scholtens J, Roozen M, Mirmiran M, Van de Poll NE, 1990. Role of noradrenaline in behavioral changes after defeat in male and female rats. Behavioural brain research 36, 199–202. [DOI] [PubMed] [Google Scholar]
- Schweinfurth MK, Neuenschwander J, Engqvist L, Schneeberger K, Rentsch AK, Gygax M, Taborsky M, 2017. Do female Norway rats form social bonds? Behav Ecol Sociobiol 71, 98 10.1007/s00265-017-2324-2 [DOI] [Google Scholar]
- Shemesh Y, Sztainberg Y, Forkosh O, Shlapobersky T, Chen A, Schneidman E, 2013. High-order social interactions in groups of mice. eLife 2, e00759 10.7554/eLife.00759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sial OK, Warren BL, Alcantara LF, Parise EM, Bolaños-Guzmán CA, 2016. Vicarious social defeat stress: Bridging the gap between physical and emotional stress. J. Neurosci. Methods 258, 94–103. 10.1016/j.jneumeth.2015.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sichilima AM, Bennett NC, Faulkes CG, 2011. Field evidence for colony size and aseasonality of breeding and in Ansell’s mole-rat, Fukomys anselli (Rodentia: Bathyergidae). African Zoology 46, 334–339. 10.1080/15627020.2011.11407506 [DOI] [Google Scholar]
- Smith AS, Wang Z, 2018. The Neurobiological influence of stress in the vole pair bond, in: Meyza KZ, Knapska E (Eds.), Neuronal Correlates of Empathy. Academic Press, pp. 79–91. 10.1016/B978-0-12-805397-3.00007-3 [DOI] [Google Scholar]
- Smith AS, Wang Z, 2014. Hypothalamic Oxytocin Mediates Social Buffering of the Stress Response. Biological Psychiatry, Neurobiological Moderators of Stress Response 76, 281–288. 10.1016/j.biopsych.2013.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P, Berdoy M, Smith RH, 1994. Body weight and social dominance in anticoagulant-resistant rats. Crop Protection 13, 311–315. 10.1016/0261-2194(94)90022-1 [DOI] [Google Scholar]
- So N, Franks B, Lim S, Curley JP, 2015. A social network approach reveals associations between mouse social dominance and brain gene expression. PLoS ONE 10, e0134509 10.1371/journal.pone.0134509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon NG, Richmond AR, Harding PA, Fries A, Jacquemin S, Schaefer RL, Lucia KE, Keane B, 2009. Polymorphism at the avpr1a locus in male prairie voles correlated with genetic but not social monogamy in field populations. Molecular Ecology 18, 4680–4695. 10.1111/j.1365-294X.2009.04361.x [DOI] [PubMed] [Google Scholar]
- Späni D, Arras M, König B, Rülicke T, 2003. Higher heart rate of laboratory mice housed individually vs in pairs. Laboratory Animals 37, 54–62. [DOI] [PubMed] [Google Scholar]
- Spencer RL, Miller AH, Moday H, McEwen BS, Blanchard RJ, Blanchard DC, Sakai RR, 1996. Chronic social stress produces reductions in available splenic type II corticosteroid receptor binding and plasma corticosteroid binding globulin levels. Psychoneuroendocrinology 21, 95–109. 10.1016/0306-4530(95)00020-8 [DOI] [PubMed] [Google Scholar]
- Stalling DT, 1990. Microtus ochrogaster. Mamm Species 1–9. 10.2307/3504103 [DOI] [Google Scholar]
- Stefanski V, Knopf G, Schulz S, 2001. Long-term colony housing in Long Evans rats: immunological, hormonal, and behavioral consequences. Journal of Neuroimmunology 114, 122–130. [DOI] [PubMed] [Google Scholar]
- Sterlemann V, Ganea K, Liebl C, Harbich D, Alam S, Holsboer F, Müller MB, Schmidt MV, 2008. Long-term behavioral and neuroendocrine alterations following chronic social stress in mice: Implications for stress-related disorders. Hormones and Behavior 53, 386–394. 10.1016/j.yhbeh.2007.11.001 [DOI] [PubMed] [Google Scholar]
- Stowe JR, Liu Y, Curtis JT, Freeman ME, Wang Z, 2005. Species differences in anxiety-related responses in male prairie and meadow voles: The effects of social isolation. Physiology & Behavior, Florida State University Special Issue 86, 369–378. 10.1016/j.physbeh.2005.08.007 [DOI] [PubMed] [Google Scholar]
- Suckow MA, Hankenson FC, Wilson RP, Foley PL, 2019. The Laboratory Rat. Academic Press. [Google Scholar]
- Swift-Gallant A, Mo K, Peragine DE, Monks DA, Holmes MM, 2015. Removal of reproductive suppression reveals latent sex differences in brain steroid hormone receptors in naked mole-rats, Heterocephalus glaber. Biology of Sex Differences 6, 31 10.1186/s13293-015-0050-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taborsky M, Hofmann HA, Beery AK, Blumstein DT, Hayes LD, Lacey EA, Martins EP, Phelps SM, Solomon NG, Rubenstein DR, 2015. Taxon matters: promoting integrative studies of social behavior: NESCent Working Group on Integrative Models of Vertebrate Sociality: Evolution, Mechanisms, and Emergent Properties. Trends in Neurosciences 38, 189–191. 10.1016/j.tins.2015.01.004 [DOI] [PubMed] [Google Scholar]
- Takahashi A, Chung J-R, Zhang S, Zhang H, Grossman Y, Aleyasin H, Flanigan ME, Pfau ML, Menard C, Dumitriu D, Hodes GE, McEwen BS, Nestler EJ, Han M-H, Russo SJ, 2017. Establishment of a repeated social defeat stress model in female mice. Scientific Reports 7, 1–12. 10.1038/s41598-017-12811-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi A, Flanigan ME, McEwen BS, Russo SJ, 2018. Aggression, Social Stress, and the Immune System in Humans and Animal Models. Front Behav Neurosci 12 10.3389/fnbeh.2018.00056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamashiro KLK, Hegeman MA, Nguyen MMN, Melhorn SJ, Ma LY, Woods SC, Sakai RR, 2007a. Dynamic body weight and body composition changes in response to subordination stress. Physiology & Behavior, Proceedings from the 2006 Meeting of the Society for the Study of Ingestive Behavior 91, 440–448. 10.1016/j.physbeh.2007.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamashiro KLK, Nguyen MMN, Fujikawa T, Xu T, Yun Ma L, Woods SC, Sakai RR, 2004. Metabolic and endocrine consequences of social stress in a visible burrow system. Physiology & Behavior 80, 683–693. 10.1016/j.physbeh.2003.12.002 [DOI] [PubMed] [Google Scholar]
- Tamashiro KLK, Nguyen MMN, Ostrander MM, Gardner SR, Ma LY, Woods SC, Sakai RR, 2007b. Social stress and recovery: implications for body weight and body composition. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology 293, R1864–R1874. 10.1152/ajpregu.00371.2007 [DOI] [PubMed] [Google Scholar]
- Tamashiro KLK, Nguyen MMN, Sakai RR, 2005. Social stress: From rodents to primates. Frontiers in Neuroendocrinology 26, 27–40. 10.1016/j.yfrne.2005.03.001 [DOI] [PubMed] [Google Scholar]
- Thorsteinsson EB, James JE, Gregg ME, 1998. Effects of video-relayed social support on hemodynamic reactivity and salivary cortisol during laboratory-based behavioral challenge. Health Psychology 17, 436. [DOI] [PubMed] [Google Scholar]
- Timmer M, Cordero MI, Sevelinges Y, Sandi C, 2011. Evidence for a Role of Oxytocin Receptors in the Long-Term Establishment of Dominance Hierarchies. Neuropsychopharmacology 36, 2349–2356. 10.1038/npp.2011.125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toor I, Kaka N, Whitney R, Ziolkowski J, Monks DA, Holmes MM, submitted. Aggression and motivation to disperse in eusocial naked mole-rats (Heterocephalus glaber). [Google Scholar]
- Torrents‐Ticó M, Bennett NC, Jarvis JUM, Zöttl M, 2018. Sex differences in timing and context of dispersal in Damaraland mole-rats (Fukomys damarensis). Journal of Zoology 306, 252–257. 10.1111/jzo.12602 [DOI] [Google Scholar]
- Turner BN, Iverson SL, Severson KL, 1983. Seasonal changes in open-field behavior in wild male meadow voles (Microtus pennsylvanicus). Behavioral and Neural Biology 39, 60–77. 10.1016/S0163-1047(83)90637-4 [DOI] [PubMed] [Google Scholar]
- Turney TH, Harmsen AG, 1984. Splenomegaly and other hematological parameters in the socially dominant mouse. Physiology & behavior 33, 559–562. [DOI] [PubMed] [Google Scholar]
- Ulrich J, 1938. The social hierarchy in albino mice. Journal of Comparative Psychology 25, 373. [Google Scholar]
- Vamvakopoulos NC, Chrousos GP, 1993. Evidence of direct estrogenic regulation of human corticotropin-releasing hormone gene expression. Potential implications for the sexual dimophism of the stress response and immune/inflammatory reaction. J Clin Invest 92, 1896–1902. 10.1172/JCI116782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Loo PLP, Mol JA, Koolhaas JM, Van Zutphen BFM, Baumans V, 2001. Modulation of aggression in male mice: influence of group size and cage size. Physiology & Behavior 72, 675–683. 10.1016/S0031-9384(01)00425-5 [DOI] [PubMed] [Google Scholar]
- Walum H, Young LJ, 2018. The neural mechanisms and circuitry of the pair bond. Nature Reviews Neuroscience 19, 643–654. 10.1038/s41583-018-0072-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidt A, Gygax L, Palme R, Touma C, König B, 2018. Impact of male presence on female sociality and stress endocrinology in wild house mice (Mus musculus domesticus). Physiology & behavior 189, 1–9. [DOI] [PubMed] [Google Scholar]
- Weissbrod A, Shapiro A, Vasserman G, Edry L, Dayan M, Yitzhaky A, Hertzberg L, Feinerman O, Kimchi T, 2013. Automated long-term tracking and social behavioural phenotyping of animal colonies within a semi-natural environment. Nat Commun 4, 2018 10.1038/ncomms3018 [DOI] [PubMed] [Google Scholar]
- Williams JR, Catania KC, Carter CS, 1992. Development of partner preferences in female prairie voles (Microtus ochrogaster): The role of social and sexual experience. Hormones and Behavior 26, 339–349. 10.1016/0018-506X(92)90004-F [DOI] [PubMed] [Google Scholar]
- Williamson CM, Franks B, Curley JP, 2016a. Mouse Social Network Dynamics and Community Structure are Associated with Plasticity-Related Brain Gene Expression. Front. Behav. Neurosci 152 10.3389/fnbeh.2016.00152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson CM, Klein IS, Lee W, Curley JP, 2019a. Immediate early gene activation throughout the brain is associated with dynamic changes in social context. Social Neuroscience 14, 253–265. 10.1080/17470919.2018.1479303 [DOI] [PubMed] [Google Scholar]
- Williamson CM, Lee W, Curley JP, 2016b. Temporal dynamics of social hierarchy formation and maintenance in male mice. Animal Behaviour 115, 259–272. 10.1016/j.anbehav.2016.03.004 [DOI] [Google Scholar]
- Williamson CM, Lee W, DeCasien AR, Lanham A, Romeo RD, Curley JP, 2019b. Social hierarchy position in female mice is associated with plasma corticosterone levels and hypothalamic gene expression. Scientific Reports 9, 1–14. 10.1038/s41598-019-43747-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson CM, Lee W, Romeo RD, Curley JP, 2017a. Social context-dependent relationships between mouse dominance rank and plasma hormone levels. Physiology & Behavior 171, 110–119. 10.1016/j.physbeh.2016.12.038 [DOI] [PubMed] [Google Scholar]
- Williamson CM, Romeo RD, Curley JP, 2017b. Dynamic changes in social dominance and mPOA GnRH expression in male mice following social opportunity. Horm Behav 87, 80–88. 10.1016/j.yhbeh.2016.11.001 [DOI] [PubMed] [Google Scholar]
- Winslow JT, Hastings N, Carter CS, Harbaugh CR, Insel TR, 1993. A role for central vasopressin in pair bonding in monogamous prairie voles. Nature 365, 545–548. 10.1038/365545a0 [DOI] [PubMed] [Google Scholar]
- Wolff JO, Lidicker WZ Jr., 1980. Population ecology of the taiga vole, Microtus xanthognathus, in interior Alaska. Can. J. Zool 58, 1800–1812. 10.1139/z80-247 [DOI] [Google Scholar]
- Wood SK, Baez MA, Bhatnagar S, Valentino RJ, 2009. Social stress-induced bladder dysfunction: potential role of corticotropin-releasing factor. Am J Physiol Regul Integr Comp Physiol 296, R1671–R1678. 10.1152/ajpregu.91013.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasukawa NJ, Monder H, Leff FR, Christian JJ, 1985. Role of female behavior in controlling population growth in mice. Aggressive Behavior 11, 49–64. [Google Scholar]
- Young AJ, Oosthuizen MK, Lutermann H, Bennett NC, 2010. Physiological suppression eases in Damaraland mole-rat societies when ecological constraints on dispersal are relaxed. Hormones and Behavior 57, 177–183. 10.1016/j.yhbeh.2009.10.011 [DOI] [PubMed] [Google Scholar]
- Young LJ, 1999. Oxytocin and Vasopressin Receptors and Species-Typical Social Behaviors. Hormones and Behavior 36, 212–221. 10.1006/hbeh.1999.1548 [DOI] [PubMed] [Google Scholar]
- Zucker I, Beery AK, 2010. Males still dominate animal studies. Nature 465, 690–690. 10.1038/465690a [DOI] [PubMed] [Google Scholar]


