Abstract
Background
Sporadic Creutzfeldt-Jakob disease (sCJD) is an extremely rare fatal and infectious neurodegenerative brain disorder characterized by rapidly progressive dementia, cerebellar ataxia, and visual disturbances. This article summarizes the retrospective analysis of 104 sCJD patients in the First Medical Center of Chinese PLA General Hospital from 2003 to 2019.
Methods
A retrospective analysis of the medical records of the 104 patients diagnosed with sCJD was performed from the aspects of demographic data, clinical manifestations, laboratory examinations, cerebrospinal fluid analysis, electroencephalograms (EEGs), diffusion-weighted imaging (DWI) scans, positron emission tomography (PET) scans, and prion protein gene mutations.
Results
In the 104 sCJD patients, pathological evidence of a spongiform change was found in 11 patients, while the remaining 93 patients were probable sCJD. The 104 patients included 57 males and 47 females, with the age of onset ranging from 29 to 82 (mean: 58, median: 60) years. The time from disease onset to death ranged from 1 to 36 months. Most of the patients died 7–12 months after the onset of sCJD. In most patients, rapidly progressive dementia appeared as the initial symptom, followed by cerebellar ataxia, visual disturbances, and neurobehavioral disorders. Most patients' DWI images showed symmetric or asymmetric hyperintensity in the cortex. In terms of EEGs, 38.2% of the patients had periodic sharp wave complexes. The sensitivity of 14-3-3 protein detection was 34.1%. The brain PET scans of 50 patients with sCJD presented 96% sensitivity for the diagnosis of sCJD.
Conclusions
This study indicated that sCJD occurred at an early age in patients in China. The sensitivity of 14-3-3 protein detection was significantly low, but brain PET was highly sensitive in the diagnosis of sCJD.
Keywords: Neuro-infectiology, Sporadic Creutzfeldt-Jakob disease, Progressive dementia, 14-3-3 protein, Periodic sharp wave complexes
Introduction
Sporadic Creutzfeldt-Jakob disease (sCJD) is a prion protein (PRNP) disease characterized by rapidly progressive dementia, visual disturbances, cerebellar ataxia, and akinetic mutism. In clinical examinations, the result of cerebrospinal fluid (CSF) 14-3-3 protein detection is positive; periodic sharp wave complexes (PSWCs) are seen in the electroencephalogram (EEG) scans; hyperintensity is observed on diffusion-weighted imaging (DWI) scans; positron emission tomography (PET) scans show hypometabolism in the cerebral cortex; and the clinical pathology report suggests the presence of spongiform change, neuronal loss, gliosis, and abnormal PRNP deposition in the brain biopsy [1]. In 1982, Stanley B. Prusiner formulated the “prion hypothesis,” which has dominated the field for the past 30 years [2]. Based on this theory, publications of relevant case reports could be found on every continent of the world. To date, sCJD has been reported to display stable morbidity, with nearly 1 case per 1 million population yearly [3]. However, in different countries, sCJD patients' conditions may vary because of regional and ethnic differences. China has monitored sCJD and has reported nearly 1,000 sCJD cases since 2004. The present study revealed a retrospective analysis of 104 sCJD cases in China and investigated differences between sCJD patients in China and those in other countries to further summarize the disease in terms of demographic data and auxiliary examinations.
Materials and Methods
All sCJD patients involved in this study were admitted to the Department of Neurology of the First Medical Center of Chinese PLA General Hospital from 2003 to 2019. The researchers obtained informed consent from all patients and their family members. In this study, 11 of the 104 patients were diagnosed pathologically after biopsy or autopsy. The enrollment diagnostic criteria of the remaining 93 patients were based on the updated clinical diagnostic criteria for sCJD published in 2009 [4], and they were all probable sCJD patients. For all patients, the diagnosis of possible Hashimoto's encephalopathy, vitamin B12 deficiency, intoxication, autoimmune encephalitis, or paraneoplastic syndrome was ruled out. The patients' conditions (e.g. progression of disease, and death) after discharge were recorded by professional physicians based on the follow-up via telephone. The detection of 14-3-3 protein in the CSF was supported by the Chinese Center for Disease Control (CDC) using Western blot (WB). Hematoxylin and eosin staining of paraffin-embedded brain samples was performed for brain pathology. The researchers performed a retrospective analysis of the cases of 104 sCJD patients from the aspects of demographic data and auxiliary examinations.
Results
Patients' Characteristics
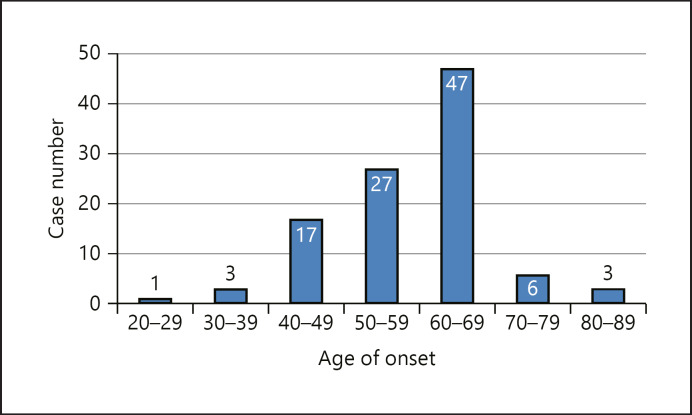
Among the 104 patients, there were 57 males and 47 females (male:female, 1.21:1). As shown in Figure 1, the age of onset of sCJD in patients was 29–82 years, with a mean age of 58 years and a median age of 60 years (20–29 years, n = 1; 30–39 years, n = 3; 40–49 years, n = 17; 50–59 years, n = 27; 60–69 years, n = 47; 70–79 years, n = 6; over 80 years, n = 3). The time from disease onset to death ranged from 1 to 36 (mean: 8.43, median: 9) months, and most patients died within 7–12 months after disease onset. A total of 104 patients came from 16 provinces in China, covering most regions in China. In this study, the number of confirmed sCJD patients increased from 2 in 2003 to 12 in 2019, showing an upward trend year by year. None of the 104 sCJD patients had a history of eating diseased livestock or food contaminated with PRNP. Telephone follow-up showed that none of the family members who contacted or cared for the sCJD patients were infected. Among the 104 patients, the predominant symptom was rapid progressive dementia (100%, 104/104), followed by cerebellar ataxia (51.9%, 54/104), akinetic mutism (45.2%, 47/104), pyramidal (43.3%, 45/104) and extrapyramidal (32.7%, 34/104) dysfunction, visual disturbances (31.7%, 33/104), psychiatric disturbances (7.7%, 8/104), and sleep disorders (4.8%, 5/104). Patients' demographic data, symptoms, and auxiliary examinations are summarized in Table 1.
Fig. 1.
Frequency histogram of onset age with a normal curve. Mean = 58, median = 60, range: 29–82, and N = 104.
Table 1.
Demographic data, symptoms, and auxiliary examinations of sCJD patients
| Demographic data, symptoms, and auxiliary examinations | Our cases |
|---|---|
| Gender (male:female) | 1.21:1 |
| Median age at onset (range), years | 60 (29–82) |
| Duration of disease (range), months | 9 (1–36) |
| Rapid progressive dementia | 100% (104/104) |
| Cerebellar ataxia | 51.9% (54/104) |
| Akinetic mutism | 45.2% (47/104) |
| Pyramidal | 43.3% (45/104) |
| Extrapyramidal | 32.7% (34/104) |
| Visual disturbance | 31.7% (33/104) |
| Psychiatric | 7.7% (8/104) |
| Sleep disorders | 4.8% (5/104) |
| DWI changes (hyperintensity) | 88.2% (90/102) |
| Cortex | 82.4% (84/102) |
| Basal ganglia | 38.2% (39/102) |
| Thalamus | 8.8% (9/102) |
| 14-3-3 protein in the CSF (positive) | 34.1% (15/44) |
| EEG changes (PSWCs) | 38.2% (39/102) |
| PET changes (hypometabolism) | 96% (48/50) |
| Biopsy | 10/104 |
| Autopsy | 1/104 |
sCJD, sporadic Creutzfeldt-Jakob disease; DWI, diffusion-weighted imaging; CSF, cerebrospinal fluid; EEG, electroencephalogram; PSWCs, periodic sharp wave complexes; PET, positron emission tomography.
Auxiliary Examination
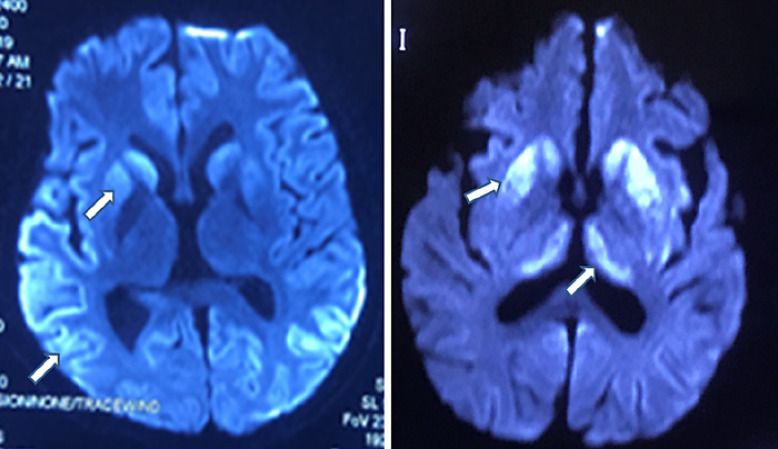
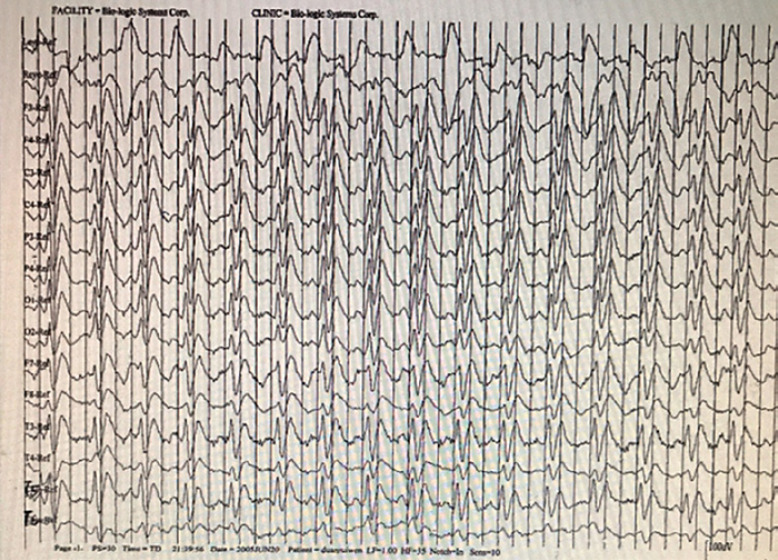
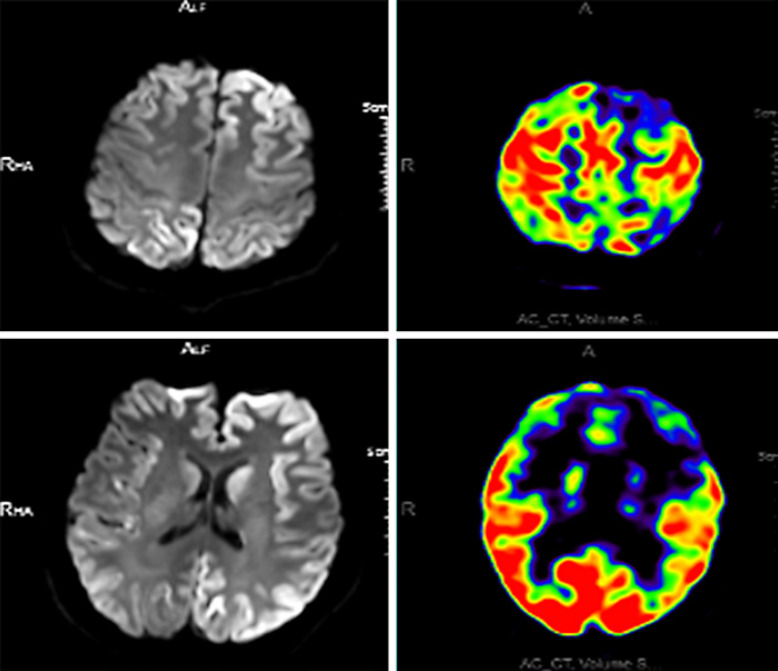
A total of 92 sCJD patients underwent lumbar puncture examination, of whom 15 showed a slight increase in CSF proteins (range: 407–937 mg/L), and the remaining 77 patients presented no significant abnormalities in white blood cell count, proteins, glucose, or chlorides in the CSF. As the detection of 14-3-3 protein in the CSF is an invasive procedure, informed consent from patients or their families is needed in advance. CSF samples should be transported at low temperature and delivered to the Chinese CDC in a timely manner for detection. There are some practical issues, such as patients or their families refusing lumbar puncture, failure to deliver CSF samples on time, or failure to store the samples properly. These are the reasons why there were only 44 CSF samples delivered to the CDC for 14-3-3 protein detection. There was no selection bias. Among them, 15 patients had positive WB results for 14-3-3 protein, with a sensitivity of 34.1% (15/44). Of the 104 sCJD patients, 102 underwent DWI. The sensitivity of DWI was 88.2% (90/102) for the diagnosis of sCJD. Table 2 shows the characteristics of patients without abnormal hyperintense signals on DWI. Among patients with abnormal hyperintense signals on DWI, 82.4% (84/102) of patients showed symmetrical or asymmetrical abnormal hyperintense signals in the cortex, 38.2% (39/102) of patients presented abnormal hyperintense signals in the basal ganglia, and 8.8% (9/102) of patients exhibited abnormal hyperintense signals in the thalamus (Fig. 2). Moreover, the three abnormal DWI signals could be displayed on the same patient's DWI scan. Of the 104 patients, 102 underwent EEG examination. Among them, 101 patients showed moderate to severe abnormal EEG changes, such as periodically distributed biphasic waves of 60–120 μV and 3–4 Hz, wide slow waves of 30–70 μV and 5–7 Hz, slow α rhythm with poor amplitude modulation and regulation, increased θ activity with some δ activity, and an EEG profile showing a low-voltage and flat background across the whole brain; however, only 38.2% (39/102) patients had PSWCs (Fig. 3). A total of 50 sCJD patients underwent brain PET-computed tomography (PET-CT) or PET-magnetic resonance imaging (PET-MRI), of whom 96.0% (48/50) had hypometabolism in the cerebral cortex. One patient underwent brain PET-MRI, which also showed hypometabolism in the cerebral cortex, and the extent of the hypometabolism was larger than that of the abnormal hyperintense signal on DWI (Fig. 4).
Table 2.
Characteristics of sCJD patients without DWI abnormal hyperintense signals
| No. | Gender | Age, years | Duration (from onset to death), months | Clinical manifestation | DWI | EEG | 14-3-3 protein | PET | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 36 | 12 | Visual, dementia | Normal | PSWCs | Undetected | Undetected | Died |
| 2 | M | 38 | 4 | Cerebellar, dementia, akinetic mutism | Normal | PSWCs | Negative | Undetected | Died |
| 3 | F | 53 | 7 | Cerebellar, dementia, akinetic mutism | Normal | PSWCs | Negative | Undetected | Died |
| 4 | M | 46 | 5 | Cerebellar, dementia | Normal | Moderate abnormal change | Positive | Undetected | Died |
| 5 | F | 62 | 7 | Cerebellar, dementia, akinetic mutism, extrapyramidal | Normal | PSWCs | Undetected | Undetected | Died |
| 6 | M | 48 | 4 | Mental disorder, dementia, pyramidal | Normal | PSWCs | Undetected | Undetected | Died |
| 7 | F | 63 | 6 | Insomnia, dementia, mental disorder, extrapyramidal | Normal | PSWCs | Undetected | Undetected | Died |
| 8 | M | 82 | 18 | Dementia, akinetic mutism | Normal | PSWCs | Positive | Undetected | Died |
| 9 | M | 81 | 12 | Dementia, akinetic mutism, pyramidal | Normal | PSWCs | Undetected | Undetected | Died |
| 10 | M | 48 | 8 | Cerebellar, dementia | Normal | Moderate abnormal change | Positive | Undetected | Died |
| 11 | M | 67 | 9 | Dementia, cerebellar | Normal | Severe abnormal change | Positive | Undetected | Died |
| 12 | M | 75 | 16 | Dementia, mental disorder, pyramidal | Normal | PSWCs | Undetected | Undetected | Died |
M, male; F, female; DWI, diffusion-weighted imaging; sCJD, sporadic Creutzfeldt-Jakob disease; PSWC, periodic sharp wave complexes; PET, positron emission tomography; EEG, electroencephalogram.
Fig. 2.
MRI-DWI images of an sCJD patient. Hyperintensity of DWI in the cerebral cortex, basal ganglia, and thalamus during the course of the disease. DWI, diffusion-weighted imaging. sCJD, sporadic Creutzfeldt-Jakob disease; MRI, magnetic resonance imaging.
Fig. 3.
PSWCs in an sCJD patient. Image of an EEG scan in an sCJD patient showing PSWCs. sCJD, sporadic Creutzfeldt-Jakob disease; PSWCs, periodic sharp wave complexes; EEG, electroencephalogram.
Fig. 4.
PET-MRI images of the same sCJD patient. Two images of PET-MRI scans in the same patient showing decreased FDG uptake in the cerebral cortex and basal ganglia. PET-MRI, positron emission tomography-magnetic resonance imaging; sCJD, sporadic Creutzfeldt-Jakob disease; FDG, fluorodeoxyglucose.
Of the 104 sCJD patients in this study, 37 underwent PRNP gene detection, in which all patients were methionine M/M homozygous at codon 129 and glutamic acid E/E homozygous at codon 219. Of the 104 sCJD patients, 11 showed evidence of diffuse spongiform changes, gliosis, and neuronal loss, which were confirmed by brain pathology. The remaining 93 patients were clinically confirmed without pathological examination.
Discussion
sCJD is a condition found worldwide, reported in both developed and developing countries. Due to the mostly subacute onset of the disease, clinical manifestations of patients in the early stage are mild but gradually worsen over time. As a result, sCJD may be easily misdiagnosed in the early stage. In this study, a systematic review was conducted on a large scale to retrospectively analyze sCJD from the perspectives of demographic data and auxiliary examinations.
In this study, the age of onset of sCJD in patients was 29–82 years, with a mean age of 58 years and a median age of 60 years, which was younger than the 65 years reported in countries in Europe, South America, and Asia [5] and the 65.5 ± 12.6 years reported in Japan [6]. It was believed that there might be some racial differences among sCJD patients in different countries. Moreover, although the sample size in this study was as large as 100 patients, there might still be bias in the research data. In recent years, an increasing number of young sCJD patients have been reported as clinicians continue to understand the disease [7, 8]. In this study, there were 4 patients aged from 20 to 39 years. None of these patients had a familial gene mutation in the PRNP gene. However, we still consider that these young patients are in urgent need of special attention from neuroscientists. Of the 104 sCJD patients, the time from disease onset to death ranged from 1 to 36 (mean: 8.43, median: 9) months, which was longer than that reported in other studies, for example, 6.6 months (Australia [9]), 7.0 months (Germany [10]), and 6.4 months (data from twelve countries [4]). It was believed that the age of disease onset in patients was younger, when their physical functions were better, and complications such as lung infection and malnutrition appeared later and progressed slowly, to a degree lower than that seen in the older patients. However, it had little to do with medical and nursing levels. Transmission of the condition between 2 patients who were nongenetic relatives had been reported in a foreign study [11, 12]. In the present study, however, individuals in close contact with the 104 patients and their nursing staff were not found to have the condition, and subsequent studies will continue to follow up these individuals.
The early misdiagnosis rate of sCJD was high. Approximately 56.7% (59/104) of the patients enrolled in this study had been misdiagnosed with viral encephalitis, anxiety and depression, cerebral infarction, or optic neuritis in other hospitals. These misdiagnoses were mostly caused by the lack of understanding of sCJD among some clinicians, especially those in primary healthcare services, due to the low incidence of the disease. In addition, the early symptoms of sCJD are not particularly typical. The most common clinical manifestation of sCJD in the early stage was rapid progressive dementia. In this study, in the early stage of the disease, most patients developed onset with rapid progressive dementia, cerebellar ataxia, visual disturbances, and psychiatric symptoms, which was similar to the onset of visual or auditory disturbances, isolated psychiatric manifestations, and isolated aphasia symptoms of sCJD reported in the literature [13].
Brain biopsy for sCJD is not as widely adopted as the diagnostic method for cerebrovascular or brain tumor diseases. Since sCJD has a high risk of disease transmission through surgical instruments, the preferred option to obtain pathological specimens is not by biopsy or autopsy in China. Therefore, it is urgent to find some examination methods with high sensitivity and specificity to improve the diagnosis of sCJD.
The relationships between sCJD and CSF proteins, such as 14-3-3, tau, and α-synuclein (α-syn), have been investigated for their potential value in premortem diagnosis [14]. In this study, we used only WB to test the level of 14-3-3 protein in the CSF, but the sensitivity was lower than the 82% reported in other countries [15]. Among the 218 sCJD patients reported by the Korean professor Jae-Sung Lin in 2015, only 106 patients were positive for CSF 14-3-3 protein, and the sensitivity was 48.6% [16]. In this regard, we consider that the lower sensitivity of 14-3-3 protein detection in this study may be related to ethnic differences. We also believe that the sensitivity and specificity of the 14-3-3 protein may be related to the progression of sCJD, during which the results of this test may be influenced [17]. In addition, in China, most patients can undergo the test for the 14-3-3 protein in the CSF only once throughout the course of the disease. It was hypothesized that if patients could undergo multiple CSF 14-3-3 protein tests during the course of the disease, the sensitivity might be increased. Recently, real-time quaking-induced conversion (RT-QuIC) assays of CSF and nasal-brushing specimens have been valuable in the diagnosis of sCJD. This technique exploits the ability of the misfolded pathological form of scrapie isoform of the prion protein (PrPSc) found in the CSF to induce the conversion of normal prion protein (PrP) to the misfolded form, which subsequently aggregates. The formation of these aggregates of misfolded PrP is monitored in real time using fluorescent dyes. RT-QuIC technology has achieved a higher detection sensitivity (95.8%) and specificity (100%) [18]. Due to its ultrahigh sensitivity and specificity, it has established itself as an important method in the diagnosis of sCJD. However, due to the special requirements for the use of equipment, this method has not been widely applied. In subsequent research, more attempts and attention should be given to the RT-QuIC detection technology. In this study, EEGs showed a sensitivity of 38.2% for PSWCs, which was significantly lower than the 53–60% [19, 20] reported in studies in other countries. In sCJD, EEGs exhibit characteristic changes depending on the stage of the disease, ranging from nonspecific findings such as diffuse slowing and frontal rhythmic delta activity in the early stages, to disease-typical PSWCs in the middle and late stages, to coma traces or even alpha coma in preterminal EEG recordings. PSWCs, either lateralized (in earlier stages) or generalized, occur in approximately two-thirds of patients with sCJD [20]. In this study, however, 1 patient underwent 6 EEG examinations during the course of the disease, with signals from normal at the beginning to moderate to severely abnormal before the typical PSWCs changed. As the disease progressed, in the later stages, the EEG of the patient became flat, and the PSWCs disappeared. It was hypothesized that repeat EEG examinations or 24-h dynamic EEG could significantly improve the sensitivity of the diagnosis and help clinicians improve their understanding of sCJD. The abnormal hyperintense signal on brain DWI corresponded to spongiform degeneration, mainly due to the small volume of vacuoles and thus reduced water diffusion. This also indicates that spongiform degeneration may be the imaging basis of nuclear magnetic changes. The DWI abnormal hyperintense signal of sCJD is mostly asymmetrical in the early stage, but as the disease progresses, it becomes more symmetrical. The abnormal hyperintense signal on DWI is most commonly found along the cortex, while the abnormal signals of the caudate nucleus and putamen and the abnormal hyperintense signal of posterior thalamic nodules are relatively rare. These signals are associated with the molecular subtype in sporadic cases, the stage and duration of the disease, and pathological findings [21]. Young et al. [22] reported that MRI had a sensitivity of 91% and a specificity of 95% in the diagnosis of sCJD, which were similar to the values in our data. PET uses radioisotopes to label tracers and to detect the level of glucose metabolism in brain cells. If neuronal damage occurs in sCJD patients, it will affect glucose metabolism in brain cells, leading to reduced fluorodeoxyglucose (FDG) uptake. However, because PET hypometabolism changes are more common in neurodegenerative diseases such as Lewy body dementia, Alzheimer's disease, and progressive supranuclear palsy, PET hypometabolism changes have not been included in the diagnostic criteria for sCJD. In our research, a total of 50 patients received PET, and the sensitivity of PET-CT and PET-MRI examinations reached as high as 96%. In addition, in this study, 1 patient underwent PET-MRI examination. In the fusion of PET images and DWI images, PET-MRI of sCJD patients showed a wider range of lesions than DWI alone. Based on the extent and location of hypometabolism, we suppose that PET can help clinicians predict the progression of the disease earlier than DWI. From the aforementioned findings, we consider that changes in PET hypometabolism may provide guiding references for the disease's early diagnosis and differential diagnosis, or even the judgment of disease progression of sCJD, if their analysis can be added to those of the patient's clinical manifestations (rapidly progressive dementia) and other auxiliary examination results (such as high-density DWI, positive results for 14-3-3 protein in the CSF, or PSWCs in EEGs). In addition, as shown in Figure 5, we found that patients with different PRNP diseases had slightly different sites of FDG-PET hypometabolism. FDG-PET hypometabolism of sCJD occurs mainly in the cerebral cortex, basal ganglia, or thalamus, among which the cerebral cortex is the most common. Gerstmann-Sträussler-Scheinker syndrome hypometabolism occurs mainly in the cerebellum, and fatal familial insomnia hypometabolism occurs mainly in the thalamus [23, 24, 25]. These findings will help clinicians identify different PRNP diseases. PRNP gene detection plays an increasingly important role in the diagnosis of PRNP disease. In this study, 35.6% (37/104) of the patients received PRNP gene detection. All 129 codons were M/M and 219 codons were E/E homozygotes. Scholars such as Kobayashi reported that there are two normally occurring polymorphisms of the human PRNP gene, methionine (M)/valine (V) at codon 129 and glutamic acid (E)/lysine (K) at codon 219, that can affect susceptibility to prion diseases. Patients who are M/M homozygous at codon 129 are overrepresented among sCJD patients [26, 27]. In this study, Chinese sCJD patients were all M/M homozygous at codon 129 and more sensitive to PRNP. Therefore, we assume that Chinese people are more susceptible to sCJD.
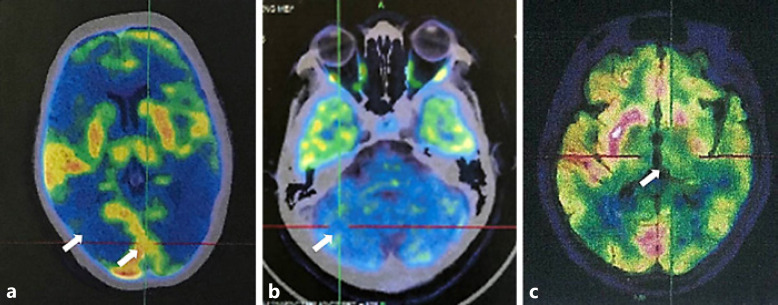
Fig. 5.
FDG-PET images of different PRNP diseases. a Decreased radioactive uptake in the occipital cortex of an sCJD patient. b Decreased radioactive uptake in the cerebellum of a GSS patient. c Decreased radioactive uptake in the thalamus of an FFI patient. FDG-PET, fluorodeoxyglucose-positron emission tomography; sCJD, sporadic Creutzfeldt-Jakob disease; PRNP, prion protein; GSS, Gerstmann-Sträussler-Scheinker syndrome; FFI, fatal familial insomnia.
In conclusion, this study retrospectively analyzed 104 patients diagnosed with sCJD pathologically and clinically. The characteristics of sCJD were summarized from the perspectives of demographic data and auxiliary examinations, and the results were compared with those from sCJD patients in other reports. This study may represent the characteristics of sCJD patients in China or even in Asia. In addition, PET-CT and PET-MRI examinations were important for the early diagnosis of sCJD due to their high sensitivity.
Statement of Ethics
The use of human clinical materials in this study was approved by the Ethical Committee of the General Hospital of the People's Liberation Army. All the patients and their caregivers gave written informed consent.
Disclosure Statement
There are no conflicts to declare.
Funding Sources
This study was supported by the Clinical Research Support Fund of General Hospital of People's Liberation Army grant 2016FC-CXYY-1002.
Author Contributions
Jia-Tang Zhang conceived and designed the experiments. Chang Qi and Jia-Tang Zhang analyzed the data. Wei Zhao, Xiao-Wei Xing, and Sheng-Yuan Yu contributed reagents/materials/analysis tools. Chang Qi wrote the manuscript.
Acknowledgements
The authors thank the Diagnostic Radiology Department and Nuclear Medicine Department of the General Hospital of the People's Liberation Army for assistance.
References
- 1.Ritchie DL, Ironside JW. Neuropathology of human prion diseases. Prog Mol Biol Transl Sci. 2017;150:319–39. doi: 10.1016/bs.pmbts.2017.06.011. [DOI] [PubMed] [Google Scholar]
- 2.Liberski PP. Historical overview of prion diseases: a view from afar. Folia Neuropathol. 2012;50((1)):1–12. [PubMed] [Google Scholar]
- 3.Kulczycki J. Creutzfeldt-Jakob disease: the past or the future. Przegl Epidemiol. 2006;60((Suppl 1)):63–7. [PubMed] [Google Scholar]
- 4.Zerr I, Kallenberg K, Summers DM, Romero C, Taratuto A, Heinemann U, et al. Updated clinical diagnostic criteria for sporadic Creutzfeldt-Jakob disease. Brain. 2009;132((10)):2659–68. doi: 10.1093/brain/awp191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Delasnerie-Laupretre N, Alperovitch A. Epidemiology of Creutzfeldt-Jakob disease. Pathol Biol. 1995;43((1)):22–4. [PubMed] [Google Scholar]
- 6.Sanjo N, Mizusawa H. Prion disease: the characteristics and diagnostic points in Japan. Rinsho Shinkeigaku. 2010;50((5)):287–300. doi: 10.5692/clinicalneurol.50.287. [DOI] [PubMed] [Google Scholar]
- 7.Yao Y, Dong X, Guan H, Lu Q. Cerebrospinal fluid real-time quaking-induced conversion test for sporadic Creutzfeldt-Jakob disease in an 18-year-old woman: a case report. Medicine. 2017;96((48)):e8699. doi: 10.1097/MD.0000000000008699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kozubski W, Wender M, Szczech J, Lenart-Jankowska D, Liberski PP. Atypical case of sporadic Creutzfeldt-Jakob disease (CJD) in a young adult. Folia Neuropathol. 1998;36((4)):225–8. [PubMed] [Google Scholar]
- 9.Collins S, Boyd A, Lee JS, Lewis V, Fletcher A, McLean CA, et al. Creutzfeldt-Jakob disease in Australia 1970–1999. Neurology. 2002 Nov 12;59((9)):1365–71. doi: 10.1212/01.wnl.0000031793.11602.8c. [DOI] [PubMed] [Google Scholar]
- 10.Meissner B, Kohler K, Bartl M, Jastrow U, Mollenhauer B, Schröter A, et al. Sporadic Creutzfeldt-Jakob disease: magnetic resonance imaging and clinical findings. Neurology. 2004 Aug 10;63((3)):450–6. doi: 10.1212/01.wnl.0000136225.80445.c9. [DOI] [PubMed] [Google Scholar]
- 11.Brown P, Cervenakova L, Mcshane L, Goldfarb LG, Bishop K, Bastian F, et al. Creutzfeldt-Jakob disease in a husband and wife. Neurology. 1998 Mar;50((3)):684–8. doi: 10.1212/wnl.50.3.684. [DOI] [PubMed] [Google Scholar]
- 12.Frontzek K, Moos R, Schaper E, Jann L, Herfs G, Zimmermann DR, et al. Iatrogenic and sporadic Creutzfeldt-Jakob disease in 2 sisters without mutation in the prion protein gene. Prion. 2015;9((6)):444–8. doi: 10.1080/19336896.2015.1121356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baiardi S, Capellari S, Bartoletti Stella A, Parchi P. Unusual clinical presentations challenging the early clinical diagnosis of Creutzfeldt-Jakob disease. J Alzheimers Dis. 2018;64((4)):1051–65. doi: 10.3233/JAD-180123. [DOI] [PubMed] [Google Scholar]
- 14.Lee SM, Hyeon JW, Kim SJ, Kim H, Noh R, Kim S, et al. Sensitivity and specificity evaluation of multiple neurodegenerative proteins for Creutzfeldt-Jakob disease diagnosis using a deep-learning approach. Prion. 2019 Jan;13((1)):141–50. doi: 10.1080/19336896.2019.1639482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang N, Marie SK, Livramento JA, Chammas R, Nitrini R. 14-3-3 Protein in the CSF of patients with rapidly progressive dementia. Neurology. 2003 Aug 12;61((3)):354–7. doi: 10.1212/01.wnl.0000078890.89473.ed. [DOI] [PubMed] [Google Scholar]
- 16.Lim JS, Kwon HM, Jang JW, Ju YR, Kim S, Park YH, et al. Characteristics of Korean patients with suspected Creutzfeldt-Jakob disease with 14-3-3 protein in cerebrospinal fluid: preliminary study of the Korean Creutzfeldt-Jakob disease active surveillance program. Prion. 2015;9((2)):136–43. doi: 10.1080/19336896.2015.1022020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Everbroeck BV, Quoilin S, Boons J, Martin J, Cras P. A prospective study of CSF markers in 250 patients with possible Creutzfeldt-Jakob disease. J Neurol Psychiatry. 2003;74((9)):1210–14. doi: 10.1136/jnnp.74.9.1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Green AJE. RT-QuIC: a new test for sporadic CJD. Pract Neurol. 2019 Feb;19((1)):49–55. doi: 10.1136/practneurol-2018-001935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giraud P, Perret-Liaudet A, Biacabe AG, Deslys JP, Laplanche JL, Chazot G, et al. Non familial Creutzfeldt-Jakob disease: a study of 53 cases. Rev Neurol. 2000 Jul;156((6–7)):616–21. [PubMed] [Google Scholar]
- 20.Wieser HG, Schindler K, Zumsteg D. EEG in Creutzfeldt-Jakob disease. Clin Neurophysiol. 2006 May;117((5)):935–51. doi: 10.1016/j.clinph.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 21.Moreno F, Larrarte LA. Neuroimaging in the diagnosis of human transmissible spongiform encephalopathies. Neurologia. 2006 Oct;21((8)):428–36. [PubMed] [Google Scholar]
- 22.Young GS, Geschwind MD, Fischbein NJ, Martindale JL, Henry RG, Liu S, et al. Diffusion-weighted and fluid-attenuated inversion recovery imaging in Creutzfeldt-Jakob disease: high sensitivity and specificity for diagnosis. AJNR Am J Neuroradiol. 2005 Jun-Jul;26((6)):1551–62. [PMC free article] [PubMed] [Google Scholar]
- 23.Tanaka Y, Minematsu K, Moriyasu H, Yamaguchi T, Yutani C, Kitamoto T, et al. A Japanese family with a variant of Gerstmann-Sträussler-Scheinker disease. J Neurol Psychiatry. 1997;62((5)):454–7. doi: 10.1136/jnnp.62.5.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cortelli P, Perani D, Parchi P, Grassi F, Montagna P, De Martin, et al. Cerebral metabolism in fatal familial insomnia: relation to duration, neuropathology, and distribution of protease-resistant prion protein. Neurology. 1997;49((1)):126. doi: 10.1212/wnl.49.1.126. [DOI] [PubMed] [Google Scholar]
- 25.He R, Hu Y, Yao L, Tian Y, Zhou Y, Yi F, et al. Clinical features and genetic characteristics of two Chinese pedigrees with fatal family insomnia. Prion. 2019;13((1)):116–23. doi: 10.1080/19336896.2019.1617027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shibuya S, Higuchi J, Shin RW, Tateishi J, Kitamoto T. Protective prion protein polymorphisms against sporadic Creutzfeldt-Jakob disease. Lancet. 1998;351((9100)):419. doi: 10.1016/S0140-6736(05)78358-6. [DOI] [PubMed] [Google Scholar]
- 27.Kobayashi A, Teruya K, Matsuura Y, Shirai T, Nakamura Y, Yamada M, et al. The influence of PRNP polymorphisms on human prion disease susceptibility: an update. Acta Neuropathol. 2015;130((2)):159–70. doi: 10.1007/s00401-015-1447-7. [DOI] [PubMed] [Google Scholar]