Abstract
Objective
The purpose of the present study was to determine the fraction of patients with mixed hearing loss who can or cannot expect benefit from power hearing aids (HAs) after stapes surgery.
Design
The audiological outcome of 374 stapes surgeries was used to calculate the patients' individual postoperative requirements in terms of gain and output of HAs. These requirements were compared to the available gain and output provided by state-of-the-art power HAs at 0.5, 1.0, 2.0, and 4.0 kHz. According to these comparisons, ears were divided into three groups. For G0, required gain and output lay within the corresponding technical limits of the HAs at all frequencies. In G1, one or both requirements could not be fulfilled at 1 frequency. G2 combined all ears where the requirements lay beyond the HA's technical limitations at 2 or more frequencies.
Results
Stapes surgery resulted in an improvement of air-bone gap (ABG) in 84.5% of the cases by 15.7 dB on average. Based on pure-tone average (0.5, 1.0, 2.0, 4.0 kHz), 40.6% of all cases showed an ABG ≤10 dB. 44.9% of all cases did no longer need a HA after stapes surgery. A power HA would fulfill both audiological criteria at all 4 frequencies in 81.6% of cases that needed a HA postoperatively. However, 18.4% would not be sufficiently treatable at 1 or more frequencies (15.0% in G1, 3.4% in G2).
Conclusions
The present study identified a subset of patients with mixed hearing loss after stapes surgery that cannot be treated sufficiently with available power HAs. As the residual ABG is an important reason for this lack of treatment success, the advancement of alternative hearing devices that circumvent the middle ear, such as powerful active middle ear implants, is indicated.
Keywords: Hearing aid usability, Stapes surgery, Gain, Output, Air-bone gap, Mixed hearing loss
Introduction
Otosclerosis is documented as one common cause of conductive and mixed hearing loss in adults [Caylakli et al., 2009]. In these patients, the hearing loss varies from mild to profound, whereby the conductive component is often accompanied by an additional sensorineural component [Shea, 1998].
For approximately 60 years, the usual treatment of hearing loss due to otosclerosis and stapes fixation has been stapes surgery, either as stapedectomy or as stapedotomy, in order to reconstitute the sound transmission of the middle ear [Meltzer et al., 1956; Shea, 1958]. During this time, numerous clinical studies have proven excellent outcomes of stapes surgery [Häusler, 2000], especially for otosclerosis patients with primarily conductive hearing loss [van Loon et al., 2014]. The surgical success is usually defined as the postoperative closure of the air-bone gap (ABG) to ≤10 dB without a deterioration of bone conduction (BC) at all frequencies [De Bruijn et al., 2001]. Although stapes surgeries could be shown to considerably improve hearing even in advanced cases of otosclerosis [Merkus et al., 2011], the surgery alone cannot yield postoperative air conduction (AC) thresholds that would require no further treatment in many cases, predominantly due to the sensorineural component of the hearing loss [Cremers et al., 1991]. Therefore, a significant number of patients with otosclerosis-related hearing loss that undergoes a stapes surgery resulting in a reduction or even closure of the ABG, still needs hearing aids (HAs) postoperatively to improve hearing [Smyth and Hassard, 1986; Vincent et al., 2006]. Alternatively, a power HA alone could serve as a noninvasive treatment option for otosclerosis in some cases. However, it is often of insufficient benefit in terms of speech discrimination [Johnson, 1993].
Thus, the combined treatment with stapes surgery and a HA for patients with postoperative AC thresholds >30 dB HL has long been recognized as the most suitable treatment for patients with mixed hearing loss [Luntz et al., 2009; Merkus et al., 2011; van Loon et al., 2014].
Although state-of-the-art power HAs provide benefit for a broad spectrum of hearing-impaired patients in terms of available gain, maximum output, and coverage of dynamic range [Rahne and Plontke, 2016], it is unclear whether all patients profit sufficiently from a combination treatment of stapes surgery and HAs.
The purpose of the present study was to investigate whether there is a subset of patients with mixed hearing loss where the conventional therapy of ear surgery plus power HA cannot satisfy the patients' requirements in terms of amplification to use the cochlear reserve to the extent necessary for sufficient restoration of communication abilities. In a first step the audiological outcome of stapes surgeries was calculated. In a second step the patients' requirements for gain and output were compared to those provided by state-of-the-art power HAs, and the respective proportions of patients who would benefit sufficiently or insufficiently was determined.
Materials and Methods
Patients and Study Design
In order to determine the clinical outcome of stapes surgery and to predict the likelihood of treatment success with state-of-the-art power HAs, a retrospective study was conducted at the Department of Otorhinolaryngology, Medical University of Hannover. The study examined all patients who had undergone stapes surgery due to primary otosclerosis, tympanosclerosis, or stapes fixation between January 2007 and November 2016 independently of previous interventions.
322 patients (123 males and 199 females) with complete pre- and postoperative audiological data sets could be included. 278 patients had received unilateral surgery, while in 22 patients, both ears had been treated. In 8 ears a revision surgery was executed. Summing up, a data set consisting of 374 stapes surgeries (191 left ears and 183 right ears) could be analyzed. At the time of surgery, the patients had an average age of 47.5 ± 12.4 years (ranging from 18.0 to 87.8 years). The study was approved by the ethics committee of Hannover Medical School (No. 2761-2015).
In a first step, the clinical outcome of the stapes surgery was determined by analyzing the patients' postoperative BC and AC thresholds. ABGs were calculated from BC and AC measurements of the same day, whereby the most recent follow-up measurements were selected (min. 6 days, max. 500 days after surgery).
In a second step, 2 criteria were established from manufacturers' technical specifications to examine whether a state-of-the-art power HA could satisfy the patients' audiological needs after stapes surgery regarding the required frequency-specific gain and power output.
Audiological Measurement
All audiological parameters had been measured monaurally in a sound-proof chamber using HDA200 headphones (Sennheiser Electronic GmbH & Co. KG, Wedemark, Germany), a KLH96 bone conductor (CB-Elmec GmbH, Radeberg, Germany) and a PC-based audiometer (AD2017 or AD17, Audio-DATA GmbH, Duvensee, Germany). The audiometer limit was 110 dB HL for AC at all measured frequencies. For BC, the audiometer limit was 55 dB HL at 6.0 kHz, 60 dB HL at 0.5 and 4.0 kHz and 70 dB HL at the remaining frequencies.
To determine the degree of hearing loss, AC and BC pure-tone thresholds had been measured in 5-dB steps at 0.5, 1.0, 1.5, 2.0, 3.0, 4.0, and 6.0 kHz. In cases where the audiometer limit had been exceeded, missing values were replaced by a best-case estimate for BC using the audiometer limit plus 5 dB.
Calculated ABGs were analyzed as frequency-specific values and as pure-tone average (ABG4) including 0.5, 1.0, 2.0, and 4.0 kHz.
Surgical success of the stapes surgery was defined according to the relevant literature as ABG4 closure ≤10 dB HL [De Bruijn et al., 2001; Shea, 1998]. Until the year 2000, the stapes surgery success rate was usually determined using a 3-frequency pure-tone average (0.5, 1.0, and 2.0 kHz) [Häusler, 2000]. In accordance with the Committee of Hearing and Equilibrium guidelines for the evaluation of results of treatment of conductive hearing loss [Committee on Hearing and Equilibrium, 1995], more recent studies now use the 4-frequency pure-tone average described above. For comparison of the success rate in this study to the large number of studies dating before 2000, the 3-frequency pure-tone average (ABG3) and respective success rate were also calculated.
Calculation of Required Gain and Output
The criteria required gain (rGAIN) and required power output (rOUTPUT) from a HA were derived from essential audiological needs of the individual patient.
Criterion 1. Speech must be audible, which can be translated to a required gain using the POGO rule for HA prescriptions [Dillon, 2012] based on the sensorineural part of the hearing loss, and a full compensation of the conductive part:
rGAINi = 1/2 BCi + ABGi + ki (1)
with the index i depicting the audiometric frequencies, and ki is −5 dB at 0.5 kHz and 0 dB at 1, 2, and 4 kHz.
Criterion 2. The amplified signal should cover enough of the dynamic range above threshold, but at least 35 dB [Rahne and Plontke, 2016; Zwartenkot et al., 2014]:
rOUTPUTi = ACi + 35 dB. (2)
Determination of Maximum Available Gain and Power Output from State-of-the-Art Power HAs
In order to determine representative maximum available gain and power output, data sheets from three popular state-of-the-art power HAs were used:
Sumo DM, Oticon A/S, Smørum, Denmark [Oticon GmbH, 2017]
Enzo, GN ReSound A/S, Ballerup, Denmark [GN ReSound A/S, 2017]
Naida S UP, Phonak AG, Stäfa, Switzerland [Phonak AG, 2017].
For the maximum available gain, the frequency-specific full-on gain (FOG [dB]) specified by the respective manufacturer with a 2-cc coupler (Type IEC 60118-7; 126 and ANSI S3.22 (2003); S.37 (1995)) was converted to real ear gain (dB) using correction factors according to Bentler and Pavlovic [1989, 1992] (Table 1). The resulting frequency-dependent FOGs measured as output sound pressure level for a 50-dB input level of the three HAs were looked up, and the lowest value at each frequency was selected for comparison to rGAIN.
Table 1.
Frequency-specific technical limits for maximum available gain and output of the three state-of-the-art power hearing aids using correction factors by Bentler and Pavlovic [1989, 1992]
| Frequency, kHz | Gain, dB | Output, dB HL | ||||
| Sumo DM |
Resound ENZO |
Phonak Naida S UP |
Sumo DM |
Resound ENZO |
Phonak Naida S UP |
|
| 0.5 | 77 | 80 | 75 | 124 | 127 | 121 |
| 1.0 | 85 | 77 | 85 | 136 | 131 | 134 |
| 2.0 | 70 | 74 | 70 | 124 | 127 | 122 |
| 4.0 | 56 | 54 | 59 | 117 | 114 | 112 |
Italicized values were taken for the calculation of the respective decision criteria.
For maximum available output, the frequency-specific maximum output (OSPL90 [dB SPL]) specified by the manufacturer with a 2-cc coupler was converted to dB HL, again using correction factors according to Bentler and Pavlovic [1989, 1992] (Table 1). As for the maximum available gain, the frequency-specific standard OSPL90 of the three power HAs were looked up, and the lowest value at each frequency was selected for comparison to rOUTPUT.
Decision Criteria for Individual Analysis of Suitability of a Power HA
For decision criterion 1 (equation 1), the claimed FOG50 of the HA had to be larger than the individually calculated required rGAIN at 0.5, 1.0, 2.0, and 4.0 kHz in order to fulfill the postoperative audiological requirements of the respective patient. By analogy, for decision criterion 2 (equation 2), the claimed OSPL90 of the HA had to be larger than the individually calculated rOUTPUT at 0.5, 1.0, 2.0, and 4.0 kHz. These decision criteria were applied to all cases considered to be candidates for HAs, having a postoperative AC threshold of >30 dB HL for at least 2 of the frequencies 0.5, 1.0, 2.0, and 4.0 kHz. This definition was taken from the pure-tone audiological qualification criterion for HA reimbursement according to the 2011 German Hilfsmittel-Richtlinie [Gemeinsamer Bundesausschuss, 2012].
According to the criteria, all included ears were divided into three groups. For the first group (G0), rGAIN and rOUTPUT lay within the corresponding technical limits of the HA at all frequencies (Table 1). For the second group (G1), either rGAIN, rOUTPUT, or both exceeded the technical limits of the HAs at 1 frequency. The third group (G2) combined all ears where rGAIN, rOUTPUT, or both lay beyond the HA's technical limits at 2 or more frequencies.
Statistical Analysis
All statistical analyses and graphical representations were performed with IBM SPSS Statistics for Windows, version 23.0 (IBM Corp., Armonk, NY, USA). The data were tested for normal distribution (Kolmogorov-Smirnov test) and homogeneity of variance (Levene's test). As all data did not show a normal distribution, the nonparametric Wilcoxon signed-rank test was used for the comparison of paired data sets. In all cases, differences were considered significant from p < 0.05.
Results
Did Stapes Surgery Close the ABG?
For determination of the clinical outcome of stapes surgery, the pre- and postoperative ABGs of the 374 surgeries were analyzed. The surgery could improve individual ABG4 in 84.5% of the cases, while 12.3% of the ears were unchanged (within ±5 dB) (Fig. 1). Only in 3.2% of the ears did the ABG increase after the surgery. On average, the ABG4 was significantly improved by 15.7 dB (5th/95th percentiles: −1.3; 32.8) (Table 2). The average ABG was significantly reduced at all frequencies after the surgery, with the highest improvement of 21.9 dB (0.0; 45.0) at 0.5 kHz. With increasing frequency, the improvement became smaller. At 6 kHz for instance, the mean ABG was reduced by 7.3 dB (−20.0; 30.0) (Table 2).
Fig. 1.
Pure-tone average of the pre- and postoperative air-bone gap (ABG4, n = 374). The solid diagonal ±5 dB (dashed diagonals) depicts the “no change” area. Symbols indicate individual results where all ABG4 frequencies were measurable (circles) or at least 1 value exceeded the audiometer limit and was estimated (triangles).
Table 2.
Frequency-specific preoperative and postoperative air-bone gaps (ABG) and their change (mean, 5th, and 95th percentiles) in n = 374 surgeries
| Frequency, kHz | Preoperative | Postoperative | Change | Residual ABG, % | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| mean | 5th | 95th | mean | 5th | 95th | mean | 5th | 95th | ≤10 dB | ≤20 dB | |
| 0.5 | 37.7 | 20 | 60 | 15.8 | 0 | 40 | 21.9*** | 0 | 45 | 44.9 | 75.9 |
| 1.0 | 28.5 | 10 | 45 | 10.7 | 0 | 30 | 17.9*** | − 1 | 35 | 67.1 | 89.8 |
| 1.5 | 24.5 | 5 | 45 | 10.1 | 0 | 30 | 14.4*** | −10 | 35 | 70.9 | 90.4 |
| 2.0 | 22.9 | 5 | 40 | 10.5 | 0 | 25 | 12.4*** | −10 | 30 | 70.3 | 92.0 |
| 3.0 | 22.1 | 5 | 40 | 10.6 | 0 | 25 | 11.5*** | −10 | 30 | 64.2 | 90.6 |
| 4.0 | 28.0 | 10 | 50 | 17.3 | 0 | 40 | 10.7*** | −15 | 35 | 37.4 | 72.5 |
| 6.0 | 33.1 | 15 | 50 | 25.8 | 10 | 50 | 7.3*** | −20 | 30 | 14.4 | 45.2 |
| ABG4 | 29.3 | 16.3 | 45.0 | 13.6 | 3.8 | 30.3 | 15.7*** | − 1.3 | 32.8 | 40.6 | 84.8 |
| ABG3 | 29.7 | 15.0 | 46.7 | 12.3 | 3.3 | 30.0 | 17.4*** | − 1.7 | 35.0 | 52.4 | 86.6 |
The level of statistically significant changes was analyzed by the Wilcoxon signed-rank test with *** p < 0.001 at all frequencies.
While stapes surgery resulted in a general improvement of ABG4, a perfect closure of ≤5 dB occurred in only 20.9% of all ears. At 1.0, 1.5, 2.0, 3.0, and 4.0 kHz, frequency-specific mean residual ABGs were near 10 dB. However, in the lower as well as higher frequencies, the residual ABGs remained considerably higher (Fig. 2, Table 2). Accordingly, stapes surgery resulted in frequency-specific success rates with ABG ≤10 dB. While the ABG4 success rate was 40.6%, success rates between 1.0 and 3.0 kHz were considerably higher and reached 64.2% (3.0 kHz) to 70.9% (1.5 kHz) (Table 2, residual ABG ≤10 dB). In 59.4% of the cases, a clinically relevant ABG4 ≥10 dB remained after stapes surgery.
Fig. 2.
Frequency-specific postoperative air-bone gaps (ABG) and ABG4 and ABG3 (n = 374). The median is depicted by black horizontal lines, and the boxes mark the interquartile ranges (i.e., 25th and 75th percentiles). After exclusion of outliers (circles), the error bars indicate the min.-max. range.
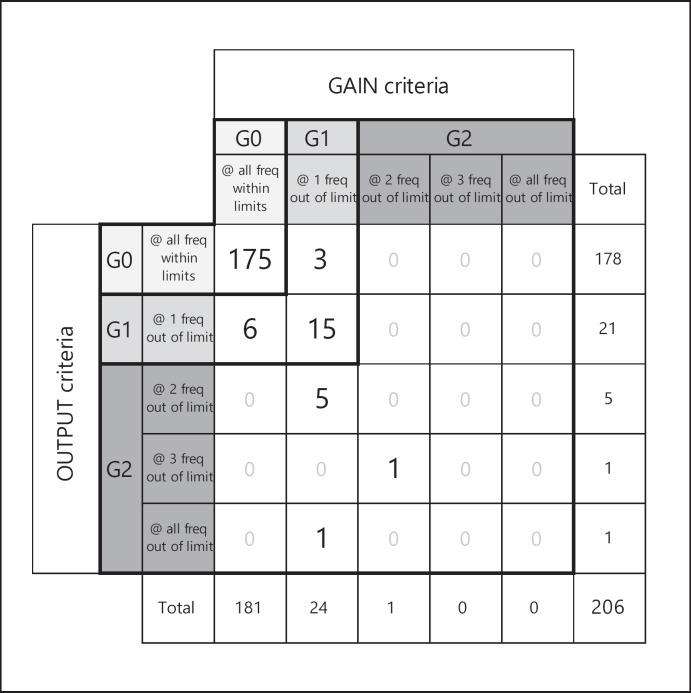
Who Can Benefit from a State-of-the-Art Power HA after Stapes Surgery?
Before stapes surgery, 95.5% (n = 357) of the examined ears had AC thresholds >30 dB HL for at least 2 of the frequencies 0.5, 1.0, 2.0, and 4.0 kHz and were candidates for a HA. After surgery, AC thresholds improved enough to no longer require amplification in 44.9% (n = 168) of ears, while 55.1% (n = 206) were still eligible for HA provision (Fig. 3).
Fig. 3.
Analysis of patients with a postoperative air conduction threshold >30 dB for at least 2 of the frequencies 0.5, 1.0, 2.0, and 4.0 kHz (n = 206). Representation patients who met audiological requirements by a state-of-the-art power hearing aid in terms of required gain and required power output at all 4 frequencies (G0), failed at 1 (G1), or at least at 2 (G2).
The previously described decision criteria rGAIN and rOUTPUT to determine whether (or not) state-of-the-art power HAs satisfy individual audiological requirements were applied to the 206 cases still needing HAs. A state-of-the-art power HA would meet both decision criteria at all frequencies in 85.0% (n = 175) of cases (group G0). However, 11.7% (n = 24) would still not meet one or both decision criteria at 1 frequency (group G1), which was 4.0 kHz in 95.8%. In most of the cases, this could be attributed to the rGAIN criterion (72.0.1%). Further 3.4% (n = 7) would lie beyond one or both decision criteria at 2 or more frequencies (group G2). In total, 15.0% would not be adequately treatable by a state-of-the-art power HA.
Discussion
For almost 60 years, stapes surgery in its various forms has been the treatment of choice for conductive hearing loss resulting from otosclerosis or stapes fixation. However, despite an overall high rate of general hearing improvement, the defined success criteria of stapes surgery, i.e. the closure of the ABG to ≤10 dB HL as well as an unchanged BC, vary among different studies in the literature, and a considerable number of patients still needs postoperative HAs, especially those who suffer from mixed hearing loss, e.g. resulting from far-advanced otosclerosis [Shea et al., 1999; van Loon et al., 2014]. The present retrospective study aimed to predict the success and failure rate of the possible treatment with HAs after stapes surgery. Postoperative clinical outcomes of 374 stapes surgeries were analyzed using 2 basic HA operation parameters [Snik et al., 2004] in order to estimate whether patients could be sufficiently treated with state-of-the-art power HAs after stapes surgery.
In the cases examined here, stapes surgery resulted in an average improvement of the postoperative ABG4 by 15.7 dB. The corresponding success rate based on ABG4 ≤10 dB was 40.6%, which is relatively low when compared to the numerous success rates documented in the literature over the past 60 years with an average of 79% (own literature review) [Häusler, 2000]. However, the range of the reported success rates between 37 and 96% is large, which might be due to e.g. differences in study design, indication, prosthesis type, surgical technique and experience. Moreover, as the success rate of the present study was highly frequency-specific (Table 2), the type of pure-tone average calculation is crucial [Niwa et al., 1992; Ueda et al., 2004; Babighian and Albu, 2009; Luntz et al., 2009; Kishimoto et al., 2015]. A considerable amount of studies reported success rates based on the calculation of ABG3 instead of ABG4[Häusler, 2000]. In the present study, an ABG3-based calculation led to an increase in the success rate to 52.4%. A further explanation might be the fact that the data set of the present study did not differentiate between stapedotomies and stapedectomies, which are known to differ in their frequency-specific success rates [Spandow et al., 2000; Kós et al., 2001; Quaranta et al., 2005]. This assumption is supported by a recent epidemiological analysis of middle ear surgery outcomes in daily routine of 9 different ear, nose and throat departments in Thuringia in Germany [Fiedler et al., 2013], which reported an overall success rate of 49%.
In addition, the lower success rate might be explained by the fact that the patients' audiological data used for analysis in the present study were not preselected for a certain, standardized medical status. Many patients had gone through a long history of revision surgeries performed at other clinics, which could not always be traced back. Thus, the proportion of revisions could not be precisely identified and analyzed in the present study; however, it is likely that a significant proportion of revisions (approx. 15%) may have contributed to the overall lower stapes surgery success rate [Schmid and Häusler, 2009]. Finally, the outcome of stapes surgery in the present study was mainly used to determine the fraction of cases with the need for a postoperative HA treatment and the proportion of insufficient benefit from HAs [De Bruijn et al., 2001; Gemeinsamer Bundesausschuss, 2012].
So far, stapes surgery alone appeared to have a less than perfect impact on overall hearing improvement in patients with mixed hearing loss. However, many experts postulate the high benefit of a combination therapy via stapes surgery followed by HA treatment [Glasscock et al., 1996; Khalifa et al., 1998; Luntz et al., 2009]. Comparison of required gain and maximum output to what is technically available was used to predict the proportion of cases which could be sufficiently treated with a power HA after stapes surgery and where HAs might fail. The corresponding estimation resulted in a high percentage of cases (85.0%) that should be successfully treatable, confirming that stapes surgery is an important therapeutic tool to enable HA benefit.
Nevertheless, the study results also demonstrated that 15.0% of cases would still not be sufficiently treatable with state-of-the-art power HAs after stapes surgery. Maximum available gain, output, or both would be too limited to serve the amplification needs and/or to cover the residual dynamic range of these patients [Rahne and Plontke, 2016; Zwartenkot et al., 2012]. In most of those cases, gain was the limiting parameter, especially at 4.0 kHz.
Although the results of the present study are based on the use of the POGO rule as criterion for required gain, the analysis has also been performed with other rules that are available in a parametric form, such as the half gain rule, POGO II, NAL-R and NAL-RP [Berger et al., 1989]. As only the required gain is affected, alternative rules led to minor changes in failure rates (13.6–15.0% insufficient results), demonstrating that the present qualitative results are mainly independent of the specific rule for required gain.
The reason why many patients with a severe sensorineural hearing loss can be treated better compared to patients with mixed hearing loss, examined in the present study, is the ABG, which cannot be overcome by most power HAs. The sample calculation in Table 3 illustrates the problem. For sensorineural or mixed hearing loss resulting in the same AC threshold, the ABG of mixed hearing loss patients considerably increases the required gain compared to patients with no ABG.
Table 3.
Examples of required and maximum available gain and output from a state-of-the-art power hearing aid in a patient with mixed hearing loss (MHL), compared to a patient with sensorineural hearing loss (SNHL) only
| SNHL | MHL | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Frequency, kHz: | 0.5 | 1.0 | 2.0 | 4.0 | 0.5 | 1.0 | 2.0 | 4.0 | |
| Situation | AC, dB HL | 70 | 75 | 80 | 85 | 70 | 75 | 80 | 85 |
| BC, dB HL | 70 | 75 | 80 | 85 | 25 | 30 | 35 | 40 | |
| ABG, dB | 0 | 0 | 0 | 0 | 45 | 45 | 45 | 45 | |
| Required gain | 1/2 BC + ABG +ki, dB | 35 | 37.5 | 40 | 42.5 | 57.5 | 60 | 62.5 | 65 |
| Available gain | dB | 75 | 77 | 70 | 54 | 75 | 77 | 70 | 54 |
| √ | √ | √ | √ | √ | √ | √ | $$ | ||
| Required output | AC + 35, dB | 105 | 110 | 115 | 120 | 105 | 110 | 115 | 120 |
| Available output | dB HL | 121 | 131 | 122 | 112 | 121 | 131 | 122 | 112 |
| √ | √ | √ | $$ | √ | √ | √ | $$ | ||
Average available gain and output have been calculated by using the marked values of Table 1.
Based on this calculation, it can be stated that patients with a mild to moderate mixed hearing loss and an ABG can either benefit from the already described combination of stapes surgery and HA or from the implantation of a percutaneous/transcutaneous BC implant. In contrast, patients with a moderate to severe mixed hearing loss (Table 3) could be treated with an alternative device, such as an active middle ear implant [Rahne and Plontke, 2016]. This alternative has to be considered for all cases in the present study where a power HA would theoretically fail to provide sufficient benefit. However, at this point, a reliable decision algorithm is lacking to predict the relative benefit of an active middle ear implant compared to stapes surgery and HAs. As stapes surgery in combination with a HA is not a fail-safe solution for all patients with mixed hearing loss, clear evidence-based criteria have to be found to determine preoperatively where chances to treat a patient sufficiently with a stapes surgery and a HA are lower compared to the implantation of an active middle ear implant with increased complexity and costs.
The present study mainly focused on the audiological status and outcomes in terms of sensorineural hearing loss and ABG based on audiometric thresholds, in order to estimate the theoretical treatment success of stapes surgery in combination with a power HA. In addition to the usually used success criterion of ABG closure, the AC and BC thresholds in combination with required gain and output can serve as reliable outcome measures in terms of need for a HA as well as predictor of benefit from a HA after stapes surgery.
Further, the applicability of the criteria has to be evaluated with regard to speech recognition (see part II: Wardenga et al. [2020]). Hence, in a subsequent step, results were validated under real-life conditions with HAs in an appropriate mixed hearing loss collective.
Conclusion
The combination of stapes surgery and subsequent power HA fitting is a successful treatment for patients with mixed hearing loss. However, the present study could identify a substantial subset of patients who would not gain sufficient benefit from this combination therapy, as even state-of-the-art power HAs would not be able to provide sufficient gain and/or output required by these patients. In some patients, the sensorineural component and the postoperative residual ABG sum up to hearing thresholds even the technically most advanced HAs cannot provide sufficient benefit for. Consequently, the advancement of alternative hearing devices that circumvent the middle ear, such as powerful active middle ear implants and direct acoustical cochlea stimulators is indicated.
Disclosure Statement
This work is part of the doctoral thesis of N.W. and was supported by a project grant from Cochlear Ltd., by the DFG Cluster of Excellence EXC1077/1 “Hearing4all”, and “RESPONSE – Partnership of Innovation in Implant Technology” as collaborative project within the program “Zwanzig20 – Partnership for Innovation” of the Federal Ministry of Education and Research, Germany. N.W., T.L., and H.M. received travel support by Cochlear Ltd. to meetings. B.W. is an employee of Cochlear Ltd. who provided the declared support.
References
- Babighian GG, Albu S. Failures in stapedotomy for otosclerosis. Otolaryngol Head Neck Surg. 2009 Sep;141((3)):395–400. doi: 10.1016/j.otohns.2009.03.028. [DOI] [PubMed] [Google Scholar]
- Bentler RA, Pavlovic CV. Transfer functions and correction factors used in hearing aid evaluation and research. Ear Hear. 1989 Feb;10((1)):58–63. doi: 10.1097/00003446-198902000-00010. [DOI] [PubMed] [Google Scholar]
- Bentler RA, Pavlovic CV. Addendum to “transfer functions and correction factors used in hearing aid evaluation and research”. Ear Hear. 1992 Aug;13((4)):284–6. doi: 10.1097/00003446-199208000-00012. [DOI] [PubMed] [Google Scholar]
- Berger KW, Hagberg EN, Rane RL. Prescription of hearing aids: Rationale, procedures, and results, 5 th. Herald Publishing House; 1989. [Google Scholar]
- Caylakli F, Yavuz H, Yilmazer C, Yilmaz I, Ozluoglu LN. Effect of preoperative hearing level on success of stapes surgery. Otolaryngol Head Neck Surg. 2009 Jul;141((1)):12–5. doi: 10.1016/j.otohns.2009.02.023. [DOI] [PubMed] [Google Scholar]
- Committee on Hearing and Equilibrium Committee on Hearing and Equilibrium guidelines for the evaluation of results of treatment of conductive hearing loss. AmericanAcademy of Otolaryngology-Head and Neck Surgery Ffoundation, Inc. Otolaryngol Head Neck Surg. 1995 Sep;113((3)):186–7. doi: 10.1016/S0194-5998(95)70103-6. [DOI] [PubMed] [Google Scholar]
- Cremers CW, Beusen JM, Huygen PL. Hearing gain after stapedotomy, partial platinectomy, or total stapedectomy for otosclerosis. Ann Otol Rhinol Laryngol. 1991 Dec;100((12)):959–61. doi: 10.1177/000348949110001201. [DOI] [PubMed] [Google Scholar]
- de Bruijn AJ, Tange RA, Dreschler WA. Efficacy of evaluation of audiometric results after stapes surgery in otosclerosis. II. A method for reporting results from individual cases. Otolaryngol Head Neck Surg. 2001 Jan;124((1)):84–9. doi: 10.1067/mhn.2001.111600. [DOI] [PubMed] [Google Scholar]
- Dillon H. Hearing aids. New York, Thieme: 2012. [Google Scholar]
- Fiedler T, Boeger D, Buentzel J, Esser D, Hoffmann K, Jecker P, et al. Middle ear surgery in Thuringia, Germany: a population-based regional study on epidemiology and outcome. Otol Neurotol. 2013 Jul;34((5)):890–7. doi: 10.1097/MAO.0b013e318280dc55. [DOI] [PubMed] [Google Scholar]
- Gemeinsamer Bundesausschuss: Richtlinie des Gemeinsamen Bundesauschusses über die Verordnung von Hilfsmitteln in der vertragsärztlichen Versorgung (Hilfsmittel-Richtlinie/HilfsM-RL) veröffentlicht im Bundesanzeiger BAnz 10042012 B2; Kraft getreten am 01042012.
- Glasscock ME, 3rd, Storper IS, Haynes DS, Bohrer PS. Stapedectomy in profound cochlear loss. Laryngoscope. 1996 Jul;106((7)):831–3. doi: 10.1097/00005537-199607000-00008. [DOI] [PubMed] [Google Scholar]
- GN ReSound A/S Enzo - Data Sheet [Internet] 2017. Available from: http://www.resoundpro.com/˜/media/REFRESH/US/00-DOWNLOADS/enzo2/ReSoundENZO2_DataSheet.ashx.
- Häusler R. Fortschritte in der Stapeschirurgie. Laryngorhinootologie. 2000;79(S2):95–139. [Google Scholar]
- Johnson EW. Hearing aids and otosclerosis. Otolaryngol Clin North Am. 1993 Jun;26((3)):491–502. [PubMed] [Google Scholar]
- Khalifa A, el-Guindy A, Erfan F. Stapedectomy for far-advanced otosclerosis. J Laryngol Otol. 1998 Feb;112((2)):158–60. doi: 10.1017/s0022215100140186. [DOI] [PubMed] [Google Scholar]
- Kishimoto M, Ueda H, Uchida Y, Sone M. Factors affecting postoperative outcome in otosclerosis patients: predictive role of audiological and clinical features. Auris Nasus Larynx. 2015 Oct;42((5)):369–73. doi: 10.1016/j.anl.2015.03.001. [DOI] [PubMed] [Google Scholar]
- Kós MI, Montandon PB, Guyot JP. Short- and long-term results of stapedotomy and stapedectomy with a teflon-wire piston prosthesis. Ann Otol Rhinol Laryngol. 2001 Oct;110((10)):907–11. doi: 10.1177/000348940111001003. [DOI] [PubMed] [Google Scholar]
- Luntz M, Yehudai N, Most T. Hearing rehabilitation counseling for patients with otosclerosis-related hearing loss. Otol Neurotol. 2009 Dec;30((8)):1037–43. doi: 10.1097/MAO.0b013e318196966f. [DOI] [PubMed] [Google Scholar]
- Meltzer PE, Lindsay JR, Goodhill V. SYMPOSIUM; the operation for the mobilization of the stapes in otosclerotic deafness. Laryngoscope. 1956 Jul;66((7)):729–84. [PubMed] [Google Scholar]
- Merkus P, van Loon MC, Smit CF, Smits C, de Cock AF, Hensen EF. Decision making in advanced otosclerosis: an evidence-based strategy. Laryngoscope. 2011 Sep;121((9)):1935–41. doi: 10.1002/lary.21904. [DOI] [PubMed] [Google Scholar]
- Niwa H, Ishida K, Tago C, Ueda H, Yanagita N. Factors affecting improvement of hearing after stapes surgery. Auris Nasus Larynx. 1992;19:S39–43. [Google Scholar]
- Oticon Gmb H. Sumo DM - Data Sheet [Internet] 2017. Available from: https://www.oticon.com/-/media/oticon/main/pdf/master/sumo/pi/159691uk_pi_sumodm.pdf.
- Phonak AG. Naida S UP - Data Sheet [Internet] 2017. Available from: https://www.phonakpro.com/content/dam/phonakpro/gc_hq/en/products_solutions/hearing_aid/naida_s/documents/datasheet_naida_s_ix_up.pdf.
- Quaranta N, Besozzi G, Fallacara RA, Quaranta A. Air and bone conduction change after stapedotomy and partial stapedectomy for otosclerosis. Otolaryngol Head Neck Surg. 2005 Jul;133((1)):116–20. doi: 10.1016/j.otohns.2005.03.011. [DOI] [PubMed] [Google Scholar]
- Rahne T, Plontke SK. Apparative Therapie bei kombiniertem Hörverlust: ein audiologischer Vergleich aktueller Hörsysteme. HNO. 2016 Feb;64((2)):91–100. doi: 10.1007/s00106-015-0087-5. [DOI] [PubMed] [Google Scholar]
- Schmid P, Häusler R. Revision stapedectomy: an analysis of 201 operations. Otol Neurotol. 2009 Dec;30((8)):1092–100. doi: 10.1097/MAO.0b013e3181b4ecb2. [DOI] [PubMed] [Google Scholar]
- Shea JJ., Jr Fenestration of the oval window. Ann Otol Rhinol Laryngol. 1958 Dec;67((4)):932–51. doi: 10.1177/000348945806700403. [DOI] [PubMed] [Google Scholar]
- Shea JJ., Jr Forty years of stapes surgery. Am J Otol. 1998 Jan;19((1)):52–5. [PubMed] [Google Scholar]
- Shea PF, Ge X, Shea JJ., Jr Stapedectomy for far-advanced otosclerosis. Am J Otol. 1999 Jul;20((4)):425–9. [PubMed] [Google Scholar]
- Smyth GD, Hassard TH. Hearing aids poststapedectomy: incidence and timing. Laryngoscope. 1986 Apr;96((4)):385–8. doi: 10.1288/00005537-198604000-00009. [DOI] [PubMed] [Google Scholar]
- Snik A, Noten J, Cremers C. Gain and maximum output of two electromagnetic middle ear implants: are real ear measurements helpful? J Am Acad Audiol. 2004 Mar;15((3)):249–57. doi: 10.3766/jaaa.15.3.7. [DOI] [PubMed] [Google Scholar]
- Spandow O, Söderberg O, Bohlin L. Long-term results in otosclerotic patients operated by stapedectomy or stapedotomy. Scand Audiol. 2000;29((3)):186–90. doi: 10.1080/010503900750042752. [DOI] [PubMed] [Google Scholar]
- Ueda H, Fujimoto T, Hibi T, Naiki M, Furuhasi A. Hearing results after stapes surgery. In: Gyo K, Wada H, Ehime M, editors. Middle Ear Mechanics in Research and Otology. Singapore, World Scientific Publishing Co. Pte. Ltd; 2004. pp. pp 279–283. [Google Scholar]
- van Loon MC, Merkus P, Smit CF, Smits C, Witte BI, Hensen EF. Stapedotomy in cochlear implant candidates with far dvanced otosclerosis. Otol Neurotol. 2014;35((10)):1707–14. doi: 10.1097/MAO.0000000000000637. [DOI] [PubMed] [Google Scholar]
- Vincent R, Sperling NM, Oates J, Jindal M. Surgical findings and long-term hearing results in 3,050 stapedotomies for primary otosclerosis: a prospective study with the otology-neurotology database. Otol Neurotol. 2006 Dec;27((8 Suppl 2)):S25–47. doi: 10.1097/01.mao.0000235311.80066.df. [DOI] [PubMed] [Google Scholar]
- Wardenga N, Snik AFM, Kludt E, Waldmann B, Lenarz T, Maier H. Hearing aid treatment in patients with mixed hearing loss. II. Speech recognition in comparison to direct acoustic cochlear stimulation. Audiol Neurotol. 2020 doi: 10.1159/000504285. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwartenkot JW, Snik AF, Kompis M, Stieger C. Gain and maximum output of implantable hearing devices in patients with moderate to severe sensorineural hearing loss. J Hear Sci. 2012;2:35–40. [Google Scholar]
- Zwartenkot JW, Snik AF, Mylanus EA, Mulder JJ. Amplification options for patients with mixed hearing loss. Otol Neurotol. 2014 Feb;35((2)):221–6. doi: 10.1097/MAO.0000000000000258. [DOI] [PubMed] [Google Scholar]