Abstract
Background/Objective
Clear cell renal cell carcinoma (ccRCC) is characterized by a high degree of functional intratumoral heterogeneity (ITH). This is highlighted by the finding that tumor cell proliferation and intracellular signaling occur preferentially in the tumor periphery. The driving forces for such a spatial organization are largely unknown. Herein, we investigate the role of the tumor microenvironment in the control of tumor cell proliferation and functional ITH.
Methods
Conditioned media (CM) derived from nonmalignant peritumoral kidney tissue were used to stimulate RCC cells in vitro. A neutralization assay was used to characterize the role of FGF-2 in the CM. The molecular mechanisms underlying the action of CM on RCC cells were investigated using immunoblotting, flow cytometry and immunofluorescence microscopy. Lastly, a series of ccRCCs were stained for Ki-67 and p27<sup>Kip1</sup>, and expression was analyzed in both tumor periphery and center.
Results
We show that CM derived from nonmalignant kidney cells adjacent to an RCC can downregulate the expression of the CDK inhibitor p27<sup>Kip1</sup> through enhanced protein degradation in an FGF-2-dependent fashion. FGF-2 functions mainly through the PI3K/AKT pathway downstream of its receptors, and RCC cells with constitutively high AKT activity show not only an enhanced degradation of p27<sup>Kip1</sup> through the Emi1-Skp2 axis, but also a subcellular mislocalization of p27<sup>Kip1</sup> to the cytoplasmic compartment. Such a mislocalization was also detected in the tumor periphery in vivo suggesting that p27<sup>Kip1</sup> plays an important role in shaping this spatial niche.
Conclusions
Our results suggest that the tumor microenvironment is involved in shaping the tumor peripheral niche by stimulating the enhanced proliferation that is characteristic for this zone.
Keywords: Tumor heterogeneity, Renal cell carcinoma, FGF-2, Tumor microenvironment, Spatial niche
Introduction
Clear cell renal cell carcinoma (ccRCC) accounts for approximately 70–75% of RCCs. Once metastatic, ccRCC is among the most lethal urological tumors despite recent therapeutic advances [1]. Genomic intratumoral heterogeneity (ITH) is a hallmark of ccRCC and contributes to the lethal disease outcome through variant tumor cells that have the ability to overcome various selection barriers [2, 3, 4].
Recent results suggest that the role of ITH in tumor progression is somewhat ambiguous since aggressive tumor growth has been reported to be associated with a low degree of ITH but a high degree of genomic instability [5]. In line with this notion, functional ITH is not a strong prognostic factor and does not correlate with tumor stage [6]. Since the latter observation implies that ITH is independent of the tumor size, it has prompted studies into the spatial organization of ccRCCs. A remarkable zonal pattern of proliferation and intracellular signaling activity has emerged from these studies, in which the tumor periphery was identified as a hotspot for both [6]. However, this functional stage-independent ITH was not associated with mutations that could explain the enhanced proliferation and activation of intracellular signaling pathways in the tumor periphery [6].
We therefore hypothesize here that extrinsic, microenvironmental factors such as growth factors contribute to functional ITH and topological niche formation in ccRCC.
We show that the modulation of expression of the cyclin-dependent kinase (CDK) inhibitor p27Kip1 is involved in spatial niche formation and that this process entails fibroblast growth factor-2 (FGF-2) derived from the tumor microenvironment. Hence, functional ITH, the formation of spatial niches and very likely other characteristics of RCC can be shaped by external cues derived from the tumor microenvironment.
Material and Methods
Patient Samples
Paraffin-embedded tissue sections were retrieved from the tissue bank of the National Center for Tumor Diseases Heidelberg and were used in accordance to the regulations of the tissue bank as well as under approval of the Ethics Committee of the University of Heidelberg School of Medicine.
Cell Culture and Treatments
The RCC cell lines ACHN and A-498 were obtained from ATCC (distributed by CLS, Eppelheim) and maintained in Eagle's minimum essential medium (EMEM) at 37°C with 5% CO2 as recommended by the distributor. The media were supplemented with 10% fetal bovine serum, 50 U/mL penicillin-streptomycin and 0.5 µg/mL amphotericin B. Cells were treated with LY294002 (Selleck; 20 µM), recombinant FGF-2 (Sigma, 100 nM) or conditioned media (CM, 25%; described below) for 24 h if not otherwise specified. Dimethyl sulfoxide was used as the solvent control for LY294002, 20 mM Tris (pH 8) was used as the solvent control for FGF-2, and phosphate-buffered saline for the neutralizing antibody.
CM and Measurement of FGF-2
Approximately 2 g of fresh nonmalignant kidney tissue directly adjacent to the tumor was obtained from 3 patients (CM1, CM2, CM3) who underwent tumor nephrectomy at the Department of Urology of the University Hospital Heidelberg. Written informed consent was obtained from each patient according to procedures approved by the University of Heidelberg School of Medicine Ethics Committee. The specimens were cut into small pieces with a scalpel. After washing to remove blood cells, the tissues were incubated in 20 mL Dulbecco's modified Eagle's medium (Thermo Fisher) for 48 h at 37°C. Afterwards, the supernatant was collected after centrifugation, aliquoted and stored at −80°C for future use.
The concentration of FGF-2 in the CM was determined with the human FGF-2 Quantikine® ELISA kit (R&D Systems) according to the manufacturer's instructions. Samples from CM and EMEM containing 10% fetal bovine serum as a control were assayed in triplicates. For each sample, 100 µL was used as input, and samples were measured at 450 nm. The optical density value at 560 nm was subtracted from the corresponding 450 nm measurement to correct for optical imperfections. FGF-2 concentrations of the samples were calculated from a standard curve with polynomial regression.
Neutralization Assay
Inhibition of FGF-2 in the CM was performed with a neutralizing FGF-2 antibody (bFM-1; Merck Millipore, 5 µg/mL). The CM with the neutralizing antibody as well as the CM with solvent control (phosphate-buffered saline) were incubated at 37°C on a shaker for 1 h before they were used to treat the cells.
Migration Assay
ACHN and A-498 cells were cultured in CM or EMEM for 24 h. Cells were seeded onto Millicell® cell culture inserts with 8 µm pore size (Millipore) in serum-free media. Cell culture media supplemented with 10% fetal bovine serum were added to the outside of the inserts. After 24 h, cells were removed from the inside of the insert with wet cotton tissue. The bottom membrane was then fixed in 4% paraformaldehyde, and cells migrated through the pores were stained with 0.1% crystal violet. The stained cells were lysed in 2% sodium dodecyl sulfate, and absorbance was measured at 560 nm. Values were normalized to controls.
Immunoblot Analysis
Cells were lysed in radioimmunoprecipitation assay lysis buffer (Sigma-Aldrich) containing protease inhibitors and phosphatase inhibitors (Roche). A total of 30 μg of protein was separated by 8% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred onto a nitrocellulose membrane. Membranes were incubated with primary antibody overnight at 4°C and with horseradish peroxidase-conjugated secondary antibody (Life Technologies) for 1 h at room temperature. Afterwards, proteins were detected with an ECLTM detection system (Thermo Scientific). Primary antibodies used were directed against AKT (Cell Signaling, 1:1,000), phospho-AKT Ser473 (p-AKT S473) (Cell Signaling, 1:1,000), Emi1 (Invitrogen, 1:100), Skp2 (Cell Signaling, 1:1,000), p27Kip1 (BD Pharmingen, 1:1,000), cyclin A (Leica Microsystems, 1:500) and GAPDH (Santa Cruz, 1:500). Immunoblots were analyzed with ImageJ (v. 1.40 g, NIH) software by measuring the intensity of each band (peak area of respective histograms). The resulting values were normalized to the corresponding GAPDH values and to corresponding controls.
Immunohistochemistry
Formalin-fixed, paraffin-embedded tissue sections were baked overnight at 37°C, deparaffinized in xylene and rehydrated in a graded ethanol series. Afterwards, the sections were immersed into the freshly prepared antigen retrieval solution (Dako) and cooked using a steam cooker. Peroxidase quenching was performed using 3% hydrogen peroxide followed by blocking using 10% goat serum.
Primary antibodies were directed against p27Kip1 (Dako, 1:25), Ki-67 (Dako, 1:100) or FGF-2 (Santa Cruz, 1:100). The slides were incubated with primary antibody overnight at 4°C, and with a corresponding biotinylated secondary antibody for 3 h at 37°C. The sections were then incubated with streptavidin-peroxidase and stained with a 3,3-diaminobenzidine tetrahydrochloride substrate kit (Thermo Scientific). Lastly, the sections were counterstained with hematoxylin (Sigma-Aldrich) and mounted with mounting media (Life Technologies) before microscopic evaluation. Each slide was scored by two independent observers (W.H. and S.D.). The number of nuclear Ki-67-positive tumor cells and cytoplasmic p27Kip1-positive cells was counted in five 40× high-power fields (HPFs). The tumor periphery was defined as the area within a 40× HPF from the outline of the tumor. The tumor center was defined as the area at least one 40× HPF apart from the tumor border toward the center of the tumor.
Flow Cytometry
After drug treatment, 1 × 106 cells were fixed with 70% ethanol for 4 h. Cells were then treated with 10 µg/mL RNAse A and stained with 50 µg/mL propidium iodide overnight at 4°C in the dark. The cells were then analyzed using a FACS Calibur flow cytometer, and data were analyzed using the Flowjo software.
Immunofluorescence Microscopy
Cells were grown on glass coverslips overnight followed by treatment with 20 µM LY294002 or 0.2% dimethyl sulfoxide as solvent control for 48 h. Then, the cells were fixed and permeabilized with 100% ice-cold methanol. After blocking with 10% donkey serum (Jackson Immunoresearch), the cells were incubated with a primary antibody directed against p27Kip1 (Cell Signaling, 1:250) overnight at 4°C and with a secondary antibody conjugated with Alexa Fluor 488 (Thermo Fisher, 1:750) in the dark for 3 h. Cell nuclei were stained with 4′,6-diamidino-2-phenylindole (Vector Laboratories). The slides were examined using an epifluorescence microscope (Leica, DM5000B; Leica, Germany). At least 300 cells were counted per slide, and at least 3 independent experiments were performed.
Quantitative Real-Time PCR
Total RNA was extracted from ACHN and A-498 renal cancer cells using the RNeasy Mini Kit (Qiagen). Afterwards, cDNA was synthesized with the MaximaTM first-strand cDNA synthesis kit (Thermo Scientific) after DNAse treatment. The quantitative PCR was performed with the SsoFastTM EvaGreen® supermix (Bio-Rad) under the following conditions: 30 s activation at 95°C, 5 s denaturation at 95°C and 5 s annealing/extension at 60°C for GAPDH for 40 cycles on a Bio-Rad CFX96 Real-Time System run on a C1000 Thermal Cycler Platform (Bio-Rad). Primers for p27Kip1 were adapted from Perearnau et al. [7] (forward 5-ATGTCAAACGTGCGAGTGTC-3; reverse 5-TCTCTGCAGTGCTTCTCCAA-3; obtained from IDT, Leuven, Belgium). GAPDH cDNA served as reference for relative quantification.
Statistical Analysis
Continuous data were summarized as means ± standard deviation. Student's t test for independent samples, two-tailed, was used wherever applicable. A p value ≤0.05 was considered significant. Data analysis was performed using the SPSS software package (SPSS v. 17.0, SPSS Inc., Chicago, IL, USA).
Results
CM Derived from Nonmalignant Peritumoral Kidney Tissue Downregulate p27Kip1 in RCC Cells
The tumor peripheral zone has previously been identified as a hotspot for RCC cell proliferation and activation of intracellular signaling pathways [6]. Since no periphery-specific mutations that could explain these findings were detected [6], we asked here whether the tumor microenvironment may be involved in the regulation of proliferation and signaling activities.
To address this question, nonmalignant peritumoral kidney tissue samples from 3 patients with advanced RCC undergoing tumor nephrectomy were cultured in vitro, and CM were collected from each specimen after 2 days (Fig. 1a).
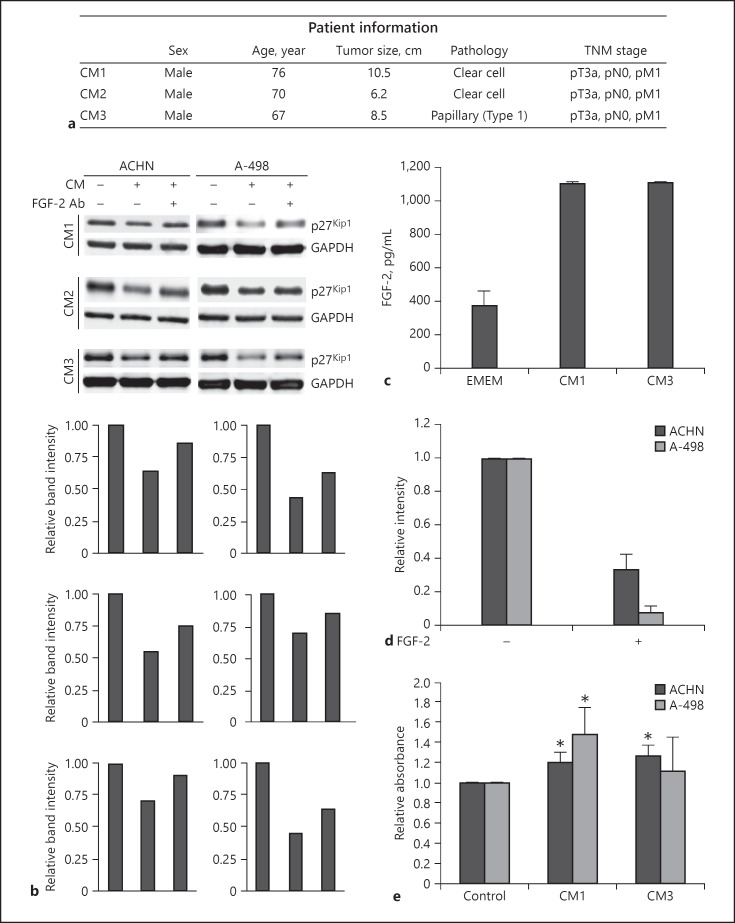
Fig. 1.
Conditioned media (CM) derived from nonmalignant kidney downregulate p27Kip1 in renal cell carcinoma (RCC) cells in a partly fibroblast growth factor-2 (FGF-2)-dependent manner. a Patient information. b Immunoblot analysis of p27Kip1 expression in ACHN and A-498 cells treated with 25% CM for 24 h with or without preincubation with a neutralizing anti-FGF-2 antibody using 3 different CM. Immunoblot for GAPDH is shown to demonstrate protein loading. The bottom panels show a quantification of the immunoblot stainings. Relative band intensity after normalization for GAPDH is shown. c Quantification of the FGF-2 concentration in CM1 and CM3 by ELISA. d Quantitative image analysis of p27Kip1 immunoblots with or without treatment with 100 nM recombinant FGF-2 for 24 h. Results from 2 (ACHN) and 3 (A-498) biological replicates are shown. e Transwell migration assay after stimulation of ACHN and A-498 RCC cells with CM1 or CM3 for 24 h. Asterisks indicate statistically significant changes (* p < 0.05).
Established RCC cell lines (ACHN and A-498) were treated with 25% CM in complete growth media for 24 h (Fig. 1b). Tumor cells were then analyzed for the expression of p27Kip1, a multifunctional CDK inhibitor that regulates cell cycle progression and that is also modulated by growth factors such as FGF-2 [8]. Remarkably, we found that CM from the 3 different patients were able to induce a downregulation of p27Kip1 protein expression (Fig. 1b).
Next, we asked whether this effect could be attributed to a specific growth factor in the CM. When we used an anti-FGF-2-neutralizing antibody (Fig. 1b) that was added to the CM prior to cell treatment, the downregulation of p27Kip1 by CM was partially blocked in both RCC cell lines (Fig. 1b).
To corroborate a role of FGF-2 in the observed effects, we first measured the concentration of FGF-2 in CM1 and CM3 and found that both contained more than 1,000 pg/mL FGF-2 (Fig. 1c).
Next, we confirmed the FGF-2-mediated downregulation of p27Kip1 through a number of immunoblot repeat experiments followed by quantitative image analysis (Fig. 1d).
A reduction of p27Kip1 and in particular its relocalization to the cytoplasm have been shown to lead to an enhanced cell migration [9, 10]. Stimulation of ACHN and A-498 cells with CM1 or CM3 was found to cause a significant increase in migratory activity with the exception of CM3 in A-498 cells (Fig. 1e).
Lastly, we performed immunohistochemical stainings of the nonmalignant tissue that was used to obtain the CM. In all 3 nephrectomy specimens, abundant expression of FGF-2 was detected in the stroma that surrounded the RCC (not shown).
These results support the notion that the nonmalignant peritumoral kidney tissue may play a role in shaping the tumor peripheral niche by releasing growth factors, in particular FGF-2, to drive cell proliferation and migration through modulation of p27Kip1 protein expression.
FGF-2 Enhances the Degradation of p27Kip1 through the Emi1-Skp2 Axis
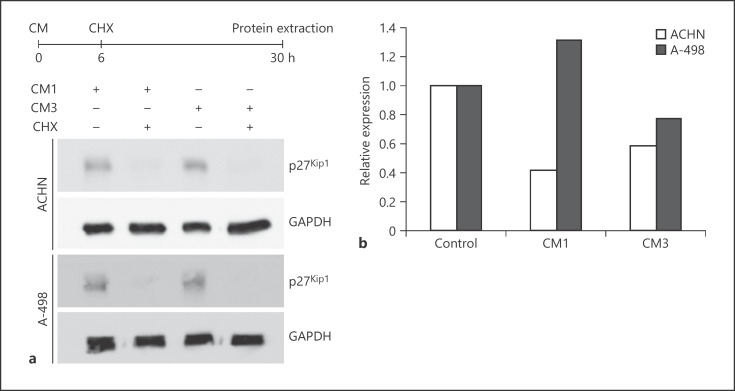
We next sought to characterize the modulation of p27Kip1 protein expression by FGF-2 in greater detail (Fig. 2). To analyze whether the downregulation was through reduced protein stability or reduced mRNA expression, we performed a cycloheximide block experiment (Fig. 2a). After 24 h treatment with cycloheximide, p27Kip1 protein expression was virtually undetectable when compared to untreated cells. At the same time, CDKN1BmRNA expression was either slightly down- or upregulated following treatment with CM (Fig. 2b). These results suggest an enhanced protein degradation as prevailing mechanism for the FGF-2-induced downregulation of p27Kip1.
Fig. 2.
Conditioned media (CM) induce enhanced degradation of p27Kip1a Cycloheximide (CHX) block experiment and immunoblot analysis for p27Kip1 expression in ACHN and A-498 cells after treatment with CM1 or CM3 with or without CHX. b Quantitative RT-PCR analysis of CDKN1B mRNA expression in ACHN and A-498 cells after treatment with CM1 or CM3 for 24 h.
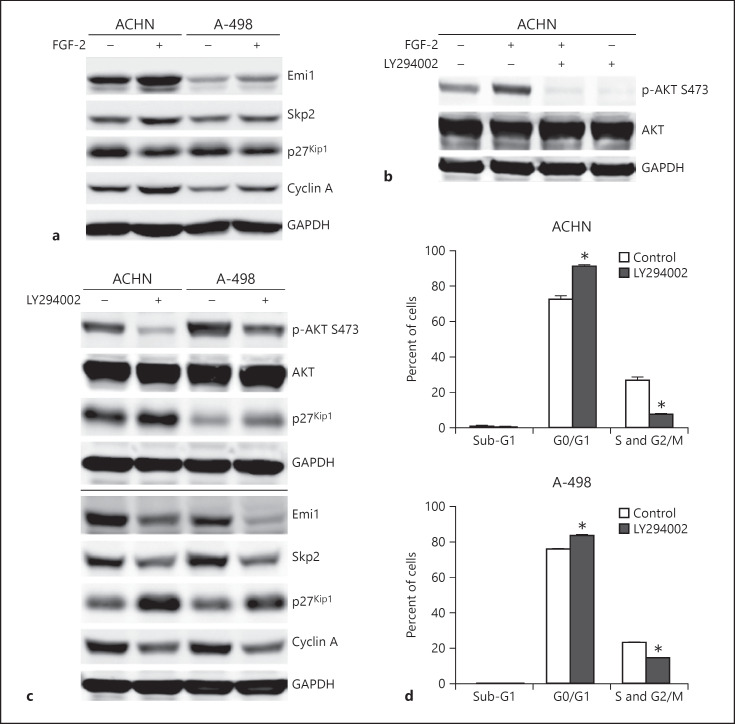
To further characterize the effects of FGF-2 on p27Kip1, we performed a series of immunoblot experiments and found that stimulation with recombinant FGF-2 not only leads to a decrease in p27Kip1 expression and an increase in the S phase marker cyclin A, but also to an increased expression of the F-box protein Skp2 and its upstream regulator Emi1 (Fig. 3a). FGF-2 is known to activate the PI3K/AKT pathway [11], and this activation can be readily abolished using a PI3K inhibitor (Fig. 3b). As shown in Figure 3c, inhibition of the high constitutive AKT activity in ACHN and A-498 RCC cells leads to an increase in p27Kip1 expression and a reduction of Emi1 and Skp2 expression. This was accompanied by a G0/G1 arrest and a decrease in cells in the S and G2/M phases (Fig. 3d). These results underscore that the p27Kip1 is dynamically regulated in RCC cells through the Emi1-Skp2 axis resulting in tumor cell proliferation.
Fig. 3.
Fibroblast growth factor-2 (FGF-2) drives renal cell carcinoma (RCC) cell proliferation via the PI3K/AKT and the Emi1-Skp2-p27Kip1 axis. a Immunoblot analysis of ACHN and A-498 RCC cells treated with 100 nM recombinant FGF-2 for 24 h for Emi, Skp2, p27Kip1 and cyclin A. GAPDH is shown as protein loading control. b Immunoblot analysis of ACHN cells for phospho (p)-AKT S473 and AKT after treatment with 100 nM recombinant FGF-2 alone or in combination with LY294002. c Immunoblot analysis of ACHN and A-498 cells for the indicated proteins after treatment with 20 μM LY294002 or solvent control (dimethyl sulfoxide) for 24 h. All immunoblot experiments shown in a–c are representative results from at least 2 biological replicates. d Cell cycle distribution of ACHN and A-498 cells treated as described in c. Asterisks indicate statistically significant changes (* p < 0.05).
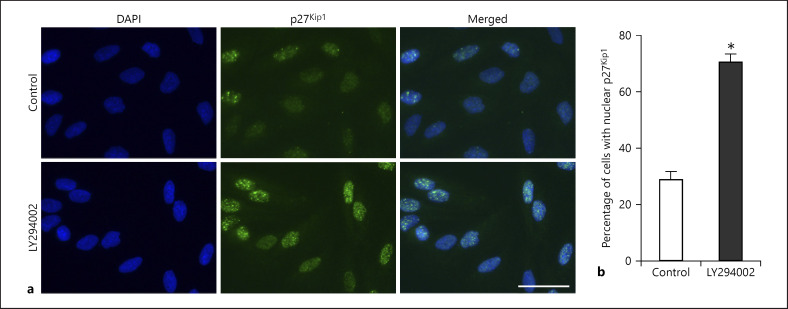
It has previously been shown that the p27Kip1 function is also regulated by its subcellular localization. There is compelling evidence that its function as a tumor suppressor can be abrogated by a cytoplasmic mislocalization [12]. When A-498 cells were treated with LY294002, an increased accumulation of p27Kip1 in the nucleus was observed (Fig. 4a). The proportion of tumor cells with nuclear p27Kip1 expression increased from 28.7% in controls to 70.5% in LY294002-treated A-498 cells (p < 0.01, Fig. 4b). These results suggest that PI3K/AKT signaling contributes to a mislocalization of p27Kip1 outside the nucleus of tumor cells, thus promoting oncogenic activities of this CDK inhibitor.
Fig. 4.
Inhibition of PI3K/AKT leads to an accumulation of p27Kip1 in the nucleus in renal cell carcinoma (RCC) cells. a Immunofluorescence microscopic analysis of A-498 cells for p27Kip1 after treatment with 20 μM Ly294002 or solvent control for 48 h. Nuclei stained with 4′,6-diamidino-2-phenylindole (DAPI). Scale bar = 50 μm. b Quantification of the proportion of cells with nuclear p27Kip1 expression after treatment described in a. Each bar represents mean and standard deviation of 3 independent experiments (* p < 0.05).
Intratumoral Spatial Niche Formation Involves the Cytoplasmic Mislocalization of p27Kip1 in the Peripheral Zone
Lastly, we sought to analyze whether p27Kip1 is also involved in functional ITH in RCC where the tumor peripheral zone is a hotspot for proliferation [6].
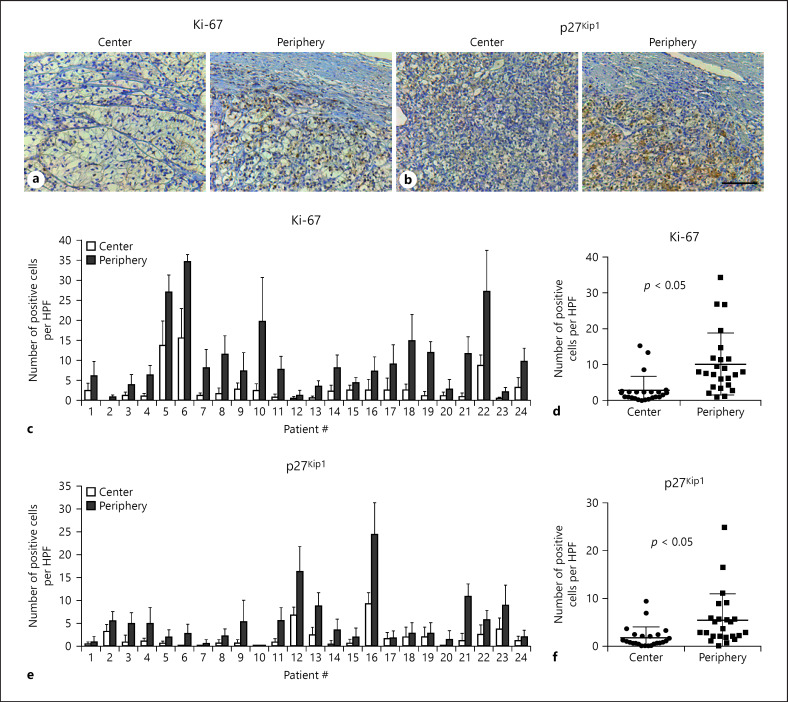
A series of 24 ccRCCs (Table 1) were analyzed for the expression of the proliferation marker Ki-67 and p27Kip1 in both the tumor center and periphery (Fig. 5a, b). We first confirmed an increased number of Ki-67-positive tumor cells in the tumor periphery compared to the center as previously described (Fig. 5c) [6]. The tumor periphery showed on average 3.6-fold more Ki-67-positive tumor cells (mean 10.2 cells/HPF) than the tumor center (mean 2.8 Ki-67-positive cells/HPF, p < 0.05; Fig. 5d).
Table 1.
Patient characteristics
| Parameters | N | % |
|---|---|---|
| Sex | ||
| Male | 16 | 66.7 |
| Female | 8 | 33.3 |
| Age at diagnosis, years | ||
| Mean ± SD (range) | 59.9±8.0 (46.5–75.8) | |
| Tumor diameter, cm | ||
| Mean ± SD (range) | 6.0±3.4 (2.0–19.0) | |
| Clear cell histology | 24 | 100 |
| pT stage | ||
| T1 | 15 | 52.5 |
| T2 | 0 | 0.0 |
| T3 | 6 | 15.0 |
| T4 | 3 | 12.5 |
| pN stage | ||
| N0 | 22 | 91.7 |
| N1 | 2 | 8.3 |
| cM stage | ||
| MO | 10 | 41.7 |
| M1 | 14 | 58.3 |
| Fuhrman grade | ||
| 1–2 | 18 | 75.0 |
| 3–4 | 6 | 25.0 |
Fig. 5.
Concomitant upregulation of Ki-67 and cytoplasmic p27Kip1 in the tumor peripheral niche. a, b Representative immunohistochemical stainings of clear cell renal cell carcinoma specimens for Ki-67 and p27Kip1 in the tumor center and periphery. Scale bar = 100 µm. c–f Quantification of the number of Ki-67- or p27Kip1-positive tumor cells per five 40× high-power fields (HPFs) in tumor center and periphery. Mean and standard deviation for each tumor are shown in c and e. Means and standard deviations across all tumors are shown in d and f with individual data points from c and e replotted as dots.
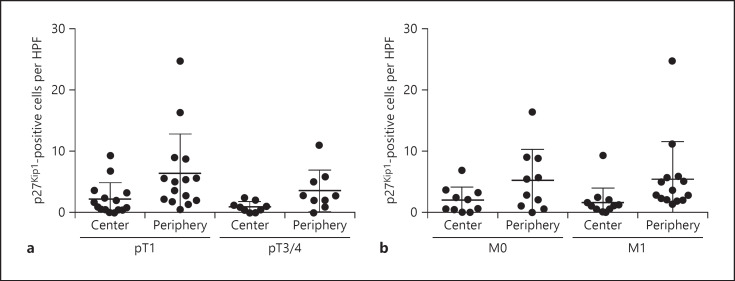
We next analyzed the expression of p27Kip1 in both compartments. Remarkably, all patients except one contained more tumor cells with cytoplasmic p27Kip1 in the tumor periphery when compared to the tumor center (Fig. 5e). In the tumor periphery, there were on average 3.1-fold more tumor cells with cytoplasmic p27Kip1 (mean 5.3 cells/HPF) when compared to the tumor center (mean 1.7 cells/HPF, p < 0.05; Fig. 5f). There were no significant differences between the frequency of p27Kip1-positive tumor cells in the center and periphery between pT1 and pT3/4 RCCs or metastatic and nonmetastatic tumors (Fig. 6).
Fig. 6.
The distribution of p27Kip1-positive renal cell carcinoma (RCC) cells is independent from the tumor stage. a, b Quantification of the number of p27Kip1-positive RCC cells in tumor center and periphery stratified into pT1 versus pT3/4 tumors (a) or M0 versus M1 tumors (b).
Taken together, these results indicate that the CDK inhibitor p27Kip1 plays a crucial role in shaping the tumor peripheral niche in RCC where it may exert oncogenic activities.
Discussion
We have previously shown that functional ITH is a key characteristic of RCC where the tumor peripheral zone represents a hotspot for proliferation and activation of intracellular signaling pathways [6]. Since our previous experiments could not identify any genetic alterations to explain this finding [6], the current study was designed to explore a potential role of the tumor microenvironment in peripheral niche formation.
Herein, we show that CM derived from nonmalignant kidney cells adjacent to an RCC can downregulate the expression of the CDK inhibitor p27Kip1 in RCC cells through enhanced protein degradation in an FGF-2-dependent fashion. FGF-2 functions mainly through the PI3K/AKT pathway, and RCC cells with constitutively high AKT activity show not only an enhanced degradation of p27Kip1 through the Emi1-Skp2 axis, but also a subcellular mislocalization of p27Kip1 to the cytoplasmic compartment. Remarkably, such a mislocalization was also detected in the tumor periphery in vivo suggesting that p27Kip1 plays an important role in shaping this spatial niche and may contribute to tumor progression.
Our findings are in line with previous reports showing that cytoplasmic p27Kip1 is associated with an unfavorable prognosis in RCC patients [13, 14]. Our findings extend previous models of tumor growth kinetics that were focused on tumor-intrinsic parameters such as genomic alterations [15].
Normal kidney tissue is rich in FGF-2, which is expressed in fibroblasts, endothelial cells, tubules and glomeruli [16]. However, we would like to point out that FGF-2 may not be the only growth factor or cytokine involved in the modulation of p27Kip1 in RCC [12]. A downregulation of p27Kip1 has also been reported in the context of hypoxia [17]. However, most RCCs have a constitutively high expression of hypoxia-inducible factors (HIFs) due to the loss of VHL. HIFs have been shown to upregulate p27Kip1 [18, 19], and there is furthermore a HIF-independent pathway to upregulate p27Kip1 following VHL loss [20]. Our finding that RCC cells in the tumor periphery, where proliferation peaks, show a cytoplasmic p27Kip1 expression may hence indicate that RCC cells are under selective pressure to subvert the negative growth regulatory effects of p27Kip1.
In addition to its role as tumor suppressor by negatively regulating CDKs in the nucleus, p27Kip1 has been implicated in promoting malignant progression. In particular, cytoplasmic p27Kip1 has been shown to stimulate tumor cell proliferation, invasion and metastasis [12, 21, 22]. Although we did not directly prove the notion that the cytoplasmic localization of p27Kip1 in the tumor peripheral zone is driven by FGF-2, our previous studies have clearly shown that this niche is enriched for tumor cells with active PI3K/AKT/mTOR signaling [6]. Since p27Kip1 can be phosphorylated by AKT on amino acid residue T157, which drives its cytoplasmic localization [13, 23], it is conceivable that this interaction contributes to the findings reported here.
Our results have a number of translational implications. First, targeting the FGF/FGF receptor pathway to reduce proliferation and potentially other oncogenic processes such as increased migratory activity in the tumor peripheral zone appears to be a promising approach. However, results from a limited number of clinical trials using FGF receptor or AKT inhibitors have not led to an integration of these compounds in clinical treatment algorithms [24, 25, 26]. Whether their limited efficacy is due to the fact that other growth factors are involved or a more general disruption of proapoptotic networks in RCC remains to be elucidated. Interestingly, cytoplasmic p27Kip1 per se has been reported to confer resistance to apoptosis [13]. Whether combination therapies will be able to thwart this intrinsic obstacle to tumor cell eradication is currently unclear.
Our results underscore the important role of the tumor microenvironment in promoting malignant progression. It is hence important to consider extrinsic factors when developing novel prognostic and/or predictive biomarkers in ccRCC. Future studies are clearly warranted to harness the tumor microenvironment for therapeutic purposes.
Disclosure Statement
The authors declare no competing interests.
Funding Sources
We are grateful to the Wilhelm Sander-Stiftung for financial support (2016.102.1). W.H. is recipient of a fellowship from the China Scholarship Council (201506210081).
Author Contributions
W.H. contributed to the design of the study, acquired data, analyzed and interpreted data and wrote a draft of the manuscript. A.K., P.L. and M.K. performed experiments and helped with data interpretation. A.K. performed data analyses. V.S., D.F. and S.C.D. helped to acquire clinical data. M.H. and S.D. supervised the study and wrote the final manuscript. All authors have read and approved the final paper.
Acknowledgments
We want to thank Yvonne Samstag and Jie Liang, Institute of Immunology, University of Heidelberg, for their kind help with flow cytometry. We are grateful to the tissue bank of the National Center for Tumor Diseases Heidelberg for tissue procurement.
References
- 1.Motzer RJ, Tannir NM, McDermott DF, Arén Frontera O, Melichar B, Choueiri TK, et al. CheckMate 214 Investigators Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. N Engl J Med. 2018 Apr;378((14)):1277–90. doi: 10.1056/NEJMoa1712126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gerlinger M, Rowan AJ, Horswell S, Math M, Larkin J, Endesfelder D, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med. 2012 Mar;366((10)):883–92. doi: 10.1056/NEJMoa1113205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gerlinger M, Horswell S, Larkin J, Rowan AJ, Salm MP, Varela I, et al. Genomic architecture and evolution of clear cell renal cell carcinomas defined by multiregion sequencing. Nat Genet. 2014 Mar;46((3)):225–33. doi: 10.1038/ng.2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gerlinger M, Catto JW, Orntoft TF, Real FX, Zwarthoff EC, Swanton C. Intratumour heterogeneity in urologic cancers: from molecular evidence to clinical implications. Eur Urol. 2015 Apr;67((4)):729–37. doi: 10.1016/j.eururo.2014.04.014. [DOI] [PubMed] [Google Scholar]
- 5.Turajlic S, Xu H, Litchfield K, Rowan A, Horswell S, Chambers T, et al. TRACERx Renal Consortium Deterministic Evolutionary Trajectories Influence Primary Tumor Growth: TRACERx Renal. Cell. 2018 Apr;173((3)):595–610.e11. doi: 10.1016/j.cell.2018.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoefflin R, Lahrmann B, Warsow G, Hübschmann D, Spath C, Walter B, et al. Spatial niche formation but not malignant progression is a driving force for intratumoural heterogeneity. Nat Commun. 2016 Jun 13;(7):ncomms11845. doi: 10.1038/ncomms11845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perearnau A, Orlando S, Islam AB, Gallastegui E, Martínez J, Jordan A, et al. p27Kip1, PCAF and PAX5 cooperate in the transcriptional regulation of specific target genes. Nucleic Acids Res. 2017 May;45((9)):5086–99. doi: 10.1093/nar/gkx075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee JG, Kay EP. Two populations of p27 use differential kinetics to phosphorylate Ser-10 and Thr-187 via phosphatidylinositol 3-Kinase in response to fibroblast growth factor-2 stimulation. J Biol Chem. 2007 Mar;282((9)):6444–54. doi: 10.1074/jbc.M607808200. [DOI] [PubMed] [Google Scholar]
- 9.Denicourt C, Saenz CC, Datnow B, Cui XS, Dowdy SF. Relocalized p27Kip1 tumor suppressor functions as a cytoplasmic metastatic oncogene in melanoma. Cancer Res. 2007 Oct;67((19)):9238–43. doi: 10.1158/0008-5472.CAN-07-1375. [DOI] [PubMed] [Google Scholar]
- 10.Rampioni Vinciguerra GL, Citron F, Segatto I, Belletti B, Vecchione A, Baldassarre G. p27kip1 at the crossroad between actin and microtubule dynamics. Cell Div. 2019 Apr;14((1)):2–7. doi: 10.1186/s13008-019-0045-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Turner N, Grose R. Fibroblast growth factor signalling: from development to cancer. Nat Rev Cancer. 2010 Feb;10((2)):116–29. doi: 10.1038/nrc2780. [DOI] [PubMed] [Google Scholar]
- 12.Chu IM, Hengst L, Slingerland JM. The Cdk inhibitor p27 in human cancer: prognostic potential and relevance to anticancer therapy. Nat Rev Cancer. 2008 Apr;8((4)):253–67. doi: 10.1038/nrc2347. [DOI] [PubMed] [Google Scholar]
- 13.Kim J, Jonasch E, Alexander A, Short JD, Cai S, Wen S, et al. Cytoplasmic sequestration of p27 via AKT phosphorylation in renal cell carcinoma. Clin Cancer Res. 2009 Jan;15((1)):81–90. doi: 10.1158/1078-0432.CCR-08-0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kruck S, Merseburger AS, Hennenlotter J, Scharpf M, Eyrich C, Amend B, et al. High cytoplasmic expression of p27(Kip1) is associated with a worse cancer-specific survival in clear cell renal cell carcinoma. BJU Int. 2012 May;109((10)):1565–70. doi: 10.1111/j.1464-410X.2011.10649.x. [DOI] [PubMed] [Google Scholar]
- 15.Waclaw B, Bozic I, Pittman ME, Hruban RH, Vogelstein B, Nowak MA. A spatial model predicts that dispersal and cell turnover limit intratumour heterogeneity. Nature. 2015 Sep;525((7568)):261–4. doi: 10.1038/nature14971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Floege J, Hudkins KL, Eitner F, Cui Y, Morrison RS, Schelling MA, et al. Localization of fibroblast growth factor-2 (basic FGF) and FGF receptor-1 in adult human kidney. Kidney Int. 1999 Sep;56((3)):883–97. doi: 10.1046/j.1523-1755.1999.00637.x. [DOI] [PubMed] [Google Scholar]
- 17.Ghafar MA, Anastasiadis AG, Chen MW, Burchardt M, Olsson LE, Xie H, et al. Acute hypoxia increases the aggressive characteristics and survival properties of prostate cancer cells. Prostate. 2003 Jan;54((1)):58–67. doi: 10.1002/pros.10162. [DOI] [PubMed] [Google Scholar]
- 18.Hackenbeck T, Knaup KX, Schietke R, Schödel J, Willam C, Wu X, et al. HIF-1 or HIF-2 induction is sufficient to achieve cell cycle arrest in NIH3T3 mouse fibroblasts independent from hypoxia. Cell Cycle. 2009 May;8((9)):1386–95. doi: 10.4161/cc.8.9.8306. [DOI] [PubMed] [Google Scholar]
- 19.Kumar S, Vaidya M. Hypoxia inhibits mesenchymal stem cell proliferation through HIF1α-dependent regulation of P27. Mol Cell Biochem. 2016 Apr;415((1-2)):29–38. doi: 10.1007/s11010-016-2674-5. [DOI] [PubMed] [Google Scholar]
- 20.Young AP, Schlisio S, Minamishima YA, Zhang Q, Li L, Grisanzio C, et al. VHL loss actuates a HIF-independent senescence programme mediated by Rb and p400. Nat Cell Biol. 2008 Mar;10((3)):361–9. doi: 10.1038/ncb1699. [DOI] [PubMed] [Google Scholar]
- 21.Podmirseg SR, Vosper J, Hengst L. p27Kip1- p(RhoB)lematic in lung cancer. J Pathol. 2019 May;248((1)):3–5. doi: 10.1002/path.5218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao D, Besser AH, Wander SA, Sun J, Zhou W, Wang B, et al. Cytoplasmic p27 promotes epithelial-mesenchymal transition and tumor metastasis via STAT3-mediated Twist1 upregulation. Oncogene. 2015 Oct;34((43)):5447–59. doi: 10.1038/onc.2014.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hong F, Larrea MD, Doughty C, Kwiatkowski DJ, Squillace R, Slingerland JM. mTOR-raptor binds and activates SGK1 to regulate p27 phosphorylation. Mol Cell. 2008 Jun;30((6)):701–11. doi: 10.1016/j.molcel.2008.04.027. [DOI] [PubMed] [Google Scholar]
- 24.Motzer RJ, Porta C, Vogelzang NJ, Sternberg CN, Szczylik C, Zolnierek J, et al. Dovitinib versus sorafenib for third-line targeted treatment of patients with metastatic renal cell carcinoma: an open-label, randomised phase 3 trial. Lancet Oncol. 2014 Mar;15((3)):286–96. doi: 10.1016/S1470-2045(14)70030-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Powles T, Foreshew SJ, Shamash J, Sarwar N, Crabb S, Sahdev A, et al. A phase Ib study investigating the combination of everolimus and dovitinib in vascular endothelial growth factor refractory clear cell renal cancer. Eur J Cancer. 2014 Aug;50((12)):2057–64. doi: 10.1016/j.ejca.2014.04.021. [DOI] [PubMed] [Google Scholar]
- 26.Escudier B, Grünwald V, Ravaud A, Ou YC, Castellano D, Lin CC, et al. Phase II results of Dovitinib (TKI258) in patients with metastatic renal cell cancer. Clin Cancer Res. 2014 Jun;20((11)):3012–22. doi: 10.1158/1078-0432.CCR-13-3006. [DOI] [PubMed] [Google Scholar]