Abstract
We updated the meta-analysis published by McDonald et al. [Chest 2002;122;1535–1542] by reviewing the effectiveness of air purification for the treatment of home-related allergic asthma (dust mite, dog, cat, and cockroach). We analysed the trials included by McDonald et al. as well as studies published since 2000. Data on asthma symptoms scores (ASS), medication use, forced expiratory volume in 1 s as a percentage of the predicted value (FEV<sub>1</sub> %pred), histamine provocative concentration causing a 20% reduction in FEV<sub>1</sub> (PC<sub>20</sub>), Asthma Quality of Life Questionnaire (AQLQ) scores, and fractional exhaled nitric oxide (FeNO) levels were extracted. The effectiveness was examined using metafor (registered in Prospero CRD42019127227). Ten trials including a total of 482 patients (baseline characteristics: mean FEV<sub>1</sub> %pred 83.2%, I<sup>2</sup> = 96.7%; mean PC<sub>20</sub> 4.93 mg/mL, I<sup>2</sup> = 44.0%; mean AQLQ 4.67 [max. 7], I<sup>2</sup> = 93.7%; mean FeNO 36.5 ppb, I<sup>2</sup> = 0%) were included. We assessed the mean differences in the AQLQ scores as +0.36 (95% CI 0.10 to 0.62, p = 0.01, n = 302, I<sup>2</sup> = 0%) and the FeNO levels as −6.67 ppb (95% CI −10.56 to −2.77, p = 0.0008, n = 304, I<sup>2</sup> = 0%). The standardised mean differences in all other health outcomes were not significant (ASS −0.68, p = 0.20; medication use: −0.01, p = 0.94; FEV<sub>1</sub> %pred −0.11, p = 0.34; PC<sub>20</sub> +0.24, p = 0.53). We found statistically significant mean differences in the AQLQ scores and FeNO levels in patients with predominantly mild to moderate asthma at baseline. A large trial reported great improvement in the subgroup of patients receiving Global Initiative for Asthma step 4 therapy. We recommend that future studies on air purification focus on patients with severe and poorly controlled allergic asthma.
keywords: Air purification, Allergy, Asthma, Meta-analysis
Introduction
Respiratory allergy is a public health problem that affects approximately 400 million people [1]. The most common home-related respiratory allergies result from house dust mite, dog, cat, and cockroach allergen (Global Initiative for Asthma, GINA, 2018). Therapies such as pharmacological treatment, immunotherapy, and avoidance of indoor allergen exposure have been developed for the treatment of allergic asthma [2]. Evidence of clinical benefits of textile-based avoidance strategies has not been demonstrated in rigorous systematic reviews [3, 4, 5]. In a scoping review, Boven et al. [6] observed potential success with the strategy of air purification for the treatment of house dust mite allergy-related asthma. Previously, McDonald et al. [7] reported improvements in asthma symptom scores (ASS) associated with air purification in a small patient subgroup (n = 88).
Whether the purification of indoor air is of clinical importance in patients with asthma remains an unanswered question. An allergic reaction is provoked in the upper airways after the deposition of aerosol particles in the epithelium. The faecal pellets of house dust mites are very small in size, at 10–40 μm (mean 22 μm), and decrease when they are partially degraded over time (diameter >0.5 μm) [8, 9]. A large proportion of cat and dog allergens are smaller than 2 μm in diameter and coagulate in the air to other aerosol dust [10]. The particle size of cockroach allergens is mainly >10 μm [11]. Industrial branches have developed specific filters (high-efficiency particulate air, HEPA, filters) that capture very small airborne particles with high efficiency (at least 85–99.999995% of particles with a diameter of 0.3 μm) [12]. These HEPA filters are applied in residential products such as housing ventilation units, mobile air cleaners, nocturnal temperature-regulated laminar airflow units, and vacuum cleaners. The strategy of air purification has a potential advantage over a textile-based control strategy because the former strategy traps airborne allergens emitted from clothes as well as emissions from indoor textiles. This advantage may explain the clinical potential of the air purification strategy. As the current evidence on the clinical effectiveness of the air purification strategy is based on small sample sizes and was obtained many years ago, there is a need to update the evidence base, as new devices for purifying the nocturnal breathing zone have been introduced [13, 14].
This study updates the existing systematic review by McDonald et al. [7] entitled “Effect of Air Filtration Systems on Asthma” by reviewing the clinical effectiveness of the air purification strategy for the treatment of home-related allergic asthma (house dust mite allergy, dog allergy, cat allergy, and cockroach allergy).
Methods
Reference Search
The starting point of this study was the systematic review by McDonald et al. [7]. This meta-analysis included ten trials. An updated search of the literature published since January 2000 was performed in EMBASE, MEDLINE, and the Cochrane Central Register of Controlled Trials (CENTRAL). The trials were limited to peer-reviewed publications in the English language, and (Congress) abstracts were excluded from the analysis. The titles and/or abstracts of the studies retrieved during the search were screened (with Endnote) by the first author (F.E.v.B.) to identify randomised trials that met the inclusion criteria outlined below. The full texts of the potentially included trials were retrieved and assessed for inclusion by the first (F.E.v.B.) and second (N.W.d.J.) authors. Any ambiguities in the selections were resolved by discussion. The inclusion criteria were as follows:
Type of study: randomised controlled trials with blinding.
Intervention: housing or mobile ventilation systems, including HEPA filters but not vacuum cleaners.
Participants: participants with physician-diagnosed bronchial allergic asthma. These participants had their sensitisation assessed by either skin testing or serum assays for specific IgE antibodies (house dust mite allergy, dog allergy, cat allergy, and cockroach allergy). The asthma assessment included a history of asthma symptoms and a pulmonary function test.
Controls: participants who received a placebo or no treatment.
Data Extractions and Outcomes
The data were extracted by the first author (F.E.v.B.). The trials included in McDonald et al. [7] were re-extracted, as this review presented only the results but not the extracted data. The data extractions yielded the following: characteristics of the study population including the baseline data; type of intervention and the control; study methodology, and outcomes. Missing data were requested from the study authors. A second author (N.W.d.J.) verified the selections and the data extraction conducted by the first author. Any ambiguities in the selection and the extraction were resolved by discussion.
The main outcome(s) were: the asthma symptom score; the number of patients with improved outcomes; medication use; forced expiratory volume in 1 s as a percentage of the predicted value (FEV1 %pred); provocative concentration that causes a 20% reduction in FEV1 (PC20); Asthma Quality of Life Questionnaire (AQLQ) score, and the fractional exhaled nitric oxide (FeNO) level. Additional outcomes included: the mite allergen load from the mattress (μg/g dust); type of patient (child or adult), and the presence of primary and cosensitisation. These additional outcomes were all tested as possible explanatory variables in the presence of at least ten trials.
For the ASS, the PC20, and the AQLQ scores, the final values were extracted (following Egbewale et al. [15]). The change scores were extracted for FEV1, medication use, and FeNO level. We defined the direction of changes as positive for an increasing FEV1 and negative for a decreasing FeNO level and medication use.
Risk of Bias (Quality) Assessment
The risk of bias was assessed for the following domains: sequence generation, allocation concealment, blinding, incomplete outcome data, and selective outcome reporting. The assessment was performed by the first author (F.E.v.B.) with the Review Manager (RevMan) computer program version 5.3 (the Cochrane Collaboration, 2014; Nordic Cochrane Centre, Copenhagen, Denmark). A second author (N.W.d.J.) verified the assessments of the first author by considering a sample. Any ambiguities in the assessments were resolved by discussion.
Strategy for Data Synthesis
The effect size was set to the standardised mean difference, excluding the number of patients showing improvement (risk ratio). We chose the mean difference as the effect size in cases in which the outcomes were all measured in the same manner (AQLQ and FeNO). First, the overall effect of the health outcomes was estimated by a random-effects meta-analysis. Additionally, the I2 was calculated for examining heterogeneity in the outcomes. In the absence of heterogeneity (I2 = 0), a fixed-effects model was used. The explanatory variables of interest included the primary sensitisation (house dust mite allergy, dog allergy, cat allergy, or cockroach allergy), the mite allergen load from the mattress at baseline, possible confounding by the type of patient (child/adult), and the presence of cosensitisation. These outcomes were analysed for a preferred minimum of ten trials per variable [16]. All the calculations were performed with the metafor package in R [17, 18]. The level of significance was set to α = 0.05.
Results
Selection of the References
We selected and included studies in two groups of publications. First, we screened the ten trials included in the meta-analysis by McDonald et al. [7]. Three trials were excluded for a lack of or only partial reporting on the treatment of asthma [19, 20] or reporting incomplete data [21]. The remaining seven trials were included in the analysis [22, 23, 24, 25, 26, 27, 28].
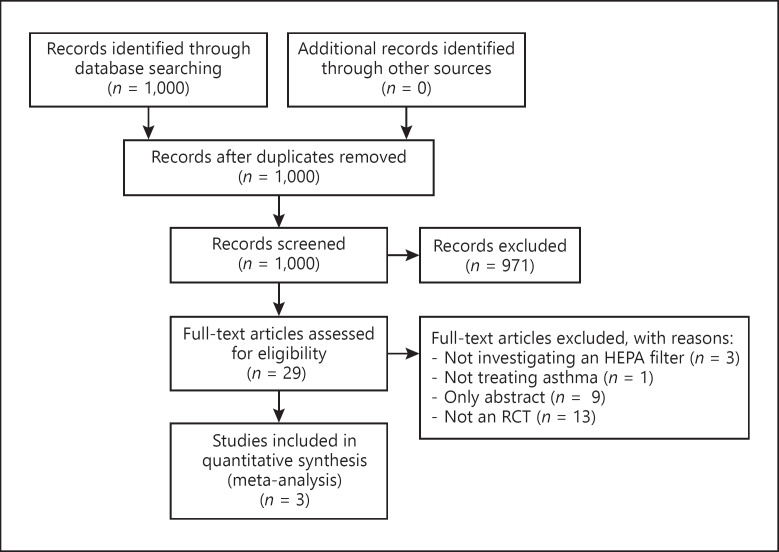
The second group consisted of studies identified in our updated search (Fig. 1) [29]. We identified a total of 1,000 titles and abstracts. A total of 971 titles were excluded for lacking randomisation and/or blinding regarding the effectiveness of air purification. Twenty-nine potentially relevant titles were selected for inclusion. We excluded twenty-six full-text articles for not meeting our inclusion criteria (online suppl. Table; for all online suppl. material, see www.karger.com/doi/10.1159/000506284). Three full-text articles were included in the analysis [13, 30, 31]. In total, ten full-text articles were included in the meta-analysis.
Fig. 1.
Flow chart of the reference search.
Description of the Trials and the Baseline Characteristics
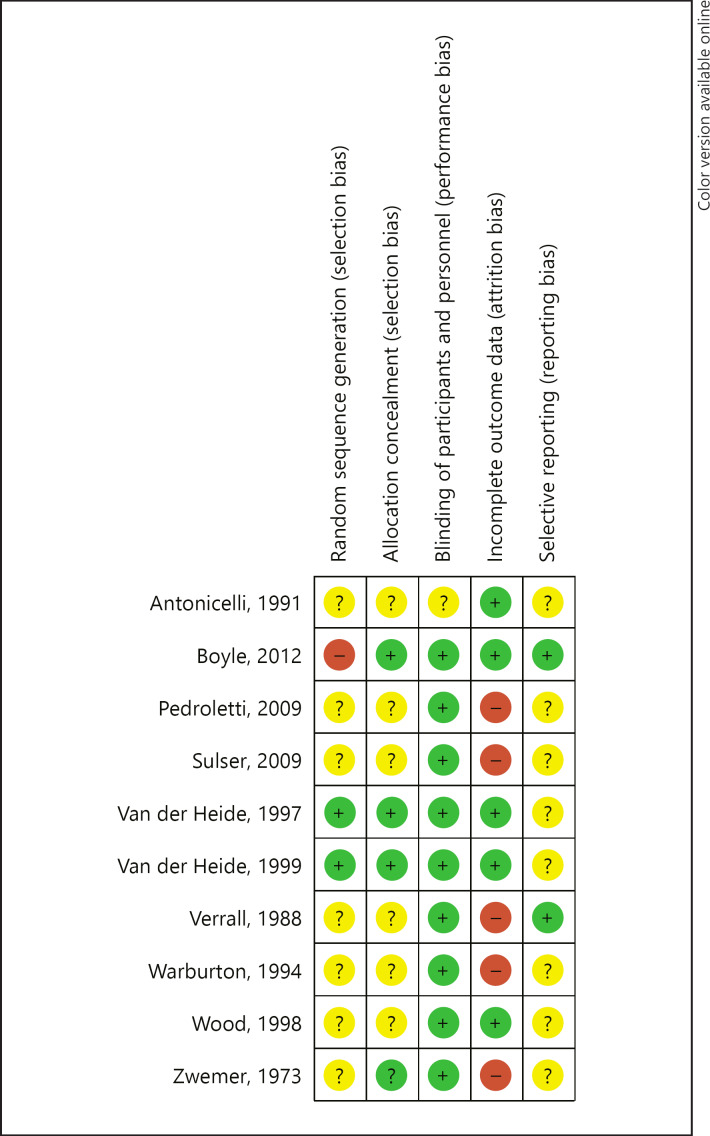
Ten trials published between 1973 and 2012 reported the treatment of asthma by air purification (Table 1). In four trials, the primary sensitisation was a pet allergy [13, 27, 28, 30]; five trials reported patients with house dust mite allergy [22, 23, 24, 25, 26], and one trial reported a mix of primary antigens [31]. None of the trials reported monosensitisation in the included patients. One trial [31] presented data on the specific IgE during the trial. Three trials reported the treatment of children with allergic asthma; the others reported the treatment of adults or both children and adults. Four trials studied nocturnal laminar airflow in the breathing zone; the other six trials studied the use of a home ventilation or mobile device with a HEPA filter. Only one trial reported on the airborne allergen exposure [28], five other trials reported on dust exposure or allergen load at baseline [24, 25, 26, 27, 30]. In the trial by Warburton et al. [25] only the data on FEV1 %pred at baseline were available for analysis. In five trials, the mean FEV1 %pred was 83.2% (I2 = 96.7%, n = 346). The mean PC20 was 4.93 mg/mL (I2 = 44.0%, 2 trials, n = 29), the mean AQLQ score was 4.67 (max. 7; I2 = 93.7%, 2 trials, n = 304), and the mean FeNO level was 36.5 ppb (I2 = 0%, 2 trials, n = 304). For the ASS and medication use, we had no (quantitative) data available at baseline. Ten trials reported on the use of medication at baseline. In four trials, the change in the use of medication was a primary outcome for measuring effectiveness [22, 25, 26, 28]. Two investigations instructed their patients not to change their medication [23, 27]. In two trials [13, 31], patients were allowed to use more medication. The risk of bias was judged as predominantly unclear with a low risk of bias in blinding (Fig. 2).
Table 1.
Characteristics of the included studies
| Trial | Use of a HEPA filter | Subjects | Primary allergy | Health outcomes extracted |
|---|---|---|---|---|
| Zwemer [22], 1973 | Nocturnal laminar airflow | Child | House dust mite | ASS |
| Verrall [23], 1988 | Nocturnal laminar airflow | Adult | House dust mite | Medication use |
| Antonicelli [23], 1991 | Mobile device | Adult | House dust mite | ASS, medication use, FEV1 %pred, PC20 |
| Warburton [24], 1994 | Mobile device | Adult | House dust mite | FEV1 %pred |
| Van der Heide [26], 1997 | Mobile device | Adult | House dust mite | PC20 |
| Wood [28], 1998 | Mobile device | Adult | Cat | ASS, medication use |
| Van der Heide [27], 1999 | Mobile device | Child | Cat or dog | Medication use, PC20 |
| Pedroletti [13], 2009 | Nocturnal laminar airflow | Adult | Cat or dog | AQLQ score, FeNO level |
| Sulser [30], 2009 | Mobile device | Adult | Cat or dog | PC20 |
| Boyle [31], 2012 | Nocturnal laminar airflow | Adult | House dust mite or cat | Medication use, FEV1 %pred, AQLQ score, FeNO level |
Fig. 2.
Summary of the judgements on the risk of bias in the trials.
Synthesis of the Efficacy Results
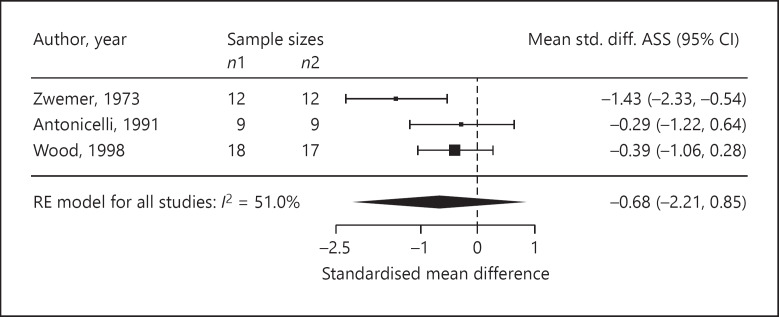
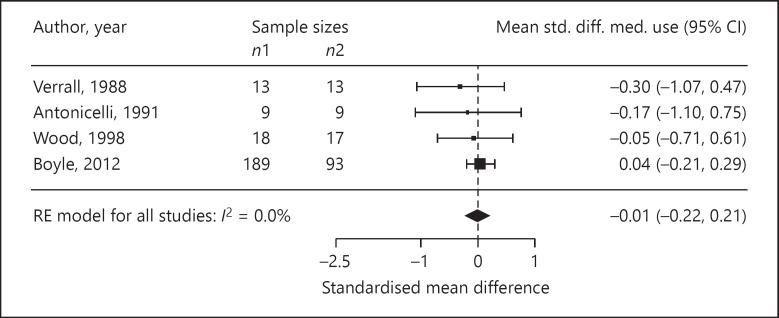
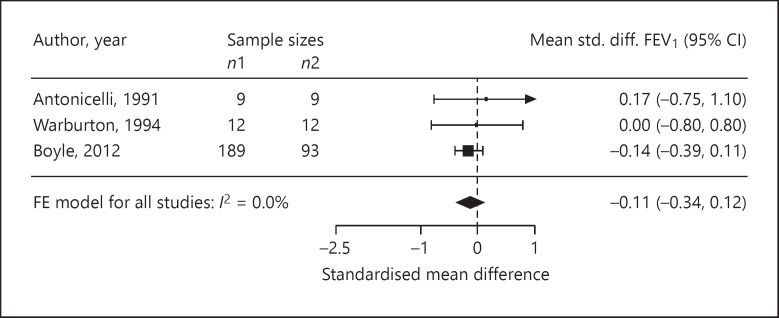
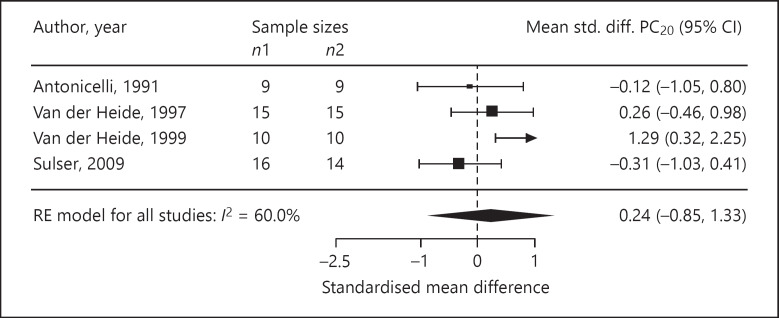
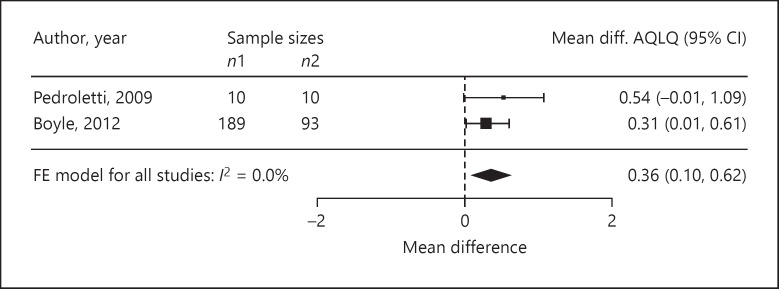
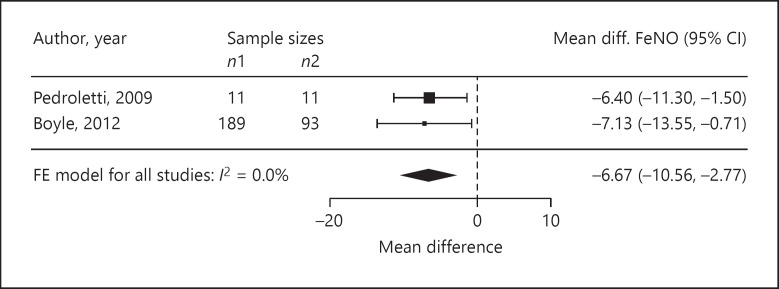
Four trials reported ASS as outcomes. We assessed the standardised mean difference in the ASS as −0.68 (95% CI −2.21 to 0.85; p = 0.20; n = 77; I2 = 51.0%; Fig. 3). The standardised mean difference in medication use was −0.01 (95% CI −0.22 to 0.21; p = 0.94; n = 401; I2 = 0%, 4 trials; Fig. 4). In three trials, the standardised mean difference in FEV1 %pred was −0.11 (95% CI −0.34 to 0.12; p = 0.34; n = 324; I2 = 0%; Fig. 5). Four trials reported on the PC20, with a standardised mean difference of +0.24 (95% CI −0.85 to 1.33; p = 0.53; n = 98; I2 = 60.0%; Fig. 6). The AQLQ scores were reported in two trials. We assessed the mean difference in the AQLQ scores as +0.36 (95% CI 0.10 to 0.62, p = 0.01, n = 302, I2 = 0%; Fig. 7). This positive increase was strongly influenced by the large trial by Boyle et al. [31] (weight 77%). The mean difference in the FeNO level was −6.67 ppb (95% CI −10.56 to −2.77, p = 0.008, n = 304, I2 = 0%; Fig. 8). None of the included trials reported on whether the physician-diagnosed numbers improved. Overall, the number of trials available was too small to allow any subgroup analysis.
Fig. 3.
Forest plot of the standardised mean differences in the ASS.
Fig. 4.
Forest plot of the standardised mean differences in medication use.
Fig. 5.
Forest plot of the standardised mean differences in the FEV1 %pred.
Fig. 6.
Forest plot of the standardised mean differences in the PC20.
Fig. 7.
Forest plot of the mean differences in the AQLQ scores.
Fig. 8.
Forest plot of the mean differences in the FeNO levels.
Discussion
We reviewed the clinical effectiveness of the air purification strategy for the treatment of home-related allergic asthma in ten trials. The mean differences in the AQLQ score (MD = +0.36; p = 0.01) and the FeNO level (MD = −6.67; p = 0.008) were statistically significant, suggesting that asthma patients may benefit from air purification. These results were obtained in patients with predominantly mild to moderate asthma outcomes at baseline (the FEV1 %pred, the AQLQ score, and the FeNO level). The overall airway hyperresponsiveness was mild at baseline, according to the classification by Cockcroft et al. [32]. The risk of bias in the trials was predominantly judged unclear; however, blinding has a low risk of bias.
The strength of this meta-analysis was the rigorous selection of trials and extraction of data. We decided a priori whether to extract change or final values considering the statistical notes by Egbewale et al. [15]. In our study, we excluded some trials that were included by McDonald et al. [7] due to a critical process in extracting the data. For instance, theyincluded the ASS by Reisman et al. [20]. After a critical review of this paper, we decided not to extract these data as only 11 of 32 patients were diagnosed with asthma; thus, we excluded this trial from the analysis. We noticed that this trial was also excluded for the same reason in the meta-analysis by Gøtzsche and Johansen [3]. While the previously analysed trials were quite old, the recent trials included the use of validated outcomes such as the AQLQ score [33]. In patients with mild to moderate disease, we observed small (not reaching the minimum clinically important difference) but significant improvements in the AQLQ scores and FeNO levels. This effect could possibly be stronger in patients with severe asthma than in those with mild to moderate asthma. This possible tendency is well presented in the large trial by Boyle et al. [31]. They studied the effectiveness of the Protexo system (a nocturnal temperature-controlled laminar airflow) and reported the outcomes of the use of medication, FEV1 %pred, AQLQ scores, and FeNO levels. They differentiated the AQLQ score, their primary outcome, and the asthma status defined by the treatment intensity of GINA and the asthma control test (ACT). After a 1-year treatment period, Boyle et al. [31] reported an AQLQ score difference of +0.31 (p = 0.04) in all the studied patients (n = 282). When limited to the patients classified as requiring GINA step 4 therapy (GINA 4) at baseline, the difference became +0.47 (p = 0.04, n = 129). In the patients receiving GINA 4 with poor control (ACT <18), the difference in the AQLQ score was +0.70 (p = 0.02, n = 87). Additionally, in the patients with a high FeNO level at baseline, the same tendency was reported by Boyle et al. [31] (mean difference in FeNO −29.7 ppb, p = 0.001).
The limitation of this meta-analysis was the relatively small number of trials included in the analysis. Our update did not result in many new included trials. In total, we included the same number of trials (n = 10) as McDonald et al. [7] included in their earlier meta-analysis. We had to exclude three trials that were included by McDonald et al. [7] because of a lack of reporting on the treatment of asthma or incompleteness of the data. McDonald et al. [7] previously reported “a small but statistically significant difference in total symptoms associated with use of domestic air filters.” They did not find benefits associated with medication use or morning peak flow values. In our update, we did not find a significant difference for the ASS outcome. The significance reported by McDonald et al. [7] was based on an analysis by the fixed-effects model. As the ASS showed moderate heterogeneity (I2 = 51%), we introduced the random-effects model and the significance was lost. The use of domestic HEPA filters will also be of relevance in the treatment of non-allergic asthma, for instance by filtering indoor air pollution. As we included only trials on the treatment of allergic asthma, this possible issue did not bias our results. The description of the allergen exposure differed in the trials and was sometimes poorly presented. Therefore, we could not analyse the degree of the exposure, and also cannot exclude the possibility that a variation of allergens from other sources affected the results.
The significant differences we found were both a result of trials sponsored by Airsonett AB (Angelholm, Sweden). One of these trials [31] was predominantly responsible for the positive AQLQ score analysis and was judged as having a risk of bias in randomisation. Their treatment group was twice the size of the control group. In principle, this creates a risk of selection bias as recruiters could “guess with greater than a 50% probability what the next treatment allocation will be” [34]. In their report, we did not find indications for baseline imbalances biasing the estimates. Another issue of relevance in both trials on the Protexo system is the possibility of changes in medication use. Pedroletti et al. [13] reported that “inhaled, short-acting beta-2 agonists were allowed as rescue treatment.” Boyle et al. [31] instructed that the patients “asthma medication were kept unchanged for the first 3 months, and thereafter adjusted to optimise asthma control.” We cannot exclude the possibility that these instructions confounded the significant results we found. Overall, the results require independent repeating, with careful monitoring of allergen exposure.
Other studies on the Protexo system resulted in (some) clinical benefits. Schauer et al. [35] observed reduced asthma exacerbation and hospitalisations in an observational study in patients with predominantly difficult-to-control asthma. In a recent pilot study, Gore et al. [36] reported the potential for the use of the Protexo system as an add-on to standard pharmacological treatment in children with difficult-to-control atopic dermatitis. These results also reflect the need to study patients with severe and uncontrolled conditions.
In brief, we reviewed the clinical effectiveness of the air purification strategy for the treatment of home-related allergic asthma (house dust mite allergy, dog allergy, cat allergy, and cockroach allergy). We found statistically significant mean differences in the AQLQ scores and FeNO levels in patients with predominantly mild to moderate asthma at baseline. A large underlying trial [31] showed potentially great improvement in the AQLQ scores in the subgroup of patients receiving GINA 4 therapy with poor control. Future studies on air purification strategies with rigorous trial designs that focus on patients with severe and poorly controlled allergic asthma are warranted.
Statement of Ethics
Ethical approval for this meta-analysis was not required.
Disclosure Statement
The authors have no conflicts of interest to declare.
Funding Sources
No sources of funding are applicable to this work.
Author Contributions
All authors contributed to the design of the work. F.E.v.B. and N.W.d.J. selected the references and extracted the data. F.E.v.B. and L.R.A. analysed the data. G.J.B. and R.G.v.W. contributed to the interpretation of the data. All authors contributed to the draft of the work, and read and approved the final manuscript.
Supplementary Material
Supplementary data
Acknowledgements
The authors thank Mr. W.M. Bramer (MSc) from Erasmus Medical Center for his assistance in the reference search.
References
- 1.Pawankar RC, Holgate ST, Lockey RF. Blaiss MS (eds) WAO white book on allergy. World Allergy Organization; 2013. [Google Scholar]
- 2.Abrams EM, Szefler SJ, Becker AB. Effect of asthma therapies on the natural course of asthma. Ann Allergy Asthma Immunol. 2016 Dec;117((6)):627–33. doi: 10.1016/j.anai.2016.09.438. [DOI] [PubMed] [Google Scholar]
- 3.Gøtzsche PC, Johansen HK. House dust mite control measures for asthma. Cochrane Database Syst Rev. 2008 Apr;((2)):CD001187. doi: 10.1002/14651858.CD001187.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kilburn S, Lasserson TJ, McKean M. Pet allergen control measures for allergic asthma in children and adults. Cochrane Database Syst Rev. 2003;((1)):CD002989. doi: 10.1002/14651858.CD002989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sheikh A, Hurwitz B, Nurmatov U, van Schayck CP. House dust mite avoidance measures for perennial allergic rhinitis. Cochrane Database Syst Rev. 2010 Jul;((7)):CD001563. doi: 10.1002/14651858.CD001563.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Boven FE, Arends LR, Braunstahl GJ, van Wijk RG. A reintroduction of environmental mite allergen control strategies for asthma treatment and the debate on their effectiveness. Clin Exp Allergy. 2019 Apr;49((4)):400–9. doi: 10.1111/cea.13340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McDonald E, Cook D, Newman T, Griffith L, Cox G, Guyatt G. Effect of air filtration systems on asthma: a systematic review of randomized trials. Chest. 2002 Nov;122((5)):1535–42. doi: 10.1378/chest.122.5.1535. [DOI] [PubMed] [Google Scholar]
- 8.van Bronswijk JE. [House dust ecosystem and house dust allergen (s)] Acta Allergol. 1972;27((3)):219–28. [PubMed] [Google Scholar]
- 9.Tovey ER, Chapman MD, Wells CW, Platts-Mills TA. The distribution of dust mite allergen in the houses of patients with asthma. Am Rev Respir Dis. 1981 Nov;124((5)):630–5. doi: 10.1164/arrd.1981.124.5.630. [DOI] [PubMed] [Google Scholar]
- 10.Luczynska CM, Li Y, Chapman MD, Platts-Mills TA. Airborne concentrations and particle size distribution of allergen derived from domestic cats (Felis domesticus). Measurements using cascade impactor, liquid impinger, and a two-site monoclonal antibody assay for Fel d I. Am Rev Respir Dis. 1990 Feb;141((2)):361–7. doi: 10.1164/ajrccm/141.2.361. [DOI] [PubMed] [Google Scholar]
- 11.De Lucca SD, Taylor DJ, O'Meara TJ, Jones AS, Tovey ER. Measurement and characterization of cockroach allergens detected during normal domestic activity. J Allergy Clin Immunol. 1999 Sep;104((3 Pt 1)):672–80. doi: 10.1016/s0091-6749(99)70341-6. [DOI] [PubMed] [Google Scholar]
- 12.Instituut SK. High efficiency air filters (EPA, HEPA and ULPA) - Part 1: Classification, performance testing, marking. NEN-EN; 2019. pp. pp.1822–1. [Google Scholar]
- 13.Pedroletti C, Millinger E, Dahlén B, Söderman P, Zetterström O. Clinical effects of purified air administered to the breathing zone in allergic asthma: A double-blind randomized cross-over trial. Respir Med. 2009 Sep;103((9)):1313–9. doi: 10.1016/j.rmed.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 14.Stillerman A, Nachtsheim C, Li W, Albrecht M, Waldman J. Efficacy of a novel air filtration pillow for avoidance of perennial allergens in symptomatic adults. Ann Allergy Asthma Immunol. 2010 May;104((5)):440–9. doi: 10.1016/j.anai.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 15.Egbewale BE, Lewis M, Sim J. Bias, precision and statistical power of analysis of covariance in the analysis of randomized trials with baseline imbalance: a simulation study. BMC Med Res Methodol. 2014 Apr;14((1)):49. doi: 10.1186/1471-2288-14-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Higgins JP, Green S. Cochrane handbook for systematic reviews of interventions. 2008. [Google Scholar]
- 17.Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36((3)) [Google Scholar]
- 18.Ihaka R, Gentleman RR. A language for data analysis and graphics. J Comput Graph Stat. 1996;5((3)):299–314. [Google Scholar]
- 19.Kooistra JB, Pasch R, Reed CE. The effects of air cleaners on hay fever symptoms in air-conditioned homes. J Allergy Clin Immunol. 1978 May;61((5)):315–9. doi: 10.1016/0091-6749(78)90053-2. [DOI] [PubMed] [Google Scholar]
- 20.Reisman RE, Mauriello PM, Davis GB, Georgitis JW, DeMasi JM. A double-blind study of the effectiveness of a high-efficiency particulate air (HEPA) filter in the treatment of patients with perennial allergic rhinitis and asthma. J Allergy Clin Immunol. 1990 Jun;85((6)):1050–7. doi: 10.1016/0091-6749(90)90050-e. [DOI] [PubMed] [Google Scholar]
- 21.Villaveces JW, Rosengren H, Evans J. Use of laminar air flow portable filter in asthmatic children. Ann Allergy. 1977 Jun;38((6)):400–4. [PubMed] [Google Scholar]
- 22.Zwemer RJ, Karibo J. Use of laminar control device as adjunct to standard environmental control measures in symptomatic asthmatic children. Ann Allergy. 1973 Jun;31((6)):284–90. [PubMed] [Google Scholar]
- 23.Verrall B, Muir DC, Wilson WM, Milner R, Johnston M, Dolovich J. Laminar flow air cleaner bed attachment: a controlled trial. Ann Allergy. 1988 Aug;61((2)):117–22. [PubMed] [Google Scholar]
- 24.Antonicelli L, Bilò MB, Pucci S, Schou C, Bonifazi F. Efficacy of an air-cleaning device equipped with a high efficiency particulate air filter in house dust mite respiratory allergy. Allergy. 1991 Nov;46((8)):594–600. doi: 10.1111/j.1398-9995.1991.tb00629.x. [DOI] [PubMed] [Google Scholar]
- 25.Warburton CJ, Niven RM, Pickering CA, Fletcher AM, Hepworth J, Francis HC. Domiciliary air filtration units, symptoms and lung function in atopic asthmatics. Respir Med. 1994 Nov;88((10)):771–6. doi: 10.1016/s0954-6111(05)80200-8. [DOI] [PubMed] [Google Scholar]
- 26.van der Heide S, Kauffman HF, Dubois AE, de Monchy JG. Allergen reduction measures in houses of allergic asthmatic patients: effects of air-cleaners and allergen-impermeable mattress covers. Eur Respir J. 1997 Jun;10((6)):1217–23. doi: 10.1183/09031936.97.10061217. [DOI] [PubMed] [Google Scholar]
- 27.van der Heide S, van Aalderen WM, Kauffman HF, Dubois AE, de Monchy JG. Clinical effects of air cleaners in homes of asthmatic children sensitized to pet allergens. J Allergy Clin Immunol. 1999 Aug;104((2 Pt 1)):447–51. doi: 10.1016/s0091-6749(99)70391-x. [DOI] [PubMed] [Google Scholar]
- 28.Wood RA, Johnson EF, Van Natta ML, Chen PH, Eggleston PA. A placebo-controlled trial of a HEPA air cleaner in the treatment of cat allergy. Am J Respir Crit Care Med. 1998 Jul;158((1)):115–20. doi: 10.1164/ajrccm.158.1.9712110. [DOI] [PubMed] [Google Scholar]
- 29.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P, PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009 Jul;6((7)):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sulser C, Schulz G, Wagner P, Sommerfeld C, Keil T, Reich A, et al. Can the use of HEPA cleaners in homes of asthmatic children and adolescents sensitized to cat and dog allergens decrease bronchial hyperresponsiveness and allergen contents in solid dust? Int Arch Allergy Immunol. 2009;148((1)):23–30. doi: 10.1159/000151502. [DOI] [PubMed] [Google Scholar]
- 31.Boyle RJ, Pedroletti C, Wickman M, Bjermer L, Valovirta E, Dahl R, et al. 4A Study Group Nocturnal temperature controlled laminar airflow for treating atopic asthma: a randomised controlled trial. Thorax. 2012 Mar;67((3)):215–21. doi: 10.1136/thoraxjnl-2011-200665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cockcroft DW, Murdock KY, Berscheid BA, Gore BP. Sensitivity and specificity of histamine PC20 determination in a random selection of young college students. J Allergy Clin Immunol. 1992 Jan;89((1 Pt 1)):23–30. doi: 10.1016/s0091-6749(05)80037-5. [DOI] [PubMed] [Google Scholar]
- 33.Juniper EF, Guyatt GH, Cox FM, Ferrie PJ, King DR. Development and validation of the Mini Asthma Quality of Life Questionnaire. Eur Respir J. 1999 Jul;14((1)):32–8. doi: 10.1034/j.1399-3003.1999.14a08.x. [DOI] [PubMed] [Google Scholar]
- 34.Kahan BC, Rehal S, Cro S. Risk of selection bias in randomised trials. Trials. 2015 Sep;16((1)):405. doi: 10.1186/s13063-015-0920-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schauer U, Bergmann KC, Gerstlauer M, Lehmann S, Gappa M, Brenneken A, et al. all members of the German Asthma Net (GAN) Improved asthma control in patients with severe, persistent allergic asthma after 12 months of nightly temperature-controlled laminar airflow: an observational study with retrospective comparisons. Eur Clin Respir J. 2015 Jul;2((1)):2. doi: 10.3402/ecrj.v2.28531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gore C, Gore RB, Fontanella S, Haider S, Custovic A. Temperature-controlled laminar airflow (TLA) device in the treatment of children with severe atopic eczema: Open-label, proof-of-concept study. Clin Exp Allergy. 2018 May;48((5)):594–603. doi: 10.1111/cea.13105. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data