Abstract
Background
Postoperative pancreatic fistula (POPF) is associated with adverse events, increased duration of stay and hospital costs. We developed perioperative care pathways stratified by POPF risk with the aims of minimizing variations in care, improving quality, and decreasing costs.
Study Design
Three unique risk-stratified pancreatectomy clinical pathways (RSPCP)- low-risk pancreatoduodenectomy (PD), high-risk PD, and distal pancreatectomy (DP) were developed and implemented. Consecutive patients treated after implementation of the RSPCPs were compared to patients treated immediately prior. Duration of stay, rates of perioperative AEs, discharge disposition and hospital readmission, as well as the associated costs of care were evaluated.
Results
The median hospital stay after pancreatectomy decreased from 10 to 6 days after implementation of the RSPCPs (p<.001), and the median cost of index hospitalization decreased by 22%. Decreased changes in median hospital stay and costs of hospitalization were observed in association with low-risk PD (p<.05) and DP (p<.05) but not high-risk PD. The incidence of 90-day adverse events, grade B/C POPF, discharge to a facility other than home, and readmission did not change after implementation.
Conclusion
Implementation of RSPCPs decreased median stay and cost of index hospitalization after pancreatectomy without unfavorably affecting rates of perioperative adverse events, readmission rates, or discharge disposition. Outcomes were most favorably improved for low-risk PD and DP. Additional work is necessary to decrease the rate of POPF, minimize variability, and improve outcomes after high-risk PD.
Keywords: pancreatectomy, ERAS, length of stay, hospital cost, POPF, pancreatoduodenectomy
Introduction
Protocols of perioperative care pathways, fast-track, and enhanced recovery after surgery (ERAS) protocols describe comprehensive, multidisciplinary strategies designed to accelerate patient recovery after operations. Typically, a single pathway is utilized to standardize care of all patients treated with a single anatomic operation or class of operations. Although the specific elements of these pathways may differ, those paathways used for abdominal operations generally encourage the use of minimally invasive operative techniques, early postoperative mobilization and per os feeding, decreased use of drainage tubes and opioid drugs, and an early target for hospital discharge.1–4 Colorectal surgeons have long demonstrated that duration of hospital stay), perioperative morbidity, and costs of hospitalization are generally decreased after the implementation of care pathways.1,5–8 More recently, surgeons have adopted clinical pathways to care for patients undergoing esophageal, gastric, hepatic, and urologic procedures, and have also reported favorable outcomes.2–4,9,10
Pancreatic resection is generally associated with greater rates of perioperative morbidity than other abdominal operations.11 Over the past several decades, mortality after pancreatectomy has decreased to <2% at high-volume treatment centers, yet up to 75% of patients still suffer from perioperative adverse events (AEs).12–14 Among these, postoperative pancreatic fistula (POPF), which develops in as many as one-third of patients, is the most feared. The association between POPF and other complications, reoperations, and mortality has been well established.15–19 In an early study, Yeo et al. found that a POPF increased the rate of reoperation from 2% to 14% and prolonged the median duration of hospitalization by 15 days.20
Almost 20 years later, despite refinements of anastomotic techniques21,22 and the development of new drugs that may decrease its incidence,23 a POPF still occurs in approximately 20% of patients after pancreatoduodenectomy (PD) and 30% after Ddistal pancreatectomy (DP)24,25 and POPF remains one of the primary drivers of additional adverse events (AEs) after pancreatic surgery.14 To the extent that POPF is relatively common and often causes patient recovery from operation to deviate from an expected pattern and timeline, the perioperative care and pace of recovery of patients undergoing pancreatic resection have been difficult to standardize. So, although clinical pathways have been designed and implemented for pancreatectomy, the associated results have generally been less dramatic than those observed for other operative procedures.26–31
The incidence of POPF is not distributed uniformly among patients who undergo pancreatectomy.17,32,33 We and others have shown that each patient’s risk of a clinically important(grade B/C) POPF can be calculated after PD or distal pancreatectomy (DP) on the basis of easily measured clinical parameters.32,34 On the basis of this observation, we hypothesized that the quality of care provided to all patients undergoing pancreatectomy would be enhanced by assigning each patient, prior to operation, to a unique care pathway designed specifically for individuals at a similar anticipated risk for grade B/C POPF. We subsequently designed and implemented three. risk-stratified, pancreatectomy clinical pathways (RSPCPs)—two for PD and one for DP—to direct patient postoperative care on this basis. In this study, we sought to describe this novel approach, to illustrate the process of development of the three pathways we used, and to provide initial results after implementation into clinical practice, including hospital stay, rates of perioperative morbidity, mortality, discharge disposition hospital readmission, and costs of care.
Methods
Risk Groups
Clinical data from 315 consecutive patients who underwent PD or DP between January 2011 and January 2014 at the University of Texas MD Anderson Cancer Center were used to develop clinical pathways appropriate to patients risk of POPF. The operative techniques of PD and DP have been standardized within our group as previously described.34 Their postoperative care was not prescribed and was generally left to the discretion of each individual surgeon. With respect to peritoneal drainage, Jackson-Pratt drains were routinely placed intraoperatively, evaluated for amylase after the patient was tolerating a diet, and removed in the absence of evidence for POPF. Pasireotide was not administered to any patients in the historic cohort prior to implementation of the pathway.
Previously, we described our use of recursive partitioning regression tree analysis (RPA) of preoperative clinical factors to retrospectively assign each of these patients to clinical subgroups based on their incidence of a grade B/C POPF.34 RPA allocated patients who underwent PD into four discrete groups with an incidence of grade B/C POPF of 0, 10, 29, and 60% based on histopathologic diagnosis, pancreatic duct diameter (measured radiographically), and BMI (p <.001). On the basis of this analysis, the four groups characterized after PD were compressed into two general categories of anticipated risk of grade B/C POPF: low (combined incidence of 6%) and high (combined incidence of 42%). RPA allocated patients who underwent DP into three groups with an incidence of grade B/C POPF of 14, 26, or 44% based on duct diameter alone (p = 0.05), but this was not a clinically relevant variable that would allow dichotomization of risk in patients who underwent DP.34
Risk-stratified clinical pathways for pancreatectomy
The pancreatic surgery service developed a unique clinical care pathway for each of these three risk groups—low-risk PD, high-risk PD and DP—based on review of the relevant literature and consensus. The objective of each risk-stratified pancreatectomy clinical pathway was standardized perioperative care and expedited postoperative recovery and discharge to an extent commensurate with each treated patient’s anticipated risk of grade B/C POPF.
The three RSPCPs were implemented by the service in October 2016 without any change to our standardized technical approach to operation. Each RSPCP included mandatory care elements and associated milestones, including: preoperative counseling, preoperative diet, pharmacologic prophylaxis against POPF (pasireotide 900 μg twice daily for 7 days), regional anesthesia, perioperative antibiotics, propyhylaxis against venous thromboembolism (VTE), tube management (e.g. nasogastric tube, urinary catheter, and peritoneal drain), restriction of intravenous fluid (IVF), early and frequent patient mobilization, initiation of diet and advancement, bowel cianregimen, pain management, pancreatic enzyme replacement, consultation with a dietit, and early discharge planning and postoperative follow-up. Operatively placed drains were removed on postoperative day (POD) 3 if the amylase level in the drain effluent was less than 3 times the upper limit of normal serum amylase and lacked any sinister qualities. Specific drug regimens that could be chosen by the attending surgeon included choice of regional anesthetic (epidural, transverse abdominal plane (TAP) block, etc.), perioperative antibiotic, composition of IVFs, use of prokinetic agents, and the oral pain regimen. In general, a multi-modal anesthetic and pain management approach was utilized in order to minimize opioid usage.
Table 1 lists some of the major pathway elements and corresponding milestones for each RSPCP. Based on our previous work demonstrating that the administration of prophylactic pasireotide was not cost effective for patients at low risk for grade B/C POPF, its use was restricted to the high-risk PD and DP pathways.34
Table 1.
Major elements and milestones of the risk-stratified pancreatectomy clinical pathways.
| Low-risk PD | High-risk PD | DP | |
|---|---|---|---|
| Regional anesthesia | + | + | + |
| POPF prophylaxis with pasireotide | − | + | + |
| NG tube removal | POD#2 | POD#3 | POD#0 |
| Urinary catheter removal | POD#2 | POD#2 | POD#2 |
| Drain removal* | POD#3 | POD#3 | POD#3 |
| CLD | POD#3 | POD#4 | POD#1 |
| Lovenox upon discharge | + | + | + |
| Anticipated dischargeˆ | POD#6 | POD#8 | POD#5 |
RSPCP = risk-stratified pancreatectomy clinical pathways; NG = nasogastric; CLD = clear liquid diet; SLIV = saline lock IV;
if the operative drain fluid amylase level measured on postoperative day 3 is <3 times the upper limit of serum amylase;
discharge with plans for a short-term follow-up.
Pathway adherence
Afterg adoption of the RSPCPs, the perioperative risk of grade B/C POPF for each patient scheduled to undergo pancreatectomy was categorized, and the patient was assigned to the appropriate RSPCP during the preoperative visit. All patients were managed according to their respective pathway. The care team, including a nurse practitioner, surgical fellow, and attending surgeon, monitored adherence to the RSPCP on a daily basis. For the purpose of this analysis, adherence to the pathway within each pathway was reported as the number and percentage of patients that met each milestone.
Cost analysis
All cost data were obtained without knowledge of the patient’s clinical status after discharge and were provided by the University of Texas MD Anderson Cancer Center financial/accounting departments. The total cost of the index hospitalization was calculated as the sum of both the direct and indirect costs for admission through discharge. Health care costs have been rapidly increasing drain effluent over the last decade. Therefore, to compare hospitalization costs between patients across the study time-period, the total cost of index hospitalization in a given fiscal year was converted to an equivalent fiscal year 2017 cost. The cost of fiscal year 2017 (FY2017) was determined by the equation: FY2017 = HY (1 + i)t, where HY = cost in the historic fiscal year, i = annual institutional cost increase, and t = time/number of compounding periods (a formula analogous to that used to calculate the time value of money 35). The accounting department at the University of Texas MD Anderson Cancer Center provided the annual institutional cost increase for patient care in every fiscal year from 2011 to 2017. The institutional increase in cost was calculated on an annual basis. Therefore, for example, a cost in fiscal year 2016 c could be adjusted to an equivalent FY2017 cost, assuming a cost increase of 3.5% that year by calculating FY2017 = FY2016 (1 + .035)1. (The specific cost data is proprietary information of the University of Texas MD Anderson Cancer Center). Therefore, cost data are reported as the percentage difference between median total cost of index hospitalization (FY2017) of the patients treated prior to and after implementation of the RSPCPs.
Adverse Events
Since 2011, we have used a prospective surveillance program to document AEs. This system, which has been described previously in detail, 14,34,36 was used to detect and grade all perioperative AEs within 90 days of pancreatectomy. The severity of all AEs were graded prospectively using the modified ACCORDION grading system37 and the pancreas-specific complications of delayed gastric emptying (DGE),38 postoperative hemorrhage (PPH),39 and POPF18 were graded prospectively using the ISGPF classification and calculators developed and published online by the Pancreas Club (www.pancreasclub.com). All patients were called by phone on the 90th postoperative day to ensure an accurate dataset. Hospital stay was calculated from the date of operation to the date of discharge.
Statistical Analysis
The 315 consecutive patients treated from July 2011 through January 2014, prior to implementation of the RSPCPs, were allocated retrospectively into the risk groups aligned with each RSPCP and were compared with the first 60 consecutive patients treated immediately after pathway implementation. Pre-existing comorbidities were categorized as none (0), mild (1), moderate (2), or severe (3) by using the Adult Comorbidity Evaluation-27 index.40 The MDACC clinical staging system was used to define potentially resectable, borderline resectable, and locally advanced pancreatic ductal adenocarcinoma (PDAC).41 Continuous variables were reported as a median with interquartile range (IQR), and categorical variables were reported with a frequency and percentage. To compare the data between groups, the Mann-Whitney test was used for quantitative data and a chi-squared or Fisher exact test for categorical data. All tests were two-sided and statistical significance was defined as p <.05. All statistical analysis was performed with SPSS, version 23 (IBM, Armonk, NY).
Results
Patient characteristics
The first 60 patients who received perioperative care on one of the three RSPCPs were compared to the 315 patients who were allocated retrospectively into analogous risk groups but who were treated prior to implementation of the pathways. The clinical characteristics of patients treated prior to and after adoption of the RSPCPs were generally similar, but a minimally invasive (MIS) DP was more common after adoption (Table 2). Patients in the low-risk PD, high-risk PD, and DP risk groups accounted for 45%, 23%, and 36%, respectively, of the entire cohort of 375 patients.
Table 2.
Clinical characteristics of consecutive patients treated prior to and following implementation of the risk-stratified pancreatectomy clinical pathways (N = 375).
| Clinical Factor, n (%) | Historic (n=315) | RSPCP (n=60) | P-value |
|---|---|---|---|
| Median Age, years (IQR) | 62 (55-69) | 60 (52-72) | .809 |
| Sex, male | 181 (57%) | 32 (53%) | .554 |
| Obese, BMI ≥ 30 | 89 (28%) | 11 (18%) | .111 |
| DM | 71 (23%) | 12 (20%) | .664 |
| Comorbidity | .078 | ||
| None | 83 (26%) | 18 (30%) | |
| Mild | 116 (37%) | 30 (50%) | |
| Moderate | 79 (25%) | 9 (15%) | |
| Severe | 37 (12%) | 3 (5%) | |
| PDAC | 147 (47%) | 26 (43%) | .635 |
| PDAC, radiographic stage | .546 | ||
| Potentially resectable | 104 (69%) | 18 (69%) | |
| Borderline resectable | 41 (27%) | 6 (23%) | |
| Locally advanced | 5 (3%) | 2 (8%) | |
| Preoperative therapy | .573 | ||
| None | 171 (54%) | 37 (62%) | |
| Chemo alone | 32 (10%) | 5 (8%) | |
| Chemoradiation +/−chemo | 112 (36%) | 18 (30%) | |
| Operation | |||
| PD | 202 (64%) | 39 (65%) | .897 |
| DP | 113 (36%) | 21 (35%) | |
| Open | 103 (91%) | 14 (67%) | .002 |
| Minimally invasive | 10 (9%) | 7 (33%) | |
| Vascular resection | .707 | ||
| Vein | 70 (22%) | 10 (17%) | |
| Artery | 2 (1%) | 0 (0%) | |
| Vein + artery | 13 (4%) | 3 (5%) | |
| Risk Group | .895 | ||
| Low-risk PD | 142 (45%) | 26 (43%) | |
| High-risk PD | 60 (19%) | 13 (22%) | |
| DP | 113 (36%) | 21 (35%) |
RSPCP, risk-stratified pancreatectomy clinical pathways; IQR, interquartile range; BMI, body mass index; DM, diabetes mellitus; PDAC, pancreatic ductal adenocarcinoma; PD, pancreatoduodenectomy; DP, distal pancreatectomy; chemo, systemic chemotherapy; LOS, length of stay.
Adherence to the Pathway
Complete (100%) adherence to the RSPCPs was uncommon. Adherence rates to the milestones specified by the low-risk PD RSPCP were generally the greatest among the three pathways, however, only 2 of the 26 patients treated in the low-risk PD pathway received care entirely as prescribed. With respect to major milestones, 20 (77%) had the NG tube removed, 16 (62%) had the urinary catheter removed, and 20 (77%) started a clear liquid diet (CLD) per pathway. Only 20 (77%) patients had the operative drain removed on POD 3, even though the drain effluent of 25 (96%) justified it per the pathway. Despite a relatively high rate of specific deviations, 19 (73%) patients treated on the low-risk PD RSPCP were discharged on POD 6 or earlier (Figure 1, see Supplemental Tables_online version only).
Figure 1.
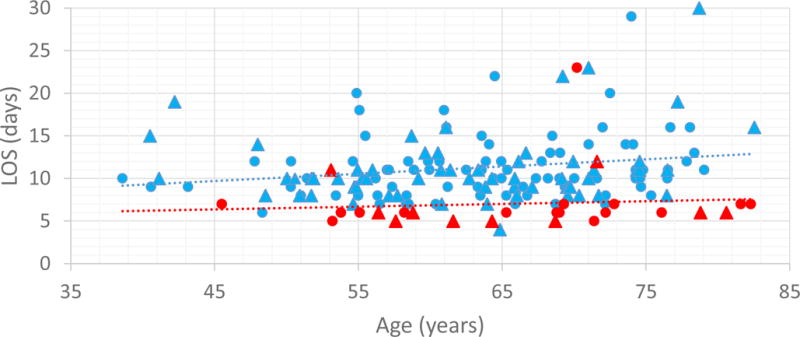
Duration of stay according to age for patients who underwent low-risk PD [historic controls, blue marker (n = 142) vs. RSPCP, red marker (n = 26)]. Patients managed on the RSPCP who underwent concomitant vascular resection are identified with triangles.
Among the 13 patients treated on the high-risk PD RSPCP, 6 (46%) had the NG tube removed, 7 (54%) had the urinary catheter removed, and 5 (39%) started a CLD per pathway. The operative drain of 2 patients was removed on POD 3, even though the drain effluent of 4 (31%) patients justified its removal per the pathway. Only 3 (23%) patients were discharged on POD 8 or earlier (Supplemental Tables).
Among the 21 patients managed on the DP pathway, 14 (67%) had the NG tube removed, 12 (57%) had the urinary catheter removed, and 14 (67%) started a CLD per pathway. The operative drain of 6 (29%) patients was removed on POD 3, but the drain effluent of 8 (38%) patients justified it per the pathway. Thirteen (62%) patients were discharged on POD 5 or earlier (Supplemental Tables).
Duration of Stay
Overall, the median duration of hospitalization of the patients treated prior to implementation of the RSPCPs was 10 (IQR 8–13) days, and that of patients treated after implementation was 6 (IQR 5–8) days (p < .001). Implementation of the RSPCPs led to a decrease in the median stay associated with low-risk PD (10 [IQR 9–12] days vs. 6 [IQR 5.75–7] days, p < .001) and DP (7 [IQR 6–10] days vs. 5 [IQR 5–7] days, p < .001). The duration of stay associated with high-risk PD did not change after implementation of the RSPCPs (12 [IQR 10–15.75] days vs. 14 [IQR 8.5–16.5] days, p = .948) (Figure 2). Nearly all patients continued to be discharged home (versus to a facility other than home [e.g., skilled nursing facility]) after implementation of the RSPCPs (Table 3).
Figure 2.
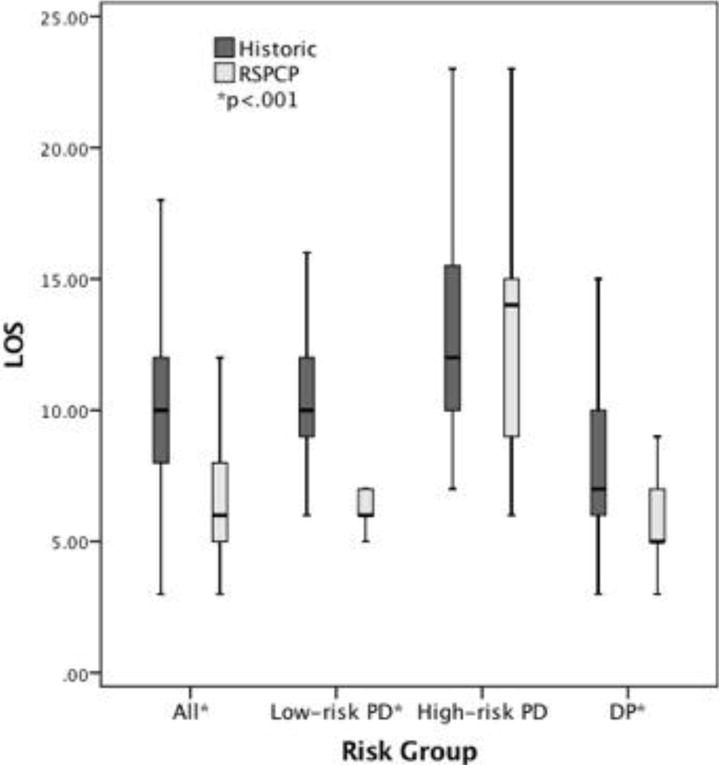
Actual median duration of stay of consecutive patients treated prior to and after implementation of the risk-stratified pancreatectomy clinical pathways (N = 375). LOS = length duration) of stay;
Table 3.
Adverse events at 90-days of consecutive patients treated prior to and after implementation of the risk-stratified pancreatectomy clinical pathways (N = 375).
| Historic (N=315) | RSPCP (N=60) | P-value | |
|---|---|---|---|
| Any AE | 239 (76%) | 48 (80%) | .489 |
| Severe AE (grade ≥ 3) | 90 (29%) | 19 (32%) | .628 |
| DGE grade B/C | 43 (14%) | 2 (3%) | .024 |
| POPF grade B/C | 64 (20%) | 14 (23%) | .598 |
| PPH grade B/C | 15 (5%) | 2 (3%) | .626 |
| Intra-abdominal abscess | 27 (9%) | 4 (7%) | .623 |
| Wound infection | 41 (13%) | 11 (18%) | .275 |
| Median stay, days (IQR) | 10 (8-13) | 6 (5-8) | <.001 |
| Discharge to home | 311 (99%) | 60 (100%) | .380 |
| 90-day readmission | 68 (22%) | 14 (23%) | .764 |
| 90-day mortality | 1 (0%) | 0 (0%) | .662 |
AE = adverse event; DGE = delayed gastric emptying; POPF = postoperative pancreatic fistula; PPH = post-pancreatectomy hemorrhage
Cost of index hospitalization
Overall, the median estimated total cost of index hospitalization for patients managed on the RSPCPs was 22% less than that of the patients treated prior to implementation of the pathways (p < .001). Use of the RSPCPs led to a decrease in the median total cost of index hospitalization associated with low-risk PD and DP by 27.1% (p < .001) and 14% (p = .012), respectively. The median total cost of the index hospitalization of patients after high-risk PD did not change (+ 9%, p = .586) (Figure 3).
Figure 3.
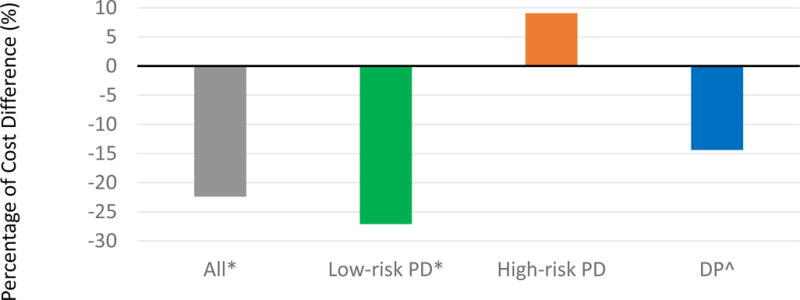
Difference in median total cost of index hospitalization between consecutive patients managed according to the risk-stratified pancreatectomy clinical pathways compared to those managed prior to implementation.
Adverse events
The incidence of any AE, severe AE, grade B/C POPF, grade B/C PPH, IAA, wound infection, readmission and mortality among patients treated prior to and after implementation of the RSPCPCs were similar (Table 3). Grade B/C DGE occurred less frequently among patients treated after implementation (p = .024).
There was no difference in the incidence of grade B/C POPF between patients treated prior to and after implementation of the RSPCPs in the low-risk PD (6% vs. 4 %, p = .710), high-risk PD (42% vs 54%, p = .422), or DP (27.% vs. 29%, p = .915) groups (Figure 4a). Similarly, there was no difference in the 90-day readmission rate of patients treated prior to and after implementation of the RSPCPs in the low-risk PD (18% vs. 15%, p = .720), high-risk PD (18% vs. 31%, p = .314), and DP (27% vs. 29%, p = .915) groups (Figure 4b).
Figure 4.
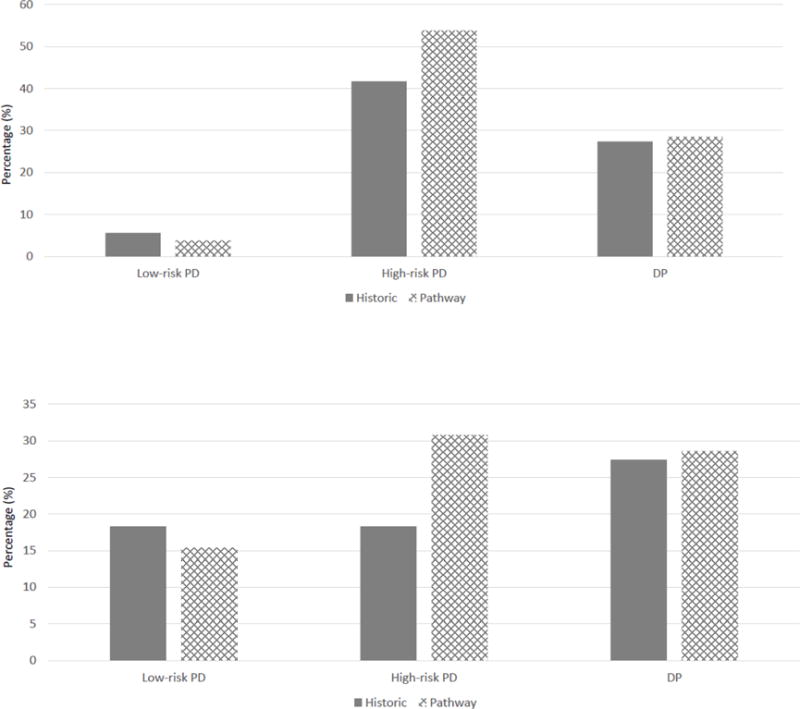
Incidence of (a) grade B/C POPF and (b) 90-day readmission rates for consecutive patients treated prior to and after implementation of the risk-stratified pancreatectomy clinical pathways.
Discussion
Here we describe both the design and successful initial implementation of three unique care pathways created to standardize the perioperative care and expedite recovery and discharge after pancreatectomy to an extent commensurate with each patient’s anticipated risk of a grade B/C POPF. Implementation of the three risk-based pathways—two for PD and one for DP—instead of two pathways based only on procedure type, appeared to decrease the median duration of hospital stay and cost of index hospitalization associated with pancreatectomy at our center by 4 days and 22%, respectively, without increasing rates of morbidity, discharge to a location other than home, hospital readmission, or mortality.
The novel strategy of using a preoperative assessment of fistula risk to assign patients to individual care pathways and thereby direct their postoperative care was developed in the context of two fundamental observations. First, a grade B/C POPF is a clinically relevant and important event that is associated with increases in rates of other adverse events, reoperations, duration of stay in the hospital, and the incidence of other deviations from the pattern of recovery associated with uncomplicated operations.20 And, per-patient hospital costs associated with a grade B/C POPF have been calculated as great as $16,300 to $27,000.42–45 Second, the risk of POPF is not uniformly distributed among patients who undergo pancreatectomy.17,32,33 Each individual patient’s risk can be predicted using factors easily measured prior to surgery; we recently characterized four discrete groups anticipated to have a risk for grade B/C fistula of 0%, 10%, 29% and 60% after PD, based on histopathologic diagnosis, duct diameter, and BMI.34
Clinical care pathways have long been used to set the pace of post-surgical recovery. Typically, a single pathway is associated with a single operation or general class of operations.3,5,9,46 But, on the basis of our data that clearly identified three discrete risk profiles for grade B/C POPF (two for PD and one for DP), we hypothesized that two risk-stratified pathways, rather than a single pathway, would be better suited to pace the recovery of all patients who undergo pancreatoduodenectomy; use of a single pathway might unnecessarily delay the recovery of patients anticipated to recover quickly given a lower-than-average risk of POPF, or might inappropriately accelerate the recovery of patients anticipated to recover more slowly given their greater-than-average POPF risk—likely with attendant risks to safety.
It must be emphasized here that the risk on which the RSPCPs are based is the anticipated risk of grade B/C POPF, not any other risk, either real or perceived, associated with the operation. Indeed, 73% of patients treated on the low-risk PD pathway were safely discharged on or prior to POD 6, even though the low-risk PD group consisted largely of patients who received operations of greater magnitude; indeed, many required venous and/or arterial resection and reconstruction, in fact, more than patients in the high-risk PD group. In this regard, it is also worth emphasizing that the incidence of POPF, both among all patients and among subgroups of patients stratified by risk, did not change after implementation of the pathways. And, decrease in duration of hospital stay and costs were realized only in the low-risk PD and DP groups—not the high-risk PD group. These observations suggest that these pathways do not change the natural history of patient disease or the perioperative recovery. They do, however, serve two critical functions: 1) they decrease variation in the care provided to patients in whom recovery is unlikely to deviate from an anticipated pattern, and 2) they provide reassurance to both patient and provider that early discharge is safe after operations unlikely to be complicated by POPF. Additional efforts are necessary to further refine each pathway and decrease the risk of POPF among patients currently at high risk for this event.
Overall, the median duration of hospitalization PD decreased from 10 days to 7 days after implementation of these RSPCPs, and the median saty for DP also decreased from 7 days to 5 days. Despite earlier discharge, we did not see an increase in rates of morbidity, discharge to a facility other than home, readmission, or mortality. These statistics are notable in the context of a recent review of pancreatectomy-specific NSQIP data which reported a mean LOS of 11 and 12.7 days after PD without or with venous resection, respectively,47 or a separate NSQIP study that reported a median duration of stay of 7 days associated with openDP.48 These statistics are also remarkable in relation to the duration of hospitalization associated with previously reported attempts to standardize perioperative care using single care pathways. Indeed, a comprehensive systematic review of ERAS for pancreatectomy (PD and DP) that reported the results of 10 studies calculated a median duration of stay of 10 days.31 Moreover, despite our overwhelming use of laparotomy for the patients included in this analysis, the metrics reported here for hospital stay are favorable even relative to those commonly associated with minimally invasive (MIS) pancreatectomy.49–51 For example, a systemic review and meta-analysis comparing open and MIS PD calculated a mean hospital stay of 9.8 and 8.2 days, respectively.52 And, the largest robotic pancreatectomy experience in the United States cites median durations of stay of10 days after PD and 6 days after DP.50 It is also worth emphasizing that the decrease in the median hospital stay was associated with a commensurate, and statistically significant decrease in the costs of hospitalization associated with pancreatectomy. Overall, hospital costs decreased by almost one-quarter, and decreases in costs associated with low-risk PD and DP were observed. Previously-described single pathways have had mixed success in this regard.26,53
It cannot be overemphasized that the risk used to assign patients to RSPCPs was calculated in the preoperative setting, because preoperative patient education and planning is well-known to have a favorable impact on postoperative outcomes,54–56 and patients who meet with their health care team prior to operation to discuss perioperative care have reported feeling more prepared for the operation, early discharge, and recovery.57–59 We assign patients to their respective RSPCP at the preoperative visit, at which time we allay their fears about the upcoming operation, outline expectations, encourage caregiver participation in the process of recovery, and even initiate discharge planning with both verbal explanations and printed materials. While it is possible that a “Hawthorne Effect” may have contributed to the success of these pathways, this effect may be better viewed as a beneficial consequence of proper pre- and perioperative counseling and goal-setting.
Active patient participation in compliance with each pathway is the goal, because improved adherence to pathways may result in better outcomes.60,61 In this regard, we acknowledge that adherence was incomplete; however, these pathways, like others, are merely guidelines, and total adherence is not only unrealistic but potentially unsafe. Indeed, a recent study evaluated 12 centers with colorectal surgery pathways and found an average rate of adherence to the pathway of only 44%.62 Further, few existing studies of pancreatectomy pathways report adherence rates; those studies that have done so also report similarly poor compliance.46 In our experience, non-adherence to the pathway typically occurred in response to deviations from an archetype pattern of recovery—the adherence rate to the low-risk PD pathway was greater than that to the high-risk PD pathway. Regardless, improving adherence when possible represents an ongoing focus of our quality improvement efforts.
This study has several notable limitations. First, like other care pathways, these were developed largely on the basis of experiential and anecdotal data, and high-level data support few of the specific elements contained therein. We view these pathways as living documents and may adapt them as dictated by the results of periodic analyses. Second, the sample size is small, predisposing the study to type II error, especially with regard to analysis of the individual RSPCP groups. Third, to the extent that a decrease in the duration of hospital stay and costs was primarily driven by success in the low-risk PD and DP risk groups, an institution’s actual case mix is an important consideration. At our free-standing cancer center, low-risk PD account for 45% of all pancreatectomies and 70% of PDs. Centers at which a greater proportion of procedures are performed for cysts or other benign diagnoses might see less impressive improvements from using these pathways. We would therefore, encourage teams at all high-volume treatment centers to critically evaluate their own data and to develop risk models and pathways most appropriate for their patient clinical profile. The risk-stratification strategy and results of this study may not be generalizable to other institutions. Fourth, we only calculated costs associated with the primary admission to avoid confoundings from costs associated with care not associated with perioperative recovery, such as the administration of adjuvant therapies. Importantly the incidence of AEs at 90 days, discharge to a location other than home, and readmission did not change after implementation, so there is no reason to assume a relevant difference in costs of care after index discharge. Fifth, it is possible that the increased utilization of MIS distal pancreatectomy after implementation of the RSPCPs (33% vs. 9% for our historic controls) contributed to the decrease in median following DP, but clearly this would not influence outcomes associated with either PD pathway. And, finally, because we did not compare this “three-pathway approach” with a Nevertheless, standard one- or two-pathway approach, we have not definitively proven its superiority, we have provided provocative data that are highly suggestive of the clinical benefit of using predictive analytics in both this and other perioperative scenarios.
Conclusions
Implementation of three unique clinical pathways after pancreatectomy linked to risk of grade B/C POPF decreased median duration of hospital stay and cost of index hospitalization without increasing rates of morbidity, discharge to a location other than home, hospital readmission, or mortality. Outcomes were most favorable for low-risk PD and DP.
Supplementary Material
Acknowledgments
We wish to thank Dr. Jason B. Fleming, Dr. Jean-Nicolas Vauthey and Dr. Timothy Vreeland for their contributions to this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
20171382R1 EDITED BY DR SARR
Conflict of interest: none
References
- 1.Wood T, Aarts MA, Okrainec A, et al. Emergency Room Visits and Readmissions Following Implementation of an Enhanced Recovery After Surgery (iERAS) Program. J Gastrointest Surg. 2017 doi: 10.1007/s11605-017-3555-2. [DOI] [PubMed] [Google Scholar]
- 2.Liang X, Ying H, Wang H, et al. Enhanced Recovery Program Versus Traditional Care in Laparoscopic Hepatectomy. Medicine (Baltimore) 2016;95(8):e2835. doi: 10.1097/MD.0000000000002835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Recart A, Duchene D, White PF, Thomas T, Johnson DB, Cadeddu JA. Efficacy and safety of fast-track recovery strategy for patients undergoing laparoscopic nephrectomy. J Endourol. 2005;19(10):1165–1169. doi: 10.1089/end.2005.19.1165. [DOI] [PubMed] [Google Scholar]
- 4.Visioni A, Shah R, Gabriel E, Attwood K, Kukar M, Nurkin S. Enhanced Recovery After Surgery for Noncolorectal Surgery?: A Systematic Review and Meta-analysis of Major Abdominal Surgery. Ann Surg. 2017 doi: 10.1097/SLA.0000000000002267. [DOI] [PubMed] [Google Scholar]
- 5.Fearon KC, Ljungqvist O, Von Meyenfeldt M, et al. Enhanced recovery after surgery: a consensus review of clinical care for patients undergoing colonic resection. Clin Nutr. 2005;24(3):466–477. doi: 10.1016/j.clnu.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 6.Lassen K, Soop M, Nygren J, et al. Consensus review of optimal perioperative care in colorectal surgery: Enhanced Recovery After Surgery (ERAS) Group recommendations. Arch Surg. 2009;144(10):961–969. doi: 10.1001/archsurg.2009.170. [DOI] [PubMed] [Google Scholar]
- 7.Ren L, Zhu D, Wei Y, et al. Enhanced Recovery After Surgery (ERAS) program attenuates stress and accelerates recovery in patients after radical resection for colorectal cancer: a prospective randomized controlled trial. World J Surg. 2012;36(2):407–414. doi: 10.1007/s00268-011-1348-4. [DOI] [PubMed] [Google Scholar]
- 8.Lemanu DP, Singh PP, Stowers MD, Hill AG. A systematic review to assess cost effectiveness of enhanced recovery after surgery programmes in colorectal surgery. Colorectal Dis. 2014;16(5):338–346. doi: 10.1111/codi.12505. [DOI] [PubMed] [Google Scholar]
- 9.Wang D, Kong Y, Zhong B, Zhou X, Zhou Y. Fast-track surgery improves postoperative recovery in patients with gastric cancer: a randomized comparison with conventional postoperative care. J Gastrointest Surg. 2010;14(4):620–627. doi: 10.1007/s11605-009-1139-5. [DOI] [PubMed] [Google Scholar]
- 10.Zhao G, Cao S, Cui J. Fast-track surgery improves postoperative clinical recovery and reduces postoperative insulin resistance after esophagectomy for esophageal cancer. Support Care Cancer. 2014;22(2):351–358. doi: 10.1007/s00520-013-1979-0. [DOI] [PubMed] [Google Scholar]
- 11.Kelly KN, Iannuzzi JC, Rickles AS, Monson JR, Fleming FJ. Risk factors associated with 30- day postoperative readmissions in major gastrointestinal resections. J Gastrointest Surg. 2014;18(1):35–43. doi: 10.1007/s11605-013-2354-7. discussion 43-34. [DOI] [PubMed] [Google Scholar]
- 12.Birkmeyer JD, Finlayson SR, Tosteson AN, Sharp SM, Warshaw AL, Fisher ES. Effect of hospital volume on in-hospital mortality with pancreaticoduodenectomy. Surgery. 1999;125(3):250–256. [PubMed] [Google Scholar]
- 13.Winter JM, Cameron JL, Campbell KA, et al. 1423 pancreaticoduodenectomies for pancreatic cancer: A single-institution experience. J Gastrointest Surg. 2006;10(9):1199–1210. doi: 10.1016/j.gassur.2006.08.018. discussion 1210-1191. [DOI] [PubMed] [Google Scholar]
- 14.Schwarz L, Bruno M, Parker NH, et al. Active Surveillance for Adverse Events Within 90 Days: The Standard for Reporting Surgical Outcomes After Pancreatectomy. Ann Surg Oncol. 2015;22(11):3522–3529. doi: 10.1245/s10434-015-4437-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gaujoux S, Cortes A, Couvelard A, et al. Fatty pancreas and increased body mass index are risk factors of pancreatic fistula after pancreaticoduodenectomy. Surgery. 2010;148(1):15–23. doi: 10.1016/j.surg.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 16.Fuks D, Piessen G, Huet E, et al. Life-threatening postoperative pancreatic fistula (grade C) after pancreaticoduodenectomy: incidence, prognosis, and risk factors. Am J Surg. 2009;197(6):702–709. doi: 10.1016/j.amjsurg.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 17.Pratt WB, Callery MP, Vollmer CM., Jr Risk prediction for development of pancreatic fistula using the ISGPF classification scheme. World J Surg. 2008;32(3):419–428. doi: 10.1007/s00268-007-9388-5. [DOI] [PubMed] [Google Scholar]
- 18.Bassi C, Dervenis C, Butturini G, et al. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. 2005;138(1):8–13. doi: 10.1016/j.surg.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 19.Denbo JW, Orr WS, Zarzaur BL, Behrman SW. Toward defining grade C pancreatic fistula following pancreaticoduodenectomy: incidence, risk factors, management and outcome. HPB (Oxford) 2012;14(9):589–593. doi: 10.1111/j.1477-2574.2012.00486.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yeo CJ, Cameron JL, Lillemoe KD, et al. Does prophylactic octreotide decrease the rates of pancreatic fistula and other complications after pancreaticoduodenectomy? Results of a prospective randomized placebo-controlled trial. Ann Surg. 2000;232(3):419–429. doi: 10.1097/00000658-200009000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grobmyer SR, Kooby D, Blumgart LH, Hochwald SN. Novel pancreaticojejunostomy with a low rate of anastomotic failure-related complications. J Am Coll Surg. 2010;210(1):54–59. doi: 10.1016/j.jamcollsurg.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 22.Keck T, Wellner UF, Bahra M, et al. Pancreatogastrostomy Versus Pancreatojejunostomy for RECOnstruction After PANCreatoduodenectomy (RECOPANC, DRKS 00000767): Perioperative and Long-term Results of a Multicenter Randomized Controlled Trial. Ann Surg. 2016;263(3):440–449. doi: 10.1097/SLA.0000000000001240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Allen PJ, Gonen M, Brennan MF, et al. Pasireotide for postoperative pancreatic fistula. N Engl J Med. 2014;370(21):2014–2022. doi: 10.1056/NEJMoa1313688. [DOI] [PubMed] [Google Scholar]
- 24.Kantor O, Talamonti MS, Pitt HA, et al. Using the NSQIP Pancreatic Demonstration Project to Derive a Modified Fistula Risk Score for Preoperative Risk Stratification in Patients Undergoing Pancreaticoduodenectomy. J Am Coll Surg. 2017;224(5):816–825. doi: 10.1016/j.jamcollsurg.2017.01.054. [DOI] [PubMed] [Google Scholar]
- 25.Diener MK, Seiler CM, Rossion I, et al. Efficacy of stapler versus hand-sewn closure after distal pancreatectomy (DISPACT): a randomised, controlled multicentre trial. Lancet. 2011;377(9776):1514–1522. doi: 10.1016/S0140-6736(11)60237-7. [DOI] [PubMed] [Google Scholar]
- 26.Vanounou T, Pratt W, Fischer JE, Vollmer CM, Jr, Callery MP. Deviation-based cost modeling: a novel model to evaluate the clinical and economic impact of clinical pathways. J Am Coll Surg. 2007;204(4):570–579. doi: 10.1016/j.jamcollsurg.2007.01.025. [DOI] [PubMed] [Google Scholar]
- 27.Kennedy EP, Rosato EL, Sauter PK, et al. Initiation of a critical pathway for pancreaticoduodenectomy at an academic institution–the first step in multidisciplinary team building. J Am Coll Surg. 2007;204(5):917–923. doi: 10.1016/j.jamcollsurg.2007.01.057. discussion 923-914. [DOI] [PubMed] [Google Scholar]
- 28.Kennedy EP, Grenda TR, Sauter PK, et al. Implementation of a critical pathway for distal pancreatectomy at an academic institution. J Gastrointest Surg. 2009;13(5):938–944. doi: 10.1007/s11605-009-0803-0. [DOI] [PubMed] [Google Scholar]
- 29.Balzano G, Zerbi A, Braga M, Rocchetti S, Beneduce AA, Di Carlo V. Fast-track recovery programme after pancreaticoduodenectomy reduces delayed gastric emptying. Br J Surg. 2008;95(11):1387–1393. doi: 10.1002/bjs.6324. [DOI] [PubMed] [Google Scholar]
- 30.Abu Hilal M, Di Fabio F, Badran A, et al. Implementation of enhanced recovery programme after pancreatoduodenectomy: a single-centre UK pilot study. Pancreatology. 2013;13(1):58–62. doi: 10.1016/j.pan.2012.11.312. [DOI] [PubMed] [Google Scholar]
- 31.Kagedan DJ, Ahmed M, Devitt KS, Wei AC. Enhanced recovery after pancreatic surgery: a systematic review of the evidence. HPB (Oxford) 2015;17(1):11–16. doi: 10.1111/hpb.12265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Callery MP, Pratt WB, Kent TS, Chaikof EL, Vollmer CM., Jr A prospectively validated clinical risk score accurately predicts pancreatic fistula after pancreatoduodenectomy. J Am Coll Surg. 2013;216(1):1–14. doi: 10.1016/j.jamcollsurg.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 33.Wellner UF, Kayser G, Lapshyn H, et al. A simple scoring system based on clinical factors related to pancreatic texture predicts postoperative pancreatic fistula preoperatively. HPB (Oxford) 2010;12(10):696–702. doi: 10.1111/j.1477-2574.2010.00239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Denbo JW, Slack RS, Bruno M, et al. Selective Perioperative Administration of Pasireotide is More Cost-Effective Than Routine Administration for Pancreatic Fistula Prophylaxis. J Gastrointest Surg. 2017;21(4):636–646. doi: 10.1007/s11605-016-3340-7. [DOI] [PubMed] [Google Scholar]
- 35.Eldenburg Leslie G, S EL, Zulauf Dwight J. Financial Management of Organized Health Care Delivery Systems. In: Wolper Lawrence F, I LW, Great Neck, New York , editors. Health Care Administration: Planning, Implementing, and Managing Organized Delivery Systems. 4th. Vol. 1. Sudbury, Massachusetts: Jones and Bartlett Publishers; 2004. pp. 217–219. [Google Scholar]
- 36.Denbo JW, Bruno ML, Cloyd JM, et al. Preoperative Chemoradiation for Pancreatic Adenocarcinoma Does Not Increase 90-Day Postoperative Morbidity or Mortality. J Gastrointest Surg. 2016;20(12):1975–1985. doi: 10.1007/s11605-016-3286-9. [DOI] [PubMed] [Google Scholar]
- 37.Strasberg SM, Linehan DC, Hawkins WG. The accordion severity grading system of surgical complications. Ann Surg. 2009;250(2):177–186. doi: 10.1097/SLA.0b013e3181afde41. [DOI] [PubMed] [Google Scholar]
- 38.Wente MN, Bassi C, Dervenis C, et al. Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the International Study Group of Pancreatic Surgery (ISGPS) Surgery. 2007;142(5):761–768. doi: 10.1016/j.surg.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 39.Wente MN, Veit JA, Bassi C, et al. Postpancreatectomy hemorrhage (PPH): an International Study Group of Pancreatic Surgery (ISGPS) definition. Surgery. 2007;142(1):20–25. doi: 10.1016/j.surg.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 40.Piccirillo JF, Tierney RM, Costas I, Grove L, Spitznagel EL., Jr Prognostic importance of comorbidity in a hospital-based cancer registry. JAMA. 2004;291(20):2441–2447. doi: 10.1001/jama.291.20.2441. [DOI] [PubMed] [Google Scholar]
- 41.Katz MH, Pisters PW, Evans DB, et al. Borderline resectable pancreatic cancer: the importance of this emerging stage of disease. J Am Coll Surg. 2008;206(5):833–846. doi: 10.1016/j.jamcollsurg.2007.12.020. discussion 846-838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ma LW, Dominguez-Rosado I, Gennarelli RL, et al. The Cost of Postoperative Pancreatic Fistula Versus the Cost of Pasireotide: Results from a Prospective Randomized Trial. Ann Surg. 2017;265(1):11–16. doi: 10.1097/SLA.0000000000001892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pratt WB, Maithel SK, Vanounou T, Huang ZS, Callery MP, Vollmer CM., Jr Clinical and economic validation of the International Study Group of Pancreatic Fistula (ISGPF) classification scheme. Ann Surg. 2007;245(3):443–451. doi: 10.1097/01.sla.0000251708.70219.d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Daskalaki D, Butturini G, Molinari E, Crippa S, Pederzoli P, Bassi C. A grading system can predict clinical and economic outcomes of pancreatic fistula after pancreaticoduodenectomy: results in 755 consecutive patients. Langenbecks Arch Surg. 2011;396(1):91–98. doi: 10.1007/s00423-010-0719-x. [DOI] [PubMed] [Google Scholar]
- 45.Cecka F, Jon B, Subrt Z, Ferko A. Clinical and economic consequences of pancreatic fistula after elective pancreatic resection. Hepatobiliary Pancreat Dis Int. 2013;12(5):533–539. doi: 10.1016/s1499-3872(13)60084-3. [DOI] [PubMed] [Google Scholar]
- 46.Robertson N, Gallacher PJ, Peel N, et al. Implementation of an enhanced recovery programme following pancreaticoduodenectomy. HPB (Oxford) 2012;14(10):700–708. doi: 10.1111/j.1477-2574.2012.00521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Beane JD, House MG, Pitt SC, et al. Pancreatoduodenectomy with venous or arterial resection: a NSQIP propensity score analysis. HPB (Oxford) 2017;19(3):254–263. doi: 10.1016/j.hpb.2016.11.013. [DOI] [PubMed] [Google Scholar]
- 48.Xourafas D, Ashley SW, Clancy TE. Comparison of Perioperative Outcomes between Open, Laparoscopic, and Robotic Distal Pancreatectomy: an Analysis of 1815 Patients from the ACS-NSQIP Procedure-Targeted Pancreatectomy Database. J Gastrointest Surg. 2017 doi: 10.1007/s11605-017-3463-5. [DOI] [PubMed] [Google Scholar]
- 49.Vijan SS, Ahmed KA, Harmsen WS, et al. Laparoscopic vs open distal pancreatectomy: a single-institution comparative study. Arch Surg. 2010;145(7):616–621. doi: 10.1001/archsurg.2010.120. [DOI] [PubMed] [Google Scholar]
- 50.Zureikat AH, Moser AJ, Boone BA, Bartlett DL, Zenati M, Zeh HJ., 3rd 250 robotic pancreatic resections: safety and feasibility. Ann Surg. 2013;258(4):554–559. doi: 10.1097/SLA.0b013e3182a4e87c. discussion 559-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rosok BI, de Rooij T, van Hilst J, et al. Minimally invasive distal pancreatectomy. HPB (Oxford) 2017;19(3):205–214. doi: 10.1016/j.hpb.2017.01.009. [DOI] [PubMed] [Google Scholar]
- 52.Pedziwiatr M, Malczak P, Pisarska M, et al. Minimally invasive versus open pancreatoduodenectomy-systematic review and meta-analysis. Langenbecks Arch Surg. 2017;402(5):841–851. doi: 10.1007/s00423-017-1583-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Porter GA, Pisters PW, Mansyur C, et al. Cost and utilization impact of a clinical pathway for patients undergoing pancreaticoduodenectomy. Ann Surg Oncol. 2000;7(7):484–489. doi: 10.1007/s10434-000-0484-0. [DOI] [PubMed] [Google Scholar]
- 54.Clarke HD, Timm VL, Goldberg BR, Hattrup SJ. Preoperative patient education reduces in-hospital falls after total knee arthroplasty. Clin Orthop Relat Res. 2012;470(1):244–249. doi: 10.1007/s11999-011-1951-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Carli F, Charlebois P, Baldini G, Cachero O, Stein B. An integrated multidisciplinary approach to implementation of a fast-track program for laparoscopic colorectal surgery. Can J Anaesth. 2009;56(11):837–842. doi: 10.1007/s12630-009-9159-x. [DOI] [PubMed] [Google Scholar]
- 56.Halaszynski TM, Juda R, Silverman DG. Optimizing postoperative outcomes with efficient preoperative assessment and management. Crit Care Med. 2004;32(4 Suppl):S76–86. doi: 10.1097/01.ccm.0000122046.30687.5c. [DOI] [PubMed] [Google Scholar]
- 57.Taylor C, Burch J. Feedback on an enhanced recovery programme for colorectal surgery. Br J Nurs. 2011;20(5):286–290. doi: 10.12968/bjon.2011.20.5.286. [DOI] [PubMed] [Google Scholar]
- 58.Burch J. Promoting enhanced recovery after colorectal surgery. Br J Nurs. 2013;22(5):S4, S6, S8–9. doi: 10.12968/bjon.2013.22.sup3.s4. [DOI] [PubMed] [Google Scholar]
- 59.Blazeby JM, Soulsby M, Winstone K, King PM, Bulley S, Kennedy RH. A qualitative evaluation of patients’ experiences of an enhanced recovery programme for colorectal cancer. Colorectal Dis. 2010;12(10):e236–242. doi: 10.1111/j.1463-1318.2009.02104.x. Online. [DOI] [PubMed] [Google Scholar]
- 60.Pecorelli N, Hershorn O, Baldini G, et al. Impact of adherence to care pathway interventions on recovery following bowel resection within an established enhanced recovery program. Surg Endosc. 2017;31(4):1760–1771. doi: 10.1007/s00464-016-5169-2. [DOI] [PubMed] [Google Scholar]
- 61.Forsmo HM, Pfeffer F, Rasdal A, et al. Compliance with enhanced recovery after surgery criteria and preoperative and postoperative counselling reduces length of hospital stay in colorectal surgery: results of a randomized controlled trial. Colorectal Dis. 2016;18(6):603–611. doi: 10.1111/codi.13253. [DOI] [PubMed] [Google Scholar]
- 62.van Zelm R, Coeckelberghs E, Sermeus W, et al. Variation in care for surgical patients with colorectal cancer: protocol adherence in 12 European hospitals. Int J Colorectal Dis. 2017;32(10):1471–1478. doi: 10.1007/s00384-017-2863-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


