Abstract
Objectives
Socioeconomic status (SES) is among the strongest determinants of body mass index (BMI), particularly for women. For older populations, selection bias due to attrition is a large barrier to assessing the accumulation of inequality. Under multiple missing data mechanisms, we investigated the extent to which childhood and midlife SES affects BMI from midlife to old age and gender differences in the association.
Method
Data come from a longitudinal national study of 2,345 U.S. adults aged 40–54 at baseline. We used latent growth models to estimate BMI trajectory over a period of 20 years. We examined results under different missing data patterns and applied methods that account for nonrandom-selection bias.
Results
Compared with individuals who had higher childhood SES, individuals who had lower childhood SES have higher BMI in midlife and experience a faster increase in BMI between midlife and old age. The observed associations remain significant even after controlling for midlife SES. After addressing nonrandom selection, the gap in BMI between high and low childhood SES widens from midlife to old age for women.
Discussion
The findings provide new evidence of cumulative inequality among older adults, documenting increasing BMI inequality from midlife to old age, particularly for women from low-SES families.
Keywords: Gender, Childhood, SES, Cumulative inequality, BMI, Life course
Socioeconomic disadvantage in early life predicts life-course trajectories of body weight. Individuals who were disadvantaged in early life tend to have higher body mass index (BMI) and greater likelihood of being overweight or obese in adolescence and young adulthood (Lee, Harris, & Gordon-Larsen, 2009), and these associations extend to midlife (Giskes et al., 2008; Pudrovska, Logan, & Richman, 2014a). Importantly, these adverse effects are stronger and more consistent among women than men, in both early adulthood (Gustafsson, Persson, & Hammarstrom, 2012; Khlat, Jusot, & Ville, 2009) and midlife (Giskes et al., 2008; Pudrovska, Reither, Logan, & Sherman-Wilkins, 2014b). For example, studies on socioeconomic status (SES) have found strong negative effects, particularly for women, of early-life SES on adult BMI; although adult SES is among the most widely studied life-course factors leading to adult BMI, researchers have shown that the effects of such early-life disadvantage are independent of the effects of adult SES (Senese, Almeida, Fath, Smith, & Loucks, 2009).
Despite extensive life-course studies on BMI, important questions remain: do BMI inequalities established in early life widen or diminish in later life? Do the adverse impacts of early disadvantage on body weight continue to be more pronounced for women than men? And what is the role of midlife SES in the associations? Using three waves (1995/1996–2013/2014) from the Midlife in the U.S. Study (MIDUS), the aim of the present study is to investigate these questions. Given the importance of body weight for later-life survival (Zajacova & Ailshire, 2013), responding to these inquiries may provide important policy-relevant guidelines and gender-specific interventions. However, assessing the accumulation of inequality for older populations is quite challenging due to nonrandom dropout across surveys (Banks, Muriel, & Smith, 2011; Ferraro, Shippee, & Schafer, 2009; O’Rand & Hamil-Luker, 2005), which can potentially lead to erroneous conclusions regarding the relationship between SES and BMI. Our study builds on prior studies by comparing the results from multiple missing data mechanisms to further examine whether the link between childhood SES and BMI becomes stronger when nonrandom selection is taken into account.
Background
Childhood SES and Adult BMI
Although the accumulation of body fat results from complex combinations of biological, behavioral, social, and environmental factors (Wyatt, Winters, & Dubbert, 2006), SES is among the strongest determinants of BMI. A large body of studies based on life-course perspectives has found that low childhood SES is associated with increased BMI among adults (Senese et al., 2009). Two models in life-course epidemiology have been widely used to explain how early-life SES affects BMI over the life course. The critical period model proposes that exposure to adverse environments during times of rapid growth and development immutably programs the structure and function of physiological systems, yielding lifelong consequences on health. Compared with critical periods, risks/resources encountered during other periods will have relatively little, if any, impact on health. According to this model, socioeconomic disadvantage in early life has a strong effect on adult BMI even after accounting for socioeconomic position during other phases of the life course. The chain of risk model or pathways model, on the contrary, suggests that early-life exposures produce an ongoing accumulation of physiological burdens through a sequence of adverse exposures. In accordance with this model, early-life disadvantage triggers a chain of socioeconomic disadvantages, and early-life SES may have no direct effect on adult body weight (Ben-Shlomo, Mishra, & Kuh, 2014).
Research based on European data has indicated that the effects of childhood SES on midlife BMI are independent of socioeconomic position in adulthood (Giskes et al., 2008; Hardy, Wadsworth, & Kuh, 2000). Findings in the United States are consistent; for example, using MIDUS, Chapman, Fiscella, Duberstein, Kawachi, & Coletta (2009) found that parental occupational prestige is inversely related to adult BMI and that the association remains significant after accounting for respondent’s own SES, particularly for middle-aged women. Similarly, using the Wisconsin Longitudinal Study (WLS), Pudrovska and colleagues (2014a) found that parental SES is inversely associated with body weight at age 65 even after controlling for midlife SES. Recent research that has used the Health and Retirement Study (HRS) has augmented the typical measures of adult SES (e.g., by including neighborhood socioeconomic characteristics) and found that the effects of parental SES on BMI still remain significant (Pavela, 2017). Overall, extant evidence supports the critical period model. Thus, we expect that early-life SES will be inversely and significantly associated with later-life BMI even after controlling for midlife SES (Hypothesis 1).
Childhood SES and BMI Trajectories in Later Life
There are two competing explanations for how and why the association between childhood SES and BMI varies over the life course. First, cumulative advantage/disadvantage theory suggests that BMI disparities between low vs. high SES will widen throughout the life course because disadvantage in early life might lead to subsequent disadvantages (Dannefer, 2003), which ultimately promote the accumulation of body fat with age. In contrast, the leveling hypothesis proposes that such BMI differentials at earlier ages become muted with increasing age through selective mortality and biological frailty among older populations (Dupre, 2007). That is, disadvantaged individuals who are in poor health are likely to be removed from the observed population through premature death, with those who remain becoming more homogenous in terms of their health status. Regarding such an apparent disappearance of inequalities in later life, cumulative inequality theory suggests that nonrandom selection may play an important role (Ferraro et al., 2009).
In testing cumulative disadvantage theory with longitudinal studies of aging, a noteworthy concern is attrition from mortality or being lost to follow-up. For example, in MIDUS, approximately half of respondents were lost to follow-up or died between 1995/1996 and 2013/2014. If the probability of attrition is systemically related to outcomes of interest, the missing-at-random assumption is no longer valid (Little & Rubin, 2014). Such nonrandom selection leads to several issues, for example, the study sample will not be representative of the population of interest and the estimated associations between covariates and the outcome may be biased (Banks et al., 2011). Given that individuals who are less healthy and of lower SES are less likely to complete surveys, life-course scholars have been concerned that nonrandom selection may affect assessments of inequality in later life (O’Rand & Hamil-Luker, 2005; Willson, Shuey, & Elder, 2007). In testing cumulative inequality theory, Ferraro and colleagues (2009) have highlighted the importance of methods that take into account potential selection bias.
Extant studies which used middle-aged populations have found supporting evidence for cumulative disadvantage theory, particularly for women. For instance, using individuals aged 40–60 from the longitudinal Dutch GLOBE study, Giskes and colleagues (2008) found that women from low-SES families show higher BMI at baseline and greater weight gain over a 13-year period than those from high-SES families. Similarly, using data from the WLS, Pudrovska and colleagues (2014a) reported that for women, low early-life SES is related to a BMI increase between age 54 and 64. However, we have little knowledge of the extent to which childhood SES affects BMI trajectories beyond midlife. Based on cumulative disadvantage theory, we expect that BMI will continue to grow steeper from midlife to old age for those from low-SES families compared with those from high-SES families (Hypothesis 2). Furthermore, guided by cumulative inequality theory (Ferraro et al., 2009), we further expect that the association between SES and changes in later-life BMI may appear stronger when nonrandom selection is taken into account (Hypothesis 3).
Gender Differences
Findings from both clinical and population-based studies have indicated that the effects of childhood SES are more consistent among women than men throughout adulthood (Giskes et al., 2008; Gustafsson et al., 2012; Pudrovska et al., 2014a; Walsemann, Ailshire, Bell, & Frongillo, 2012). This gendered pattern might be partially attributed to biological differences because women tend to expend less energy than men and accumulate more abdominal fat (Lovejoy & Sainsbury, 2009). Cumulative inequality theory, however, suggests that gender differences in the accumulation of inequality may produce differential vulnerability to early-life disadvantage (Ferraro et al., 2009). Early-life environments penalize women more than men, thereby reinforcing relationships between SES and body weight (Pudrovska et al., 2014b). That is, socioeconomic disadvantage has a greater impact on BMI for girls than for boys; girls who are overweight during adolescence are likely to have low educational attainment and in turn have high BMI in midlife. Moreover, some studies have reported that low SES in adulthood is more closely linked with higher BMI among women than men (Drewnowski, 2009; Khlat et al., 2009; Pudrovska et al., 2014b). Accordingly, we expect that the adverse effects of childhood SES on later-life BMI will be more pronounced for women than for men (Hypothesis 4). In addition, the mediating role of midlife SES in the association between childhood SES and later-life BMI is stronger for women than for men (Hypothesis 5).
Data and Methods
Sample
Data for this study are obtained from the MIDUS study, a national survey designed to assess the role of social, psychological, and behavioral factors in understanding differences in mental and physical health (n = 7,108; 52% women). MIDUS began in 1995/1996 (Wave 1 [W1]) with noninstitutionalized, English-speaking adults aged 25–74 in the 48 contiguous states (Brim, Ryff, & Kessler, 2004). MIDUS consists of a two-stage survey: a telephone interview and a self-administered questionnaire (SAQ). Approximately 89% of the sample completed both the telephone interview and SAQ at W1 (n = 6,325). Follow-up interviews with MIDUS respondents were completed every 9–10 years: n = 4,963 in 2004–2006 (W2) and n = 3,294 in 2013–2014 (W3). The mortality data currently available to researchers were obtained from multiple sources (e.g., National Death Index reports, mortality closeout interviews, longitudinal sample maintenance), providing information on date-of-death up to October 31, 2015. Over the course of the survey, 1,140 respondents from the baseline SAQ (18% of the 6,325 respondents) were known to have died.
Although MIDUS was designed to assess the health and well-being of middle-aged individuals over time, it includes a wide age range of respondents (aged 25–74). After sensitivity analysis of age cutoffs, we limited the analytic sample to those respondents who were 40–54 years old at baseline (in 1995/1996), which includes 1,140 men and 1,205 women (37% of SAQ respondents at W1). This sampling restriction allows us to (a) minimize confounding of age and cohort patterns in BMI (for details, see Supplementary Figure S1), (b) track BMI from midlife to early old age (40s to early 70s), and (c) compare our findings with those from prior studies, which focused on similar age groups (e.g., Giskes et al., 2008).
Measures
Socioeconomic status
To capture socioeconomic circumstances over the life course, we used an extensive number of indicators that represent both subjective and objective SES. To compare results from measures that have different distributions, each indicator was standardized based on the distribution of the pooled sample and coded so that higher values represent more resources. We then created two SES indexes, both of which are measured at baseline (W1). The index of childhood SES (Cronbach’s α = .74) is an average of six indicators: (a) mother’s and (b) father’s education (1 = no school/some grade school to 12 = PhD, MD, or other professional degree), (c) mother’s and (d) father’s occupational prestige score (observed range 7.1–80.5) measured by Duncan’s Socioeconomic Index (Hauser & Warren, 1997), (e) welfare status (0 = never on welfare, 1 = ever on welfare), and (f) financial level growing up (1 = a lot better off than the average family to 7 = a lot worse off).
The index of midlife SES (α = .78) is an average of eight indicators: (a) educational degree (1 = no school/some grade school to 12 = PhD, MD, or other professional degree), (b) household income ($0–$300,000 or more), (c) wage/salary income ($0–$100,000 or more), (d) current or previous occupation (1 = never employed or manual labor, 2 = service/sales/administrative, 3 = management/business/financial, 4 = professional), (e) current financial situation (0 = worst possible through 10 = best possible), (f) control over financial situation (0 = worst possible through 10 = best possible), (g) availability of money to meet basic needs (1 = more than enough through 3 = not enough, reverse coded), and (h) level of difficulty paying bills (1 = very difficult through 4 = not at all difficult).
Body mass index
At W1, respondents were asked to recall their weight at age 21, and at all three waves, respondents reported their current height and weight, providing measures of BMI (i.e., weight in kilograms divided by the square of height in meters). Prior work has indicated a strong correlation between self-reported weight and measurements by research staff, yet some studies have reported that respondents at the tails of the weight distribution tend to slightly self-normalize their weight (Bowman & DeLucia, 1992). To confirm the reliability of the self-reported measures of weight and height, we compared data from self-reports to those from the MIDUS biomarker study. We found that self-reported weight is slightly underreported while self-reported height is overreported. Although BMI is not always accurate, particularly for muscular individuals (Huxley, Mendis, Zheleznyakov, Reddy, & Chan, 2010), it is the most frequently used measure of body fat.
We controlled for age, race/ethnicity, and gender (gender-stratified model) at baseline. Body weight (e.g., obesity) is a highly heritable trait (Willyard, 2014). Some studies have indicated that weight gain during parenthood is likely to persist and accumulate, even after children become independent (Lee & Ryff, 2016). Thus, we included both number of children and retrospective reports of body weight at age 21 as biodemographic confounders.
Analytic Strategies
Latent growth model
To examine the relationship between childhood SES and BMI, we applied a latent growth modeling approach (see, e.g., Bollen & Curran, 2006). The growth model estimates the effect of childhood SES on BMI measured at W1 (intercept) and on the rate of change in BMI between W1 and W3 (slope). The outcome model consists of two levels: time and individual levels. The first level explores the relationship between time (different waves) and BMI, expressed as follows:
where is the BMI for case i at time t, and is a time-specific error. There are two latent factors that vary across individuals: intercept and slope . We used an approach that does not assume a linear or quadratic relationship but models the rate of change without assuming a linear or quadratic shape (see Bollen & Curran, 2006 for more information). In our sample, BMI increased between W1 (aged 40–54) and W3 (aged 60–74), with the rate of change slowing down after W2 (aged 50–64) for both genders.
The second level explores the relationship between these latent factors (intercept and slope) and childhood SES after accounting for individual-level confounders (age, race/ethnicity, body weight at age 21, and number of children at W1).
where represents individual covariates and and are individual errors for intercept and slope, respectively. The coefficients and represent changes in the intercept and slope associated with a one-unit increase in childhood SES.
The analytic model has two stages. First, we estimated the effect of childhood SES on the baseline BMI (intercept) and the change in BMI over time (slope; Model 1). We then added midlife SES into Model 1 to test whether the effect of childhood SES on the intercept and slope remained significant even after adjusting for midlife SES (Model 2). We tested gender differences in the effects of childhood SES on the growth trajectory of BMI using the gender interaction effects in the pooled sample of women and men. The significance of indirect effects (the mediating effects of midlife SES) was tested using the multiplication of regression coefficients approach (Baron & Kenny, 1986), and gender differences in the indirect pathway were examined by the gender interaction terms on the indirect effects in the pooled sample from both genders.
Missing data patterns and mechanisms
In our analytic sample, 58% of respondents (1,364 out of 2,345) remained in the study throughout all three waves, whereas 42% of respondents had died or were lost to follow-up (LFU) following W1 or W2. The profiles of these groups’ missing patterns differ substantially in terms of their SES, BMI, and health-related conditions, as well as demographic characteristics (see Supplementary Table S1). Compared with individuals who participated in the entire study, those who dropped out (died or LFU) following W1 or W2 showed lower childhood and midlife SES, worse health, and higher BMI (particularly for women). Among those who dropped out following W1 or W2, those who died were older and had higher BMI than those who were LFU. This indicates potential problems of selective attrition when we limit our sample to those who participated in all three waves.
To reach robust conclusions, we compared the results from the three different approaches to evaluate the extent to which our estimates change under different missing data mechanisms. We first estimated the effect of childhood SES on BMI using listwise deletion (also called complete case analysis); that is, we only included respondents who had no missing score on BMI (n = 1,038). Listwise deletion is among the most common methods for handling missing data. This approach provides a valid result only if the size of missing data is small and if data are missing completely at random (MCAR), which seems implausible given the missing data pattern shown in Supplementary Table S1. Second, we included all respondents at baseline (n = 2,345) and estimated the effect of childhood SES on BMI using full information maximum likelihood (FIML). This approach accommodates missing data by calculating each parameter of particular statistics using all data available in the sample (Geiser, 2012). FIML estimates are known to be unbiased if attrition is consistent with data being missing at random (MAR; Enders & Bandalos, 2001). MAR assumes that, after controlling for observed variables, such as age, SES, health-related indicators, and demographic characteristics, the chance of missing data on the outcome (i.e., BMI) does not depend on the value of the outcome. Although the MAR assumption is plausible, there might be important variables that were not observed. Finally, we used a pattern mixture model in which respondents are classified into different groups based on their missing data patterns and estimates are obtained by averaging across different missing patterns (see, e.g., Glynn, Laird, & Rubin, 1986; Hedeker & Gibbons, 2006). This approach assumes that attrition was consistent with a missing not at random (MNAR) mechanism, that is, that the chance of missing data on BMI is related to BMI itself. For example, those who have high BMI values may tend to drop out or die before a study ends. We cannot exclude this scenario because our data show systemic missing data for BMI due to mortality, especially for women.
Given that the missing data patterns differ substantially by gender, we analyzed gender-stratified models. All control variables have 1%–2% of data missing on average. We handled missing data for these confounders by using FIML, assuming that values were MAR. Descriptive statistics were calculated using Stata 15.0 (StataCorp, 2018), and latent growth models were analyzed in Mplus 8.0 (Muthén & Muthén, 2017).
Results
Descriptive Statistics
For both genders, the mean sample BMI at baseline was above the overweight threshold (25 kg/m2), with a greater BMI for men than women (27.5 vs. 26.7 kg/m2, p < .01). Although there was no gender difference in childhood SES, women had lower midlife SES than men (p < .001). Compared with men, women were more likely to participate in all waves of the survey (61% vs. 56%, p < .05). Such gender differences in participation were partially attributed to greater mortality risk for men than women during the survey period (Table 1).
Table 1.
Characteristics of the Sample at Baseline (mean [SD] or proportion) by Gender
| Variables | Men (n = 1,140) | Women (n = 1,205) | Gender difference |
|---|---|---|---|
| Life course SES | |||
| Childhood SES index | 0.01 (1.00) | −0.06 (0.95) | ns |
| Adult SES index | 0.18 (0.60) | −0.04 (0.62) | p < .001 |
| Body mass index at W1 | 27.46 (4.43) | 26.72 (6.35) | p < .01 |
| Biodemographic covariates | |||
| Age | 46.57 (4.15) | 46.80 (4.21) | ns |
| White | 0.92 | 0.92 | ns |
| Body weight at age 21 | 166.20 (26.83) | 126.89 (23.98) | p < .001 |
| Number of children | 1.98 (1.30) | 2.12 (1.12) | p < .05 |
| Attendance from W 1 to W3 | |||
| Attended W1, W2, and W3 | 0.56 | 0.61 | p < .05 |
| Attended W1 and W2 and LFU | 0.15 | 0.15 | ns |
| Attended W1 and W2 and died | 0.06 | 0.03 | p < .01 |
| Attended W1 and LFU | 0.17 | 0.16 | ns |
| Attended W1 and died | 0.06 | 0.05 | ns |
Notes: ns = statistically not significant; LFU = lost to follow-up; SES = socioeconomic status; W = wave. SD are in parentheses.
MCAR-Based Effects of Life-Course SES on Trajectory of BMI
Table 2 shows results from a latent growth model using respondents who participated in all three surveys. The results support cumulative inequality theory for both genders. Specifically, the effect of childhood SES on the intercept of BMI is negative and significant, meaning that those who have lower childhood SES tend to have higher BMI measured at baseline. Similarly, the effect of childhood SES on the slope of BMI is negative and significant, suggesting that those who have lower childhood SES tend to increase their BMI level faster between midlife (W1) and old age (W3) than those who have higher childhood SES. Although the gender difference is not statistically significant, we found that the effect of childhood SES on the intercept of BMI is greater for women than men (−0.88 vs. −0.61 in Model 1). The effect of childhood SES on the slope of BMI is steeper for women than men (−0.34 vs. −0.23 in Model 1). After accounting for midlife SES in Model 2, the effect size of childhood SES decreased for both genders for the intercept and the slope. There was no gender difference in indirect effects, either on the intercept or on the slope, although the indirect effect of childhood SES on the intercept of BMI via midlife SES appeared greater for women than men. The findings from the MCAR mechanism, however, are subject to selection bias because individuals who dropped out differ from those who remained in the study.
Table 2.
MCAR-Based Effects of Life-Course SES on Trajectory of BMI for Women (n = 555) and Men (n = 483)
| Women | Men | |||
|---|---|---|---|---|
| Model 1 | Model 2 | Model 1 | Model 2 | |
| Predictors of BMI intercept | ||||
| Childhood SES | −0.88*** (0.25) | −0.73*** (0.25) | −0.61*** (0.13) | −0.52*** (0.13) |
| Adult SES | −0.95* (0.35) | −0.77* (0.32) | ||
| Predictors of BMI slope | ||||
| Childhood SES | −0.34* (0.15) | −0.29 (0.16) | −0.23** (0.09) | −0.23** (0.09) |
| Adult SES | −0.29 (0.26) | −0.03 (0.17) |
Notes: BMI = body mass index; MCAR = missing completely at random; SES = socioeconomic status. Controls: age, race/ethnicity, body weight at age 21, number of children.
*p < .05, **p < .01, ***p < .001.
MAR-Based Effects of Life-Course SES on Trajectory of BMI
To address problems related to missing data, we next fitted a latent growth model by assuming MAR (Table 3). Consistent with the findings from the MCAR mechanism, the results from the MAR mechanism support cumulative inequality theory for both genders. That is, individuals from low-SES families had higher BMI at baseline and steeper increases in BMI between midlife and old age (Model 1). Similar gendered patterns appeared—the effect of childhood SES is greater for women than men for both intercept (−0.75 vs. −0.45; p < .05) and slope (−0.39 vs. −0.25; ns), which support Hypothesis 4. After accounting for midlife SES in Model 2, the effect size of childhood SES decreased, but there was little change in significance levels between models (Hypotheses 1 and 2). Noticeably, the effect size of midlife SES on the intercept of BMI is around twice as large for women than men (−1.13 vs. −0.40; p < .05). The indirect effect of childhood SES on the intercept of BMI via midlife SES was significantly greater for women than men (−0.19 vs. −0.06, p < .05), but there was no significant gender difference in the indirect effect via midlife SES on the slope via midlife SES (Hypothesis 5).
Table 3.
MAR-Based Effects of Life-Course SES on Trajectory of BMI for Women (n = 1,205) and Men (n = 1,140)
| Women | Men | |||
|---|---|---|---|---|
| Model 1 | Model 2 | Model 1 | Model 2 | |
| Predictors of BMI intercept | ||||
| Childhood SES | −0.75***a (0.16) | −0.58*** (0.16) | −0.45***a (0.11) | −0.39** (0.11) |
| Adult SES | −1.13***a (0.24) | −0.40a (0.22) | ||
| Predictors of BMI slope | ||||
| Childhood SES | −0.39** (0.13) | −038** (0.14) | −0.25** (0.09) | −0.21* (0.09) |
| Adult SES | −0.08 (0.22) | −0.31 (0.16) |
Notes: BMI = body mass index; MAR = missing at random; SES = socioeconomic status. Controls: age, race/ethnicity, body weight at age 21, number of children.
aSignificant gender differences in the effects of childhood SES (p < .05).
*p < .05, **p < .01, ***p < .001.
MNAR-Based Effects of Life-Course SES on Trajectory of BMI
Finally, we estimated a pattern mixture model to address issues related to MNAR. To carry out a pattern mixture model, we divided respondents into five subsamples based on different missing patterns. Group 1: responded in all three waves (58% of the sample), Group 2: responded in W1 and W2 and LFU (15%), Group 3: responded in W1 and W2 and died (5%), Group 4: responded in W1 and LFU, and Group 5 (16%): responded in W1 and died (6%). We then estimated the overall trajectory by averaging estimates across different missing patterns. The pattern mixture model employed in this study is inherently underidentified (some parameters are not known) because the rate of change in BMI (slope) and the effect of childhood SES on the slope are inestimable if there is only one observation (Groups 4 and 5). One way to circumvent this issue is to substitute inestimable parameters with estimable parameters (Enders, 2011). For this procedure, we implemented three approaches: completed cases, neighboring cases, and weighted cases.
For completed cases, we replaced the inestimable parameters in both Groups 4 and 5 (attend W1 and LFU or died) with their counterparts from Group 1 (those who completed all three waves). This approach assumes that dropout cases and completed cases will follow a similar trajectory. We found that the results were consistent with the estimates under the MAR assumption. There was a significant and negative effect of childhood SES on baseline BMI and the rate of change in BMI for both genders (left column in Table 4).
Table 4.
MNAR-Based Effects of Life-Course SES on Trajectory of BMI for Women (n = 1,205) and Men (n = 1,140)
| Women | Men | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Complete cases | Neighboring cases | Weighted cases | Complete cases | Neighboring cases | Weighted cases | |||||||
| Model 1 | Model 2 | Model 1 | Model 2 | Model 1 | Model 2 | Model 1 | Model 2 | Model 1 | Model 2 | Model 1 | Model 2 | |
| Predictors of BMI intercept | ||||||||||||
| Childhood SES | −0.74***a (0.16) | −0.57*** (0.19) | −0.74***a (0.16) | −0.57*** (0.19) | −0.74***a (0.16) | −0.57*** (0.19) | −0.44***a (0.11) | −0.41** (0.11) | −0.44***a (0.11) | −0.41** (0.11) | −0.44***a (0.11) | −0.41** (0.11) |
| Adult SES | −1.19***a (0.25) | −1.19***a (0.25) | −1.19***a (0.25) | −0.46*a (0.22) | −0.46*a (0.22) | −0.46*a (0.22) | ||||||
| Predictors of BMI slope | ||||||||||||
| Childhood SES | −0.40** (0.13) | −0.38** (0.14) | −0.43** (0.15) | −0.37* (0.18) | −0.42** (0.15) | −0.37* (0.18) | −0.21** (0.09) | −0.17* (0.09) | −0.23* (0.11) | −0.18 (0.11) | −0.24* (0.11) | −0.18 (0.13) |
| Adult SES | −0.17 (0.22) | −0.17 (0.34) | −0.15 (0.26) | −0.24 (0.16) | −0.28 (0.19) | −0.28 (0.19) |
Notes: BMI = body mass index; MNAR = missing not at random; SES = socioeconomic status. Controls: age, race/ethnicity, body weight at age 21, number of children.
aSignificant gender differences in the effects of childhood SES (p < .05).
*p < .05, **p < .01, ***p < .001.
For neighboring cases, we replaced the inestimable parameters in Group 4 (attended W1 and LFU) with their counterparts from Group 2 (attended W1 and W2 and LFU), and we replaced the inestimable parameters in Group 5 (attended W1 and died) with their counterparts from Group 3 (attended W1, W2, and died). This approach assumes that the growth trajectory for those who died will be similar while the trajectory for those who were LFU will be similar regardless of when they dropped out (W2 or W3). We found that the effect of childhood SES on the level of BMI was significant and negative. However, after replacing neighboring cases, the effect differed from the MAR-based result. More specifically, the effect sizes for women regarding the effect of childhood SES on the slope of BMI are slightly larger when replacing neighboring cases than the MCAR-based and MAR-based results (−0.34 vs. −0.39 vs. −0.43 for Model 1 in Table 2 vs. Table 3 vs. Table 4). In contrast, the effect sizes for men in terms of the effect of childhood SES on the slope of BMI changed (0.23 vs. −0.25 vs. −0.23 for Model 1 in Table 2 vs. Table 3 vs. Table 4). Regarding the role of midlife SES, the findings are similar to those based on the MAR mechanism except that after controlling for midlife SES, for men, the effect of childhood SES on the slope of BMI was no longer statistically significant. The indirect effect of childhood SES on the intercept of BMI was greater for women than men (−0.19 vs. −0.06, p < .05), and there was no significant gender difference in the indirect effect on the slope via midlife SES.
Last, for weighted cases, we replaced the inestimable parameters in Groups 4 and 5 with the weighted average of parameters in Groups 2 and 3. This assumes that the growth trajectory for those who dropped out following W1 will be similar to either those who died or were LFU following W2. We found that the results from using weighted cases were almost identical to those from using neighboring cases. Among these three approaches, replacing the neighboring or weighted cases represents a more plausible scenario than using completed cases given the difference in profiles of those who completed all waves of the study and those who died or were LFU as shown in Supplementary Table S1.
Overall, findings from all three approaches (MCAR, MAR, and MNAR-based approaches) support the cumulative inequality theory (Hypothesis 3), particularly for women. We found that, for women only, the results from MCAR mechanisms underestimated the slope of BMI compared with MAR- and MNAR-based results. MAR-based results underestimated the slope of BMI relative to MNAR-based results, more so for women than men. Overall, the results imply that after addressing the confounding effects of selective attrition, and the effect of cumulative inequality appears stronger for women.
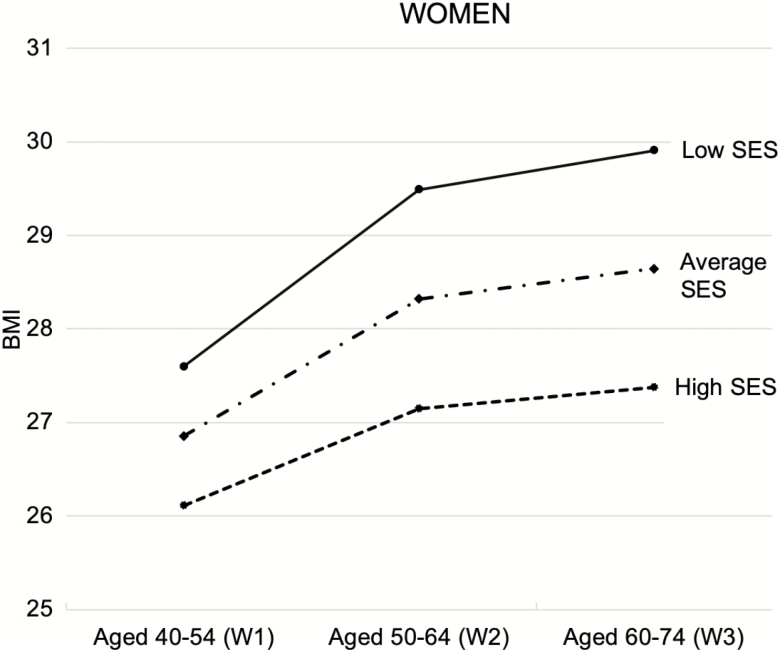
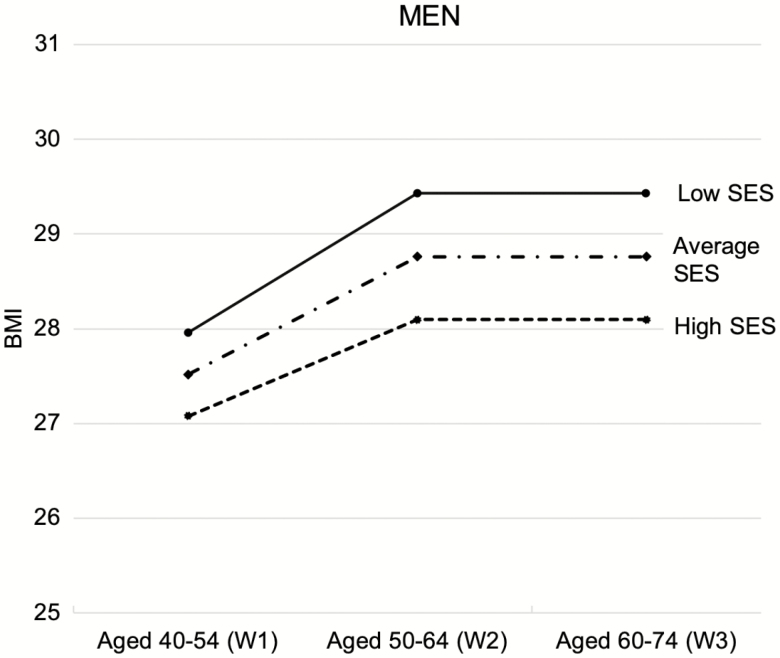
To present the results in an intuitive way, we plotted predicted BMI trajectories. Figure 1 (women) and Figure 2 (men) provide a graphical representation of the effect of childhood SES on the trajectory of BMI by gender using neighboring cases. Specifically, we illustrate the association between three values of childhood SES (1 SD above the average, the average, and 1 SD below the average) and the trajectories of BMI for men and women. Women who have low childhood SES (1 SD below the average) have a predicted BMI score of 27.6 at W1 (aged 40–54); those who have high childhood SES (1 SD above the average) are predicted to have a BMI score of 26.1. Similarly, among men, those from low-SES families show higher BMI than those from high-SES families (28.0 vs. 27.1). The gap between high vs. low childhood SES was 1.5 for women and 0.9 for men at age 40–54. The gap, however, widens as age increases, particularly for women, so that by W3 (aged 60–74), the difference in BMI between high and low childhood SES was 2.5 for women, but only 1.3 for men.
Figure 1.
Predicted trajectories of BMI by childhood SES for women. The plots are based on Model 1 from Table 4 (MNAR-neighboring cases). Low SES = 1 SD lower than the average childhood SES; high SES = 1 SD higher than the average. BMI = body mass index; MNAR = missing not at random; SES = socioeconomic status; W = wave.
Figure 2.
Predicted trajectories of BMI by childhood SES for men. The plots are based on Model 1 from Table 4 (MNAR-neighboring cases). Low SES = 1 SD lower than the average childhood SES; high SES = 1 SD higher than the average. BMI = body mass index; MNAR = missing not at random; SES = socioeconomic status; W = wave.
Discussion
Early-life socioeconomic position and gender have been consistently shown to be strongly associated with BMI over the life course. However, few studies have examined how these factors shape BMI disparities from midlife to old age. This lack of research may be partially attributed to the large barriers posed by high attrition and selective survival in evaluating the accumulation of inequality among older populations. Using a national sample of U.S. middle-aged adults, the purpose of this study was to examine the extent to which early-life SES produces inequalities in midlife BMI that widen or diminish in later life, whether these associations differ by gender, and the role of midlife SES in the associations. To address issues related to nonrandom selection, we examined results under multiple missing data mechanisms and applied analytic techniques that take into account selection bias. Our study yielded several main findings.
Based on the critical period model (Ben-Shlomo et al., 2014), we expected that socioeconomic circumstances in early life would affect individuals’ body weight in later life. Our findings show that older adults from low-SES families had higher BMI than those from high-SES families and the association remained significant even after controlling for midlife SES, indicating an independent and robust effect of early-life conditions. Our findings are in line with evidence from WLS, HRS, and European studies suggesting that parental SES has an independent association with BMI among middle-aged adults, even after taking into account their own SES (Giskes et al., 2008; Pavela, 2017; Pudrovska et al., 2014b). Motivated by cumulative disadvantage/advantage theory (Dannefer, 2003), we further expected that such BMI inequalities would widen as individuals age. Our findings showed that the gap in BMI between individuals from low- and high-SES families increased in later life for both men and women.
Overall, our findings are consistent with two studies that investigated the association between childhood SES on changes in BMI (Giskes et al., 2008; Pudrovska et al., 2014a). However, these studies relied on changes in BMI across two points in time, which might be inadequate for assessing underlying growth. In addition, Giskes and colleagues (2008) did not explicitly address issues related to selection bias despite high attrition rates, which might have contributed to an attenuation of early-life SES gradients in midlife BMI. Given that our sample has high attrition and nonrandom selection, we explicitly compared the results from three analytic approaches assuming different missing data mechanisms. The findings, indeed, indicated that BMI disparities widened from midlife to old age, particularly for women. That the observed pattern was even more pronounced when we considered selection bias lends support to cumulative inequality theory (Ferraro et al., 2009).
The gender difference in the impact of childhood SES is noteworthy. Socioeconomic disadvantage in early life is significantly and inversely associated with body weight in midlife and rapid weight gain between midlife and old age, particularly for women. Our estimates showed that the BMI difference between those from high-SES vs. low-SES backgrounds was 0.9 for men and 1.5 for women at baseline but increased to 1.3 for men and 2.5 for women about 20 years later. More intuitively, for the average man (5 ft. 9 in. tall), the BMI difference of 1.5 amounts to a roughly 10-pound difference between those from high-SES vs. low-SES backgrounds. For the average woman (5 ft. 4 in. tall), the BMI difference of 2.5 amounts to a roughly 15-pound difference between those from high-SES vs. low-SES backgrounds. Given that women tend to be about 5 inches shorter than men, each pound may have stronger health-compromising effects for women than men. It is important to note in Figure 1 that, among those from low-SES backgrounds, the average female had a lower BMI at W1 than the average male but showed higher BMI at W3. This finding echoes those from prior studies of younger populations (Gustafsson et al., 2012; Hardy et al., 2000; Walsemann et al., 2012) and also provided new evidence that cumulative BMI inequality continues even in old age, particularly for women from socioeconomically disadvantaged families.
Consistent with prior work (e.g., Giskes et al., 2008; Pudrovska, Reither, et al., 2014), we found that midlife SES partially explains the association between childhood SES and BMI in later life, yet the role of midlife SES differs by gender. Specifically, the effect of midlife SES on BMI in midlife was significantly larger for women than men, which is consistent with prior findings (Drewnowski, 2009; Khlat et al., 2009; Pudrovska et al., 2014; Salonen et al., 2009). Furthermore, the mediating role of midlife SES in the association between childhood SES and midlife BMI (the intercept) was larger for women. This suggests that economic hardship in midlife may have an even more detrimental impact on women than men (in addition to childhood disadvantage). For women, therefore, improving financial conditions in midlife may help reduce the BMI disparities rooted in early-life SES. Yet, the finding should be interpreted cautiously. Given that overweight/obesity is more strongly associated with employment discrimination for women (Shinall, 2015), we cannot rule out the possibility that the finding may result from reverse causation.
Limitations and Conclusions
The limitations of our study should be noted. First, although the data covers a follow-up period of about 20 years, MIDUS only has three data points, with a wide age range at baseline. Although it is impossible to disentangle age and cohort effects within MIDUS, future research could better estimate the growth curve model by using data that have more data points and a smaller age range. Second, our study relied on retrospective reports of childhood SES and BMI at age 21, which potentially produces some recall bias; yet, prior studies support the validity of recall of childhood SES (Krieger, Okamoto, & Selby, 1998) and a strong correlation between recalled past weight and previously measured weight (Perry, Byers, Mokdad, Serdula, & Williamson, 1995). Third, unmeasured factors in this study may potentially affect our estimates, a common limitation in observational research. For example, both childhood SES and adult BMI may be affected by parental BMI; thus, poor BMI profiles of parents might produce the association between low childhood SES and high BMI in adulthood.
Similarly, the indirect effect transmitted through midlife SES might be overestimated due to other midlife variables if the variables are associated with both the adult SES and later BMI trajectories. Sensitivity analysis suggests that there is no substantial difference in findings if other midlife variables are included, for example, chronic conditions, unhealthy behaviors, and marital status (see Supplementary Table S2). Nonetheless, we do not attempt to claim a causal relationship between childhood SES, midlife SES, and later-life BMI due to possible unmeasured variables that may confound the relationships. Finally, given that our sample consists of predominantly white participants, it is important for future researchers to look at a more heterogenous population. Focusing on minorities might reveal, for instance, that Black women from low-SES families have worse BMI profiles due to their intersecting subaltern statuses (of gender, race, and social class).
Despite these limitations, our study provides further evidence that the important life-course periods when socioeconomic conditions substantially affect body weight vary for men and women. Policy programs initiated in early life that minimize exposure to socioeconomic deprivation could improve healthy BMI profiles in later life for disadvantaged children. Moreover, our findings suggest that older adults from disadvantaged families are likely to be overweight or obese in early old age and may gain more weight in later life. Given clear connections between high BMI, chronic conditions, and weight loss, some of these older adults might lose weight via the development of chronic illnesses. Such BMI trajectories (“obese gaining” and “obese losing”) are linked with elevated risk of later-life mortality (Zajacova & Ailshire, 2013). Thus, maintaining a healthy weight is important for a long and healthy life, particularly for people from disadvantaged families.
Funding
Research reported in this publication was supported by grants from the National Institute on Aging of the National Institutes of Health (R00AG052458). The work was also supported by the National Institute on Aging (P01AG020166) to conduct a longitudinal follow-up of the MIDUS investigation. The original study was supported by the John D. and Catherine T. MacArthur Foundation Research Network on Successful Midlife Development. Support also came from M01RR023942 (Georgetown) and M01RR00865 (UCLA) from the General Clinical Research Centers Program. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Conflict of Interest
None reported.
Supplementary Material
Author Contributions
C. Lee initiated and designed the study, conducted parts of the analysis, and wrote the entire manuscript. S. Park designed and conducted the main part of the analysis and drafted the Data and Methods, and Result sections of the manuscript. The authors thank Dana A. Glei (consultant) for helpful comments on analytic strategies.
References
- Banks J., Muriel A., & Smith J. P (2011). Attrition and health in ageing studies: Evidence from ELSA and HRS. Longitudinal and Life Course Studies, 2, 101–126. doi:10.14301/llcs.v2i2.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron R. M., & Kenny D. A (1986). The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. Journal of Personality and Social Psychology, 51, 1173–1182. [DOI] [PubMed] [Google Scholar]
- Ben-Shlomo Y., Mishra G., & Kuh D (2014). Life course epidemiology. In Ahrens W. & Pigeot I. (Eds.), Handbook of epidemiology (pp. 1521–1549). New York, NY: Springer. doi:10.1007/978-0-387-09834-0-56 [Google Scholar]
- Bollen K. A., & Curran P. J (2006). Latent curve models: A structural equation perspective (Vol. 467). Hoboken, NJ: John Wiley & Sons, Inc. doi:10.1002/0471746096 [Google Scholar]
- Bowman R. L., & DeLucia J. L (1992). Accuracy of self-reported weight: A meta-analysis. Behavior Therapy, 23, 637–655. doi:10.1016/S0005-7894(05)80226-6 [Google Scholar]
- Brim O. G., Ryff C. D., & Kessler R. C (2004). How healthy are we? A national study of well-being at midlife. Chicago, IL: University of Chicago Press. [Google Scholar]
- Chapman B. P., Fiscella K., Duberstein P., Kawachi I., & Coletta M (2009). Can the influence of childhood socioeconomic status on men’s and women’s adult body mass be explained by adult socioeconomic status or personality? Findings from a national sample. Health Psychology, 28, 419–427. doi:10.1037/a0015212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dannefer D. (2003). Cumulative advantage/disadvantage and the life course: Cross-fertilizing age and social science theory. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 58, S327–S337. doi:10.1093/geronb/58.6.s327 [DOI] [PubMed] [Google Scholar]
- Drewnowski A. (2009). Obesity, diets, and social inequalities. Nutrition Reviews, 67(Suppl 1), S36–S39. doi:10.1111/j.1753-4887.2009.00157.x [DOI] [PubMed] [Google Scholar]
- Dupre M. E. (2007). Educational differences in age-related patterns of disease: Reconsidering the cumulative disadvantage and age-as-leveler hypotheses. Journal of Health and Social Behavior, 48, 1–15. doi:10.1177/002214650704800101 [DOI] [PubMed] [Google Scholar]
- Enders C. K. (2011). Missing not at random models for latent growth curve analyses. Psychological Methods, 16, 1–16. doi:10.1037/a0022640 [DOI] [PubMed] [Google Scholar]
- Enders C. K., & Bandalos D. L (2001). The relative performance of full information maximum likelihood estimation for missing data in structural equation models. Structural Equation Modeling, 8, 430–457. doi:10.1207/S15328007SEM0803_5 [Google Scholar]
- Ferraro K. F., Shippee T. P., & Schafer M. H (2009). Cumulative inequality theory for research on aging and the life course. In Bengtson V. L. & Settersten R. (Eds.), Handbook of theories of aging (pp. 413–433). New York, NY: Springer Publishing Company. [Google Scholar]
- Geiser C. (2012). Data analysis with Mplus (methodology in the social sciences). New York, NY: Guilford Press. [Google Scholar]
- Giskes K., van Lenthe F. J., Turrell G., Kamphuis C. B., Brug J., & Mackenbach J. P (2008). Socioeconomic position at different stages of the life course and its influence on body weight and weight gain in adulthood: A longitudinal study with 13-year follow-up. Obesity, 16, 1377–1381. doi:10.1038/oby.2008.54 [DOI] [PubMed] [Google Scholar]
- Glynn R. J., Laird N. M., & Rubin D. B (1986). Selection modeling versus mixture modeling with nonignorable nonresponse. In Wainer H. (Ed.), Drawing inferences from self-selected samples (pp. 115–142). New York, NY: Springer. doi:10.1007/978-1-4612-4976-4_10 [Google Scholar]
- Gustafsson P. E., Persson M., & Hammarstrom A (2012). Socio-economic disadvantage and body mass over the life course in women and men: Results from the Northern Swedish Cohort. European Journal of Public Health, 22, 322–327. doi:10.1093/eurpub/ckr061 [DOI] [PubMed] [Google Scholar]
- Hardy R., Wadsworth M., & Kuh D (2000). The influence of childhood weight and socioeconomic status on change in adult body mass index in a British national birth cohort. International Journal of Obesity, 24, 725–734. doi:10.1038/sj.ijo.0801238 [DOI] [PubMed] [Google Scholar]
- Hauser R. M., & Warren J. R (1997). Socioeconomic indexes for occupations: A review, update, and critique. Sociological Methodology, 27, 177–298. doi:10.1111/1467-9531.271028 [Google Scholar]
- Hedeker D., & Gibbons R. D (2006). Longitudinal data analysis (Vol. 451). Hoboken, NJ: John Wiley & Sons. Inc. [Google Scholar]
- Huxley R., Mendis S., Zheleznyakov E., Reddy S., & Chan J (2010). Body mass index, waist circumference and waist: Hip ratio as predictors of cardiovascular risk—a review of the literature. European Journal of Clinical Nutrition, 64, 16–22. doi:10.1038/ejcn.2009.68 [DOI] [PubMed] [Google Scholar]
- Khlat M., Jusot F., & Ville I (2009). Social origins, early hardship and obesity: A strong association in women, but not in men? Social Science and Medicine, 68, 1692–1699. doi:10.1016/j.socscimed.2009.02.024 [DOI] [PubMed] [Google Scholar]
- Krieger N., Okamoto A., & Selby J. V (1998). Adult female twins’ recall of childhood social class and father’s education: A validation study for public health research. American Journal of Epidemiology, 147, 704–708. doi:10.1093/oxfordjournals.aje.a009512 [DOI] [PubMed] [Google Scholar]
- Lee H., Harris K. M., & Gordon-Larsen P (2009). Life course perspectives on the links between poverty and obesity during the transition to young adulthood. Population Research and Policy Review, 28, 505–532. doi:10.1007/s11113-008-9115-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C., & Ryff C. D (2016). Early parenthood as a link between childhood disadvantage and adult heart problems: A gender-based approach. Social Science and Medicine, 171, 58–66. doi:10.1016/j.socscimed.2016.10.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little R. J., & Rubin D. B (2014). Statistical analysis with missing data (Vol. 333). Hoboken, NJ: John Wiley & Sons, Inc. [Google Scholar]
- Lovejoy J., & Sainsbury A (2009). Sex differences in obesity and the regulation of energy homeostasis. Obesity Reviews, 10, 154–167. doi:10.1111/j.1467-789X.2008.00529.x [DOI] [PubMed] [Google Scholar]
- Muthén L. K., & Muthén B. O (2017). Mplus Version 8.0 (Computer file). Los Angeles, CA: Muthén and Muthén. [Google Scholar]
- O’Rand A. M., & Hamil-Luker J (2005). Processes of cumulative adversity: Childhood disadvantage and increased risk of heart attack across the life course. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 60, 117–124. doi:10.1093/geronb/60.Special_Issue_2.S117 [DOI] [PubMed] [Google Scholar]
- Pavela G. (2017). Is childhood socioeconomic status independently associated with adult BMI after accounting for adult and neighborhood socioeconomic status? PLoS One, 12, e0168481. doi:10.1371/journal.pone.0168481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry G. S., Byers T. E., Mokdad A. H., Serdula M. K., & Williamson D. F (1995). The validity of self-reports of past body weights by U.S. adults. Epidemiology, 6, 61–66. [DOI] [PubMed] [Google Scholar]
- Pudrovska T., Logan E. S., & Richman A (2014a). Early-life social origins of later-life body weight: The role of socioeconomic status and health behaviors over the life course. Social Science Research, 46, 59–71. doi:10.1016/j.ssresearch.2014.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pudrovska T., Reither E. N., Logan E. S., & Sherman-Wilkins K. J (2014b). Gender and reinforcing associations between socioeconomic disadvantage and body mass over the life course. Journal of Health and Social Behavior, 55, 283–301. doi:10.1177/0022146514544525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salonen M. K., Kajantie E., Osmond C., Forsén T., Ylihärsilä H., Paile-Hyvärinen M.,…Eriksson J. G (2009). Role of socioeconomic indicators on development of obesity from a life course perspective. Journal of Environmental and Public Health, 2009, 625168. doi:10.1155/2009/625168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senese L. C., Almeida N. D., Fath A. K., Smith B. T., & Loucks E. B (2009). Associations between childhood socioeconomic position and adulthood obesity. Epidemiologic Reviews, 31, 21–51. doi:10.1093/epirev/mxp006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinall J. B. (2015). Occupational characteristics and the obesity wage penalty Retrieved from https://papers.ssrn.com/sol3/papers.cfm?abstract_id=2379575
- StataCorp. (2018). Stata statistical software: Release 15. College Station, TX: StataCorp LP. [Google Scholar]
- Walsemann K. M., Ailshire J. A., Bell B. A., & Frongillo E. A (2012). Body mass index trajectories from adolescence to midlife: Differential effects of parental and respondent education by race/ethnicity and gender. Ethnicity and Health, 17, 337–362. doi:10.1080/13557858.2011.635374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willson A. E., Shuey K. M., & Elder G. H (2007). Cumulative advantage processes as mechanisms of inequality in life course health. American Journal of Sociology, 112, 1886–1924. doi:10.1086/512712 [Google Scholar]
- Willyard C. (2014). Heritability: The family roots of obesity. Nature, 508, S58–S60. doi:10.1038/508S58a [DOI] [PubMed] [Google Scholar]
- Wyatt S. B., Winters K. P., & Dubbert P. M (2006). Overweight and obesity: Prevalence, consequences, and causes of a growing public health problem. The American Journal of the Medical Sciences, 331, 166–174. [DOI] [PubMed] [Google Scholar]
- Zajacova A., & Ailshire J (2013). Body mass trajectories and mortality among older adults: A joint growth mixture-discrete-time survival analysis. Gerontologist, 54, 221–231. doi:10.1093/geront/gns164 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.