Abstract
Objectives
We investigated the prevalence of anosmia and ageusia in adult patients with a laboratory-confirmed diagnosis of infection with severe acute respiratory distress syndrome coronavirus-2 (SARS-CoV-2).
Methods
This was a retrospective observational analysis of patients infected with SARS-CoV-2 admitted to hospital or managed in the community and their household contacts across a London population during the period March 1st to April 1st, 2020. Symptomatology and duration were extracted from routinely collected clinical data and follow-up telephone consultations. Descriptive statistics were used.
Results
Of 386 patients, 141 (92 community patients, 49 discharged inpatients) were included for analysis; 77/141 (55%) reported anosmia and ageusia, nine reported only ageusia and three only anosmia. The median onset of anosmia in relation to onset of SARS-CoV-2 disease (COVID-19) symptoms (as defined by the Public Health England case definition) was 4 days (interquartile range (IQR) 5). Median duration of anosmia was 8 days (IQR 16). Median duration of COVID-19 symptoms in community patients was 10 days (IQR 8) versus 18 days (IQR 13.5) in admitted patients. As of April 1, 45 patients had ongoing COVID-19 symptoms and/or anosmia; 107/141 (76%) patients had household contacts, and of 185 non-tested household contacts 79 (43%) had COVID-19 symptoms with 46/79 (58%) reporting anosmia. Six household contacts had anosmia only.
Conclusions
Over half of the positive patients reported anosmia and ageusia, suggesting that these should be added to the case definition and used to guide self-isolation protocols. This adaptation may be integral to case findings in the absence of population-level testing. Until we have successful population-level vaccination coverage, these steps remain critical in the current and future waves of this pandemic.
Keywords: Anosmia, Ageusia, Coronavirus, COVID-19, Ear nose and throat [MeSH], SARS-CoV-2
Introduction
Since the outbreak of the severe acute respiratory syndrome coronavirus (SARS-CoV-2) pandemic—reported first from Wuhan, China in December 2019—there have been increasing reports of anosmia (total or partial loss of smell) and ageusia (total or partial loss of taste) amongst patients presenting with suspected or confirmed infection [1]. Early reports from Italy and South Korea showed anosmia in up to 34% of patients [[2], [3], [4]]. A more recent cross-European analysis looking at patients with mild to moderate SARS-CoV-2 disease (COVID-19) put this number at 85.6% [5].
Anosmia can occur in a wide range of viral infections; published literature estimates the prevalence of olfactory disorders, including anosmia, to be 11–40% [1,[6], [7], [8]]. The higher estimates (20–40%) were generated using data from patients in specialized smell and taste centres, and the lower estimate (11%) was based on data from general ear, nose, and throat clinics [8]. SARS-CoV-2 does not generate clinically significant nasal congestion or rhinorrhoea that would typically be associated with anosmia in other upper respiratory tract infections, and it has also been observed that anosmia manifests either early in the disease process or in patients with mild symptoms [1]. Early analysis of the ‘Anosmia Reporting Tool’ by the American Academy of Otolaryngology showed anosmia in 73% of patients prior to COVID-19 diagnosis, and was the initial symptom in 26.6%, suggesting that anosmia may be a presenting symptom of COVID-19 [9]. In the absence of a widespread population testing strategy, understanding the symptomatology of this new illness is critical to ensuring that the correct advice is given to patients and the public in relation to self-isolation leading to reduced population transmission.
In the United Kingdom (UK), the transmission of SARS-CoV-2 was first confirmed in February 2020. From March 1, SARS-CoV-2 was reported across England, Wales, Scotland and Ireland, indicating widespread community transmission. Public Health England guidance currently recognizes symptoms of COVID-19 to include fever ≥37.8°C and at least one of the symptoms persistent cough (with or without sputum), hoarseness, nasal discharge or congestion, shortness of breath, sore throat, wheezing or sneezing [10]. To date, anosmia and ageusia are not recognised symptoms in the disease case definition. However, there is increasing evidence of these symptoms presenting in patients who are otherwise asymptomatic, highlighting the possibility that new-onset anosmia and/or ageusia may be useful as a component of screening for the virus [4]. This is critical at this stage in the pandemic, as adding olfactory symptoms to the case definition of COVID-19 would be especially useful should anosmia and/or ageusia be shown to present early and in otherwise asymptomatic patients who may go on to require hospital admission.
In this retrospective analysis of both community and secondary-care patients in a London population diagnosed with SARS-CoV-2 infection, we aimed to establish the prevalence of new-onset anosmia and ageusia and place them within disease symptom progression. We also investigated whether the olfactory symptoms experienced by COVID-19 patients are accompanied by other nasal symptoms (congestion or rhinorrhoea) as with other post-viral olfactory disorders. Additionally, we investigated the presence of COVID-19 symptoms, anosmia and ageusia in the household contacts of these patients.
Methods
Ethics approval and consent to participate
Data were collected as part of routine care by the responsible clinical team. No patient-identifiable data are reported in this analysis. The need for written informed consent was waived by the Research Governance Office of Chelsea and Westminster NHS Foundation Trust.
Study design and participants
This was a retrospective, observational analysis of patients diagnosed either as inpatients at a 430-bed London acute teaching hospital or in the surrounding community. As well as receiving unwell patients warranting admission, the hospital adopted a community testing strategy to identify suspected COVID-19 cases. Unwell patients were screened through call centres and general practitioners, who referred patients suspected of having COVID-19 to a centralized response team. Depending on mobility, these patients were either directed to drive through SARS-CoV-2 testing clinics or visited at home by community testing teams. Patients were also assessed through a bespoke section of the emergency department. This community testing ceased on March 13th, 2020.
Nasopharyngeal and oral swabs were taken from all patients (both community and secondary care) and tested at a central reference laboratory using real-time reverse transcriptase polymerase chain reaction (PCR) (initially using a proprietary assay run by Public Health England, then from March 6th, 2020 onwards a commercial assay from AusDiagnostics®, Australia) for SARS-CoV-2. Patients who were considered to be clinically stable were allowed to self-isolate at home. Community patients were informed of their result by telephone. Inpatients had their symptoms and clinical course documented in their electronic healthcare record (Millennium: Cerner Corporation, Kansas City, Missouri, USA) by the admitting clinical team. Demographic and clinical data were collected from electronic health records for all patients included in the analysis. Details of onset of COVID-19 symptoms, anosmia and/or ageusia were extracted where present.
All patients with a laboratory diagnosis of COVID-19 between March 1 and April 1, 2020 were identified. Patients were included if they (a) had a positive diagnosis for COVID-19 based on real-time PCR detection of SARS-CoV-2, and (b) were tested in the community OR admitted to and discharged from hospital. Patients were excluded if they (a) died post-diagnosis, (b) were <16 years of age, (c) did not have an accurate record of symptom history (e.g. due to confusion or lack of memory), or (d) were readmitted to hospital or transferred to another healthcare facility. We excluded current inpatients because a large proportion had current oxygen requirements either through nasal cannulae or via face-masks. These devices could influence the assessment of anosmia. Additionally, these patients were in isolation wards with only essential care being given to limit onward transmission, which made them inaccessible for the purposes of this study.
Between April 13th and April 17th, 2020, telephone consultations were conducted with all identified patients to verify symptomatology and timeline to resolution of clinical symptoms. All patients were asked a series of standardized questions on the presence of COVID-19 symptoms and also diarrhoea, vomiting, myalgia, and any change in their sense of smell and taste. If changes in smell and/or taste were reported, further standardized questions were asked regarding time of onset relative to onset of COVID-19 symptoms, duration of change, and whether these symptoms had resolved. Only complete recovery from anosmia was considered as resolution of the symptom. Partial recovery was considered as hyposmia. At the time of this study there was little evidence associating anosmia and COVID-19, therefore the primary outcome was new-onset anosmia and the questionnaire was not scored [11]. Details of the presence of household contacts and their symptomatology were also routinely collected in line with public health guidance. Specifically, patients were asked whether household contacts had experienced COVID-19 symptoms and whether any of them had experienced anosmia.
Study outcome measures
The primary outcome measure was the prevalence of new-onset anosmia and/or associated ageusia. Secondary outcome measures included analysis of duration of COVID-19 symptoms (as outlined by the current Public Health England case definition) [10] and new-onset anosmia and/or associated ageusia. Clinical presentation was defined as mild versus severe depending on whether patients were admitted to hospital or isolated in the community. An additional outcome measure was the prevalence of new-onset anosmia amongst household contacts.
Results
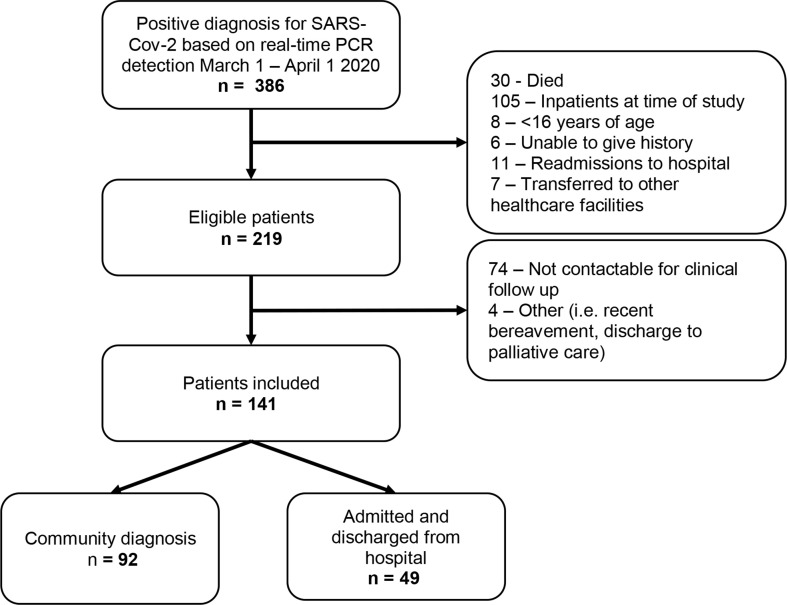
Between March 1st and April 1st 2020, 386 patients were diagnosed as SARS-CoV-2-positive using real-time PCR detection (Fig. 1 ). Of these, 167 were excluded as they did not fit the study inclusion criteria. Of the remaining 219, 74 were not contactable for the follow-up telephone consultations after three attempts across multiple dates. The final analysis included 141 patients. Of these, 92 were treated in the community and 49 were admitted to and discharged from hospital.
Fig. 1.
Flow diagram of participant selection for patients positive for severe acute respiratory distress syndrome coronavirus-2 (SARS-CoV-2) from a London community and a secondary-care population between March 1st and April 1st, 2020.
Table 1 summarizes the most commonly reported COVID-19 symptoms. Of the 141 patients, 77 (55%) reported anosmia and ageusia. Three patients reported only anosmia. Nine patients reported only ageusia. No patients reported pre-existing anosmia. Nasal congestion was reported in 39/80 patients (49%) with anosmia and 43/89 patients (48%) with ageusia. Nasal symptoms in the absence of anosmia and/or ageusia were reported in 16/52 patients (31%).
Table 1.
Patient demographics and frequency of COVID-19 symptoms in patients from a London community and secondary-care population between March 1st and April 1st, 2020
| Total | Community | Admitted | |
|---|---|---|---|
| n | 141 | 92 (65.2%) | 49 (34.8%) |
| Mean age (range) | 45.6 (20–93) | 40.7 (20–87) | 54.9 (22–93) |
| Sex (male/female) | 83/58 | 58/34 | 27/22 |
| Most common reported symptoms | |||
| Fever | 111 (75.7%) | 70 (76.1%) | 41 (83.7%) |
| Cough | 102 (72.3%) | 68 (73.9%) | 34 (69.4%) |
| Myalgia | 93 (66.0%) | 67 (72.8%) | 26 (53.1%) |
| Ageusia | 89 (63.1%) | 57 (62.0%) | 32 (65.3%) |
| Shortness of breath | 86 (61.0%) | 54 (58.7%) | 32 (65.3%) |
| Anosmia | 80 (56.7%) | 56 (60.9%) | 24 (49.0%) |
| Nasal congestion | 60 (42.6%) | 43 (46.7%) | 17 (34.7%) |
| Diarrhoea | 45 (31.9%) | 23 (25.0%) | 22 (44.9%) |
| Vomiting | 19 (13.5%) | 11 (12.0%) | 8 (16.3%) |
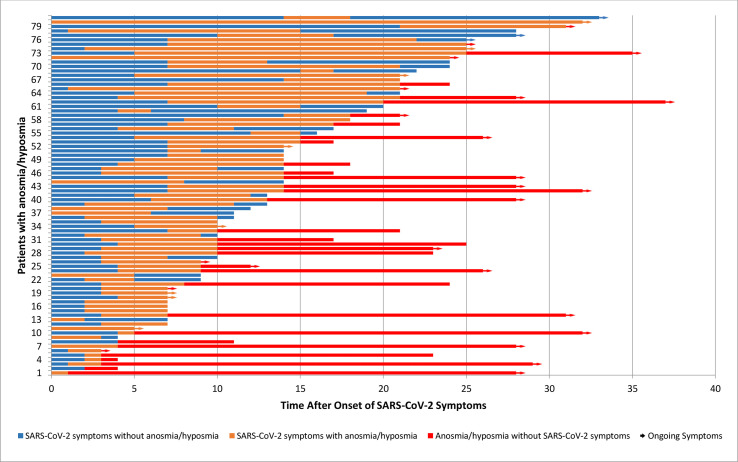
Table 2 shows the median duration of COVID-19 symptoms and anosmia (duration of symptoms was not normally distributed). The data in the table exclude 14 patients who were clear about the presence of both COVID-19 symptoms and anosmia but were unable to give an accurate duration of the anosmia. Fig. 2 charts the onset and duration of anosmia in relation to onset of COVID-19 symptoms. The onset of anosmia ranged between 1 and 21 days, and the duration was reported to be between 1 and 30 days with 32/81 patients experiencing ongoing anosmia or hyposmia at the end of the study period.
Table 2.
Natural history of COVID-19 symptoms and anosmia in patients from a London community and secondary-care population between March 1st and April 1st, 2020
| Total | Community | Admitted | |
|---|---|---|---|
| Patients with resolved COVID-19 symptoms | 114 | 83 | 31 |
| Patients with unresolved COVID-19 symptoms | 13 | 6 | 7 |
| Median duration of COVID-19 symptoms in days (interquartile range) | 12 (11.5) | 10 (8) | 118 (13.5) |
| Patients with resolved anosmia | 49 | 34 | 15 |
| Patients with unresolved anosmia/ongoing hyposmia | 32 | 21 | 11 |
| Median lag for onset of anosmia in days (IQR) | 4 (5) | 3 (3) | 5 (4) |
| Median duration of anosmia in days (IQR) | 8 (16) | 14 (16) | 7 (8.5) |
IQR, interquartile range.
Fig. 2.
Onset and duration (in days) of anosmia in relation to COVID-19 symptoms in patients from a London community and a secondary-care population between March 1st and April 1st, 2020. Arrow indicates ongoing symptoms at the time of telephone consultations.
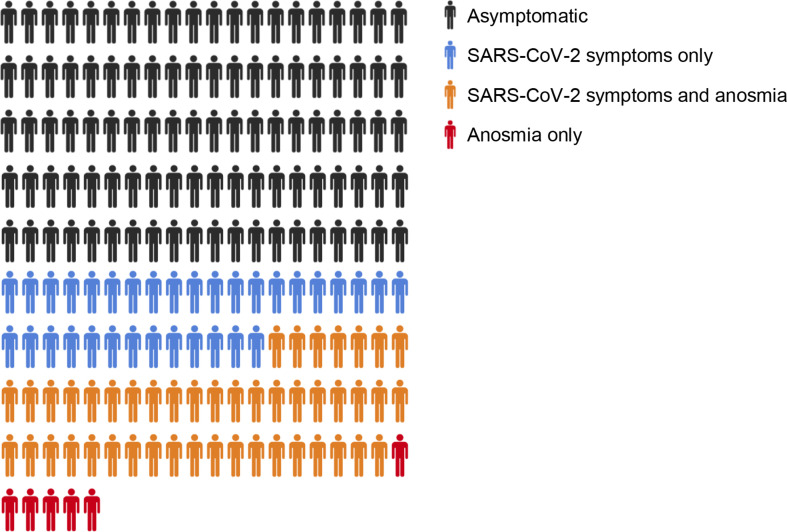
Of the 141 patients, 107 (76%) had one or more household contacts (total number of household contacts = 195) during their isolation period. Five households contained two study participants each (n = 10), leaving 185 non-tested household contacts. Of these, 79 (43%) had COVID-19 symptoms and 46 (58%) had anosmia. Six household contacts had anosmia in the absence of other symptoms (Fig. 3 ).
Fig. 3.
The presence of COVID-19 symptoms and anosmia in non-tested household contacts (n = 185) of patients from a London community and a secondary-care population tested positive for severe acute respiratory distress syndrome coronavirus-2 (SARS-CoV-2) between March 1st and April 1st, 2020.
Discussion
This analysis reports that over half of patients with COVID-19 experienced anosmia and/or ageusia. These findings are important as they support the increasing evidence associating anosmia and ageusia with SARS-CoV-2. They also represent a snapshot of the community setting at the early stages of the spread of this pandemic in the UK, which is valuable given the early cessation of community testing.
The prevalence of anosmia in post-viral respiratory tract infections seen in specialist clinics is greater than in general ear nose and throat clinics [8]. This suggests that the pickup rates in specialist clinics are higher. Our analysis, though more comparable to that of a general clinic setting, found the prevalence of anosmia to be greater than that found in specialist anosmia clinics. This higher prevalence of anosmia in COVID-19 is broadly in line with the current literature, where anosmia has been identified as one of the most predictive symptoms of COVID-19 [12]. Of all patients with anosmia, the majority (52%) did not report concurrent nasal congestion. This supports data from a large cross-European analysis that showed olfactory disorders are prevalent in COVID-19 patients, who may not have nasal symptoms [5].
Patients who reported ageusia only could not accurately differentiate between losing their sense of taste or their sense of smell. This may be due to retronasal olfactory function being labelled as taste [13]. The gustatory system (transmitted via the glossopharyngeal, facial and vagal nerves) only recognizes the basic tastes (sweet, sour, salty, bitter and umami), but most of the culinary experiences are recognized by the olfactory nerve [14]. Indeed, there is a close association between anosmia and ageusia, which may make it difficult for patients to differentiate between the two [15]. In our analysis we therefore made the assumption that ageusia was unlikely to be present in the absence of anosmia, and we therefore considered these patients to have anosmia also.
In this study a sizeable proportion of patients reported anosmia and ageusia extending beyond the resolution of COVID-19 symptoms. Additionally, three patients reported anosmia in the absence of any other symptoms. Mild community-treated patients were more likely to report anosmia than those admitted to hospital, which supports emerging evidence associating new-onset anosmia with mild or absent COVID-19 symptoms [1,5]. Prospective studies are needed to investigate the epidemiological significance of this in the context of potential spread of disease by individuals with mild atypical presentations.
The relative short time span of onset of anosmia in relation to other COVID-19 symptoms suggests that anosmia may be a useful early diagnostic factor in this viral disease and may subsequently have a role in guiding isolation practice. Duration and time of onset of anosmia were twice as long in the hospital group, although this analysis was not powered to investigate the significance of the variation in findings between hospitalized and community patients. Severe symptoms in hospitalized patients may initially overshadow the presence of anosmia, possibly explaining the delay in perceived onset. It is worth mentioning that, as of April 17th (end of the data collection period), 45 patients had ongoing COVID-19 symptoms and/or anosmia/hyposmia. This means the reported duration times are likely to be underrepresented.
All patients in this study were tested for SARS-CoV-2 due to clinical suspicion based on symptomatology. Therefore, by the nature of the selection process, it is unlikely that any COVID-19 patients with anosmia alone would have been tested. Over half of the symptomatic (but not tested) household contacts, however, reported anosmia, with a further six experiencing anosmia alone. Being close contacts of confirmed COVID-19 patients, it is likely the high prevalence of anosmia in this group is related to transmission of SARS-CoV-2. This shows consistency in prevalence of anosmia in an exposed (but not confirmed) population, but also that mild versions of the illness may present with anosmia alone. To obtain a true understanding of the clinical significance of anosmia in SARS-CoV-2, we need to prospectively investigate new-onset anosmia in the general population, potentially coupled with serological testing.
The strength of this study is that it provides an early insight into the chronological sequence of anosmia in COVID-19 and also the association between symptoms and household transmission. Since this analysis provides a snapshot of symptomatology in SARS-CoV-2-positive patients, it is not possible to draw population-wide conclusions. Recall bias of patients may also have influenced the clinically recorded data, especially of those hospitalized. Similarly, the absence of objective testing meant we were not able to clinically define the extent and severity of the anosmia and ageusia. Furthermore, physical examination was not possible in the community due to social distancing rules and the potential for onwards transmission. The use of a structured questionnaire, however, helped to ameliorate this possible reporting bias (Supplementary Material). It was not possible to estimate the number of asymptomatic COVID-19 patients in our community population as asymptomatic patients may have been less likely to present to hospital, and may not have been eligible for community testing as per PHE diagnostic criteria. Several patients had ongoing anosmia, and further follow-up will be required to further discern the duration of these symptoms.
This analysis did not include patients under the age of 16, and it has been widely reported that children do not appear to present in the same way as adults but may still be asymptomatic. The symptomatology of COVID-19 in children needs to be examined.
More than half the patients with confirmed COVID-19 suffered anosmia and ageusia. This is significant when compared with the prevalence of anosmia and ageusia in other post-viral upper respiratory tract infections. These findings suggest that anosmia and ageusia be added to existing case definitions for COVID-19 and used to guide self-isolation procedures. This is critical in the absence of population-level testing.
The findings of this research highlight the need to investigate new-onset anosmia in the general population, particularly in those without other symptoms. A better understanding of the long-term outcomes of anosmia in COVID-19 patients is needed. Until a time when we have successful population-level vaccination coverage, these steps remain critical to managing the current and subsequent waves of this pandemic.
Author contributions
AP and EC developed the study design. AP, EC, DA, and AA were responsible for data collection. AP, EC and AA assisted with data interpretation. AP and EC performed the literature search and wrote the first draft of the paper. All authors have critically read and commented on draft versions of the manuscript and approved the final version.
Transparency declaration
All authors have completed the ICMJE Form for Disclosure of Potential Conflicts of Interest and declare the following. EC has received speaker fees from bioMerieux (2019). NM has received speaker fees from Beyer (2016) and Pfizer (2019) and received educational support from Eumedica (2016) and Baxter (2017). LSPM has consulted for bioMerieux (2013), DNAelectronics (2015–18), Dairy Crest (2017–2018), Umovis Lab (2020), received speaker fees from Profile Pharma (2018–2019) and Pfizer (2018–2020), received research grants from the National Institute for Health Research (2013–2020), CW+ Charity (2018–2019), and Leo Pharma (2016), and received educational support from Eumedica (2016–2018). AP, DLA, and AA have no conflicts of interest to declare. This research did not receive any grant from funding agencies in the public or commercial sectors.
Acknowledgements
EC is supported by Economic and Social Science Research Council (ESRC) and the National Institute for Health Research (NIHR) Health Protection Research Unit (HPRU) in Healthcare Associated Infections and Antimicrobial Resistance, UK Department of Health (HPRU-2012-10047) in partnership with Public Health England. LSPM acknowledges support from the National Institute of Health Research (NIHR) Imperial Biomedical Research Centre (BRC) and the National Institute for Health Research HPRU in Healthcare Associated Infection and Antimicrobial Resistance at Imperial College London in partnership with Public Health England. The views expressed in this publication are those of the authors and not necessarily those of the NHS, the National Institute for Health Research, or the UK Department of Health.
Editor: L. Leibovici
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cmi.2020.05.026.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Xydakis M.S., Dehgani-Mobaraki P., Holbrook E.H., Geisthoff U.W., Bauer C., Hautefort C. Smell and taste dysfunction in patients with COVID-19. Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30293-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mao L., Jin H., Wang M., Hu Y., Chen S., He Q. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020 doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giacomelli A., Pezzati L., Conti F., Bernacchia D., Siano M., Oreni L. Self-reported olfactory and taste disorders in SARS-CoV-2 patients: a cross-sectional study. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hopkins C., Kumar N. British Rhinological Society and ENT-UK; London: 2020. Loss of sense of smell as marker of COVID-19 infection: joint statement from the. [Google Scholar]
- 5.Lechien J.R., Chiesa-Estomba C.M., De Siati D.R., Horoi M., Le Bon S.D., Rodriguez A. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Oto-Rhino-Laryngology. 2020;2 doi: 10.1007/s00405-020-05965-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hummel T., Landis B., Huttenbrink K. Smell and taste disorders. GMS Curr Top Otorhinolaryngol Head Neck Surg. 2011;10:Doc04. doi: 10.1002/9781119952930.ch88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Riel D., Verdijk R., Kuiken T. The olfactory nerve: a shortcut for influenza and other viral diseases into the central nervous system. J Pathol. 2015;235:277–287. doi: 10.1002/path.4461. [DOI] [PubMed] [Google Scholar]
- 8.Welge-Lüssen A., Wolfensberger M. Olfactory disorders following upper respiratory tract infections. Adv Otorhinolaryngol. 2006;63:125–132. doi: 10.1159/000093758. [DOI] [PubMed] [Google Scholar]
- 9.Kaye R., Chang C.W.D., Kazahaya K., Brereton J., Denneny J.C. Otolaryngol Neck Surg; 2020. COVID-19 anosmia reporting Tool: initial findings. [DOI] [PubMed] [Google Scholar]
- 10.Public Health England . 2020. Guidance: COVID-19: investigation and initial clinical management of possible cases. [Google Scholar]
- 11.Donovan J.O., Tanveer S., Jones N., Hopkins C., Senior B.A., Wise S.K. What is the evidence for anosmia (loss of smell) as a clinical feature of COVID-19? Cent Evidence-Based Med. 2020 [Google Scholar]
- 12.Menni C., Valdes A.M., Freidin M.B., Sudre C.H., Nguyen L.H., Drew D.A. Real-time tracking of self-reported symptoms to predict potential COVID-19. Nat Med. 2020 doi: 10.1038/s41591-020-0916-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deems D.A., Doty R.L., Settle R.G., Moore-Gillon V., Shaman P., Mester A.F. Smell and taste disorders, a study of 750 patients from the university of Pennsylvania smell and taste center. Arch Otolaryngol Head Neck Surg. 1991;117:519–528. doi: 10.1001/archotol.1991.01870170065015. [DOI] [PubMed] [Google Scholar]
- 14.Hummel T., Heilmann S., Landis B.N., Reden J., Frasnelli J., Small D.M. Perceptual differences between chemical stimuli presented through the ortho- or retronasal route. Flavour Fragr J. 2006 doi: 10.1002/ffj.1700. [DOI] [Google Scholar]
- 15.Doty R.L. Sensory aging: chemical senses. Encycl Neurosci. 2009 doi: 10.1016/B978-008045046-9.00147-9. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.