Clinical Practice Points.
-
•
Early detection of COVID-19 is essential, even more in patients with non–small-cell lung cancer, who are already at higher risk of developing severe pneumonitis.
-
•
Differential diagnosis from toxicities induced by radiotherapy or immunotherapy is challenging, as clinical and radiologic presentation might almost completely overlap.
-
•
As stage III non–small-cell lung cancer is curable in about 40% of cases, an aggressive and timely treatment appears mandatory.
Immune-checkpoint inhibitors could possibly augment the detrimental cytokine release, crucial in Covid-19 pathogenesis.
Introduction
The Coronavirus-disease-2019 (Covid-19) outbreak is currently generating an overwhelming burden for public health worldwide: as of May 16, 2020, 4,425,485 confirmed cases had been listed and 302,059 deaths reported.1 The clinical presentation of Covid-19 is heterogeneous, lacks pathognomonic signs, and mostly overlaps with other affections of the respiratory system.2 Management of this condition is even more challenging in patients with non–small-cell lung cancer (NSCLC) because they are more vulnerable to develop severe disease, and manifestations of the tumor and side effects of anticancer therapy could resemble Covid-19. Maintenance therapy with the programmed death-ligand 1 (PD-L1) inhibitor durvalumab is currently the standard of care for stage III unresectable NSCLC after concurrent radio-chemotherapy (RCT), with a reported incidence of pneumonitis of 33.9% (grade ≥ 3 in 3.4%).3 Herein, we present the first report to date of a case of Covid-19 during durvalumab, focused on the differential diagnosis between radiation-induced pneumonitis and PD-L1 immune checkpoint inhibitor toxicity in a patient with NSCLC.
Case Report
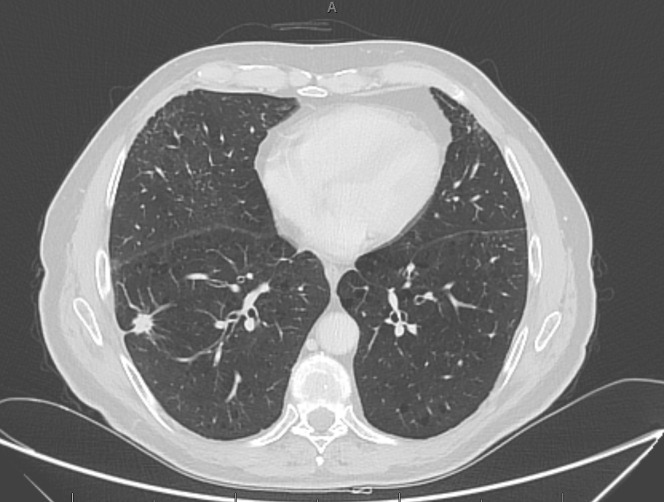
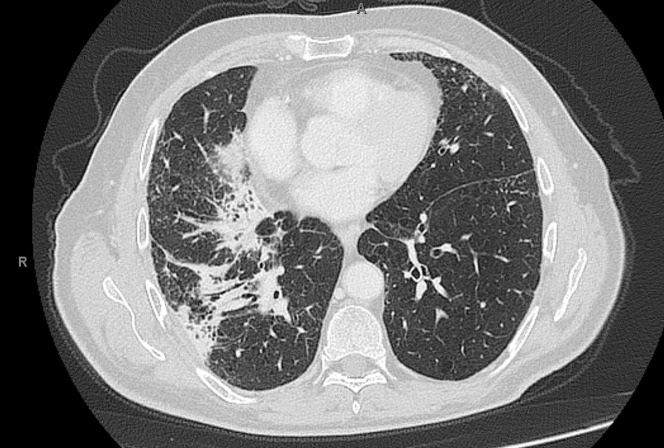
A 75-year-old man underwent an abdominal computed tomography (CT) scan for diverticulitis, with the incidental finding of a lesion of the lower right lung lobe (17 × 17 mm) with ipsilateral hilar-mediastinal lymphadenopathies. A bronchoscopic biopsy was performed, with histologic diagnosis of adenocarcinoma with PD-L1 expression > 90% and wild-type EGFR, ALK, and ROS1. A positron emission tomography-CT scan showed pathologic uptake of the known neoplastic sites; the tumor was staged cT1c cN2 M0, IIIA. Comorbidities included recurring diverticulitis, GOLD stage I chronic obstructive pulmonary disease, hypertension, and gastritis. Performance status was Eastern Cooperative Oncology Group grade 1, as the only symptom was dyspnea on exertion, and the patient was a smoker (about 100 pack/year). Our institutional tumor board advised for concurrent chemo-radiotherapy (cCRT), that was delivered with volumetric modulated arc therapy at a dose of 60 Gy in 30 fractions to the primary tumor and mediastinal nodes (levels 7 and 10R). Four cycles of concurrent chemotherapy with paclitaxel and carboplatin were administered. A total body CT scan was performed 15 days after cCRT, showing a partial response of both the primary tumor and the subcarinal adenopathy, with the disappearance of the hilar lymphadenopathy (Figure 1 ); no ground-glass opacities or consolidations were detected. Maintenance with durvalumab was started 27 days after cCRT end, and 4 cycles were administered without associated toxicities or alteration of blood tests (including liver, thyroid, and hypophysis function). On January 8, 2020, he presented for the fifth cycle, reporting fatigue, worsening of the dyspnea, and non-productive cough; no pathologic findings were detected at physical examination. Immunotherapy was discontinued, and a chest CT scan was requested. A pattern of atypical immune-related pneumonitis was detected, with multifocal consolidations in the right lung involving the upper, middle, and lower lobes, and surrounding the known lesion (Figure 2 ) and bilateral diffuse interstitial thickening. It should be noted that, at that time, Covid-19 was not considered among the differential diagnoses, as the first direct transmission in Italy was reported on February 18, 2020. Oral prednisone 50 mg daily was started: after a week of treatment, dyspnea markedly improved and returned to the baseline condition. The steroid was gradually tapered, halving the dose every week, and suspended after 4 weeks. Chest CT scan was repeated on February 17, and findings were stable compared with the previous evaluation. On March 4, he was admitted to restart durvalumab, reporting the onset 2 days earlier of fever, unremitting dry cough, and diarrhea, with dyspnea at rest. The physical examination revealed bilateral diffuse crackles; peripheral oxygen saturation in ambient air was 88%, heart rate was 120 bpm; arterial blood-gas test showed normocapnic hypoxemia (PaO2 62 mmHg). Chest radiography showed a diffuse alveolar and interstitial infiltrate, affecting the right lung and the inferior field of the left lung (Figure 3 ). Complete blood count, creatinine, and liver function were normal. Nasopharyngeal swab resulted negative for influenza A-B, adenovirus, and respiratory syncytial virus; reverse-transcription polymerase-chain-reaction (RT-PCR) test resulted positive for SARS-CoV-2, confirming the suspect of Covid-19. The patient was then hospitalized and started treatment with lopinavir/ritonavir, oseltamivir, and hydroxychloroquine and prophylactic antibiotic therapy with azithromycin and piperacillin/tazobactam. Clinical conditions abruptly deteriorated, and dyspnea did not improve despite oxygen therapy at increasing volumes. Owing to the overwhelmingly high number of patients treated at our hospital intensive care unit during the Covid-19 outbreak, mechanical ventilation could not be promptly performed. His respiratory function worsened, and the patient died of respiratory failure on March 7, 2020.
Figure 1.
Total-Body Ct Scan was Performed 15 Days After Radio-Chemotherapy, Showing Partial Response Of Disease
Figure 2.
Chest Ct-Scan Showing a Pattern of Atypical Immune-Related Pneumonitis After 4 Cycles of Durvalumab
Figure 3.
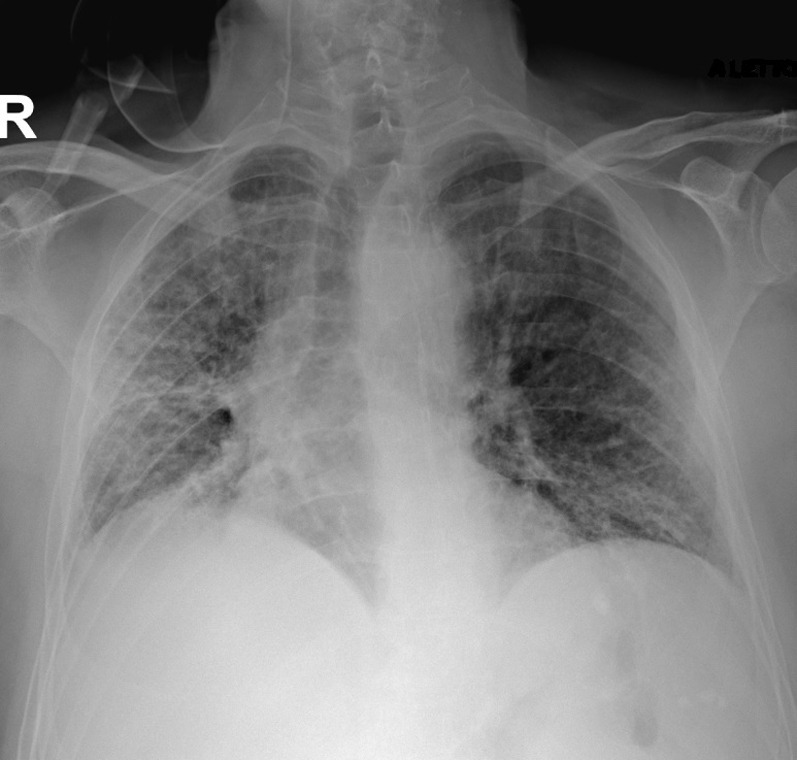
Chest Radiograph at Covid-19 Presentation, Presenting Bilateral Diffuse Alveolar and Interstitial Infiltrate
Discussion
Pneumonitis is quite common in advanced NSCLC and can be a consequence of the disease itself, super-infections, coexisting lung dysfunctions, or side effects of the treatment. Recently, Covid-19 has been added to the list of possible causes, emerging as a real threat for already frail patients.
Covid-19 Pneumonitis
Clinical presentation of Covid-19 is aspecific. Many patients are asymptomatic or express mild symptoms, resulting in underdetection of the total cases.4 Fever is present in most patients, and common symptoms encompass cough, malaise, and myalgia.3, 4, 5 Laboratory findings include lymphopenia, thrombocytopenia, and increased C-reactive protein.5 Up to 20% of the cases might develop severe disease, characterized by dyspnea, tachypnea, and hypoxemia requiring hospitalization; about 5% to 10% of the patients precipitate, often suddenly, into critical conditions, including respiratory failure and multi-organ dysfunction.3, 4, 5 Lung involvement is typically bilateral, peripheral, and basal in the vast majority of the cases. It usually presents with multifocal ground-glass opacities (GGO) and interstitial infiltrations, which may evolve into consolidations and ‘crazy paving’ patterns; complete clearing usually needs several weeks.5 , 6 Current treatment includes antiviral agents, and the antimalarial immune-modulator chloroquine.3, 4, 5, 6, 7 In severe cases, oxygen therapy and prophylactic antibiotics are administered, whereas the use of glucocorticoids is debated.3, 4, 5, 6, 7, 8 About 5% to 10% of the patients necessitate admission in intensive care units, most of them requiring mechanical ventilation.3
Radiation-induced Pneumonitis
The incidence of pneumonitis in patients undergoing radical RT for NSCLC varies from 5% to 25%.9, 10, 11 Chest CT is the elective technique both for diagnosis and follow-up.10 , 11 Damage is usually limited to the irradiated volume and surrounding tissue. Nevertheless, a minority of the cases develop ‘out-of-field’ or bilateral involvement owing to immune-mediated reactions.10 , 11 Initially, a patchy ground-glass attenuation can be observed, with a possible gradual evolution over weeks or months towards consolidation and finally to fibrosis.10 , 11 Radiologic and clinical changes may appear many months after RT and resemble disease progression.10 The mainstay of the treatment of symptomatic cases is oral glucocorticoids. Most of the patients ameliorate after a few weeks of treatment, followed by a very slow tapering to avoid relapses.10 , 11 Less than 5% of cases are grade ≥ 3 and necessitate hospitalization.9 Recover of pulmonary function requires several months, and a few patients develop irreversible and occasionally fatal respiratory failure.11
Immune Checkpoint Inhibitor-induced Pneumonitis
The incidence of pneumonitis caused by programmed cell death protein-1 (PD-1)/PD-L1 inhibitors is 3% to 5% in clinical trials, and up to 19% in ‘real-world’ cohorts, with increased risk for previous radiotherapy.12, 13, 14 Immune-related events appear within an ample range of time to onset and even months after treatment discontinuation.12 , 14 , 15 Chest CT scan usually shows a bilateral multifocal interstitial pattern with non-segmental GGO or consolidations involving especially the lower lungs, septal thickening, and traction bronchiectasis.12, 13, 14 Atypical patterns are frequent and encompass interstitial pneumonitis, GGO around the tumor (peritumoral infiltration), opacities in the ipsilateral lung, and aggravation, or ‘recall,’ of radiation-induced fibrosis.14 , 16 In grade 1 to 2 pneumonitis, immunotherapy must be temporarily withheld, and oral steroids should be administered with close clinical and radiologic monitoring and gradually tapered after response. In the absence of improvement or grade ≥ 3 toxicity, the drug should be permanently suspended, and high-dose steroids started in a hospital setting.12 , 13 The steroid-refractory disease is uncommon; it may require the addition of further immunosuppressors but is characterized by high mortality.12
Pathogenesis
The data summarized above highlight the remarkable similarity between different patterns of pneumonitis caused by either radiation or immunotherapy and Covid-19. A plausible assumption could be that Covid-19 pneumonia is at least partly owing to a disproportionate release of inflammatory molecules. This phenomenon could explain the higher levels of cytokines including interleukin-6 and tumor necrosis factor measured in patients developing severe respiratory symptoms17 and the investigation of immunosuppressants such as chloroquine and tocilizumab (a humanized monoclonal antibody targeting interleukin-6 receptor, also used to manage steroid-refractory pneumonitis induced by PD-L1 inhibitors) for Covid-19 treatment.18 , 19 The activity of the PD-1/PD-L1 axis does not only induce immunotolerance towards neoplastic cells but also avoids excessive and unregulated activation of the immune system during the acute phase of viral infections.20 Therefore, immune-checkpoint inhibitors could also augment the detrimental cytokine release induced by Covid-19.
Conclusions
This report outlines the complexity and the importance of early detection of Covid-19, even more in patients with NSCLC, who are already at higher risk of developing severe pneumonitis on a multi-factorial basis. Nevertheless, differential diagnosis from toxicities induced by radiotherapy or immunotherapy is challenging, as clinical and radiologic presentation might almost completely overlap. In order to face the Covid-19 emergency, we implemented a dedicated workflow to allow a timely diagnosis. In case of contacts at risk or at the onset of respiratory symptoms or fever, RT-PCR for Sars-Cov-2 on nasopharyngeal swab should be immediately performed. Although chest radiograph assessed with a validated score21 could be useful as a first-line test, CT scan is recommended in all the suspect cases owing to its elevated sensitivity and should be repeated to monitor the evolution of disease. Considering that stage III NSCLC is curable in about 40% of cases, an aggressive and prompt treatment appears mandatory, also taking into account the presumptive susceptibility to develop severe Covid-19 presentations.
Disclosure
The authors have stated that they have no conflicts of interest.
References
- 1.World Health Organization Coronavirus disease (COVID-19) pandemic. https://www.who.int/emergencies/diseases/novel-coronavirus-2019 Available at: (update May 16). Accessed May 16, 2020.
- 2.Guan W.J., Ni Z.Y., Hu Y. China Medical Treatment Expert Group for Covid-19. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Antonia S.J., Villegas A., Daniel D., PACIFIC Investigators Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med. 2017;377:1919–1929. doi: 10.1056/NEJMoa1709937. [DOI] [PubMed] [Google Scholar]
- 4.Arabi Y.M., Murthy S., Webb S. COVID-19: a novel coronavirus and a novel challenge for critical care. Intensive Care Med. 2020;46:833–836. doi: 10.1007/s00134-020-05955-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kanne J.P., Little B.P., Chung J.H., Elicker B.M., Ketai L.H. Essentials for radiologists on COVID-19: an update-Radiology scientific expert panel. Radiology. 2020 Feb 27:200527. doi: 10.1148/radiol.2020200527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ai T., Yang Z., Hou H. Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology. 2020 Feb 26:200642. doi: 10.1148/radiol.2020200642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li G., De Clercq E. Therapeutic options for the 2019 novel coronavirus (2019-nCoV) Nat Rev Drug Discov. 2020;19:149–150. doi: 10.1038/d41573-020-00016-0. [DOI] [PubMed] [Google Scholar]
- 8.Shang L., Zhao J., Hu Y., Du R., Cao B. On the use of corticosteroids for 2019-nCoV pneumonia. Lancet. 2020;395:683–684. doi: 10.1016/S0140-6736(20)30361-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li M., Gan L., Song A., Xue J., Lu Y. Rethinking pulmonary toxicity in advanced non-small cell lung cancer in the era of combining anti-PD-1/PD-L1 therapy with thoracic radiotherapy. Biochim Biophys Acta Rev Cancer. 2019;1871:323–330. doi: 10.1016/j.bbcan.2019.02.004. [DOI] [PubMed] [Google Scholar]
- 10.Hanania A.N., Mainwaring W., Ghebre Y.T., Hanania N.A., Ludwig M. Radiation-induced lung injury: assessment and management. Chest. 2019;156:150–162. doi: 10.1016/j.chest.2019.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Keffer S., Guy C.L., Weiss E. Fatal radiation pneumonitis: literature review and case series. Adv Radiat Oncol. 2019;5:238–249. doi: 10.1016/j.adro.2019.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suresh K., Voong K.R., Shankar B. Pneumonitis in non–small cell lung cancer patients receiving immune checkpoint immunotherapy: incidence and risk factors. J Thorac Oncol. 2018;13:1930–1939. doi: 10.1016/j.jtho.2018.08.2035. [DOI] [PubMed] [Google Scholar]
- 13.Haanen J.B.A.G., Carbonnel F., Robert C., ESMO Guidelines Committee Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28(Suppl 4):iv119–i142. doi: 10.1093/annonc/mdx225. [DOI] [PubMed] [Google Scholar]
- 14.Widmann G., Nguyen V.A., Plaickner J., Jaschke W. Imaging features of toxicities by immune checkpoint inhibitors in cancer therapy. Curr Radiol Rep. 2016;5:59. doi: 10.1007/s40134-017-0256-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Couey M.A., Bell R.B., Patel A.A. Delayed immune-related events (DIRE) after discontinuation of immunotherapy: diagnostic hazard of autoimmunity at a distance. J Immunother Cancer. 2019;7:165. doi: 10.1186/s40425-019-0645-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baba T., Sakai F., Kato T. Radiologic features of pneumonitis associated with nivolumab in non-small-cell lung cancer and malignant melanoma. Future Oncol. 2019;15:1911–1920. doi: 10.2217/fon-2019-0102. [DOI] [PubMed] [Google Scholar]
- 17.Qin C., Zhou L., Hu Z. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin Infect Dis. 2020 Mar 12 doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sahraei Z., Shabani M., Shokouhi S., Saffaei A. Aminoquinolines against coronavirus disease 2019 (COVID-19): chloroquine or hydroxychloroquine. Int J Antimicrob Agents. 2020;55:105945. doi: 10.1016/j.ijantimicag.2020.105945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stroud C.R., Hegde A., Cherry C. Tocilizumab for the management of immune mediated adverse events secondary to PD-1 blockade. J Oncol Pharm Pract. 2019;25:551–557. doi: 10.1177/1078155217745144. [DOI] [PubMed] [Google Scholar]
- 20.Schönrich G., Raftery M.J. The PD-1/PD-L1 axis and virus infections: a delicate balance. Front Cell Infect Microbiol. 2019;9:207. doi: 10.3389/fcimb.2019.00207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Borghesi A., Maroldi R. COVID-19 outbreak in Italy: experimental chest x-ray scoring system for quantifying and monitoring disease progression. Radiol Med. 2020;125:509–513. doi: 10.1007/s11547-020-01200-3. [DOI] [PMC free article] [PubMed] [Google Scholar]