Graphical abstract
Keywords: COVID-19, SARS-CoV-2, Chemokines, Growth factors, Cytokine storm
Highlights
-
•
A wide range of cytokines are involved in the development of COVID-19 disease.
-
•
Some of these biomolecules are related to the progression and even to the prognosis of the infection.
-
•
Findings on the role of cytokine storm associated with SARS-CoV-2 infection can be useful in order to manage this highly virulent disease.
Abstract
COVID-19 disease, caused by infection with SARS-CoV-2, is related to a series of physiopathological mechanisms that mobilize a wide variety of biomolecules, mainly immunological in nature. In the most severe cases, the prognosis can be markedly worsened by the hyperproduction of mainly proinflammatory cytokines, such as IL-1, IL-6, IL-12, IFN-γ, and TNF-α, preferentially targeting lung tissue. This study reviews published data on alterations in the expression of different cytokines in patients with COVID-19 who require admission to an intensive care unit. Data on the implication of cytokines in this disease and their effect on outcomes will support the design of more effective approaches to the management of COVID-19.
1. Introduction
COVID-19 is a is a novel β-coronavirus caused by infection with SARS-CoV-2 and is closely related to SARS-CoV. It is the third zoonotic disease by coronavirus to affect humans, following Severe Acute Respiratory Syndrome (SARS) and Middle-East respiratory syndrome (MERS) [1,2].
The first cases of infection by this virus were reported in a shellfish market in south Wuhan in December 2019 [3], and it proved impossible to rule out human-human transmission. SARS-CoV-2 samples were detected in this market, but the infected animal species has not been definitively established at the time of writing [4]. One study proposed that snakes were more likely than other animals to be carriers [5], but subsequent research indicated that bats may be responsible for its transmission, given the close similarity (96.2 % of genome sequence) between SARS-CoV-2 in bats and humans [[6], [7], [8], [9]].
The basic reproductive number (R0) in China was reported to reach at least 2.2 [10,11]. Human-human transmission started among family members, observing that 31.3 % of infected individuals in this first wave resided in Wuhan and that 72.3 % did not live there but had contact with people who did [12]. The mortality rate of the disease ranged between 2.2 and 2.84 %, and the mean age of non-survivors was 75 years [13,14]. The mean interval between initial symptoms and death was 14 days (range, 6–41 days) and was shorter (11.5 days) in patients aged ≥70 years [14].
The clinical manifestations of COVID-19 appear after an incubation period of around 5–6 days and most frequently include fever, coughing, and fatigue, with the possible onset of sputum production, headache, hemoptysis, diarrhea, dyspnea, and/or lymphopenia, among others [12,[14], [15], [16], [17], [18], [19]]. Computed tomography images of patients with severe complications of COVID-19 reveal the presence of pneumonia although with abnormal characteristics, including RNAemia, acute respiratory distress syndrome, acute cardiac damage, and evidence of pulmonary ground-glass opacities. In some patients, ground-glass opacities were detected in subpleural regions in both lungs, which may lead to both systemic and localized immune responses, exacerbating inflammation. In some cases, treatment with inhalers and interferon not only had no positive effects but also worsened the clinical symptoms, with the progression of pulmonary opacities [17,20]. Patients of advanced age and those with underlying conditions (e.g., hypertension, chronic obstructive pulmonary disease, diabetes, and/or cardiovascular disease, etc.) are at higher risk of a rapid progression to acute respiratory distress syndrome, septic shock, metabolic acidosis, coagulation dysfunction, arrhythmia, kidney damage, heart failure, liver dysfunction, and/or secondary infection, often resulting in death [17,19].
Treatment options against this new disease are mainly limited to the mitigation of clinical symptoms, especially those affecting the respiratory system, including the application of oxygen therapy, with the provision of extracorporeal membrane oxygenation for patients with refractory hypoxemia. Treatments with hyperimmune plasma and immunoglobulin G have also been received by some critically ill patients [21,22], while the administration of antivirals and corticosteroids is contraindicated [19,23]. Remdesivir, an adenosine nucleotide analog, has proven effective in in vitro and animal studies and in one patient in the USA [24,25]. Antiretrovirals used against the human immunodeficiency virus (HIV), such as lopinavir/ritonavir, have also been found to reduce the viral load of SARS-CoV-2 [26]. Chloroquine has also been proposed as a candidate drug for the treatment of COVID-19 based on its immunomodulatory properties and capacity to suppress tumor necrosis factor α (TNF-α) and interleukin-6 (IL-6) and to inhibit autophagy [27,28]. In general, and especially in patients suffering from a cytokine storm, clinicians should focus on the reduction of uncontrolled inflammation by blocking IL-6 and TNF-α or eliminating cytokines via hemoperfusion [29] (Table 1 ). Cytokine hyperproduction is often followed by edema, gas exchange dysfunction, acute respiratory syndrome, acute cardiac damage, and secondary infection [17].
Table 1.
Experimental treatments available for SARS-CoV-2 infection.
| COVID19 Treatment | Cytokine Target | Clinical effect | Reference |
|---|---|---|---|
| Tocilizumab | IL-6 | Block Il-6 receptor, and revert the cytokine storm production | Luo et al. [84] |
| Xu et al. [95] | |||
| Dholaria et al. [96] | |||
| Zhang et al. [97] | |||
| Turn back inflammation and pulmonary fibrosis | Wang & Han [98] | ||
| Shieh et al. [99] | |||
| Blood purification | Cytokines | Eliminate cytokines | Ma et al. [85] |
| Myo-inositol | IL-6 | Il-6 levels reduction, and prevent the cascade inflammation response | Bizzarri et al. [102] |
| Azithromycin | IL-6 | IL-6 and TNF-α Blockage | Schultz [103] |
| TNF-α | Gautret et al. [104] | ||
| Chloroquine | IL-6 | IL-6 & TNF-α supression | Wang et al. [105] |
| TNF-α | |||
| Fedratinib | IL-17 | Decreasing of IL-17 | Wu and Yang [163] |
| (Assayed in murine model) | |||
| Certolizumab & antiviral therapy | TNF-α | Antibody anti- TNF-α | Zhang et al. [188] |
| MSC | IL-1 | Downregulation of IL-1, VEGF, IL-12, IFN-γ & TNF-α | Chen et al. [144] |
| VEGF | Chen et al. [43] | ||
| IL-12 | Leng et al. [145] | ||
| IFN-γ | |||
| TNF-α |
With regard to the pathophysiological mechanism of the virus, it enters the cell via angiotensin-converting enzyme-2 (ACE-2), mainly through the Toll-like receptor-7 (TLR-7) present in endosomes. TLR-7 activation requires the production of TNF-α, IL-12, and IL-6 to enable the generation of specific cytotoxic CD8+ T cells. This involves the formation of antigen-specific B cells and antibody production through CD4+ helper T cells [29].
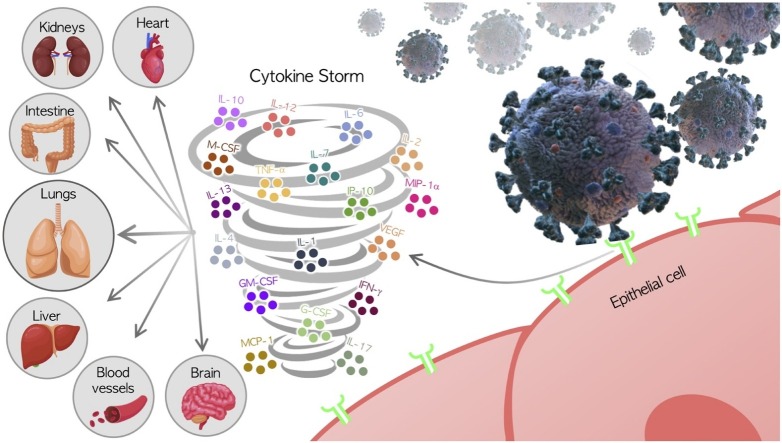
The majority of patients infected with COVID-19 have normal or reduced white cell counts and lymphocytopenia, and those with severe disease have shown significantly elevated levels of neutrophils, dimer-D, and urea in blood, with a continuing decrease in lymphocytes. Increases in certain cytokines and chemokines (e.g., IL-6, IL-10, and TNF-α) have also been observed in these patients. Thus, patients admitted to intensive care units (ICUs) have been found to have elevated serum levels of IL-2, IL-7, IL-10, macrophage colony-stimulating factor (M-CSF), granulocyte colony-stimulating factor (G-CSF), granulocyte-macrophage colony stimulating factor (GM-CSF), 10 kD interferon-gamma-induced protein (IP-10), monocyte chemoattractant protein-1 (MCP-1), macrophage inflammatory protein 1-α (MIP 1-α), and TNF-α [17,30,31] (Fig. 1 ).
Fig. 1.
Cytokine storm and severity of the COVID-19 disease.
It is essential to analyze the factors underlying the physiopathology of this pandemic disease, and certain cytokines appear to play a key role. The objective of this study was to review data on the cytokines that influence the progression of COVID-19 in order to support efforts to manage this highly virulent disease.
2. SARS-CoV-2 and cytokines
The immediate immune response to infection by viruses, bacteria, or other microorganisms involves the mobilization of cells and molecules and draws on energetic, enzymatic, and biosynthetic resources; i.e., metabolic resources [[32], [33], [34]]. Metabolic dysfunctions caused by viral infection requires a reprograming of the host metabolism to generate effective antiviral defense responses. Data published on interferences between the actions of viruses and cytokines reveal the molecular mechanisms underlying the innate immune response against viral infection [[35], [36], [37]].
Cytokines are a group of polypeptide signaling molecules responsible for regulating a large number of biological processes via cell surface receptors [38]. Key cytokines include those involved in adaptive immunity (e.g., IL-2 and IL-4), proinflammatory cytokines and interleukins (ILs) (e.g., interferon (IFN)-I, -II, and -III; IL-1, IL-6, and IL-17; and TNF-α); and anti-inflammatory cytokines (e.g., IL-10). In response to stress-generating internal processes (e.g., cancer or microbial infection), host cells secrete cytokines with a highly important role in cell metabolism reprogramming as a defensive response [32,39,40].
Concerning COVID-19 disease, Blanco-Mello et al. described a distinctive and unsuitable inflammatory response related to SARS-CoV-2 infection. These authors revealed that an “inappropriate and weak immune response” appears more frequently in patients with comorbidities. Thus, this could favor virus replication and enhance complications related to severe cases of the disease [41].
In the short time since the emergence of COVID-19, numerous studies have described abnormal levels of the following cytokines and chemokines in the patients: IL-1, IL-2, IL-4, IL-6, IL-7, IL-10, IL-12, IL-13, IL-17, M-CSF, G-CSF, GM-CSF, IP-10, IFN-γ, MCP-1, MIP 1-α, hepatocyte growth factor (HGF), TNF-α, and vascular endothelial growth factor (VEGF) [17,30,31,42,43] (Table 2 ). The key point in SARS-CoV-2 infection could be the depletion of antiviral defenses related to innate immune response as well as an elevated production of inflammatory cytokines [41].
Table 2.
Cytokines involved in SARS-CoV-2 infection.
| Reference | Methodology | Objective | Main Results |
|---|---|---|---|
| Chen et al. [16] | Retrospective study | To describe the epidemiological and clinical features of SARS-CoV-2 pneumonia in patients from Wuhan Jinyintan Hospital | Approximately 50 % of the subjects who developed the disease had been exposed to the Huanan seafood market. It affects men more often, with an average age of 55. Approximately half of the subjects had other comorbidities. The main symptomatology is characterized by fever, cough, shortness of breath, muscle pain among others. CT scan revealed that 75% of patients developed bilateral pneumonia and some cases evolved into acute respiratory distress syndrome (ARDS) or died from multiple organ failure. |
| Huang et al. [17] | Descriptive study | To describe the epidemiological, clinical, laboratory and radiological characteristics, treatment and outcomes of patients infected with COVID-19 in Wuhan and make a comparison between patients in the intensive care unit (ICU) and those who are not | Infection by SARS-CoV-2 caused clusters of severe respiratory disease and was associated with ICU admission and high mortality. Most of the infected patients were male, with different comorbidities and an average age of 49 years. The disease is mainly manifested by fever, cough, and myalgia or fatigue. Dyspnea occurred in more than 50 % of the patients. Lymphopenia was present in 63 % of the cases. Patients admitted to the ICU were found to have higher plasma levels of IL2, IL7, IL10, GSCF, IP10, MCP1, MIP1A, and TNFα. |
| Liu et al. [30] | Descriptive study | To describe the epidemiology, clinical features, possible treatments and prognosis of patients infected with SARS-CoV-2 in Hubei | The initial manifestations of SARS-CoV-2 were fever, cough and muscle pain or fatigue. Most patients had a normal or decreased white blood cell count, and 72.3 % had lymphocytopenia. Lung involvement was observed in all cases. Treatment was based on symptom control and respiratory support. Immunoglobulin G was administered to some critically ill patients while systemic corticosteroids showed no significant benefit. The risk of death was related to age, comorbidities and the period between initial symptoms and dyspnea. |
| Wang et al. [31] | Retrospective study | To describe the Cytokine release syndrome-like (CRSL) that occurs in patients affected by COVID-19 pneumonia and to identify risk factors and possible treatments. | Average age: 58 years old. Gender: men mostly. Extensive pulmonary inflammation and ARDS: 83,3%. Symptoms: fever, hypoxia and shock (28,6%). Laboratory findings: Decrease of CD3, CD4, CD8, NK cells. Increase of IL-6, CD4/CD8 ratio. CRSL: 72, 7%, manifested by: pulmonary inflammation, decrease of CD4, CD8 and NK, increase of IL-6 and dysfunction of non-pulmonary organs. Management: ventilation, anti-inflammatory therapy, mechanical-ventilation |
| Chen et al. [43] | Literature review | To review the literature on the relationship between COVID-19 and the cytokine storm as well as the possible immutherapic treatments available | The effectiveness of some therapies was demonstrated such as the use of stem cells that inhibit the activation of T-lymphocytes, macrophages and induce their differentiation into regulatory subpopulations of T-cells and anti-inflammatory macrophages, but also inhibit the secretion of IL-1α, TNF-α, IL-6, IL-12, and γ-interferon, controlling the cytokine storm. |
| Conti et al. [52] | Literature review | To clarify the relationship between IL-1 and IL-6 pro-inflammatory cytokines and lung inflammation. Anti-inflammatory strategies | Infection with COVID-19 causes a release of IL-1β and IL-6 which will lead to lung inflammation, fever and fibrosis. The therapeutic potential of several cytokines, such as IL-37 and 38, capable of inhibiting molecules such as those previously mentioned, has been demonstrated |
| Mehta et al. [53] | Correspondence | To define the cytokine storm syndrome that occurs in severe COVID-19 states and to identify possible treatments | Cytokine storm syndrome is characterized by increased interleukin (IL)-2, IL-7, IL-6 granulocyte- colony stimulating factor, interferon-γ inducible protein 10, monocyte chemo- attractant protein 1, macrophage inflammatory protein 1-α, and tumour necrosisfactor-α. Immunosuppressors such as tozulimab or anakinra appear to work in states of hyperinflammation as described in severe COVID-19 states |
| Wan et al. [54] | Observational study | To characterize the state of the immune system and the implications of different cytokines in SARS-CoV-2 patients and to study their relationship with the severity of the process | Lower levels of CD4 + T and CD8 + T and higher levels of IL-6 and Il-10 were observed in the more severe NCPs. These markers may help predict the worsening of mild patients. |
| Yang et al. [55] | Observational study | To study the different cytokine profiles in patients infected with SARS-CoV-2 and their relationship to the severity of the disease. | A total of 14 cytokines were shown to be elevated in patients with COVID-19, with particularly high levels of IP-10, MCP-3, and IL-1ra in severe patients. These markers were predictors of the evolution of the disease towards more severe and even fatal states. |
| Liu et al. [56] | Retrospective study | To characterize the cytokine storm that occurs in patients with COVID-19 | Patients with COVID 19 have hypercitokinaemia that manifests itself with an elevation of 38 of the 48 cytokines measured. Patients with lung lesions were observed to have an upregulation of M-CSF, IL-10, IFN-2, IL-17, IL-4, IP-10, IL-7, IL-1ra, G-CSF, IL-12, IFN-γ, IL-1, IL-2, HGF, and PDGF-BB. These biomarkers may be useful as predictors of the severity of the pathology. |
| Qin et al. [57] | Retrospective study | To analyze the expression of different biomarkers, inflammatory cytokines and lymphocyte subsets in patients infected with COVID-19, and to determine their relationship with the severity of the process. | An increase in neutrophil-lymphocyte-ratio and T lymphocytopenia (especially CD4 + T cells) was observed, which was more pronounced in severe patients. Elevated serum levels of TNF-α, IL-1 and IL-6 and IL-8 were also found in severe cases. |
| Zhang et al. [58] | Literature review | To study the use of anti-inflammatory drugs in the therapeutic approach of patients infected with COVID-19 | Treatment with anti-inflammatory drugs may be useful in managing the cytokine storm that develops in critically ill patients. However, possible immunological alterations of the host should be considered before starting therapy and their individual characteristics should also be considered. |
| Kritas et al. [60] | Literature review | To determine the cytokine potential of various cytokines of the IL-1 family as a new strategy in the management of inflammation | IL-37, which inhibits IL-1, may be considered in the treatment of patients with COVID-19 because of its anti-inflammatory activity that would help control fever and inflammation. |
| Chen et al. [64] | Retrospective study | To establish and compare cytokine profiles between moderate and severe stages of COVID-19 in patients from Tongji Hospital. | Severe patients more frequently presented dyspnea, lymphopenia and hypoalbuminemia, with higher levels of alanine aminotransferase, lactate dehydrogenase, C-reactive protein, ferritin and D-dimer, as well as significantly higher levels of IL-2R, IL-6, IL-10 and TNF-α. The numbers of T-lymphocytes, CD4 + T-cells and CD8 + T-cells were decreased in all cases but more markedly in severe patients. Expression of IFN-γ by CD4 + T cells was lower in severe cases |
| Chen et al. [65] | Retrospective study | To establish the clinical features of COVID-19 and to study the relationship between the cytokine storm detected in serum and the severity of the process in patients from Tongji Hospital. | The main symptoms of 2019-nCoV pneumonia were fever with or without respiratory symptoms and other systemic symptoms. Serum white blood cell counts were normal or decreased, lymphocyte count decreased, hs-CRP increased, procalcitonin normal, LDH increased and albumin decreased. High resolution CT showed single or multiple frosted glass shadows accompanied by septal thickening. Significantly higher levels of interleukin-2 receptor (IL-2R) and IL-6 were found in the most severe patients. No differences in serum TNF-α, IL-1, IL-8, IL-10, hs-CRP, lymphocyte count and LDH levels were found between the groups |
| Chu et al. [81] | Ex vivo study | To study replication, cell tropism and the route of immune activation by SARS-CoV-2 in human lung tissues | SARS-CoV-2 infected and replicated in human lung tissues more efficiently than SARS-CoV. In lung tissue, SARS-CoV-2 infected type I and II pneumocytes and alveolar macrophages but did not stimulate production of type I, II, or III interferons and only increased expression of IL6, MCP1, CXCL1, CXCL5, and IP10 |
| Diao et al. [82] | Retrospective study | To analyze figures and markers of T-cell exhaustion in patients with COVID-19 | A marked reduction in the overall T-cell count was observed in patients with COVID-19. Total T-cell, CD8 + T-cell, or CD4 + T-cell counts below 800/μL, 300/μL, or 400/μL are negatively correlated with patient survival and serum levels of IL-6, IL-10, and TNF-α. In addition, it was observed that PD-1 (a marker of T-cell exhaustion) is higher in patients with COVID 19 and that its expression is related to the severity of the process. |
| Dong et al. [83] | Retrospective study | Study the possible vertical transmission of the SARS-CoV-2 | A newborn presented IgM antibody to SARS-CoV-2 as well as cytokine alterations although he was negative for RT-PCRs |
| Pedersen & Ho [86] | Literature review | To describe the cytokine storm that occurs in severe SARS-Cov-2 infected patients | Increased levels of IL-6, IL-10 and TNF-α, lymphopenia (in CD4+ and CD8 + T cells) and decreased expression of IFN-γ in CD4 + T cells in severe patients were evidenced. |
| Ruan et al. [87] | Retrospective study | To establish predictors of mortality in patients infected with COVID-19 | Predictors of mortality in patients infected with SARS-Cov-2 include age, presence of comorbidities, presence of secondary infection, and elevated inflammatory markers in the blood such as lymphocytes, platelets, albumin, total bilirubin, blood urea nitrogen, blood creatinine, myoglobin, cardiac troponin, C-reactive protein (CRP), and interleukin-6 |
| Sun et al. [88] | Retrospective study | To describe epidemiological and clinical features, imaging and laboratory data, clinical treatments and outcomes of severely or critically ill pediatric patients infected with COVID-19 in Wuhan. | Most of the subjects were males between the ages of 2 months and 15 years. The main manifestations included polypnea, fever and cough. Patch-like shadows and ground glass opacity were observed in most patients on chest CT scans. The analyses showed an increase in C-reactive protein, procalcitonin and lactate dehydrogenase, CD3, CD4, CD8, IL-6, IL-10 and IFN-γ and a decrease in CD16 + CD56 and Th/Ts. Treatment was based on symptom control and respiratory support. Two subjects required invasive mechanical ventilation. |
| Wu et al. [90] | Retrospective cohort study | To describe the clinical features and outcomes in patients with ARDS or who died from COVID-19 Pneumonia | Average age: 51 years old. Gender: men mostly. Background: Hypertension and diabetes. ARDS: 41.8 % manifested by dyspnea. Death: 52.4 %. Risk factors for ARDS and death: age, neutrophilia, organ dysfunction and coagulation, high fever (for ARDS). Treatment: Among patients with ARDS, methylprednisolone |
| Chen et al. [91] | Retrospective study | To analyze the relationships between the incidence of RNAaemia and the cytokine storm as well as the severity of the disease | There seems to be a relationship between RNAaemia and the severity of the patient's condition, as well as with IL-6 levels, which is particularly high in this group of subjects and may act as a therapeutic target for the management of critically ill patients. |
| Zhou et al. [92] | Retrospective multicenter study | To describe risk factors for mortality anD clinical course of illness in patients infected with COVID-19 in Wuhan. | Survival: 137/191 subjects. Background: hypertension, diabetes and coronary heart disease. Mortality risk factors: advanced age, high scores on the Sequential Assessment of Organ Failure scale and d-dimer greater than 1 μg/mL on admission. Time of virus excretion: 20 days in survivors although in the deceased it was detectable until death. |
| Zhou et al. [93] | Retrospective study | To analyze blood samples from patients with SARS-CoV-2 severe pneumonia in order to identify their immune characteristics. | COVID-19 disease leads the activation of CD4+ T cells and generate GM-CSF, among others. The infection generates the secretion of several cytokines that induce inflammatory CD14+ and CD16+ monocytes with the consequent increase of IL-6 expression and the acceleration of inflammatory process. |
| Akhmerov & Marban [94] | Literature review | To describe the impact of COVID-19 at the cardiovascular level | The mechanisms linking cardiac involvement and SARS-CoV-2 appear to be related to respiratory failure and hypoxemia, direct myocardial infection by the virus, indirect injury by the systemic inflammatory response, or a combination of all three. COVID-19 has been associated with myocarditis, blood pressure abnormalities and arrhythmias, acute coronary syndromes and acute myocardial infarction. |
| Saghazadeh & Rezaei [131] | Literature review | To describe the epidemiological and immunological features of COVID-19 | COVID-19 mainly affects men. One of the main risk factors for infection and death is old age. 30 % of patients affected by COVID-19 had previous pathologies such as cardiovascular disease, diabetes, chronic respiratory disease, hypertension, and cancer. All these factors condition the transmission of the virus. The first and last alteration caused by SARS-CoV-2 is a lung lesion accompanied by the pro-inflammatory cytokine storm. |
| Rio & Malani [169] | Viewpoint | To describe the most relevant features of COVID-19 | R0: 2.68. Compared to SARS-CoV and MERS, SARS-CoV-2 has a higher infectivity and lower-case fatality rate. Incubation period: between 5.2 days to 14 days. Clinical features: fever, dry cough, difficulty in breathing. Other symptoms are myalgia, headache, sore throat and diarrhoea. Average age: 49–56 years. Severity: most of the cases are mild, all patients admitted to the hospital have pneumonia with infiltrates on chest X-ray and ground glass opacities on chest CT scan. Evolution: ARDS and ICU admission. Treatment: symptomatic and respiratory support |
| Lin et al. [185] | Literature review | To clarify the pathogenesis of SARS-CoV-2 and compare it with other infections caused by coronavirus | The virus enters the body through the nasal and pharyngeal mucous membranes and reaches the lung parenchyma where it can be incorporated into the circulation causing viremia. Once in the bloodstream it can affect organs that express ACE2. The period from the first symptoms to ARDS is approximately 8 days. At this point a second, much more aggressive stage of the infection begins. In infected patients, lymphopenia and an increase in pro-inflammatory cytokines are detected. |
| Xiong et al. [186] | Retrospective study | To study transcriptional changes in bronchoalveolar lavage fluid (BALF) and peripheral blood mononuclear cells (PBMC) specimens from COVID-19 patients | An association was observed between the pathogenesis of COVID-19 and the excessive release of cytokines such as CCL2/MCP-1, CXCL10/IP-10, CCL3/MIP-1A and CCL4/MIP1B. In addition, it was postulated that the ability of the virus to activate apoptosis and the P53 signaling pathway in lymphocytes may be the cause of lymphopenia in patients. |
2.1. IL-1
IL-1 actively participates in the inflammatory response to infection [39], and its main sources are activated monocytes and macrophages [44]. SARS-CoV-2 appears to act on the activation and maturation of IL-1β, which in turn activates other proinflammatory cytokines, such as IL-6 and TNF-α [[45], [46], [47]]. Hence, IL-1β forms part of the cytokine storm produced by coronavirus infections [[48], [49], [50], [51], [52], [53], [54]]. Yang et al. detected elevated levels of the antagonistic receptor of IL-1 (IL-1Ra) in 14 severe cases of COVID-19, and this marker has been associated with increased viral load, loss of pulmonary function, lung damage, and mortality risk [55]. Liu et al. also found elevated IL-1α levels in patients with severe COVID-19, and these were strongly associated with lung injury [56]. IL-1 levels are related to the virulence of the process, and significantly higher serum levels have been observed in SARS-CoV-2 cases with severe symptoms than in mild cases or in those infected with the 2003 SARS-CoV or 2012 MERS coronavirus [57]. Most COVID-19 patients with severe symptoms have elevated levels ofIL-1β, which has been associated with SARS, hypercoagulation, and disseminated intravascular coagulation [58]. For this reason, some therapeutic strategies have used the inhibition of IL-1 in an attempt to avoid the cytokine storm [59,60]. In this way, mesenchymal stem cells (MSCs) have been used to inhibit proinflammatory cytokines such as IL-1α and TNF-α [43].
2.2. IL-2
IL-2 plays a key role in the proliferation of T cells and in the generation of effector and memory T cells [61]. It is involved in adaptive immunity and increases glucose metabolism to promote the proliferation and activation of T, B, and NK cells [39]. Hence, IL-2 participates in the prevention of autoimmune diseases and is essential to control immune responses and maintain self-tolerance [62]. The absence of this interleukin has been associated with a poor control of effector cells and the consequent development of autoimmunity [63].
Huang et al. detected elevated levels of IL-2 or its receptor IL-2R in patients with COVID-19, and it has been reported that these increases are directly proportional to the severity of the disease [17,30,43,[52], [53], [54],56,57,64,65]. Elevation of this interleukin and its association with disease severity have also been reported in patients with other types of coronavirus [[66], [67], [68]].
2.3. IL-4
IL-4 is also involved in adaptive immunity, playing a crucial role in the immune regulation governed by activated T helper (Th) cells. It preferentially acts through activation, proliferation, and differentiation of B lymphocytes and promotion of the immunoglobulin E isotype. It therefore decisively intervenes in the induction of humoral immunity-regulating Th2 cells [39,69].
It has been proposed that IL-4 has an anti-inflammatory function that is specific to the tissue in which it is present, reflecting the metabolic plasticity of different tissues [70]. With respect to viral infections that target the respiratory system, Bot et al. observed that its expression during infection with an influenza virus had negative effects on CD8+ memory T cells [71].Various studies of COVID-19 patients have detected elevated IL-4 levels as part of the cytokine storm associated with severe respiratory symptoms [16,17,43,72].
2.4. L-6
IL-6 is involved in inflammation, the immune response, and hematopoiesis [73]. This pleiotropic biomolecule is secreted by multiple cell types and regulates a wide variety of physiological processes [74]. During initial stages of inflammation, secreted IL-6 travels to the liver and induces a large number of acute-phase proteins, including C-reactive protein (CRP), serum amyloid A (SAA), fibrinogen, haptoglobin, and α1-antitrypsin. In addition, IL-6 has been found to diminish the production of fibronectin, albumin, and transferrin [75]. It has been reported that it has regenerative and anti-inflammatory effects mediated by the conventional signaling process, although it also exerts proinflammatory effects mediated by trans-signaling, as in the case of viral infections [76].
In relation to the different types of coronavirus, elevated IL-6 levels have been observed in SARS cases and related to the severity of symptoms [48,77,78] and in SARS-CoV, being implicated in possible T-cell dysfunctionality. It has been observed that SARS-CoV-induced cytokines may damage the capacity of T cells in relation to dendritic cells, compromising the viability of these cells and macrophages to eliminate the pathogen [49,79]. Similar observations have been made in relation to MERS [50,80].
Elevated IL-6 levels have been found in patients with COVID-19 and related to a poor prognosis [16,31,53,65,[81], [82], [83], [84], [85], [86], [87], [88], [89], [90]]. Wan et al. detected elevated IL-6 levels in one-third of patients with mild symptoms and three-quarters of those with severe symptoms, concluding that IL-6, alongside IL-10, may be of prognostic value in patients with COVID-19 [54]. Among patients admitted to ICU, Diao et al. found an inverse proportional association between elevated IL-6 levels and T cell counts [82], and another study of patients with severe symptoms described elevated serum levels of IL-6 and CRP [91]. A study of 452 patients infected with SARS-CoV-2 also reported that the elevation of IL-6 levels was more marked with more severe symptoms [57]. These levels have been higher than those observed in patients with SARS-CoV or MERS [77,80]. IL-6 levels were also found to be markedly higher in patients who died from COVID-19 than in those who recovered [92]. As noted above, activation of IL-1β by SARS-CoV-2 in turn activates IL-6 and TNF-α [[45], [46], [47]]. It has also been demonstrated that a high expression of IL-6 in patients with COVID-19 can accelerate the inflammatory process, contributing to the cytokine storm and worsening the prognosis [93]. The cytokine storm, including elevated levels of IL-6, has also been associated with cardiac damage in these patients [94].
With regard to the important role of IL-6 in the SARS-CoV-2-induced cytokine storm and its evasion, it has been reported that the monoclonal antibody tocilizumab acts by blocking the receptor of IL-6 and has been reported to reverse cytokine hyperproduction [84,[95], [96], [97]], inflammation, and pulmonary fibrosis [98,99]. In fact, a multicenter clinical trial is under way in China on the usefulness of tocilizumab to treat patients with COVID-19 (NCT04252664 & NCT04257656), and it has already been included in clinical practice guidelines in China and Italy [100,101]. The polyol myo-inositol was also described by Bizzarri et al. as a potential candidate drug to reduce IL-6 levels and the risk of cytokine storm [102]. In addition, the capacity of the macrolide azithromycin in combination with hydroxychloroquine, to reduce nasopharyngeal levels of SARS-CoV-2 has been attributed to its capacity to block IL-6 and TNF-α [103,104]. Chloroquine also has the capacity to inhibit IL-6 and TNF-α, and is being tested against COVID-19 [104,105]. Finally, blood purification therapy has been proposed to eliminate pathological antibodies and IL-6, among other cytokines, given its successful application against other diseases [85].
2.5. IL-7
IL-7 plays an important role in lymphocyte differentiation, participating in the development of T cells and peripheral homeostasis [106]. All of the main CD4 T cell subgroups (CD4+ immature, memory, and Th17 cells) depend on this cytokine for peripheral homeostasis [[107], [108], [109], [110]]. IL-7 activates T cells, increases the production of proinflammatory cytokines, and negatively regulates transforming growth factor beta (TGF-β). The role of this biomolecule depends on IL-6 [111], and it has been suggested that IL-7 secretion can be induced by viral infections [112].
As in the case of other cytokines, it has been reported that IL-7 levels are elevated in patients with COVID-19 and directly related to disease severity [17,53,54].
2.6. IL-10
IL-10 is a type 2 cytokine that inhibits the production of proinflammatory cytokines (e.g., IFNγ, TNFα, IL-1β, and IL-6) in various cell types and prevents dendritic cell maturation by blocking IL-12. It hampers expression of the major histocompatibility complex and costimulatory molecules, which have an important role in cell immunity. However, IL-10 can have immunostimulatory effects, including the stimulation of IFNγ production by CD8+ T cells. It is also a powerful factor for the growth and differentiation of B cells, mast cells, and thymocytes [[113], [114], [115], [116], [117], [118], [119]]. Numerous authors have described viruses (Epstein Barr virus, cytomegalovirus and herpesvirus) that contain IL-10 homologs, which may contribute to the presentation of different binding profiles to receptors and biological activities that can increase viral resistance [72,114,[120], [121], [122], [123], [124], [125]].
However, IL-10 regulation is decreased in infection with some viruses, such as HIV, contributing to T-cell depletion [[126], [127], [128]]. Animal studies have shown that the antibody-mediated inhibition of IL-10 signaling improves the T-cell response and contributes to eliminating viral resistance. Hence, the effective blocking of IL-10 signaling may be useful against resistant viral infections [129,130].
Various authors have detected this interleukin in patients with COVID-19 and related its levels to disease severity and progression, as in the case of other cytokines [17,43,57,64,82,83,86,88,89] and it has been reported to have possible prognostic value [54]. In fact, some authors indicate that IL-10 may be hyper-expressed in anti-SARS-CoV-2 immunity, being higher in patients of advanced age with respect to a “hyperinflammatory response”, possibly related to the reduction of T-cell receptors in the elderly [131]. As with other cytokines, IL-10 levels were found to be higher in patients with COVID-19 than in those with SARS-CoV or MERS [17].
2.7. IL-12
IL-12 is one of a group of heterodimeric biomolecules with distinctive characteristics, including pairing versality (also observed for IL-23, IL-27, and IL-35), which are involved in molecular processes and functions with a crucial role in positive and negative feedback [132,133]. IL is a proinflammatory/prostimulatory cytokine produced in response to microbial agents by dendritic cells, B cells, monocytes, and macrophages, among others. It has key functions in the development of Th1 and Th17 cells [[134], [135], [136], [137]]. IL-12 also induces the production of IFN-γ by T and NK cells in a positive feedback mechanism [138].
The action of this cytokine in viral infections is based on its direct chemotactic effects on the infiltration of NK cells, increasing their binding to vascular endothelial cells. NK cells secrete IFN-γ, which participates in positive feedback by increasing the production of IL-12 [139,140]. Viral infections rapidly induce the gene expression of IL-12, which also acts after viral replication [[141], [142], [143]]. For instance, IL-12 is endogenously induced during the pneumonia produced by influenza and activates NK cells, which secrete IFN-γ and thereby inhibit viral replication. IL-12 has been found to produce some improvement in the response of CD8 + T cells [140].
Elevated serum IL-12 levels have been observed in patients infected with SARS-CoV-2, [16,17,43] and in those infected with other coronaviruses such as SARS-CoV [77].
MSCs inhibit the secretion of IL-12, as well as IFN-γ and TNF-α, and have been proposed as an effective therapy against COVID-19 [144,145].
2.8. IL-13
IL-13 is secreted by activated Th2 cells, constituting a counter-regulatory system for the Th1-type immune response. It is considered an important regulator of immune responses mediated by Th2-type cytokines [146]. IL-13 has varied functions and has been implicated in the development of bronchial asthma by inducing the production of TGF-β, eotaxin-3, and mucin [[147], [148], [149], [150]]. It also participates in the activation of mast cells (de Vries JE, 1996). Both IL-13 and IL-4 are involved in allergic processes, asthma, and the regulation of Th2 lymphocytes [151]. IL-13 mediates tissue responses to infections, including the mobilization of eosinophils and the expulsion of parasites [152].
Few data are available on the presence of IL-13 in patients with COVID-19. Huang et al. found no difference in serum IL-13 levels between those requiring ICU admission and those who did not [17]. However, Liu et al. observed a directly proportional association between IL-13 levels and the viral load of SARS-CoV-2 [56].
2.9. IL-17
IL-17 is synthetized by Th17 lymphocytes and is elevated in inflammatory processes and autoimmune diseases [[153], [154], [155], [156]]. It is also produced by CD8+ cells and by various sets of immature lymphocytes, including gamma-delta T cells, NK cells, and group 3 innate lymphoid cells [157]. IL-17, alongside IL-22 and TNF-α, also induces the production of antimicrobial peptides [158]. Hence, IL-17 is a proinflammatory cytokine that plays a role in tissue damage, physiological stress, and infection. These functions vary according to the tissue in which IL-17 is expressed, being particularly important in the gastrointestinal tract and skin [159,160].
Elevated IL-17 levels have been reported in patients with SARS-CoV-2 as part of the cytokine storm [17], and they have been associated with the viral load and disease severity [56]. In contrast, Wan et al. found that IL-17 levels were normal in patients with COVID-19, with no significant differences between patients with severe versus mild symptoms [54]. Elevated IL-17 levels were previously described in patients with SARS-CoV or MERS [161,162]. The fact that Th17 cells can produce IL-17, among others, has led to proposals for a therapeutic approach to COVID-19 focused on Janus kinase 2 (JAK2) inhibitor named Fedratinib. This JAK2 inhibitor decreases IL-17 expression by Th17 cells in murine models [163].
2.10. M-CSF
M-CSF, also known as colony-stimulating factor-1, is a primary growth factor that belongs to the family of colony-stimulating factors [164]. It regulates the growth, proliferation, and differentiation of hematopoietic cells, including monoblasts, promonocytes, monocytes, macrophages, and osteoclasts. It is secreted by various cell types, including monocytes, fibroblasts, osteoblasts, stromal cells, endothelial cells, and tumor cells. The actions of M-CSF are mediated by a type III tyrosine-kinase receptor. It requires the synergic action of IL-1 and IL-3 during the early differentiation of myeloid line cells, while at subsequent stages it can directly control the proliferation and differentiation of mononuclear cells of the phagocytic system [165]. M-CSF expression increases during infectious processes, augmenting the production of myeloid cells [166].
Liu et al. found significantly elevated levels of this factor in patients with COVID-19 and associated the hyperexpression of this and other cytokines with lung damage, which may assist in predicting disease severity [56].
2.11. G-CSF
G-CSF is essential for the proliferation and maturation of polymorphonuclear granulocyte cells (PMNs), preparing the organism for defense against invasion by certain pathogens. The immunological functions of PMNs in infections include chemotaxis, phagocytosis, and the release of lysosomal enzymes and other signaling molecules. Thus, G-CSF has hematopoietic growth factor properties and simultaneously functions as a mediator of anti-infectious and anti-inflammatory responses [167]. It is the main determinant of the number of circulating neutrophils and is a key mediator in physiological responses that require the action of these cells. Increased levels of endogenous G-CSF have been reported in infectious and traumatic processes and in patients with neutropenia or fever with/without neutropenia. G-CSF levels have also been correlated with bacteremia by Gram-negative organisms [168].
Huang et al. found that G-CSF levels were elevated in patients with COVID-19 and even higher in those requiring ICU admission [17]. This could lead the multiorgan failure related to severe cases [90,169]. Wu D and Yang XO (2020) associated G-CSF levels with the response of Th17 lymphocytes in patients with SARS-CoV-2, finding that the IL-17 produced by these cells can induce the production of G-CSF, among others. They also reported that Th17 contributes to the cytokine storm triggered by SARS-CoV-2 [163]. Liu et al. directly related G-CSF levels to the viral load of SARS-CoV-2 and the associated lung damage [56].
2.12. GM-CSF
GM-CSF is a heterodimeric complex that consists of a specific alpha chain of GM-CSF and a signal transduction subunit shared with IL-3 and IL-5 receptors. It is a single-membrane protein that homodimerizes after binding to G-CSF [[170], [171], [172]]. The main sources of this biomolecule are fibroblasts and endothelial, epithelial, stromal, and hematopoietic cells [164], and it is secreted by epithelial cells in the lung [173,174]. Unlike M-CSF and G-CSF, GM-CSF is virtually undetectable in the blood, although it has been found to be locally produced and activated in tissues affected by inflammatory processes. Serum GM-CSF levels can also be elevated in response to endotoxins. Hence, GM-CSF plays an important role in the inflammatory process [[175], [176], [177]], stimulating the proliferation and activation of macrophages, eosinophils, neutrophils, monocytes, dendritic cells, and microglial cells. Besides its role as a hematopoietic growth factor, this cytokine increases the production of proinflammatory cytokines, favors antigenic presentation and phagocytosis, and promotes chemotaxis and leukocyte adhesion. It is of major importance in maintaining immune homeostasis in lung and gut [178].
Elevated serum GM-CSF levels have been detected during the acute phase of infection by SARS-CoV-2 in comparison to healthy individuals, both in those who required ICU admission and those who did not [17]. Th17 cells produce GM-CSF, and high Th17 counts have been associated with elevated GM-CSF levels [163]. It has also been observed that the activation of Th1 cells by pathogens generate GM-CSF, among other cytokines [93].
2.13. IP-10
IP-10 was initially identified as a chemokine whose secretion is induced by IFN-γ. IP-10 is secreted by neutrophils, endothelial cells, keratinocytes, fibroblasts, dendritic cells, astrocytes, and hepatocytes. Through its binding to chemokine receptor 3 (CXCR3), it regulates immune system responses by activating and recruiting leukocytes, including T cells, monocytes, and NK cells. Therefore, IP-10 and CXCR3 play a key role in recruiting leukocytes to inflamed tissues and in perpetuating inflammation, thereby making a major contribution to tissue damage [179]. Elevated IP-10 concentrations have been found in numerous infections, mainly viral infections [180].
Serum IP-10 levels were found to be elevated in patients with COVID-19 and even higher in those who required ICU admission, suggesting their relationship with lung damage and disease severity [17]. High levels of this biomolecule were previously found in patients with SARS-CoV [77], and the levels are even higher in those with SARS-CoV-2 [81]. Thus, the hyperproduction of IP-10, among others, is considered to contribute to disease progression [17]. Liu et al. associated elevated serum IP-10 levels with a higher viral load and greater lung damage in patients with SARS-CoV-2 [56]. Yang et al. also found especially high levels of this cytokine in the most severe cases of COVID-19, and they associated IP-10 expression with disease progression and mortality, alongside the expressions of monocyte chemotactic protein-3 (MCP-3) and IL-1Ra [55].
2.14. IFN-γ
IFN-γ is a type-II IFN produced by a wide variety of lymphocyte cells, including CD4+ and CD8+ T cells, Treg cells, FoxP3+ CD8− T cells, B cells, and NK cells. Monocytes, macrophages, dendritic cells, and neutrophil granulocytes can also produce this cytokine. Although numerous cells can be the source of IFN-γ, it is mainly produced by T and NK cells. MSCs can also secrete low IFN-γ levels to regulate hematopoiesis [181]. IFN-γ participates in numerous immune and adaptive immunological functions and in inflammatory processes. It promotes macrophage activation and antigen presentation and is highly involved in anti-bacteria and anti-virus immunity and in signal transduction. It is difficult to classify IFN-γ as a pro- or anti-inflammatory cytokine, given its complex and varied roles [182].
Huang et al. found that serum IFN-γ levels were higher in patients with COVID-19 than in healthy individuals and proposed that the elevation of this and other cytokines might result from the activation of Th1 and Th2 cells. Also, elevated serum IFN-γ levels were previously reported in patients with SARS-CoV or MERS [17,77,183]. Liu et al. (2020) observed that elevated IFN-γ levels were associated with greater viral load and lung damage [56]. Sun et al. found that IFN-γ, IL-6, and IL-10 levels were higher in patients with infection by SARS-CoV-2 but did not differ between patients who required ICU admission and those who did not [88]. In fact, these authors found that levels of this cytokine were lower in CD4+ T-cells from patients with severe versus mild symptoms and suggested that the infection may initially affect CD4+ and CD8+ T-cells, reducing the production of IFN-γ [64].
2.15. MCP-1
This protein belongs the C-C chemokine family and is a powerful monocyte chemotactic factor that is constitutively produced or induced by oxidative stress, cytokines, or growth factors. It can be expressed by endothelial cells, fibroblasts, epithelial cells, smooth muscle cells, mesangial cells, astrocytes, monocytes, and microglial cells, which play an important role in the antiviral response in the peripheral circulation and tissues. Monocytes and macrophages are the main source of MCP-1, which regulates the migration and infiltration of monocytes, memory T cells, and NK cells [184].
Huang et al. found that MCP-1 levels were higher in patients with COVID-19 and even higher among those admitted to ICU [17]. It has been reported that MCP-1 increases rapidly in the early acute phase of infection and then progressively decreases with the advance of the disease [185]. Xiong et al. detected elevated levels of MCP-1 and other cytokines in the bronchoalveolar lavage fluid of patients with COVID-19 and associated the pathogeny of the virus with these cytokines [186]. Elevated levels of this protein have also been detected in the lung tissue of patients infected with SARS-CoV-2 [81].
2.16. TNF-α
TNF-α is produced by various cell types, such as monocytes, macrophages, and T cells, among others. This cytokine has been related to proinflammatory responses mediated by IL-1β and IL-6. Alongside other cytokines, TNF-α is involved in the regulation of inflammatory processes, infectious diseases, and malignant tumors [187].
It has been observed that serum TNF-α levels are elevated in patients with COVID-19 and are higher with more severe disease [17,57,64]. Diao et al. reported similar results in a sample of 522 patients with COVID-19 and found an inverse relationship between TNF-α levels and T-cell counts [82]. In contrast, Wan et al. described normal TNF-α levels in patients with COVID-19 [54]. TNF-α was one of the cytokines whose overproduction was related to a poor prognosis in patients with SARS-CoV and MERS [[48], [49], [50],183]. Zhang et al. proposed that the administration of certolizumab, an anti-TNF-α antibody, might have beneficial effects on patients with COVID-19 [188]. Another possible therapeutic approach is to use MSCs to inhibit TNF-α and IL-1α, among other cytokines [145].
2.17. SARS-CoV-2 and other biomolecules
Other biomolecules besides cytokines have shown increased expression in patients with COVID-19, including growth factors.
2.18. VEGF
VEGF is essential for vascular endothelial homeostasis and is present in numerous cells and tissues. It has also been found to participate in the pathogenesis of tumor growth and metastasis. VEGF plays an essential role in endothelial cell activation by binding to cell surface receptors (VEGFR). The integrity of the endothelial barrier in lung tissue is crucial for alveolar immune regulation. Severe inflammation and the associated immune responses induce the apoptosis of epithelial and endothelial cells, increasing the production of VEGF and exacerbating edema and the extravasation of immune cells [188]. The pathogenic effects of this factor are related to its action on vascular permeability and neoangiogenesis [189,190]. VEGF hyper-regulation is observed in various viral infections, and attempts have been made to stimulate this factor by using VEGF homologs present in some viruses or by activating inflammatory mediators that trigger the overexpression of VEGF [189].
Serum VEGF levels were found to be higher in patients with SARS-CoV-2, although they did not differ between those who were admitted to ICU and those who were not [17]. As noted above, the therapeutic application of MSCs is under consideration due to their regenerative and immunomodulatory potential. Besides VEGF, MSCs can also secrete VEGF, among others, which would be useful in the approach to respiratory distress syndrome and in the regeneration of lung tissue and treatment of lung fibrosis induced by infections [145].
2.19. HGF
Hepatocyte growth factor (HGF) is synthesized by fibroblasts and by endothelial and hepatic cells, among other cell populations. The active form is produced by the action of a serine protease enzyme released by damaged tissues. In the lungs, HGF is sequested by pulmonary fibroblasts, and its secretion is increased when the tissue is damaged [191].
Serum HGF and MIP 1-α levels were found to be elevated in patients with COVID-19, and even higher MIP 1-α levels were found in those requiring ICU admission [17].
3. Conclusions
The immunological reaction triggered by infection with SARS-CoV-2 mobilizes numerous cytokines, mainly of proinflammatory character. Changes in their levels are associated with the presence of the disease and a more severe prognosis. This study summarizes findings on the role of these cytokines in the onset and outcomes of SARS-CoV-2 infection and on possible therapeutic approaches involving the inhibition of their activity.
Declaration of Competing Interest
The authors declare no competing or financial interests.
Acknowledgements
This study was supported by research group BIO277 (Junta de Andalucía) and the Department of Nursing of the University of Granada.
Biographies

Victor J. Costela Ruiz recieved his Ph.D in Clinical Medicine and Public Health from the University of Granada, Spain. He is currently working at the university research group BIO277 in several issues related to cells activity as well as the role of certain biomolecules involved in cells metabolism, such as cytokines and growth factors. He also works in the clinical field as nurse practitioner in the Andalusian Health System.

Rebeca Illescas-Montes completed her Ph.D in Clinical Medicine and Public Health in 2018. She is Assistant Professor at the University of Granada, Spain. She is also a researcher member of the Biomedical group BIO277. Her multidisciplinary research experience lie in life sciences, cell biology and signaling, tissue regeneration and immunology.

Jose M. Puerta Puerta, M.D. Ph.D, obtained his medical degree from Granada University, Spain in 2006 and a PhD degree in Clinical Medicine in 2017 from the same University. He is Hematologist and Coordinator of the Hematology and Hemotherapy Outpatients Hospital in Granada, Spain. He is also Coordinator of the Andalusian Group of Chronic Myeloid Leukemia as well as member of the Spanish Society of Hematology and Hemotherapy. His clinical work and research field is focus on myeloproliferative and lymphoproliferative tumors, high grade lymphomas and quality of life of hematological patients.

Concepción Ruiz is a professor of the Faculty of Health Sciences (University of Granada). She got her Ph.D degree in Pharmacy in 1983 (University of Granada). She is the leader of the Biomedical group (BIO277). His research experience is multidisciplinary in microbiology, immunology and molecular, biochemical and cellular aspects of different cell populations related to growth factors, cytokines, extracellular matrix proteins in tissues under repair.

Lucía Melguizo Rodríguez, Ph.D. Graduated with PhD degree in Clinical Medicine and Public Health from the University of Granada. She is an active member of the Biomedical research group BIO277. Currently, she is an assistant professor at the Faculty of Nursing at the University of Granada. Her research focuses on biochemical and cellular aspects of different cell populations, phytochemicals, tissue regeneration and wound healing.
References
- 1.Chen Y., Liu Q., Guo D. Emerging coronaviruses: genome structure, replication, and pathogenesis. J. Med. Virol. 2020;92:418–423. doi: 10.1002/jmv.25681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R., Niu P., Zhan F., Ma X., Wang D., Xu W., Wu G., Gao G.F., Tan W. China novel coronavirus investigating and research team, a novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guo Y.-R., Cao Q.-D., Hong Z.-S., Tan Y.-Y., Chen S.-D., Jin H.-J., Tan K.-S., Wang D.-Y., Yan Y. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak - an update on the status. Mil. Med. Res. 2020;7:11. doi: 10.1186/s40779-020-00240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sun P., Lu X., Xu C., Sun W., Pan B. Understanding of COVID-19 based on current evidence. J. Med. Virol. 2020 doi: 10.1002/jmv.25722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ji W., Wang W., Zhao X., Zai J., Li X. Cross-species transmission of the newly identified coronavirus 2019-nCoV. J. Med. Virol. 2020;92:433–440. doi: 10.1002/jmv.25682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benvenuto D., Giovanetti M., Ciccozzi A., Spoto S., Angeletti S., Ciccozzi M. The 2019-new coronavirus epidemic: evidence for virus evolution. J. Med. Virol. 2020;92:455–459. doi: 10.1002/jmv.25688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chan J.F.-W., Kok K.-H., Zhu Z., Chu H., To K.K.-W., Yuan S., Yuen K.-Y. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg. Microbes Infect. 2020;9:221–236. doi: 10.1080/22221751.2020.1719902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hui D.S., Azhar E.I., Madani T.A., Ntoumi F., Kock R., Dar O., Ippolito G., Mchugh T.D., Memish Z.A., Drosten C., Zumla A., Petersen E. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health - the latest 2019 novel coronavirus outbreak in Wuhan, China. Int. J. Infect. Dis. 2020;91:264–266. doi: 10.1016/j.ijid.2020.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou P., Yang X.-L., Wang X.-G., Hu B., Zhang L., Zhang W., Si H.-R., Zhu Y., Li B., Huang C.-L., Chen H.-D., Chen J., Luo Y., Guo H., Jiang R.-D., Liu M.-Q., Chen Y., Shen X.-R., Wang X., Zheng X.-S., Zhao K., Chen Q.-J., Deng F., Liu L.-L., Yan B., Zhan F.-X., Wang Y.-Y., Xiao G.-F., Shi Z.-L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu Y., Gayle A.A., Wilder-Smith A., Rocklöv J. The reproductive number of COVID-19 is higher compared to SARS coronavirus. J. Travel Med. 2020;27 doi: 10.1093/jtm/taaa021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Riou J., Althaus C.L. Pattern of early human-to-human transmission of Wuhan 2019 novel coronavirus (2019-nCoV), December 2019 to January 2020. Eurosurveillance. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.4.2000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guan W.-J., Ni Z.-Y., Hu Y., Liang W.-H., Ou C.-Q., He J.-X., Liu L., Shan H., Lei C.-L., Hui D.S.C., Du B., Li L.-J., Zeng G., Yuen K.-Y., Chen R.-C., Tang C.-L., Wang T., Chen P.-Y., Xiang J., Li S.-Y., Wang J.-L., Liang Z.-J., Peng Y.-X., Wei L., Liu Y., Hu Y.-H., Peng P., Wang J.-M., Liu J.-Y., Chen Z., Li G., Zheng Z.-J., Qiu S.-Q., Luo J., Ye C.-J., Zhu S.-Y., Zhong N.-S. China Medical Treatment Expert Group for Covid-19, Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Velavan T.P., Meyer C.G. The COVID-19 epidemic. Trop. Med. Int. Health. 2020;25:278–280. doi: 10.1111/tmi.13383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang W., Tang J., Wei F. Updated understanding of the outbreak of 2019 novel coronavirus (2019-nCoV) in Wuhan, China. J. Med. Virol. 2020;92:441–447. doi: 10.1002/jmv.25689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carlos W.G., Dela Cruz C.S., Cao B., Pasnick S., Jamil S. Novel Wuhan (2019-nCoV) coronavirus. Am. J. Respir. Crit. Care Med. 2020;201:P7–P8. doi: 10.1164/rccm.2014P7. [DOI] [PubMed] [Google Scholar]
- 16.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., Qiu Y., Wang J., Liu Y., Wei Y., Xia J., Yu T., Zhang X., Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ren L.-L., Wang Y.-M., Wu Z.-Q., Xiang Z.-C., Guo L., Xu T., Jiang Y.-Z., Xiong Y., Li Y.-J., Li X.-W., Li H., Fan G.-H., Gu X.-Y., Xiao Y., Gao H., Xu J.-Y., Yang F., Wang X.-M., Wu C., Chen L., Liu Y.-W., Liu B., Yang J., Wang X.-R., Dong J., Li L., Huang C.-L., Zhao J.-P., Hu Y., Cheng Z.-S., Liu L.-L., Qian Z.-H., Qin C., Jin Q., Cao B., Wang J.-W. Identification of a novel coronavirus causing severe pneumonia in human: a descriptive study. Chin. Med. J. 2020 doi: 10.1097/CM9.0000000000000722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., Wang B., Xiang H., Cheng Z., Xiong Y., Zhao Y., Li Y., Wang X., Peng Z. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020 doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lei J., Li J., Li X., Qi X. CT imaging of the 2019 novel coronavirus (2019-nCoV) pneumonia. Radiology. 2020;295:18. doi: 10.1148/radiol.2020200236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.2020. Novel Coronavirus (2019-nCoV) Situation Reports.https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports (n.d.). (Accessed 4 April 2020) [Google Scholar]
- 22.Chen L., Xiong J., Bao L., Shi Y. Convalescent plasma as a potential therapy for COVID-19. Lancet Infect. Dis. 2020;20:398–400. doi: 10.1016/S1473-3099(20)30141-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li H., Wang Y.M., Xu J.Y., Cao B. [Potential antiviral therapeutics for 2019 Novel Coronavirus] Zhonghua Jie He He Hu Xi Za Zhi. 2020;43:E002. doi: 10.3760/cma.j.issn.1001-0939.2020.0002. [DOI] [PubMed] [Google Scholar]
- 24.Agostini M.L., Andres E.L., Sims A.C., Graham R.L., Sheahan T.P., Lu X., Smith E.C., Case J.B., Feng J.Y., Jordan R., Ray A.S., Cihlar T., Siegel D., Mackman R.L., Clarke M.O., Baric R.S., Denison M.R. Coronavirus susceptibility to the antiviral remdesivir (GS-5734) is mediated by the viral polymerase and the proofreading exoribonuclease. MBio. 2018;9 doi: 10.1128/mBio.00221-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holshue M.L., DeBolt C., Lindquist S., Lofy K.H., Wiesman J., Bruce H., Spitters C., Ericson K., Wilkerson S., Tural A., Diaz G., Cohn A., Fox L., Patel A., Gerber S.I., Kim L., Tong S., Lu X., Lindstrom S., Pallansch M.A., Weldon W.C., Biggs H.M., Uyeki T.M., Pillai S.K. Washington state 2019-nCoV case investigation team, first case of 2019 novel coronavirus in the United States. N. Engl. J. Med. 2020;382:929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lim J., Jeon S., Shin H.Y., Kim M.J., Seong Y.M., Lee W.J., Choe K.W., Kang Y.M., Lee B., Park S.J. Case of the index patient who caused tertiary transmission of COVID-19 infection in Korea: the application of lopinavir/ritonavir for the treatment of COVID-19 infected pneumonia monitored by quantitative RT-PCR. J. Korean Med. Sci. 2020;35:e79. doi: 10.3346/jkms.2020.35.e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vincent M.J., Bergeron E., Benjannet S., Erickson B.R., Rollin P.E., Ksiazek T.G., Seidah N.G., Nichol S.T. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol. J. 2005;2:69. doi: 10.1186/1743-422X-2-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Golden E.B., Cho H.-Y., Hofman F.M., Louie S.G., Schönthal A.H., Chen T.C. Quinoline-based antimalarial drugs: a novel class of autophagy inhibitors. Neurosurg. Focus. 2015;38:E12. doi: 10.3171/2014.12.FOCUS14748. [DOI] [PubMed] [Google Scholar]
- 29.Ahmadpoor P., Rostaing L. Why the immune system fails to mount an adaptive immune response to a Covid -19 infection. Transpl. Int. 2020 doi: 10.1111/tri.13611. [DOI] [PubMed] [Google Scholar]
- 30.Liu K., Fang Y.-Y., Deng Y., Liu W., Wang M.-F., Ma J.-P., Xiao W., Wang Y.-N., Zhong M.-H., Li C.-H., Li G.-C., Liu H.-G. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chin. Med. J. 2020 doi: 10.1097/CM9.0000000000000744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang W., He J., Lie puyi, Huang liyan, Wu S., lin yongping, liu xiaoqing. The definition and risks of cytokine release syndrome-like in 11 COVID-19-Infected pneumonia critically ill patients: disease characteristics and retrospective analysis. Intensive Care Crit. Care Med. 2020 doi: 10.1101/2020.02.26.20026989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O’Neill L.A.J. How low cholesterol is good for anti-viral immunity. Cell. 2015;163:1572–1574. doi: 10.1016/j.cell.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 33.Wu D., Sanin D.E., Everts B., Chen Q., Qiu J., Buck M.D., Patterson A., Smith A.M., Chang C.-H., Liu Z., Artyomov M.N., Pearce E.L., Cella M., Pearce E.J. Type 1 interferons induce changes in core metabolism that are critical for immune function. Immunity. 2016;44:1325–1336. doi: 10.1016/j.immuni.2016.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bambouskova M., Gorvel L., Lampropoulou V., Sergushichev A., Loginicheva E., Johnson K., Korenfeld D., Mathyer M.E., Kim H., Huang L.-H., Duncan D., Bregman H., Keskin A., Santeford A., Apte R.S., Sehgal R., Johnson B., Amarasinghe G.K., Soares M.P., Satoh T., Akira S., Hai T., de Guzman Strong C., Auclair K., Roddy T.P., Biller S.A., Jovanovic M., Klechevsky E., Stewart K.M., Randolph G.J., Artyomov M.N. Electrophilic properties of itaconate and derivatives regulate the IκBζ-ATF3 inflammatory axis. Nature. 2018;556:501–504. doi: 10.1038/s41586-018-0052-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Agalioti T., Lomvardas S., Parekh B., Yie J., Maniatis T., Thanos D. Ordered recruitment of chromatin modifying and general transcription factors to the IFN-beta promoter. Cell. 2000;103:667–678. doi: 10.1016/s0092-8674(00)00169-0. [DOI] [PubMed] [Google Scholar]
- 36.Cheng S.-C., Joosten L.A.B., Netea M.G. The interplay between central metabolism and innate immune responses. Cytokine Growth Factor Rev. 2014;25:707–713. doi: 10.1016/j.cytogfr.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 37.Sanchez E.L., Lagunoff M. Viral activation of cellular metabolism. Virology. 2015;479–480:609–618. doi: 10.1016/j.virol.2015.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bartee E., McFadden G. Cytokine synergy: an underappreciated contributor to innate anti-viral immunity. Cytokine. 2013;63:237–240. doi: 10.1016/j.cyto.2013.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Turner M.D., Nedjai B., Hurst T., Pennington D.J. Cytokines and chemokines: at the crossroads of cell signalling and inflammatory disease. Biochim. Biophys. Acta. 2014;1843:2563–2582. doi: 10.1016/j.bbamcr.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 40.Vabret N., Britton G.J., Gruber C., Hegde S., Kim J., Kuksin M., Levantovsky R., Malle L., Moreira A., Park M.D., Pia L., Risson E., Saffern M., Salomé B., Selvan M.E., Spindler M.P., Tan J., van der Heide V., Gregory J.K., Alexandropoulos K., Bhardwaj N., Brown B.D., Greenbaum B., Gümüş Z.H., Homann D., Horowitz A., Kamphorst A.O., Curotto de Lafaille M.A., Mehandru S., Merad M., Samstein R.M. Immunology of COVID-19: current state of the science. Immunity. 2020 doi: 10.1016/j.immuni.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Blanco-Melo D., Nilsson-Payant B.E., Liu W.-C., Uhl S., Hoagland D., Møller R., Jordan T.X., Oishi K., Panis M., Sachs D., Wang T.T., Schwartz R.E., Lim J.K., Albrecht R.A., tenOever B.R. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell. 2020 doi: 10.1016/j.cell.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.-Novel Coronavirus (2019-nCoV) Infections Trigger an Exaggerated Cytokine Response Aggravating Lung Injury, http://www.chinaxiv.org/abs/202002.00018 (n.d.) (Accessed 30 April 2020).
- 43.Chen C., Zhang X.R., Ju Z.Y., He W.F. Advances in the research of cytokine storm mechanism induced by Corona Virus Disease 2019 and the corresponding immunotherapies. Zhonghua Shao Shang Za Zhi. 2020;36:E005. doi: 10.3760/cma.j.cn501120-20200224-00088. [DOI] [PubMed] [Google Scholar]
- 44.van den Borne B.E., Dijkmans B.A., de Rooij H.H., le Cessie S., Verweij C.L. Chloroquine and hydroxychloroquine equally affect tumor necrosis factor-alpha, interleukin 6, and interferon-gamma production by peripheral blood mononuclear cells. J. Rheumatol. 1997;24:55–60. [PubMed] [Google Scholar]
- 45.DeDiego M.L., Nieto-Torres J.L., Regla-Nava J.A., Jimenez-Guardeño J.M., Fernandez-Delgado R., Fett C., Castaño-Rodriguez C., Perlman S., Enjuanes L. Inhibition of NF-κB-mediated inflammation in severe acute respiratory syndrome coronavirus-infected mice increases survival. J. Virol. 2014;88:913–924. doi: 10.1128/JVI.02576-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nieto-Torres J.L., DeDiego M.L., Verdiá-Báguena C., Jimenez-Guardeño J.M., Regla-Nava J.A., Fernandez-Delgado R., Castaño-Rodriguez C., Alcaraz A., Torres J., Aguilella V.M., Enjuanes L. Severe acute respiratory syndrome coronavirus envelope protein ion channel activity promotes virus fitness and pathogenesis. PLoS Pathog. 2014;10:e1004077. doi: 10.1371/journal.ppat.1004077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Siu K.-L., Yuen K.-S., Castaño-Rodriguez C., Ye Z.-W., Yeung M.-L., Fung S.-Y., Yuan S., Chan C.-P., Yuen K.-Y., Enjuanes L., Jin D.-Y. Severe acute respiratory syndrome coronavirus ORF3a protein activates the NLRP3 inflammasome by promoting TRAF3-dependent ubiquitination of ASC. FASEB J. 2019;33:8865–8877. doi: 10.1096/fj.201802418R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cheung C.Y., Poon L.L.M., Ng I.H.Y., Luk W., Sia S.-F., Wu M.H.S., Chan K.-H., Yuen K.-Y., Gordon S., Guan Y., Peiris J.S.M. Cytokine responses in severe acute respiratory syndrome coronavirus-infected macrophages in vitro: possible relevance to pathogenesis. J. Virol. 2005;79:7819–7826. doi: 10.1128/JVI.79.12.7819-7826.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Law H.K.W., Cheung C.Y., Ng H.Y., Sia S.F., Chan Y.O., Luk W., Nicholls J.M., Peiris J.S.M., Lau Y.L. Chemokine up-regulation in SARS-coronavirus–infected, monocyte-derived human dendritic cells. Blood. 2005;106:2366–2374. doi: 10.1182/blood-2004-10-4166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chu H., Zhou J., Wong B.H.-Y., Li C., Chan J.F.-W., Cheng Z.-S., Yang D., Wang D., Lee A.C.-Y., Li C., Yeung M.-L., Cai J.-P., Chan I.H.-Y., Ho W.-K., To K.K.-W., Zheng B.-J., Yao Y., Qin C., Yuen K.-Y. Middle east respiratory syndrome coronavirus efficiently infects human primary T lymphocytes and activates the extrinsic and intrinsic apoptosis pathways. J. Infect. Dis. 2016;213:904–914. doi: 10.1093/infdis/jiv380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hui D.S.C., Zumla A. Severe acute respiratory syndrome: historical, epidemiologic, and clinical features. Infect. Dis. Clin. North Am. 2019;33:869–889. doi: 10.1016/j.idc.2019.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Conti P., Ronconi G., Caraffa A., Gallenga C., Ross R., Frydas I., Kritas S. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (COVI-19 or SARS-CoV-2): anti-inflammatory strategies. J. Biol. Regul. Homeost. Agents. 2020;34 doi: 10.23812/CONTI-E. [DOI] [PubMed] [Google Scholar]
- 53.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. HLH Across Speciality Collaboration, UK, COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wan S., Yi Q., Fan S., Lv J., Zhang X., Guo L., Lang C., Xiao Q., Xiao K., Yi Z., Qiang M., Xiang J., Zhang B., Chen Y. Characteristics of lymphocyte subsets and cytokines in peripheral blood of 123 hospitalized patients with 2019 novel coronavirus pneumonia (NCP) Hematology. 2020 doi: 10.1101/2020.02.10.20021832. [DOI] [Google Scholar]
- 55.Yang Y., Shen C., Li J., Yuan J., Yang M., Wang F., Li G., Li Y., Xing L., Peng L., Wei J., Cao M., Zheng H., Wu W., Zou R., Li D., Xu Z., Wang H., Zhang M., Zhang Z., Liu L., Liu Y. Exuberant elevation of IP-10, MCP-3 and IL-1ra during SARS-CoV-2 infection is associated with disease severity and fatal outcome. Infectious Diseases (except HIV/AIDS) 2020 doi: 10.1101/2020.03.02.20029975. [DOI] [Google Scholar]
- 56.Liu Y., Zhang C., Huang F., Yang Y., Wang F., Yuan J., Zhang Z., Qin Y., Li X., Zhao D., Li S., Tan S., Wang Z., Li J., Shen C., Li J., Peng L., Wu W., Cao M., Xing L., Xu Z., Chen L., Zhou C., Liu W.J., Liu L., Jiang C. 2020. 2019-novel Coronavirus (2019-nCoV) Infections Trigger an Exaggerated Cytokine Response Aggravating Lung Injury.http://www.chinaxiv.org/abs/202002.00018 (accessed April 30, 2020) [Google Scholar]
- 57.Qin C., Zhou L., Hu Z., Zhang S., Yang S., Tao Y., Xie C., Ma K., Shang K., Wang W., Tian D.-S. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang W., Zhao Y., Zhang F., Wang Q., Li T., Liu Z., Wang J., Qin Y., Zhang X., Yan X., Zeng X., Zhang S. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): the perspectives of clinical immunologists from China. Clin. Immunol. 2020;214 doi: 10.1016/j.clim.2020.108393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tanaka T., Narazaki M., Kishimoto T. Immunotherapeutic implications of IL-6 blockade for cytokine storm. Immunotherapy. 2016;8:959–970. doi: 10.2217/imt-2016-0020. [DOI] [PubMed] [Google Scholar]
- 60.Kritas S.K., Ronconi G., Caraffa A., Gallenga C.E., Ross R., Conti P. Mast cells contribute to coronavirus-induced inflammation: new anti-inflammatory strategy. J. Biol. Regul. Homeost. Agents. 2020;34 doi: 10.23812/20-Editorial-Kritas. [DOI] [PubMed] [Google Scholar]
- 61.Rosenberg S.A. IL-2: the first effective immunotherapy for human cancer. J. Immunol. 2014;192:5451–5458. doi: 10.4049/jimmunol.1490019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yuan X., Cheng G., Malek T.R. The importance of regulatory T-cell heterogeneity in maintaining self-tolerance. Immunol. Rev. 2014;259:103–114. doi: 10.1111/imr.12163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Abbas A.K., Trotta E., Simeonov D.R., Marson A., Bluestone J.A. Revisiting IL-2: biology and therapeutic prospects. Sci. Immunol. 2018;3 doi: 10.1126/sciimmunol.aat1482. [DOI] [PubMed] [Google Scholar]
- 64.Chen G., Wu D., Guo W., Cao Y., Huang D., Wang H., Wang T., Zhang X., Chen H., Yu H., Zhang X., Zhang M., Wu S., Song J., Chen T., Han M., Li S., Luo X., Zhao J., Ning Q. Clinical and immunologic features in severe and moderate Coronavirus Disease 2019. J. Clin. Invest. 2020 doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen L., Liu H.G., Liu W., Liu J., Liu K., Shang J., Deng Y., Wei S. [Analysis of clinical features of 29 patients with 2019 novel coronavirus pneumonia] Zhonghua Jie He He Hu Xi Za Zhi. 2020;43:E005. doi: 10.3760/cma.j.issn.1001-0939.2020.0005. [DOI] [PubMed] [Google Scholar]
- 66.Huang K.-J., Su I.-J., Theron M., Wu Y.-C., Lai S.-K., Liu C.-C., Lei H.-Y. An interferon-gamma-related cytokine storm in SARS patients. J. Med. Virol. 2005;75:185–194. doi: 10.1002/jmv.20255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li C.K., Wu H., Yan H., Ma S., Wang L., Zhang M., Tang X., Temperton N.J., Weiss R.A., Brenchley J.M., Douek D.C., Mongkolsapaya J., Tran B.-H., Lin C.S., Screaton G.R., Hou J., McMichael A.J., Xu X.-N. T cell responses to whole SARS coronavirus in humans. J. Immunol. 2008;181:5490–5500. doi: 10.4049/jimmunol.181.8.5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Channappanavar R., Fett C., Zhao J., Meyerholz D.K., Perlman S. Virus-specific memory CD8 t cells provide substantial protection from lethal severe acute respiratory syndrome coronavirus infection. J. Virol. 2014;88:11034–11044. doi: 10.1128/JVI.01505-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yang X.-X., Li F.-X., Wu Y.-S., Wu D., Tan J.-Y., Li M. Association of TGF-beta1, IL-4 and IL-13 gene polymerphisms with asthma in a Chinese population. Asian Pac. J. Allergy Immunol. 2011;29:273–277. [PubMed] [Google Scholar]
- 70.Tsao C.-H., Shiau M.-Y., Chuang P.-H., Chang Y.-H., Hwang J. Interleukin-4 regulates lipid metabolism by inhibiting adipogenesis and promoting lipolysis. J. Lipid Res. 2014;55:385–397. doi: 10.1194/jlr.M041392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bot A., Holz A., Christen U., Wolfe T., Temann A., Flavell R., von Herrath M. Local IL-4 expression in the lung reduces pulmonary influenza-virus-specific secondary cytotoxic T cell responses. Virology. 2000;269:66–77. doi: 10.1006/viro.2000.0187. [DOI] [PubMed] [Google Scholar]
- 72.Liu Y., de Waal Malefyt R., Briere F., Parham C., Bridon J.M., Banchereau J., Moore K.W., Xu J. The EBV IL-10 homologue is a selective agonist with impaired binding to the IL-10 receptor. J. Immunol. 1997;158:604–613. [PubMed] [Google Scholar]
- 73.Tanaka T., Narazaki M., Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol. 2014;6:a016295. doi: 10.1101/cshperspect.a016295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rincon M., Irvin C.G. Role of IL-6 in asthma and other inflammatory pulmonary diseases. Int. J. Biol. Sci. 2012;8:1281–1290. doi: 10.7150/ijbs.4874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Heinrich P.C., Castell J.V., Andus T. Interleukin-6 and the acute phase response. Biochem. J. 1990;265:621–636. doi: 10.1042/bj2650621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rose-John S. IL-6 trans-signaling via the soluble IL-6 receptor: importance for the pro-inflammatory activities of IL-6. Int. J. Biol. Sci. 2012;8:1237–1247. doi: 10.7150/ijbs.4989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wong C.K., Lam C.W.K., Wu A.K.L., Ip W.K., Lee N.L.S., Chan I.H.S., Lit L.C.W., Hui D.S.C., Chan M.H.M., Chung S.S.C., Sung J.J.Y. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin. Exp. Immunol. 2004;136:95–103. doi: 10.1111/j.1365-2249.2004.02415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Okabayashi T., Kariwa H., Yokota S., Iki S., Indoh T., Yokosawa N., Takashima I., Tsutsumi H., Fujii N. Cytokine regulation in SARS coronavirus infection compared to other respiratory virus infections. J. Med. Virol. 2006;78:417–424. doi: 10.1002/jmv.20556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yoshikawa T., Hill T., Li K., Peters C.J., Tseng C.-T.K. Severe acute respiratory syndrome (SARS) coronavirus-induced lung epithelial cytokines exacerbate SARS pathogenesis by modulating intrinsic functions of monocyte-derived macrophages and dendritic cells. J. Virol. 2009;83:3039–3048. doi: 10.1128/JVI.01792-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Min C.-K., Cheon S., Ha N.-Y., Sohn K.M., Kim Y., Aigerim A., Shin H.M., Choi J.-Y., Inn K.-S., Kim J.-H., Moon J.Y., Choi M.-S., Cho N.-H., Kim Y.-S. Comparative and kinetic analysis of viral shedding and immunological responses in MERS patients representing a broad spectrum of disease severity. Sci. Rep. 2016;6 doi: 10.1038/srep25359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chu H., Chan J.F.-W., Wang Y., Yuen T.T.-T., Chai Y., Hou Y., Shuai H., Yang D., Hu B., Huang X., Zhang X., Cai J.-P., Zhou J., Yuan S., Kok K.-H., To K.K.-W., Chan I.H.-Y., Zhang A.J., Sit K.-Y., Au W.-K., Yuen K.-Y. Comparative replication and immune activation profiles of SARS-CoV-2 and SARS-CoV in human lungs: an ex vivo study with implications for the pathogenesis of COVID-19. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Diao B., Wang C., Tan Y., Chen X., Liu Y., Ning L., Chen L., Li M., Liu Y., Wang G., Yuan Z., Feng Z., Wu Y., Chen Y. Infectious Diseases (except HIV/AIDS); 2020. Reduction and Functional Exhaustion of T Cells in Patients With Coronavirus Disease 2019 (COVID-19) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dong L., Tian J., He S., Zhu C., Wang J., Liu C., Yang J. Possible vertical transmission of SARS-CoV-2 from an infected mother to her newborn. JAMA. 2020 doi: 10.1001/jama.2020.4621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Luo P., Liu Y., Qiu L., Liu X., Liu D., Li J. Tocilizumab treatment in COVID-19: a single center experience. J. Med. Virol. 2020 doi: 10.1002/jmv.25801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ma J., Xia P., Zhou Y., Liu Z., Zhou X., Wang J., Li T., Yan X., Chen L., Zhang S., Qin Y., Li X. Potential effect of blood purification therapy in reducing cytokine storm as a late complication of severe COVID-19. Clin. Immunol. 2020 doi: 10.1016/j.clim.2020.108408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pedersen S.F., Ho Y.-C. SARS-CoV-2: a storm is raging. J. Clin. Invest. 2020 doi: 10.1172/JCI137647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020 doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sun D., Li H., Lu X.-X., Xiao H., Ren J., Zhang F.-R., Liu Z.-S. Clinical features of severe pediatric patients with coronavirus disease 2019 in Wuhan: a single center’s observational study. World J. Pediatr. 2020 doi: 10.1007/s12519-020-00354-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang Z., Yang B., Li Q., Wen L., Zhang R. Clinical Features of 69 Cases with Coronavirus Disease 2019 in Wuhan, China. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wu C., Chen X., Cai Y., Xia J., Zhou X., Xu S., Huang H., Zhang L., Zhou X., Du C., Zhang Y., Song J., Wang S., Chao Y., Yang Z., Xu J., Zhou X., Chen D., Xiong W., Xu L., Zhou F., Jiang J., Bai C., Zheng J., Song Y. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern. Med. 2020 doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chen X., Zhao B., Qu Y., Chen Y., Xiong J., Feng Y., Men D., Huang Q., Liu Y., Yang B., Ding J., Li F. Infectious Diseases (except HIV/AIDS); 2020. Detectable Serum SARS-CoV-2 Viral Load (RNAaemia) is Closely Associated With Drastically Elevated Interleukin 6 (IL-6) Level in Critically Ill COVID-19 Patients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X., Guan L., Wei Y., Li H., Wu X., Xu J., Tu S., Zhang Y., Chen H., Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhou Y., Fu B., Zheng X., Wang D., Zhao C., Qi Y., Sun R., Tian Z., Xu X., Wei H. Aberrant pathogenic GM-CSF+ T cells and inflammatory CD14+CD16+ monocytes in severe pulmonary syndrome patients of a new coronavirus. BioRxiv. 2020 doi: 10.1101/2020.02.12.945576. 2020.02.12.945576. [DOI] [Google Scholar]
- 94.Akhmerov A., Marban E. COVID-19 and the heart. Circ. Res. 2020 doi: 10.1161/CIRCRESAHA.120.317055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Xu X., Han M., Li T., Sun W., Wang D., Fu B., Zheng X., Yang Y., Li X., Zhang X., Pan A., Wei H. 2020. Effective Treatment of Severe COVID-19 Patients with Tocilizumab; p. 12. (n.d.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dholaria B.R., Bachmeier C.A., Locke F. Mechanisms and management of chimeric antigen receptor T-cell therapy-related toxicities. BioDrugs. 2019;33:45–60. doi: 10.1007/s40259-018-0324-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhang C., Wu Z., Li J.-W., Zhao H., Wang G.-Q. The cytokine release syndrome (CRS) of severe COVID-19 and Interleukin-6 receptor (IL-6R) antagonist Tocilizumab may be the key to reduce the mortality. Int. J. Antimicrob. Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang Z., Han W. Biomarkers of cytokine release syndrome and neurotoxicity related to CAR-T cell therapy. Biomark. Res. 2018;6:4. doi: 10.1186/s40364-018-0116-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Shieh J.-M., Tseng H.-Y., Jung F., Yang S.-H., Lin J.-C. Elevation of IL-6 and IL-33 levels in serum associated with lung fibrosis and skeletal muscle wasting in a bleomycin-induced lung injury mouse model. Mediators Inflamm. 2019;2019 doi: 10.1155/2019/7947596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.2020. Stanford Medicine COVID-19 Pharmacotherapy Information.http://med.stanford.edu/id/covid19/jcr:content/main/panel_builder/panel_0/accordion/accordion_content2/download/file.res/Pharmacotherapy%2004.01.pdf [Google Scholar]
- 101.2020. Vademecum per la cura delle persone con malattia da COVI-19.http://www.simit.org/medias/1569-covid19-vademecum-13-03-202.pdf [Google Scholar]
- 102.Bizzarri M., Laganà A.S., Aragona D., Unfer V. Inositol and pulmonary function. Could myo-inositol treatment downregulate inflammation and cytokine release syndrome in SARS-CoV-2? Eur. Rev. Med. Pharmacol. Sci. 2020;24:3426–3432. doi: 10.26355/eurrev_202003_20715. [DOI] [PubMed] [Google Scholar]
- 103.Schultz M.J. Macrolide activities beyond their antimicrobial effects: macrolides in diffuse panbronchiolitis and cystic fibrosis. J. Antimicrob. Chemother. 2004;54:21–28. doi: 10.1093/jac/dkh309. [DOI] [PubMed] [Google Scholar]
- 104.Gautret P., Lagier J.-C., Parola P., Hoang V.T., Meddeb L., Mailhe M., Doudier B., Courjon J., Giordanengo V., Vieira V.E., Dupont H.T., Honoré S., Colson P., Chabrière E., La Scola B., Rolain J.-M., Brouqui P., Raoult D. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int. J. Antimicrob. Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 105.Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M., Shi Z., Hu Z., Zhong W., Xiao G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Nodland S.E., Berkowska M.A., Bajer A.A., Shah N., de Ridder D., van Dongen J.J.M., LeBien T.W., van Zelm M.C. IL-7R expression and IL-7 signaling confer a distinct phenotype on developing human B-lineage cells. Blood. 2011;118:2116–2127. doi: 10.1182/blood-2010-08-302513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Schluns K.S., Kieper W.C., Jameson S.C., Lefrançois L. Interleukin-7 mediates the homeostasis of naïve and memory CD8 T cells in vivo. Nat. Immunol. 2000;1:426–432. doi: 10.1038/80868. [DOI] [PubMed] [Google Scholar]
- 108.Tan J.T., Dudl E., LeRoy E., Murray R., Sprent J., Weinberg K.I., Surh C.D. IL-7 is critical for homeostatic proliferation and survival of naive T cells. Proc. Natl. Acad. Sci. U. S. A. 2001;98:8732–8737. doi: 10.1073/pnas.161126098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Seddon B., Tomlinson P., Zamoyska R. Interleukin 7 and T cell receptor signals regulate homeostasis of CD4 memory cells. Nat. Immunol. 2003;4:680–686. doi: 10.1038/ni946. [DOI] [PubMed] [Google Scholar]
- 110.Fry T.J., Mackall C.L. The many faces of IL-7: from lymphopoiesis to peripheral T cell maintenance. J. Immunol. 2005;174:6571–6576. doi: 10.4049/jimmunol.174.11.6571. [DOI] [PubMed] [Google Scholar]
- 111.Pellegrini M., Calzascia T., Toe J.G., Preston S.P., Lin A.E., Elford A.R., Shahinian A., Lang P.A., Lang K.S., Morre M., Assouline B., Lahl K., Sparwasser T., Tedder T.F., Paik J.-H., DePinho R.A., Basta S., Ohashi P.S., Mak T.W. IL-7 engages multiple mechanisms to overcome chronic viral infection and limit organ pathology. Cell. 2011;144:601–613. doi: 10.1016/j.cell.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 112.Sawa Y., Arima Y., Ogura H., Kitabayashi C., Jiang J.-J., Fukushima T., Kamimura D., Hirano T., Murakami M. Hepatic interleukin-7 expression regulates T cell responses. Immunity. 2009;30:447–457. doi: 10.1016/j.immuni.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 113.Thompson-Snipes L., Dhar V., Bond M.W., Mosmann T.R., Moore K.W., Rennick D.M. Interleukin 10: a novel stimulatory factor for mast cells and their progenitors. J. Exp. Med. 1991;173:507–510. doi: 10.1084/jem.173.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rousset F., Garcia E., Defrance T., Péronne C., Vezzio N., Hsu D.H., Kastelein R., Moore K.W., Banchereau J. Interleukin 10 is a potent growth and differentiation factor for activated human B lymphocytes. Proc. Natl. Acad. Sci. U. S. A. 1992;89:1890–1893. doi: 10.1073/pnas.89.5.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Santin A.D., Hermonat P.L., Ravaggi A., Bellone S., Pecorelli S., Roman J.J., Parham G.P., Cannon M.J. Interleukin-10 increases Th1 cytokine production and cytotoxic potential in human papillomavirus-specific CD8(+) cytotoxic T lymphocytes. J. Virol. 2000;74:4729–4737. doi: 10.1128/jvi.74.10.4729-4737.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Moore K.W., de Waal Malefyt R., Coffman R.L., O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 117.Chang W.L.W., Baumgarth N., Yu D., Barry P.A. Human cytomegalovirus-encoded Interleukin-10 homolog inhibits maturation of dendritic cells and alters their functionality. J. Virol. 2004;78:8720–8731. doi: 10.1128/JVI.78.16.8720-8731.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Saraiva M., O’Garra A. The regulation of IL-10 production by immune cells. Nat. Rev. Immunol. 2010;10:170–181. doi: 10.1038/nri2711. [DOI] [PubMed] [Google Scholar]
- 119.Mumm J.B., Emmerich J., Zhang X., Chan I., Wu L., Mauze S., Blaisdell S., Basham B., Dai J., Grein J., Sheppard C., Hong K., Cutler C., Turner S., LaFace D., Kleinschek M., Judo M., Ayanoglu G., Langowski J., Gu D., Paporello B., Murphy E., Sriram V., Naravula S., Desai B., Medicherla S., Seghezzi W., McClanahan T., Cannon-Carlson S., Beebe A.M., Oft M. IL-10 elicits IFNγ-dependent tumor immune surveillance. Cancer Cell. 2011;20:781–796. doi: 10.1016/j.ccr.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 120.Moore K.W., Vieira P., Fiorentino D.F., Trounstine M.L., Khan T.A., Mosmann T.R. Homology of cytokine synthesis inhibitory factor (IL-10) to the Epstein-Barr virus gene BCRFI. Science. 1990;248:1230–1234. doi: 10.1126/science.2161559. [DOI] [PubMed] [Google Scholar]
- 121.Ding Y., Qin L., Kotenko S.V., Pestka S., Bromberg J.S. A single amino acid determines the immunostimulatory activity of interleukin 10. J. Exp. Med. 2000;191:213–224. doi: 10.1084/jem.191.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ding Y., Qin L., Zamarin D., Kotenko S.V., Pestka S., Moore K.W., Bromberg J.S. Differential IL-10R1 expression plays a critical role in IL-10-mediated immune regulation. J. Immunol. 2001;167:6884–6892. doi: 10.4049/jimmunol.167.12.6884. [DOI] [PubMed] [Google Scholar]
- 123.Kotenko S.V., Saccani S., Izotova L.S., Mirochnitchenko O.V., Pestka S. Human cytomegalovirus harbors its own unique IL-10 homolog (cmvIL-10) Proc. Natl. Acad. Sci. U. S. A. 2000;97:1695–1700. doi: 10.1073/pnas.97.4.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Raftery M.J., Wieland D., Gronewald S., Kraus A.A., Giese T., Schönrich G. Shaping phenotype, function, and survival of dendritic cells by cytomegalovirus-encoded IL-10. J. Immunol. 2004;173:3383–3391. doi: 10.4049/jimmunol.173.5.3383. [DOI] [PubMed] [Google Scholar]
- 125.Yoon S.I., Jones B.C., Logsdon N.J., Walter M.R. Same structure, different function crystal structure of the Epstein-Barr virus IL-10 bound to the soluble IL-10R1 chain. Structure. 2005;13:551–564. doi: 10.1016/j.str.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 126.Blackburn S.D., Wherry E.J. IL-10, T cell exhaustion and viral persistence. Trends Microbiol. 2007;15:143–146. doi: 10.1016/j.tim.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 127.Wilson E.B., Brooks D.G. The role of IL-10 in regulating immunity to persistent viral infections. Curr. Top. Microbiol. Immunol. 2011;350:39–65. doi: 10.1007/82_2010_96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Duell B.L., Tan C.K., Carey A.J., Wu F., Cripps A.W., Ulett G.C. Recent insights into microbial triggers of interleukin-10 production in the host and the impact on infectious disease pathogenesis. FEMS Immunol. Med. Microbiol. 2012;64:295–313. doi: 10.1111/j.1574-695X.2012.00931.x. [DOI] [PubMed] [Google Scholar]
- 129.Brooks D.G., Trifilo M.J., Edelmann K.H., Teyton L., McGavern D.B., Oldstone M.B.A. Interleukin-10 determines viral clearance or persistence in vivo. Nat. Med. 2006;12:1301–1309. doi: 10.1038/nm1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ejrnaes M., Filippi C.M., Martinic M.M., Ling E.M., Togher L.M., Crotty S., von Herrath M.G. Resolution of a chronic viral infection after interleukin-10 receptor blockade. J. Exp. Med. 2006;203:2461–2472. doi: 10.1084/jem.20061462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Saghazadeh A., Rezaei N. Immune-epidemiological parameters of the novel coronavirus – a perspective. Expert Rev. Clin. Immunol. 2020;0:1–6. doi: 10.1080/1744666X.2020.1750954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Collison L.W., Vignali D.A.A. Interleukin-35: odd one out or part of the family? Immunol. Rev. 2008;226:248–262. doi: 10.1111/j.1600-065X.2008.00704.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Jones L.L., Vignali D.A.A. Molecular interactions within the IL-6/IL-12 cytokine/receptor superfamily. Immunol. Res. 2011;51:5–14. doi: 10.1007/s12026-011-8209-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ma X., Trinchieri G. Regulation of interleukin-12 production in antigen-presenting cells. Adv. Immunol. 2001;79:55–92. doi: 10.1016/s0065-2776(01)79002-5. [DOI] [PubMed] [Google Scholar]
- 135.O’Shea J.J., Paul W.E. Regulation of T(H)1 differentiation--controlling the controllers. Nat. Immunol. 2002;3:506–508. doi: 10.1038/ni0602-506. [DOI] [PubMed] [Google Scholar]
- 136.Langrish C.L., McKenzie B.S., Wilson N.J., de Waal Malefyt R., Kastelein R.A., Cua D.J. IL-12 and IL-23: master regulators of innate and adaptive immunity. Immunol. Rev. 2004;202:96–105. doi: 10.1111/j.0105-2896.2004.00214.x. [DOI] [PubMed] [Google Scholar]
- 137.Hunter C.A. New IL-12-family members: IL-23 and IL-27, cytokines with divergent functions. Nat. Rev. Immunol. 2005;5:521–531. doi: 10.1038/nri1648. [DOI] [PubMed] [Google Scholar]
- 138.Vignali D.A.A., Kuchroo V.K. IL-12 family cytokines: immunological playmakers. Nat. Immunol. 2012;13:722–728. doi: 10.1038/ni.2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Allavena P., Paganin C., Zhou D., Bianchi G., Sozzani S., Mantovani A. Interleukin-12 is chemotactic for natural killer cells and stimulates their interaction with vascular endothelium. Blood. 1994;84:2261–2268. [PubMed] [Google Scholar]
- 140.Komastu T., Ireland D.D., Reiss C.S. IL-12 and viral infections. Cytokine Growth Factor Rev. 1998;9:277–285. doi: 10.1016/s1359-6101(98)00017-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Coutelier J.P., Van Broeck J., Wolf S.F. Interleukin-12 gene expression after viral infection in the mouse. J. Virol. 1995;69:1955–1958. doi: 10.1128/jvi.69.3.1955-1958.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Barna M., Komatsu T., Reiss C.S. Activation of type III nitric oxide synthase in astrocytes following a neurotropic viral infection. Virology. 1996;223:331–343. doi: 10.1006/viro.1996.0484. [DOI] [PubMed] [Google Scholar]
- 143.Kanangat S., Thomas J., Gangappa S., Babu J.S., Rouse B.T. Herpes simplex virus type 1-mediated up-regulation of IL-12 (p40) mRNA expression. Implications in immunopathogenesis and protection. J. Immunol. 1996;156:1110–1116. [PubMed] [Google Scholar]
- 144.Chen H.-W., Chen H.-Y., Wang L.-T., Wang F.-H., Fang L.-W., Lai H.-Y., Chen H.-H., Lu J., Hung M.-S., Cheng Y., Chen M.-Y., Liu S.-J., Chong P., Lee O.K.-S., Hsu S.-C. Mesenchymal stem cells tune the development of monocyte-derived dendritic cells toward a myeloid-derived suppressive phenotype through growth-regulated oncogene chemokines. J. Immunol. 2013;190:5065–5077. doi: 10.4049/jimmunol.1202775. [DOI] [PubMed] [Google Scholar]
- 145.Leng Z., Zhu R., Hou W., Feng Y., Yang Y., Han Q., Shan G., Meng F., Du D., Wang S., Fan J., Wang W., Deng L., Shi H., Li H., Hu Z., Zhang F., Gao J., Liu H., Li X., Zhao Y., Yin K., He X., Gao Z., Wang Y., Yang B., Jin R., Stambler I., Lim L.W., Su H., Moskalev A., Cano A., Chakrabarti S., Min K.-J., Ellison-Hughes G., Caruso C., Jin K., Zhao R.C. Transplantation of ACE2- mesenchymal stem cells improves the outcome of patients with COVID-19 pneumonia. Aging Dis. 2020;11:216–228. doi: 10.14336/AD.2020.0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Rengarajan J., Szabo S.J., Glimcher L.H. Transcriptional regulation of Th1/Th2 polarization. Immunol. Today. 2000;21:479–483. doi: 10.1016/s0167-5699(00)01712-6. [DOI] [PubMed] [Google Scholar]
- 147.Yi T., Mui A.L., Krystal G., Ihle J.N. Hematopoietic cell phosphatase associates with the interleukin-3 (IL-3) receptor beta chain and down-regulates IL-3-induced tyrosine phosphorylation and mitogenesis. Mol. Cell. Biol. 1993;13:7577–7586. doi: 10.1128/mcb.13.12.7577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Klingmüller U., Lorenz U., Cantley L.C., Neel B.G., Lodish H.F. Specific recruitment of SH-PTP1 to the erythropoietin receptor causes inactivation of JAK2 and termination of proliferative signals. Cell. 1995;80:729–738. doi: 10.1016/0092-8674(95)90351-8. [DOI] [PubMed] [Google Scholar]
- 149.Chen H.E., Chang S., Trub T., Neel B.G. Regulation of colony-stimulating factor 1 receptor signaling by the SH2 domain-containing tyrosine phosphatase SHPTP1. Mol. Cell. Biol. 1996;16:3685–3697. doi: 10.1128/mcb.16.7.3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Paulson R.F., Vesely S., Siminovitch K.A., Bernstein A. Signalling by the W/Kit receptor tyrosine kinase is negatively regulated in vivo by the protein tyrosine phosphatase Shp1. Nat. Genet. 1996;13:309–315. doi: 10.1038/ng0796-309. [DOI] [PubMed] [Google Scholar]
- 151.Paul W.E., Zhu J. How are TH2-type immune responses initiated and amplified? Nat. Rev. Immunol. 2010;10:225–235. doi: 10.1038/nri2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Liang H.-E., Reinhardt R.L., Bando J.K., Sullivan B.M., Ho I.-C., Locksley R.M. Divergent expression patterns of IL-4 and IL-13 define unique functions in allergic immunity. Nat. Immunol. 2012;13:58–66. doi: 10.1038/ni.2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Harrington L.E., Hatton R.D., Mangan P.R., Turner H., Murphy T.L., Murphy K.M., Weaver C.T. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat. Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 154.Langrish C.L., Chen Y., Blumenschein W.M., Mattson J., Basham B., Sedgwick J.D., McClanahan T., Kastelein R.A., Cua D.J. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J. Exp. Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Duerr R.H., Taylor K.D., Brant S.R., Rioux J.D., Silverberg M.S., Daly M.J., Steinhart A.H., Abraham C., Regueiro M., Griffiths A., Dassopoulos T., Bitton A., Yang H., Targan S., Datta L.W., Kistner E.O., Schumm L.P., Lee A.T., Gregersen P.K., Barmada M.M., Rotter J.I., Nicolae D.L., Cho J.H. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science. 2006;314:1461–1463. doi: 10.1126/science.1135245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.McInnes I.B., Mease P.J., Ritchlin C.T., Rahman P., Gottlieb A.B., Kirkham B., Kajekar R., Delicha E.-M., Pricop L., Mpofu S. Secukinumab sustains improvement in signs and symptoms of psoriatic arthritis: 2 year results from the phase 3 FUTURE 2 study. Rheumatology (Oxford). 2017;56:1993–2003. doi: 10.1093/rheumatology/kex301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Cua D.J., Tato C.M. Innate IL-17-producing cells: the sentinels of the immune system. Nat. Rev. Immunol. 2010;10:479–489. doi: 10.1038/nri2800. [DOI] [PubMed] [Google Scholar]
- 158.Zenewicz L.A. IL-22: There Is a Gap in Our Knowledge. Immunohorizons. 2018;2:198–207. doi: 10.4049/immunohorizons.1800006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Isailovic N., Daigo K., Mantovani A., Selmi C. Interleukin-17 and innate immunity in infections and chronic inflammation. J. Autoimmun. 2015;60:1–11. doi: 10.1016/j.jaut.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 160.McGeachy M.J., Cua D.J., Gaffen S.L. The IL-17 family of cytokines in health and disease. Immunity. 2019;50:892–906. doi: 10.1016/j.immuni.2019.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Josset L., Menachery V.D., Gralinski L.E., Agnihothram S., Sova P., Carter V.S., Yount B.L., Graham R.L., Baric R.S., Katze M.G. Cell host response to infection with novel human coronavirus EMC predicts potential antivirals and important differences with SARS coronavirus. MBio. 2013;4:e00165–00113. doi: 10.1128/mBio.00165-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Faure E., Poissy J., Goffard A., Fournier C., Kipnis E., Titecat M., Bortolotti P., Martinez L., Dubucquoi S., Dessein R., Gosset P., Mathieu D., Guery B. Distinct immune response in two MERS-CoV-infected patients: can we go from bench to bedside? PLoS One. 2014;9:e88716. doi: 10.1371/journal.pone.0088716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Wu D., Yang X.O. TH17 responses in cytokine storm of COVID-19: an emerging target of JAK2 inhibitor Fedratinib. J. Microbiol. Immunol. Infect. 2020 doi: 10.1016/j.jmii.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Becher B., Tugues S., Greter M. GM-CSF: From Growth Factor to Central Mediator of Tissue Inflammation. Immunity. 2016;45:963–973. doi: 10.1016/j.immuni.2016.10.026. [DOI] [PubMed] [Google Scholar]
- 165.Chockalingam S., Ghosh S.S. Macrophage colony-stimulating factor and cancer: a review. Tumour Biol. 2014;35:10635–10644. doi: 10.1007/s13277-014-2627-0. [DOI] [PubMed] [Google Scholar]
- 166.Chiba Y., Mizoguchi I., Hasegawa H., Ohashi M., Orii N., Nagai T., Sugahara M., Miyamoto Y., Xu M., Owaki T., Yoshimoto T. Regulation of myelopoiesis by proinflammatory cytokines in infectious diseases. Cell. Mol. Life Sci. 2018;75:1363–1376. doi: 10.1007/s00018-017-2724-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Hartung T., von Aulock S., Wendel A. Role of granulocyte colony-stimulating factor in infection and inflammation. Med. Microbiol. Immunol. 1998;187:61–69. doi: 10.1007/s004300050075. [DOI] [PubMed] [Google Scholar]
- 168.Kuritzkes D.R. Neutropenia, neutrophil dysfunction, and bacterial infection in patients with human immunodeficiency virus disease: the role of granulocyte colony-stimulating factor. Clin. Infect. Dis. 2000;30:256–260. doi: 10.1086/313642. [DOI] [PubMed] [Google Scholar]
- 169.del Rio C., Malani P.N. Novel coronavirus—important information for clinicians. JAMA. 2019;323(2020):1039–1040. doi: 10.1001/jama.2020.1490. [DOI] [PubMed] [Google Scholar]
- 170.Kitamura T., Hayashida K., Sakamaki K., Yokota T., Arai K., Miyajima A. Reconstitution of functional receptors for human granulocyte/macrophage colony-stimulating factor (GM-CSF): evidence that the protein encoded by the AIC2B cDNA is a subunit of the murine GM-CSF receptor. Proc. Natl. Acad. Sci. U. S. A. 1991;88:5082–5086. doi: 10.1073/pnas.88.12.5082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Tavernier J., Devos R., Cornelis S., Tuypens T., Van der Heyden J., Fiers W., Plaetinck G. A human high affinity interleukin-5 receptor (IL5R) is composed of an IL5-specific alpha chain and a beta chain shared with the receptor for GM-CSF. Cell. 1991;66:1175–1184. doi: 10.1016/0092-8674(91)90040-6. [DOI] [PubMed] [Google Scholar]
- 172.Avalos B.R. Molecular analysis of the granulocyte colony-stimulating factor receptor. Blood. 1996;88:761–777. [PubMed] [Google Scholar]
- 173.Guilliams M., De Kleer I., Henri S., Post S., Vanhoutte L., De Prijck S., Deswarte K., Malissen B., Hammad H., Lambrecht B.N. Alveolar macrophages develop from fetal monocytes that differentiate into long-lived cells in the first week of life via GM-CSF. J. Exp. Med. 2013;210:1977–1992. doi: 10.1084/jem.20131199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Schneider C., Nobs S.P., Kurrer M., Rehrauer H., Thiele C., Kopf M. Induction of the nuclear receptor PPAR-γ by the cytokine GM-CSF is critical for the differentiation of fetal monocytes into alveolar macrophages. Nat. Immunol. 2014;15:1026–1037. doi: 10.1038/ni.3005. [DOI] [PubMed] [Google Scholar]
- 175.Sheridan J.W., Metcalf D. Studies on the bone marrow colony stimulating factor (CSF): relation of tissue CSF to serum CSF. J. Cell. Physiol. 1972;80:129–140. doi: 10.1002/jcp.1040800114. [DOI] [PubMed] [Google Scholar]
- 176.Kay A.B., Ying S., Varney V., Gaga M., Durham S.R., Moqbel R., Wardlaw A.J., Hamid Q. Messenger RNA expression of the cytokine gene cluster, interleukin 3 (IL-3), IL-4, IL-5, and granulocyte/macrophage colony-stimulating factor, in allergen-induced late-phase cutaneous reactions in atopic subjects. J. Exp. Med. 1991;173:775–778. doi: 10.1084/jem.173.3.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Hamilton J.A., Anderson G.P. GM-CSF biology. Growth Factors. 2004;22:225–231. doi: 10.1080/08977190412331279881. [DOI] [PubMed] [Google Scholar]
- 178.Shiomi A., Usui T. Pivotal roles of GM-CSF in autoimmunity and inflammation. Mediators Inflamm. 2015;2015 doi: 10.1155/2015/568543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Ruffilli I., Ferrari S.M., Colaci M., Ferri C., Fallahi P., Antonelli A. IP-10 in autoimmune thyroiditis. Horm. Metab. Res. 2014;46:597–602. doi: 10.1055/s-0034-1382053. [DOI] [PubMed] [Google Scholar]
- 180.van der Does Y., Tjikhoeri A., Ramakers C., Rood P.P.M., van Gorp E.C.M., Limper M. TRAIL and IP-10 as biomarkers of viral infections in the emergency department. J. Infect. 2016;72:761–763. doi: 10.1016/j.jinf.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 181.Tang M., Tian L., Luo G., Yu X. Interferon-gamma-Mediated osteoimmunology. Front. Immunol. 2018;9:1508. doi: 10.3389/fimmu.2018.01508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Lees J.R. Interferon gamma in autoimmunity: a complicated player on a complex stage. Cytokine. 2015;74:18–26. doi: 10.1016/j.cyto.2014.10.014. [DOI] [PubMed] [Google Scholar]
- 183.Mahallawi W.H., Khabour O.F., Zhang Q., Makhdoum H.M., Suliman B.A. MERS-CoV infection in humans is associated with a pro-inflammatory Th1 and Th17 cytokine profile. Cytokine. 2018;104:8–13. doi: 10.1016/j.cyto.2018.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Deshmane S.L., Kremlev S., Amini S., Sawaya B.E. Monocyte chemoattractant protein-1 (MCP-1): an overview. J. Interferon Cytokine Res. 2009;29:313–326. doi: 10.1089/jir.2008.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Lin L., Lu L., Cao W., Li T. Hypothesis for potential pathogenesis of SARS-CoV-2 infection-a review of immune changes in patients with viral pneumonia. Emerg. Microbes Infect. 2020;9:727–732. doi: 10.1080/22221751.2020.1746199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Xiong Y., Liu Y., Cao L., Wang D., Guo M., Jiang A., Guo D., Hu W., Yang J., Tang Z., Wu H., Lin Y., Zhang M., Zhang Q., Shi M., Liu Y., Zhou Y., Lan K., Chen Y. Transcriptomic characteristics of bronchoalveolar lavage fluid and peripheral blood mononuclear cells in COVID-19 patients. Emerg. Microbes Infect. 2020;9:761–770. doi: 10.1080/22221751.2020.1747363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Pasquereau S., Kumar A., Herbein G. Targeting TNF and TNF Receptor Pathway in HIV-1 Infection: from Immune Activation to Viral Reservoirs. Viruses. 2017;9 doi: 10.3390/v9040064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Zhang R., Wang X., Ni L., Di X., Ma B., Niu S., Liu C., Reiter R.J. COVID-19: Melatonin as a potential adjuvant treatment. Life Sci. 2020;250 doi: 10.1016/j.lfs.2020.117583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Alkharsah K.R. VEGF upregulation in viral infections and its possible therapeutic implications. Int. J. Mol. Sci. 2018;19 doi: 10.3390/ijms19061642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Apte R.S., Chen D.S., Ferrara N. VEGF in signaling and disease: beyond discovery and development. Cell. 2019;176:1248–1264. doi: 10.1016/j.cell.2019.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Libetta C., Esposito P., Martinelli C., Grosjean F., Gregorini M., Rampino T., Dal Canton A. Hepatocyte growth factor (HGF) and hemodialysis: physiopathology and clinical implications. Clin. Exp. Nephrol. 2016;20:371–378. doi: 10.1007/s10157-015-1211-. [DOI] [PubMed] [Google Scholar]