Abstract
Background
During the coronavirus disease 2019 pandemic, several acral chilblain-like lesions were observed in young patients with suspected, but mostly unconfirmed, infection with severe acute respiratory syndrome coronavirus 2. The histopathologic aspect of these lesions is as yet poorly known.
Objective
To investigate the pathologic features of chilblain-like lesions.
Methods
Biopsies were obtained from 17 cases of chilblain-like lesions during the coronavirus disease 2019 pandemic in France and were studied by routine histologic examination, immunohistochemistry, and direct immunofluorescence. The patients had suspected but unconfirmed infection with severe acute respiratory syndrome coronavirus 2 (negative nasopharyngeal polymerase chain reaction and serologic test results).
Results
Chilblain-like lesions showed many features in common with those reported in idiopathic and autoimmune-related chilblains, including epidermal necrotic keratinocytes, dermal edema, perivascular and perieccrine sweat gland lymphocytic (predominantly CD3/CD4+) inflammation, and frequent vascular changes (endothelialitis, microthromboses, fibrin deposition, and immunoreactant deposits on vessels).
Conclusions
Chilblain-like lesions show histopathologic features similar to those of idiopathic and autoimmune-related chilblains, with a high rate of vascular changes and direct immunofluorescence positivity. The role of severe acute respiratory syndrome coronavirus 2 in the development of these puzzling lesions remains to be elucidated.
Key words: chilblains, COVID-19, dermatopathology, direct immunofluorescence, eosinophils, immunohistochemistry, SARS-CoV-2
Capsule Summary.
-
•
Several acral chilblain-like lesions have been observed during the coronavirus disease 2019 pandemic. Their histopathologic features are poorly known.
-
•
Chilblain-like lesions show findings comparable to those of idiopathic and autoimmune-related chilblains, and frequently contain vascular changes and immune deposits (immunoglobulins, complement, or both) on dermal vessels by direct immunofluorescence.
Introduction
Coronavirus disease 2019 (COVID-19) is a multisystemic infection manifesting mainly with fever, cough, and pneumonia that may be severe and lethal. The disease is due to a new zoonotic-transmitted coronavirus named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). This was initially isolated in Wuhan, China, in December 2019 but has since spread worldwide, causing a pandemic disease with hundreds of thousands of fatalities.1 , 2 COVID-19 is a multisystemic disease affecting several systems in addition to the respiratory one, such as the gastrointestinal tract3 and the central nervous system.4 Several skin manifestations have been reported in patients with confirmed or suspected COVID-19, including morbilliform rashes, a papulovesicular varicella-like eruption, urticaria, livedoid rashes, and petechial and purpuric lesions.5, 6, 7, 8, 9 In addition, several puzzling cases of chilblain-like lesions of the extremities (mainly the toes) have been recorded in several countries in Europe and the Middle East contemporarily with the COVID-19 spread.10, 11, 12, 13, 14, 15 These lesions accounted for 19% to 38% of all cutaneous lesions associated with known or strongly suspected COVID-19 in 2 recent studies from France10 and Spain13; they were observed in young patients with no systemic symptoms or only mild ones, and appeared rather late in the course of the (suspected) infection. These lesions have been termed COVID-toes,16 even though their causal relationship with SARS-CoV-2 has not been convincingly proven. Among 63 such Italian cases, a swab test result was positive in merely 3.2% of patients.11 Another report on chilblain-like lesions included 2 patients with COVID-19–positive test results, but another patient had negative results by polymerase chain reaction and rapid antibody test.15 The pathologic features of chilblain-like lesions in this setting have not been extensively studied. Until now, a histopathologic study of chilblain-like lesions had been published in only 1 COVID-19–confirmed case17 and in 4 possible but unconfirmed COVID-19–positive cases.10 , 16 We performed a histopathologic, immunohistochemical, and immunofluorescence study of a series of 17 chilblain-like lesions to obtain further insight into their pathogenesis, and especially to assess whether these lesions have diagnostic microscopic features allowing their differentiation from clinically similar lesions, such as idiopathic chilblains and those associated with autoimmune disorders (namely, lupus erythematosus).
Patients and methods
This case series study included 17 patients (11 men and 6 women; mean age 32 years; range 15-63 years) who were referred to our dermatology department in April 2020 for cutaneous, red-violaceous, edematous, rarely necrotic, chilblain-like lesions localized on the toes (n = 9), the feet (heel and sole; n = 6), and fingers (n = 2). Among these patients, 5 mentioned recent nonspecific general symptoms preceding the onset of cutaneous lesions (cough, fever, or weakness) and 6 suspected a possible contamination from a family member (although only 2 of them had had positive test results for SARS-CoV-2). No clinical or biological evidence in favor of an underlying autoimmune disease was present (basic evaluation included routine blood tests and levels of inflammatory markers, D-dimers, antinuclear antibodies, complement, cryoglobulins, and antiphospholipid antibodies). A nasopharyngeal polymerase chain reaction test for SARS-CoV-2 and serologic testing for antibodies to this virus were performed concomitantly with the skin biopsies; all these tests had negative results.
Two skin biopsies were obtained from chilblain-like lesions from each of these patients. Formalin-fixed, paraffin-embedded skin specimens were studied microscopically for various epidermal and dermal changes associated with idiopathic and autoimmune-related chilblains. To characterize cells participating in the inflammation, the sections were immunolabeled for CD3, CD4, CD8 (T, T-helper, and T-suppressor/cytotoxic cells), CD20 (B cells), CD79a (plasma cells), CD30 (activated T cells), CD68 (histiomonocytic cells), and CD303/BDCA-2 (plasmacytoid dendritic cells) with an immunoperoxidase technique and diaminobenzidine as chromogen. Snap-frozen biopsies were studied by direct immunofluorescence for the presence of immunoreactants (IgA, IgG, IgM, and C3).
Results
Hematoxylin-eosin–stained tissue sections showed several common features between most chilblain-like lesion cases, with some quantitative variations. The detailed findings are shown in Supplemental Table I (available via Mendeley at https://data.mendeley.com/datasets/8k44cy638x/1). Briefly, the most common epidermal changes included deep horizontal zones of parakeratosis within the horny layer (71%), possibly because of preceding bullae, and scattered or confluent necrotic or apoptotic keratinocytes (41%), which in 3 cases resulted in areas of epidermal necrosis (Fig 1 ). The dermis invariably contained a perivascular inflammatory cell infiltrate, which was made predominantly of lymphocytes; it was also localized around eccrine sweat glands, producing an aspect of lymphocytic eccrine hidradenitis, in 47% of cases (Fig 2 ), and extended occasionally to the subcutaneous adipose tissue as perivascular cuffs. Rare eosinophils were admixed in 4 cases (23.5%) (Fig 3 ). Additional frequent findings included erythrocyte extravasation (82%) (Fig 3); papillary dermis edema (76%), which was massive in 4 cases, resulting in the formation of subepidermal pseudobullae (Fig 1); endothelial cell swelling (65%) (Fig 3 ); and moderately increased interstitial mucin deposition (41%). Less frequent, although remarkable, findings included the presence of vascular microthrombi within superficial dermal capillaries and more rarely in dermal venules (Fig 4) and fibrinoid deposits in the upper dermis and in the wall of dermal venules (Fig 5 ).
Fig 1.

Chilblain-like lesion. Scanning magnification shows diffuse upper dermal edema and a dense dermal (perivascular and perieccrine sweat gland) inflammatory infiltrate. (Hematoxylin-eosin-saffron stain; original magnification: ×40.) esg, Eccrine sweat gland.
Fig 2.
Chilblain-like lesion. Dense lymphocytic infiltrate around an eccrine sweat gland. Inset: perieccrine clusters of CD303+/BDCA-2 plasmacytoid dendritic cells. (Hematoxylin-eosin-saffron stain; original magnification: ×250; inset [immunoperoxidase revealed with diaminobenzidine]; original magnification: ×250.)
Fig 3.
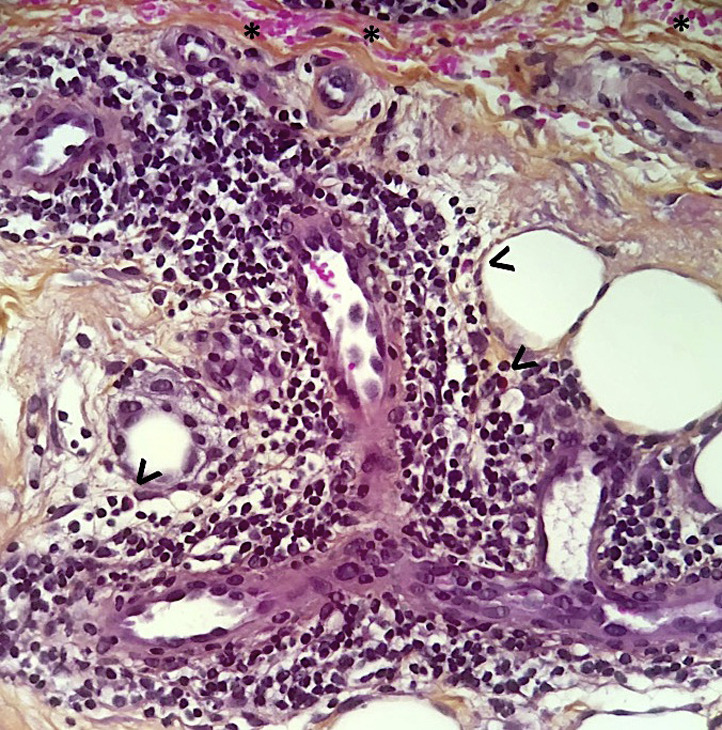
Chilblain-like lesion. Dense lymphocytic dermal perivascular infiltrate admixed with occasional eosinophils (arrowheads). Endothelial cell swelling and extravasated red blood cells (asterisks).
Fig 4.
Chilblain-like lesion. Eosinophilic thrombi within the lumen of a dermal venule. (Hematoxylin-eosin-saffron stain; original magnification: ×400.)
Fig 5.
Chilblain-like lesion. Eosinophilic fibrin deposits on the wall of a dermal venule. Inset shows vascular deposits of immunoglobulin M. (Hematoxylin-eosin-saffron stain; original magnification: ×400; inset [direct immunofluorescence]; original magnification: ×400.)
The immunohistochemical study, performed in 5 representative cases, revealed that the majority (>75%) of dermal infiltrating cells were CD3+ T cells, with a CD4:CD8 ratio of approximately 3:2. Few B or plasma cells (CD20+/CD79a+) were found (<10% of all lymphocytes). Scattered CD30+-activated T cells (accounting for <15% of all lymphocytes) were found in the upper dermis. All biopsies contained CD303/BDCA-2+ plasmacytoid dendritic cells, usually in small perivascular or perieccrine clusters in the mid dermis (Fig 2, inset) and occasionally also in the papillary dermis. Several CD68+ histiomonocytic cells were scattered throughout the dermis.
Direct immunofluorescence examination result was positive in 14 of 17 frozen biopsy specimens (82%). The examination showed vascular deposits of IgM, IgA, and C3 in 9, 5, and 5 cases, respectively (Fig 5, inset). In the 3 remaining cases, direct immunofluorescence result was either negative or showed nonspecific findings (such as C3 microgranular deposits at the dermal-epidermal junction or IgM deposits on colloid bodies of the papillary dermis).
Discussion
Although the clinical appearance of the chilblain-like lesions we investigated in this study is similar, if not identical, to that of both idiopathic and autoimmune-related chilblains, these lesions are distinct from an etiologic point of view. Indeed, the chilblain-like lesions appeared during March 2020 (which was in France one of the warmest months ever recorded), a fact that is against the role of cold and humid weather, as is the case for idiopathic chilblains. Furthermore, the autoimmunity evaluation performed for our patients (namely, for antinuclear antibodies) ruled out an underlying autoimmune disease, such as lupus erythematosus, which may be associated with pernio-like lesions (lupus pernio). The term COVID toes reflects the particular circumstances under which the outbreak of chilblain-like lesions was recorded, although the term COVID chilblains (by analogy to idiopathic or autoimmune chilblains) would be more correct.
Our study, which to our knowledge includes the largest series of pathologically studied chilblain-like lesions to date, showed that these lesions share several pathologic features with both idiopathic and autoimmune-associated chilblains. Indeed, despite some claimed differences, idiopathic and autoimmune-related chilblains are difficult to separate pathologically. Papillary edema and perieccrine sweat gland localization of the dermal infiltrate have been claimed to favor the diagnosis of idiopathic chilblains over autoimmune-related chilblains,18, 19, 20 although an earlier study did not find such differences.21 Conversely, basal cell layer vacuolization and interface changes were claimed to be more common in autoimmune-related chilblains versus idiopathic chilblains,18 , 19 but the differences are not discriminating from a statistical point of view. More recently, it was reported that the presence of abundant dermal mucin and interstitial fibrin was associated with lupus erythematosus, but that the number and distribution of CD123+ plasmacytoid dendritic cells and CD30+ lymphocytes had no discriminatory role.20
Our results show that the chilblain-like lesions exhibit, to various degrees, the most common pathologic features of idiopathic chilblains and autoimmune-related chilblains, including necrotic epidermal keratinocytes, papillary edema, dense perivascular and perieccrine sweat gland inflammation, predominance of CD3+/CD4+ T cells, and presence of CD303+ plasmacytoid dendritic cells and CD30+-activated cells in the dermal infiltrate. Rarer but remarkable findings include the presence of vascular microthromboses and fibrin deposition in the wall of dermal venules. The presence of eosinophils in the dermal infiltrate has not been, as far as we know, reported in idiopathic chilblains or autoimmune-related chilblains18, 19, 20, 21, 22 and is remarkable, even though eosinophils were found in small numbers and in a minority of our cases. Similarly, the high rate of positive-result direct immunofluorescence showing vascular deposits of IgM, IgA, or C3 is an original finding. Indeed, direct immunofluorescence result was negative in a case of COVID-19–associated chilblain,17 showed a lupus band test in 21% of cases of autoimmune-related chilblains,22 and showed nonspecific findings in cases of idiopathic chilblains associated with myelomonocytic leukemia23; these studies did not mention specific vascular deposits.
The pathogenesis of these chilblain-like lesions remains unclear. Our findings—namely, the deposits of immunoglobulins and C3 on dermal vessels and the presence of vascular microthrombi, swollen endothelial cells (endothelialitis), and fibrin deposits within the wall of dermal venules—suggest a vascular involvement in the genesis of chilblain-like lesions. So far, the presence of capillary microthrombi has been highlighted in 3 cases with probable COVID-1910 , 16 and could be related to the altered coagulation status observed in patients with (severe) COVID-19.24 Nevertheless, the precise role of SARS-CoV-2 in the development of chilblain-like lesions, although possible from an epidemiologic point of view, remains unclear. The presence of endothelialitis in chilblain-like lesions is consistent with a role of the SARS-CoV-2 because endothelialitis in several organs has been reported in the course of COVID-1925; however, few patients with chilblain-like lesions had confirmed infection with SARS-CoV-2,11 , 15 , 17 and most of them (including the patients in this study) had negative test results.11 Furthermore, a search for SARS-Cov-2 performed with polymerase chain reaction in 3 of the skin biopsies included in our study had negative results (data not shown). It can be speculated that chilblain-like lesions develop as an indirect consequence of viral infection, via an exaggerated immune response that can contain the infectious potential of the virus, but induces vascular injury that is the pathologic substratum of chilblain-like lesions. If this hypothesis is confirmed, chilblain-like lesions could be regarded as a paraviral manifestation, as recently suggested for an erythematous-scaly eruption in a patient with COVID-19, in which skin polymerase chain reaction did not detect SARS-CoV-2 in the skin lesions.26
In conclusion, our findings suggest that the pathologic features of chilblain-like lesions (COVID toes) are similar to those of idiopathic and autoimmune-related chilblains. Novel findings may include the presence of eosinophils in the dermal infiltrate and the high positivity rate of direct immunofluorescence examination, which highlights the involvement of vascular injury in the genesis of these lesions. If confirmed by a larger number of observations, these findings could shed more light on the pathogenesis of chilblain-like lesions. However, the precise role of SARS-CoV-2 in the development of these lesions remains to be elucidated.
Acknowledgments
We thank our colleagues who referred patients to us, and Dr Georgia Karayannopoulou (AHEPA Hospital, Thessaloniki, Greece) for fruitful discussions.
Footnotes
Drs Kanitakis and Lesort contributed equally to the article.
Funding sources: None.
Conflicts of interest: None disclosed.
Reprints not available from the authors.
References
- 1.Zhu N., Zhang D., Wang W., et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Novel Coronavirus Pneumonia Emergency Response Epidemiology Team The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in ChinaZhonghua Liu Xing Bing Xue Za Zhi. 2020;41(2):145–151. [Google Scholar]
- 3.Hajifathalian K., Mahadev S., Schwartz R.E., et al. SARS-COV-2 infection (coronavirus disease 2019) for the gastrointestinal consultant. World J Gastroenterol. 2020;26(14):1546–1553. doi: 10.3748/wjg.v26.i14.1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Helms J., Kremer S., Merdji H., et al. Neurologic features in severe SARS-CoV-2 infection. N Engl J Med. 2020;382(23):2268–2270. doi: 10.1056/NEJMc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sachdeva M., Gianotti R., Shah M., et al. Cutaneous manifestations of COVID-19: report of three cases and a review of literature. J Dermatol Sci. 2020;98(2):75–81. doi: 10.1016/j.jdermsci.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marzano A.V., Genovese G., Fabbrocini G., et al. Varicella-like exanthem as a specific COVID-19-associated skin manifestation: multicenter case series of 22 patients. J Am Acad Dermatol. 2020;83(1):280–285. doi: 10.1016/j.jaad.2020.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diaz-Guimaraens B., Diaz-Guimaraens B., Dominguez-Santas M., et al. Petechial skin rash associated with severe acute respiratory syndrome coronavirus 2 infection. JAMA Dermatol. 2020 doi: 10.1001/jamadermatol.2020.1741. [DOI] [PubMed] [Google Scholar]
- 8.Manalo I.F., Smith M.K., Cheeley J., Jacobs R.A. Dermatologic manifestation of COVID-19: transient livedo reticularis. J Am Acad Dermatol. 2020;83(2):700. doi: 10.1016/j.jaad.2020.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joob B., Wiwanitkit V. COVID-19 can present with a rash and be mistaken for dengue. J Am Acad Dermatol. 2020;82(5):E177. doi: 10.1016/j.jaad.2020.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Masson A., Bouaziz J.-D., Sulimovic L., et al. on behalf of the SNDV (French Union of Dermatologists-Venereologists) Chilblains are a common cutaneous finding during the COVID-19 pandemic: a retrospective nationwide study from France. J Am Acad Dermatol. 2020;83(2):667–670. doi: 10.1016/j.jaad.2020.04.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Piccolo V., Neri I., Filippeschi C., et al. Chilblain-like lesions during COVID-19 epidemic: a preliminary study on 63 patients. J Eur Acad Dermatol Venereol. 2020 doi: 10.1111/jdv.16526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Recalcati S., Barbagallo T., Frasin L.A., et al. Acral cutaneous lesions in the time of COVID-19. J Eur Acad Dermatol Venereol. 2020 doi: 10.1111/jdv.16533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galván Casas C., Català A., Carretero Hernández G., et al. Classification of the cutaneous manifestations of COVID-19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol. 2020 doi: 10.1111/bjd.19163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alramthan A., Aldaraji W. A case of COVID-19 presenting in clinical picture resembling chilblains disease. First report from the Middle East. Clin Exp Dermatol. 2020 doi: 10.1111/ced.14243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Landa N., Mendieta-Eckert M., Fonda-Pascual P., Aguirre T. Chilblain-like lesions on feet and hands during the COVID-19 pandemic. Int J Dermatol. 2020;59(6):739–743. doi: 10.1111/ijd.14937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sigal A., Zirn J., Lipper G., Shapiro P. A case of pernio-like lesions (“COVID toes”) with histologic confirmation of microthrombi. https://docs.google.com/document/d/1QrCmMYX1eXRfhDewC-Y6lugEfGPwOq-U4ppUAnhlibg/mobilebasic Accessed June 26, 2020.
- 17.Kolivras A., Dehavay F., Delplace D., et al. Coronavirus (COVID-19) infection–induced chilblains: a case report with histopathologic findings. JAAD Case Rep. 2020;6(6):489–492. doi: 10.1016/j.jdcr.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boada A., Bielsa I., Fernández-Figueras M.T., Ferrándiz C. Perniosis: clinical and histopathological analysis. Am J Dermatopathol. 2010;32(1):19–23. doi: 10.1097/DAD.0b013e3181af1d24. [DOI] [PubMed] [Google Scholar]
- 19.Crowson A.N., Magro C.M. Idiopathic perniosis and its mimics: a clinical and histological study of 38 cases. Hum Pathol. 1997;28(4):478–484. doi: 10.1016/s0046-8177(97)90038-1. [DOI] [PubMed] [Google Scholar]
- 20.Wang L., Chan P. Comparative analysis of chilblain lupus erythematosus and idiopathic perniosis: histopathologic features and immunohistochemistry for CD123 and CD30. Am J Dermatopathol. 2018;40(4):265–271. doi: 10.1097/DAD.0000000000000945. [DOI] [PubMed] [Google Scholar]
- 21.Cribier B., Djeridi N., Peltre B., Grosshans E. A histologic and immunohistochemical study of chilblains. J Am Acad Dermatol. 2001;45(6):924–929. doi: 10.1067/mjd.2001.117861. [DOI] [PubMed] [Google Scholar]
- 22.Viguier M., Pinquier L., Cavelier-Balloy B., et al. Clinical and histopathologic features and immunologic variables in patients with severe chilblains. A study of the relationship to lupus erythematosus. Medicine (Baltimore) 2001;80(3):180–188. doi: 10.1097/00005792-200105000-00004. [DOI] [PubMed] [Google Scholar]
- 23.Nazzaro G., Genovese G., Marzano A.V. Idiopathic chilblains in myelomonocytic leukemia: not a simple association. Int J Dermatol. 2018;57(5):596–598. doi: 10.1111/ijd.13896. [DOI] [PubMed] [Google Scholar]
- 24.Tang N., Bai H., Chen X., Gong J., Li D., Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18(5):1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Varga Z., Flammer A.J., Steiger P., et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395(10234):1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sanchez A., Sohier P., Benghanem S., et al. Digitate papulosquamous eruption associated with severe acute respiratory syndrome coronavirus 2 infection. JAMA Dermatol. 2020 doi: 10.1001/jamadermatol.2020.1704. [DOI] [PubMed] [Google Scholar]





