Abstract
Lavender is a medicinal and aromatic plant that is widely grown in Turkey. Gall symptoms were observed on roots of lavender (Lavandula angustifolia Mill.) collected from Kırklareli and Edirne provinces. Egg masses were collected from galled roots. DNA isolated from all samples was screened by species-specific primers belonging to the most common species of root-knot nematodes and M. arenaria was the only species that was identified in all of the samples analyzed. This is the first report of M. arenaria infecting lavender in Turkey.
Keywords: Lavandula angustifolia, Molecular marker, PCR
Root-knot nematodes are the most damaging group of plant-parasitic nematodes (Gill and McSorley, 2011). Since they have a wide host range worldwide, root-knot nematodes cause serious economic losses in plants (Jones et al., 2013). In addition, they can cause more serious damage by forming disease complexes with soil pathogens (Siddiqui et al., 2014; Lobna et al., 2016).
Medicinal and aromatic plants are widely used in pharmacy and perfumery industries. Lavender (Lavandula spp.) is mainly grown and used in medicine, perfumes, cosmetics and food (Lis-Balchin, 2002). Many pests and pathogens attack lavenders during growing periods. Root-knot nematodes are the most common and most important nematodes causing damage to lavender. Meloidoyne incogita (Kofoid and White, 1912; Chitwood, 1949) was found infecting L. hybrida Rev. from Argentina (Gorustovich et al., 1997) and L. officinalis Chaix et Vill. from Egypt (Ibrahim and Mokbel, 2009). In another study, M. luci Carneiro, Carneiro et al. (2014) was reported on L. spica L. (Carneiro et al., 2014). Lavender species, L. spica L. was inoculated with M. arenaria (Neal, 1892; Chitwood, 1949) and was a suitable host for this root-knot nematode (Moreno et al., 1990). However, there is no report on root-knot nematodes infecting lavender in Turkey.
In 2019, a survey was carried out in the lavender growing areas in Kırklareli and Edirne provinces of Turkey. The roots of lavender plants with symptoms of stunting were observed and examined. Galled roots were collected from seedlings and examined with a stereo-binocular microscope. The galls were symptoms caused by root-knot nematodes (Fig. 1). Egg masses were separately collected from roots of L. angustifolia cultivars, Raya, Hemus, Sevtopolis, Hebar and Yubileina using a small needle. For molecular identification, DNA was extracted from egg masses using the High Pure PCR Template Preparation Kit (Roche). Then, DNA samples were screened by species-specific primers belonging to the most common species of root-knot nematodes. PCR amplifications were carried out using inc-K14F/inc-K14R primers (Randig et al., 2002) and MincF1/MincR1 (Devran et al., 2018) for M. incognita; Fjav/Rjav (Zijlstra et al., 2000) for M. javanica (Treub, 1885; Chitwood, 1949); Far/Rar (Zijlstra et al., 2000) for M. arenaria; JMV1/JMV2/JMVhapla (Wishart et al., 2002) for M. hapla (Chitwood, 1949), M. fallax Karssen 1996, and M. chitwoodi (Golden et al., 1980).
Figure 1:
Root-knot nematode galls caused by Meloidogyne arenaria (Neal, 1892; Chitwood, 1949) on lavender (Lavandula angustifolia Mill.).
PCR was performed in a total volume of 25 μL containing the following: 2.5 μL 10X PCR buffer, 2 mM MgCl2, 200 μM dNTPs, 0.4 μM of each primer, 1 U Taq DNA Polymerase (ABM), 20 ng of DNA and molecular grade water. PCR was performed in a SimpliAmp™ Thermal Cycler (Applied Biosystems, CA, USA) using reaction conditions detailed in cited publications. PCR products were electrophoresed on a 1.5% agarose gel in 1X TAE buffer. Agarose gels were stained with ethidium bromide (0.5 μg/μL) and visualized using the Gel iX Imager (Intas Science, Göttingen, Germany).
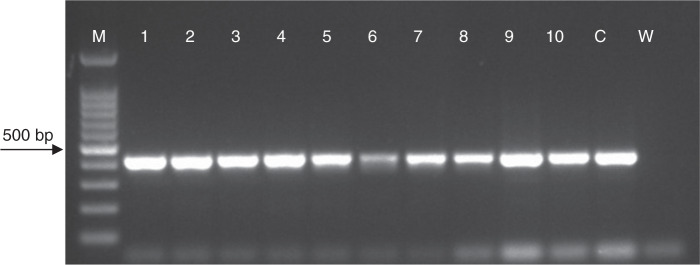
In the present study, a total of 10 DNA samples were screened with 5 different primer sets. M. arenaria-specific Far/Rar primers only produced expected approximately 420 bp PCR product all samples but other primer sets did not yield in sampled analyzed (Fig. 2). This is the first report of root-knot nematode infecting lavender in Turkey.
Figure 2:
PCR products amplified using primers Far/Rar. M: marker (100 bp DNA Ladder HibriGen), 1–4: samples collected from Kırklareli, 5–10: samples collected from Edirne, C: positive control, W: water.
In conclusion, the present study is the first to report information on the identification root-knot nematodes in lavender crops in Turkey using molecular markers. The results showed that lavender growing fields in Kırıklareli and Edirne provinces were infested with M. arenaria. These findings could be used to control root-knot nematodes by screening for resistant cultivars or utilizing crop rotations with non-host plants.
References
- Carneiro R. M., Correa V. R., Almeida M. R. A., Gomes A. C. M., Deimi A. M., Castagnone-Sereno P. and Karssen G.. 2014. Meloidogyne luci n. sp. (Nematoda: Meloidogynidae), a root-knot nematode parasitising different crops in Brazil, Chile and Iran. Nematology 16:289–301. [Google Scholar]
- Chitwood B. G.. 1949. Root-knot nematodes-Part 1. A revision of the genus Meloidogyne Goeldi, 1987. Proceedings of the helminthological Society of Washington 16:90–104. [Google Scholar]
- Devran Z., Polat İ., Mıstanoğlu İ. and Baysal Ö.,. 2018. A novel multiplex PCR tool for simultaneous detection of three root-knot nematodes. Australasian Plant Pathology 47:389–392. [Google Scholar]
- Gill H. K. and McSorley R.. 2011. Cover crops for managing root-knot nematodes., University of Florida, IFAS Extension, ENY-063, Gainesville, FL, pp. 1–6. [PMC free article] [PubMed]
- Golden A. M., O’Bannon H., Santo G. S. and Ftnley A. M.. 1980. Description and SEM observations of Meloidogyne chilwoodi n. sp. (Meloidogynidae), a root-knot nematode on potato in the Pacifie Northwest. Journal of Nematology 12:319–327 [PMC free article] [PubMed] [Google Scholar]
- Gorustovich M. N. A., Otero M. D. C., Rossi E. R. and Boldrini C.. 1997. Meloidogyne species in rosemary (Rosmarinus officinalis L.) and lavender (Lavandula hybrida Rev.) in the Cerrillos department, Province of Salta, Argentina. II WOCMAP Congress Medicinal and Aromatic Plants, Part 3: Agricultural Production, Post Harvest Techniques, Biotechnology, pp. 209–12.
- Ibrahim I. K. and Mokbel A. A.. 2009. Occurrence and distribution of the root-knot nematodes Meloidogyne spp. and their host plants in Northern Egypt. Egyptian Journal of Experimental Biology 5:1–7. [Google Scholar]
- Jones J. T., Haegeman A., Danchın E. G., Gaur H. S., Helder J., Jones M. G., Kikuchi T., Manzanılla-López R., Palomares-Rıus J. E., Wesemael W. M. L. and Perry R. N.. 2013. Top 10 plant-parasitic nematodes in molecular plant pathology. Molecular Plant Pathology 14:946–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karssen G. 1996. Description of Meloidogynefallax n. sp. (Nematoda: Heteroderidae), a root-knot nematode from The Netherlands. Fundamental and Applied Nematology 19:593–599 [Google Scholar]
- Lis-Balchin M. 2002. General introduction to the genus Lavandula. In Lis-Balchin M. (Ed.), Lavender. Taylor & Francis, London, p. 1. [Google Scholar]
- Lobna H., Hajer R., Naima M. B. and Najet H. R.. 2016. Studies on disease complex incidence of Meloidogyne javanica and Fusarium oxysporum f.sp. lycopersici on resistant and susceptible tomato cultivars. Journal of Agricultural Science and Food Technology 2:41–48. [Google Scholar]
- Moreno J. E., French E. C., Prine G. M. and Dunn R. A.. 1990. Evaluation of selected herbs for resistance to root-knot nematodes infection. Proceedings of the 26th Annual Meeting 534–540. [Google Scholar]
- Randig O., Bongiovanni M., Carneiro R. M. and Castagnone-Sereno P.. 2002. Genetic diversity of root-knot nematodes from Brazil and development of SCAR marker specific for the coffee damaging species. Genome 45:862–870. [DOI] [PubMed] [Google Scholar]
- Siddiqui Z. A., Shehzad M. and Alam S.. 2014. Interactions of Ralstonia solanacearum and Pectobacterium carotovorum with Meloidogyne incognita on potato. Archives of Phytopathology and Plant Protection 47:449–455. [Google Scholar]
- Wishart J, Phillips M. S. and Blok V. C.. 2002. Ribosomal Intergenic Spacer: a Polymerase chain reaction diagnostic for Meloidogyne chitwoodi, M. fallax, and M. hapla . Nematology 92:884–892. [DOI] [PubMed] [Google Scholar]
- Zijlstra C., Donkers-Venne D. T. H. M. and Fargette M.. 2000. Identification of Meloidogyne incognita, M. javanica and M. arenaria using sequence characterized amplifed region (SCAR) based PCR assays. Nematology 2:847–853. [Google Scholar]