Abstract
The rising consumer demand for alternative and sustainable protein sources drives the popularity of the use of plant-based proteins in the pet food industry. Pulse crops, which include beans, peas, lentils, and chickpeas, have become an important addition to both human and animal diets due to their protein content and functional properties. However, knowledge of their nutrient composition and protein quality is necessary for the proper formulation of these ingredients in pet foods. The objective of this study was to determine the macronutrient composition and standardized amino acid digestibility and to describe the protein quality through the use of digestible indispensable amino acid scores (DIAAS-like) of five pulse ingredients. Black bean (BB) grits, garbanzo beans (GB), green lentils (GL), navy bean (NB) powder, and yellow peas (YP) were analyzed for dry matter (DM), ash and organic matter (OM), crude protein (CP), gross energy (GE), acid hydrolyzed fat (AHF), and total dietary fiber (TDF) to determine the macronutrient composition. Precision-fed rooster assays were conducted using cecectomized roosters to calculate standardized amino acid digestibility and true metabolizable energy corrected for nitrogen (TMEn). The essential amino acids, with the exception of methionine, were highly digestible with digestibility values of 80% to 90% (dry matter basis) for all selected pulse ingredients. BB grits had the lowest (P < 0.05) digestibility of arginine (86.5%) and histidine (80.6%) in contrast to GB (94.9% and 89.9%, respectively). The TMEn of GB was highest (P < 0.05) at 3.56 kcal/g compared with the other pulses. The DIAAS-like values for adult dogs were consistently the lowest for methionine for all pulses, making it the first-limiting amino acid in these ingredients. The DIAAS-like values for adult cats showed GL had lowest (P < 0.05) score in tryptophan compared with other pulses when using both AAFCO values and NRC recommended allowances as reference proteins. Methionine was the first-limiting amino acid for YP and tryptophan for GL. Based on macronutrient composition, protein quality, and amino acid digestibility, it can be concluded that pulse ingredients have the required nutritional characteristics to be viable protein sources in canine and feline foods. However, the use of complementary protein sources is recommended to counterbalance any potential limiting amino acids in pulse ingredients.
Keywords: amino acid digestibility, cat, cecectomized rooster, dog, protein, pulses
Introduction
Traditional protein sources in pet foods include the use of animal proteins and animal byproducts. Consumer demand has been focused on novel protein sources in order to provide a high-quality product for their pet (Dust et al., 2005). However, due to the rapidly growing human population, it is estimated that the global meat consumption will increase by an additional 233 million tons by 2050, creating a need for alternative protein sources (Fiacco et al., 2017). Therefore, plant-based proteins, such as pulses, have gained popularity as an inexpensive and sustainable alternative to animal proteins (FAO, 2016).
Pulses, a subset of legumes, are harvested specifically for the dry grain portion, including peas, lentils, and beans, contain a high concentration of protein (McCrory et al., 2010). With protein supply increasingly becoming a global issue, the inclusion of alternative proteins becomes important in the sustainability of the food system (Duranti et al., 1997). Pulses also have diverse functional qualities, including a large water-holding capacity and gelling agents that broaden their use in multiple food and feed industries (Boye et al., 2010). In the pet food industry, pulses have appeared in grain-free diets, along with vegetarian diets, as consumers look for novel protein sources and nontraditional food options.
Like many plant-based proteins, there is little information available about the protein quality, protein content, nutrient digestibility, and energy content of pulse ingredients for dogs and cats which limits their use in pet food formulations (Deng et al., 2016). Because of this, the objective of this study was to evaluate the macronutrient composition, standardized amino acid digestibility, and protein quality of black bean (BB) grits, garbanzo beans (GB), green lentils (GL), navy bean (NB) powder, and yellow peas (YP) for use in canine and feline diets. It was hypothesized that the amino acids in select pulses would be well digested using the cecectomized rooster model, and that methionine would be the first-limiting amino acid in these ingredients for dogs and cats.
Materials and Methods
The protocols used in this study were approved by the Institutional Animal Care and Use Committee at the University of Illinois at Urbana-Champaign. All methods were performed in accordance with the U. S. Public Health Service Policy on Humane Care and Use of Laboratory Animals.
Sample preparation and chemical analysis
All pulse ingredients were supplied as raw ingredients except BB grits that were pre-cooked to remove oligosaccharides (ADM, Decatur, IL). The pulse ingredients were ground through a 2-mm screen in a Wiley mill (model 4; Thomas Scientific, Swedesboro, NJ) and were analyzed in duplicate for dry matter (DM), ash, and organic matter (OM) according to AOAC (2006; methods 934.01 and 942.05). Crude protein (CP) was calculated from Leco (TruMac N, Leco Corporation, St. Joseph, MI) and total nitrogen values were determined according to AOAC (2006; method 992.15). Gross energy (GE) was measured using bomb calorimetry (model 6200, Parr Instrument Co., Moline, IL). Acid hydrolyzed fat (AHF) was used to measure the total fat content according to AACC (1983) and Budde (1952). Total dietary fiber (TDF) was measured according to Prosky et al. (1992). Lastly, a complete amino acid profile was determined for each of the five pulses according to AOAC (2007). A coefficient of variance of 5% was used for all assays.
Precision-fed rooster assay
A precision-fed rooster assay was conducted to determine the standardized amino acid digestibility and true metabolizable energy corrected to zero nitrogen retention (TMEn) of the pulse ingredients (Parsons, 1985). The assay was conducted using 20 cecectomized, single-comb White Leghorn roosters with four roosters per treatment. The roosters were housed individually in cages with wire floors in a temperature-controlled room. The room was kept on a 16:8 (L:D) h cycle. The roosters were fasted for 26 h and then crop intubated 30 g of a 1:1 ratio of a pulse and corn mixture. After 48 h, excreta were collected, freeze-dried, ground to a consistent particle size, and later analyzed for a complete amino acid profile (AOAC, 2007). To calculate the standardized amino acid digestibility, endogenous values were calculated from roosters that were fasted for 48 h. Standardized amino acid digestibility was calculated according to Sibbald (1979) using the following equations:
In the above equations, standardized amino acid digestibility was first calculated for the corn and pulse ingredient mixture where FAA is the total amino acids fed; EAA is the total amino acids voided in the excreta, and EndAA is the total endogenous amino acids voided in the excreta of fasted roosters. Standardized amino acid digestibility for the pulse ingredient was then calculated by difference by correcting for the addition of corn where AADc is the amino acid digestibility of the corn; AADm is the mixture amino acid digestibility; and FAA ratio is the ratio of amino acid content (%) of the pulse ingredient to the sum of amino acid content (%) of the pulse ingredient and corn.
The TMEn values were calculated as outlined by Parsons et al. (1982) which used the following equations:
First, a mixed TMEn value was calculated for the combination of the corn and pulse ingredient. In the above equation, FEfed is the total GE that was fed (kcal); EEfed and EEfasted are the total excreta energy (kcal) that was voided by the fed and fasted roosters, respectively; 8.22 is the GE per gram of nitrogen of uric acid, Nfed and Nfasted are the amount of nitrogen (g) retained by the fed and fasted birds, respectively; and FI is feed intake. To correct for the added corn, the TMEn of the pulse ingredients themselves was calculated by difference where TMEnc is the TMEn of the corn, TMEnm is the TMEn of the corn:pulse ingredient mixture, and 0.5 corrects for the 1:1 (or 50:50 substitution) ratio of corn to pulse ingredient in the mixture.
Digestible indispensable amino acid scores-like values
A version of digestible indispensable amino acid scores (DIAAS-like) was calculated to determine the protein quality of the pulse ingredients based on the standardized amino acid digestibility from the cecectomized roosters. The DIAAS-like values were calculated according to Mathai et al. (2017). A reference protein (mg/g) was calculated from both the Association of American Feed Control Officials (AAFCO) and the National Research Council (NRC) by determining how much of each indispensable amino acid (mg) was present in 1 g of protein based on canine and feline adult maintenance requirements. Similarly, the amount of each indispensable amino acid (mg) present in 1 g of protein of the pulse ingredient was calculated. The DIAAS-like score could then be calculated as follows:
A DIAAS-like score of 100% or above is considered to be high protein quality for dogs and cats, scores less than 100% but higher than 50% are moderate protein quality, and scores below 50% are considered to be insufficient as a primary source for the respective amino acid (Mathai et al., 2017). For each ingredient, the amino acid with the lowest DIAAS-like score is the first-limiting amino acid. Ingredients with DIAAS-like scores over 100% for all amino acids meet the requirements of the animal and, therefore have no amino acid that is limiting.
Statistical analyses
All data were analyzed in SAS (SAS Institute Inc., version 9.4, Cary, NC) using the Mixed Models procedure. The model for the precision-fed rooster assay was performed with a fixed effect of treatment and a random effect of rooster. Differences between treatments were reported using a Fisher-protected least significant difference test with a Tukey adjustment to control for a type-1 experiment-wise error. Differences between treatments were considered statistically significant using a probability of P < 0.05. The standard errors of the mean (SEM) were reported based on the Mixed Models procedure in SAS.
Results and Discussion
Chemical composition of pulses
The chemical composition of the pulse ingredients was similar (Table 1). The DM content of the pulses ranged from 89.6% to 92.4%. The remaining proximate analyses were measured on a dry matter basis (DMB). The OM content was approximately 97% for the five pulses. CP was consistent with the BB and NB having the lowest CP content of 20.8% and GL having the highest at 27.2%. Boye et al. (2010) analyzed the chemical composition of several varieties of raw peas, chickpeas, lentils, and beans. The CP content of these pulses ranged from 16.9% to 31.4%. Kabuli type chickpeas, from three different countries of origin, had an average CP content of 22.1%, the lentils had an average CP content of 28%, and the beans (two varieties of kidney beans) had an average of 20.2% (Boye et al., 2010).
Table 1.
Chemical composition of select pulse proteins
| Treatments | |||||
|---|---|---|---|---|---|
| Item | YP | GL | BB grits | NB powder | GB |
| DM, % | 90.5 | 89.6 | 92.4 | 92.3 | 91.7 |
| ---------- %, DMB ---------- | |||||
| OM, % | 97.1 | 97.2 | 96.1 | 96.5 | 96.7 |
| CP, % | 22.2 | 27.2 | 20.8 | 20.8 | 22.6 |
| AHF, % | 2.1 | 2.6 | 2.9 | 3.6 | 5.4 |
| TDF, % | 34.6 | 34.7 | 29.1 | 29.3 | 33.4 |
| GE, kcal/g; measured | 4.3 | 4.4 | 4.5 | 4.4 | 4.6 |
More variation was seen within the analyzed pulses for TDF (Table 1). The TDF content was similar in YP, GL, and GB at approximately 34%. The BB and NB had a TDF content that was slightly lower at about 29%. In one study that analyzed multiple cultivars of chickpeas, beans, peas, and lentils, the TDF content was slightly lower than what was measured in this study. The average TDF content for the cultivars of chickpeas was 21.8%, beans were 25.8%, peas were 24.7%, and lentils were 20.0% (Chen et al., 2016). However, a different study showed legumes, including green peas, soybeans, and chickpeas, typically contain 20.9% to 46.9% TDF (Mallillin et al., 2008). Because fibrous components were not the main focus of the current study, soluble and insoluble fractions were not measured due to the use of these pulses as protein sources.
The AHF content was lower in the YP, GL, and BB with an average of 2.5% (Table 1). The NB was slightly higher at 3.6% and GB was the highest at 5.4%. The total lipid content of lentils, peas, and beans typically ranges from 1% to 3% (Hall et al., 2017). However, the higher fat content in the GB is consistent with the values previously reported. Raw chickpeas typically have a fat content ranging from 2.7% to 6.5% crude fat (Shad et al., 2009). The GE content was approximately 4.5 kcal/g and is reflective of the AHF content of the individual pulse ingredients. The chemical composition of these ingredients, with high protein and fiber and low-fat content, has made pulses easily incorporated into pet foods and a popular ingredient choice for several decades (Mansilla et al., 2019).
The essential amino acid profile (Table 2) showed that the pulses have a comparable essential amino acid content. The essential amino acid content can vary based on growing environment and genotype but most pulse proteins are consistently deficient in methionine and cysteine (Agarwal, 2017). According to the NRC (2006), the minimum requirement for methionine for adult dogs at maintenance is 0.26% DMB. The YP (0.23%), GL (0.21%), and NB (0.23%) fell below the minimum requirement set by the NRC (2006) for adult dogs and would, therefore, require supplementation with an additional protein source when used in canine diets. Pulses, with their high lysine concentration, have a complementary amino acid profile to cereal grains, which are traditionally low in lysine and high in methionine (Robinson et al., 2019). Similar to methionine, the concentration of tryptophan in GL (0.10% DMB) fell below the NRC minimum requirement of 0.11%. However, the remaining essential amino acids in these pulses exceeded the recommended allowances for adult dogs at maintenance. Nosworthy et al. (2017) analyzed the amino acid profile of a variety of pulses and reported similar methionine and tryptophan concentrations. In their study, the methionine content of whole GL was the same at 0.21% (DMB), whereas a higher tryptophan content of 0.21% (DMB) was observed in contrast with the GL analyzed in the current study. Those same authors also reported the lowest methionine content of chickpeas (0.15%; DMB) whereas GB in the current study had the highest methionine content at 0.33% (DMB). Of the 10 pulses analyzed by Nosworthy et al. (2017), only 2 (split red lentils and split green peas) met the NRC minimum requirement for methionine, both of which had a concentration of 0.26%. Traditional animal protein sources, such as chicken-based ingredients, have higher concentrations of all essential amino acids compared with pulse ingredients and generally exceed the NRC recommended allowances (Faber et al., 2010; Oba et al., 2019).
Table 2.
Essential amino acid profile of select pulse proteins
| %, DMB | Treatment | ||||
|---|---|---|---|---|---|
| Essential amino acids | YP | GL | BB grits | NB powder | GB |
| Arginine | 1.82 | 2.00 | 1.25 | 1.16 | 2.31 |
| Histidine | 0.56 | 0.64 | 0.66 | 0.63 | 0.58 |
| Isoleucine | 1.04 | 1.18 | 1.15 | 1.08 | 1.04 |
| Leucine | 1.66 | 1.89 | 1.85 | 1.75 | 1.64 |
| Lysine | 1.77 | 1.82 | 1.63 | 1.55 | 1.56 |
| Methionine | 0.23 | 0.21 | 0.28 | 0.23 | 0.33 |
| Phenylalanine | 1.13 | 1.30 | 1.35 | 1.26 | 1.32 |
| Threonine | 0.85 | 0.89 | 1.02 | 0.96 | 0.76 |
| Tryptophan | 0.17 | 0.10 | 0.25 | 0.26 | 0.20 |
| Valine | 1.17 | 1.32 | 1.36 | 1.30 | 1.07 |
Precision-fed rooster assay
The standardized amino acid digestibility (Table 3) was calculated for the five pulse ingredients based on excreta collected from the cecectomized roosters. The use of cecectomized roosters and the precision-fed rooster assay has proven to be an appropriate model for estimating canine in vivo digestibility, as the model has shown similar results to ileal-cannulated dogs (Johnson et al., 1998). The suitability of cecectomized roosters as a model for feline nutrition has not been definitively established, although it has been accepted that differences between dogs and cats are negligible when protein digestibility is greater than 90% (Kendall et al., 1982; Kerr et al., 2013). Additionally, because all pulse ingredients but BB were raw, the impact of commonly used processing methods in companion animal nutrition (e.g., extrusion) on nutrient digestibility and amino acid bioavailability should be taken into consideration. While the evaluation of raw ingredients can be seen as a limitation, as it does not account for potential changes in nutrient digestibility due to processing, it should be acknowledged that the variety of processing methods used in pet food manufacturing would impact the nutrient bioavailability differently. Therefore, understanding the nutritional value of each raw ingredient is beneficial for diet formulation. Any additional benefit or detriment due to processing can be accounted for separately, based on specific processing validation tests. Pulses were mixed in a 1:1 ratio with raw ground corn in order to minimize the adherence of pulses to the sides of the tube during crop intubation. The addition of corn ensured that all of the pulse ingredients were deposited into the crop of the rooster. The complete amino acid profile and endogenous value for the corn used were analyzed from a single harvest and could, therefore, be factored out of the equation to provide the standardized digestibility of only the pulse ingredient.
Table 3.
Standardized amino acid digestibility of select pulse proteins calculated using precision-fed rooster assay1
| %, DMB | Treatment | |||||
|---|---|---|---|---|---|---|
| Essential amino acids | YP | GL | BB grits | NB powder | GB | SEM |
| Arginine | 93.6ab | 92.3ab | 86.5b | 91.8ab | 94.8a | 2.41 |
| Histidine | 89.1ab | 81.4ab | 80.6b | 88.0ab | 89.9a | 2.93 |
| Isoleucine | 87.2 | 88.1 | 83.3 | 87.9 | 85.8 | 3.29 |
| Leucine | 88.0 | 86.4 | 83.5 | 88.6 | 86.9 | 3.58 |
| Lysine | 89.0 | 88.4 | 82.5 | 86.6 | 84.1 | 3.13 |
| Methionine | 79.1 | 72.9 | 74.6 | 80.6 | 81.9 | 5.22 |
| Phenylalanine | 88.1 | 89.7 | 86.6 | 89.8 | 90.3 | 2.73 |
| Threonine | 90.3 | 88.9 | 81.7 | 84.2 | 88.4 | 4.27 |
| Tryptophan | 86.5 | 87.9 | 85.9 | 90.1 | 85.4 | 3.42 |
| Valine | 87.7 | 86.1 | 80.9 | 85.4 | 85.7 | 4.12 |
1 n = 4 cecectomized roosters per treatment.
a,bMeans within a row with different superscripts are significantly different at P < 0.05.
The essential amino acids, with the exception of methionine, were highly digestible with digestibility values of 80% to 90% (DMB). The digestibility of methionine for YP, GL, and BB ranged from 72% to 79% (DMB). The lowered amino acid digestibility could also be due to the presence of anti-nutritional factors commonly present in pulses, such as trypsin inhibitors, tannins, and lectins, that could negatively impact protein utilization (Jain et al., 2009). The methionine digestibility for NB and GB were 80.6% and 81.9%, respectively, and could be considered highly digestible. BB grits had the lowest digestibility (P < 0.05) of arginine (86.5%) and histidine (80.6%) with GB having the highest (P < 0.05) arginine (94.8%) and histidine (89.9%) digestibility. One study measured the standardized amino acid digestibility of soybean meal, a legume and traditional plant protein source, using cecectomized roosters and all essential amino acids were highly digestible, ranging from 85.1% to 90.9% (Kim et al., 2012).
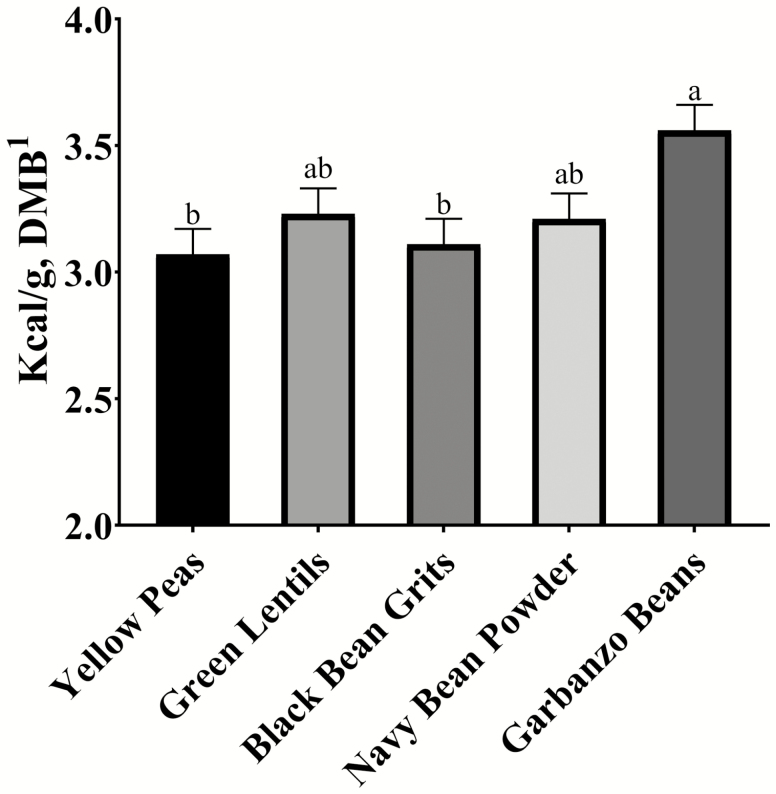
The TMEn values were calculated for each of the pulse proteins (Figure 1) by accounting for endogenous energy losses and higher nitrogen losses in fasted birds when compared with fed birds. The correction for these losses provides a more accurate measure of true metabolizable energy (Parsons et al., 1982). The TMEn of the corn was analyzed from the same harvest that was used in the trial and could, therefore, be factored out of the equation. For the pulse ingredients, GB had a higher (P < 0.05) TMEn value of 3.56 kcal/g (DMB) than YP (3.07 kcal/g) and BB (3.11 kcal/g). The GL and NB ingredients were not significantly different (P > 0.05) than any of the other pulse ingredients. The GB ingredient is comparable to the TMEn of chicken meal, which was reported by Oba et al. (2019) to be 3.72 kcal/g (DMB). However, retorted, steamed, and raw chicken had higher TMEn values, with an average of 5.59 kcal/g (DMB), which indicates that different processing methods could have an effect (Oba et al., 2019). In the current study, the cooked BB was significantly lower (P < 0.05) than the raw GB and GL, but not significantly different (P > 0.05) from the remaining raw pulses. Another study found other animal protein sources (pork peptone, lamb, calamari, alligator, venison, duck, and chicken meals) had TMEn values that ranged from 3.12 kcal/g (DMB) to 4.82 kcal/g (DMB), demonstrating the large amount of variability that exists within protein sources. Lamb meal had the most similar TMEn value (3.12 kcal/g) to the analyzed pulse ingredients (Deng et al., 2016). In terms of plant proteins, the TMEn value of dehulled soybean meal was calculated to be 2.95 kcal/g (DMB) which is slightly lower than the pulses (Parsons et al., 1982).
Figure 1.
TMEn of selected pulse proteins. a,bMeans with different superscripts are significantly different at P < 0.05.
DIAAS-like values
Traditional DIAAS values are calculated using standardized amino acid digestibility from ileal-cannulated pigs and the estimated average requirement of 2-5-yr-old children used as a reference protein (Marinangeli and House, 2017). In this study, the DIAAS-like values were calculated using standardized amino acid digestibility from the cecectomized roosters and reference proteins of AAFCO recommended values and NRC recommended allowances for adult maintenance dogs (Tables 4 and 5) and cats (Tables 6 and 7). Unlike the FAO-established method of determining protein quality, known as protein digestibility-corrected amino acid scores, DIAAS scores are not truncated at 100% to avoid underestimating the quality of high-quality protein sources (Mathai et al., 2017; FAO/WHO, 1991).
Table 4.
DIAAS-like1 for select pulse proteins compared with AAFCO recommended values requirements for adult dogs at maintenance
| %, DMB | Treatment | |||||
|---|---|---|---|---|---|---|
| Essential amino acids | YP | GL | BB grits | NB powder | GB | SEM |
| Arginine | 270.2b | 238.6c | 172.3d | 179.7d | 340.6a | 6.062 |
| Histidine | 210.9b | 179.4c | 225.8ab | 249.4a | 216.1b | 7.899 |
| Isoleucine | 193.8abc | 181.1c | 205.5ab | 215.8a | 186.8bc | 7.441 |
| Leucine | 174.5bc | 158.9c | 185.3ab | 197.0a | 166.7bc | 7.176 |
| Lysine | 202.4a | 168.4b | 173.6b | 183.5ab | 165.2b | 6.415 |
| Methionine | 44.5b | 30.5c | 51.3b | 48.3b | 64.8a | 3.012 |
| Phenylalanine | 178.6b | 170.7b | 210.9a | 216.2a | 209.8a | 6.107 |
| Threonine | 129.9ab | 109.1c | 141.6a | 145.5a | 111.3bc | 6.274 |
| Tryptophan | 74.6d | 36.4e | 109.5b | 126.5a | 84.9c | 3.158 |
| Valine | 169.3bc | 152.7c | 182.4ab | 194.8a | 148.4c | 7.999 |
1DIAAS-like (%) = [(mg of digestible indispensable amino acid in 1 g of dietary protein)/mg of same indispensable AA in 1 g of reference protein)] × 100.
a–eMeans within a row with different superscripts are significantly different at P < 0.05.
Table 5.
DIAAS-like1 for select pulse proteins compared with NRC recommended allowances for adult dogs at maintenance
| %, DMB | Treatment | |||||
|---|---|---|---|---|---|---|
| Essential amino acids | YP | GL | BB grits | NB powder | GB | SEM |
| Arginine | 219.6b | 193.9c | 139.9d | 146.0d | 276.8a | 4.925 |
| Histidine | 118.4b | 100.8c | 126.8ab | 140.1a | 121.4b | 4.436 |
| Isoleucine | 107.7ab | 100.6b | 114.2a | 119.9a | 103.8ab | 4.134 |
| Leucine | 96.9bc | 88.3c | 102.9ab | 109.5a | 92.6bc | 3.986 |
| Lysine | 203.0a | 168.9b | 174.2b | 184.1ab | 165.7b | 6.435 |
| Methionine | 24.9b | 17.1c | 28.7b | 26.9b | 36.2a | 1.684 |
| Phenylalanine | 99.7b | 95.3b | 117.7a | 120.6a | 117.0a | 3.409 |
| Threonine | 80.6ab | 67.7c | 87.8a | 90.2a | 69.0bc | 3.890 |
| Tryptophan | 47.4d | 23.1e | 69.5b | 80.3a | 53.9c | 2.006 |
| Valine | 94.4bc | 85.1c | 101.7ab | 108.7a | 82.8c | 4.461 |
1DIAAS-like (%) = [(mg of digestible indispensable amino acid in 1 g of dietary protein)/mg of same indispensable AA in 1 g of reference protein)] × 100.
a–eMeans within a row with different superscripts are significantly different at P < 0.05.
Table 6.
DIAAS-like1 for select pulse proteins compared with AAFCO recommended values for adult cats at maintenance
| %, DMB | Treatment | |||||
|---|---|---|---|---|---|---|
| Essential amino acids | YP | GL | BB grits | NB powder | GB | SEM |
| Arginine | 192.1b | 169.7c | 122.5d | 127.8d | 242.2a | 4.311 |
| Histidine | 187.5b | 159.5c | 200.8ab | 221.8a | 192.1b | 7.025 |
| Isoleucine | 204.5abc | 191.1c | 216.9ab | 227.7a | 197.2bc | 7.851 |
| Leucine | 138.2bc | 125.9c | 146.8ab | 156.1a | 132.1bc | 5.684 |
| Lysine | 222.1a | 184.8b | 190.5b | 201.4ab | 181.2b | 7.038 |
| Methionine | 106.8b | 73.2c | 123.1b | 115.7b | 155.4a | 7.222 |
| Phenylalanine | 277.7b | 265.4b | 327.9a | 336.2a | 326.1a | 9.496 |
| Threonine | 123.0ab | 103.3c | 134.1a | 137.8a | 105.5bc | 5.944 |
| Tryptophan | 107.8d | 52.5e | 158.2b | 182.8a | 122.8c | 4.566 |
| Valine | 193.9bc | 174.9c | 209.0ab | 223.3a | 170.1c | 9.166 |
1DIAAS-like (%) = [(mg of digestible indispensable amino acid in 1 g of dietary protein)/mg of same indispensable AA in 1 g of reference protein)] × 100.
a–eMeans within a row with different superscripts are significantly different at P < 0.05.
Table 7.
DIAAS-like1 for select pulse proteins compared with NRC recommended allowances for adult cats at maintenance
| %, DMB | Treatment | |||||
|---|---|---|---|---|---|---|
| Essential amino acids | YP | GL | BB grits | NB powder | GB | SEM |
| Arginine | 199.6b | 176.3c | 127.3d | 132.8d | 251.6a | 4.477 |
| Histidine | 173.1b | 147.3c | 185.3ab | 204.7a | 177.4b | 6.484 |
| Isoleucine | 190.3abc | 177.8c | 201.8ab | 211.9a | 183.4bc | 7.304 |
| Leucine | 129.3bc | 117.7c | 137.3ab | 145.9a | 123.5bc | 5.315 |
| Lysine | 418.0a | 347.8b | 358.6b | 379.0ab | 341.1b | 13.249 |
| Methionine | 96.6b | 66.2c | 111.4b | 104.7b | 140.6a | 6.535 |
| Phenylalanine | 224.2b | 214.3b | 264.8a | 271.4a | 263.4a | 7.668 |
| Threonine | 133.2ab | 111.9c | 145.2a | 149.2a | 114.2bc | 6.435 |
| Tryptophan | 102.0d | 49.7e | 149.7b | 172.9a | 116.2c | 4.321 |
| Valine | 181.4bc | 163.6c | 195.5ab | 208.8a | 159.1c | 8.573 |
1DIAAS-like (%) = [(mg of digestible indispensable amino acid in 1 g of dietary protein)/mg of same indispensable AA in 1 g of reference protein)] × 100.
a–eMeans within a row with different superscripts are significantly different at P < 0.05.
The DIAAS-like scores for dogs compared with the AAFCO (2018) recommended values showed scores ranging from 109.1% to 270.2% for all essential amino acids except methionine and tryptophan. For YP, GL, and NB, the methionine scores fell below 50% indicating a need for a supplemental protein source to meet the maintenance requirement for dogs. As the lowest DIAAS-like score, methionine is the first-limiting amino acid in all five of these pulse ingredients when compared with AAFCO values for adult dogs. For tryptophan, YP and GB had moderate scores of 74.6% and 84.9%, respectively, while GL had the lowest score for tryptophan at 36.4% (P < 0.05). The DIAAS-like scores for dogs compared with the NRC recommended allowances showed low methionine and tryptophan scores for all five pulse sources, with GL as the lowest (P < 0.05) at 17.1% and 23.1%, respectively. Methionine had the lowest DIAAS-like score and was the first-limiting amino acid for all five pulse ingredients when compared with NRC values for adult dogs. All five pulse ingredients had moderate scores for threonine. Additionally, moderate scores were seen for leucine and valine in YP, GL, and GB and for phenylalanine in both YP and GL. Minimal data utilizing DIAAS-like values in companion animal nutrition have been published. A recent study examined DIAAS-like values for adult dogs and cats at maintenance for chicken meal, retorted chicken, steamed chicken, and raw chicken, common ingredients used in pet food formulations. When compared with AAFCO values for adult dogs, methionine was the first-limiting amino acid for chicken meal while tryptophan was the first-limiting amino acid for the other chicken-based ingredients (Oba et al., 2019). These findings corroborate with ours, as when compared with the NRC recommended allowances for adult dogs, the first-limiting amino acid for all five selected pulses was methionine with several other amino acids, including tryptophan, not sufficiently meeting maintenance requirements based on the individual pulse ingredient.
The DIAAS-like scores for adult cats at maintenance compared with the AAFCO recommended values showed all pulse ingredients to be high-quality protein with only GL having a moderate score for methionine (73.2%) and tryptophan (52.5%). Therefore, tryptophan is the first-limiting amino acid for GL. The rest of the DIAAS-like scores ranged from 103.3% to 336.3%, and thus a first-limiting amino acid could not be identified for the remaining ingredients. Compared with the NRC recommended allowances for adult cats at maintenance, the GL had an insufficient score of 49.7% for tryptophan and a moderate score of 66.2% for methionine. For both of these amino acids, GL was the lowest (P < 0.05). Similarly, YP had a moderate methionine score of 96.6%. The remaining DIAAS-like scores for the ingredients ranged from 102.0% to 418.0%. The first-limiting amino acids could be identified for YP (methionine) and GL (tryptophan). Oba et al. (2019) reported that threonine was the first-limiting amino acid for chicken-based ingredients when using the AAFCO values and NRC recommended allowances for adult cats as reference proteins. Specifically, chicken meal had the lowest DIAAS-like value of 91.5% (AAFCO) and 98.8% (NRC) for threonine.
Nosworthy et al. (2017) calculated DIAAS values for 10 varieties of pulses using fecal nitrogen digestibility compared with the reference protein pattern for children 6 mo to 3 yr of age. They report the first-limiting amino acids for NB, whole GL, split YP, and BB were the sulfur-containing amino acids, methionine and cysteine, with an average DIAAS value of 61%. The first-limiting amino acid for GB was tryptophan, with a DIAAS value of 67%. While similar to the results of the pulse ingredients used in our study, differences can be attributed to the use of fecal nitrogen digestibility instead of standardized ileal amino acid digestibility and reference protein pattern. Mathai et al. (2017) calculated traditional DIAAS scores for experimental swine diets containing either 25% pea protein concentrate, 21% soy protein isolate, or 35% soy flour. The first-limiting amino acids for all three diets were the sulfur-containing amino acids (methionine and cysteine) with DIAAS values ranging from 62% (pea protein concentrate) to 89% (soy flour).
Conclusions
The results of this study demonstrate that pulse ingredients have a macronutrient composition that is beneficial in many pet food formulas. With high protein and fiber concentrations and a low fat content, pulse ingredients can provide nutritional benefits in areas such as weight control (Kim et al., 2016). Pulse ingredients have an essential amino acid profile that is adequate to meet the majority of the amino acid requirements established by the NRC with the exception of methionine. Cereal grains have complementary amino acid profiles that can provide supplemental methionine and a more complete essential amino acid profile (Samaranayaka et al., 2017). The results of the DIAAS-like calculations show that pulse proteins have the capability to provide high-quality protein in both canine and feline foods. The inclusion of plant-based proteins may contribute to the sustainability of the pet food industry by providing inexpensive, low-input alternatives to animal protein sources, especially those classified as human-grade animal protein sources directly competing with human food system. Based on macronutrient composition, protein quality, and amino acid digestibility, it can be concluded that pulse ingredients have the potential to be viable, novel protein sources in companion animal foods. However, the use of complementary protein sources is recommended to counterbalance any potential limiting amino acids in pulse ingredients. Future studies should evaluate the nutritional adequacy of pulse-rich foods that are assumed to be nutritionally complete and balanced for dogs and cats.
Acknowledgments
We thank Archer Daniels Midland Company for the financial support. C.M.P. and M.R.C.G. designed the experiment. L.M.R., P.C.S., and P.L.U. performed the animal trials and laboratory analyses. L.M.R. and M.R.C.G. performed the statistical analyses. L.M.R wrote the manuscript. All authors provided intellectual input and reviewed this manuscript.
Glossary
Abbreviations
- AAFCO
Association of American Feed Control Officials
- AHF
acid hydrolyzed fat
- BB
black bean grit
- CP
crude protein
- DIAAS-like
digestible indispensable amino acid score
- DM
dry matter
- DMB
dry matter basis
- GB
garbanzo bean
- GE
gross energy
- GL
green lentil
- NB
navy bean powder
- NRC
National Research Council
- OM
organic matter
- TDF
total dietary fiber
- TMEn
true metabolizable energy corrected for nitrogen
- YP
yellow peas
Conflict of interest statement
L.M.R., P.C.S., P.L.U., C.M.P., and M.R.C.G. have no conflict of interest to declare. J.M.H. and G.M.D. are employed by ADM, company that supported this research.
Literature Cited
- Agarwal A. 2017. Proteins in pulses. J. Nutr. Disorders Ther. 7:1. doi: 10.4172/2161-0509.1000e129 [DOI] [Google Scholar]
- American Association of Cereal Chemists (AACC) 1983. Approved methods. 8th ed. St. Paul (MN):AACC. [Google Scholar]
- AOAC 2006. Official methods of analysis. 17th ed. Arlington (VA):AOAC. [Google Scholar]
- AOAC 2007. Official methods of analysis. 17th ed. Arlington (VA):AOAC. [Google Scholar]
- Association of American Feed Control Officials (AAFCO) 2018. Official publication. Champaign (IL):AAFCO. [Google Scholar]
- Boye J., Zare F., and Pletch A.. . 2010. Pulse proteins: processing, characterization, functional properties and applications in food and feed. Food Res. Int. 43:414–431. doi: 10.1016/j.foodres.2009.09.003 [DOI] [Google Scholar]
- Budde E. F. 1952. The determination of fat in baked biscuit type of dog foods. J. AOAC Int. 35:799–805. doi: 10.1093/jaoac/35.3.799 [DOI] [Google Scholar]
- Chen Y., McGee R., Vandemark G., Brick M., and Thompson H. J.. . 2016. Dietary fiber analysis of four pulses using AOAC 2011.25: implications for human health. Nutrients 8:E829. doi: 10.3390/nu8120829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng P., Utterback P. L., Parsons C. M., Hancock L., and Swanson K. S.. . 2016. Chemical composition, true nutrient digestibility, and true metabolizable energy of novel pet food protein sources using the precision-fed cecectomized rooster assay. J. Anim. Sci. 94:3335–3342. doi: 10.2527/jas.2016-0473 [DOI] [PubMed] [Google Scholar]
- Duranti M., and Gius C.. . 1997. Legume seeds: protein content and nutritional value. Field Crops Res. 53:31–45. doi: 10.1016/S0378-4290(97)00021-X [DOI] [Google Scholar]
- Dust J. M., Grieshop C. M., Parsons C. M., Karr-Lilienthal L. K., Schasteen C. S., Quigley J. D. 3rd, Merchen N. R., and Fahey G. C. Jr. 2005. Chemical composition, protein quality, palatability, and digestibility of alternative protein sources for dogs. J. Anim. Sci. 83:2414–2422. doi: 10.2527/2005.83102414x [DOI] [PubMed] [Google Scholar]
- Faber T. A., Bechtel P. J., Hernot D. C., Parsons C. M., Swanson K. S., Smiley S., and Fahey G. C. Jr. 2010. Protein digestibility evaluations of meat and fish substrates using laboratory, avian, and ileally cannulated dog assays. J. Anim. Sci. 88:1421–1432. doi: 10.2527/jas.2009-2140 [DOI] [PubMed] [Google Scholar]
- Fiacco D. C., Lowe J. A., Wiseman J., and White G. A.. . 2017. Evaluation of vegetable protein in canine diets: assessment of performance and apparent ilea amino acid digestibility using a broiler model. J. Anim. Physiol. Anim. Nutr. 102:e442–e448. doi: 10.1111/jpn.12764 [DOI] [PubMed] [Google Scholar]
- Food and Agriculture Organization of the United Nations. 1991. Report of a joint FAO/WHO expert consultation. Protein quality evaluation. Available from https://apps.who.int/iris/bitstream/handle/10665/38133/9251030979_eng.pdf;jsessionid=6952945D411B84EE71035A9C120AF970?sequence=1 [accessed December 10, 2019].
- Food and Agricultural Organization of the United Nations 2016. International year of the pulses Available from http://www.fao.org/zhc/detail-events/en/c/462585/ [accessed January 10, 2019].
- Hall C., Hillen C., and Robinson J. G.. . 2017. Composition, nutritional value, and health benefits of pulses. Cereal Chem. 94:11–31. doi: 10.1094/CCHEM-03-16-0069-FI [DOI] [Google Scholar]
- Jain A. K., Kumar S., and Panwar J. D. S.. . 2009. Antinutritional factors and their detoxification in pulses: a review. Agric. Rev. 30:64–70. [Google Scholar]
- Johnson M. L., Parsons C. M., Fahey G. C. Jr, Merchen N. R., and Aldrich C. G.. . 1998. Effects of species raw material source, ash content, and processing temperature on amino acid digestibility of animal by-product meals by cecectomized roosters and ileally cannulated dogs. J. Anim. Sci. 76:1112–1122. doi: 10.2527/1998.7641112x [DOI] [PubMed] [Google Scholar]
- Kendall P. T., Holme D. W., and Smith P. M.. . 1982. Comparative evaluation of net digestive and absorptive efficiency in dogs and cats fed a variety of contrasting diet types. J. Small Anim. Prac. 23:577–587. doi: 10.1111/j.1748-5827.1982.tb02518.x [DOI] [Google Scholar]
- Kerr K. R., Beloshapka A. N., and Swanson K. S.. . 2013. 2011 and 2012 Early Careers Achievement Awards: use of genomic biology to study companion animal intestinal microbiota. J. Anim. Sci. 91:2504–2511. doi: 10.2527/jas.2012-6225 [DOI] [PubMed] [Google Scholar]
- Kim S. J., de Souza R. J., Choo V. L., Ha V., Cozma A. I., Chiavaroli L., Mirrahimi A., Blanco Mejia S., Di Buono M., Bernstein A. M., . et al. 2016. Effects of dietary pulse consumption on body weight: a systematic review and meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 103:1213–1223. doi: 10.3945/ajcn.115.124677 [DOI] [PubMed] [Google Scholar]
- Kim E. J., Utterback P. L., and Parsons C. M.. . 2012. Comparison of amino acid digestibility coefficients for corn, corn gluten meal, and corn distillers dried grains with solubles among 3 different bioassays. Poult. Sci. 91:3141–3147. doi: 10.3382/ps.2012-02418 [DOI] [PubMed] [Google Scholar]
- Mallillin A. C., Trinidad T. P., Raterta R., Dagbay K., and Loyola A. S.. . 2008. Dietary fibre and fermentability characteristics of root crops and legumes. Br. J. Nutr. 100:485–488. doi: 10.1017/S000711450891151X [DOI] [PubMed] [Google Scholar]
- Mansilla W. D., Marinangeli C. P. F., Ekenstedt K. J., Larsen J. A., Aldrich G., Columbus D. A., Weber L., Abood S. K., and Shoveller A. K.. . 2019. Special Topic: The association between pulse ingredients and canine dilated cardiomyopathy: addressing the knowledge gaps before establishing causation1. J. Anim. Sci. 97:983–997. doi: 10.1093/jas/sky488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinangeli C. P. F., and House J. D.. . 2017. Potential impact of the digestible indispensable amino acid score as a measure of protein quality on dietary regulation and health. Nutr. Rev. 75:658–667. doi: 10.1093/nutrit/nux025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathai J. K., Liu Y., and Stein H. H.. . 2017. Values for digestible indispensable amino acid scores (DIAAS) for some dairy and plant proteins may better describe protein quality than values calculated using the concept for protein digestibility-corrected amino acid scores (PDCAAS). Br. J. Nutr. 117:490–499. doi: 10.1017/S0007114517000125 [DOI] [PubMed] [Google Scholar]
- McCrory M. A., Hamaker B. R., Lovejoy J. C., and Eichelsdoerfer P. E.. . 2010. Pulse consumption, satiety, and weight management. Adv. Nutr. 1:17–30. doi: 10.3945/an.110.1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council (NRC). 2006. Nutrient requirements of dogs and cats. 2nd ed. Washington (DC):National Academies Press. [Google Scholar]
- Nosworthy M. G., Neufeld J., Frohlich P., Young G., Malcolmson L., and House J. D.. . 2017. Determination of the protein quality of cooked Canadian pulses. Food Sci. Nutr. 5:896–903. doi: 10.1002/fsn3.473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oba P. M., Utterback P. L., Parsons C. M., de Godoy M. R. C., and Swanson K. S.. . 2019. Chemical composition, true nutrient digestibility, and true metabolizable energy of chicken-based ingredients differing by processing method using the precision-fed cecectomized rooster assay1. J. Anim. Sci. 97:998–1009. doi: 10.1093/jas/sky461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons C. M. 1985. Influence of caecectomy on digestibility of amino acids by roosters fed distillers’ dried grains with solubles. J. Agric. Sci. 104:469–472. doi: 10.1017/S0021859600044178 [DOI] [Google Scholar]
- Parsons C. M., Potter L. M., and Bliss B. A.. . 1982. True metabolizable energy corrected to nitrogen equilibrium. Poult. Sci. 61:2241–2246. doi: 10.3382/ps.0612241 [DOI] [Google Scholar]
- Prosky L., Asp N. G., Schweizer T. F., De Vries J. W., and Furda I.. . 1992. Determination of insoluble and soluble dietary fiber in foods and food products: collaborative study. J. Assoc. Off. Anal. Chem. 75:360–367. doi: 10.1093/jaoac/71.5.1017 [DOI] [Google Scholar]
- Robinson G. H. J., Balk J., and Domoney C.. . 2019. Improving pulse crops as a source of protein, starch, and micronutrients. Nutr. Bull. 44:202–215. doi: 10.1111/nbu.12399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaranayaka A. 2017. Lentil: revival of poor man’s meat. In: Nadathur, S. R., J. P. D. Wanasundara, and L. Scanlin, editors. Sustainable protein sources; Cambridge (MA): Academic Press; p. 185–196. doi: 10.1016/B978-0-12-802778-3.00011-1. [DOI] [Google Scholar]
- Shad M. A., Pervez H., Zafar Z. I., Zia-Ul-Haq M., and Nawaz H.. . 2009. Evaluation of biochemical composition and physiochemical parameters of oil from seeds of desi chickpea varieties cultivated in arid zone of Pakistan. Pak. J. Bot. 41:655–662. [Google Scholar]
- Sibbald I. R. 1979. A bioassay for available amino acids and true metabolizable energy in feedingstuffs. Poult. Sci. 58:668–673. doi: 10.3382/ps.0580668 [DOI] [PubMed] [Google Scholar]