Abstract
OBJECTIVE
Von Hippel-Lindau disease (VHL) is a tumor predisposition syndrome characterized by CNS hemangioblastomas (HBs) and clear cell renal cell carcinomas (RCCs) due to hypoxia-inducible factor activation (pseudohypoxia). Because of the lack of effective medical therapies for VHL, HBs and RCCs account for significant morbidity and mortality, ultimately necessitating numerous neurological and renal surgeries. Propranolol is an FDA-approved pan-beta adrenergic antagonist with antitumor effects against infantile hemangiomas (IHs) and possibly VHL HBs. Here, the authors investigated the antitumor efficacy of propranolol against pseudohypoxia-driven VHL-HBs and VHL-RCCs.
METHODS
Patient-derived VHL-associated HBs (VHL-HBs) or 786-O-VHL−/− RCC cells were treated with clinically relevant concentrations of propranolol in vitro and assessed with viability assays, flow cytometry, quantitative real-time polymerase chain reaction, and western blotting. In vivo confirmation of propranolol antitumor activity was confirmed in athymic nude mice bearing 786-O xenograft tumors. Lastly, patients enrolled in a VHL natural history study (NCT00005902) were analyzed for incidental propranolol intake. Propranolol activity against VHL-HBs was assessed retrospectively with volumetric HB growth kinetic analysis.
RESULTS
Propranolol decreased HB and RCC viability in vitro with IC50 (half maximal inhibitory concentration) values of 50 μM and 200 μM, respectively. Similar to prior reports in infantile hemangiomas, propranolol induced apoptosis and paradoxically increased VEGF-A mRNA expression in patient-derived VHL-HBs and 786-O cells. While intracellular VEGF protein levels were not affected by propranolol treatment, propranolol decreased HIF expression in 786-O cells (7.6-fold reduction, p < 0.005). Propranolol attenuated tumor progression compared with control (33% volume reduction at 7 days, p < 0.005) in 786-O xenografted tumor-bearing mice. Three patients (harboring 25 growing CNS HBs) started propranolol therapy during the longitudinal VHL-HB study. HBs in these patients tended to grow slower (median growth rate 27.1 mm3/year vs 13.3 mm3/year) during propranolol treatment (p < 0.0004).
CONCLUSIONS
Propranolol decreases VHL-HB and VHL-related RCC viability in vitro likely by modulation of VEGF expression and by inducing apoptosis. Propranolol abrogates 786-O xenograft tumor progression in vivo, and retrospective clinical data suggest that propranolol curtails HB growth. These results suggest that propranolol may play a role in the treatment of VHL-related tumors.
Keywords: von Hippel-Lindau disease, VHL, hemangioblastoma, renal cell carcinoma, propranolol, translational medicine, oncology
VON Hippel-Lindau (VHL) disease is an inherited tumor predisposition syndrome that is caused by germline mutations in the VHL tumor suppressor gene found on the short arm of chromosome 3.5,11,17 Patients with VHL have a propensity to develop hemangioblastomas (HBs) of the CNS and retina as well as renal cell carcinomas (RCCs), endolymphatic sac tumors, pheochromocytomas, and pancreatic neuroendocrine tumors.5,17 Although the manifestations of the disorder can vary significantly across individuals, the pathophysiology of tumor formation for RCCs, HBs, and pancreatic neuroendocrine tumors is believed to result from inappropriate upregulation of hypoxia response elements.8,25 Patients with VHL harbor a quantitative or qualitative deficiency of the VHL protein, which, under normoxic conditions, fosters the degradation of hypoxia-inducible factors (HIFs).8,17,36 In susceptible tissues, loss of heterozygosity of the wild-type VHL allele leads to constitutive, inappropriate upregulation of HIFs that upregulate expression of a variety of genes, including erythropoietin, vascular endothelial growth factor (VEGF), and transforming growth factor–alpha, which are believed to play important roles in tumorgenesis.3,5, 9, 11,17
As a result of the development of a variety of benign and malignant neoplasms, patients with VHL have a median survival ranging between 40 and 52 years, the shortest of any tumor predisposition syndrome.34 The leading causes of morbidity and mortality in patients with VHL are RCC and CNS HB.5,8,16 Both RCC and HB are common manifestations of VHL, occurring in 30% and 72% of affected individuals, respectively.8,17 The treatment for VHL-related RCC (VHL-RCC) and HBs is surgery. As patients with VHL can develop upward of 600 RCC foci per kidney and 10 or more symptomatic HBs over the course of their lifetimes, they often require multiple renal and neurological surgeries.8,16,33 Although there have been reports on the efficacy of certain antitumor agents for VHL-related HBs (VHL-HBs), no systemic therapy has gained widespread use in curtailing the progression of VHL-HBs or VHL-RCCs.22,28,29 Thus, the identification of a well-tolerated, safe, and easily administered systemic therapy that curbs VHL-HB or VHL-RCC progression would represent a significant advancement in the treatment of patients with VHL disease.
Léauté-Labrèze et al. demonstrated that propranolol, an FDA-approved, nonselective, β1 and β2 adrenergic antagonist used for the treatment of hypertension and cardiac arrhythmias, is effective in treating infantile hemangiomas (IHs), i.e., benign, vascular tumors of infancy.12,13 Given the histological and pathophysiological similarities between IHs and VHL-HBs, it has been hypothesized that propranolol may be an effective treatment in patients with VHL disease. Albiñana et al. showed that propranolol is able to reduce HIF-1α expression in patient-derived HB cells while inducing apoptosis and decreasing cell viability in vitro.2 As a result, we sought to confirm these findings and further expand on these results to determine if propranolol may harbor broader treatment effects in VHL-HBs and VHL-RCCs.
Methods
Clinical Sample Collection
HB samples were obtained under a National Institutes of Health institutional review board–approved protocol (clinicaltrials.gov identifier NCT00060541). All specimens were obtained from individuals undergoing surgery of symptom-producing HBs. Informed consent was obtained from all patients.
HB Stromal Cell Extraction and Cell Culture
HB tumor cells were isolated as previously described.24,30 Briefly, isolated HB cells were incubated at 37°C for 24 hours after isolation in DMEM supplemented with 10% fetal bovine serum, l-glutamine, penicillin, streptomycin, and F-12K. All subsequent experiments were performed within 24–48 hours of HB extraction. The 786-O RCC cell line was obtained from America Type Culture Collection and grown per the distributer’s specification.
Cell Viability Assays
Cells (1 × 104) were plated on flat-bottom 96-well plates and subsequently exposed to 0–400 μM (S)-(−)-propranolol hydrochloride (Sigma-Aldrich) for 24–48 hours at 37°C.7,38 After incubation, the Cell Counting Kit-8 cytotoxicity assay (Dojindo Molecular Technologies) was subsequently performed according to the manufacturer’s protocol. An ELx800 96-well spectrometer (BioTek Instruments) was used to perform all absorbance measurements.
RNA Extraction and Quantitative Real-Time Polymerase Chain Reaction
RNA was extracted from cells using the RNeasy Mini Kit (Qiagen) according to manufacturer’s instructions. Complementary DNA libraries were subsequently created using SuperScript III First-Strand Synthesis SuperMix (Invitrogen Life Technologies). Real-time polymerase chain reaction (RT-PCR) was then performed using QuantStudio 6 Flex RT-PCR system (Applied Biosystems). Gene expression was determined using primers designed for vascular endothelial growth factor (VEGF, Qiagen), glucose transporter 1 (GLUT1, Qiagen), and hypoxia-inducible factor 1α (HIF1α, Qiagen), with β-actin as internal control (Sigma-Aldrich). In all instances, the Power SYBR Green PCR Master Mix was utilized (Applied Biosystems).
Apoptosis Assays
The 786-O cells were cultured in the presence of 0–200 μM (S)-(−)-propranolol hydrochloride (Sigma-Aldrich) for 24–48 hours at 37°C. The Vybrant Apoptosis Assay Kit #5 (Hoechst 33342/propidium iodide, ThermoFisher Scientific) was then used to determine the percentage of necrotic and apoptotic cells in culture according to the manufacturer’s specification.
Immunoblot Analysis
For protein extraction, whole-cell lysates were collected in radioimmunoprecipitation assay buffer containing a protease inhibitor cocktail and centrifuged at 15,295g for 15 minutes at 4°C. Protein concentration was determined by Nanodrop 2000 (Thermo Fisher Scientific). Proteins were electrophoretically separated on 4%–12% Bis-Tris gels (Invitrogen) and subsequently electroblotted onto polyvinylidene difluoride membranes with the Trans-Blot Transfer Turbo System (Bio-Rad). After blocking, the membranes were probed with anti-VEGF (Thermo Fisher Scientific) or anti-HIF2α (sc-46691, Santa Cruz), and the immunoreactive signal was detected with the ChemiDoc MP Imaging System (Bio-Rad). Densitometry was performed using Image Lab software (Bio-Rad).
786-O Xenograft Mouse Model
All animal experiments were approved by the National Institute of Neurological Disorders and Stroke Animal Care and Use Committee. Five- to 6-week-old (n = 10) female athymic nude mice (Jackson Laboratory) were injected in the right flank with 5 × 106 786-O cells as previously described.10 Tumor volume was determined using the formula V = (L × W)/2, where L and W represent the length and width of the tumor, respectively.10 When tumors grew to between 80 and 150 mm3, the mice were randomized to receive daily intraperitoneal injections of propranolol (20 mg/kg) or phosphate-buffered saline (PBS).31 Flank tumor growth was monitored over time. At the end of the study, blood was collected from mice, and the serum was analyzed for levels of mouse and human VEGF-A using VEGF ELISA kits (Thermo Fisher Scientific) per the manufacturer’s instructions.
Immunohistochemistry
When PBS or propranolol-treated mice reached their endpoint (tumor volumes greater than 1000 mm3 or evidence of ulceration), the flank tumors were harvested and fixed in formalin. The fixed tumors were paraffin embedded, and blocks were sectioned at 5-μm thickness. An automated immunostainer (Bond-Max, Leica) was used for reproducible staining of paraffin sections. Tissue was probed using antibodies against HIF2α (Bioss), VEGF (Proteintech), and GLUT1 (Proteintech). Antibodies were optimized using positive control tissue with high pH (EDTA) epitope retrieval.
Incidental use of Propranolol
Patients with VHL disease and HBs who were enrolled in a natural history study (clinicaltrials.gov identifier NCT00005902) were retrospectively assessed for oral propranolol usage. Patients who underwent clinical and radiographic follow-up before and after propranolol implementation were assessed. HBs were assessed using postcontrast T1-weighted MRI. HB volumes were calculated using a modified ellipse formula.16,18 The growth rates of tumors with evidence of growth were determined before and after propranolol therapy was implemented as previously described.16
Statistical Analysis
All in vitro studies were subjected to at least 3 independent biological experiments. Data are presented as the mean ± SEM. Statistical analysis was performed using Prism Software (GraphPad). One-way ANOVA was used for comparing results of RT-PCR. The t-test was used for continuous variables for in vitro and in vivo experiments; p < 0.05 was considered statistically significant.
For the HB-volumetric analysis, the volumetric growth rate per year during a period was estimated as the difference in tumor volume between the first and last clinic visit divided by the tumor volume at the first visit and the duration of tumor follow-up (in years). For each tumor, the growth rate per year was determined before and after propranolol implementation. Since each patient harbored multiple tumors and propranolol was a within-subject factor, a nested model was applied to model both patient and tumor (nested within patient) as random effects while propranolol was treated as a fixed effect.15 Natural logarithm transformation was applied to the growth rates, and the Shapiro-Wilk test was used to test the normality of the residuals. SAS (version 9.4, SAS Institute) was used for the above analyses; p < 0.05 was considered statistically significant.
Results
Propranolol Reduces the Viability of Patient-Derived VHL-HB Cells
In order to confirm the results of Albiñana et al., patient-derived HB cells were cultured from tumors resected from 4 separate individuals with VHL disease. Patient-derived HB primary cultures were then treated with propranolol (0–400 μM) for 24 or 48 hours. Cultured HB viability tended to decrease in both a dose- and time-dependent manner (Fig. 1A–D). HB viability was reduced by 50% (half maximal inhibitory concentration; IC50) with exposure to 50 μM of propranolol within 24 hours. Cells cultured with at least 50 μM of propranolol also demonstrated morphological changes within 48 hours after exposure (Fig. 1B). Cells became rounded, which has been observed in cells undergoing apoptosis in the presence propranolol.2 Treatment with 100 μM of propranolol led to the loss of viability of almost all cells in culture at 24 and 48 hours (Fig. 1C and D).
FIG. 1.
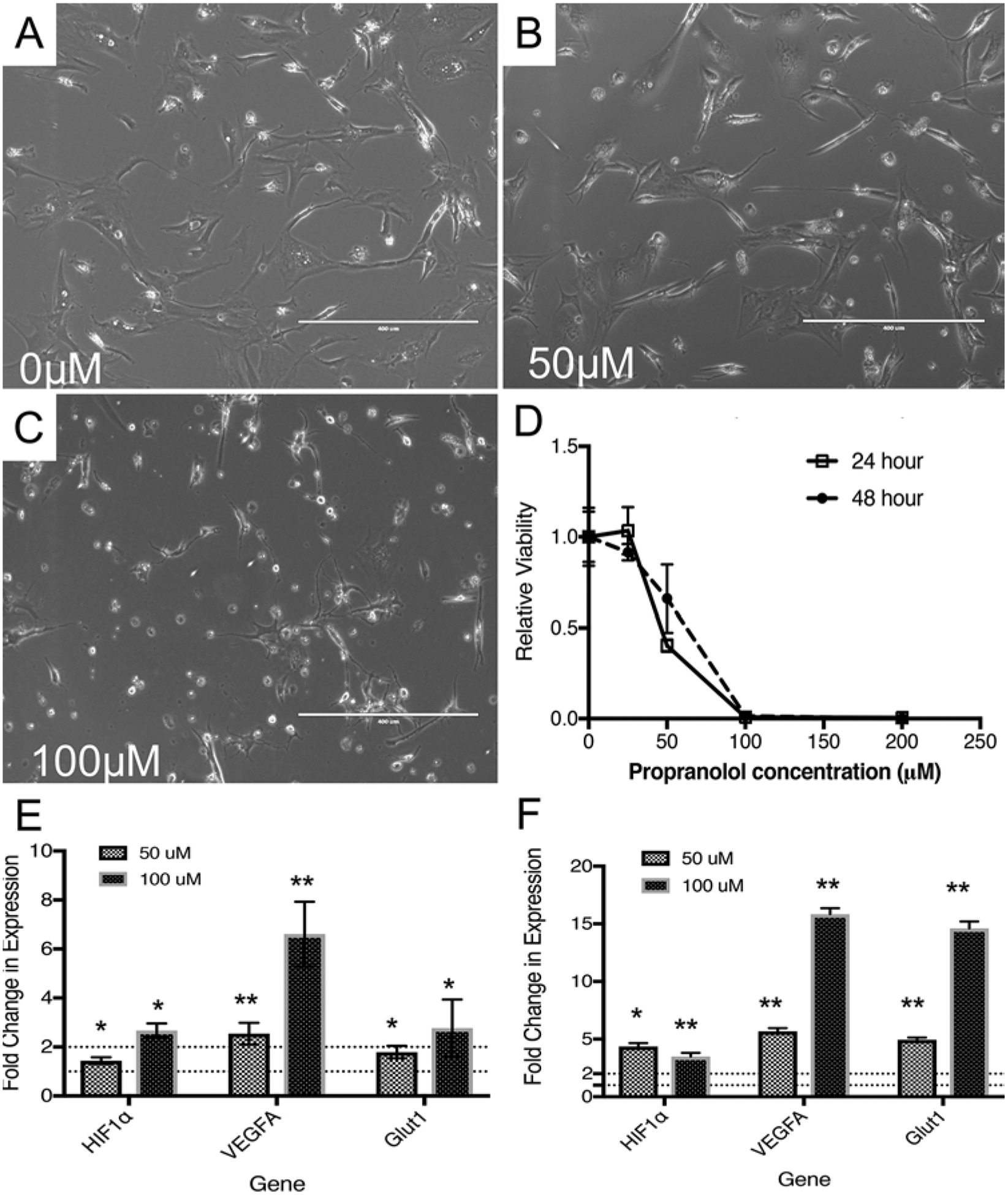
Propranolol leads to decreased HB viability ex vivo. Patient-derived HB cells were cultured and treated with propranolol (0–400 μM). A–C: Representative photomicrographs (×10) of treated HB cells exposed to 0 μM (A), 50 μM (B), and 100 μM (C) of propranolol are shown. Cells exposed to propranolol become rounded (B) prior to completely detaching from the cultureware (C) with increasing concentrations of propranolol. D: Representative viability assays are shown. Propranolol decreases HB viability with an approximate IC50 of 50 μM after 24 or 48 hours of propranolol exposure (n = 3 for each data point, experiments repeated over 4 different HB specimens). E and F: Gene expression analysis of the pseudohypoxia-signaling pathway was performed using RT-PCR (E and F). Propranolol induces HIF-1α, VEGF-A, and GLUT1 mRNA levels at 24 (E) and 48 (F) hours, which has been observed previously in propranolol-treated IHs (n = 3, for each data point). *p < 0.05, **p < 0.0001.
Propranolol Induces HIF, VEGF, and GLUT-1 mRNA Expression in VHL-HB Cells
Propranolol leads to a discordant increase in VEGF mRNA expression while also reducing intracellular VEGF protein expression due to interference with VEGF mRNA translation.7 However, other studies have demonstrated that propranolol may lead to a decrease in VEGF mRNA and protein expression.2 We therefore investigated the effect of propranolol on gene expression within the hypoxia pathway in 2 patient-derived HB primary cultures. On treatment with propranolol (50 μM and 100 μM) for 24 or 48 hours, HIF1α, VEGF-A, and GLUT1 mRNA were overexpressed (Fig. 1E and F). The largest increase in expression was evident after 48 hours of treatment with 100 μM of propranolol (approximately 3.5-fold increase in HIF1α, approximately 15-fold increase in VEGF expression, and approximately 15-fold increase in GLUT1 expression; p < 0.0001).
Propranolol Reduces the Viability of a VHL−/− RCC Cell Line by Inducing Apoptosis
As VHL-HBs are not malignant tumors and no established cell line for HBs exist, we further explored the mechanisms of propranolol activity in the immortalized human RCC cell line (786-O), which lacks a functional VHL gene (VHL−/−). Thus, the 786-O line represents a useful probe for studying VHL-related tumors in vitro.36 Propranolol decreased 786-O viability in vitro, albeit with an IC50 at 200 μM within 24 hours (Fig. 2A). As propranolol has been shown to induce apoptosis in VHL-HBs and IHs, we investigated whether the decreased viability observed in propranolol-treated 786-O cells could be secondary to apoptosis. Using the Hoechst propidium iodide assay, we found that there was a 4.5-fold increase in the population of cells undergoing apoptosis when exposed to 200 μM of propranolol for 24 hours (Fig. 2B, p < 0.0001). Consistent with our viability assays, flow cytometry depicted a substantial reduction in the number of viable cells (72% reduction, p < 0.0001). While this was partly explained by the increased rates of apoptosis, 786-O cells exposed to propranolol had a 6-fold increase in the percentage of necrotic cells (p < 0.0001), suggesting a potential direct toxic effect of propranolol on these cells.
FIG. 2.
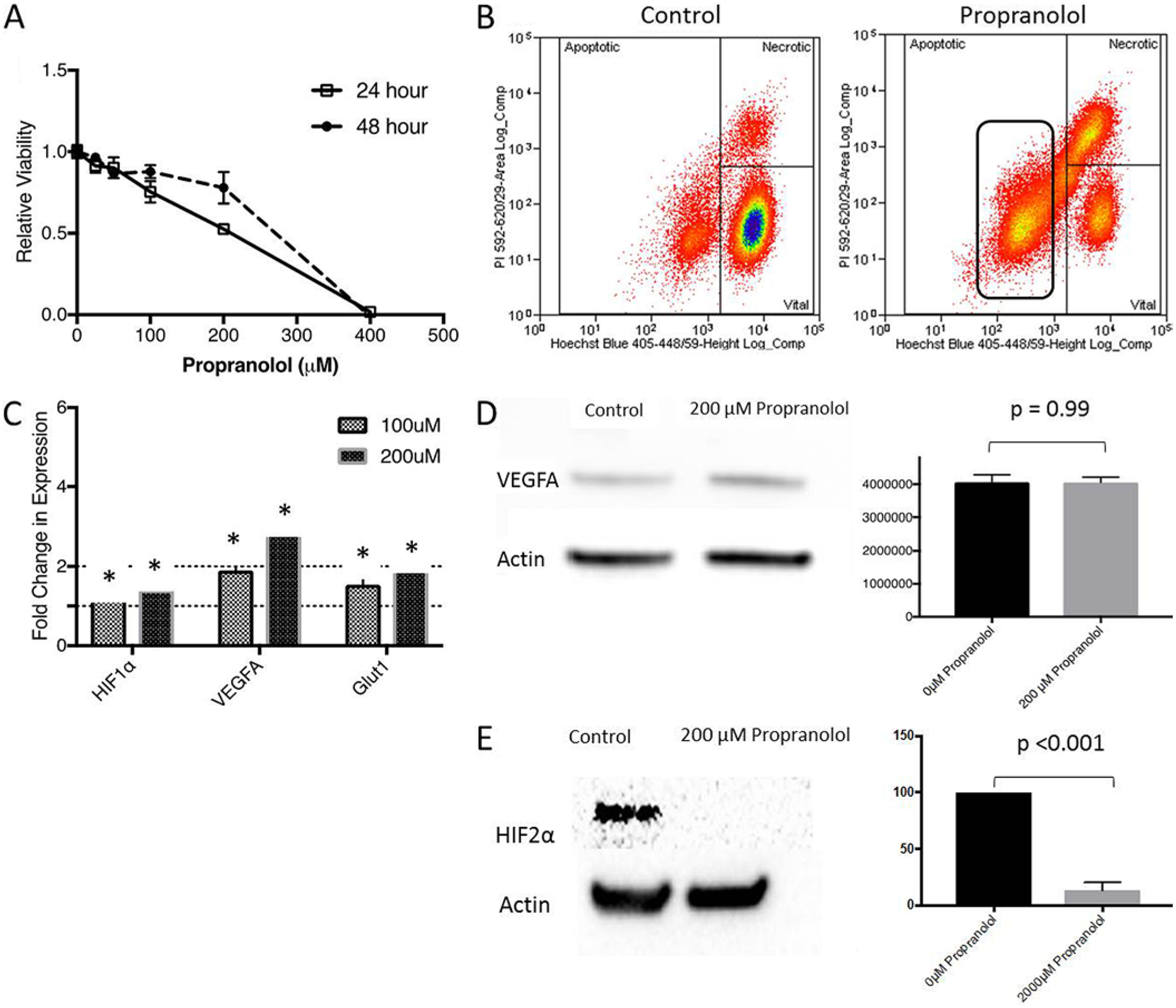
Propranolol decreases RCC cell line viability in vitro by inducing apoptosis. A: Results of viability assays exhibit decreased 786-O viability at 24 or 48 hours in the presence of 200 μM of propranolol. Biological triplicates were performed for each data point shown. B: Flow cytometry was used to investigate the degree of apoptotic, necrotic, and viable cells after treatment with 200 μM of propranolol. Results suggest that treated cells had a 4.5-fold increase in rates of apoptosis (p < 0.0001), a 6-fold increase in rates of necrosis (p < 0.0001), and a 72% reduction in viability (n = 3, p < 0.0001). C: RT-PCR (n = 3) was then performed to investigate gene expression in 786-O cells exposed to 200 μM of propranolol. There were increases in HIF-1α, VEGF-A, and GLUT1 mRNA levels in a dose-dependent manner. This is similar to results observed in IHs. Propranolol had no effect on intracellular VEGF-A protein levels, but did decrease HIF2α expression in 786-O cells (n = 3). D and E: Representative western blots for VEGF (D) and HIF2α (E) are shown. Normalized relative intensity values are shown next to representative immunoblots. *p < 0.05. Figure is available in color online only.
Propranolol Decreases HIF Expression Without Affecting VEGF Expression in 786-O Cells
As propranolol has been shown to affect the hypoxia-signaling pathway in IHs, we investigated whether HIF, VEGF, or GLUT1 expression was affected in 786-O cells treated with propranolol. As was the trend in VHL-HBs, propranolol led to 1.9- and 1.4-fold increases in GLUT1 and HIF mRNA, respectively (p < 0.05, Fig. 2C). In a less dramatic fashion compared with VHL-HBs, propranolol increased VEGF-A mRNA expression approximately 2-fold in 786-O cells (Fig. 2C). We tested whether propranolol inhibits the translation of VEGF-A from its mRNA template.7 Surprisingly, unlike reports in VHL-HBs and IHs, VEGF protein levels were unchanged (Fig. 2D), suggesting that at least in this RCC cell line, the changes in viability observed in 786-O cells seen with increasing propranolol concentrations are not affected by VEGF protein expression in vitro. As other authors have suggested that propranolol may decrease HIF expression in IHs, we assayed HIF2α levels in 786-O cells exposed to propranolol, as HIF2α is the predominant isoform found in 786-O cells.7 We found that propranolol treatment led to a 7.6-fold reduction in HIF2α expression in 786-O cells treated with 200 μM of propranolol (p < 0.001, Fig. 2E).
Propranolol Slows RCC Tumor Growth in a Xenograft Model and Decreases Serum VEGF-A
Next, we sought to confirm these results in an in vivo model of VHL-deficient tumors. As no established animal model exists for CNS HBs, we utilized an RCC-derived flank xenograft model in athymic nude mice.31 Mice were randomized to receive daily intraperitoneal injections of propranolol (20 mg/kg/day) versus PBS after tumors were between 80 and 150 mm3 in volume. Daily administration of propranolol led to slower tumor xenograft tumor progression, which was maintained through 9 days of propranolol therapy (p < 0.005) (Fig. 3A). Prior to euthanizing mice in the study, peripheral blood and serum were harvested from each mouse and analyzed for VEGF-A levels. Treated mice had a 3-fold reduction in circulating mouse VEGF-A levels as determined by ELISA (Fig. 3B, p < 0.0001). Circulating human VEGF-A levels, which may have potentially been secreted by the tumor, were not detectable in either control or propranolol-treated mice. To confirm these findings, we measured the serum VEGF levels of tumor-bearing mice (treated with PBS) versus mice without xenografted tumors (also treated with PBS). We found no statistically significant difference in the serum VEGF levels of tumor-bearing versus non–tumor-bearing mice (Fig. 3C). Interestingly, non–tumor-bearing mice treated with propranolol had no change in their serum VEGF levels (Fig. 3C). Thus, it appears that propranolol selectively decreases the amount of serum VEGF in the setting of xenografted 786-O tumors.
FIG. 3. A:
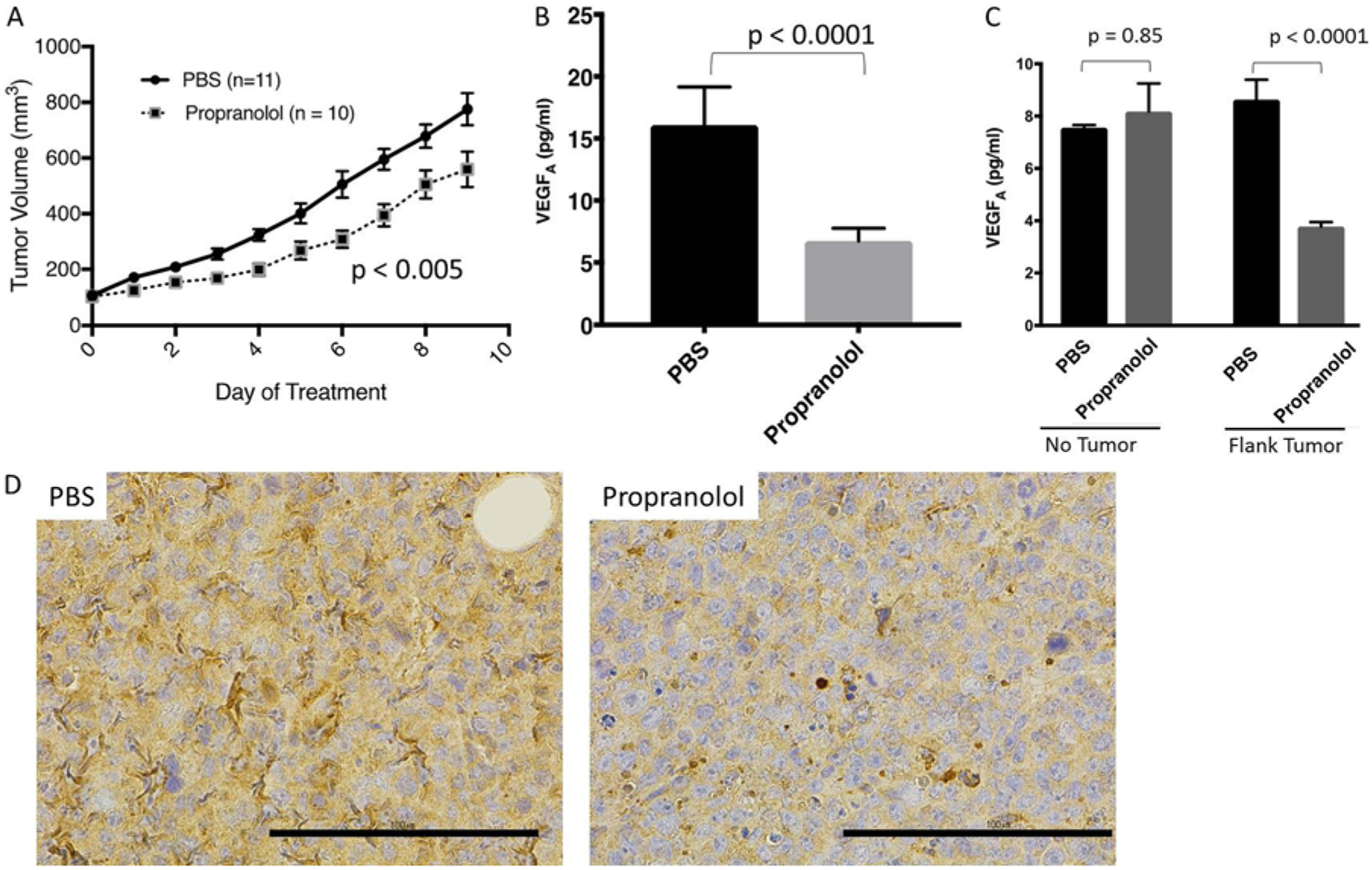
Athymic nude mice bearing 786-O flank tumors have slower rates of tumor progression when treated with propranolol (n = 10) versus PBS (n = 11, p < 0.005). B: Circulating VEGF-A levels in propranolol-treated mice are reduced (p < 0.0001). Human VEGF-A levels are undetectable in both PBS/propranolol treatment groups. C: An additional cohort of mice (n = 12) was treated with PBS or propranolol with (n = 6) or without flank tumors (n = 6). Circulating murine levels of VEGF-A were not affected by the addition of the tumor (p = 0.85). Propranolol treatment decreased the serum levels of murine VEGF-A only in the setting of xenografted 786-O tumors (p < 0.0001). D: There was a qualitative decrease in HIF2α expression in flank tumors, as determined by immunohistochemical analysis in the propranolol-treated mice. Representative photomicrographs are shown (PBS and propranolol, ×40).
We further analyzed the expression of GLUT1, VEGF, and HIF2α in the flank tumors from xenografted mice receiving with PBS versus propranolol. On immunohistochemical analysis, there was no difference in VEGF or GLUT1 expression between both groups. There was a qualitative decrease in the staining for HIF2α, however, which would be consistent with our in vitro western blots (Fig. 3D).
Retrospective Analysis Suggests Propranolol Slows HB Growth in Patients With VHL Disease
Lastly, we explored whether or not propranolol has any utility in the treatment of patients with VHL disease. We identified 9 patients who were enrolled in an ongoing natural history study of VHL-related HBs at the National Institutes of Health (NCT00005902) who were administered propranolol (for other clinical reasons) for at least 30 days. Dosing ranged between 10 and 100 mg twice daily of propranolol. Of the 9 patients identified, 3 patients had radiographic follow-up data before and after propranolol administration. In all 3 patients, propranolol was started during the longitudinal study for other clinical indications (hypertension, migraine prophylaxis). These 3 patients harbored 66 HBs throughout the CNS. Of the identified tumors, 25 tumors had evidence of growth (at least a 10 mm3 volume increase) prior to implementation of propranolol and were included in subsequent analysis. Tumors in this cohort had a 2.1-year median follow-up on propranolol. The tumors grew slower when patients were taking propranolol (median growth rate 13.3 mm3/yr) than when they were not taking propranolol (median growth rate 27.1 mm3/yr, p < 0.0004). Thus, retrospective clinical data are consistent with our other preclinical data that propranolol may slow HB progression in patients with VHL disease.
Discussion
VHL disease remains a chronic disorder characterized by the formation of CNS HBs, RCCs, pheochromocytomas, endolymphatic sac tumors, and pancreatic neuroendocrine tumors.5,16,17 Despite advances in the understanding of the pathophysiology of tumor formation in patients with VHL, no effective systemic therapies have been developed. Herein, we provide in vitro, in vivo, and retrospective clinical data that suggest that propranolol may be effective in the treatment of VHL-related tumors, including HBs and RCCs.
Propranolol’s activity against IHs was first demonstrated clinically.12 Following this fortuitous observation, several authors have investigated the mechanism underpinning propranolol’s antitumor properties. Propranolol, unlike other beta-receptor antagonists, functions as an inverse agonist against the beta-receptor, a G-protein coupled receptor.4 Thus, when bound to the β1 or β2 receptor, propranolol not only prevents the binding of epinephrine, but also induces a conformational change that downregulates the receptors endogenous activity, thereby decreasing intracellular cAMP (cyclic adenosine monophosphate) levels.4 This property of propranolol activity has been suggested by some to underlie propranolol’s antiproliferative effects in IHs. Hemangioma stem cells, for example, when exposed to propranolol have been shown to undergo apoptosis, which has been correlated with decreased intracellular cAMP levels with subsequent activation of the extracellular signal-regulated kinase (ERK)1/2 pathway.20
Other authors have suggested that propranolol downregulates the PI3-K (phosphatidylinositol 3-kinase) pathway, leading to reduced VEGF levels in IHs.23 In other studies, propranolol exposure decreased IH HIF expression, thereby leading to reduced VEGF expression.7 Furthermore, local effects of beta-receptor inhibition leading to vasoconstriction and decreased IH perfusion have been hypothesized as an alternative mechanism of propranolol-mediated IH regression.20 Thus, while the precise mechanism of propranolol activity in IHs remains a subject of ongoing investigation, it appears that propranolol has the potential to 1) induce apoptosis and 2) augment HIF/ VEGF expression while 3) exerting systemic cardiovascular effects that lead to reduced tumor perfusion.7,14,20,23
Albiñana et al. demonstrated that propranolol reduces primarily cultured HB cells in vitro by inducing apoptosis and augmenting VEGF expression.2 Our work confirms these findings in a separate cohort of VHL-derived HB cells and provides further preclinical data that propranolol may be efficacious in treating VHL-HBs and VHL-RCCs. As reported herein, propranolol decreased the viability of cultured HBs while also inducing apoptosis and reducing the viability of an RCC cell line in vitro. Our data further suggest that propranolol is able to decrease HIF2α expression in 786-O cells. HIF inhibitors have been reported to reverse the VHL phenotype in a zebrafish model of VHL.19 In our work, propranolol did not lead to a decrease in 786-O VEGF protein expression, however. One possibility is that VEGF expression would be expected to occur after HIF protein expression was reduced. In IHs, propranolol led to decreased VEGF expression only after 4 days of propranolol exposure.7 The 786-O cells had limited viability after 48 hours of treatment and thus we may be limited in our ability to detect a significant change in expression of VEGF in 786-O cells treated with propranolol. Furthermore, although one may expect the VEGF mRNA levels to decrease, and not increase, if HIF expression is reduced, this paradoxical finding has been reported in IHs treated with propranolol.7
Our work further suggests that propranolol slows RCC tumor progression in vivo while decreasing serum VEGFA levels in treated tumor-bearing mice. In our experiments, human VEGF, which would have otherwise been secreted by the RCC xenograft tumors, was not detected in mouse sera. Additionally, the presence of a 786-O flank tumor did not increase in the serum level of VEGF independently (Fig. 3C). Interestingly, propranolol did not affect the serum VEGF levels of mice that were not bearing 786-O tumors (Fig. 3C). Thus, it appears that propranolol reduced murine VEGF secretion only in the presence of 786-O xenografted tumors. We hypothesize that this may be one reason that propranolol-treated RCC-bearing mice had slower rates of tumor progression. In this way, while propranolol may harbor a direct antitumor effect, it may also induce systemic changes that lead to an inhospitable tumor environment. Our data do not suggest a clear mechanism for why propranolol may reduce serum VEGF selectively in xenografted mice, and further work on this needs to be performed.
Plasma VEGF levels have recently been reported to serve as a biomarker in patients with VHL disease taking propranolol for ocular VHL.1 In a recent study, patients with VHL disease who harbored retinal hemangioblastomas received propranolol up to 120 mg/day. Although no retinal HBs regressed in the study, systemic VEGF levels decreased with prolonged treatment with propranolol, which is consistent with our in vivo findings. In our study, the approximate IC50 of propranolol against VHL-HBs and 786-O cells ranged between 50 μM and 200 μM. This is similar to the concentrations at which IHs and VHL-HBs have responded to propranolol therapy in vitro.2,7,38 Based on prior pharmacological studies, plasma serum propranolol concentrations would be unlikely to achieve concentrations this high, however.32 Nevertheless, although IH growth is inhibited by propranolol treatment between 50 and 300 μM in vitro, propranolol has shown clear clinical efficacy for patients with IHs treated with oral propranolol.7,12,13,38 Furthermore, a recent study examined the role of VEGF-A expression in 786-O cells. The 786-O cells transfected with siRNA directed against VEGF mRNA had decreased viability, increased rates of apoptosis, and decreased migratory potential.37 Similarly, VEGF has been shown to promote the growth of other solid tumors such as colorectal cancer and cervical cancer.27,35 Thus, we hypothesize that the reduction in systemic VEGF levels induced by propranolol may be one mechanism underlying propranolol’s efficacy in vivo.
In addition to our in vitro and animal work, our analysis of VHL-HB growth kinetics in a retrospective analysis of a VHL-HB natural history study suggests that patients had slower-growing CNS HBs while receiving propranolol. Although retrospective and derived from a small population of patients, these data are consistent with our cell culture and murine studies and thus provide further biological plausibility that propranolol may have a role in the treatment of VHL-related neoplasms. The protective effect of propranolol and other beta blockers has been reported in other retrospective population-based studies examining hepatocellular carcinoma, prostate cancer, and other malignancies.6,21,26 Nevertheless, as HBs have stochastic growth patterns and variable growth rates that can fluctuate over time, further prospective studies are needed to validate propranolol as a viable treatment option for VHL-HBs.
Conclusions
Overall, our study provides consistent in vitro, in vivo, and retrospective clinical data that propranolol harbors antitumor properties in VHL-HBs and VHL-RCCs, 2 tumors that lead to a majority of morbidity and mortality in patients with VHL disease.5,8,17,34 While further work is necessary to identify the mechanisms underlying propranolol’s antitumor properties, given the fact that propranolol is a safe, well-tolerated, FDA-approved oral medication that freely crosses the blood-brain barrier, our results encourage the development of clinical trials to test propranolol as a novel treatment agent for VHL disease.
Acknowledgments
This study was supported by the Intramural Research Program of the National Institute for Neurological Diseases and Stroke, and by the National Institutes of Health Clinical Center in Bethesda, MD. This research was also made possible through the National Institutes of Health Medical Research Scholars Program.
ABBREVIATIONS
- ELISA
enzyme linked immunosorbent assay
- GLUT1
glucose transporter 1
- HB
hemangioblastoma
- HIF
hypoxia-inducible factor
- IC50
half maximal inhibitory concentration
- IH
infantile hemangioma
- PBS
phosphate-buffered saline
- RCC
renal cell carcinoma
- RT-PCR
real-time polymerase chain reaction
- VEGF
vascular endothelial growth factor
- VHL
von Hippel-Lindau
- VHL-HB
VHL-related HB
- VHL-RCC
VHL-related RCC
Footnotes
Disclosures
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Supplemental Information
Previous Presentations
Portions of this manuscript were previously presented as posters at the 2017 AANS Annual Scientific Meeting, Los Angeles, California, April 22–26, 2017, and at the 22nd Annual Meeting and Education Day, Society of Neuro-Oncology, San Francisco, California, November 16–19, 2017.
References
- 1.Albiñana V, Jiménez Escribano RM, Soler I, Rodríguez-Padial L, Recio-Poveda L, Villar Gómez de las Heras K, et al: Repurposing propranolol as a drug for the treatment of retinal hemangioblastomas in von Hippel-Lindau disease. Orhanet J Rare Dis 12:122, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albiñana V, Villar Gómez de las Heras K, Serrano-Heras G, Segura T, Perona-Moratalla AB, Mota-Pérez M, et al. : Propranolol reduces viability and induces apoptosis in hemangioblastoma cells from von Hippel-Lindau patients. Orphanet J Rare Dis 10:118, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bader HL, Hsu T: Systemic VHL gene functions and the VHL disease. FEBS Lett 586:1562–1569, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baker JG, Hall IP, Hill SJ: Agonist and inverse agonist actions of β-blockers at the human β2-adrenoceptor provide evidence for agonist-directed signaling. Mol Pharmacol 64:1357–1369, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Butman JA, Linehan WM, Lonser RR: Neurologic manifestations of von Hippel-Lindau disease. JAMA 300:1334–1342, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang PY, Huang WY, Lin CL, Huang TC, Wu YY, Chen JH, et al. : Propranolol reduces cancer risk: a population-based cohort study. Medicine (Baltimore) 94:e1097, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chim H, Armijo BS, Miller E, Gliniak C, Serret MA, Gosain AK: Propranolol induces regression of hemangioma cells through HIF-1α-mediated inhibition of VEGF-A. Ann Surg 256:146–156, 2012 [DOI] [PubMed] [Google Scholar]
- 8.Chittiboina P, Lonser RR: Von Hippel-Lindau disease. Handb Clin Neurol 132:139–156, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gossage L, Eisen T, Maher ER: VHL, the story of a tumour suppressor gene. Nat Rev Cancer 15:55–64, 2015 [DOI] [PubMed] [Google Scholar]
- 10.Joshi S, Singh AR, Durden DL: Pan-PI-3 kinase inhibitor SF1126 shows antitumor and antiangiogenic activity in renal cell carcinoma. Cancer Chemother Pharmacol 75:595–608, 2015 [DOI] [PubMed] [Google Scholar]
- 11.Latif F, Tory K, Gnarra J, Yao M, Duh FM, Orcutt ML, et al. : Identification of the von Hippel-Lindau disease tumor suppressor gene. Science 260:1317–1320, 1993 [DOI] [PubMed] [Google Scholar]
- 12.Léauté-Labrèze C, Dumas de la Roque E, Hubiche T, Boralevi F, Thambo JB, Taïeb A: Propranolol for severe hemangiomas of infancy. N Engl J Med 358:2649–2651, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Léauté-Labrèze C, Hoeger P, Mazereeuw-Hautier J, Guibaud L, Baselga E, Posiunas G, et al. : A randomized, controlled trial of oral propranolol in infantile hemangioma. N Engl J Med 372:735–746, 2015 [DOI] [PubMed] [Google Scholar]
- 14.Li P, Guo Z, Gao Y, Pan W: Propranolol represses infantile hemangioma cell growth through the β2-adrenergic receptor in a HIF-1α-dependent manner. Oncol Rep 33:3099–3107, 2015 [DOI] [PubMed] [Google Scholar]
- 15.Littell RC: Repeated measures analysis with clustered subjects, in Proceedings of the SAS Global Forum 2007 Conference. Cary, NC: SAS Institute, 2007. (http://www2.sas.com/proceedings/forum2007/178-2007.pdf) [Accessed June 27, 2018] [Google Scholar]
- 16.Lonser RR, Butman JA, Huntoon K, Asthagiri AR, Wu T, Bakhtian KD, et al. : Prospective natural history study of central nervous system hemangioblastomas in von Hippel-Lindau disease. J Neurosurg 120:1055–1062, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lonser RR, Glenn GM, Walther M, Chew EY, Libutti SK, Linehan WM, et al. : von Hippel-Lindau disease. Lancet 361:2059–2067, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Lundin P, Pedersen F: Volume of pituitary macroadenomas: assessment by MRI. J Comput Assist Tomogr 16:519–528, 1992 [DOI] [PubMed] [Google Scholar]
- 19.Metelo AM, Noonan HR, Li X, Jin Y, Baker R, Kamentsky L, et al. : Pharmacological HIF2α inhibition improves VHL disease-associated phenotypes in zebrafish model. J Clin Invest 125:1987–1997, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Munabi NCO, England RW, Edwards AK, Kitajewski AA, Tan QK, Weinstein A, et al. : Propranolol targets hemangioma stem cells via cAMP and mitogen-activated protein kinase regulation. Stem Cells Transl Med 5:45–55, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nkontchou G, Aout M, Mahmoudi A, Roulot D, Bourcier V, Grando-Lemaire V, et al. : Effect of long-term propranolol treatment on hepatocellular carcinoma incidence in patients with HCV-associated cirrhosis. Cancer Prev Res (Phila) 5:1007–1014, 2012 [DOI] [PubMed] [Google Scholar]
- 22.Omar AI: Bevacizumab for the treatment of surgically unresectable cervical cord hemangioblastoma: a case report. J Med Case Reports 6:238, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pan WK, Li P, Guo ZT, Huang Q, Gao Y: Propranolol induces regression of hemangioma cells via the down-regulation of the PI3K/Akt/eNOS/VEGF pathway. Pediatr Blood Cancer 62:1414–1420, 2015 [DOI] [PubMed] [Google Scholar]
- 24.Park DM, Zhuang Z, Chen L, Szerlip N, Maric I, Li J, et al. : von Hippel-Lindau disease-associated hemangioblastomas are derived from embryologic multipotent cells. PLoS Med 4:e60, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peng S, Shepard MJ, Wang J, Li T, Ning X, Cai L, et al. : Genotype-phenotype correlations in Chinese von Hippel-Lindau disease patients. Oncotarget 8:38456–38465, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perron L, Bairati I, Harel F, Meyer F: Antihypertensive drug use and the risk of prostate cancer (Canada). Cancer Causes Control 15:535–541, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Qi L, Xing LN, Wei X, Song SG: Effects of VEGF suppression by small hairpin RNA interference combined with radiotherapy on the growth of cervical cancer. Genet Mol Res 13:5094–5106, 2014 [DOI] [PubMed] [Google Scholar]
- 28.Rogers LR, LoRusso P, Nadler P, Malik G, Shields A, Kaelin W: Erlotinib therapy for central nervous system hemangioblastomatosis associated with von Hippel-Lindau disease: a case report. J Neurooncol 101:307–310, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sardi I, Sanzo M, Giordano F, Buccoliero AM, Mussa F, Aricò M, et al. : Monotherapy with thalidomide for treatment of spinal cord hemangioblastomas in a patient with von Hippel-Lindau disease. Pediatr Blood Cancer 53:464–467, 2009 [DOI] [PubMed] [Google Scholar]
- 30.Sizdahkhani S, Feldman MJ, Piazza MG, Ksendzovsky A, Edwards NA, Ray-Chaudhury A, et al. : Somatostatin receptor expression on von Hippel-Lindau-associated hemangioblastomas offers novel therapeutic target. Sci Rep 7:40822, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stiles JM, Amaya C, Rains S, Diaz D, Pham R, Battiste J, et al. : Targeting of beta adrenergic receptors results in therapeutic efficacy against models of hemangioendothelioma and angiosarcoma. PLoS One 8:e60021, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Von Bahr C, Hermansson J, Tawara K: Plasma levels of (+) and (−)-propranolol and 4-hydroxypropranolol after administration of racemic (±)-propranolol in man. Br J Clin Pharmacol 14:79–82, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wanebo JE, Lonser RR, Glenn GM, Oldfield EH: The natural history of hemangioblastomas of the central nervous system in patients with von Hippel-Lindau disease. J Neurosurg 98:82–94, 2003 [DOI] [PubMed] [Google Scholar]
- 34.Wilding A, Ingham SL, Lalloo F, Clancy T, Huson SM, Moran A, et al. : Life expectancy in hereditary cancer pre-disposing diseases: an observational study. J Med Genet 49:264–269, 2012 [DOI] [PubMed] [Google Scholar]
- 35.Xing X, Gu X, Ma T, Ye H: Biglycan up-regulated vascular endothelial growth factor (VEGF) expression and promoted angiogenesis in colon cancer. Tumour Biol 36:1773–1780, 2015 [DOI] [PubMed] [Google Scholar]
- 36.Yang C, Huntoon K, Ksendzovsky A, Zhuang Z, Lonser RR: Proteostasis modulators prolong missense VHL protein activity and halt tumor progression. Cell Reports 3:52–59, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zeng FC, Zeng MQ, Huang L, Li YL, Gao BM, Chen JJ, et al. : Downregulation of VEGFA inhibits proliferation, promotes apoptosis, and suppresses migration and invasion of renal clear cell carcinoma. OncoTargets Ther 9:2131–2141, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang L, Mai HM, Zheng J, Zheng JW, Wang YA, Qin ZP, et al. : Propranolol inhibits angiogenesis via down-regulating the expression of vascular endothelial growth factor in hemangioma derived stem cell. Int J Clin Exp Pathol 7:48–55, 2013 [PMC free article] [PubMed] [Google Scholar]


