Abstract
Low-pressure hydrocephalus (LPH) is a rare clinical diagnosis, characterized by neurologic decline and ventriculomegaly that persists despite normal to low intracranial pressure. LPH is typically managed by negative-pressure drainage via ventriculostomy, followed by low-resistance shunt insertion. We present the case of a middle-aged man with a history of hemangioblastomatosis who had spontaneous subarachnoid hemorrhage. He was treated with a ventriculoperitoneal shunt and then underwent resection of a Meckel’s cave hemangioblastoma and whole brain irradiation. One month later, he presented to us with worsening symptoms and hydrocephalus despite shunt interrogations and revisions revealing no malfunction. Ventriculostomy drainage at negative-pressure was required for resolution of symptoms and ventriculomegaly, leading us to a diagnosis of LPH. This was successfully treated using an improvised ultra-low pressure valveless ventriculoperitoneal shunt, with maintained resolution of LPH for over one year. The system was created by ligating the distal slit valve end of a peritoneal catheter to prevent reflux and allow sub-zero pressure drainage by siphoning.
Keywords: Low-pressure hydrocephalus, Negative-pressure hydrocephalus, Hemangioblastoma, CSF drainage
INTRODUCTION
Low-pressure hydrocephalus (LPH) is a rare clinical diagnosis, characterized by: (1) neurologic decline with medium-pressure cerebrospinal fluid (CSF) diversion, (2) persistent ventriculomegaly and neurologic decline with low intraventricular pressures, and (3) clinical and radiographic improvement of ventriculomegaly with CSF-diversion at negative pressure.[1,2] We present a case of LPH associated with hemangioblastomatosis, which was successfully treated with an improvised negative-pressure shunt.
CASE DESCRIPTION
A 45-year-old male, who previously underwent an uncomplicated cerebellar hemangioblastoma (Hb) resection several years ago, presented with numbness of the left side of his face, worsening headaches, nausea and blurred vision over the past week. Following his previous Hb resection, surveillance neuro-axis imaging revealed the spontaneous development of multiple hemangioblastomas in the posterior fossa and spinal cord. He had no relevant family history and genetic testing excluded Von Hippel-Lindau disease. At presentation, his neurological examination was remarkable for left facial paresthesia and a left abducens nerve palsy.
Computed tomography (CT) of the head revealed communicating hydrocephalus (Figure 1A–1B). A lumbar puncture revealed an opening pressure of 36cm H2O, and CSF analysis demonstrated elevated protein and xanthochromia. Given the clinical presentation, we attributed the results to spontaneous hemorrhage from multiple hemangioblastomas (Figure 1C–1D).
Figure 1:
Axial CT head (A and B) at presentation showing communicating hydrocephalus. Axial (C) and coronal (D) T1-Weighted post-contrast Magnetic Resonance Imaging (MRI) showing a left-sided hemangioblastoma extending into Meckel's cave. There was resolution of hydrocephalus, five days after initial ventriculoperitoneal shunt insertion.
A right frontal ventriculoperitoneal shunt was placed using a fixed medium-pressure valve, resulting in resolution of ventriculomegaly (Figure 1D). The patient then underwent resection of the Meckel’s cave hemangioblastoma combined with left anterior petrosectomy and retrosigmoid approaches. Following initial convalescence, the patient underwent external beam irradiation (4500 cGy in 25 fractions) of the brain and cervical spine with concurrent valproic acid as a radiosensitizer. One month later, he presented again with worsening headaches. CT of the head revealed ventriculomegaly with a trapped left lateral ventricle (Figure 2A). The ventriculoperitoneal shunt was interrogated in the operating room and found to be functional. The fixed medium-pressure valve was changed to a programmable Medtronic Strata® valve set at 0.5 (equivalent to 3 – 4 mmHg). Despite this, the patient failed to show radiographic or clinical improvement even after the valve was dialed down to the lowest setting (Figure 2B). Over the next month, the patient became increasingly confused with urinary incontinence and worsening ventriculomegaly. A shunt tap showed no evidence of proximal or distal obstruction, but there was clinical improvement after aspiration of 30mL of CSF.
Figure 2:
Axial CT head (A) on readmission showing trapped left lateral ventricle and ventriculomegaly with ventriculoperitoneal shunt in-situ. Axial CT head (B) showing ventriculomegaly after shunt revision with programmable Medtronic Strata® valve at 0.5. Axial CT head (C) showing improved ventriculomegaly with drainage of 15–20cc per hour with external ventricular drain at 4cm below the tragus. Axial CT head (D) showing worsened ventriculomegaly after raising ventriculostomy to level of tragus. Plain lateral radiograph of skull (E) showing valveless improvised shunt in-situ. Axial CT head (F) showing resolution of ventriculomegaly with improvised shunt. Axial T2-weighted MRI showing maintained resolution of ventriculomegaly six-months (G) and one-year (H) after insertion of improvised shunt.
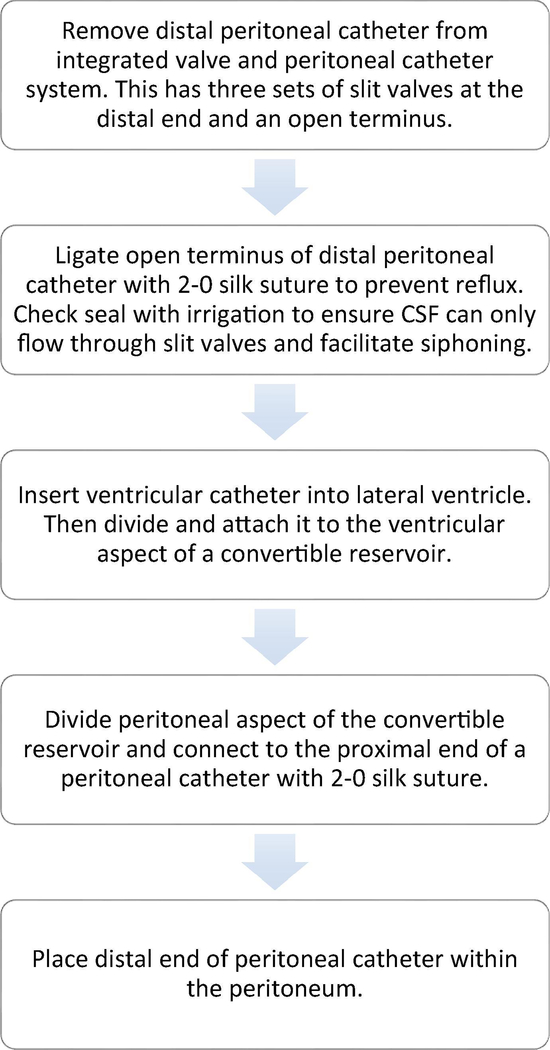
The ventriculoperitoneal shunt was replaced with an external ventricular drain, and it was determined after several failed slow-weaning attempts that draining 15–20cc of CSF per hour was necessary for clinical and radiographic improvement. This was possible when the ventriculostomy was lowered to 4cm below the tragus (Figure 2C). Attempts to raise the ventriculostomy to the level of the tragus was accompanied by progressive ventriculomegaly and somnolence, consistent with LPH (Figure 2D). For long-term management, we used an improvised right parietal valveless negative-pressure shunt system (Figure 2E). This was made utilizing a Medtronic barium impregnated ventricular catheter and convertible reservoir (Figure 3). Using this improvised low-pressure shunt, clinical and radiological improvement has been maintained beyond one-year follow-up (Figures 2F–2G).
Figure 3:
Steps in making the improvised negative-pressure shunt
DISCUSSION
LPH has been associated with subarachnoid hemorrhage, infection, posterior fossa tumors, congenital aqueductal stenosis and spinal arachnoid cysts.[3] How these conditions lead to LPH is unclear, however various models leading to reduced elasticity of brain parenchyma have been proposed.[1] Most convincingly, the poroelastic model suggests that increased permeability of the brain is triggered by an inflammatory process causing the brain to become saturated and more compliant, ultimately leading to a low-pressure hydrocephalic state. This is supported by the common appearance of transependymal flow on neuroimaging of LPH patients as seen in our case (Figure 2C), as well as evidence suggesting that the initial development of LPH occurs at normal intracranial pressure.[1,2] Subarachnoid hemorrhage is a rare complication of hemangioblastomatosis that has only rarely been reported in the context of extramedullary hemangioblastomas.[4] To our knowledge, our case is the first reported of negative-pressure hydrocephalus associated with hemangioblastomatosis.
Management of LPH involves identifying CSF leaks which can be closed surgically.[5] A mainstay of treatment is the use of negative-pressure drainage techniques via ventriculostomy with CSF drainage at below the level of the external auditory meatus (subzero method).[2] LPH is often refractory to initial therapy, necessitating negativepressure shunt systems for long-term management.[3,5] Our case demonstrates that LPH can be managed with a simple improvised negative-pressure shunt made using a valveless reservoir and modified ventriculoperitoneal shunt to prevent reflux and allow negativepressure siphoning.
Highlights.
Negative-pressure hydrocephalus is associated with subarachnoid hemorrhage
Subarachnoid hemorrhage is a rare complication of extramedullary hemangioblastomas
Increased brain compliance is likely responsible for low-pressure hydrocephalus
Negative-pressure hydrocephalus may be managed with a simple improvised shunt
Acknowledgments
This project was supported by the Intramural Research Program of the National Institute of Neurological Diseases and Stroke.
Footnotes
Declaration of interest:
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Akins PT, Guppy KH, Axelrod YV, Chakrabarti I, Silverthorn J, Williams AR. The genesis of low pressure hydrocephalus. Neurocrit Care 2011;15:461–8. doi: 10.1007/s12028-011-9543-6. [DOI] [PubMed] [Google Scholar]
- [2].Pang D, Altschuler E. Low-Pressure Hydrocephalic State and Viscoelastic Alterations in the Brain. Neurosurgery 1994;35:643–56. doi: 10.1227/00006123-199410000-00010. [DOI] [PubMed] [Google Scholar]
- [3].Filippidis AS, Kalani MYS, Nakaji P, Rekate HL. Negative-pressure and low-pressure hydrocephalus: the role of cerebrospinal fluid leaks resulting from surgical approaches to the cranial base. J Neurosurg 2011;115:1031–7. doi: 10.3171/2011.6.JNS101504. [DOI] [PubMed] [Google Scholar]
- [4].de San Pedro JR, Rodríguez FA, Níguez BF, Sánchez JFM-L, López-Guerrero AL, Murcia MF, et al. Massive hemorrhage in hemangioblastomas Literature review. Neurosurg Rev 2010;33:11–26. doi: 10.1007/s10143-009-0217-1. [DOI] [PubMed] [Google Scholar]
- [5].Kalani MYS, Turner JD, Nakaji P. Treatment of refractory low-pressure hydrocephalus with an active pumping negative-pressure shunt system. J Clin Neurosci 2013;20:462–6. doi: 10.1016/j.jocn.2012.04.027. [DOI] [PubMed] [Google Scholar]