Abstract
Immuno-positron emission tomography (immunoPET) is a paradigm-shifting molecular imaging modality combining the superior targeting specificity of monoclonal antibody (mAb) and the inherent sensitivity of PET technique. A variety of radionuclides and mAbs have been exploited to develop immunoPET probes, which has been driven by the development and optimization of radiochemistry and conjugation strategies. In addition, tumor-targeting vectors with a short circulation time (e.g., Nanobody) or with an enhanced binding affinity (e.g., bispecific antibody) are being used to design novel immunoPET probes. Accordingly, several immunoPET probes, such as 89Zr-Df-pertuzumab and 89Zr-atezolizumab, have been successfully translated for clinical use. By noninvasively and dynamically revealing the expression of heterogeneous tumor antigens, immunoPET imaging is gradually changing the theranostic landscape of several types of malignancies. ImmunoPET is the method of choice for imaging specific tumor markers, immune cells, immune checkpoints, and inflammatory processes. Furthermore, the integration of immunoPET imaging in antibody drug development is of substantial significance because it provides pivotal information regarding antibody targeting abilities and distribution profiles. Herein, we present the latest immunoPET imaging strategies and their preclinical and clinical applications. We also emphasize current conjugation strategies that can be leveraged to develop next-generation immunoPET probes. Lastly, we discuss practical considerations to tune the development and translation of immunoPET imaging strategies.
Graphical Abstract

1. INTRODUCTION
Molecular imaging is defined as “visualization, characterization, and measurement of biological processes at the molecular and cellular levels in humans and other living systems” by using molecular imaging agents and tools.1 Positron emission tomography (PET) imaging is the foundation of molecular imaging and has drastically improved global healthcare since its inception in the clinical practice.2–4 With the gradual discovery of the molecular pathogenesis of cancers and contemporaneous understanding of the host immune system, molecularly targeted therapies (e.g., small-molecule inhibitors and monoclonal antibodies [mAbs]) and immunotherapies (e.g., immune checkpoint inhibitors) have been developed. The clinical use of these novel regimens is changing the therapeutic landscape for numerous cancers.5–7 In the era of molecularly targeted therapy and cancer immunotherapy, it is clear that PET imaging with traditional radiotracers is inadequate.8 For instance, 18F-fluorodeoxyglucose (18F-FDG) PET/computed tomography (CT) has been integrated into several criteria in predicting and assessing responses to targeted therapies or immunotherapies.9,10 However, several studies have reported that 18F-FDG PET/CT parameters, such as SUVmax and SUVmean, did not correlate with clinical responses for immunotherapy regimens.11,12 Additionally, it is challenging to differentiate immune-related adverse events (e.g., sarcoidosis) and pseudoprogression on 18F-FDG PET images,13,14 leading to misinterpretation.
To further improve the clinical management of cancers and noncancerous diseases, the integration of novel molecular imaging approaches into routine diagnostic toolbox is critically important.15 Antibody-derived molecular imaging probes have been instrumental in visualizing target expression and pharmacokinetics of therapeutic mAbs in living subjects. Although several antibody-based tracers for single-photon emission computed tomography (SPECT) imaging exist in the clinic,16 PET imaging with antibody-based tracers has distinct advantages in terms of image quality, spatial resolution, and quantification.17
2. CONCEPT OF IMMUNOPET
Immuno-positron emission tomography (immunoPET or iPET), which exquisitely fuses the extraordinary targeting specificity of mAb and the superior sensitivity and resolution of PET, is a paradigm shift for molecular imaging modalities.18 The concept of immunoPET was manifested more than two decades ago,19,20 but its development rapidly accelerated in recent years with the increasing approval of therapeutic antibodies and the more widespread production of long half-life radionuclides. Meanwhile, the concept of immunoPET has evolved over the years with the incorporation of antibody fragments or mimetics as targeting moieties. More importantly, the clinical application of immunoPET imaging has increased our understanding of tumor heterogeneity and refined clinical disease management. For instance, the status of programmed death ligand-1 (PD-L1) assessed by 89Zr-atezolizumab immunoPET, but not by immunohistochemistry (IHC) or RNA sequencing, predicted the therapeutic response of atezolizumab in patients with three types of tumors.21
Despite the existence of several reviews on immunoPET, there are none that comprehensively describe the design strategies and the application landscape of this novel imaging modality. In this review, we first elaborate on the development of immunoPET imaging strategies by introducing positron-emitting radionuclides, associated chelators, targeting vectors (e.g., mAbs and antibody fragments), as well as traditional and novel conjugation strategies. We then introduce the role of immunoPET in imaging cancers and noncancerous diseases, followed by a recapitulation of how immunoPET imaging aids in the development of antibody and antibody-based therapeutics. In the last part of the review, we discuss practical considerations for future development and translation of immunoPET imaging tracers.
3. DESIGN AND CONJUGATION STRATEGIES OF IMMUNOPET
ImmunoPET applications require simple, fast, and specific radiolabeling of antibody vectors under mild conditions. Optimal immunoPET imaging is attributed to a highly specific tumor uptake and low background retention. Toward this end, it is essential for a tracer to specifically saturate its target as fast as possible, with the unbound tracer cleared out rapidly from the blood circulation. Generally, the successful development of immunoPET probes is highly dependent on the choice of tumor-targeting vectors, radionuclides, bifunctional chelators, and conjugation strategies as discussed below.
3.1. Antibodies, Antibody Fragments, and VHHs
3.1.1. Full-Length Antibodies.
The development and use of mAbs have achieved considerable success, and various kinds of mAbs have been adapted to treat solid tumors, hematological malignancies, as well as noncancerous diseases.5,22–24 In 2018, the Food and Drug Administration (FDA) and European Medicines Agency (EMA) approved 13 antibody therapeutics for clinical use.25 Although only five new antibody therapeutics were approved in 2019, it is anticipated that at least 13 products will be granted approval in 2020.26 The therapeutic mechanisms of mAbs mainly include antibody-dependent cell-mediated cytotoxicity (ADCC), antibody-dependent cellular phagocytosis (ADCP), complement-dependent cytotoxicity (CDC), interruption of a signaling pathway, inhibition of enzymatic activity, and inhibition of immune checkpoint, which are discussed extensively in other reviews.27,28 For therapeutic purposes, immunoglobulin G (IgG) is considered to have the most favorable balance between clearance and tumor uptake.29 Over the past decade, immunoPET imaging with radiolabeled mAbs has progressed rapidly together with the development of antibody engineering and production of long-lived PET radionuclides.18 Currently, mAb-based immunoPET imaging is being actively investigated in preclinical models and has attracted considerable attention in clinical practice. The tumor-targeting and treatment efficacy of mAbs can be maximized by generating bispecific antibodies (BsAb) or trispecific antibodies.30,31 By targeting multiple tumor antigens, these novel polyspecific antibodies are alternatives for developing immunoPET probes.32
3.1.2. Limitations of Full-Length mAbs in Immuno-PET Imaging.
Despite the clinical success, mAb-derived immunoPET probes suffer from several disadvantages. First, the size of mAb (150 kDa; Figure 1a) exceeds the clearance cutoff value (60 kDa) of glomerular filtration. Additionally, the interaction between the Fc domain of IgG and the neonatal Fc receptor (FcRn) in endothelial cells further protects serum IgG from degradation.33–35 Consequently, long-lived radionuclides that match the serum half-life of mAbs are required to develop the radiotracers. These factors synergistically contribute to the typical features of mAb-based immunoPET imaging, such as slow blood clearance, less optimal target-to-background [T/B] ratio, and the necessity to image repeatedly after administration of a single dose of the tracer. In addition, the pharmacology of antibody–antigen binding and the internalization of the antibody–antigen complex must also be considered when developing mAb-derived PET imaging tracers.36–38 To maximize tumor uptake and detect liver malignancies or metastases, preloading or coadministration of unlabeled antibody is required in the course of immunoPET imaging.39 However, the required blocking dose differs in a target- and antibody-dependent manner40 and is greatly affected by the antibody treatment schedule at the time of tracer injection.41 Therefore, a feasible and reproducible imaging protocol needs to be carefully established before carrying out regular immunoPET scanning. Antibodies are produced in eukaryotic cell lines due to their complex expression and post-translational modifications.42 Because of this, the use of large amounts of antibodies is costly, further increased from the expenses of producing radiometals. Therefore, high costs may limit the widespread use of mAb-based immunoPET imaging tracers.
Figure 1.
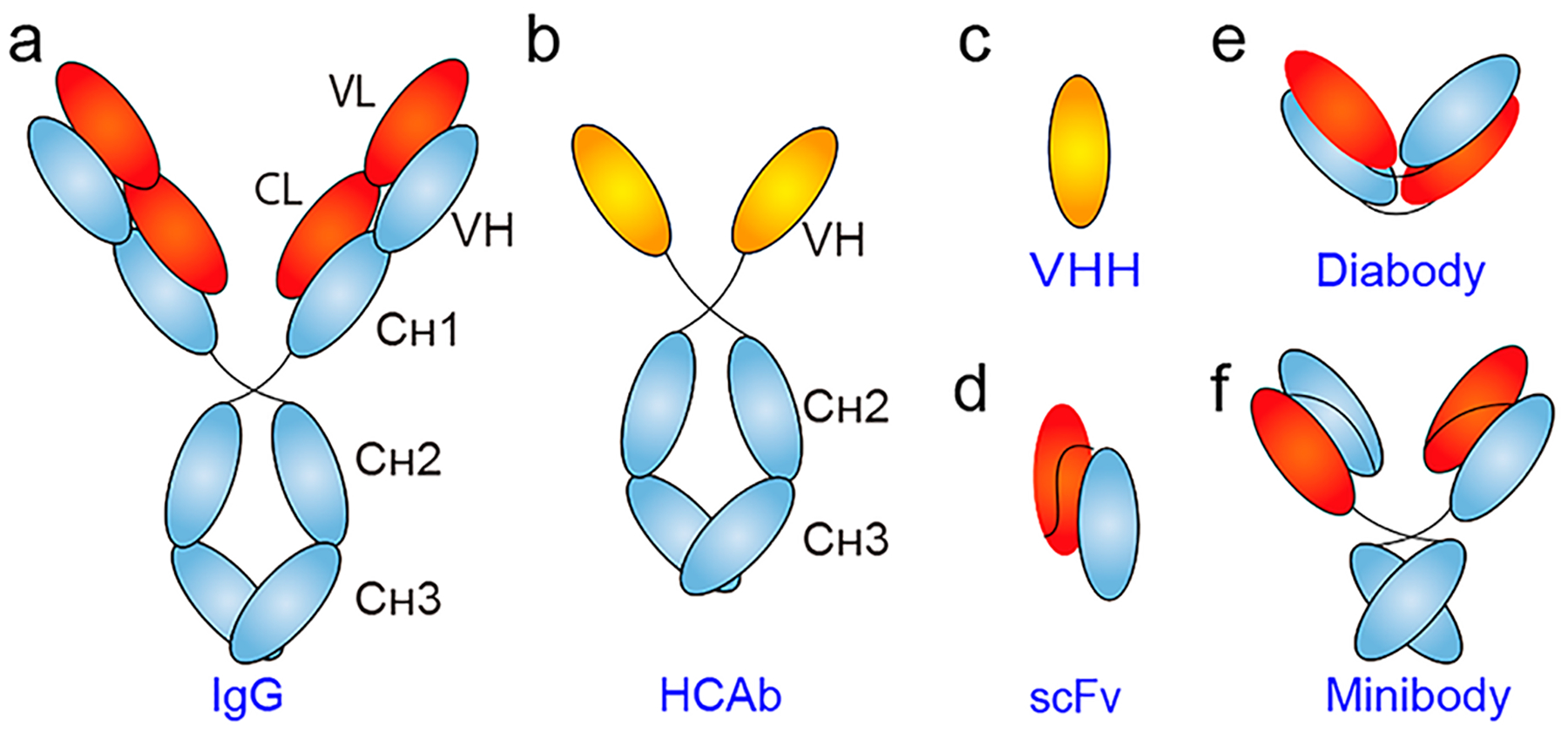
Schematic of representative antibody and antibody fragments. (a) Conventional IgG is composed of two identical heavy chains and two identical light chains. While each heavy chain consists of three constant domains (i.e., CH1, CH2, and CH3) and a variable domain (VH), an IgG light chain has one constant domain (CL) and one variable domain (VL). (b) Heavy-chain-only antibody (HCAb) lacks the light chains and the typical CH1 domain. (c) The antigen-binding specificity of a HCAb is due to the single VHH domain. (d) Single-chain variable fragment (scFv) is the smallest unit of the IgG molecule that retains antigen-binding capacity. Using scFv as the building block, (e) diabody (dimers of scFv), and (f) minibody (dimers of scFv–CH3) can be constructed.
3.1.3. VHHs.
Because mAbs tend to circulate in the blood and deposit in normal organs such as the liver, spleen, and bone marrow, smaller antibody constructs have been employed to accelerate the clearance of unbound radiotracer from systemic circulation and correspondingly achieve higher T/B ratios. Furthermore, small antibody fragments may penetrate solid tumors more efficiently and homogeneously.43 Several types of smaller targeting vectors are available, including camelid heavy-chain-only antibodies (HCAbs) and shark-derived immunoglobulin new antigen receptors (IgNARs).44 HCAbs (Figure 1b) are naturally occurring antigen-binding antibodies in Camelidae. HCAbs can be obtained by immunization of camels, llamas, or dromedaries or from näive or synthetic phage libraries.45 HCAbs have only two constant domains, as opposed to the three constant domains of an IgG. HCAbs are generally humanized for theranostic purposes.46,47
The variable domain of the heavy chain of a HCAb (VHH, Figure 1c), often referred to as Nanobody (a trade name of Ablynx) or single-domain antibody (sdAb), is the smallest antigen-binding derivative. With its molecular weight around 15 kDa and diameter <4 nm, VHH can penetrate deeply into tumor tissues while retaining its antigen recognition ability.48 VHHs targeting various cellular or subcellular receptors or oncogenic proteins have also been generated.49,50 Manifold techniques are available to achieve chemical functionalization of VHHs, which indubitably facilitates more sophisticated applications of VHHs.51,52 Specifically, strategies like PEGylation and albumin hitchhiking may be used to prolong the circulatory half-life of VHH,53–55 enabling more thorough and efficient targeting of the targets.
It has been suggested that VHHs are “magic bullets” for molecular cancer imaging.56 For radiometal labeling, cysteine (Cys) or lysine (Lys) residues on VHHs are chemically modified with bifunctional chelators. Whereas for radio-iodination of VHHs, either direct electrophilic radioiodination or indirect radiolabeling methods can be used.57 Because of the variable number of Lys or Cys residues in the complementary-determining region 3 (CDR3) of VHHs, it is challenging to radiolabel VHHs homogeneously using these standard methods. Methodologies that enable site-specific radiolabeling are readily available to prepare homogeneous VHH-based radiotracers (described in section 3.3.3.).58,59 Despite the favorable pharmacokinetics, radiotracers based on VHHs have very high kidney accumulation due to the renal clearance of the excess material, limiting their role in detecting lesions located in the urinary system or in the vicinity of kidneys. Several factors (e.g., the sequence of the VHH, conjugation method, as well as specific receptors in the glomeruli) may all contribute to the high kidney retention of the developed radiotracers.60,61 However, there are strategies to circumvent this phenomenon, including coinfusion of gelofusine and Lys,62 removal of polyhistidine tag (His-tag),63 PEGylation,64 and site-specific radiohalogen labeling.65 Furthermore, VHHs are also being actively exploited for therapeutic purposes,66 either in the form of radioimmunotherapy (RIT),67–69 or in the form of photoimmunotherapy (PIT).70,71
3.1.4. Other Engineered Antibody Fragments and Proteins.
Several other antibody fragments have been engineered for imaging purposes.72 In general, these antibody fragments lack the Fc region and are smaller in size. Single-chain variable fragment (scFv, Figure 1d) is one of the most popular antibody fragments with a molecular size of ~25 kDa. A scFv clears exceptionally fast from the bloodstream and creates much higher T/B ratios compared with an intact IgG. scFv is composed of variable light and variable heavy chains that are joined by a flexible peptide linker. As such, the length and amino acid composition of the peptide linker between the two domains significantly affect the binding affinity and size of the engineered scFv.73 A significant drawback of scFv molecules is their monovalent antigen-binding specificity. In certain cases, engineering a monovalent scFv into multivalent constructs may enhance the avidity and optimize the tumor-targeting capability. Diabody (~60 kDa, Figure 1e) is a divalent variant of the monovalent scFv. Typically, a Cysmodified diabody (Cys-diabody) is constructed and used for site-specific radiolabeling.74 Similar to radiolabeled VHHs, radiolabeled scFv, and diabody are rapidly cleared by the urinary system, resulting in high accumulation of the radiotracers in the kidneys. Other larger divalent forms, such as minibody (Mb, Figure 1f) and (scFv)2-Fc constructs, have also been developed as targeting vectors.75,76 Several multivalent scFv fragments, such as triabody (~90 kDa) and trimerbody (110 kDa), also showed potential as ligands for immunoPET imaging.77,78
In the pursuit of proteins with enhanced or novel functions, a multitude of protein scaffolds has been generated and used in the field of molecular imaging. These low-molecular-weight proteins lack disulfide bonds and glycosylation, so they can be expressed in bacterial systems with proper conformation rapidly.79 Currently, one of the most commonly engineered protein scaffolds for PET imaging is the Affibody (6 kDa).80 For instance, ZHER2:342 is an Affibody molecule targeting human epidermal growth factor receptor 2 (HER2) and has been widely studied for PET imaging. The most attractive advantages of HER2-targeting Affibodies are their unique binding sites on HER2, which are distinct from HER2-targeted therapeutics (e.g., trastuzumab).81,82 Therefore, novel imaging approaches employing these Affibodies may help discriminate the downregulation and saturation of HER2 following HER2-targeted therapies. Other similar molecules that have already been used for molecular imaging include adnectins,83 fibronectin,84 knottins,85,86 and anticalins.87,88 Like VHHs, the primary benefits of these small antigen-targeting moieties are to permit same-day molecular imaging.89 The advantages from accelerated clearance are compensated by lower tumor uptake, yielding modest imaging quality. To enhance tumor retention and decrease kidney retention, several approaches (e.g., PEGylation) can be used to modify the targeting vectors.90–92
Of the BsAbs, bispecific T-cell engager (BiTE) antibody constructs (~55 kDa) are designed to induce context-dependent anticancer immune responses by cross-linking tumor cells with cytotoxic T cells.93–95 One successful example is the blinatumomab, which simultaneously targets CD19-positive B cells and then recruits CD3-positive cytotoxic T cells.96 It is much more challenging to design T-cell-dependent BsAb constructs because each arm of the antibody has a different antigen-binding affinity. Moreover, the T-cell-targeting arm substantially affects the in vivo distribution of the antibody and, therefore, the fate of the developed molecular imaging tracers.97,98
3.2. Radionuclides and Chelators
In recent years, various antibodies targeting diverse antigens have been labeled with gamma-emitting radionuclides (e.g., 131I, 123I, 111In, or 99mTc) and used for diagnosis by SPECT or planar imaging or for therapeutic applications. Because of their poor diagnostic performance, very few are routinely used in the clinic. With the global installation of cyclotrons, a variety of novel positron-emitting radionuclides is being produced.99,100 High-purity radiometals, a fundamental component in immunoPET imaging probes, are increasingly being produced and used in recent years.101,102 Traditionally, radiometals are eluted from generators or produced using solid targets with cyclotrons.103 As a supplement to solid targets, liquid targets (solution targets) can also be used to produce radiometals upon optimization.104 Several factors need to be considered before exploiting them for radiolabeling, which include physical properties (e.g., half-life [T1/2] and decay mode), chemical properties, production efficiency, safety profiles, and price. The T1/2 of a chosen positron emitter has to closely match the biological half-life of the targeting vector. In conjugating immunoPET imaging probes, the positron emitter is generally complexed with an inert chelator that is attached to the targeting antibody. The major principle is that the binding affinity, stability, and pharmacokinetic characteristics of the final radiopharmaceutical are in concert with the naive antibody. There are several review articles describing various radiometals and their coordination chemistry101,105,106 and 18F radiolabeling of heat-sensitive molecules.107 In this section, we will confine to the most promising radionuclides and related chelators used for immunoPET imaging (Table 1).
Table 1.
Representative Radionuclides Used in Developing ImmunoPET Imaging Probesa
| isotope | T1/2 | emission profiles | production methods | ref |
|---|---|---|---|---|
| 89Zr | 78.4 h | β+: 22.8%, Eβ+max = 901 keV; EC: 77%, Eγ = 909 keV | 89Y(d,2n)89Zr, 89Y(p,n)89Zr, natSr(α,xn)89Zr, etc. | 111,120 |
| 64Cu | 12.7 h | β+: 19%, Eβ+max = 656 keV; EC 41%, Eγ = 1346 keV; β−: 40%, Eβ−max = 579 keV | 64Ni(p,n)64Cu 64Ni(d, 2n)64Cu 68Zn(p,α)64Cu | 141 |
| 124I | 4.18 d | β+: 22%, Eβ+max = 2.13 MeV | 124Te(p,n)124I | 180 |
| 86Y | 14.7 h | β+: 34%, Eβ+max = 3.153 MeV; EC: 66%, Eγ = 1043 keV | 86Sr(p,n)86Y 86Sr(d,2n)86Y | 163,164 |
| 68Ga | 1.1 h | β+: 89%, Eβ+max = 1899 keV; EC: 11%, Eγ = 1077 keV | 68Ge/68Ga generator 68Zn(p,n)68Ga | 225,226 |
| 44Sc | 3.9 h | β+: 94%, Eβ+max = 1474 keV; EC: 6%, Eγ = 1157 keV | 44Ti/44Sc generator natCa(p,n)44Sc 44Ca(p,n)44Sc | 981–983 |
| 18F | 109.8 min | β+: 97%, Eβ+max = 635 keV | 18O(p,n)18F | 196 |
| 52Mn | 5.591 d | β+: 29.4%, Eβ+max = 575 keV; Eγ = 1434 | 52Cr(p,n)52 Mn natCr(p,x)52 Mn | 246 |
Abbreviations: T1/2, half-life; EC, electron capture (e.c.). The methods given in this Table are commonly used approaches to produce radionuclides. The readers are recommended to refer to the cited references for production details.
3.2.1. Zirconium-89.
Zirconium-89 (89Zr, T1/2 = 78.4 h) has been extensively used in the biomedical imaging field due to its fitting emission energy properties and long half-life, which matches the circulation half-life of mAbs.108,109 89Zr can be produced via several different nuclear reaction pathways, such as the natSr(α,xn)89Zr reaction, 89Y(d,2n)89Zr reaction, or 89Y(p,n)89Zr reaction.110 However, the production of 89Zr using solid targets with a small medical cyclotron might still be challenging. Recent studies have reported the production of 89Zr via solution targets, which are filled with a yttrium nitrate solution (Y(NO3)3·6H2O). 111,112 Further refinement of the irradiation procedures of liquid targets may potentially broaden the availability of 89Zr and therefore the development of 89Zr-based PET imaging. In developing 89Zr-mAb conjugates, 89Zroxalate (89Zr–Zr(ox)2) can be converted to 89Zr-chloride (89Zr-ZrCl4), which tends to undergo aquation and chelation with chelator-modified mAbs more rapidly.113,114 Moreover, 89Zr-chloride lacks the toxic oxalic acid of 89Zr-oxalate.115
89Zr is coupled to a mAb of interest through a bifunctional chelator, which possesses a ligand for capturing 89Zr and a reactive group for conjugating Lys or Cys residues on the mAb surface. Desferrioxamine (Figure 2a), denoted as Df or DFO, is a clinically used chelator for complexation of 89Zr. Traditionally, the preparation of 89Zr-mAb conjugates involves a multistep synthesis, in which a succinylated-derivative of desferrioxamine B (N-sucDf) was used to modify mAbs.116–118 This pioneering work paved the way for subsequent preclinical and more importantly, the clinical success of 89Zr-mAb immunoPET imaging. The development of a novel p-isothiocyanatobenzyl-derivative of desferrioxamine B (known variously as p-SCN-Bn-deferoxamine, Df-Bz-NCS or DFO-pPhe-NCS; Figure 2b) further allowed efficient and rapid preparation of 89Zr-mAb conjugates. This process involves the first coupling of Df–Bz–NCS to the lysine-NH2 groups of a mAb under alkaline conditions (pH 8.9–9.1) followed by radiolabeling with 89Zr-oxalate.119,120
Figure 2.

Chemical structures of chelators used in 89Zr-labeling of antibody vectors.
Despite their attractive characteristics, such chelators are unable to saturate the octavalent demands of the Zr4+ cation,121 which results in less stable 89Zr-immunoconjugates as indicated by accumulation of free 89Zr in the bone. Preclinical studies suggested that 89Zr’s tropism for bone may introduce undesirable radiation to bone marrow and confound imaging conclusions of bone malignancies or joint inflammation.122,123 The clinical impact of unbound 89Zr remains to be determined. A family of novel bifunctional chelators that impart enhanced stabilities has been developed to surmount this problem. One of these, the tetrahydroxamate chelator called DFO* (Figure 2c) and its derivative DFO*-pPhe-NCS (Figure 2d) were synthesized successfully.124,125 89Zr-DFO*-trastuzumab was thermodynamically more stable and had significantly lower bone uptake compared to the DFO modified mAb.125 More recently, DFOcyclo*-pPhe-NCS (Figure 3a), a novel DFO* derivative, was developed.126 When competed with excess DFO, this novel chelator was more stable than DFO and DFO* for chelating 89Zr. ImmunoPET imaging and biodistribution studies further demonstrated significantly lower bone uptake of 89Zr-DFOcyclo*-trastuzumab than 89Zr-DFO-trastuzumab. Despite this, 89Zr-DFOcyclo*-trastuzumab and 89Zr-DFO*-trastuzumab showed comparable imaging performance (Figure 3b). Desferrichromes (DFC) and related compounds have also been explored for coordinating 89Zr, but the DFC system did not show a dramatic advantage over DFO in in vivo imaging studies.127
Figure 3.

Comparison DFO, DFO*, and DFOcyclo* in immunoPET imaging. (a) Chemical structure of DFOcyclo*-pPhe-NCS. (b) ImmunoPET imaging with 89Zr-DFO-trastuzumab (left), 89Zr-DFOcyclo*-trastuzumab (middle), and 89Zr-DFO*-trastuzumab (right) in HER2+ SKOV-3 models. The results showed bone uptake in mice injected with 89Zr-DFO-trastuzumab but not with 89Zr-DFOcyclo*-trastuzumab or 89Zr-DFO*-trastuzumab at 168 h after injection of the radiotracers. Reproduced with permission from ref 126. Copyright 2019 Springer Berlin Heidelberg under [CC LICENSE] [http://creativecommons.org/licenses/by/4.0/].
Other novel 89Zr chelators include those that do not contain hydroxamate moieties, such as p-SCN-Bn-H6phospa,128 3,4,3-(LI-1,2-HOPO),129 p-SCN-Bn-HOPO,130,131 and many other novel chelators containing hydroxamate moieties.132–137 For instance, DFO-1-hydroxy-2-pyridone ligand (DFO-HOPO) is an octadentate chelator for 89Zr. 89Zr-DFO-HOFO showed excellent renal clearance and significantly lower bone uptake compared with 89Zr-DFO.135 Meanwhile, other novel 89Zr chelators with cyclic structures have been developed.136,138,139 Most recently, ligands containing carboxylate or amino donors have been tested as 89Zr chelators.140 Of these, 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA, vide infra) has been shown to outperform other analogues because 89Zr-DOTA demonstrated exceptional stability and more rapid systemic clearance. However, the high temperature (90 °C), longer reaction duration (45 min), and the need to use 89Zr-ZrCl4 may hinder its application in the immunoPET imaging field.
3.2.2. Copper-64.
Copper-64 (64Cu, T1/2 = 12.7 h) stands out as an immunoPET imaging radionuclide because of its ready availability and favorable properties. 64Cu is typically produced by bombardment of an enriched nickel target via the 64Ni(p,n)64Cu nuclear reaction.141 Interestingly, a recent study reported the possibility of 64Cu production using a liquid target.142 Because 64Cu undergoes β− emission in addition to β+ emission, it is a promising theranostic radionuclide. Furthermore, the combination of 64Cu and 67Cu (T1/2 = 61.8 h, β−: 100%) results in an attractive theranostic pair.143 To avoid nonspecific deposition of 64Cu in healthy tissues, various macrocyclic ligands and their derivatives have been developed. DOTA and its derivatives (Figure 4a–d) are the most commonly used ones to chelate 64Cu for PET imaging.144,145 However, it has been shown that DOTA is not the chelator of choice to develop immunoPET tracers.146,147 NOTA (1,4,7-triazacyclononane-1,4,7-triacetic acid)-based chelators (Figure 4e,f) are the most successful for chelating both 64Cu and 68Ga (vide infra) and are well-suited for radiolabeling of heat-sensitive antibody vectors at room temperature (rt). A comparison of the NOTA derivative 1,4,7-triazacyclononane,1-glutaric acid-4,7-acetic acid (NODAGA, Figure 4g) and DOTA for labeling a mAb with 64Cu demonstrated better in vivo performance of 64Cu-NODAGA-mAb than that of 64Cu-DOTA-mAb.148 p-SCN-Bz-MANOTA (Figure 4h), another NOTA derivative, outperformed DOTA and NODAGA as 64Cu-MANOTA-Fab showed the highest stability and the lowest background uptake in immunoPET imaging studies.149
Figure 4.
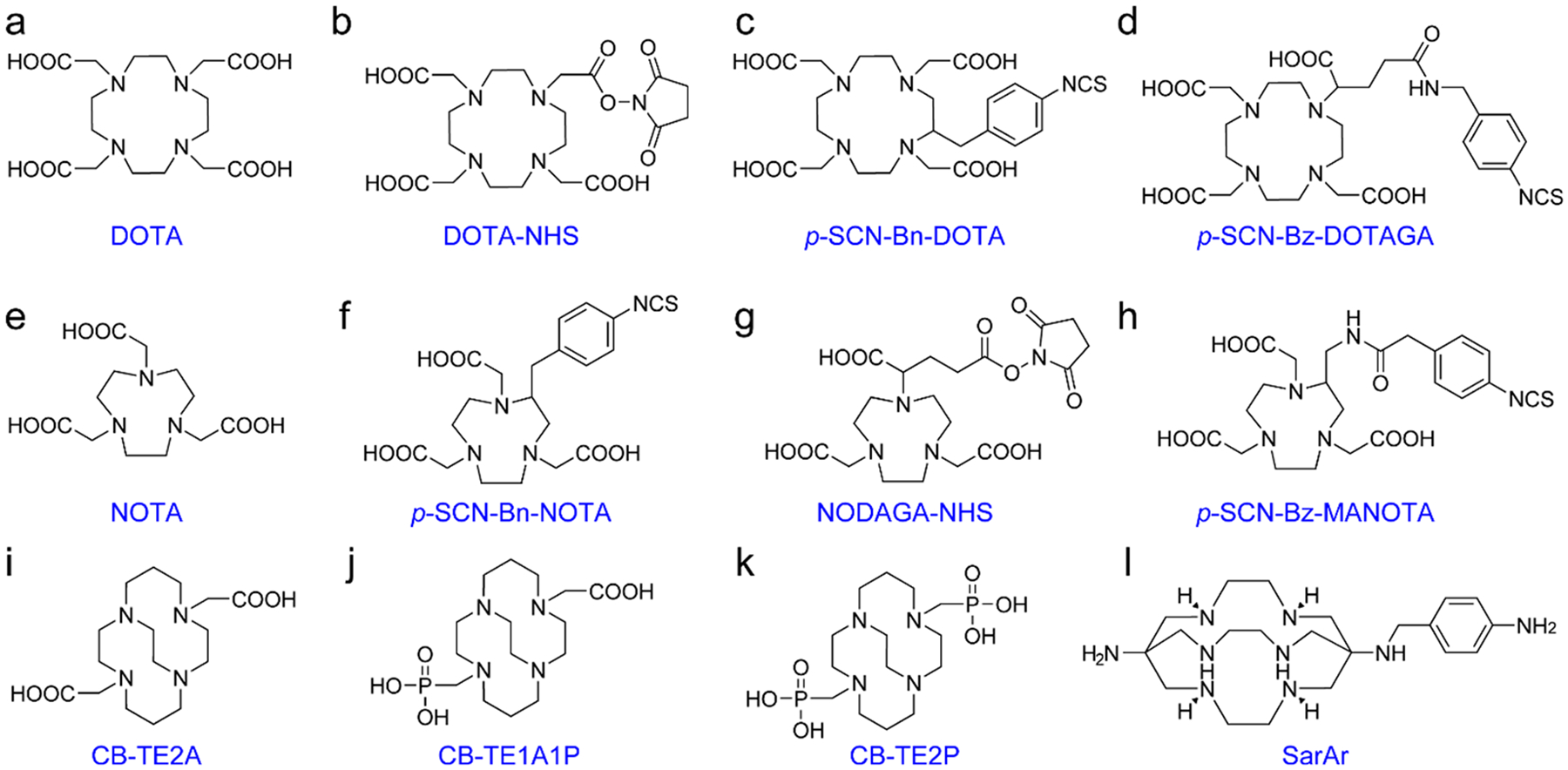
Chemical structures of chelators used in 64Cu-labeling of antibody vectors.
In addition, a series of cyclam-based macrocycles have been devised for 64Cu-labeling of antibodies. One of such agents, CB - TE2A (4, 11 - bis (carboxymethyl) - 1, 4, 8, 11 - tetraazabicyclo[6.6.2]hexadecane, Figure 4i), has shown its merits as an effective chelator for 64Cu.150 Unfortunately, the unfriendly labeling conditions (95 °C, 60 min, pH 6–7) limit the use of CB-TE2A in applications with antibody-based agents.151 CB-TE1A1P [(1,4,8,11-tetraazacyclotetradecane-1-(methanephosphonic acid)-8-(methanecarboxylic acid), Figure 4j] bearing a methanephosphonic acid and a carboxymethyl pendant arm can be radiolabeled with 64Cu at rt.152,153 While CB-TE2P [1,4,8,11-tetraazacyclotetradecane-1,8-di-(methanephosphonic acid), Figure 4k] can also be used for 64Cu-labeling at mild conditions,154 CB-TE1A1P is more favorable for antibody labeling because the carboxylate group allows for facile bioconjugation. Indeed, several studies have used 64Cu-CB-TE1A1P for immunoPET imaging.155,156 To further capitalize the better radiochemical yield (RCY) of cyclam derivatives, several other cyclam-based bis-(phosphinate)-bearing ligands for conventional or click chemistry-based 64Cu-labeling were developed.157,158 These novel cyclam-based bifunctional ligands are highly promising for developing immunoPET probes with 64Cu under mild conditions (25–37 °C, 10–20 min, pH 5.5–6.2).158 SarAr (Figure 4l) is a sarcophagine-based chelator used for developing immunoPET probes and can be labeled with radiometals under mild conditions (20–37 °C, 5–30 min, pH 5–5.5).159,160
The use of different chelators may result in varied accumulation patterns of the 64Cu-labeled mAb in the blood pool and in other healthy organs (e.g., liver) despite the similar tumor uptake.161 Generally, 64Cu undergoes hepatobiliary clearance which may result in increased liver and intestine signals, limiting the detection of diseases at these sites as well as diseases at the adjacent organs or tissues (e.g., pancreas). However, this problem can be resolved with 64Cu-labeled VHHs, which precisely detected small pancreatic tumors with clarity and high T/B ratio.162
3.2.3. Yttrium-86.
Yttrium-86 (86Y, T1/2 = 14.7 h) decays via electron capture (ec) and positron emission, accompanied by the emission of γ rays. Several nuclear reactions have been explored to produce 86Y. To date, the recommended reaction is 86Sr(p,n)86Y reaction,163–165 where the target material SrCO3 or SrO is enriched for irradiation with energies from 8–15 MeV. Although a liquid target has also been used to produce 86Y,104 the yield is generally low and methods for separating radiation-induced chemical species need to be established. With the refinement of methods for separating radioyttrium,166 the large scale production of 86Y is feasible with hospital-based cyclotrons. The most appealing application of 86Y is in tandem with yttrium-90 (90Y, T1/2 = 64.1 h), which is a pure beta emitter with excellent therapeutic properties.167,168 The advantage of this theranostic pair is that quantitative PET imaging with 86Y allows precise dosimetry of 90Y-based radiopharmaceuticals.169 Both clinical and preclinical studies have suggested that sequential use of 86Y and 90Y is an attractive theranostic pair if proper targeting vectors are used.170,171 Derivatives of ethylenediaminetetraacetic acid (EDTA, Figure 5a,b), diethylenetriamine pentaacetic acid (DTPA, Figure 5c), and DOTA are the most widely used chelators for yttrium radiolabeling of mAbs.172,173 Several studies have shown that incorporating the isothiocyanatobenzyl group (SCN-Bz) into the backbone of DTPA (Figure 5d) may sterically hinder the release of yttrium from the radiopharmaceuticals.174,175 To further improve the coordination efficiency of DTPA derivatives, CHX-A′′-DTPA (Figure 5e) and p-SCN-Bn-CHX-A′′-DTPA (Figure 5f) bearing a cyclohexyl were developed, and these chelators possessed improved stability over DTPA in radiolabeling mAbs.176–179
Figure 5.

Chemical structures of chelators used in 86Y-labeling of antibody vectors.
3.2.4. Radioiodine-124.
Radioisotopes of iodine have long been used as theranostic agents in the field of thyroid cancer.180 One among these, 124I (T1/2 = 4.18 d) can be produced through the 124Te(p,n)124I reaction.181,182 Iodine-124 has gained interest in radiolabeling mAbs since the clinical feasibility of immunoPET imaging with a 124I-labeled HMFGI mAb was first demonstrated in 1991.183–186 ImmunoPET imaging with 124I-labeled antibody agents are useful for evaluating bone metastases, another major benefit compared to those labeled with bone-seeking radiometals (e.g., 89Zr). It is important to mention that 124I-labeled immunoPET probes may not be appropriate for detecting primary thyroid cancers, stomach cancers, and urinary malignancies (e.g., bladder cancer and prostate cancer) because thyroid and stomach can scavenge iodide produced by deiodination and iodide is cleared via the urinary system. In addition to its role in PET imaging, 124I is a theranostic agent because of Auger electrons produced during its decay.187 ImmunoPET imaging using 124I-mAb is fully concordant with 131I-mAb RIT, where immunoPET imaging acts as a scouting procedure prior to RIT.188 Currently, the IODO-GEN method is the method of choice for radioiodination of noninternalizing mAbs.189 In an attempt to trap radioiodinated mAbs inside the tumor cells for improved molecular imaging, residualizing prosthetic agents for radioiodination have been developed and used by several groups.190–195
3.2.5. Fluorine-18.
With a high positron yield of 97%, a low mean positron range of 0.5 mm, and no simultaneous γ ray emission, fluorine-18 (18F, T1/2 = 110 min) is an ideal radionuclide for PET imaging.196 Diabodies and VHHs labeled with 18F have short plasma half-lives and can permit same-day imaging,197 a practical advantage over mAb-based imaging tracers. However, 18F has not been used to develop immunoPET probes until recently due to the harsh radio-labeling conditions and low RCY.
With the development of automated chemistry stations, several prosthetic groups for radiofluorination have been reported.198 [18F]Fluorobenzaldehyde ([18F]FBA, Figure 6a) is among the most popular prosthetic groups used for radio-fluorination of biomolecules.199 N-Succinimidyl-4-[18F]-fluorobenzoate ([18F]SFB, Figure 6b) is another prosthetic group which can form a stable amide bond with the Lys residue on proteins or peptides.200–202 Generally, an average RCY of 30%–35% will be obtained when [18F]SFB is used for labeling proteins or peptides.203 N-[2-(4-[18F]-Fluorobenzamido)-ethyl]maleimide ([18F]FBEM, Figure 6c) is a thiol-reactive 18F-labeling agent that can be site-specifically conjugated to Cys residues.204–207 However, the radiosynthesis of [18F]FEBM is a multistep and time-consuming process and often results in low RCY. It has been reported that the preparation of [18F]FBEM using automated radiochemical procedures requires less time and provides higher RCY (~17%).208 Another prosthetic group that facilitates radio-labeling of biomolecules under mild conditions (37–40 °C, 15 min, pH 8.5–9.0) is 2,3,5,6-tetrafluorophenyl 6-[18F]-fluoronicotinate ([18F]TFPFN, Figure 6d).209,210 Recent studies simplified the synthesis of [18F]TFPFN without drying [18F]fluoride,211,212 but the unfavorable RCY (~5%) in labeling VHHs may limit its applications.213 Direct 18F-labeling approaches include the silicon–fluoride acceptor approach (18F-SiFA),214–217 and the organotrifluoroborate ([18F]BF3) method,218 but one concern is that the solvents (e.g., acetonitrile) or the acidic conditions (pH 2.0–2.5) used in these methods are detrimental for sensitive antibodies.
Figure 6.

Chemical structures of prosthetic groups and chelators in 18F-labeling of antibody vectors.
In 2009, McBride et al. reported the aluminum-fluoride (Al18F) chelation strategy where fluorine is firmly bound to Al3+ by forming Al18F, which is complexed to NOTA with the resultant complex conjugated to the biomolecule of interest.219 This method has since been widely used for radiofluorination of various biomolecules.220 Although this procedure allows rapid fluorination of peptides, the high temperature (~100 °C) necessary for the complexation is unsuitable for most antibodies and some peptides. To overcome this drawback, a facile two-step procedure was described,221 where [18F]AlF was first complexed to NODA-MPAEM at high temperature (105–109 °C, 15–20 min; Figure 6e) and the purified intermediate then conjugated to antibodies via the maleimide–thiol reaction at rt for 10 min. Building upon this work, a series of novel acyclic polydentate ligands permitting facile Al18F radiolabeling of antibodies have been developed.222,223 RESCA-tetrafluorophenyl ester ((±)-H3RESCA-TFP, Figure 6f) and RESCA-maleimide ((±)-H3RESCA-Mal, Figure 6g) are two chelators that can be used to conjugate biomolecules via the Lys or Cys residues, respectively.224 Future studies are warranted to evaluate the diagnostic value of Al18F-RESCA-labeled antibodies.
3.2.6. Gallium-68.
Gallium-68 (68Ga, T1/2 = 1.1 h) is an attractive positron-emitting radionuclide because it is readily available from an affordable in-house 68Ge/68Ga generator. Because 68Ge has a half-life of 270.8 days, the shelf life of the generator is about 6–12 months based on elution schedules.225 Meanwhile, other means have been explored to produce 68Ga on a large scale.226–228 Although extensively studied for 67Ga/68Ga complexation, the DOTA-Gallium complex is less stable than its counterpart NOTA analogue. Currently, NOTA and its derivatives are the “gold standard” for 67Ga/68Ga complexation because of their fast and efficient radiolabeling at rt and high in vivo stability.229 HBED and its derivatives (Figure 7a–c) enabled 68Ga-labeling of heat-labile antibodies and antibody fragments at ambient temperatures.230,231 H2dedpa and its bifunctional derivative p-SCN-Bn-H2dedpa (Figure 7d,e) were developed for labeling peptides with 68Ga or 64Cu and tracers based on these chelators have shown promising imaging potentials.232–234 CP256 (Figure 7f) and YM103 (Figure 7g) are two other acyclic ligands that have yielded encouraging imaging results.235 PCTA and its derivative (Figure 7h,i) were superior with respect to kinetics and RCY for radiolabeling mAbs with 64Cu.236,237 Similarly, 68Ga-PCTA complex also showed improvement over 68Ga-NOTA for conjugating peptides.238 TRAP-Pr, a derivative of NOTA bearing phosphinic acid groups, showed significantly improved specificity for Ga3+ in radiolabeling peptides,239 but the harsh radiolabeling conditions (95 °C, pH 3.2) prohibit its use in radiolabeling of antibody vectors. To the best of our knowledge, many of the chelators mentioned above (e.g., p-SCN-Bn-H2dedpa, YM103, and PCTA) have not been utilized in developing immunoPET probes, but it is plausible that these chelators may have a certain value for 68Ga-based immunoPET probes.
Figure 7.

Chemical structures of chelators used in 68Ga-labeling of antibody vectors.
3.2.7. Other Radiometals.
Scandium-44 (44Sc, T1/2 = 3.9h) is a positron-emitting isotope and can be produced from a generator or a cyclotron source.240–242 Our team showed that CHX-A”-DTPA as opposed to other conventional chelators(i.e., DOTA, NOTA, DTPA), achieved 44Sc-labeling of a Fab fragment at rt.243 We are positive that the development of novel chelation strategies will further expand applications of 44Sc in the biomedical imaging field.244,245
Manganese-52 (52Mn, T1/2 = 5.591 d) can be produced via several nuclear reactions including the natCr(p,x)52Mn reaction.246,247 Manganese-52 has a higher β+ branching ratio of 29.4% and a lower β+ energy of 575 keV when compared to 89Zr (β+: 22.8%, Eβ+max = 901 keV), making it a promising alternative to 89Zr for immunoPET imaging. In a proof-of-concept study, we reported that the chelation of 52Mn via DOTA is possible and immunoPET imaging with 52Mn-DOTA-mAb is feasible over the course of several days.248 Other radiometals that can be incorporated into immunoPET imaging include 152Tb (T1/2 = 17.5 h),249 76Br (T1/2 = 16.2h),250,251 and 132La (T1/2 = 4.59 h).252,253
3.3. New Conjugation Strategies
Lysine-based random conjugation is the most prevalent method used for chemical modification of antibodies, followed by nonspecific Cys-based conjugation. Indeed, many clinical-grade radiolabeled antibodies have been produced via lysine functionalization. However, modification of antibodies at undesirable sites may compromise the immunoreactivity and distribution profiles of the radiolabeled antibodies. Hence, continuous efforts have been devoted to developing site-specific conjugation strategies to produce well-defined radio-tracers for high-quality imaging.254
3.3.1. Conventional Site-Specific Conjugation.
For site-specific modification purposes, proteins and antibodies are produced with short peptide tags via standard protein engineering and recombinant expression protocols. Generally, tags are introduced at the C-terminal end of the mAbs or sdAbs to avoid antigen-binding interference. Cys engineering is the most frequently used method for site-specific radiolabeling of antibody vectors (Figure 8a).255,256 The most commonly used strategy for Cys modification is via the maleimide conjugation, which is reversible and may result in the release of the maleimide scaffold in plasma (retro-Michael reaction).257 Recently, a novel strategy that may realize irreversible Cys bioconjugation was described.258 Thanks to the continuous advancement of the biomedical field, more sophisticated techniques are being exponentially developed for site-specific modification of proteins.259 Future studies may synthesize novel immunoPET agents taking advantage of these emerging techniques.
Figure 8.
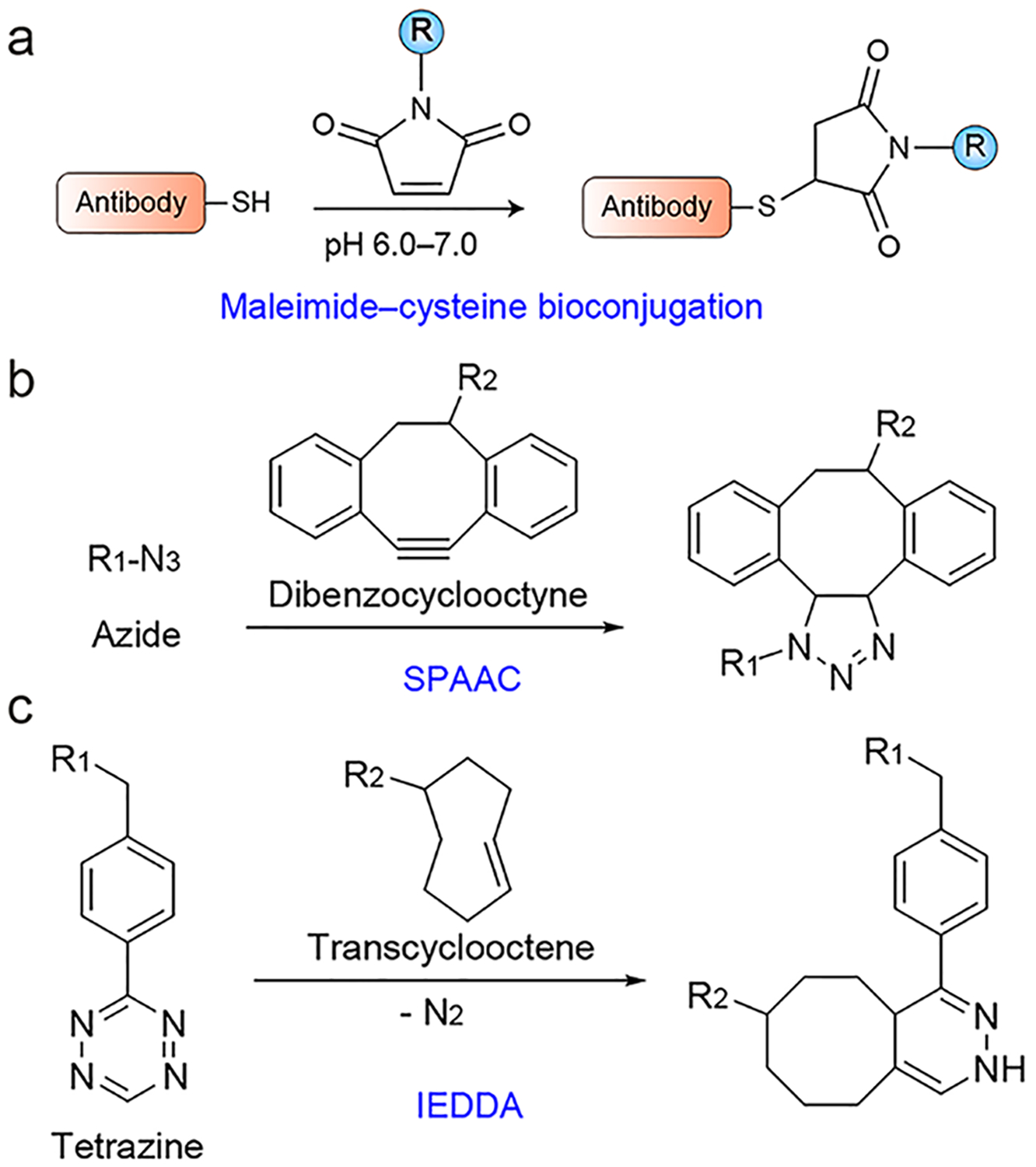
Site-specific radiolabeling strategies. (a) The maleimide–cysteine reaction is among the most commonly used strategies for site-specific radiolabeling of antibody vectors. R = chelator of interest. (b) The strain-promoted azide–alkyne cycloaddition (SPAAC) reaction. R1 = antibody of interest, R2 = chelator of interest. (c) The inverse electron demand Diels–Alder (IEDDA) cycloaddition reaction. R1 = chelator of interest, R2 = antibody of interest. It is worth noting that radiolabeling via the click chemistry reaction goes both ways.
3.3.2. Click Chemistry-Mediated Radiolabeling.
Click chemistry has been increasingly applied to develop new molecular imaging probes.260,261 Of various click chemistry reactions, the Cu(I)-catalyzed 1,3-dipolar cycloaddition between azides and alkynes (CuAAC) is frequently used to develop radiopharmaceuticals. 18F-labeled small-molecule probes prepared via the CuAAC reaction are actively assessed in clinical settings.262,263 If it is necessary to avoid the use of Cu(I) catalyst, particularly when developing immunoPET probes with radiometals, the catalyst-free strain-promoted azide–alkyne cycloaddition (SPAAC) reaction is a bioorthogonal alternative (Figure 8b).264,265 However, the synthetic complexity and hydrophobicity of the cyclooctyne precursors in the SPAAC system may potentially limit its widespread application. The inverse electron demand Diels–Alder (IEDDA) reaction between strained trans-cyclooctene (TCO) and electron-deficient tetrazine (Tz) is a giant step forward in the field of bioorthogonal chemistry in terms of reactivity and application possibilities (Figure 8c).266 As such, this chemistry has been applied in a myriad of uses developing molecular imaging probes.267–269 Moreover, the axial TCO isomers were found to be more reactive than their equatorial analogues.270
Photoclick chemistry has been used in the field of chemical biology for years.271,272 On the basis of the previous success, several groups have used photochemical methods to develop immunoPET probes recently.273–275 Upon further refinement, these novel bioconjugation methods may eliminate time-consuming purification steps and maximize RCY, which is particularly needed for short-lived radionuclides such as 11C (T1/2 ≈ 20 min) and 68Ga (T1/2 = 1.1 h).
3.3.3. Enzyme-Mediated Radiolabeling.
Enzymatic methods are well suited to achieve site-specific labeling of antibody vectors. Prominent one among them utilizes sortase A (SrtA), an enzyme derived primarily from Gram-positive Staphylococcus aureus. Typically, SrtA recognizes substrates containing C-terminal LPXTG motifs (where X = any amino acid except proline) and cleaves the peptide between threonine and glycine (Gly), leading to loss of the downstream part of the substrates (e.g., His-tag) and formation of new peptide bonds with nucleophilic substrates containing N-terminal Gly residues.276 While SrtA is a well-established enzyme responsible for anchoring LPXTG-containing proteins to the growing cell wall and pili of various Gram-positive bacteria, recombinant SrtA has been developed into a valuable protein engineering tool in recent years.277 By employing sortase-mediated transpeptidation, it is facile to install functional moieties (e.g., chelator and dye) onto the N- or C-terminus of an antibody in a site-specific fashion (Figure 9). The use of SrtA has facilitated site-specific radiolabeling of VHHs using either 18F55,278 or radiometals.64,162 A unique two-step modular system is also available to conjugate immunoPET probes. In this system, SrtA is used to incorporate the strained cyclooctyne functional groups into the targeting vector of interest, followed by azide–alkyne cycloaddition reaction between the click chemistry handles.279,280 With further improvement of the catalytic activity of SrtA and evolution of Ca2+ independent SrtA mutants,281–283 SrtA will serve as a versatile platform for developing more sophisticated immuno-PET probes.
Figure 9.

Sortase-catalyzed site-specific labeling of antibody moieties. (a) For C-terminal labeling, the LPXTG motif is expressed at the C-terminus of the targeting vector (e.g., VHH and antibody fragment). (b) For N-terminal labeling, sortase recognition tag (i.e., LPXTG) is positioned at the C-terminus of the modification (e.g., chelator and dye) with the oligoglycine nucleophile inserted at the N-terminus of the targeting vector.
Butelase-1 is another transpeptidase found in Clitoria ternatea (butterfly pea) and recognizes a tripeptide motif, Asn-His-Val.284,285 Butelase-1 efficiently cyclizes peptides and proteins with a high yield. Although it is the fastest peptide ligase, its biological applications are limited because it cannot be produced as of now using recombinant techniques. Theoretically, a combination of butelase-1 and SrtA may facilitate the labeling of proteins at two distinct sites. This was proven in a recent work by Harmand et al.,286 which reported that the combination of SrtA and butelase 1 allowed facile preparation of C-to-C fusion proteins and dual-labeling of an IgG1 molecule with two fluorescent dyes. A combination of butelase-1 with other transpeptidases may be possible in the future.
Recently, a chemoenzymatic strategy combining glycan engineering and click chemistry was invented. The combination of these strategies allowed site-specific attachment of molecules to the heavy chain glycans. This methodology involves the following steps: (1) removal of galactose residues on the CH2 domain of the heavy chains of an antibody using β−1,4-galactosidase, (2) attachment of azide-modified monosaccharide to the heavy chain glycans using β-galactosyltransferase mutant to Gal-T(Y289L), (3) synthesis of chelator or dye-containing cyclic dibenzocyclooctyne (DIBO or DBCO), (4) catalyst-free click chemistry between azide-bearing antibody and DIBO-bearing payload (e.g., chelator or dye), and (5) radiolabeling of the site selectively modified antibody using radionuclides of interest (Figure 10).287 This method has been applied to design dual-labeled agents for PET and optical imaging of colorectal cancers.288 A recent study demonstrated that 89Zr-DFO-trastuzumab developed using this chemoenzymatic strategy outperformed its counterpart developed by random conjugation method because the site-specifically modified 89Zr-DFO-trastuzumab showed enhanced immunoreactivity and stability in immunoPET imaging studies.289 Although being able to yield homogeneous and well-defined products, this method suffers from a lengthy and relatively tedious protocol.
Figure 10.

Schematic of a chemoenzymatic methodology for site-specifically grafting cargoes (e.g., chelator) to the heavy-chain glycans of an antibody of interest. Reproduced with permission from ref 261. Copyright 2016 American Chemical Society.
HaloTag is a genetic construct with multifunctional and versatile capabilities. The molecular mechanism of this system is based on a mutant bacterial haloalkane dehalogenase enzyme, which is obtained from Rhodococcus rhodochrous.290,291 Use of the HaloTag technology begins with the fusion of the HaloTag (33 kDa) to the protein of interest. A HaloTag-specific ligand is then introduced, resulting in the formation of an irreversible covalent bond between the HaloTag-modified protein and the ligand.292,293 As we all know, His-tag is ubiquitously introduced in protein production, but its role is merely limited to the isolation and purification of proteins.294,295 In comparison, HaloTag can be employed to rapidly purify proteins and the isolated proteins may further enable multimodal molecular imaging. Preliminary evidence has demonstrated the feasibility of HaloTag-based pretargeted imaging. This strategy involves first administering a HaloTag-modified antibody for pretargeting and then a small radiolabeled molecule with a short circulation time for imaging.296
Analogous to these, Schibli et al. reported that microbial transglutaminase (mTGase) can modify mAbs in a stoichio-metric manner and the site-specifically engineered mAbs are of particular interest for molecular imaging.297,298 Another bacterial enzyme lipoic acid ligase (LplA) was also used with [18F]fluorooctanoic acid ([18F]FA) for site-specific radio-labeling of a Fab fragment.299 Although mTGase and LplA have shown potential in mediating site-specific radiolabeling of biomolecules, their robustness needs to be confirmed by future studies.
3.3.4. Pretargeted ImmunoPET Imaging Strategies.
The slow blood clearance of mAb is problematic because it leads to high background activity and radiation exposure, especially to the red bone marrow. Aside from reducing the molecular size and removing or blocking the Fcγ receptors (FcγRs), pretargeted immunoPET imaging holds promise to improve the imaging quality while decreasing radiation exposure.300 In addition, this imaging approach enables the use of short-lived PET radionuclides (e.g., the widely available 68Ga and 18F).301 Pretargeted imaging was initially achieved using the biotin–streptavidin interaction and BsAbs.302,303 In the avidin–biotin pretargeting approach, a streptavidinmodified immunoconjugate, and radiolabeled biotin were used.304,305 Streptavidin and biotin-based pretargeting systems have been investigated in several clinical studies.306–308 However, the bacterially derived streptavidin constructs are prone to have immunogenicity, which may limit repeated imaging or therapy.309 A second concern for this system is that endogenous biotin in patient blood and tissues may competitively occupy and block the binding sites of streptavidin, thus preventing the binding of radiolabeled biotin.
In a BsAb-based pretargeted imaging system, a BsAb that can bind to target antigen and radiolabeled hapten is first injected, enabling the saturation of the target and washing out of the unbound antibodies. Once the unbound antibody is cleared from the blood and normal tissues, a radiolabeled chelate is injected and a portion will be captured by the BsAbs at the tumor sites with the remainder eliminated rapidly from the body. This strategy has been refined over the years and used in clinical studies for pretargeted radioimmunotherapy (pRIT),310 as well as for pretargeted immunoSPECT and immunoPET imaging.311–314 Traditionally, BsAbs used for pretargeted imaging and therapy were produced by chemical methods315,316 or by recombinant expression from Escherichia coli or myeloma cell cultures.317,318 Although several clinical studies have validated the therapeutic effect of pRIT using the antibodies produced this way,319,320 the murine or chimeric property of such agents limit their clinical use. To further advance the clinical translation and application of pretargeted imaging and therapy, a more innovative Dock-and-Lock method is now being used to develop humanized recombinant BsAbs on a large scale.321 One such example is the anti-CEA × anti-HSG TF2 BsAb, which contains a humanized antihist-amine-succinyl-glycine (HSG) Fab fragment from the anti-HSG mAb 679 and two humanized anti-CEA Fab fragments derived from the hMN14 mAb (labetuzumab).219,321 As the hapten peptides of the pretargeted system, the radiolabeled small molecules bear two HSG groups and various chelators (Figure 11), allowing versatile labeling with radionuclides of interest (e.g., 68Ga and [18F]AlF). Currently, several other such BsAbs (e.g., TF4 for CD20 and TF10 for a mucin antigen) produced via this approach are also being actively investigated for theranostic purposes.322–324 Using the directed evolution and yeast surface display,325 Orcutt and coauthors at the Massachusetts Institute of Technology (MIT) constructed another pretargeted system,326,327 which exploits a principle similar to the streptavidin–biotin system but replaces streptavidin with a C825 scFv capturing benzyl(Bn)-DOTA-radiometal complex. In this system, use of a clearing agent (e.g., dextran-(Y)-DOTA-Bn conjugate) further improved the imaging quality and therapeutic outcome.328,329
Figure 11.

Chemical structures of DOTA-HSG and NOTA-HSG hapten peptides used in pretargeted immunoPET imaging.
In recent years, bioorthogonal chemical reactions have been developed as alternatives to biologic pretargeting interactions for recruiting radiolabeled probes to the tumor-bound mAb, as excellently described in several reviews.330–332 Since its initial report,266 the IEDDA reaction has been widely used for pretargeted tumor imaging. Various TCO-conjugated antibodies and 111In-, 18F-, or 64Cu-labeled Tz probes have been used to achieve pretargeted imaging.333–335 These novel strategies substantially improved T/B ratios and reduced radiation dose to the bone marrow. Ideal imaging results were achieved when the tagged biomolecules (e.g., TCO-modified mAb) were completely cleared from the circulation before injecting the radiolabeled Tz, which could be accomplished by injecting a clearing agent (e.g., Tz-galactose-albumin).336,337 Furthermore, sequential use of the enzyme-mediated site-specific modification and click chemistry produced improved imaging results.338 On the basis of the available evidence, the added value of this strategy is confined to the production of homogeneous immunoconjugate and reduced use of the antibody because the imaging performance was comparable to that of the randomly labeled analogous construct.339 Along with this, several other Tz-modified chelators have been developed for labeling peptides with radiometals,340,341 but their performance with antibodies remains to be determined. It is also notable that some chemical reactions are not suitable for in vivo pretargeted imaging due either to the interactions of the radioligand with serum albumin or to the slow reaction kinetics.342,343
As discussed above, high-affinity Affibodies have been successfully applied for molecular imaging of cancers. The unfavorable part is that their rapid renal clearance, reabsorption, and internalization unavoidably lead to high accumulation of the radiotracers in the proximal tubule of the kidneys. Pretargeted imaging approaches have been harnessed to overcome this disadvantage.344–347 One such approach is the peptide nucleic acid (PNA)-mediated hybridization system, where the primary PNA strand used for tumor targeting can selectively and rapidly hybridize with the secondary complementary PNA strand equipped with radio-nuclides. For instance, Vorobyeva et al. developed a modular system consisting of ZHER2:342-SR-HP1 (primary targeting agent) and 68Ga-HP2 (secondary targeting agent).348 They reported that this Affibody-based imaging approach yielded increased tumor uptake and decreased kidney uptake in preclinical ovary cancer models.
3.3.5. Clearance-Enhanced ImmunoPET Imaging.
Other than the above-mentioned methods for increasing image contrast and improving image quality, use of urokinase and a urokinase-cleavable bifunctional chelator (CB-TE1A1PUSL-DBCO) is a promising radionuclide clearance enhancement system.349 This system has been used to develop 64Cu-CB-TE1A1P-USL-trastuzumab,156 where injection of urokinase triggers urokinase-responsive cleavage of the radiotracer, leading to enhanced elimination of radioactivity from the blood circulation, enhanced hepatic radioactivity clearance, and significantly increased tumor-to-blood ratio. These studies indicate that urokinase and urokinase substrate linkers can be used to induce clearance of radioactivity from the nontargeted tissues and shorten the time required to obtain optimal immunoPET imaging contrast.
4. IMMUNOPET IMAGING OF CANCERS
The major application of immunoPET imaging is to facilitate better management of cancer patients. According to several clinical reports, immunoPET imaging provided excellent specificity and sensitivity in detecting primary tumors.184,186 ImmunoPET imaging is also an appealing option for detecting lymph node and distant metastases.118,350 More importantly, accumulating clinical evidence suggests that immunoPET can detect previously unknown lymph node and distant meta-stases.39,41 These impressive results indicate that immunoPET may complement IHC staining in clinical dilemmas when suspected lesions are inaccessible for biopsy. However, suboptimal imaging conditions (e.g., imaging protocol and facility performance) and low expression of the target in small tumor lesions may lead to underestimation of the tumor burden and target abundance. Following immunoPET imaging, patients with positive findings can be selected for subsequent therapies (e.g., antibody therapy and antibody-based RIT), whereas patients with negative or heterogeneous findings may need multidisciplinary treatments. ImmunoPET is a useful diagnostic tool but also a theranostic companion for radiation dosimetry prior to administering the therapeutic radiopharmaceuticals (discussed in section 8). Moreover, immunoPET imaging is useful for improved triage during early disease stages and to facilitate image-guided surgery.351,352 The information provided by immunoPET will significantly enhance the existing diagnostic methods for better tumor characterization. One can envision that tumors may be classified not only according to their origins and mutation status but also according to the expression of specific tumor antigens in the future.
4.1. Receptor Tyrosine Kinases
Receptor tyrosine kinases (RTKs) are often overexpressed and/or mutated in a variety of cancers.353 As the best-studied oncogenic drivers, RTKs have been among the most explored targets for developing anticancer therapeutics. Indeed, mAbs and small-molecule tyrosine kinase inhibitors (TKIs) suppressing RTKs or their ligands are the most typical examples of targeted cancer therapies. Along with this success, substantial efforts have been dedicated to developing immunoPET imaging approaches for revealing the heterogeneous status of RTKs in cancers.354
4.1.1. Epidermal Growth Factor Receptor.
Human epidermal growth factor receptor (EGFR) is a RTK regulated by at least seven activating ligands in humans.355 Several mAbs (e.g., cetuximab, panitumumab, and nimotuzumab) targeting the extracellular domain of EGFR and TKIs (e.g., erlotinib) targeting the intracellular domain of EGFR have been approved for treating EGFR-positive cancers. An initial clinical study demonstrated that 89Zr-Df-cetuximab immunoPET imaging could visualize EGFR expression and predict the treatment efficacy of cetuximab in advanced colorectal cancers.356 However, a follow-up study showed that 89Zr-Dfcetuximab uptake failed to predict the efficacy of cetuximab monotherapy in patients with RAS wild-type metastatic colorectal cancer.357 89Zr-Df-cetuximab was further investigated in nine patients with head and neck squamous cell carcinoma (HNSCC) or nonsmall-cell lung cancer (NSCLC). The results showed no direct relationship between EGFR expression and tumor uptake of the radiotracer in terms of the T/B ratio,358 which was in concert with the results reported by two other studies.359,360 Because cetuximab irreversibly binds to EGFR expressed in liver cells, van Loon et al. reasoned that an optimal preloading of unlabeled cetuximab is needed to first saturate liver EGFRs.358 In addition, Pool et al. found that shed EGFR ectodomain levels in liver and plasma interfere with EGFR-targeted immunoPET imaging agents, and increased administration of radiotracer could improve tumor visualization.361
Panitumumab is a fully human mAb targeting EGFR.362 Niu et al. initially reported that 64Cu-DOTA-panitumumab immunoPET imaging failed to quantify EGFR protein expression in three different HNSCC xenografts,363 probably due to the poor penetration of the antibody and varying tumor vasculature in the used models. However, several other studies showed that uptake of 89Zr-panitumumab was associated with EGFR expression in other tumor models.364–366 Additionally, a group reported the clinical safety and feasibility of 89Zrpanitumumab immunoPET in noninvasively characterizing EGFR expression.367,368 Scott et al. screened a variety of mouse mAbs and found that mAb 806 specifically targets the overexpressed or activated forms of EGFR.369,370 ch806, a chimeric form of mAb 806, has been validated as an effective therapeutic antibody and ch806-based molecular imaging showed specific accumulation of the antibody at multiple tumor sites.371,372 In an attempt to trap the radioiodinated ch806 in lysosomes, ch806 was further radiolabeled with residualizing peptides 124I-IMP-R4 and 124I-PEG4-tptddYddtpt. The tumor uptake of 124I-IMP-R4-ch806 (Figure 12) and 124I PEG4-tptddYddtpt-ch806 was apparent in preclinical glioma models.373,374 More recently, two other preclinical studies have demonstrated the value of 89Zr-Df-nimotuzumab immunoPET diagnosing epidermoid carcinomas and gliomas.375,376
Figure 12.
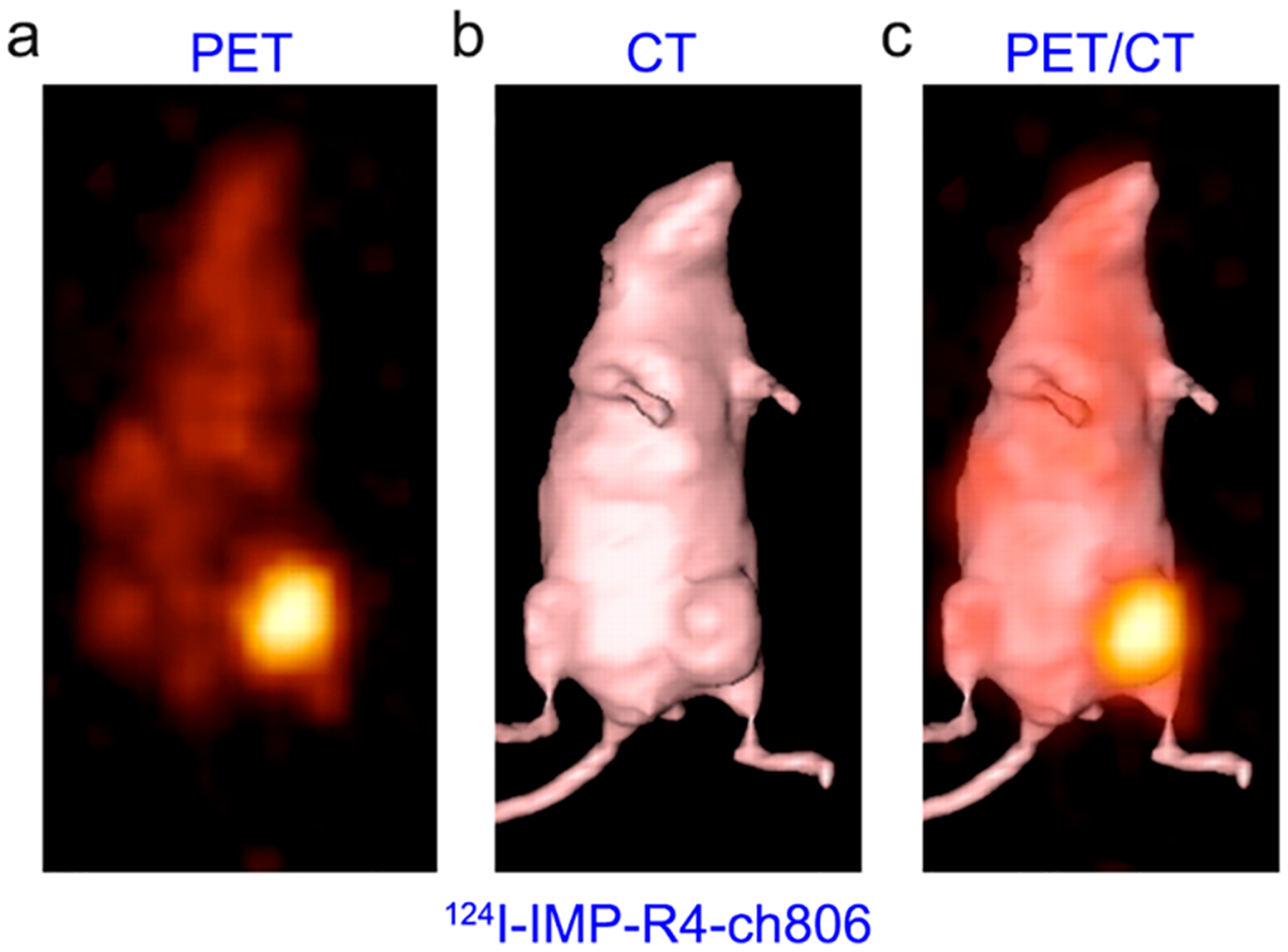
ImmunoPET imaging of EGFR expression using 124I-labeled residualizing radiotracer. (a–c) 124I-IMP-R4-ch806 immuno-PET imaging clearly delineated EGFR-positive gliomas with negligible uptake in normal tissues. Reproduced with permission from ref 373. Copyright 2010 SNMMI.
Affibody-based PET imaging probes are also being developed to image EGFR expression.377–381 Burley et al. developed two Affibody-based EGFR-targeting radioligands 89Zr-DFO-ZEGFR:03115 and 18F-AlF-NOTA-ZEGFR:03115).382 The authors found that 18F-AlF-NOTA-ZEGFR:03115 PET imaging could correlate EGFR downregulation in response to cetuximab treatment in preclinical HNSCC models. These probes may have clinical utility because of the poor performance of 89Zr-Df-cetuximab and the dichotomous role 64Cu-DOTA-panitumumab in mapping EGFR levels. Although the development of next-generation EGFR-targeting mAbs may overcome cetuximab-induced resistance,383,384 VHH-based EGFR-targeting therapeutics may also overcome cetuximab-induced resistance. One such VHH, 7D12, penetrates more deeply and homogeneously into tumors than cetuximab.385,386 Therefore, 7D12-based nuclear medicine imaging approaches may serve as promising tools in selecting patients suitable for VHH-based therapies.387,388
4.1.2. Human Epidermal Growth Factor Receptor 2.
Human epidermal growth factor receptor 2 (HER2/ErbB2) has attracted much interest as a molecular imaging target in the past two decades. Along with the clinical approval of HER2-targeted antibody therapeutics (e.g., trastuzumab, trastuzumab emtansine [T-DM1], and pertuzumab), several antibody-based radiotracers have been developed for imaging HER2 expression.389 Of them, two initial clinical studies using 111In-DTPA-trastuzumab reported uptake of the tracer in the myocardial wall and detection of new HER2-positive breast cancer lesions.16,390 Since 89Zr became clinically available,118 successive translational studies have reported the value of 89Zr-Df-trastuzumab immunoPET in detecting both previously known and unknown metastatic breast cancer lesions,41 detecting heterogeneous HER2 expression in breast cancer lesions before T-DM1 treatment and predicting T-DM1 treatment outcomes.391–393 64Cu-DOTA-trastuzumab is an alternative that has also been tested in the clinic.394,395 In addition to trastuzumab, pertuzumab is another FDA-approved mAb targeting HER2. 89Zr-Df-pertuzumab has been successfully translated into the clinic and 89Zr-Df-pertuzumab immunoPET imaging was able to detect primary breast cancers and distant breast cancer metastases including brain metastases (Figure 13a–d).396 However, no clinical studies have directly compared the diagnostic efficacies of 89Zr-Dftrastuzumab and 64Cu-DOTA-trastuzumab.
Figure 13.
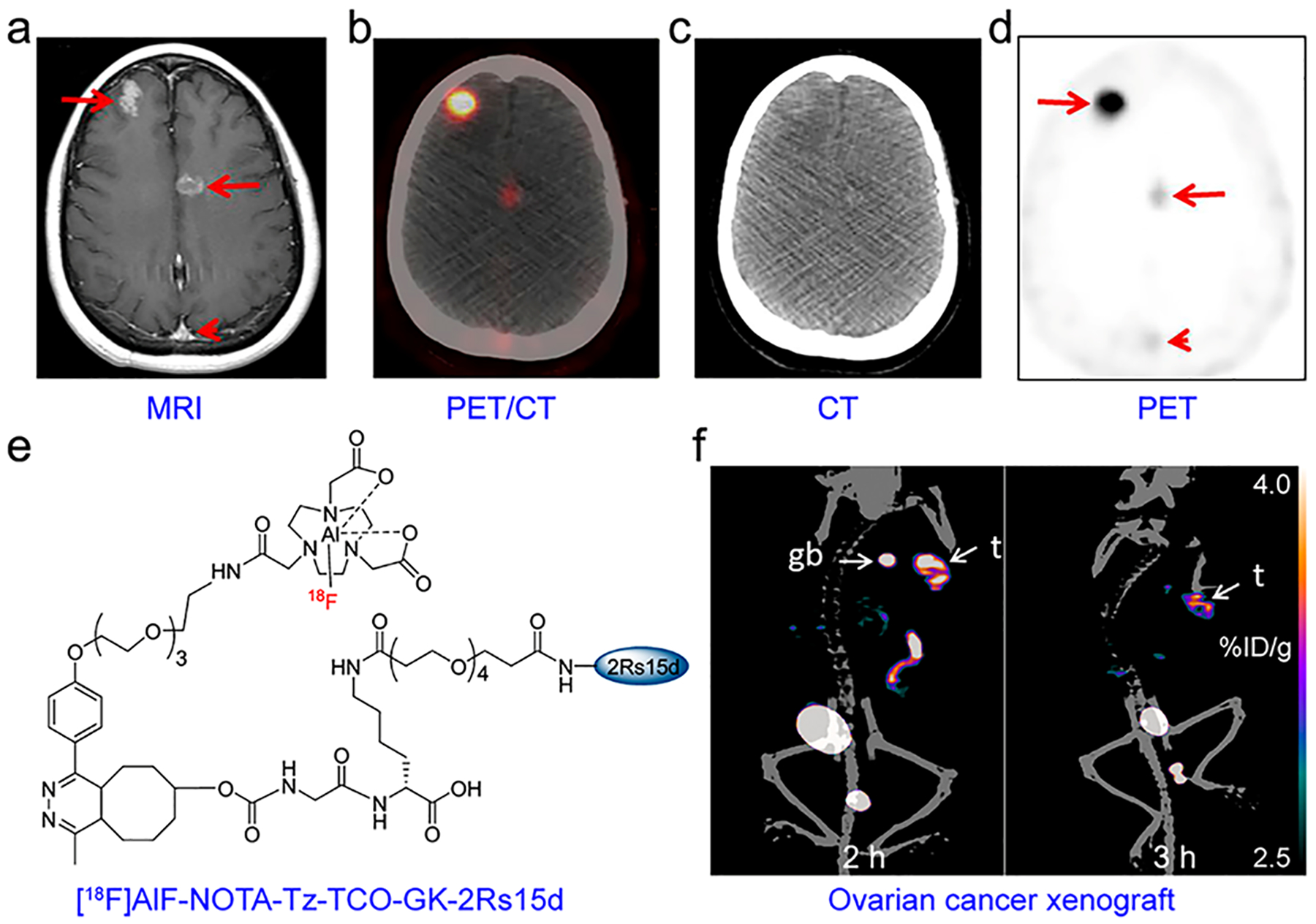
ImmunoPET imaging of HER2 expression. (a) T1-weighed MR imaging of a 46-year-old woman showed brain metastases from breast cancer (red arrows). (b–d) 89Zr-Dfpertuzumab immunoPET/CT imaging of the same patient demonstrated varying uptake of the radiotracer in brain metastases (red arrows) and minimal uptake in the superior sagittal sinus (red arrowhead). Reproduced with permission from ref 396. Copyright 2018 SNMMI. (e) Chemical structure of [18F]AlF-NOTA-Tz-TCOGK-2Rs15d. (f) ImmunoPET/CT imaging of a human ovarian cancer xenograft at 2 and 3 h after injection of [18F]AlF-NOTA-Tz-TCOGK-2Rs15d. Reproduced with permission from ref 416. Copyright 2018 American Chemical Society.
Increasing evidence supports HER2 as a broad tumor biomarker beyond its established role in breast cancers.397 Preliminary studies have reported the value of HER2-specific immunoPET in elucidating HER2 expression levels in gastric cancer and esophagogastric adenocarcinoma.398–400 Because HER2 serves as a biomarker for ovarian cancer and also potentially for advanced thyroid cancers, it is rational that HER2-targeted immunoPET imaging was able to map HER2 expression in these solid tumors.401–404
Although a combination of 18F-FDG PET and 89Zr-Dftrastuzumab immunoPET robustly predicted the treatment efficacy of T-DM1, 89Zr-Df-trastuzumab did not accumulate in a proportion of HER2-positive lesions.391 Temporal modulation of HER2 expression with mucolytic treatment enhanced tumor uptake of 89Zr-Df-trastuzumab in a preclinical breast cancer model.405 Moreover, it has been shown that caveolin-1 mediates HER2 internalization and depletion of caveolin-1 with lovastatin increased tumor uptake of 89Zr-DFO-trastuzumab.406 This effect was further validated by a more recent study where oral administration of lovastatin enhanced tumor accumulation of 89Zr-DFO-pertuzumab in preclinical gastric cancer models.407 More importantly, image-guided modulation of HER2 expression and internalization could improve the efficacy of trastuzumab treatment.408 These results together demonstrate that modulation of HER2 expression or internalization could increase tumor uptake of HER2-targeted immunoPET probes and also the efficacies of HER2-targeted therapeutic regimens.
Small biomolecules (e.g., antibody fragments, sdAbs, and Affibodies) are also being used as HER2-targeting vectors.409,410 Beylergil et al. developed 68Ga-DOTA-F(ab′)2-trastuzumab and investigated the diagnostic utility of this radiotracer in 15 patients with breast cancer. Although less optimal than radiolabeled trastuzumab, this imaging approach detected diseases in 4/8 patients with HER2-positive breast cancers.411 2Rs15d is a sdAb developed against HER2 and has shown excellent targeting of HER2 in both preclinical settings,63 and clinical settings.412 In both cases, 68Ga was randomly conjugated on Lys residues of 2Rs15d without compromising the targeting affinity of 2Rs15d, in part due to the absence of Lys residues in its antigen-binding domains. (Note: 2Rs15d contains six lysines that are dispersed in the framework and away from the receptor-binding regions.) A recent study revealed that SrtA-mediated site-specific radiolabeling of 2Rs15d yielded homogeneous 68Ga-NOTA-2Rs15d, and immunoPET imaging with this radiotracer readily visualized HER2-positive breast cancers.413 However, the renal clearance of 68Ga-NOTA-2Rs15d and the resultant high tracer retention in bilateral kidneys is problematic, which was also observed in several other 18F-labeled VHHs.202,414 In this setting, two HER2-specific VHHs (i.e., 2Rs15d and 5F7) were radiolabeled with [18F]TFPFN and subsequent immunoPET imaging with the synthesized probes successfully detected tumors with prominent tumor uptake and substantially lower renal uptake.415 Furthermore, [18F]AlF-NOTA-Tz-TCO-GK-2Rs15d was developed by the IEDDA reaction and incorporation of a renal brush border enzyme-cleavable Gly-Lys (GK) linker in the prosthetic moiety. This strategy achieved quite high RCY (~17%) while also maintaining high T/B ratios (Figure 13e,f).416 2Rs15d has been validated useful as a vehicle for RIT after labeling with 177Lu or 131I,67,417 providing valuable therapeutic options accompanying the above-mentioned immunoPET imaging. A clinical trial evaluating the safety and distribution of 131I-SGMIB-2Rs15d in breast cancers has completed patient recruitment (NCT02683083).
ZHER2:342 is a second-generation HER2-targeting Affibody and has been radiolabeled nonselectively with 125I and 111In418,419 or site-specifically with 18F-FBEM.205,420,421 Xu et al. further modified ZHER2:342 with a hydrophilic linker (Cys-Gly-Gly-Gly-Arg-Asp-Asn) that is conjugated with maleimidomonoamide-NOTA and radiolabeled the derivative with Al18F.422 The authors found that this modification produced excellent imaging contrast and low abdomen uptake of the developed tracer. DOTA-ZHER2:342‑pep2 (ABY-002) is a chemically synthesized derivative of ZHER2:342 with a DOTA coupled to its NH2 terminus. Therefore, this agent could be site-specifically radiolabeled with 111In or 68Ga.423–425 Furthermore, 111In-ABY-002 and 68Ga-ABY-002 demonstrated the potential in visualizing HER2-expressing metastatic lesions in patients with breast cancer.426 Another group of HER2-specific molecular imaging tracers is based on the Affibody MMA-DOTA-Cys61-ZHER2:2891-Cys (ABY-025).427–429 Of them, 68Ga-ABY-025 can be produced in compliance with Good Manufacturing Practice (GMP)430 and can be used to accurately image HER2 expression in metastatic breast cancers.431–434 Because 18F is a radionuclide validated for clinical use and its longer half-life enables imaging over a longer time window compared to 68Ga, Glaser et al. used three methods (i.e., 18F-SiFA, 18F-AlF-NOTA, and 18F-FBA) to radiolabel ABY-025. They found that 18F-FBA-ZHER2:2891 (GE226) emerged as a highly specific candidate for imaging HER2 expression in pre- and post-treatment settings.435,436 ZHER2:2395 is another variant of ZHER2:342 and has a C-terminal Cys.437 This Affibody molecule was site-specifically labeled with Al18F-NOTA (90 °C, 15 min) with an acceptable RCY (21% ± 5.7%).438
Aiming to improve the therapeutic effect of HER2-targeted therapies, BsAbs are being increasingly produced,439–442 which may serve as appealing immunoPET imaging components. Because of the progress in preclinical and clinical studies, we are optimistic that HER2-targeted immunoPET imaging will provide a noninvasive and dynamic visualization and quantification of HER2 expression in heterogeneous tumors, which will refine clinical management of HER2-targeted therapeutics. Apart from detecting the heterogeneous HER2 expression in tumor tissues for initial patient selection,443 HER2-specific immunoPET may serve as a useful tool in predicting therapeutic responses following HER2-targeted therapies.444,445 This will be especially useful when the applied HER2-specific vector binds to epitopes different from that of the clinically approved antibodies (e.g., trastuzumab and pertuzumab).446
4.1.3. Human Epidermal Growth Factor Receptor 3.
Human epidermal growth factor receptor 3 (HER3/ErbB3) has weak intracellular kinase activity and does not form homodimers, but it is a signal amplifier after forming heterodimers with other EGFR family members (i.e., HER2 and EGFR). It has been well established that HER3 is related to the development and progression of several types of cancers.447 With the broad clinical application of HER2- and EGFR-targeting agents, increasing evidence indicates that HER3 is also implicated in the resistance of HER2- or EGFR-targeting therapeutics.448 As such, more than 13 HER3-targeting mAbs (e.g., patritumab, lumretuzumab, KTN3379, REGN1400) are under clinical investigation as either monotherapy agents or components of combination therapies.449 Additionally, duligotuzumab (MEHD7945A) is a human IgG1 mAb dually targeting HER3 and EGFR.450,451
Patritumab is a fully human anti-HER3 mAb and has shown to have an excellent safety profile in clinical trials. Although a preclinical study showed the feasibility of 64Cu-DOTA-patritumab immunoPET in imaging HER3 expression,452 the clinical use of 64Cu-DOTA-patritumab was not satisfactory because tumor uptake of 64Cu-DOTA-patritumab was not robust.453 The discrepancy observed in these two studies may possibly be caused by the substantial uptake of the radiotracer in human livers and less potent targeting property of the antibody. GSK2849330 is another fully human HER3-specific mAb and dose-dependent, saturable uptake of the agent was reported in a preclinical study where 89Zr-GSK2849330 was employed.454 More recently, van Oordt et al. characterized the value of 89Zr-GSK2849330 immunoPET in clinical settings.455 The authors validated 89Zr-Df-GSK2849330 saturation after preloading with unlabeled GSK2849330 and also observed a significant uptake of the tracer in the liver and spleen. Lumretuzumab (RG7116) is a humanized mAb targeting the extracellular domain of HER3.456 ImmunoPET imaging with 89Zr-lumretuzumab provided useful information on HER3 expression in multiple tumor-bearing mouse models.457 A follow-up clinical study using 89Zr-lumretuzumab further demonstrated tumor uptake of the tracer.458 The study also revealed significant liver uptake of the tracer, which was partially caused by Kupffer cell-mediated capture and clearance of the glycoengineered antibody.
Several other HER3-targeting vectors, including HER3 specific antibody fragments and Affibody, have been developed and employed as PET and fluorescent imaging probes.459–462 However, probes of long retention time are favored for imaging HER3 because this receptor has a low density on the tumor cells but high abundance in the normal organs and tissues, such as the small intestine. Furthermore, Affibody-based imaging probes have very fast blood clearance due to their small sizes (~7 kDa), which results in substantial kidney retention that interferes with image interpretation.463,464 To this end, a biparatopic VHH construct MSB0010853 (39.5 kDa), which blocks two different HER3 epitopes, was developed (Figure 14a).465 Warnders et al. reported that tumor uptake of 89Zr-MSB0010853 correlated with HER3 expression (Figure 14b), and its uptake in tissues was dose-dependent. Owing to the relatively larger size and the albumin-binding capacity of 89Zr-MSB0010853, bloodstream circulation of this tracer was relatively longer and renal uptake is relatively lower (<15% ID/g).465
Figure 14.
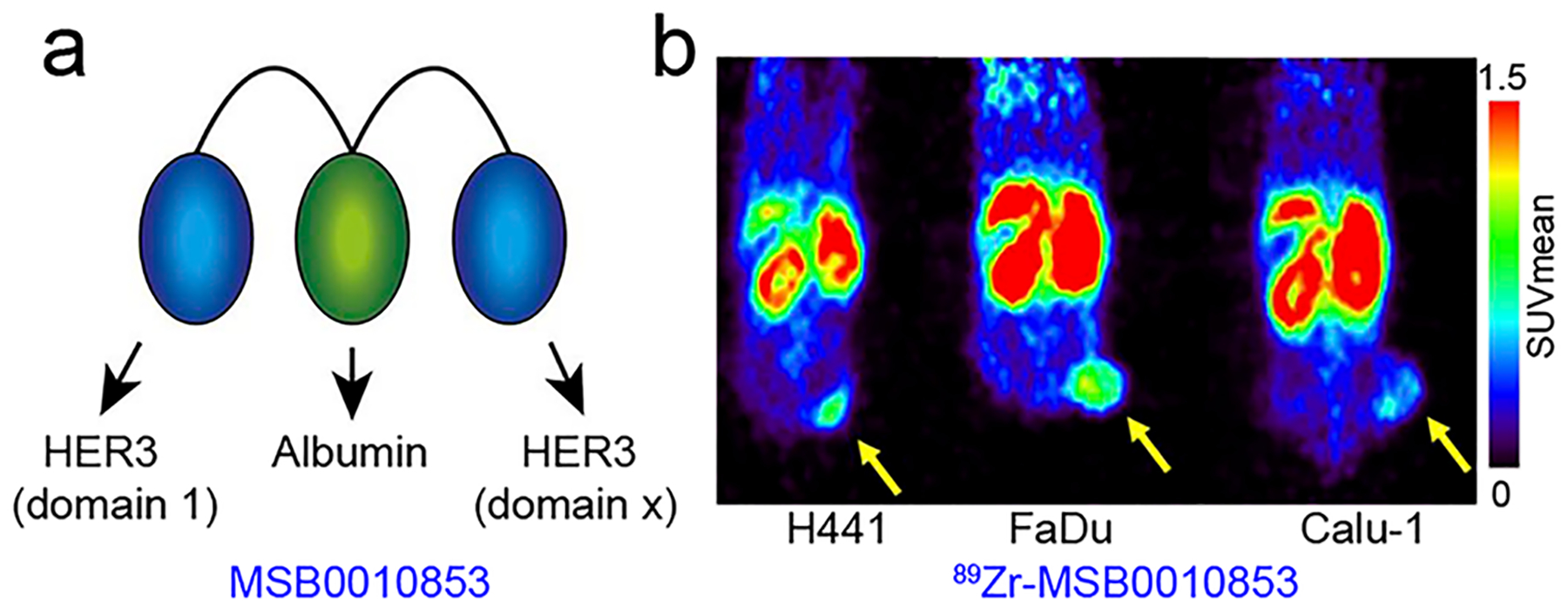
ImmunoPET imaging of HER3 expression. (a) MSB0010853 is composed of two Nanobodies targeting two different epitopes of HER3 and an additional Nanobody targeting albumin. (b) 89Zr-MSB0010853 immunoPET imaging of HER3-positive mouse xenografts (H441 and FaDu) and HER3-negative mouse xenograft (Calu-1) demonstrated the ability of this imaging approach to reveal varying HER3 expression levels. Reproduced with permission from ref 465. Copyright 2017 SNMMI.
4.1.4. Vascular Endothelial Growth Factor Receptor.
Vascular endothelial-derived growth factor (VEGF)/VEGF receptor (VEGFR) signaling pathway is the key pathway regulating vasculogenesis and angiogenesis during physiologic homeostasis and diseases.466 A number of therapeutic agents targeting VEGF (e.g., bevacizumab and ramucirumab) and VEGFR (e.g., sorafenib and sunitinib) have been approved for clinical use around the world.467 As a neutralizing mAb targeting VEGF-A, the benefits of bevacizumab have been validated for different oncological indications.468 To date, clinical immunoPET studies using 89Zr-Df-bevacizumab were performed in a variety of tumors, including breast cancer,469 neuroendocrine tumors,470 renal cell carcinoma (RCC),471 NSCLC,472 and glioma.473,474 89Zr-Df-bevacizumab immuno-PET imaging detected VEGF-A downregulation induced either by the mammalian target of rapamycin inhibitor (everolimus) in patients with neuroendocrine tumors,470 or by the HSP90 inhibitor, luminespib (NVP-AUY922), in ovarian cancer xenografts.475 However, clinical 89Zr-Df-bevacizumab immunoPET imaging failed to monitor VEGF reduction in patients with breast cancer following NVP-AUY922 treatment.476 To enhance the penetration of bevacizumab across the blood–brain barrier (BBB), intra-arterial administration and blood–brain barrier opening (BBBO) are two emerging strategies.477 In agreement with this clinical evidence, BBBO with mannitol followed by intra-arterial administration of 89Zr-Df-bevacizumab resulted in significantly higher accumulation of the tracer in the ipsilateral hemisphere.478
To gain a more thorough insight into tumor response following antiangiogenic treatment, ranibizumab (a humanized Fab fragment targeting all isoforms of VEGF-A) was radiolabeled with 89Zr.479 Uptake of 89Zr-ranibizumab in the tumor center reduced substantially following sunitinib treatment. However, immunoPET scanning performed 7 days after the termination of the treatment showed that tracer accumulation in the tumor centers increased and returned to baseline. It is worthwhile to note that uptake of 89Zr-Dfbevacizumab in RCC and normal organs may also rebound following sunitinib treatment,471 but a similar phenomenon was not observed following sorafenib or everolimus treatment of RCC.480,481 All of the clinical evidence indicates that expression of VEGF not only varies in different patients but also among the metastases and within the tumor in a single patient. VEGF-directed immunoPET is useful for visualizing the dynamic changes of VEGF before and after VEGF-targeted therapies. It has been postulated that antiangiogenic therapies induce the apoptosis of the endothelial cells and “normalize” the hyper-permeability of the tumor vasculature.482 Therefore, disruption of the tumor vasculature may lead to reduced tumor uptake of the radiotracer, regardless of the VEGF levels. This factor should be taken into consideration when interpreting the imaging results.
Although VEGF-A binds to both VEGFR-1 and VEGFR-2, VEGFR-2 plays a key role in regulating angiogenesis and vascular permeability. Results from several preclinical studies demonstrated that it was also feasible to image tumor vasculature by targeting VEGFR-2.483–485
4.1.5. Other Receptor Tyrosine Kinases.
Other than the EGFR family members, there are many other RTKs implicated in the pathogenesis and progression of cancers and non-cancerous diseases.486 Of them, c-MET (also known as mesenchymal–epithelial transition factor), platelet-derived growth factor (PDGF) receptor (PDGFR), and insulin-like growth factor-1 receptor (IGF-1R) are emerging therapeutic and diagnostic targets.
c-MET is the receptor for the hepatocyte growth factor (HGF). The HGF/c-MET pathway is vital for the development and metastatic progression of gastrointestinal cancers,487 NSCLC,488 and several other malignancies.489 Furthermore, c-MET is closely related to the acquired resistance of EGFR-targeted or VEGFR2-targeted therapies in a broad range of solid tumors.490 Current efforts are directed to validate c-MET as a biomarker and HGF/c-MET pathway inhibitors as anticancer therapeutics. However, several clinical trials have failed to demonstrate the synergistic effect of c-MET or HGF inhibitors in combination therapies, such as onartuzumab plus erlotinib in NSCLC,491 and rilotumumab plus epirubicin, cisplatin, and capecitabine in gastric or gastro-esophageal junction adenocarcinoma.492 Therefore, development and clinical translation of companion diagnostic probes may underpin clinical investigation of HGF/c-MET pathway inhibition for cancer therapies. Luo et al. produced a recombinant human HGF (rh-HGF) and demonstrated that 64Cu-NOTA-rh-HGF PET imaging indirectly visualized c-MET-positive human glioblastoma in mouse models.493 Using a fully human mAb rilotumumab (AMG102) which selectively targets HGF, Price et al. developed 89Zr-DFO-AMG102 and reported that immunoPET imaging with this tracer determined HGF in the local tumor microenvironment (TME).494 To directly delineate c-MET abundance, several radiotracers have been developed and tested in preclinical mouse models.495,496 Onartuzumab is a one-armed monovalent antibody targeting the c-MET.497 Pool et al. demonstrated that 89Zr-Dfonartuzumab immunoPET imaging could visualize erlotinib-induced c-MET upregulation and luminespib-induced c-MET downregulation in NSCLC models.498 Escorcia et al. reported the theranostic value of 89Zr-Df-onartuzumab and 177Lu-DTPA-onartuzumab in pancreatic ductal adenocarcinoma (PDAC) xenograft models. In this theranostic scenario, immunoPET imaging with 89Zr-Df-onartuzumab identified c-MET-positive PDAC xenografts and targeted radioligand therapy with 177Lu-DTPA-onartuzumab efficiently delayed the growth of the selected tumors.499 A more recent study by Klingler et al. reported the synthesis of 89Zr-DFO-azepinonartuzumab within 10 min via the one-pot photoradiochemical conjugation reaction.500 When compared to the conventional 89Zr-DFO-Bn-NCS-onartuzumab, immunoPET imaging with 89Zr-DFO-azepin-onartuzumab resulted in comparable T/B ratios but a lower uptake in the liver. These results highlight the feasibility of immunoPET in assessing the dynamics of the HGF/c-MET signaling pathway and selecting candidates most likely to benefit from HGF/c-MET-targeted therapies.
PDGF and PDGF receptors (i.e., PDGFRα and PDGFRβ) stimulate the growth of tumor cells and regulate tumor angiogenesis as well as tumor stromal fibroblasts.501,502 Although no PDGF-specific TKIs have been approved, olaratumab (LY3012207, IMC-3G3), a human IgG1 mAb targeting PDGFRα, was approved by the FDA in 2016 for treating soft-tissue sarcomas.503 To directly image stromal PDGFR, PDGFRβ-targeting Affibodies have been radiolabeled with 111In or 68Ga and tested in preclinical glioma models.504–506 By using a dual cysteine disulfide bond linker, a dimeric Affibody molecule ZPDGFRβ was produced and 89Zr-DFO-ZPDGFRβ immunoPET imaging visualized PDGFRβ-expressing colorectal adenocarcinomas.507 Overexpression of PDGFRa has been reported to be associated with lymph node metastases of papillary thyroid cancers.508–511 Accordingly, Wagner et al. developed 64Cu-NOTA-D13C6 and reported that immunoPET imaging with this tracer identified papillary thyroid cancers that have the potential to metastasize (Figure 15).512
Figure 15.

ImmunoPET imaging of PDGFRa expression using 64Cu-NOTA-D13C6. While lower uptake of 64Cu-NOTA-D13C6 was seen in the PDGFRα-negative B-CPAP tumor (left flank), higher accumulation of the radiotracer was observed in the transfected PDGFRα-positive B-CPAP tumor (right flank) at late time-points. Reproduced with permission from ref 512. Copyright 2018 Elsevier Inc.
IGF-1R is universally expressed in hematologic and solid tumors.513,514 Insulin-like growth factors and IGF-1R are attractive targets for cancer therapy and imaging.515 R1507 is a mAb targeting IGF-1R and molecular imaging with R1507-based radiotracers, 111In-R1507 and 89Zr-Df-R1507, successfully determined IGF-1R expression in breast cancer xenografts.516 We screened an IGF-1R-specific mAb 1A2G11 and developed an immunoPET probe 89Zr-Df-1A2G11,517 which specifically accumulated in IGF-1R-positive pancreatic cancers.518 In accordance with the antibody-based imaging findings, an Affibody-based immunoPET tracer also specifically delineated U87MG tumors with enhanced clearance from the renal-urine system.519 These preclinical results imply that IGF-1R-specific immunoPET may help identify patients that would benefit from anti-IGF-1R therapies and enable dynamic monitoring of the IGF-1R expression following the therapies.520
4.2. Clusters of Differentiation
Clusters of differentiation (CD) antigens have long been investigated as either diagnostic or therapeutic targets for a broad spectrum of diseases. In this review, we will describe in-depth some selected markers (i.e., CD20, CD38, CD146, and CD105). However, there are many other CD antigens that have shown potential as molecular imaging targets, including CD54 (known as intercellular adhesion molecule, ICAM-1),521 CD44,118,522–524 CD47,525–527 and CD138.528,529
4.2.1. CD20.
Lymphoma is an umbrella term for a large group of cancers that often arise from the lymph nodes. CD20 and CD30 are two common biomarkers for molecular imaging of lymphoma.530–532 Rituximab, a CD20-specific chimeric mAb, was approved by the FDA for the treatment of non-Hodgkin’s lymphoma (NHL) in 1997 and rheumatoid arthritis (RA) in 2006.533 Studies have reported the feasibility of 64Cu-DOTA-rituximab and 89Zr-rituximab immunoPET in revealing CD20 expression in NHL-bearing humanized mouse models.534,535 Of them, 89Zr-rituximab has been translated for clinical use. Muylle et al. have reported that 89Zr-rituximab immunoPET/CT scanning without preloading of cold rituximab enabled clearer tumor visualization and higher tumor uptake (Figure 16).536
Figure 16.

ImmunoPET imaging of non-Hodgkin’s lymphomas. (a) In a patient with circulating CD20+ lymphocytes, significant uptake of 89Zr-rituximab was observed in the spleen, which was blocked by preloading with unlabeled rituximab (250 mg/m2) prior to injection of 89Zr-rituximab. The spleen is indicated with black arrows. (b) In the same patient, preloading reduced 89Zr-rituximab uptake in the involved lymph nodes (white arrows), but enhanced uptake of the radiotracer in the visceral lesions (blue arrows). Reproduced with permission from ref 536. Copyright 2015 Springer Berlin Heidelberg.
Recently, antibody fragments targeting human CD20 have been engineered and investigated after being labeled with 124I, 89Zr, or 64Cu. Olafsen et al. engineered two rituximab fragments of different sizes (a Mb of 80 kDa and a scFv-Fc fragment of 105 kDa). The authors reported that both fragments offered CD20-specific imaging, but the Mb-based radiotracer resulted in images of higher contrast.537 Humanized obinutuzumab (GA101) and fully human ofatumumab are two other CD20-targeting mAbs showing superior activity when compared to rituximab.538 Yoon et al. radiolabeled these two mAbs with 89Zr and compared their diagnostic efficacies with 89Zr-rituximab.539 The authors reported that both 89Zrobinituzumab and 89Zr-ofatumumab localized lymphoma xenografts more clearly than 89Zr-rituximab. Zettlitz et al. further engineered antibody fragments from obinutuzumab and radiolabeled these CD20-specific vectors with 89Zr, 124I, and 18F.197,540 Similarly, the authors found that obinutuzumab-based radiotracers outperformed rituximab-based radiotracers in delineating CD20 expression.540 To further conquer the poor tumor penetration and undesirable pharmacokinetics of full-length antibodies, a set of hCD20-targeting sdAbs were generated.68 One of these sdAbs, sdAb 9079 was radiolabeled with 68Ga and 177Lu and used for immunoPET imaging and targeted therapy of lymphoma, respectively.
Both 124I-rituximab and 89Zr-rituximab immunoPET/CT imaging was able to visualize CD20 expression in patients with RA.541,542 In addition to imaging NHL and RA, recent studies have reported the value of 89Zr-rituximab immunoPET imaging in diagnosing orbital inflammatory diseases and interstitial pneumonitis,543–545 but rituximab treatment has not been approved for these diseases.
4.2.2. CD38.
CD38 is a transmembrane glycoprotein highly and uniformly expressed on multiple myeloma (MM), NHL, and several types of solid tumor cells.546,547 Several CD38-specific mAbs (e.g., daratumumab, isatuximab, and MOR202) have been developed. Daratumumab is a fully human mAb and has been proven a clinical success for treating MM.548 Recently, both 64Cu- and 89Zr-labeled daratumumab have shown excellent imaging attributes for detecting subcutaneous and disseminated MM (Figure 17a–c).549,550 Preclinical applications of CD38-targeted immunoPET imaging has also been extended to detecting lung cancer, hepatocellular carcinoma, and lymphoma (Figure 17d).551–553 Additionally, CD38-specific sdAbs were generated for imaging and treating MM.554,555 Small agents like sdAbs penetrate more effectively into the disseminated MM lesions than the conventional mAbs. Because daratumumab administration interferes with CD38 detection and some of the sdAbs binds to CD38 independently of daratumumab,555,556 immunoPET probes derived from sdAbs will be very useful for detecting MM at early stages and evaluating the efficacy of daratumumab treatment.
Figure 17.

ImmunoPET imaging of CD38 expression. (a) 89Zr-DFO-daratumumab immunoPET/CT imaging of a mouse bearing bilateral MM1.S tumors (T1 and T2). (b) 89Zr-DFO-daratumumab immuno-PET/CT imaging of a mouse bearing a unilateral MM1.S tumor (T3) in the presence of unlabeled daratumumab as a blocking agent. (c) Representative bioluminescence imaging of the mice in the blocking group receiving an injection of cold daratumumab. The bioluminescent signal indicates the successful establishment of the tumor on the right flank of the mouse. Reproduced with permission from ref 550. Copyright 2018 SNMMI. (d) 89Zr-DFO-daratumumab immunoPET imaging of lymphoma (Ramos tumor) at 120 h after administration of the tracer. Reproduced with permission from ref 552. Copyright 2018 Springer Berlin Heidelberg.
Accompanying the above imaging success, substantial preclinical evidence has demonstrated that CD38-targeted RIT could achieve the eradication of disseminated MM.557–559 Using a less immunogenic two-step pRIT strategy consisting of a novel 028-Fc-C825 bispecific protein (targeting CD38 antigen and yttrium-DOTA ligand) and a 90Y-DOTA-biotin dramatically reduced tumor growth and increased survival in both MM and NHL models.560 These results indicate the superiority of CD38 for both immunoPET imaging and pRIT. Clinical translation of these CD38-targeted theranostic agents will likely improve the management of patients with MM (NCT03665155).561
4.2.3. CD146.
CD146, also known as MUC18 or MCAM, was originally identified as a marker for melanoma and now is a novel biomarker for several cancers.562 Recently, Jiang et al. demonstrated that CD146 interacts with VEGFR-2 in the endothelium and promotes angiogenesis. Additionally, they found that an anti-CD146 mAb (AA98) or CD146 siRNA could successfully inhibit the CD146/VEGFR-2 pathway.563 It has been proven that AA98 has anticancer effects in several types of cancer, including leiomyosarcoma, pancreatic cancer, hepatocellular carcinoma, breast cancer, among others.564–567 The work is of importance because intrinsic or acquired resistance to bevacizumab occurs in clinical practice,568 and novel agents targeting CD146 may have therapeutic benefits.
Our group initially generated a murine anti-CD146 mAb (denoted as YY146) and radiolabeled it for immunoPET imaging of glioblastoma multiforme.569 In the work, we reported that 64Cu-NOTA-YY146 immunoPET imaging could delineate U87MG tumors as small as 2 mm in diameter. Additionally, we explored the potential therapeutic effects of YY146 on U87MG cells. Another immunoPET imaging agent based on YY146 was developed by labeling the YY146 with 89Zr.570 Follow-up studies from our group revealed that 64Cu-NOTA-YY146 immunoPET could clearly visualize both subcutaneous and metastatic lung cancer xenografts.571,572 Furthermore, 89Zr-Df-YY146-ZW800, a dual-modality imaging tool, enabled immunoPET and near-infrared fluorescence (NIRF) imaging of CD146-positive hepatocellular carcinomas (Figure 18a,b).573 The prominent and persistent uptake of 89Zr-Df-YY146-ZW800 also facilitated image-guided resection of the orthotopic HepG2 tumors (Figure 18c). On the basis of the previous success, a more recent study further developed a theranostic pair consisting of 89Zr-Df-YY146 and IR700-YY146.122 While immunoPET imaging with 89Zr-Df-YY146 precisely diagnosed CD146-positive melanomas, PIT with IR700-YY146 efficiently eradicated a large portion of CD146-positive small melanomas in an image-guided manner. These promising results imply that the development of humanized YY146 (huYY146) would be worthwhile. In future clinical scenarios, immunoPET imaging with 64Cu-NOTA-huYY146 or 89Zr-Df-huYY146 may identify patients with an increased likelihood of responding to CD146-targeted therapies. CD146-targeted immunoPET imaging will be able to monitor early treatment responses to such therapies.
Figure 18.

ImmunoPET probes targeting CD146 and CD105. (a) ImmunoPET and (b) near-infrared fluorescence (NIRF) imaging performed at different time-points after intravenous injection of 89Zr-Df-YY146-ZW800 demonstrated prominent and persistent uptake of the tracer in HepG2 tumors but not in the YY146 blocking group. H (heart), L (liver), and T (tumor). (c) Clear delineation of orthotopic HepG2 tumors by both PET and NIRF imaging was enabled through 89Zr-Df-YY146-ZW800, which further facilitated image-guided resection of the multiple tumors (red and yellow arrows). Reproduced with permission from ref 573. Copyright 2016 Ivyspring International Publisher. (d) Serial coronal immunoPET imaging using a tissue factor and CD105 dual-targeting 64Cu-NOTA-heterodimer at 3, 15, 24, and 30 h postinjection of the tracer clearly detected the BxPC-3 tumor. (e) Coronal PET images of mice bearing an orthotopic BxPC-3 tumor at 3, 15, 24, and 30 h following injection of 64Cu-NOTA-heterodimer. This imaging technique realized an easy diagnosis of the orthotopic BxPC-3 tumor with negligible radioactivity around the surrounding tissues. Reproduced with permission from ref 590. Copyright 2016 American Association for Cancer Research.
4.2.4. CD105.
Endoglin (CD105) is a transforming growth factor-β (TGF-β) coreceptor expressed on proliferating vascular endothelium in solid tumors.574,575 A recent study elucidated that increased tumoral cytoplasmic and endothelial expression of CD105 was significantly associated with advanced stage, renal vein invasion, and microvascular invasion of RCC.576 TRC105, a therapeutic mAb that binds to human CD105 with high avidity, has been demonstrated to be safe and effective in patients with advanced solid tumors such as RCC and hepatocellular carcinoma.577–581
The first successful immunoPET imaging of CD105 expression in murine breast tumor models was reported by our group in 2011.582,583 64Cu-DOTA-TRC105 and 89Zr-Df-TRC105 were used in these two studies. 66Ga-NOTATRC105,584 and multimodality imaging probes targeting CD105,585–587 have since been developed and validated in various solid tumor models. Of note, 89Zr-Df-TRC105–800CW immunoPET imaging could noninvasively monitor lung metastases from breast cancer and facilitate straightforward image-guided surgical removal of the tumors.585 We also generated and characterized a Fab fragment from the TRC105 and then investigated its potential utility for PET imaging of tumor angiogenesis in breast cancer models.588,589 To develop highly specific noninvasive imaging probes for pancreatic cancer, we synthesized a bispecific heterodimer by conjugating an antitissue factor (TF) Fab with an anti-CD105 Fab and then labeled the heterodimer with 64Cu. This dual-targeting technique provided synergistic improvements in binding affinity, and immunoPET imaging with the developed 64Cu-NOTA-heterodimer clearly delineated both subcutaneous and orthotopic pancreatic tumors (Figure 18d,e).590 After dual-labeling of the heterodimer with 64Cu and fluorescent dye, dual-modality PET/NIRF imaging using 64Cu-NOTA-heterodimer-ZW800 also specifically and readily detected pancreatic tumors.591
These studies strongly demonstrated the feasibility of noninvasive imaging of CD105 expression using PET or PET/NIRF technologies. Broad clinical applications of TRC105-based imaging will enable noninvasive detection of both primary and small metastatic tumor nodules, intra-operative guidance for tumor removal, selective patient stratification for TRC105 therapy, and image-guided RIT.592 In addition, CD105 expression is upregulated on tumor endothelial cells following inhibition of the VEGF signaling pathway.593,594 As such, TRC105-based immunoPET imaging may select patients who will potentially benefit from the combinational therapy with TRC105 and VEGF inhibitors or antibodies.
4.3. Carbohydrate Antigens
4.3.1. Carbohydrate Antigen 19.9.
Carbohydrate antigen 19.9 (CA19.9) is an established biomarker for several epithelial tumors, lung cancer, breast cancer, and PDAC. 5B1 is a fully human IgG targeting CA19.9 and has been widely used for theranostic purposes.595–597 In a first-in-human clinical trial, immunoPET imaging with 89Zr-DFO-5B1 detected known PDACs, metastases, and small LN metastases,39 which remained undetected on conventional imaging studies.
By using the chemoenzymatic methodology described above,287 5B1 was also site-specifically modified with DFO and radiolabeled with 89Zr. ImmunoPET imaging with 89Zr-ssDFO-5B1 showed exceptional uptake of the radiotracer in the CA19.9-positive BxPC-3 models but not the CA19.9-negative MIAPaCa-2 models. Moreover, dual-modal imaging with 89Zr-ssdual-5B1 delineated both primary and metastatic tumors in an orthotopically implanted PDAC model.598 Because tumors shed CA19.9 into the bloodstream, it is sometimes necessary to inject cold antibody to saturate the shed antigen.599 By utilizing the IEDDA reaction with TCO-conjugated antibodies and Tz-conjugated radioligands, several pretargeted immunoPET imaging strategies have been explored. One such system with 5B1-TCO and Tz-PEG11-Al[18F]-NOTA resulted in clear delineation of the CA19.9-expressing PDAC xenografts. However, radioactivity in the intestine retained over the imaging course. Another pretargeting approach consisting of 5B1-TCO and 64Cu-NOTA-PEG7-Tz or 64Cu-NOTA-Tz showed improved diagnostic value, with the former combination showed better T/B contrast at the earlier imaging time-points (Figure 19).600
Figure 19.

Pretargeted immunoPET imaging of pancreatic cancer. 5B1-TCO was first administered to target CA19.9-expressing orthotopic Capan-2 xenograft followed by injection of 64Cu-NOTAPEG7-Tz 3 days after the previous injection. (a) Coronal and (b) maximum-intensity projection (MIP) images demonstrated that this pretargeted imaging approach clearly delineated the Capan-2 tumor.(c) Immunohistochemistry (top left), autoradiography (bottom left), and fused PET/CT image (right) from the same mouse further showed precise colocalization of CA19.9-expressing tumor cells and 64Cu-NOTA-PEG7-Tz. Reproduced with permission from ref 600. Copyright 2016 SNMMI.
4.3.2. Carbohydrate Antigen 125.
Mucin 16 (MUC16) is a glycoprotein highly expressed in several types of cancers (e.g., ovarian, endometrial, and fallopian tube cancers). Carbohydrate antigen 125 (CA-125) is the released extracellular region of MUC16 following proteolytic cleavage and is regularly used to screen for ovarian cancer, to monitor cancer treatment efficacy, and to check for cancer recurrence.601 B43.13 (oregovomab) is a high-affinity murine mAb and has been employed as an immunotherapeutic agent in the treatment of advanced ovarian cancers. To facilitate early detection of ovarian cancers, 64Cu-NOTA-mAb-B43.13 and 89Zr-DFO-mAb-B43.13 were developed sequentially.602,603 ImmunoPET imaging with these two tracers clearly delineated CA125-positive OVCAR3 tumors. 89Zr-DFO-mAb-B43.13 immunoPET imaging is particularly attractive because it also detected LN involvement with high contrast and accuracy.603 REGN4018 is a human BsAb that specifically binds to MUC16 and CD3.604 Although circulating CA-125 serves as an antigen sink and interferes with MUC16-targeted therapies, the potency of REGN4018 is not hampered by the circulating CA-125. It has been shown that the site-specifically labeled 89Zr-DFO-REGN4018 localized to the spleen and lymph nodes of nontumor-bearing mice. The imaging capabilities of 89Zr-DFO-REGN4018 was further investigated in humanized mouse models with two humanized targets (CD3 and MUC16). In these models, the bispecific 89Zr-DFOREGN4018 specifically accumulated in the secondary lymphoid organs as well as the MUC16-expressing tumors (Figure 20). Currently, REGN4018 is undergoing a phase I clinical trial (NCT03564340) either as a monotherapy agent or in combination with cemiplimab (an anti-PD-1 antibody).
Figure 20.
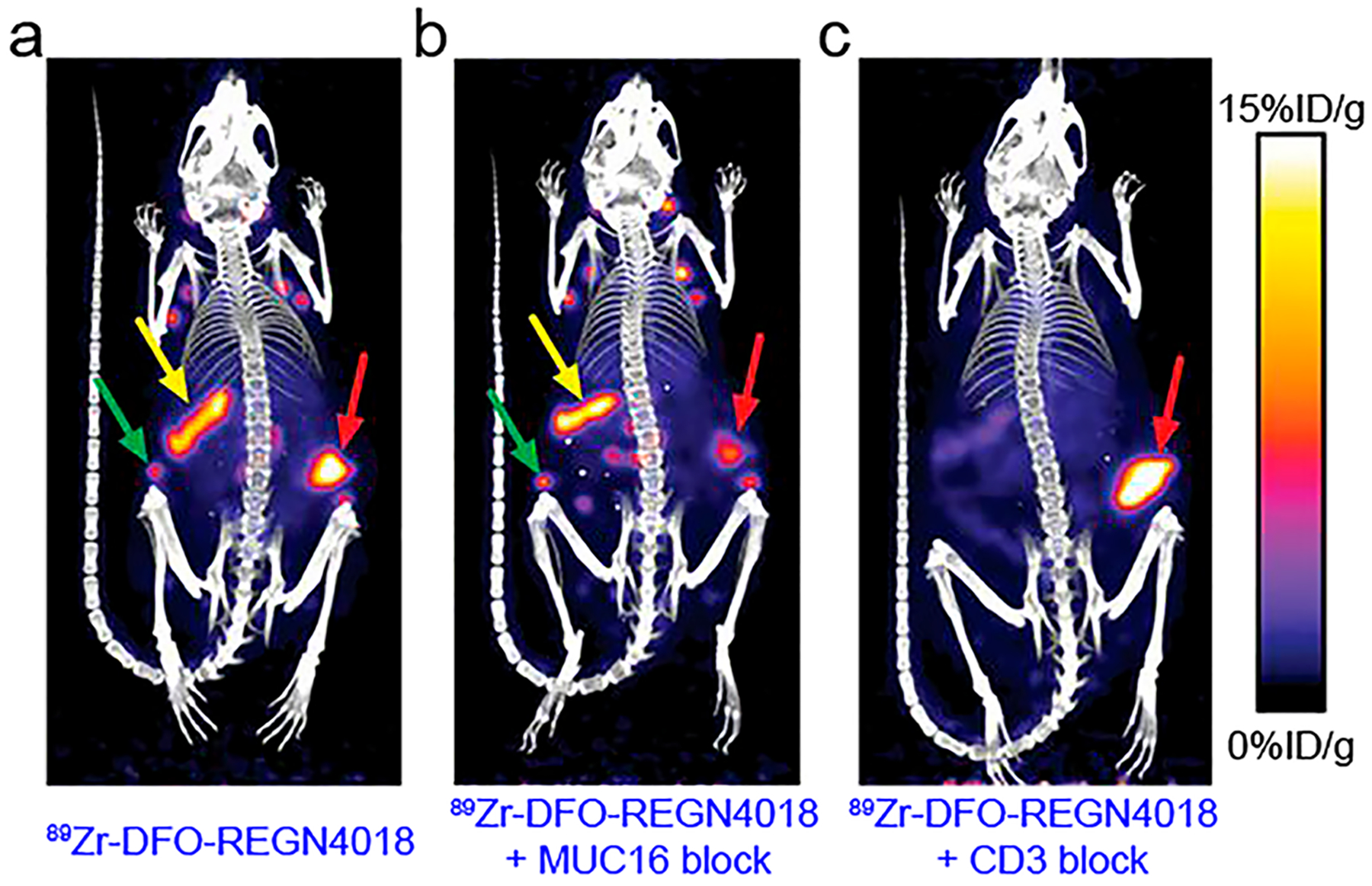
ImmunoPET imaging of ovarian cancers with a bispecific radiotracer 89Zr-DFO-REGN4018. (a) 89Zr-DFO-REGN4018 immunoPET/CT imaging of humanized tumor-bearing mice showed the distribution of the tracer to the spleen (yellow arrow), lymph nodes (green arrow), and tumor (red arrow). (b) Blocking with a MUC16 parental antibody reduced the tumor uptake of 89Zr-DFO-REGN4018 without influencing the spleen and lymph node uptake. (c) Blocking with an anti-CD3 antibody substantially reduced the spleen and lymph node uptake of 89Zr-DFO-REGN4018 without influencing the tumor uptake. Reproduced with permission from ref 604. Copyright 2019 American Association for the Advancement of Science.
4.4. Prostate-Specific Membrane Antigen
Using 18F-FDG or 11C-choline PET tracers to image prostate cancer (PCa) often yields false negative or false positive uptake due to the slow growth and the low glycolytic rate of most PCas. Interpretation of 18F-FDG PET images in PCa is often difficult or even impossible because of the spillover effects from the accumulation of the tracer in the bladder and the intestine.605 Prostate-specific membrane antigen (PSMA) is a transmembrane glycoprotein that is highly expressed on most prostate adenocarcinomas and has gained increasing interest as a target molecule for imaging and therapy in the past five years.606–608 A recent study has elucidated a novel oncogenic signaling role of PSMA and reported that suppression of PSMA inhibited the PI3K signaling pathway and promoted tumor regression in preclinical PCa models.609 Studies using both antibodies and small-molecule agents have been conducted to develop PSMA-targeted SPECT and PET imaging platforms. PSMA-targeted theranostic approaches have been extensively reviewed elsewhere.610–613 Herein, we only focus on PSMA-specific immunoPET imaging probes.
A variety of mAbs specific for intracellular and extracellular epitopes of PSMA have been developed.605,614–616 J591, a humanized mAb which binds to an extracellular domain of PSMA, has been clinically investigated for both imaging and therapy.617–621 Recently, Fung et al. demonstrated that 124IJ591 and 89Zr-J591 had comparable surface binding and internalization rates in preclinical prostate models.622 These studies imply that PCa theranostics using 177Lu- and 124I-or 89Zr- labeled J591 is feasible, safe and may have superior targeting toward bone lesions relative to conventional imaging modalities. This may refine management strategies for patients with PSMA-positive PCas. Capromab (7E11) is a murine mAb, which has been investigated clinically as a SPECT imaging agent for recurrent and metastatic PCas623 and a therapeutic agent after labeling with 90Y.624 Because capromab binds to an epitope on the intracellular domain of PSMA, tracers derived from this mAb are limited to detecting dead cells and do not possess advantages in imaging soft-tissue and bone metastases from PCas when compared to tracers binding to the extracellular domain.625 D2B is another PSMA-specific murine mAb for developing theranostic agents.626,627 More recently, Barinka and co-workers reported the characterization of four novel murine PSMA-specific mAbs. One of these, 5D3, demonstrated 10-fold higher affinity compared to J591.628 A follow-up study further revealed that 5D3 may serve as a promising surrogate for imaging PSMA expression.629
When compared to mAbs, VHHs and engineered antibody fragments offer faster delivery and similar tumor delineating properties.630,631 Viola-Villegas et al. engineered a Mb and a diabody from the intact antibody J591 and reported that immunoPET imaging with these smaller antibody fragments offered rapid tumor accumulation and accelerated clearance in PSMA-expressing PCas.632 One such radiotracer, 89Zr-IAB2M, was further translated in a dose-escalation clinical trial that included 18 patients with metastatic PCas.633 In this clinical study, immunoPET imaging with 89Zr-IAB2M delineated metastatic PCa lesions in 17 of 18 patients 48 h after the intravenous injection of the radiotracer (10 mg). Moreover, 89Zr-IAB2M immunoPET outperformed 99mTc-MDP and CT in detecting bone lesions and magnetic resonance imaging (MRI) and 18F-FDG PET in detecting LN/soft tissue metastases (Figure 21).633 A phase I/IIa trial further confirmed the diagnostic efficacy of 89Zr-IAB2M.634 Another advantage of this probe was its negligible accumulation in lacrimal and salivary glands because a major side effect of PSMA-targeting agents is the xerostomia.635 Beile et al. initially generated three mAbs against cell-adherent PSMA from spleen cells of mice636–638 and more recently developed multimeric antibody fragments from these murine antibodies.639 They showed that the radiolabeled antibody fragments had stable tumor uptake and faster serum clearance.
Figure 21.
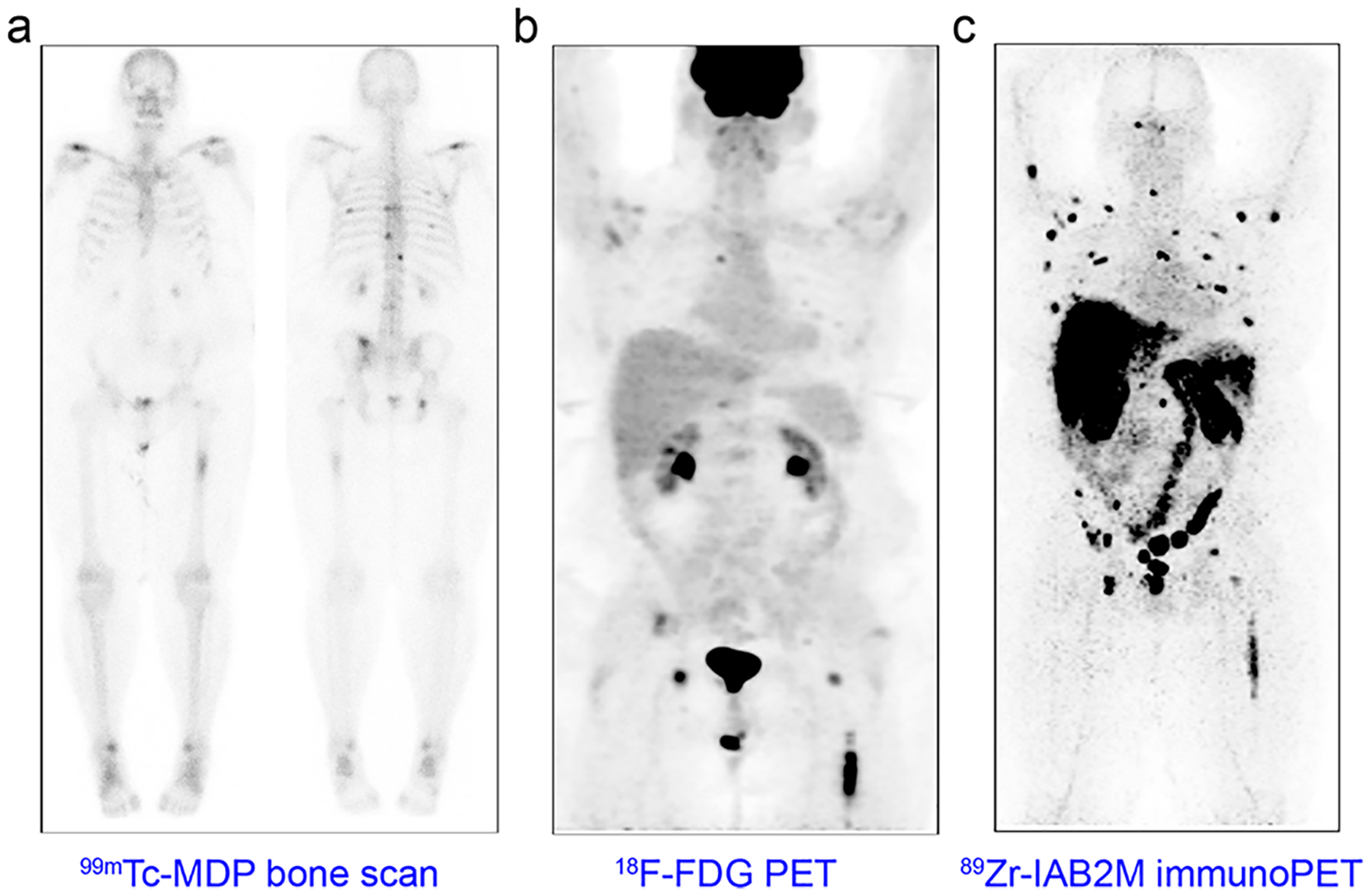
ImmunoPET imaging of prostate cancers with the minibody-based 89Zr-IAB2M. (a) 99mTc-MDP bone scan of a PCa patient showed multiple metastatic lesions in ribs, vertebrae, and left femur. (b) An 18F-FDG PET scanning showed the lesion in the left femur but failed to clearly detect the vertebral lesions. (c) 89Zr-IAB2M immunoPET imaging of the same patient detected more lesions than either conventional imaging modalities. Reproduced with permission from ref 633. Copyright 2016 SNMMI.
Amassing clinical studies investigating PSMA-targeted PET imaging have demonstrated uptake of the tracers in non-prostate malignancies.640,641 It has been proposed that uptake of PSMA-targeted tracer in nonprostate malignancies is due to the significant angiogenesis in tumor tissues.642 Indeed, PSMA is expressed by the neovasculature endothelium but not by the tumor cells or the normal vasculature endothelium in most solid tumors.643,644 In this context, several clinical studies have suggested that PSMA is another promising surrogate for molecular imaging of tumor neovasculature.645,646 As such, PSMA-targeted PET and immunoPET imaging will help select patients for subsequent PSMA-targeted therapies and/or antiangiogenesis therapies (e.g., bevacizumab).
4.5. Carcinoembryonic Antigen
As a key member of carcinoembryonic antigen-related cell adhesion molecules (CEACAMs), carcinoembryonic antigen (CEA) serves as a vital tumor antigen and a serum tumor marker.647 Arcitumomab (CEAScan) is a 99mTc-labeled hapten-peptide pretargeted imaging probe approved by the FDA and EMA for detecting colonic cancer metastases.648 However, this agent was withdrawn from the market because of competition from the more cost-effective 18F-FDG. To improve the imaging quality and specificity, several CEA-specific pretargeted immunoSPECT imaging agents were investigated. These efforts are exemplified in a study by Sharkey et al.,649 in which a superior tumor-to-blood ratio (~100:1) was obtained in human colon cancer xenografts. Alternatively, CEA-directed pretargeted immunoPET imaging was designed by first pretargeting CEA with a multivalent BsAb (which also targets HSG) followed by the injection of a radiolabeled hapten peptide. In this strategy, the DOTA-containing IMP288 peptide allowed 68Ga complexation and the NOTA-containing IMP-449 peptide allowed facile [18F]AlF chelation.311,650 In addition to detecting subcutaneous tumors, two recent studies demonstrated that TF2/68Ga-IMP288 pretargeted immunoPET outperformed 18F-FDG PET in detecting disseminated human colorectal cancers (Figure 22).651,652 Moreover, this highly sensitive pretargeting technique could be used to visualize CEA-containing human colorectal cancer tissues and normal epithelial cells.653 The study also demonstrated that pretargeted immunoPET has superior accuracy than 18F-FDG PET in delineating CEA+ tumors due to the highly specific tumor uptake and low background activity. Upon clinical translation, this novel imaging method may be useful to identify tumor lesions during surgical dissection. A recent first-in-human clinical trial has reported that TF2/68Ga-IMP288 pretargeted immunoPET imaging revealed abnormal foci in all patients with relapsed medullary thyroid cancer.654 This study also demonstrated that a 30 h time lag between the injection of TF2 and 68Ga-IMP288 and a TF2-to-IMP288 molar ratio of 20 were the most favorable conditions for imaging. Moreover, an ongoing clinical trial is evaluating the diagnostic role of TF2/68Ga-IMP288 pretargeted immunoPET imaging in patients with HER2-negative but CEA+ breast cancers (NCT01730612).
Figure 22.
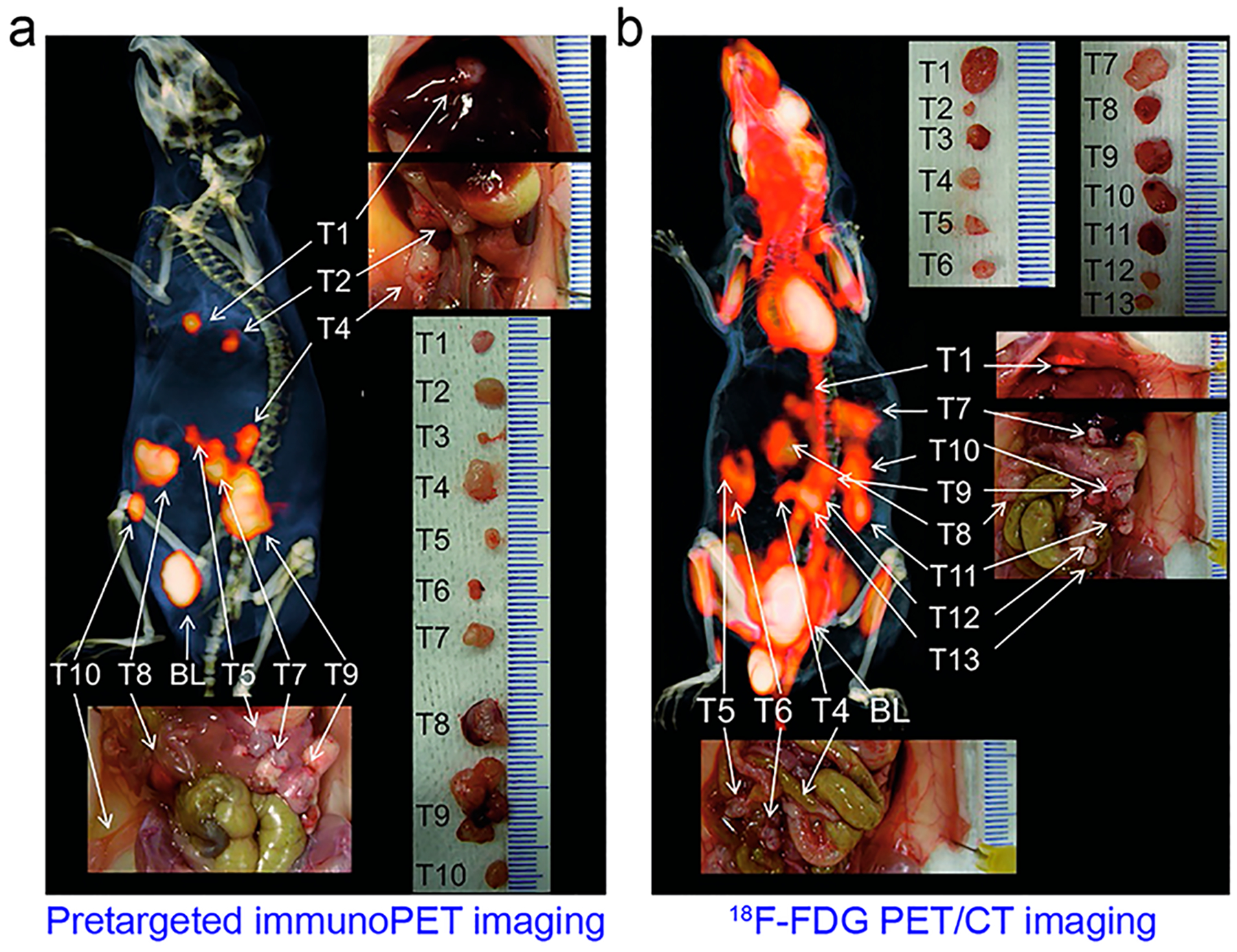
Pretargeted immunoPET imaging of metastatic colorectal cancers. (a) In this approach, CEA- and HSG-targeting BsAb TF2 was given first to saturate the LS174T tumors, followed by administration of DOTA- and HSG-containing 68Ga-IMP288 16 h later. This imaging approach clearly delineated tumors, except for two small tumor lesions (T3 and T6). Bladder (BL) uptake indicates excellent excretion of the 68Ga-IMP288 through the urinary system. (b) 18FFDG PET/CT imaging of the same mouse showed less optimal image contrast due to uptake in the intestines. Reproduced with permission from ref 651. Copyright 2012 Springer Nature.
Along with this progress, a series of antibody fragments were engineered from a murine mAb T84.66 and have been radiolabeled with radiometals such as 64Cu and 124I.655–657 To improve the T/B ratio of the engineered antibody fragments, mutation of the residues in the Fc fragment essential for FcRn binding can be performed. For instance, Kenanova et al. formatted a series of anti-CEA scFv-Fc fragments658,659 and found that PET imaging with a 124I-labeled scFv-Fc bearing one double mutation (H310A/H435Q) showed the highest imaging quality.658 To further permit same-day immunoPET imaging, several types of labetuzumab fragments have been radiolabeled with [18F]AlF using the chelating ligand NODAMPAEM.660 Despite the facile and rapid procedure, high kidney and liver accumulation of the developed tracers may hinder the clinical translation.660
AMG 211 (MEDI-565) is a BiTE composed of a humanized anti-CEA arm and a deimmunized anti-CD3 arm. AMG 211 could efficiently activate human T cells which lysed CEA+ colorectal tumor cells in a preclinical model.661 However, this treatment effect was not observed in patients with advanced gastrointestinal adenocarcinoma.662 To uncover the underlying reasons for the poor efficacy, the in vivo biodistribution and tumor targeting ability of AMG 211 was investigated with 89Zr-AMG 211 immunoPET imaging.663,664 The results revealed the accumulation of the tracer in CD3-rich lymphoid organs (e.g., spleen and bone marrow). Tumor uptake of 89Zr-AMG 211 was evident yet varied strikingly within and between patients, attributed to the heterogeneous expression of CEA in different tumor lesions and varying tumor vasculature and tissue permeability.664 To specifically deliver interleukin-2 (IL-2) to CEA+ tumors and overcome the adverse effects of IL-2 monotherapy, cergutuzumab amunaleukin (CEA-IL2v) has been designed and is actively undergoing a clinical investigation in combination with the anti-PD-L1 atezolizumab in CEA-expressing advanced solid tumors (NCT02350673).665 Recently, a pilot immunoPET imaging study with 89Zr-CEAIL2v showed a preferential drug accumulation in CEA+ tumors, and two cycles of CEA-IL2v administration reduced the number of tumor lesions and tumor uptake of the radiotracer (Figure 23).666 This exploratory study also indicated nonspecific uptake of 89Zr-CEA-IL2 in nonmalignant lymphoid organs, suggesting the in vivo distribution of CEA-IL2v is driven by the synergistic effect of CEA targeting and IL-2 binding on immune cells.
Figure 23.

ImmunoPET imaging of solid tumors using 89Zr-CEAIL2v. 89Zr-CEA-IL2v immunoPET imaging of a patient with CEA+ colorectal cancer at cycle 1, day 5 (left) showed uptake of the radiotracer in the bilateral hilar lymph nodes and the left dorsal lung metastasis (white arrows). The uptake in these malignant lesions and a nonpathological lymph node (red arrows) decreased after the fourth cycle of CEA-IL2v treatment (right). Notably, uptake in the liver (yellow arrows) increased and uptake in the spleens (orange arrows) decreased following the treatments. Reproduced with permission from ref 666. Copyright 2018 Impact Journals, LLC.
4.6. Carbonic Anhydrase IX
Carbonic anhydrase IX (CAIX) is a cytosolic and trans-membrane enzyme belonging to the zinc-containing metal-loenzyme family.667 By catalyzing the conversion of carbon dioxide and water to carbonic acid (CO2 + H2O ⇄ HCO3− + H+), CAIX contributes to the acidic extracellular environment of hypoxic tissues, predominantly hypoxic tumors and metastases.668 CAIX is homogeneously overexpressed in 95% of clear cell renal cell carcinoma (ccRCC) cases, so it is an optimal theranostic target for ccRCC. Several mAbs (e.g., cG250 or girentuximab) targeting CAIX are undergoing clinical investigations.669,670 On the basis of preclinical studies, where 124I-cG250 and 89Zr-cG250 immunoPET imaging clearly visualized CAIX-expressing ccRCC xenografts,671–673 a recent clinical study reported that 89Zr-girentuximab immunoPET imaging precisely predicted the pathology of all the immunoPET-positive primary renal lesions. This result changed the clinical decision for 36% of patients with recurrent/metastatic ccRCC (Figure 24).674 Several proof-of-concept clinical studies have also validated the safety and superior diagnostic value of 124I-cG250 in ccRCC,184,186,675 with an average sensitivity and specificity of 86.2% and 85.9%, respectively.186 Additionally, multimodal imaging with 124I-cG250 could realize precise intraoperative localization of ccRCC, which further guided and confirmed complete surgical resection of the diseases.676 In contrast to 124I-cG250, which rapidly releases 124I after being internalized into tumor cells, the residual radiometal 89Zr from 89Zr-cG250 will be trapped inside the tumor cells.672
Figure 24.

ImmunoPET imaging of clear cell renal cell carcinoma (ccRCC) with 89Zr-girentuximab. (a) A patient with ccRCC who previously had undergone nephrectomy was subjected to a CT scan that showed neoplasms in the right kidney and the adjacent adrenal (white circle). (b) 89Zr-girentuximab immunoPET/CT imaging of the same patient showed that both the lesions had an uptake of the tracer. Additional uptake in the proximal radius was seen (insert), which changed the management strategy of the patient from a futile radical nephrectomy to radiotherapy. Reproduced with permission from ref 674. Copyright 2018 European Association of Urology.
M75, another mAb targeting CAIX, is regularly used in IHC studies to detect CAIX.677 In recent studies this mAb has been radiolabeled with 64Cu and 61Cu, and the developed radio-tracers revealed specific binding in CAIX-expressing colorectal cancer models.678,679 Moreover, several other CAIX targeting mAbs have shown preliminary anticancer effects.680–682 Up to now, they have not been used for immunoPET imaging. Lastly, it is worthwhile to note that CAIX is highly expressed in a plethora of other tumor cells, tumor-associated stromal cells, and cancer stem cells.668 Because of the above-described merits and limited presence of CAIX in normal tissues, CAIX may serve as an ideal target for developing advanced theranostic agents.
4.7. Trophoblast Cell Surface Antigen 2
Trophoblast cell surface antigen 2 (TROP-2), a 46 kDa transmembrane glycoprotein, is overexpressed in a broad range of cancers.683,684 Sacituzumab govitecan (IMMU-132) is an antibody–drug conjugate (ADC) targeting TROP-2 and has been approved as a third-line therapy for metastatic triple-negative breast cancers.685 Moreover, its application is being extended for the treatment of several other malignancies. hRS7 is a humanized IgG1 mAb targeting TROP-2. hRS7-based immunoPET or immunoSPECT imaging clearly visualized TROP2 expression in PCa models.686 TF12 (157 kDa) is a trivalent BsAb composed of an anti-HSG Fab and two anti-TROP-2 Fabs derived from the hRS7.687 Using TF12, pretargeted immunoPET imaging, pRIT, and image-guided surgery of human PCas have all been achieved.688–690 While TROP-2 immunoPET imaging is of clinical interest for selecting TROP-2-positive patients, its value in evaluating the therapeutic response following IMMU-132 treatment is limited because TROP-2 is not a predictive biomarker for response.691
4.8. Stem Cell Antigens
Cancer stem cells (CSCs) are characterized by undifferentiated features, which may include self-renewal, long-term replication, and diverse differentiation abilities.692 It has been proposed that CSCs are a major driving force of tumor occurrence and metastasis.693 Additionally, chemotherapy and radiotherapy resistances are mediated in part by CSCs.694 Therefore, noninvasive imaging of CSCs is clinically relevant and may aid the identification and eradication of CSCs. Of the various stem cell markers, leucine-rich repeat-containing G-protein coupled receptor 5 (LGR5), prostate stem cell antigen (PSCA), and CD133 are three attractive biomarkers that have been exploited for immunoPET imaging.
LGR5, a marker of adult stem cells, is highly expressed in some human cancers.695 Noninvasive LGR5 assessment was achieved with two LGR5-targeting immunoPET probes (i.e., 89Zr-DFO-8F2 and 89Zr-DFO-9G5). 89Zr-DFO-8F2 showed higher specificity over 89Zr-DFO-9G5 in visualizing LGR5 expression in colorectal tumors.696 While PSMA-targeting agents have shown great promise in patients with PCas, PSCA is an alternative target for designing theranostic agents. Using an anti-PSCA mAb, 7F5, and a newly developed chelator, L5-NCS, David et al. showed that 64Cu-L5-7F5 immunoPET imaging clearly visualized PSCA-positive PC3 tumors with minute activity in the liver. In comparison, 64Cu-NODAGA-7F5 immunoPET imaging showed much less tumor accumulation but significantly higher liver uptake (Figure 25).158 A11 is a humanized anti-PSCA Mb and 124I or 89Zr-labeled A11 was able to detect PSCA-expressing PCas697 as well as to evaluate the treatment response.698 Additionally, PSCA-targeted fluorescence or dual-modal immunoPET/fluorescence imaging agents hold great promise for intraoperatively visualizing PSCA-positive PCas and pancreatic cancers.699–701
Figure 25.
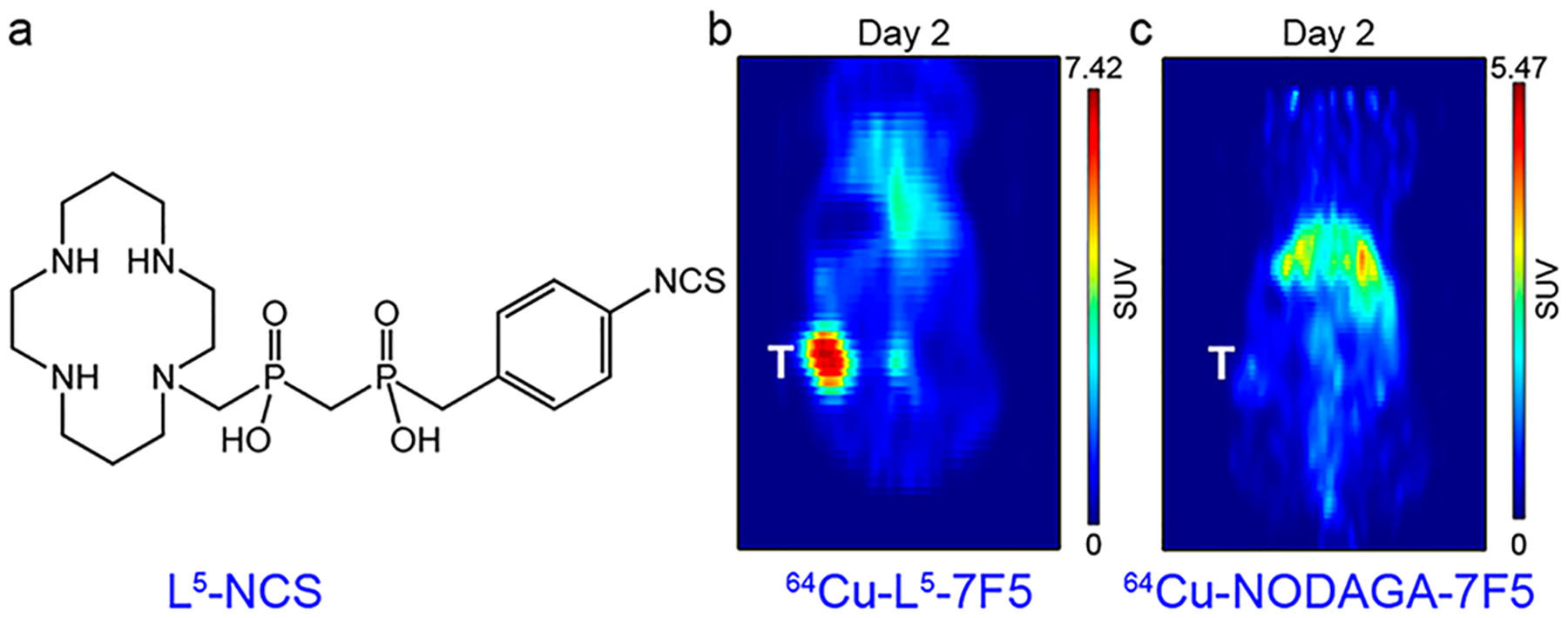
ImmunoPET imaging of cancer stem cell markers. (a) Chemical structure of a novel chelator L5-NCS. (b) 64Cu-L5-7F5 immunoPET imaging clearly detected PSCA-expressing PC3 tumor 2 days after injection of the tracer. (c) In comparison, 64Cu-NODAGA-7F5 showed much lower tumor uptake and higher liver uptake. Reproduced with permission from ref 158. Copyright 2018 American Chemical Society.
AC133, an epitope of the second extracellular loop of CD133, is one of the most extensively investigated markers for CSCs.702 An initial study showed that anti-AC133 mAb-based fluorescence imaging agents visualized CD133-overexpressing tumors.703 More recently, immunoPET imaging with an AC133-targeted radiotracer (64Cu-NOTA-AC133 mAb) permitted successful detection of CD133+ U251 tumors and glioma stem cells. More importantly, the 64Cu-NOTA-AC133 mAb immunoPET imaging patterns correlated well with the cytoarchitecture of the orthotopically growing gliomas.704 In the efforts for imaging CSCs, one recurring concern is that CSCs are not abundant in the TME. Taking this fact into consideration, we advocate the use of PET imaging over other imaging modalities for detecting and quantifying CSCs.
4.9. Proteases
Proteases are a group of evolutionarily conserved enzymes and can be classified into six groups by their mechanisms of action: Cys, serine, threonine, aspartate, glutamic acid proteases, and metalloprotease. Proteases play essential roles in tumor angiogenesis, invasion, and metastasis.705 Therefore, proteases are attractive targets for developing molecular imaging probes. Urokinase plasminogen activator (uPA) receptor (uPAR) is one of the validated targets. AE105, a high-affinity peptide antagonist against uPAR, has been radiolabeled and investigated extensively in preclinical studies.706 Inspired by the preclinical success, 64Cu-DOTA-AE105 and 68Ga-NOTA-AE105 have been successfully translated into the clinic for PET imaging of uPAR in patients with solid tumors.707,708 Using an IgG1 mAb (ATN-291) specifically targeting human uPA, Yang and co-workers developed a radiotracer 89Zr-Df-ATN-291.709 The authors reported the utility of 89Zr-Df-ATN-291 immunoPET in monitoring the expression of uPA/uPAR in several mouse xenografts. Matrix metalloproteinases (MMPs) have been heralded as attractive targets for cancer therapy for decades.710 LEM-2/15 is a mAb specific for membrane type 1-matrix metalloproteinase (MT1-MMP, MMP14), and immunoPET imaging with 89Zr-DFO-LEM 2/15 specifically localized the intracranial patient-derived xenograft tumors.711 Moreover, 89Zr-DFO-LEM 2/15 immunoPET imaging showed a higher specificity than 68Ga-DOTAAF7p, a MT1-MMP-specific peptide-based tracer, in identifying PDACs.712
Prostate-specific antigen (PSA) is a target in the androgen receptor (AR) pathway. PSA level in the blood is a reliable readout of AR pathway activity. “Free” PSA (fPSA) is a catalytically active serine protease and has been exploited as an immunoPET imaging target. 5A10 is a mAb that selectively binds fPSA. 89Zr-5A10 immunoPET detected intratumoral fPSA expression following different pharmacological interventions.713 However, 89Zr-5A10 immunoPET yielded less favorable imaging contrast because 89Zr-5A10 targeted secreted PSA with only a small proportion of the tracer directed to the tumor sites. Therefore, an immunoPET probe with increased internalization into tumor cells would image the AR pathway activity more clearly. To achieve this, two mAbs (i.e., 11B6 and its humanized version hu11B6) targeting human kallikrein-related peptidase 2 (hK2), another serine protease regulated by the AR pathway, were used for immunoPET imaging.714 In contrast to the transient uptake of 89Zr-5A10 in tumors, 89Zr-11B6 internalized into the tumor cells and showed steadily increasing tumor uptake. Moreover, 89Zr-11B6 immunoPET imaging delineated AR-driven hK2 expression in mice bearing subcutaneous or metastatic human PCa xenografts.714
4.10. Membrane-Bound Surface Glycoprotein Mesothelin
Membrane-bound surface glycoprotein mesothelin (MSLN) is overexpressed in mesothelioma and several other solid tumors such as ovarian, lung, breast, and pancreatic cancers.715,716 It is worth mentioning that soluble mesothelin is a superior tumor marker for monitoring tumor burden and therapeutic response in patients with malignant pleural mesothelioma.717 Substantial studies have reported the feasibility of MSLN imaging using several MSLN-targeting mAbs such as a murine mAb 11–25, a chimeric mAb amatuximab, and an ADC DMOT4039A (composed of MMOT0530A and MMAE).718–720 In a clinical study evaluating the treatment efficacy of DMOT4039A in patients with pancreatic or ovarian cancer, Lamberts et al. found that uptake of 89Zr-MMOT0530A correlated with MSLN expression from IHC staining.721 These results indicate that this imaging technique may guide individualized antibody-based therapies. As observed in a previous study,361 several recent studies have demonstrated that shed antigen MSLN in blood circulation negatively regulates the circulation and tumor-targeting efficacy of amatuximab.722,723 These results indicate the role of MSLN-targeted immunoPET in assessing the performance of mesothelin-targeted therapeutic antibodies and selecting patients for mesothelin-targeted therapies.724
4.11. Glycoprotein A33
Glycoprotein A33 (GPA33) is a cell surface target uniformly overexpressed in over 90% of colorectal cancers and a subset of gastric and pancreatic cancers.725 This antigen has been extensively studied as a target for both RIT and SPECT imaging using a murine mAb A33.726,727 Following the humanization of A33 (huA33) and preclinical evaluation of huA33-based immunoPET tracers,728,729 recent studies have translated 124I-huA33 to the clinic. This immunoPET imaging technique enabled the quantitative assessment of GPA33 status in colorectal cancers.185,730 Pretargeted immunoPET imaging has been successfully employed to delineate GPA33-expressing human colorectal carcinoma xenografts. In such a work by Zeglis et al., huA33-TCO was administered 24 h prior to 64Cu-Tz-SarAr to reach the tumors and permit blood clearance.339 Owing to the superior stability and exclusive renal clearance of 64Cu-Tz-SarAr, the in vivo pretargeting yielded specific uptake of the radioligand in the colorectal tumors with high T/B ratios (Figure 26). This imaging strategy also outperformed a previous pretargeted imaging strategy, in which substantial uptake of 64Cu-Tz-NOTA was observed in the gastrointestinal tract.335 In the future, the combination of GPA33-specific immunoPET imaging and pRIT may allow precise diagnosis and treatment of colorectal cancers.329,731,732
Figure 26.

Pretargeted immunoPET imaging of colorectal cancers. (a) Schematic of the imaging strategy, in which huA33-TCO was first administered to accumulate in the tumor followed by injection of 64Cu-Tz-SarAr 24 h later. (b) Coronal image and (c) fused PET/CT images 24 h postinjection of the radioligand showed the effective delineation of the subcutaneous SW1222 xenografts. Reproduced with permission from ref 339. Copyright 2015 American Chemical Society.
4.12. Other Promising Tumor Biomarkers
Several other cell surface antigens have shown promise for imaging cancers at the cellular and molecular levels in either preclinical or clinical settings, such as folate receptor alpha,733,734 tumor vascular markers,735–737 death receptor 5,738–740 glypican-3,741–743 cell adhesion molecules,744,745 six-transmembrane epithelial antigen of prostate-1 (STEAP1),746–749 hormone receptors,750–752 TGF-β pathway,753–755 chemokine receptors,756,757 and extracellular matrix-like fibronectin.162,188 Here, we also discuss some of the promising targets briefly.
Tumor-associated glycoprotein (TAG)-72 is another glyco-protein highly expressed in the majority of adenocarcinomas. 3E8 is a humanized mAb against TAG-72 and has shown theranostic potential in colorectal cancer xenografts.758 For antibody fragment-based immunoPET imaging of TAG-72, PEGylated targeting vectors may serve as favorable surrogates for efficiently delivering the radionuclide to the tumor site while lowering kidney uptake.759–761 Delta-like 3 (DLL3), a Notch pathway ligand, has been identified as a marker for pulmonary neuroendocrine tumors (i.e., small cell lung cancer and large cell neuroendocrine carcinoma) and neuroendocrine PCas.762–764 As such, DLL3 may also serve as a tractable immunoPET imaging target.765,766 Similar to the GPA33-targeted theranostic scenario, GD2-specific immunoPET imaging may help assess GD2 expression in patients with neuroblastomas.767,768 Along with the clinical use of GD2-targeted antibody therapy and RIT,769–771 and future implementation of pRIT,772 GD2-targeted immunoPET imaging may guide precise anti-GD2 therapies and complement other imaging modalities to refine the management of neuroblastomas.
5. IMMUNOPET IMAGING OF IMMUNE SYSTEM
Cancer immunotherapy is increasingly becoming the standard-of-care treatment for a broad spectrum of cancers. IHC, polymerase-chain-reaction (PCR)-based assays and serum or blood biomarkers are regularly used to predict the therapeutic responses of immunotherapy regimens. Despite these approaches, it is still very challenging to properly select patients suitable for immunotherapy and precisely evaluate the treatment responses. Furthermore, immune-related adverse events are increasingly being reported. Currently, there are no reliable surveillance strategies to diagnose those unexpected complications.773,774 Accumulating evidence has suggested that immunoPET imaging may substantially improve the clinical immunotherapy by dynamically visualizing immune responses across the whole body.775–777 To achieve this goal, radiotracers have been developed to image interleukins and specific immune cells, including B cells,778 natural killer cells,779,780 macrophages,781–783 myeloid cells,278,784 and T cells. In this section, we mainly present the most recent evidence of immunoPET strategies in delineating T cells by targeting lineage-associated antigens, immune checkpoint molecules, OX40, and interferon gamma (IFNγ).
5.1. Lineage-Associated Antigens
In addition to tracking T cells via ex vivo direct labeling or reporter gene imaging,785–787 it is advantageous to track T cells using novel immunoPET techniques by targeting general T cell markers (e.g., CD3, CD4, CD8, CD2, and CD7). ImmunoPET imaging with a 89Zr-labeled antimouse CD3 antibody (clone 17A2; R&D Systems) revealed a correlation between high tumor uptake of the radiotracer and better therapeutic response of anticytotoxic T lymphocyte antigen 4 (CTLA-4) therapy in a preclinical colorectal cancer model.788 However, significant liver accumulation of the radiotracer was found on immunoPET images, which was explained as liver clearance of the radiolabeled antibody. Another study also labeled the same clone (clone 17A2; Bio X Cell) with 89Zr and confirmed the uptake of the developed radiotracer in lymphoid organs (i.e., spleen, lymph nodes, and thymus) and tumor-infiltrating lymphocytes (TILs) in syngeneic bladder cancer models.789 But what was different in this study was that the tracer was largely deposited in the spleen rather than in the liver, similar to that reported in another study.790 CD2 and CD7 are pan T cell markers and immunoPET tracers targeting these two markers have been developed to track adoptively transferred T cells.791 However, the safety profiles, long-term effects, and impact of these tracers on the functionality of T cells remain to be determined.
To noninvasively detect CD8+ T cells, two Mbs (i.e., 2.43 Mb and YTS169 Mb) were engineered from two parental rat antimouse CD8 mAbs. Both 64Cu-NOTA-Mbs retained high antigen specificity in imaging CD8+ T cells.792 IAB22M2C is a clinical-stage Mb that targets human CD8 with high affinity. A recent phase I study has demonstrated the safety profile of 89Zr-Df-IAB22M2C immunoPET imaging in patients with solid tumors.793 This study also reported a specific accumulation of the radiotracer in CD8+ T cell-rich tissues, such as lymph nodes, spleen, and tumors. A phase II clinical trial with this immunoPET probe investigating the correlation between 89Zr-Df-IAB22M2C immunoPET signal and CD8 status assessed by IHC in patients with metastatic cancers is currently underway (NCT03802123).
Two diabody-based anti-CD4 and anti-CD8 tracers, 89ZrmalDFO-GK1.5 cDb and 89Zr-malDFO-2.43 cDb, also specifically targeted various lymphoid organs and allowed longitudinal monitoring of transplanted T cells.794 To thoroughly assess CD8-targeted immunoPET imaging, 89ZrmalDFO-169 cDb binding to CD8a of all mouse strains was assessed.795 ImmunoPET imaging with this radiotracer detected intratumoral CD8+ T cells after anti-CD137 or anti-PD-L1 therapy in immune-competent mice bearing CT26 tumors. Afterward, 64Cu-TETA-169 cDb was developed and used to image CD8+ T cells after immunotherapy using different treatment protocols.796 As reported by the work, serial 64Cu-TETA-169 cDb immunoPET imaging mapped T cell distribution and also detected treatment-associated hypertrophy of liver and spleen following multiple cycles of immunotherapy.
Other types of antibody vectors used for imaging T cells include VHH and monovalent antibody. Rashidian et al. produced a CD8-specific mouse VHH (VHH-X118) and fabricated 89Zr-PEGylatedVHH-X118 using sortase-catalyzed site-specific conjugation.64 This immunoPET probe robustly detected thymus, secondary lymphoid structures as well as CD8+ T cells in B16 melanomas. This study also highlighted the value of CD8-targeted immunoPET in predicting the treatment response of anti-CTLA-4 immunotherapy, where the homogeneous distribution of immunoPET signal within the tumors predicted a favorable response to the anti-CTLA-4 immunotherapy. Moreover, another recent study radiolabeled a CD8-specific monovalent antibody with 89Zr and immuno-PET imaging with the developed agent (denoted as ZED8) efficiently detected human CD8-expressing tumors.797 This tracer was developed under quality standards appropriate for regulatory approval and is currently under clinical investigation (NCT04029181).
5.2. Immune Checkpoints
Immune checkpoints are critical components of inhibitory immune signaling pathways. The first-generation immune checkpoint inhibitors are immunomodulatory mAbs that block immune checkpoints. Notably, immune checkpoint inhibitors blocking CTLA-4, programmed death receptor 1 (PD1), and PD-L1 have emerged as trailblazers in treating various kinds of cancers.6 Accordingly, immunoPET probes targeting CTLA-4, PD1, or PD-L1, are extensively investigated.
CTLA-4 is a negative immune regulator highly expressed on regulatory T cells (Treg) and on activated T cells. Recent studies have elucidated that CTLA-4 and PD-1 may share one pathway by inhibiting signaling through CD28, which is a costimulatory receptor that promotes T cell activation and proliferation.798 Several studies have reported the feasibility of CTLA-4-targeted immunoPET in imaging T cells in humanized mice799 and in immune-competent mice.800 H11 is a VHH targeting mouse CTLA-4 and immunoPET with 89Zr-H11-PEG clearly delineated CTLA-4 expression within the TME.801 Moreover, several studies have shown the expression of CTLA-4 on tumor cells,802 which was corroborated by an imaging study where 64Cu-DOTA-ipilimumab showed persistent accumulation in the CTLA-4-expressing A549 tumors.803
PD1 is a characteristic marker expressed on exhausted CD8+ T cells. Pembrolizumab and nivolumab are anti-PD-1 checkpoint inhibitors used for treating a variety of solid tumors. Several immunoPET probes using pembrolizumab/ nivolumab and 89Zr/64Cu have been developed and investigated in preclinical studies.804–808 Of them, 89Zr-pembrolizumab and 64Cu-pembrolizumab PET/CT examinations showed prominent uptake of the radiotracers in the humanized mice bearing A375 melanomas, indicating infiltration of PD-1-positive human lymphocytes into the tumors.806 Similarly, 89Zr-Df-nivolumab immunoPET imaging delineated PD-1-positive human lymphocytes in A549 tumors and salivary glands in humanized mice.808 More recently, a seminal study by Niemeijer et al. investigated the clinical value of 89Zr-Dfnivolumab immunoPET imaging in 13 patients with NSCLC prior to the nivolumab treatment.809 For the first time, this study reported a correlation between 89Zr-Df-nivolumab uptake and PD-1 expression in the TILs assessed by IHC in clinical settings (Figure 27a,b). This study also indicated the value of 89Zr-Df-nivolumab uptake in predicting the treatment efficacy of nivolumab. However, this predictive value needs to be confirmed in a larger patient cohort.
Figure 27.

ImmunoPET imaging of immune checkpoints in nonsmall-cell lung cancer (NSCLC). (a) 18F-FDG PET/CT scan of a patient with NSCLC showed lung tumors and mediastinal lymph node metastases with high glucose metabolism. (b) PD-1-specific 89Zr-Dfnivolumab immunoPET/CT imaging demonstrated heterogeneous uptake of the radiotracer within and between the tumor lesions. (c) Similarly, heterogeneous uptake of PD-L1-specific 18F-BMS-986192, a 18F-labeled adnectin protein, was seen within and between the tumor lesions. Reproduced with permission from ref 809. Copyright 2018 Springer Nature.
Two IHC methods for determining PD-L1 expression have been approved to predict patient response before anti–PD-L1 therapies. However, sampling limitations and multifaceted expression of PD-L1 may lead to underestimation of the target. Substantial preclinical evidence has suggested that immuno-PET imaging can assess the heterogeneous status of PD-L1 throughout the whole body and thus can overcome above drawbacks.810–813 In a recent first-in-human clinical trial (NCT02478099), 89Zr-atezolizumab was assessed in 22 patients with progressive bladder cancer, NSCLC or triple-negative breast cancer.21 ImmunoPET imaging with 89Zratezolizumab showed deposition of the radiotracer in non-malignant lymph nodes, spleens, and sites of inflammation. More importantly, 89Zr-atezolizumab immunoPET visualized all the metastatic tumor lesions by imaging heterogeneous PD-L1 expression. High tumor uptake of 89Zr-atezolizumab correlated with better response to atezolizumab treatment, whereas PD-L1 IHC failed to predict the treatment outcome. 89Zr-atezolizumab immunoPET imaging could also select RCC patients who may benefit from nivolumab therapy.814 Moreover, there are two ongoing clinical trials evaluating the diagnostic value of PD-L1-targeted immunoPET in advanced thoracic malignancies (NCT03746704) and in locally advanced or metastatic solid tumors (NCT02453984).
Engineered small proteins (e.g., fibronectin, ~10 kDa) are alternative targeting moieties that have been used for imaging PD-L1 expression.815 Following a preclinical study which discovered the high binding affinity of 18F-BMS-986192 (an 18F-labeled adnectin) to human and cynomolgus PD-L1,816 a recent clinical study reported that 18F-BMS-986192 immuno-PET could noninvasively image the heterogeneous PD-L1 status in lung cancers (Figure 27c). High-affinity consensus (HAC) PD1 (14 kDa) is another high-affinity binder engineered from PD-1 protein and can be used for imaging human PD-L.817 In the course of optimizing HAC-PD1 variants for PD-L1 imaging, aglycosylated HAC-PD1 showed increased tumor uptake and decreased glandular uptake.818 Other targeting vectors that have been integrated into immunoPET for imaging PD-L1 include an Affibody molecule,819 VHHs,820–822 and a Fab fragment.823
Beyond imaging PD-L1 expression pre- and postimmuno-therapy, PD-L1-targeted immunoPET is an innovative approach to monitor PD-L1 upregulation following radiation therapy824,825 or PD-L1 downregulation after molecular-targeted therapy.826 Although anti-PD-L1 therapy has become the first-line treatment option for patients with NSCLC, therapeutic resistance occurs in some patients. Among many potential reasons, secreted PD-L1 splicing variants may induce resistance to anti-PD-L1 therapy by acting as decoys in plasma.827 Similar to that observed in other conditions,722,723 PD-L1-targeted immunoPET may predict and assess anti-PDL1 resistance because soluble PD-L1 will relocate the radiotracer to the plasma and liver but not to the tumor sites. Besides its expression on tumor cells, PD-L1 is also expressed in normal lymphoid organs and is an independent marker for brown adipose tissue (BAT) (Figure 28),828–830 indicating that PD-L1-targeted immunoPET may as serve as a cutting-edge imaging technique to aid basic research.
Figure 28.
ImmunoPET imaging of programmed death-ligand 1 (PDL1) in brown adipose tissue (BAT). B3 is a single domain antibody specific for mouse PD-L1 and (a) 18F-B3 immunoPET/CT imaging of a 6-week-old wild-type C57BL/6 mouse showed deposition of the radiotracer in the BAT. (b) 18F-B3 immunoPET/CT imaging of an age-matched PD-L1 knockout mouse showed the absence of PD-L1 signal in the BAT, confirming the specificity of the developed radiotracer. Reproduced with permission from ref 830. Copyright 2017 Springer Nature.
5.3. Other Emerging T Cell Markers
OX40, also known as CD134, is a 50 kDa type I membrane glycoprotein belonging to the tumor necrosis factor (TNF) receptor superfamily.831 Binding of OX40 by its ligand OX40L results in the activation of T cells, indicating OX40 is a promising candidate for monitoring clinical immunotherapies. By radiolabeling a murine mAb (clone: OX-86; Bio X Cell) with 64Cu, Alam et al. developed an OX40-targeted radiotracer 64Cu-DOTA-AbOX40.832 The authors found that early immunoPET imaging with 64Cu-DOTA-AbOX40 characterized the spatiotemporal expression of OX40+ T cells and also predicted the response of CpG vaccination in lymphoma models. These results demonstrated that OX40-targeted immunoPET could adequately visualize the heterogeneous dynamics of OX40+ T cells in immune responses across different subjects. For further clinical translation, this murine antibody-based immunoPET imaging strategy needs to be optimized with human or humanized antibodies.
IFNγ is a soluble immunomodulatory factor that exerts effects on both innate and adaptive immunity. IFNγ is primarily secreted by activated lymphocytes such as CD4+ and CD8+ T cells.833 Gibson et al. recently labeled an IFNγ-targeting rat mAb (AN-18) with 89Zr and found that tumor cell uptake of 89Zr-anti-IFNγ was IFNγ-dependent.790 After a series of CpG vaccination and mAb treatment experiments, they further demonstrated the robust ability of 89Zr-anti-IFNγ immunoPET in detecting T cell activation and exhaustion in spontaneous tumor models. These results together support the future development of IFNγ-targeted immunoPET for clinical use. In addition to the targets discussed above, there are several other targets successfully leveraged for imaging T cells, including granzyme B834,835 and IL-2 receptor.836 However, peptides and interleukins rather than antibodies were used as the targeting vectors in these platforms.
6. IMMUNOPET IMAGING OF INFLAMMATION
Although 18F-FDG PET is a clinically viable method to noninvasively quantify inflammation, it lacks specificity due to the uptake of the tracer by metabolically active tissues. Inflammation is decisive in tumor progression, and inflammation and cancer share certain signaling molecules.837 Therefore, some of the aforementioned immunoPET probes could reasonably be extended to detect and evaluate inflammatory diseases.838 In this section, we highlight the role of immunoPET in detecting several inflammatory diseases.
6.1. Rheumatoid Arthritis
Rheumatoid arthritis (RA) is a polygenic and multifactorial joint disease characterized by autoantibodies to various molecules such as IgG and citrullinated proteins.839 Treatment strategies for RA mainly include disease-modifying antirheumatic drugs, which can be further categorized into small molecules (e.g., methotrexate) and biologic agents (e.g., anti-TNFα mAbs).840 Although RA is incurable, it is important to identify and treat RA patients at earlier stages to delay the joint damage.
To track autoantibody to glucose-6-phosphate isomerase (GPI), Wipke et al. initially radiolabeled an anti-GPI IgG with 64Cu and found that the developed probe specifically localized to the diseased joints.841 This highlighted the possibility that immunoPET imaging may help us understand the sophisticated autoimmunity responses involved in human RA. F8-IL10 is a novel treatment option for RA patients. F8-IL10 was developed by fusing the Fv fragment of the human antibody F8 with the anti-inflammatory cytokine IL10, resulting in selective delivery of IL10 to the fibronectin-expressing inflammatory sites.842 A translational study investigating [124I]I-F8-IL10 found that this radiotracer accumulated readily in the arthritic joints of RA patients. However, this immunoPET study also revealed very rapid blood clearance of the radiotracer and unexpected high uptake in the liver and spleen.843 Certolizumab pegol (CZP) is a PEGylated fab fragment targeting TNFα and has shown remarkable efficacy in controlling the symptoms of RA. 89Zr-DFO-CZP was recently synthesized and immunoPET imaging with this tracer specifically located diseased joints and paws of transgenic mice expressing human TNFα.844
Fibroblast activation protein (FAP) is a type II trans-membrane glycoprotein belonging to the family of serine prolyl oligopeptidases. In addition to being a theranostic target for cancers,845–847 FAP is closely associated with the progression of RA. A fully human noninternalizing anti-FAP antibody 28H1 that binds to both murine and human FAP has been reported.848 When labeled with 89Zr, it specifically visualized inflamed joints of RA models (Figure 29).849 More importantly, 28H1-based molecular imaging tracers could monitor the response of RA to different treatment options at molecular levels.850–852
Figure 29.

ImmunoPET imaging of rheumatoid arthritis (RA). (a) 89Zr-28H1 immunoPET/CT imaging of a mouse with collagen-II-induced arthritis 72 h after injection of the radiotracer. 89Zr-28H1 accumulated in the inflamed joints with high contrast. (b) 18F-FDG PET/CT imaging also showed uptake in the inflamed joints but the uptake was lower than that of 89Zr-28H1. Reproduced with permission from ref 849. Copyright 2015 SNMMI.
6.2. Inflammatory Bowel Disease
Inflammatory bowel disease (IBD) is a chronic inflammatory disorder composed of two major subtypes: Crohn’s disease and ulcerative colitis.853 In current clinical practice, endoscopy is the most commonly applied technique to diagnose and monitor IBD. However, this invasive examination fails to provide information regarding molecular markers involved in the development and progression of IBD. By targeting integrins and immune mediators, immunoPET imaging approaches have been adapted to sensitively grade the disease severity.854 An initial study radiolabeled a β7 integrin-specific mAb (FIB504.64) and showed specific uptake of 64Cu-DOTAFIB504.64 in the gut of mice with dextran sulfate sodium (DSS)-induced colitis.855 To lower background signals in nontarget organs, immunoPET probes were developed using FIB504.64 fragments.856 In these studies, 64Cu-NOTA-FIB504.64-F(ab′)2 outperformed 64Cu-NOTA-FIB504.64-Fab in detecting DSS-induced colitis. Moreover, 64Cu-NOTA-FIB504.64-F(ab′)2 immunoPET also demonstrated better imaging contrast than 64Cu-DOTA-DATK32, a mAb-derived tracer targeting the integrin heterodimer α4β7.
More recently, several immunoPET tracers targeting innate immune cells, interleukins, and CD4+ T cells have been characterized in murine models of colitis. Dmochowska et al. reported increased IL-1β and increased infiltration of CD11b+ CD3− innate immune cells in the inflamed colon of colitic mice.857 ImmunoPET imaging studies in this work further corroborated the above observations. While 89Zr-α-CD11b (clone M1/70) immunoPET detected colonic inflammation with higher sensitivity than MRI, 89Zr-α-IL-1β (clone B122) immunoPET correlated the disease severity, although less robustly than 18F-FDG (Figure 30a–c). 89Zr-malDFO-GK1.5 cDb is an antimouse CD4 radiotracer initially used for imaging immune system reconstitution.794 Freise et al. recently assessed the diagnostic value of 89Zr-malDFO-GK1.5 cDb in tracking CD4+ T cell infiltration in DSS-induced colitis mouse models.858 ImmunoPET imaging showed radiotracer uptake in mesenteric lymph nodes and colons of the colitic mice (Figure 30d,e), which correlated with increased infiltration of CD4+ T cells into the inflamed colons revealed by IHC staining. Taken together, immunoPET is less intrusive and could provide useful information regarding the dynamics of immune cells throughout the intestines. This may in turn help monitor the disease severity and predict the treatment responses.
Figure 30.

ImmunoPET imaging of inflammatory bowel disease (IBD). (a) 89Zr-a-IL-1b, (b) 89Zr-α-CD11b immunoPET imaging, and (c) conventional 18F-FDG PET imaging all detected dextran sulfate sodium (DSS)-induced colonic inflammations, which was indicated by uptake in the colons. Reproduced with permission from ref 857. Copyright 2019 SNMMI. (d) 89Zr-malDFO-GK1.5 cDb, an immunoPET probe targeting mouse CD4, was used to image IBD by capturing CD4+ T cells. Ex vivo 89Zr-malDFO-GK1.5 cDb immunoPET imaging showed increased radiotracer concentration in the DSS-treated colons, ceca, and mesenteric lymph nodes (MLNs).(e) Corresponding gross specimens obtained from the normal mice and from the colitic mice. Note that colons of the DSS-treated mice were shorter than that of the control mice. Reproduced with permission from ref 858. Copyright 2018 SNMMI.
6.3. Other Inflammatory Diseases
With the development of specific mAbs for various inflammation-related antigens, immunoPET probes have been developed to image several other inflammatory diseases such as graft versus host disease,859,860 atherosclerotic plaques,861–863 and inflammation-induced lymphangiogenesis.864,865 In addition, immunoPET or immunoPET/MRI hybrid imaging are being used to detect bacterial, fungal, or viral infections.866–870 In the case of invasive pulmonary aspergillosis, a mouse mAb mJF5 and its humanized derivative hJF5 have proven to be highly specific to mannoprotein antigens produced by Aspergillus. Preclinical studies have validated the accuracy of 64Cu-DOTA-mJF5 and 64Cu-NODAGA-hJF5 in detecting Aspergillus lung infection.871–873 From these preclinical reports, it is conceivable that upon continuous investigation and clinical translation, immunoPET imaging holds enormous potential to diagnose the abovementioned inflammatory diseases.
7. IMMUNOPET IMAGING OF BETA CELL MASS
Diabetes mellitus (DM) is a metabolic disease characterized by a functional loss of beta cell mass (β cell mass, BCM) or the insufficient response of beta cells to insulin. It has been estimated that over 550 million people will suffer from diabetes by 2030.874 To monitor the early and dynamic change of BCM, various molecular imaging probes have been developed, including several antibody-based probes.875 Transmembrane protein 27 (TMEM27) is a validated BCM biomarker, and TMEM27-targeted immunoPET and fluorescent imaging probes have shown promise in imaging BCM.876 Dipeptidyl peptidase 6 (DPP6) is a newly identified biomarker for pancreatic alpha and beta cells. A VHH (i.e., 4hD29) targeting DPP6 was produced, initially radiolabeled with 99mTc, and used for immunoSPECT imaging of endocrine cell mass (ECM).877 More recently, 68Ga-NOTA-4hD29 was developed and immunoPET imaging with this agent successfully detected DPP6-positive tumors.878 Upon clinical translation, this agent holds promise for detecting ECM, transplanted human islets, and DPP6-positive tumors.
ImmunoPET imaging of BCM is advantageous compared to other imaging modalities because of its targeting sensitivity and specificity. In preclinical models, beta cell-specific immunoPET has been used to image insulinomas and grafted human islets. However, it is very challenging for immunoPET to detect islets scattered across the pancreas in preclinical models, due either to the high background signal or to the restricted species reactivity of the antibodies. It remains to be determined whether immunoPET imaging can visualize beta cell islets in humans. If so, this novel imaging modality may improve the management of diabetes by monitoring the dynamic change of BCM over time. As we mentioned above, 52Mn is a relatively new radiometal used for immunoPET. Two interesting studies have elucidated that 52Mn alone serves as a sensitive tracer for measuring and quantifying BCM.879,880 Clinical trials are warranted to confirm the unique application of 52Mn PET in quantifying and monitoring BCM.
8. IMMUNOPET IMAGING-GUIDED ADVANCED THERAPEUTICS
The landscape of theranostics has evolved over time. The management of thyroid diseases (e.g., differentiated thyroid cancer and hyperthyroidism) has been revolutionized since the use of theranostic radioiodine isotopes in the 1940s.881,882 With the identification of highly specific cancer antigens and the concomitant development of radiopharmaceuticals, PSMA-targeted and somatostatin-derived theranostic agents have achieved remarkable success in diagnosing and treating advanced PCas and neuroendocrine tumors, respectively.883,884 This trend is expected to flourish with the discovery of newer molecular targets and synchronous advance in radiochemistry.885
ImmunoPET imaging can visualize the spatial heterogeneity of target expression and thus predict the responses of therapeutic antibodies, including the immune checkpoint inhibitors.21,809,814 Besides selecting patients for antibody therapies, immunoPET imaging is a robust approach to select patients for antibody-based therapies, particularly RIT. Most of the aforementioned biomarkers can be leveraged to develop RIT agents. Traditional RIT has proven useful for treating radiosensitive tumors and disseminated hematologic malignancies.886–888 However, it is less effective for radioresistant or bulky tumors. It is anticipated that innovative pRIT (e.g., agents produced via the Dock-and-Lock technology) may deliver a higher therapeutic dose to the tumor while reducing hematologic toxicity. Several exemplary targets with clinical theranostic evidence are discussed below.
B7-H3 (CD276) is an immunoregulatory glycoprotein that is also overexpressed in a broad spectrum of solid tumors.889 Preclinical studies have reported that immunoPET imaging was able to delineate CD276 expression on tumor cells and blood vessels.890,891 8H9 (Omburtamab) is a murine mAb against B7-H3,892 and 131I-8H9 has been used to treat several types of solid tumors after intrathecal administration.893,894 A recent study elucidated that pretherapy immunoPET imaging with 124I-8H9 allowed for noninvasive estimation of the therapeutic index of 131I-8H9.895 Interestingly, 124I itself may serve as a theranostic radioisotope due to the emission of high-energy positrons. To test the clinical feasibility, a phase I clinical trial was carried out to assess the theranostic value of 124I-8H9 in patients with diffuse intrinsic pontine glioma.896 The results demonstrated that 124I-8H9 was precisely delivered to the brainstem lesions after intratumoral infusion (Figure 31). Moreover, immunoPET/MR imaging following 124I-8H9 infusion quantitated the absorbed dose in the tumors and other organs. These clinical studies together demonstrate that intrathecal administration of 124I-8H9 and 131I-8H9 in tandem (or 124I-8H9 alone) may maximize the therapeutic outcome while limiting the systemic toxicity.
Figure 31.

Representative immunoPET/MR imaging of a patient with diffuse intrinsic pontine glioma after convection-enhanced delivery of 124I-8H9. The axial (upper sections) and sagittal (lower sections) fused PET/MR images showed predominant retention of 124I-8H9 in the brainstem. In this case, 124I-8H9 servers as a theranostic agent allowing for concurrent imaging, dosimetry, and therapy. Reproduced with permission from ref 896. Copyright 2018 Elsevier Inc.
CEA is another biomarker extensively studied for cancer theranostics over the years. CEA-directed pRIT was initially investigated in patients with primary colorectal cancer897 and then in patients with medullary thyroid cancer.319,320 However, the frequent hematological toxicity and immune responses of these first-generation approaches limited their broad applications. The IMP288 peptide allows facile radiolabeling with either therapeutic (such as 177Lu, 90Y, and 213Bi) or diagnostic (such as 68Ga, 111In, and 86Y) radiometals, providing a perfect platform for designing theranostic agents. A preclinical study has shown the survival benefit of TF2/177Lu-IMP288 pRIT in colorectal cancer models.898 A follow-up clinical trial further reported that TF2/177Lu-IMP288 pRIT was feasible in patients with colorectal cancer, but the efficacy was limited because all the patients showed progressive disease eight weeks later.899,900 Use of 213Bi (T1/2 = 45.6 min) instead of 177Lu in this pretargeted system showed comparable therapeutic efficacy in colorectal cancer models, but the dosing schedule needs to be optimized to reduce nephrotoxicity before clinical translation.901 With further development of humanized antibodies,902 dual-modal imaging probes,903 and the pRIT system,904 CEA-targeted theranostic toolbox holds excellent prospects for improving the management of CEA-positive human malignancies.
As mentioned earlier (section 4.3.1), a recent clinical trial has demonstrated the clinical feasibility of CA19.9-targeted immunoPET in localizing PDAC.39 This study also indicated that 5B1 might deliver therapeutic doses to PDAC after labeled with beta emitters. A preclinical study explored the therapeutic benefit of CA19.9-directed pRIT, in which 5B1-TCO was administered 72 h before the injection of 177Lu-DOTA-PEG7-Tz or 177Lu-CHX-A′′-DTPA-PEG7 -Tz.905 The results demonstrated that the former combination showed a dose-dependent therapeutic response in PDAC models. Along with this preclinical evidence, an ongoing clinical trial (NCT03118349) is evaluating the safety and dosimetry of 177Lu-CHX-A′′-DTPA-5B1 in patients with CA19.9-positive malignancies. 5B1 as a standalone monotherapy or in combination with chemotherapy is also under clinical investigation (NCT02672917). On the basis of the reported evidence and future clinical trial results, the 5B1-based theranostic toolbox may hopefully improve the clinical management of CA19.9-positive malignancies.
RIT is an established tool in the treatment of hematologic malignancies, such as NHL.906,907 CD20 is a classical theranostic target since two radiolabeled murine mAbs (131I-tositumomab, Bexxar; 90Y-ibritumomab tiuxetan, Zevalin) were approved for the treatment of B-cell NHL.908 Nonetheless, the clinical use of Bexxar and Zevalin is stagnant. Bexxar has not been commercially available since 2014 for multiple reasons (mainly due to the disappointing profits). In two recent studies, researchers reported the feasibility and satisfactory treatment efficacy of 90Y-rituximab in patients with relapsed or refractory NHL.909,910 Furthermore, several preclinical and clinical studies have reported the superior effectiveness of pRIT or RIT in treating lymphomas by targeting several targets, such as CD20,911,912 CD38,560 and CD45.913 CD33 is another alternative target for RIT of hematological malignancies914,915 and for immunoPET imaging.916 Continuous innovation of the pRIT systems and incorporation of immunoPET techniques may reinvigorate the enthusiasm for managing hematologic malignancies with nuclear medicine approaches. Readers are recommended to refer to an excellent review parsing RIT for more information.167
9. IMMUNOPET IMAGING-GUIDED DRUG DEVELOPMENT
In the development of antibody- and antibody-based therapeutics, iterative approaches are needed, including the identification of antigens and screening and selection of optimal antibodies. Traditionally, several analytical and structural techniques (e.g., mass spectrometry, liquid chromatography, and electrophoresis) are used to assess the developed antibody therapeutics. Apart from these procedures, it is necessary to examine the pharmacodynamic properties and safety profiles of antibodies before clinical translation for human use.917 Complementary to their role as diagnostic methods in the clinic, molecular imaging approaches are increasingly being used for fundamental research and antibody drug development.918–920 Molecular imaging can assist antibody drug discovery (e.g., target selection and antibody optimization) as well as the clinical assessment of antibody drugs (e.g., distribution, clearance, safety profile, and therapeutic efficacy). As a result, the translation of promising antibody candidates can be accelerated from preclinical prototype to bedside reality.921,922
With the development and use of total-body PET scanners, immunoPET imaging will facilitate the thorough assessment of mAb pharmacokinetics up to 30 days at the preclinical stage.923–925 ImmunoPET imaging can reveal both dose-dependent and dose-independent uptake of mAb in normal organs and in tumors. This is particularly useful when the target antigen is expressed in tumors as well as normal tissues.926 While the dose-dependent uptake is largely mediated by relevant receptors expressed on the surface of tumor cells, the dose-independent uptake is mainly caused by osmosis and retention of mAb in the TME. ImmunoPET imaging will help estimate the therapeutic effect of antibody therapeutics in phase I dose-escalation studies and calculate the amount of unlabeled antibody required for preloading or coadministration,41,927 which will saturate target antigens in normal organs and maximize the binding of mAb to the target antigens in tumors.
In addition to mAbs, ADCs are among the most effective targeted cancer therapeutics. Third-generation ADCs with increased potency and stability are under clinical investigation.928 During ADC development, cytotoxic drugs are linked to mAbs via bifunctional linkers. ImmunoPET imaging has shown its value in assessing the in vivo stability of novel linkers. This was exemplified by two recent studies where a novel platinum(II) linker was developed for ADC conjugates.929,930 More importantly, immunoPET imaging can characterize the effect of drug-to-antibody ratio on the overall blood retention and tumor-targeting efficacies of the developed ADCs (Figure 32).930–932 As another therapeutic alternative, RIT delivers therapeutic radionuclides to the tumor site by taking advantage of antibody specificity.933 ImmunoPET imaging permits dosimetry calculations for these therapies and further predicts dose-limiting organs prior to the RIT,934 optimizing the development and use of RIT agents.
Figure 32.
ImmunoPET imaging guides antibody drug development. (a) Trastuzumab-Lx-AF is an antibody–drug conjugate developed by linking trastuzumab with auristatin F (AF) via the linker Lx. To evaluate the influence of drug-to-antibody ratios (DARs), 89Zr-DFO-trastuzumab-Lx-AF immunoPET/CT imaging was carried out at 96 h postinjection of the radiotracer. The imaging results demonstrated the varying stabilities of the Lx-based ADCs. Importantly, a DAR of 2.6 did compromise the tumor targeting. (b) Biodistribution studies further confirmed the immunoPET imaging results (black bars, DAR of 0; red bars, DAR of 2.6; blue bars, DAR of 5.2; *, P < 0.05). Reproduced with permission from ref 930. Copyright 2018 SNMMI.
ImmunoPET-guided drug development provides insightful information regarding the distribution and targeting potential of new antibody drugs or antibody-based therapeutics. However, it should not be ignored that radionuclides, chelators, and radiolabeling methods may alter the inferred pharmacokinetics of the conjugated antibody tracers.
10. FUTURE PERSPECTIVES AND CONCLUSIONS
As described in this review, immunoPET is an invaluable companion diagnostic tool actively changing the management of cancers and noncancerous diseases (Table 2). However, it must be noted that most of the immunoPET probes have only been assessed in preclinical stages or in small cohorts of patients. Further studies are needed to translate some of the promising immunoPET probes and to confirm the diagnostic value of the clinically used ones. The development of antibody therapeutics will continue to reshape the therapeutic landscape of human diseases, and more sophisticated immunoPET imaging strategies will be designed accordingly. By making the right diagnoses and optimizing subsequent therapeutic decisions, immunoPET will hopefully help clinicians refine clinical practice and realize truly personalized medicine. The ultimate purpose of designing and using immunoPET is to facilitate better management of patients and lessen the financial burden for them and society.935,936
Table 2.
Representative Clinical-Stage ImmunoPET Imaging Probesa
| probe | target | targeting moiety | cancer types | ref |
|---|---|---|---|---|
| 89Zr-Df-cetuximab | EGFR | mAb | solid tumors | 356,358 |
| 89Zr-panitumumab | EGFR | mAb | colorectal cancer | 368 |
| 89Zr-Df-trastuzumab | HER2 | mAb | breast cancer, EGA | 41,391,399 |
| 64Cu-DOTA-trastuzumab | HER2 | mAb | breast cancer | 394,395 |
| 89Zr-Df-pertuzumab | HER2 | mAb | breast cancer | 396 |
| 124I-trastuzumab | HER2 | mAb | GC, GEC | 400 |
| 68Ga-HER2-Nanobody | HER2 | Nanobody | breast cancer | 412 |
| 68Ga-ABY-025 | HER2 | Affibody | breast cancer | 430,433 |
| 64Cu-DOTA-patritumab | HER3 | mAb | solid tumors | 453 |
| 89Zr-GSK2849330 | HER3 | mAb | solid tumors | 455 |
| 89Zr-lumretuzumab | HER3 | mAb | solid tumors | 458 |
| 89Zr-Df-bevacizumab | VEGF | mAb | solid tumors | 469,471,474 |
| 124I-huA33 | A33 | mAb | colorectal cancer | 185 |
| 89Zr-cmAb U36 | CD44v6 | mAb | HNSCC | 118 |
| 89Zr-RG7356 | CD44 | mAb | solid tumors | 523,524 |
| 89Zr-rituximab | CD20 | mAb | lymphoma | 536 |
| 89Zr-DFO-5B1 | CA19.9 | mAb | pancreatic cancer | 39 |
| 89Zr-huJ591 | PSMA | mAb | prostate cancer | 619,620 |
| 89Zr-Df-IAB2M | PSMA | Mb | prostate cancer | 633,634 |
| 68Ga-IMP288 | CEA | BsAb | medullary thyroid cancer | 654 |
| 89Zr-AMG 211 | CEA/CD3 | BiTE | gastrointestinal adenocarcinomas | 664 |
| 89Zr-CEA-IL2 | CEA | immunocytokine | solid tumors | |
| 89Zr-girentuximab | CAIX | mAb | renal cell carcinoma | 674 |
| 124I-cG250 | CAIX | mAb | renal cell carcinoma | 675 |
| 89Zr-DF0-MSTP2109A | STEAP1 | mAb | prostate cancer | 748,749 |
| 89Zr-fresolimumab | TGF-β | mAb | glioma | 754 |
| 89Zr-Df-IAB22M2C | CD8 | Mb | solid tumors | 793 |
| 89Zr-atezolizumab | PD-L1 | mAb | NSCLC, TNBC, bladder cancer | 21 |
| 18F-BMS-986192 | PD-L1 | adnectin | lung cancer | 809 |
| 89Zr-nivolumab | PD1 | mAb | lung cancer | 809 |
| [124I]I-F8-IL10 | fibronectin | Fv fragment | rheumatoid arthritis | 843 |
Abbreviations: mAb, monoclonal antibody; EGA, esophagogastric adenocarcinoma; GC, gastric cancer; GEC, gastroesophageal junction cancer; HNSCC, squamous cell carcinoma of the head and neck; Mb, minibody; Fv fragment, single-chain antibody variable domain (Fv) fragment; BsAb, bispecific antibody; BiTE, bispecific T-cell engager; PSMA, prostate-specific membrane antigen; CEA, carcinoembryonic antigen; CAIX, carbonic anhydrase IX; STEAP1, six-transmembrane epithelial antigen of prostate-1; TGF-β, transforming growth factor-β; NSCLC, nonsmall cell lung cancer; TNBC, triple-negative breast cancer; PD-L1, programmed death ligand-1; PD1, programmed death receptor 1.
There are several concerns about the immunoPET technique. A fundamental question that needs to be addressed is under which settings immunoPET may be integrated into the clinical diagnostic toolbox. In our view, immunoPET is a companion diagnostic tool that should be used together with clinically approved or clinical-stage therapeutic regimens. An initial immunoPET imaging is preferred to be performed before the commencement of targeted antibody or small-molecule inhibitor treatment because it will provide pivotal information on the baseline expression level of the target. The unique information obtained at the baseline will further allow adequate restaging and evaluation of the disease. Repeated immunoPET imaging after the treatments will help evaluate the therapeutic response and also the change of the target, especially for patients with multiple biopsy-inaccessible tumor lesions. Several other concerns might be gradually resolved with the progress of the field. Specifically speaking, the development of GMP-compliant production and purification processes may spur the clinical use of this imaging technique while minimizing unnecessary radiation dose to the health care staff.937,938 ImmunoPET imaging with total-body PET scanners will further decrease radiation exposure without compromising the image quality.939–941 Furthermore, advances in PET technology and reconstruction algorithms will lead to improved spatial and temporal resolution of immunoPET images.942 In most instances, immunoPET imaging is performed for patients with metastatic diseases. Consequently, another misgiving is the lack of histochemical confirmation of some of the tracer-avid lesions. However, the primary purpose of the initial immunoPET imaging is to stratify patients by mapping the expression of a specific biomarker. If the uptake or sizes of the tracer-avid lesions decrease on post-treatment immunoPET images, it is rational to consider these lesions as histopathology-positive.
For developing antibody-based radiopharmaceuticals, we would like to emphasize the importance of multidisciplinary collaboration. Specifically, multiple approaches (e.g., genomic, serological, proteomic, biological, and bioinformatical approaches) should be leveraged to identify antigens highly or exclusively overexpressed on the surface of the tumor cells, tumor stromal cells, tumor vasculature endothelial cells, immune cells, or beta cells.943 While the abundance and specificity are key factors when selecting suitable imaging targets, the function and stability of the targets need also to be considered.944 Of the diverse targets that are currently being investigated in preclinical studies or in clinical trials, some are relatively specific for certain tumor types (for example, TROP-2 for breast cancer and PCa, and CD138 for MM). When using antibodies as targeting vectors, it is important to remember that different IgG types have varying circulation time,945 and the interactions between the Fc domain and FcγR/FcRn dynamically regulate the immunological functions of the developed antibody.946–948 For instance, tislelizumab (BGBA317) is a humanized IgG4 that binds to PD-1 but not to FcγRI.949 This improvement results in enhanced tumor growth inhibition when compared to BGB-A317/IgG4S228P, which has a high affinity to FcγR. Thus, Fc engineering could be used to introduce mutations that may abrogate the binding of IgG with FcγR. This strategy could also be exploited to increase the cellular accumulation of an antibody–antigen complex when targeting secreted antigens714 or to enhance the persistence of VHHs in circulation.950 In addition, strategies like deglycosylation of antibodies may further improve the immunoPET imaging quality.951,952 A high-affinity antibody does not always guarantee high tumor uptake because other factors such as circulating antigens and tumor vasculature may affect the accessibility of the radiolabeled antibody. Aside from the singly targeted imaging probes, antibody-based heterodimers, or dual-targeting probes may have higher targeting efficacy and superior specificity than their monospecific peers.30,32,953 With the advent and evolvement of click chemistry,261 future studies may further harness this powerful method to synthesize modular immunoPET probes with improved in vivo performance. Furthermore, pretargeting strategies may also be harnessed to optimize the imaging quality.954,955
In the characterization of antibody-based diagnostic or theranostic probes, attention should be paid whether immunodeficient strains of animals are used in the imaging studies and how this may impact the imaging performance of the investigated probes. For example, a recent study reported that immunoPET radiotracers had inefficient tumor targeting and high off-target binding to the spleen in the highly immunodeficient mouse strains.766 This was consistent with a previous study which reported that ADCs had limited antitumor activity in the ultra immunodeficient NSG mice.956 In this setting, the use of humanized mouse models or nonhuman primates is very necessary to assess the imaging performance of the immunoPET probes prior to pilot clinical investigations.957 In addition, antibodies cross-reactive with human, nonhuman primate, and mouse antigens would be beneficial in preclinical immunoPET imaging studies.
Other than their use in immunoPET, antibodies are actively investigated as either monotherapy agents or antibody conjugates for effective cancer treatment. Antibodies can be modified with therapeutic radionuclides and photosensitizers for RIT167 and PIT,958,959 respectively, as novel treatment options. Two radiolabeled therapeutic anti-CD20 antibodies, 131I-tositumomab and 90Y-ibritumomab tiuxetan, have been approved by the FDA for the treatment of B-cell lymphoma.531,960 To maximize the therapeutic index, pRIT strategies may be used. For detailed information on RIT, readers are recommended to refer to other excellent reviews.302,954,961 RTKs and oncogenic proteins are ideal candidates for RIT.237,618,962 For instance, a recent study reported that pRIT with anti-HER2-DOTA-pRIT + 177Lu-DOTA-Bn inhibited HER-2 positive breast cancers and substantially improved survival without inducing toxicity in normal tissues.963 Therefore, radiolabeled antibodies or antibody fragments targeting some of the selected makers discussed above may provide additional therapeutic options for cancer patients in the era of precision medicine.964,965 The use of pairs of β+ and β− emitting radionuclides (e.g., 86Y/90Y) is desirable as a promising theranostic platform for sequential imaging, dosimetry, and therapy. In addition to the traditional β-emitting therapeutic isotopes, accumulating evidence is supporting the clinical application of targeted alpha therapy, where mAbs or small molecules are labeled with therapeutic α-emitters. 225Ac (T1/2 = 10.0 d) and 213Bi are both attractive therapeutic α-emitters, but the therapeutic index of 213Bilabeled agents was inferior to 225Ac-labeled agents.966,967 In translating these radiotherapeutics to the clinic, the safety profiles of the agents need to be carefully assessed. For therapeutic radionuclides without intrinsic imaging capabilities, immunoPET imaging may help estimate the pharmacokinetics of the therapeutic radiopharmaceuticals and evaluate the dose-limiting organs.
Optical fluorescence imaging using dye-labeled mAbs or sequential PET/NIR imaging with dual-labeled mAbs has the potential to improve cancer surgery outcomes.968–970 However, the payload of fluorophores needs to be carefully determined because the ratio of the fluorophore to mAb greatly affects both the pharmacokinetics and tumor-targeting efficiency of the developed probes.971,972 Besides, the emission of charged particles from radionuclides traveling through dielectric materials, such as in living subjects, results in the production of Cerenkov luminescence.973 Cerenkov luminescence has been validated effective for triggering photodynamic therapy.974,975 More interestingly, Cerenkov luminescence imaging (CLI) with optical imaging systems after the administration of radioactive tracers is an emerging imaging paradigm and can be exploited for image-guided surgery.976 While applications involving Cerenkov luminescence are still in their infancy, they hold great potential.977,978
While several advantages exist over other imaging modalities, the broad application of immunoPET may be restrained by a scarcity of radiometals and antibodies in less-developed countries. Therefore, immunoSPECT imaging may alternatively fill the gap. To this end, γ-emitting radionuclides like 99mTc, 123I, and 111In may be used to develop immunoSPECT imaging probes.979 It should be noted that the high-energy γ emission from 131I makes it unfavorable for developing immunoSPECT probes. The use of novel radiolabeling methods may improve the stability, binding affinity, internalization, and intracellular track of the radioiodinated mAbs.980
In summary, the development of immunoPET imaging strategies has achieved great success in the past decade. With continuous improvement and clinical translation, immunoPET imaging holds great promise in optimizing clinical management of human diseases, especially cancers.
ACKNOWLEDGMENTS
We apologize to authors whose work we could not cite due to the limited space of this review. We thank Zhoumi Hu and Dawei Jiang for their insightful comments on this paper. We also appreciate all the reviewers for their input and insightful comments. The study was supported by research grants from the Natural Science Foundation of China (grant nos. 81830052, 81530053, and 81974271), Shanghai Key Laboratory of Molecular Imaging (grant no. 18DZ2260400), University of Wisconsin—Madison, and the National Institutes of Health (P30 CA014520).
ABBREVIATIONS USED
- ADCP
antibody-dependent cellular phagocytosis
- ADCC
antibody-dependent cell-mediated cytotoxicity
- ADC
antibody–drug conjugate
- AR
androgen receptor
- BBB
blood–brain barrier
- BiTE
bispecific T-cell engager
- BCM
beta cell mass
- CDC
complement-dependent cytotoxicity
- CB-TE2A
4,11-bis(carboxymethyl)-1,4,8,11-tetraazabicyclo[6.6.2]hexadecane
- CA19.9
carbohydrate antigen 19.9
- CA-125
carbohydrate antigen 125
- CEA
carcinoembryonic antigen
- CAIX
carbonic anhydrase IXcc
- RCC
clear cell renal cell carcinoma
- CSC
cancer stem cell
- CTLA-4
cytotoxic T lymphocyte antigen 4
- CLI
Cerenkov luminescence imaging
- CuAAC
Cu(I)-catalyzed 1,3-dipolar cycloaddition between azides and alkynes
- DSS
dextran sulfate sodium
- DLL3
delta-like 3
- EDTA
ethylenediaminetetraacetic acid
- EGFR
human epidermal growth factor receptor
- FcγR
Fcγ receptor
- FcRn
neonatal Fc receptor
- 18F-FDG
18F-fluorodeoxyglucose
- GPI
glucose-6-phosphate isomerase
- GPA33
glycoprotein A33
- HNSCC
head and neck squamous cell carcinoma
- HCAb
heavy-chain-only antibody
- HER2/ErbB2
human epidermal growth factor receptor 2
- HER3/ErbB3
Human epidermal growth factor receptor 3
- HGF
hepatocyte growth factor
- IHC
immunohistochemistry
- IGF-1R
insulin-like growth factor-1 receptor
- IBD
inflammatory bowel disease
- ImmunoPET
immuno-positron emission tomography
- LGR5
leucine-rich repeat-containing G-protein coupled receptor 5
- mAb
monoclonal antibody
- MM
multiple myeloma
- MSLN
membrane-bound surface glycoprotein mesothelin
- NSCLC
nonsmall-cell lung cancer
- NHL
non-Hodgkin’s lymphoma
- NOTA
1,4,7-triazacyclononane-1,4,7-triacetic acid
- NODAGA
1,4,7-triazacyclononane,1-glutaric acid-4,7-acetic acid
- PDGFR
platelet-derived growth factor receptor
- PD-L1
programmed death ligand-1
- PET
positron emission tomography
- pRIT
pretargeted radioimmunotherapy
- PDAC
pancreatic ductal adenocarcinoma
- PSCA
prostate stem cell antigen
- PSA
prostate-specific antigen
- PD1
programmed death receptor 1
- PIT
photoimmunotherapy
- PSMA
prostate-specific membrane antigen
- RCY
radiochemical yield
- RTKs
receptor tyrosine kinases
- RIT
radioimmunotherapy
- RA
rheumatoid arthritis
- SPECT
single-photon emission computed tomography
- sdAb
single-domain antibody
- scFv
single-chain variable fragment
- SPAAC
strain-promoted azide–alkyne cycloaddition
- SrtA
sortase A
- STEAP1
six-transmembrane epithelial antigen of prostate-1
- TKIs
tyrosine kinase inhibitors
- TME
tumor microenvironment
- TGF-β
transforming growth factor-β
- TROP-2
trophoblast cell-surface antigen 2
- TAG-72
tumor-associated glycoprotein-72
- Treg
regulatory T cells
- VHH
variable domain of the heavy chain of a HCAb
- VEGF
vascular endothelial-derived growth factor
- VEGFR
vascular endothelial-derived growth factor receptor
Biographies
Weijun Wei obtained his M.D. and Ph.D. degrees with distinction in nuclear medicine and molecular imaging from the School of Medicine, Shanghai Jiao Tong University, in 2019. Dr. Wei was a joint graduate at the University of Wisconsin–Madison under the supervision of Prof. Weibo Cai during 09/2017–09/2018. Currently, Dr. Wei serves as a nuclear medicine physician at Renji Hospital, School of Medicine, Shanghai Jiao Tong University, where he is focusing on developing novel antibody- and nanobody-based probes for imaging and treating cancers. Dr. Wei has published more than 20 articles in several world-renowned journals.
Zachary Rosenkrans is currently a Ph.D. student in the Pharmaceutical Sciences program at the University of Wisconsin–Madison under the supervision of Dr. Weibo Cai. He previously received a B.S. in Chemical Engineering from the University of Kansas in Lawrence, KS. His research is focused on developing nanomaterial-based platforms for image-guided drug delivery and theranostics.
Prof. Jianjun Liu is the chief nuclear medicine physician at Renji Hospital, School of Medicine, Shanghai Jiao Tong University. As the director of the department, Prof. Liu leads a multidisciplinary team exploring novel therapeutic approaches for cancers on one hand and developing next-generation molecular imaging probes on the other hand. One of his specific interests is to design, validate, and translate antibody- and nanobody-based immunoPET imaging tracers. Prof. Liu has authored more than 40 research papers and tutored over 10 graduates.
Prof. Gang Huang is a well-recognized professor and leader of nuclear medicine and molecular imaging in China. His research and clinical work are focused on the diagnosis and treatment of malignancies, especially theranostics using nuclear medicine and molecular imaging approaches. He is the Elected President of the Asia Oceania Federation of Nuclear Medicine and Biology, Dean of the Asia Oceania School of Nuclear Medicine, the Ninth President of the Chinese Nuclear Medicine Society, and Editor-in-Chief of the Chinese Journal of Nuclear Medicine and Molecular Imaging. Prof. Huang has authored more than 200 articles, edited more than 30 books, given over 500 talks and received over 10 awards, such as the State Scientific and Technological Progress Award (the second place) and the Shanghai Medical Science and Technology Award (the first place), etc.
Prof. Quan-Yong Luo obtained his M.D. degree in nuclear medicine and molecular imaging from Shanghai Jiao Tong University in 2006. Currently, he is the director and chief physician at the Department of Nuclear Medicine, Shanghai Jiao Tong University Affiliated Sixth People’s Hospital. Besides his interest in clinical PET and SPECT imaging, he has been actively involved in radioiodine treatment of differentiated thyroid cancers for two decades. Prof. Luo has published more than 70 articles, in which his team has thoroughly investigated the diagnostic efficacies of SPECT and PET imaging, as well as the treatment efficacy of radioiodine in patients with differentiated thyroid cancer.
Prof. Weibo Cai is a Vilas Distinguished Achievement Professor of Radiology/Medical Physics/Biomedical Engineering/Materials Science & Engineering/Pharmaceutical Sciences at the University of Wisconsin–Madison, USA. He received a Ph.D. degree in Chemistry from UCSD in 2004. Prof. Cai’s research at UW–Madison (http://mi.wisc.edu/) is mainly focused on antibody-based molecular imaging and nanotechnology. He has authored more than 300 articles (H-index: 79), edited three books, and received many awards (e.g., Fellow of AIMBE in 2018 and Fellow of SNMMI in 2019). Prof. Cai’s trainees at UW–Madison have received over 100 awards.
Footnotes
The authors declare no competing financial interest.
Contributor Information
Weijun Wei, Department of Nuclear Medicine, Renji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai 200127, China;; Departments of Radiology and Medical Physics, University of Wisconsin—Madison, Madison, Wisconsin 53705, United States
Zachary T. Rosenkrans, Department of PharmaceuticalSciences, University of Wisconsin—Madison, Madison, Wisconsin 53705, United States
Jianjun Liu, Department of Nuclear Medicine, Renji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai 200127, China.
Gang Huang, Department of Nuclear Medicine, Renji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai 200127, China;; Shanghai Key Laboratory of Molecular Imaging, Shanghai University of Medicine and Health Sciences, Shanghai 201318, China
Quan-Yong Luo, Department of Nuclear Medicine, Shanghai Jiao Tong University Affiliated Sixth People’s Hospital, Shanghai 200233, China.
Weibo Cai, Departments of Radiology and Medical Physics and Department of Pharmaceutical Sciences, University of Wisconsin—Madison, Madison, Wisconsin 53705, United States;; University of Wisconsin Carbone Cancer Center, Madison, Wisconsin 53705, United States
REFERENCES
- (1).Mankoff DA A definition of molecular imaging. J. Nucl. Med 2007, 48, 18N–21N. [PubMed] [Google Scholar]
- (2).Ametamey SM; Honer M; Schubiger PA Molecular imaging with PET. Chem. Rev 2008, 108, 1501–1516. [DOI] [PubMed] [Google Scholar]
- (3).James ML; Gambhir SS A molecular imaging primer: modalities, imaging agents, and applications. Physiol. Rev 2012, 92, 897–965. [DOI] [PubMed] [Google Scholar]
- (4).Rahmim A; Lodge MA; Karakatsanis NA; Panin VY; Zhou Y; McMillan A; Cho S; Zaidi H; Casey ME; Wahl RL Dynamic whole-body PET imaging: principles, potentials and applications. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 501–518. [DOI] [PubMed] [Google Scholar]
- (5).Scott AM; Wolchok JD; Old LJ Antibody therapy of cancer. Nat. Rev. Cancer 2012, 12, 278–287. [DOI] [PubMed] [Google Scholar]
- (6).Khalil DN; Smith EL; Brentjens RJ; Wolchok JD The future of cancer treatment: immunomodulation, CARs and combination immunotherapy. Nat. Rev. Clin. Oncol 2016, 13, 273–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Sanmamed MF; Chen L A paradigm shift in cancer immunotherapy: from enhancement to normalization. Cell 2018, 175, 313–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Pimlott SL; Sutherland A Molecular tracers for the PET and SPECT imaging of disease. Chem. Soc. Rev 2011, 40, 149–162. [DOI] [PubMed] [Google Scholar]
- (9).Cho SY; Lipson EJ; Im HJ; Rowe SP; Gonzalez EM; Blackford A; Chirindel A; Pardoll DM; Topalian SL; Wahl RL Prediction of response to immune checkpoint inhibitor therapy using early-Time-Point (18)F-FDG PET/CT imaging in patients with advanced melanoma. J. Nucl. Med 2017, 58, 1421–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Aide N; Hicks RJ; Le Tourneau C; Lheureux S; Fanti S; Lopci E FDG PET/CT for assessing tumour response to immunotherapy: Report on the EANM symposium on immune modulation and recent review of the literature. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 238–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Sachpekidis C; Anwar H; Winkler J; Kopp-Schneider A; Larribere L; Haberkorn U; Hassel JC; Dimitrakopoulou-Strauss A The role of interim (18)F-FDG PET/CT in prediction of response to ipilimumab treatment in metastatic melanoma. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1289–1296. [DOI] [PubMed] [Google Scholar]
- (12).Anwar H; Sachpekidis C; Winkler J; Kopp-Schneider A; Haberkorn U; Hassel JC; Dimitrakopoulou-Strauss A Absolute number of new lesions on (18)F-FDG PET/CT is more predictive of clinical response than SUV changes in metastatic melanoma patients receiving ipilimumab. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 376–383. [DOI] [PubMed] [Google Scholar]
- (13).Wong ANM; McArthur GA; Hofman MS; Hicks RJ The Advantages and challenges of using FDG PET/CT for response assessment in melanoma in the era of targeted agents and immunotherapy. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 67–77. [DOI] [PubMed] [Google Scholar]
- (14).Rossi S; Toschi L; Castello A; Grizzi F; Mansi L; Lopci E Clinical characteristics of patient selection and imaging predictors of outcome in solid tumors treated with checkpoint-inhibitors. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 2310–2325. [DOI] [PubMed] [Google Scholar]
- (15).de Vries EGE; Kist de Ruijter L; Lub-de Hooge MN; Dierckx RA; Elias SG; Oosting SF Integrating molecular nuclear imaging in clinical research to improve anticancer therapy. Nat. Rev. Clin. Oncol 2019, 16, 241–255. [DOI] [PubMed] [Google Scholar]
- (16).Behr TM; Behe M; Wormann B Trastuzumab and breast cancer. N. Engl. J. Med 2001, 345, 995–998. [DOI] [PubMed] [Google Scholar]
- (17).Rahmim A; Zaidi H PET versus SPECT: strengths, limitations and challenges. Nucl. Med. Commun 2008, 29, 193–207. [DOI] [PubMed] [Google Scholar]
- (18).Knowles SM; Wu AM Advances in immuno-positron emission tomography: antibodies for molecular imaging in oncology. J. Clin. Oncol 2012, 30, 3884–3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Philpott GW; Schwarz SW; Anderson CJ; Dehdashti F; Connett JM; Zinn KR; Meares CF; Cutler PD; Welch MJ; Siegel BA RadioimmunoPET: detection of colorectal carcinoma with positron-emitting copper-64-labeled monoclonal antibody. J. Nucl. Med 1995, 36, 1818–1824. [PubMed] [Google Scholar]
- (20).Goldenberg DM; Nabi HA Breast cancer imaging with radiolabeled antibodies. Semin. Nucl. Med 1999, 29, 41–48. [DOI] [PubMed] [Google Scholar]
- (21).Bensch F; van der Veen EL; Lub-de Hooge MN; Jorritsma-Smit A; Boellaard R; Kok IC; Oosting SF; Schroder CP; Hiltermann TJN; van der Wekken AJ; et al. 89)Zratezolizumab imaging as a non-invasive approach to assess clinical response to PD-L1 blockade in cancer. Nat. Med 2018, 24, 1852–1858. [DOI] [PubMed] [Google Scholar]
- (22).Ribas A; Wolchok JD Cancer immunotherapy using checkpoint blockade. Science 2018, 359, 1350–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Carter PJ; Lazar GA Next generation antibody drugs: pursuit of the ‘high-hanging fruit. Nat. Rev. Drug Discovery 2018, 17, 197–223. [DOI] [PubMed] [Google Scholar]
- (24).Sehlin D; Syvanen S Engineered antibodies: new possibilities for brain PET? Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 2848–2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Kaplon H; Reichert JM Antibodies to watch in 2019. MAbs 2019, 11, 219–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Kaplon H; Muralidharan M; Schneider Z; Reichert JM Antibodies to watch in 2020. MAbs 2020, 12, 1703531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Bournazos S; Wang TT; Dahan R; Maamary J; Ravetch JV Signaling by antibodies: Recent Progress. Annu. Rev. Immunol 2017, 35, 285–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Hafeez U; Gan HK; Scott AM Monoclonal antibodies as immunomodulatory therapy against cancer and autoimmune diseases. Curr. Opin. Pharmacol 2018, 41, 114–121. [DOI] [PubMed] [Google Scholar]
- (29).Wittrup KD; Thurber GM; Schmidt MM; Rhoden JJ Practical theoretic guidance for the design of tumor-targeting agents. Methods Enzymol 2012, 503, 255–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Brinkmann U; Kontermann RE The making of bispecific antibodies. MAbs 2017, 9, 182–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Runcie K; Budman DR; John V; Seetharamu N Bi-specific and tri-specific antibodies- the next big thing in solid tumor therapeutics. Mol. Med 2018, 24, 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Luo H; Hong H; Yang SP; Cai W Design and applications of bispecific heterodimers: molecular imaging and beyond. Mol. Pharmaceutics 2014, 11, 1750–1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Ward ES; Zhou J; Ghetie V; Ober RJ Evidence to support the cellular mechanism involved in serum IgG homeostasis in humans. Int. Immunol 2003, 15, 187–195. [DOI] [PubMed] [Google Scholar]
- (34).Ober RJ; Martinez C; Vaccaro C; Zhou J; Ward ES Visualizing the site and dynamics of IgG salvage by the MHC class I-related receptor, FcRn. J. Immunol 2004, 172, 2021–2029. [DOI] [PubMed] [Google Scholar]
- (35).Suzuki T; Ishii-Watabe A; Tada M; Kobayashi T; Kanayasu-Toyoda T; Kawanishi T; Yamaguchi T Importance of neonatal FcR in regulating the serum half-life of therapeutic proteins containing the Fc domain of human IgG1: a comparative study of the affinity of monoclonal antibodies and Fc-fusion proteins to human neonatal FcR. J. Immunol 2010, 184, 1968–1976. [DOI] [PubMed] [Google Scholar]
- (36).Graff CP; Wittrup KD Theoretical analysis of antibody targeting of tumor spheroids: importance of dosage for penetration, and affinity for retention. Cancer Res 2003, 63, 1288–1296. [PubMed] [Google Scholar]
- (37).Rudnick SI; Lou J; Shaller CC; Tang Y; Klein-Szanto AJ; Weiner LM; Marks JD; Adams GP Influence of affinity and antigen internalization on the uptake and penetration of Anti-HER2 antibodies in solid tumors. Cancer Res 2011, 71, 2250–2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Bergstrom M The use of microdosing in the development of small organic and protein therapeutics. J. Nucl. Med 2017, 58, 1188–1195. [DOI] [PubMed] [Google Scholar]
- (39).Lohrmann C; O’Reilly EM; O’Donoghue JA; Pandit-Taskar N; Carrasquillo JA; Lyashchenko SK; Ruan S; Teng R; Scholz W; Maffuid PW; et al. Retooling a blood-based biomarker: phase I assessment of the high-affinity CA19–9 antibody HuMab-5B1 for immuno-PET imaging of pancreatic cancer. Clin. Cancer Res 2019, 25, 7014–7023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Bensch F; Smeenk MM; van Es SC; de Jong JR; Schroder CP; Oosting SF; Lub-de Hooge MN; Menke-van der Houven van Oordt CW; Brouwers AH; Boellaard R; et al. Comparative biodistribution analysis across four different (89)Zrmonoclonal antibody tracers-The first step towards an imaging warehouse. Theranostics 2018, 8, 4295–4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Dijkers EC; Oude Munnink TH; Kosterink JG; Brouwers AH; Jager PL; de Jong JR; van Dongen GA; Schroder CP; Lub-de Hooge MN; de Vries EG Biodistribution of 89Zr-trastuzumab and PET imaging of HER2-positive lesions in patients with metastatic breast cancer. Clin. Pharmacol. Ther 2010, 87, 586–592. [DOI] [PubMed] [Google Scholar]
- (42).Steinmeyer DE; McCormick EL The art of antibody process development. Drug Discovery Today 2008, 13, 613–618. [DOI] [PubMed] [Google Scholar]
- (43).Orcutt KD; Adams GP; Wu AM; Silva MD; Harwell C; Hoppin J; Matsumura M; Kotsuma M; Greenberg J; Scott AM; et al. Molecular simulation of receptor occupancy and tumor penetration of an antibody and smaller scaffolds: application to molecular Imaging. Mol. Imaging Biol 2017, 19, 656–664. [DOI] [PubMed] [Google Scholar]
- (44).Konning D; Zielonka S; Grzeschik J; Empting M; Valldorf B; Krah S; Schroter C; Sellmann C; Hock B; Kolmar H Camelid and shark single domain antibodies: structural features and therapeutic potential. Curr. Opin. Struct. Biol 2017, 45, 10–16. [DOI] [PubMed] [Google Scholar]
- (45).Yan J; Li G; Hu Y; Ou W; Wan Y Construction of a synthetic phage-displayed Nanobody library with CDR3 regions randomized by trinucleotide cassettes for diagnostic applications. J. Transl. Med 2014, 12, 343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Vincke C; Loris R; Saerens D; Martinez-Rodriguez S; Muyldermans S; Conrath K General strategy to humanize a camelid single-domain antibody and identification of a universal humanized nanobody scaffold. J. Biol. Chem 2009, 284, 3273–3284. [DOI] [PubMed] [Google Scholar]
- (47).Huang H-F; Zhu H; Li G-H; Xie Q; Yang X-T; Xu X-X; Tian X-B; Wan Y-K; Yang Z Construction of anti-hPD-L1 HCAb Nb6 and in situ (124)I labeling for noninvasive detection of PD-L1 expression in human bone sarcoma. Bioconjugate Chem 2019, 30, 2614–2623. [DOI] [PubMed] [Google Scholar]
- (48).Muyldermans S Nanobodies: natural single-domain antibodies. Annu. Rev. Biochem 2013, 82, 775–797. [DOI] [PubMed] [Google Scholar]
- (49).Ingram JR; Schmidt FI; Ploegh HL Exploiting nanobodies’ singular traits. Annu. Rev. Immunol 2018, 36, 695–715. [DOI] [PubMed] [Google Scholar]
- (50).Heukers R; De Groof TWM; Smit MJ Nanobodies detecting and modulating GPCRs outside in and inside out. Curr. Opin. Cell Biol 2019, 57, 115–122. [DOI] [PubMed] [Google Scholar]
- (51).Holliger P; Hudson PJ Engineered antibody fragments and the rise of single domains. Nat. Biotechnol 2005, 23, 1126–1136. [DOI] [PubMed] [Google Scholar]
- (52).Schumacher D; Helma J; Schneider AFL; Leonhardt H; Hackenberger CPR Nanobodies: chemical functionalization strategies and intracellular applications. Angew. Chem., Int. Ed 2018, 57, 2314–2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Tijink BM; Laeremans T; Budde M; Stigter-van Walsum M; Dreier T; de Haard HJ; Leemans CR; van Dongen GA Improved tumor targeting of anti-epidermal growth factor receptor Nanobodies through albumin binding: taking advantage of modular Nanobody technology. Mol. Cancer Ther 2008, 7, 2288–2297. [DOI] [PubMed] [Google Scholar]
- (54).Vugmeyster Y; Entrican CA; Joyce AP; Lawrence-Henderson RF; Leary BA; Mahoney CS; Patel HK; Raso SW; Olland SH; Hegen M; et al. Pharmacokinetic, biodistribution, and biophysical profiles of TNF nanobodies conjugated to linear or branched poly(ethylene glycol). Bioconjugate Chem 2012, 23, 1452–1462. [DOI] [PubMed] [Google Scholar]
- (55).Rashidian M; Wang L; Edens JG; Jacobsen JT; Hossain I; Wang Q; Victora GD; Vasdev N; Ploegh H; Liang SH Enzyme-mediated modification of single-domain antibodies for imaging modalities with different characteristics. Angew. Chem., Int. Ed 2016, 55, 528–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Chakravarty R; Goel S; Cai W Nanobody: the “magic bullet” for molecular imaging? Theranostics 2014, 4, 386–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Pruszynski M; Koumarianou E; Vaidyanathan G; Revets H; Devoogdt N; Lahoutte T; Lyerly HK; Zalutsky MR Improved tumor targeting of anti-HER2 nanobody through N-succinimidyl 4-guanidinomethyl-3-iodobenzoate radiolabeling. J. Nucl. Med 2014, 55, 650–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).De Vos J; Devoogdt N; Lahoutte T; Muyldermans S Camelid single-domain antibody-fragment engineering for (pre)-clinical in vivo molecular imaging applications: adjusting the bullet to its target. Expert Opin. Biol. Ther 2013, 13, 1149–1160. [DOI] [PubMed] [Google Scholar]
- (59).Crauwels M; Massa S; Martin C; Betti C; Ballet S; Devoogdt N; Xavier C; Muyldermans S Site-specific radioactive labeling of nanobodies. Methods Mol. Biol 2018, 1827, 505–540. [DOI] [PubMed] [Google Scholar]
- (60).Behr TM; Goldenberg DM; Becker W Reducing the renal uptake of radiolabeled antibody fragments and peptides for diagnosis and therapy: present status, future prospects and limitations. Eur. J. Nucl. Med. Mol. Imaging 1998, 25, 201–212. [DOI] [PubMed] [Google Scholar]
- (61).Vegt E; de Jong M; Wetzels JF; Masereeuw R; Melis M; Oyen WJ; Gotthardt M; Boerman OC Renal toxicity of radiolabeled peptides and antibody fragments: mechanisms, impact on radionuclide therapy, and strategies for prevention. J. Nucl. Med 2010, 51, 1049–1058. [DOI] [PubMed] [Google Scholar]
- (62).Tchouate Gainkam LO; Caveliers V; Devoogdt N; Vanhove C; Xavier C; Boerman O; Muyldermans S; Bossuyt A; Lahoutte T Localization, mechanism and reduction of renal retention of technetium-99m labeled epidermal growth factor receptor-specific nanobody in mice. Contrast Media Mol. Imaging 2011, 6, 85–92. [DOI] [PubMed] [Google Scholar]
- (63).Xavier C; Vaneycken I; D’Huyvetter M; Heemskerk J; Keyaerts M; Vincke C; Devoogdt N; Muyldermans S; Lahoutte T; Caveliers V Synthesis, preclinical validation, dosimetry, and toxicity of 68Ga-NOTA-anti-HER2 Nanobodies for iPET imaging of HER2 receptor expression in cancer. J. Nucl. Med 2013, 54, 776–784. [DOI] [PubMed] [Google Scholar]
- (64).Rashidian M; Ingram JR; Dougan M; Dongre A; Whang KA; LeGall C; Cragnolini JJ; Bierie B; Gostissa M; Gorman J; et al. Predicting the response to CTLA-4 blockade by longitudinal noninvasive monitoring of CD8 T cells. J. Exp. Med 2017, 214, 2243–2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (65).Vaneycken I; D’huyvetter M; Hernot S; De Vos J; Xavier C; Devoogdt N; Caveliers V; Lahoutte T Immuno-imaging using nanobodies. Curr. Opin. Biotechnol 2011, 22, 877–881. [DOI] [PubMed] [Google Scholar]
- (66).Debie P; Devoogdt N; Hernot S Targeted nanobody-based molecular tracers for nuclear imaging and image-guided surgery. Antibodies 2019, 8, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (67).D’Huyvetter M; Vincke C; Xavier C; Aerts A; Impens N; Baatout S; De Raeve H; Muyldermans S; Caveliers V; Devoogdt N; et al. Targeted radionuclide therapy with A 177Lu-labeled anti-HER2 nanobody. Theranostics 2014, 4, 708–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (68).Krasniqi A; D’Huyvetter M; Xavier C; Van der Jeught K; Muyldermans S; Van Der Heyden J; Lahoutte T; Tavernier J; Devoogdt N Theranostic radiolabeled anti-CD20 sdAb for targeted radionuclide therapy of non-Hodgkin lymphoma. Mol. Cancer Ther 2017, 16, 2828–2839. [DOI] [PubMed] [Google Scholar]
- (69).Pruszynski M; D’Huyvetter M; Bruchertseifer F; Morgenstern A; Lahoutte T Evaluation of an anti-HER2 nanobody labeled with (225)Ac for targeted alpha-particle therapy of cancer. Mol. Pharmaceutics 2018, 15, 1457–1466. [DOI] [PubMed] [Google Scholar]
- (70).Heukers R; van Bergen en Henegouwen PM; Oliveira S Nanobody-photosensitizer conjugates for targeted photodynamic therapy. Nanomedicine 2014, 10, 1441–1451. [DOI] [PubMed] [Google Scholar]
- (71).van Driel P; Boonstra MC; Slooter MD; Heukers R; Stammes MA; Snoeks TJA; de Bruijn HS; van Diest PJ; Vahrmeijer AL; van Bergen En Henegouwen PMP; et al. EGFR targeted nanobody-photosensitizer conjugates for photodynamic therapy in a pre-clinical model of head and neck cancer. J. Controlled Release 2016, 229, 93–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (72).Kenanova V; Wu AM Tailoring antibodies for radionuclide delivery. Expert Opin. Drug Delivery 2006, 3, 53–70. [DOI] [PubMed] [Google Scholar]
- (73).Long NE; Sullivan BJ; Ding H; Doll S; Ryan MA; Hitchcock CL; Martin EW Jr.; Kumar K; Tweedle MF; Magliery TJ Linker engineering in anti-TAG-72 antibody fragments optimizes biophysical properties, serum half-life, and high-specificity tumor imaging. J. Biol. Chem 2018, 293, 9030–9040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (74).Olafsen T; Cheung CW; Yazaki PJ; Li L; Sundaresan G; Gambhir SS; Sherman MA; Williams LE; Shively JE; Raubitschek AA; et al. Covalent disulfide-linked anti-CEA diabody allows site-specific conjugation and radiolabeling for tumor targeting applications. Protein Eng., Des. Sel 2004, 17, 21–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (75).Hu S; Shively L; Raubitschek A; Sherman M; Williams LE; Wong JY; Shively JE; Wu AM Minibody: A novel engineered anti-carcinoembryonic antigen antibody fragment (single-chain Fv-CH3) which exhibits rapid, high-level targeting of xenografts. Cancer Res 1996, 56, 3055–3061. [PubMed] [Google Scholar]
- (76).Wu AM Engineered antibodies for molecular imaging of cancer. Methods 2014, 65, 139–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (77).Eder M; Knackmuss S; Le Gall F; Reusch U; Rybin V; Little M; Haberkorn U; Mier W; Eisenhut M 68Ga-labelled recombinant antibody variants for immuno-PET imaging of solid tumours. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 1397–1407. [DOI] [PubMed] [Google Scholar]
- (78).Rios X; Compte M; Gomez-Vallejo V; Cossio U; Baz Z; Morcillo MA; Ramos-Cabrer P; Alvarez-Vallina L; Llop J Immuno-PET imaging and pharmacokinetics of an anti-CEA scFv-based trimerbody and its monomeric counterpart in human gastric carcinoma-bearing mice. Mol. Pharmaceutics 2019, 16, 1025–1035. [DOI] [PubMed] [Google Scholar]
- (79).Banta S; Dooley K; Shur O Replacing antibodies: engineering new binding proteins. Annu. Rev. Biomed. Eng 2013, 15, 93–113. [DOI] [PubMed] [Google Scholar]
- (80).Stahl S; Graslund T; Eriksson Karlstrom A; Frejd FY; Nygren PA; Lofblom J Affibody molecules in biotechnological and medical applications. Trends Biotechnol 2017, 35, 691–712. [DOI] [PubMed] [Google Scholar]
- (81).Kramer-Marek G; Kiesewetter DO; Capala J Changes in HER2 expression in breast cancer xenografts after therapy can be quantified using PET and (18)F-labeled affibody molecules. J. Nucl. Med 2009, 50, 1131–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (82).Orlova A; Wallberg H; Stone-Elander S; Tolmachev V On the selection of a tracer for PET imaging of HER2-expressing tumors: direct comparison of a 124I-labeled affibody molecule and trastuzumab in a murine xenograft model. J. Nucl. Med 2009, 50, 417–425. [DOI] [PubMed] [Google Scholar]
- (83).Lipovsek D Adnectins: engineered target-binding protein therapeutics. Protein Eng., Des. Sel 2011, 24, 3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (84).Hackel BJ; Kimura RH; Gambhir SS Use of (64)Cu-labeled fibronectin domain with EGFR-overexpressing tumor xenograft: molecular imaging. Radiology 2012, 263, 179–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (85).Kimura RH; Cheng Z; Gambhir SS; Cochran JR Engineered knottin peptides: a new class of agents for imaging integrin expression in living subjects. Cancer Res 2009, 69, 2435–2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (86).Kintzing JR; Cochran JR Engineered knottin peptides as diagnostics, therapeutics, and drug delivery vehicles. Curr. Opin. Chem. Biol 2016, 34, 143–150. [DOI] [PubMed] [Google Scholar]
- (87).Terwisscha van Scheltinga AG; Lub-de Hooge MN; Hinner MJ; Verheijen RB; Allersdorfer A; Hulsmeyer M; Nagengast WB; Schroder CP; Kosterink JG; de Vries EG; et al. In vivo visualization of MET tumor expression and anticalin biodistribution with the MET-specific anticalin 89Zr-PRS-110 PET tracer. J. Nucl. Med 2014, 55, 665–671. [DOI] [PubMed] [Google Scholar]
- (88).Schiefner A; Skerra A The menagerie of human lipocalins: a natural protein scaffold for molecular recognition of physiological compounds. Acc. Chem. Res 2015, 48, 976–985. [DOI] [PubMed] [Google Scholar]
- (89).Krasniqi A; D’Huyvetter M; Devoogdt N; Frejd FY; Sorensen J; Orlova A; Keyaerts M; Tolmachev V Same-day imaging using small proteins: Clinical Experience and Translational Prospects in Oncology. J. Nucl. Med 2018, 59, 885–891. [DOI] [PubMed] [Google Scholar]
- (90).Tsai SW; Li L; Williams LE; Anderson AL; Raubitschek AA; Shively JE Metabolism and renal clearance of 111In-labeled DOTA-conjugated antibody fragments. Bioconjugate Chem 2001, 12, 264–270. [DOI] [PubMed] [Google Scholar]
- (91).Yang K; Basu A; Wang M; Chintala R; Hsieh MC; Liu S; Hua J; Zhang Z; Zhou J; Li M; et al. Tailoring structure-function and pharmacokinetic properties of single-chain Fv proteins by site-specific PEGylation. Protein Eng., Des. Sel 2003, 16, 761–770. [DOI] [PubMed] [Google Scholar]
- (92).Kontermann RE Strategies for extended serum half-life of protein therapeutics. Curr. Opin. Biotechnol 2011, 22, 868–876. [DOI] [PubMed] [Google Scholar]
- (93).Klinger M; Benjamin J; Kischel R; Stienen S; Zugmaier G Harnessing T cells to fight cancer with BiTE(R) antibody constructs–past developments and future directions. Immunol. Rev 2016, 270, 193–208. [DOI] [PubMed] [Google Scholar]
- (94).Yuraszeck T; Kasichayanula S; Benjamin JE Translation and clinical development of bispecific T-cell engaging antibodies for cancer treatment. Clin. Pharmacol. Ther 2017, 101, 634–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (95).Krishnamurthy A; Jimeno A Bispecific antibodies for cancer therapy: A review. Pharmacol. Ther 2018, 185, 122–134. [DOI] [PubMed] [Google Scholar]
- (96).Topp MS; Gokbuget N; Stein AS; Zugmaier G; O’Brien S; Bargou RC; Dombret H; Fielding AK; Heffner L; Larson RA; et al. Safety and activity of blinatumomab for adult patients with relapsed or refractory B-precursor acute lymphoblastic leukaemia: a multicentre, single-arm, phase 2 study. Lancet Oncol 2015, 16, 57–66. [DOI] [PubMed] [Google Scholar]
- (97).Warnders FJ; Waaijer SJ; Pool M; Lub-de Hooge MN; Friedrich M; Terwisscha van Scheltinga AG; Deegen P; Stienen SK; Pieslor PC; Cheung HK; et al. Biodistribution and PET imaging of labeled bispecific T Cell-engaging antibody targeting EpCAM. J. Nucl. Med 2016, 57, 812–817. [DOI] [PubMed] [Google Scholar]
- (98).Mandikian D; Takahashi N; Lo AA; Li J; Eastham-Anderson J; Slaga D; Ho J; Hristopoulos M; Clark R; Totpal K; et al. Relative target affinities of T-cell-dependent bispecific antibodies determine biodistribution in a solid tumor mouse model. Mol. Cancer Ther 2018, 17, 776–785. [DOI] [PubMed] [Google Scholar]
- (99).Zhou Y; Baidoo KE; Brechbiel MW Mapping biological behaviors by application of longer-lived positron emitting radio-nuclides. Adv. Drug Delivery Rev 2013, 65, 1098–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (100).Boros E; Packard AB Radioactive transition metals for imaging and therapy. Chem. Rev 2019, 119, 870–901. [DOI] [PubMed] [Google Scholar]
- (101).Price EW; Orvig C Matching chelators to radiometals for radiopharmaceuticals. Chem. Soc. Rev 2014, 43, 260–290. [DOI] [PubMed] [Google Scholar]
- (102).Kostelnik TI; Orvig C Radioactive main group and rare earth metals for imaging and therapy. Chem. Rev 2019, 119, 902–956. [DOI] [PubMed] [Google Scholar]
- (103).Aluicio-Sarduy E; Ellison PA; Barnhart TE; Cai W; Nickles RJ; Engle JW PET radiometals for antibody labeling. J. Labelled Compd. Radiopharm 2018, 61, 636–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (104).Oehlke E; Hoehr C; Hou X; Hanemaayer V; Zeisler S; Adam MJ; Ruth TJ; Celler A; Buckley K; Benard F; et al. Production of Y-86 and other radiometals for research purposes using a solution target system. Nucl. Med. Biol 2015, 42, 842–849. [DOI] [PubMed] [Google Scholar]
- (105).Cutler CS; Hennkens HM; Sisay N; Huclier-Markai S; Jurisson SS Radiometals for combined imaging and therapy. Chem. Rev 2013, 113, 858–883. [DOI] [PubMed] [Google Scholar]
- (106).Wadas TJ; Wong EH; Weisman GR; Anderson CJ Coordinating radiometals of copper, gallium, indium, yttrium, and zirconium for PET and SPECT imaging of disease. Chem. Rev 2010, 110, 2858–2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (107).Morris O; Fairclough M; Grigg J; Prenant C; McMahon A A review of approaches to (18) F radiolabelling affinity peptides and proteins. J. Labelled Compd. Radiopharm 2019, 62, 4–23. [DOI] [PubMed] [Google Scholar]
- (108).Zhang Y; Hong H; Cai W PET tracers based on Zirconium-89. Curr. Radiopharm 2011, 4, 131–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (109).Dilworth JR; Pascu SI The chemistry of PET imaging with zirconium-89. Chem. Soc. Rev 2018, 47, 2554–2571. [DOI] [PubMed] [Google Scholar]
- (110).La MT; Tran VH; Kim HK Progress of coordination and utilization of Zirconium-89 for positron emission tomography (PET) studies. Nucl. Med. Mol. Imaging 2019, 53, 115–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (111).Pandey MK; Bansal A; Engelbrecht HP; Byrne JF; Packard AB; DeGrado TR Improved production and processing of (8)(9)Zr using a solution target. Nucl. Med. Biol 2016, 43, 97–100. [DOI] [PubMed] [Google Scholar]
- (112).Dias GM; Ramogida CF; Rousseau J; Zacchia NA; Hoehr C; Schaffer P; Lin KS; Benard F 89)Zr for antibody labeling and in vivo studies - a comparison between liquid and solid target production. Nucl. Med. Biol 2018, 58, 1–7. [DOI] [PubMed] [Google Scholar]
- (113).Graves SA; Kutyreff C; Barrett KE; Hernandez R; Ellison PA; Happel S; Aluicio-Sarduy E; Barnhart TE; Nickles RJ; Engle JW Evaluation of a chloride-based (89)Zr isolation strategy using a tributyl phosphate (TBP)-functionalized extraction resin. Nucl. Med. Biol 2018, 64–65, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (114).Pandya DN; Bhatt NB; Almaguel F; Rideout-Danner S; Gage HD; Solingapuram Sai KK; Wadas TJ 89)Zr-chloride can be used for immuno-PET radiochemistry without loss of antigen reactivity in vivo. J. Nucl. Med 2019, 60, 696–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (115).Holland JP; Sheh Y; Lewis JS Standardized methods for the production of high specific-activity zirconium-89. Nucl. Med. Biol 2009, 36, 729–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (116).Verel I; Visser GWM; Boellaard R; Stigter-van Walsum M; Snow GB; van Dongen GAMS 89Zr immuno-PET: comprehensive procedures for the production of 89Zr-labeled monoclonal antibodies. J. Nucl. Med 2003, 44, 1271–1281. [PubMed] [Google Scholar]
- (117).Verel I; Visser GW; Boerman OC; van Eerd JE; Finn R; Boellaard R; Vosjan MJ; Stigter-van Walsum M; Snow GB; van Dongen GA Long-lived positron emitters zirconium-89 and iodine-124 for scouting of therapeutic radioimmunoconjugates with PET. Cancer Biother.Radiopharm 2003, 18, 655–661. [DOI] [PubMed] [Google Scholar]
- (118).Borjesson PK; Jauw YW; Boellaard R; de Bree R; Comans EF; Roos JC; Castelijns JA; Vosjan MJ; Kummer JA; Leemans CR; et al. Performance of immuno-positron emission tomography with zirconium-89-labeled chimeric monoclonal antibody U36 in the detection of lymph node metastases in head and neck cancer patients. Clin. Cancer Res 2006, 12, 2133–2140. [DOI] [PubMed] [Google Scholar]
- (119).Perk LR; Vosjan MJ; Visser GW; Budde M; Jurek P; Kiefer GE; van Dongen GA p-Isothiocyanatobenzyl-desferrioxamine: a new bifunctional chelate for facile radiolabeling of monoclonal antibodies with zirconium-89 for immuno-PET imaging. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 250–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (120).Vosjan MJ; Perk LR; Visser GW; Budde M; Jurek P; Kiefer GE; van Dongen GA Conjugation and radiolabeling of monoclonal antibodies with zirconium-89 for PET imaging using the bifunctional chelate p-isothiocyanatobenzyl-desferrioxamine. Nat. Protoc 2010, 5, 739–743. [DOI] [PubMed] [Google Scholar]
- (121).Holland JP; Divilov V; Bander NH; Smith-Jones PM; Larson SM; Lewis JS 89Zr-DFO-J591 for immunoPET of prostate-specific membrane antigen expression in vivo. J. Nucl. Med 2010, 51, 1293–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (122).Wei W; Jiang D; Ehlerding EB; Barnhart TE; Yang Y; Engle JW; Luo QY; Huang P; Cai W CD146-targeted multimodal image-guided photoimmunotherapy of melanoma. Adv. Sci. (Weinh) 2019, 6, 1801237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (123).Bailly C; Gouard S; Guerard F; Chalopin B; Carlier T; Faivre-Chauvet A; Remaud-Le Saec P; Bourgeois M; Chouin N; Rbah-Vidal L What is the best radionuclide for immuno-PET of multiple myeloma? a comparison study between (89)Zr- and (64)Cu-labeled anti-CD138 in a preclinical syngeneic model. Int. J. Mol. Sci 2019, 20, 2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (124).Patra M; Bauman A; Mari C; Fischer CA; Blacque O; Haussinger D; Gasser G; Mindt TL An octadentate bifunctional chelating agent for the development of stable zirconium-89 based molecular imaging probes. Chem. Commun. (Cambridge, U. K.) 2014, 50, 11523–11525. [DOI] [PubMed] [Google Scholar]
- (125).Vugts DJ; Klaver C; Sewing C; Poot AJ; Adamzek K; Huegli S; Mari C; Visser GWM; Valverde IE; Gasser G; et al. Comparison of the octadentate bifunctional chelator DFO*-pPhe-NCS and the clinically used hexadentate bifunctional chelator DFO-pPhe-NCS for (89)Zr-immuno-PET. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 286–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (126).Raave R; Sandker G; Adumeau P; Jacobsen CB; Mangin F; Meyer M; Moreau M; Bernhard C; Da Costa L; Dubois A; et al. Direct comparison of the in vitro and in vivo stability of DFO, DFO* and DFOcyclo* for (89)Zr-immunoPET. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 1966–1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (127).Adams CJ; Wilson JJ; Boros E Multifunctional desferrichrome analogues as versatile (89)Zr(IV) chelators for immunoPET probe development. Mol. Pharmaceutics 2017, 14, 2831–2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (128).Price EW; Zeglis BM; Lewis JS; Adam MJ; Orvig C H6phospa-trastuzumab: bifunctional methylenephosphonate-based chelator with 89Zr, 111In and 177Lu. Dalton Trans 2014, 43, 119–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (129).Deri MA; Ponnala S; Zeglis BM; Pohl G; Dannenberg JJ; Lewis JS; Francesconi LC Alternative chelator for (8)(9)Zr radiopharmaceuticals: radiolabeling and evaluation of 3,4,3-(LI-1,2-HOPO). J. Med. Chem 2014, 57, 4849–4860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (130).Deri MA; Ponnala S; Kozlowski P; Burton-Pye BP; Cicek HT; Hu C; Lewis JS; Francesconi LC p-SCN-Bn-HOPO: A superior bifunctional chelator for (89)Zr immunoPET. Bioconjugate Chem 2015, 26, 2579–2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (131).Tinianow JN; Pandya DN; Pailloux SL; Ogasawara A; Vanderbilt AN; Gill HS; Williams SP; Wadas TJ; Magda D; Marik J Evaluation of a 3-hydroxypyridin-2-one (2,3-HOPO) based macrocyclic chelator for (89)Zr(4+) and its use for immunoPET imaging of HER2 positive model of ovarian carcinoma in mice. Theranostics 2016, 6, 511–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (132).Guérard F; Lee Y-S; Tripier R; Szajek LP; Deschamps JR; Brechbiel MW Investigation of Zr(iv) and89Zr(iv) complexation with hydroxamates: progress towards designing a better chelator than desferrioxamine B for immuno-PET imaging. Chem. Commun 2013, 49, 1002–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (133).Guerard F; Lee YS; Brechbiel MW Rational design, synthesis, and evaluation of tetrahydroxamic acid chelators for stable complexation of zirconium(IV). Chem. - Eur. J 2014, 20, 5584–5591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (134).Boros E; Holland JP; Kenton N; Rotile N; Caravan P Macrocycle-based hydroxamate ligands for complexation and immunoconjugation of (89)Zirconium for positron emission tomography (PET) imaging. ChemPlusChem 2016, 81, 274–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (135).Allott L; Da Pieve C; Meyers J; Spinks T; Ciobota D; Kramer-Marek G; Smith G Evaluation of DFO-HOPO as an octadentate chelator for zirconium-89. Chem. Commun 2017, 53, 8529–8532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (136).Zhai C; Summer D; Rangger C; Franssen GM; Laverman P; Haas H; Petrik M; Haubner R; Decristoforo C Novel bifunctional cyclic chelator for (89)Zr labeling-radiolabeling and targeting properties of RGD Conjugates. Mol. Pharmaceutics 2015, 12, 2142–2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (137).Rudd SE; Roselt P; Cullinane C; Hicks RJ; Donnelly PSJCC A desferrioxamine B squaramide ester for the incorporation of zirconium-89 into antibodies. Chem. Commun 2016, 52, 11889–11892. [DOI] [PubMed] [Google Scholar]
- (138).Summer D; Garousi J; Oroujeni M; Mitran B; Andersson KG; Vorobyeva A; Lofblom J; Orlova A; Tolmachev V; Decristoforo C Cyclic versus noncyclic chelating scaffold for (89)Zr-Labeled ZEGFR:2377 affibody bioconjugates targeting epidermal growth factor receptor overexpression. Mol. Pharmaceutics 2018, 15, 175–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (139).Zhai C; He S; Ye Y; Rangger C; Kaeopookum P; Summer D; Haas H; Kremser L; Lindner H; Foster J; et al. Rational design, synthesis and preliminary evaluation of novel fusarinine C-based chelators for radiolabeling with Zirconium-89. Biomolecules 2019, 9, 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (140).Pandya DN; Bhatt N; Yuan H; Day CS; Ehrmann BM; Wright M; Bierbach U; Wadas TJ Zirconium tetraazamacro-cycle complexes display extraordinary stability and provide a new strategy for zirconium-89-based radiopharmaceutical development. Chem. Sci 2017, 8, 2309–2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (141).McCarthy DW; Shefer RE; Klinkowstein RE; Bass LA; Margeneau WH; Cutler CS; Anderson CJ; Welch MJ Efficient production of high specific activity 64Cu using a biomedical cyclotron. Nucl. Med. Biol 1997, 24, 35–43. [DOI] [PubMed] [Google Scholar]
- (142).Alves F; Alves VHP; Do Carmo SJC; Neves ACB; Silva M; Abrunhosa AJ Production of copper-64 and gallium-68 with a medical cyclotron using liquid targets. Mod. Phys. Lett. A 2017, 32, 1740013. [Google Scholar]
- (143).Smith NA; Bowers DL; Ehst DA The production, separation, and use of 67Cu for radioimmunotherapy: a review. Appl. Radiat. Isot 2012, 70, 2377–2383. [DOI] [PubMed] [Google Scholar]
- (144).Cai W; Wu Y; Chen K; Cao Q; Tice DA; Chen X In vitro and in vivo characterization of 64Cu-labeled Abegrin, a humanized monoclonal antibody against integrin alpha v beta 3. Cancer Res 2006, 66, 9673–9681. [DOI] [PubMed] [Google Scholar]
- (145).Cai W; Chen K; He L; Cao Q; Koong A; Chen X Quantitative PET of EGFR expression in xenograft-bearing mice using 64Cu-labeled cetuximab, a chimeric anti-EGFR monoclonal antibody. Eur. J. Nucl. Med. Mol. Imaging 2007, 34, 850–858. [DOI] [PubMed] [Google Scholar]
- (146).Ferreira CL; Yapp DT; Lamsa E; Gleave M; Bensimon C; Jurek P; Kiefer GE Evaluation of novel bifunctional chelates for the development of Cu-64-based radiopharmaceuticals. Nucl. Med. Biol 2008, 35, 875–882. [DOI] [PubMed] [Google Scholar]
- (147).Cooper MS; Ma MT; Sunassee K; Shaw KP; Williams JD; Paul RL; Donnelly PS; Blower PJ Comparison of (64)Cu-complexing bifunctional chelators for radioimmunoconjugation: labeling efficiency, specific activity, and in vitro/in vivo stability. Bioconjugate Chem 2012, 23, 1029–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (148).Ghosh SC; Pinkston KL; Robinson H; Harvey BR; Wilganowski N; Gore K; Sevick-Muraca EM; Azhdarinia A Comparison of DOTA and NODAGA as chelators for (64)Cu-labeled immunoconjugates. Nucl. Med. Biol 2015, 42, 177–183. [DOI] [PubMed] [Google Scholar]
- (149).Moreau M; Poty S; Vrigneaud JM; Walker P; Guillemin M; Raguin O; Oudot A; Bernhard C; Goze C; Boschetti F; et al. MANOTA: a promising bifunctional chelating agent for copper-64 immunoPET. Dalton Trans 2017, 46, 14659–14668. [DOI] [PubMed] [Google Scholar]
- (150).Wong EH; Weisman GR; Hill DC; Reed DP; Rogers ME; Condon JS; Fagan MA; Calabrese JC; Lam K-C; Guzei IA; et al. Synthesis and characterization of cross-bridged cyclams and pendant-armed derivatives and structural studies of their copper(II) complexes. J. Am. Chem. Soc 2000, 122, 10561–10572. [Google Scholar]
- (151).Wadas TJ; Anderson CJ Radiolabeling of TETA- and CBTE2A-conjugated peptides with copper-64. Nat. Protoc 2006, 1, 3062–3068. [DOI] [PubMed] [Google Scholar]
- (152).Ferdani R; Stigers DJ; Fiamengo AL; Wei L; Li BT; Golen JA; Rheingold AL; Weisman GR; Wong EH; Anderson CJ Synthesis, Cu(II) complexation, 64Cu-labeling and biological evaluation of cross-bridged cyclam chelators with phosphonate pendant arms. Dalton Trans 2012, 41, 1938–1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (153).Guo Y; Ferdani R; Anderson CJ Preparation and biological evaluation of (64)Cu labeled Tyr(3)-octreotate using a phosphonic acid-based cross-bridged macrocyclic chelator. Bioconju-gate Chem 2012, 23, 1470–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (154).Stigers DJ; Ferdani R; Weisman GR; Wong EH; Anderson CJ; Golen JA; Moore C; Rheingold AL A new phosphonate pendant-armed cross-bridged tetraamine chelator accelerates copper(ii) binding for radiopharmaceutical applications. Dalton Trans 2010, 39, 1699–1701. [DOI] [PubMed] [Google Scholar]
- (155).Zeng D; Guo Y; White AG; Cai Z; Modi J; Ferdani R; Anderson CJ Comparison of conjugation strategies of cross-bridged macrocyclic chelators with cetuximab for copper-64 radiolabeling and PET imaging of EGFR in colorectal tumor-bearing mice. Mol. Pharmaceutics 2014, 11, 3980–3987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (156).Ren Q; Mohri K; Warashina S; Wada Y; Watanabe Y; Mukai H Improved immuno-PET imaging of HER2-positive tumors in mice: urokinase injection-triggered clearance enhancement of(64)Cu-trastuzumab. Mol. Pharmaceutics 2019, 16, 1065–1073. [DOI] [PubMed] [Google Scholar]
- (157).David T; Kubicek V; Gutten O; Lubal P; Kotek J; Pietzsch HJ; Rulisek L; Hermann P Cyclam derivatives with a bis(phosphinate) or a phosphinato-phosphonate pendant arm: ligands for fast and efficient copper(II) complexation for nuclear medical applications. Inorg. Chem 2015, 54, 11751–11766. [DOI] [PubMed] [Google Scholar]
- (158).David T; Hlinova V; Kubicek V; Bergmann R; Striese F; Berndt N; Szollosi D; Kovacs T; Mathe D; Bachmann M; et al. Improved conjugation, 64-Cu radiolabeling, in vivo stability, and imaging using nonprotected bifunctional macrocyclic ligands: bis-(Phosphinate) cyclam (BPC) chelators. J. Med. Chem 2018, 61, 8774–8796. [DOI] [PubMed] [Google Scholar]
- (159).Di Bartolo N; Sargeson AM; Smith SV New 64Cu PET imaging agents for personalised medicine and drug development using the hexa-aza cage, SarAr. Org. Biomol. Chem 2006, 4, 3350–3357. [DOI] [PubMed] [Google Scholar]
- (160).Voss SD; Smith SV; DiBartolo N; McIntosh LJ; Cyr EM; Bonab AA; Dearling JL; Carter EA; Fischman AJ; Treves ST; et al. Positron emission tomography (PET) imaging of neuroblastoma and melanoma with 64Cu-SarAr immunoconjugates. Proc. Natl. Acad. Sci. U. S. A 2007, 104, 17489–17493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (161).Dearling JL; Voss SD; Dunning P; Snay E; Fahey F; Smith SV; Huston JS; Meares CF; Treves ST; Packard AB Imaging cancer using PET–the effect of the bifunctional chelator on the biodistribution of a (64)Cu-labeled antibody. Nucl. Med. Biol 2011, 38, 29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (162).Jailkhani N; Ingram JR; Rashidian M; Rickelt S; Tian C; Mak H; Jiang Z; Ploegh HL; Hynes RO Noninvasive imaging of tumor progression, metastasis, and fibrosis using a nanobody targeting the extracellular matrix. Proc. Natl. Acad. Sci. U.S. A 2019, 116, 14181–14190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (163).Yoo J; Tang L; Perkins TA; Rowland DJ; Laforest R; Lewis JS; Welch MJ Preparation of high specific activity (86)Y using a small biomedical cyclotron. Nucl. Med. Biol 2005, 32, 891–897. [DOI] [PubMed] [Google Scholar]
- (164).Avila-Rodriguez MA; Nye JA; Nickles RJ Production and separation of non-carrier-added 86Y from enriched 86Sr targets. Appl. Radiat. Isot 2008, 66, 9–13. [DOI] [PubMed] [Google Scholar]
- (165).Qaim SM; Spahn I Development of novel radionuclides for medical applications. J. Labelled Compd. Radiopharm 2018, 61, 126–140. [DOI] [PubMed] [Google Scholar]
- (166).Aluicio-Sarduy E; Hernandez R; Valdovinos HF; Kutyreff CJ; Ellison PA; Barnhart TE; Nickles RJ; Engle JW Simplified and automatable radiochemical separation strategy for the production of radiopharmaceutical quality (86)Y using single column extraction chromatography. Appl. Radiat. Isot 2018, 142, 28–31. [DOI] [PubMed] [Google Scholar]
- (167).Larson SM; Carrasquillo JA; Cheung NK; Press OW Radioimmunotherapy of human tumours. Nat. Rev. Cancer 2015, 15, 347–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (168).Hernandez R; Walker KL; Grudzinski JJ; Aluicio-Sarduy E; Patel R; Zahm CD; Pinchuk AN; Massey CF; Bitton AN; Brown RJ; et al. (90)Y-NM600 targeted radionuclide therapy induces immunologic memory in syngeneic models of T-cell Non-Hodgkin’s Lymphoma. Commun. Biol 2019, 2, 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (169).Rosch F; Herzog H; Qaim SM The beginning and development of the theranostic approach in nuclear medicine, as exemplified by the radionuclide pair (86)Y and (90)Y. Pharmaceuticals 2017, 10, 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (170).Herzog H; Rosch F; Stocklin G; Lueders C; Qaim SM; Feinendegen LE Measurement of pharmacokinetics of yttrium-86 radiopharmaceuticals with PET and radiation dose calculation of analogous yttrium-90 radiotherapeutics. J. Nucl. Med 1993, 34, 2222–2226. [PubMed] [Google Scholar]
- (171).Ehlerding EB; Ferreira CA; Aluicio-Sarduy E; Jiang D; Lee HJ; Theuer CP; Engle JW; Cai W 86/90Y-based theranostics targeting angiogenesis in a murine breast cancer model. Mol. Pharmaceutics 2018, 15, 2606–2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (172).Ikotun OF; Lapi SE The rise of metal radionuclides in medical imaging: copper-64, zirconium-89 and yttrium-86. Future Med. Chem 2011, 3, 599–621. [DOI] [PubMed] [Google Scholar]
- (173).Nayak TK; Brechbiel MW 86Y based PET radiopharmaceuticals: radiochemistry and biological applications. Med. Chem 2011, 7, 380–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (174).Roselli M; Schlom J; Gansow OA; Raubitschek A; Mirzadeh S; Brechbiel MW; Colcher D Comparative biodistributions of yttrium- and indium-labeled monoclonal antibody B72.3 in athymic mice bearing human colon carcinoma xenografts. J. Nucl. Med 1989, 30, 672–682. [PubMed] [Google Scholar]
- (175).Camera L; Kinuya S; Garmestani K; Brechbiel MW; Wu C; Pai LH; McMurry TJ; Gansow OA; Pastan I; Paik CH; et al. Comparative biodistribution of indium- and yttrium-labeled B3 monoclonal antibody conjugated to either 2-(p-SCN-Bz)-6-methyl-DTPA (1B4M-DTPA) or 2-(p-SCN-Bz)-1,4,7,10-tetraazacyclodode-cane tetraacetic acid (2B-DOTA). Eur. J. Nucl. Med 1994, 21, 640–646. [DOI] [PubMed] [Google Scholar]
- (176).Palm S; Enmon RM Jr.; Matei C; Kolbert KS; Xu S; Zanzonico PB; Finn RL; Koutcher JA; Larson SM; Sgouros G Pharmacokinetics and biodistribution of (86)Y-trastuzumab for(90)Y dosimetry in an ovarian carcinoma model: correlative MicroPET and MRI. J. Nucl. Med 2003, 44, 1148–1155. [PubMed] [Google Scholar]
- (177).Parry R; Schneider D; Hudson D; Parkes D; Xuan JA; Newton A; Toy P; Lin R; Harkins R; Alicke B; et al. Identification of a novel prostate tumor target, mindin/RG-1, for antibody-based radiotherapy of prostate cancer. Cancer Res 2005, 65, 8397–8405. [DOI] [PubMed] [Google Scholar]
- (178).Nayak TK; Regino CA; Wong KJ; Milenic DE; Garmestani K; Baidoo KE; Szajek LP; Brechbiel MW PET imaging of HER1-expressing xenografts in mice with 86Y-CHX-A′′-DTPA-cetuximab. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 1368–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (179).Nayak TK; Garmestani K; Milenic DE; Baidoo KE; Brechbiel MW HER1-targeted 86Y-panitumumab possesses superior targeting characteristics than 86Y-cetuximab for PET imaging of human malignant mesothelioma tumors xenografts. PLoS One 2011, 6, e18198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (180).Silberstein EB Radioiodine: the classic theranostic agent. Semin. Nucl. Med 2012, 42, 164–170. [DOI] [PubMed] [Google Scholar]
- (181).Sajjad M; Bars E; Nabi HA Optimization of 124I production via 124Te(p,n)124I reaction. Appl. Radiat. Isot 2006, 64, 965–970. [DOI] [PubMed] [Google Scholar]
- (182).Nye JA; Avila-Rodriguez MA; Nickles RJ A new binary compound for the production of 124I via the 124Te(p,n)124I reaction. Appl. Radiat. Isot 2007, 65, 407–412. [DOI] [PubMed] [Google Scholar]
- (183).Wilson CB; Snook DE; Dhokia B; Taylor CV; Watson IA; Lammertsma AA; Lambrecht R; Waxman J; Jones T; Epenetos AA Quantitative measurement of monoclonal antibody distribution and blood flow using positron emission tomography and 124iodine in patients with breast cancer. Int. J. Cancer 1991, 47, 344–347. [DOI] [PubMed] [Google Scholar]
- (184).Divgi CR; Pandit-Taskar N; Jungbluth AA; Reuter VE; Gonen M; Ruan S; Pierre C; Nagel A; Pryma DA; Humm J; et al. Preoperative characterisation of clear-cell renal carcinoma using iodine-124-labelled antibody chimeric G250 (124I-cG250) and PET in patients with renal masses: a phase I trial. Lancet Oncol 2007, 8, 304–310. [DOI] [PubMed] [Google Scholar]
- (185).Carrasquillo JA; Pandit-Taskar N; O’Donoghue JA; Humm JL; Zanzonico P; Smith-Jones PM; Divgi CR; Pryma DA; Ruan S; Kemeny NE; et al. (124)I-huA33 antibody PET of colorectal cancer. J. Nucl. Med 2011, 52, 1173–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (186).Divgi CR; Uzzo RG; Gatsonis C; Bartz R; Treutner S; Yu JQ; Chen D; Carrasquillo JA; Larson S; Bevan P; et al. Positron emission tomography/computed tomography identification of clear cell renal cell carcinoma: results from the REDECT trial. J. Clin. Oncol 2013, 31, 187–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (187).Cascini GL; Niccoli Asabella A; Notaristefano A; Restuccia A; Ferrari C; Rubini D; Altini C; Rubini G 124 Iodine: a longer-life positron emitter isotope-new opportunities in molecular imaging. BioMed Res. Int 2014, 2014, 672094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (188).Tijink BM; Perk LR; Budde M; Stigter-van Walsum M; Visser GW; Kloet RW; Dinkelborg LM; Leemans CR; Neri D; van Dongen GA (124)I-L19-SIP for immuno-PET imaging of tumour vasculature and guidance of (131)I-L19-SIP radioimmuno-therapy. Eur. J. Nucl. Med. Mol. Imaging 2009, 36, 1235–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (189).Visser GW; Klok RP; Gebbinck JW; ter Linden T; van Dongen GA; Molthoff CF Optimal quality (131)I-monoclonal antibodies on high-dose labeling in a large reaction volume and temporarily coating the antibody with IODO-GEN. J. Nucl. Med 2001, 42, 509–519. [PubMed] [Google Scholar]
- (190).Foulon CF; Reist CJ; Bigner DD; Zalutsky MR Radioiodination via D-amino acid peptide enhances cellular retention and tumor xenograft targeting of an internalizing anti-epidermal growth factor receptor variant III monoclonal antibody. Cancer Res 2000, 60, 4453–4460. [PubMed] [Google Scholar]
- (191).Stein R; Govindan SV; Mattes MJ; Chen S; Reed L; Newsome G; McBride BJ; Griffiths GL; Hansen HJ; Goldenberg DM Improved iodine radiolabels for monoclonal antibody therapy. Cancer Res 2003, 63, 111–118. [PubMed] [Google Scholar]
- (192).Govindan SV; Griffiths GL; Stein R; Andrews P; Sharkey RM; Hansen HJ; Horak ID; Goldenberg DM Clinical-scale radiolabeling of a humanized anticarcinoembryonic antigen monoclonal antibody, hMN-14, with residualizing 131I for use in radioimmunotherapy. J. Nucl. Med 2005, 46, 153–159. [PubMed] [Google Scholar]
- (193).Vaidyanathan G; Alston KL; Bigner DD; Zalutsky MR Nepsilon-(3-[*I]Iodobenzoyl)-Lys5-Nalpha-maleimido-Gly1-GEEEK ([*I]IB-Mal-D-GEEEK): a radioiodinated prosthetic group containing negatively charged D-glutamates for labeling internalizing monoclonal antibodies. Bioconjugate Chem 2006, 17, 1085–1092. [DOI] [PubMed] [Google Scholar]
- (194).Vaidyanathan G; Zalutsky MR Synthesis of N-succinimidyl 4-guanidinomethyl-3-[*I]iodobenzoate: a radio-iodination agent for labeling internalizing proteins and peptides. Nat. Protoc 2007, 2, 282–286. [DOI] [PubMed] [Google Scholar]
- (195).Boswell CA; Marik J; Elowson MJ; Reyes NA; Ulufatu S; Bumbaca D; Yip V; Mundo EE; Majidy N; Van Hoy M; et al. Enhanced tumor retention of a radiohalogen label for site-specific modification of antibodies. J. Med. Chem 2013, 56, 9418–9426. [DOI] [PubMed] [Google Scholar]
- (196).Fowler JS; Ido T Initial and subsequent approach for the synthesis of 18FDG. Semin. Nucl. Med 2002, 32, 6–12. [DOI] [PubMed] [Google Scholar]
- (197).Zettlitz KA; Tavare R; Tsai WK; Yamada RE; Ha NS; Collins J; van Dam RM; Timmerman JM; Wu AM 18)F-labeled anti-human CD20 cys-diabody for same-day immunoPET in a model of aggressive B cell lymphoma in human CD20 transgenic mice. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 489–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (198).Krishnan HS; Ma L; Vasdev N; Liang SH (18)F-labeling of sensitive biomolecules for positron emission tomography. Chem. - Eur. J 2017, 23, 15553–15577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (199).Speranza A; Ortosecco G; Castaldi E; Nardelli A; Pace L; Salvatore M Fully automated synthesis procedure of 4-[18F]fluorobenzaldehyde by commercial synthesizer: amino-oxi peptide labelling prosthetic group. Appl. Radiat. Isot 2009, 67, 1664–1669. [DOI] [PubMed] [Google Scholar]
- (200).Cai W; Olafsen T; Zhang X; Cao Q; Gambhir SS; Williams LE; Wu AM; Chen X PET imaging of colorectal cancer in xenograft-bearing mice by use of an 18F-labeled T84.66 anti-carcinoembryonic antigen diabody. J. Nucl. Med 2007, 48, 304–310. [PubMed] [Google Scholar]
- (201).Sharma SK; Wuest M; Way JD; Bouvet VR; Wang M; Wuest FR Synthesis and pre-clinical evaluation of an (18)F-labeled single-chain antibody fragment for PET imaging of epithelial ovarian cancer. Am. J. Nucl. Med. Mol. Imaging 2016, 6, 185–198. [PMC free article] [PubMed] [Google Scholar]
- (202).Vaidyanathan G; McDougald D; Choi J; Koumarianou E; Weitzel D; Osada T; Lyerly HK; Zalutsky MR Preclinical evaluation of 18F-labeled anti-HER2 nanobody conjugates for imaging HER2 receptor expression by immuno-PET. J. Nucl. Med 2016, 57, 967–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (203).Vaidyanathan G; Zalutsky MR Synthesis of N-succinimidyl 4-[18F]fluorobenzoate, an agent for labeling proteins and peptides with 18F. Nat. Protoc 2006, 1, 1655–1661. [DOI] [PubMed] [Google Scholar]
- (204).Cai W; Zhang X; Wu Y; Chen X A thiol-reactive 18F-labeling agent, N-[2-(4–18F-fluorobenzamido)ethyl]maleimide, and synthesis of RGD peptide-based tracer for PET imaging of alpha v beta 3 integrin expression. J. Nucl. Med 2006, 47, 1172–1180. [PMC free article] [PubMed] [Google Scholar]
- (205).Kramer-Marek G; Kiesewetter DO; Martiniova L; Jagoda E; Lee SB; Capala J [18F]FBEM-Z(HER2:342)-affibody molecule-a new molecular tracer for in vivo monitoring of HER2 expression by positron emission tomography. Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 1008–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (206).Li W; Niu G; Lang L; Guo N; Ma Y; Kiesewetter DO; Backer JM; Shen B; Chen X PET imaging of EGF receptors using [18F]FBEM-EGF in a head and neck squamous cell carcinoma model. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 300–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (207).Lim K; Ropchan J; Kiesewetter DO; Chen X; Huang Y Automated radiosynthesis of [(18)F]FBEM, a sulfhydryl site specific labeling agent for peptides and proteins. Appl. Radiat. Isot 2018, 140, 294–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (208).Kiesewetter DO; Jacobson O; Lang L; Chen X Automated radiochemical synthesis of [18F]FBEM: a thiol reactive synthon for radiofluorination of peptides and proteins. Appl. Radiat. Isot 2011, 69, 410–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (209).Olberg DE; Arukwe JM; Grace D; Hjelstuen OK; Solbakken M; Kindberg GM; Cuthbertson A One step radiosynthesis of 6-[(18)F]fluoronicotinic acid 2,3,5,6-tetrafluorophenyl ester ([(18)F]F-Py-TFP): a new prosthetic group for efficient labeling of biomolecules with fluorine-18. J. Med. Chem 2010, 53, 1732–1740. [DOI] [PubMed] [Google Scholar]
- (210).Basuli F; Li C; Xu B; Williams M; Wong K; Coble VL; Vasalatiy O; Seidel J; Green MV; Griffiths GL; et al. Synthesis of fluorine-18 radio-labeled serum albumins for PET blood pool imaging. Nucl. Med. Biol 2015, 42, 219–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (211).Basuli F; Zhang X; Jagoda EM; Choyke PL; Swenson RE Facile room temperature synthesis of fluorine-18 labeled fluoronicotinic acid-2,3,5,6-tetrafluorophenyl ester without azeotropic drying of fluorine-18. Nucl. Med. Biol 2016, 43, 770–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (212).Basuli F; Zhang X; Woodroofe CC; Jagoda EM; Choyke PL; Swenson RE Fast indirect fluorine-18 labeling of protein/peptide using the useful 6-fluoronicotinic acid-2,3,5,6-tetrafluorophenyl prosthetic group: A method comparable to direct fluorination. J. Labelled Compd. Radiopharm 2017, 60, 168–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (213).Zhou Z; McDougald D; Devoogdt N; Zalutsky MR; Vaidyanathan G Labeling single domain antibody fragments with fluorine-18 using 2,3,5,6-tetrafluorophenyl 6-[18F]fluoronicotinate resulting in high tumor-to-kidney ratios. Mol. Pharmaceutics 2019, 16, 214–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (214).Schirrmacher E; Wangler B; Cypryk M; Bradtmoller G; Schafer M; Eisenhut M; Jurkschat K; Schirrmacher R Synthesis of p-(di-tert-butyl[(18)F]fluorosilyl)benzaldehyde ([(18)F]SiFA-A) with high specific activity by isotopic exchange: a convenient labeling synthon for the (18)F-labeling of N-amino-oxy derivatized peptides. Bioconjugate Chem 2007, 18, 2085–2089. [DOI] [PubMed] [Google Scholar]
- (215).Iovkova L; Könning D; Wängler B; Schirrmacher R; Schoof S; Arndt H-D; Jurkschat K SiFA-modified phenylalanine: a key compound for the efficient synthesis of 18F-labelled peptides. Eur.J. Inorg. Chem 2011, 2011, 2238–2246. [Google Scholar]
- (216).Wangler B; Kostikov AP; Niedermoser S; Chin J; Orchowski K; Schirrmacher E; Iovkova-Berends L; Jurkschat K; Wangler C; Schirrmacher R Protein labeling with the labeling precursor [(18)F]SiFA-SH for positron emission tomography. Nat. Protoc 2012, 7, 1964–1969. [DOI] [PubMed] [Google Scholar]
- (217).Wangler C; Niedermoser S; Chin J; Orchowski K; Schirrmacher E; Jurkschat K; Iovkova-Berends L; Kostikov AP; Schirrmacher R; Wangler B One-step (18)F-labeling of peptides for positron emission tomography imaging using the SiFA methodology. Nat. Protoc 2012, 7, 1946–1955. [DOI] [PubMed] [Google Scholar]
- (218).Liu Z; Lin KS; Benard F; Pourghiasian M; Kiesewetter DO; Perrin DM; Chen X One-step (18)F labeling of biomolecules using organotrifluoroborates. Nat. Protoc 2015, 10, 1423–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (219).McBride WJ; Sharkey RM; Karacay H; D’Souza CA; Rossi EA; Laverman P; Chang CH; Boerman OC; Goldenberg DM A novel method of 18F radiolabeling for PET. J. Nucl. Med 2009, 50, 991–998. [DOI] [PubMed] [Google Scholar]
- (220).Kumar K; Ghosh A (18)F-AlF Labeled Peptide and Protein conjugates as positron emission tomography imaging pharmaceuticals. Bioconjugate Chem 2018, 29, 953–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (221).McBride WJ; D’Souza CA; Sharkey RM; Goldenberg DM The radiolabeling of proteins by the [18F]AlF method. Appl. Radiat. Isot 2012, 70, 200–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (222).Cleeren F; Lecina J; Billaud EM; Ahamed M; Verbruggen A; Bormans GM New chelators for low temperature Al(18)F-labeling of biomolecules. Bioconjugate Chem 2016, 27, 790–798. [DOI] [PubMed] [Google Scholar]
- (223).Cleeren F; Lecina J; Ahamed M; Raes G; Devoogdt N; Caveliers V; McQuade P; Rubins DJ; Li W; Verbruggen A; et al. 18)F-labeling of heat-sensitive biomolecules for positron emission tomography imaging. Theranostics 2017, 7, 2924–2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (224).Cleeren F; Lecina J; Bridoux J; Devoogdt N; Tshibangu T; Xavier C; Bormans G Direct fluorine-18 labeling of heat-sensitive biomolecules for positron emission tomography imaging using the Al(18)F-RESCA method. Nat. Protoc 2018, 13, 2330–2347. [DOI] [PubMed] [Google Scholar]
- (225).Zhernosekov KP; Filosofov DV; Baum RP; Aschoff P; Bihl H; Razbash AA; Jahn M; Jennewein M; Rosch F Processing of generator-produced 68Ga for medical application. J. Nucl. Med 2007, 48, 1741–1748. [DOI] [PubMed] [Google Scholar]
- (226).Pandey MK; Byrne JF; Jiang H; Packard AB; DeGrado TR Cyclotron production of (68)Ga via the (68)Zn(p,n)(68)Ga reaction in aqueous solution. Am. J. Nucl. Med. Mol. Imaging 2014, 4, 303–310. [PMC free article] [PubMed] [Google Scholar]
- (227).Alves F; Alves VH; Neves ACB; do Carmo SJC; Nactergal B; Hellas V; Kral E; Gonçalves-Gameiro C; Abrunhosa AJ Cyclotron production of Ga-68 for human use from liquid targets: From theory to practice. AIP Conf. Proc 2011, 1845, 020001. [Google Scholar]
- (228).Tieu W; Hollis CA; Kuan KKW; Takhar P; Stuckings M; Spooner N; Malinconico M Rapid and automated production of [(68)Ga]gallium chloride and [(68)Ga]Ga-DOTA-TATE on a medical cyclotron. Nucl. Med. Biol 2019, 74–75, 12–18. [DOI] [PubMed] [Google Scholar]
- (229).Fani M; Andre JP; Maecke HR 68Ga-PET: a powerful generator-based alternative to cyclotron-based PET radiopharmaceuticals. Contrast Media Mol. Imaging 2008, 3, 67–77. [DOI] [PubMed] [Google Scholar]
- (230).Eder M; Wangler B; Knackmuss S; LeGall F; Little M; Haberkorn U; Mier W; Eisenhut M Tetrafluorophenolate of HBED-CC: a versatile conjugation agent for 68Ga-labeled small recombinant antibodies. Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 1878–1886. [DOI] [PubMed] [Google Scholar]
- (231).Fay R; Gut M; Holland JP Photoradiosynthesis of(68)Ga-labeled HBED-CC-Azepin-MetMAb for immuno-PET of c-MET receptors. Bioconjugate Chem 2019, 30, 1814–1820. [DOI] [PubMed] [Google Scholar]
- (232).Boros E; Ferreira CL; Cawthray JF; Price EW; Patrick BO; Wester DW; Adam MJ; Orvig C Acyclic chelate with ideal properties for (68)Ga PET imaging agent elaboration. J. Am. Chem. Soc 2010, 132, 15726–15733. [DOI] [PubMed] [Google Scholar]
- (233).Boros E; Ferreira CL; Yapp DT; Gill RK; Price EW; Adam MJ; Orvig C RGD conjugates of the H2dedpa scaffold: synthesis, labeling and imaging with 68Ga. Nucl. Med. Biol 2012, 39, 785–794. [DOI] [PubMed] [Google Scholar]
- (234).Boros E; Cawthray JF; Ferreira CL; Patrick BO; Adam MJ; Orvig C Evaluation of the H2)dedpa scaffold and its cRGDyK conjugates for labeling with 64Cu. Inorg. Chem 2012, 51, 6279–6284. [DOI] [PubMed] [Google Scholar]
- (235).Berry DJ; Ma Y; Ballinger JR; Tavare R; Koers A; Sunassee K; Zhou T; Nawaz S; Mullen GE; Hider RC; et al. Efficient bifunctional gallium-68 chelators for positron emission tomography: tris(hydroxypyridinone) ligands. Chem. Commun. (Cambridge, U. K.) 2011, 47, 7068–7070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (236).Ferreira CL; Yapp DT; Crisp S; Sutherland BW; Ng SS; Gleave M; Bensimon C; Jurek P; Kiefer GE Comparison of bifunctional chelates for (64)Cu antibody imaging. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 2117–2126. [DOI] [PubMed] [Google Scholar]
- (237).Song IH; Lee TS; Park YS; Lee JS; Lee BC; Moon BS; An GI; Lee HW; Kim KI; Lee YJ; et al. Immuno-PET imaging and radioimmunotherapy of 64Cu-/177Lu-labeled anti-EGFR antibody in esophageal squamous cell carcinoma model. J. Nucl. Med 2016, 57, 1105–1111. [DOI] [PubMed] [Google Scholar]
- (238).Ferreira CL; Lamsa E; Woods M; Duan Y; Fernando P; Bensimon C; Kordos M; Guenther K; Jurek P; Kiefer GE Evaluation of bifunctional chelates for the development of gallium-based radiopharmaceuticals. Bioconjugate Chem 2010, 21, 531–536. [DOI] [PubMed] [Google Scholar]
- (239).Notni J; Pohle K; Wester HJ Comparative gallium-68 labeling of TRAP-, NOTA-, and DOTA-peptides: practical consequences for the future of gallium-68-PET. EJNMMI Res 2012, 2,28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (240).Hernandez R; Valdovinos HF; Yang Y; Chakravarty R; Hong H; Barnhart TE; Cai W (44)Sc: an attractive isotope for peptide-based PET imaging. Mol. Pharmaceutics 2014, 11, 2954–2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (241).Singh A; van der Meulen NP; Muller C; Klette I; Kulkarni HR; Turler A; Schibli R; Baum RP First-in-human PET/CT imaging of metastatic neuroendocrine neoplasms with cyclotron-produced (44)Sc-DOTATOC: a proof-of-concept study. Cancer Biother.Radiopharm 2017, 32, 124–132. [DOI] [PubMed] [Google Scholar]
- (242).Chaple IF; Lapi SE Production and use of the first-row transition metal PET radionuclides (43,44)Sc, (52)Mn, and (45)Ti. J. Nucl. Med 2018, 59, 1655–1659. [DOI] [PubMed] [Google Scholar]
- (243).Chakravarty R; Goel S; Valdovinos HF; Hernandez R; Hong H; Nickles RJ; Cai W Matching the decay half-life with the biological half-life: ImmunoPET imaging with (44)Sc-labeled cetuximab Fab fragment. Bioconjugate Chem 2014, 25, 2197–2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (244).Nagy G; Szikra D; Trencsenyi G; Fekete A; Garai I; Giani AM; Negri R; Masciocchi N; Maiocchi A; Uggeri F; et al. AAZTA: an ideal chelating agent for the development of (44) Sc PET imaging agents. Angew. Chem., Int. Ed 2017, 56, 2118–2122. [DOI] [PubMed] [Google Scholar]
- (245).Li L; Jaraquemada-Peláez M. d. G.; Aluicio-Sarduy E; Wang X; Jiang D; Sakheie M; Kuo H-T; Barnhart TE; Cai W; Radchenko V; et al. [nat/44Sc(pypa)]–: thermodynamic stability, radiolabeling, and biodistribution of a prostate-specific-membrane-antigen-targeting conjugate. Inorg. Chem 2020, 59, 1985–1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (246).Fonslet J; Tietze S; Jensen AI; Graves SA; Severin GW Optimized procedures for manganese-52: Production, separation and radiolabeling. Appl. Radiat. Isot 2017, 121, 38–43. [DOI] [PubMed] [Google Scholar]
- (247).Brandt M; Cardinale J; Rausch I; Mindt TL Manganese in PET imaging: opportunities and challenges. J. Labelled Compd. Radiopharm 2019, 62, 541–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (248).Graves SA; Hernandez R; Fonslet J; England CG; Valdovinos HF; Ellison PA; Barnhart TE; Elema DR; Theuer CP; Cai W; et al. Novel preparation methods of (52)Mn for immunoPET imaging. Bioconjugate Chem 2015, 26, 2118–2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (249).Cicone F; Gnesin S; Denoel T; Stora T; van der Meulen NP; Muller C; Vermeulen C; Benesova M; Koster U; Johnston K; et al. Internal radiation dosimetry of a (152)Tb-labeled antibody in tumor-bearing mice. EJNMMI Res 2019, 9, 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (250).Hoglund J; Tolmachev V; Orlova A; Lundqvist H; Sundin A Optimized indirect (76)Br-bromination of antibodies using N-succinimidyl para-[76Br]bromobenzoate for radioimmuno PET. Nucl. Med. Biol 2000, 27, 837–843. [DOI] [PubMed] [Google Scholar]
- (251).Rossin R; Berndorff D; Friebe M; Dinkelborg LM; Welch MJ Small-animal PET of tumor angiogenesis using a (76)Br-labeled human recombinant antibody fragment to the ED-B domain of fibronectin. J. Nucl. Med 2007, 48, 1172–1179. [DOI] [PubMed] [Google Scholar]
- (252).Aluicio-Sarduy E; Hernandez R; Olson AP; Barnhart TE; Cai W; Ellison PA; Engle JW Production and in vivo PET/ CT imaging of the theranostic pair (132/135)La. Sci. Rep 2019, 9, 10658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (253).Aluicio-Sarduy E; Thiele NA; Martin KE; Vaughn BA; Devaraj J; Olson AP; Barnhart TE; Wilson JJ; Boros E; Engle JW Establishing radiolanthanum chemistry for targeted nuclear medicine applications. Chem. - Eur. J 2020, 26, 1238–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (254).Fay R; Holland JP The impact of emerging bioconjugation chemistries on radiopharmaceuticals. J. Nucl. Med 2019, 60, 587–591. [DOI] [PubMed] [Google Scholar]
- (255).Tinianow JN; Gill HS; Ogasawara A; Flores JE; Vanderbilt AN; Luis E; Vandlen R; Darwish M; Junutula JR; Williams SP; et al. Site-specifically 89Zr-labeled monoclonal antibodies for ImmunoPET. Nucl. Med. Biol 2010, 37, 289–297. [DOI] [PubMed] [Google Scholar]
- (256).Tavare R; Wu WH; Zettlitz KA; Salazar FB; McCabe KE; Marks JD; Wu AM Enhanced immunoPET of ALCAM-positive colorectal carcinoma using site-specific (6)(4)Cu-DOTA conjugation. Protein Eng., Des. Sel 2014, 27, 317–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (257).Ravasco J; Faustino H; Trindade A; Gois PMP Bioconjugation with maleimides: a useful tool for chemical biology. Chem. - Eur. J 2019, 25, 43–59. [DOI] [PubMed] [Google Scholar]
- (258).Bernardim B; Matos MJ; Ferhati X; Companon I; Guerreiro A; Akkapeddi P; Burtoloso ACB; Jimenez-Oses G; Corzana F; Bernardes GJL Efficient and irreversible antibody-cysteine bioconjugation using carbonylacrylic reagents. Nat. Protoc 2019, 14, 86–99. [DOI] [PubMed] [Google Scholar]
- (259).Boutureira O; Bernardes GJ Advances in chemical protein modification. Chem. Rev 2015, 115, 2174–2195. [DOI] [PubMed] [Google Scholar]
- (260).Zeng D; Zeglis BM; Lewis JS; Anderson CJ The growing impact of bioorthogonal click chemistry on the development of radiopharmaceuticals. J. Nucl. Med 2013, 54, 829–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (261).Meyer JP; Adumeau P; Lewis JS; Zeglis BM Click chemistry and radiochemistry: the first 10 years. Bioconjugate Chem 2016, 27, 2791–2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (262).Nguyen QD; Smith G; Glaser M; Perumal M; Arstad E; Aboagye EO Positron emission tomography imaging of drug-induced tumor apoptosis with a caspase-3/7 specific [18F]-labeled isatin sulfonamide. Proc. Natl. Acad. Sci. U. S. A 2009, 106, 16375–16380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (263).Dubash SR; Merchant S; Heinzmann K; Mauri F; Lavdas I; Inglese M; Kozlowski K; Rama N; Masrour N; Steel JF; et al. Clinical translation of [(18)F]ICMT-11 for measuring chemotherapy-induced caspase 3/7 activation in breast and lung cancer. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 2285–2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (264).de Almeida G; Sletten EM; Nakamura H; Palaniappan KK; Bertozzi CR Thiacycloalkynes for copper-free click chemistry. Angew. Chem., Int. Ed 2012, 51, 2443–2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (265).Chen K; Wang X; Lin W-Y; Shen CK-F; Yap L-P; Hughes LD; Conti PS Strain-promoted catalyst-free click chemistry for rapid construction of 64Cu-labeled PET imaging probes. ACS Med. Chem. Lett 2012, 3, 1019–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (266).Blackman ML; Royzen M; Fox JM Tetrazine ligation: fast bioconjugation based on inverse-electron-demand Diels-Alder reactivity. J. Am. Chem. Soc 2008, 130, 13518–13519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (267).Zeglis BM; Mohindra P; Weissmann GI; Divilov V; Hilderbrand SA; Weissleder R; Lewis JS Modular strategy for the construction of radiometalated antibodies for positron emission tomography based on inverse electron demand Diels-Alder click chemistry. Bioconjugate Chem 2011, 22, 2048–2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (268).Kumar A; Hao G; Liu L; Ramezani S; Hsieh JT; Oz OK; Sun X Click-chemistry strategy for labeling antibodies with copper-64 via a cross-bridged tetraazamacrocyclic chelator scaffold. Bioconjugate Chem 2015, 26, 782–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (269).Shi X; Gao K; Huang H; Gao R Pretargeted immuno-PET based on bioorthogonal chemistry for imaging EGFR positive colorectal cancer. Bioconjugate Chem 2018, 29, 250–254. [DOI] [PubMed] [Google Scholar]
- (270).Rossin R; van den Bosch SM; Ten Hoeve W; Carvelli M; Versteegen RM; Lub J; Robillard MS Highly reactive transcyclooctene tags with improved stability for Diels-Alder chemistry in living systems. Bioconjugate Chem 2013, 24, 1210–1217. [DOI] [PubMed] [Google Scholar]
- (271).Lim RK; Lin Q Photoinducible bioorthogonal chemistry: a spatiotemporally controllable tool to visualize and perturb proteins in live cells. Acc. Chem. Res 2011, 44, 828–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (272).Herner A; Lin Q Photo-triggered click chemistry for biological applications. Top Curr. Chem. (Cham) 2016, 374, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (273).Sun L; Ding J; Xing W; Gai Y; Sheng J; Zeng D Novel strategy for preparing dual-modality optical/PET imaging probes via photo-click chemistry. Bioconjugate Chem 2016, 27, 1200–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (274).Patra M; Eichenberger LS; Fischer G; Holland JP Photochemical conjugation and one-pot radiolabelling of antibodies for immuno-PET. Angew. Chem., Int. Ed 2019, 58, 1928–1933. [DOI] [PubMed] [Google Scholar]
- (275).Patra M; Klingler S; Eichenberger LS; Holland JP Simultaneous photoradiochemical labeling of antibodies for immuno-positron emission tomography. iScience 2019, 13, 416–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (276).Mazmanian SK; Liu G; Ton-That H; Schneewind OJS Staphylococcus aureus sortase, an enzyme that anchors surface proteins to the cell wall. Science 1999, 285, 760–763. [DOI] [PubMed] [Google Scholar]
- (277).Pishesha N; Ingram JR; Ploegh HL Sortase A: a model for transpeptidation and its biological applications. Annu. Rev. Cell Dev. Biol 2018, 34, 163–188. [DOI] [PubMed] [Google Scholar]
- (278).Rashidian M; Keliher EJ; Bilate AM; Duarte JN; Wojtkiewicz GR; Jacobsen JT; Cragnolini J; Swee LK; Victora GD; Weissleder R; et al. Noninvasive imaging of immune responses. Proc. Natl. Acad. Sci. U. S. A 2015, 112, 6146–6151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (279).Paterson BM; Alt K; Jeffery CM; Price RI; Jagdale S; Rigby S; Williams CC; Peter K; Hagemeyer CE; Donnelly PS Enzyme-mediated site-specific bioconjugation of metal complexes to proteins: sortase-mediated coupling of copper-64 to a single-chain antibody. Angew. Chem., Int. Ed 2014, 53, 6115–6119. [DOI] [PubMed] [Google Scholar]
- (280).Alt K; Paterson BM; Westein E; Rudd SE; Poniger SS; Jagdale S; Ardipradja K; Connell TU; Krippner GY; Nair AK; et al. A versatile approach for the site-specific modification of recombinant antibodies using a combination of enzyme-mediated bioconjugation and click chemistry. Angew. Chem., Int. Ed 2015, 54, 7515–7519. [DOI] [PubMed] [Google Scholar]
- (281).Chen I; Dorr BM; Liu DR A general strategy for the evolution of bond-forming enzymes using yeast display. Proc. Natl. Acad. Sci. U. S. A 2011, 108, 11399–11404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (282).Chen L; Cohen J; Song X; Zhao A; Ye Z; Feulner CJ; Doonan P; Somers W; Lin L; Chen PR Improved variants of SrtA for site-specific conjugation on antibodies and proteins with high efficiency. Sci. Rep 2016, 6, 31899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (283).Jeong HJ; Abhiraman GC; Story CM; Ingram JR; Dougan SK Generation of Ca2+-independent sortase A mutants with enhanced activity for protein and cell surface labeling. PLoS One 2017, 12, e0189068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (284).Nguyen GK; Wang S; Qiu Y; Hemu X; Lian Y; Tam JP Butelase 1 is an asx-specific ligase enabling peptide macro-cyclization and synthesis. Nat. Chem. Biol 2014, 10, 732–738. [DOI] [PubMed] [Google Scholar]
- (285).Nguyen GK; Qiu Y; Cao Y; Hemu X; Liu CF; Tam JP Butelase-mediated cyclization and ligation of peptides and proteins. Nat. Protoc 2016, 11, 1977–1988. [DOI] [PubMed] [Google Scholar]
- (286).Harmand TJ; Bousbaine D; Chan A; Zhang X; Liu DR; Tam JP; Ploegh HL One-pot dual labeling of IgG 1 and preparation of C-to-C fusion proteins through a combination of Sortase A and Butelase 1. Bioconjugate Chem 2018, 29, 3245–3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (287).Zeglis BM; Davis CB; Aggeler R; Kang HC; Chen A; Agnew BJ; Lewis JS Enzyme-mediated methodology for the site-specific radiolabeling of antibodies based on catalyst-free click chemistry. Bioconjugate Chem 2013, 24, 1057–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (288).Zeglis BM; Davis CB; Abdel-Atti D; Carlin SD; Chen A; Aggeler R; Agnew BJ; Lewis JS Chemoenzymatic strategy for the synthesis of site-specifically labeled immunoconjugates for multimodal PET and optical imaging. Bioconjugate Chem 2014, 25, 2123–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (289).Kristensen LK; Christensen C; Jensen MM; Agnew BJ; Schjoth-Frydendahl C; Kjaer A; Nielsen CH Site-specifically labeled (89)Zr-DFO-trastuzumab improves immuno-reactivity and tumor uptake for immuno-PET in a subcutaneous HER2-positive xenograft mouse model. Theranostics 2019, 9, 4409–4420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (290).Los GV; Encell LP; McDougall MG; Hartzell DD; Karassina N; Zimprich C; Wood MG; Learish R; Ohana RF; Urh M; et al. HaloTag: a novel protein labeling technology for cell imaging and protein analysis. ACS Chem. Biol 2008, 3, 373–382. [DOI] [PubMed] [Google Scholar]
- (291).England CG; Luo H; Cai W HaloTag technology: a versatile platform for biomedical applications. Bioconjugate Chem 2015, 26, 975–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (292).Urh M; Rosenberg M HaloTag, a platform technology for protein analysis. Curr. Chem. Genomics 2013, 6, 72–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (293).Encell LP; Ohana RF; Zimmerman K; Otto P; Vidugiris G; Wood MG; Los GV; McDougall MG; Zimprich C; Karassina N Suppl 1: Development of a dehalogenase-based protein fusion tag capable of papid, selective and covalent attachment to customizable ligands. Curr. Chem. Genomics 2013, 6, 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (294).Young CL; Britton ZT; Robinson AS Recombinant protein expression and purification: a comprehensive review of affinity tags and microbial applications. Biotechnol. J 2012, 7, 620–634. [DOI] [PubMed] [Google Scholar]
- (295).Wood DW New trends and affinity tag designs for recombinant protein purification. Curr. Opin. Struct. Biol 2014, 26, 54–61. [DOI] [PubMed] [Google Scholar]
- (296).Knight JC; Mosley M; Uyeda HT; Cong M; Fan F; Faulkner S; Cornelissen B In vivo pretargeted imaging of HER2 and TAG-72 expression using the HaloTag enzyme. Mol. Pharmaceutics 2017, 14, 2307–2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (297).Jeger S; Zimmermann K; Blanc A; Grunberg J; Honer M; Hunziker P; Struthers H; Schibli R Site-specific and stoichiometric modification of antibodies by bacterial transglutaminase. Angew. Chem., Int. Ed 2010, 49, 9995–9997. [DOI] [PubMed] [Google Scholar]
- (298).Spycher PR; Amann CA; Wehrmuller JE; Hurwitz DR; Kreis O; Messmer D; Ritler A; Kuchler A; Blanc A; Behe M; et al. Dual, site-specific modification of antibodies by using solid-phase immobilized microbial transglutaminase. ChemBioChem 2017, 18, 1923–1927. [DOI] [PubMed] [Google Scholar]
- (299).Drake CR; Sevillano N; Truillet C; Craik CS; VanBrocklin HF; Evans MJ Site-specific radiofluorination of biomolecules with 8-[(18)F]-fluorooctanoic acid catalyzed by lipoic acid ligase. ACS Chem. Biol 2016, 11, 1587–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (300).Goldenberg DM; Sharkey RM; Paganelli G; Barbet J; Chatal JF Antibody pretargeting advances cancer radioimmunodetection and radioimmunotherapy. J. Clin. Oncol 2006, 24, 823–834. [DOI] [PubMed] [Google Scholar]
- (301).Summer D; Mayr S; Petrik M; Rangger C; Schoeler K; Vieider L; Matuszczak B; Decristoforo C Pretargeted imaging with Gallium-68-improving the binding capability by increasing the number of tetrazine motifs. Pharmaceuticals 2018, 11, 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (302).Goldenberg DM; Chang CH; Rossi EA; McBride WJ; Sharkey RM Pretargeted molecular imaging and radio-immunotherapy. Theranostics 2012, 2, 523–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (303).Altai M; Membreno R; Cook B; Tolmachev V; Zeglis BM Pretargeted imaging and therapy. J. Nucl. Med 2017, 58, 1553–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (304).Hnatowich DJ; Virzi F; Rusckowski M Investigations of avidin and biotin for imaging applications. J. Nucl. Med 1987, 28, 1294–1302. [PubMed] [Google Scholar]
- (305).Sakahara H; Saga T Avidin-biotin system for delivery of diagnostic agents. Adv. Drug Delivery Rev 1999, 37, 89–101. [DOI] [PubMed] [Google Scholar]
- (306).Kalofonos HP; Rusckowski M; Siebecker DA; Sivolapenko GB; Snook D; Lavender JP; Epenetos AA; Hnatowich DJ Imaging of tumor in patients with indium-111-labeled biotin and streptavidin-conjugated antibodies: preliminary communication. J. Nucl. Med 1990, 31, 1791–1796. [PubMed] [Google Scholar]
- (307).Weiden PL; Breitz HB Pretargeted radioimmunotherapy (PRIT) for treatment of non-Hodgkin’s lymphoma (NHL). Crit. Rev. Oncol. Hematol 2001, 40, 37–51. [DOI] [PubMed] [Google Scholar]
- (308).Forero A; Weiden PL; Vose JM; Knox SJ; LoBuglio AF; Hankins J; Goris ML; Picozzi VJ; Axworthy DB; Breitz HB; et al. Phase 1 trial of a novel anti-CD20 fusion protein in pretargeted radioimmunotherapy for B-cell non-Hodgkin lymphoma. Blood 2004, 104, 227–236. [DOI] [PubMed] [Google Scholar]
- (309).Paganelli G; Chinol M Radioimmunotherapy: is avidinbiotin pretargeting the preferred choice among pretargeting methods? Eur. J. Nucl. Med. Mol. Imaging 2003, 30, 773–776. [DOI] [PubMed] [Google Scholar]
- (310).Chang CH; Sharkey RM; Rossi EA; Karacay H; McBride W; Hansen HJ; Chatal JF; Barbet J; Goldenberg DM Molecular advances in pretargeting radioimunotherapy with bispecific antibodies. Mol. Cancer Ther 2002, 1, 553–563. [PubMed] [Google Scholar]
- (311).McBride WJ; Zanzonico P; Sharkey RM; Noren C; Karacay H; Rossi EA; Losman MJ; Brard PY; Chang CH; Larson SM; et al. Bispecific antibody pretargeting PET (immunoPET) with an 124I-labeled hapten-peptide. J. Nucl. Med 2006, 47, 1678–1688. [PubMed] [Google Scholar]
- (312).Sharkey RM; Karacay H; McBride WJ; Rossi EA; Chang C-H; Goldenberg DM Bispecific antibody pretargeting of radionuclides for immuno–single-photon emission computed tomography and immuno–positron emission tomography molecular imaging: An update. Clin. Cancer Res 2007, 13, 5577s–5585s. [DOI] [PubMed] [Google Scholar]
- (313).Sharkey RM; Karacay H; Vallabhajosula S; McBride WJ; Rossi EA; Chang CH; Goldsmith SJ; Goldenberg DM Metastatic human colonic carcinoma: molecular imaging with pretargeted SPECT and PET in a mouse model. Radiology 2008, 246, 497–507. [DOI] [PubMed] [Google Scholar]
- (314).Karacay H; Sharkey RM; McBride WJ; Rossi EA; Chang CH; Goldenberg DM Optimization of hapten-peptide labeling for pretargeted immunoPET of bispecific antibody using generator-produced 68Ga. J. Nucl. Med 2011, 52, 555–559. [DOI] [PubMed] [Google Scholar]
- (315).Karacay H; Sharkey RM; McBride WJ; Griffiths GL; Qu Z; Chang K; Hansen HJ; Goldenberg DM Pretargeting for cancer radioimmunotherapy with bispecific antibodies: role of the bispecific antibody’s valency for the tumor target antigen. Bioconjugate Chem 2002, 13, 1054–1070. [DOI] [PubMed] [Google Scholar]
- (316).Sharkey RM; McBride WJ; Karacay H; Chang K; Griffiths GL; Hansen HJ; Goldenberg DM A universal pretargeting system for cancer detection and therapy using bispecific antibody. Cancer Res 2003, 63, 354–363. [PubMed] [Google Scholar]
- (317).Rossi EA; Sharkey RM; McBride W; Karacay H; Zeng L; Hansen HJ; Goldenberg DM; Chang CH Development of new multivalent-bispecific agents for pretargeting tumor localization and therapy. Clin. Cancer. Res 2003, 9, 3886S–3896S. [PubMed] [Google Scholar]
- (318).Rossi EA; Chang CH; Losman MJ; Sharkey RM; Karacay H; McBride W; Cardillo TM; Hansen HJ; Qu Z; Horak ID; et al. Pretargeting of carcinoembryonic antigen-expressing cancers with a trivalent bispecific fusion protein produced in myeloma cells. Clin. Cancer Res 2005, 11, 7122s–7129s. [DOI] [PubMed] [Google Scholar]
- (319).Chatal JF; Campion L; Kraeber-Bodere F; Bardet S; Vuillez JP; Charbonnel B; Rohmer V; Chang CH; Sharkey RM; Goldenberg DM; et al. Survival improvement in patients with medullary thyroid carcinoma who undergo pretargeted anti-carcinoembryonic-antigen radioimmunotherapy: a collaborative study with the French Endocrine Tumor Group. J. Clin. Oncol 2006, 24, 1705–1711. [DOI] [PubMed] [Google Scholar]
- (320).Salaun PY; Campion L; Bournaud C; Faivre-Chauvet A; Vuillez JP; Taieb D; Ansquer C; Rousseau C; Borson-Chazot F; Bardet S; et al. Phase II trial of anticarcinoembryonic antigen pretargeted radioimmunotherapy in progressive metastatic medullary thyroid carcinoma: biomarker response and survival improvement. J. Nucl. Med 2012, 53, 1185–1192. [DOI] [PubMed] [Google Scholar]
- (321).Rossi EA; Goldenberg DM; Cardillo TM; McBride WJ; Sharkey RM; Chang CH Stably tethered multifunctional structures of defined composition made by the dock and lock method for use in cancer targeting. Proc. Natl. Acad. Sci. U. S. A 2006, 103, 6841–6846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (322).Goldenberg DM; Rossi EA; Sharkey RM; McBride WJ; Chang CH Multifunctional antibodies by the Dock-and-Lock method for improved cancer imaging and therapy by pretargeting. J. Nucl. Med 2007, 49, 158–163. [DOI] [PubMed] [Google Scholar]
- (323).Karacay H; Sharkey RM; Gold DV; Ragland DR; McBride WJ; Rossi EA; Chang CH; Goldenberg DM Pretargeted radioimmunotherapy of pancreatic cancer xenografts: TF10–90Y-IMP-288 alone and combined with gemcitabine. J. Nucl. Med 2009, 50, 2008–2016. [DOI] [PubMed] [Google Scholar]
- (324).Sharkey RM; Rossi EA; McBride WJ; Chang CH; Goldenberg DM Recombinant bispecific monoclonal antibodies prepared by the dock-and-lock strategy for pretargeted radio-immunotherapy. Semin. Nucl. Med 2010, 40, 190–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (325).Boder ET; Wittrup KD Yeast surface display for directed evolution of protein expression, affinity, and stability. Methods Enzymol 2000, 328, 430–444. [DOI] [PubMed] [Google Scholar]
- (326).Orcutt KD; Ackerman ME; Cieslewicz M; Quiroz E; Slusarczyk AL; Frangioni JV; Wittrup KD A modular IgG-scFv bispecific antibody topology. Protein Eng., Des. Sel 2010, 23, 221–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (327).Orcutt KD; Slusarczyk AL; Cieslewicz M; Ruiz-Yi B; Bhushan KR; Frangioni JV; Wittrup KD Engineering an antibody with picomolar affinity to DOTA chelates of multiple radionuclides for pretargeted radioimmunotherapy and imaging. Nucl. Med. Biol 2011, 38, 223–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (328).Orcutt KD; Rhoden JJ; Ruiz-Yi B; Frangioni JV; Wittrup KD Effect of small-molecule-binding affinity on tumor uptake in vivo: a systematic study using a pretargeted bispecific antibody. Mol. Cancer Ther 2012, 11, 1365–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (329).Cheal SM; Xu H; Guo HF; Lee SG; Punzalan B; Chalasani S; Fung EK; Jungbluth A; Zanzonico PB; Carrasquillo JA; et al. Theranostic pretargeted radioimmunotherapy of colorectal cancer xenografts in mice using picomolar affinity (8)(6)Y-or (1)(7)(7)Lu-DOTA-Bn binding scFv C825/GPA33 IgG bispecific immunoconjugates. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 925–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (330).Knight JC; Cornelissen B Bioorthogonal chemistry: implications for pretargeted nuclear (PET/SPECT) imaging and therapy. Am. J. Nucl. Med. Mol. Imaging 2014, 4, 96–113. [PMC free article] [PubMed] [Google Scholar]
- (331).Rossin R; Robillard MS Pretargeted imaging using bioorthogonal chemistry in mice. Curr. Opin. Chem. Biol 2014, 21, 161–169. [DOI] [PubMed] [Google Scholar]
- (332).Bailly C; Bodet-Milin C; Rousseau C; Faivre-Chauvet A; Kraeber-Bodere F; Barbet J Pretargeting for imaging and therapy in oncological nuclear medicine. EJNMMI Radiopharm Chem 2017, 2, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (333).Rossin R; Renart Verkerk P; van den Bosch SM; Vulders RC; Verel I; Lub J; Robillard MS In vivo chemistry for pretargeted tumor imaging in live mice. Angew. Chem., Int. Ed 2010, 49, 3375–3378. [DOI] [PubMed] [Google Scholar]
- (334).Devaraj NK; Thurber GM; Keliher EJ; Marinelli B; Weissleder R Reactive polymer enables efficient in vivo bioorthogonal chemistry. Proc. Natl. Acad. Sci. U. S. A 2012, 109, 4762–4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (335).Zeglis BM; Sevak KK; Reiner T; Mohindra P; Carlin SD; Zanzonico P; Weissleder R; Lewis JS A pretargeted PET imaging strategy based on bioorthogonal Diels-Alder click chemistry. J. Nucl. Med 2013, 54, 1389–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (336).Rossin R; Lappchen T; van den Bosch SM; Laforest R; Robillard MS Diels-Alder reaction for tumor pretargeting: in vivo chemistry can boost tumor radiation dose compared with directly labeled antibody. J. Nucl. Med 2013, 54, 1989–1995. [DOI] [PubMed] [Google Scholar]
- (337).Rossin R; van Duijnhoven SM; Lappchen T; van den Bosch SM; Robillard MS Trans-cyclooctene tag with improved properties for tumor pretargeting with the diels-alder reaction. Mol. Pharmaceutics 2014, 11, 3090–3096. [DOI] [PubMed] [Google Scholar]
- (338).Cook BE; Adumeau P; Membreno R; Carnazza KE; Brand C; Reiner T; Agnew BJ; Lewis JS; Zeglis BM Pretargeted PET imaging using a site-specifically labeled immuno-conjugate. Bioconjugate Chem 2016, 27, 1789–1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (339).Zeglis BM; Brand C; Abdel-Atti D; Carnazza KE; Cook BE; Carlin S; Reiner T; Lewis JS Optimization of a pretargeted strategy for the PET imaging of colorectal carcinoma via the modulation of radioligand pharmacokinetics. Mol. Pharmaceutics 2015, 12, 3575–3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (340).Seibold U; Wangler B; Wangler C Rational design, development, and stability assessment of a macrocyclic four-hydroxamate-bearing bifunctional chelating agent for (89) Zr. ChemMedChem 2017, 12, 1555–1571. [DOI] [PubMed] [Google Scholar]
- (341).Litau S; Seibold U; Wangler B; Schirrmacher R; Wangler C iEDDA Conjugation reaction in radiometal labeling of peptides with (68)Ga and (64)Cu: unexpected findings. ACS Omega 2018, 3, 14039–14053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (342).Vugts DJ; Vervoort A; Stigter-van Walsum M; Visser GW; Robillard MS; Versteegen RM; Vulders RC; Herscheid JK; van Dongen GA Synthesis of phosphine and antibody-azide probes for in vivo Staudinger ligation in a pretargeted imaging and therapy approach. Bioconjugate Chem 2011, 22, 2072–2081. [DOI] [PubMed] [Google Scholar]
- (343).van den Bosch SM; Rossin R; Renart Verkerk P; Ten Hoeve W; Janssen HM; Lub J; Robillard MS Evaluation of strained alkynes for Cu-free click reaction in live mice. Nucl. Med. Biol 2013, 40, 415–423. [DOI] [PubMed] [Google Scholar]
- (344).Westerlund K; Honarvar H; Tolmachev V; Eriksson Karlstrom A Design, preparation, and characterization of PNA-based hybridization probes for affibody-molecule-mediated pretargeting. Bioconjugate Chem 2015, 26, 1724–1736. [DOI] [PubMed] [Google Scholar]
- (345).Honarvar H; Westerlund K; Altai M; Sandstrom M; Orlova A; Tolmachev V; Karlstrom AE Feasibility of affibody molecule-based PNA-mediated radionuclide pretargeting of malignant tumors. Theranostics 2016, 6, 93–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (346).Altai M; Perols A; Tsourma M; Mitran B; Honarvar H; Robillard M; Rossin R; ten Hoeve W; Lubberink M; Orlova A; et al. Feasibility of affibody-based bioorthogonal chemistry-mediated radionuclide pretargeting. J. Nucl. Med 2016, 57, 431–436. [DOI] [PubMed] [Google Scholar]
- (347).Westerlund K; Altai M; Mitran B; Konijnenberg M; Oroujeni M; Atterby C; de Jong M; Orlova A; Mattsson J; Micke P; et al. Radionuclide therapy of HER2-expressing human xenografts using affibody-based peptide nucleic acid-mediated pretargeting: in vivo proof of principle. J. Nucl. Med 2018, 59, 1092–1098. [DOI] [PubMed] [Google Scholar]
- (348).Vorobyeva A; Westerlund K; Mitran B; Altai M; Rinne S; Sorensen J; Orlova A; Tolmachev V; Karlstrom AE Development of an optimal imaging strategy for selection of patients for affibody-based PNA-mediated radionuclide therapy. Sci. Rep 2018, 8, 9643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (349).Matsumura K; Zouda M; Wada Y; Yamashita F; Hashida M; Watanabe Y; Mukai H Urokinase injection-triggered clearance enhancement of a 4-arm PEG-conjugated (64)Cu-bombesin analog tetramer: a novel approach for the improvement of PET imaging contrast. Int. J. Pharm 2018, 545, 206–214. [DOI] [PubMed] [Google Scholar]
- (350).Borjesson PK; Jauw YW; de Bree R; Roos JC; Castelijns JA; Leemans CR; van Dongen GA; Boellaard R Radiation dosimetry of 89Zr-labeled chimeric monoclonal antibody U36 as used for immuno-PET in head and neck cancer patients. J. Nucl. Med 2009, 50, 1828–1836. [DOI] [PubMed] [Google Scholar]
- (351).Freise AC; Wu AM In vivo imaging with antibodies and engineered fragments. Mol. Immunol 2015, 67, 142–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (352).Zhang RR; Schroeder AB; Grudzinski JJ; Rosenthal EL; Warram JM; Pinchuk AN; Eliceiri KW; Kuo JS; Weichert JP Beyond the margins: real-time detection of cancer using targeted fluorophores. Nat. Rev. Clin. Oncol 2017, 14, 347–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (353).Du Z; Lovly CM Mechanisms of receptor tyrosine kinase activation in cancer. Mol. Cancer 2018, 17, 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (354).Pool M; de Boer HR; Hooge MNL; van Vugt M; de Vries EGE Harnessing integrative omics to facilitate molecular imaging of the human epidermal growth factor receptor family for precision medicine. Theranostics 2017, 7, 2111–2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (355).Lemmon MA; Schlessinger J; Ferguson KM The EGFR family: not so prototypical receptor tyrosine kinases. Cold Spring Harbor Perspect. Biol 2014, 6, a020768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (356).Menke-van der Houven van Oordt CW; Gootjes EC; Huisman MC; Vugts DJ; Roth C; Luik M; Mulder ER; Schuit RC; Boellaard R; Hoekstra OS; et al. 89Zr-cetuximab PET imaging in patients with advanced colorectal cancer. Oncotarget 2015, 6, 30384–30393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (357).van Helden EJ; Elias SG; Gerritse SL; van Es SC; Boon E; Huisman MC; van Grieken NCT; Dekker H; van Dongen GAMS; Vugts DJ; et al. [89Zr]Zr-cetuximab PET/CT as biomarker for cetuximab monotherapy in patients with RAS wild-type advanced colorectal cancer. Eur. J. Nucl. Med. Mol. Imaging 2019, DOI: 10.1007/s00259-019-04555-6. [DOI] [Google Scholar]
- (358).van Loon J; Even AJG; Aerts H; Ollers M; Hoebers F; van Elmpt W; Dubois L; Dingemans AC; Lalisang RI; Kempers P; et al. PET imaging of zirconium-89 labelled cetuximab: a phase I trial in patients with head and neck and lung cancer. Radiother. Oncol 2017, 122, 267–273. [DOI] [PubMed] [Google Scholar]
- (359).Aerts HJ; Dubois L; Perk L; Vermaelen P; van Dongen GA; Wouters BG; Lambin P Disparity between in vivo EGFR expression and 89Zr-labeled cetuximab uptake assessed with PET. J. Nucl. Med 2008, 50, 123–131. [DOI] [PubMed] [Google Scholar]
- (360).Even AJ; Hamming-Vrieze O; van Elmpt W; Winnepenninckx VJ; Heukelom J; Tesselaar ME; Vogel WV; Hoeben A; Zegers CM; Vugts DJ; et al. Quantitative assessment of Zirconium-89 labeled cetuximab using PET/CT imaging in patients with advanced head and neck cancer: a theragnostic approach. Oncotarget 2017, 8, 3870–3880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (361).Pool M; Kol A; Lub-de Hooge MN; Gerdes CA; de Jong S; de Vries EG; Terwisscha van Scheltinga AGT Extracellular domain shedding influences specific tumor uptake and organ distribution of the EGFR PET tracer 89Zr-imgatuzumab. Oncotarget 2016, 7, 68111–68121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (362).Yang XD; Jia XC; Corvalan JR; Wang P; Davis CG Development of ABX-EGF, a fully human anti-EGF receptor monoclonal antibody, for cancer therapy. Crit. Rev. Oncol. Hematol 2001, 38, 17–23. [DOI] [PubMed] [Google Scholar]
- (363).Niu G; Li Z; Xie J; Le QT; Chen X PET of EGFR antibody distribution in head and neck squamous cell carcinoma models. J. Nucl. Med 2009, 50, 1116–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (364).Chang AJ; De Silva RA; Lapi SE Development and characterization of 89Zr-labeled panitumumab for immuno-positron emission tomographic imaging of the epidermal growth factor receptor. Mol. Imaging 2013, 12, 17–27. [PMC free article] [PubMed] [Google Scholar]
- (365).Bhattacharyya S; Kurdziel K; Wei L; Riffle L; Kaur G; Hill GC; Jacobs PM; Tatum JL; Doroshow JH; Kalen JD Zirconium-89 labeled panitumumab: a potential immuno-PET probe for HER1-expressing carcinomas. Nucl. Med. Biol 2013, 40, 451–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (366).Nayak TK; Garmestani K; Milenic DE; Brechbiel MW PET and MRI of metastatic peritoneal and pulmonary colorectal cancer in mice with human epidermal growth factor receptor 1-targeted 89Zr-labeled panitumumab. J. Nucl. Med 2012, 53, 113–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (367).Do K; Cao L; Kang Z; Turkbey B; Lindenberg ML; Larkins E; Holkova B; Steinberg SM; Raffeld M; Peer CJ; et al. A phase II study of sorafenib combined with cetuximab in EGFR-expressing, KRAS-mutated metastatic colorectal cancer. Clin. Colorectal Cancer 2015, 14, 154–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (368).Lindenberg L; Adler S; Turkbey IB; Mertan F; Ton A; Do K; Kummar S; Gonzalez EM; Bhattacharyya S; Jacobs PM; et al. Dosimetry and first human experience with (89)Zrpanitumumab. Am. J. Nucl. Med. Mol. Imaging 2017, 7, 195–203. [PMC free article] [PubMed] [Google Scholar]
- (369).Johns TG; Stockert E; Ritter G; Jungbluth AA; Huang HJ; Cavenee WK; Smyth FE; Hall CM; Watson N; Nice EC; et al. Novel monoclonal antibody specific for the de2–7 epidermal growth factor receptor (EGFR) that also recognizes the EGFR expressed in cells containing amplification of the EGFR gene. Int. J. Cancer 2002, 98, 398–408. [DOI] [PubMed] [Google Scholar]
- (370).Jungbluth AA; Stockert E; Huang HJ; Collins VP; Coplan K; Iversen K; Kolb D; Johns TJ; Scott AM; Gullick WJ; et al. A monoclonal antibody recognizing human cancers with amplification/overexpression of the human epidermal growth factor receptor. Proc. Natl. Acad. Sci. U. S. A 2003, 100, 639–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (371).Panousis C; Rayzman VM; Johns TG; Renner C; Liu Z; Cartwright G; Lee FT; Wang D; Gan H; Cao D; et al. Engineering and characterisation of chimeric monoclonal antibody 806 (ch806) for targeted immunotherapy of tumours expressing de2–7 EGFR or amplified EGFR. Br. J. Cancer 2005, 92, 1069–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (372).Scott AM; Lee FT; Tebbutt N; Herbertson R; Gill SS; Liu Z; Skrinos E; Murone C; Saunder TH; Chappell B; et al. A phase I clinical trial with monoclonal antibody ch806 targeting transitional state and mutant epidermal growth factor receptors. Proc. Natl. Acad. Sci. U. S. A 2007, 104, 4071–4076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (373).Lee FT; O’Keefe GJ; Gan HK; Mountain AJ; Jones GR; Saunder TH; Sagona J; Rigopoulos A; Smyth FE; Johns TG; et al. Immuno-PET quantitation of de2–7 epidermal growth factor receptor expression in glioma using 124I-IMP-R4-labeled antibody ch806. J. Nucl. Med 2010, 51, 967–972. [DOI] [PubMed] [Google Scholar]
- (374).Lee FT; Burvenich IJ; Guo N; Kocovski P; Tochon-Danguy H; Ackermann U; O’Keefe GJ; Gong S; Rigopoulos A; Liu Z; et al. L-tyrosine confers residualizing properties to a d-amino acid-rich residualizing peptide for radioiodination of internalizing antibodies. Mol. Imaging 2016, 15, 153601211664753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (375).Chekol R; Solomon VR; Alizadeh E; Bernhard W; Fisher D; Hill W; Barreto K; DeCoteau JF; Parada AC; Geyer CR; Fonge H 89)Zr-nimotuzumab for immunoPET imaging of epidermal growth factor receptor I. Oncotarget 2018, 9, 17117–17132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (376).Tang Y; Hu Y; Liu W; Chen L; Zhao Y; Ma H; Yang J; Yang Y; Liao J; Cai J; et al. A radiopharmaceutical [(89)Zr]Zr-DFO-nimotuzumab for immunoPET with epidermal growth factor receptor expression in vivo. Nucl. Med. Biol 2019, 70, 23–31. [DOI] [PubMed] [Google Scholar]
- (377).Miao Z; Ren G; Liu H; Jiang L; Cheng Z Small-animal PET imaging of human epidermal growth factor receptor positive tumor with a 64Cu labeled affibody protein. Bioconjugate Chem 2010, 21, 947–954. [DOI] [PubMed] [Google Scholar]
- (378).Miao Z; Ren G; Liu H; Qi S; Wu S; Cheng Z PET of EGFR expression with an 18F-labeled affibody molecule. J. Nucl. Med 2012, 53, 1110–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (379).Su X; Cheng K; Jeon J; Shen B; Venturin GT; Hu X; Rao J; Chin FT; Wu H; Cheng Z Comparison of two site-specifically (18)F-labeled affibodies for PET imaging of EGFR positive tumors. Mol. Pharmaceutics 2014, 11, 3947–3956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (380).Cheng Q; Wallberg H; Grafstrom J; Lu L; Thorell JO; Hagg Olofsson M; Linder S; Johansson K; Tegnebratt T; Arner ES; et al. Preclinical PET imaging of EGFR levels: pairing a targeting with a non-targeting Sel-tagged Affibody-based tracer to estimate the specific uptake. EJNMMI Res 2016, 6, 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (381).Garousi J; Andersson KG; Mitran B; Pichl ML; Stahl S; Orlova A; Lofblom J; Tolmachev V PET imaging of epidermal growth factor receptor expression in tumours using 89Zr-labelled ZEGFR:2377 affibody molecules. Int. J. Oncol 2016, 48, 1325–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (382).Burley TA; Da Pieve C; Martins CD; Ciobota DM; Allott L; Oyen WJG; Harrington KJ; Smith G; Kramer-Marek G Affibody-based PET imaging to guide EGFR-targeted cancer therapy in Head and Neck squamous cell cancer models. J. Nucl. Med 2019, 60, 353–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (383).Reilly EB; Phillips AC; Buchanan FG; Kingsbury G; Zhang Y; Meulbroek JA; Cole TB; DeVries PJ; Falls HD; Beam C; et al. Characterization of ABT-806, a humanized tumor-specific anti-EGFR monoclonal antibody. Mol. Cancer Ther 2015, 14, 1141–1151. [DOI] [PubMed] [Google Scholar]
- (384).Phillips AC; Boghaert ER; Vaidya KS; Falls HD; Mitten MJ; DeVries PJ; Benatuil L; Hsieh CM; Meulbroek JA; Panchal SC; et al. Characterization of ABBV-221, a tumor-selective EGFR-targeting antibody drug conjugate. Mol. Cancer Ther 2018, 17, 795–805. [DOI] [PubMed] [Google Scholar]
- (385).Oliveira S; van Dongen GA; Stigter-van Walsum M; Roovers RC; Stam JC; Mali W; van Diest PJ; van Bergen en Henegouwen, P. M. Rapid visualization of human tumor xenografts through optical imaging with a near-infrared fluorescent anti-epidermal growth factor receptor nanobody. Mol. Imaging 2012, 11, 33–46. [PubMed] [Google Scholar]
- (386).Tintelnot J; Baum N; Schultheiss C; Braig F; Trentmann M; Finter J; Fumey W; Bannas P; Fehse B; Riecken K; et al. Nanobody targeting of epidermal growth factor receptor (EGFR) ectodomain variants overcomes resistance to therapeutic EGFR antibodies. Mol. Cancer Ther 2019, 18, 823–833. [DOI] [PubMed] [Google Scholar]
- (387).Vosjan MJ; Perk LR; Roovers RC; Visser GW; Stigter-van Walsum M; van Bergen En Henegouwen PM; van Dongen GA Facile labelling of an anti-epidermal growth factor receptor Nanobody with 68Ga via a novel bifunctional desferal chelate for immuno-PET. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 753–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (388).Beltrán Hernández I; Rompen R; Rossin R; Xenaki KT; Katrukha EA; Nicolay K; van Bergen en Henegouwen P; Grüll H; Oliveira S Imaging of tumor spheroids, dual-isotope SPECT, and autoradiographic analysis to assess the tumor uptake and distribution of different nanobodies. Mol. Imag. Biol 2019, 21, 1079–1088. [DOI] [PubMed] [Google Scholar]
- (389).Wei W; Ni D; Ehlerding EB; Luo QY; Cai W PET imaging of receptor tyrosine kinases in cancer. Mol. Cancer Ther 2018, 17, 1625–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (390).Perik PJ; Lub-De Hooge MN; Gietema JA; van der Graaf WT; de Korte MA; Jonkman S; Kosterink JG; van Veldhuisen DJ; Sleijfer DT; Jager PL; et al. Indium-111-labeled trastuzumab scintigraphy in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer. J. Clin. Oncol 2006, 24, 2276–2282. [DOI] [PubMed] [Google Scholar]
- (391).Gebhart G; Lamberts LE; Wimana Z; Garcia C; Emonts P; Ameye L; Stroobants S; Huizing M; Aftimos P; Tol J; et al. Molecular imaging as a tool to investigate heterogeneity of advanced HER2-positive breast cancer and to predict patient outcome under trastuzumab emtansine (T-DM1): the ZEPHIR trial. Ann. Oncol 2016, 27, 619–624. [DOI] [PubMed] [Google Scholar]
- (392).Ulaner GA; Hyman DM; Ross DS; Corben A; Chandarlapaty S; Goldfarb S; McArthur H; Erinjeri JP; Solomon SB; Kolb H; et al. Detection of HER2-positive metastases in patients with HER2-negative primary breast cancer using 89Zr-trastuzumab PET/CT. J. Nucl. Med 2016, 57, 1523–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (393).Ulaner GA; Hyman DM; Lyashchenko SK; Lewis JS; Carrasquillo JA 89Zr-trastuzumab PET/CT for detection of human epidermal growth factor receptor 2-positive metastases in patients with human epidermal growth factor receptor 2-negative primary breast cancer. Clin. Nucl. Med 2017, 42, 912–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (394).Tamura K; Kurihara H; Yonemori K; Tsuda H; Suzuki J; Kono Y; Honda N; Kodaira M; Yamamoto H; Yunokawa M; et al. 64Cu-DOTA-trastuzumab PET imaging in patients with HER2-positive breast cancer. J. Nucl. Med 2013, 54, 1869–1875. [DOI] [PubMed] [Google Scholar]
- (395).Mortimer JE; Bading JR; Park JM; Frankel PH; Carroll MI; Tran TT; Poku EK; Rockne RC; Raubitschek AA; Shively JE; et al. Tumor uptake of (64)Cu-DOTA-trastuzumab in patients with metastatic breast cancer. J. Nucl. Med 2018, 59, 38–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (396).Ulaner GA; Lyashchenko SK; Riedl C; Ruan S; Zanzonico PB; Lake D; Jhaveri K; Zeglis B; Lewis JS; O’Donoghue JA First-in-human human epidermal growth factor receptor 2-targeted imaging using (89)Zr-pertuzumab PET/CT: dosimetry and clinical application in patients with breast cancer. J. Nucl. Med 2018, 59, 900–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (397).Meric-Bernstam F; Johnson AM; Dumbrava EEI; Raghav K; Balaji K; Bhatt M; Murthy RK; Rodon J; Piha-Paul SA Advances in HER2-targeted therapy: novel agents and opportunities beyond breast and gastric Cancer. Clin. Cancer Res 2019, 25, 2033–2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (398).Guo X; Zhu H; Zhou N; Chen Z; Liu T; Liu F; Xu X; Jin H; Shen L; Gao J; et al. Noninvasive detection of HER2 expression in gastric cancer by (64)Cu-NOTA-trastuzumab in PDX mouse model and in patients. Mol. Pharmaceutics 2018, 15, 5174–5182. [DOI] [PubMed] [Google Scholar]
- (399).O’Donoghue JA; Lewis JS; Pandit-Taskar N; Fleming SE; Schoder H; Larson SM; Beylergil V; Ruan S; Lyashchenko SK; Zanzonico PB; et al. Pharmacokinetics, biodistribution, and radiation dosimetry for (89)Zr-trastuzumab in patients with esophagogastric cancer. J. Nucl. Med 2018, 59, 161–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (400).Guo X; Zhou N; Chen Z; Liu T; Xu X; Lei X; Shen L; Gao J; Yang Z; Zhu H Construction of (124)I-trastuzumab for noninvasive PET imaging of HER2 expression: from patient-derived xenograft models to gastric cancer patients. Gastric Cancer 2020, DOI: 10.1007/s10120-019-01035-6. [DOI] [PubMed] [Google Scholar]
- (401).Jiang D; Im HJ; Sun H; Valdovinos HF; England CG; Ehlerding EB; Nickles RJ; Lee DS; Cho SY; Huang P; et al. Radiolabeled pertuzumab for imaging of human epidermal growth factor receptor 2 expression in ovarian cancer. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1296–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (402).Yanai A; Harada R; Iwata R; Yoshikawa T; Ishikawa Y; Furumoto S; Ishida T; Yanai K Site-specific labeling of F-18 proteins using a supplemented cell-free protein synthesis system and O-2-[(18)F]Fluoroethyl-L-Tyrosine: [(18)F]FET-HER2 affibody molecule. Mol. Imaging Biol 2019, 21, 529–537. [DOI] [PubMed] [Google Scholar]
- (403).Qi S; Hoppmann S; Xu Y; Cheng Z PET imaging of HER2-positive tumors with Cu-64-Labeled affibody molecules. Mol. Imag. Biol 2019, 21, 907–916. [DOI] [PubMed] [Google Scholar]
- (404).Wei W; Jiang D; Rosenkrans ZT; Barnhart TE; Engle JW; Luo Q; Cai W HER2-targeted multimodal imaging of anaplastic thyroid cancer. Am. J. Cancer Res 2019, 9, 2413–2427. [PMC free article] [PubMed] [Google Scholar]
- (405).Wimana Z; Gebhart G; Guiot T; Vanderlinden B; Morandini R; Doumont G; Sherer F; Van Simaeys G; Goldman S; Ghanem G; et al. Mucolytic agents can enhance HER2 receptor accessibility for [(89)Zr]trastuzumab, improving HER2 imaging in a mucin-overexpressing breast cancer xenograft mouse model. Mol. Imaging Biol 2015, 17, 697–703. [DOI] [PubMed] [Google Scholar]
- (406).Pereira PMR; Sharma SK; Carter LM; Edwards KJ; Pourat J; Ragupathi A; Janjigian YY; Durack JC; Lewis JS Caveolin-1 mediates cellular distribution of HER2 and affects trastuzumab binding and therapeutic efficacy. Nat. Commun 2018, 9, 5137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (407).Pereira PMR; Mandleywala K; Ragupathi A; Carter LM; Goos J; Janjigian YY; Lewis JS Temporal modulation of HER2 membrane availability increases pertuzumab uptake and pretargeted molecular imaging of gastric tumors. J. Nucl. Med 2019, 60, 1569–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (408).Pereira PMR; Ragupathi A; Shmuel S; Mandleywala K; Viola NT; Lewis JS HER2-targeted PET imaging and therapy of hyaluronan-masked HER2-overexpressing breast cancer. Mol. Pharmaceutics 2020, 17, 327–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (409).Cheng Z; De Jesus OP; Namavari M; De A; Levi J; Webster JM; Zhang R; Lee B; Syud FA; Gambhir SS Small-animal PET imaging of human epidermal growth factor receptor type 2 expression with site-specific 18F-labeled protein scaffold molecules.J. Nucl. Med 2008, 49, 804–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (410).Cheng Z; De Jesus OP; Kramer DJ; De A; Webster JM; Gheysens O; Levi J; Namavari M; Wang S; Park JM; et al. 64Cu-labeled affibody molecules for imaging of HER2 expressing tumors. Mol. Imaging Biol 2010, 12, 316–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (411).Beylergil V; Morris PG; Smith-Jones PM; Modi S; Solit D; Hudis CA; Lu Y; O’Donoghue J; Lyashchenko SK; Carrasquillo JA; Larson SM; Akhurst TJ Pilot study of 68Ga-DOTA-F(ab′)2-trastuzumab in patients with breast cancer. Nucl. Med. Commun 2013, 34, 1157–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (412).Keyaerts M; Xavier C; Heemskerk J; Devoogdt N; Everaert H; Ackaert C; Vanhoeij M; Duhoux FP; Gevaert T; Simon P; et al. Phase I study of 68Ga-HER2-nanobody for PET/CT assessment of HER2 expression in breast carcinoma. J. Nucl. Med 2016, 57, 27–33. [DOI] [PubMed] [Google Scholar]
- (413).Massa S; Vikani N; Betti C; Ballet S; Vanderhaegen S; Steyaert J; Descamps B; Vanhove C; Bunschoten A; van Leeuwen FW; et al. Sortase A-mediated site-specific labeling of camelid single-domain antibody-fragments: a versatile strategy for multiple molecular imaging modalities. Contrast Media Mol. Imaging 2016, 11, 328–339. [DOI] [PubMed] [Google Scholar]
- (414).Zhou Z; Chitneni SK; Devoogdt N; Zalutsky MR; Vaidyanathan G Fluorine-18 labeling of an anti-HER2 VHH using a residualizing prosthetic group via a strain-promoted click reaction: chemistry and preliminary evaluation. Bioorg. Med. Chem 2018, 26, 1939–1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (415).Zhou Z; McDougald D; Devoogdt N; Zalutsky MR; Vaidyanathan G Labeling single domain antibody fragments with fluorine-18 using 2,3,5,6-tetrafluorophenyl 6-[18 F]fluoronicotinate resulting in high tumor to kidney ratios. Mol. Pharmaceutics 2019, 16, 214–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (416).Zhou Z; Devoogdt N; Zalutsky MR; Vaidyanathan G An efficient method for labeling single domain antibody fragments with (18)F using tetrazine-trans-cyclooctene ligation and a renal brush border enzyme-cleavable linker. Bioconjugate Chem 2018, 29, 4090–4103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (417).D’Huyvetter M; De Vos J; Xavier C; Pruszynski M; Sterckx YGJ; Massa S; Raes G; Caveliers V; Zalutsky MR; Lahoutte T; et al. (131)I-labeled anti-HER2 camelid sdAb as a theranostic tool in cancer treatment. Clin. Cancer Res 2017, 23, 6616–6628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (418).Orlova A; Magnusson M; Eriksson TL; Nilsson M; Larsson B; Höidén-Guthenberg I; Widström C; Carlsson J; Tolmachev V; Ståhl S. J. C. r.; Nilsson FY Tumor imaging using a picomolar affinity HER2 binding affibody molecule. Cancer Res 2006, 66, 4339–4348. [DOI] [PubMed] [Google Scholar]
- (419).Tolmachev V; Nilsson FY; Widstrom C; Andersson K; Rosik D; Gedda L; Wennborg A; Orlova A 111In-benzyl-DTPAZHER2:342, an affibody-based conjugate for in vivo imaging of HER2 expression in malignant tumors. J. Nucl. Med 2006, 47, 846–853. [PubMed] [Google Scholar]
- (420).Kiesewetter DO; Kramer-Marek G; Ma Y; Capala J Radiolabeling of HER2 specific affibody(R) molecule with F-18. J. Fluorine Chem 2008, 129, 799–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (421).Kramer-Marek G; Bernardo M; Kiesewetter DO; Bagci U; Kuban M; Omer A; Zielinski R; Seidel J; Choyke P; Capala J PET of HER2-positive pulmonary metastases with 18F-ZHER2:342 affibody in a murine model of breast cancer: comparison with 18FFDG. J. Nucl. Med 2012, 53, 939–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (422).Xu Y; Bai Z; Huang Q; Pan Y; Pan D; Wang L; Yan J; Wang X; Yang R; Yang M PET of HER2 expression with a novel(18)FAl labeled affibody. J. Cancer 2017, 8, 1170–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (423).Orlova A; Tolmachev V; Pehrson R; Lindborg M; Tran T; Sandstrom M; Nilsson FY; Wennborg A; Abrahmsen L; Feldwisch J Synthetic affibody molecules: a novel class of affinity ligands for molecular imaging of HER2-expressing malignant tumors. Cancer Res 2007, 67, 2178–2186. [DOI] [PubMed] [Google Scholar]
- (424).Tolmachev V; Velikyan I; Sandstrom M; Orlova A A HER2-binding Affibody molecule labelled with 68Ga for PET imaging: direct in vivo comparison with the 111In-labelled analogue. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 1356–1367. [DOI] [PubMed] [Google Scholar]
- (425).Tolmachev V; Wallberg H; Sandstrom M; Hansson M; Wennborg A; Orlova A Optimal specific radioactivity of anti-HER2 affibody molecules enables discrimination between xenografts with high and low HER2 expression levels. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 531–539. [DOI] [PubMed] [Google Scholar]
- (426).Baum RP; Prasad V; Muller D; Schuchardt C; Orlova A; Wennborg A; Tolmachev V; Feldwisch J Molecular imaging of HER2-expressing malignant tumors in breast cancer patients using synthetic 111In- or 68Ga-labeled affibody molecules. J. Nucl. Med 2010, 51, 892–897. [DOI] [PubMed] [Google Scholar]
- (427).Sorensen J; Sandberg D; Sandstrom M; Wennborg A; Feldwisch J; Tolmachev V; Astrom G; Lubberink M; Garske-Roman U; Carlsson J; et al. First-in-human molecular imaging of HER2 expression in breast cancer metastases using the 111In-ABY-025 affibody molecule. J. Nucl. Med 2014, 55, 730–735. [DOI] [PubMed] [Google Scholar]
- (428).Ahlgren S; Orlova A; Wallberg H; Hansson M; Sandstrom M; Lewsley R; Wennborg A; Abrahmsen L; Tolmachev V; Feldwisch J Targeting of HER2-expressing tumors using 111In-ABY-025, a second-generation affibody molecule with a fundamentally reengineered scaffold. J. Nucl. Med 2010, 51, 1131–1138. [DOI] [PubMed] [Google Scholar]
- (429).Feldwisch J; Tolmachev V; Lendel C; Herne N; Sjoberg A; Larsson B; Rosik D; Lindqvist E; Fant G; Hoiden-Guthenberg I; et al. Design of an optimized scaffold for affibody molecules. J. Mol. Biol 2010, 398, 232–247. [DOI] [PubMed] [Google Scholar]
- (430).Sorensen J; Velikyan I; Sandberg D; Wennborg A; Feldwisch J; Tolmachev V; Orlova A; Sandstrom M; Lubberink M; Olofsson H; et al. Measuring HER2-receptor expression in metastatic breast cancer using [68Ga]ABY-025 affibody PET/CT. Theranostics 2016, 6, 262–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (431).Velikyan I; Wennborg A; Feldwisch J; Lindman H; Carlsson J; Sorensen J Good manufacturing practice production of [(68)Ga]Ga-ABY-025 for HER2 specific breast cancer imaging. Am. J. Nucl. Med. Mol. Imaging 2016, 6, 135–153. [PMC free article] [PubMed] [Google Scholar]
- (432).Sandstrom M; Lindskog K; Velikyan I; Wennborg A; Feldwisch J; Sandberg D; Tolmachev V; Orlova A; Sorensen J; Carlsson J; et al. Biodistribution and radiation dosimetry of the anti-HER2 aaffibody molecule 68Ga-ABY-025 in breast cancer patients. J. Nucl. Med 2016, 57, 867–871. [DOI] [PubMed] [Google Scholar]
- (433).Sandberg D; Tolmachev V; Velikyan I; Olofsson H; Wennborg A; Feldwisch J; Carlsson J; Lindman H; Sorensen J Intra-image referencing for simplified assessment of HER2-expression in breast cancer metastases using the affibody molecule ABY-025 with PET and SPECT. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1337–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (434).Velikyan I; Schweighofer P; Feldwisch J; Seemann J; Frejd FY; Lindman H; Sorensen J Diagnostic HER2-binding radiopharmaceutical, [(68)Ga]Ga-ABY-025, for routine clinical use in breast cancer patients. Am. J. Nucl. Med. Mol. Imaging 2019, 9, 12–23. [PMC free article] [PubMed] [Google Scholar]
- (435).Glaser M; Iveson P; Hoppmann S; Indrevoll B; Wilson A; Arukwe J; Danikas A; Bhalla R; Hiscock D Three methods for 18F labeling of the HER2-binding affibody molecule Z(HER2:2891) including preclinical assessment. J. Nucl. Med 2013, 54, 1981–1988. [DOI] [PubMed] [Google Scholar]
- (436).Trousil S; Hoppmann S; Nguyen QD; Kaliszczak M; Tomasi G; Iveson P; Hiscock D; Aboagye EO Positron emission tomography imaging with 18F-labeled ZHER2:2891 affibody for detection of HER2 expression and pharmacodynamic response to HER2-modulating therapies. Clin. Cancer Res 2014, 20, 1632–1643. [DOI] [PubMed] [Google Scholar]
- (437).Ahlgren S; Wallberg H; Tran TA; Widstrom C; Hjertman M; Abrahmsen L; Berndorff D; Dinkelborg LM; Cyr JE; Feldwisch J; et al. Targeting of HER2-expressing tumors with a site-specifically 99mTc-labeled recombinant affibody molecule, ZHER2:2395, with C-terminally engineered cysteine. J. Nucl. Med 2009, 50, 781–789. [DOI] [PubMed] [Google Scholar]
- (438).Heskamp S; Laverman P; Rosik D; Boschetti F; van der Graaf WT; Oyen WJ; van Laarhoven HW; Tolmachev V; Boerman OC Imaging of human epidermal growth factor receptor type 2 expression with 18F-labeled affibody molecule ZHER2:2395 in a mouse model for ovarian cancer. J. Nucl. Med 2012, 53, 146–153. [DOI] [PubMed] [Google Scholar]
- (439).Yin W; Zhu J; Gonzalez-Rivas D; Okumura M; Rocco G; Pass H; Jiang G; Yang Y Construction of a novel bispecific antibody to enhance antitumor activity against lung cancer. Adv. Mater 2018, 30, 1805437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (440).Slaga D; Ellerman D; Lombana TN; Vij R; Li J; Hristopoulos M; Clark R; Johnston J; Shelton A; Mai E Avidity-based binding to HER2 results in selective killing of HER2-overexpressing cells by anti-HER2/CD3. Sci. Transl. Med 2018, 10, eaat5775. [DOI] [PubMed] [Google Scholar]
- (441).Rius Ruiz I; Vicario R; Morancho B; Morales CB; Arenas EJ; Herter S; Freimoser-Grundschober A; Somandin J; Sam J; Ast O; et al. p95HER2-T cell bispecific antibody for breast cancer treatment. Sci. Transl. Med 2018, 10, eaat1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (442).Huang S; Li F; Liu H; Ye P; Fan X; Yuan X; Wu Z; Chen J; Jin C; Shen B; et al. Structural and functional characterization of MBS301, an afucosylated bispecific anti-HER2 antibody. MAbs 2018, 10, 864–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (443).Bensch F; Brouwers AH; Lub-de Hooge MN; de Jong JR; van der Vegt B; Sleijfer S; de Vries EGE; Schroder CP 89)Zr-trastuzumab PET supports clinical decision making in breast cancer patients, when HER2 status cannot be determined by standard work up. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 2300–2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (444).Kramer-Marek G; Gijsen M; Kiesewetter DO; Bennett R; Roxanis I; Zielinski R; Kong A; Capala J Potential of PET to predict the response to trastuzumab treatment in an ErbB2-positive human xenograft tumor model. J. Nucl. Med 2012, 53, 629–637. [DOI] [PubMed] [Google Scholar]
- (445).Lam K; Chan C; Reilly RM Development and preclinical studies of (64)Cu-NOTA-pertuzumab F(ab′)2 for imaging changes in tumor HER2 expression associated with response to trastuzumab by PET/CT. MAbs 2017, 9, 154–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (446).Zhou Z; Vaidyanathan G; McDougald D; Kang CM; Balyasnikova I; Devoogdt N; Ta AN; McNaughton BR; Zalutsky MR Fluorine-18 labeling of the HER2-targeting single-domain antibody 2Rs15d using a residualizing label and preclinical evaluation. Mol. Imaging Biol 2017, 19, 867–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (447).Gala K; Chandarlapaty S Molecular pathways: HER3 targeted therapy. Clin. Cancer Res 2014, 20, 1410–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (448).Pool M; Kol A; de Jong S; de Vries EGE; Lub-de Hooge MN; Terwisscha van Scheltinga AGT 89)Zr-mAb3481 PET for HER3 tumor status assessment during lapatinib treatment. MAbs 2017, 9, 1370–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (449).Jacob W; James I; Hasmann M; Weisser M Clinical development of HER3-targeting monoclonal antibodies: Perils and progress. Cancer Treat. Rev 2018, 68, 111–123. [DOI] [PubMed] [Google Scholar]
- (450).Schaefer G; Haber L; Crocker LM; Shia S; Shao L; Dowbenko D; Totpal K; Wong A; Lee CV; Stawicki S; et al. A two-in-one antibody against HER3 and EGFR has superior inhibitory activity compared with monospecific antibodies. Cancer Cell 2011, 20, 472–486. [DOI] [PubMed] [Google Scholar]
- (451).McKnight BN; Kuda-Wedagedara ANW; Sevak KK; Abdel-Atti D; Wiesend WN; Ku A; Selvakumar D; Carlin SD; Lewis JS; Viola-Villegas NT Imaging EGFR and HER3 through(89)Zr-labeled MEHD7945A (Duligotuzumab). Sci. Rep 2018, 8, 9043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (452).Sharp T; Glaus C; Fettig N; Hewig A; Ogbagabriel S; Freeman D; Beaupre D; Hwang D; Welch M Pharmacological evaluation of 64Cu - DOTA - AMG 888 (U3–1287) in control and tumor bearing mice using biodistribution and microPET imaging. Proceedings of the World Molecular Imaging Congress, 2011. [Google Scholar]
- (453).Lockhart AC; Liu Y; Dehdashti F; Laforest R; Picus J; Frye J; Trull L; Belanger S; Desai M; Mahmood S; et al. Phase 1 evaluation of [(64)Cu]DOTA-patritumab to assess dosimetry, apparent receptor occupancy, and safety in subjects with advanced solid tumors. Mol. Imaging Biol 2016, 18, 446–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (454).Alsaid H; Skedzielewski T; Rambo MV; Hunsinger K; Hoang B; Fieles W; Long ER; Tunstead J; Vugts DJ; Cleveland M; et al. Non invasive imaging assessment of the biodistribution of GSK2849330, an ADCC and CDC optimized anti HER3 mAb, and its role in tumor macrophage recruitment in human tumor-bearing mice. PLoS One 2017, 12, e0176075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (455).Menke-van der Houven van Oordt CW; McGeoch A; Bergstrom M; McSherry I; Smith DA; Cleveland M; Al-Azzam W; Chen L; Verheul H; Hoekstra OS; et al. Immuno-PET imaging to assess target engagement: experience from (89)Zr-Anti-HER3 mAb (GSK2849330) in patients with solid tumors. J. Nucl. Med 2019, 60, 902–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (456).Mirschberger C; Schiller CB; Schraml M; Dimoudis N; Friess T; Gerdes CA; Reiff U; Lifke V; Hoelzlwimmer G; Kolm I; et al. RG7116, a therapeutic antibody that binds the inactive HER3 receptor and is optimized for immune effector activation. Cancer Res 2013, 73, 5183–5194. [DOI] [PubMed] [Google Scholar]
- (457).Terwisscha van Scheltinga AG; Lub-de Hooge MN; Abiraj K; Schroder CP; Pot L; Bossenmaier B; Thomas M; Holzlwimmer G; Friess T; Kosterink JG; et al. ImmunoPET and biodistribution with human epidermal growth factor receptor 3 targeting antibody (8)(9)Zr-RG7116. MAbs 2014, 6, 1051–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (458).Bensch F; Lamberts LE; Smeenk MM; Jorritsma-Smit A; Lub-de Hooge MN; Terwisscha van Scheltinga AGT; de Jong JR; Gietema JA; Schroder CP; Thomas M; et al. (89)Zrlumretuzumab PET Imaging before and during HER3 antibody lumretuzumab treatment in patients with solid tumors. Clin. Cancer Res 2017, 23, 6128–6137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (459).Da Pieve C; Allott L; Martins CD; Vardon A; Ciobota DM; Kramer-Marek G; Smith G Efficient [(18)F]AlF radio-labeling of ZHER3:8698 affibody molecule for imaging of HER3 positive tumors. Bioconjugate Chem 2016, 27, 1839–1849. [DOI] [PubMed] [Google Scholar]
- (460).Maruthachalam BV; El-Sayed A; Liu J; Sutherland AR; Hill W; Alam MK; Pastushok L; Fonge H; Barreto K; Geyer CR A single-framework synthetic antibody library containing a combination of canonical and variable complementarity-determining regions. ChemBioChem 2017, 18, 2247–2259. [DOI] [PubMed] [Google Scholar]
- (461).El-Sayed A; Bernhard W; Barreto K; Gonzalez C; Hill W; Pastushok L; Fonge H; Geyer CR Evaluation of antibody fragment properties for near-infrared fluorescence imaging of HER3-positive cancer xenografts. Theranostics 2018, 8, 4856–4869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (462).Martins CD; Da Pieve C; Burley TA; Smith R; Ciobota DM; Allott L; Harrington KJ; Oyen WJG; Smith G; Kramer-Marek G HER3-mediated resistance to Hsp90 inhibition detected in breast cancer xenografts by affibody-based PET imaging. Clin. Cancer Res 2018, 24, 1853–1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (463).Orlova A; Malm M; Rosestedt M; Varasteh Z; Andersson K; Selvaraju RK; Altai M; Honarvar H; Strand J; Stahl S; et al. Imaging of HER3-expressing xenografts in mice using a (99m)Tc-(CO) 3-HEHEHE-Z HER3:08699 affibody molecule. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 1450–1459. [DOI] [PubMed] [Google Scholar]
- (464).Rosestedt M; Andersson KG; Mitran B; Tolmachev V; Lofblom J; Orlova A; Stahl S Affibody-mediated PET imaging of HER3 expression in malignant tumours. Sci. Rep 2015, 5, 15226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (465).Warnders FJ; Terwisscha van Scheltinga AGT; Knuehl C; van Roy M; de Vries EFJ; Kosterink JGW; de Vries EGE; Lub-de Hooge MN Human epidermal growth factor receptor 3-specific tumor uptake and biodistribution of (89)Zr-MSB0010853 visualized by real-time and noninvasive PET imaging. J. Nucl. Med 2017, 58, 1210–1215. [DOI] [PubMed] [Google Scholar]
- (466).Ferrara N; Adamis AP Ten years of anti-vascular endothelial growth factor therapy. Nat. Rev. Drug Discovery 2016, 15, 385–403. [DOI] [PubMed] [Google Scholar]
- (467).Apte RS; Chen DS; Ferrara N VEGF in signaling and disease: beyond discovery and development. Cell 2019, 176, 1248–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (468).Ferrara N; Hillan KJ; Gerber HP; Novotny W Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat. Rev. Drug Discovery 2004, 3, 391–400. [DOI] [PubMed] [Google Scholar]
- (469).Gaykema SB; Brouwers AH; Lub-de Hooge MN; Pleijhuis RG; Timmer-Bosscha H; Pot L; van Dam GM; van der Meulen SB; de Jong JR; Bart J; et al. 89Zr-bevacizumab PET imaging in primary breast cancer. J. Nucl. Med 2013, 54, 1014–1018. [DOI] [PubMed] [Google Scholar]
- (470).van Asselt SJ; Oosting SF; Brouwers AH; Bongaerts AH; de Jong JR; Lub-de Hooge MN; Oude Munnink TH; Fiebrich HB; Sluiter WJ; Links TP; et al. Everolimus reduces(89)Zr-bevacizumab tumor uptake in patients with neuroendocrine tumors. J. Nucl. Med 2014, 55, 1087–1092. [DOI] [PubMed] [Google Scholar]
- (471).Oosting SF; Brouwers AH; van Es SC; Nagengast WB; Oude Munnink TH; Lub-de Hooge MN; Hollema H; de Jong JR; de Jong IJ; de Haas S; et al. 89Zr-bevacizumab PET visualizes heterogeneous tracer accumulation in tumor lesions of renal cell carcinoma patients and differential effects of antiangiogenic treatment. J. Nucl. Med 2015, 56, 63–69. [DOI] [PubMed] [Google Scholar]
- (472).Bahce I; Huisman MC; Verwer EE; Ooijevaar R; Boutkourt F; Vugts DJ; van Dongen GA; Boellaard R; Smit EF Pilot study of (89)Zr-bevacizumab positron emission tomography in patients with advanced non-small cell lung cancer. EJNMMI Res 2014, 4, 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (473).Jansen MH; Veldhuijzen van Zanten SEM; van Vuurden DG; Huisman MC; Vugts DJ; Hoekstra OS; van Dongen GA; Kaspers GL Molecular drug imaging: (89)Zr-bevacizumab PET in children with diffuse intrinsic pontine glioma. J. Nucl. Med 2017, 58, 711–716. [DOI] [PubMed] [Google Scholar]
- (474).Veldhuijzen van Zanten SEM; Sewing ACP; van Lingen A; Hoekstra OS; Wesseling P; Meel MH; van Vuurden DG; Kaspers GJL; Hulleman E; Bugiani M Multiregional tumor drug-uptake imaging by PET and microvascular morphology in end-stage diffuse intrinsic pontine glioma. J. Nucl. Med 2018, 59, 612–615. [DOI] [PubMed] [Google Scholar]
- (475).Nagengast WB; de Korte MA; Oude Munnink TH; Timmer-Bosscha H; den Dunnen WF; Hollema H; de Jong JR; Jensen MR; Quadt C; Garcia-Echeverria C; et al. 89Zrbevacizumab PET of early antiangiogenic tumor response to treatment with HSP90 inhibitor NVP-AUY922. J. Nucl. Med 2010, 51, 761–767. [DOI] [PubMed] [Google Scholar]
- (476).Gaykema SB; Schroder CP; Vitfell-Rasmussen J; Chua S; Oude Munnink TH; Brouwers AH; Bongaerts AH; Akimov M; Fernandez-Ibarra C; Lub-de Hooge MN; et al. 89Zrtrastuzumab and 89Zr-bevacizumab PET to evaluate the effect of the HSP90 inhibitor NVP-AUY922 in metastatic breast cancer patients. Clin. Cancer Res 2014, 20, 3945–3954. [DOI] [PubMed] [Google Scholar]
- (477).Chakraborty S; Filippi CG; Burkhardt JK; Fralin S; Ray A; Wong T; Ortiz R; Langer DJ; Boockvar JA Durability of single dose intra-arterial bevacizumab after blood/brain barrier disruption for recurrent glioblastoma. J. Exp. Ther. Oncol 2016, 11, 261–267. [PubMed] [Google Scholar]
- (478).Lesniak WG; Chu C; Jablonska A; Du Y; Pomper MG; Walczak P; Janowski M A Distinct advantage to intraarterial delivery of (89)Zr-bevacizumab in PET imaging of mice with and without osmotic opening of the blood-brain barrier. J. Nucl. Med 2019, 60, 617–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (479).Nagengast WB; Lub-de Hooge MN; Oosting SF; den Dunnen WF; Warnders FJ; Brouwers AH; de Jong JR; Price PM; Hollema H; Hospers GA; et al. VEGF-PET imaging is a noninvasive biomarker showing differential changes in the tumor during sunitinib treatment. Cancer Res 2011, 71, 143–153. [DOI] [PubMed] [Google Scholar]
- (480).Desar IM; Stillebroer AB; Oosterwijk E; Leenders WP; van Herpen CM; van der Graaf WT; Boerman OC; Mulders PF; Oyen WJ 111In-bevacizumab imaging of renal cell cancer and evaluation of neoadjuvant treatment with the vascular endothelial growth factor receptor inhibitor sorafenib. J. Nucl. Med 2010, 51, 1707–1715. [DOI] [PubMed] [Google Scholar]
- (481).van Es SC; Brouwers AH; Mahesh SVK; Leliveld-Kors AM; de Jong IJ; Lub-de Hooge MN; de Vries EGE; Gietema JA; Oosting SF (89)Zr-bevacizumab PET: potential early indicator of everolimus efficacy in patients with metastatic renal cell carcinoma. J. Nucl. Med 2017, 58, 905–910. [DOI] [PubMed] [Google Scholar]
- (482).Willett CG; Boucher Y; di Tomaso E; Duda DG; Munn LL; Tong RT; Chung DC; Sahani DV; Kalva SP; Kozin SV; et al. Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat. Med 2004, 10, 145–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (483).Luo H; England CG; Graves SA; Sun H; Liu G; Nickles RJ; Cai W PET imaging of VEGFR-2 expression in lung cancer with 64Cu-labeled ramucirumab. J. Nucl. Med 2016, 57, 285–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (484).Meyer JP; Edwards KJ; Kozlowski P; Backer MV; Backer JM; Lewis JS Selective imaging of VEGFR-1 and VEGFR-2 using 89Zr-labeled single-chain VEGF mutants. J. Nucl. Med 2016, 57, 1811–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (485).Li M; Jiang D; Barnhart TE; Cao T; Engle JW; Chen W; Cai W Immuno-PET imaging of VEGFR-2 expression in prostate cancer with (89)Zr-labeled ramucirumab. Am. J. Cancer Res 2019, 9, 2037–2046. [PMC free article] [PubMed] [Google Scholar]
- (486).Lemmon MA; Schlessinger J Cell signaling by receptor tyrosine kinases. Cell 2010, 141, 1117–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (487).Bradley CA; Salto-Tellez M; Laurent-Puig P; Bardelli A; Rolfo C; Tabernero J; Khawaja HA; Lawler M; Johnston PG; Van Schaeybroeck S Targeting c-MET in gastrointestinal tumours: rationale, opportunities and challenges. Nat. Rev. Clin. Oncol 2017, 14, 562–576. [DOI] [PubMed] [Google Scholar]
- (488).Wu YL; Soo RA; Locatelli G; Stammberger U; Scagliotti G; Park K Does c-Met remain a rational target for therapy in patients with EGFR TKI-resistant non-small cell lung cancer? Cancer Treat. Rev 2017, 61, 70–81. [DOI] [PubMed] [Google Scholar]
- (489).Hughes VS; Siemann DW Have clinical trials properly assessed c-Met inhibitors? Trends Cancer 2018, 4, 94–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (490).Corso S; Giordano S Cell-autonomous and non-cell-autonomous mechanisms of HGF/MET-driven resistance to targeted therapies: from basic research to a clinical perspective. Cancer Discovery 2013, 3, 978–992. [DOI] [PubMed] [Google Scholar]
- (491).Spigel DR; Edelman MJ; O’Byrne K; Paz-Ares L; Mocci S; Phan S; Shames DS; Smith D; Yu W; Paton VE; et al. Results from the phase III randomized trial of onartuzumab plus erlotinib versus erlotinib in previously treated stage IIIB or IV non-small-cell lung cancer: METLung. J. Clin. Oncol 2017, 35, 412–420. [DOI] [PubMed] [Google Scholar]
- (492).Catenacci DVT; Tebbutt NC; Davidenko I; Murad AM; Al-Batran SE; Ilson DH; Tjulandin S; Gotovkin E; Karaszewska B; Bondarenko I; et al. Rilotumumab plus epirubicin, cisplatin, and capecitabine as first-line therapy in advanced MET-positive gastric or gastro-oesophageal junction cancer (RILOMET-1): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2017, 18, 1467–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (493).Luo H; Hong H; Slater MR; Graves SA; Shi S; Yang Y; Nickles RJ; Fan F; Cai W PET of c-Met in cancer with (6) (4)Cu-labeled hepatocyte growth factor. J. Nucl. Med 2015, 56, 758–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (494).Price EW; Carnazza KE; Carlin SD; Cho A; Edwards KJ; Sevak KK; Glaser JM; de Stanchina E; Janjigian YY; Lewis JS (89)Zr-DFO-AMG102 immuno-PET to determine local hepatocyte growth factor protein levels in tumors for enhanced patient selection. J. Nucl. Med 2017, 58, 1386–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (495).Jagoda EM; Lang L; Bhadrasetty V; Histed S; Williams M; Kramer-Marek G; Mena E; Rosenblum L; Marik J; Tinianow JN; et al. Immuno-PET of the hepatocyte growth factor receptor Met using the 1-armed antibody onartuzumab. J. Nucl. Med 2012, 53, 1592–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (496).Li K; Tavare R; Zettlitz KA; Mumenthaler SM; Mallick P; Zhou Y; Marks JD; Wu AM Anti-MET immunoPET for non-small cell lung cancer using novel fully human antibody fragments. Mol. Cancer Ther 2014, 13, 2607–2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (497).Merchant M; Ma X; Maun HR; Zheng Z; Peng J; Romero M; Huang A; Yang NY; Nishimura M; Greve J; et al. Monovalent antibody design and mechanism of action of onartuzumab, a MET antagonist with anti-tumor activity as a therapeutic agent. Proc. Natl. Acad. Sci. U. S. A 2013, 110, E2987–2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (498).Pool M; Terwisscha van Scheltinga AGT; Kol A; Giesen D; de Vries EGE; Lub-de Hooge MN 89)Zr-onartuzumab PET imaging of c-MET receptor dynamics. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1328–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (499).Escorcia F; Houghton J; Abdel-Atti D; Pereira P; Cho A; Gutsche N; Baidoo K; Lewis J ImmunoPET predicts response to Met-targeted radioligand therapy in models of pancreatic cancer resistant to Met kinase inhibitors. Theranostics 2020, 10, 151–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (500).Klingler S; Fay R; Holland JP Light-induced radiosyn-thesis of (89)ZrDFO-azepin-onartuzumab for imaging the hepatocyte growth factor receptor. J. Nucl. Med 2020, 237180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (501).Ostman A PDGF receptors in tumor stroma: biological effects and associations with prognosis and response to treatment. Adv. Drug Delivery Rev 2017, 121, 117–123. [DOI] [PubMed] [Google Scholar]
- (502).Heldin CH; Lennartsson J; Westermark B Involvement of platelet-derived growth factor ligands and receptors in tumorigenesis. J. Intern. Med 2018, 283, 16–44. [DOI] [PubMed] [Google Scholar]
- (503).Tap WD; Jones RL; Van Tine BA; Chmielowski B; Elias AD; Adkins D; Agulnik M; Cooney MM; Livingston MB; Pennock G; et al. Olaratumab and doxorubicin versus doxorubicin alone for treatment of soft-tissue sarcoma: an open-label phase 1b and randomised phase 2 trial. Lancet 2016, 388, 488–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (504).Lindborg M; Cortez E; Hoiden-Guthenberg I; Gunneriusson E; von Hage E; Syud F; Morrison M; Abrahmsen L; Herne N; Pietras K; et al. Engineered high-affinity affibody molecules targeting platelet-derived growth factor receptor beta in vivo. J. Mol. Biol 2011, 407, 298–315. [DOI] [PubMed] [Google Scholar]
- (505).Strand J; Varasteh Z; Eriksson O; Abrahmsen L; Orlova A; Tolmachev V Gallium-68-labeled affibody molecule for PET imaging of PDGFRbeta expression in vivo. Mol. Pharmaceutics 2014, 11, 3957–3964. [DOI] [PubMed] [Google Scholar]
- (506).Tolmachev V; Varasteh Z; Honarvar H; Hosseinimehr SJ; Eriksson O; Jonasson P; Frejd FY; Abrahmsen L; Orlova A Imaging of platelet-derived growth factor receptor beta expression in glioblastoma xenografts using affibody molecule 111In-DOTAZ09591. J. Nucl. Med 2014, 55, 294–300. [DOI] [PubMed] [Google Scholar]
- (507).Cai H; Shi Q; Tang Y; Chen L; Chen Y; Tao Z; Yang H; Xie F; Wu X; Liu N; et al. Positron emission tomography imaging of platelet-derived growth factor receptor beta in colorectal tumor xenograft using Zirconium-89 labeled dimeric affibody molecule. Mol. Pharmaceutics 2019, 16, 1950–1957. [DOI] [PubMed] [Google Scholar]
- (508).Zhang J; Wang P; Dykstra M; Gelebart P; Williams D; Ingham R; Adewuyi EE; Lai R; McMullen T Platelet-derived growth factor receptor-alpha promotes lymphatic metastases in papillary thyroid cancer. J. Pathol 2012, 228, 241–250. [DOI] [PubMed] [Google Scholar]
- (509).Lopez-Campistrous A; Adewuyi EE; Benesch MGK; Ko YM; Lai R; Thiesen A; Dewald J; Wang P; Chu K; Ghosh S; et al. PDGFRalpha regulates follicular cell differentiation driving treatment resistance and disease recurrence in papillary thyroid cancer. EBioMedicine 2016, 12, 86–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (510).Ekpe-Adewuyi E; Lopez-Campistrous A; Tang X; Brindley DN; McMullen TP Platelet derived growth factor receptor alpha mediates nodal metastases in papillary thyroid cancer by driving the epithelial-mesenchymal transition. Oncotarget 2016, 7, 83684–83700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (511).Adewuyi EE; Deschenes J; Lopez-Campistrous A; Kattar MM; Ghosh S; McMullen TPW Autocrine activation of platelet-derived growth factor receptor alpha in metastatic papillary thyroid cancer. Hum. Pathol 2018, 75, 146–153. [DOI] [PubMed] [Google Scholar]
- (512).Wagner M; Wuest M; Hamann I; Lopez-Campistrous A; McMullen TPW; Wuest F Molecular imaging of platelet-derived growth factor receptor-alpha (PDGFRalpha) in papillary thyroid cancer using immuno-PET. Nucl. Med. Biol 2018, 58, 51–58. [DOI] [PubMed] [Google Scholar]
- (513).Mitsiades CS; Mitsiades NS; McMullan CJ; Poulaki V; Shringarpure R; Akiyama M; Hideshima T; Chauhan D; Joseph M; Libermann TA; et al. Inhibition of the insulin-like growth factor receptor-1 tyrosine kinase activity as a therapeutic strategy for multiple myeloma, other hematologic malignancies, and solid tumors. Cancer Cell 2004, 5, 221–230. [DOI] [PubMed] [Google Scholar]
- (514).Belfiore A; Malaguarnera R; Vella V; Lawrence MC; Sciacca L; Frasca F; Morrione A; Vigneri R Insulin receptor isoforms in physiology and disease: an updated view. Endocr. Rev 2017, 38, 379–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (515).Sun Y; Sun X; Shen B Molecular imaging of IGF-1R in cancer. Mol. Imaging 2017, 16, 1536012117736648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (516).Heskamp S; van Laarhoven HW; Molkenboer-Kuenen JD; Franssen GM; Versleijen-Jonkers YM; Oyen WJ; van der Graaf WT; Boerman OC ImmunoSPECT and immunoPET of IGF-1R expression with the radiolabeled antibody R1507 in a triple-negative breast cancer model. J. Nucl. Med 2010, 51, 1565–1572. [DOI] [PubMed] [Google Scholar]
- (517).Hong H; Nayak TR; Shi S; Graves SA; Fliss BC; Barnhart TE; Cai W Generation and screening of monoclonal antibodies for immunoPET imaging of IGF1R in prostate cancer. Mol. Pharmaceutics 2014, 11, 3624–3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (518).England CG; Kamkaew A; Im HJ; Valdovinos HF; Sun H; Hernandez R; Cho SY; Dunphy EJ; Lee DS; Barnhart TE; et al. ImmunoPET imaging of insulin-Like growth factor 1 receptor in a subcutaneous mouse model of pancreatic cancer. Mol. Pharmaceutics 2016, 13, 1958–1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (519).Su X; Cheng K; Liu Y; Hu X; Meng S; Cheng Z PET imaging of insulin-like growth factor type 1 receptor expression with a 64Cu-labeled Affibody molecule. Amino Acids 2015, 47, 1409–1419. [DOI] [PubMed] [Google Scholar]
- (520).Terwisscha van Scheltinga AG; Berghuis P; Nienhuis HH; Timmer-Bosscha H; Pot L; Gaykema SB; Lub-de Hooge MN; Kosterink JG; de Vries EG; Schroder CP Visualising dual downregulation of insulin-like growth factor receptor-1 and vascular endothelial growth factor-A by heat shock protein 90 inhibition effect in triple negative breast cancer. Eur. J. Cancer 2014, 50, 2508–2516. [DOI] [PubMed] [Google Scholar]
- (521).You L; Wang X; Guo Z; Zhang D; Zhang P; Li J; Su X; Pan W; Zhang X MicroSPECT imaging of triple negative breast cancer cell tumor xenografted in athymic mice with radioiodinated anti-ICAM-1 monoclonal antibody. Appl. Radiat. Isot 2018, 139, 20–25. [DOI] [PubMed] [Google Scholar]
- (522).Vugts DJ; Heuveling DA; Stigter-van Walsum M; Weigand S; Bergstrom M; van Dongen GA; Nayak TK Preclinical evaluation of 89Zr-labeled anti-CD44 monoclonal antibody RG7356 in mice and cynomolgus monkeys: Prelude to Phase 1 clinical studies. MAbs 2014, 6, 567–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (523).Menke-van der Houven van Oordt CW; Gomez-Roca C; van Herpen C; Coveler AL; Mahalingam D; Verheul HM; van der Graaf WT; Christen R; Ruttinger D; Weigand S; et al. First-in-human phase I clinical trial of RG7356, an anti-CD44 humanized antibody, in patients with advanced, CD44-expressing solid tumors. Oncotarget 2016, 7, 80046–80058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (524).Jauw YWS; Huisman MC; Nayak TK; Vugts DJ; Christen R; Naegelen VM; Ruettinger D; Heil F; Lammertsma AA; Verheul HMW; et al. Assessment of target-mediated uptake with immuno-PET: analysis of a phase I clinical trial with an anti-CD44 antibody. EJNMMI Res 2018, 8, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (525).Zheleznyak A; Ikotun OF; Dimitry J; Frazier WA; Lapi SE Imaging of CD47 expression in xenograft and allograft tumor models. Mol. Imaging 2013, 12, 7290.2013.00069. [DOI] [PubMed] [Google Scholar]
- (526).Pan Y; Volkmer JP; Mach KE; Rouse RV; Liu JJ; Sahoo D; Chang TC; Metzner TJ; Kang L; van de Rijn M; et al. Endoscopic molecular imaging of human bladder cancer using a CD47 antibody. Sci. Transl. Med 2014, 6, 260ra148. [DOI] [PubMed] [Google Scholar]
- (527).Frost SH; Miller BW; Back TA; Santos EB; Hamlin DK; Knoblaugh SE; Frayo SL; Kenoyer AL; Storb R; Press OW; et al. Alpha-imaging confirmed efficient targeting of CD45-positive cells after 211At-radioimmunotherapy for hematopoietic cell transplantation. J. Nucl. Med 2015, 56, 1766–1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (528).Bailly C; Gouard S; Lacombe M; Remaud-Le Saec P; Chalopin B; Bourgeois M; Chouin N; Tripier R; Halime Z; Haddad F; et al. Comparison of immuno-PET of CD138 and PET imaging with (64)CuCl2 and (18)F-FDG in a preclinical syngeneic model of multiple myeloma. Oncotarget 2018, 9, 9061–9072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (529).Navarro AS; Le Bihan T; Le Saec P; Bris NL; Bailly C; Sai-Maurel C; Bourgeois M; Cherel M; Tripier R; Faivre-Chauvet A TE1PA as innovating chelator for (64)Cu immuno-TEP imaging: a comparative in vivo study with DOTA/NOTA by conjugation on 9E7.4 mAb in a syngeneic multiple myeloma model. Bioconjugate Chem 2019, 30, 2393–2403. [DOI] [PubMed] [Google Scholar]
- (530).Rylova SN; Del Pozzo L; Klingeberg C; Tonnesmann R; Illert AL; Meyer PT; Maecke HR; Holland JP Immuno-PET imaging of CD30-positive lymphoma using 89Zr-desferrioxamine-labeled CD30-specific AC-10 antibody. J. Nucl. Med 2016, 57, 96–102. [DOI] [PubMed] [Google Scholar]
- (531).England CG; Rui L; Cai W Lymphoma: current status of clinical and preclinical imaging with radiolabeled antibodies. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 517–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (532).Kang L; Jiang D; Ehlerding EB; Barnhart TE; Ni D; Engle JW; Wang R; Huang P; Xu X; Cai W Noninvasive trafficking of brentuximab vedotin and PET imaging of CD30 in lung cancer murine models. Mol. Pharmaceutics 2018, 15, 1627–1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (533).Leget GA; Czuczman MS Use of rituximab, the new FDA-approved antibody. Curr. Opin. Oncol 1998, 10, 548–551. [DOI] [PubMed] [Google Scholar]
- (534).Natarajan A; Gowrishankar G; Nielsen CH; Wang S; Iagaru A; Goris ML; Gambhir SS Positron emission tomography of 64Cu-DOTA-Rituximab in a transgenic mouse model expressing human CD20 for clinical translation to image NHL. Mol. Imaging Biol 2012, 14, 608–616. [DOI] [PubMed] [Google Scholar]
- (535).Natarajan A; Gambhir SS Radiation dosimetry study of[(89)Zr]rituximab tracer for clinical translation of B cell NHL imaging using positron emission tomography. Mol. Imaging Biol 2015, 17, 539–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (536).Muylle K; Flamen P; Vugts DJ; Guiot T; Ghanem G; Meuleman N; Bourgeois P; Vanderlinden B; van Dongen GA; Everaert H; et al. Tumour targeting and radiation dose of radioimmunotherapy with (90)Y-rituximab in CD20+ B-cell lymphoma as predicted by (89)Zr-rituximab immuno-PET: impact of preloading with unlabelled rituximab. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 1304–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (537).Olafsen T; Betting D; Kenanova VE; Salazar FB; Clarke P; Said J; Raubitschek AA; Timmerman JM; Wu AM Recombinant anti-CD20 antibody fragments for small-animal PET imaging of B-cell lymphomas. J. Nucl. Med 2009, 50, 1500–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (538).Freeman CL; Sehn LH A tale of two antibodies: obinutuzumab versus rituximab. Br. J. Haematol 2018, 182, 29–45. [DOI] [PubMed] [Google Scholar]
- (539).Yoon JT; Longtine MS; Marquez-Nostra BV; Wahl RL Evaluation of next-generation anti-CD20 antibodies labeled with(89)Zr in human lymphoma xenografts. J. Nucl. Med 2018, 59, 1219–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (540).Zettlitz KA; Tavare R; Knowles SM; Steward KK; Timmerman JM; Wu AM ImmunoPET of malignant and normal B cells with (89)Zr- and (124)I-labeled obinutuzumab antibody fragments reveals differential CD20 internalization in vivo. Clin. Cancer Res 2017, 23, 7242–7252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (541).Tran L; Huitema AD; van Rijswijk MH; Dinant HJ; Baars JW; Beijnen JH; Vogel WV CD20 antigen imaging with (1)(2)(4)I-rituximab PET/CT in patients with rheumatoid arthritis. Hum. Antibodies 2011, 20, 29–35. [DOI] [PubMed] [Google Scholar]
- (542).Bruijnen S; Tsang-A-Sjoe M; Raterman H; Ramwadhdoebe T; Vugts D; van Dongen G; Huisman M; Hoekstra O; Tak PP; Voskuyl A; van der Laken C B-cell imaging with zirconium-89 labelled rituximab PET-CT at baseline is associated with therapeutic response 24 weeks after initiation of rituximab treatment in rheumatoid arthritis patients. Arthritis Res. Ther 2016, 18, 266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (543).Laban KG; Kalmann R; Leguit RJ; de Keizer B Zirconium-89-labelled rituximab PET-CT in orbital inflammatory disease. EJNMMI Res 2019, 9, 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (544).de Keizer B; Laban KG; Kalmann R Zirconium-89 labelled rituximab PET-CT imaging of Graves’ orbitopathy. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 738–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (545).Adams H; van de Garde EM; van Moorsel CH; Vugts DJ; van Dongen GA; Grutters JC; Keijsers RG [(89)Zr]Zrrituximab PET/CT activity in patients with therapy refractory interstitial pneumonitis: a feasibility study. Am. J. Nucl. Med. Mol. Imaging 2019, 9, 296–308. [PMC free article] [PubMed] [Google Scholar]
- (546).van de Donk NW; Janmaat ML; Mutis T; Lammerts van Bueren JJ; Ahmadi T; Sasser AK; Lokhorst HM; Parren PW Monoclonal antibodies targeting CD38 in hematological malignancies and beyond. Immunol. Rev 2016, 270, 95–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (547).Morandi F; Horenstein AL; Costa F; Giuliani N; Pistoia V; Malavasi F CD38: a target for immunotherapeutic approaches in multiple myeloma. Front. Immunol 2018, 9, 2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (548).Lokhorst HM; Plesner T; Laubach JP; Nahi H; Gimsing P; Hansson M; Minnema MC; Lassen U; Krejcik J; Palumbo A; et al. Targeting CD38 with daratumumab monotherapy in multiple myeloma. N. Engl. J. Med 2015, 373, 1207–1219. [DOI] [PubMed] [Google Scholar]
- (549).Caserta E; Chea J; Minnix M; Viola D; Vonderfecht S; Yazaki P; Crow D; Khalife J; Sanchez JF; Palmer JM; et al. Copper 64-labeled daratumumab as a PET/CT imaging tracer for multiple myeloma. Blood 2018, 131, 741–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (550).Ghai A; Maji D; Cho N; Chanswangphuwana C; Rettig M; Shen D; DiPersio J; Akers W; Dehdashti F; Achilefu S; et al. Preclinical development of CD38-targeted [(89)Zr]Zr-DFO-daratumumab for imaging multiple myeloma. J. Nucl. Med 2018, 59, 216–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (551).Ehlerding EB; England CG; Jiang D; Graves SA; Kang L; Lacognata S; Barnhart TE; Cai W CD38 as a PET imaging target in lung cancer. Mol. Pharmaceutics 2017, 14, 2400–2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (552).Kang L; Jiang D; England CG; Barnhart TE; Yu B; Rosenkrans ZT; Wang R; Engle JW; Xu X; Huang P; et al. ImmunoPET imaging of CD38 in murine lymphoma models using(89)Zr-labeled daratumumab. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1372–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (553).Li S; England CG; Ehlerding EB; Kutyreff CJ; Engle JW; Jiang D; Cai W ImmunoPET imaging of CD38 expression in hepatocellular carcinoma using 64Cu-labeled daratumumab. Am. J. Transl. Res 2019, 11, 6007–6015. [PMC free article] [PubMed] [Google Scholar]
- (554).Li T; Qi S; Unger M; Hou YN; Deng QW; Liu J; Lam CMC; Wang XW; Xin D; Zhang P; et al. Immuno-targeting the multifunctional CD38 using nanobody. Sci. Rep 2016, 6, 27055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (555).Fumey W; Koenigsdorf J; Kunick V; Menzel S; Schutze K; Unger M; Schriewer L; Haag F; Adam G; Oberle A; et al. Nanobodies effectively modulate the enzymatic activity of CD38 and allow specific imaging of CD38(+) tumors in mouse models in vivo. Sci. Rep 2017, 7, 14289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (556).Oberle A; Brandt A; Alawi M; Langebrake C; Janjetovic S; Wolschke C; Schutze K; Bannas P; Kroger N; Koch-Nolte F; et al. Long-term CD38 saturation by daratumumab interferes with diagnostic myeloma cell detection. Haematologica 2017, 102, e368–e370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (557).Green DJ; Orgun NN; Jones JC; Hylarides MD; Pagel JM; Hamlin DK; Wilbur DS; Lin Y; Fisher DR; Kenoyer AL; et al. A preclinical model of CD38-pretargeted radioimmunotherapy for plasma cell malignancies. Cancer Res 2014, 74, 1179–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (558).Teiluf K; Seidl C; Blechert B; Gaertner FC; Gilbertz KP; Fernandez V; Bassermann F; Endell J; Boxhammer R; Leclair S; et al. alpha-Radioimmunotherapy with (2)(1)(3)Bi-anti-CD38 immunoconjugates is effective in a mouse model of human multiple myeloma. Oncotarget 2015, 6, 4692–4703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (559).O’Steen S; Comstock ML; Orozco JJ; Hamlin DK; Wilbur DS; Jones JC; Kenoyer A; Nartea ME; Lin Y; Miller BW; et al. The alpha-emitter astatine-211 targeted to CD38 can eradicate multiple myeloma in a disseminated disease model. Blood 2019, 134, 1247–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (560).Green DJ; O’Steen S; Lin Y; Comstock ML; Kenoyer AL; Hamlin DK; Wilbur DS; Fisher DR; Nartea M; Hylarides MD; et al. CD38-bispecific antibody pretargeted radioimmunotherapy for multiple myeloma and other B-cell malignancies. Blood 2018, 131, 611–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (561).Jamet B; Bailly C; Carlier T; Touzeau C; Nanni C; Zamagni E; Barre L; Michaud AV; Cherel M; Moreau P; et al. Interest of PET imaging in multiple myeloma. Front. Med 2019, 6, 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (562).Wang Z; Yan X CD146, a multi-functional molecule beyond adhesion. Cancer Lett 2013, 330, 150–162. [DOI] [PubMed] [Google Scholar]
- (563).Jiang T; Zhuang J; Duan H; Luo Y; Zeng Q; Fan K; Yan H; Lu D; Ye Z; Hao J; et al. CD146 is a coreceptor for VEGFR-2 in tumor angiogenesis. Blood 2012, 120, 2330–2339. [DOI] [PubMed] [Google Scholar]
- (564).Yan X; Lin Y; Yang D; Shen Y; Yuan M; Zhang Z; Li P; Xia H; Li L; Luo D; et al. A novel anti-CD146 monoclonal antibody, AA98, inhibits angiogenesis and tumor growth. Blood 2003, 102, 184–191. [DOI] [PubMed] [Google Scholar]
- (565).Bu P; Gao L; Zhuang J; Feng J; Yang D; Yan X Anti-CD146 monoclonal antibody AA98 inhibits angiogenesis via suppression of nuclear factor-kappaB activation. Mol. Cancer Ther 2006, 5, 2872–2878. [DOI] [PubMed] [Google Scholar]
- (566).Ma X; Liu J; Wu J; Yan X; Wu P; Liu Y; Li S; Tian Y; Cao Y; Chen G; et al. Synergistic killing effect between vorinostat and target of CD146 in malignant cells. Clin. Cancer Res 2010, 16, 5165–5176. [DOI] [PubMed] [Google Scholar]
- (567).Zeng Q; Li W; Lu D; Wu Z; Duan H; Luo Y; Feng J; Yang D; Fu L; Yan X CD146, an epithelial-mesenchymal transition inducer, is associated with triple-negative breast cancer. Proc. Natl. Acad. Sci. U. S. A 2012, 109, 1127–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (568).Van Cutsem E; Lambrechts D; Prenen H; Jain RK; Carmeliet P Lessons from the adjuvant bevacizumab trial on colon cancer: what next? J. Clin. Oncol 2011, 29, 1–4. [DOI] [PubMed] [Google Scholar]
- (569).Yang Y; Hernandez R; Rao J; Yin L; Qu Y; Wu J; England CG; Graves SA; Lewis CM; Wang P; et al. Targeting CD146 with a 64Cu-labeled antibody enables in vivo immunoPET imaging of high-grade gliomas. Proc. Natl. Acad. Sci. U. S. A 2015, 112, E6525–6534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (570).Hernandez R; Sun H; England CG; Valdovinos HF; Barnhart TE; Yang Y; Cai W ImmunoPET imaging of CD146 expression in malignant brain tumors. Mol. Pharmaceutics 2016, 13, 2563–2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (571).Sun H; England CG; Hernandez R; Graves SA; Majewski RL; Kamkaew A; Jiang D; Barnhart TE; Yang Y; Cai W ImmunoPET for assessing the differential uptake of a CD146-specific monoclonal antibody in lung cancer. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 2169–2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (572).England CG; Jiang D; Hernandez R; Sun H; Valdovinos HF; Ehlerding EB; Engle JW; Yang Y; Huang P; Cai W ImmunoPET imaging of CD146 in murine models of intrapulmonary metastasis of non-small cell lung cancer. Mol. Pharmaceutics 2017, 14, 3239–3247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (573).Hernandez R; Sun H; England CG; Valdovinos HF; Ehlerding EB; Barnhart TE; Yang Y; Cai W CD146-targeted immunoPET and NIRF imaging of hepatocellular carcinoma with a dual-labeled monoclonal antibody. Theranostics 2016, 6, 1918–1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (574).Seon BK; Haba A; Matsuno F; Takahashi N; Tsujie M; She X; Harada N; Uneda S; Tsujie T; Toi H; et al. Endoglin-targeted cancer therapy. Curr. Drug Delivery 2011, 8, 135–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (575).Dourado KMC; Baik J; Oliveira VKP; Beltrame M; Yamamoto A; Theuer CP; Figueiredo CAV; Verneris MR; Perlingeiro RCR Endoglin: a novel target for therapeutic intervention in acute leukemias revealed in xenograft mouse models. Blood 2017, 129, 2526–2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (576).Saeednejad Zanjani L; Madjd Z; Abolhasani M; Shariftabrizi A; Rasti A; Asgari M Expression of CD105 cancer stem cell marker in three subtypes of renal cell carcinoma. Cancer Biomarkers 2018, 21, 821–837. [DOI] [PubMed] [Google Scholar]
- (577).Rosen LS; Hurwitz HI; Wong MK; Goldman J; Mendelson DS; Figg WD; Spencer S; Adams BJ; Alvarez D; Seon BK; et al. A phase I first-in-human study of TRC105 (Anti-Endoglin Antibody) in patients with advanced cancer. Clin. Cancer Res 2012, 18, 4820–4829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (578).Gordon MS; Robert F; Matei D; Mendelson DS; Goldman JW; Chiorean EG; Strother RM; Seon BK; Figg WD; Peer CJ; et al. An open-label phase Ib dose-escalation study of TRC105 (anti-endoglin antibody) with bevacizumab in patients with advanced cancer. Clin. Cancer Res 2014, 20, 5918–5926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (579).Karzai FH; Apolo AB; Cao L; Madan RA; Adelberg DE; Parnes H; McLeod DG; Harold N; Peer C; Yu Y; et al. A phase I study of TRC105 anti-endoglin (CD105) antibody in metastatic castration-resistant prostate cancer. BJU Int 2015, 116, 546–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (580).Duffy AG; Ma C; Ulahannan SV; Rahma OE; Makarova-Rusher O; Cao L; Yu Y; Kleiner DE; Trepel J; Lee MJ; et al. Phase I and preliminary phase II study of TRC105 in combination with sorafenib in hepatocellular carcinoma. Clin. Cancer Res 2017, 23, 4633–4641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (581).Dorff TB; Longmate JA; Pal SK; Stadler WM; Fishman MN; Vaishampayan UN; Rao A; Pinksi JK; Hu JS; Quinn DI; et al. Bevacizumab alone or in combination with TRC105 for patients with refractory metastatic renal cell cancer. Cancer 2017, 123, 4566–4573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (582).Hong H; Yang Y; Zhang Y; Engle JW; Barnhart TE; Nickles RJ; Leigh BR; Cai W Positron emission tomography imaging of CD105 expression during tumor angiogenesis. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 1335–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (583).Hong H; Severin GW; Yang Y; Engle JW; Zhang Y; Barnhart TE; Liu G; Leigh BR; Nickles RJ; Cai W Positron emission tomography imaging of CD105 expression with 89Zr-Df-TRC105. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 138–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (584).Engle JW; Hong H; Zhang Y; Valdovinos HF; Myklejord DV; Barnhart TE; Theuer CP; Nickles RJ; Cai W Positron emission tomography imaging of tumor angiogenesis with a 66Ga-labeled monoclonal antibody. Mol. Pharmaceutics 2012, 9, 1441–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (585).Hong H; Zhang Y; Severin GW; Yang Y; Engle JW; Niu G; Nickles RJ; Chen X; Leigh BR; Barnhart TE; et al. Multimodality imaging of breast cancer experimental lung metastasis with bioluminescence and a monoclonal antibody dual-labeled with 89Zr and IRDye 800CW. Mol. Pharmaceutics 2012, 9, 2339–2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (586).Zhang Y; Hong H; Severin GW; Engle JW; Yang Y; Goel S; Nathanson AJ; Liu G; Nickles RJ; Leigh BR; et al. ImmunoPET and near-infrared fluorescence imaging of CD105 expression using a monoclonal antibody dual-labeled with (89)Zr and IRDye 800CW. Am. J. Transl. Res 2012, 4, 333–346. [PMC free article] [PubMed] [Google Scholar]
- (587).Zhang Y; Hong H; Nayak TR; Valdovinos HF; Myklejord DV; Theuer CP; Barnhart TE; Cai W Imaging tumor angiogenesis in breast cancer experimental lung metastasis with positron emission tomography, near-infrared fluorescence, and bioluminescence. Angiogenesis 2013, 16, 663–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (588).Zhang Y; Hong H; Orbay H; Valdovinos HF; Nayak TR; Theuer CP; Barnhart TE; Cai W PET imaging of CD105/ endoglin expression with a (6)(1)/(6)(4)Cu-labeled Fab antibody fragment. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 759–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (589).Hong H; Zhang Y; Orbay H; Valdovinos HF; Nayak TR; Bean J; Theuer CP; Barnhart TE; Cai W Positron emission tomography imaging of tumor angiogenesis with a (61/64)Cu-labeled F(ab′)(2) antibody fragment. Mol. Pharmaceutics 2013, 10, 709–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (590).Luo H; England CG; Shi S; Graves SA; Hernandez R; Liu B; Theuer CP; Wong HC; Nickles RJ; Cai W Dual targeting of tissue factor and CD105 for preclinical PET imaging of pancreatic cancer. Clin. Cancer Res 2016, 22, 3821–3830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (591).Luo H; England CG; Goel S; Graves SA; Ai F; Liu B; Theuer CP; Wong HC; Nickles RJ; Cai W ImmunoPET and near-Infrared fluorescence imaging of pancreatic cancer with a dual-labeled bispecific antibody fragment. Mol. Pharmaceutics 2017, 14, 1646–1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (592).Ehlerding EB; Lacognata S; Jiang D; Ferreira CA; Goel S; Hernandez R; Jeffery JJ; Theuer CP; Cai W Targeting angiogenesis for radioimmunotherapy with a (177)Lu-labeled antibody. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 123–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (593).Bockhorn M; Tsuzuki Y; Xu L; Frilling A; Broelsch CE; Fukumura D Differential vascular and transcriptional responses to anti-vascular endothelial growth factor antibody in orthotopic human pancreatic cancer xenografts. Clin. Cancer. Res 2003, 9, 4221–4226. [PubMed] [Google Scholar]
- (594).Davis DW; Inoue K; Dinney CP; Hicklin DJ; Abbruzzese JL; McConkey DJ Regional effects of an antivascular endothelial growth factor receptor monoclonal antibody on receptor phosphorylation and apoptosis in human 253J B-V bladder cancer xenografts. Cancer Res 2004, 64, 4601–4610. [DOI] [PubMed] [Google Scholar]
- (595).Sawada R; Sun SM; Wu X; Hong F; Ragupathi G; Livingston PO; Scholz WW Human monoclonal antibodies to sialyl-Lewis (CA19.9) with potent CDC, ADCC, and antitumor activity. Clin. Cancer Res 2011, 17, 1024–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (596).Viola-Villegas NT; Rice SL; Carlin S; Wu X; Evans MJ; Sevak KK; Drobjnak M; Ragupathi G; Sawada R; Scholz WW; et al. Applying PET to broaden the diagnostic utility of the clinically validated CA19.9 serum biomarker for oncology. J. Nucl. Med 2013, 54, 1876–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (597).Escorcia FE; Steckler JM; Abdel-Atti D; Price EW; Carlin SD; Scholz WW; Lewis JS; Houghton JL Tumor-specific Zr-89 immuno-PET imaging in a human bladder cancer model. Mol. Imaging Biol 2018, 20, 808–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (598).Houghton JL; Zeglis BM; Abdel-Atti D; Aggeler R; Sawada R; Agnew BJ; Scholz WW; Lewis JS Site-specifically labeled CA19.9-targeted immunoconjugates for the PET, NIRF, and multimodal PET/NIRF imaging of pancreatic cancer. Proc. Natl. Acad. Sci. U. S. A 2015, 112, 15850–15855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (599).Houghton JL; Abdel-Atti D; Scholz WW; Lewis JS Preloading with unlabeled CA19.9 targeted human monoclonal antibody leads to improved PET imaging with (89)Zr-5B1. Mol. Pharmaceutics 2017, 14, 908–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (600).Houghton JL; Zeglis BM; Abdel-Atti D; Sawada R; Scholz WW; Lewis JS Pretargeted immuno-PET of pancreatic cancer: overcoming circulating antigen and internalized antibody to reduce radiation doses. J. Nucl. Med 2016, 57, 453–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (601).Clarke-Pearson DL Clinical practice. screening for ovarian cancer. N. Engl. J. Med 2009, 361, 170–177. [DOI] [PubMed] [Google Scholar]
- (602).Sharma SK; Wuest M; Wang M; Glubrecht D; Andrais B; Lapi SE; Wuest F Immuno-PET of epithelial ovarian cancer: harnessing the potential of CA125 for non-invasive imaging. EJNMMI Res 2014, 4, 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (603).Sharma SK; Sevak KK; Monette S; Carlin SD; Knight JC; Wuest FR; Sala E; Zeglis BM; Lewis JS Preclinical 89Zr immuno-PET of high-grade serous ovarian cancer and lymph node metastasis. J. Nucl. Med 2016, 57, 771–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (604).Crawford A; Haber L; Kelly MP; Vazzana K; Canova L; Ram P; Pawashe A; Finney J; Jalal S; Chiu D; et al. A Mucin 16 bispecific T cell-engaging antibody for the treatment of ovarian cancer. Sci. Transl. Med 2019, 11, eaau7534. [DOI] [PubMed] [Google Scholar]
- (605).Bander NH Technology insight: monoclonal antibody imaging of prostate cancer. Nat. Clin. Pract. Urol 2006, 3, 216–225. [DOI] [PubMed] [Google Scholar]
- (606).Maurer T; Eiber M; Schwaiger M; Gschwend JE Current use of PSMA-PET in prostate cancer management. Nat. Rev. Urol 2016, 13, 226–235. [DOI] [PubMed] [Google Scholar]
- (607).Donin NM; Reiter RE Why Targeting PSMA is a game changer in the management of prostate cancer. J. Nucl. Med 2018, 59, 177–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (608).Wustemann T; Haberkorn U; Babich J; Mier W Targeting prostate cancer: prostate-specific membrane antigen based diagnosis and therapy. Med. Res. Rev 2019, 39, 40–69. [DOI] [PubMed] [Google Scholar]
- (609).Kaittanis C; Andreou C; Hieronymus H; Mao N; Foss CA; Eiber M; Weirich G; Panchal P; Gopalan A; Zurita J; et al. Prostate-specific membrane antigen cleavage of vitamin B9 stimulates oncogenic signaling through metabotropic glutamate receptors. J. Exp. Med 2018, 215, 159–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (610).Lutje S; Heskamp S; Cornelissen AS; Poeppel TD; van den Broek SAMW; Rosenbaum-Krumme S; Bockisch A; Gotthardt M; Rijpkema M; Boerman OC PSMA ligands for radionuclide imaging and therapy of prostate cancer: clinical status. Theranostics 2015, 5, 1388–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (611).Afshar-Oromieh A; Babich JW; Kratochwil C; Giesel FL; Eisenhut M; Kopka K; Haberkorn U The rise of PSMA ligands for diagnosis and therapy of prostate cancer. J. Nucl. Med 2016, 57, 79S–89S. [DOI] [PubMed] [Google Scholar]
- (612).Ceci F; Herrmann K; Hadaschik B; Castellucci P; Fanti S Therapy assessment in prostate cancer using choline and PSMA PET/CT. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 78–83. [DOI] [PubMed] [Google Scholar]
- (613).Sheikhbahaei S; Afshar-Oromieh A; Eiber M; Solnes LB; Javadi MS; Ross AE; Pienta KJ; Allaf ME; Haberkorn U; Pomper MG; et al. Pearls and pitfalls in clinical interpretation of prostate-specific membrane antigen (PSMA)-targeted PET imaging. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 2117–2136. [DOI] [PubMed] [Google Scholar]
- (614).Liu H; Moy P; Kim S; Xia Y; Rajasekaran A; Navarro V; Knudsen B; Bander NH Monoclonal antibodies to the extracellular domain of prostate-specific membrane antigen also react with tumor vascular endothelium. Cancer Res 1997, 57, 3629–3634. [PubMed] [Google Scholar]
- (615).Chang SS; Reuter VE; Heston WD; Bander NH; Grauer LS; Gaudin PB Five different anti-prostate-specific membrane antigen (PSMA) antibodies confirm PSMA expression in tumor-associated neovasculature. Cancer Res 1999, 59, 3192–3198. [PubMed] [Google Scholar]
- (616).Liu H; Rajasekaran AK; Moy P; Xia Y; Kim S; Navarro V; Rahmati R; Bander NH Constitutive and antibody-induced internalization of prostate-specific membrane antigen. Cancer Res 1998, 58, 4055–4060. [PubMed] [Google Scholar]
- (617).Milowsky MI; Nanus DM; Kostakoglu L; Vallabhajosula S; Goldsmith SJ; Bander NH Phase I trial of yttrium-90-labeled anti-prostate-specific membrane antigen monoclonal antibody J591 for androgen-independent prostate cancer. J. Clin. Oncol 2004, 22, 2522–2531. [DOI] [PubMed] [Google Scholar]
- (618).Tagawa ST; Milowsky MI; Morris M; Vallabhajosula S; Christos P; Akhtar NH; Osborne J; Goldsmith SJ; Larson S; Taskar NP; et al. Phase II study of lutetium-177-labeled anti-prostate-specific membrane antigen monoclonal antibody J591 for metastatic castration-resistant prostate cancer. Clin. Cancer Res 2013, 19, 5182–5191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (619).Pandit-Taskar N; O’Donoghue JA; Beylergil V; Lyashchenko S; Ruan S; Solomon SB; Durack JC; Carrasquillo JA; Lefkowitz RA; Gonen M; et al. (8)(9)ZrhuJ591 immuno-PET imaging in patients with advanced metastatic prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 2093–2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (620).Pandit-Taskar N; O’Donoghue JA; Durack JC; Lyashchenko SK; Cheal SM; Beylergil V; Lefkowitz RA; Carrasquillo JA; Martinez DF; Fung AM; et al. A phase I/II study for analytic validation of 89Zr-J591 immunoPET as a molecular imaging agent for metastatic prostate cancer. Clin. Cancer Res 2015, 21, 5277–5285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (621).Pandit-Taskar N; O’Donoghue JA; Divgi CR; Wills EA; Schwartz L; Gonen M; Smith-Jones P; Bander NH; Scher HI; Larson SM; Morris MJ Indium 111-labeled J591 anti-PSMA antibody for vascular targeted imaging in progressive solid tumors. EJNMMI Res 2015, 5, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (622).Fung EK; Cheal SM; Fareedy SB; Punzalan B; Beylergil V; Amir J; Chalasani S; Weber WA; Spratt DE; Veach DR; et al. Targeting of radiolabeled J591 antibody to PSMA-expressing tumors: optimization of imaging and therapy based on non-linear compartmental modeling. EJNMMI Res 2016, 6, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (623).Petronis JD; Regan F; Lin K Indium-111 capromab pendetide (ProstaScint) imaging to detect recurrent and metastatic prostate cancer. Clin. Nucl. Med 1998, 23, 672–677. [DOI] [PubMed] [Google Scholar]
- (624).Deb N; Goris M; Trisler K; Fowler S; Saal J; Ning S; Becker M; Marquez C; Knox S Treatment of hormone-refractory prostate cancer with 90Y-CYT-356 monoclonal antibody. Clin. Cancer. Res 1996, 2, 1289–1297. [PubMed] [Google Scholar]
- (625).Tagawa ST; Beltran H; Vallabhajosula S; Goldsmith SJ; Osborne J; Matulich D; Petrillo K; Parmar S; Nanus DM; Bander NH Anti-prostate-specific membrane antigen-based radio-immunotherapy for prostate cancer. Cancer 2010, 116, 1075–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (626).Lutje S; Rijpkema M; Franssen GM; Fracasso G; Helfrich W; Eek A; Oyen WJ; Colombatti M; Boerman OC Dual-modality image-guided surgery of prostate cancer with a radiolabeled fluorescent anti-PSMA monoclonal antibody. J. Nucl. Med 2014, 55, 995–1001. [DOI] [PubMed] [Google Scholar]
- (627).Lutje S; Gerrits D; Molkenboer-Kuenen JD; Herrmann K; Fracasso G; Colombatti M; Boerman OC; Heskamp S Characterization of site-specifically conjugated monomethyl auristatin E- and duocarmycin-based anti-PSMA antibody-drug conjugates for treatment of PSMA-expressing tumors. J. Nucl. Med 2018, 59, 494–501. [DOI] [PubMed] [Google Scholar]
- (628).Novakova Z; Foss CA; Copeland BT; Morath V; Baranova P; Havlinova B; Skerra A; Pomper MG; Barinka C Novel monoclonal antibodies recognizing human prostate-specific membrane antigen (PSMA) as research and theranostic Tools. Prostate 2017, 77, 749–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (629).Banerjee SR; Kumar V; Lisok A; Plyku D; Novakova Z; Brummet M; Wharram B; Barinka C; Hobbs R; Pomper MG Evaluation of (111)In-DOTA-5D3, a surrogate SPECT imaging agent for radioimmunotherapy of prostate-specific membrane antigen. J. Nucl. Med 2019, 60, 400–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (630).Chatalic KL; Veldhoven-Zweistra J; Bolkestein M; Hoeben S; Koning GA; Boerman OC; de Jong M; van Weerden WM A Novel (1)(1)(1)In-labeled anti-prostate-specific membrane antigen nanobody for targeted SPECT/CT imaging of prostate cancer. J. Nucl. Med 2015, 56, 1094–1099. [DOI] [PubMed] [Google Scholar]
- (631).Nawaz S; Mullen GED; Sunassee K; Bordoloi J; Blower PJ; Ballinger JR Simple, mild, one-step labelling of proteins with gallium-68 using a tris(hydroxypyridinone) bifunctional chelator: a (68)Ga-THP-scFv targeting the prostate-specific membrane antigen. EJNMMI Res 2017, 7, 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (632).Viola-Villegas NT; Sevak KK; Carlin SD; Doran MG; Evans HW; Bartlett DW; Wu AM; Lewis JS Noninvasive Imaging of PSMA in prostate tumors with (89)Zr-Labeled huJ591 engineered antibody fragments: the faster alternatives. Mol. Pharmaceutics 2014, 11, 3965–3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (633).Pandit-Taskar N; O’Donoghue JA; Ruan S; Lyashchenko SK; Carrasquillo JA; Heller G; Martinez DF; Cheal SM; Lewis JS; Fleisher M; et al. First-in-human imaging with 89Zr-Df-IAB2M anti-PSMA minibody in patients with metastatic prostate cancer: pharmacokinetics, biodistribution, dosimetry, and lesion uptake. J. Nucl. Med 2016, 57, 1858–1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (634).Joraku A; Hatano K; Kawai K; Kandori S; Kojima T; Fukumitsu N; Isobe T; Mori Y; Sakata M; Hara T; et al. Phase I/IIa PET imaging study with (89)zirconium labeled anti-PSMA minibody for urological malignancies. Ann. Nucl. Med 2019, 33, 119–127. [DOI] [PubMed] [Google Scholar]
- (635).Langbein T; Chausse G; Baum RP Salivary gland toxicity of PSMA radioligand therapy: relevance and preventive strategies. J. Nucl. Med 2018, 59, 1172–1173. [DOI] [PubMed] [Google Scholar]
- (636).Elsasser-Beile U; Wolf P; Gierschner D; Buhler P; Schultze-Seemann W; Wetterauer U A new generation of monoclonal and recombinant antibodies against cell-adherent prostate specific membrane antigen for diagnostic and therapeutic targeting of prostate cancer. Prostate 2006, 66, 1359–1370. [DOI] [PubMed] [Google Scholar]
- (637).Elsasser-Beile U; Reischl G; Wiehr S; Buhler P; Wolf P; Alt K; Shively J; Judenhofer MS; Machulla HJ; Pichler BJ PET imaging of prostate cancer xenografts with a highly specific antibody against the prostate-specific membrane antigen. J. Nucl. Med 2009, 50, 606–611. [DOI] [PubMed] [Google Scholar]
- (638).Alt K; Wiehr S; Ehrlichmann W; Reischl G; Wolf P; Pichler BJ; Elsasser-Beile U; Buhler P High-resolution animal PET imaging of prostate cancer xenografts with three different 64Cu-labeled antibodies against native cell-adherent PSMA. Prostate 2010, 70, 1413–1421. [DOI] [PubMed] [Google Scholar]
- (639).Wiehr S; Buhler P; Gierschner D; Wolf P; Rolle AM; Kesenheimer C; Pichler BJ; Elsasser-Beile U Pharmacokinetics and PET imaging properties of two recombinant anti-PSMA antibody fragments in comparison to their parental antibody. Prostate 2014, 74, 743–755. [DOI] [PubMed] [Google Scholar]
- (640).Rowe SP; Gorin MA; Pomper MG Imaging of prostate-specific membrane antigen with small-molecule PET radiotracers: from the bench to advanced clinical applications. Annu. Rev. Med 2019, 70, 461–477. [DOI] [PubMed] [Google Scholar]
- (641).Salas Fragomeni RA; Amir T; Sheikhbahaei S; Harvey SC; Javadi MS; Solnes LB; Kiess AP; Allaf ME; Pomper MG; Gorin MA; et al. Imaging of nonprostate cancers using PSMA-targeted radiotracers: rationale, current state of the field, and a Call to Arms. J. Nucl. Med 2018, 59, 871–877. [DOI] [PubMed] [Google Scholar]
- (642).Malik D; Kumar R; Mittal BR; Singh H; Bhattacharya A; Singh SK 68Ga-labeled PSMA uptake in nonprostatic malignancies: has the time come to remove “PS” From PSMA? Clin. Nucl. Med 2018, 43, 529–532. [DOI] [PubMed] [Google Scholar]
- (643).Silver DA; Pellicer I; Fair WR; Heston WD; Cordon-Cardo C Prostate-specific membrane antigen expression in normal and malignant human tissues. Clin. Cancer. Res 1997, 3, 81–85. [PubMed] [Google Scholar]
- (644).Chang SS; O’Keefe DS; Bacich DJ; Reuter VE; Heston WD; Gaudin PB Prostate-specific membrane antigen is produced in tumor-associated neovasculature. Clin. Cancer. Res 1999, 5, 2674–2681. [PubMed] [Google Scholar]
- (645).Matsuda M; Ishikawa E; Yamamoto T; Hatano K; Joraku A; Iizumi Y; Masuda Y; Nishiyama H; Matsumura A Potential use of prostate specific membrane antigen (PSMA) for detecting the tumor neovasculature of brain tumors by PET imaging with (89)Zr-Df-IAB2M anti-PSMA minibody. J. Neuro-Oncol 2018, 138, 581–589. [DOI] [PubMed] [Google Scholar]
- (646).Milowsky MI; Nanus DM; Kostakoglu L; Sheehan CE; Vallabhajosula S; Goldsmith SJ; Ross JS; Bander NH Vascular targeted therapy with anti-prostate-specific membrane antigen monoclonal antibody J591 in advanced solid tumors. J. Clin. Oncol 2007, 25, 540–547. [DOI] [PubMed] [Google Scholar]
- (647).Beauchemin N; Arabzadeh A Carcinoembryonic antigen-related cell adhesion molecules (CEACAMs) in cancer progression and metastasis. Cancer Metastasis Rev 2013, 32, 643–671. [DOI] [PubMed] [Google Scholar]
- (648).Moffat FL Jr.; Pinsky CM; Hammershaimb L; Petrelli NJ; Patt YZ; Whaley FS; Goldenberg DM Clinical utility of external immunoscintigraphy with the IMMU-4 technetium-99m Fab′ antibody fragment in patients undergoing surgery for carcinoma of the colon and rectum: results of a pivotal, phase III trial. the immunomedics study group. J. Clin. Oncol 1996, 14, 2295–2305. [DOI] [PubMed] [Google Scholar]
- (649).Sharkey RM; Cardillo TM; Rossi EA; Chang CH; Karacay H; McBride WJ; Hansen HJ; Horak ID; Goldenberg DM Signal amplification in molecular imaging by pretargeting a multivalent, bispecific antibody. Nat. Med 2005, 11, 1250–1255. [DOI] [PubMed] [Google Scholar]
- (650).Schoffelen R; Sharkey RM; Goldenberg DM; Franssen G; McBride WJ; Rossi EA; Chang CH; Laverman P; Disselhorst JA; Eek A; et al. Pretargeted immuno-positron emission tomography imaging of carcinoembryonic antigen-expressing tumors with a bispecific antibody and a 68Ga- and 18F-labeled hapten peptide in mice with human tumor xenografts. Mol. Cancer Ther 2010, 9, 1019–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (651).Schoffelen R; van der Graaf WT; Sharkey RM; Franssen GM; McBride WJ; Chang CH; Laverman P; Goldenberg DM; Oyen WJ; Boerman OC Pretargeted immuno-PET of CEA-expressing intraperitoneal human colonic tumor xenografts: a new sensitive detection method. EJNMMI Res 2012, 2, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (652).Foubert F; Gouard S; Sai-Maurel C; Cherel M; Faivre-Chauvet A; Goldenberg DM; Barbet J; Bailly C; Bodet-Milin C; Carlier T; et al. Sensitivity of pretargeted immunoPET using(68)Ga-peptide to detect colonic carcinoma liver metastases in a murine xenograft model: Comparison with (18)FDG PET-CT. Oncotarget 2018, 9, 27502–27513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (653).Hall H; Velikyan I; Blom E; Ulin J; Monazzam A; Pahlman L; Micke P; Wanders A; McBride W; Goldenberg DM; et al. In vitro autoradiography of carcinoembryonic antigen in tissue from patients with colorectal cancer using multifunctional antibody TF2 and (67/68Ga)-labeled haptens by pretargeting. Am. J. Nucl. Med. Mol. Imaging 2012, 2, 141–150. [PMC free article] [PubMed] [Google Scholar]
- (654).Bodet-Milin C; Faivre-Chauvet A; Carlier T; Rauscher A; Bourgeois M; Cerato E; Rohmer V; Couturier O; Drui D; Goldenberg DM; et al. Immuno-PET using anticarcinoembryonic antigen bispecific antibody and 68Ga-labeled peptide in metastatic medullary thyroid carcinoma: clinical optimization of the pretargeting parameters in a first-in-human trial. J. Nucl. Med 2016, 57, 1505–1511. [DOI] [PubMed] [Google Scholar]
- (655).Wu AM; Yazaki PJ; Tsai S; Nguyen K; Anderson AL; McCarthy DW; Welch MJ; Shively JE; Williams LE; Raubitschek AA; et al. High-resolution microPET imaging of carcinoembryonic antigen-positive xenografts by using a copper-64-labeled engineered antibody fragment. Proc. Natl. Acad. Sci. U. S. A 2000, 97, 8495–8500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (656).Sundaresan G; Yazaki PJ; Shively JE; Finn RD; Larson SM; Raubitschek AA; Williams LE; Chatziioannou AF; Gambhir SS; Wu AM 124I-labeled engineered anti-CEA minibodies and diabodies allow high-contrast, antigen-specific small-animal PET imaging of xenografts in athymic mice. J. Nucl. Med 2003, 44, 1962–1969. [PMC free article] [PubMed] [Google Scholar]
- (657).Li L; Yazaki PJ; Anderson AL; Crow D; Colcher D; Wu AM; Williams LE; Wong JY; Raubitschek A; Shively JE Improved biodistribution and radioimmunoimaging with poly-(ethylene glycol)-DOTA-conjugated anti-CEA diabody. Bioconjugate Chem 2006, 17, 68–76. [DOI] [PubMed] [Google Scholar]
- (658).Kenanova V; Olafsen T; Crow DM; Sundaresan G; Subbarayan M; Carter NH; Ikle DN; Yazaki PJ; Chatziioannou AF; Gambhir SS; et al. Tailoring the pharmacokinetics and positron emission tomography imaging properties of anti-carcinoembryonic antigen single-chain Fv-Fc antibody fragments. Cancer Res 2005, 65, 622–631. [PMC free article] [PubMed] [Google Scholar]
- (659).Girgis MD; Olafsen T; Kenanova V; McCabe KE; Wu AM; Tomlinson JS Targeting CEA in pancreas cancer xenografts with a mutated scFv-Fc antibody fragment. EJNMMI Res 2011, 1, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (660).Lutje S; Franssen GM; Sharkey RM; Laverman P; Rossi EA; Goldenberg DM; Oyen WJ; Boerman OC; McBride WJ Anti-CEA antibody fragments labeled with [(18)F]AlF for PET imaging of CEA-expressing tumors. Bioconjugate Chem 2014, 25, 335–341. [DOI] [PubMed] [Google Scholar]
- (661).Osada T; Hsu D; Hammond S; Hobeika A; Devi G; Clay TM; Lyerly HK; Morse MA Metastatic colorectal cancer cells from patients previously treated with chemotherapy are sensitive to T-cell killing mediated by CEA/CD3-bispecific T-cell-engaging BiTE antibody. Br. J. Cancer 2010, 102, 124–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (662).Pishvaian M; Morse MA; McDevitt J; Norton JD; Ren S; Robbie GJ; Ryan PC; Soukharev S; Bao H; Denlinger CS Phase 1 dose escalation study of MEDI-565, a bispecific T-cell engager that targets human carcinoembryonic antigen, in patients with advanced gastrointestinal adenocarcinomas. Clin. Colorectal Cancer 2016, 15, 345–351. [DOI] [PubMed] [Google Scholar]
- (663).Waaijer SJH; Warnders FJ; Stienen S; Friedrich M; Sternjak A; Cheung HK; van Scheltinga A; Schroder CP; de Vries EGE; Lub-de Hooge MN Molecular imaging of radiolabeled bispecific T-cell engager (89)Zr-AMG211 targeting CEA-positive tumors. Clin. Cancer Res 2018, 24, 4988–4996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (664).Moek KL; Waaijer SJH; Kok IC; Suurs FV; Brouwers AH; Menke-van der Houven van Oordt CW; Wind TT; Gietema JA; Schroder CP; Mahesh SVK; et al. 89)Zr-labeled bispecific T-cell engager AMG 211 PET shows AMG 211 accumulation in CD3-rich tissues and clear, heterogeneous tumor Uptake. Clin. Cancer Res 2019, 25, 3517–3527. [DOI] [PubMed] [Google Scholar]
- (665).Klein C; Waldhauer I; Nicolini VG; Freimoser-Grundschober A; Nayak T; Vugts DJ; Dunn C; Bolijn M; Benz J; Stihle M; et al. Cergutuzumab amunaleukin (CEA-IL2v), a CEA-targeted IL-2 variant-based immunocytokine for combination cancer immunotherapy: Overcoming limitations of aldesleukin and conventional IL-2-based immunocytokines. Oncoimmunology 2017, 6, e1277306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (666).van Brummelen EMJ; Huisman MC; de Wit-van der Veen LJ; Nayak TK; Stokkel MPM; Mulder ER; Hoekstra OS; Vugts DJ; Van Dongen G; Verheul HM; et al. (89)Zr-labeled CEA-targeted IL-2 variant immunocytokine in patients with solid tumors: CEA-mediated tumor accumulation and role of IL-2 receptor-binding. Oncotarget 2018, 9, 24737–24749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (667).Corbet C; Feron O Tumour acidosis: from the passenger to the driver’s seat. Nat. Rev. Cancer 2017, 17, 577–593. [DOI] [PubMed] [Google Scholar]
- (668).Supuran CT; Alterio V; Di Fiore A; D’Ambrosio K; Carta F; Monti SM; De Simone G Inhibition of carbonic anhydrase IX targets primary tumors, metastases, and cancer stem cells: Three for the price of one. Med. Res. Rev 2018, 38, 1799–1836. [DOI] [PubMed] [Google Scholar]
- (669).Oosterwijk-Wakka JC; Boerman OC; Mulders PF; Oosterwijk E Application of monoclonal antibody G250 recognizing carbonic anhydrase IX in renal cell carcinoma. Int. J. Mol. Sci 2013, 14, 11402–11423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (670).Lau J; Lin KS; Benard F Past, Present, and Future: Development of theranostic agents targeting carbonic anhydrase IX. Theranostics 2017, 7, 4322–4339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (671).Lawrentschuk N; Lee FT; Jones G; Rigopoulos A; Mountain A; O’Keefe G; Papenfuss AT; Bolton DM; Davis ID; Scott AM Investigation of hypoxia and carbonic anhydrase IX expression in a renal cell carcinoma xenograft model with oxygen tension measurements and (1)(2)(4)I-cG250 PET/CT. Urol. Oncol 2011, 29, 411–420. [DOI] [PubMed] [Google Scholar]
- (672).Stillebroer AB; Franssen GM; Mulders PF; Oyen WJ; van Dongen GA; Laverman P; Oosterwijk E; Boerman OC ImmunoPET imaging of renal cell carcinoma with (124)I- and(89)Zr-labeled anti-CAIX monoclonal antibody cG250 in mice. Cancer Biother.Radiopharm 2013, 28, 510–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (673).Cheal SM; Punzalan B; Doran MG; Evans MJ; Osborne JR; Lewis JS; Zanzonico P; Larson SM Pairwise comparison of 89Zr- and 124I-labeled cG250 based on positron emission tomography imaging and nonlinear immunokinetic modeling: in vivo carbonic anhydrase IX receptor binding and internalization in mouse xenografts of clear-cell renal cell carcinoma. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 985–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (674).Hekman MCH; Rijpkema M; Aarntzen EH; Mulder SF; Langenhuijsen JF; Oosterwijk E; Boerman OC; Oyen WJG; Mulders PFA Positron emission tomography/computed tomography with (89)Zr-girentuximab can aid in diagnostic dilemmas of clear cell renal cell carcinoma suspicion. Eur. Urol 2018, 74, 257–260. [DOI] [PubMed] [Google Scholar]
- (675).Pryma DA; O’Donoghue JA; Humm JL; Jungbluth AA; Old LJ; Larson SM; Divgi CR Correlation of in vivo and in vitro measures of carbonic anhydrase IX antigen expression in renal masses using antibody 124I-cG250. J. Nucl. Med 2011, 52, 535–540. [DOI] [PubMed] [Google Scholar]
- (676).Povoski SP; Hall NC; Murrey DA Jr.; Sharp DS; Hitchcock CL; Mojzisik CM; Bahnson EE; Knopp MV; Martin EW Jr.; Bahnson RR Multimodal imaging and detection strategy with 124 I-labeled chimeric monoclonal antibody cG250 for accurate localization and confirmation of extent of disease during laparoscopic and open surgical resection of clear cell renal cell carcinoma. Surg. Innov 2013, 20, 59–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (677).Jensen HK; Nordsmark M; Donskov F; Marcussen N; von der Maase H Immunohistochemical expression of carbonic anhydrase IX assessed over time and during treatment in renal cell carcinoma. BJU Int 2008, 101, 41–44. [DOI] [PubMed] [Google Scholar]
- (678).Cepa A; Ralis J; Kral V; Paurova M; Kucka J; Humajova J; Laznicek M; Lebeda O In vitro evaluation of the monoclonal antibody (64)Cu-IgG M75 against human carbonic anhydrase IX and its in vivo imaging. Appl. Radiat. Isot 2018, 133, 9–13. [DOI] [PubMed] [Google Scholar]
- (679).Cepa A; Ralis J; Maresova L; Kleinova M; Seifert D; Sieglova I; Kral V; Polasek M; Paurova M; Laznicek M; et al. Radiolabeling of the antibody IgG M75 for epitope of human carbonic anhydrase IX by (61)Cu and (64)Cu and its biological testing. Appl. Radiat. Isot 2019, 143, 87–97. [DOI] [PubMed] [Google Scholar]
- (680).Ahlskog JK; Schliemann C; Marlind J; Qureshi U; Ammar A; Pedley RB; Neri D Human monoclonal antibodies targeting carbonic anhydrase IX for the molecular imaging of hypoxic regions in solid tumours. Br. J. Cancer 2009, 101, 645–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (681).Zatovicova M; Jelenska L; Hulikova A; Csaderova L; Ditte Z; Ditte P; Goliasova T; Pastorek J; Pastorekova S Carbonic anhydrase IX as an anticancer therapy target: preclinical evaluation of internalizing monoclonal antibody directed to catalytic domain. Curr. Pharm. Des 2010, 16, 3255–3263. [DOI] [PubMed] [Google Scholar]
- (682).Murri-Plesko MT; Hulikova A; Oosterwijk E; Scott AM; Zortea A; Harris AL; Ritter G; Old L; Bauer S; Swietach P; et al. Antibody inhibiting enzymatic activity of tumour-associated carbonic anhydrase isoform IX. Eur. J. Pharmacol 2011, 657, 173–183. [DOI] [PubMed] [Google Scholar]
- (683).van Rij CM; Frielink C; Goldenberg DM; Sharkey RM; Lutje S; McBride WJ; Oyen WJ; Boerman OC Pretargeted radioimmunotherapy of prostate cancer with an anti-TROP-2xanti-HSG bispecific antibody and a (177)Lu-labeled peptide. Cancer Biother.Radiopharm 2014, 29, 323–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (684).Goldenberg DM; Stein R; Sharkey RM The emergence of trophoblast cell-surface antigen 2 (TROP-2) as a novel cancer target. Oncotarget 2018, 9, 28989–29006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (685).Bardia A; Mayer IA; Vahdat LT; Tolaney SM; Isakoff SJ; Diamond JR; O’Shaughnessy J; Moroose RL; Santin AD; Abramson VG; et al. Sacituzumab Govitecan-hziy in refractory metastatic triple-negative breast cancer. N. Engl. J. Med 2019, 380, 741–751. [DOI] [PubMed] [Google Scholar]
- (686).van Rij CM; Sharkey RM; Goldenberg DM; Frielink C; Molkenboer JD; Franssen GM; van Weerden WM; Oyen WJ; Boerman OC Imaging of prostate cancer with immuno-PET and immuno-SPECT using a radiolabeled anti-EGP-1 monoclonal antibody. J. Nucl. Med 2011, 52, 1601–1607. [DOI] [PubMed] [Google Scholar]
- (687).Sharkey RM; van Rij CM; Karacay H; Rossi EA; Frielink C; Regino C; Cardillo TM; McBride WJ; Chang CH; Boerman OC; et al. A new Tri-Fab bispecific antibody for pretargeting Trop-2-expressing epithelial cancers. J. Nucl. Med 2012, 53, 1625–1632. [DOI] [PubMed] [Google Scholar]
- (688).van Rij CM; Lutje S; Frielink C; Sharkey RM; Goldenberg DM; Franssen GM; McBride WJ; Rossi EA; Oyen WJ; Boerman OC Pretargeted immuno-PET and radioimmunotherapy of prostate cancer with an anti-TROP-2 x anti-HSG bispecific antibody. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 1377–1383.23674207 [Google Scholar]
- (689).Lutje S; Rijpkema M; Goldenberg DM; van Rij CM; Sharkey RM; McBride WJ; Franssen GM; Frielink C; Helfrich W; Oyen WJ; et al. Pretargeted dual-modality immuno-SPECT and near-infrared fluorescence imaging for image-guided surgery of prostate cancer. Cancer Res 2014, 74, 6216–6223. [DOI] [PubMed] [Google Scholar]
- (690).van Rij CM; Frielink C; Goldenberg DM; Sharkey RM; Franssen GM; Lutje S; McBride WJ; Oyen WJ; Boerman OC Pretargeted immunoPET of prostate cancer with an anti-TROP-2 x anti-HSG bispecific antibody in mice with PC3 xenografts. Mol. Imaging Biol 2015, 17, 94–101. [DOI] [PubMed] [Google Scholar]
- (691).Heist RS; Guarino MJ; Masters G; Purcell WT; Starodub AN; Horn L; Scheff RJ; Bardia A; Messersmith WA; Berlin J; et al. Therapy of advanced non-small-cell lung cancer with an SN-38-anti-Trop-2 drug conjugate, Sacituzumab Govitecan. J. Clin. Oncol 2017, 35, 2790–2797. [DOI] [PubMed] [Google Scholar]
- (692).Batlle E; Clevers H Cancer stem cells revisited. Nat. Med 2017, 23, 1124–1134. [DOI] [PubMed] [Google Scholar]
- (693).Medema JP Cancer stem cells: the challenges ahead. Nat. Cell Biol 2013, 15, 338–344. [DOI] [PubMed] [Google Scholar]
- (694).Steinbichler TB; Dudas J; Skvortsov S; Ganswindt U; Riechelmann H; Skvortsova II. Therapy resistance mediated by cancer stem cells. Semin. Cancer Biol 2018, 53, 156–167. [DOI] [PubMed] [Google Scholar]
- (695).Leung C; Tan SH; Barker N Recent advances in Lgr5(+) stem cell research. Trends Cell Biol 2018, 28, 380–391. [DOI] [PubMed] [Google Scholar]
- (696).Azhdarinia A; Voss J; Ghosh SC; Simien JA; Hernandez Vargas S; Cui J; Yu WA; Liu Q; Carmon KS Evaluation of anti-LGR5 antibodies by immunoPET for imaging colorectal tumors and development of antibody-drug conjugates. Mol. Pharmaceutics 2018, 15, 2448–2454. [DOI] [PubMed] [Google Scholar]
- (697).Knowles SM; Zettlitz KA; Tavare R; Rochefort MM; Salazar FB; Stout DB; Yazaki PJ; Reiter RE; Wu AM Quantitative immunoPET of prostate cancer xenografts with 89Zrand 124I-labeled anti-PSCA A11 minibody. J. Nucl. Med 2014, 55, 452–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (698).Knowles SM; Tavare R; Zettlitz KA; Rochefort MM; Salazar FB; Jiang ZK; Reiter RE; Wu AM Applications of immunoPET: using 124I-anti-PSCA A11 minibody for imaging disease progression and response to therapy in mouse xenograft models of prostate cancer. Clin. Cancer Res 2014, 20, 6367–6378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (699).Sonn GA; Behesnilian AS; Jiang ZK; Zettlitz KA; Lepin EJ; Bentolila LA; Knowles SM; Lawrence D; Wu AM; Reiter RE Fluorescent image-guided surgery with an anti-prostate stem cell antigen (PSCA) diabody enables targeted resection of mouse prostate cancer xenografts in real time. Clin. Cancer Res 2016, 22, 1403–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (700).Tsai WK; Zettlitz KA; Tavare R; Kobayashi N; Reiter RE; Wu AM Dual-modality immunoPET/fluorescence imaging of prostate cancer with an anti-PSCA cys-minibody. Theranostics 2018, 8, 5903–5914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (701).Zettlitz KA; Tsai WK; Knowles SM; Kobayashi N; Donahue TR; Reiter RE; Wu AM Dual-modality immuno-PET and near-Infrared fluorescence imaging of pancreatic cancer using an anti-prostate stem cell antigen cys-diabody. J. Nucl. Med 2018, 59, 1398–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (702).Kemper K; Sprick MR; de Bree M; Scopelliti A; Vermeulen L; Hoek M; Zeilstra J; Pals ST; Mehmet H; Stassi G; et al. The AC133 epitope, but not the CD133 protein, is lost upon cancer stem cell differentiation. Cancer Res 2010, 70, 719–729. [DOI] [PubMed] [Google Scholar]
- (703).Tsurumi C; Esser N; Firat E; Gaedicke S; Follo M; Behe M; Elsasser-Beile U; Grosu AL; Graeser R; Niedermann G Non-invasive in vivo imaging of tumor-associated CD133/prominin. PLoS One 2010, 5, e15605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (704).Gaedicke S; Braun F; Prasad S; Machein M; Firat E; Hettich M; Gudihal R; Zhu X; Klingner K; Schuler J; et al. Noninvasive positron emission tomography and fluorescence imaging of CD133+ tumor stem cells. Proc. Natl. Acad. Sci. U. S. A 2014, 111, E692–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (705).Lopez-Otin C; Hunter T The regulatory crosstalk between kinases and proteases in cancer. Nat. Rev. Cancer 2010, 10, 278–292. [DOI] [PubMed] [Google Scholar]
- (706).Skovgaard D; Persson M; Kjaer A PET imaging of urokinase-type plasminogen activator receptor (uPAR) in prostate cancer: current status and future perspectives. Clin Transl Imaging 2016, 4, 457–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (707).Persson M; Skovgaard D; Brandt-Larsen M; Christensen C; Madsen J; Nielsen CH; Thurison T; Klausen TL; Holm S; Loft A; et al. First-in-human uPAR PET: imaging of cancer aggressiveness. Theranostics 2015, 5, 1303–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (708).Skovgaard D; Persson M; Brandt-Larsen M; Christensen C; Madsen J; Klausen TL; Holm S; Andersen FL; Loft A; Berthelsen AK; et al. Safety, dosimetry, and tumor detection ability of (68)Ga-NOTA-AE105: first-in-human study of a novel radioligand for uPAR PET imaging. J. Nucl. Med 2017, 58, 379–386. [DOI] [PubMed] [Google Scholar]
- (709).Yang D; Severin GW; Dougherty CA; Lombardi R; Chen D; Van Dort ME; Barnhart TE; Ross BD; Mazar AP; Hong H Antibody-based PET of uPA/uPAR signaling with broad applicability for cancer imaging. Oncotarget 2016, 7, 73912–73924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (710).Kessenbrock K; Plaks V; Werb Z Matrix metal-loproteinases: regulators of the tumor microenvironment. Cell 2010, 141, 52–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (711).de Lucas AG; Schuhmacher AJ; Oteo M; Romero E; Camara JA; de Martino A; Arroyo AG; Morcillo MA; Squatrito M; Martinez-Torrecuadrada JL; et al. Targeting MT1-MMP as an immunoPET-based strategy for imaging gliomas. PLoS One 2016, 11, e0158634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (712).Morcillo MA; Garcia de Lucas A; Oteo M; Romero E; Magro N; Ibanez M; Martinez A; Garaulet G; Arroyo AG; Lopez-Casas PP; et al. MT1-MMP as a PET imaging biomarker for pancreas cancer management. Contrast Media Mol. Imaging 2018, 2018, 8382148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (713).Ulmert D; Evans MJ; Holland JP; Rice SL; Wongvipat J; Pettersson K; Abrahamsson PA; Scardino PT; Larson SM; Lilja H; et al. Imaging androgen receptor signaling with a radiotracer targeting free prostate-specific antigen. Cancer Discovery 2012, 2, 320–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (714).Thorek DL; Watson PA; Lee SG; Ku AT; Bournazos S; Braun K; Kim K; Sjostrom K; Doran MG; Lamminmaki U; et al. Internalization of secreted antigen-targeted antibodies by the neonatal Fc receptor for precision imaging of the androgen receptor axis. Sci. Transl. Med 2016, 8, 367ra167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (715).Pastan I; Hassan R Discovery of mesothelin and exploiting it as a target for immunotherapy. Cancer Res 2014, 74, 2907–2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (716).Hassan R; Thomas A; Alewine C; Le DT; Jaffee EM; Pastan I Mesothelin immunotherapy for cancer: ready for prime time? J. Clin. Oncol 2016, 34, 4171–4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (717).Creaney J; Francis RJ; Dick IM; Musk AW; Robinson BW; Byrne MJ; Nowak AK Serum soluble mesothelin concentrations in malignant pleural mesothelioma: relationship to tumor volume, clinical stage and changes in tumor burden. Clin. Cancer Res 2011, 17, 1181–1189. [DOI] [PubMed] [Google Scholar]
- (718).Kobayashi K; Sasaki T; Takenaka F; Yakushiji H; Fujii Y; Kishi Y; Kita S; Shen L; Kumon H; Matsuura E A novel PET imaging using (6)(4)Cu-labeled monoclonal antibody against mesothelin commonly expressed on cancer cells. J. Immunol. Res 2015, 2015, 268172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (719).Lindenberg L; Thomas A; Adler S; Mena E; Kurdziel K; Maltzman J; Wallin B; Hoffman K; Pastan I; Paik CH; et al. Safety and biodistribution of 111In-amatuximab in patients with mesothelin expressing cancers using single photon emission computed tomography-computed tomography (SPECT-CT) imaging. Oncotarget 2015, 6, 4496–4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (720).Terwisscha van Scheltinga AG; Ogasawara A; Pacheco G; Vanderbilt AN; Tinianow JN; Gupta N; Li D; Firestein R; Marik J; Scales SJ; et al. Preclinical efficacy of an antibody-drug conjugate targeting mesothelin correlates with quantitative 89ZrimmunoPET. Mol. Cancer Ther 2017, 16, 134–142. [DOI] [PubMed] [Google Scholar]
- (721).Lamberts LE; Menke-van der Houven van Oordt CW; ter Weele EJ; Bensch F; Smeenk MM; Voortman J; Hoekstra OS; Williams SP; Fine BM; Maslyar D; et al. ImmunoPET with anti-mesothelin antibody in patients with pancreatic and ovarian cancer before anti-mesothelin antibody-drug conjugate treatment. Clin. Cancer Res 2016, 22, 1642–1652. [DOI] [PubMed] [Google Scholar]
- (722).Shin IS; Lee SM; Kim HS; Yao Z; Regino C; Sato N; Cheng KT; Hassan R; Campo MF; Albone EF; et al. Effect of chelator conjugation level and injection dose on tumor and organ uptake of 111In-labeled MORAb-009, an anti-mesothelin antibody. Nucl. Med. Biol 2011, 38, 1119–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (723).Lee JH; Kim H; Yao Z; Szajek LP; Grasso L; Kim I; Paik CH Tumor-Shed Antigen Affects Antibody Tumor Targeting: Comparison of two (89)Zr-labeled antibodies directed against shed or nonshed antigens. Contrast Media Mol. Imaging 2018, 2018, 2461257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (724).Wickstroem K; Hagemann UB; Cruciani V; Wengner AM; Kristian A; Ellingsen C; Siemeister G; Bjerke RM; Karlsson J; Ryan OB; et al. Synergistic effect of a mesothelin-targeted (227) Th conjugate in combination with DNA damage response inhibitors in ovarian cancer xenograft models. J. Nucl. Med 2019, 60, 1293–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (725).Sakamoto J; Kojima H; Kato J; Hamashima H; Suzuki H Organ-specific expression of the intestinal epithelium-related antigen A33, a cell surface target for antibody-based imaging and treatment in gastrointestinal cancer. Cancer Chemother. Pharmacol 2000, 46 (Suppl), S27–32. [DOI] [PubMed] [Google Scholar]
- (726).Welt S; Scott AM; Divgi CR; Kemeny NE; Finn RD; Daghighian F; Germain JS; Richards EC; Larson SM; Old LJ Phase I/II study of iodine 125-labeled monoclonal antibody A33 in patients with advanced colon cancer. J. Clin. Oncol 1996, 14, 1787–1797. [DOI] [PubMed] [Google Scholar]
- (727).Welt S; Divgi CR; Kemeny N; Finn RD; Scott AM; Graham M; Germain JS; Richards EC; Larson SM; Oettgen HF; et al. Phase I/II study of iodine 131-labeled monoclonal antibody A33 in patients with advanced colon cancer. J. Clin. Oncol 1994, 12, 1561–1571. [DOI] [PubMed] [Google Scholar]
- (728).King DJ; Antoniw P; Owens RJ; Adair JR; Haines AM; Farnsworth AP; Finney H; Lawson AD; Lyons A; Baker TS; et al. Preparation and preclinical evaluation of humanised A33 immunoconjugates for radioimmunotherapy. Br. J. Cancer 1995, 72, 1364–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (729).Lee FT; Hall C; Rigopoulos A; Zweit J; Pathmaraj K; O’Keefe GJ; Smyth FE; Welt S; Old LJ; Scott AM Immuno-PET of human colon xenograft- bearing BALB/c nude mice using 124I-CDR-grafted humanized A33 monoclonal antibody. J. Nucl. Med 2001, 42, 764–769. [PubMed] [Google Scholar]
- (730).O’Donoghue JA; Smith-Jones PM; Humm JL; Ruan S; Pryma DA; Jungbluth AA; Divgi CR; Carrasquillo JA; Pandit-Taskar N; Fong Y; et al. 124I-huA33 antibody uptake is driven by A33 antigen concentration in tissues from colorectal cancer patients imaged by immuno-PET. J. Nucl. Med 2011, 52, 1878–1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (731).Cheal SM; Fung EK; Patel M; Xu H; Guo HF; Zanzonico PB; Monette S; Wittrup KD; Cheung NV; Larson SM Curative multicycle radioimmunotherapy monitored by quantitative SPECT/CT-based theranostics, using bispecific antibody pretargeting strategy in colorectal cancer. J. Nucl. Med 2017, 58, 1735–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (732).Keinanen O; Brennan JM; Membreno R; Fung K; Gangangari K; Dayts EJ; Williams CJ; Zeglis BM Dual radionuclide theranostic pretargeting. Mol. Pharmaceutics 2019, 16, 4416–4421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (733).Brand C; Sadique A; Houghton JL; Gangangari K; Ponte JF; Lewis JS; Pillarsetty NVK; Konner JA; Reiner T Leveraging PET to image folate receptor alpha therapy of an antibody-drug conjugate. EJNMMI Res 2018, 8, 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (734).Heo GS; Detering L; Luehmann HP; Primeau T; Lee YS; Laforest R; Li S; Stec J; Lim KH; Lockhart AC; et al. Folate receptor alpha-targeted (89)Zr-M9346A immuno-PET for image-guided intervention with Mirvetuximab Soravtansine in triple-negative breast cancer. Mol. Pharmaceutics 2019, 16, 3996–4006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (735).Cai W; Ebrahimnejad A; Chen K; Cao Q; Li ZB; Tice DA; Chen X Quantitative radioimmunoPET imaging of EphA2 in tumor-bearing mice. Eur. J. Nucl. Med. Mol. Imaging 2007, 34, 2024–2036. [DOI] [PubMed] [Google Scholar]
- (736).Chacko AM; Li C; Nayak M; Mikitsh JL; Hu J; Hou C; Grasso L; Nicolaides NC; Muzykantov VR; Divgi CR; et al. Development of 124I immuno-PET targeting tumor vascular TEM1/endosialin. J. Nucl. Med 2014, 55, 500–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (737).Li C; Chacko AM; Hu J; Hasegawa K; Swails J; Grasso L; El-Deiry WS; Nicolaides N; Muzykantov VR; Divgi CR; et al. Antibody-based tumor vascular theranostics targeting endosialin/TEM1 in a new mouse tumor vascular model. Cancer Biol. Ther 2014, 15, 443–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (738).Burvenich IJ; Lee FT; Cartwright GA; O’Keefe GJ; Makris D; Cao D; Gong S; Chueh AC; Mariadason JM; Brechbiel MW; et al. Molecular imaging of death receptor 5 occupancy and saturation kinetics in vivo by humanized monoclonal antibody CS-1008. Clin. Cancer Res 2013, 19, 5984–5993. [DOI] [PubMed] [Google Scholar]
- (739).Ciprotti M; Tebbutt NC; Lee FT; Lee ST; Gan HK; McKee DC; O’Keefe GJ; Gong SJ; Chong G; Hopkins W; et al. Phase I imaging and pharmacodynamic trial of CS-1008 in patients with metastatic colorectal cancer. J. Clin. Oncol 2015, 33, 2609–2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (740).Burvenich IJ; Lee FT; Guo N; Gan HK; Rigopoulos A; Parslow AC; O’Keefe GJ; Gong SJ; Tochon-Danguy H; Rudd SE; et al. In vitro and in vivo evaluation of (89)Zr-DS-8273a as a theranostic for anti-death receptor 5 therapy. Theranostics 2016, 6, 2225–2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (741).Sham JG; Kievit FM; Grierson JR; Miyaoka RS; Yeh MM; Zhang M; Yeung RS; Minoshima S; Park JO Glypican-3-targeted 89Zr PET imaging of hepatocellular carcinoma. J. Nucl. Med 2014, 55, 799–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (742).Sham JG; Kievit FM; Grierson JR; Chiarelli PA; Miyaoka RS; Zhang M; Yeung RS; Minoshima S; Park JO Glypican-3-targeting F(ab′)2 for 89Zr PET of hepatocellular carcinoma. J. Nucl. Med 2014, 55, 2032–2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (743).Carrasquillo JA; O’Donoghue JA; Beylergil V; Ruan S; Pandit-Taskar N; Larson SM; Smith-Jones PM; Lyashchenko SK; Ohishi N; Ohtomo T; Abou-Alfa GK I-124 codrituzumab imaging and biodistribution in patients with hepatocellular carcinoma. EJNMMI Res 2018, 8, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (744).Hall MA; Pinkston KL; Wilganowski N; Robinson H; Ghosh P; Azhdarinia A; Vazquez-Arreguin K; Kolonin AM; Harvey BR; Sevick-Muraca EM Comparison of mAbs targeting epithelial cell adhesion molecule for the detection of prostate cancer lymph node metastases with multimodal contrast agents: quantitative small-animal PET/CT and NIRF. J. Nucl. Med 2012, 53, 1427–1437. [DOI] [PubMed] [Google Scholar]
- (745).Campbell DO; Noda A; Verlinsky A; Snyder J; Fujita Y; Murakami Y; Fushiki H; Miyoshi S; Lacayo S; Cabral E; et al. Preclinical evaluation of an anti-nectin-4 immunoPET reagent in tumor-bearing mice and biodistribution studies in cynomolgus monkeys. Mol. Imaging Biol 2016, 18, 768–775. [DOI] [PubMed] [Google Scholar]
- (746).Doran MG; Watson PA; Cheal SM; Spratt DE; Wongvipat J; Steckler JM; Carrasquillo JA; Evans MJ; Lewis JS Annotating STEAP1 regulation in prostate cancer with 89Zr immuno-PET. J. Nucl. Med 2014, 55, 2045–2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (747).Williams SP; Ogasawara A; Tinianow JN; Flores JE; Kan D; Lau J; Go M; Vanderbilt AN; Gill HS; Miao L; et al. ImmunoPET helps predicting the efficacy of antibody-drug conjugates targeting TENB2 and STEAP1. Oncotarget 2016, 7, 25103–25112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (748).O’Donoghue JA; Danila DC; Pandit-Taskar N; Beylergil V; Cheal SM; Fleming SE; Fox JJ; Ruan S; Zanzonico PB; Ragupathi G; et al. Pharmacokinetics and biodistribution of a[(89)Zr]Zr-DFO-MSTP2109A anti-STEAP1 antibody in metastatic castration-resistant prostate cancer patients. Mol. Pharmaceutics 2019, 16, 3083–3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (749).Carrasquillo JA; Fine BM; Pandit-Taskar N; Larson SM; Fleming SE; Fox JJ; Cheal SM; O’Donoghue JA; Ruan S; Ragupathi G; et al. Imaging patients with metastatic castration-resistant prostate cancer using 89Zr-DFO-MSTP2109A anti-STEAP1 antibody. J. Nucl. Med 2019, 60, 1517–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (750).Hong H; Yan Y; Shi S; Graves SA; Krasteva LK; Nickles RJ; Yang M; Cai W PET of follicle-stimulating hormone receptor: broad applicability to cancer imaging. Mol. Pharmaceutics 2015, 12, 403–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (751).Cheal SM; Ruan S; Veach DR; Longo VA; Punzalan BJ; Wu J; Fung EK; Kelly MP; Kirshner JR; Giurleo JT; et al. ImmunoPET imaging of endogenous and transfected prolactin receptor tumor xenografts. Mol. Pharmaceutics 2018, 15, 2133–2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (752).Deshayes E; Ladjohounlou R; Le Fur P; Pichard A; Lozza C; Boudousq V; Sevestre S; Jarlier M; Kashani R; Koch J; et al. Radiolabeled antibodies against mullerian-inhibiting substance receptor, type II: new tools for a theranostic approach in ovarian cancer. J. Nucl. Med 2018, 59, 1234–1242. [DOI] [PubMed] [Google Scholar]
- (753).Oude Munnink TH; Arjaans ME; Timmer-Bosscha H; Schroder CP; Hesselink JW; Vedelaar SR; Walenkamp AM; Reiss M; Gregory RC; Lub-de Hooge MN; et al. PET with the 89Zr-labeled transforming growth factor-beta antibody fresolimumab in tumor models. J. Nucl. Med 2011, 52, 2001–2008. [DOI] [PubMed] [Google Scholar]
- (754).den Hollander MW; Bensch F; Glaudemans AW; Oude Munnink TH; Enting RH; den Dunnen WF; Heesters MA; Kruyt FA; Lub-de Hooge MN; Cees de Groot J; et al. TGF-beta antibody uptake in recurrent high-grade glioma imaged with 89Zrfresolimumab PET. J. Nucl. Med 2015, 56, 1310–1314. [DOI] [PubMed] [Google Scholar]
- (755).Rotteveel L; Poot AJ; Bogaard HJ; Ten Dijke P; Lammertsma AA; Windhorst AD In vivo imaging of TGFbeta signalling components using positron emission tomography. Drug Discovery Today 2019, 24, 2258–2272. [DOI] [PubMed] [Google Scholar]
- (756).Weiss ID; Jacobson O Molecular imaging of chemokine receptor CXCR4. Theranostics 2013, 3, 76–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (757).Azad BB; Chatterjee S; Lesniak WG; Lisok A; Pullambhatla M; Bhujwalla ZM; Pomper MG; Nimmagadda S A fully human CXCR4 antibody demonstrates diagnostic utility and therapeutic efficacy in solid tumor xenografts. Oncotarget 2016, 7, 12344–12358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (758).Yoon SO; Lee TS; Kim SJ; Jang MH; Kang YJ; Park JH; Kim KS; Lee HS; Ryu CJ; Gonzales NR; et al. Construction, affinity maturation, and biological characterization of an anti-tumor-associated glycoprotein-72 humanized antibody. J. Biol. Chem 2006, 281, 6985–6992. [DOI] [PubMed] [Google Scholar]
- (759).Li L; Turatti F; Crow D; Bading JR; Anderson AL; Poku E; Yazaki PJ; Williams LE; Tamvakis D; Sanders P; et al. Monodispersed DOTA-PEG-conjugated anti-TAG-72 diabody has low kidney uptake and high tumor-to-blood ratios resulting in improved 64Cu PET. J. Nucl. Med 2010, 51, 1139–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (760).Li L; Crow D; Turatti F; Bading JR; Anderson AL; Poku E; Yazaki PJ; Carmichael J; Leong D; Wheatcroft D; et al. Site-specific conjugation of monodispersed DOTA-PEGn to a thiolated diabody reveals the effect of increasing peg size on kidney clearance and tumor uptake with improved 64-copper PET imaging. Bioconjugate Chem 2011, 22, 709–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (761).Ding H; Carlton MM; Povoski SP; Milum K; Kumar K; Kothandaraman S; Hinkle GH; Colcher D; Brody R; Davis PD; et al. Site specific discrete PEGylation of (124)I-labeled mCC49 Fab′ fragments improves tumor MicroPET/CT imaging in mice. Bioconjugate Chem 2013, 24, 1945–1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (762).Saunders LR; Bankovich AJ; Anderson WC; Aujay MA; Bheddah S; Black K; Desai R; Escarpe PA; Hampl J; Laysang A; et al. A DLL3-targeted antibody-drug conjugate eradicates high-grade pulmonary neuroendocrine tumor-initiating cells in vivo. Sci. Transl. Med 2015, 7, 302ra136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (763).Rudin CM; Pietanza MC; Bauer TM; Ready N; Morgensztern D; Glisson BS; Byers LA; Johnson ML; Burris HA 3rd; Robert F; et al. Rovalpituzumab tesirine, a DLL3-targeted antibody-drug conjugate, in recurrent small-cell lung cancer: a first-inhuman, first-in-class, open-label, phase 1 study. Lancet Oncol 2017, 18, 42–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (764).Puca L; Gavyert K; Sailer V; Conteduca V; Dardenne E; Sigouros M; Isse K; Kearney M; Vosoughi A; Fernandez L Delta-like protein 3 expression and therapeutic targeting in neuroendocrine prostate cancer. Sci. Transl. Med 2019, 11, eaav0891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (765).Sharma SK; Pourat J; Abdel-Atti D; Carlin SD; Piersigilli A; Bankovich AJ; Gardner EE; Hamdy O; Isse K; Bheddah S; et al. Noninvasive Interrogation of DLL3 Expression in Metastatic Small Cell Lung Cancer. Cancer Res 2017, 77, 3931–3941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (766).Sharma SK; Chow A; Monette S; Vivier D; Pourat J; Edwards KJ; Dilling TR; Abdel-Atti D; Zeglis BM; Poirier JT; et al. Fc-Mediated anomalous biodistribution of therapeutic antibodies in immunodeficient mouse models. Cancer Res 2018, 78, 1820–1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (767).Vavere AL; Butch ER; Dearling JL; Packard AB; Navid F; Shulkin BL; Barfield RC; Snyder SE 64Cu-p-NH2-Bn-DOTA-hu14.18K322A, a PET radiotracer targeting neuroblastoma and melanoma. J. Nucl. Med 2012, 53, 1772–1778. [DOI] [PubMed] [Google Scholar]
- (768).Maier FC; Schmitt J; Maurer A; Ehrlichmann W; Reischl G; Nikolaou K; Handgretinger R; Pichler BJ; Thaiss WM Correlation between positron emission tomography and Cerenkov luminescence imaging in vivo and ex vivo using 64Cu-labeled antibodies in a neuroblastoma mouse model. Oncotarget 2016, 7, 67403–67411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (769).Kramer K; Humm JL; Souweidane MM; Zanzonico PB; Dunkel IJ; Gerald WL; Khakoo Y; Yeh SD; Yeung HW; Finn RD; et al. Phase I study of targeted radioimmunotherapy for leptomeningeal cancers using intra-Ommaya 131-I-3F8. J. Clin. Oncol 2007, 25, 5465–5470. [DOI] [PubMed] [Google Scholar]
- (770).Kramer K; Pandit-Taskar N; Humm JL; Zanzonico PB; Haque S; Dunkel IJ; Wolden SL; Donzelli M; Goldman DA; Lewis JS A phase II study of radioimmunotherapy with intraventricular (131) I-3F8 for medulloblastoma. Pediatr. Blood Cancer 2018, 65, e26754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (771).Ladenstein R; Potschger U; Valteau-Couanet D; Luksch R; Castel V; Yaniv I; Laureys G; Brock P; Michon JM; Owens C; et al. Interleukin 2 with anti-GD2 antibody ch14.18/CHO (dinutuximab beta) in patients with high-risk neuroblastoma (HRNBL1/SIOPEN): a multicentre, randomised, phase 3 trial. Lancet Oncol 2018, 19, 1617–1629. [DOI] [PubMed] [Google Scholar]
- (772).Cheal SM; Xu H; Guo HF; Zanzonico PB; Larson SM; Cheung NK Preclinical evaluation of multistep targeting of diasialoganglioside GD2 using an IgG-scFv bispecific antibody with high affinity for GD2 and DOTA metal complex. Mol. Cancer Ther 2014, 13, 1803–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (773).Martins F; Sofiya L; Sykiotis GP; Lamine F; Maillard M; Fraga M; Shabafrouz K; Ribi C; Cairoli A; Guex-Crosier Y; et al. Adverse effects of immune-checkpoint inhibitors: epidemiology, management and surveillance. Nat. Rev. Clin. Oncol 2019, 16, 563–580. [DOI] [PubMed] [Google Scholar]
- (774).Pauken KE; Dougan M; Rose NR; Lichtman AH; Sharpe AH Adverse events following cancer immunotherapy: obstacles and opportunities. Trends Immunol 2019, 40, 511–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (775).Wei W; Jiang D; Ehlerding EB; Luo Q; Cai W Noninvasive PET imaging of T cells. Trends Cancer 2018, 4, 359–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (776).van der Veen EL; Bensch F; Glaudemans A; Lub-de Hooge MN; de Vries EGE Molecular imaging to enlighten cancer immunotherapies and underlying involved processes. Cancer Treat. Rev 2018, 70, 232–244. [DOI] [PubMed] [Google Scholar]
- (777).Mayer AT; Gambhir SS The immunoimaging toolbox. J. Nucl. Med 2018, 59, 1174–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (778).Walther M; Gebhardt P; Grosse-Gehling P; Wurbach L; Irmler I; Preusche S; Khalid M; Opfermann T; Kamradt T; Steinbach J; et al. Implementation of 89Zr production and in vivo imaging of B-cells in mice with 89Zr-labeled anti-B-cell antibodies by small animal PET/CT. Appl. Radiat. Isot 2011, 69, 852–857. [DOI] [PubMed] [Google Scholar]
- (779).Galli F; Rapisarda AS; Stabile H; Malviya G; Manni I; Bonanno E; Piaggio G; Gismondi A; Santoni A; Signore A In vivo imaging of natural killer cell trafficking in tumors. J. Nucl. Med 2015, 56, 1575–1580. [DOI] [PubMed] [Google Scholar]
- (780).Sato N; Stringaris K; Davidson-Moncada JK; Reger R; Adler SS; Dunbar C; Choyke PL; Childs RW In-vivo tracking of adoptively transferred natural killer-cells in rhesus macaques using(89)Zirconium-oxine cell labeling and PET imaging. Clin. Cancer Res 2020, DOI: 10.1158/1078-0432.CCR-19-2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (781).Blykers A; Schoonooghe S; Xavier C; D’Hoe K; Laoui D; D’Huyvetter M; Vaneycken I; Cleeren F; Bormans G; Heemskerk J; et al. PET imaging of macrophage mannose receptor-expressing macrophages in tumor stroma using 18F-radiolabeled camelid single-domain antibody fragments. J. Nucl. Med 2015, 56, 1265–1271. [DOI] [PubMed] [Google Scholar]
- (782).Xavier C; Blykers A; Laoui D; Bolli E; Vaneyken I; Bridoux J; Baudhuin H; Raes G; Everaert H; Movahedi K; et al. Clinical translation of [68Ga]Ga-NOTA-anti-MMR-sdAb for PET/CT imaging of protumorigenic macrophages. Mol. Imag. Biol 2019, 21, 898–906. [DOI] [PubMed] [Google Scholar]
- (783).Cao Q; Huang Q; Mohan C; Li C Small-animal PET/CT imaging of local and systemic immune response using (64)CualphaCD11b. J. Nucl. Med 2019, 60, 1317–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (784).Nigam S; McCarl L; Kumar R; Edinger RS; Kurland BF; Anderson CJ; Panigrahy A; Kohanbash G; Edwards WB Preclinical immunoPET imaging of glioblastoma-infiltrating myeloid cells using Zirconium-89 labeled Anti-CD11b antibody. Mol. Imag. Biol 2019, DOI: 10.1007/s11307-019-01427-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (785).Mall S; Yusufi N; Wagner R; Klar R; Bianchi H; Steiger K; Straub M; Audehm S; Laitinen I; Aichler M; et al. Immuno-PET imaging of engineered human T cells in tumors. Cancer Res 2016, 76, 4113–4123. [DOI] [PubMed] [Google Scholar]
- (786).Keu KV; Witney TH; Yaghoubi S; Rosenberg J; Kurien A; Magnusson R; Williams J; Habte F; Wagner JR; Forman S; et al. Reporter gene imaging of targeted T cell immunotherapy in recurrent glioma. Sci. Transl. Med 2017, 9, eaag2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (787).Yusufi N; Mall S; Bianchi HO; Steiger K; Reder S; Klar R; Audehm S; Mustafa M; Nekolla S; Peschel C; et al. In-depth characterization of a TCR-specific tracer for sensitive detection of tumor-directed transgenic T cells by immuno-PET. Theranostics 2017, 7, 2402–2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (788).Larimer BM; Wehrenberg-Klee E; Caraballo A; Mahmood U Quantitative CD3 PET imaging predicts tumor growth response to anti-CTLA-4 therapy. J. Nucl. Med 2016, 57, 1607–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (789).Beckford Vera DR; Smith CC; Bixby LM; Glatt DM; Dunn SS; Saito R; Kim WY; Serody JS; Vincent BG; Parrott MC Immuno-PET imaging of tumor-infiltrating lymphocytes using zirconium-89 radiolabeled anti-CD3 antibody in immune-competent mice bearing syngeneic tumors. PLoS One 2018, 13, e0193832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (790).Gibson HM; McKnight BN; Malysa A; Dyson G; Wiesend WN; McCarthy CE; Reyes J; Wei WZ; Viola-Villegas NT IFNgamma PET imaging as a predictive tool for monitoring response to tumor immunotherapy. Cancer Res 2018, 78, 5706–5717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (791).Mayer KE; Mall S; Yusufi N; Gosmann D; Steiger K; Russelli L; Bianchi HO; Audehm S; Wagner R; Braunlein E; et al. T-cell functionality testing is highly relevant to developing novel immuno-tracers monitoring T cells in the context of immunotherapies and revealed CD7 as an attractive target. Theranostics 2018, 8, 6070–6087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (792).Tavare R; McCracken MN; Zettlitz KA; Knowles SM; Salazar FB; Olafsen T; Witte ON; Wu AM Engineered antibody fragments for immuno-PET imaging of endogenous CD8+ T cells in vivo. Proc. Natl. Acad. Sci. U. S. A 2014, 111, 1108–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (793).Pandit-Taskar N; Postow M; Hellmann M; Harding J; Barker C; O’Donoghue J; Ziolkowska M; Ruan S; Lyashchenko S; Tsai F; et al. First-in-human imaging with (89)Zr-Df-IAB22M2C anti-CD8 minibody in patients with solid malignancies: preliminary pharmacokinetics, biodistribution, and lesion targeting. J. Nucl. Med 2019, 59, jnumed.119.229781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (794).Tavare R; McCracken MN; Zettlitz KA; Salazar FB; Olafsen T; Witte ON; Wu AM Immuno-PET of murine T cell reconstitution postadoptive stem cell transplantation using anti-CD4 and anti-CD8 cys-diabodies. J. Nucl. Med 2015, 56, 1258–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (795).Tavare R; Escuin-Ordinas H; Mok S; McCracken MN; Zettlitz KA; Salazar FB; Witte ON; Ribas A; Wu AM An effective immuno-PET imaging method to monitor CD8-dependent responses to immunotherapy. Cancer Res 2016, 76, 73–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (796).Seo JW; Tavare R; Mahakian LM; Silvestrini MT; Tam S; Ingham ES; Salazar FB; Borowsky AD; Wu AM; Ferrara KW CD8(+) T-cell density imaging with (64)Cu-labeled cys-diabody informs immunotherapy protocols. Clin. Cancer Res 2018, 24, 4976–4987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (797).Gill H; Seipert R; Carroll VM; Gouasmat A; Yin J; Ogasawara A; de Jong I; Phan MM; Wang X; Yang J; et al. The production, quality control, and characterization of ZED8, a CD8-specific (89)Zr-labeled immuno-PET clinical imaging agent. AAPS J 2020, 22, 22. [DOI] [PubMed] [Google Scholar]
- (798).Mitsuiki N; Schwab C; Grimbacher B What did we learn from CTLA-4 insufficiency on the human immune system? Immunol. Rev 2019, 287, 33–49. [DOI] [PubMed] [Google Scholar]
- (799).Ehlerding EB; Lee HJ; Jiang D; Ferreira CA; Zahm CD; Huang P; Engle JW; McNeel DG; Cai W Antibody and fragment-based PET imaging of CTLA-4+ T-cells in humanized mouse models. Am. J. Cancer Res 2019, 9, 53–63. [PMC free article] [PubMed] [Google Scholar]
- (800).Higashikawa K; Yagi K; Watanabe K; Kamino S; Ueda M; Hiromura M; Enomoto S 64Cu-DOTA-anti-CTLA-4 mAb enabled PET visualization of CTLA-4 on the T-cell infiltrating tumor tissues. PLoS One 2014, 9, e109866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (801).Ingram JR; Blomberg OS; Rashidian M; Ali L; Garforth S; Fedorov E; Fedorov AA; Bonanno JB; Le Gall C; Crowley S; et al. Anti-CTLA-4 therapy requires an Fc domain for efficacy. Proc. Natl. Acad. Sci. U. S. A 2018, 115, 3912–3917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (802).Contardi E; Palmisano GL; Tazzari PL; Martelli AM; Fala F; Fabbi M; Kato T; Lucarelli E; Donati D; Polito L; et al. CTLA-4 is constitutively expressed on tumor cells and can trigger apoptosis upon ligand interaction. Int. J. Cancer 2005, 117, 538–550. [DOI] [PubMed] [Google Scholar]
- (803).Ehlerding EB; England CG; Majewski RL; Valdovinos HF; Jiang D; Liu G; McNeel DG; Nickles RJ; Cai W ImmunoPET imaging of CTLA-4 expression in mouse models of non-small cell lung cancer. Mol. Pharmaceutics 2017, 14, 1782–1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (804).Natarajan A; Mayer AT; Xu L; Reeves RE; Gano J; Gambhir SS Novel Radiotracer for immunoPET imaging of PD-1 checkpoint expression on tumor infiltrating lymphocytes. Bioconjugate Chem 2015, 26, 2062–2069. [DOI] [PubMed] [Google Scholar]
- (805).England CG; Ehlerding EB; Hernandez R; Rekoske BT; Graves SA; Sun H; Liu G; McNeel DG; Barnhart TE; Cai W Preclinical pharmacokinetics and biodistribution studies of 89Zr-labeled pembrolizumab. J. Nucl. Med 2017, 58, 162–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (806).Natarajan A; Mayer AT; Reeves RE; Nagamine CM; Gambhir SS Development of novel immunoPET tracers to image human PD-1 checkpoint expression on tumor-infiltrating lymphocytes in a humanized mouse model. Mol. Imaging Biol 2017, 19, 903–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (807).Cole EL; Kim J; Donnelly DJ; Smith RA; Cohen D; Lafont V; Morin PE; Huang RY; Chow PL; Hayes W; et al. Radiosynthesis and preclinical PET evaluation of (89)Zr-nivolumab (BMS-936558) in healthy non-human primates. Bioorg. Med. Chem 2017, 25, 5407–5414. [DOI] [PubMed] [Google Scholar]
- (808).England CG; Jiang D; Ehlerding EB; Rekoske BT; Ellison PA; Hernandez R; Barnhart TE; McNeel DG; Huang P; Cai W (89)Zr-labeled nivolumab for imaging of T-cell infiltration in a humanized murine model of lung cancer. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 110–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (809).Niemeijer AN; Leung D; Huisman MC; Bahce I; Hoekstra OS; van Dongen G; Boellaard R; Du S; Hayes W; Smith R; et al. Whole body PD-1 and PD-L1 positron emission tomography in patients with non-small-cell lung cancer. Nat. Commun 2018, 9, 4664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (810).Lesniak WG; Chatterjee S; Gabrielson M; Lisok A; Wharram B; Pomper MG; Nimmagadda S PD-L1 detection in tumors using [(64)Cu]atezolizumab with PET. Bioconjugate Chem 2016, 27, 2103–2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (811).Truillet C; Oh HLJ; Yeo SP; Lee CY; Huynh LT; Wei J; Parker MFL; Blakely C; Sevillano N; Wang YH; et al. Imaging PD-L1 expression with immunoPET. Bioconjugate Chem 2018, 29, 96–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (812).Moroz A; Lee CY; Wang YH; Hsiao JC; Sevillano N; Truillet C; Craik CS; Fong L; Wang CI; Evans MJ A preclinical assessment of (89)Zr-atezolizumab identifies a requirement for carrier added formulations not observed with (89)Zr-C4. Bioconjugate Chem 2018, 29, 3476–3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (813).Xu M; Han Y; Liu G; Xu Y; Duan D; Liu H; Du F; Luo P; Liu Z Preclinical study of a fully human anti-PD-L1 antibody as a theranostic agent for cancer immunotherapy. Mol. Pharmaceutics 2018, 15, 4426–4433. [DOI] [PubMed] [Google Scholar]
- (814).Vento J; Mulgaonkar A; Woolford L; Nham K; Christie A; Bagrodia A; de Leon AD; Hannan R; Bowman I; McKay RM; et al. PD-L1 detection using Zr-atezolizumab immuno-PET in renal cell carcinoma tumorgrafts from a patient with favorable nivolumab response. J. Immunother Cancer 2019, 7, 144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (815).Natarajan A; Patel CB; Ramakrishnan S; Panesar PS; Long SR; Gambhir SS A novel engineered small protein for positron emission tomography imaging of human programmed death ligand-1: validation in mouse models and human cancer tissues. Clin. Cancer Res 2019, 25, 1774–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (816).Donnelly DJ; Smith RA; Morin P; Lipovsek D; Gokemeijer J; Cohen D; Lafont V; Tran T; Cole EL; Wright M; et al. Synthesis and biologic evaluation of a novel (18)F-labeled adnectin as a PET radioligand for imaging PD-L1 expression. J. Nucl. Med 2018, 59, 529–535. [DOI] [PubMed] [Google Scholar]
- (817).Maute RL; Gordon SR; Mayer AT; McCracken MN; Natarajan A; Ring NG; Kimura R; Tsai JM; Manglik A; Kruse AC; et al. Engineering high-affinity PD-1 variants for optimized immunotherapy and immuno-PET imaging. Proc. Natl. Acad. Sci. U. S.A 2015, 112, E6506–6514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (818).Mayer AT; Natarajan A; Gordon SR; Maute RL; McCracken MN; Ring AM; Weissman IL; Gambhir SS Practical immuno-PET radiotracer design considerations for human immune checkpoint imaging. J. Nucl. Med 2017, 58, 538–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (819).Gonzalez Trotter DE; Meng X; McQuade P; Rubins D; Klimas M; Zeng Z; Connolly BM; Miller PJ; O’Malley SS; Lin SA; et al. In vivo imaging of the programmed death ligand 1 by (18)F PET. J. Nucl. Med 2017, 58, 1852–1857. [DOI] [PubMed] [Google Scholar]
- (820).Li D; Cheng S; Zou S; Zhu D; Zhu T; Wang P; Zhu X Immuno-PET imaging of (89)Zr labeled anti-PD-L1 domain antibody. Mol. Pharmaceutics 2018, 15, 1674–1681. [DOI] [PubMed] [Google Scholar]
- (821).Lv G; Sun X; Qiu L; Sun Y; Li K; Liu Q; Zhao Q; Qin S; Lin J PET imaging of tumor PD-L1 expression with a highly specific non-blocking nanobody. J. Nucl. Med 2020, 61, 117–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (822).Broos K; Lecocq Q; Xavier C; Bridoux J; Nguyen TT; Corthals J; Schoonooghe S; Lion E; Raes G; Keyaerts M; et al. Evaluating a single domain antibody targeting human PD-L1 as a nuclear imaging and therapeutic agent. Cancers 2019, 11, 872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (823).Wissler HL; Ehlerding EB; Lyu Z; Zhao Y; Zhang S; Eshraghi A; Buuh ZY; McGuth JC; Guan Y; Engle JW; et al. Site-specific immuno-PET tracer to image PD-L1. Mol. Pharmaceutics 2019, 16, 2028–2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (824).Kikuchi M; Clump DA; Srivastava RM; Sun L; Zeng D; Diaz-Perez JA; Anderson CJ; Edwards WB; Ferris RL Preclinical immunoPET/CT imaging using Zr-89-labeled anti-PD-L1 monoclonal antibody for assessing radiation-induced PD-L1 upregulation in head and neck cancer and melanoma. Oncoimmunology 2017, 6, e1329071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (825).Ehlerding EB; Lee HJ; Barnhart TE; Jiang D; Kang L; McNeel DG; Engle JW; Cai W Noninvasive imaging and quantification of radiotherapy-induced PD-L1 upregulation with(89)Zr-Df-atezolizumab. Bioconjugate Chem 2019, 30, 1434–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (826).Li D; Zou S; Cheng S; Song S; Wang P; Zhu X Monitoring the response of PD-L1 expression to epidermal growth factor receptor tyrosine kinase inhibitors in nonsmall-cell lung cancer xenografts by immuno-PET imaging. Mol. Pharmaceutics 2019, 16, 3469–3476. [DOI] [PubMed] [Google Scholar]
- (827).Gong B; Kiyotani K; Sakata S; Nagano S; Kumehara S; Baba S; Besse B; Yanagitani N; Friboulet L; Nishio M; et al. Secreted PD-L1 variants mediate resistance to PD-L1 blockade therapy in non-small cell lung cancer. J. Exp. Med 2019, 216, 982–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (828).Josefsson A; Nedrow JR; Park S; Banerjee SR; Rittenbach A; Jammes F; Tsui B; Sgouros G Imaging, biodistribution, and dosimetry of radionuclide-labeled PD-L1 antibody in an immunocompetent mouse model of breast cancer. Cancer Res 2016, 76, 472–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (829).Hettich M; Braun F; Bartholoma MD; Schirmbeck R; Niedermann G High-resolution PET imaging with therapeutic antibody-based PD-1/PD-L1 checkpoint tracers. Theranostics 2016, 6, 1629–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (830).Ingram JR; Dougan M; Rashidian M; Knoll M; Keliher EJ; Garrett S; Garforth S; Blomberg OS; Espinosa C; Bhan A; et al. PD-L1 is an activation-independent marker of brown adipocytes. Nat. Commun 2017, 8, 647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (831).Willoughby J; Griffiths J; Tews I; Cragg MS OX40: structure and function - what questions remain? Mol. Immunol 2017, 83, 13–22. [DOI] [PubMed] [Google Scholar]
- (832).Alam IS; Mayer AT; Sagiv-Barfi I; Wang K; Vermesh O; Czerwinski DK; Johnson EM; James ML; Levy R; Gambhir SS Imaging activated T cells predicts response to cancer vaccines. J. Clin. Invest 2018, 128, 2569–2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (833).Burke JD; Young HA IFN-gamma: A cytokine at the right time, is in the right place. Semin. Immunol 2019, 43, 101280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (834).Larimer BM; Wehrenberg-Klee E; Dubois F; Mehta A; Kalomeris T; Flaherty K; Boland G; Mahmood U Granzyme B PET imaging as a predictive biomarker of immunotherapy response. Cancer Res 2017, 77, 2318–2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (835).Larimer BM; Bloch E; Nesti S; Austin EE; Wehrenberg-Klee E; Boland G; Mahmood U The effectiveness of checkpoint inhibitor combinations and administration timing can be measured by granzyme B PET imaging. Clin. Cancer Res 2019, 25, 1196–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (836).van der Veen EL; Antunes IF; Maarsingh P; Hessels-Scheper J; Zijlma R; Boersma HH; Jorritsma-Smit A; Hospers GAP; de Vries EGE; Lub-de Hooge MN; de Vries EFJ Clinical-grade N-(4-[18F]fluorobenzoyl)-interleukin-2 for PET imaging of activated T-cells in humans. EJNMMI Radiopharm. Chem 2019, 4, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (837).Grivennikov SI; Greten FR; Karin M Immunity, inflammation, and cancer. Cell 2010, 140, 883–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (838).Lee HJ; Ehlerding EB; Cai W Antibody-based tracers for PET/SPECT imaging of chronic inflammatory diseases. ChemBio-Chem 2019, 20, 422–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (839).Smolen JS; Aletaha D; Barton A; Burmester GR; Emery P; Firestein GS; Kavanaugh A; McInnes IB; Solomon DH; Strand V; Yamamoto K Rheumatoid arthritis. Nat. Rev. Dis Primers 2018, 4, 18001. [DOI] [PubMed] [Google Scholar]
- (840).Aletaha D; Smolen JS Diagnosis and management of rheumatoid arthritis: a review. JAMA 2018, 320, 1360–1372. [DOI] [PubMed] [Google Scholar]
- (841).Wipke BT; Wang Z; Kim J; McCarthy TJ; Allen PM Dynamic visualization of a joint-specific autoimmune response through positron emission tomography. Nat. Immunol 2002, 3, 366–372. [DOI] [PubMed] [Google Scholar]
- (842).Schwager K; Kaspar M; Bootz F; Marcolongo R; Paresce E; Neri D; Trachsel E Preclinical characterization of DEKAVIL (F8-IL10), a novel clinical-stage immunocytokine which inhibits the progression of collagen-induced arthritis. Arthritis Res. Ther 2009, 11, R142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (843).Bruijnen STG; Chandrupatla D; Giovanonni L; Neri D; Vugts DJ; Huisman MC; Hoekstra OS; Musters RJP; Lammertsma AA; van Dongen G; et al. F8-IL10: a new potential antirheumatic drug evaluated by a PET-guided translational approach. Mol. Pharmaceutics 2019, 16, 273–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (844).Beckford-Vera DR; Gonzalez-Junca A; Janneck JS; Huynh TL; Blecha JE; Seo Y; Li X; VanBrocklin HF; Franc BL PET/CT imaging of human TNFalpha using [(89)Zr]-certolizumab pegol in a transgenic preclinical model of rheumatoid arthritis. Mol. Imaging Biol 2020, 22, 105–114. [DOI] [PubMed] [Google Scholar]
- (845).Loktev A; Lindner T; Burger EM; Altmann A; Giesel F; Kratochwil C; Debus J; Marme F; Jager D; Mier W; et al. Development of fibroblast activation protein-targeted radiotracers with improved tumor retention. J. Nucl. Med 2019, 60, 1421–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (846).Giesel FL; Kratochwil C; Lindner T; Marschalek MM; Loktev A; Lehnert W; Debus J; Jager D; Flechsig P; Altmann A; et al. 68)Ga-FAPI PET/CT: biodistribution and preliminary dosimetry estimate of 2 DOTA-containing FAP-targeting agents in patients with various cancers. J. Nucl. Med 2019, 60, 386–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (847).Lindner T; Loktev A; Giesel F; Kratochwil C; Altmann A; Haberkorn U Targeting of activated fibroblasts for imaging and therapy. EJNMMI Radiopharm Chem 2019, 4, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (848).Bacac M; Freimoser-Grundschober A; Hosse R; Klein C; Moessner E; Nicolini VG; Umaña P Anti-FAP antibodies and methods of use US US9011847B2, 2015.
- (849).Laverman P; van der Geest T; Terry SY; Gerrits D; Walgreen B; Helsen MM; Nayak TK; Freimoser-Grundschober A; Waldhauer I; Hosse RJ; et al. Immuno-PET and immuno-SPECT of rheumatoid arthritis with radiolabeled anti-fibroblast activation protein antibody correlates with severity of arthritis. J. Nucl. Med 2015, 56, 778–783. [DOI] [PubMed] [Google Scholar]
- (850).Terry SY; Koenders MI; Franssen GM; Nayak TK; Freimoser-Grundschober A; Klein C; Oyen WJ; Boerman OC; Laverman P Monitoring therapy response of experimental arthritis with radiolabeled tracers targeting fibroblasts, macrophages, or integrin alphavbeta3. J. Nucl. Med 2016, 57, 467–472. [DOI] [PubMed] [Google Scholar]
- (851).van der Geest T; Laverman P; Gerrits D; Walgreen B; Helsen MM; Klein C; Nayak TK; Storm G; Metselaar JM; Koenders MI; et al. Liposomal treatment of experimental arthritis can be monitored noninvasively with a radiolabeled anti-fibroblast activation protein antibody. J. Nucl. Med 2017, 58, 151–155. [DOI] [PubMed] [Google Scholar]
- (852).van der Geest T; Roeleveld DM; Walgreen B; Helsen MM; Nayak TK; Klein C; Hegen M; Storm G; Metselaar JM; van den Berg WB; et al. Imaging fibroblast activation protein to monitor therapeutic effects of neutralizing interleukin-22 in collagen-induced arthritis. Rheumatology (Oxford, U. K.) 2018, 57, 737–747. [DOI] [PubMed] [Google Scholar]
- (853).Liu TC; Stappenbeck TS Genetics and pathogenesis of inflammatory bowel disease. Annu. Rev. Pathol.: Mech. Dis 2016, 11, 127–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (854).Dmochowska N; Wardill HR; Hughes PA Advances in imaging specific mediators of inflammatory bowel disease. Int. J. Mol. Sci 2018, 19, 2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (855).Dearling JL; Park EJ; Dunning P; Baker A; Fahey F; Treves ST; Soriano SG; Shimaoka M; Packard AB; Peer D Detection of intestinal inflammation by MicroPET imaging using a(64)Cu-labeled anti-beta(7) integrin antibody. Inflamm. Bowel Dis 2010, 16, 1458–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (856).Dearling JL; Daka A; Veiga N; Peer D; Packard AB Colitis immunoPET: defining target cell populations and optimizing pharmacokinetics. Inflamm. Bowel Dis 2016, 22, 529–538. [DOI] [PubMed] [Google Scholar]
- (857).Dmochowska N; Tieu W; Keller MD; Wardill HR; Mavrangelos C; Campaniello MA; Takhar P; Hughes PA Immuno-PET of innate immune markers CD11b and IL-1beta detects inflammation in murine colitis. J. Nucl. Med 2019, 60, 858–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (858).Freise AC; Zettlitz KA; Salazar FB; Tavare R; Tsai WK; Chatziioannou AF; Rozengurt N; Braun J; Wu AM Immuno-PET in inflammatory bowel disease: imaging CD4-positive T cells in a murine model of colitis. J. Nucl. Med 2018, 59, 980–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (859).Van Elssen C; Rashidian M; Vrbanac V; Wucherpfennig KW; Habre ZE; Sticht J; Freund C; Jacobsen JT; Cragnolini J; Ingram J; et al. Noninvasive imaging of human immune responses in a human xenograft model of graft-versus-host disease. J. Nucl. Med 2017, 58, 1003–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (860).Pektor S; Schloder J; Klasen B; Bausbacher N; Wagner DC; Schreckenberger M; Grabbe S; Jonuleit H; Miederer M Using immuno-PET imaging to monitor kinetics of T cell-mediated inflammation and treatment efficiency in a humanized mouse model for GvHD. Eur. J. Nucl. Med. Mol. Imaging 2019, DOI: 10.1007/s00259-019-04507-0. [DOI] [PubMed] [Google Scholar]
- (861).De Vos J; Mathijs I; Xavier C; Massa S; Wernery U; Bouwens L; Lahoutte T; Muyldermans S; Devoogdt N Specific targeting of atherosclerotic plaques in ApoE(−/−) mice using a new Camelid sdAb binding the vulnerable plaque marker LOX-1. Mol. Imaging Biol 2014, 16, 690–698. [DOI] [PubMed] [Google Scholar]
- (862).Senders ML; Que X; Cho YS; Yeang C; Groenen H; Fay F; Calcagno C; Meerwaldt AE; Green S; Miu P; et al. PET/MR imaging of malondialdehyde-acetaldehyde epitopes with a human antibody detects clinically relevant atherothrombosis. J. Am. Coll. Cardiol 2018, 71, 321–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (863).Senders ML; Hernot S; Carlucci G; van de Voort JC; Fay F; Calcagno C; Tang J; Alaarg A; Zhao Y; Ishino S; et al. Nanobody-facilitated multiparametric PET/MRI phenotyping of atherosclerosis. JACC Cardiovasc. Imaging 2019, 12, 2015–2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (864).Mumprecht V; Honer M; Vigl B; Proulx ST; Trachsel E; Kaspar M; Banziger-Tobler NE; Schibli R; Neri D; Detmar M In vivo imaging of inflammation- and tumor-induced lymph node lymphangiogenesis by immuno-positron emission tomography. Cancer Res 2010, 70, 8842–8851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (865).Mumprecht V; Roudnicky F; Detmar M Inflammation-induced lymph node lymphangiogenesis is reversible. Am. J. Pathol 2012, 180, 874–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (866).Santangelo PJ; Rogers KA; Zurla C; Blanchard EL; Gumber S; Strait K; Connor-Stroud F; Schuster DM; Amancha PK; Hong JJ; et al. Whole-body immunoPET reveals active SIV dynamics in viremic and antiretroviral therapy-treated macaques. Nat. Methods 2015, 12, 427–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (867).Morad HOJ; Wild AM; Wiehr S; Davies G; Maurer A; Pichler BJ; Thornton CR Pre-clinical imaging of invasive candidiasis using immunoPET/MR. Front. Microbiol 2018, 9, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (868).Pickett JE; Thompson JM; Sadowska A; Tkaczyk C; Sellman BR; Minola A; Corti D; Lanzavecchia A; Miller LS; Thorek DL Molecularly specific detection of bacterial lipoteichoic acid for diagnosis of prosthetic joint infection of the bone. Bone Res 2018, 6, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (869).Santangelo PJ; Cicala C; Byrareddy SN; Ortiz KT; Little D; Lindsay KE; Gumber S; Hong JJ; Jelicic K; Rogers KA; et al. Early treatment of SIV+ macaques with an alpha4beta7 mAb alters virus distribution and preserves CD4(+) T cells in later stages of infection. Mucosal Immunol 2018, 11, 932–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (870).Duvenhage J; Ebenhan T; Garny S; Hernandez Gonzalez I; Leyva Montana R; Price R; Birkholtz LM; Zeevaart JR Molecular imaging of a Zirconium-89 labeled antibody targeting plasmodium falciparum-infected human erythrocytes. Mol. Imaging Biol 2020, 22, 115–123. [DOI] [PubMed] [Google Scholar]
- (871).Rolle AM; Hasenberg M; Thornton CR; Solouk-Saran D; Mann L; Weski J; Maurer A; Fischer E; Spycher PR; Schibli R; et al. ImmunoPET/MR imaging allows specific detection of Aspergillus fumigatus lung infection in vivo. Proc. Natl. Acad. Sci. U.S. A 2016, 113, E1026–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (872).Davies G; Rolle AM; Maurer A; Spycher PR; Schillinger C; Solouk-Saran D; Hasenberg M; Weski J; Fonslet J; Dubois A; et al. Towards translational immunoPET/MR imaging of invasive pulmonary aspergillosis: the humanised monoclonal antibody JF5 detects aspergillus lung infections in vivo. Theranostics 2017, 7, 3398–3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (873).Thornton CR Molecular imaging of invasive pulmonary aspergillosis using immunoPET/MRI: the future looks bright. Front. Microbiol 2018, 9, 691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (874).Whiting DR; Guariguata L; Weil C; Shaw J IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res. Clin. Pract 2011, 94, 311–321. [DOI] [PubMed] [Google Scholar]
- (875).Wei W; Ehlerding EB; Lan X; Luo QY; Cai W Molecular imaging of beta-cells: diabetes and beyond. Adv. Drug Delivery Rev 2019, 139, 16–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (876).Vats D; Wang H; Esterhazy D; Dikaiou K; Danzer C; Honer M; Stuker F; Matile H; Migliorini C; Fischer E; et al. Multimodal imaging of pancreatic beta cells in vivo by targeting transmembrane protein 27 (TMEM27). Diabetologia 2012, 55, 2407–2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (877).Balhuizen A; Massa S; Mathijs I; Turatsinze JV; De Vos J; Demine S; Xavier C; Villate O; Millard I; Egrise D; et al. A nanobody-based tracer targeting DPP6 for non-invasive imaging of human pancreatic endocrine cells. Sci. Rep 2017, 7, 15130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (878).Demine S; Garcia Ribeiro R; Thevenet J; Marselli L; Marchetti P; Pattou F; Kerr-Conte J; Devoogdt N; Eizirik DL A nanobody-based nuclear imaging tracer targeting dipeptidyl peptidase 6 to determine the mass of human beta cell grafts in mice. Diabetologia 2020, 63, 825–836. [DOI] [PubMed] [Google Scholar]
- (879).Graves SA; Hernandez R; Valdovinos HF; Ellison PA; Engle JW; Barnhart TE; Cai W; Nickles RJ Preparation and in vivo characterization of 51MnCl2 as PET tracer of Ca2+ channel-mediated transport. Sci. Rep 2017, 7, 3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (880).Hernandez R; Graves SA; Gregg T; VanDeusen HR; Fenske RJ; Wienkes HN; England CG; Valdovinos HF; Jeffery JJ; Barnhart TE; et al. Radiomanganese PET detects changes in functional beta-cell mass in mouse models of diabetes. Diabetes 2017, 66, 2163–2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (881).Kolbert KS; Pentlow KS; Pearson JR; Sheikh A; Finn RD; Humm JL; Larson SM Prediction of absorbed dose to normal organs in thyroid cancer patients treated with 131I by use of 124I PET and 3-dimensional internal dosimetry software. J. Nucl. Med 2007, 48, 143–149. [PubMed] [Google Scholar]
- (882).Ho AL; Grewal RK; Leboeuf R; Sherman EJ; Pfister DG; Deandreis D; Pentlow KS; Zanzonico PB; Haque S; Gavane S; et al. Selumetinib-enhanced radioiodine uptake in advanced thyroid cancer. N. Engl. J. Med 2013, 368, 623–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (883).Werner RA; Weich A; Kircher M; Solnes LB; Javadi MS; Higuchi T; Buck AK; Pomper MG; Rowe SP; Lapa C The theranostic promise for Neuroendocrine Tumors in the late 2010s -Where do we stand, where do we go? Theranostics 2018, 8, 6088–6100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (884).Ahmadzadehfar H; Rahbar K; Essler M; Biersack HJ PSMA-based theranostics: a step-by-step practical approach to diagnosis and therapy for mCRPC patients. Semin. Nucl. Med 2020, 50, 98–109. [DOI] [PubMed] [Google Scholar]
- (885).Langbein T; Weber WA; Eiber M Future of theranostics: an outlook on precision oncology in nuclear medicine. J. Nucl. Med 2019, 60, 13S–19S. [DOI] [PubMed] [Google Scholar]
- (886).Liersch T; Meller J; Kulle B; Behr TM; Markus P; Langer C; Ghadimi BM; Wegener WA; Kovacs J; Horak ID; et al. Phase II trial of carcinoembryonic antigen radioimmunotherapy with 131I-labetuzumab after salvage resection of colorectal metastases in the liver: five-year safety and efficacy results. J. Clin. Oncol 2005, 23, 6763–6770. [DOI] [PubMed] [Google Scholar]
- (887).Liersch T; Meller J; Bittrich M; Kulle B; Becker H; Goldenberg DM Update of carcinoembryonic antigen radio-immunotherapy with (131)I-labetuzumab after salvage resection of colorectal liver metastases: comparison of outcome to a contemporaneous control group. Ann. Surg. Oncol 2007, 14, 2577–2590. [DOI] [PubMed] [Google Scholar]
- (888).Morschhauser F; Radford J; Van Hoof A; Vitolo U; Soubeyran P; Tilly H; Huijgens PC; Kolstad A; d’Amore F; Gonzalez Diaz M; et al. Phase III trial of consolidation therapy with yttrium-90-ibritumomab tiuxetan compared with no additional therapy after first remission in advanced follicular lymphoma. J. Clin. Oncol 2008, 26, 5156–5164. [DOI] [PubMed] [Google Scholar]
- (889).Flem-Karlsen K; Fodstad O; Tan M; Nunes-Xavier CE B7-H3 in cancer - beyond immune regulation. Trends Cancer 2018, 4, 401–404. [DOI] [PubMed] [Google Scholar]
- (890).Bao R; Wang Y; Lai J; Zhu H; Zhao Y; Li S; Li N; Huang J; Yang Z; Wang F; et al. Enhancing anti-PD-1/PD-L1 immune checkpoint inhibitory cancer therapy by CD276-targeted photodynamic ablation of tumor cells and tumor vasculature. Mol. Pharmaceutics 2019, 16, 339–348. [DOI] [PubMed] [Google Scholar]
- (891).Burvenich IJG; Parakh S; Lee FT; Guo N; Liu Z; Gan HK; Rigopoulos A; O’Keefe GJ; Gong SJ; Goh YW; et al. Molecular imaging of T cell co-regulator factor B7-H3 with(89)Zr-DS-5573a. Theranostics 2018, 8, 4199–4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (892).Modak S; Kramer K; Gultekin SH; Guo HF; Cheung NK Monoclonal antibody 8H9 targets a novel cell surface antigen expressed by a wide spectrum of human solid tumors. Cancer Res 2001, 61, 4048–4054. [PubMed] [Google Scholar]
- (893).Kramer K; Kushner BH; Modak S; Pandit-Taskar N; Smith-Jones P; Zanzonico P; Humm JL; Xu H; Wolden SL; Souweidane MM; et al. Compartmental intrathecal radio-immunotherapy: results for treatment for metastatic CNS neuroblastoma. J. Neuro-Oncol 2010, 97, 409–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (894).Bailey K; Pandit-Taskar N; Humm JL; Zanzonico P; Gilheeney S; Cheung NV; Kramer K Targeted radioimmuno-therapy for embryonal tumor with multilayered rosettes. J. Neuro-Oncol 2019, 143, 101–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (895).Pandit-Taskar N; Zanzonico PB; Kramer K; Grkovski M; Fung EK; Shi W; Zhang Z; Lyashchenko SK; Fung AM; Pentlow KS; et al. Biodistribution and dosimetry of intraventricularly administered (124)I-omburtamab in patients with metastatic leptomeningeal tumors. J. Nucl. Med 2019, 60, 1794–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (896).Souweidane MM; Kramer K; Pandit-Taskar N; Zhou Z; Haque S; Zanzonico P; Carrasquillo JA; Lyashchenko SK; Thakur SB; Donzelli M; et al. Convection-enhanced delivery for diffuse intrinsic pontine glioma: a single-centre, dose-escalation, phase 1 trial. Lancet Oncol 2018, 19, 1040–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (897).Le Doussal JM; Chetanneau A; Gruaz-Guyon A; Martin M; Gautherot E; Lehur PA; Chatal JF; Delaage M; Barbet J Bispecific monoclonal antibody-mediated targeting of an indium-111-labeled DTPA dimer to primary colorectal tumors: pharmacokinetics, biodistribution, scintigraphy and immune response. J. Nucl. Med 1993, 34, 1662–1671. [PubMed] [Google Scholar]
- (898).Schoffelen R; van der Graaf WT; Franssen G; Sharkey RM; Goldenberg DM; McBride WJ; Rossi EA; Eek A; Oyen WJ; Boerman OC Pretargeted 177Lu radioimmunotherapy of carcinoembryonic antigen-expressing human colonic tumors in mice.J. Nucl. Med 2010, 51, 1780–1787. [DOI] [PubMed] [Google Scholar]
- (899).Schoffelen R; Boerman OC; Goldenberg DM; Sharkey RM; van Herpen CM; Franssen GM; McBride WJ; Chang CH; Rossi EA; van der Graaf WT; et al. Development of an imaging-guided CEA-pretargeted radionuclide treatment of advanced colorectal cancer: first clinical results. Br. J. Cancer 2013, 109, 934–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (900).Schoffelen R; Woliner-van der Weg W; Visser EP; Goldenberg DM; Sharkey RM; McBride WJ; Chang CH; Rossi EA; van der Graaf WT; Oyen WJ; Boerman OC Predictive patient-specific dosimetry and individualized dosing of pretargeted radioimmunotherapy in patients with advanced colorectal cancer. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 1593–1602. [DOI] [PubMed] [Google Scholar]
- (901).Heskamp S; Hernandez R; Molkenboer-Kuenen JDM; Essler M; Bruchertseifer F; Morgenstern A; Steenbergen EJ; Cai W; Seidl C; McBride WJ; et al. Alpha- versus beta-emitting radionuclides for pretargeted radioimmunotherapy of carcinoembryonic antigen-expressing human colon cancer xenografts. J. Nucl. Med 2017, 58, 926–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (902).Nittka S; Krueger MA; Shively JE; Boll H; Brockmann MA; Doyon F; Pichler BJ; Neumaier M Radioimmunoimaging of liver metastases with PET using a 64Cu-labeled CEA antibody in transgenic mice. PLoS One 2014, 9, e106921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (903).Lu Z; Pham TT; Rajkumar V; Yu Z; Pedley RB; Arstad E; Maher J; Yan R A dual reporter Iodinated labeling reagent for cancer positron emission tomography imaging and fluorescence-guided surgery. J. Med. Chem 2018, 61, 1636–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (904).Bodet-Milin C; Ferrer L; Rauscher A; Masson D; Rbah-Vidal L; Faivre-Chauvet A; Cerato E; Rousseau C; Hureaux J; Couturier O; et al. Pharmacokinetics and dosimetry studies for optimization of pretargeted radioimmunotherapy in CEA-expressing advanced lung cancer patients. Front. Med 2015, 2, 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (905).Houghton JL; Membreno R; Abdel-Atti D; Cunanan KM; Carlin S; Scholz WW; Zanzonico PB; Lewis JS; Zeglis BM Establishment of the in vivo efficacy of pretargeted radio-immunotherapy utilizing inverse electron demand diels-alder click chemistry. Mol. Cancer Ther 2017, 16, 124–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (906).Witzig TE; Gordon LI; Cabanillas F; Czuczman MS; Emmanouilides C; Joyce R; Pohlman BL; Bartlett NL; Wiseman GA; Padre N; et al. Randomized controlled trial of yttrium-90-labeled ibritumomab tiuxetan radioimmunotherapy versus rituximab immunotherapy for patients with relapsed or refractory low-grade, follicular, or transformed B-cell non-Hodgkin’s lymphoma. J. Clin. Oncol 2002, 20, 2453–2463. [DOI] [PubMed] [Google Scholar]
- (907).Kaminski MS; Tuck M; Estes J; Kolstad A; Ross CW; Zasadny K; Regan D; Kison P; Fisher S; Kroll S; et al. 131I-tositumomab therapy as initial treatment for follicular lymphoma. N. Engl. J. Med 2005, 352, 441–449. [DOI] [PubMed] [Google Scholar]
- (908).Tennvall J; Fischer M; Bischof Delaloye A; Bombardieri E; Bodei L; Giammarile F; Lassmann M; Oyen W; Brans BE; et al. EANM procedure guideline for radio-immunotherapy for B-cell lymphoma with 90Y-radiolabelled ibritumomab tiuxetan (Zevalin). Eur. J. Nucl. Med. Mol. Imaging 2007, 34, 616–622. [DOI] [PubMed] [Google Scholar]
- (909).Thakral P; Singla S; Vashist A; Yadav MP; Gupta SK; Tyagi JS; Sharma A; Bal CS; Snehlata EY; Malhotra A Preliminary experience with yttrium-90-labelled rituximab (chimeric anti CD-20 antibody) in patients with relapsed and refractory B cell non-Hodgkins lymphoma. Curr. Radiopharm 2016, 9, 160–168. [DOI] [PubMed] [Google Scholar]
- (910).Vaes M; Bron D; Vugts D; Paesmans M; Meuleman M; Ghanem G; Guiot T; Vanderlinden B; Thielemans K; van Dongen G Safety and efficacy of radioimmunotherapy with 90Yttrium-rituximab in patients with relapsed CD20+ B cell lymphoma: a feasibility study. J. Cancer Sci. Ther 2012, 4, 394–400. [Google Scholar]
- (911).Green DJ; Frayo SL; Lin Y; Hamlin DK; Fisher DR; Frost SH; Kenoyer AL; Hylarides MD; Gopal AK; Gooley TA; et al. Comparative analysis of bispecific antibody and streptavidin-targeted radioimmunotherapy for B-cell cancers. Cancer Res 2016, 76, 6669–6679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (912).Park SI; Shenoi J; Frayo SM; Hamlin DK; Lin Y; Wilbur DS; Stayton PS; Orgun N; Hylarides M; Buchegger F; et al. Pretargeted radioimmunotherapy using genetically engineered antibody-streptavidin fusion proteins for treatment of non-hodgkin lymphoma. Clin. Cancer Res 2011, 17, 7373–7382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (913).Cassaday RD; Press OW; Pagel JM; Rajendran JG; Gooley TA; Fisher DR; Holmberg LA; Miyaoka RS; Sandmaier BM; Green DJ; et al. Phase I study of a CD45-targeted antibody–radionuclide conjugate for high-risk lymphoma. Clin. Cancer Res 2019, 25, 6932–6938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (914).Petrich T; Korkmaz Z; Krull D; Fromke C; Meyer GJ; Knapp WH In vitro experimental (211)At-anti-CD33 antibody therapy of leukaemia cells overcomes cellular resistance seen in vivo against gemtuzumab ozogamicin. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 851–861. [DOI] [PubMed] [Google Scholar]
- (915).Hagemann UB; Wickstroem K; Wang E; Shea AO; Sponheim K; Karlsson J; Bjerke RM; Ryan OB; Cuthbertson AS In vitro and in vivo efficacy of a novel CD33-targeted thorium-227 conjugate for the treatment of acute myeloid leukemia. Mol. Cancer Ther 2016, 15, 2422–2431. [DOI] [PubMed] [Google Scholar]
- (916).Srideshikan SM; Brooks J; Zuro D; Kumar B; Sanchez J; Echavarria Parra L; Orellana M; Vishwasrao P; Nair I; Chea J; et al. ImmunoPET, [(64)Cu]Cu-DOTA-Anti-CD33 PET-CT, imaging of an AML xenograft model. Clin. Cancer Res 2019, 25, 7463–7474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (917).de Vries EG; de Jong S; Gietema JA Molecular imaging as a tool for drug development and trial design. J. Clin. Oncol 2015, 33, 2585–2587. [DOI] [PubMed] [Google Scholar]
- (918).Willmann JK; van Bruggen N; Dinkelborg LM; Gambhir SS Molecular imaging in drug development. Nat. Rev. Drug Discovery 2008, 7, 591–607. [DOI] [PubMed] [Google Scholar]
- (919).Larson SM Cancer drug development with the help of radiopharmaceuticals: academic experience. Curr. Pharm. Des 2009, 15, 950–956. [DOI] [PubMed] [Google Scholar]
- (920).Miller MA; Weissleder R Imaging of anticancer drug action in single cells. Nat. Rev. Cancer 2017, 17, 399–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (921).Cai W; Rao J; Gambhir SS; Chen X How molecular imaging is speeding up antiangiogenic drug development. Mol. Cancer Ther 2006, 5, 2624–2633. [DOI] [PubMed] [Google Scholar]
- (922).Lamberts LE; Williams SP; Terwisscha van Scheltinga AG; Lub-de Hooge MN; Schroder CP; Gietema JA; Brouwers AH; de Vries EG Antibody positron emission tomography imaging in anticancer drug development. J. Clin. Oncol 2015, 33, 1491–1504. [DOI] [PubMed] [Google Scholar]
- (923).Berg E; Zhang X; Bec J; Judenhofer MS; Patel B; Peng Q; Kapusta M; Schmand M; Casey ME; Tarantal AF; et al. Development and evaluation of mini-EXPLORER: a long axial field-of-view PET scanner for nonhuman primate imaging. J. Nucl. Med 2018, 59, 993–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (924).Berg E; Gill H; Marik J; Ogasawara A; Williams SP; van Dongen G; Vugts DJ; Cherry SR; Tarantal AF Total-body PET and highly stable chelators together enable meaningful (89)Zr-antibody-PET studies up to 30 days post-injection. J. Nucl. Med 2020, 61, 453–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (925).Rosenkrans ZT; Cai W Total-body PET imaging for up to 30 days after injection of (89)Zr-labeled antibodies. J. Nucl. Med 2020, 61, 451–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (926).Jauw YWS; O’Donoghue JA; Zijlstra JM; Hoekstra OS; Menke-van der Houven van Oordt CW; Morschhauser F; Carrasquillo JA; Zweegman S; Pandit-Taskar N; Lammertsma AA; et al. 89)Zr-Immuno-PET: toward a noninvasive clinical tool to measure target engagement of therapeutic antibodies in vivo. J. Nucl. Med 2019, 60, 1825–1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (927).Oude Munnink TH; Dijkers EC; Netters SJ; Lub-de Hooge MN; Brouwers AH; Haasjes JG; Schroder CP; de Vries EG Trastuzumab pharmacokinetics influenced by extent human epidermal growth factor receptor 2-positive tumor load. J. Clin. Oncol 2010, 28, e355–356 author reply e357.. [DOI] [PubMed] [Google Scholar]
- (928).Beck A; Goetsch L; Dumontet C; Corvaia N Strategies and challenges for the next generation of antibody-drug conjugates. Nat. Rev. Drug Discovery 2017, 16, 315–337. [DOI] [PubMed] [Google Scholar]
- (929).Sijbrandi NJ; Merkul E; Muns JA; Waalboer DC; Adamzek K; Bolijn M; Montserrat V; Somsen GW; Haselberg R; Steverink PJ; et al. A novel platinum(II)-based bifunctional ADC linker benchmarked using 89Zr-desferal and auristatin F-conjugated trastuzumab. Cancer Res 2017, 77, 257–267. [DOI] [PubMed] [Google Scholar]
- (930).Muns JA; Montserrat V; Houthoff HJ; Codee-van der Schilden K; Zwaagstra O; Sijbrandi NJ; Merkul E; van Dongen G In vivo characterization of platinum (II)-based linker technology for the development of antibody-drug conjugates: taking advantage of dual labeling with (195m)Pt and (89)Zr. J. Nucl. Med 2018, 59, 1146–1151. [DOI] [PubMed] [Google Scholar]
- (931).Jacobson O; Li Q; Chen H; Niu G; Kiesewetter DO; Xu L; Cook K; Yang G; Dall’Acqua W; Tsui P; et al. PET-guided evaluation and optimization of internalized antibody-drug conjugates targeting erythropoietin-producing hepatoma A2 receptor.J. Nucl. Med 2017, 58, 1838–1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (932).Adumeau P; Vivier D; Sharma SK; Wang J; Zhang T; Chen A; Agnew BJ; Zeglis BM Site-specifically labeled antibody-drug conjugate for simultaneous therapy and immunoPET. Mol. Pharmaceutics 2018, 15, 892–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (933).Teicher BA; Chari RV Antibody conjugate therapeutics: challenges and potential. Clin. Cancer Res 2011, 17, 6389–6397. [DOI] [PubMed] [Google Scholar]
- (934).Rizvi SN; Visser OJ; Vosjan MJ; van Lingen A; Hoekstra OS; Zijlstra JM; Huijgens PC; van Dongen GA; Lubberink M Biodistribution, radiation dosimetry and scouting of 90Y-ibritumomab tiuxetan therapy in patients with relapsed B-cell non-Hodgkin’s lymphoma using 89Zr-ibritumomab tiuxetan and PET. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 512–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (935).Moek KL; Giesen D; Kok IC; de Groot DJA; Jalving M; Fehrmann RSN; Lub-de Hooge MN; Brouwers AH; de Vries EGE Theranostics using antibodies and antibody-related therapeutics. J. Nucl. Med 2017, 58, 83S–90S. [DOI] [PubMed] [Google Scholar]
- (936).Schwarz SW; Decristoforo C; Goodbody AE; Singhal N; Saliba S; Ruddock P; Zukotynski K; Ross AA Harmonization of United States, European Union and Canadian first-in-human regulatory requirements for radiopharmaceuticals-is this possible? J. Nucl. Med 2019, 60, 158. [DOI] [PubMed] [Google Scholar]
- (937).Wright BD; Whittenberg J; Desai A; DiFelice C; Kenis PJ; Lapi SE; Reichert DE Microfluidic preparation of a 89Zr-labeled trastuzumab single-patient dose. J. Nucl. Med 2016, 57, 747–752. [DOI] [PubMed] [Google Scholar]
- (938).Poot AJ; Adamzek KWA; Windhorst AD; Vosjan M; Kropf S; Wester HJ; van Dongen G; Vugts DJ Fully automated(89)Zr labeling and purification of antibodies. J. Nucl. Med 2019, 60, 691–695. [DOI] [PubMed] [Google Scholar]
- (939).Cherry SR; Badawi RD; Karp JS; Moses WW; Price P; Jones T Total-body imaging: Transforming the role of positron emission tomography. Sci. Transl. Med 2017, 9, eaaf6169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (940).Karp JS; Viswanath V; Geagan MJ; Muehllehner G; Pantel AR; Parma MJ; Perkins AE; Schmall JP; Werner ME; Daube-Witherspoon ME PennPET explorer: design and preliminary performance of a whole-body imager. J. Nucl. Med 2020, 61, 136–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (941).Badawi RD; Shi H; Hu P; Chen S; Xu T; Price PM; Ding Y; Spencer BA; Nardo L; Liu W; et al. First human imaging studies with the EXPLORER total-body PET scanner. J. Nucl. Med 2019, 60, 299–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (942).Zaidi H; Alavi A; Naqa IE Novel quantitative PET techniques for clinical decision support in oncology. Semin. Nucl. Med 2018, 48, 548–564. [DOI] [PubMed] [Google Scholar]
- (943).Haberkorn U; Mier W; Kopka K; Herold-Mende C; Altmann A; Babich J Identification of ligands and translation to clinical applications. J. Nucl. Med 2017, 58, 27S–33S. [DOI] [PubMed] [Google Scholar]
- (944).Lee ST; Burvenich I; Scott AM Novel target selection for nuclear medicine studies. Semin. Nucl. Med 2019, 49, 357–368. [DOI] [PubMed] [Google Scholar]
- (945).Jefferis R Antibody therapeutics: isotype and glycoform selection. Expert Opin. Biol. Ther 2007, 7, 1401–1413. [DOI] [PubMed] [Google Scholar]
- (946).Jefferis R Isotype and glycoform selection for antibody therapeutics. Arch. Biochem. Biophys 2012, 526, 159–166. [DOI] [PubMed] [Google Scholar]
- (947).Bournazos S; Ravetch JV Fcgamma receptor function and the design of vaccination strategies. Immunity 2017, 47, 224–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (948).Warnders FJ; Lub-de Hooge MN; de Vries EGE; Kosterink JGW Influence of protein properties and protein modification on biodistribution and tumor uptake of anticancer antibodies, antibody derivatives, and non-Ig scaffolds. Med. Res. Rev 2018, 38, 1837–1873. [DOI] [PubMed] [Google Scholar]
- (949).Zhang T; Song X; Xu L; Ma J; Zhang Y; Gong W; Zhang Y; Zhou X; Wang Z; Wang Y; et al. The binding of an anti-PD-1 antibody to FcgammaRIota has a profound impact on its biological functions. Cancer Immunol. Immunother 2018, 67, 1079–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (950).Ingram JR; Blomberg OS; Sockolosky JT; Ali L; Schmidt FI; Pishesha N; Espinosa C; Dougan SK; Garcia KC; Ploegh HL; et al. Localized CD47 blockade enhances immunotherapy for murine melanoma. Proc. Natl. Acad. Sci. U. S. A 2017, 114, 10184–10189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (951).Vivier D; Sharma SK; Adumeau P; Rodriguez C; Fung K; Zeglis BM The impact of FcgammaRI binding on immuno-PET.J. Nucl. Med 2019, 60, 1174–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (952).Vivier D; Fung K; Rodriguez C; Adumeau P; Ulaner GA; Lewis JS; Sharma SK; Zeglis BM The influence of glycans-specific bioconjugation on the FcγRI binding and in vivo performance of 89Zr-DFO-pertuzumab. Theranostics 2020, 10, 1746–1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (953).Ehlerding EB; Sun L; Lan X; Zeng D; Cai W Dual-targeted molecular imaging of cancer. J. Nucl. Med 2018, 59, 390–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (954).Kraeber-Bodere F; Rousseau C; Bodet-Milin C; Frampas E; Faivre-Chauvet A; Rauscher A; Sharkey RM; Goldenberg DM; Chatal JF; Barbet J A pretargeting system for tumor PET imaging and radioimmunotherapy. Front. Pharmacol 2015, 6, 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (955).Keinanen O; Fung K; Pourat J; Jallinoja V; Vivier D; Pillarsetty NK; Airaksinen AJ; Lewis JS; Zeglis BM; Sarparanta M Pretargeting of internalizing trastuzumab and cetuximab with a (18)F-tetrazine tracer in xenograft models. EJNMMI Res 2017, 7, 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (956).Stefan N; Gebleux R; Waldmeier L; Hell T; Escher M; Wolter FI; Grawunder U; Beerli RR Highly potent, anthracycline-based antibody-drug conjugates generated by enzymatic, site-specific conjugation. Mol. Cancer Ther 2017, 16, 879–892. [DOI] [PubMed] [Google Scholar]
- (957).De La Rochere P; Guil-Luna S; Decaudin D; Azar G; Sidhu SS; Piaggio E Humanized mice for the study of immunooncology. Trends Immunol 2018, 39, 748–763. [DOI] [PubMed] [Google Scholar]
- (958).Fernandes SRG; Fernandes R; Sarmento B; Pereira PMR; Tome JPC Photoimmunoconjugates: novel synthetic strategies to target and treat cancer by photodynamic therapy. Org. Biomol. Chem 2019, 17, 2579–2593. [DOI] [PubMed] [Google Scholar]
- (959).Kobayashi H; Choyke PL Near-infrared photoimmuno-therapy of cancer. Acc. Chem. Res 2019, 52, 2332–2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (960).Green DJ; Press OW Whither radioimmunotherapy: to be or not to be? Cancer Res 2017, 77, 2191–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (961).Patra M; Zarschler K; Pietzsch HJ; Stephan H; Gasser G New insights into the pretargeting approach to image and treat tumours. Chem. Soc. Rev 2016, 45, 6415–6431. [DOI] [PubMed] [Google Scholar]
- (962).Levy A; Nigro G; Sansonetti PJ; Deutsch E Candidate immune biomarkers for radioimmunotherapy. Biochim. Biophys. Acta, Rev. Cancer 2017, 1868, 58–68. [DOI] [PubMed] [Google Scholar]
- (963).Cheal SM; Xu H; Guo HF; Patel M; Punzalan B; Fung EK; Lee SG; Bell M; Singh M; Jungbluth AA; et al. Theranostic pretargeted radioimmunotherapy of internalizing solid tumor antigens in human tumor xenografts in mice: curative treatment of HER2-positive breast carcinoma. Theranostics 2018, 8, 5106–5125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (964).Kraeber-Bodere F; Bodet-Milin C; Rousseau C; Eugene T; Pallardy A; Frampas E; Carlier T; Ferrer L; Gaschet J; Davodeau F; et al. Radioimmunoconjugates for the treatment of cancer. Semin. Oncol 2014, 41, 613–622. [DOI] [PubMed] [Google Scholar]
- (965).Ehlerding EB; Lan X; Cai W “Albumin Hitchhiking” with an evans blue analog for cancer theranostics. Theranostics 2018, 8, 812–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (966).Kratochwil C; Bruchertseifer F; Giesel FL; Weis M; Verburg FA; Mottaghy F; Kopka K; Apostolidis C; Haberkorn U; Morgenstern A 225Ac-PSMA-617 for PSMA-targeted alpha-radiation therapy of metastatic castration-resistant prostate cancer. J. Nucl. Med 2016, 57, 1941–1944. [DOI] [PubMed] [Google Scholar]
- (967).Kratochwil C; Schmidt K; Afshar-Oromieh A; Bruchertseifer F; Rathke H; Morgenstern A; Haberkorn U; Giesel FL Targeted alpha therapy of mCRPC: dosimetry estimate of (213)Bismuth-PSMA-617. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 31–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (968).Vahrmeijer AL; Hutteman M; van der Vorst JR; van de Velde CJ; Frangioni JV Image-guided cancer surgery using near-infrared fluorescence. Nat. Rev. Clin. Oncol 2013, 10, 507–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (969).Linssen MD; Ter Weele EJ; Allersma DP; Lub-de Hooge MN; van Dam GM; Jorritsma-Smit A; Nagengast WB Roadmap for the development and clinical translation of optical tracers cetuximab-800CW and trastuzumab-800CW. J. Nucl. Med 2019, 60, 418–423. [DOI] [PubMed] [Google Scholar]
- (970).Zettlitz KA; Waldmann CM; Tsai WK; Tavare R; Collins J; Murphy JM; Wu AM A dual-modality linker enables site-specific conjugation of antibody fragments for (18)F-immuno-PET and fluorescence imaging. J. Nucl. Med 2019, 60, 1467–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (971).Cohen R; Vugts DJ; Stigter-van Walsum M; Visser GW; van Dongen GA Inert coupling of IRDye800CW and zirconium-89 to monoclonal antibodies for single- or dual-mode fluorescence and PET imaging. Nat. Protoc 2013, 8, 1010–1018. [DOI] [PubMed] [Google Scholar]
- (972).Lee HJ; Ehlerding EB; Jiang D; Barnhart TE; Cao T; Wei W; Ferreira CA; Huang P; Engle JW; Cai W Dual-labeled pertuzumab for multimodality image-guided ovarian tumor resection. Am. J. Cancer Res 2019, 9, 1454–1468. [PMC free article] [PubMed] [Google Scholar]
- (973).Shaffer TM; Pratt EC; Grimm J Utilizing the power of Cerenkov light with nanotechnology. Nat. Nanotechnol 2017, 12, 106–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (974).Ferreira CA; Ni D; Rosenkrans ZT; Cai W Radionuclide-activated nanomaterials and their biomedical applications. Angew. Chem., Int. Ed 2019, 58, 13232–13252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (975).Kotagiri N; Sudlow GP; Akers WJ; Achilefu S Breaking the depth dependency of phototherapy with Cerenkov radiation and low-radiance-responsive nanophotosensitizers. Nat. Nanotechnol 2015, 10, 370–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (976).Maier FC; Wild AM; Kirchen N; Holm F; Fuchs K; Schwenck J; Maurer A; Wiehr S Comparative immuno-Cerenkov luminescence and -PET imaging enables detection of PSMA(+) tumors in mice using (64)Cu-radiolabeled monoclonal antibodies. Appl. Radiat. Isot 2019, 143, 149–155. [DOI] [PubMed] [Google Scholar]
- (977).Ni D; Ferreira CA; Barnhart TE; Quach V; Yu B; Jiang D; Wei W; Liu H; Engle JW; Hu P; et al. Magnetic targeting of nanotheranostics enhances Cerenkov radiation-induced photodynamic therapy. J. Am. Chem. Soc 2018, 140, 14971–14979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (978).Yu B; Wei H; He Q; Ferreira CA; Kutyreff CJ; Ni D; Rosenkrans ZT; Cheng L; Yu F; Engle JW; et al. Efficient uptake of (177) Lu-porphyrin-PEG nanocomplexes by tumor mitochondria for multimodal-imaging-guided combination therapy. Angew. Chem., Int. Ed 2018, 57, 218–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (979).Xing Y; Chand G; Liu C; Cook GJR; O’Doherty J; Zhao L; Wong NCL; Meszaros LK; Ting HH; Zhao J Early phase I study of a (99m)Tc-labeled anti-programmed death ligand-1 (PD-L1) single-domain antibody in SPECT/CT assessment of PD-L1 expression in non-small cell lung cancer. J. Nucl. Med 2019, 60, 1213–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (980).Zhang P; Zhuang R; Guo Z; Su X; Chen X; Zhang X A highly efficient copper-mediated radioiodination approach using aryl boronic acids. Chem. - Eur. J 2016, 22, 16783–16786. [DOI] [PubMed] [Google Scholar]
- (981).Pruszynski M; Loktionova NS; Filosofov DV; Rosch F Post-elution processing of (44)Ti/(44)Sc generator-derived (44)Sc for clinical application. Appl. Radiat. Isot 2010, 68, 1636–1641. [DOI] [PubMed] [Google Scholar]
- (982).Alliot C; Kerdjoudj R; Michel N; Haddad F; Huclier-Markai S Cyclotron production of high purity (44m,44)Sc with deuterons from (44)CaCO3 targets. Nucl. Med. Biol 2015, 42, 524–529. [DOI] [PubMed] [Google Scholar]
- (983).van der Meulen NP; Bunka M; Domnanich KA; Muller C; Haller S; Vermeulen C; Turler A; Schibli R Cyclotron production of (44)Sc: From bench to bedside. Nucl. Med. Biol 2015, 42, 745–751. [DOI] [PubMed] [Google Scholar]


