Supplemental Digital Content is available in the text.
Key Words: optic disc pit, maculopathy, whole-exome sequencing, glaucoma
Abstract
Optic disc pit maculopathy (ODP-M) is a subtype of ODP, characterized by a serous retinal detachment and/or macular retinoschisis. Currently, ODP and ODP-M pathogenesis remain unknown although many hypotheses exist about their clinical features. In this study, we report a case of new ODP-M detected after surgical iridectomy in a patient with primary angle-closure glaucoma (PACG) with a preoperative normal retina and optic nerve. Fine optic disc and the macular area structures were investigated using several imaging techniques. Findings revealed that the course of ODP and ODP-M provide us with some insights and understanding of their underlying pathogenesis.
Optic disc pit (ODP) is a rare anomaly of the optic nerve head (ONH) characterized by gray, round, or oval atrophy in the disc, which is often associated with strands of attached and condensed vitreous collagen at the retinal surface.1–3 ODP can be associated with serous retinal detachments at the macula (ODP-M) in 25% to 75% of cases, causing a significant visual loss at 20/200 or worse.4,5 Its clinical features include intraretinal and subretinal fluid accumulation, retinoschisis, and retinal pigment changes.4 The clinical use of high-resolution optical coherence tomography (OCT) has enabled us to examine the fine retinal structure in ODP/ODP-M.
Based on OCT findings, a hypothesis was once proposed that a 3-directional connection between subretinal/intraretinal space, perineural space, and the vitreous cavity is the pathogenesis underlying fluid accumulation in ODP/ODP-M. The fluid may originally come from vitreous through hidden small retinal holes, from the choroid, or from retinal vascular leakage, or from cerebrospinal fluid.2,6–9 However, all these ideas have not yet been definitively confirmed with anatomic imaging because of limited resolution and retinal penetration capacity in previous generation imaging equipment. Besides, all previous cases reported were ODP/ODP-M pathologies in a mature state, when we never have got an opportunity to observe what happens at the initial disease stage and how it evolves. Therefore, a theories/hypothesis covering and explains a complete disease evolving process is very difficult to reproduce.
We know in some cases, ODP is a congenital anomaly related to the PAX2 gene mutation10 with variable expressivity of a single autosomal dominantly inherited defect.11 Until now, no triggering factors have ever been reported to be associated with the development of maculopathy.12 Here we report a case of ODP-M triggered by surgical iridectomy in a patient with primary angle-closure glaucoma with a preoperative normal retina and optic nerve, therefore, the first case that was ever reported in revealing ODP/ODP-M development at the very early disease stage. Using swept-source OCT (SS-OCT), we were able to investigate the fine and deep retinal structures. Besides, a whole-exome sequencing of the patient and her core family members were performed, and comprehensive bioinformatics tools sets were used to identify known and unknown genes that may cause the pathology of OPD/OPD-M.
CASE REPORT
A 37-year-old female individual complained of left eye pain and swelling for about 1 year. The best-corrected visual acuity of the left eye was 20/30 and 20/20 in the right eye. The intraocular pressure (IOP) measured by the noncontact tonometer was 34.7 and 14.7 mm Hg for the left and right eyes, respectively. An ocular examination of the left eye revealed slight corneal epithelial edema, a very shallow peripheral anterior chamber depth, a dilated pupil of 7×7 mm diameter, and a transparent lens. The optic disc looked pale, and the cup to disc (C/D) ratio was 0.9. There were no pathologies identified in the right eye with a C/D ratio of 0.3, except that a shallow peripheral anterior chamber was present. Azarga (Brinzolamide and Timolol Maleate Eye Drops) and Alphagan (Brimonidine Tartrate) were prescribed for the left eye, and the IOP was controlled at around 17 mm Hg. Synechia angle closure in 9 of 12 clock hours (from 5 to 3 o’clock, of 75% total angle) was detected under gonioscopy in the left eye, whereas the right eye was II to IV degree narrow. A tunnel visual field and temporal visual island were detected during the Humphrey visual field test for the left eye. A diagnosis of primary angle-closure glaucoma was made for the left eye and primary angle closure suspect for the right one. Surgical iridectomy was performed on the left eye and laser peripheral iridotomy on the right eye, together with postoperative antiglaucoma medications. Preoperatively, there was no ODP/ODP-M in either eye. Images of fundus photography, ultrasound biomicroscopy, and OCT are summarized in Figures 1A–D. The best-corrected visual acuity and IOP changes during the follow-up period up to 1 year postoperatively are shown in Figures 2A and B. Postoperative IOP was well controlled as shown in Figure 2B.
FIGURE 1.
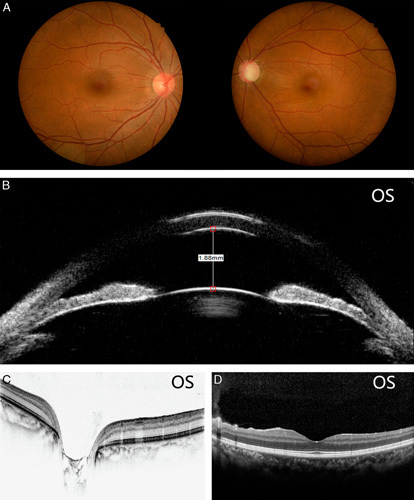
A–D, Preoperative images of fundus photography, ultrasound biomicroscopy, and optical coherence tomography.
FIGURE 2.
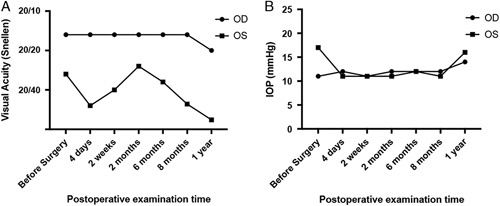
A and B, The best-corrected visual acuity and intraocular pressure change during the follow-up period up to 1 year postoperatively.
From postoperative month 2, the patient started to notice a slightly reduced vision in the left eye. The deterioration in visual acuity continued at each follow-up. SS-OCT was prescribed when the visual acuity dropped to 20/100. Posterior vitreous detachment, retinal edema, and retinoschisis ranging from the optic disc to the macula were detected (Fig. 3A). To illustrate the origins of accumulated intraretinal fluid, fluorescein fundus angiography, and indocyanine green angiography were performed, and no leakage was from either retinal blood vessels, retinal pigment epithelium, or choroidal blood vessels was identified. The only finding was enhanced fluorescence accumulated over the optic disc at later fluorescein fundus angiography stages (Figs. 4A, B). Diffuse reduction of retinal signals was detected in multifocal electroretinogram (mf-ERG) (Fig. 4C). A very faint fluorescence in the macular retinoschisis area was detected as a result of fundus autofluorescence (Fig. 4D). There were no pathologic signs noted within the contralateral right eye (Fig. 4E). We used SS-OCT to perform 320 consecutive scans of the optic disc area and 512 consecutive scans of the entire retina (Supplementary Video 1, Supplemental Digital Content 1, http://links.lww.com/IJG/A383 and Supplementary Video 2, Supplemental Digital Content 2, http://links.lww.com/IJG/A384). Of all of these scans, a small channel-like connection was found between the vitreous cavity and retinoschisis, but the 2 ends had not yet communicated directly. This suggests that there may be a flap-like structure that opens only when pressure changes on both sides creating a gradient.9 The same regimen of intensive scans was repeated 2 months after follow-up, and there were no obvious changes in the extent of retinoschisis or ODP, conforming to the idea that there is little or no self-recovery process in OPD/OPD-M (Supplement Video 3, Supplemental Digital Content 3, http://links.lww.com/IJG/A385).
FIGURE 3.
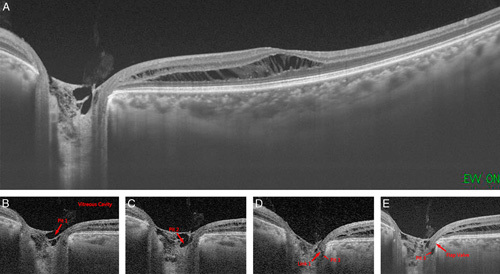
A, Postoperatively optical coherence tomography images revealed posterior vitreous detachment, retinal edema, and retinoschisis ranging from the optic disc to the macula. B–E, Vitreous cavity was connected to the retinoschisis through pits 1 to 3 and a valve/flap-like structure. Arrow indicates the pits and the links between them.
FIGURE 4.
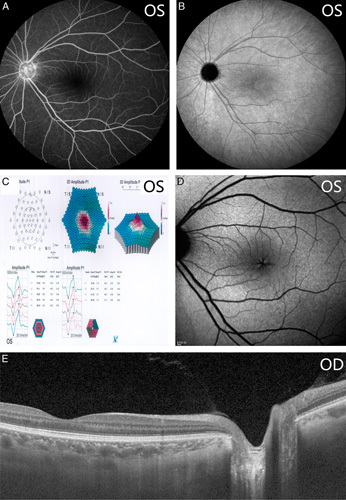
A–D, Postoperative images of fundus photography, multifocal electroretinogram, fundus autofluorescence of the left eye. E, The postoperative optical coherence tomography image of the contralateral right eye.
DISCUSSION
ODP is currently considered a congenital disease, whose etiology is still unclear. It is generally believed that ODP is caused by imperfect closure of the superior edge of the embryonic fissure.13 However, considering that the ODP and ODP-M in our case only appeared after glaucoma surgery, it is necessary to identify whether it was caused as a pathology secondary to glaucomatous optic neuropathy (GON) or intraocular surgery.
Currently, there were several case reports describing peripapillary retinoschisis in patients with different types of glaucoma. Bayraktar et al reported that the prevalence of peripapillary retinoschisis in 940 various types of glaucoma patients was 3.1%,14 and Lee et al15 reported their prevalence was 5.9%. However, in these reports, retinoschisis happens in the advanced stage of glaucoma. In Lee and colleagues’ study, an acquired optic nerve pit was found in 8 patients but not in Bayraktar and colleagues’s study. In other studies, optic nerve pit was not identified.16–19 These observations suggest that a link may exist during the development of retinoschisis in GON. It was proposed that there may be a microscopic interconnection within the nerve fiber layer (NFL) in patients with progressive glaucoma, acting as a conduit through which vitreous fluid may enter the retina.20 Therefore, the pathologic changes associated with retinoschisis caused by glaucoma are mainly connected to the NFL around the optic disc, which is not associated with breaking the lamina cribrosa (LC). In brief, the channel and depression changes on the disc rim are detectable and obvious, whereas the LC is not affected.21
In contrast, our case is different in that structural changes have developed in the retina while postoperative IOP was well controlled and no manifest changes were identified between preoperative and postoperative optic nerve cupping (stable C/D ratios). Furthermore, the retinoschisis, in the form of atrophy and cavities, was detected at or behind the level of LC, whereas there were no changes detected within the NFL similar to those reported in other cases of GON-related pathology. Although it cannot be ruled out completely in our case that GON could be a contributing factor leading to the development of the disc pit, there is no evidence to prove that the occurrence of OPD/OPD-M is secondary to a progressive GON.15,22
In contrast, classic ODP/ODP is believed to be the result of incomplete closure of the superior end of the embryonic fissure, which seems not to be suitable for explaining our case either.3,23,24 Preoperatively, we had completely normal retinal and optic nerve structures as documented by OCT, therefore completely eliminating the possibility of an inborn pathology. To further test whether there is a genetic factor involved in our case, we performed whole-exome sequencing on this patient and her core family. All specific known or unknown gene mutations that may be related to retinoschisis were scrutinized. To rule out de novo mutation as both parents were unaffected, de novo mutation screening was according to several criteria: (1) variants classified as pathogenic or likely pathogenic following the American College of Medical Genetics and Genomics (ACMG) guidelines were included; (2) variants with frequencies in Genome Aggregation Database (gnomAD) and Exome Aggregation Consortium (ExAC) <1×10−3 were included; and (3) nonsense and frameshift mutations, and splice site or predicted damaging missense mutations by SIFT or polymorphism phenotyping (PolyPhen)2 were included. There were no identified genetic mutations that could be potentially pathologic.
Therefore, we suspected the pathology in our case was acquired and secondary in nature and most importantly is a stage in which the disease was still in an early, developing process. However, there are not many acquired ODP reported in the literature, and these articles are not completely consistent with the clinical manifestations of congenital ODP16,25–27; they are either simple retinoschisis or pit of the optic nerve, retinoschisis caused by optic disc depression during glaucoma progression, or a companion symptom.
Using high-resolution SS-OCT imaging, we can detect very small atrophic cavities within the ONH. A thorough screening of 320 consecutive imaging of ONH together with 512 consecutive images of the retina indicates that there may be direct connecting channels for the pathology and fluid accumulation (Supplementary Video 1, Supplemental Digital Content 1, http://links.lww.com/IJG/A383 and Supplementary Video 2, Supplemental Digital Content 2, http://links.lww.com/IJG/A384). From Figure 3B–E, we could detect that the vitreous cavity was connected directly to pit 1, and pit 1 was connected to pits 2 and 3 directly. However, the direct channel between pit 3 and the retinoschisis was not found in the consecutive scanning although they are physically very close. There could be 3 explanations: (1) the connecting tunnel is not a straight line; therefore, its complete formation cannot be detected on a facet imaging. However, in this situation, the tunnel section image would present the movement path from 1 cavity to another in the video of the intensive scanning; (2) the diameter of the connecting tunnel is too small to be detectable by current imaging resolution or imaging intervals of the existing program is not fine enough. However, if such a minimal tunnel truly exists, there is doubt of how such an almost undetectable tunnel produces such extensive retinal edema and retinoschisis; and (3) the most likely explanation is that there are no direct connecting tunnels in a manner described by Johnson’s theory.9 Instead, there is a valve/flap-like structure, which is sometimes open and sometimes closed upon a pressure gradient9 along 2 sides of the cavities. Therefore, the source of the fluid might be a potential cavity that communicates between the vitreous cavity and the retina, and the created pressure gradient presses water into the retina, causing retinoschisis but deserves further confirmation with more clinical cases.
Based on existing theories/hypothesis, we propose a unifying pathophysiological explanation for this acquired ODP/OPD-M: (1) the formation of ODP is because of optic disc atrophy caused by glaucoma and (2) fluids comes from the vitreous flow through valves into the intraoptic nerve and subretinal spaces. However, in the early stage, small cavities caused by atrophy are gradually formed and have not been completely connected in the retina but form potential channels and a flap-like structure. With the gradual expansion of the cavities, the vitreous cavity can directly communicate with subretinal/intraretinal space, and finally, expanded cavities connect the cerebrospinal fluid and the subretinal/intraretinal space. Our scrutiny, in this case, allows us to rule out the possibility of a potential fluid leak from the retinal or choroidal vessels.
Altogether, this case allows us to observe the early pathologic changes and disease progression of ODP using high-definition SS-OCT. Based on our findings, we propose a theory that unifies the 3 different explains for the source of liquid in ODP and reveals the different stages in the same disease. These findings could lead us to further understanding of the nature of acquired ODP.
Supplementary Material
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.glaucomajournal.com.
Footnotes
Supported by Major Project of National Natural Science Foundation of China (NSFC)-Guangdong Province Joint Fund (grant number: 3030902113080), the Science and Technology Planning Project of Guangdong Province (grant number: 303090100502050-18; Guangzhou, China), Guangzhou Science and Technology Plan Project (grant number: No. 2018- 1202-SF-0019; Guangzhou, China), Research Funds of the State Key Laboratory of Ophthalmology (grant numbers: 30306020240020153, 30306020240020192, 3030902113058, 3030902113118, PT1001022; Guangzhou, China), and Fundamental Research Funds of Sun Yat-sen University (grant number: 16ykjc31; Guangzhou, China).
Disclosure: The authors declare no conflict of interest.
REFERENCES
- 1.Steel DHW, Suleman J, Murphy DC, et al. Optic disc pit maculopathy: a two-year nationwide prospective population-based study. Ophthalmology. 2018;125:1757–1764. [DOI] [PubMed] [Google Scholar]
- 2.Kalogeropoulos D, Ch’ng SW, Lee R, et al. Optic disc pit maculopathy: a review. Asia Pac J Ophthalmol (Phila). 2019;8:247–255. [DOI] [PubMed] [Google Scholar]
- 3.Georgalas I, Ladas I, Georgopoulos G, et al. Optic disc pit: a review. Graefes Arch Clin Exp Ophthalmol. 2011;249:1113–1122. [DOI] [PubMed] [Google Scholar]
- 4.Moisseiev E, Moisseiev J, Loewenstein A. Optic disc pit maculopathy: when and how to treat? A review of the pathogenesis and treatment options. Int J Retina Vitreous. 2015;1:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Uzel MM, Karacorlu M. Optic disk pits and optic disk pit maculopathy: a review. Surv Ophthalmol. 2019;64:595–607. [DOI] [PubMed] [Google Scholar]
- 6.Michalewski J, Michalewska Z, Nawrocki J. Spectral domain optical coherence tomography morphology in optic disc pit associated maculopathy. Indian J Ophthalmol. 2014;62:777–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gowdar JP, Rajesh B, Giridhar A, et al. An insight into the pathogenesis of optic disc pit-associated maculopathy with enhanced depth imaging. JAMA Ophthalmol. 2015;133:466–469. [DOI] [PubMed] [Google Scholar]
- 8.Johnson TM, Johnson MW. Pathogenic implications of subretinal gas migration through pits and atypical colobomas of the optic nerve. Arch Ophthalmol. 2004;122:1793–1800. [DOI] [PubMed] [Google Scholar]
- 9.Jain N, Johnson MW. Pathogenesis and treatment of maculopathy associated with cavitary optic disc anomalies. Am J Ophthalmol. 2014;158:423–435. [DOI] [PubMed] [Google Scholar]
- 10.Samimi S, Antignac C, Combe C, et al. Bilateral macular detachment caused by bilateral optic nerve malformation in a papillorenal syndrome due to a new PAX2 mutation. Eur J Ophthalmol. 2008;18:656–658. [DOI] [PubMed] [Google Scholar]
- 11.Slusher MM, Weaver RG, Jr, Greven CM, et al. The spectrum of cavitary optic disc anomalies in a family. Ophthalmology. 1989;96:342–347. [DOI] [PubMed] [Google Scholar]
- 12.Sadun AA, Khaderi KR.Sadda S. Optic disc anomalies, pits, and associated serous macular detachment. Retina, 5th edn UK: Elsevier Health Sciences; 2013:1583–1588. [Google Scholar]
- 13.Chen MS, Tsai WF. Congenital optic pits and central serous chorioretinopathy. Aust N Z J Ophthalmol. 1997;25:165–166. [DOI] [PubMed] [Google Scholar]
- 14.Bayraktar S, Cebeci Z, Kabaalioglu M, et al. Peripapillary Retinoschisis in Glaucoma Patients. J Ophthalmol. 2016;2016:1612720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee EJ, Kim TW, Kim M, et al. Peripapillary retinoschisis in glaucomatous eyes. PloS One. 2014;9:e90129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arranz-Marquez E, Jarrin Hernandez E, Pastor A, et al. Retinoschisis and neurosensory detachment in advanced focal glaucoma. Arch Soc Esp Oftalmol. 2017;92:495–498. [DOI] [PubMed] [Google Scholar]
- 17.Hollander DA, Barricks ME, Duncan JL, et al. Macular schisis detachment associated with angle-closure glaucoma. Arch Ophthalmol. 2005;123:270–272. [DOI] [PubMed] [Google Scholar]
- 18.Kahook MY, Noecker RJ, Ishikawa H, et al. Peripapillary schisis in glaucoma patients with narrow angles and increased intraocular pressure. Am J Ophthalmol. 2007;143:697–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hwang JC. Regional practice patterns for retinal detachment repair in the United States. Am J Ophthalmol. 2012;153:1125-1128. [DOI] [PubMed] [Google Scholar]
- 20.Woo R, Akil H, Koulisis N, et al. Sustained resolution of macular retinoschisis after trabeculectomy in a patient with progressive glaucoma. J Glaucoma. 2017;26:e180–e186. [DOI] [PubMed] [Google Scholar]
- 21.Omodaka K, Horii T, Takahashi S, et al. 3D evaluation of the lamina cribrosa with swept-source optical coherence tomography in normal tension glaucoma. PloS One. 2015;10:e0122347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Farjad H, Besada E, Frauens BJ. Peripapillary schisis with serous detachment in advanced glaucoma. Optom Vis Sci. 2010;87:E205–E217. [DOI] [PubMed] [Google Scholar]
- 23.Chatziralli I, Theodossiadis P, Theodossiadis GP. Optic disk pit maculopathy: current management strategies. Clin Ophthalmol. 2018;12:1417–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Teke MY, Elgin U, Ozdal P, et al. Autofluorescence and optical coherence tomography findings in optic disc pit-associated maculopathy: case series. Intern Ophthalmol. 2011;31:485–491. [DOI] [PubMed] [Google Scholar]
- 25.Haruta M, Yamakawa R. Vitrectomy for macular retinoschisis without a detectable optic disk pit. Retina. 2017;37:915–920. [DOI] [PubMed] [Google Scholar]
- 26.Lee SH, Lee EJ, Kim TW. Structural characteristics of the acquired optic disc pit and the rate of progressive retinal nerve fiber layer thinning in primary open-angle glaucoma. JAMA Ophthalmol. 2015;133:1151–1158. [DOI] [PubMed] [Google Scholar]
- 27.Yoshitake T, Nakanishi H, Setoguchi Y, et al. Bilateral papillomacular retinoschisis and macular detachment accompanied by focal lamina cribrosa defect in glaucomatous eyes. Jpn J Ophthalmol. 2014;58:435–442. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.glaucomajournal.com.


