Abstract:
Latifolin, one of the major flavonoids extracted from lignum dalbergiae odoriferae, has been documented to protect the heart from acute myocardial ischemia induced by pituitrin and isoproterenol in rats and has also been found to inhibit inflammation. In this study, we aimed to investigate whether latifolin could protect the heart from doxorubicin (DOX)-induced cardiotoxicity and elucidate its underlying mechanisms. Male mice were treated with an intraperitoneal dose of DOX (20 mg/kg) plus oral latifolin at a dose of 50 or 100 mg/kg for 12 days. After exposure, we assessed cardiac function, myocardial injury, and macrophage polarization in excised cardiac tissue. Our results demonstrated that latifolin prevented DOX-induced cardiac dysfunction and produced macrophage polarization in mice challenged with latifolin. In cultured peritoneal macrophages, latifolin significantly reduced inflammatory cytokines (P < 0.05). Furthermore, latifolin remarkably decreased the percentage of macrophage M1/M2 polarization (P < 0.05). The results from the present study highlight the benefits of treatment with latifolin in DOX-induced cardiotoxicity, and the mechanism involved in mediating the polarization phenotype change of M1/M2 macrophages.
Key Words: latifolin, doxorubicin, cardiotoxicity, macrophage, polarization
INTRODUCTION
Doxorubicin (DOX), a broad-spectrum chemotherapeutic, is one of the most preferred agents for the treatment of various malignancies.1 Regrettably, its application is limited because of its dose-dependent and time-dependent cardiotoxicity, leading to cardiomyopathy and heart failure.2,3 Heart failure is a major health burden, affecting 40 million people globally. The mechanism of DOX-induced cardiotoxicity is not fully understood, but there is evidence that the mitochondrial dysfunction, excessive production of proinflammatory cytokines, and cardiac membrane injuries are potentially associated with DOX-induced pathogenesis of cardiotoxicity.4–6 Furthermore, additional reports have shown that DOX can enhance the inflammatory responses in the myocardium by activating expression of NF-κB, a key regulator for immune/inflammatory responses.7 Therefore, anti-inflammation strategies that may relieve the cardiotoxicity exerted by DOX have received considerable attention.
Macrophages were on behalf of immune cell in the myocardium.8 It was found that macrophages are able to attain either M1 or M2 phenotype in the microenvironment, with macrophages M1 and M2 playing a proinflammatory and anti-inflammatory role, respectively. Proinflammatory macrophage M1 expresses inflammatory cytokines that aggravate tissue damage, whereas anti-inflammatory macrophage, M2, produces anti-inflammatory cytokines to facilitate tissue repair.9,10 The ratio of M1 to M2 determines inflammation damage or tissue repair, a predictive biomarker that is gaining increasing recognition in the field.
Traditional Chinese medicine lignum dalbergiae odoriferae is the dried heart wood or root of Dalbergia odorifera T. Chen, a leguminous plant belonging to the family Papilionaceae. It has been shown to have the functions of removing blood stasis, regulating “qi,” and relieving pain. Lignum dalbergiae odoriferae is primarily composed of volatile oils and flavonoids. Pharmacological studies have shown that flavonoids of lignum dalbergiae odoriferae exhibit antithrombotic, anti-inflammatory, and antioxidant biological effects.11–13 Our previous study demonstrated that latifolin, a flavonoid extracted from lignum dalbergiae odoriferae, exerts a protective effect on acute myocardial ischemia induced by pituitrin and isoproterenol in rats.14 It has also been deduced that Latifolin could improve cardiac function and reduce inflammatory cell infiltration in myocardial tissue. Therefore, it was further hypothesized that latifolin could attenuate doxorubicin-induced cardiotoxicity by inhibiting the inflammatory response and that the mechanism might be involved in the polarization of macrophages M1 and M2. The current study aimed to investigate this hypothesis.
MATERIAL AND METHODS
All procedures involving animals were conducted in compliance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Animal care and housing, as well as the experimental protocol conducted in compliance with and approved by the Animal Care and Ethics Review Committee at the Faculty of Jiangxi University of Traditional Chinese Medicine, Nanchang, China (The approval no. JXLLSC-2019-23).
Chemicals
Latifolin (chemical name (−)-(R)-latifolin, PubChem No. 340211) was provided by the Jiangxi University of Traditional Chinese Medicine (Broadwood Dalbergia was purchased from Fangchenggang City, Guangxi Province, China). Latifolin was isolated and prepared from the core material of lignum dalbergiae odoriferae. The preparation process entailed crushing lignum dalbergiae odoriferae heartwood and extracting with 70% ethanol by heated reflux extraction. The extract was then concentrated and extracted with water to make a suspension, followed by petroleum ether extraction. The extract was then analyzed by silica gel chromatography. The gradient elution was carried out with petroleum ether-ethyl acetate as the eluent to obtain the monomeric compound. The structure was determined by 1H-NMR, 13C-NMR (Fig. 1A). The purity of the extract was determined to be >95%. Latifolin samples were dissolved in dimethylsulfoxide (DMSO) for in vitro experiments, and DMSO were obtained from Solarbio (Peking, China); the purity being >99.5%, and samples were evenly dispersed in 0.5% sodium carboxyl methyl cellulose (CMC-Na) as a suspension for in vivo experiments. CMC-Na were purchased from Yuanye Biological Technology Co, Ltd (Shanghai, China), and the degree of substitution (DS) is 0.7.
FIGURE 1.
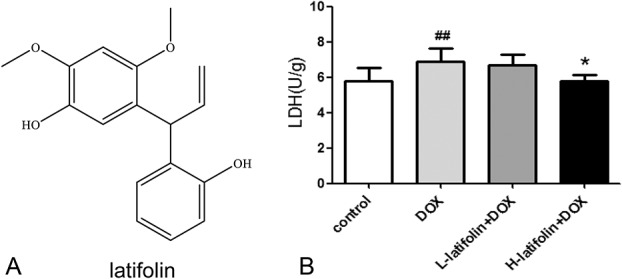
Latifolin protect cardiac tissue from DOX-induced injury. A, The structure of latifolin. B, Compared with the control group, DOX significantly increased LDH levels in cardiac tissue; compared with the DOX group, latifolin significantly reduced LDH levels in cardiac tissue. Data were shown as mean ± SD, n = 6. ##P < 0.01 versus control group, *P < 0.05 versus DOX group.
Animals and Experimental Design
Male C57BL/6 mice (10 weeks age, 25 ± 2 g) were purchased from Hunan Slac Jingda Laboratory Animal Co, Ltd Animals were housed in cages under controlled environmental conditions (24 ± 2°C temperature, 50 ± 5% relative humidity, and 12 hours light/dark cycle). All animals were fed standard laboratory diet and received water ad libitum during the experimentation.
After acclimatization for 5 days, mice were randomly divided into 4 groups (each composed of 16 animals). Group I (control) orally received 0.5% CMC-Na for 12 days. Group II (DOX) received an oral dose of 0.5% CMC-Na for 12 days with an intraperitoneal 20 mg/kg single dose of DOX on the fifth day. Group III (DOX + latifolin 50 mg/kg) and group IV (DOX + latifolin 100 mg/kg) received 20 mg/kg intraperitoneal DOX injections on the fifth day (the same dose administered to group II) plus oral latifolin at a dose of 50 or 100 mg/kg, respectively, for 12 days. After 12 days of treatment, mice were anesthetized with 1% pentobarbital sodium (40 mg/kg) and sacrificed, following which heart tissues were harvested.
Echocardiography
Cardiac function was tested by echocardiography (VisualSonics Vevo2100, Ottawa, Canada) using a Vivo Imaging System ultrasound with a 10-MHz linear array ultrasound transducer. Mice were anesthetized with 2% isoflurane in an oxygen mix. Both parasternal short-axis and long-axis views were performed to assess the left ventricle. M-mode images of the left ventricle end systole and end diastole were recorded and then left ventricle ejection fraction (LVEF) and left ventricle fractional shortening (LVFS) were measured.
Analysis of LDH in Cardiac Tissues
Myocardial injury was assessed by LDH in cardiac tissue. Using an electric homogenizer, tissue samples were weighed and homogenized in normal saline on ice, followed by centrifugation at 3500 rpm for 15 minutes at 4°C. The resulting supernatants were tested with LDH assay kits (A020-2-2; Jiancheng Bioengineering Institute, Nanjing, China) in accordance with the providers' manual.
Histological Analysis
Myocardial pathological changes were evaluated by hematoxylin and eosin staining. Excised heart samples were collected, and cardiac tissue specimens were immediately fixed in 4% paraformaldehyde for 24 hours, then processed using a paraffin-embedding technique. Subsequently, 5-µm-thick sections were deparaffinized with xylene, stained with hematoxylin and eosin for histopathology, and finally examined by light microscopy (Leica, Wetzlar, Germany).
Detection of M1/M2 Biomarkers in Cardiac Tissues
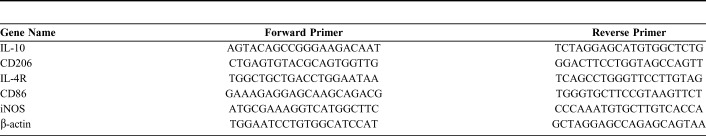
M1/M2 biomarkers in the heart tissue were measured by real-time polymerase chain reaction (RT-PCR) according to standard protocols. In summary, heart tissues were harvested, total RNA was extracted using Trizol reagent (182807; Life Technologies, Carlsbad, CA), and complementary DNAs were synthesized with RevertAid First Strand cDNA Synthesis Kit (AE311-03; TransGen Biotech, Peking, China). The validation and optimization of the standard curves and melting curves with every primer set were carried out. The primers for RT-PCR are presented in Table 1.
TABLE 1.
The Sequence of Primer Used in RT-PCR
Peritoneal Macrophage Collection and Identification
Four days after intraperitoneal injection of 100 μg ConA in C57BL/6 mice, peritoneal accumulational cells were harvested from the peritoneal cavity with 5 mL of phosphate-buffered saline and centrifuged. The cells were then washed with phosphate-buffered saline after centrifugation. Total cell numbers were determined, and the cells were resuspended at a concentration of 106 cells/mL. Single-cell suspensions were then incubated with 5 μL CD11b/c-PE (1:200, cat. no. 12-0110-82; eBioscience) for identification using flow cytometry (Gallios Beckman).
Analysis of Macrophage Secretome
Peritoneal macrophages were harvested and cultured in Roswell Park Memorial Institute 1640 (RPMI 1640) medium supplemented with 10% fetal bovine serum, and the macrophages were morphologically identified under a microscope (CKX41 OLYMPUS, Tokyo, Japan). Cells were plated in 6-well plates at a density of 1 × 106 cells and divided into the following groups: (1) control group; (2) model group [lipopolysaccharides (LPS)/interferon-γ (IFN-γ) stimulation]: 100 ng/mL LPS and 30 ng/mL IFN-γ induced for 12 hours; and (3) latifolin group (latifolin 1.25, 2.5, 5, 10 μg/mL): Before LPS/IFN-γ stimulation, latifolin were given for 24 hours. At the end of LPS/IFN-γ stimulation, the cell supernatant was collected and centrifuged at 3500 rpm for 10 minutes. The supernatant was analyzed to determine the level of interleukin (IL) 6 (cat. no. RK00020;Abclonal), IL-1β (cat. no. RK00009; Abclonal), and tumor necrosis factor α (TNF-α) (cat. no. RK00029;Abclonal) using an ELISA Kit. Optical density (OD) values were determined using a microplate reader (Spectra Max i3 Molecular Devices).
Macrophage Polarization
Peritoneal macrophages were collected from all groups. To accomplish polarization, we used F4/80-FITC (1:200, eBioscience, cat. no. 11-4801-81), CD16/32-PEcy7 (1:200, eBioscience, cat. no. A14719), and CD206-APC (1:200, eBioscience, cat. no.17-2061-82) in a cocktail. The cells were primarily gated with F4/80+ markers for macrophages. M1 macrophages were gated on F4/80+ and CD16/32+ cell surface markers, whereas M2 macrophages were gated on F4/80+ and CD206+ cell surface markers. Cells were counted using a Gallios Flow cytometer (Beckman, NY), and data were analyzed using Kaluza Analysis 2.0.
Statistical Analysis
All data is presented as mean ± SD, and analyses were performed with SPSS 20.0 software. The multigroup comparisons were conducted by a one-way analysis of variance followed by Fisher's least significant difference (LDS) post hoc test. Values of P < 0.05 were considered to indicate a statistically significant difference.
RESULTS
Latifolin Attenuates LDH Activity in Cardiac Tissue
Myocardial injury was assessed by LDH in cardiac tissue. The result showed that LDH was significantly elevated (P < 0.01) after intraperitoneal administration of DOX compared with the control group. However, latifolin significantly reduced (P < 0.05) LDH levels at 100 mg/kg compared with the DOX group (Fig. 1B). LDH results suggest that latifolin has a protective effect on heart damage caused by DOX.
Latifolin Improved Cardiac Function
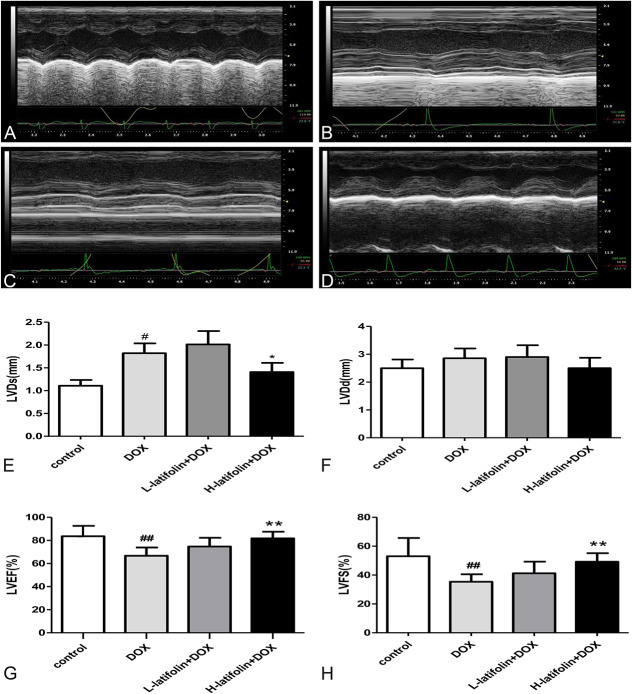
Echocardiography was used to evaluate cardiac function 1 day before the animals were sacrificed. As shown in Figure 2, cardiac systolic and diastolic functions were high in the control group. DOX was shown to decrease cardiac systolic function compared with the control group (Fig. 2B), as demonstrated by significant decreases (P < 0.01) in LVEF and LVFS. Compared with the DOX group, however, latifolin (100 mg/kg) significantly improved cardiac systolic function (Fig. 2D), whereby LVEF and LVFS having increased significantly (P < 0.01, Figs. 2G, H).
FIGURE 2.
Echocardiography was used to evaluate cardiac function. Latifolin significantly improved cardiac function, and LVEF and LVFS were increased significantly. A, Normal heart function of control group; (B) DOX decreased cardiac systolic function; (C) low-dose latifolin (50 mg/kg) group; (D) high-dose latifolin (100 mg/kg) group; (E) left ventricular systolic diameter result; (F) left ventricular diastolic diameter result. (G) left ventricular ejection fraction result; (H) left ventricular fractional shortening result. Data were shown as mean ± SD, n = 8. ##P < 0.01 versus control group, **P < 0.01 versus DOX group.
Histological Analysis
In the control group, histological analysis of heart tissues revealed normal myocardial architecture with anastomosing cardiac myofibers, and each cardiomyocyte contained a central, oval, and euchromatic nuclei (Fig. 3A). The DOX-treated group showed dispersed vacuoles (black arrow) in the sarcoplasm, slight fragmentation, and degeneration of the myofibrillar (Fig. 3B). The histological sections of the latifolin groups showed reorganization of myocardial architecture with less vacuoles in the sarcoplasm and myofibrillar loss (Figs. 3C, D).
FIGURE 3.
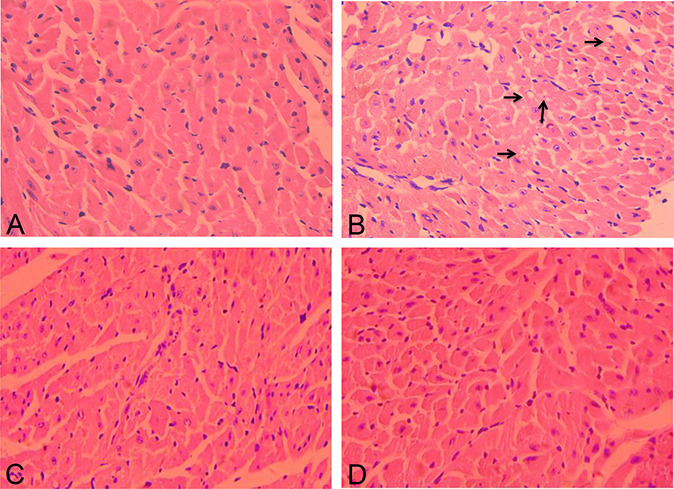
The pathological structure indicated by hematoxylin and eosin staining in control and different treatment groups (hematoxylin and eosin, ×200) (n = 3). A, Normal myocardial architecture of control group. (B) The DOX group showed vacuoles sarcoplasm, fragmentation, and degeneration of the myofibrillar, nuclei fade and detachment. (C) The latifolin (50 mg/kg) group showed mild degeneration of myocardial tissues; (D) the latifolin (100 mg/kg) group showed well-organized cardiac myofibers and nuclei profile.
Latifolin Influences Macrophage M1/M2 Polarization in Cardiac Tissue
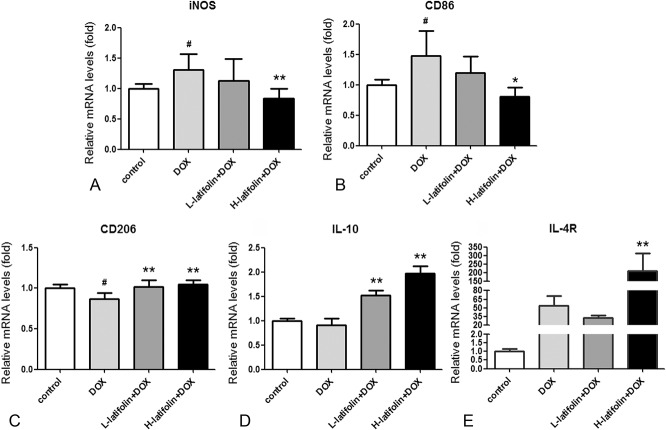
The messenger RNA (mRNA) expression of macrophage M1/M2 polarized marker in cardiac tissue was detected by RT-PCR. The results showed that the DOX group significantly increased (P < 0.05) the expression levels of M1 markers iNOS and CD86, whereas the M2 marker CD206 was significantly decreased (P < 0.05), compared with the control group. Compared with the DOX group, the high-dose latifolin (100 mg/kg) group significantly decreased iNOS and CD86 gene expression (P < 0.01; P < 0.05; Figs. 4A, B) and significantly increased the expression of CD206, IL-10, and IL-4R in myocardial tissue (P < 0.01; Figs. 4C–E).
FIGURE 4.
The mRNA expression levels of macrophages M1/M2 polarization marker in cardiac tissue. Latifolin reduced mRNA expression of M1 polarization marker (A) iNOS and (B) CD86 and increased mRNA expression of M2 polarization marker (C) CD206, (D) IL-10, and (E) IL-4R. Data were shown as mean ± SD, n = 3. #P < 0.05 versus control group, *P < 0.05, **P < 0.01 versus model group.
Peritoneal Macrophage Identification
CD11b-PE fluorescence signal was collected by flow cytometry to identify macrophages. The results showed an abundance of macrophages, which accounted for 90.0% of the cell population (Fig. 5A). Macrophages (gate A) were positive for CD11b-PE fluorescence (Fig. 5B).
FIGURE 5.
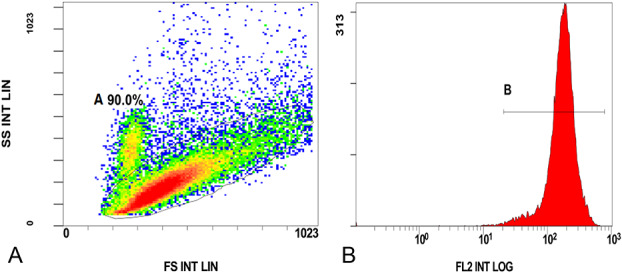
Flow cytometry identification of peritoneal macrophages. A, Peritoneal macrophages accounted for 90.0% in the population cells. (B) Macrophages were positive for CD11b-PE fluorescence.
Latifolin Influenced Macrophage Secretome in Vitro
The effects of latifolin on macrophage secretome were assessed in vitro and analyzed by nzyme-linked immunosorbent assay. The results are shown in Figure 6. Compared with the control group, the model group significantly increased (P < 0.05) inflammatory cytokines IL-6, IL-1β, and TNF-α level. However, compared with the model group, IL-6 and IL-1β were significantly decreased in the 10 μg/mL latifolin group (P < 0.05, Figs. 6A, B). The 2.5 μg/mL latifolin group was also shown to significantly decrease the TNF-α level (P < 0.05; Fig. 6C).
FIGURE 6.
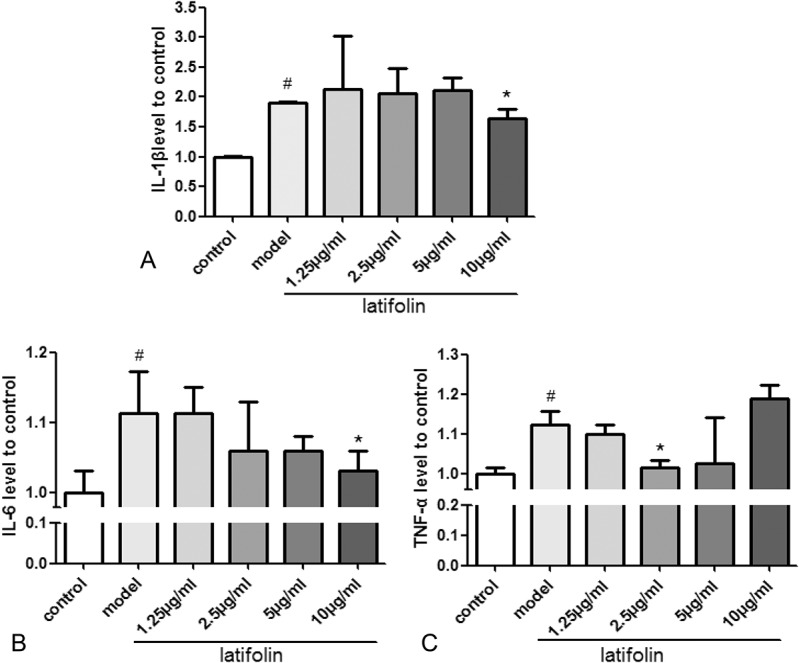
The effects of latifolin on macrophage secretome in vitro. Latifolin reduces inflammatory cytokine (A) IL-6; (B) IL-1β; (C) TNF-α level. Data were shown as mean ± SD, n = 3. #P < 0.05 versus control, *P < 0.05 versus model group.
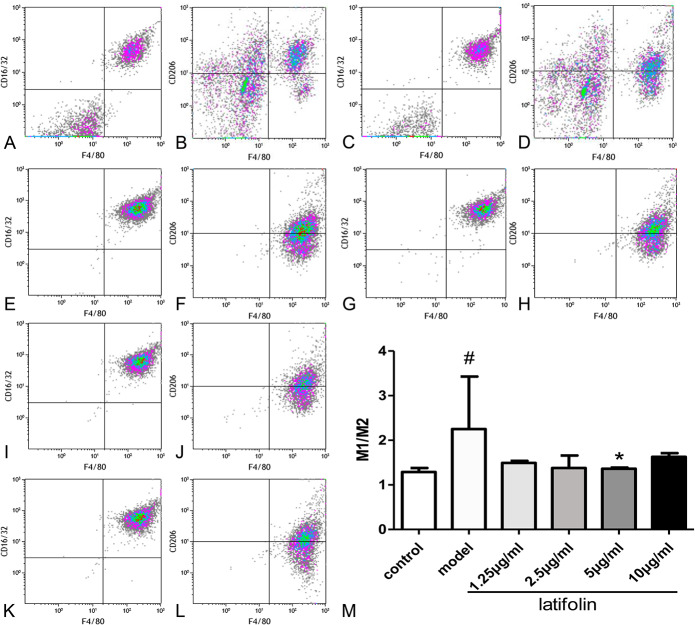
Latifolin Modulated Macrophage Polarization
Flow cytometry was used to determine the effect of latifolin on macrophage polarization. F4/80-FICT (M1, M2), CD16/32-PE cy7 (M1), and CD206-APC (M2) fluorescence signal were collected to analyze macrophage polarization. As shown in Figure 7, the M1:M2 ratio in the model group was significantly higher (P < 0.05) than that in the normal group. However, the M1:M2 ratio was significantly decreased after treatment of latifolin at 5 μg/mL (Fig. 7M; P < 0.05).
FIGURE 7.
The effect of latifolin on the M1 and M2 polarization of peritoneal macrophages. Latifolin decreased the M1:M2 ratio on the concentration of 5 μg/mL. A, M1 polarization of control group; (B) M2 polarization of control group; (C) M1 polarization of model group; (D) M2 polarization of model group; (E) M1 polarization of 10 μg/mL group; (F) M2 polarization of 10 μg/mL group; (G) M1 polarization of 5 μg/mL group; (H) M2 polarization of 5 μg/mL group; (I) M1 polarization of 2.5 μg/mL group; (J) M2 polarization of 2.5 μg/mL group; (K) M1 polarization of 1.25 μg/mL group; (L) M2 polarization of 1.25 μg/mL group; (M) M1:M2 ratio. Data were shown as mean ± SD, n = 3. #P < 0.05, versus control, *P < 0.05, versus model group.
DISCUSSION
This results from this study demonstrated that latifolin, a new flavonoid, exhibited a protective effect on myocardial injury induced by DOX. Latifolin was shown to reduce LDH levels in cardiac tissue and increased LVEF and LVFS. RT-PCR was used to detect the expression of M1 and M2 markers in the tissue. It was shown that latifolin decreased the gene expression of M1 markers iNOS and CD86 and increased the genetic expression of M2 markers CD206, IL-10, and IL-4R. Furthermore, our in vitro studies showed that latifolin significantly reduced the secretion of IL-6, IL-1β, and TNF-α in LPS + IFN-γ–stimulated peritoneal macrophages (P < 0.05). The results from the flow cytometry assessment showed that latifolin significantly reduced the ratio of M1 to M2 in peritoneal macrophages.
In the first few days after DOX-induced myocardial injury, inflammatory activity is high in the injured myocardial tissue, and the mononuclear-macrophage system is particularly active during the development and progression of inflammation.15 Under such conditions, monocytes from blood are recruited into the tissues and are differentiated into macrophages. Macrophages can adopt 1 of the 2 well-established polarized phenotypes, depending on the microenvironment. Polarized phenotypes are often described as classically activated macrophages (M1 macrophages) or alternatively activated macrophages (M2 macrophages).16 Continued dominance of M1 macrophages can obstruct tissue regeneration and may have serious consequences such as ventricular septal defect, infarct rupture, acute mitral regurgitation, aneurysm formation, and heart failure. M2 macrophages reduce inflammation and support tissue regeneration.17 It is known that the M1/M2 macrophage polarization balance regulates the fate of an organ in inflammation or injury.18 Jadapalli et al19 reported that the LL-DOX (7.5 mg/kg per week) and AH-DOX (15 mg/kg per week) caused myocardial apoptosis and cardiac dysfunction. It has also been shown that DOX triggers splenic contraction and irreversible dysregulation of cyclooxygenase (COX) and lipoxygenase (LOX), which alter the inflammation-resolution program in the myocardium with decreasing CD169+ metallophilic macrophages. Huang et al20 also reported that glabridin, one of the most studied licorice flavonoid, prevents doxorubicin-induced cardiotoxicity through colonic macrophage polarization in mice. In this study, glabridin was shown to decrease the ratio of M1 to M2 colonic macrophages and also decreased the production of M1 cytokines, such as IL-1β and TNF-α, whereas increasing the production of M2 cytokines, such as IL-10 and TGF-β, in the colonic macrophages by downregulating NF-κB and upregulating STAT6. In our current study, it was also found that latifolin significantly increased LVEF and LVFS percentages (P < 0.01) and significantly decreased the LDH content (P < 0.05), thereby demonstrating that latifolin can significantly improve cardiac function and reduce myocardial damage. Moreover, we showed that latifolin decreased the genetic expression of M1 markers, iNOS and CD86, and increased the genetic expression of M2 markers CD206, IL-10, and IL-4R in myocardial tissue. Furthermore, flow cytometry results also showed that latifolin can significantly reduce the ratio of M1 to M2 of peritoneal macrophages. These results indicate that the protective effect of latifolin on DOX-induced cardiac injury is related to its influence on macrophage M1/M2 polarization.
The M1/M2 nomenclature originated from the cytokines that are connected to these macrophage phenotypes. Classically activated M1 macrophages are proinflammatory and produce proinflammatory cytokines such as IL-1β, IL-6, IL-12, IL-23, and TNF-α. Alternatively, activated M2 macrophages are anti-inflammatory and produce anti-inflammatory cytokines such as IL-10 and TGF-β.9 Furthermore, reports showed that DOX also enhances inflammatory responses in the myocardium by activating the expression of NF-κB, which is a key regulator for immune/inflammatory responses. DOX induces the production of cytokines, such as TNF-α and nitric oxides, which are closely related to M1 polarization.21 Koreachina et al22 found that latifolin played a critical role in anti-inflammatory effects in macrophages, and it significantly inhibited TNF-α, IL-1βproduction in primary murine peritoneal macrophages exposed to LPS. In our current study, it was also found that latifolin significantly reduced the secretion of IL-6, IL-1β, and TNF-α in LPS + IFN-γ–stimulated peritoneal macrophages. It indicates that latifolin reduced proinflammatory cytokines and blocked inflammatory responses caused by DOX.
The surface markers of M1 macrophages include CD11b, F4/80, CD68, CD16/32, and CD86, whereas those of M2 macrophage include CD11b, F4/80, and CD206. M1 and M2 can be transformed into each other.23 However, there is evidence to believe that M1 and M2 phenotypes might not be stably differentiated subsets. In vitro assessments showed that macrophages activated by LPS for a few hours are unable to reactivate a large part of the proinflammatory genes. However, they maintain the ability to stimulate the expression of many other genes, including IL-10.24 This altered state of reactivity, commonly described as endotoxin tolerance, results in an overall and continuous switch of the gene expression program from proinflammatory M1 signature to M2 anti-inflammatory phenotype. Ben-Mordechai et al25 studied the protective effects of Mesenchymal stem cells on infarct repair mediated by macrophages. They found that the percent of M1 plus M2 was sometimes >100%, suggesting that some “intermediate” macrophages expressed both M1 and M2 markers during transition from M1 to M2. In our present study, we found that the percentage of M1 plus M2 was about 100% in the normal group, whereas the percentage of M1 plus M2 was significantly >100% after LPS+ IFN-γ stimulation. In addition, the DOX group did not significantly decrease the expression levels of the M2 marker IL-10 and IL-4R compared with the normal group. Our results suggest that M1 and M2 phenotypes might not be stably differentiated and that some “intermediate” macrophages expressed both M1 and M2 markers.
Although our data provide strong evidence that latifolin could prevent DOX-induced cardiotoxicity by regulating M1 and M2 polarization, there are some limitations of this study. Although the polarization of M1 and M2 can be directly differentiated by mononuclear macrophages, and M1 and M2 can also transform each other; it cannot be ascertained from this study whether latifolin directly affects mononuclear macrophages or the transformation between M1 and M2. The mechanism through which latifolin exerts its effects on M1 and M2 polarization is also still unclear. Further investigations are therefore required in this area.
Collectively, the results from the current study demonstrate the cardioprotective effects of latifolin against DOX-induced cardiotoxicity through anti-inflammatory properties by M1 and M2 polarization. Latifolin (100 mg/kg) not only was effective in preventing cardiac dysfunction that was stimulated by DOX but also provided evidence of macrophage polarization in mice. These findings suggest that the benefits of using latifolin in chemotherapeutic treatments to prevent DOX-induced cardiotoxicity, which if left unabated could lead to serious cardiac malfunctions.
ACKNOWLEDGMENTS
The authors thank R. Liu and L. Chen. They are the guarantors of this work and had full access to all the data in the study and took responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Supported by National Key R&D Program of China (2018YFC1706102), the National Natural Science Foundation of China (No. 81660676; 81360629), Natural Science Foundation of Jiangxi Support (20171BAB205096) and Provincial Key R&D Projects of Jiangxi (JXSYLXK-ZHYAO124).
The authors report no conflicts of interest.
N. Zhang and B. Shou contribute equally to this article. N. Zhang, B. Shou, and X. Lai performed the research. N. Zhang, B. Shou, and L. Chen designed the research study. Y. Luo, W. Meng, and R. Liu contributed essential reagents or tools. N. Zhang, B. Shou, X. Lai, Y. Cui, and L. Chen analyzed the data. N. Zhang and L. Chen wrote the article.
REFERENCES
- 1.Bisogno G, Jenney M, Bergeron C, et al. Addition of dose-intensified doxorubicin to standard chemotherapy for rhabdomyosarcoma (EpSSG RMS 2005): a multicentre, open-label, randomised controlled, phase 3 trial. Lancet Oncol. 2018;19:1061–1071. [DOI] [PubMed] [Google Scholar]
- 2.Li DL, Wang ZV, Ding G, et al. Doxorubicin blocks cardiomyocyte autophagic flux by inhibiting lysosome acidification. Circulation. 2016;133:1668–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Galán-Arriola C, Lobo M, Vílchez-Tschischke JP, et al. Serial magnetic resonance imaging to identify early stages of anthracycline-induced cardiotoxicity. J Am Coll Cardiol. 2019;73:779–791. [DOI] [PubMed] [Google Scholar]
- 4.Varga ZV, Ferdinandy P, Liaudet L, et al. Drug-induced mitochondrial dysfunction and cardiotoxicity. Am J Physiol Heart Circ Physiol. 2015;309:H1453–H1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hajra S, Patra AR, Basu A, et al. Prevention of doxorubicin (DOX)-induced genotoxicity and cardiotoxicity: effect of plant derived small molecule indole-3-carbinol (I3C) on oxidative stress and inflammation. Biomed Pharmacother. 2018;101:228–243. [DOI] [PubMed] [Google Scholar]
- 6.Du Q, Zhu B, Zhai Q, et al. Sirt3 attenuates doxorubicin-induced cardiac hypertrophy and mitochondrial dysfunction via suppression of Bnip3. Am J Transl Res. 2017;9:3360–3373. [PMC free article] [PubMed] [Google Scholar]
- 7.Guo RM, Xu WM, Lin JC, et al. Activation of the p38 MAPK/NF-κB pathway contributes to doxorubicin-induced inflammation and cytotoxicity in H9c2 cardiac cells. Mol Med Rep. 2013;8:603–608. [DOI] [PubMed] [Google Scholar]
- 8.Chen B, Frangogiannis NG. Immune cells in repair of the infarcted myocardium. Microcirculation. 2017;24:e12305. [DOI] [PubMed] [Google Scholar]
- 9.Shapouri-Moghaddam A, Mohammadian S, Vazini H, et al. Macrophage plasticity, polarization, and function in health and disease. J Cel Physiol. 2018;233:6425–6440. [DOI] [PubMed] [Google Scholar]
- 10.Horckmans M, Ring L, Duchene J, et al. Neutrophils orchestrate post-myocardial infarction healing by polarizing macrophages towards a reparative phenotype. Eur Heart J. 2017;38:187–197. [DOI] [PubMed] [Google Scholar]
- 11.Yang ZH, Mei C, He XH, et al. Advance in studies on chemical constitutions, pharmacological mechanism and pharmacokinetic profile of dalbergiae odoriferae lignum. China J Chin Mater Med. 2013;38:1679–1683. [PubMed] [Google Scholar]
- 12.Mu F, Duan J, Bian H, et al. Cardioprotective effects and mechanism of Radix Salviae miltiorrhizae and lignum Dalbergiaeodoriferae on rat myocardial ischemia/reperfusion injury. Mol Med Rep. 2017;16:1759–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin R, Duan J, Mu F, et al. Cardioprotective effects and underlying mechanism of Radix Salvia miltiorrhiza and Lignum Dalbergia odorifera in a pig chronic myocardial ischemia model. Int J Mol Med. 2018;42:2628–2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li XL, Chen LY, Guan ZY, et al. Effect of neoflavonoid latifolin isolated from Dalbergia odorifera on acute myocardial ischemia in rats and its mechanism of Nrf2 signaling pathway. Zhongguo Zhong Yao Za Zhi. 2017;42:3974–3982. [DOI] [PubMed] [Google Scholar]
- 15.Sanmarco LM, Eberhardt N, Ponce NE, et al. New insights into the immunobiology of mononuclear phagocytic cells and their relevance to the pathogenesis of cardiovascular diseases. Front Immunol. 2018;8:1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Courties G, Heidt T, Sebas M, et al. In vivo silencing of the transcription factor IRF5 reprograms the macrophage phenotype and improves infarct healing. J Am Coll Cardiol. 2014;63:1556–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferrante CJ, Leibovich SJ. Regulation of macrophage polarization and wound healing. Adv Wound Care. 2012;1:10–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alvarez MM, Liu JC, Trujillo-de Santiago G, et al. Delivery strategies to control inflammatory response: modulating M1-M2 polarization in tissue engineering applications. J Control Release. 2016;240:349–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jadapalli JK, Wright GW, Kain V, et al. Doxorubicin triggers splenic contraction and irreversible dysregulation of COX and LOX that alters inflammation-resolution program in the myocardium [J]. Am J Physiol Heart Circ Physiol. 2018;315:H1091–H1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang K, Liu Y, Tang H, et al. Glabridin prevents doxorubicin-induced cardiotoxicity through gut microbiota modulation and colonic macrophage polarization in mice. Front Pharmacol. 2019;10:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Montecucco F, Liberale L, Bonaventura A, et al. The role of inflammation in cardiovascular outcome. Curr Atheroscler Rep. 2017;19:11. [DOI] [PubMed] [Google Scholar]
- 22.Koreachina I, Inve W. The neoflavonoid latifolin isolated from MeOH extract of Dalbergia odorifera attenuates inflammatory responses by inhibiting NF-κB activation via Nrf2-mediated Heme oxygenase-1 expression[J]. Phytotherapy Res. 2014;28:1216–1223. [DOI] [PubMed] [Google Scholar]
- 23.Stafeev IS, Menshikov MY, Tkachuk VA, et al. The role of macrophages in repair of injured myocardium and perspectives of metabolic reprogramming of immune cells for myocardial post-infarction recovery. Kardiologiia. 2017;57:53–59. [PubMed] [Google Scholar]
- 24.Lawrence T, Natoli G. Ranscriptional regulation of macrophage polarization: enabling diversity with identity. Nat Rev Immunol. 2011;11:750–761. [DOI] [PubMed] [Google Scholar]
- 25.Ben Mordechai T, Holbova R, Landa Rouben N, et al. Macrophage subpopulations are essential for infarct repair with and without stem cell therapy. J Am Coll Cardiol. 2013;62:1890–1901. [DOI] [PubMed] [Google Scholar]